Abstract
The study aimed to explore the potential of Kromalgin, a cost-effective and readily available dental material, as a sustainable solution for the removal of U(VI) metal ions from aqueous solutions. In this context, Kromalgin, a single-use dental impression material discarded after each application, was employed for uranium adsorption, addressing the environmental concern of radioactive pollution while simultaneously providing a method for recycling dental waste. The parameters affecting the adsorption of U(VI) ions on the adsorbent, such as pH, amount of adsorbent, concentration of uranium ions, temperature, and contact time, were examined. Under optimum conditions, a 94.56% adsorption efficiency was achieved. The experimental results of adsorption of U(VI) ions on Kromalgin were evaluated by applying the Langmuir, Freundlich, and Dubinin–Radushkevich isotherm models. According to the obtained data, the adsorption of U(VI) ions on Kromalgin complies with all three isotherm models. Thermodynamic parameters such as enthalpy change (ΔH 0), free energy change (ΔG 0), and entropy change (ΔS 0) were calculated, indicating that the adsorption process is spontaneous and endothermic in nature. The results indicate an adsorption capacity of 142.86 mg g−1 and an adsorption energy of 17.81 kJ mol−1. The abundance, cost-effectiveness, and highly effective results of Kromalgin have positioned it as a reasonable option for U(VI) removal.
1 Introduction
Uranium, a toxic and radioactive heavy metal, is found in small amounts in all components of the environment [1]. Uranium plays a pivotal role in both scientific and industrial fields. Its significance stems primarily from its unique properties as a fissile material, making it a cornerstone of nuclear energy production [2]. Apart from nuclear energy, uranium plays a crucial role, with significant applications in military operations, various industries, and other domains [3]. However, its radioactivity and toxicity present notable risks to both the environment and human health [4,5].
Contamination of various components of the environment with uranium is a critical environmental concern, prompting the scientific community, particularly those in wastewater treatment and environmental research, to develop more efficient remediation methods for uranium removal and protection of clean water resources [6,7,8]. In the environment, uranium primarily exists in the form of oxides, silicate minerals, and phosphate minerals, often bound to various metals. The two predominant oxidation states of uranium are U(IV) and U(VI) [9]. The environmental toxicity of uranium is closely related to the morphology and solubility of uranyl ions (
Alginate, a naturally derived product extracted from brown-green algae, is a sustainable and renewable material widely used in various industries, including food, cosmetics, agriculture, and biomedical applications, particularly in tissue engineering [23,24]. One such example is its common use in dentistry to create impressions of teeth and oral tissues for various dental procedures [25]. It is an environmentally friendly and affordable natural polysaccharide, abundant in free hydroxyl and carboxyl groups, and it exhibits the capability to chelate or form complexes with metal ions, showcasing significant potential as a material for adsorption [26,27]. Alginate-based materials could serve as a cost-effective adsorbent for applications such as wastewater treatment or the removal of contaminant ions including uranium [28]. These materials represent a promising avenue for heavy metal adsorption due to their biodegradability, non-toxicity, and high metal-binding capacity, making them both efficient and environmentally sustainable [29]. Numerous studies in the literature have demonstrated the use of alginate-based materials in the separation and recovery of uranium [28,29,30,31,32,33,34,35,36,37].
Dental alginates are primarily composed of sodium alginate; however, they also contain filler materials (such as diatomite and calcium sulfate) and additives [27]. Kromalgin is a dust-free alginate used for dental impressions with a phased chromatic indicator. After use in dentistry, it is discarded without being recycled [35]. It can be obtained affordably from any pharmaceutical store and is disposed of as waste in dental practices, making it available from there as well. Unlike pristine alginate, dental alginates contain various additives, including calcium sulfate as a setting agent, diatomaceous earth to enhance the mechanical properties, and trisodium phosphate to improve the shelf life [27]. These components may influence the material’s adsorption capacity and overall chemical behavior. Therefore, prior to being used as an adsorbent, dental alginates would require appropriate pretreatment or purification processes.
In this study, the adsorption characteristics of U(VI) ions from aqueous solutions by an alginate-based commercial dental material (Kromalgin) were investigated. Kromalgin was selected as a representative dental alginate to evaluate the adsorption behavior of commercially available formulations rather than pristine alginate. Additionally, this study provides valuable insights into the potential utilization of dental waste materials for adsorption applications. The factors influencing the adsorption of U(VI) ions on the adsorbent, including pH, adsorbent quantity, initial concentration, temperature, and contact time, were examined. The thermodynamic parameters for the adsorption of uranium on the adsorbent were determined by analyzing experimental data obtained at various temperatures. For better interpretation, the mechanism of uranium adsorption processes was modeled by Langmuir, Freundlich, and Dubinin–Radushkevich (D–R) isotherms, which are commonly used isotherms for adsorption in the solid–liquid phase. Additionally, Fourier transform infrared (FTIR) spectroscopy and scanning electron microscopy (SEM) analyses were conducted to further investigate the structural and morphological changes in the adsorbent material before and after U(VI) adsorption, which provided deeper insights into the adsorption mechanism.
2 Materials and methods
2.1 Reagents
2.2 Instrumentation
A thermostated shaker bath (ST402, Nuve, Ankara, Turkey) was used to perform batch adsorption tests. The pH of each solution was measured using a microprocessor pH meter (Inolab pH 537, WTW, Weilheim, Germany). The samples were centrifuged using a digital centrifuge (Universal 16A, Hettich, Tuttlingen, Germany). The samples were dried in a laboratory oven (M420, Electro-Mag, İstanbul, Turkey). The concentration of U(VI) ions in the solution was determined using an ultraviolet–visible (UV–vis) spectrophotometer (UV–vis 1601, Shimadzu, Kyoto, Japan). FT-IR spectra were recorded using an FT-IR spectrometer (Spectrum BX, PerkinElmer, Waltham, MA, USA). The surface properties and morphology of the adsorbent were examined using a scanning electron microscope (Thermo Scientific Phenom, Waltham, MA, USA).
2.3 Preparation of the adsorbent
The adsorbent material used in the study was Kromalgin, which was obtained commercially. The composition of the material is sodium alginate (10–12%), calcium sulfate (∼10%), magnesium oxide (3–5%), diatomaceous earth (60–70%), dipotassium hexafluorotitanate (0.5–2.5%), zinc oxide (0.5–2.5%), and minor components such as retardants, stabilizers, pigments, and flavoring agents. To purify the adsorbent from undesirable materials, Kromalgin was washed with distilled water and alcohol and then stirred thoroughly until a uniform density was achieved. Subsequently, it was dried in an oven at 105 ± 5°C for 6 h. After grinding, the resulting adsorbent was crushed using a mortar and then passed through a 150 μm sieve. The stages of the experimental procedure are illustrated in Figure 1.
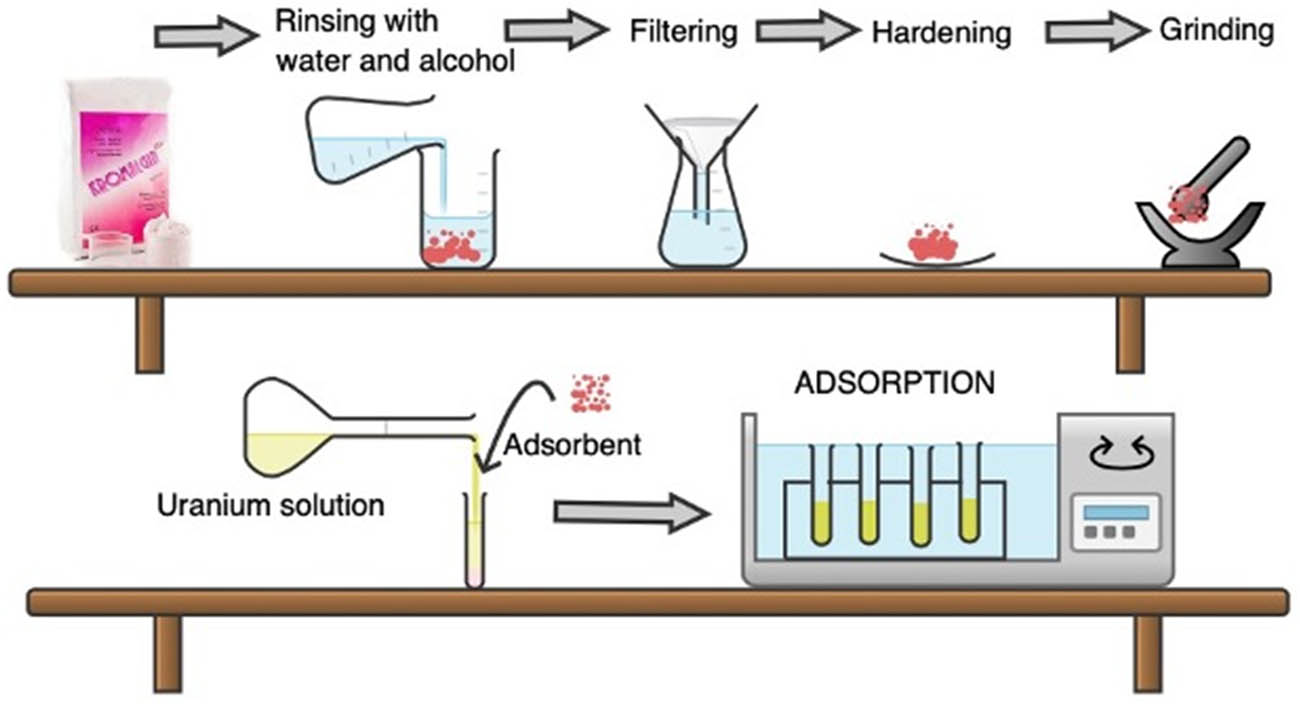
Flowsheet of the preparation of the adsorbent and experimental procedure.
2.4 Batch sorption experiments
Batch procedures were used to conduct experimental studies. A polyethylene (PE) bottle containing 0.05 g of adsorbent was stirred with 10 mL of a known concentration of U(VI) solution in a thermostatically controlled shaking water bath at constant temperature. After shaking for a specific duration, the adsorbent was separated, and the amount of adsorbed U(VI) ions was analyzed spectrophotometrically using tri-n-octylphosphine oxide–dibenzoylmethane (TOPO–DBM) method at 405 nm [38].
2.5 Data analysis
The following equations were used to calculate the adsorption percentage (
The symbols used in the above equations represent the following: C i (mg L−1) is the concentration of uranium ions before adsorption, C e (mg L−1) is the concentration of uranium ions remaining in the solution after adsorption, V (mL) is the volume of the liquid phase, and m (g) is the weight of the adsorbent.
Langmuir, Freundlich, and D–R isotherm models were used in the adsorption studies. Constant values in these models were calculated using the equations given below.
Langmuir isotherm equation [40,41]:
The symbols in the equation represent the following: C e is the equilibrium concentration of metal ions in the solution, q e is the amount of metal ions adsorbed onto the adsorbent Q max is the monolayer adsorption capacity, and b L is the adsorption energy.
Freundlich isotherm equation [42]:
In the Freundlich equation, q e is the amount of adsorbate adsorbed per mass of adsorbent at equilibrium. K F and n F are Freundlich constants, which express the adsorption capacity and intensity, respectively.
D–R isotherm equation [43]:
The mean energy of adsorption E (kJ mol−1) was calculated using the following equation [43]:
The symbols used in the above equations are as follows: C ads is the concentration of the adsorbed metal ion, X m is the maximum adsorption capacity, β is the activity coefficient related to adsorption energy, ε is the Polanyi potential, R is the universal gas constant, (8.314 J mol−1 K−1), and T is the absolute temperature.
The equations used to calculate the thermodynamic parameters are given by equations (8) (van’t Hoff equation) and (9) (Gibbs free energy equation) [44].
In the above equations, K d is the distribution coefficient, ∆H 0 is the enthalpy, ∆S 0 is the entropy, K is the absolute temperature, R is the gas constant, and ∆G 0 is the Gibbs free energy.
3 Results and discussion
3.1 Characterization of the adsorbent
Characterization of the adsorbent has been performed using FTIR spectroscopy and SEM analysis. FT-IR spectra were recorded in the range of 4,000–400 cm−1 at a resolution of 2 cm−1. The FTIR spectra presented in Figure 2 reveal structural variations between commercial Kromalgin and its U(VI)-loaded water/alcohol-treated forms. A prominent peak around 1,068 cm⁻1, observed in all spectra, is attributed to C–O [31,45,46] or C═O stretching vibrations within the carboxylate groups of sodium alginate [46] originating from the structure of Kromalgin. The common peaks at 620 and 791 cm⁻1 are assigned to Si–O–Si bending and symmetric stretching vibrations, respectively, with similar vibrations reported in the range of 600–825 cm⁻1 [47,48], attributable to the silicate structures originating from the diatomite content in Kromalgin. The peaks around 1,412 and 1,616 cm−1 correspond to the symmetric and asymmetric stretching vibrations of the carboxylate (–COO⁻) groups from sodium alginate [32,45,46]. The O–H stretching vibration appears within the range of 3,400–3,700 cm⁻1, corresponding to hydroxyl groups [31,32,37,45,46,47], and the peaks at 2,903 and 2,978 cm⁻1 suggest interactions involving alkyl groups [37,46,47,49,50].

IR spectra of Kromalgin before and after U(VI) adsorption.
Following the adsorption of uranium(vi), a significant change in the intensity of several peaks in the spectrum was observed, indicating enhanced interactions of the functional groups and structural changes in the material. The peaks at 3,677 cm⁻1 suggest a possible interaction between hydroxyl groups and U(VI) ions. The increased intensity observed at 2,903 and 2,978 cm⁻1 is notable, and a similar enhancement is seen at 1,412 and 1,616 cm⁻1, collectively suggesting changes in the surface structure of alginate upon U(VI) binding. These results suggest a possible role of hydroxyl and carboxylate groups in the adsorption process, while alkyl groups may influence the interaction indirectly by modifying the surface properties of the adsorbent. In summary, the FTIR spectra presented in Figure 2 suggest the adsorption of U(VI) ions by the alginate-based Kromalgin. The observed changes in the characteristic functional group peaks imply coordination interactions between
SEM analysis was performed to investigate the surface morphology of Kromalgin before and after adsorption. Figure 3(a) and (b) presents SEM images of Kromalgin before adsorption at 300 and 150 µm scales, respectively, and Figure 3(c, e, and f) presents SEM images of Kromalgin samples treated with water and Figure 3(d) of Kromalgin samples treated with alcohol after U(VI) adsorption. The images reveal morphological differences that arise from both the treatment method and the comparison between the states before and after adsorption, highlighting the pore structures of the water-treated sample in greater detail.

SEM images of Kromalgin samples (a) and (b) Kromalgin before adsorption (300 and 150 µm); (c) water-treated Kromalgin after adsorption (150 µm); (d) alcohol-treated Kromalgin after adsorption (150 µm); (e) water-treated Kromalgin after adsorption (200 µm); (f) water-treated Kromalgin after adsorption, showing pore structures (200 µm).
As shown in Figure 3(a) and (b), before the adsorption of U(VI) ions, Kromalgin exhibited a less compact structure with numerous surface pores, which diminished after adsorption. The rough and non-uniform structure of the adsorbent plays a role in providing more binding sites and increasing the contact area, which reduce the mass transfer resistance and facilitate the diffusion of metal ions during the adsorption process [47]. After U(VI) ion adsorption, as shown in Figure 3(c)–(e), the surface of Kromalgin becomes smoother, and the pores are relatively reduced, which can be attributed to the adsorption of U(VI) ions. In the SEM images after adsorption, more wrinkles and small particles are observed on the surface. These particles can be attributed to the adsorption of U(VI) ions, suggesting their attachment to the surface of Kromalgin during the adsorption process. Particle size measurements were conducted on a representative SEM image (Figure 3f), revealing particle diameters ranging from 6.81 to 35.07 μm, suggesting a heterogeneous structure with a broad particle size distribution.
3.2 Effect of pH
Because pH affects the charge on the active site of the adsorbent surface, it is the key variable in the metal adsorption process in solution. Throughout the adsorption of U(VI), the surface charge of the adsorbent may undergo changes depending on the pH. This variability in pH can influence the ability of
Batch adsorption experiments were performed at various pH levels to investigate the impact of pH on the adsorption of U(VI) ions onto Kromalgin. Three parallel series of experiments were conducted on the adsorbent: (1) commercially available form; (2) alcohol-treated adsorbent; (3) water-treated adsorbent. Washing with water aimed to remove water-soluble impurities and residual salts that could block active adsorption sites, while washing with alcohol targeted the removal of organic contaminants and enhanced surface cleanliness. The untreated sample served as a control to observe the baseline adsorption capacity. This approach allowed a comparative assessment of how different pretreatment methods influence the availability of binding sites and the overall efficiency of uranium adsorption. Experiments were carried out at pH 2.5, 3, 4, 5, 6, 7, and 8 with an initial concentration of 150 mg L−1 U(VI), at a temperature of 30°C for 60 min of adsorption time (Figure 4).

Effect of pH on adsorption of U(VI) on Kromalgin (C i: 150 mg L−1, m: 0.05 g, V: 10 mL, t: 1.0 h, and T: 30°C).
According to the experimental study results, commercial, alcohol-treated, and water-treated adsorbents showed maximum adsorption at pH 3 under the specified conditions with the adsorption percentage of 75.06, 86.15, and 89.70%, respectively. The small difference in adsorption efficiencies is due to the fact that the Kromalgin adsorbent material, free of water-soluble impurities, exhibits better adsorption performance compared to when alcohol-soluble impurities are present. Therefore, the subsequent experiments were conducted using the water-treated adsorbent.
When the distribution profiles of uranium species at different pH values reported by Zhang et al. are examined; it can be observed that pH 3 is a value where the hydrolysis of uranium(vi) ions is minimal, and thus UO2 +2 ions are the dominant species in the solution [52]. Therefore, experiments conducted at pH 3 provide ideal conditions for investigating the adsorption efficiency of uranium(vi) ions.
3.3 Effect of time
The effect of contact time on U(VI) adsorption was investigated by experiments conducted at varying contact times (5–1,440 min) with an initial concentration of 150 mg L−1 U(VI) solution at pH 3 (Figure 5). Figure 5 shows that as the contact time increases, the adsorption percentage and equilibrium constant of the ion increase for the Kromalgin adsorbent. During a 30 min agitation period, the adsorption percentage and distribution coefficient of U(VI) ions were found to be 92.35% and 2415.73 mL g⁻1, respectively. Subsequent periods showed only a slight increase in the adsorption percentage reaching 94.56% adsorption and the equilibrium constant reaching a 3473.25 mL g⁻1 distribution coefficient after 300 min. In the agitation periods of 600–1,440 min, there was little change in the adsorption percentage and equilibrium constant. Considering the economy of the applied process, a 1-hour mixing time is deemed efficient.

Effect of contact time on the adsorption of U(VI) on Kromalgin (C i: 150 mg L−1, m: 0.05 g, V: 10 mL, pH: 3, and T: 30°C).
3.4 Effect of temperature
To examine the effect of various temperatures on U(VI) adsorption on Kromalgin, 0.05 g of adsorbent was shaken with a U(VI) solution in a thermostat water bath at temperatures of 298.15, 303.15, 308.15, and 313.15 K for 24 h. The results are shown in Figure 6.

Effect of temperature on the adsorption of U(VI) on Kromalgin (C i: 150 mg L−1, m: 0.05 g, V: 10 mL, pH: 3, and t: 1.0 h).
Thermodynamic parameters of adsorption were calculated using van’t Hoff graphs (Figure 7) drawn on the basis of the Nernst distribution law and the van’t Hoff equation. The results are given in Table 1. The rise in temperature increased the adsorption capacity, indicating that the adsorption process is endothermic. This was also supported by the positive standard enthalpy (52.71 kJ mol−1). The positive value of enthalpy change (ΔH 0) between 298.15 and 313.15 K reflects the difference in bond energy between coordinated water and the adsorbent [53]. The sorption-free energy value indicates that the chemisorption process is the dominant mechanism [54] in the U(VI) adsorption process on water-treated Kromalgin. The entropy change (ΔS 0) value was found to be 0.234 kJ mol−1 K−1 for the adsorption of U(VI) ions. A positive entropy value indicates that random adhesion at the solid/solution interface increases during the adsorption process [55]. The fact that the free energy change (ΔG) reaches smaller values with increasing temperature explains that the adsorption process is spontaneous and favorable at high temperatures. In addition, negative values of ΔG 0 indicate that the adsorption nature is thermodynamically feasible.

Van’t Hoff plot for adsorption of U(VI) on Kromalgin in aqueous solution.
Thermodynamic parameters for the adsorption of U(VI) on Kromalgin
| ΔH 0 (kJ mol−1) | ΔS 0 (kJ mol−1 K−1) | ΔG 0 (kJ mol−1) | |||
|---|---|---|---|---|---|
| 298.15 K | 303.15 K | 308.15 K | 313.15 K | ||
| 52.71 | 0.234 | −17.06 | −18.23 | −19.40 | −20.57 |
3.5 Effect of concentration
To examine the effect of initial concentration on

Effect of concentration on the adsorption of U(VI) on Kromalgin (m: 0.05 g, V: 10 mL, pH: 3, t: 1.0 h, and T: 30°C).
3.6 Sorption isotherms
The adsorption isotherm serves as a method for characterizing the equilibrium state between the concentration of solutes adsorbed on a solid surface and the corresponding amount of adsorption under constant temperature conditions. Adsorption continues until the concentration of solute remaining in the solution reaches a dynamic equilibrium with the concentration of solute adsorbed on the surface. In this state of equilibrium, there is a distinct distribution between the solid and liquid phases of the solute. This distribution rate is a measure of the equilibrium state in the adsorption process. The interaction between the adsorbent and the adsorbate can be described by various adsorption isotherms, including the Freundlich, Langmuir, and D–R isotherms. These three adsorption isotherms are commonly used for solid–liquid phase adsorption. Specifically, the Langmuir and Freundlich models are commonly used to correlate experimental equilibrium adsorption data of heavy metals onto alginate-based adsorbents [26]. The Langmuir adsorption isotherm model is commonly used to describe adsorption on a homogeneous surface, assuming monolayer coverage without interactions between the adsorbed molecules [40]. The Freundlich isotherm is employed to characterize heterogeneous adsorption, which assumes a surface that is not energetically uniform [42]. The D–R isotherm serves as an empirical model that aids in understanding the adsorption process, distinguishing between chemical and physical adsorption [43].
Graphs of Langmuir, Freundlich, and D–R isotherms were created with the equilibrium data obtained for U(VI) ions in the concentration range of 150 to 500 mg L−1 at 30°C (Figure 9). The linearity of the curves represented by R 2 was found to be 0.9968, 0.9825, and 0.9836 for Langmuir, Freundlich, and D–R isotherms, respectively. This indicates a good correlation between the experimental data and the mathematical model equations for the three isotherm models. Isotherm data derived from the adsorption of U(VI) ions onto Kromalgin are presented in Table 2.

Sorption isotherms of adsorption of
Parameters of Langmuir, Freundlich, and D–R isotherms for the adsorption of U(VI) on Kromalgin
| Isotherm models | Parameters | |
|---|---|---|
| Langmuir | Q max (mg g−1) | 142.86 |
| b L (L g−1) | 0,086 | |
| R 2 | 0.9968 | |
| Freundlich | K F (mg g−1) | 2159.23 |
| n F | 5.67 | |
| R 2 | 0.9825 | |
| D–R | X m (mg g−1) | 4.58 |
| β (mol kJ−1)2 | −0.1575 × 10−8 | |
| E (kJ mol−1) | 17.81 | |
| R 2 | 0.9836 | |
According to the Langmuir isotherm, the adsorption capacity for U(VI) ions was determined to be 142.86. The perfect fit of the isotherm graphs to the Langmuir isotherm indicates that the adsorption is homogeneous, single-layered, and that chemical adsorption is dominant. This result is compatible with that obtained from the adsorption enthalpy. The D–R isotherm was employed to estimate the mean free energy of adsorption (E). If the E value is between 1.00 and 16.00 kJ mol−1, adsorption is physical, and when it is above 16.00 kJ mol−1, it is chemical adsorption [54]. The mean energy of adsorption was calculated as 17.81 kJ mol−1. The adsorption energy E is greater than 16.00 kJ mol−1 for U(VI) ions, showing that the adsorption of U(VI) ions on Kromalgin proceeds through the chemical interaction mechanism.
Various adsorbents, including activated carbon [56], metal–organic frameworks (MOFs) [57], clays [58], and bio-based materials [59] have been extensively studied for U(VI) removal. Among these, activated carbon is widely used due to its high surface area and well-developed porosity, with adsorption capacities ranging from 2.3 to 639.77 mg g⁻1 under optimal conditions, as reported by Mittal et al. [60]. However, alginate-based materials offer advantages such as cost-effectiveness, biodegradability, and ease of modification [61]. The alginate-based adsorbent materials can be used as unmodified or functionalized with various compounds. Studies have shown that the enhancement in characteristics and functional properties, including mechanical strength and adsorption capacity, is evident compared when with its unmodified form when alginate is functionalized with organic compounds [26]. Characteristics of alginate can also be improved by compounding with organic substances, oxides, or nanoparticles [62,63,64]. Many studies have been conducted on U(VI) adsorption using alginate-based adsorbents. In Table 3, some examples from U(VI) adsorption studies using alginate-based adsorbents in the literature are presented in comparison with the current study. Although a direct comparison is not possible, the Kromalgin adsorbent used in this study provides significant results, rivaling the alginate-based adsorbent materials in the literature.
Comparison of experimental results of U(VI) adsorption using different alginate-based adsorbents in the literature
| Adsorbent | pH | Q max (mg g−1) | Thermodynamic | Adsorption efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Calcium alginate beads | 3.0 | 23.4 | Endothermic | — | [30] |
| Calcium alginate beads | 7.0 | 237.15 | Exothermic | 82.24 | [31] |
| Calcium alginate beads | 4.0 | 400 | Endothermic | 91 | [28] |
| La–BDC/rGO/GQDs@mingled hydrogel nanocomposite | 7.12 | 39.2 | Endothermic | 91.19 | [32] |
| Floating macroporous alginate–agarose–magnetite cryobeads | 5.0 | 120 | Endothermic | 97 | [33] |
| B. subtilis/alginate–chitosan microcapsule | 6.0 | 376.64 | Endothermic | — | [34] |
| SCX 37 composite | 4.0 | 41.39 | Endothermic | 97.56 | [35] |
| Kromalgin | 3.0 | 142.86 | Endothermic | 94.56 | This study |
3.7 Desorption studies
Recovering uranium from the loaded adsorbent is crucial for cost-effectiveness and plays a key role in the overall efficiency of the uranium removal process from aqueous media [65]. Desorption experiments were carried out by treating 0.05 g of U(VI)-loaded adsorbents (150 mg L⁻1) with 10 mL of HNO₃ and NaOH solutions at concentrations of 0.5 and 1.0 mol L⁻1. After a contact time of 1 h, the aqueous phase was sampled for analysis. Experiments with NaOH at concentrations of 0.5 and 1.0 mol L−1 resulted in relatively low desorption efficiencies of 28 and 41%, respectively. In contrast, HNO3 achieved significantly higher efficiencies of 72.35 and 81.41% at concentrations of 0.50 and 1.0 mg L⁻1, respectively. These findings demonstrate that HNO3 is an effective desorbing agent for uranium, achieving significantly higher recovery rates compared to NaOH based on a single desorption cycle.
4 Conclusions
The increasing presence of uranium in water sources due to industrial and nuclear activities poses significant environmental and health risks. Therefore, the development of cost-effective and efficient adsorbents for uranium removal remains a critical area of research. This study aimed to evaluate the potential of Kromalgin, a commercially available dental alginate material, as an alternative adsorbent for U(VI) removal from aqueous solutions. The data obtained from the experimental studies can be summarized as follows:
The commercial dental filling material Kromalgin is an effective means of adsorption of U(VI) from aqueous solutions.
To ensure the removal of U(VI) ions from aqueous solutions at the highest possible level, the parameters affecting adsorption were investigated, and the optimum conditions were determined. The maximum uptake of U(VI) ions (94.56%) was achieved under optimum conditions: at pH 3 and 303.15 K and with 150 mg L−1 initial concentration.
By examining the effect of temperature, the thermodynamic parameters (∆H, ∆S, and ∆G) were calculated. As the temperature increased, it was found that the adsorption percentage increased and the Gibbs free energy change decreased. Adsorption was spontaneous and effective at higher temperatures.
The adsorption of U(VI) ions on Kromalgin conforms to Langmuir, Freundlich, and D–R adsorption isotherms. Chemical interactions are dominant in the adsorption mechanism. Adsorption capacity and the adsorption energy were found to be 142.86 mg g−1 and 17.81 kJ mol−1, respectively.
Desorption experiments showed that 1.0 mol L−1 HNO₃ effectively desorbed the uranium adsorbed by 0.05 g Kromalgin, achieving a uranium recovery of 81.1% after 1 h, indicating the material’s ability to release adsorbed uranium in a single desorption cycle.
This study highlights Kromalgin as a promising adsorbent for uranium removal; however, further research is necessary to optimize its application under real-world conditions. Future investigations should focus on evaluating its long-term stability, adsorption efficiency in complex water matrices, and potential for regeneration and reuse. Additionally, modifying Kromalgin with functionalized materials or composite structures could enhance its adsorption performance, broadening its applicability in environmental remediation. The repurposing of this single-use dental waste material also contributes to sustainability efforts. These findings contribute to the ongoing development of cost-effective and sustainable adsorbents, supporting global efforts in radioactive waste management and water purification technologies.
Acknowledgments
The author would like to thank Pamukkale University, Faculty of Science, Department of Chemistry, for their support and contributions in providing the chemicals and devices used in the experimental studies.
-
Funding information: The author states no funding involved.
-
Author contribution: K.E.E.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing – original draft, writing – review and editing.
-
Conflict of interest: The author states no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wang YQ, Zhang Z, Li Q, Liu YH. Adsorption of uranium from aqueous solution using HDTMA+-pillared bentonite: Isotherm, kinetic and thermodynamic aspects. J Radioanal Nucl Chem. 2012;293:231–9. 10.1007/s10967-012-1659-4.Search in Google Scholar
[2] Zhu J, Zhao L, Song D, Yu J, Liu Q, Liu J, et al. Graphene oxide based ion-imprinted polymers for selective uranium adsorption from seawater. Appl Surf Sci. 2023;640:158378. 10.1016/j.apsusc.2023.158378.Search in Google Scholar
[3] Li L, Li H, Lin M, Wen J, Hu S. Effects of chain conformation on uranium adsorption performance of amidoxime adsorbents. Sep Purif Technol. 2023;307:122777. 10.1016/j.seppur.2022.122777.Search in Google Scholar
[4] Wu L, Yang X, Chen T, Li Y, Meng Q, Zhu L, et al. Three-dimensional C3N5/RGO aerogels with enhanced visible-light response and electron-hole separation efficiency for photocatalytic uranium reduction. Chem Eng J. 2022;427:131773. 10.1016/j.cej.2021.131773.Search in Google Scholar
[5] Chen T, Zhang J, Ge H, Li M, Li Y, Liu B, et al. Efficient extraction of uranium in organics-containing wastewater over g-C3N4/GO hybrid nanosheets with type-II band structure. J Hazard Mater. 2020;384:121383. 10.1016/j.jhazmat.2019.121383.Search in Google Scholar PubMed
[6] Pan N, Jin Y, Wang X, Hu X, Chi F, Zou H, et al. A self-assembled supramolecular material containing phosphoric acid for ultrafast and efficient capture of uranium from acidic solutions. ACS Sustain Chem Eng. 2019;7(1). 10.1021/acssuschemeng.8b04596.Search in Google Scholar
[7] Saksham BSK, Rakesh K, Rohit M, Manpreet SB. Synthesis of nanocellulose-pectin based hybrid adsorbent for efficient uranium removal: A sustainable approach towards environmental remediation. J Env Chem Eng. 2025;13(1):114976. 10.1016/j.jece.2024.114976.Search in Google Scholar
[8] Ting X, Qichen L, Jun L, Yong Z, Wenkun Z. Highly enhanced adsorption performance to uranium(vi) by facile synthesized hydroxyapatite aerogel. J Hazard Mater. 2022;423(B):127184. 10.1016/j.jhazmat.2021.127184.Search in Google Scholar PubMed
[9] Han J, Zou J, Li X, Ding A, Shang Z, Sun H, et al. Study on the remediation of uranium-contaminated soils by compound leaching: Screening of leaching agents and a pilot-scale application. J Clean Prod. 2024;450:141918. 10.1016/j.jclepro.2024.141918.Search in Google Scholar
[10] Chen Y, Zhang Q, Fu X, Liu Y, Wang R, Zeng Q. A comprehensive review on progress and prospects of modified hydroxyapatite for uranium fixation from water. Sep Purif Technol. 2025;364:132599. 10.1016/j.seppur.2025.132599.Search in Google Scholar
[11] Wang J, Zhou W, Shi Y, Li Y, Xian D, Guo N, et al. Uranium sorption on oxyhydroxide minerals by surface complexation and precipitation. Chin Chem Lett. 2022;33(7):3461–7. 10.1016/j.cclet.2022.01.019.Search in Google Scholar
[12] Wang C, Helal AS, Wang Z, Zhou J, Yao X, Shi Z, et al. Uranium in situ electrolytic deposition with a reusable functional graphene-foam electrode. Adv Mater. 2021;33:2102633. 10.1002/adma.202102633.Search in Google Scholar PubMed
[13] Lin KL, Chu ML, Shieh MC. Treatment of uranium containing effluents with reverse osmosis process. Desalination. 1987;61(2):125–36. 10.1016/0011-9164(87)80013-9.Search in Google Scholar
[14] Shen J, Schäfer A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review. Chemosphere. 2014;117:679–91. 10.1016/j.chemosphere.2014.09.090.Search in Google Scholar PubMed
[15] Wang Y, Peng B, Gong Y, Xie Y, Feng T, Liu X, et al. Carboxyl and amino acid functionalized indole-based polymer for ultrafast uranium extraction in aqueous solution. J Mol Liq. 2023;383:122143. 10.1016/j.molliq.2023.122143.Search in Google Scholar
[16] Liu Y, Li T, Xu C, Yin J, Zhang M, Wang J, et al. A highly selective bisamidoxime polymer adsorbent developed for the efficient extraction of uranium from seawater. Colloids Surf A: Physicochem Eng Asp. 2025;714:136564. 10.1016/j.colsurfa.2025.136564.Search in Google Scholar
[17] Chen J, Gao J, Lv H, Wen Q, Han J, Liu P, et al. Preparation of polydopamine-functionalized polyamidoxime membrane for uranium recovery from seawater. Appl Surf Sci. 2023;634:157604. 10.1016/j.apsusc.2023.157604.Search in Google Scholar
[18] He X, Dugas MP, Hodul JN, Boudouris BW, Phillip WA. Porous block polymer composite membranes for uranium uptake. Appl Surf Sci. 2024;643:158650. 10.1016/j.apsusc.2023.158650.Search in Google Scholar
[19] Aslani CK, Amik O. Active Carbon/PAN composite adsorbent for uranium removal: Modeling adsorption isotherm data, thermodynamic and kinetic studies. Appl Radiat Isot. 2021;168:109474. 10.1016/j.apradiso.2020.109474.Search in Google Scholar PubMed
[20] Boussouga YA, Stryhanyuk H, Richnow HH, Schäfer AI. Adsorption of uranium(vi) complexes with polymer-based spherical activated carbon. Water Res. 2023;249:120825. 10.1016/j.watres.2023.120825.Search in Google Scholar PubMed
[21] Singh NB, Nagpal G, Agrawal S, Rachna. Water purification by using adsorbents: A review. Env Technol Innov. 2018;11:187–240. 10.1016/j.eti.2018.05.006.Search in Google Scholar
[22] Wu Y, Pang H, Liu L, Wang X, Yu S, Fu D, et al. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Env Pollut. 2019;246:608–20. 10.1016/j.envpol.2018.12.076.Search in Google Scholar PubMed
[23] Riseh RF, Fathi F, Vatankhah M, Kennedy JF. Alginate supramolecular for encapsulation of plant biocontrol bacteria: A review. Carbohydr Polym. 2025;358:123511. 10.1016/j.carbpol.2025.123511.Search in Google Scholar PubMed
[24] Tammina SK, Priyadarshi R, Khan A, Manzoor A, Abdel Rahman RSH, Banat F. Recent developments in alginate-based nanocomposite coatings and films for biodegradable food packaging applications. Int J Biol Macromol. 2025;295:139480. 10.1016/j.ijbiomac.2025.139480.Search in Google Scholar PubMed
[25] Nandini VV, Venkatesh KV, Nair KC. Alginate impressions: A practical perspective. J Conserv Dent. 2008;11(1):37–41. 10.4103/0972-0707.43416.Search in Google Scholar PubMed PubMed Central
[26] Ou M, Li W, Zhang Z, Xu X. β-Cyclodextrin and diatomite immobilized in sodium alginate biosorbent for selective uranium(vi) adsorption in aqueous solution. Int J Biol Macromol. 2022;222(B):2006–16. 10.1016/j.ijbiomac.2022.09.290.Search in Google Scholar PubMed
[27] Sutirman ZA, Sanagi MM, Wan Aini WI. Alginate-based adsorbents for removal of metal ions and radionuclides from aqueous solutions: A review. Int J Biol Macromol. 2021;174:216–28. 10.1016/j.ijbiomac.2021.01.150.Search in Google Scholar PubMed
[28] Gok C, Aytas S. Biosorption of uranium(vi) from aqueous solution using calcium alginate beads. J Hazard Mater. 2009;168(1):369–75. 10.1016/j.jhazmat.2009.02.063.Search in Google Scholar PubMed
[29] Wang D, Zhang J, Li J. Phosphate-functionalized magnetic calcium alginate for the engineering remediation of uranium-contaminated water and soil. J Chem Eng. 2023;475:145910. 10.1016/j.cej.2023.145910.Search in Google Scholar
[30] Bai J, Fan F, Wu X, Tian W, Zhao L, Yin X, et al. Equilibrium, kinetic and thermodynamic studies of uranium biosorption by calcium alginate beads. J Env Radioact. 2013;126:226–31. 10.1016/j.jenvrad.2013.08.010.Search in Google Scholar PubMed
[31] Yu J, Wang J, Jiang Y. Removal of uranium from aqueous solution by alginate beads. Nucl Eng Technol. 2017;49(3):534–40. 10.1016/j.net.2016.09.004.Search in Google Scholar
[32] Tharwat RM, Mahmoud ME, Abdelfattah AM, Hassan SSM. Decorated xanthan gum/alginate mingled hydrogel beads@La(III)-MOFs@reduced graphene oxide@graphene quantum dots nanohybrid for adsorptive capture and recovery of U(VI). J Mol Liq. 2023;309(5):122960. 10.1016/j.molliq.2023.122960.Search in Google Scholar
[33] Tripathi A, Melo JS, D’Souza SF. Uranium(vi) recovery from aqueous medium using novel floating macroporous alginate-agarose-magnetite cryobeads. J Hazard Mater. 2013;246–247:87–95. 10.1016/j.jhazmat.2012.12.002.Search in Google Scholar PubMed
[34] Tong K. Preparation and biosorption evaluation of Bacillus subtilis/alginate–chitosan microcapsule. Nanotechnol Sci Appl. 2017;10:35–43. 10.2147/NSA.S104808.Search in Google Scholar PubMed PubMed Central
[35] Erden KE, Donat R. Eco-friendly biosorption of uranium from aqueous solutions using sepiolite-Cavex CA37 composite biosorbent. Clean Soil Air Water. 2023;51(6):2200313. 10.1002/clen.202200313.Search in Google Scholar
[36] Basu H, Singhal RK, Pimple MV, Saha S. Graphene oxide encapsulated in alginate beads for enhanced sorption of uranium from different aquatic environments. J Env Chem Eng. 2018;6(2):1625–33. 10.1016/j.jece.2018.01.065.Search in Google Scholar
[37] Akhtar K, Khalid AM, Akhtar MW, Ghauri MA. Removal and recovery of uranium from aqueous solutions by Ca-alginate immobilized Trichoderma harzianum. Bioresour Technol. 2009;100(20):4551–8. 10.1016/j.biortech.2009.03.073.Search in Google Scholar PubMed
[38] Francois CA. Rapid spectrophotometric determination of submilligram quantities of uranium. Anal Chem. 1958;30(1):50–4. 10.1021/ac60133a012.Search in Google Scholar
[39] Donat R, Esen K, Cetisli H, Aytas S. Adsorption of uranium(vi) onto Ulva sp.-sepiolite composite. J Radioanal Nucl Chem. 2009;279:253–61. 10.1007/s10967-007-7243-7.Search in Google Scholar
[40] Langmuir I. The constitution and fundamental properties of solids and liquids, Part I Solids. J Am Chem Soc. 1916;38:2221–95. 10.1021/ja02268a002.Search in Google Scholar
[41] Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–403. 10.1021/ja02242a004.Search in Google Scholar
[42] Freundlich HMF. Over the adsorption in solution. J Phys Chem. 1906;57:385–70.Search in Google Scholar
[43] Dubinin MM, Raduskhevich LV. Equation of the characteristic curve of activated charcoal. Proc Acad Sci. 1987;55:331–3.Search in Google Scholar
[44] Khan SA, Rehman R, Khan MA. Adsorption of chromium (III), chromium (VI) and silver (I) on bentonite. Waste Manag. 1995;15(4):271–82. 10.1016/0956-053X(95)00025-U.Search in Google Scholar
[45] Li T, Xu Y, Wang F, Xia L. Effective separation of U(VI) in acidic uranium-containing wastewater by potassium manganese ferrocyanide and carbon nanotube encapsulated calcium alginate beads. J Env Chem Eng. 2023;11(5):111133. 10.1016/j.jece.2023.111133.Search in Google Scholar
[46] Wang Q, Li L, Tian Y, Kong L, Cai G, Zhang H, et al. Shapeable amino-functionalized sodium alginate aerogel for high-performance adsorption of Cr(VI) and Cd(II): Experimental and theoretical investigations. J Chem Eng. 2022;446(4):137430. 10.1016/j.cej.2022.137430.Search in Google Scholar
[47] Khajavi P, Keshtkar AR, Moosavian MA. The optimization of U(VI) removal by a novel amidoximated modified calcium alginate gel bead with entrapped functionalized SiO2 nanoparticles. Prog Nucl Energy. 2021;140:103887. 10.1016/j.pnucene.2021.103887.Search in Google Scholar
[48] Vogel H, Meyer-Jacob C, Thöle L, Lippold JA, Jaccard SL. Quantification of biogenic silica by means of Fourier transform infrared spectroscopy (FTIRS) in marine sediments. Limnol Oceanogr Methods. 2016;14:828–38. 10.1002/lom3.10129.Search in Google Scholar
[49] Peighambardoust SJ, Safarzadeh H. Swelling behavior study of poly(methacrylic acid-co-acrylamide) nano-composite hydrogel adsorbents containing different nanoparticles. Desalin Water Treat. 2023;298:44–52. 10.5004/dwt.2023.29610.Search in Google Scholar
[50] Dhanya V, Rajesh N. Carbonized black pepper spike and its alginate composite for effective batch and column adsorption of uranium from water. Groundw Sustain Dev. 2024;26:101209. 10.1016/j.gsd.2024.101209.Search in Google Scholar
[51] Gumber N, Pai RV, Sanyal K, Dutta B, Hassan PA. Synthesis and uranium adsorption studies of UiO-66 (Ce) based metal organic frameworks from aqueous solutions. Microporous Mesoporous Mater. 2022;341:112108. 10.1016/j.micromeso.2022.112108.Search in Google Scholar
[52] Zhang Y, Zhao H, Fan Q, Zheng X, Li P, Liu S, et al. Sorption of U(VI) onto a decarbonated calcareous soil. J Radioanal Nucl Chem. 2011;288:395–404. 10.1007/s10967-010-0948-z.Search in Google Scholar
[53] Saeed MM. Adsorption profile and thermodynamic parameters of the preconcentration of Eu (III) on 2-thenoyltrifluoroacetone loaded polyurethane (PUR) foam. Radioanal Nucl Chem. 2003;256(1):73–80. 10.1023/A:1023300109423.Search in Google Scholar
[54] Saeed MM. Uptake of Tm(III) ions onto polyurethane foam from H2O – ethanol mixture containing 1-(2-pyridylazo)-2-naphthol (PAN). J Radioanal Nucl Chem. 2006;267(2):427–33. 10.1007/s10967-006-0066-0.Search in Google Scholar
[55] Yavuz O, Altunkaynak Y, Guzel F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003;37:948–52. 10.1016/S0043-1354(02)00409-8.Search in Google Scholar
[56] Boussouga YA, Joseph J, Stryhanyuk H, Richnow HH, Schäfer AI. Adsorption of uranium(vi) complexes with polymer-based spherical activated carbon. Water Res. 2024;249:120825. 10.1016/j.watres.2023.120825.Search in Google Scholar PubMed
[57] Ma L, Huang C, Yao Y, Fu M, Han F, Li Q, et al. Self-assembled MOF microspheres with hierarchical porous structure for efficient uranium adsorption. Sep Purif Technol. 2023;314:123526. 10.1016/j.seppur.2023.123526.Search in Google Scholar
[58] Donat R. The removal of uranium(vi) from aqueous solutions onto natural sepiolite. J Chem Thermodyn. 2009;41(7):829–35. 10.1016/j.jct.2009.01.009.Search in Google Scholar
[59] Smječanin N, Bužo D, Mašić E, Nuhanović M, Sulejmanović J, Azhar O, et al. Algae based green biocomposites for uranium removal from wastewater: Kinetic, equilibrium and thermodynamic studies. Mater Chem Phys. 2022;283:125998. 10.1016/j.matchemphys.2022.125998.Search in Google Scholar
[60] Mittal H, Alfantazi AM, Alhassan SM. Recent developments in the adsorption of uranium ions from wastewater/seawater using carbon-based adsorbents. J Env Chem Eng. 2024;12(1):111705. 10.1016/j.jece.2023.111705.Search in Google Scholar
[61] Liao J, Ding C, Shi J, Jiang L, Wang Q, Wang L, et al. A sodium alginate gel bead adsorbent doping with amidoxime-modified hydroxyapatite for the efficient adsorption of uranium. Int J Biol Macromol. 2024;266(2):131112. 10.1016/j.ijbiomac.2024.131112.Search in Google Scholar PubMed
[62] Yue Y, Wang X, Wu Q, Han J, Jiang J. Assembly of polyacrylamide-sodium alginate-based organic-inorganic hydrogel with mechanical and adsorption properties. Polymers. 2019;11:1239. 10.3390/polym11081239.Search in Google Scholar PubMed PubMed Central
[63] Lotfy D, El-Sayyad GS, Shehata N. Hexamethylenetetramine functionalized graphene oxide-alginate beads nanocomposite as efficient sorbent for dye from aqueous solution. Int J Biol Macromol. 2023;228:754–72. 10.1016/j.ijbiomac.2022.12.208.Search in Google Scholar PubMed
[64] Hou X, Xue Z, Xia Y, Qin Y, Zhang G, Liu H, et al. Effect of SiO2 nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. Int J Biol Macromol. 2019;125:1289–98. 10.1016/j.ijbiomac.2018.09.109.Search in Google Scholar PubMed
[65] Zhu J, Wang J, Liu Q, Yu J, Liu J, Chen R, et al. Amidoxime-functionalized MXene/graphene oxide aerogel for sunlight enhanced uranium adsorption. J Env Chem Eng. 2025;13(3):116254. 10.1016/j.jece.2025.116254.Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies

