Abstract
C28H40N6NiO12S2, monoclinic, P21/c (no. 14), a = 6.202(1) Å, b = 7.126(1) Å, c = 38.499(2) Å, β = 91.258(2)°, V = 1701.1(4) Å3, Z = 2, Rgt(F) = 0.0698, wRref(F2) = 0.1515, T = 300 K.
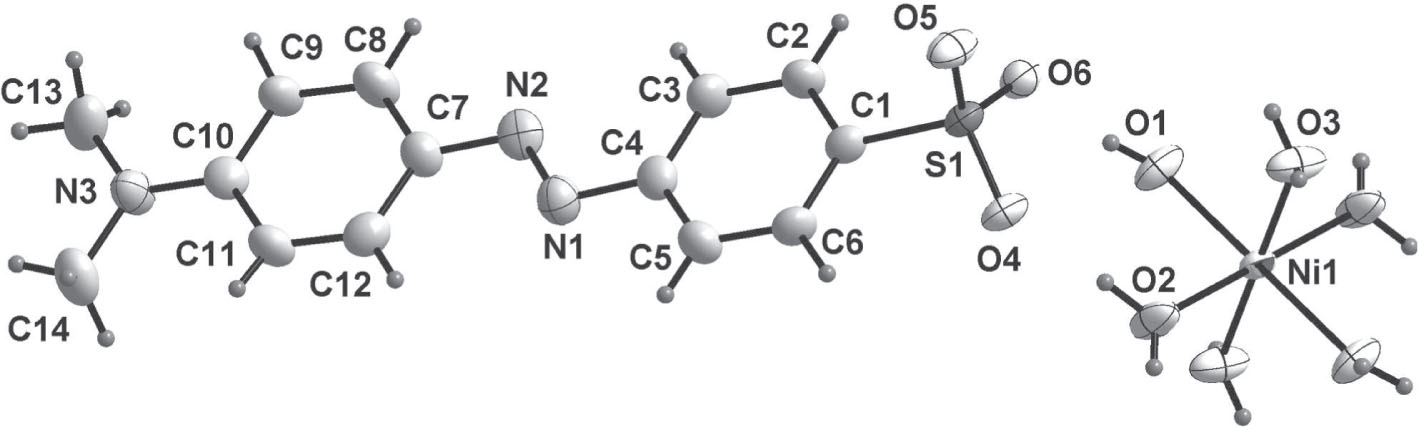
The cation and the anion forming the title crystal structure are shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red-brown prism |
| Size: | 0.57 × 0.48 × 0.37 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 7.6 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 50°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 34163, 3000, 0.119 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2062 |
| N(param)refined: | 283 |
| Programs: | SHELX [1], Bruker programs [2], SIR92 [3], DIAMOND [4], publCIF [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Ni1 | 1.0000 | 0.5000 | 0.5000 | 0.0265(3) |
| S1 | 0.61159(17) | −0.0048(2) | 0.43946(3) | 0.0311(3) |
| O1 | 0.7066(6) | 0.4952(7) | 0.47613(11) | 0.0443(10) |
| H1O1 | 0.654(12) | 0.588(10) | 0.4697(19) | 0.066* |
| H2O1 | 0.641(12) | 0.410(10) | 0.4665(19) | 0.066* |
| O2 | 1.1044(7) | 0.2850(7) | 0.46915(14) | 0.0510(14) |
| H1O2 | 1.007(12) | 0.209(11) | 0.4599(19) | 0.077* |
| H2O2 | 1.213(13) | 0.261(12) | 0.465(2) | 0.077* |
| O3 | 0.8978(8) | 0.3037(7) | 0.53500(15) | 0.0494(13) |
| H1O3 | 0.783(13) | 0.256(11) | 0.535(2) | 0.074* |
| H2O3 | 0.958(13) | 0.234(12) | 0.538(2) | 0.074* |
| O4 | 0.8461(5) | −0.0042(6) | 0.44346(9) | 0.0405(9) |
| O5 | 0.5172(7) | −0.1773(6) | 0.45212(11) | 0.0444(11) |
| O6 | 0.5158(6) | 0.1616(6) | 0.45484(10) | 0.0413(10) |
| N1 | 0.4072(8) | 0.0013(8) | 0.28748(12) | 0.0491(12) |
| N2 | 0.2172(8) | 0.0427(5) | 0.27879(12) | 0.0403(12) |
| N3 | −0.0081(7) | 0.0152(8) | 0.13739(11) | 0.0467(12) |
| C1 | 0.5524(7) | 0.0051(8) | 0.39443(13) | 0.0329(11) |
| C2 | 0.3503(9) | 0.0698(8) | 0.38326(16) | 0.0389(14) |
| H2 | 0.255(10) | 0.095(8) | 0.4010(15) | 0.047* |
| C3 | 0.2987(10) | 0.0709(9) | 0.34817(16) | 0.0448(15) |
| H3 | 0.159(10) | 0.115(9) | 0.3399(16) | 0.054* |
| C4 | 0.4466(9) | 0.0127(9) | 0.32395(14) | 0.0393(12) |
| C5 | 0.6476(10) | −0.0542(8) | 0.33564(16) | 0.0430(15) |
| H5 | 0.738(10) | −0.084(9) | 0.3192(16) | 0.052* |
| C6 | 0.7007(9) | −0.0555(8) | 0.37060(15) | 0.0394(14) |
| H6 | 0.845(10) | −0.091(8) | 0.3788(15) | 0.047* |
| C7 | 0.1724(9) | 0.0309(8) | 0.24258(14) | 0.0405(14) |
| C8 | −0.0348(11) | 0.0781(10) | 0.23174(17) | 0.0489(16) |
| H8 | −0.135(10) | 0.107(9) | 0.2493(17) | 0.059* |
| C9 | −0.0968(10) | 0.0725(9) | 0.19712(15) | 0.0435(15) |
| H9 | −0.250(10) | 0.101(9) | 0.1916(15) | 0.052* |
| C10 | 0.0481(9) | 0.0158(8) | 0.17194(14) | 0.0386(12) |
| C11 | 0.2549(10) | −0.0420(9) | 0.18356(16) | 0.0462(16) |
| H11 | 0.337(10) | −0.103(9) | 0.1699(16) | 0.055* |
| C12 | 0.3165(10) | −0.0368(8) | 0.21804(16) | 0.0430(15) |
| H12 | 0.444(10) | −0.051(9) | 0.2238(16) | 0.052* |
| C13 | −0.2199(12) | 0.0699(12) | 0.1255(2) | 0.059(2) |
| H13A | −0.330(13) | −0.018(12) | 0.133(2) | 0.088* |
| H13B | −0.257(13) | 0.180(12) | 0.134(2) | 0.088* |
| H13C | −0.244(13) | 0.020(11) | 0.104(2) | 0.088* |
| C14 | 0.1426(14) | −0.0469(12) | 0.11170(19) | 0.061(2) |
| H14A | 0.270(13) | 0.032(12) | 0.111(2) | 0.092* |
| H14B | 0.183(13) | −0.170(12) | 0.115(2) | 0.092* |
| H14C | 0.061(13) | −0.048(11) | 0.092(2) | 0.092* |
Source of materials
NiCl2⋅6H2O (0.364 g) was added to a solution of C14H14N3NaO3S (0.491 g) in methanol (200 mL) and the mixture was slowly evaporated. Small red-brown crystals were obtained after approximately 1 week.
Experimental details
All H atoms were located in a difference Fourier map and refined with Uiso(H) = 1.2 of their parent atoms with the exception of H atoms in the methyl groups and water molecules. H atoms in methyl groups and water molecules were refined using Uiso(H) = 1.5Ueq(C) or 1.5Ueq(O).
Comment
Recently, owing to the various properties, for example, a low-dimensional magnetism, photovoltaic effects, and thermoelectric applications, inorganic-organic hybrid systems have attracted considerable interest [6], [7], [8]. As a part of this approach, we prepared a series of inorganic-organic layered materials [9], [10], [11].
In the layered title structure Ni(H2O)6 layers are placed between organic bilayers. The crystal structure is similar to the previously reported Mn analogue [11]. Many crystal structures containing methyl-orange have been reported [12], [13], [14], [15]. Through the hydrogen bonds all water ligands are linked and as a result hydrogen bonded layers are in an ab plane. Based on the bond lengths, each nickel(II) is almost octahedrally surrounded by six water molecules. The distances between Ni—O range from 2.02(3) Å to 2.052(5) Å. Compared to the Mn compound, the difference is very tiny. Organic chains are parallel to the c axis. The hydrogen bonds in the title compound are classified as medium strong [16, 17] . Similar to the Mn compound, the C—N bond lengths are shorter than the sum of the covalent radii, 1.47 Å (N1—C4 = 1.422 Å, N2—C7 = 1.418 Å, N3—C10 = 1.367 Å). Some ‘sacrificial’ structures can explain this shortening of the bond lengths [11, 18, 19, 20].
Acknowledgement
The work at KAERI was supported by the Nuclear R&D Programs (NRF-2012M2A2A6004261).
References
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Bruker. SMART, SADABS and SAINT. Bruker AXS Inc., Madison, WI (2007).Search in Google Scholar
Altomare, A.; Cascarano, G.; Giavcovazzo, C.; Guagliardi, A.; Burla, M. C.; Plidori, G.; Camali, M.: SIR92. J. Appl. Crystallogr. 36 (1994) 435.10.1107/S002188989400021XSearch in Google Scholar
Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Version 3.2i. Crystal Impact, Bonn, Germany (2012).Search in Google Scholar
Westrip, S. P.: publCIF: software for editing, validating and formating crystallographic informations files. J. Appl. Cryst. 43 (2010) 920–925.10.1107/S0021889810022120Search in Google Scholar
De Jongh, L. J.: In magnetic properties of layered transition metal compounds. Dordrecht: Kluwer (1990); ISBN:0-7923-0236-9.10.1007/978-94-009-1860-3Search in Google Scholar
Mitzi, D. B.: Synthesis, structure, and properties of organic-inorganic perovskites and related materials. Prog. Inorg. Chem. 48 (1999) 1–121.10.1002/9780470166499.ch1Search in Google Scholar
He, Y.; Galli, G.: Perovskites for solar thermoelectric applications: a first principle study of CH3NH3AI3 (A = Pb and Sn). Chem. Mat. 26 (2014) 5394–5400.10.1021/cm5026766Search in Google Scholar
Oh, I. H.; Kim, D.; Huh, Y. D.; Park, Y.; Park, J. M. S.; Park, S. H.: Bis(2-phenylethylammonium) tetrachloridocobaltate(II). Acta Crystallogr. E67 (2011) m522–m523.10.1107/S1600536811011603Search in Google Scholar PubMed PubMed Central
Park, S. H.; Oh, I. H.; Park, S.; Park, Y.; Kim, J. H.; Huh, Y. D.: Canted antiferromagnetism and spin reorientation transition in layered inorganic-organic perovskite (C6H5CH2NH3)2MnCl4. Dalton Trans. 41 (2012) 1237–1242.10.1039/C1DT11544HSearch in Google Scholar
Oh, I. H.; Kim, J. Y.; Park, S. H.: Crystal structure of hexaaquamanganese(II) bis((E)-4((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6O12S2. Z. Kristallogr. − NCS 231 (2016) 369–371.10.1515/ncrs-2014-9088Search in Google Scholar
Hanson, A. W.: The crystal structure of methyl orange monohydrate monoethanolate. Acta Crystallogr. B29 (1976) 454–460.10.1107/S0567740873002748Search in Google Scholar
Shibata, H.; Sato, K.; Mizuguchi, J.: Crystal structure of methyl orange derivatives and their electronic spectra. J. Imaging Sci. Technol. 53 (2009) 50302-1–50302-4.10.2352/J.ImagingSci.Technol.2009.53.5.050302Search in Google Scholar
Burke, N. J.; Burrows, A. D.; Mahon, M. F.; Teat, S. J.: Incorporation of sulfonate dyes into hydrogen-bonded networks. CrystEngComm 6 (2004) 429–436.10.1039/b406614fSearch in Google Scholar
Burke, N. J.; Burrows, A. D.; Mahon, M. F.; Warren, J. E.: Incorporation of dyes into hydrogen-bond networks: the structures and properties of guanidinium sulfonate derivatives containg ethyl orange and 4-aminoazobenzene-4’-sulfonate. Cryst. Growth Des. 6 (2006) 546–554.10.1021/cg050499eSearch in Google Scholar
Steiner, T.: Hydrogen-bond distances to halide ions in organic and organometaallic crystal structures: up-to-date database study. Acta Crystallogr. B54 (1998) 456–463.10.1107/S0108768197014821Search in Google Scholar
Steiner, T.: The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 41 (2002) 48–76.10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-USearch in Google Scholar
Sakurai, T.; Sundaralingan, M.; Jeffrey, G. A.: A nuclear quadrupole resonance and X-ray study of the crystal structure of 2,5-dichloroaniline. Acta Crystallogr. 16 (1963) 354–363.10.1107/S0365110X63000979Search in Google Scholar
Trotter, J.; Whitlow, S. H.; Zobel, T.: The crystal and molecular structure of p-chloroaniline. J. Chem Soc. (A) (1966) 353–356.10.1039/j19660000353Search in Google Scholar
Oh, I. H.; Park, S. H.: Crystal structure of (E)-4-(2-(4-(diethylamino)phenyl)diazen-1-ium-1-yl)benzenesulfonate monohydrate. Z. Kristallogr. − NCS 231 (2016) 409–410.10.1515/ncrs-2015-0042Search in Google Scholar
©2017 In-Hwan Oh et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2-(2-ethoxyphenyl)-7-propyl-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one, C17H20N4O2
- Crystal structure of catena-poly[aqua-(μ3-1,3,5-benzenetricarboxylato-κ3O:O′:O′′)-[μ3hydroxy-(1,3-di-(μ2-1,2,4-triazole-4-yl)benzoato-κ2N:N′)copper(II)], C19H16Cu2N6O9
- Crystal structure of poly[aqua-(μ3-3,5-di(4H-1,2,4-triazolyl-4-κ3N,N′:N′′)benzenecarboxylato)silver(I)], C11H9AgN6O3
- Crystal structure of tetrapropylammonium hydrogen carbonate, C13H29NO3
- Crystal structure of poly[μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)yttrium(III)] monohydrate, C20H24O17Y2
- Crystal structure of catena-poly[dichlorido-(μ2-4-(1H-pyrazol-3-yl)-pyridine-κ2N,N′)]cadmium(II), C48H42Cd3Cl16N18
- Crystal structure of bis(tetraethylammonium) [1,1′-biphenyl]-2,2′-dicarboxylate trihydrate, C30H54N2O7
- Crystal structure of poly[(thiophene-3,4-dicarboxylato-κ1O)bis[1,2-bis(4-pyridyl)ethane-κ2N:N′]silver(I)] octahydrate, C30H42Ag2N4O12S
- The crystal structure of amine-(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)silver(I) dihydrate, C9H13AgN4O4
- Crystal structure of poly[tetrakis(μ2-cyanido-κ2N:O)-cyanido-tris(pyridine)dicobalt(II/III)], C20H15Co2N8
- Crystal structure of bis(pyridine)-bis(2-formyl-4,6-dichlorophenolato)cobalt(II), C24H16Cl4CoN2O4
- Crystal structure of (E)-1-(4-(((E)-5-bromo-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H15BrN2O2
- Crystal structure of catena-poly[(μ2-3-(1H-pyrazol-4-yl)-5-(pyridin-4-yl)-1,2,4-triazole-κ N:N′)-bis(benzoato-κO)zinc(II)], C24H18N6O4Zn
- Hydrothermal synthesis and crystal structure of a poly[aqua-(μ4-4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′) dimanganese(II)], [Mn2(C9H6O4)2(C12H11N5)(H2O)]
- Crystal structure of diaqua-catena-poly[diaqua-bis(μ2-5-(4-(1H-1,2,4-triazol-1-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)] dihydrate, C20H24CoN14O4
- Crystal structure of bis(μ3-2,2′-azanediylbis(ethan-1-olato)-κ5O:O,N,O′:O′)-tetrachlorido-bis(μ2-2-((2-hydroxyethyl)amino)ethan-1-olato-κ3N,O:O)dicobalt(II)dicobalt(III), C16H38Cl4Co4N4O8
- Crystal structure of poly[μ4-(4-(carboxylatomethyl)benzoato-κ4O:O′:O′′:O′′′)-(2-(4-(1H-imidazol-1-yl)phenyl)-1H-benzo[d]imidazole-κN)manganese(II)] [Mn(C9H6O4)(C16H12N4)]
- Crystal structure of 4-chloro-6-phenylpyrimidine, C10H7ClN2
- The crystal structure of [6-methoxy-2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1(2H)-one]difluoroborane, C13H10BF5O3
- Crystal structure of 3,3′-(butane-1,4-diylbis(azanylylidene))bis(1-phenylbut-1-en-1-olato)-κ4N,N′,O,O′]copper(II), C24H26N2O2Cu
- Crystal structure of tetraaqua-bis((E)-N′-(2-bromobenzylidene)isonicotinohydrazide-κN)zinc(II) dinitrate, C26H28N8O12Br2Zn
- Crystal structure of 2-amino-4-(4-bromophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13BrN2O2
- A single crystal study on tert-butyl-4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate, C26H30BrFN4O4
- Crystal structure of 1,1′-((1E,1′E)-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(naphthalen-2-olato-κ3O,O′,N)zinc(II), C36H26N2O4Zn
- Crystal structure of diaqua-bis(5′-(pyridin-1-ium-4-yl)-1H-[3,3′-bi(1,2,4-triazol)]-2′-ide-κ2N,N′)cobalt(II) — bis(5-(pyridin-4-yl-κN)-1H,1′H-3,3′-bi(1,2,4-triazole))octamolybdate – water (2/1/8), C27H33CoMo4N21O19
- Crystal structure of 3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-6,8-diiodo-4H-1,3-benzoxazin-4-one, C20H24I2N2O2
- Crystal structure of dinitrato-κO-bis(tris((1H-benzo[d]imidazol-2-yl)methyl)amine-κ4N,N′,N′′,N′′′)-(μ2-cyclohexane-1,4-dicarboxylato-κ4O,O′:O′′,O′′′)dimanganese(II) – methanol – water (1/6/2), C62H80Mn2N16O18
- Crystal structure of bis(2-hydroxyethyl(phenyl)carbamodithioate)nickel(II), C18H20N2NiO2S4
- Crystal structure of methyl 1-(4-fluorobenzyl)-4-methoxy-5-oxopyrrolidine-3-carboxylate, C14H16FNO4
- Crystal structure of di-μ-iodido-bis(6-(p-tolyl)-2,2′-bipyridine-κ2N,N′)dicopper(I) — 2-(diphenylphosphoryl)benzoic acid (1/2), C36H29CuIN2O3P
- Crystal structure of 2-amino-4-(3-bromo-4-fluoro-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16BrFN2O2
- Crystal structure of bis(μ2-2-chlorobenzoato-κ3O,O′:O′)-(2-chlorobenzoato-κO)-(2-chlorobenzoato-κO,O′)-bis(1,10-phenanthroline-κ2N,N′)-dicadmium(II) monohydrate, C52H36Cd2Cl4N4O10
- Crystal structure of 2-(8a-methyl-5-oxo-hexahydroimidazo [1,2-a]pyridin-1(5H)-yl)-2-oxoethyl acetate, C12H18N2O4
- Crystal structure of (E)-N,N-diethyl-2-(5-nitrothiazol-2-yl)-1-phenylethen-1-amine, C15H17N3O2S
- Crystal structure of diazido-dimethanolato-bis(μ2-2-(((3-oxidopropyl)imino)methyl)phenolato-κ4O:O,O′,N)dimanganese(III), C22H28Mn2N8O6
- The crystal structure of bis(2-(2,2,2-trifluoroacetyl)-3,4-dihydronaphthalen-1-olato-κ2O,O′)copper(II), C24H16CuF6O4
- Crystal structure of hexaaquanickel(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40N6NiO12S2
- Crystal structure of catena-poly[aqua-(μ2-hexamethylenetetramine-κ2N:N′)-bis(2,6-difluorobenzoato-κ2O:O′)cadmium(II)]monohydrate, C20H22CdF4N4O6
- Crystal structure of 3-benzyl-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C15H12N2OS
- Crystal structure of bis(μ2-ferrocenecarboxylato-κ2O:O′)-bis(1,10-phenanthroline-κ2N,N′)-(μ2-methanolato-κ2O,O)dicopper(II) tetrafluoroborate – acetonitrile (1/1), C49H40BCu2F4Fe2N5O5
- The crystal structure of tetrakis(1,3,5-triaza-7-phosphatricyclo[3.3.1.13,7]decane-κP)silver(I) chloride dihydrate, C24H60AgClN12O6P4
- Crystal structure of 5-ethyl-2-(p-tolyl)-1,3-dioxane-5-carboxylic acid, C14H18O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16ClIN2
- Crystal structure of catena-poly[(μ2-hexamethylenetetramine-κ2N:N′)-tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)dicopper(II)], C34H24Cu2F8N4O8
- Crystal structure of ethyl 3-hydroxy-5-methyl-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)-[1,1′-biphenyl]-4- carboxylate, C25H24N3O3
- The crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)-(diphenylcyclohexylphosphine-κP)rhodium(I), C24H25NO3PRh
- Crystal structure of bis((pyrazin-2-ylmethyl)(pyrazine-2-carbonyl)amido-κ3N,N′,N′′)copper(II), C20H16CuN10O2
- Crystal structure of catena-poly[tetraaqua-(μ2-succinonitrile-κ2N:N′)cobalt(II)] dinitrate, C4H12CoN4O10
- The crystal structure of 1,1′-bisisoquinoline, C18H12N2
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′,O)cobalt(III) perchlorate dihydrate, C22H22ClCoN4O10

