Abstract
Breast cancer is the second leading cause of mortality among women, accounting for 12.5% of all new cancer cases worldwide. By 2030, the estimated number of deaths will escalate to 11.4 million. The research objective is to design and synthesize safe and effective anti-breast cancer agents. A series of substituted chromone-2-carboxamide derivatives (5a–n) were synthesized in moderate to high yields. These compounds’ (5a–n) antiproliferative activity was evaluated against human ER(+) MCF-7 (breast), ER(−) MDA-MB-231 (breast), and Ishikawa (endometrial) cancer cell lines using CellTiter-Glo assay at concentrations ranging from 0.01 to 100,000 nM. Seven compounds showed significant cytotoxicity against at least one cancer cell line with an IC50 value of 25.7–87.8 µM. Compound 5g showed high activity on MCF-7 (IC50 = 25.7 µM), MDA-MB-231 (IC50 = 48.3 µM), and Ishikawa (IC50 = 25.7 µM) cell lines. The newly synthesized molecules were docked in the active sites of the human estrogen receptors (ERs), ER-α (7KBS), and ER-β (2FSZ) crystal structures to determine the locations of the active compounds’ probable binding sites (bioactive conformations).
1 Introduction
According to the World Health Organization, breast cancer is the second leading cause of mortality among women, accounting for 12.5% of all new cancer cases worldwide. By 2030, the estimated number of deaths will escalate to 11.4 million [1,2]. Furthermore, the incidence rates of breast cancer have been rising by 3% each year. Despite the advancement in breast cancer treatment with currently available drugs, the recovery rate is still unacceptably low. A novel heterocyclic scaffold incorporating many natural product structural features provides a new approach to developing safe and effective anti-breast cancer agents. Chromones (4-H-chromen-4-ones) with a benzo-γ-pyrone framework have emerged as privileged structures in drug discovery, showcasing favorable drug-like characteristics [3,4,5]. They exhibit a diverse array of pharmacological activities, including anti-inflammatory [6], anticancer [7,8], antioxidant [9], antibacterial [10,11], antiviral [12], and antimicrobial [13,14,15], monoamine oxidase B inhibitors [16,17,18,19], and possess A3 adenosine receptor activities [20,21]. Numerous investigators have pursued the development of more diverse and complex bioactive molecules based on the chromone template, as they serve as valuable targets for exploring the structure–activity relationship (SAR) studies in new drug discovery projects. Estrogen receptor (ER), progesterone receptor, and their corresponding steroid hormones play a crucial role in the development, differentiation, and function of normal breast and endometrial cells [22,23]. The two subtypes of human ERs, ER-α and ER-β, show different tissue distribution patterns and transcriptional activities. In ER-positive (ER+) breast cancer cells, blocking the binding of estrogen (E2) to the ER receptors with selective antagonists effectively stops cell growth and proliferation. Since both ER-α and ER-β are often over-expressed in breast cancer cells, they represent promising therapeutic targets for treating this type of cancer [24,25,26,27,28]. Tamoxifen TAM has been the leading drug in treating breast cancer for over two decades, showing significant effectiveness in ER(+) breast cancer, particularly in post-menopausal women [29,30,31]. However, a notable limitation of TAM is that it acts as an ER antagonist in the breast tissue while functioning as an agonist in other tissues with an increased risk of developing endometrial cancer [32,33]. Hussien et al. [34] reported that several new coumarin and chromene prototype derivatives have been synthesized and evaluated for their ERα and ERβ selective activities. Coumarin prototype compounds were found to be ERα selective and the most active, exhibiting potential antiproliferative activity against both ER+ and ER− breast cancer cell lines. Similarly, Saquib et al. [35] showed that a few novel coumarin-chalcone chimeric molecules were synthesized and evaluated for in vitro antiproliferative activity against MDA-MB-231, MCF-7, Ishikawa, and Hela cancer cell lines. One compound showed very good activity against MCF-7 (IC50 = 7.42 μM) and MDA-MB-231 (IC50 = 12.58 μM), better than standard drugs tamoxifen and raloxifene. Our approach was to use the information we learned in designing molecular structures that maximize the safety and efficacy of anti-breast cancer activities. The substitution pattern on chromone scaffolds plays a vital role in determining their affinities toward different biologically significant targets, presenting the possibility of new pathways for developing pharmacologically active compounds. In the present study, chromone-2-carboxamides with electron-donating and electron-withdrawing substituents on the rings were synthesized and evaluated for their anticancer activities against breast cancer cell lines. In silico docking analysis and probable binding modes of these compounds were determined using the Glide XP (extra precision) method by mapping the active site of the human ERs ER-α (7KBS) and ER-β (1QKM) crystal structures to estimate the most probable and stable conformation of the molecules.
2 Materials and methods
All chemicals and solvents were purchased from Sigma-Aldrich and used without further purification. 1H NMR and 13C NMR spectra were recorded on the Varian Gemini HX 300 MHz instrument in DMSO-d6 and CDCl3. The chemical shifts are expressed in parts per million (δ, ppm) relative to tetramethyl silane as an internal standard. Elemental analyses were carried out by Galbraith Laboratories Inc, Knoxville, TN, USA, and are within ±0.4% of theoretical values unless otherwise noted. Melting points were determined on a Mel-Temp 3.0 melting point apparatus and were uncorrected. Column chromatography was performed on silica gel (200–425 mesh). Analytical thin-layer chromatography was performed on 250 µm-layer flexible plates, and spots were detected under UV light. Human MCF-7 and MDA-MB-231 breast cancer cell lines were purchased from the NCI. The human Ishikawa endometrial cancer cell line was purchased from Sigma Aldrich. All three cell lines were cultured in phenol red-free RPMI-1640 (Hyclone, 500 mL) supplemented with l-glutamine-dipeptide (Hyclone, 5 mL) and 50 mL of 10% fetal bovine serum (Atlanta Biologicals).
2.1 General procedure for the synthesis of substituted chromone-2-carboxamides (5a–n)
The substituted chromone-2-carboxamide analogs (5a–n) were synthesized through a four-step process, starting from the readily available 2′,4′,6′-trihydroxyacetophenone, and obtained in moderate to high yields. The synthetic route is depicted in Scheme 1. The dimethoxy-2-hydroxyacetophenones 2a–b were prepared according to the previously described method [36] with an excess of dimethyl sulfate in the presence of anhydrous potassium carbonate in acetone and refluxed for 30 min. The dimethoxy-2-hydroxyacetophenones 2a–b were obtained in good yields. The carboxylic esters 3a–b were prepared by condensation of substituted 2-hydroxy acetophenone 2a–b with diethyl oxalate in the presence of sodium in ethanol. The product underwent cyclization to yield the chromone carboxylic esters [37]. The hydrolysis of the compounds 3a–b with a mixture of conc. HCl and acetic acid (1:2 v/v) gave corresponding chromone-2-carboxylic acids 4a–b in moderate to good yields [38]. The substituted chromone-2-carboxamides 5a–n were obtained by the reaction of 4a–b with differently substituted amines in the presence of diphenylphosphoryl azide (DPPA) and triethylamine (TEA) in moderate to good yields [39]. All the compounds 5a–n were characterized using 1H, 13C NMR, and elemental analyses.

Synthesis of substituted chromone-2-carboxamide derivative 5a–n.
Reaction conditions: (i) DMS, K2CO3, acetone, 60°C, 30 min; (ii) Na, EtOH, diethyl oxalate, conc. HCl, 100°C, 4 h; (iii) acetic acid, conc. HCl, 100°C, 4 h; and (iv) substituted aniline or amine, DMF, DPPA, TEA, R.T. 24 h.
2.2 Biological activity
2.2.1 In vitro antiproliferative activity
The antiproliferative activities of fourteen substituted chromone-2-carboxamide derivatives (5a–n) were evaluated at the Southern Research Institute (SRI, Birmingham, Alabama, USA). The compounds were screened against human MDA-MB-231 (ER−), MCF-7 (ER+) and Ishikawa (endometrial) cancer cell lines in comparison to Tamoxifen (TAM). The cell lines were cultured and treated with compounds 5a–n, including the standard TAM ranging from 0.01 to 100,000 nM concentration in the presence of 10 nM estradiol using the previously reported method [40,41,42]. The results expressed as IC50 were the average of three data points for each concentration and were calculated using GraphPad Prism 4.0.
2.2.2 Molecular docking and pharmacophore generation
The ligands under study (5a–n) were sketched using a 2D sketcher in Schrodinger software’s Maestro interface and were optimized using the LigPrep tool. All possible states at target pH 7.0 ± 2.0 were generated using Epik, and energy was minimized using the OPLS4 force field in Schrodinger’s Small Molecule Drug Discovery Suite [30]. The ER-alpha (ER-α) ligand-binding domain (LBD) in complex with raloxifene (PDB: 7KBS) and the ER-beta (ER-β) LBD in complex with 4-hydroxytamoxifen (2FSZ) were downloaded from RCSB Protein Data Bank using Protein Preparation Workflow in Maestro. The downloaded receptor ligand complexes were prepossessed by capping the terminus, filling in any missed sidechains or loops using Prime. Hydrogen atoms were added to the proteins and ligands, and the entire receptor ligand complex was minimized using the OPLS4 force field. Hydrogen bond assignments were optimized using PROPKA. The water molecules in the crystal structure were removed and later retained close to the bound ligand (5 Å distance) to identify any possible interaction of the compounds under study with water molecules in the active site of the receptors. For thorough experimentation, the centroid of the co-crystallized bound ligand (Raloxifene) was used in receptor grid generation. The size of the grid was subject to small increments to give more room for the ligands to bind as part of the experimentation. Ligand flexibility was also taken into consideration in docking experiments. To validate the docking procedure, re-docking of the co-crystallized ligands (RAL and OHT) was done in the LBD of both the receptors. A Pharmacophore was generated using the PHASE module in Schrodinger Discovery Suite.
3 Results and discussion
3.1 Synthesis of substituted 2-hydroxy acetophenones (2a–b)
A solution of 1-(2,4-dihydroxyphenyl) ethanone (1a, 10 mmol), dimethyl sulfate (20 mmol), and anhydrous K2CO3 (20 mmol) in acetone (30 ml) was refluxed at 50–60°C for 30 min. The reaction mixture was filtered, and the solid residue was washed with warm acetone. The solvent was evaporated in vacuo, and the residue was purified by silica gel column chromatography to obtain compound 2a in good yield. The remaining compound 2b was synthesized similarly.
3.1.1 Synthesis of substituted chromone-2-carboxylic esters (3a–b)
Sodium ethanolate was prepared by dissolving 10 mmol of sodium metal in 15 mL of anhydrous ethanol. At room temperature, the sodium ethanolate solution and diethyl oxalate (1.2 equiv) were added to the corresponding compounds (2a–b, 5 mmol), and the reaction mixture was heated at 90–100°C for 4 h. After this period, 1 mL of conc. HCl was added, and the reaction continued for 30 min. The mixture was then cooled to room temperature, washed with water, and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure using a rotary evaporator. The resulting crude product was purified by silica gel chromatography, yielding compounds 3a–b in 70–90% yields.
3.1.2 Synthesis of substituted chromones-2-carboxylic acids (4a–b)
Hydrolysis of ethyl esters 3a–b was carried out using the reported method [37,38]. A solution of ethyl ester (3a–b, 9 mmol) was added to a mixture of glacial acetic acid and conc. HCl (2:1 v/v), and the reaction was stirred at 100°C for 4 h. Upon completion, the reaction mixture was cooled to room temperature. The resulting precipitates were separated by filtration, washed with methylene chloride, and the solid was dried under a high vacuum. The compounds 4a–b were obtained in 70–80% yields.
3.2 Synthesis of substituted chromones-2-carboxamides (5a–n)
The substituted chromone-2-carboxylic acids (4a–b, 0.2 mmol, 1 eq) were dissolved in DMF (2 mL), and DPPA (82 mg, 1.5 eq), and TEA (30 mg, 1.5 eq). The reaction mixture was cooled to 0°C and stirred for 30 min. The corresponding aniline (28 mg, 1.5 eq) was then added, and the temperature was gradually increased to room temperature. The reaction was allowed to proceed for 24 h. After completion, the reaction mixtures were diluted with ethyl acetate, washed with 5% HCl, and followed with water. The organic layers were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residues were purified by silica gel column chromatography and eluted in 20% ethyl acetate/hexane to afford the final product 5a–n in fair to good yields. In the 1H NMR spectra, the –NH proton in compounds 5a–n appeared as a singlet 8.1–10.58 ppm, confirmed by the D2O exchange.
3.2.1 7,8-Dimethoxy-4-oxo-N-phenyl-4H-chromene-2-carboxamide (5a)
Yield: 90%; solid; m.p. 196–197°C, 1H NMR (CDCl3): δ 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 3.0 Hz, 1H), 7.14 (s, 1H), 7.18 (d, J = 3.0 Hz, 1H), 7.36 (t, 2H), 7.64 (d, J = 3.0 Hz, 2H), 7.91 (d, J = 3.0 Hz, 1H), 8.67(s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 56.10, 60.86, 110.07, 111.37, 119.42, 119.43, 120.47, 123.95, 128.96, 135.57, 138.65, 148.51, 153.09, 157.21, 157.93, 177.22. Anal. calcd. for C18H15NO5 (325.32): C, 66.46; H, 4.65; N, 4.31%. Found: C, 66.58; H, 4.63; N, 4.20%.
3.2.2 6,8-Dichloro-4-oxo-N-phenyl-4H-chromene-2-carboxamide (5b)
Yield 49%; solid; m.p. 225°C (decompose); 1H NMR (CDCl3): δ 7.29 (s, 1H), 7.68 (d, J = 4.0 Hz, 2H), 7.42 (t, J = 6.5 Hz, 2H), 7.24 (t, J = 7.1 Hz, 1H), 7.81 (s, 1H), 7.44 (s, 1H), 8.10 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 113.50, 121.47, 122.03, 123.95, 125.66, 127.94, 128.86, 134.42, 138.65, 150.33, 153.86, 157.47, 173.62. Anal. calcd. for C16H9Cl2NO3 0.2 H2O: (337.763): C, 56.90; H, 2.69; N, 4.15%. Found: C, 56.86; H, 2.82; N, 4.07%.
3.2.3 6,8-Dichloro-N-(2,4-dihydroxy phenyl)-4-oxo-4H-chromene-2-carboxamide (5c)
Yield 27%; m.p. 270°C (decompose); 1H NMR (DMSO-D6): δ 6.25 (dd, J = 1.0, 3.0 Hz, 1H), 6.41 (s, 1H), 6.99 (s, 1H), 7.86 (d, J = 3.0Hz, 1H), 7.97 (s, 1H), 8.29 (s, 1H), 9.40 (brs, 2H, OH, D2O exchangeable), 10.30 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 95.5, 98.14, 113.52, 122.03, 125.66, 126.98, 127.95, 134.54, 140.93, 150.33, 154.20, 157.43, 159.34, 173.62. Anal. calcd. for C16H9Cl2NO5 (366.16): C, 52.48; H, 2.48; N, 3.83%. Found: C, 52.38; H, 2.56; N, 3.67%.
3.2.4 N-(4-Hydroxyphenyl)-7-methoxy-4-oxo-4H-chromene-2-carboxamide (5d)
Yield 82%; m.p. 271–272°C; 1H NMR (DMSO-d6): δ 3.93 (s, 3H, OCH3), 6.78 (d, J = 3.0 Hz, 2H), 6.85 (s, 1H), 7.12 (dd, J = 1.0,3.0 Hz, 1H), 7.29 (d, J = 1.0 Hz, 1H), 7.55 (d, J = 3.0 Hz, 2H), 7.96 (d, J = 3.0 Hz, 1H), 9.45 (s, 1H, NH D2O exchangeable), 10.50 (s,1H, OH, D2O exchangeable); 13C NMR (DMSO-D6): δ 55.88, 101.06, 112.51, 114.55, 115.80, 117.65, 122.77, 125.91, 131.00, 151.25, 154.67, 157.26, 157.88, 163.77, 176.82. Anal. calcd. for C17H13NO5 (311.29): C, 65.59; H, 4.21; N, 4.50%. Found: C, 65.13; H, 4.25; N, 4.29%.
3.2.5 N-(4-Hydroxyphenyl)-7,8-dimethoxy-4-oxo-4H-chromene-2-carboxamide (5e)
Yield 26%; m.p. 295–296°C; 1H NMR (DMSO-d6): δ 3.92 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 6.77 (d, J = 3.0 Hz, 2H), 6.88 (s, 1H), 7.32 (d, J = 3.0 Hz, 1H), 7.53 (d, J = 3.0 Hz, 2H), 7.79 (d, J = 3.0 Hz, 1H), 9.43 (s, 1H, NH, D2O exchangeable), 10.36 (s, 1H, OH, D2O exchangeable); 13C NMR (DMSO-D6): δ 56.10, 60.86, 110.07, 111.37, 115.80, 119.43, 122.77, 131.00, 135.57, 148.51, 151.25, 153.20, 157.21, 157.93, 177.22. Anal. calcd. for C18H15NO6 0.2 H2O (344.92): C, 62.68; H, 4.38; N, 4.06%. Found: C, 62.73; H, 4.47; N, 3.97%.
3.2.6 N-(3,5-Dihydroxyphenyl)-5,7-dimethoxy-4-oxo-4H-chromene-2-carboxamide (5f)
Yield 40%; m.p. 300°C (decompose); 1H NMR (DMSO-d6)): δ 3.83 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 6.24 (dd, J = 2.1, 6.6 Hz, 1H), 6.40 (s, 1H), 6.55 (d, J = 2.1 Hz, 1H), 6.61 (s, 1H), 6.83 (s, 1H), 7.28 (d, J = 8.7 Hz, 1H), 9.35 (s, 2H, OH, D2O exchangeable), 9.70 (s, 1H), 9.76 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 56.38, 56.56, 95.50, 96.45, 98.14, 98.28, 109.03, 110.44, 140.93, 155.03, 153.37, 159.34, 160.04, 164.77, 175.20. Anal. calcd. for C18H15NO7 (357.31): C, 60.50; H, 4.23; N, 3.92%. Found: C, 60.35; H, 4.46; N, 4.12%.
3.2.7 N-(4-Hydroxyphenyl)-5,7-dimethoxy-4-oxo-4H-chromene-2-carboxamide (5g)
Yield 69 %; m.p. 300°C (decompose); 1H NMR (DMSO-d6): δ 3.83 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 6.55 (d, J = 2.4 Hz, 1H), 6.65 (s, 1H), 6.76 (d, J = 8.7 Hz, 2H), 6.85 (d, J = 2.1 Hz, 1H), 7.53 (dd, J = 6.6, 2.1 Hz, 2H), 9.45 (s, 1H, NH, D2O exchangeable), 10.41 (s, 1H, OH, D2O exchangeable); 13C NMR (DMSO-D6): δ 56.45, 56.63, 94.00, 97.08, 109.33, 112.93, 115.60, 123.39, 129.38, 153.69, 155.11, 157.56, 159.17, 160.81, 164.59, 176.01. Anal. calcd. for C18H15NO6 (341.31): C, 63.34; H, 4.43; N, 4.10%. Found: C, 63.45; H, 4.47; N, 3.97%.
3.2.8 N-(3,5-Dihydroxyphenyl)-7,8-dimethoxy-4-oxo-4H-chromene-2-carboxamide (5h)
Yield 70%; m.p. 300°C (decompose); 1H NMR (DMSO-d6): δ 3.96 (s, 6H, OCH3), 6.26 (d, J = 3.0 Hz, 1H), 6.43 (s, 1H), 6.82 (s, 1H), 7.32 (d, J = 3.0 Hz, 1H), 7.79 (d, J = 3.0 Hz, 1H), 7.91 (d, J = 3.0 Hz, 1H), 9.34 (s, 2H, OH, D2O exchangeable), 9.42 (s, 1H), 10.32 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 56.10, 60.85, 95.50, 98.14, 111.37, 112.43, 119.43, 135.57, 140.93, 148.51, 153.43, 157.21, 158.02, 159.34, 177.22. Anal. calcd. for C18H15NO7 (357.31): C, 60.50; H, 4.23; N, 3.92%. Found: C, 60.12; H, 4.44; N, 4.02%.
3.2.9 6,8-Dichloro-N-(4-hydroxyphenyl)-4-oxo-4H-chromene-2-carboxamide (5i)
Yield 35%; m.p. 270°C (decompose); 1H NMR (DMSO-D6): δ 6.76 (d, J = 8.7 Hz, 2H), 7.07 (s, 1H), 7.52 (d, J = 2.7 Hz, 2H), 7.96 (d, J = 2.7 Hz, 1H), 8.26 (d, J = 2.4 Hz, 1H), 9.45 (s, 1H, OH, D2O exchangeable), 10.41 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 113.50, 115.8, 122.03, 122.77, 125.66, 127.93, 127.94, 131.00, 134.42, 150.33, 151.25, 153.96, 157.32, 173.62. Anal. calcd. for C16H9Cl2NO4 0.5 H2O (359.17): C, 53.18; H, 2.56; N, 3.86%. Found: C, 53.51; H, 2.53; N, 3.90%.
3.2.10 6,8-Dichloro-N-(3,5-dimethoxyphenyl)-4-oxo-4H-chromene-2-carboxamide (5j)
Yield 37%; m.p. 290°C (decompose); 1H NMR (DMSO-D6): δ 3.74 (s, 6H, OCH3), 6.34 (t, J = 2.4 Hz, 1H), 7.01 (d, J = 3.9 Hz, 2H), 7.13 (s, 1H), 7.96 (dd, J = 0.6, 2.1 Hz, 1H), 8.28 (dd, J = 0.6, 2.1 Hz, 1H), 10.57 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 55.22, 55.31, 93.55, 98.75, 98.81, 113.52, 122.03, 125.66, 127.93, 127.98, 134.42, 140.81, 150.33, 153.88, 157.15, 161.50, 161.57, 173.62. Anal. calcd. for C18H13Cl2NO5 (394.21): C, 54.84; H, 3.32; N, 3.55%. Found: C, 54.56; H, 3.39; N, 3.91%.
3.2.11 4-{[(6,8-Dichloro-4-oxo-4H-chromen-2-yl)carbonyl]amino}phenyl acetate (5k)
Yield 34%; m.p. 250°C (decompose); 1H NMR (CDCl3): δ 2.29 (s, 3H, –CH3), 7.13 (dd, J = 2.1, 4.8 Hz, 2H), 7.24 (s, 1H), 7.69 (dd, J = 2.1, 4.8 Hz, 2H), 7.81 (d, J = 2.4 Hz, 1H), 8.11 (d, J = 2.4 Hz, 1H), 8.56 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 20.85, 113.50, 120.42, 120.85, 121.71, 121.92, 122.18, 15.66, 127.58, 127.94, 134.42, 136.15, 146.85, 150.33, 153.96, 157.31, 169.15, 173.68. Anal. calcd. for C18H11Cl2NO5 (392.19): C, 55.12; H, 2.83; N, 3.57%. Found: C, 54.85; H, 3.15; N, 3.87%.
3.2.12 6,8-Dichloro-N-((furan-2-yl)methyl)-4-oxo-4H-chromene-2-carboxamide (5l)
Yield 50%; m.p. 135°C; 1H NMR (CDCl3): δ 4.63 (d, J = 2.0 Hz, 2H), 6.30 (t, J = 7.2 Hz, 2H), 7.14 (m, 1H), 7.15 (s, 1H), 7.35 (s, 1H), 7.71 (s, 1H), 8.02 (s, 1H, NH, D2O exchangeable); 13C NMR (CDCl3): δ 35.33, 108.12, 110.56, 113.52, 122.03, 125.39, 127.54, 127.93, 134.42, 141.27, 150.21, 151.78, 152.08, 160.85, 173.56. Anal. calcd. for C15H9Cl2NO4 (338.14): C, 53.28; H, 2.68; N, 4.14%. Found: C, 53.38; H, 2.67; N, 4.04%.
3.2.13 6,8-Dichloro-4-oxo-N-(pyridin-4-yl)-4H-chromene-2-carboxamide (5m)
Yield 40%; m.p. 270°C (decompose); 1H NMR (DMSO-D6): δ 7.20 (s, 1H), 7.74 (d, J = 2.0 Hz, 2H), 7.97 (s, 1H), 8.30 (s, 1H), 8.54 (d, J = 2.0 Hz, 2H), 11.00 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-D6): δ 113.50, 114.04, 122.03, 125.66, 127.93, 128.02, 134.42, 146.09, 150.01, 150.35, 154.89, 157.39, 173.62. Anal. calcd. for C15H8Cl2N2O3 (335.14): C, 53.76; H, 2.41; N, 8.36%. Found: C, 54.28; H, 2.90; N, 7.82%.
3.2.14 6,8-Dichloro-4-oxo-N-((thiophen-2-yl)methyl)-4H-chromene-2-carboxamide (5n)
Yield 44%; m.p. 225°C (decompose); 1H NMR (CDCl3): δ 4.80 (d, J = 2.0 Hz, 2H), 6.94 (dd, J = 1.0, 2.0 Hz, 1H), 7.03 (s, 1H), 7.17 (s, 2H), 7.23 (d, J = 2.0 Hz, 1H), 7.70 (s, 1H), 8.02 (s, 1H, NH, D2O exchangeable); 13C NMR (CDCl3): δ 36.22, 113.32, 122.03, 123.79, 123.79, 125.37, 125.50, 126.85, 127.54, 127.93, 134.42, 141.43, 150.21, 152.74, 160.82, 173.52. Anal. calcd. for C15H9Cl2NO3S (354.21): C, 50.86; H, 2.56; N, 3.95%. Found: C, 50.99; H, 2.71; N, 3.90%.
3.3 SAR
The in vitro antiproliferative activity of the substituted chromone-2-carboxamide derivatives (5a–n) was evaluated against human MDA-MB-231 (ER−), MCF-7 (ER+), and Ishikawa (endometrial) cancer cell lines to establish a SAR for the development of more potent compounds. The results were expressed as IC50 values, which are the concentrations of the test compounds, where a 50% reduction is observed in cell growth compared to the untreated control after a 72 h period of exposure to the test compounds, as shown in Table 1. Six of the 14 compounds showed antiproliferative activity against at least one cancer cell line with an IC50 value of 25.7–87.8 µM. Five compounds were active against MCF-7, in which compound 5g showed more significant antiproliferative activity with IC50 25.7 µM. Four compounds were active against MDA-MB-231 cell lines, and compound 5h showed the lowest IC50, 43.6 µM compared to other compounds. Only two compounds, 5d and 5g, were active against Ishikawa endometrial cancer cell lines, in which compound 5g showed the best activity with IC50 25.7 µM. A detailed analysis of the antiproliferative activity of the substituted chromone-2-carboxamide derivatives revealed several structure-activity trends. Compounds containing dichloro-substitutions on the chromone benzene ring, with either OCH3 groups (5j) or hydroxyl groups in the para-position (5i, 5c) and compounds with direct five- and six-membered heterocyclic rings attached to carboxamide nitrogen or with one carbon apart (5l, 5m, 5n) exhibited complete inactivity against all three cancer cell lines (>100 μM). However, compound 5b, with no substitution on the carboxamide ring, and compound 5k, with a para-acyl substitution, exhibited good selectivity towards MCF-7 cell lines, with IC50 values of 35.8 and 32.8 µM, respectively. Methoxy-substituted compounds on the chromone ring displayed improved activity and selectivity. In particular, compound 5h, with hydroxyl substitutions at the meta-position of the carboxamide aromatic ring, showed very good selectivity on MDA-MB-231 (IC50 = 43.6 µM), and no activity against MCF-7 (>100 µM) and Ishikawa (>100 µM) cell lines, suggesting a potential candidate for a triple-negative anti-breast cancer agent. Compound (5g) showed the best activity for MCF-7 (IC50 = 25.7 μM), which expresses high levels of ER-α. However, compound (5d) with only one -OCH3 group on the chromone ring with hydroxyl substitutions at the para-position of the carboxamide aromatic ring showed good selectivity towards MCF-7 and Ishikawa cell lines (IC50 = 38.2 and 36.2 μM, respectively), albeit with lower activity than compound (5g). Compound (5a) with no substitutions on the carboxamide benzene ring showed no activity on three cell lines.
In vitro antiproliferative activities of substituted chromone-2-carboxamides 5a–n
| Compounds | Structure | Yield (%) | M.P. (°C) | IC50 (µM) | ||
|---|---|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | ISHIKAWA | ||||
| 5a |
 |
90 | 196–197 | >100 | >100 | >100 |
| 5b |
 |
49 | 225* | 35.8 | 93.1 | >100 |
| 5c |
 |
27 | 270* | > 100 | >100 | >100 |
| 5d |
 |
82 | 271–272 | 38.2 | >100 | 36.2 |
| 5e |
 |
26 | 295–296 | >100 | >100 | >100 |
| 5f |
 |
40 | 300* | 87.8 | >100 | >100 |
| 5g |
 |
69 | 300* | 25.7 | 48.3 | 25.7 |
| 5h |
 |
70 | 300* | >100 | 43.6 | >100 |
| 5i |
 |
35 | 270* | >100 | >100 | >100 |
| 5j |
 |
37 | 290* | >100 | >100 | >100 |
| 5k |
 |
34 | 250* | 32.8 | 81.6 | >100 |
| 5l |
 |
50 | 135–136 | >100 | >100 | >100 |
| 5m |
 |
40 | 270* | >100 | >100 | >100 |
| 5n |
 |
44 | 225* | >100 | >100 | >100 |
| Tamoxifen | — | — | 12.7 | 22.8 | 18.1 | |
*Compound decomposed.
3.4 Molecular docking studies
3.4.1 Human ER-α
The individual compounds under study (5a–n) were docked into the active site of the ER-α receptor (PDB ID 7KBS) using the Glide XP (extra precision) workflow as implemented in the Schrodinger Small-Molecule Drug Discovery Suite [43]. For the best results, the centroid of the co-crystallized bound ligand (Raloxifene) was used in receptor grid generation, and a room of 20–25 Å was chosen to give more space and conformational flexibility for the compounds to bind in the active site. Binding affinities of the synthesized compounds with the receptor were visually compared using the Ligand Interaction Diagrams.
A close observation of the top-ranked binding pose of the most active compound in the series toward MCF-7 cell lines (5g) reveals strong hydrogen bonding interactions between the phenyl ring OH group with ARG 394 and LEU 387, π–π stacking interactions of the amide phenyl ring with PHE 404, and amide NH with LEU 346. The interaction of phenyl OH with ARG 394 in the active site is considered the key interaction for a biological response [44]. Figures 1 and 2 show the 2-D and 3-D protein–ligand interaction diagram of the active compound 5g under the current study. In another docking experiment, water molecules in the active site close to the co-crystallized ligand (5 Å) were retained to notice any role of water molecules contributing to binding. A three-way interaction between H2O, the phenylic OH group on the compound, and ARG 394 and LEU 387 of the receptor was observed. (Figure 3). These key interactions might have contributed to the tight binding of the compound 5g in the active site of the receptor and hence the best activity in the present study. A close look at the Glide docking score values of the compounds understudy on ER-α active site (7KBS) (Table 2) show that the majority of least active compounds on all cell lines (5a, 5i, 5j, 5l, 5m, 5n) scored very poorly (−4 to −5) whereas some of the most active compounds in the list (5d, 5f, 5g, 5h) scored better (−6 to −7) as compared to the standard compound under study, i.e., Tamoxifen (−8.02).
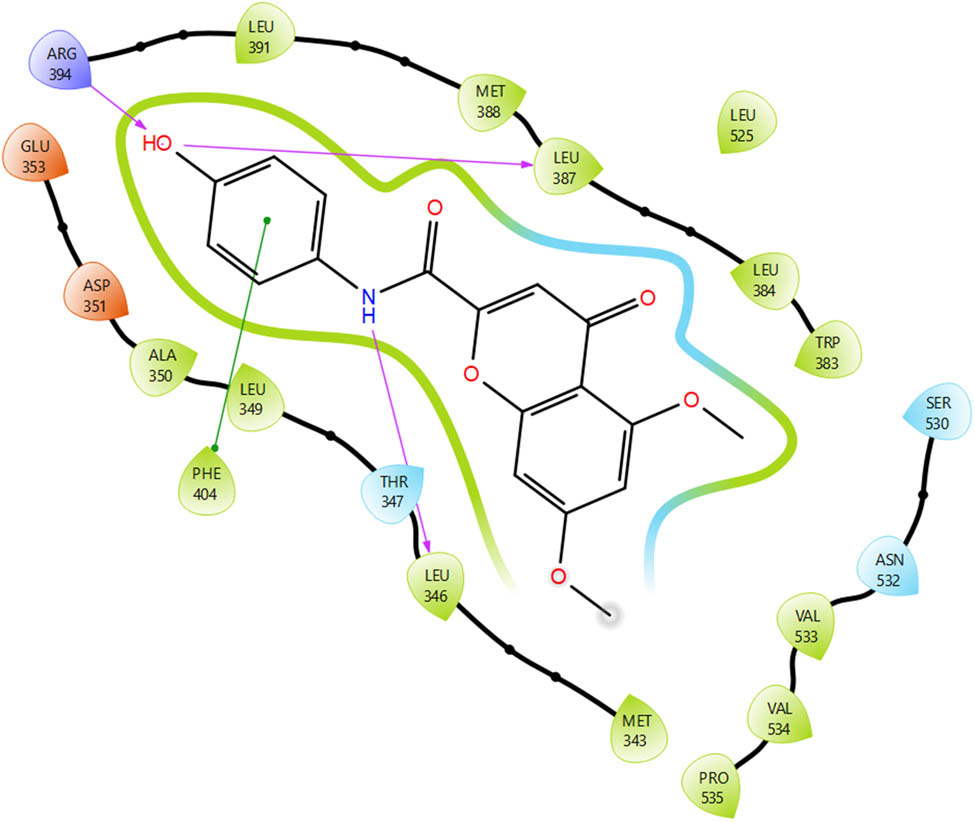
2-D protein–ligand interaction of compound 5g in the active site of 7KBS.

3-D protein–ligand interaction of compound 5g in the active site of 7KBS. Reference ligand (raloxifene) in green.

2-D protein–ligand interaction of compound 5g in the active site of 7KBS with waters retained in the active site.
Glide docking scores of substituted chromone-2-carboxamides 5a–n at the active site of ER-α (PDB ID: 7KBS) and ER-β (PDB ID: 2FSZ)
| Compounds | Structure | Glide docking scores of ER-α (7KBS) | Glide docking scores of ER-β (2FSZ) |
|---|---|---|---|
| 5a |
 |
−5.25 | −5.17 |
| 5b |
 |
−5.22 | −6.16 |
| 5c |
 |
−6.80 | −4.24 |
| 5d |
 |
−6.02 | −6.16 |
| 5e |
 |
−6.12 | −7.54 |
| 5f |
 |
−7.10 | −7.09 |
| 5g |
 |
−6.44 | −6.16 |
| 5h |
 |
−7.10 | −7.05 |
| 5i |
 |
−4.76 | −5.12 |
| 5j |
 |
−4.82 | −5.11 |
| 5k |
 |
−1.485 | −2.70 |
| 5l |
 |
−4.71 | −6.68 |
| 5m |
 |
−4.88 | −6.87 |
| 5n |
 |
−4.83 | −5.84 |
| Tamoxifen | −8.02 | −10.55 |
Based on the docking poses of raloxifene (7KBS ligand) and compound 5g in the active site of the ER-α receptor, the pharmacophore hypothesis was generated using PHASE [43]. The best hypothesis generated is ADHRR, where acceptor, donor, hydrophobic groups, and aromatic rings were hypothesized to be essential for the biological activity (Figure 4).

Overlay of raloxifene (7KBS ligand, green) and compound 5g to generate PHASE hypothesis.
3.4.2 Human ER-β
Similar to the human ER-α docking procedures, the individual compounds under study (5a–n) were docked into the active site of the ER-β receptor (PDB ID: 2FSZ) using the Glide XP (extra precision) workflow as implemented in the Schrodinger Small-Molecule Drug Discovery Suite [43]. The binding affinities of the synthesized compounds with the receptor were compared using the Ligand Interaction Diagrams. The docking scores of the compounds range from −2.7 to −7.4 (Table 2). The crucial π–π stacking interactions between one of the phenyl rings in the compounds under study with PHE 356 of the receptor were not observed. This interaction was believed to be crucial for biological activity, as was observed in Tamoxifen. Also, no good correlation of the docking scores with the activity data was noticed. Interestingly, the crystal structure of the receptor 2FSZ showed to have an alternative binding site, and hence, the possibility of the compounds binding in that active site was explored. Here, the binding scores were very low (−1 to −3) and, hence, the compounds binding there were deemed to be ruled out.
4 Conclusion
In this study, 14 chromone-2-carboxamide derivatives were synthesized in moderate to high yields. The antiproliferative activities of these compounds, 5a–n, against MCF-7, MDA-MB-231, and Ishikawa cell lines were evaluated by CellTiter-Glo assay. Seven compounds showed IC50 values of 25.7–87.8 µM against at least one cancer cell line. Compound 5g showed more significant activity against all three cell lines, with methoxy groups on chromone and a hydroxyl group at the para position of the phenyl carboxamide. Molecular docking was carried out on receptors ER-α and ER-β to identify the key interactions of the compounds understudy with the receptor amino acids. The study on the ER-α showed strong hydrogen bonding interactions between the phenyl ring OH group with ARG 394 and LEU 387, which might be responsible for the biological activity. Good docking score correlations were noticed in this study. Docking studies of the compounds on ER-b did not show any significant interactions or docking score correlations. During this study, we have identified a few molecules that are selective toward MCF-7 over MDA-MB-231 cell lines (5d and 5f) and MDA-MB-231 over MCF-7 cell lines (5h). Efforts are on to synthesize more analogs based on the above data to improve the biological activity of these molecules over those cell lines.
Acknowledgments
This research was supported by the National Institute of Minority Health and Health Disparities of the National Institutes of Health under Grant Number U54MD007582. We also thank Dr. Michael J. Roberts, Cell Biology and Immunology, Southern Research, Birmingham, AL, for testing cytotoxicity on three cell lines. The authors also appreciate the support of National Institutes of Health, NCI under award numbers U54CA233396, U54CA233444, and U54CA233465, which supported the Florida-California Cancer Research, Education and Engagement (CaRE2) Health Equity Center.
-
Funding information: This research was supported by the National Institute of Minority Health and Health Disparities of the National Institutes of Health under Grant Number U54MD007582.
-
Author contributions: M.G.: writing and data curation; M.A.G.: methodology and experiment; S.E.: docking studies; B.M.: writing review and editing; and K.K.R.: supervision, project administration, funding acquisition.
-
Conflict of interest: The authors declare no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data presented in this study are available upon request from the corresponding author. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
References
[1] Tran KB, Lang JJ, Compton K, Xu R, Acheson AR, Henrikson HJ, et al. The global burden of cancer attributable to risk factors, 2010–19: A systematic analysis for the global burden of disease study 2019. Lancet. 2022;400(10352):563–91. 10.1016/S0140-6736(22)01438-6.Search in Google Scholar PubMed PubMed Central
[2] Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660.Search in Google Scholar PubMed
[3] Keri RS, Budagumpi S, Pai RK, Balakrishna RG. Chromones as a privileged scaffold in drug discovery: A review. Eur J Med Chem. 2014;78:340–74. 10.1016/j.ejmech.2014.03.047.Search in Google Scholar PubMed
[4] Gaspar A, Matos MJ, Garrido J, Uriarte E, Borges F. Chromone: A valid scaffold in medicinal chemistry. Chem Rev. 2014;114(9):4960–92. 10.1021/cr400265z.Search in Google Scholar PubMed
[5] Gomes LR, Low JN, Cagide F, Gaspar A, Reis J, Borges F. Structural characterization of some N-phenyl-4-oxo-4H-2-chromone carboxamides. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2013;69(Pt 3):294–309. 10.1107/S2052519213009676.Search in Google Scholar PubMed
[6] Shaveta, Singh A, Kaur M, Sharma S, Bhatti R, Singh P. Rational design, synthesis and evaluation of chromone-indole and chromone-pyrazole based conjugates: Identification of a lead for anti-inflammatory drug. Eur J Med Chem. 2014;77:185–92. 10.1016/j.ejmech.2014.03.003.Search in Google Scholar PubMed
[7] Patil VM, Masand N, Verma S, Masand V. Chromones: Privileged scaffold in anticancer drug discovery. Chem Biol Drug Des. 2021;98(5):943–53. 10.1111/cbdd.13951.Search in Google Scholar PubMed
[8] Nam DH, Lee KY, Moon CS, Lee YS. Synthesis and anticancer activity of chromone-based analogs of lavendustin A. Eur J Med Chem. 2010;45(9):4288–92. 10.1016/j.ejmech.2010.06.030.Search in Google Scholar PubMed
[9] Kim SH, Lee YH, Jung SY, Kim HJ, Jin C, Lee YS. Synthesis of chromone carboxamide derivatives with antioxidative and calpain inhibitory properties. Eur J Med Chem. 2011;46(5):1721–8. 10.1016/j.ejmech.2011.02.025.Search in Google Scholar PubMed
[10] Anjum NF, Aleem A, Nayeem N, Asdaq SM. Synthesis and antibacterial activity of substituted 2-phenyl-4-chromones. Der Pharm Chem. 2011;3(5):56–62.Search in Google Scholar
[11] Raju BC, Rao RN, Suman P, Yogeeswari P, Sriram D, Shaik TB, et al. Synthesis, structure-activity relationship of novel substituted 4H-chromen-1,2,3,4-tetrahydropyrimidine-5-carboxylates as potential anti-mycobacterial and anticancer agents. Bioorg Med Chem Lett. 2011;21(10):2855–9. 10.1016/j.bmcl.2011.03.079.Search in Google Scholar PubMed
[12] Kim MK, Yoon H, Barnard DL, Chong Y. Design, synthesis and antiviral activity of 2-(3-amino-4-piperazinylphenyl)chromone derivatives. Chem Pharm Bull (Tokyo). 2013;61(4):486–8. 10.1248/cpb.c12-01050.Search in Google Scholar PubMed
[13] Dengle RV, Deshmukh RN. Synthesis and antimicrobial evaluation of chromones bearing 1, 5-benzo thiazepinyl moiety. Int J Pharml Sci Res. 2013;4:1495–8. 10.13040/IJPSR.0975-8232.4(4).1495-98.Search in Google Scholar
[14] Gaspar A, Teixeira F, Uriarte E, Milhaze N, Melo A, Cordeiro MN, et al. Towards the discovery of a novel class of monoamine oxidase inhibitors: Structure–Property–Activity and docking studies on chromone amides. Chem Med Chem. 2011;6(4):628–32. 10.1002/cmdc.201000452.Search in Google Scholar PubMed
[15] Reis J, Gaspar A, Milhazes N, Borges F. Chromone as a privileged scaffold in drug discovery: recent advances: Miniperspective. J Med Chem. 2017;60(19):7941–57. 10.1021/acs.jmedchem.6b01720.Search in Google Scholar PubMed
[16] Rao YJ, Abhijit K, Mallikarjun G, Hemasri Y. Design and synthesis of novel benzyloxy-tethered-chromone-carboxamide derivatives as potent and selective human monoamine oxidase-b inhibitors. Chem Pap. 2021;75:703–16. org/10.1007/s11696-020-01332-w.Search in Google Scholar
[17] Alcaro S, Gaspar A, Ortuso F, Milhazes N, Orallo F, Uriarte E, et al. Chromone-2- and -3-carboxylic acids inhibit differently monoamine oxidases A and B. Bioorg Med Chem Lett. 2010;20(9):2709–12. 10.1016/j.bmcl.2010.03.081.Search in Google Scholar PubMed
[18] Gaspar A, Reis J, Fonseca A, Milhazes N, Viña D, Uriarte E, et al. Chromone 3-phenylcarboxamides as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2011;21(2):707–9. 10.1016/j.bmcl.2010.11.128.Search in Google Scholar PubMed
[19] Cagide F, Silva T, Reis J, Gaspar A, Borges F, Gomes LR, et al. Discovery of two new classes of potent monoamine oxidase-B inhibitors by tricky chemistry. Chem Commun (Camb). 2015;51(14):2832–5. 10.1039/c4cc08798d.Search in Google Scholar PubMed
[20] Cagide F, Gaspar A, Reis J, Chavarria D, Vilar S, Hripcsak G, et al. Navigating in chromone chemical space: discovery of novel and distinct A3 adenosine receptor ligands. RSC Adv. 2015;5:78572–85. 10.1039/C5RA14988F.Search in Google Scholar
[21] Gaspar A, Reis J, Kachler S, Paoletta S, Uriarte E, Klotz KN, et al. Discovery of novel A3 adenosine receptor ligands based on chromone scaffold. Biochem Pharmacol. 2012;84(1):21–9. 10.1016/j.bcp.2012.03.007.Search in Google Scholar PubMed
[22] Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: Understanding how progesterone receptor can be used to select endocrine therapy. Breast. 2005;14(6):458–65. 10.1016/j.breast.2005.08.024.Search in Google Scholar PubMed
[23] Johnston S. Fulvestrant and the sequential endocrine cascade for advanced breast cancer. Br J Cancer. 2004;90(Suppl 1):S15–8. 10.1038/sj.bjc.6601632.Search in Google Scholar PubMed PubMed Central
[24] Jordan VC. The role of Tamoxifen in the treatment and prevention of breast cancer. Curr Probl Cancer. 1992;16(3):129–76. 10.1016/0147-0272(92)90002-6.Search in Google Scholar PubMed
[25] Seema B, Shakti S, Puneet K, Swati K, Manan KD, Akshara S, et al. Synthesis and docking studies on styryl chromones exhibiting cytotoxicity in human breast cancer cell line. Bioorg Med Chem Lett. 2010;20(16):4945–50. org/10.1016/j.bmcl.2010.05.108.Search in Google Scholar
[26] Ettinger B. Overview of estrogen replacement therapy: a historical perspective. Proc Soc Exp Biol Med. 1998;217(1):2–5. 10.3181/00379727-217-44198.Search in Google Scholar PubMed
[27] Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64(4):1522–33. 10.1158/0008-5472.can-03-3326.Search in Google Scholar PubMed
[28] Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor alpha mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67(11):5337–44. 10.1158/0008-5472.CAN-06-4582.Search in Google Scholar PubMed
[29] Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–70. 10.1210/endo.138.3.4979.Search in Google Scholar PubMed
[30] Seeger H, Huober J, Wallwiener D, Mueck AO. Inhibition of human breast cancer cell proliferation with estradiol metabolites is as effective as with Tamoxifen. Horm Metab Res. 2004;36(5):277–80. 10.1055/s-2004-814480.Search in Google Scholar PubMed
[31] Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–88. 10.1093/jnci/90.18.1371.Search in Google Scholar PubMed
[32] Phillips DH, Venitt S. Safety of prophylactic Tamoxifen. Lancet. 1993;341(8858):1485–6. 10.1016/0140-6736(93)90934-9.Search in Google Scholar PubMed
[33] Jordan VC. Tamoxifen: A most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205–13. 10.1038/nrd1031.Search in Google Scholar PubMed
[34] Hussain MK, Ansari MI, Yadav N, Gupta PK, Gupta AK, Saxena R, et al. Design and synthesis of ERα/ERβ selective coumarin and chromene derivatives as potential anti-breast cancer and anti-osteoporotic agents. RSC Adv. 2014;4:8828–45. 10.1039/C3RA45749D.Search in Google Scholar
[35] Saquib M, Baig MH, Khan MF, Azmi S, Khatoon S, Rawat AK, et al. Design and synthesis of bioinspired benzocoumarin-chalcones chimeras as potential anti-breast cancer agents. ChemistrySelect. 2021;6:8754–65. 10.1002/slct.202101853.Search in Google Scholar
[36] Tong YF, Chen S, Cheng YH, Wu S. A convenient synthesis of 6-demethoxycapillarisin. Chin Chem Lett. 2007;18(4):407–8. 10.1016/j.cclet.2007.01.049.Search in Google Scholar
[37] Zagorevskii VA, Zykov DA, Orliva EK. Synthesis of Chromone-2-carboxylic Acids and Their Esters. J Gen Chem USSR. 1960;30(12):3850–94.Search in Google Scholar
[38] Paulvannan K, Hale R, Mesis R, Chen T. Tandem N-acyliminium/Pictet–Spengler/intramolecular Diels–Alder reaction: an expedient route to hexacyclic tetrahydro-β-carbolines. Tetrahedron Lett. 2002;43(2):203–7. 10.1016/S0040-4039(01)02074-3.Search in Google Scholar
[39] Lynch JK, Freeman JC, Judd AS, Iyengar R, Mulhern M, Zhao G, et al. Optimization of chromone-2-carboxamide melanin concentrating hormone receptor 1 antagonists: assessment of potency, efficacy, and cardiovascular safety. J Med Chem. 2006;49(22):6569–84. 10.1021/jm060683e.Search in Google Scholar PubMed
[40] Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–8. 10.1016/0022-1759(93)90011-u.Search in Google Scholar PubMed
[41] Gangapuram M, Eyunni S, Redda KK. Synthesis and pharmacological evolution of tetrahydroisoquinolines as anti breast cancer agents. J Cancer Sci Ther. 2014;6:161–9. 10.4172/1948-5956.1000266.Search in Google Scholar PubMed PubMed Central
[42] Suresh VK, Madhavi G, Redda KK. In-vitro antiproliferative activity of new tetrahydroisoquinolines (THIQs) on Ishikawa cells and their 3D pharmacophore models. Lett Drug Des Discov. 2014;11:428–36. 10.2174/1570180811666131203002502.Search in Google Scholar PubMed PubMed Central
[43] Schrödinger Release 2024-1: Maestro. New York, NY: Schrödinger, LLC;s 2024.Search in Google Scholar
[44] McCullough C, Neumann TS, Gone JR, He Z, Herrild C, Wondergem Nee Lukesh J, et al. Probing the human estrogen receptor-α binding requirements for phenolic mono- and di-hydroxyl compounds: A combined synthesis, binding and docking study. Bioorg Med Chem. 2014;22(1):303–10. 10.1016/j.bmc.2013.11.024.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies

