Abstract
Aristolochic acid I (AA-I) has been proven to be toxic, and there has been a global research frenzy to test it. To simplify the detection steps and enhance detection efficiency, this study proposes a detection method utilizing magnetic carriers such as Fe3O4 and gold nanoclusters. To enrich the AA-I in the sample, this study first designs a magnetic carrier based on ferric oxide, and then synthesizes a porcine serum albumin gold nanocluster fluorescent needle for the detection of AA substances. The magnetic molecularly imprinted polymer of AA-I could be completely separated from ultrapure water within 8 s. The maximum imprinting efficiency factor value was obtained when the mass ratio of “template molecule: crosslinking agent: functional monomer” was 1:20:4. The methyl acrylic acid was used as the functional monomer, and acetonitrile methanol was used as the solvents. As the temperature increased from 30 to 80°C, the fluorescence quenching intensity briefly increased and then gradually decreased from about 0.4 to nearly 0.1. The most suitable temperature was approximately 38°C. The proposed method can effectively collect and detect AA-I, has practicality, and can provide ideas for the development of AA-I detection methods.
1 Introduction
Aristolochic acid I (AA-I) is widely distributed in plants of the Aristolochiaceae family. Based on its excellent pharmacological effects, such as anti-tumor and anti-infection, it has been used in the treatment of arthritis, snake bites, and other cases [1]. After 1990, studies have found that AA-I has strong nephrotoxicity and is prone to causing AA nephropathy. Nowadays, the main causes of this disease include excessive use of drugs containing AA-I in medication, as well as mixing or misuse of medicinal materials. Although there have been many studies on AA-I detection methods, most of them focus on AA-I detection methods in pharmaceuticals or plants. There is very little detection of it in biological samples, and there are many detection instruments and processes that need to be prepared, and the steps are cumbersome. At present, the detection methods for AA-I are not yet perfect, so it is necessary to find a fast and effective method for detecting AA-I. In detection methods, molecular imprinting technology combines carriers with the analyte to form molecularly imprinted polymers (MIPs), which helps to collect the analyte and improve detection accuracy. In terms of detection methods, gold nanoclusters (AuNCs) are currently a hot research topic in optoelectronics and medicine due to their stability and antioxidant properties. AA-I is the most toxic and carcinogenic component among AA compounds. Its unique nitrophenanthrene structure can cause irreversible kidney damage and gene mutations; therefore, using it as a specific detection target has important toxicological significance [2]. Existing studies generally use AA-I as the core marker for evaluating AA toxicity. High-purity AA-I standards are used in experiments to ensure the template specificity of molecular imprinting polymers and the standardization of detection methods, avoiding the influence of cross-interference of homologues on quantitative accuracy [3,4,5].
There is already a wealth of research on using Fe3O4 as a magnetic carrier. Ashuri et al. prepared an acid-base responsive system using magnetic nanocomposites of folate coupled chitosan grafted with Fe3O4-oxidized graphene (FCCG-Fe3O4-OG) and used it for loading and controlled release of the anticancer drug gemcitabine. This composite material had a mesoporous structure with an average pore size of 14.78 nm. The drug release results indicated that the FCCG-Fe3O4-OG magnetic bio-composite material was a potential sustained-release carrier for gemcitabine. Sadighian et al. synthesized graphene oxide magnetite nanocomposites as carriers and magnetic resonance contrast agents. They also used composite materials with acid-base dependent release characteristics to design new colon drug delivery systems. The results proved the superiority of the synthesized composite material and also found that it is a good magnetic resonance transverse relaxation time contrast agent under the transverse relaxation time imaging sequence [6]. Meng et al. synthesized a new collectible pesticide delivery carrier by combining metal organic framework (MOF) materials with polydopamine-coated Fe3O4. The experiment found that magnetic MOF nanocomposites loaded with imidacloprid had better insecticidal activity against apple aphids than free imidacloprid, indicating its potential as a magnetic and collectable green pesticide carrier in agriculture [7]. Shi et al. synthesized a novel magnetic targeted drug carrier, Fe3O4 polyvinyl alcohol, to improve the hydrophilicity of Fe3O4. The drug carrier performance of Fe3O4 polyvinyl alcohol was measured using doxorubicin hydrochloride as a model drug. The obtained drug reagents exhibit high drug loading levels and excellent release levels [8].
Numerous scholars are researching the use of AuNCs as a detection method. Yin et al. developed a hydrogen peroxide-responsive nanoenzyme to achieve safe and efficient photodynamic therapy. A large amount of AuNCs were loaded into mesoporous silica to form nano assemblies, and manganese dioxide nanosheets were wrapped as switch shielding shells. Experimental results have shown that synthetic materials exhibit excellent on/off modulation and enhancement in magnetic resonance imaging and photodynamic therapy, making them suitable for safe and efficient therapeutic diagnostics [9]. Kuo et al. used AuNCs as antibacterial agents to understand the antibacterial mechanism, and observed their interaction with bacteria in real-time through in situ transmission electron microscopy. The successful preparation of glutathione-coupled AuNCs was confirmed by optical and structural characterization. The high antibacterial activity of the surface ligand was confirmed in the intracellular reactive oxygen species generation experiment induced in Vinegar Tail Grass [10]. Yuan et al. developed a surface geometric mismatch strategy by using mixed ligands with different types of obstacles. The determination of the single crystal structure showed that AuNCs had eight uncoordinated gold atoms in a twisted hexagonal prism shape. It was found that the cluster has excellent performance in selectively oxidizing benzyl alcohol to benzaldehyde, and due to its protection of the negatively charged multidentate ligand diphenylamine, it also exhibited excellent stability [11].
In summary, there has been a wealth of research on using Fe3O4 as a magnetic carrier, and numerous experiments have verified its superior performance as a carrier. The research on using AuNCs as a detection method is also relatively mature, and it is mostly applied in medicine and optics. Based on these advantages of both, this study innovatively combines Fe3O4 as a magnetic carrier with AuNCs to accurately detect the content of AA-I in the sample, and proposes an AA-I detection method based on magnetic carriers Fe3O4 and AuNCs. The experiment uses magnetic carrier Fe3O4 to collect AA-I and then uses AuNCs to detect it to simplify the detection time and improve the detection accuracy.
Given the low content and high interference of AA-I in complex biological samples, magnetic MIPs with specific recognition ability for AA-I are synthesized by magnetic molecular imprinting technology. AA-I is selectively captured by specific binding sites generated by molecular imprinting. Combining the advantages of magnetic separation, the target can be quickly enriched and separated within seconds, simplifying the tedious preprocessing steps and laying the foundation for subsequent high-sensitivity detection. Given the problems of expensive instruments, complex operations, and longtime consumption in existing AA-I detection methods, porcine serum albumin (PSA)-AuNCs are innovatively synthesized as fluorescent probes. AuNCs are selected based on their excellent optical properties and good biocompatibility and stability. To further improve the reliability and robustness of the detection results, especially in actual samples where multiple structural analogs or complex matrices may exist, a stacking ensemble classification model is constructed. Ensemble learning methods can effectively reduce the variance and bias of individual models by combining the prediction results of multiple base learners, improving the overall model’s generalization ability and recognition accuracy.
2 Methods and materials
This study first synthesized AA-I magnetic MIPs for the enrichment of AA-I for detection purposes. To explore a convenient detection method, this study synthesized PSA-AuNCs as a detection tool. The study has redesigned a stacking integrated classification model to identify AA substances in samples.
2.1 Preparation of AA-I magnetic MIPs
To achieve precise detection of AA-I, it was necessary to first separate and enrich the AA-I molecules in the sample to be tested, and then proceed with the detection. Supercritical fluid extraction (SFE) and solid-phase extraction (SPE) were currently common separation and enrichment methods. SPE included MIPs, macroporous adsorption resin, MOF, and carbon nanotubes. The principle was to enrich and separate the samples by adding adsorbents, achieving the goal of enriching the required substances [12,13,14,15]. The working principle of SFE was to control the polarity of supercritical fluid by controlling the temperature and pressure of the fluid, thereby selectively separating and extracting the required substances from the sample. SFE was often used in research on the removal of AA substances [16]. Figure 1 shows the advantages and disadvantages of the above method.
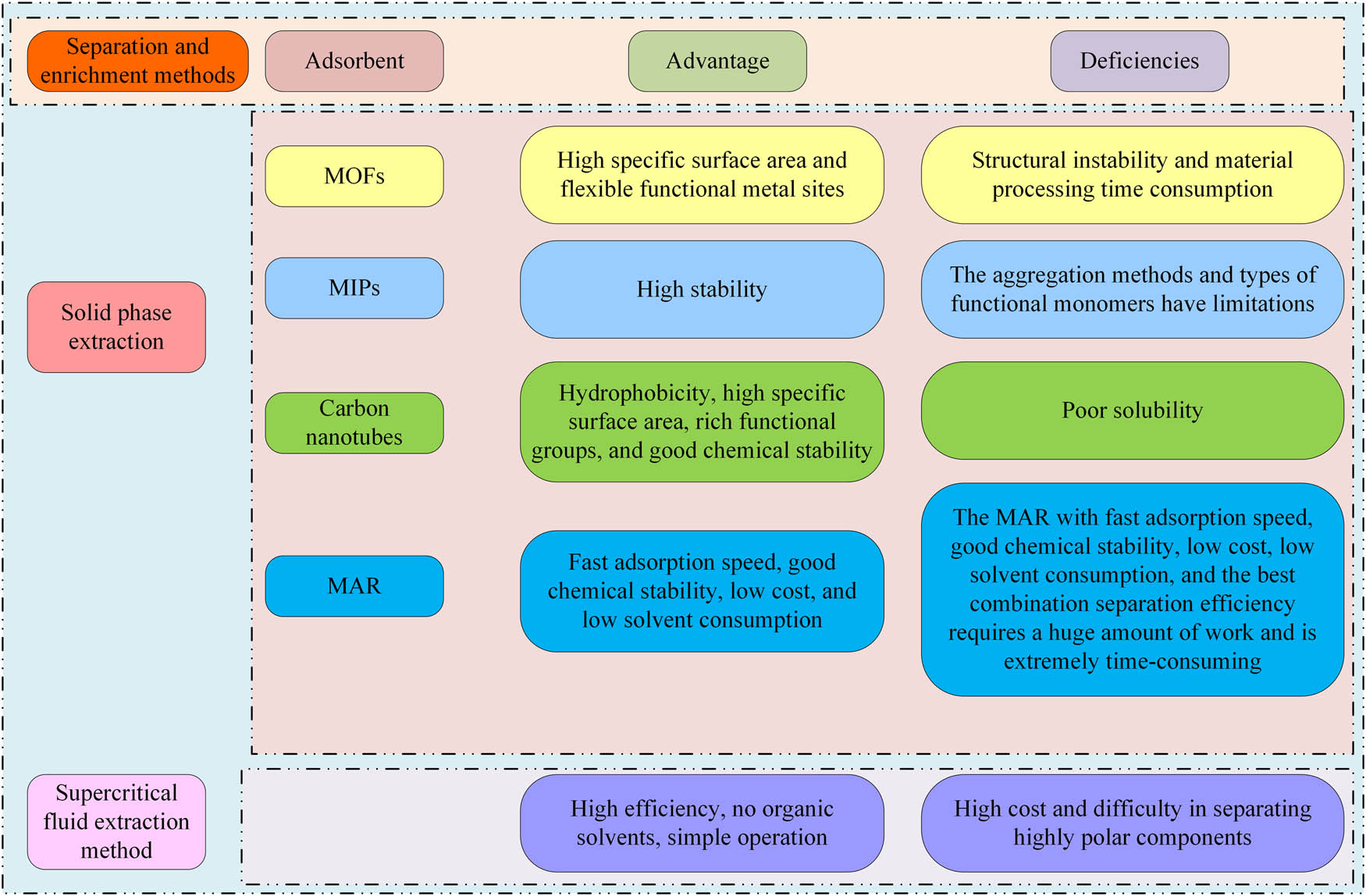
Characteristics of separation and enrichment methods for AA-I.
In Figure 1, MIPs were selected as adsorbents, which were the best choice for separating and enriching AA-I. Therefore, this study combined magnetic material Fe3O4 nanoparticles with molecular surface imprinting technology to synthesize AA-I magnetic MIPs based on Fe3O4. The working principle was to polymerize functional monomers, crosslinking agents, and template molecules to form specific polymers, MIPs. Then, by eluting the template molecules in the polymer, a cavity with the same volume size and contour structure as the desired enriched substance could be obtained, allowing MIPs to have a specific recognition and adsorption effect on the substance to be enriched in the experiment [17,18,19]. The specific preparation process is shown in Figure 2.

Schematic diagram of MIPs preparation process.
The experiment aimed to create magnetic MIP microspheres that combine the advantages of good physical and chemical properties, strong recognition ability, large adsorption capacity, and easy detachment to improve the extraction efficiency of AA-I molecules and facilitate subsequent detection, saving detection time. In the selection of experimental drugs, the purity of AA-I reagent was 98%, while the purity of other reagents was analytical grade. The manufacturers were from Shanghai and Chengdu, respectively. Figure 3 shows the detailed information.

Main reagent information for the experiment.
By analyzing existing literature, the process of preparing AA-I magnetic MIPs was divided into three steps [20,21]. In the preparation of AA-I magnetic MIPs, it was necessary to synthesize Fe3O4@SiO2 particles. The materials to be prepared included electronic balance, three-neck flask, isopropyl alcohol, methanol, ultra-pure water, ammonia water, and ethyl orthosilicate. 40 mL of isopropyl alcohol, 500 mg of dried Fe3O4 particles, and 8 mL of ultra-pure water were successively added to the three-necked flask. Ultrasonic mixing was used for 25 min to ensure that the components were well mixed. Then, 15 mL of 25% ammonia and 6 mL of ethyl orthosilicate were added, and the solution was fully stirred by a mechanical agitator and reacted at room temperature for 16 h. After the reaction was complete, the black precipitate produced by the reaction was collected using an external magnet and washed several times with ultra-pure water and methanol to remove impurities. Finally, the black precipitate after cleaning was dried in a vacuum environment of 60°C, and finally Fe3O4@SiO2 particles were obtained.
For the preparation of Fe3O4@SiO2@MPS particles, 50 μL of 3-MPS and 500 mg of Fe3O4@SiO2 particles were added to 80 mL of ultra-pure water containing 15% acetic acid using a pipette. The solution was heated to 70°C in a water bath while stirring for 4 h to ensure adequate reaction. After the reaction was over, the reaction products were collected using an external magnet and washed with ultra-pure water until the pH became neutral. After washing, the product was dried in a vacuum at 55°C to obtain Fe3O4@SiO2@MPS particles.
To prepare Fe3O4@SiO2@MPS@MIP particles, as shown in Figure 4, a functional monomer prepolymer was first produced by adding 0.040 mmoL AA-I reagent to 50 mL of nitrile-methanol solution. It was necessary to ensure sufficient dissolution after cooling. Then, 0.160 mmoL of methacrylic acid was added and allowed to react at room temperature for 6 h. 0.52 mmol of ethylene glycol dimethacrylate, 50 mg Fe3O4@SiO2@MPS particles, and 0.06 mmol azodiisobutyronitrile were added. To remove oxygen from the solution, it was treated with ultrasound for 20 min and then aerated with nitrogen for 12 min. In a nitrogen environment, the solution was heated in a water bath to 75°C and stirred for 30 h to promote the completion of the reaction. After the reaction, the products were collected again with external magnets and washed by 8:2 methanol-acetic acid solution for many times until no residue of Fe3O4@SiO2@MPS@MIPs particles could be detected by HPLC. The preparation of Fe3O4@SiO2@MPS@non-imprinted polymers (NIPs) particles was similar, but the AA-I reagent was not required, thus simplifying the process.

Preparation process of Fe3O4@SiO2@MPS@MIPs particles.
The adsorption capacity and final morphology of MIPs were related to the amount of reagents used in the synthesis process, the proportion of each reagent, and the type of drug. Therefore, to select the Fe3O4@SiO2@MPS@MIPs particles with the strongest adsorption capacity, it was necessary to compare polymers made with different ratios and types of reagents. The comparison method was to conduct adsorption experiments on all polymers using standard AA-I reagents, and then high-performance liquid chromatography was used to detect the concentration of AA-I in the remaining solution, to reflect the adsorption capacity of the polymers. The lower the concentration of AA-I in the remaining solution, the stronger the adsorption capacity of the polymer. Moreover, the specific recognition ability of polymers determined their specific adsorption ability, which was influenced by the binding force between functional monomers and template molecules. It was crucial to determine the proportion of template molecules, crosslinking agents, and functional monomers used. Based on existing literature, the dosage ratios of the three were set to 1:40:4 [22]. The calculation formula for imprinting efficiency is given in equation (1).
where
where
2.2 Construction of AA-I identification method
The enrichment of AA-I has been achieved, and the next step was to design the detection of AA-I. At present, the analysis and detection methods for AA mainly included thin-layer chromatography, UV visible spectrophotometry, high-performance liquid chromatography, and mass spectrometry. When the above methods had different drawbacks, such as UV visible spectrophotometry being only suitable for trace analysis, thin layer chromatography had poor accuracy, sensitivity, and precision. The equipment for using high-performance liquid chromatography was expensive and requires professional personnel to operate. The combination of high-performance liquid chromatography and mass spectrometry was costly and time-consuming. The specific advantages and disadvantages of the above method are shown in Figure 5.

Characteristics of classical detection methods for AA-I.
Figure 5 shows that existing methods have certain limitations. Currently, the excellent electrical, optical, and catalytic properties of fluorescent nanomaterials were gradually emerging in the scientific community [23]. The most widely studied fluorescent nanomaterials currently included fluorescent metal nanoclusters, quantum dots, etc. Since quantum dots were mostly prepared from heavy metals, they were highly toxic and prone to environmental damage. Therefore, although they had many excellent properties such as narrow emission spectra and strong resistance to photobleaching, their medical applications were also limited [24,25]. Metal nanoclusters had the advantages of high compatibility with biomolecules, simple preparation, and good stability. They were widely used in many fields such as catalysis, environmental monitoring, optics, and electronics, especially in the research of silver nanoclusters and AuNCs. However, the chemical properties of silver nanoclusters were more active than those of AuNCs, and they were prone to form larger nanoparticles in water [26]. Therefore, this study used AuNCs with more stable chemical properties, lower toxicity, and better biocompatibility as fluorescent probes. Its working principle was that the substance under test quenched its fluorescence by changing the system in which AuNCs were located, which could be seen as a sensor that converts chemical signals into optical signals. The specific working principle is displayed in Figure 6.

The working principle of fluorescent probes.
The experimental instruments mainly selected included an electronic balance with an accuracy of one hundred thousandth, a circulating water multi-purpose vacuum pump, a collector type constant temperature heating stirrer, a fluorescence spectrometer, a UV visible absorption spectrometer, a transmission electron microscope, an ultrasonic cleaner, an electric hot air drying oven, a centrifuge, a high-performance liquid chromatography, a Fourier transform spectrometer, and a dynamic light scattering instrument. The manufacturers of the main instruments for the above experiment included companies in Shanghai, Beijing, the United States, Japan, and the United Kingdom. The detailed information of their models and manufacturers is exhibited in Figure 7.

Main instruments and equipment for the experiment.
In the selection of templates for synthesizing AuNCs, this study selected PSA as the template for synthesis based on the characteristic that functional groups of biomolecules could have a good affinity with gold [27,28,29]. In terms of preparation, the first step was to prepare a solution of tetrachloroauric acid. The method was to dissolve 1 g of tetrachloroauric acid in 50 mL of pure water. 5 mL of the obtained solution and 50 mg/mL of PSA solution separately were taken and placed in a three necked flask. The mixture was stirred evenly with magnetic force under water bath heating conditions, and the temperature was controlled at 37°C. The pH value of the solution was adjusted to 11.0, and 0.5 mL of sodium hydroxide solution was added with a concentration of 1 mol/L. The reaction in the water bath was continued to heat for 12 h, and the obtained solution at 4°C was finally stored. To better detect AA-I, the experimental conditions were set as follows: the pH value of the solution system was 7.4, the detection temperature was 37°C. The machine could be immediately tested.
3 Results
This study first verified the superiority of the preparation experiment and synthesis conditions of AA-I magnetic MIPs, and then verified the detection performance of PSA-AuNCs. To verify its identification effect in biological samples, AA-I in rat urine was also detected.
3.1 Performance verification of AA-I magnetic MIPs
A series of experiments were conducted to verify the performance of the synthesized AA-I magnetic MIPs. First, the AA-I standard solution lay the foundation for the dynamic and static adsorption experiments of Fe3O4@SiO2@MPS@MIPs and Fe3O4@SiO2@MPS@NIPs particles. An AA-I standard stock solution with a concentration of 300 μg/mL was prepared. Method: 15 mg of AA-I standard was dissolved in 50 mL of methanol solution, and then, this stock solution was used to prepare a standard working solution of 50–400 μg/mL.
Figure 8 showed the characterization results of Fe3O4@SiO2@MPS@MIPs particles and Fe3O4@SiO2@MPS@NIPs particles. In Figure 8(a), the surface of Fe3O4@SiO2@MPS@MIPs particles was uneven and had a 3D hole structure. In Figure 8(b), the individual Fe3O4@SiO2@MPS@NIPs particles were relatively smooth and did not have a 3D hole structure. Overall, there was not much difference in particle size between the two types of particles, both of which were clustered together. In further transmission electron microscopy and atomic force microscopy analysis, the dark Fe3O4 magnetic core was surrounded by a light-colored SiO2 intermediate layer, and the outermost layer was an MIP-imprinted polymer shell layer with a thickness of about 20–30 nm. The surface roughness of Fe3O4@SiO2@MPS@MIPs particles was significantly higher than that of non-imprinted polymer Fe3O4@SiO2@MPS@NIPs particles, with a root mean square roughness value of about 8.5 nm. The root mean square roughness value of NIPs particles was about 3.2 nm. The significantly increased irregular surface morphology and higher roughness directly confirmed the successful formation of rich imprinting cavity structures on the surface of the MIP layer.

Characterization results of two synthesized particles. (a) Scanning electron micrographs of MIPs. (b) Scanning electron micrographs of NIPs.
The results of isothermal static and dynamic adsorption experiments are shown in Figure 9. In Figure 9(a), in the static adsorption experiment, the adsorption capacity of both materials gradually increased as the concentration of AA-I increased from 50 to 400 mg/mL. 400 mg/mL was significantly higher than that of Fe3O4@SiO2@MPS@NIPs (1.8 mg/g), indicating that MIPs had strong molecular recognition ability and efficient adsorption capacity. The adsorption capacity of Fe3O4@SiO2@MPS@MIPs was relatively low, with a maximum of only about 0.5 mg/g, indicating that materials without specific molecular recognition sites had limited adsorption capacity. In Figure 9(b), in the dynamic experiment, as the time increased from 0 to 20 h, the adsorption capacity of both materials tended to equilibrium. At 20 h, the equilibrium of Fe3O4@SiO2@MPS@MIPs was reached with an adsorption capacity of 0.8 mg/g, and the initial adsorption rate was relatively fast (after about 5 h, the adsorption capacity approached 0.7 mg/g). The adsorption capacity of Fe3O4@SiO2@MPS@NIPs increased slowly, and the final equilibrium adsorption capacity was only about 0.3 mg/g. The specific recognition performance of MIPs enabled them to quickly achieve high adsorption capacity under high concentration and dynamic conditions, demonstrating great potential in adsorption separation technology.

Results of isothermal static and dynamic adsorption experiments. (a) Static experiments. (b) Dynamic experiments.
Next the superiority of the synthesis conditions for AA-I magnetic MIPs was verified. The effects of different synthesis parameters were analyzed, as shown in Figure 10. In Figure 10(a), different ratios of “template molecules: crosslinking agent: functional monomer” (1:20:4, 1:20:6, 1:15:3, 1:25:5, 1:10:4, 1:40:4) had a significant impact on the IF value. Among them, the IF values of 1:10:4 and 1:40:4 were relatively high, close to 3 and 2.5, respectively, while the IF value of 1:15:3 was the lowest, close to 1. In Figure 10(b), there were significant differences in the effect of using different functional monomers on imprinting efficiency. The IF value of methacrylic acid (MAA) was the highest, close to 3.5, followed by methacrylamide (MAM) at around 2. The IF values of AA and 2-vinylpyridine (VP) were relatively low, approaching 1.5 and 1, respectively. MAA exhibited the best imprinting effect as a functional monomer. Figure 10(c) showed that the IF values of different solvents exhibited a certain variation pattern. The IF value of acetonitrile methanol mixed solvent was the highest, close to 4; Methanol ethanol mixed solvent was second, with a value of about 2.5; The IF value of chloroform was the lowest, only 1. This indicated that mixed solvents could provide a more suitable dissolution environment during the molecular imprinting polymerization, thereby improving imprinting efficiency. Choosing MAA as the functional monomer with a reasonable ratio and using acetonitrile methanol mixed solvent could significantly improve the imprinting efficiency.

Analysis of the effects of different synthesis parameters. (a) Imprinting efficiency of different ratios of template molecules, crosslinkers, and functional monomers. (b) Imprinting efficiency of different functional monomers. (c) Stamping efficiency of different solvents.
3.2 Performance verification of AuNCs
The characterization results of PSA-AuNCs are shown in Figure 11. From Figure 11(a), the peak positions of the excitation spectrum and emission spectrum of PSA-AuNCs were approximately 300 and 700 nm, respectively, indicating that the nanoclusters had typical photoluminescence characteristics. The larger stokes shift (∼400 nm) indicated a significant energy loss between photon absorption and emission, which may be related to the surface states of metal clusters and the ligand interactions of proteins. From Figure 11(b), the spectral lines of PSA-AuNCs showed significant changes compared to PSA, indicating that there was an interaction between protein molecules and AuNCs, which may involve deformation of amide bonds or changes in bond energy in the protein. After introducing AA-I, the spectrum of PSA-AuNCs of AA further changed, especially in the absorption peaks at 3,000–3,500 cm−1 (corresponding to the stretching vibration of –OH or −NH groups) and 1,500–1,700 cm−1 (amide I and amide II regions), indicating that AA binds to the nanoclusters through hydrogen bonding or other weak interactions. In Figure 11(c), the nanoparticles were uniformly dispersed with a diameter of approximately 7 nm, exhibiting good monodispersity. This small-sized characteristic provided advantages for its applications in optics, catalysis, and biomedical fields.

Characterization results of PSA-AuNCs. (a) Excitation wavelength and emission wavelength. (b) Comparison of infrared spectra. (c) Particle size image.
The experimental results of the detection conditions were analyzed, as shown in Figure 12. From Figure 12(a), there was not much difference in fluorescence intensity within the pH range of 5.5–9.0, indicating that the solution of PSA-AuNCs can remain stable in this environment. From Figure 12(b), the fluorescence intensity decreased within this pH range when AA-I solution was added. When the pH was 7.4, the fluorescence of PSA-AuNCs was most quenched by AA-I. This confirmed that the detection of AA-I was most sensitive when the pH was 7.4, and this pH matched the pH of the human body environment, making it a potential fluorescent probe for detecting AA-I in biological samples. In Figure 12(c), as the temperature increased from 30 to 80°C, the fluorescence quenching intensity first briefly increased and then gradually decreased, from about 0.4 to nearly 0.1. This indicated that the increase in temperature led to a decrease in fluorescence quenching efficiency, and the most suitable temperature was about 38°C. From the analysis of Figure 12(d), there was not much difference in the degree of quenching with the increase in time, indicating that the detection of AA-I using PSA-AuNCs could be carried out immediately, saving time.

Experimental results of testing conditions. (a) Fluorescence intensities of porcine serum albumin-gold nanoclusters at different pH. (b) Fluorescence intensities of porcine serum albumin-gold nanoclusters at different pH values after the addition of AA-I. (c) Effect of different temperatures on the degree of quenching. (d) Effect of different times on the degree of quenching.
Figure 13 shows the response performance of PSA-AuNCs to AA-I. In Figure 13(a), when a concentration of 0.1 μg/mL of AA-I solution was added, PSA-AuNCs could undergo fluorescence quenching reaction. This indicated that the detection sensitivity of PSA-AuNCs fluorescent probes was good. As the concentration of AA-I solution increased, the fluorescence intensity gradually weakened, indicating that the quenching intensity is also gradually increasing. In Figure 13(b), the concentration of AA-I solution was positively correlated with the fluorescence quenching degree of PSA-AuNCs, with a correlation coefficient of 0.9923. This indicated that the PSA-AuNCs fluorescence probe detection method was suitable for analyzing actual samples.

Detection of the responsiveness of PSA-AuNCs to AA-I. (a) Emission wavelength spectra at different concentrations. (b) The relationship between the concentration of AA-I and the degree of fluorescence quenching of pig serum albumin gold nanoclusters.
The anti-interference experiment is shown in Figure 14. As shown in Figure 14, regardless of the presence of any interfering substances in the sample, PSA-AuNCs only exhibited a significant quenching reaction toward AA-I, with a quenching degree of over 0.40. The results indicated that the system had good anti-interference ability, especially when targeting most metal ions and organic molecules, the signal was not significantly affected.

Anti interference experiment. (a) Quenching strength. (b) Anti-interference degree.
Figure 15 shows the detection results of AA-I in rat urine. In Figure 15(a), the sample recovery rates of rats using the PSA-AuNCs detection method were 116.43, 105.1, and 93.2%, respectively. The recovery rates using high-performance liquid chromatography were higher, at 128.6, 114.5, and 105.7%, respectively. The recovery rate results of the PSA-AuNCs detection method were better. In Figure 15(b), for the three concentrations of AA-I, the detection time using PSA-AuNCs was significantly shorter than that using high-performance liquid chromatography. As the concentration increased, the increase in time required for the research method was not significant, indicating that the method had good detection performance and saved detection time, making it suitable for detecting AA-I components in actual biological samples.

Detection results of AA-I in rat urine. (a) Sample recovery rates. (b) Detection time.
4 Discussion and conclusion
To find a more concise AA-I detection method, this study proposed a detection method based on magnetic carrier Fe3O4 and AuNCs, and designed a discrimination model for it. Its effectiveness has been verified through experiments. The IF of the Fe3O4 magnetic carrier prepared by Wang was 2.16. Compared to this, the prepared carrier had a larger IF of 3.24, indicating better adsorption performance [30,31]. This was because the functional monomer used in the study was methacrylic acid, which could promote its formation of complexes with AA-I, thereby providing more adsorption sites. Deng used high-performance liquid chromatography to detect AA-I, it showed good detection results, but the research method took significantly shorter time [32]. This was because this method could be directly carried out after the collection was completed, without the need for additional instruments, simplifying the detection steps. There were also areas that needed improvement in this study. Due to the limitations of research methods in the selection and aggregation of functional monomers, there might still be faster enrichment methods that can further improve the enrichment efficiency of detecting target substances.
Due to the toxicity of AA-I, existing detection methods mostly focused on the detection of AA-I in pharmaceuticals and medicinal materials. Such detection methods were relatively cumbersome, and there was also a lack of research on detection methods in biological samples. Therefore, to explore a fast and effective method for detecting AA-I, this study proposed a detection method based on magnetic carriers Fe3O4 and AuNCs. This study conducted relevant experiments to verify the effectiveness of the proposed method. In the experimental results, MIPs showed that the adsorption capacity of AA-I in the static adsorption experiment reached 1.8 mg/g at a concentration of 400 µg/mL, which was higher than the 0.5 mg/g of NIPs; The adsorption capacity reached equilibrium within 20 h, and the MIPs exhibited rapid and efficient adsorption characteristics. The influence of synthesis conditions on imprinting efficiency was analyzed, and it was found that the optimal ratio of template molecule, cross-linking agent, and functional monomer was 1:20:4. At pH 7.4 and 37°C, AuNCs exhibited the best fluorescence quenching effect on AA-I, making them suitable for detection in biological samples. The minimum detectable concentration was 0.1 µg/mL, and there was a good linear relationship between the degree of fluorescence quenching and the concentration of AA-I. The recovery rates of rat samples using the PSA-AuNCs detection method were 116.4.3, 105.1, and 93.2%, respectively. In summary, the research method can effectively detect and distinguish AA-I, simplify the testing steps, and shorten the testing time. The limitation of this study lies in the limitations of the aggregation methods and types of functional monomers for MIPs. Future research will focus on developing better separation and enrichment methods.
-
Funding information: The author states no funding involved.
-
Author contributions: The author confirms the sole responsibility for the conception of the study, the presentation of results and manuscript preparation.
-
Conflict of interest: The author states no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: Data will be made available on request to the author.
References
[1] Das S, Thakur S, Korenjak M, Chung F, Zavadil J. Aristolochic acid-associated cancers: a public health risk in need of global action. Nat Rev Cancer. July 2022;22(10):576–91. 10.1038/s41568-022-00494-x.Search in Google Scholar PubMed
[2] Kwok HC, Tse HT, Ng KK, Wang S, Au CK, Cai Z, et al. Absorptivity is an important determinant in the toxicity difference between aristolochic acid I and aristolochic acid II. J Agric Food Chem. Jan. 2025;73(4):2551–61. 10.1021/acs.jafc.4c10765.Search in Google Scholar PubMed PubMed Central
[3] Cui T, Che S, Yan X, Yang R, Xu Z, Liu S, et al. Clinical and safety outcomes associated with aristolochic acid exposure: a systematic review and meta-analysis. Toxicol Mech Methods. Feb. 2025;35(5):513–23. 10.1080/15376516.2025.2457340.Search in Google Scholar PubMed
[4] Wang Z, Li K, Yi X, Wu Y, Zhao Y, He P, et al. Mechanisms of aristolochic acid I hepatotoxicity: Central role of PDK4-induced mitochondrial dysfunction and hepatic inflammation. Food Chem Toxicol. Sept. 2025;186:115592. 10.1016/j.fct.2025.115592.Search in Google Scholar PubMed
[5] Reinoso-Calle JJ, Laso-Barrera CA, Segovia-Valdiviezo JM. Aristolochic acid nephropathy: molecular mechanisms, clinical impact and therapeutic challenges in a progressive renal disease. Iberoam J Med. April 2025;7(2):53–63. 10.53986/ibjm.2025.0012.Search in Google Scholar
[6] Sadighian S, Bayat N, Najaflou S. Preparation of graphene oxide/Fe3O4 nanocomposite as a potential magnetic nanocarrier and MRI contrast agent. Chemistryselect. March 2021;6(12):2862–8. 10.1002/slct.202100195.Search in Google Scholar
[7] Meng W, Gao Y, Tian Z, Xu W, Cheng J, Li S, et al. Fe3O4 magnetic cores coated with metal–organic framework shells as collectable composite nanoparticle vehicles for sustained release of the pesticide imidacloprid. ACS Appl Nano Mater. May 2021;4(6):5864–70. 10.1021/acsanm.1c00800.Search in Google Scholar
[8] Shi Z, Wang Y, Xiao T, Dong S, Lan T. Preparation and thermal decomposition kinetics of a new type of a magnetic targeting drug carrier. ACS Omega. Jan. 2021;6(4):3427–33. 10.1021/acsomega.0c06075.Search in Google Scholar PubMed PubMed Central
[9] Yin Z, Ji Q, Wu D. H2O2-responsive gold nanoclusters@ mesoporous silica@ manganese dioxide nanozyme for “off/on” modulation and enhancement of magnetic resonance imaging and photodynamic therapy. ACS Appl Mater Interfaces. March 2021;13(13):14928–37. 10.1021/acsami.1c00430.Search in Google Scholar PubMed
[10] Kuo J, Tan S, Hsiao C, Chinmaya M, Chen H, Yougbaré S, et al. Unveiling the antibacterial mechanism of gold nanoclusters via in situ transmission electron microscopy. ACS Sustain Chem Eng. Dec 2021;10(1):464–71. 10.1021/acssuschemeng.1c06714.Search in Google Scholar
[11] Yuan S, Lei Z, Guan Z, Wang Q. Atomically precise preorganization of open metal sites on gold nanoclusters with high catalytic performance. Nanomedicine-UK. Dec 2021;60(10):5225–9. 10.1002/anie.202012499.Search in Google Scholar PubMed
[12] Lei Z, Li J, Nan A, Jiang Z, Wang Q. Cluster from cluster: A quantitative approach to magic gold nanoclusters. Angew Chem. April 2021;133(26):14536–40. 10.1002/ange.202103290.Search in Google Scholar
[13] Ren D, Jiang S, Fu L, Wang Z, Zhang S, Zhang X, et al. Laccase immobilized on amino-functionalized magnetic Fe3O4-SiO2 core–shell material for 2, 4-dichlorophenol removal. Environ Technol. March 2022;43(17):2697–711. 10.1080/09593330.2021.1895323.Search in Google Scholar PubMed
[14] Qin K, Shi X, Chen Y, Feng Q, Guo R, Liu Q. Enhanced bio-affinity of magnetic QD-P (St-GMA) @ Fe3O4 micro-particles via surface-quaternized modification. Environ Sci Pollut R. April 2023;30(23):64168–78. 10.1007/s11356-023-26907-4.Search in Google Scholar PubMed
[15] Mohammadi M, Pourseyed A. Magnetite Fe3O4 surface as an effective drug delivery system for cancer treatment drugs: Density functional theory study. J Biomol Struct Dyn. April 2021;39(8):2798–805. 10.1080/07391102.2020.1754915.Search in Google Scholar PubMed
[16] Thong P, Huong L, Tu N, Hoang T, Khanh L, Hong P, et al. Multifunctional nanocarriers of Fe3O4@ PLA-PEG/curcumin for MRI, magnetic hyperthermia and drug delivery. Nanomedicine-UK. May 2022;17(22):1677–93. 10.2217/nnm-2022-0070.Search in Google Scholar PubMed
[17] Williams A. Human-centric functional computing as an approach to human-like computation. ACS Appl Mater Interfaces. Dec. 2023;1(2):118–37. 10.47852/bonviewAIA2202331.Search in Google Scholar
[18] Zhou Y, Yang Z, Zhou R, Zeng B, Liu X, Li X, et al. Peptide-inspired one-step synthesis of surface-functionalized Fe3O4 magnetic nanoparticles for oriented enzyme immobilization and biocatalytic applications. ACS Appl Nano Mater. June 2022;5(6):8260–70. 10.1021/acsanm.2c01346.Search in Google Scholar
[19] Shi Z, Wang Y, Dong S, Lan T. Comparison of the performance of magnetic targeting drug carriers prepared using two synthesis methods. RSC Adv. June 2021;11(34):20670–8. 10.1039/D1RA04256D.Search in Google Scholar PubMed PubMed Central
[20] Groumpos P. A critical historic overview of artificial intelligence: Issues, challenges, opportunities, and threats. ACS Appl Nano Mater. Dec. 2023;1(4):197–213. 10.47852/bonviewAIA3202689.Search in Google Scholar
[21] Wang Z, Li Q, Tan L, Zang S, Mak T. Metal–organic frameworks-mediated assembly of gold nanoclusters for sensing applications. J Anal Test. Dec. 2022;6(2):163–77. 10.1002/ange.202013027.Search in Google Scholar
[22] Li Q, Zeman J, Schatz G, Gui X. Source of bright near-infrared luminescence in gold nanoclusters. ACS Nano. Oct. 2021;15(10):16095–105. 10.1021/acsnano.1c04759.Search in Google Scholar PubMed
[23] Seong H, Efremov V, Park G, Kim H, Yoo J. Atomically precise gold nanoclusters as model catalysts for identifying active sites for electroreduction of CO2. Angew Chem. April 2021;133(26):14684–91. 10.1002/ange.202102887.Search in Google Scholar
[24] Wang Y, Bürgi T. Ligand exchange reactions on thiolate-protected gold nanoclusters. Nanoscale Adv. March 2021;3(10):2710–27. 10.1039/D1NA00178G.Search in Google Scholar PubMed PubMed Central
[25] Liang H, Chen Q, Mo Q, Wu Y, Fang X. Atomically precise thiolate-protected gold nanoclusters: Current advances in solar-powered photoredox catalysis. J Mater Chem A. July 2023;11(17):9401–26. 10.1039/D4TA01662A.Search in Google Scholar
[26] Li S, Nagarajan A, Alfonso D, Sun M, Douglas R, Mpourmpakis G, et al. Boosting CO2 electrochemical reduction with atomically precise surface modification on gold nanoclusters. RSC Adv. Dec. 2021;60(12):6351–6. 10.1002/anie.202016129.Search in Google Scholar PubMed
[27] Wang J, Wang Z, Li S, Liu C, Shang L. Carboranealkynyl‐protected gold nanoclusters: Size conversion and UV/Vis–NIR optical properties. Angew Chem. April 2021;133(11):6024–9. 10.1007/s41664-022-00224-0.Search in Google Scholar PubMed PubMed Central
[28] Si W, Li Y, Zhang S, Wang S, Feng L, Gao Z, et al. Toward controlled syntheses of diphosphine-protected homochiral gold nanoclusters through precursor engineering. ACS Nano. Sept. 2021;15(10):16019–29. 10.1021/acsnano.1c04421.Search in Google Scholar PubMed
[29] Kurani A, Doshi P, Vakharia A. A comprehensive comparative study of artificial neural network (ANN) and support vector machines (SVM) on stock forecasting. Ann Data Sci. June 2023;10(1):183–208. 10.1007/s40745-021-00344-x.Search in Google Scholar
[30] Abedi R, Costache R, Shafizadeh H, Pham Q. Flash-flood susceptibility mapping based on XGBoost, random forest and boosted regression trees. Geocarto Int. May 2022;37(19):5479–96. 10.1080/10106049.2021.1920636.Search in Google Scholar
[31] Wang Y, Liu Y, Cao Z, Zhao X. Effect of doping ratio and amount of polyaniline, cobalt ferrite, and carbon fiber powder on the electromagnetic properties of coated polyester-cotton fabrics. Text Res J. Dec. 2022;92(20):3816–25. 10.1177/00405175221095738.Search in Google Scholar
[32] Deng G, Yun H, Bootharaju M, Hyeon T. Copper doping boosts electrocatalytic CO2 reduction of atomically precise gold nanoclusters, J Am Chem Soc. Dec. 2023;145(50):27407–14. 10.1021/jacs.3c08438.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies

