Abstract
C24H25NO3PRh, orthorhombic, P212121 (no. 19), a = 9.5498(1) Å, b = 13.4996(2) Å, c = 17.428(3) Å, V = 2246.7(5) Å3, Z = 4, Rgt(F) = 0.0163, wRref(F2) = 0.0376, T = 100 K.
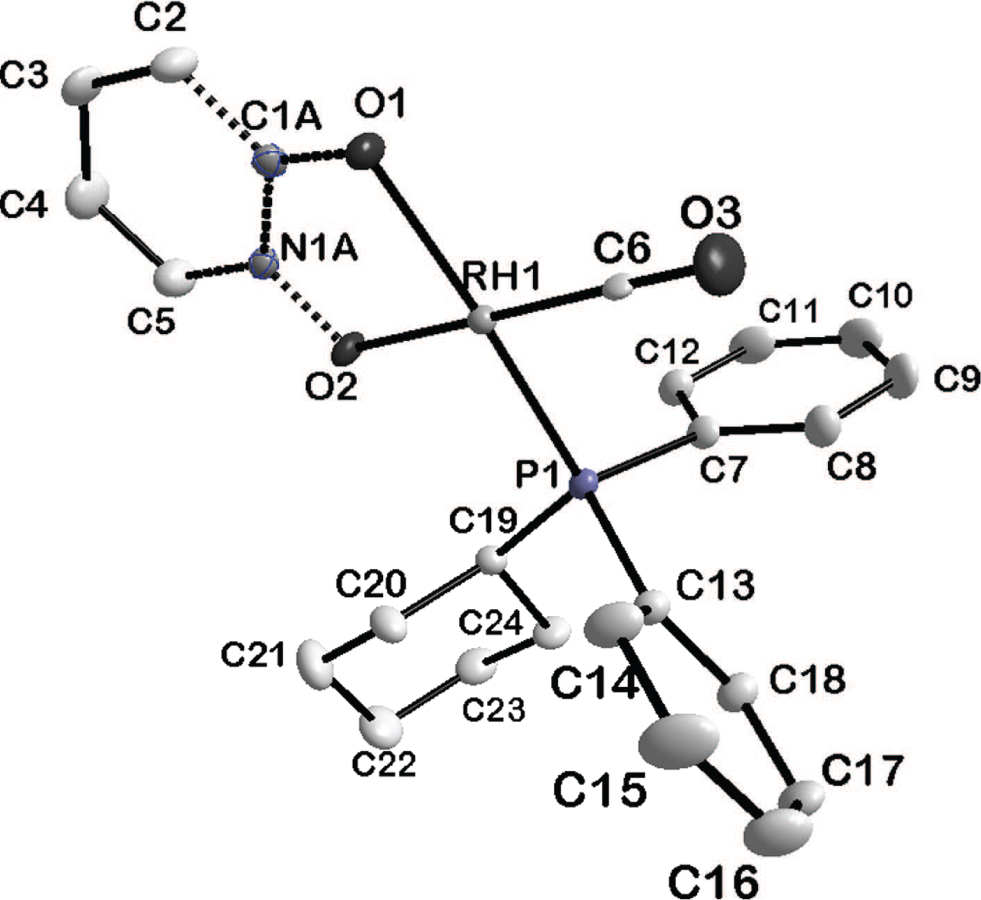
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow cuboid |
| Size: | 0.25 × 0.21 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 8.6 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 56°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 62189, 5394, 0.040 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5214 |
| N(param)refined: | 273 |
| Programs: | SHELX [1], Bruker programs [2], DIAMOND [3], WinGX [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C18 | 0.20115(18) | 0.35714(12) | 0.21110(10) | 0.0194(3) |
| H18 | 0.1819 | 0.3509 | 0.2644 | 0.023* |
| C19 | −0.11103(18) | 0.21929(12) | 0.22526(9) | 0.0166(3) |
| H19 | −0.1653 | 0.1590 | 0.2403 | 0.020* |
| C8 | 0.29728(19) | 0.14995(13) | 0.28919(11) | 0.0232(4) |
| H8 | 0.3478 | 0.1871 | 0.2521 | 0.028* |
| C9 | 0.3669(2) | 0.11000(14) | 0.35180(11) | 0.0307(4) |
| H9 | 0.4645 | 0.1210 | 0.3578 | 0.037* |
| C24 | −0.11277(19) | 0.28726(12) | 0.29514(10) | 0.0205(4) |
| H24A | −0.0619 | 0.2553 | 0.3380 | 0.025* |
| H24B | −0.0645 | 0.3501 | 0.2827 | 0.025* |
| C12 | 0.08191(19) | 0.07910(13) | 0.33483(10) | 0.0214(4) |
| H12 | −0.0156 | 0.0675 | 0.3290 | 0.026* |
| C16 | 0.3001(2) | 0.44907(14) | 0.10631(11) | 0.0325(4) |
| H16 | 0.3500 | 0.5051 | 0.0879 | 0.039* |
| C6 | 0.22432(18) | 0.05540(13) | 0.09787(10) | 0.0203(3) |
| C17 | 0.27413(19) | 0.43927(12) | 0.18420(10) | 0.0231(4) |
| H17 | 0.3060 | 0.4884 | 0.2191 | 0.028* |
| C13 | 0.15609(17) | 0.28388(12) | 0.16034(10) | 0.0163(3) |
| C23 | −0.2624(2) | 0.30874(14) | 0.31938(11) | 0.0262(4) |
| H23A | −0.3075 | 0.2465 | 0.3364 | 0.031* |
| H23B | −0.2618 | 0.3552 | 0.3634 | 0.031* |
| C21 | −0.3409(2) | 0.29003(16) | 0.18212(12) | 0.0319(5) |
| H21A | −0.3924 | 0.2275 | 0.1911 | 0.038* |
| H21B | −0.3878 | 0.3256 | 0.1396 | 0.038* |
| C10 | 0.2951(2) | 0.05411(15) | 0.40569(10) | 0.0325(4) |
| H10 | 0.3437 | 0.0261 | 0.4480 | 0.039* |
| C14 | 0.1805(2) | 0.29567(13) | 0.08229(11) | 0.0257(4) |
| H14 | 0.1470 | 0.2475 | 0.0470 | 0.031* |
| C20 | −0.19155(19) | 0.26614(13) | 0.15879(10) | 0.0220(4) |
| H20A | −0.1437 | 0.3277 | 0.1424 | 0.026* |
| H20B | −0.1926 | 0.2199 | 0.1147 | 0.026* |
| C22 | −0.3471(2) | 0.35338(15) | 0.25441(12) | 0.0326(5) |
| H22A | −0.3108 | 0.4204 | 0.2428 | 0.039* |
| H22B | −0.4459 | 0.3603 | 0.2709 | 0.039* |
| C7 | 0.15315(17) | 0.13577(12) | 0.28049(9) | 0.0166(3) |
| C15 | 0.2541(2) | 0.37814(15) | 0.05559(11) | 0.0345(5) |
| H15 | 0.2725 | 0.3852 | 0.0023 | 0.041* |
| C11 | 0.1529(2) | 0.03927(14) | 0.39786(9) | 0.0277(4) |
| H11 | 0.1033 | 0.0020 | 0.4353 | 0.033* |
| O1 | −0.00822(11) | −0.08180(8) | 0.05211(6) | 0.0177(2) |
| O2 | −0.17128(11) | 0.03295(9) | 0.13536(6) | 0.0171(2) |
| O3 | 0.34334(13) | 0.06222(11) | 0.08705(9) | 0.0361(4) |
| P1 | 0.06165(4) | 0.17455(3) | 0.19372(2) | 0.01312(8) |
| Rh1 | 0.039656(12) | 0.045707(8) | 0.115510(7) | 0.01218(3) |
| N1 | −0.22715(14) | −0.04344(11) | 0.09621(7) | 0.0166(3) |
| C1 | −0.14052(17) | −0.10247(11) | 0.05412(9) | 0.0127(3) |
| C5 | −0.36880(18) | −0.05673(13) | 0.09785(9) | 0.0212(4) |
| H5 | −0.4259 | −0.0133 | 0.1272 | 0.025* |
| C2 | −0.20179(19) | −0.18112(13) | 0.01427(10) | 0.0216(4) |
| H2 | −0.1438 | −0.2248 | −0.0144 | 0.026* |
| C3 | −0.3429(2) | −0.19665(13) | 0.01551(11) | 0.0239(4) |
| H3 | −0.3827 | −0.2506 | −0.0118 | 0.029* |
| C4 | −0.42852(19) | −0.13216(13) | 0.05752(11) | 0.0250(4) |
| H4 | −0.5272 | −0.1409 | 0.0579 | 0.030* |
Source of materials
[Rh(hopo)(CO)2] (hopo = 2-oxopyridine-N-oxide) was synthesized according to the method described previously [5]. [Rh(hopo)(CO)2(PPh2Cy)] was synthesized by dissolving [Rh(hopo)(CO)2] (0.1001 g, 0.3721 mmol) in 5 cm3 of acetone. Diphenylcyclohexylphosphine (PPh2Cy) (0.1198 g, 0.4465 mmol) was added to the aforementioned solution with stirring. Some ice water was added dropwise to precipitate the product. Yellow cuboid crystals, suitable for X-ray diffraction were obtained from recrystallization in acetone and a few drops of water.
IR: νCO 1974 cm−1. 1H-NMR (600 MHz, CD2Cl2) δ 8.06 (d, 1J = 6.4 Hz, 1H), 7.90 – 7.77 (m, 10H), 7.74 (d, 1J = 6.4 Hz, 10H), 7.47 (s, 1H), 7.31 (dd, 2J = 14.6, 1J = 7.0 Hz, 1H), 7.27 (t, 1J = 7.6 Hz, 1H), 6.93 (d, 1J = 8.6 Hz, 1H), 6.71 (d, 1J = 8.4 Hz, 1H), 6.55 (t, 1J = 6.5 Hz, 1H), 6.46 (t, 1J = 6.5 Hz, 1H), 1.96 (dd, 2J = 534.0, 1J = 200.8 Hz, 22H).13C-NMR (75 MHz, CD2Cl2) δ 191.41 – 188.70 (m), 162.69 (s), 133.57 (d, J = 10.4 Hz), 133.21 (s), 132.57 (s), 131.91 (s), 130.05 (s), 128.09 (d, J = 10.0 Hz), 116.07 (s), 109.89 (d, J = 67.7 Hz), 35.97 (s), 28.57 (s), 26.88 (d, J = 13.0 Hz), 26.18 (s). 31P-NMR (243 MHz, CD2Cl2) δ 54.07 (d, 1J (PRh major isomer) = 175.3 Hz), 53.83 (d, 1J (PRh minor isomer) = 166.8 Hz).
Experimental details
The H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with Uiso(H) = 1.2Ueq (Caromatic). The highest peak is located 1.18 Å from O3 and the deepest hole is situated 1.01 Å from Rh1. The C1 and N1 atoms are disordered in a 0.8/0.2 ratio with the higher occupancy attributed to the isomer with PPh2Cy cis to N. Some unusual ellipsoids in the direct vicinity of the Rh metal are caused by some small problems with the absorption correction.
Comment
The oxidative addition of iodomethane to Rh(I) complexes to form alkyl and acyl product is an important step in the catalytic process and is used on a massive scale in industry to convert methanol and carbon monoxide to acetic acid in Monsanto process [6, 7] . In the precursor [Rh(BID)(CO)2] complexes (where BID represents different monoanionic bidentate ligands such as cupferrate and 2-oxopyridine N-oxide, etc.) one of the two carbonyl can be substituted by tertiary phosphine ligands (PR3) to form [Rh(BID)(CO)(PR3)] complexes. These complexes have been intensively studied as possible candidates for catalytic processes [8], [9], [10]. In this study the complex [Rh(hopo)(CO)(PPh2Cy)] (hopo = 2-oxopyridine N-oxide) has been synthesized to study the electronic and steric influence of the phosphine ligands on the oxidative addition of methyl iodide.
The complex has a distorted square planar geometry around the Rh centre indicated by the small bite angle of 78.7(1)° of the five membered ring. Two isomers are present in a 80/20 ratio, with the principal isomer PPh2Cy cis to the N atom. This was confirmed by 1H, 13C, and 31P NMR spectra of the complex. The effective cone angle, θE [11], of 156.48(2)° is similar to related compounds angles which are in the range of 154–157° [12], [13], [14], [15].
Acknowledgement
This work is based on the research supported in part by the National Research Foundation of South Africa for the grant, Unique Grant No. 93957, The authors would like to thank Prof. Andreas Roodt and the University of the Free State for financial support.
References
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
Bruker. SAINT-Plus (Version 7.12) and SADABS (Version 2004/1). Bruker AXS Inc., Madison, Wisconsin, USA, (2004).Search in Google Scholar
Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Version 3.0c Crystal Impact, Bonn, Germany, (2005).Search in Google Scholar
Farrugia, L. J.: WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 32 (1999) 837–838.10.1107/S0021889899006020Search in Google Scholar
Elmakki, M. A.; Koen, R.; Venter, J. A.; Drost, R.: Crystal structure of dicarbonyl(pyridine-2-olate-1-oxido-κ2O,O′)rhodium(I), C7H4NO4Rh. Z. Kristallogr. − NCS 231 (2016) 703–705.10.1515/ncrs-2015-0240Search in Google Scholar
Venter, J. A.; Leipoldt, J. G.; Eldik, R. V.: Solvent, temperature, and pressure dependence of oxidative addition of iodomethane to complexes of the type RhI(β-diketone)(CO)(PPh3). Inorg. Chim. 30 (1991) 2207–2209.10.1021/ic00009a046Search in Google Scholar
Hallinan, N.; Hinnenkamp, J.: Catalysis of organic reactions. Chem. Ind. 82 (2001) 545.Search in Google Scholar
Basson, S. S.; Leipoldt, J. G.; Roodt, A.; Venter, J. A.: Mechanism for the oxidative addition of iodomethane to carbonyl (N-hydroxy-N-nitrosobenzenaminato-O,O′)-triarylphosphinerhodium(I) complexes and crystal structure of [Rh(cupf)(CO)(CH3)(I)(PPh3)]. Inorg. Chim. Acta 128 (1987) 31–37.10.1002/chin.198731295Search in Google Scholar
Uguagliati, P.; Deganello, G.; Belluco, U.: The system μ-dichlorotetracarbonyldirhodium − tertiary phosphines. Inorg. Chim. Acta 9 (1974) 203–207.10.1016/S0020-1693(00)89906-5Search in Google Scholar
Brink, A.; Roodt, A.; Steyl, G.; Visser, H. G.: Steric vs. electronic anomaly observed from iodomethane oxidative addition to tertiary phosphine modified rhodium(I) acetylacetonato complexes following progressive phenyl replacement by cyclohexyl [PR3 = PPh3, PPh2Cy, PPhCy2, PCy3]. Dalton Trans. 39 (2010) 5572–5578.10.1039/b922083fSearch in Google Scholar
Tolman, C. A.: Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev. 77 (1977) 313–348.10.1021/cr60307a002Search in Google Scholar
Venter, G. J. S.; Steyl, G.; Roodt, A.: Carbonyl[4-(2,6-dimethylphenylamino)pent-3-en-2-onato-κ2N,O] (triphenylphosphine-κP)rhodium(I). Acta Crystallogr. E65 (2009) m1606–1607.10.1107/S160053680904817XSearch in Google Scholar PubMed PubMed Central
Venter, G. J. S.; Steyl, G.; Roodt, A.: Carbonyl[4-(2,3-dimethylphenylamino)pent-3-en-2-onatoκ2N,O] (triphenylphosphine-κP)rhodium(I). Acta Crystallogr. E65 (2009) m1321–m1322.10.1107/S1600536809039816Search in Google Scholar PubMed PubMed Central
Venter, G. J. S.; Steyl, G.; Roodt, A.: Carbonyl-(4-(2,6-dichlorophenylamino)pent-3-en-2-onato-κ2N,O)-(triphenylphosphine-κP)-rhodium(I) acetonesolvate, C315H28Cl2NO2⋅5PRh. Z. Kristallogr. − NCS 228 (2013) 410–412.10.1524/ncrs.2013.0172Search in Google Scholar
Elmakki, M. A.; Koen, R.; Drost, R. M.; Alexander, O. T.; Venter, G. J. S.; Venter, J. A.: Crystal structure of carbonyl(2-oxopyridin-1(2H-olato-κ2O,O′)(triphenylphosphine-κP)rhodium(I), C24H19NO3PRh. Z. Kristallogr. − NCS 231 (2016) 781–783.10.1515/ncrs-2015-0266Search in Google Scholar
©2017 Mohammed A. Elmakki et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10

