Abstract
C52H36Cd2Cl4N4O10, triclinic, P1̄ (no. 2), a = 11.3651(8) Å, b = 12.9707(10) Å, c = 18.7281(14) Å, α = 79.327(1)°, β = 74.543(1)°, γ = 70.703(1)°, V = 2497.1(3) Å3, Z = 2, Rgt(F) = 0.0393, wRref(F2) = 0.0933, T = 293 K.
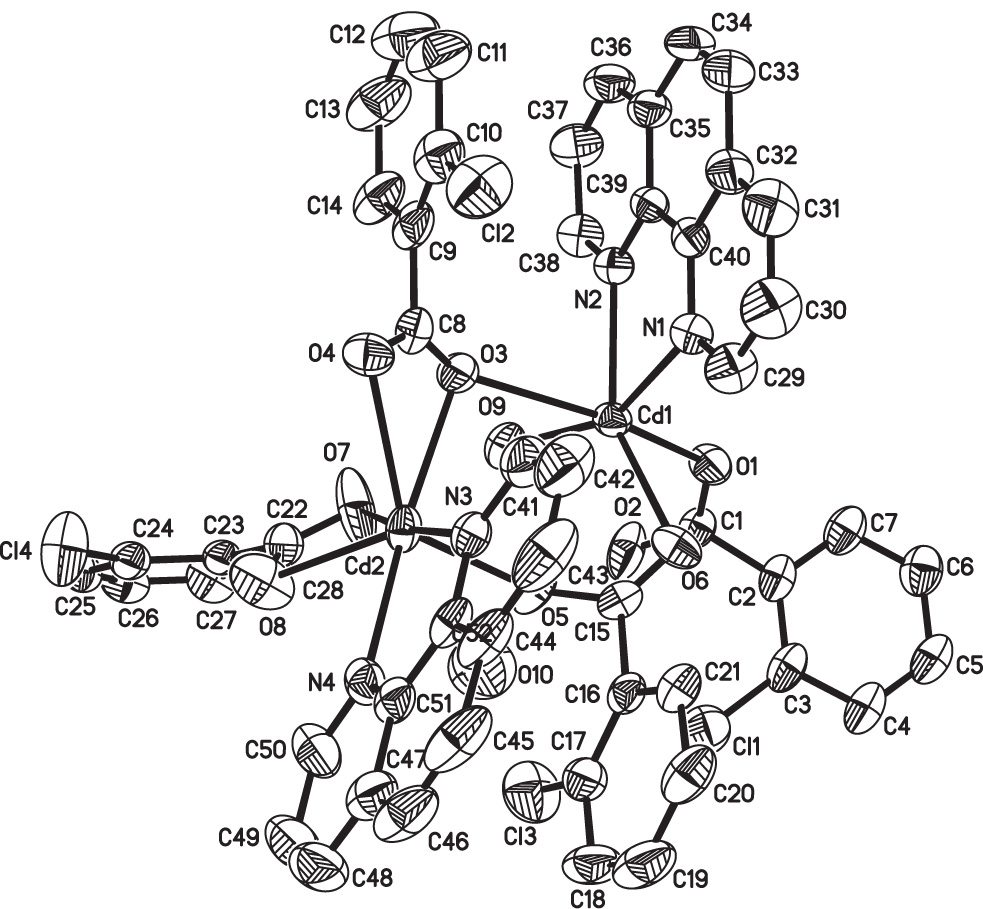
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.25 × 0.20 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 11.3 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 50.2°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 13176, 8786, 0.050 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 6947 |
| N(param)refined: | 782 |
| Programs: | SHELX [1], Bruker programs [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cd1 | 0.21644(3) | 0.17774(2) | 0.337088(15) | 0.05170(10) |
| Cd2 | 0.15039(3) | 0.41437(2) | 0.180239(16) | 0.05440(10) |
| Cl1a | 0.6810(2) | 0.02429(19) | 0.19125(12) | 0.1009(8) |
| C2a | 0.5952(8) | −0.0585(5) | 0.3312(5) | 0.0598(19) |
| C3a | 0.6808(8) | −0.0774(4) | 0.2630(4) | 0.0721(19) |
| C4a | 0.7653(6) | −0.1816(5) | 0.2520(3) | 0.088(2) |
| H4Aa | 0.8225 | −0.1942 | 0.2063 | 0.106* |
| C5a | 0.7642(6) | −0.2669(4) | 0.3091(4) | 0.085(2) |
| H5Aa | 0.8207 | −0.3366 | 0.3017 | 0.102* |
| C6a | 0.6786(6) | −0.2480(5) | 0.3772(3) | 0.087(2) |
| H6Aa | 0.6779 | −0.3051 | 0.4154 | 0.104* |
| C7a | 0.5942(7) | −0.1438(6) | 0.3883(4) | 0.079(2) |
| H7Aa | 0.5369 | −0.1312 | 0.4339 | 0.095* |
| Cl1Bb | 0.5643(4) | −0.1497(4) | 0.4607(3) | 0.124(2) |
| C2Bb | 0.5947(16) | −0.0565(12) | 0.3213(11) | 0.070(4) |
| C3Bb | 0.6269(14) | −0.1531(13) | 0.3680(8) | 0.076(3) |
| C4Bb | 0.7281(12) | −0.2421(9) | 0.3415(7) | 0.076(3) |
| H4Bb | 0.7497 | −0.3067 | 0.3728 | 0.092* |
| C5Bb | 0.7969(10) | −0.2346(9) | 0.2683(7) | 0.082(3) |
| H5Bb | 0.8646 | −0.2942 | 0.2506 | 0.099* |
| C6Bb | 0.7647(13) | −0.1380(11) | 0.2215(7) | 0.089(3) |
| H6Bb | 0.8107 | −0.1329 | 0.1725 | 0.107* |
| C7Bb | 0.6635(17) | −0.0489(9) | 0.2480(10) | 0.082(3) |
| H7Bb | 0.6420 | 0.0157 | 0.2167 | 0.098* |
| Cl2 | −0.28452(15) | 0.36103(15) | 0.32795(10) | 0.1148(5) |
| Cl4 | 0.15106(17) | 0.81331(12) | 0.16508(12) | 0.1258(6) |
| O1 | 0.3842(2) | 0.0457(2) | 0.36781(17) | 0.0729(9) |
| O2 | 0.5292(3) | 0.1337(3) | 0.3355(2) | 0.1020(13) |
| O3c | 0.0663(11) | 0.3670(9) | 0.3171(6) | 0.076(3) |
| O4c | −0.0826(9) | 0.4691(9) | 0.2574(6) | 0.071(2) |
| O5c | 0.3212(8) | 0.2561(8) | 0.1914(5) | 0.068(2) |
| O6c | 0.2806(10) | 0.0978(9) | 0.2181(7) | 0.076(3) |
| O7c | 0.2555(12) | 0.4756(11) | 0.2581(8) | 0.132(5) |
| O8c | 0.2204(11) | 0.5755(8) | 0.1575(5) | 0.113(3) |
| O3Bc | 0.0581(11) | 0.3317(8) | 0.2952(6) | 0.055(2) |
| O4Bc | −0.0363(8) | 0.5010(7) | 0.2553(5) | 0.067(2) |
| O5Bc | 0.2660(7) | 0.2367(7) | 0.2142(5) | 0.0509(18) |
| O6Bc | 0.3368(10) | 0.0575(8) | 0.2195(7) | 0.070(3) |
| O7Bc | 0.3091(10) | 0.4598(10) | 0.2170(6) | 0.073(3) |
| O8Bc | 0.1381(7) | 0.5924(5) | 0.1995(5) | 0.0719(18) |
| O9 | 0.3100(3) | 0.3001(2) | 0.35879(16) | 0.0715(8) |
| H9A | 0.3147 | 0.3557 | 0.3273 | 0.107* |
| H9B | 0.3854 | 0.2564 | 0.3554 | 0.107* |
| N1 | 0.0519(3) | 0.1025(3) | 0.34581(18) | 0.0562(8) |
| N2 | 0.0881(3) | 0.1883(3) | 0.45823(17) | 0.0526(8) |
| N3 | 0.0452(3) | 0.3390(2) | 0.11973(18) | 0.0544(8) |
| N4 | 0.2251(3) | 0.4427(3) | 0.0502(2) | 0.0632(9) |
| C1 | 0.4947(4) | 0.0503(3) | 0.3457(2) | 0.0577(10) |
| C8 | −0.0386(4) | 0.4211(3) | 0.3102(3) | 0.0599(10) |
| C9 | −0.1336(5) | 0.4324(4) | 0.3837(3) | 0.0725(13) |
| C10 | −0.2453(5) | 0.4081(4) | 0.3961(3) | 0.0873(15) |
| C11 | −0.3359(6) | 0.4182(6) | 0.4650(4) | 0.116(2) |
| H11A | −0.4102 | 0.3981 | 0.4736 | 0.139* |
| C12 | −0.3068(7) | 0.4596(7) | 0.5181(4) | 0.130(3) |
| H12A | −0.3646 | 0.4694 | 0.5638 | 0.156* |
| C13 | −0.1979(8) | 0.4870(6) | 0.5069(3) | 0.125(3) |
| H13A | −0.1850 | 0.5143 | 0.5459 | 0.150* |
| C14 | −0.1040(5) | 0.4772(4) | 0.4418(3) | 0.0927(17) |
| H14A | −0.0299 | 0.4968 | 0.4352 | 0.111* |
| C15 | 0.3198(4) | 0.1581(4) | 0.1781(2) | 0.0567(10) |
| Cl3a | 0.5485(2) | 0.2392(2) | 0.06877(17) | 0.1194(10) |
| C16a | 0.3561(7) | 0.1466(6) | 0.0939(2) | 0.055(2) |
| C17a | 0.4513(6) | 0.1844(5) | 0.0438(3) | 0.070(2) |
| C18a | 0.4765(6) | 0.1723(6) | −0.0315(3) | 0.095(3) |
| H18Aa | 0.5402 | 0.1976 | −0.0650 | 0.114* |
| C19a | 0.4064(7) | 0.1222(6) | −0.0567(2) | 0.102(3) |
| H19Aa | 0.4232 | 0.1140 | −0.1070 | 0.122* |
| C20a | 0.3112(6) | 0.0844(5) | −0.0065(3) | 0.086(2) |
| H20Aa | 0.2643 | 0.0509 | −0.0234 | 0.103* |
| C21a | 0.2861(6) | 0.0965(6) | 0.0687(3) | 0.067(2) |
| H21Aa | 0.2224 | 0.0712 | 0.1023 | 0.080* |
| Cl3Bb | 0.2167(5) | 0.0574(4) | 0.0808(3) | 0.0967(15) |
| C16Bb | 0.3703(14) | 0.1649(13) | 0.0995(5) | 0.054(4) |
| C17Bb | 0.3284(12) | 0.1236(11) | 0.0506(7) | 0.071(4) |
| C18Bb | 0.3798(13) | 0.1359(12) | −0.0255(6) | 0.096(5) |
| H18Bb | 0.3518 | 0.1083 | −0.0582 | 0.116* |
| C19Bb | 0.4730(13) | 0.1895(13) | −0.0528(5) | 0.104(5) |
| H19Bb | 0.5073 | 0.1977 | −0.1037 | 0.125* |
| C20Bb | 0.5149(11) | 0.2307(10) | −0.0039(7) | 0.099(5) |
| H20Bb | 0.5772 | 0.2666 | −0.0222 | 0.119* |
| C21Bb | 0.4635(13) | 0.2184(12) | 0.0722(6) | 0.084(5) |
| H21Bb | 0.4916 | 0.2461 | 0.1049 | 0.101* |
| C22 | 0.2515(5) | 0.5581(4) | 0.2149(3) | 0.0653(11) |
| C23 | 0.3118(4) | 0.6368(3) | 0.2305(2) | 0.0555(10) |
| C24 | 0.2748(4) | 0.7477(3) | 0.2107(2) | 0.0642(11) |
| C25 | 0.3357(5) | 0.8158(4) | 0.2268(3) | 0.0760(14) |
| H25A | 0.3087 | 0.8912 | 0.2135 | 0.091* |
| C26 | 0.4345(5) | 0.7710(5) | 0.2621(3) | 0.0817(15) |
| H26A | 0.4760 | 0.8155 | 0.2727 | 0.098* |
| C27 | 0.4717(5) | 0.6614(5) | 0.2817(3) | 0.0887(15) |
| H27A | 0.5390 | 0.6307 | 0.3058 | 0.106* |
| C28 | 0.4117(5) | 0.5950(4) | 0.2666(3) | 0.0740(13) |
| H28A | 0.4389 | 0.5199 | 0.2809 | 0.089* |
| C29 | 0.0326(5) | 0.0626(4) | 0.2915(3) | 0.0789(13) |
| H29A | 0.0947 | 0.0544 | 0.2474 | 0.095* |
| C30 | −0.0754(6) | 0.0323(5) | 0.2972(3) | 0.0970(17) |
| H30A | −0.0867 | 0.0064 | 0.2572 | 0.116* |
| C31 | −0.1641(5) | 0.0408(5) | 0.3616(4) | 0.0916(16) |
| H31A | −0.2369 | 0.0200 | 0.3663 | 0.110* |
| C32 | −0.1477(4) | 0.0804(4) | 0.4211(3) | 0.0687(12) |
| C33 | −0.2354(4) | 0.0894(4) | 0.4912(3) | 0.0850(15) |
| H33A | −0.3074 | 0.0662 | 0.4986 | 0.102* |
| C34 | −0.2184(4) | 0.1292(4) | 0.5461(3) | 0.0808(15) |
| H34A | −0.2787 | 0.1342 | 0.5911 | 0.097* |
| C35 | −0.1092(4) | 0.1647(4) | 0.5378(2) | 0.0658(12) |
| C36 | −0.0878(5) | 0.2104(4) | 0.5933(3) | 0.0826(14) |
| H36A | −0.1473 | 0.2196 | 0.6384 | 0.099* |
| C37 | 0.0192(5) | 0.2414(5) | 0.5819(3) | 0.0859(15) |
| H37A | 0.0345 | 0.2707 | 0.6190 | 0.103* |
| C38 | 0.1060(4) | 0.2284(4) | 0.5129(3) | 0.0709(12) |
| H38A | 0.1798 | 0.2490 | 0.5054 | 0.085* |
| C39 | −0.0175(3) | 0.1560(3) | 0.4701(2) | 0.0506(9) |
| C40 | −0.0379(3) | 0.1120(3) | 0.4109(2) | 0.0523(10) |
| C41 | −0.0411(5) | 0.2885(4) | 0.1530(3) | 0.0784(13) |
| H41A | −0.0610 | 0.2780 | 0.2048 | 0.094* |
| C42 | −0.1037(5) | 0.2503(5) | 0.1145(4) | 0.104(2) |
| H42A | −0.1649 | 0.2154 | 0.1398 | 0.125* |
| C43 | −0.0741(5) | 0.2648(5) | 0.0388(4) | 0.104(2) |
| H43A | −0.1152 | 0.2394 | 0.0121 | 0.125* |
| C44 | 0.0179(5) | 0.3177(4) | 0.0004(3) | 0.0764(14) |
| C45 | 0.0528(6) | 0.3382(5) | −0.0792(3) | 0.100(2) |
| H45A | 0.0107 | 0.3182 | −0.1082 | 0.120* |
| C46 | 0.1427(7) | 0.3844(5) | −0.1119(3) | 0.107(2) |
| H46A | 0.1661 | 0.3926 | −0.1637 | 0.129* |
| C47 | 0.2053(5) | 0.4222(4) | −0.0709(3) | 0.0837(16) |
| C48 | 0.2993(7) | 0.4739(6) | −0.1032(4) | 0.116(2) |
| H48A | 0.3241 | 0.4856 | −0.1548 | 0.139* |
| C49 | 0.3544(7) | 0.5069(6) | −0.0602(4) | 0.124(3) |
| H49A | 0.4189 | 0.5398 | −0.0816 | 0.149* |
| C50 | 0.3142(5) | 0.4916(4) | 0.0165(3) | 0.0917(16) |
| H50A | 0.3513 | 0.5167 | 0.0457 | 0.110* |
| C51 | 0.1710(4) | 0.4069(3) | 0.0075(2) | 0.0593(11) |
| C52 | 0.0762(4) | 0.3532(3) | 0.0437(2) | 0.0565(10) |
| O10 | 0.5701(5) | 0.3055(5) | 0.2163(3) | 0.184(2) |
| H10A | 0.4947 | 0.3122 | 0.2129 | 0.277* |
| H10B | 0.5754 | 0.3652 | 0.2257 | 0.277* |
aOccupancy: 0.662(3); bOccupancy: 0.338(3); cOccupancy: 0.5.
Source of materials
A mixture of Cd(CH3COO)2⋅2H2O (0.1 mmol), 2-chlorobenzoic acid (0.2 mmol), 1,10-phenanthroline (0.2 mmol), triethylamine (0.1 mL) and 15 mL CH3OH/H2O (1:2, v/v) was sealed in a 25 mL teflon-lined stainless-steel reactor and heated to 393 K for 72 h. The colorless block crystals suitable for X-ray diffraction analysis were obtained after the autoclave was cooled to room temperature.
Experimental details
Aromatic hydrogen atoms were placed in calculated positions (C—H = 0.93 Å) and refined as riding atoms with Uiso(H) = 1.2Ueq(C). Water H atoms were permitted to ride at the positions located in difference maps with Uiso(H) = 1.5Ueq(O), giving the O—H distances 0.85 Å. Some oxygen atoms of the carboxylate groups are disordered over two positions in this structure (ratio: 1/1). The two aromatic rings of the two 2-chlorobenzoic acids were disordered over opposite direction and overlapped each other with site occupation factors of 0.662 and 0.338. The disorder is omitted in the figure for clarity.
Comment
In the last two decades, the design and synthesis of metal-organic frameworks is one of the most active areas in coordination chemistry not only because of their intriguing variety of structures but also due to their potential applications in gas storage and separation, magnetism, catalysis, sensor, luminescence, and so forth [3], [4], [5], [6], [7], [8], [9], [10], [11], [12].
2-Chlorobenzoic acid and the deprotonated corresponding carboxylate anion is an excellent ligand and widely used in construction of coordination polymers because it posseses rich coordination modes, such as monodentate, chelating, and various modes of bridging coordination of two, three, or even more metal centers. It can also act as hydrogen-bond acceptor and donor in assembling supramolecular complexes [13, 14] . 1,10-Phenanthroline (phen) is a typical N-donor ligand and also widely used in coordination polymers, due to its rigidity, planarity, aromaticity, basicity and chelating capability [15, 16] .
The asymmetric unit of the title complex consists of two Cd(II), four chlorobenzoato ligands, two phen ligands, one coordinated water molecule, and one free water molecule. Both Cd1 and Cd2 exhibit distorted pentagonal-bipyramidal coordination geometries. Cd1 is coordinated by two nitrogen atoms from one phen ligand, four oxygen atoms from three carboxylate ligands, and one oxygen atom from one water molecule. Cd2 is coordinated by two nitrogen atoms from one phen ligand and five oxygen atoms from three carboxylate ligands. The two Cd atoms are bridged by O3 and O5 to form a dinuclear complex. Each dinuclear unit is connected to adjacent ones through O—H⋯O hydrogen bonds.
Acknowledgement
This work was financially supported by Changsha Enviromental Protection College.
References
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
Bruker. APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA (2005).Search in Google Scholar
Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O. M.: Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402 (1999) 276–279.10.1038/46248Search in Google Scholar
Kitagawa, S.; Kitaura, R.; Noro, S.: Functional porous coordination polymers. Angew. Chem. Int. Ed. 43 (2004) 2334–2375.10.1002/anie.200300610Search in Google Scholar PubMed
Li, J. R.; Sculley, J.; Zhou, H. C.: Metal-organic frameworks for separations. Chem. Rev. 112 (2012) 869–932.10.1021/cr200190sSearch in Google Scholar PubMed
Aresta, M.; Dibenedetto, A.; Angelini, A.: Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological Use of CO2. Chem. Rev. 114 (2014) 1709–1742.10.1021/cr4002758Search in Google Scholar PubMed
Yu, F.; Li, D. D.; Cheng, L.; Yin, Z.; Zeng, M. H.; Kurmoo, M.: Porous supramolecular networks constructed of onedimensional metal organic chains: carbon dioxide and iodine capture. Inorg. Chem. 54 (2015) 1655–1660.10.1021/ic502650zSearch in Google Scholar PubMed
Ni, Z.-P.; Liu, J.-L.; Hoque, M. N.; Liu, W.; Li, J.-Y.; Chen, Y.-C.; Tong, M.-L.: Recent advances in guest effects on spin-crossover behavior in Hofmann-type metal-organic frameworks. Coord. Chem. Rev. 335 (2017) 28–43.10.1016/j.ccr.2016.12.002Search in Google Scholar
Wang, H.-R.; Meng, W.; Wu, J.; Ding, J.; Hou, H.-W.; Fan, Y.-T.: Crystalline central-metal transformation in metal-organic frameworks. Coord. Chem. Rev. 307 (2016) 130–146.10.1016/j.ccr.2015.05.009Search in Google Scholar
Wang, Z.; Chen, G.; Ding, K. L.: Self-supported catalysts. Chem. Rev. 109 (2009) 322–359.10.1021/cr800406uSearch in Google Scholar PubMed
Kreno, L. E.; Leong, K.; Farha, O. K.; Allendorf, M. R.; Van Duyne, R. P.; Hupp, J. T.: Metal-organic framework materials as chemical sensors. Chem. Rev. 112 (2012) 1105–1125.10.1021/cr200324tSearch in Google Scholar PubMed
Cu, Y.; Yue, Y.; Qian, G.; Chen, B.: Luminescent functional metal-organic frameworks. Chem. Rev. 112 (2012) 1126–1162.10.1021/cr200101dSearch in Google Scholar PubMed
Wang, F.-Q.; Weng, D.-F.; Zheng, X.-J.; Zhang, J.-J.; Ma, H.; Jin, L.-P.: 3D supramolecular network assembly and thermal decomposition of new copper(II) complexes with pyrazine-2,6-dicarboxylic acid. Inorg. Chim. Acta 360 (2007) 2029–2038.10.1016/j.ica.2006.10.023Search in Google Scholar
Dey, D.; Roy, P.; Purkayastha, R. N. D.; Pallepogu, R.; Male, L.; Mckee, V.: Syntheses, characterization, and crystal structures of two zinc(II) carboxylates containing pyridine. J. Coord. Chem. 64 (2011) 1165–1176.10.1080/00958972.2011.564278Search in Google Scholar
Dabb, S. L.; Fletcher, N. C.: mer and fac isomerism in tris chelate diimine metal complexes. Dalton Trans. 44 (2015) 4406–4422.10.1039/C4DT03535FSearch in Google Scholar PubMed
Chen, X.; Han, S.; Wang, R.; Li, Y.: Four supra-molecular isomers of di-chlorido-bis-(1,10-phenanthroline)cobalt(II): synthesis, structure characterization and isomerization. Acta Crystallogr. C72 (2016) 6–13.10.1107/S2053229615022779Search in Google Scholar
©2017 Chao Liu, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10

