Abstract
C20H22CdF4N4O6, orthorhombic, P212121 (no. 19), a = 7.475(3) Å, b = 12.246(5) Å, c = 24.615(11) Å, V =2253.0(17) Å3, Z = 4, Rgt(F) = 0.0245, wRref(F2) = 0.0706, T = 296(2) K.
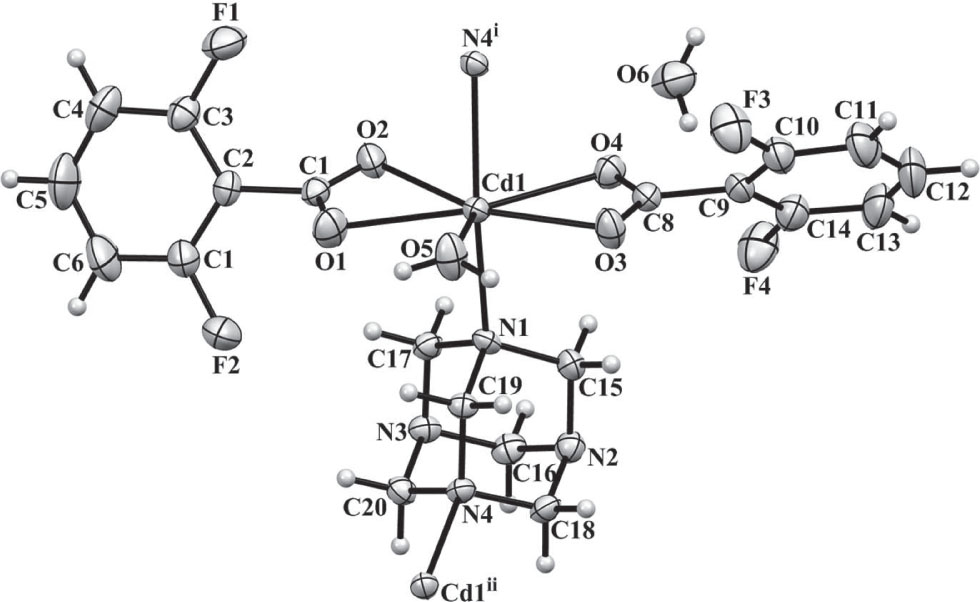
A part of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.28 × 0.24 × 0.19 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 10.5 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 56.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 13694, 5379, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5272 |
| N(param)refined: | 332 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cd1 | −0.05369(2) | 0.280356(14) | 0.244183(7) | 0.02140(6) |
| O1 | 0.0575(3) | 0.23144(18) | 0.15766(9) | 0.0383(5) |
| C1 | 0.1827(4) | 0.3007(2) | 0.15616(10) | 0.0253(5) |
| O2 | 0.2120(3) | 0.36609(17) | 0.19342(8) | 0.0343(5) |
| C2 | 0.3018(4) | 0.3003(2) | 0.10697(10) | 0.0264(6) |
| C3 | 0.3096(4) | 0.3877(3) | 0.07116(12) | 0.0317(6) |
| F1 | 0.2027(3) | 0.47473(16) | 0.08168(9) | 0.0505(5) |
| C4 | 0.4179(5) | 0.3903(3) | 0.02646(14) | 0.0485(9) |
| H7 | 0.4172 | 0.4497 | 0.0030 | 0.058* |
| C5 | 0.5275(6) | 0.3026(4) | 0.01732(16) | 0.0635(11) |
| H8 | 0.6041 | 0.3035 | −0.0125 | 0.076* |
| C6 | 0.5270(6) | 0.2127(4) | 0.05133(15) | 0.0583(10) |
| H9 | 0.6025 | 0.1536 | 0.0451 | 0.070* |
| C7 | 0.4113(4) | 0.2140(3) | 0.09450(11) | 0.0367(6) |
| F2 | 0.4067(3) | 0.12527(16) | 0.12726(9) | 0.0527(6) |
| O3 | −0.2439(3) | 0.26013(17) | 0.31871(8) | 0.0367(5) |
| C8 | −0.1676(4) | 0.3296(2) | 0.34744(10) | 0.0259(5) |
| O4 | −0.0276(3) | 0.37923(16) | 0.33295(8) | 0.0311(4) |
| C9 | −0.2492(4) | 0.3550(2) | 0.40176(11) | 0.0290(6) |
| C10 | −0.4301(5) | 0.3750(3) | 0.40820(12) | 0.0366(7) |
| F3 | −0.5341(3) | 0.37500(19) | 0.36333(9) | 0.0528(5) |
| C11 | −0.5098(6) | 0.3999(3) | 0.45711(16) | 0.0533(10) |
| H10 | −0.6312 | 0.4157 | 0.4594 | 0.064* |
| C12 | −0.4026(6) | 0.4003(4) | 0.50239(15) | 0.0593(11) |
| H11 | −0.4538 | 0.4143 | 0.5361 | 0.071* |
| C13 | −0.2207(6) | 0.3803(3) | 0.49929(13) | 0.0523(10) |
| H12 | −0.1493 | 0.3816 | 0.5302 | 0.063* |
| C14 | −0.1490(5) | 0.3586(3) | 0.44894(12) | 0.0380(7) |
| F4 | 0.0286(3) | 0.3376(2) | 0.44587(8) | 0.0550(5) |
| N1 | 0.1461(3) | 0.14677(17) | 0.28256(8) | 0.0208(4) |
| C15 | 0.1637(4) | 0.1461(2) | 0.34344(10) | 0.0258(5) |
| H13 | 0.2103 | 0.2159 | 0.3555 | 0.031* |
| H14 | 0.0465 | 0.1362 | 0.3596 | 0.031* |
| N2 | 0.2827(3) | 0.05890(19) | 0.36178(9) | 0.0272(5) |
| C16 | 0.4609(4) | 0.0729(2) | 0.33745(10) | 0.0281(5) |
| H15 | 0.5393 | 0.0149 | 0.3499 | 0.034* |
| H16 | 0.5114 | 0.1420 | 0.3491 | 0.034* |
| N3 | 0.4503(3) | 0.07057(16) | 0.27768(9) | 0.0241(4) |
| C17 | 0.3298(3) | 0.1587(2) | 0.26042(10) | 0.0240(5) |
| H17 | 0.3239 | 0.1597 | 0.2211 | 0.029* |
| H18 | 0.3788 | 0.2281 | 0.2722 | 0.029* |
| C18 | 0.2091(4) | −0.0461(2) | 0.34448(11) | 0.0256(5) |
| H19 | 0.0924 | −0.0563 | 0.3610 | 0.031* |
| H20 | 0.2866 | −0.1044 | 0.3570 | 0.031* |
| C19 | 0.0753(3) | 0.03885(19) | 0.26643(10) | 0.0210(5) |
| H21 | 0.0631 | 0.0366 | 0.2272 | 0.025* |
| H22 | −0.0428 | 0.0294 | 0.2821 | 0.025* |
| N4 | 0.1915(3) | −0.05240(17) | 0.28418(9) | 0.0212(4) |
| C20 | 0.3738(3) | −0.0350(2) | 0.26082(11) | 0.0243(5) |
| H23 | 0.4521 | −0.0935 | 0.2726 | 0.029* |
| H24 | 0.3667 | −0.0374 | 0.2215 | 0.029* |
| O5 | −0.2730(3) | 0.1654(2) | 0.21132(10) | 0.0402(5) |
| H5A | −0.250(7) | 0.135(4) | 0.1824(12) | 0.09(2)* |
| H5B | −0.339(6) | 0.135(4) | 0.2336(15) | 0.092(18)* |
| O6 | 0.1471(4) | 0.5586(2) | 0.38381(11) | 0.0479(6) |
| H6A | 0.109(10) | 0.498(3) | 0.394(4) | 0.22(5)* |
| H6B | 0.075(5) | 0.609(3) | 0.3872(19) | 0.071(16)* |
Source of materials
An aqueous solution (10 mL) of 2,6-difluorobenzoic acid (110 mg, 0.70 mmol), NaHCO3 (60 mg, 0.70 mmol), and hexamethylenetetramine (50 mg, 0.36 mmol) was mixed with an ethanolic solution (10 mL) of Cd(NO3)2⋅6H2O (250 mg, 0.81 mmol). The resulting colorless solution was allowed to stand at room temperature to give colorless needle crystals within two weeks (yield 66 mg, 31%).
Experimental details
H atoms bound to C atoms were places in calculated positions and refined as riding on their parent atoms, with C—H = 0.93 Å (aromatic), C—H = 0.96 Å (methylene), C—H = 0.97 Å (ethylene), with Uiso(H) = 1.2 Ueq(C) for all H atoms. H atoms bound to water molecules were found from the Fourier difference map.
Comment
Coordination polymers have received much attention in recent years because of their potential applications such as catalysis, separation, gas storage, luminescence, and magnetism [3], [4], [5]. The traditional strategy of construction of coordination polymers is the self-assembly of organic molecules and metal ions. Bi- or polytopic molecules such as anionic aromatic acids and neutral N-containing heterocyclic molecules are usually used as organic ligands for extending the structures. Among various aromatic acids, the “parent” acid, namely, benzoic acid or its substituted derivatives, have been utilized rather rarely in literature because it has only one carboxyl group, although some polynuclear clusters have been reported so far [6], [7], [8]. The fluorine substituted molecules are good candidates for construction of functional coordination polymers, because they are more stable to oxidation and show enhanced thermal stability [9]. Usage of pentafluorobenzoate as ligand has been reported in some studies, while only few complexes with 2,6-difluorobenzoate (dfba) as ligand have been reported [10, 11] . On the other hand, for the N-heterocyclic ligands, hexamethylenetetramine (hmt), which has four coordinating N atoms, is a good neutral bridging ligand, not only because it has various coordination modes that span from terminal monodentate to μ2-, μ3-, or μ4-bridging mode; but also because it can act as hydrogen bonding acceptor to generate supramolecular networks [12, 13] .
The asymmetric unit of the title complex contains one Cd2+ ion, two dfba− ligands, one hmt ligand, one coordinated and one not coordinated water molecule. The Cd2+ ion is seven coordinated in a distorted pentagonal bipyramidal geometry with four oxygen atoms from two dfba− ligands, two nitrogen atoms from two hmt molecules, and one water molecule. N1 and N4i are located at the axial positions, and O1, O2, O3, O4, and O5 are in the equatorial plane. It is obviously that the distances of Cd1—O2 (2.571(2) Å) and Cd1—O4 (2.506(2) Å) are longer than those of Cd1—O1, Cd1—O3, and Cd1—O5 (av. 2.307(2) Å). The carboxyl groups of two dfba− ligands adopt chelating coordination modes. Each hmt ligand bridges two Cd2+ ions through N1 and N4 atoms with the Cd⋯Cd distance of 6.182 Å to form a chain along b. The uncoordinated water molecule is hydrogen bonded with the water ligand (O5—H5A⋯O6 = 2.842(4) Å), and two oxygen atoms of different carboxylate groups (O6—H6B⋯O1 = 2.803(4), O6—H6A⋯O4 = 2.845(4) Å). The neighboring chains along a are hydrogen bonded with each other through the coordinated water molecules and the nitrogen atoms of hmt ligands (O5—H5B⋯N3 = 2.881(3) Å) to form a 2D network.
Funding source: Natural Science Foundation of Jiangxi Province
Award Identifier / Grant number: 20112BBF60024
Award Identifier / Grant number: 20151BAB204014
Funding statement: This work was supported by the National Nature Foundation of China [No. 21461011 and 31560712], the Natural Science Foundation of Jiangxi Province [No. 20112BBF60024, 20151BAB204014].
References
Bruker. SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA, (2012).Search in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Cook, T. R.; Zheng, Y.-R.; Stang, P. J.: Metal–organic frameworks and self–assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal–organic materials. Chem. Rev. 113 (2013) 734–777.10.1021/cr3002824Search in Google Scholar PubMed PubMed Central
O’Keeffe, M.; Yaghi, O. M.: Deconstructing the crystal structures of metal organic frameworks and related materials into their underlying nets. Chem. Rev. 112 (2012) 675–702.10.1021/cr200205jSearch in Google Scholar PubMed
Zhang, W.; Xiong, R.-G.: Ferroelectric metal–organic framworks. Chem. Rev. 112 (2012) 1163–1195.10.1021/cr200174wSearch in Google Scholar PubMed
Leng, J.-D.; Liu, J.-L.; Tong, M.-L.: Unique nanoscale {CuII36LnIII24} (Ln = Dy and Gd) metallo-rings. Chem. Comm. 48 (2012) 5286–5288.10.1039/c2cc30521fSearch in Google Scholar PubMed
Majeed, Z.; Mondal, K. C.; Kostakis, G. E.; Lan, Y.; Anson, C. E.; Powell, A. K.: [LnNa(PhCO2)4] (Ln = Ho, Dy): the first examples of chiral srs 3D networks constructed using the monotopic benzoate ligand. Chem. Comm. 46 (2010) 2551–2553.10.1039/b923998gSearch in Google Scholar PubMed
Zeng, Y.-F.; Xu, G.-C.; Hu, X.; Chen, Z.; Bu, X.-H.; Gao, S.; Sanudo, E. C.: Single–molecule–magnet behavior in a Fe12Sm4 cluster. Inorg. Chem. 49 (2010) 9734–9736.10.1021/ic1009708Search in Google Scholar PubMed
Peikert, K.; Hoffmann, F.; Froba, M.: Fluorine magic: one new organofluorine linker leads to three new metal–organic frameworks. CrystEngComm 17 (2015) 353–360.10.1039/C4CE00408FSearch in Google Scholar
Yu, L.-L.; Liu, Q.; Xi, H.-T.; Zhu, E.-J.; Sun, X.-Q.; Xu, Z.: Synthesis, crystal structure and electrochemical properties of complex [Co(O2CC6HF4)2(phen)2] ((O2CC6HF4) = 2, 3, 5, 6-tetrafluorobenzoate; phen = 1, 10-phenanthroline). Chin. J. Inorg. Chem. 26 (2010) 621–626.Search in Google Scholar
Hulvey, Z.; Melot, B. C.; Cheetham, A. K.: Structure and magnetic field–induced transition in a one–dimensional hybrid inorganic–organic chain system, Co2(4,4′-bpy)(tfhba)2⋅4,4′-bpy (4,4′-bpy = 4,4′-Bipyridine; tfhba = 2,3,5,6-Tetrafluoro-4-hydroxybenzoate). Inorg. Chem. 49 (2010) 4594–4598.10.1021/ic100164kSearch in Google Scholar PubMed
Hazra, S.; Biswas, S.; Kirillov, A. M.; Ghosh,A.: Nickel(II) complexes self-assembled from hexamethylenetetramine and isomeric nitrobenzoates: structural diversity and supramolecular features. Polyhedron 79 (2014) 66–71.10.1016/j.poly.2014.04.038Search in Google Scholar
Kirillov, A. M.: Hexamethylenetetramine: an old new building block for design of coordination polymers. Coord. Chem. Rev. 255 (2011) 1603–1622.10.1016/j.ccr.2011.01.023Search in Google Scholar
©2017 Yuan Hou-Qun et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10

