Abstract
The aim of this study was to provide a comprehensive overview of the botanical characteristics, chemical composition, pharmacological activities, toxicology, and metabolic pathways of the primary active compounds, especially flavonoids, found in Apocynum venetum and offer new insights and scientific evidence for further research on this plant. Information on A. venetum was obtained from traditional Chinese medicine books and electronic databases such as PubMed, CNKI, ScienceDirect, Scifinder, the Chinese Pharmacopoeia (2020), and the Flora of China. A. venetum, with a long history of use, was valuable across multiple. A total of 174 compounds were identified; flavonoids were the primary active constituents. Numerous pharmacological studies demonstrated the leaf extract’s antidepressant, hepatoprotective, antihypertensive, lipid-lowering, cardiotonic, anxiolytic, and antioxidant properties, with a high safety profile. A. venetum leaves contain diverse chemical constituents with broad pharmacological activities. Modern pharmacological research provides reliable evidence supporting the plant’s traditional uses, such as calming the liver and promoting diuresis. However, several issues remain unresolved. Further research on non-flavonoid compounds is needed. Additionally, the potential of A. venetum leaves in the treatment of epilepsy is discussed, highlighting future prospects for the plant’s development and utilization.
Abbreviations
- DA
-
dopamine
- CYP
-
cytochrome P450 enzyme
- FST
-
forced swimming test
- TST
-
tail suspension test
- OFT
-
open field test
- S100A10
-
S100 calcium-binding protein A10
- 5-HT
-
serotonin
- ALT
-
alanine aminotransferase
- TNF-α
-
tumor necrosis factor-α
- APAP
-
acetaminophen
- LDH
-
lactate dehydrogenase
- Ang-II
-
carbachol angiotensin II
- l-NAME
-
an inhibitor of nitric oxide [NO] synthase
- TG
-
triglycerides
- MDA
-
malondialdehyde
- SOD
-
superoxide dismutase
- SREBP-1c
-
sterol regulatory element-binding protein
- GLUT
-
glucose transporters
- PDE3
-
phosphodiesterase 3
- GABA
-
γ-aminobutyric acid
- TT
-
thrombin time
- MYD
-
maximum tolerated dose
- TCMs
-
traditional Chinese medicines
1 Introduction
Apocynum venetum L., commonly known as luobuma in Chinese, is a perennial herbaceous plant belonging to the Apocynaceae family. It is also referred to as “tea flower,” “wild hemp,” and “zeqi hemp,” and has two varieties: red hemp and white hemp [1]. In China, A. venetum is mainly distributed in regions such as East China and Northwest China, typically growing wild in saline-alkaline wastelands, desert fringes, and barren lands, as depicted in Figures 1 and 2.

Taxonomy of A. venetum L.
![Figure 2
Different parts of A. venetum L. [(a) plant, (b) stem and leaf, (c) flower, and (d) fruit].](/document/doi/10.1515/chem-2025-0156/asset/graphic/j_chem-2025-0156_fig_002.jpg)
Different parts of A. venetum L. [(a) plant, (b) stem and leaf, (c) flower, and (d) fruit].
The bast fiber of A. venetum is characterized by its fine, soft, corrosion-resistant, wear-resistant, and tensile-resistant properties, which make it widely applicable in the textile, paper, aviation, and maritime industries. Additionally, A. venetum leaves are used as a tea, offering benefits such as heat clearing, dizziness prevention, and cardiotonic effects. Various parts of the plant, including the roots, stems, leaves, and flowers, are used in traditional medicine. The plant is considered slightly cold in nature, with a bitter and sweet taste. A. venetum contains a wide range of important phytochemicals, including flavonoids and their glycosides, phenylpropanoids, steroids and their glycosides, terpenoids, volatile oils, organic acids, pyrrolizidine alkaloids, and polysaccharides. These compounds have demonstrated pharmacological activities such as antidepressant, hepatoprotective, antihypertensive, lipid-lowering, cardiotonic, anxiolytic, and antioxidant effects [2]. This review aimed to summarize recent advances in the chemical composition and pharmacological activities of A. venetum, exploring its active compounds and mechanisms of action to provide a scientific basis for future research.
2 Materials and methods
This study utilized data from electronic databases, including PubMed, CNKI, ScienceDirect, Scifinder, and the Flora of China, focusing on information related to A. venetum. The search terms included “Apocynum venetum L.,” “Apocynum venetum L. leaves,” “Apocynum venetum L. flowers,” and “Apocynum venetum L. roots.” Additionally, the 2020 edition of the Chinese Pharmacopoeia and other relevant books, such as the Dictionary of Chinese Medicine, were consulted to further supplement the content of this review.
3 Results
3.1 Study on the pharmacological action and mechanism of A. venetum
Previous studies have reported that various parts of A. venetum, including the roots, stems, leaves, and flowers, possess biological activities such as antidepressant, hepato-protective, antihypertensive, lipid-lowering, cardiotonic, and anxiolytic effects. It may play a role by inhibiting inflammation and regulating oxidative stress response (Figure 3).
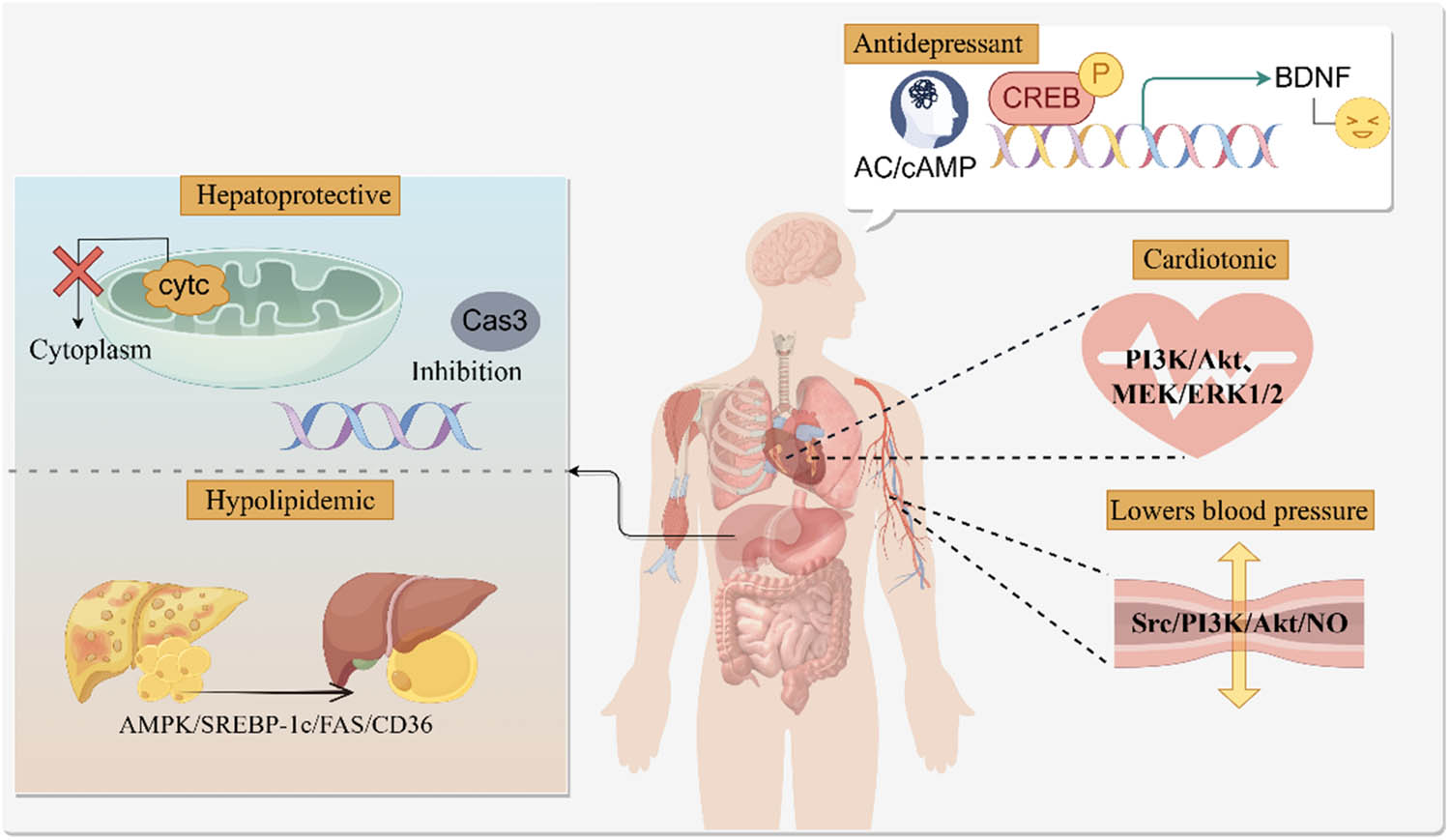
The graphical abstract of pharmacological action.
3.1.1 A. venetum flavonoids demonstrate antidepressant effects through the regulation of calcium ion channels, which enhances neurohormone levels and inhibits neuroinflammatory responses
It has been reported that the antidepressant activity of A. venetum is mainly attributed to its flavonoid compounds, and the total flavonoid content is positively correlated with its antidepressant effects [3]. Studies have shown that treatment with A. venetum extracts at doses of 30–125 mg/kg for 14 days significantly shortened the immobility time of male SD rats in the forced swimming test (FST). Hyperoside and isoquercitrin, at doses of 0.6–1.3 mg/kg, produced similar effects. Moreover, treatment with A. venetum extract at doses of 15, 60, and 250 mg/kg for 8 weeks resulted in a reduction in norepinephrine and dopamine (DA) concentrations in rats. Through radioligand binding assays, it was found that treatment with A. venetum extract did not alter the binding of [125I]CYP (cytochrome P450 enzyme) to β-adrenergic receptors in the frontal cortex of rats, suggesting that A. venetum extract may exert its antidepressant effects by modulating the presynaptic α2-receptor sensitivity through the adrenergic system [4].
Further research revealed that A. venetum flavonoid extract (25, 50, and 100 μg/mL) or hyperoside (2.5, 5, and 10 g/mL) could protect PC12 cells from cortisol-induced neurotoxicity by upregulating BDNF through reducing intracellular Ca2+ levels and activating the AC-cAMP-CREB signaling pathway, thereby exerting antidepressant effects [5,6] (Figure 4). Additionally, male ICR mice were pretreated with 50 mg/kg sulpiride (DA D2 receptor antagonist), 0.05 mg/kg SCH23390 (DA D1 receptor antagonist), and 50 mg/kg A. venetum extract for 10 days before being subjected to the FST, tail suspension test (TST), and open field test (OFT). The DA receptor antagonists blocked the anti-depressant effects of A. venetum extract in the TST, indicating that DA D1 receptors are involved in the antidepressant mechanism of A. venetum extract [6]. Furthermore, studies found that treatment with A. venetum water extract at doses of 0.58, 1.17, and 2.34 g/kg for 4 weeks increased the sleep occurrence rate, sleep duration, and brain coefficient in male ICR mice. Treatment with 1.62 g/kg A. venetum water extract for 4 weeks elevated the brain coefficient in male SD rats, decreased DA levels, and increased 5-hydroxyindoleacetic acid levels. These results suggest that A. venetum water extract improves sleep, potentially through mechanisms involving the upregulation of hypothalamic 5-HT (serotonin) levels and downregulation of DA levels [7,8,9].
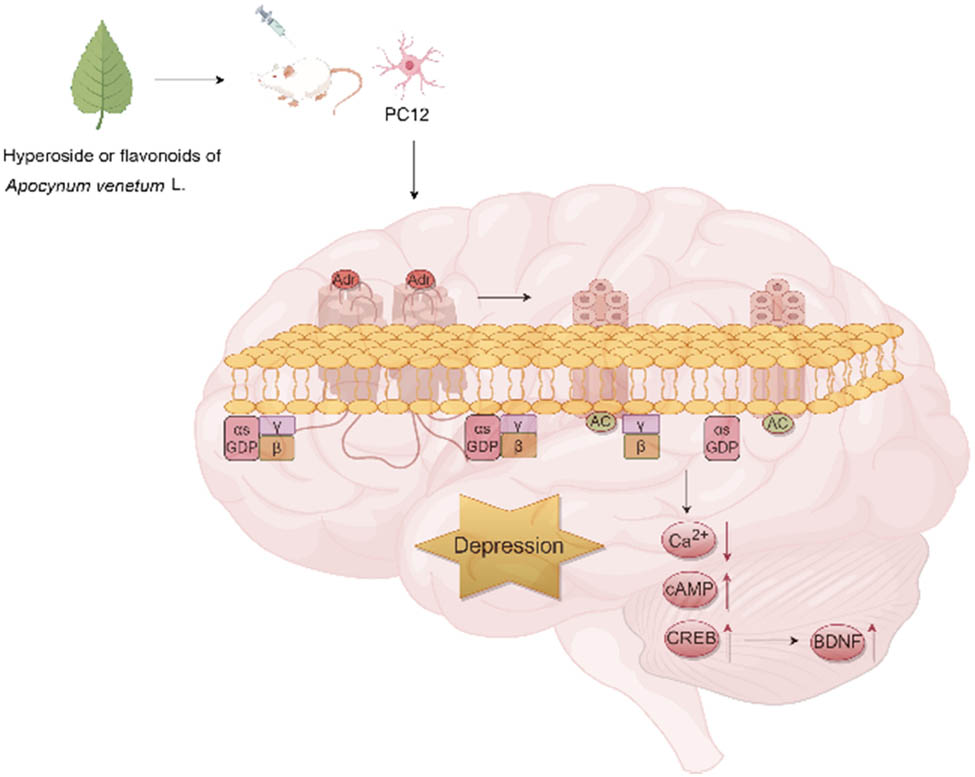
Antidepressant effects of A. venetum flavonoids and hyperoside.
Ion channels play a crucial role in the antidepressant effects of A. venetum leaves. Through whole-cell patch-clamp recording on N2A cells, researchers have found that A. venetum water extract exerts neuropharmacological effects by regulating voltage-gated Na+ and K+ channels [10]. To further explore the antidepressant mechanism of A. venetum leaves, the chronic unpredictable mild stress model was used, wherein female SD rats were subjected to mild stress for 4 weeks, followed by an OFT. It was observed that pretreatment with 40 or 80 mg/kg of total flavonoid extract from A. venetum significantly increased the total horizontal movement distance and vertical activity counts of the rats. Reverse transcription polymerase chain reaction analysis revealed a reduction in the expression level of the S100A10 gene. S100A10, a member of the calcium-binding protein S100 family, is a widely distributed protein involved in various intracellular and extracellular processes. The total flavonoid components of A. venetum may exert antidepressant effects by regulating the expression of the S100A10 (S100 calcium-binding protein A10) gene.
3.1.2 The extract of A. venetum leaves has the potential to protect the liver by modulating oxidative stress and inhibiting cellular apoptosis
The use of A. venetum leaves as a hepatoprotective agent has a history of over 1,000 years. Modern pharmacological studies have shown that pretreatment with A. venetum extracts at doses of 50 and 500 mg/kg for 1 week inhibits the elevation of alanine aminotransferase (ALT) levels induced by CCL4 or d-galactosamine/lipopolysaccharide (LPS) in male ddY mice, and reduced tumor necrosis factor-α (TNF-α) levels. The flavonoid compounds in A. venetum were found to inhibit TNF-α-induced cell death, suggesting that flavonoids might be the hepatoprotective components in A. venetum leaves [11]. Furthermore, pretreatment with 50 or 100 mg/kg A. venetum leaf extract for 3 days alleviated acetaminophen (APAP)-induced liver damage in male Kunming mice. Histopathological examination, DNA ladder assay, and Western blot analysis showed that A. venetum leaf extract inhibited APAP-induced hepatocyte apoptosis by suppressing the release of cytochrome c, activation of caspase-3, and DNA fragmentation, thereby exerting its hepatoprotective effects [12] (Figure 5). To further investigate the hepatoprotective mechanism of A. venetum leaves, in vitro studies using 25, 50, and 100 μg/mL of total flavonoid extract from A. venetum showed significant protection against CCL4-induced damage in HepG2 cells. Cell viability was increased, the number of apoptotic cells decreased, and lactate dehydrogenase (LDH) release was reduced in a dose-dependent manner. Subsequent in vivo experiments revealed that pretreatment with 100, 200, and 400 mg/kg of total flavonoid extract from A. venetum for 14 days significantly reduced the elevation of ALT and aspartate aminotransferase levels in male Kunming mice, and provided dose-dependent protection against the reduction in peroxidase activity induced by CCL4 [13]. These findings suggest that the total flavonoid components of A. venetum may exert hepatoprotective effects by scavenging free radicals, inhibiting apoptosis, and enhancing the antioxidant defense system.
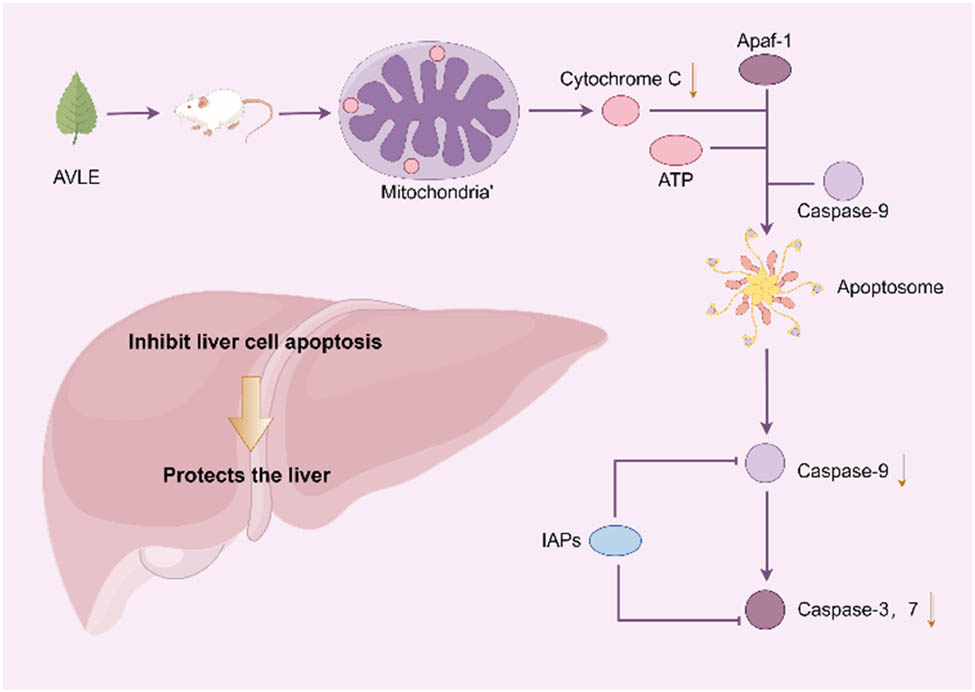
Hepatoprotective effects of A. venetum leaf extract.
3.1.3 The leaf extract of A. venetum has been shown to lower blood pressure through the inhibition of inflammation and the enhancement of vascular endothelial function
It has been reported that the aqueous extract of A. venetum leaves exhibits significant antihypertensive effects. Pretreatment with 10 mg/mL of A. venetum extract for 15 min effectively inhibited the contraction of aortic rings in male SD rats induced by KCl, carbachol, angiotensin II (Ang-II), and phenylephrine [14,15]. Further studies revealed that this inhibitory effect could be reversed by l-NAME (an inhibitor of nitric oxide [NO] synthase), suggesting that the antihypertensive effect of A. venetum extract may be mediated through the scavenging of superoxide anions and the release of NO [15]. An in-depth investigation into the antihypertensive mechanism of A. venetum leaves showed that treatment with 10 μg/mL of A. venetum extract increased NO levels in the aorta of male SD rats, and this effect was abolished by inhibitors of Src kinase and PI3K. Moreover, in the presence of Src kinase inhibitors, PI3K inhibitors, and NO synthase inhibitors, the same concentration of A. venetum extract led to the upregulation of Akt and eNOS expression in human umbilical vein endothelial cells. The expression of these two proteins was inhibited by Src kinase and PI3K inhibitors, which subsequently reduced NO levels [16]. Therefore, A. venetum extract induces NO-mediated vasodilation through the Src/PI3K/Akt signaling pathway, as illustrated in Figure 6. Network pharmacology studies on the anti-hypertensive mechanisms of A. venetum suggest that its active components primarily exert therapeutic effects on hypertension by inhibiting inflammatory responses, improving vascular endothelial function, and influencing hemorheology [17].
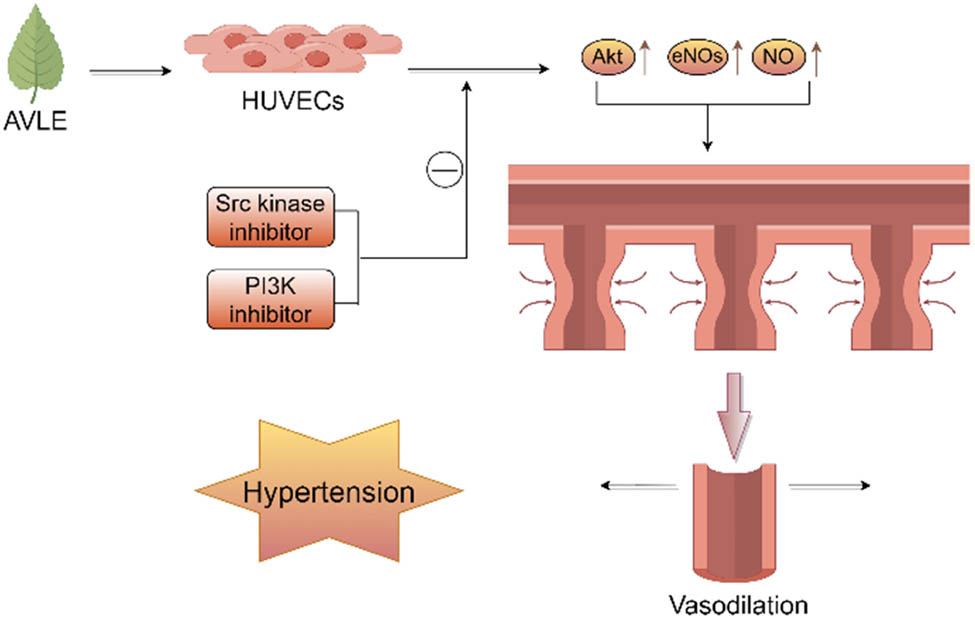
Vasodilatory effects of A. venetum leaf extract.
3.1.4 The leaf extract of A. venetum contributes to the reduction of blood lipid levels by enhancing oxidative stress management and suppressing inflammation
A. venetum leaves exhibit significant lipid-lowering effects. After 1 month of treatment with total flavonoid extracts of A. venetum at doses of 5, 50, and 100 mg/kg, or with isoquercitrin at doses of 0.1, 0.5, and 5 mg/kg, male Wistar rats, male SD rats, or male ICR mice fed a high-fat diet showed reduced lipid deposition, aortic thickening, and a decrease in subendothelial foam cells and lipid vacuoles in the aorta. In addition, the levels of total cholesterol, triglycerides (TG), low-density lipoprotein cholesterol, vascular cell adhesion molecule-1, malondialdehyde (MDA), and Ang-II in serum decreased, while high-density lipoprotein cholesterol levels and superoxide dismutase (SOD) activity increased. The atherosclerosis index and NO levels were also elevated [18,19,20]. In summary, the total flavonoids in A. venetum may exert lipid-lowering effects by improving endothelial damage and oxidative stress caused by hyperlipidemia.
Moreover, genetic studies on male ICR mice using SREBP-1c (sterol regulatory element-binding protein) and AMPK inhibitors, along with glucose uptake experiments, revealed that inhibition of SREBP-1c and AMPK for 2 weeks attenuated the anti-obesity effects of isoquercitrin. Administration of isoquercitrin reduced blood glucose levels and increased the gene expression of glucose transporters (GLUT1, GLUT2, and GLUT4) in obese mice. Additionally, the levels of SREBP-1c, fatty acid synthase (FAS), stearoyl-CoA de-saturase-1, and CD36 were significantly elevated in obese mice. These findings suggest that A. venetum water extract and its main component isoquercitrin may improve obesity symptoms by inhibiting the AMPK/SREBP-1c/FAS/CD36 signaling pathway and promoting glucose uptake [20] (Figure 7). Network pharmacology studies investigating the anti-atherosclerosis and lipid-lowering mechanisms of A. venetum leaves suggest that the plant primarily exerts its therapeutic effects through inhibiting inflammatory responses, improving vascular endothelial function, and influencing hemorheology in the treatment of atherosclerosis. It treats hyperlipidemia through processes such as lipid biosynthesis, regulation of inflammatory responses, and oxidative stress [18,21].
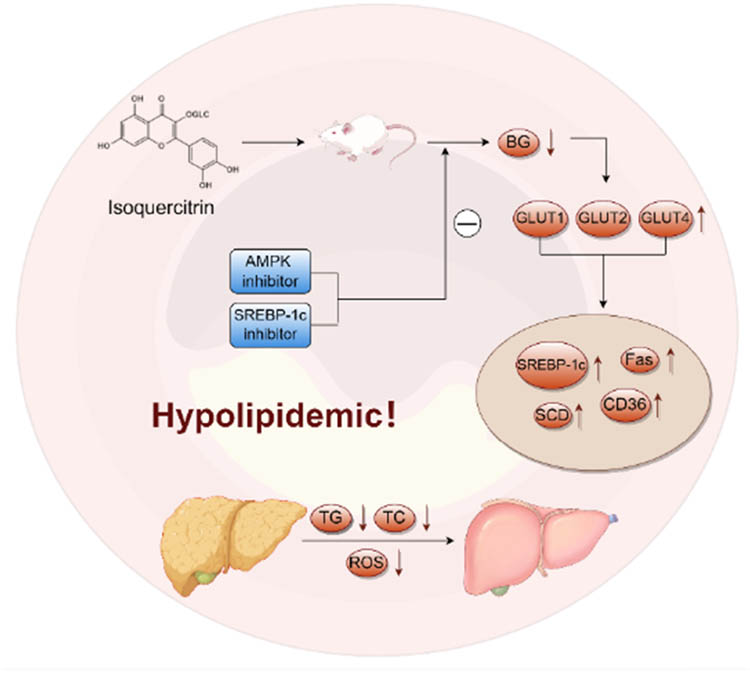
Lipid-lowering effects of isoquercitrin.
3.1.5 The leaf extract of A. venetum may exert a cardiotonic effect by interacting with proteins and pathways associated with inflammation and the immune system
A. venetum leaves contain various cardiac glycosides, which exhibit significant cardiotonic effects. Studies have shown that treatment with 1 mg/mL of A. venetum extract significantly increased the contractile force and pulse rate of the right atrium in male Hartley guinea pigs. In vitro assays on human platelet phosphodiesterase 3 (PDE3) revealed that A. venetum extract at this concentration inhibited PDE3 activity, suggesting that the cardiotonic effects of A. venetum extract may be mediated through PDE3 inhibition [22]. Further research demonstrated that pretreatment with 500 mg/kg of A. venetum leaf extract for 7 days reduced myocardial infarction size and the number of apoptotic cardiomyocytes in male SD rats subjected to myocardial ischemia/reperfusion injury. The treatment also decreased the AI and reduced the levels of creatine kinase, LDH, SOD, caspase-3, and gp91phox. Additionally, A. venetum leaf extract inhibited the production of superoxide and MDA while increasing the phosphorylation levels of Akt and ERK1/2. These protective effects were blocked by the PI3K inhibitor wortmannin or the ERK1/2 inhibitor PD98059, indicating that A. venetum leaf extract exerts its cardioprotective effects through the PI3K/Akt and MEK/ERK1/2 signaling pathways [23,24] (Figure 8).
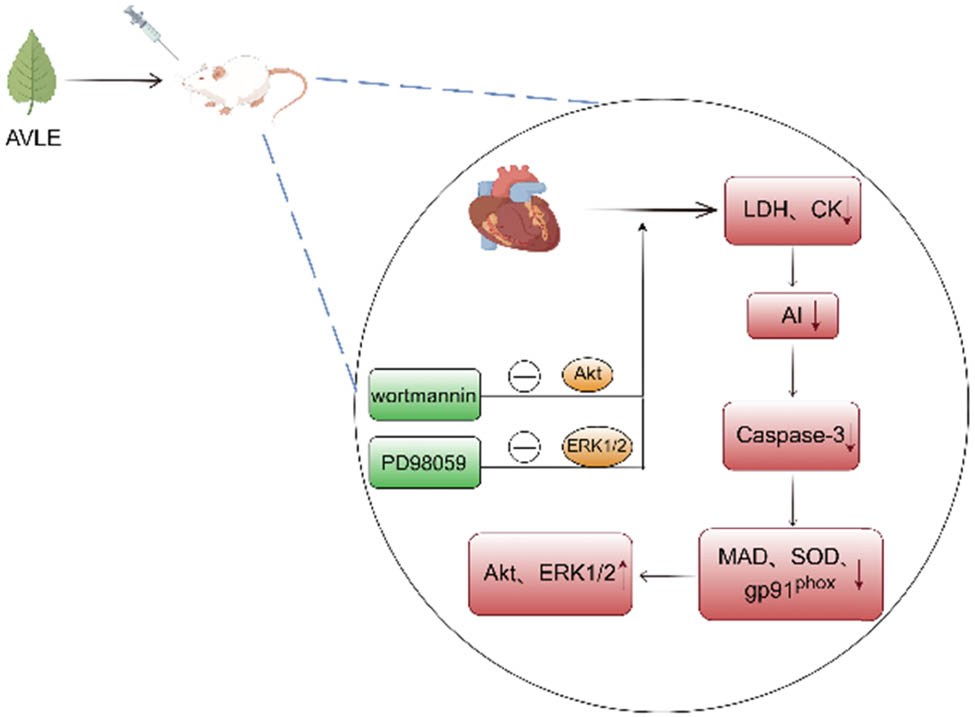
Cardioprotective effects of A. venetum leaf extract.
Moreover, network pharmacology studies on the cardioprotective mechanisms of A. venetum leaves suggest that the plant may treat cardiovascular diseases by targeting proteins and pathways related to inflammation, the immune system, endocrine system, signal transduction, hormone regulation, smooth muscle relaxation, and angiogenesis [21]. Experimental validation has shown that pretreatment with A. venetum leaf extract for 30 min alleviated doxorubicin-induced apoptosis in rat cardiomyocyte H9C2 cells and human cardiomyocyte HCM cells. The survival rate of cardiomyocytes increased in a dose-dependent manner from 0 to 70 µg/mL, with the highest survival rate observed at 70 µg/mL. However, this effect was reversed by the Akt inhibitor, suggesting that A. venetum leaf extract may protect cardiomyocytes by inhibiting apoptosis through the Akt/Bcl-2 signaling pathway [25].
3.1.6 A. venetum extract exerts anti-anxiety effects by mediating GABA system
Traditionally, A. venetum has been known for its calming effects, and modern pharmacological studies have shown that ethanol extracts of A. venetum at doses of 22.5–30 and 100–125 mg/kg, as well as kaempferol at 0.02–1.0 mg/kg, increased the time spent and the number of entries into the open arms of male C57BL/6 mice in the elevated plus maze test. However, this anxiolytic effect was blocked by the GABA (γ-aminobutyric acid) receptor antagonists flumazenil and WAY-100635, which effectively inhibited the effects of A. venetum extracts at doses of 125 and 30 mg/kg, respectively [26,27]. These findings suggest that kaempferol contributes to the anxiolytic activity of A. venetum and that the anxiolytic effect of its ethanol extract is mediated through the GABAergic system.
3.1.7 Other effects
In addition to the aforementioned activities, A. venetum exhibits inhibiting oxidative stress, anti-inflammatory, and anticoagulant effects.
A. venetum extract is abundant in polyphenolic compounds and may function as an in vitro aldose reductase inhibitor. It has the potential to restore the polyol pathway, inhibit oxidative stress, and maintain intracellular autophagy through the AMPK/mTOR/ULK1 signaling pathway. These mechanisms may underlie the therapeutic effects of A. venetum in the treatment of diabetic retinopathy [22]. A. venetum polysaccharide, which mainly consists of arabinose, galactose, rhamnose, glucose, xylose, caramel, and mannose, can inhibit the expression of cyclooxygenase-2, tumor necrosis factor-α, and interleukin-6 mRNA in RAW 264.7 macrophages induced by LPS. It also significantly reduced paw edema in mice after carrageenan injection. It can be developed as a potential antioxidant and anti-inflammatory agent [28].
In terms of anticoagulant activity, A. venetum has unique effects. Studies have shown that A. venetum extracts at concentrations of 200–500 μg/mL can significantly inhibit ADP-induced platelet aggregation in venous blood samples from male rabbits in a dose-dependent manner. The ethyl acetate fraction of A. venetum extract exhibited the strongest anti-platelet aggregation activity, suggesting that the flavonoid glycosides in A. venetum possess potent anti-platelet properties [29]. Additionally, polysaccharides such as Vp3, at a concentration of 1 mg/200 μL, were found to prolong prothrombin time and thrombin time (TT), while Vp2a-II at the same concentration prolonged activated partial thromboplastin time and TT. Vp3 exerts anticoagulant effects through the extrinsic pathway, whereas Vp2a-II inhibits coagulation via both intrinsic and extrinsic pathways [30].
Network pharmacology studies on the antiseptic effects of A. venetum leaves revealed that compounds such as kaempferol, luteolin, proanthocyanidin B1, and stigmasterol may target predictive biological markers such as AKT1, VEGFA, MAPK3, EGFR, SRC, and PTGS2 to regulate signal transduction, hormone levels, and anti-infection processes. These compounds may treat sepsis through pathways such as VEGF, EGFR, ErbB, HIF-1, and Ras, playing an important role in controlling inflammation and infection [24].
3.2 Toxicological analysis of A. venetum
A. venetum tea, made from the dried leaves of the plant, has a long history of use for lowering blood pressure, reducing blood lipids, and delaying aging. It remains widely consumed today, with a recommended daily dosage of 0.3 g/kg. Modern studies have shown that A. venetum tea contains flavonoids, sterols, glycosides, amino acids, and other chemical constituents, with the total flavonoid content ranging from 0.20 to 2.5%. Flavonoids are the primary active compounds in A. venetum, providing antihypertensive, cardiotonic, diuretic, and lipid-lowering effects. Acute toxicity experiments with total flavonoid extracts of A. venetum in mice revealed that the maximum tolerated dose (MTD) was 10.24 g/(kg d), which is 161 times the recommended human daily dose, indicating a high level of safety [31].
Although there are few safety evaluations of A. venetum both domestically and internationally, a safety assessment was conducted following the national standard “Procedures and Methods for Toxicological Evaluation of Food Safety (GB15193.1-1994).” This assessment indicated that when consumed as a natural health tea, A. venetum tea did not produce toxic effects on any toxicological parameters in experimental animals at doses up to 10 times the human intake [32]. Additionally, acute toxicity tests in mice showed that the oral MTD of A. venetum tea was greater than 30.0 g/kg bw, classifying it as a non-toxic substance [33]. However, A. venetum water extract administered at a maximum dose of 24 g/(kg day), equivalent to 800 times the intended human dose, exhibited some acute toxic effects. Subacute toxicity tests indicated that the safe dose of A. venetum water extract was 40–80 times the clinical dose [34]. Overall, the safety margin of A. venetum extract is relatively high.
Therefore, A. venetum is considered a safe natural plant for consumption and has potential for further development and utilization. However, due to the relatively high content of alkaloids, which have certain toxic effects, A. venetum tea should not be consumed in large quantities over prolonged periods to avoid toxicity. It is also not recommended for individuals with weak digestive systems or those with heart conditions.
4 Active components
To date, various chemical constituents have been identified and isolated from A. venetum, including flavonoids, phenylpropanoids, steroids, terpenoids, volatile oils, organic acids, alkaloids, and polysaccharides. Each of these compounds plays distinct pharmacological roles.
4.1 Flavonoids and their glycosides
Over the past two decades, several flavonoid compounds have been isolated from A. venetum, including flavones, flavonols, flavanols, dihydroflavonoids, biflavonoids, isoflavones, chalcones, anthocyanins, and proanthocyanidins. Increasing research has reported the biological activities of these flavonoids. For instance, hyperoside and isoquercitrin have been shown to exhibit antihypertensive effects. Moreover, many traditional Chinese medicines (TCMs) used to treat coronary heart disease or promote blood circulation, such as rutin and quercetin, contain flavonoid compounds, which have demonstrated significant coronary dilation effects. Some flavonoids have also been shown to reduce blood lipid levels and prevent atherosclerosis. The names and structures of these flavonoid compounds are listed in Table 1 and Figure 9.
Flavonoid compounds identified in A. venetum
| Classification | Name | Source | References |
|---|---|---|---|
| Flavonoids | (1) Luteolin | Leaf | [35] |
| (2) Apigenin | Leaf | [35] | |
| (3) Isoorientin | Leaf | [35] | |
| (4) Acacetin | Leaf | [35] | |
| (5) Chrysoeriol | Leaf | [36] | |
| Flavonols | (6) Kaempferol | Leaves, flowers | [37] |
| (7) Quercetin | Leaves, flowers | [38] | |
| (8) Rutin | Leaf | [39] | |
| (9) Tamarixetin | Leaf | [40] | |
| (10) Isorhamnetin | Leaf | [39,41] | |
| (11) Myricetin | Leaf | [42] | |
| (12) Baicalein | Leaf | [43–45] | |
| (13) Avicularin | Leaf | [44] | |
| (14) Hyperoside | Leaf | [38] | |
| (15) Isoquercitrin | Leaf | [38] | |
| (16) Quercetin-3-glucuronide | Leaf | [43] | |
| (17) Trifolin | Leaf | [43,44] | |
| (18) Quercetin 7-O-rutinoside | Leaf | [35] | |
| (19) Acetylated hyperoside | Leaf | [43,44] | |
| (20) Acetylated isoquercetin | Leaf | [38] | |
| (21) Malonated hyperoside | Leaf | [45] | |
| (22) Malonated isoquercetin | Leaf | [45] | |
| (23) Kaempferol-6′-O-acetate | Leaf | [11] | |
| (24) Isoquercetin-6′-O-acetate | Leaf | [11] | |
| (25) Kaempferol-3-O-α-d-galactopyranoside | Leaf | [46] | |
| (26) Quercetin-3-O-α-d-glucosy-α-d-glucopyranoside | Toast leaves | [43,44] | |
| (27) Astragalin | Leaf | [47] | |
| Dihydroflavones | (28) Hesperetin | Leaf | [36] |
| (29) Bavachin | Leaf | [37] | |
| Double flavonoids | (30) Amentoflavone | Leaf | [11,12] |
| (31) Biapigenin | Leaf | [11,12] | |
| Isoflavones | (32) 8-O-methylretusin | Leaf | [48] |
| Chalcones | (33) Carthamin | Leaf | [36] |
| Anthocyanins | (34) Delphinidin | Leaf | [36] |
| (35) Pelargonidin | Leaf | [36] | |
| (36) Malvidin chloride | Leaf | [36] | |
| (37) Peonidin chloride | Leaf | [36] | |
| (38) Cyanidin | Leaf | [36] | |
| Flavanols | (39) Epicatechin | Leaf | [49] |
| (40) Catechin | Leaf | [49] | |
| (41) Gallic catechin | Leaf | [49] | |
| (42) Epigallocatechin | Leaf | [49] | |
| Proanthocyanidins | (43) Procyanidin C1 | Leaf | [3] |
| (44) Procyanidin B2 | Leaf | [50,51] | |
| (45) Procyanidin B1 | Leaf | [52] | |
| (46) Procyanidin | Leaf | [53] |
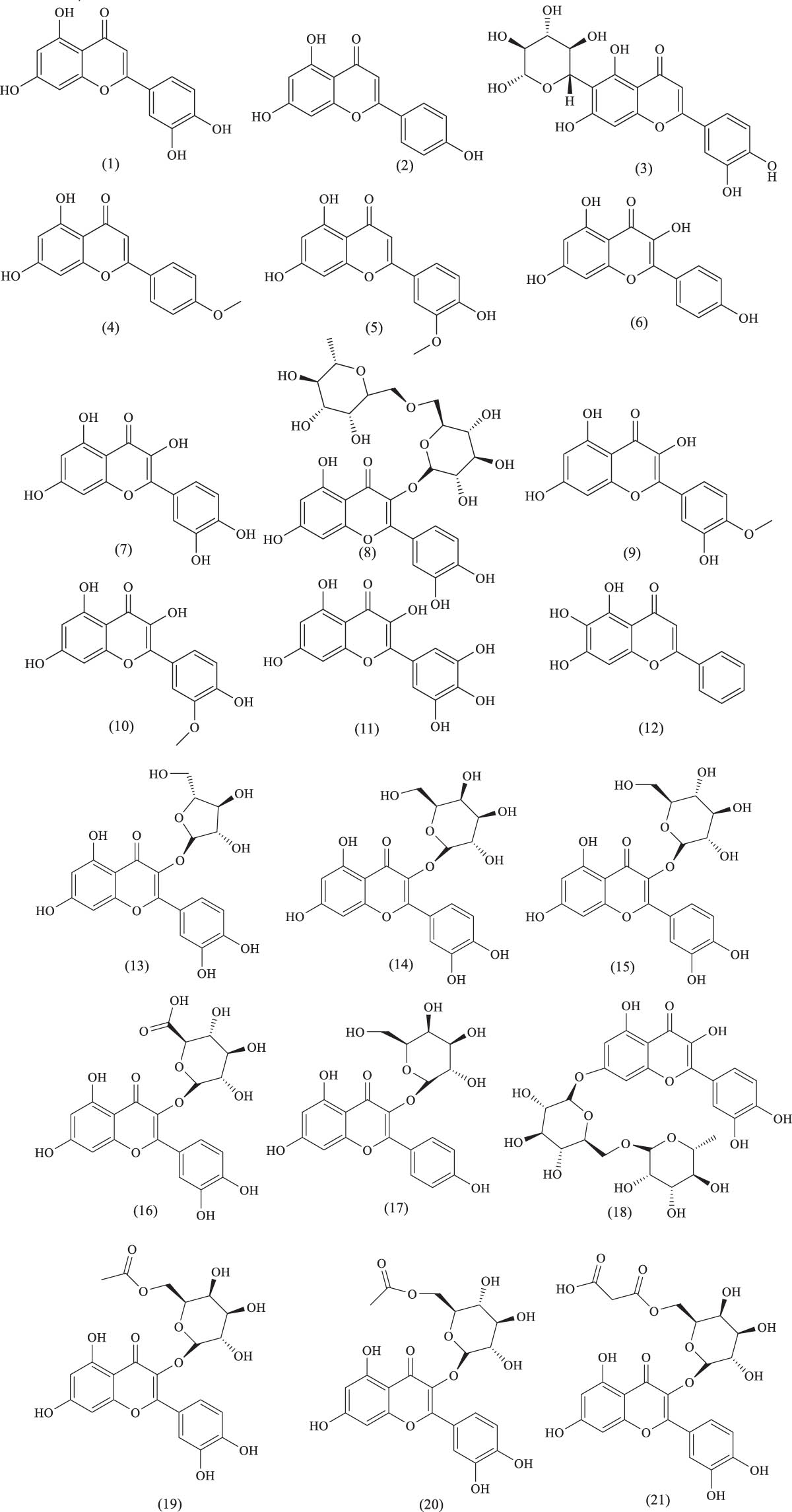
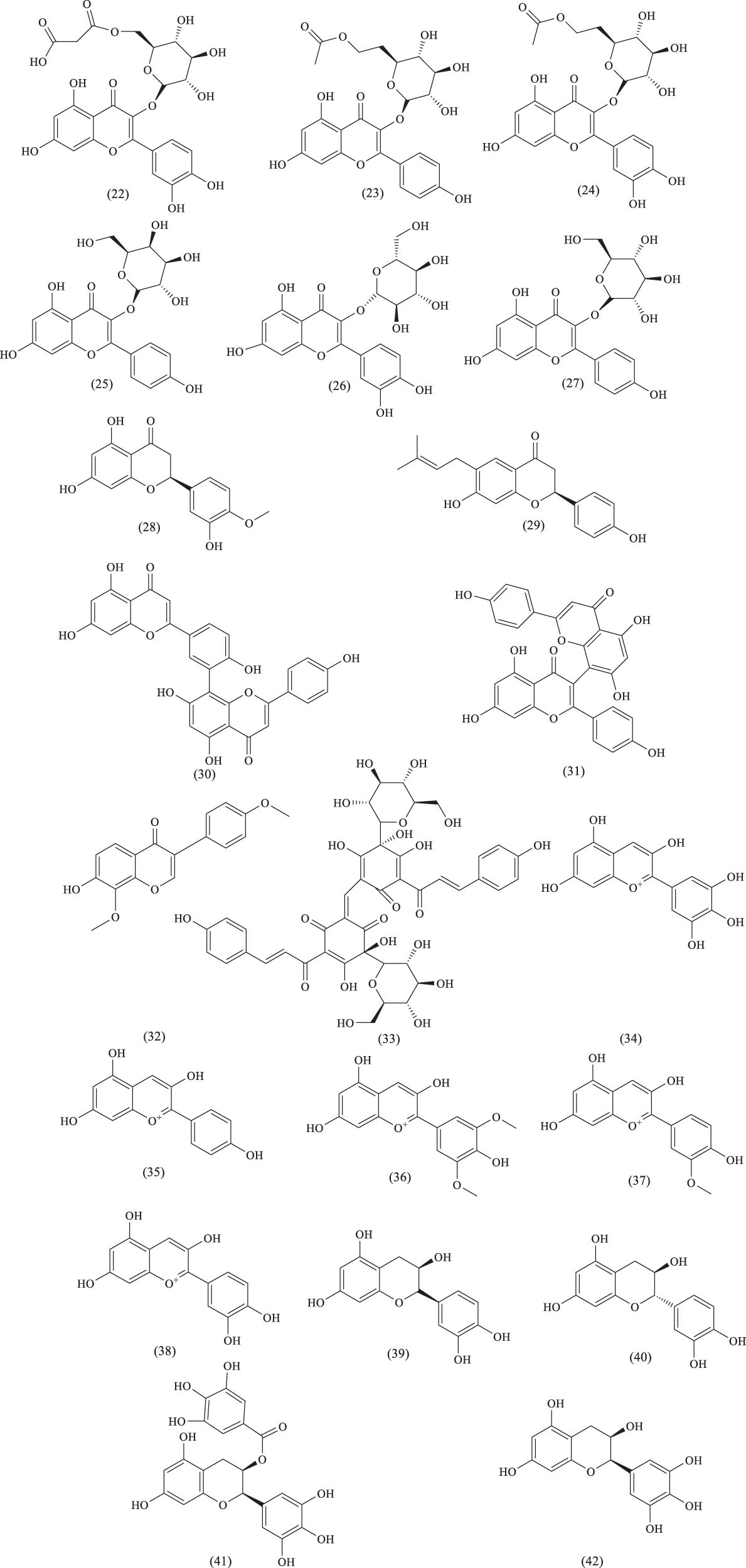
Chemical structures of flavonoids and their glycosides.
4.2 Phenylpropanoids
The phenylpropanoids isolated from A. venetum to date primarily include simple phenylpropanoids and coumarins. Coumarins, which are widely found in nature, have become a research hotspot in recent years for the development of new drugs due to their strong biological activities, such as antiviral, antitumor, anti-osteoporotic, and anticoagulant effects. The names and structures of these phenylpropanoid compounds are listed in Table 2 and Figure 10.
Phenylpropanoid compounds identified in A. venetum
| Serial number | Name | Source | References |
|---|---|---|---|
| (1) | Scopoletin | Leaf | [54] |
| (2) | Esculetin | Leaf | [55] |
| (3) | Esculin | Leaf | [54] |
| (4) | Isofraxidin | Leaf | [54] |
| (5) | 2-Methyl-5-acetonyl-7-hydroxychromone | Leaf | [56] |
| (6) | Neochlorogenic acid | Leaf | [57] |
| (7) | Chlorogenic acid | Leaf | [55] |
| (8) | Cryptochlorogenic acid | Leaf | [57] |
| (9) | 3-O-Caffeoylquinic acid methyl ester | Leaf | [55] |
| (10) | Isochlorogenic acid C | Leaf | [53] |
| (11) | 1-CQA | Leaf | [53] |
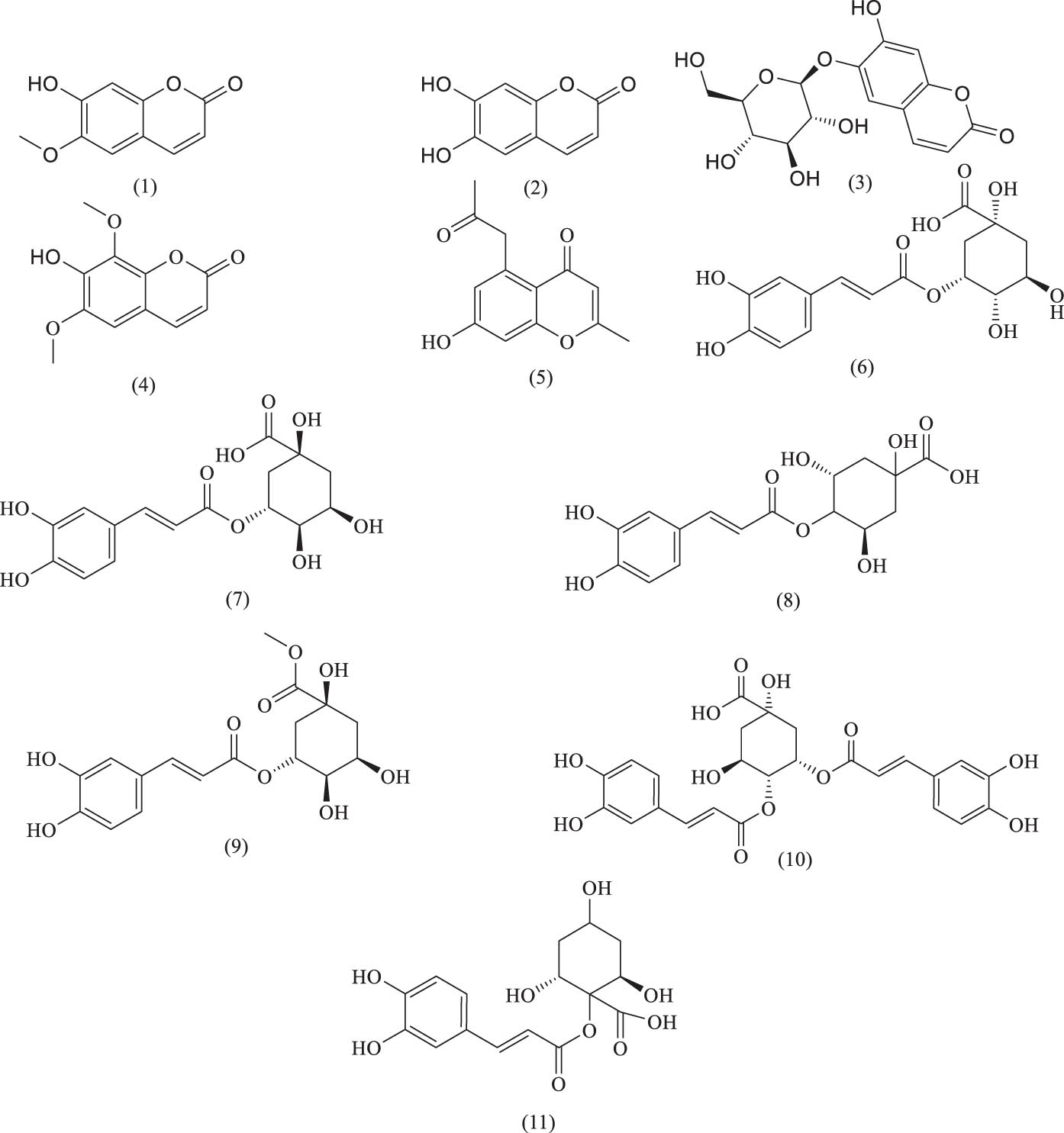
Chemical structures of phenylpropanoids.
4.3 Steroids and their glycosides
Among the steroid compounds isolated from A. venetum, β-sitosterol and lupeol are the most prominent active components, known for their antibacterial and antiviral activities. Cardiac glycosides, which are essential for the treatment of heart failure, have their biological activity and toxicity influenced by the type and number of sugar moieties attached. Certain cardiac glycosides also exhibit antitumor effects in animals, such as hyrcanoside, which has demonstrated significant inhibitory effects on human nasopharyngeal carcinoma. The names and structures of these steroid compounds are listed in Table 3 and Figure 11.
Steroid compounds identified in A. venetum
| Serial number | Name | Source | References |
|---|---|---|---|
| (1) | β-Sitosterol | Stems | [58] |
| (2) | Daucosterol | Flowers | [43] |
| (3) | Stigmasterol | Leaf | [58] |
| (4) | Campesterol | Leaf | [58] |
| (5) | Cholesterol | Leaf | [58] |
| (6) | Uvaol | Leaf | [58] |
| (7) | Cardenolide B-1 | Leaves, stems, roots | [59] |
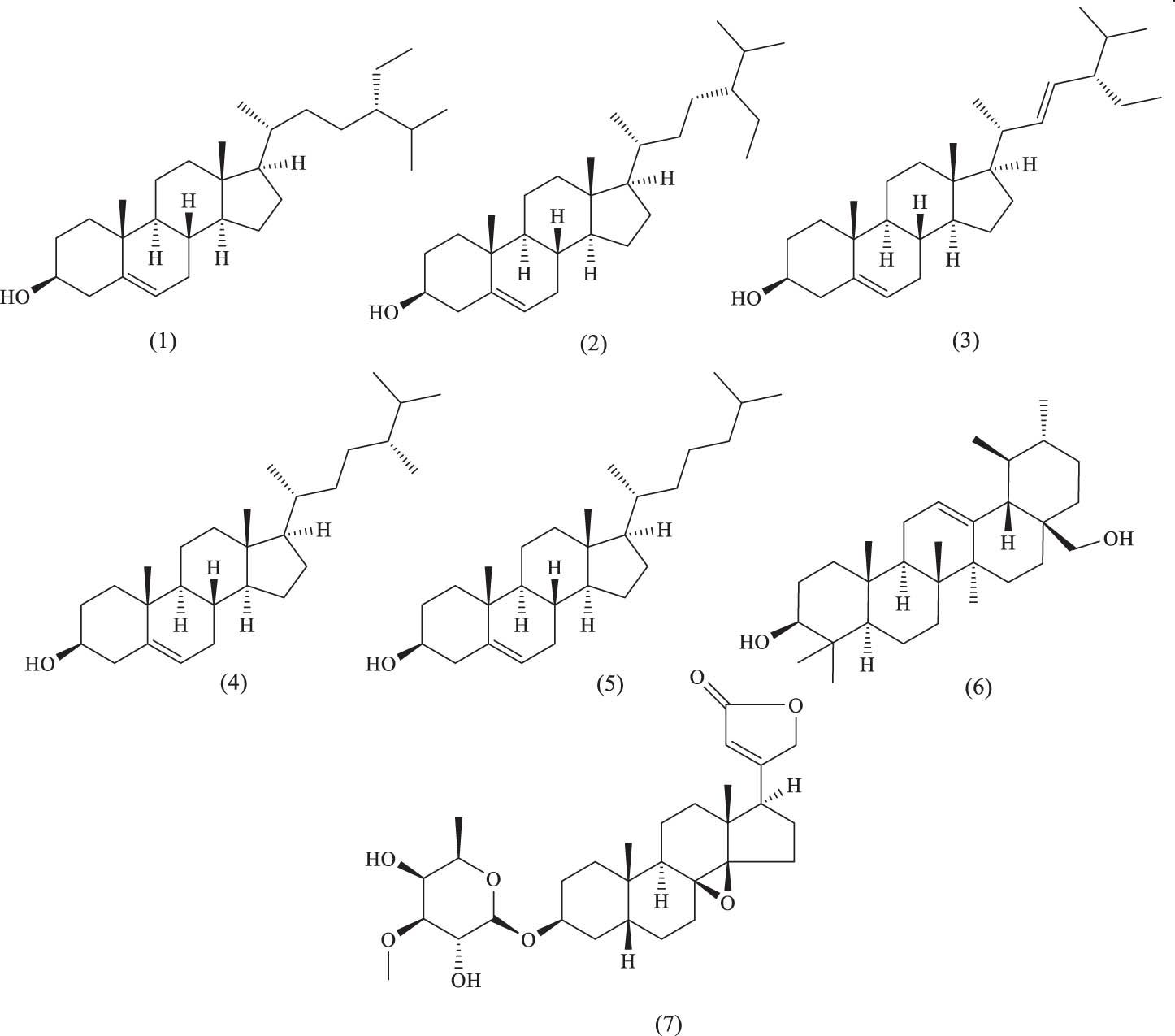
Steroids and their glycosides.
4.4 Terpenoids and volatile oils
To date, two terpenoid compounds, lupeol and phytol, have been isolated from the leaves of A. venetum. Additionally, 19 volatile compounds have been identified from the leaves of A. venetum. Furthermore, 88 aliphatic and aromatic compounds have been isolated from the flowers and leaves. According to ancient medical texts, volatile oils possess various medicinal properties, including expectorant, antitussive, antiasthmatic, antipyretic, analgesic, antibacterial, and anti-inflammatory effects. The names and structures of these terpenoid compounds and volatile oils are listed in Table 4 and Figure 12.
Terpenoids and volatile oils identified in A. venetum
| Classification | Serial number | Name | References |
|---|---|---|---|
| Terpenoids | (1) | Lupeol | [60] |
| (2) | Phytol | [60] | |
| Aromatic compounds | (3) | P-xylene | [61] |
| (4) | 4-Ethylphenol | [61] | |
| (5) | 3,5-diMethylphenol | [61] | |
| (6) | 3-Methylphenol | [61] | |
| (7) | 1,2-Benzenedimethanol | [61] | |
| (8) | 5,6,7,7a-Tetrahidro-4,4,7a-trimetil-(4H)-benzofuranosa | [61] | |
| (9) | Dibutyl phthalate | [62] | |
| (10) | Phenethyl alcohol | [62] | |
| (11) | Benzyl alcohol | [62] | |
| (12) | 1,1-Dimetoxi-etanoacetofenona | [62] | |
| (13) | 2-Feniletil acetato | [62] | |
| (14) | Benzoin acid 2 phenyl ethyl ester | [62] | |
| (15) | Ethyl formate | [62] | |
| (16) | Diisobutyl phthalate | [62] | |
| (17) | Alcohol cis-oxoaromatico | [61] | |
| (18) | Alcoholes aromaticos transoxidados | [61] | |
| (19) | Benzaldehyde | [61] | |
| (20) | Anethol | [61] | |
| (21) | 1,2-Dihidro-1,5,8-trimetil-naftaleno | [61] | |
| (22) | 2-Penten-1-ol | [61] | |
| (23) | 4,4-Dimetil-2-ciclopenten-1-ona | [61] | |
| (24) | Metilciclohexano | [61] | |
| (25) | 2-Metildihidro-3(2H)-furanona | [61] | |
| (26) | 1,2,5,5-Tetrametil-1,3-ciclopentadieno | [61] | |
| (27) | N-Hexen-3-ol | [61] | |
| (28) | 1,3,5-Octatrieno | [61] | |
| (29) | 2-Furanmetanol | [61] | |
| (30) | 1,3,5,7-Cicloocteno | [61] | |
| (31) | 2-Acetilfuran | [61] | |
| (32) | 6-Methyl-5-hepten-2-one | [61] | |
| (33) | 2-Pentilfurano | [61] | |
| (34) | Limoneno | [61] | |
| (35) | 2,2,6-Trimetilciclohexanona | [61] | |
| (36) | 1-Octanol | [61] | |
| (37) | α-Terpinene | [61] | |
| (38) | 2,6-Dimetilciclohexanol | [61] | |
| (39) | 6-Methyl-3,5-pentadien-2-one | [61] | |
| (40) | Camphene | [61] | |
| (41) | Menthone | [61] | |
| (42) | Menthol | [61] | |
| (43) | α-Terpineol | [61] | |
| (44) | Safranal | [61] | |
| (45) | Decyl aldehyde | [61] | |
| (46) | β-Cyclocitraldehyde | [61] | |
| (47) | α-Purineno | [61] | |
| (48) | Damascenone | [61] | |
| (49) | Tetradecane | [61] | |
| (50) | δ-Eudesmene | [61] | |
| (51) | 1,2,4-Triethylcyclohexane | [61] | |
| (52) | β-Caryophyllene | [61] | |
| (53) | Damascenone | [61] | |
| (54) | Pristane | [61] | |
| Aromatic compounds | (55) | N-Eicosane | [61] |
| (56) | 2,6,11,15-Tetrametilhexadecano | [61] | |
| (57) | 10-Methylnonadecane | [61] | |
| (58) | Ácido E-15-heptadecenoico | [61] | |
| (59) | 6,10,14-Trimetil-2-pentadecanona | [62] | |
| (60) | Methyl palmitate | [62] | |
| (61) | Palmitic acid | [62] | |
| (62) | 1-Octadeceno | [62] | |
| (63) | N-eicosane | [62] | |
| (64) | Oleic acid | [62] | |
| (65) | 10,13-Octadecadienoato de metilo | [62] | |
| (66) | N-heneicosane | [62] | |
| (67) | Linoleic acid | [62] | |
| (68) | 1-(1,5-Dimetilhexil)-4-(4-metilpentil) ciclohexano | [62] | |
| (69) | 2-Octilciclopropanocarboxaldehído | [62] | |
| (70) | (Z)-9,17-Octadecadienal | [62] | |
| (71) | Olealdehyde | [62] | |
| (72) | Linolenic alcohol | [62] | |
| (73) | Tetracosene | [62] | |
| (74) | N-tetracosane | [62] | |
| (75) | Ethyl acetate | [62] | |
| (76) | Leaf alcohol | [62] | |
| (77) | 15-Nonacosanone | [62] | |
| (78) | 2-Methoxy-1,3-dioxolane | [62] | |
| (79) | Squalene | [62] | |
| (80) | Octadecanaldehyde | [62] | |
| (81) | Pentadecane | [62] | |
| (82) | Hexadecane | [62] | |
| (83) | N-heptadecane | [62] | |
| (84) | Nineteenth carbon dioxide | [62] | |
| (85) | Methyl oleate | [62] | |
| (86) | N-hentriacontane | [62] | |
| (87) | Palmitic acid | [58] | |
| (88) | Linolenic acid | [55] |
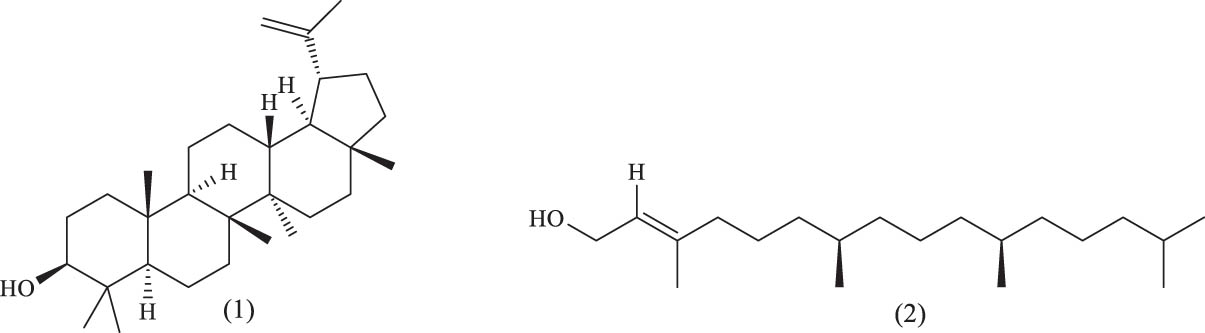
Chemical structures of terpenoids.
4.5 Organic acids
Six organic acid compounds have been isolated from A. venetum, exhibiting biological activities, such as antioxidant, anticancer, hepatoprotective, and immunomodulatory effects. The names and structures of these organic acids are listed in Table 5 and Figure 13.
Organic acids identified in A. venetum
| Serial number | Name | Source | References |
|---|---|---|---|
| (1) | Vanillic acid | flowers | [58] |
| (2) | Caffeic acid | Leaf | [63] |
| (3) | Succinic acid | Leaf | [64] |
| (4) | Fumaric acid | Leaf | [65] |
| (5) | 3-Hydroxy-4-methoxybenzoic acid | Leaf | [58] |
| (6) | Protocatechuic acid | Leaf | [58] |
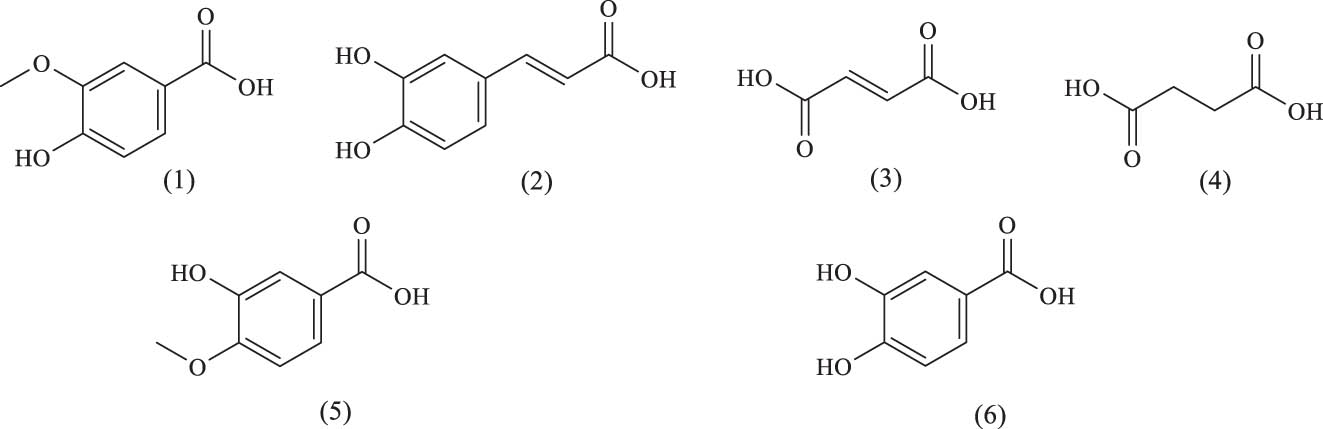
Chemical structures of organic acids.
4.6 Alkaloids
Alkaloids are often the active ingredients in many medicinal plants, capable of regulating the activities of various metabolic enzymes and transporters, making them an important class of natural organic compounds. The alkaloids isolated from A. venetum are primarily pyrrolizidine alkaloids and tropane alkaloids. Pyrrolizidine alkaloids are naturally occurring compounds widely distributed across plant species worldwide. However, most pyrrolizidine alkaloid N-oxides and their metabolically activated products, dehydropyrrolizidine alkaloids, exhibit various toxic effects, including multi-organ damage, carcinogenicity, and developmental toxicity [67]. The pyrrolizidine alkaloid content in A. venetum leaves has been measured, showing that the concentrations of indicine, lycopsamine, indicine N-oxide, and lycopsamine N-oxide range from 10.51 to 27.56, 20.66 to 42.54, 40.54 to 76.89, and 61.45 to 164.23 µg/kg, respectively. These alkaloid levels exceed the limits set by some countries or organizations, indicating a potential safety risk with long-term use. Further research on the hepatotoxicity of these alkaloids is necessary to ensure the safe use of this medicinal plant. The names and structures of these alkaloid compounds are listed in Table 6 and Figure 14.
Alkaloid compounds identified in A. venetum
| Serial number | Name | Source | References |
|---|---|---|---|
| (1) | Intermedine | Leaf | [66] |
| (2) | Lycopsamine | Leaf | [66] |
| (3) | Intermedine N-oxide | Leaf | [66] |
| (4) | Lycopsamine N-oxide | Leaf | [66] |
| (5) | Scopolamine | Root | [52] |
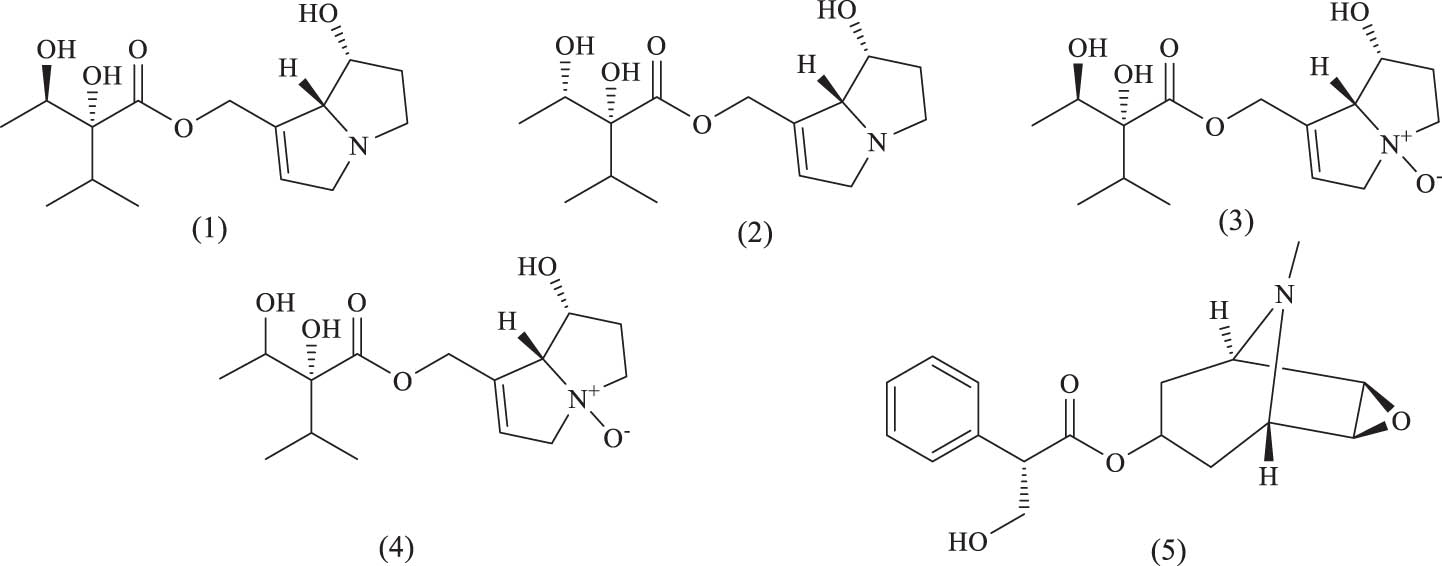
Chemical structures of alkaloids.
4.7 Polysaccharides and other components
In addition to alkaloids, several polysaccharides have also been isolated from A. venetum. One such polysaccharide, AVRP-N [67], is composed of mannose, arabinose, glucose, and galactose in a molar ratio of 35.10:34.18:23.67:7.04, with a molecular weight of 6.43 kDa [67]. Vp2a-II, another polysaccharide, consists of rhamnose, arabinose, xylose, mannose, glucose, and galactose in a molar ratio of 1.94:16.7:0.38:1.76:0.17:15.4, with a molecular weight of 7 kDa [68]. Vp3 comprises rhamnose, arabinose, xylose, mannose, glucose, and galactose in a molar ratio of 8.09:7.87:2.12:2.46:0.41:8.43, with a molecular weight of 9 kDa. Another notable compound, ATPC-A, is a polysaccharide conjugate extracted from A. venetum tea residue under alkaline conditions, with strong emulsification properties [69]. Alkaline phosphatase is a water-soluble crude polysaccharide extracted from dried A. venetum leaves through hot water extraction, ethanol precipitation, and the Sevag method for protein removal. It is composed of mannose, rhamnose, glucuronic acid, glucose, galactose, and arabinose in a molar ratio of 0.29:1.0:0.55:3.88:1.12:2.20:1.29. Many reports have highlighted the biological activities of plant polysaccharides, including hypoglycemic, lipid-lowering, anti-thrombosis, antibacterial, anti-inflammatory, immunomodulatory, and anti-fatigue effects. Polysaccharides in natural medicines often possess strong bioactivities and are considered the active ingredients of these medicines. Their biological activities are influenced by associated proteins, pigments, metal ions, and stereochemical structures. This article also explored the potential role of polysaccharides from A. venetum in treating central nervous system (CNS) diseases, such as epilepsy. Additionally, A. venetum contains essential amino acids, inorganic elements, and trace elements, whose names and structures are presented in Table 7 and Figure 15.
Polysaccharide and other compounds identified in A. venetum
| Classification | Serial number | Name | Source | References |
|---|---|---|---|---|
| Polysaccharide | (1) | AVRP-N | Root | [65] |
| (2) | Vp2a-II | Flowers | [68] | |
| (3) | Vp3 | Flowers | [68] | |
| (4) | ATPC-A mixture | Leaf | [69] | |
| (5) | ALP | Leaf | [67] | |
| Other ingredients | (6) | Grasshopper ketone | Leaf | [36] |
| (7) | Inositol | Leaf | [58] | |
| (8) | Phytol | Leaf | [58] | |
| (9) | Methoxytocopherol | Leaf | [58] | |
| (10) | Caryophyllene oxide | Leaf | [56] | |
| (11) | Anthraquinone | Leaf | [56] |
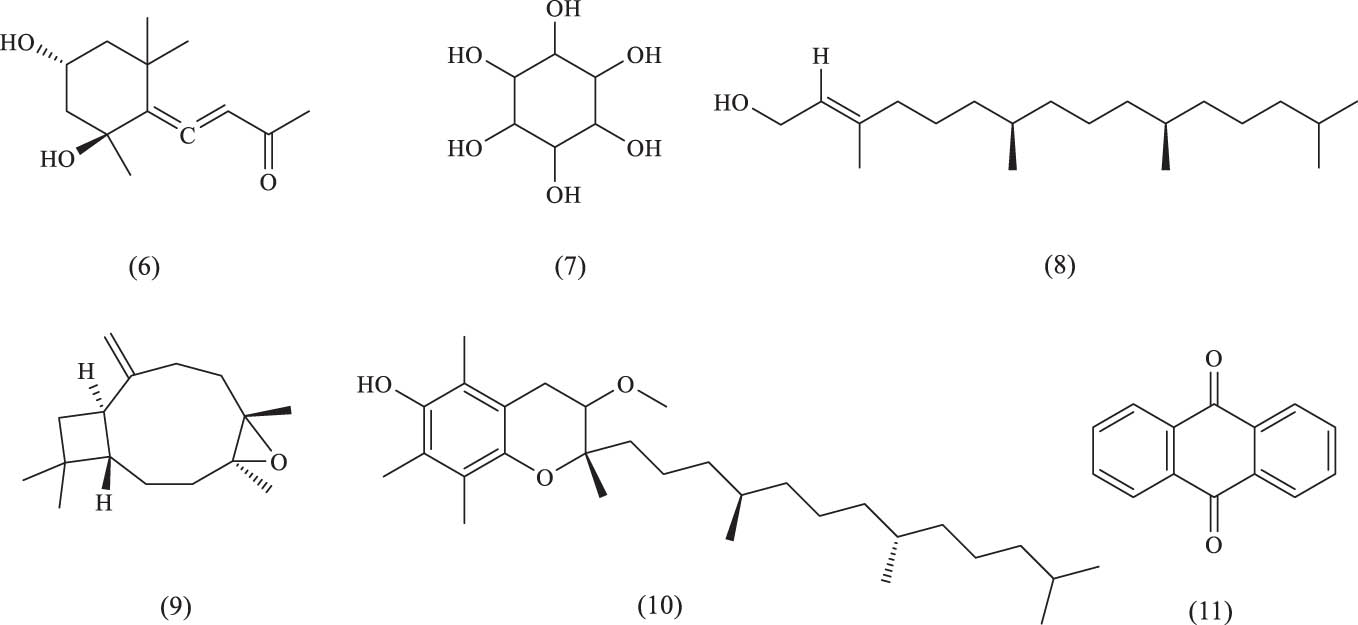
Chemical structures of other components.
5 Metabolic study of flavonoids in A. venetum leaves
The metabolism of TCM in the body involves both prototype compounds and metabolites. The former are referred to as the “chemical constituents of TCM,” while the latter are known as the “metabolome of TCM.” In a study [52], A. venetum leaf extract (2 mL) was administered orally to rats, and urine was collected 12 h after administration. Eight prototype compounds were identified, including chlorogenic acid, scopoletin, hyperoside, isoquercitrin, trifolirhizin, formononetin, quercetin, and kaempferol. Among these, hyperoside, isoquercitrin, trifolirhizin, formononetin, quercetin, and kaempferol are flavonoid compounds. Additionally, 34 metabolites were detected, 30 of which were related to flavonoid metabolism. The urinary metabolite profile indicated that A. venetum leaf extract underwent the following metabolic reactions to form these 34 metabolites: (1) Phase I reactions, including methylation, hydroxylation, hydration, reduction, and hydrolysis, and (2) Phase II reactions, primarily sulfate conjugation and glucuronidation. Methylation, sulfate conjugation, and glucuronidation were the major metabolic pathways. The metabolic process of the key active compounds is illustrated in Figure 16. Both A. venetum leaf extract and urine containing the extract showed flavonoid compounds as the primary constituents. Hyperoside is the most abundant compound in A. venetum, while isoquercitrin, an isomer of hyperoside, is also present in significant amounts. Hyperoside lacks hydrolytic enzymes in the intestine, so upon ingestion, it is rapidly absorbed into the bloodstream and metabolized into other forms in the plasma, resulting in low bioavailability. Isoquercitrin, on the other hand, may undergo enterohepatic circulation, but its absorption in the small intestine is poor, and the unabsorbed portion is metabolized by intestinal microbiota, also resulting in low bioavailability. Isoquercitrin is readily metabolized into glucuronidated quercetin in the body, although the presence of other components in A. venetum extract may enhance its bioavailability.
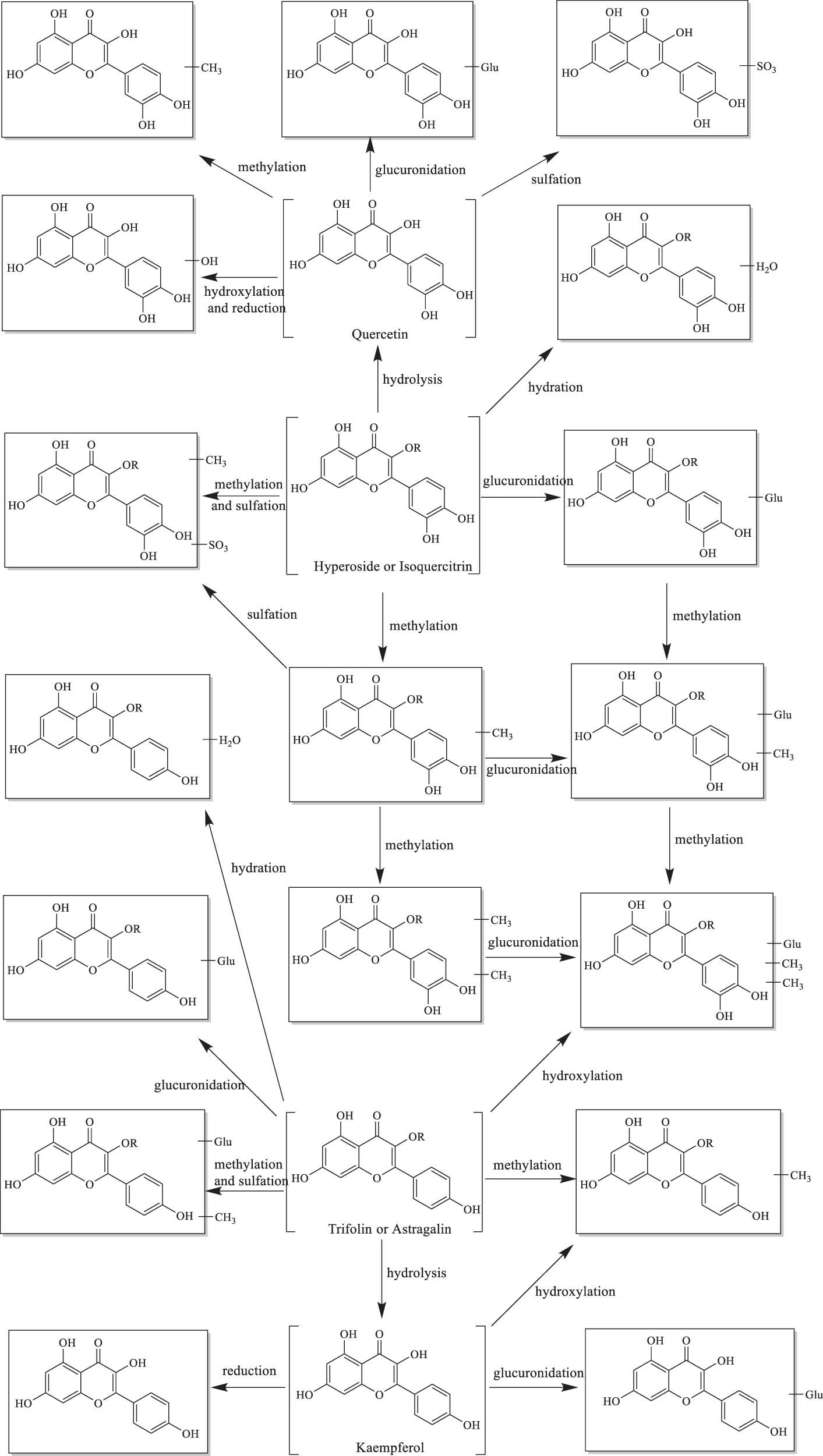
Chemical structures of flavonoid metabolites and their major metabolic pathways.
It is known that the flavonoid compounds hyperoside, quercetin, isoquercitrin, trifolirhizin, and kaempferol are the primary active ingredients responsible for the pharmacological effects of A. venetum. Additionally, A. venetum leaves have been found to improve TG metabolism, regulate blood lipids, reduce lipid peroxidation levels in rats, and enhance the immune system and amino acid metabolism in Tan sheep, promoting carbohydrate absorption. Furthermore, A. venetum leaves also influence serum metabolite levels in Tan sheep. Through UPLC-Q-TOF/MS, differential metabolites such as chlorophenol, β-hydroxybutyrate, catechol, fumarate, ethylmalonate, 2-hydroxyphenylacetic acid, gentisic acid, and protocatechuic acid were identified [48].
6 Potential of A. venetum L. leaves in the treatment of epilepsy
Existing studies have demonstrated that A. venetum leaves exhibit anxiolytic, antidepressant, and sedative effects, but further research is required to explore their potential in treating CNS disorders such as epilepsy. In the development of antiepileptic drugs, traditional Chinese medicines have garnered widespread attention for their natural, safe, and low-toxicity profiles. Research has shown that LPS exacerbates hippocampal neuronal damage and induces hippocampal neuroinflammation in epileptic mice, with an increased expression of interleukin-1 receptor type 1 (IL-1R1) signaling during this process. Blocking central IL-1R1 can reduce seizure susceptibility and severity in epilepsy [66]. This indicates that IL-1R1 mediates LPS-induced central inflammatory responses, increasing seizure susceptibility.
Polysaccharides from A. venetum leaves, such as ALRPN-1 (composed of glucose, ga-lactose, and arabinose) and ALRPN-2 (composed of glucose, galactose, and mannose), have demonstrated significant anti-inflammatory activity in LPS-induced macrophages by regulating the levels of pro-inflammatory mediators (NO) and cytokines (TNF-α, IL-6, and IL-1β) and activating the ERK/MAPK signaling pathway. It is hypothesized that A. venetum polysaccharides may reduce LPS-induced seizure susceptibility. Furthermore, A. venetum leaf extract has been shown to exert neuroprotective effects by inhibiting neuronal apoptosis in rat cortical neurons via the downregulation of caspase-3 expression and modulation of the Bcl-2/Bax ratio [68]. Additionally, the extract directly inhibits superoxide production by significantly reducing the expression of gp91phox, a key component of NADPH oxidase, enhancing SOD activity, and reducing the formation of MDA, thereby inhibiting neuronal apoptosis [69]. Based on this, it can be concluded that A. venetum leaves may exert neuroprotective effects through oxidative stress modulation, providing a foundation for further research into the pharmacodynamics and mechanisms of A. venetum in epilepsy treatment.
7 Conclusions
This article systematically reviews the chemical constituents isolated from A. venetum leaves, their pharmacological mechanisms of action, and the metabolism of the major active compounds. A. venetum leaves are rich in essential nutrients and represent a safe, healthy, and eco-friendly herbal medicine with medicinal uses, offering great potential for development in the medical and textile industries. Numerous studies have reported on the pharmacological effects of the primary active compounds in A. venetum leaves, particularly flavonoids. However, there has been relatively little research on other constituents, such as steroids, phenylpropanoids, and organic acids. Further studies could explore the medicinal and economic potential of A. venetum leaves, as well as the possibility of developing new drug formulations by combining A. venetum with other herbal or Western medicines to enhance therapeutic efficacy.
-
Funding information: This research was funded by a grant from Natural Science Research of Jiangsu Higher Education Institutions of China (No. 23KJD360002), Science and Technology Program of Huai’an City, China (Nos. HABZ202226, HAB202368), Engineering Research Center for the Development and Processing Technology of Precision Health Pharmaceutical Food Products of Jiangsu Province, “Huai Shang Elite” project [Grant number (2023)8], Heilongjiang Provincial Science Foundation Project (No. LH2021C076), and Jiangsu Food & Pharmaceutical Science College college-level Fund (No. JSFPC2024003).
-
Author contributions: Writing – original draft preparation, Yi Xiu and Xiaomao Li; validation, Siyi Wang and Jiaqi Yu; resources, Lijia Jing and Na Guo; and project administration, Yuzhi Jiao and Weiwei Zhai. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Editorial board of Flora of China of Chinese Academy of Sciences. Flora of China. Beijing: Science Press; 1977.Search in Google Scholar
[2] Xiang T, Wu L, Isah MB, Chen C, Zhang X. Apocynum venetum, a medicinal, economical and ecological plant: a review update. PeerJ 2023;11:e14966.10.7717/peerj.14966Search in Google Scholar PubMed PubMed Central
[3] Guo HL, Guo SB, Zhao XJ, Hu LJ, Hu ZF, Xiao XM, et al. Experimental comparison of antidepressant pharmacological effects of Apocynum venetum L. Jiangxi J Tradit Chin Med. 2014;45:55–7.Search in Google Scholar
[4] Butterweck V, Nishibe S, Sasaki T, Uchida M. Antidepressant effects of Apocynum venetum leaves in a forced swimming test. Biol Pharm Bull. 2001;24:848–51.10.1248/bpb.24.848Search in Google Scholar PubMed
[5] Zheng M, Liu C, Pan F, Shi D, Ma F, Zhang Y, et al. Protective effects of flavonoid extract from Apocynum venetum leaves against corticosterone-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2011;31:421–8.10.1007/s10571-010-9635-4Search in Google Scholar PubMed PubMed Central
[6] Zheng M, Liu C, Pan F, Shi D, Zhang Y. Antidepressant-like effect of hyperoside isolated from Aapocynum venetum leaves: possible cellular mechanisms. Phytomedicine. 2012;19:145–9.10.1016/j.phymed.2011.06.029Search in Google Scholar PubMed
[7] Wang J, Xie J, Chen YM. Antidepressant effect of flavonoids extracted from Apocynum venetum leaves and expression of S100A10 in rat model of depression. Zhejiang Prev Med 2016;28:574–7.Search in Google Scholar
[8] Wang J, Xie J, Chen YM, Gao LS, Zhang HS. Antidepressant effect of flavonoids extracted from Apocynum venetum leaves in rat model of depression. Zhejiang Med 2016;38:14–6.Search in Google Scholar
[9] He W, Wang L, Huang JF, Liu L, Liu XY, Du TF, et al. Sedative and hypnotic effects of water extract of Apocynum venetum leaves. Drug Eval Respir. 2022;45:274–80.Search in Google Scholar
[10] Kuo CS, Kwan CY, Gong CL, Tsai MF, Nishibe S, Tatsuzaki J, et al. Apocynum venetum leaf aqueous extract inhibits voltage-gated sodium channels of mouse neuroblastoma N2A cells. J Ethnopharmacol. 2011;136:149–55.10.1016/j.jep.2011.04.035Search in Google Scholar PubMed
[11] Xiong Q, Fan W, Tezuka Y, Adnyana IK, Stampoulis P, Hattori M, et al. Hepatoprotective effect of Apocynum venetum and its active constituents. Planta Med. 2000;66:127–33.Search in Google Scholar
[12] Xie W, Chen C, Jiang Z, Wang J, Melzig MF, Zhang X. Apocynum venetum attenuates acetaminophen-induced liver injury in mice. Am J Chin Med. 2015;43:457–76.10.1142/S0192415X15500299Search in Google Scholar PubMed
[13] Zhang W, Dong Z, Chang X, Zhang C, Rong G, Gao X, et al. Protective effect of the total flavonoids from Apocynum venetum L. On carbon tetrachloride-induced hepatotoxicity in vitro and in vivo. J Physiol Biochem. 2018;74:301–12.10.1007/s13105-018-0618-0Search in Google Scholar PubMed
[14] Kwan CY, Zhang WB, Nishibe S, Seo S. A novel in vitro endothelium-dependent vascular relaxant effect of apocynum venetum leaf extract. Clin Exp Pharmacol Physiol. 2005;32:789–95.10.1111/j.1440-1681.2005.04255.xSearch in Google Scholar PubMed
[15] Lau YS, Kwan CY, Ku TC, Hsieh WT, Wang HD, Nishibe S, et al. Apocynum venetum leaf extract, an antihypertensive herb, inhibits rat aortic contraction induced by angiotensin ii: a nitric oxide and superoxide connection. J Ethnopharmacol. 2012;143:565–71.10.1016/j.jep.2012.07.012Search in Google Scholar PubMed
[16] Lau YS, Ling WC, Murugan D, Kwan CY, Mustafa MR. Endothelium-dependent relaxation effect of Apocynum venetum leaf extract via SRC/PI3K/AKT signalling pathway. Nutrients. 2015;7:5239–53.10.3390/nu7075220Search in Google Scholar PubMed PubMed Central
[17] Pei Y, Wang R, Zhou HW. Network pharmacology mechanism in treating hypertension with Apocynum venetum. Cent South Pharm. 2019;17:1997–2001.Search in Google Scholar
[18] Xu ZG, Bai RY, Yan XM, Li Y, Zhou Y, Zhang J, et al. Effect of total flavonoids from Apocynum venetum L. leaves on blood lipid metabolism in hyperlipidemic rats. 2021;32:208–13.Search in Google Scholar
[19] Zhang Y, Leng XW, Li YJ, Liu K, Yan MT, Ren LQ. Anti-atherosclerotic effect of flavonoids in Folium Apocyni Veneti. Chin J Public Health. 2016;32:1696–9.Search in Google Scholar
[20] Manzoor M, Muroi M, Ogawa N, Kobayashi H, Nishimura H, Chen D, et al. Isoquercitrin from apocynum venetum L. Produces an anti-obesity effect on obese mice by targeting c-1-tetrahydrofolate synthase, carbonyl reductase, and glutathione s-transferase p and modification of the AMPK/SREBP-1c/FAS/CD36 signaling pathway in mice in vivo. Food Funct. 2022;13:10923–36.10.1039/D2FO02438ASearch in Google Scholar
[21] Qin MS, Yu H, Zhang XL, Liu HZ. Network pharmacologic analysis of the mechanism of Apocyni Veneti Folium in the treatment of cardiovascular disease. Tradit Chin Drug Res Clin Pharmacol. 2020;31:576–82.Search in Google Scholar
[22] Li Q, Zhang YC, Liu XH, Song JP, Wang LJ. Analysis of essential components of folium Apocyni Veneti from different habitats by HSGC-MS. Res Pract Chin Med. 2009;23:34–7.Search in Google Scholar
[23] Song R, Zhou J. Microemulsion liquid chromatographic method for simultaneous separation and determination of six flavonoids of Apocynum venetum leaf extract. J Chromatogr B. 2015;995–996:8–14.10.1016/j.jchromb.2015.05.019Search in Google Scholar PubMed
[24] Qu XL, Wang WQ, Liang XY, Tie R, Liu FE, Li R, et al. Effects of Apocynum Venetum leaf extract on myocardial ischemia reperfusion injury rat. Chin J Microc. 2015;25:15–8.Search in Google Scholar
[25] Zhang Y, Liu S, Ma JL, Chen C, Huang P, Ji JH, et al. Apocynum venetum leaf extract alleviated doxorubicin-induced cardiotoxicity through the akt/bcl-2 signaling pathway. Phytomedicine. 2022;94:153815.Search in Google Scholar
[26] Grundmann O, Nakajima J, Seo S, Butterweck V. Anti-anxiety effects of apocynum venetum L. In the elevated plus maze test. J Ethnopharmacol. 2007;110:406–11.10.1016/j.jep.2006.09.035Search in Google Scholar PubMed
[27] Grundmann O, Nakajima J, Kamata K, Seo S, Butterweck V. Kaempferol from the leaves of apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine. 2009;16:295–302.10.1016/j.phymed.2008.12.020Search in Google Scholar PubMed
[28] Abubakar AS, Ahmad B, Ahmad N, Liu L, Liu B, Qu Y, et al. Physicochemical evaluation, structural characterization, in vitro and in vivo bioactivities of water-soluble polysaccharides from Luobuma (Apocynum L.) tea. Food Chem. 2024;460(Pt 2):140453.10.1016/j.foodchem.2024.140453Search in Google Scholar PubMed
[29] Kasimu R, Fan Z, Wang X, Hu J, Wang P, Wang J. Anti-platelet aggregation activities of different fractions in leaves of apocynum venetum L. J Ethnopharmacol. 2015;168:116–21.10.1016/j.jep.2015.03.013Search in Google Scholar PubMed
[30] Wang L, Zhang X, Niu Y, Ahmed AF, Wang J, Kang W. Anticoagulant activity of two novel polysaccharides from flowers of Apocynum venetum L. Int J Biol Macromol. 2019;124:1230–7.10.1016/j.ijbiomac.2018.12.015Search in Google Scholar PubMed
[31] Hu MN, Liang TG. Experimental study on acute toxicity of total flavonoids in Apocynum venetum. Guide China Med. 2016;14:37–8.Search in Google Scholar
[32] Yu YY. Biological effects of Apocynum Venetum tea on cardiovascular system and it’s safety assessment. Hangzhou, China: Zhejiang University; 2006.Search in Google Scholar
[33] Li L, Wang QS, Chen DF, Zhang M, Wang WY. Toxicological assessment on safety of tea of Apoeynum venetum leaf. Pract Prev Med. 2010;17:2081–4.Search in Google Scholar
[34] Guo Y, He W, Luo FX, Abudukeremu Ma FC, Wang L, Huang JF, et al. Study the acute? Toxicity and sub-acute toxicity of Apocynum extract in mice by intragastric administration. Xinjiang Med J. 2019;49:770–3.Search in Google Scholar
[35] Gao G, Chen P, Chen J, Chen K, Wang X, Abubakar AS, et al. Genomic survey, transcriptome, and metabolome analysis of Apocynum venetum and Apocynum hendersonii to reveal major flavonoid biosynthesis pathways. Metabolites. 2019;9(12):296. 10.3390/metabo9120296.Search in Google Scholar PubMed PubMed Central
[36] Fu HM, Yin CL, Shen ZY, Yang MH. Flavonoids from the leaves of Apocynum venetum and their anti-inflammatory activity. J Chem Res. 2022;46. 10.1177/17475198211073871.Search in Google Scholar
[37] Cheng XL, Zhang SQ, Li QS. Chemical constituents of flavonoids from Apocynum venetum. J Chin Med Mater 2007;30(9):1086–8.Search in Google Scholar
[38] Li LH, Yuan Z. Study on flavonoids in leaves of Apocynum ventum. J Chin Med Mater. 2006;31(16):1337–40.Search in Google Scholar
[39] Zeng HS. Study on the chemical composition of Apocynum venetum leaves. Xian, China: Northwest University; 2009.Search in Google Scholar
[40] Hou JJ, Han LW, Yang GE, Li QS. Research progress on chemical components and pharmacological activities of Apocynum venetum leaves. Chin Tradit Herb Drugs. 2006;1603–5. 10.3321/j.issn:0253-2670.2006.10.056.Search in Google Scholar
[41] Zhang Y, Liu S, Ma JL, Chen C, Huang P, Ji JH, et al. Apocynum venetum leaf extract alleviated doxorubicin-induced cardiotoxicity through the akt/bcl-2 signaling pathway. Phytomedicine 2022;94:153815.10.1016/j.phymed.2021.153815Search in Google Scholar PubMed
[42] Chen L, Du LJ, Ding Y, Xing DM, Wang W. Studies on chemical constituents from flowers of Apocynum venetum. Zhongguo Zhong Yao Za Zhi. 2005;30:1340–2.Search in Google Scholar
[43] Zhang Y, Liu C, Zhang Z, Qi Y, Wu G, Li S. Solvent gradient elution for comprehensive separation of constituents with wide range of polarity in Apocynum venetum leaves by high-speed counter-current chromatography. J Sep Sci. 2010;33:2743–8.10.1002/jssc.201000308Search in Google Scholar PubMed
[44] Zhang Y, Liu C, Zhang Z, Wang J, Wu G, Li S. Comprehensive separation and identification of chemical constituents from Apocynum venetum leaves by high-performance counter-current chromatography and high-performance liquid chromatography coupled with mass spectrometry. J Chromatogr B. 2010;878:3149–55.10.1016/j.jchromb.2010.09.027Search in Google Scholar PubMed
[45] Kamata K, Seo S, Nakajima J. Constituents from leaves of Apocynum venetum L. J Nat Med. 2008;62:160–3.10.1007/s11418-007-0202-3Search in Google Scholar PubMed
[46] Murakami T, Kishi A, Matsuda H, Hattori M, Yoshikawa M. Medicinal foodstuffs. Xxiv. Chemical constituents of the processed leaves of Apocynum venetum L.: Absolute stereostructures of Apocynosides i and ii. Chem Pharm Bull. 2001;49:845–8.10.1248/cpb.49.845Search in Google Scholar PubMed
[47] Kong NN, Fang ST, Liu Y, Xia CH. Nonflavonoid constituents from leaves of Apocynum venetum. Chin Tradit Herb Drugs. 2013;44:3114–8.Search in Google Scholar
[48] Fan WZ, Tezuka Y, Xiong QB, Hattori M, Namba T, Kadota S, et al. New Phenylpropanoid-substituted flavan-3-ols isolated from leaves of Apocynum venetum (Luobuma-Ye). Chem Pharm Bull. 1999;47:1049–50.10.1248/cpb.47.1049Search in Google Scholar
[49] Yokozawa T, Nakagawa T. Inhibitory effects of luobuma tea and its components against glucose-mediated protein damage. Food Chem Toxicol. 2004;42:975–81.10.1016/j.fct.2004.02.010Search in Google Scholar PubMed
[50] Xiong Q, Fan W, Tezuka Y, Adnyana IK, Stampoulis P, Hattori M, et al. Hepatoprotective effect of Apocynum venetum and its active constituents. Planta Med. 2000;66:127–33.10.1055/s-2000-11135Search in Google Scholar PubMed
[51] Xu ZG, Bai RY, Yan XM, Li Y, Zhou Y, Zhang J, et al. Effect of total flavonoids from Apocynum venetum L. leaves on blood lipid metabolism in hyperlipidemic rats. Tradit Chin Drug Res Clin Pharmacol. 2021;32:208–13.Search in Google Scholar
[52] Wei NN. Isolation, purification and structure analysis of the polysaccharides from Apocynum venetum leaves and its interaction with Galectin-3. Changchun: Northeast Normal University; 2010.Search in Google Scholar
[53] Cai YX, MaLiKe A, Xiao ZH. Chemical constituents from flower of Apocynum venetum. Chin Tradit Herb Drugs. 2007;1306–7.Search in Google Scholar
[54] Kong NN, Fang ST, Liu Y, Wang JH, Yang CY, Xia CH. Flavonoids from the halophyte Apocynum venetum and their antifouling activities against marine biofilm-derived bacteria. Nat Prod Res. 2014;28:928–31.10.1080/14786419.2014.886205Search in Google Scholar PubMed
[55] Pei Y, Wang R, Zhou HW. Network pharmacology mechanism in treating hypertension with Apocynum venetum. Cent South Pharm. 2019;17:1997–2001.Search in Google Scholar
[56] Wu SZ, He MY, Li GW, Wu WP, Qiu YJ, Zeng H, et al. UPLC characteristic maps and determination of flavonoids in Apocyni Veneti Folium from different producing areas. Mod Chin Med. 2021;23:619–26.Search in Google Scholar
[57] Du S. Studies on chemical components and anti-inflammatory activity of Apocynum venetum L. Shenzhen, China: Shenzhen University; 2020.Search in Google Scholar
[58] Lv JR. Analysis of chemical constituents and activity studies on essential oil in flower and leaf of Apocynum venetum L. Xianyang, China: Northwest A&F University; 2007.Search in Google Scholar
[59] Wang LL, Zhao C, He N, Liu YN, Li WQ, Wang YH, et al. Analysis of Fat-soluble constituents from flowers of Apocynum venetum L. J Henan Univ. 2017;36:260–2.Search in Google Scholar
[60] Zhao L, Liang S, Lv L, Zhang H, Guo-Tan G, Chai Y, et al. Screening and analysis of metabolites in rat urine after oral administration of Apocynum venetum L. Extracts using HPLC-TOF-MS. J Sep Sci. 2014;37(5):515–26.10.1002/jssc.201301036Search in Google Scholar PubMed
[61] Liang SS. Study on quality control and metabolism of pharmacodynamic constituents in vivo for ApocynumVetenum L. Shanghai, China: Naval Medical University; 2012.Search in Google Scholar
[62] Zhang YF, Wei D, Guo SY, Chen F, Du NS. Research on the chemical components of Apocynum dahuricum. Nat Prod Res Dev. 2006;18:954.Search in Google Scholar
[63] Qu XL, Wang WQ, Liang XY, Tie R, Liu FE, Li R, et al. Effects of Apocynum Venetum leaf extract on myocardial ischemia reperfusion injury rat. Chin J Microcirc. 2015;25:15–8.Search in Google Scholar
[64] Sun XX, Xiang E, Qiu SK, Wang H, Guo Y. Research progress on toxicity of pyrrolizidine alkaloids. Chin J Pharmacovigilance. 2019;16:76–80.Search in Google Scholar
[65] Chen XQ, Wang CP, Wang CY, Liu CX, Yuan Y, Wang BJ, et al. The emulsification properties of alkaline-extracted polysaccharide conjugates from Apocynum venetum L. tea residues. Food Hydrocoll. 2022;124:107315. 10.1016/j.foodhyd.2021.107315.Search in Google Scholar
[66] Jin CQ. Effects of Apocynum venetum feeding for Tan-sheep based on metabolomics and transcriptomics. Ningxia, China: Ningxia University; 2019.Search in Google Scholar
[67] Liu D, Wang SY, Wang GN, Zheng LH, Sun Y, Liu L, et al. Structural characterization and immunoregulatory activity of a neutral polysaccharide from the roots of Apocynum venetum L. Int J Biol Macromol. 2022;222:90–100.10.1016/j.ijbiomac.2022.09.158Search in Google Scholar PubMed
[68] Hu A, Yuan H, Qin Y, Zhu Y, Zhang L, Chen Q, et al. Lipopolysaccharide (LPS) increases susceptibility to epilepsy via interleukin-1 type 1 receptor signaling. Brain Res. 2022;1793:148052.10.1016/j.brainres.2022.148052Search in Google Scholar PubMed
[69] Xiang J, Tang YP, Zhou ZY, Wu P, Wang Z, Mori M, et al. Apocynum venetum leaf extract protects rat cortical neurons from injury induced by oxygen and glucose deprivation in vitro. Can J Physiol Pharmacol. 2010;88:907–17.10.1139/Y10-069Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Juglone’s apoptotic impact against eimeriosis-induced infection: a bioinformatics, in-silico, and in vivo approach
- Potential anticancer agents from genus Aerva based on tubulin targets: an in-silico integration of quantitative structure activity relationship (QSAR), molecular docking, simulation, drug-likeness, and density functional theory (DFT) analysis
- Hepatoprotective and PXR-modulating effects of Erodium guttatum extract in propiconazole-induced toxicity
- Studies on chemical composition of medicinal plants collected in natural locations in Ecuador
- A study of different pre-treatment methods for cigarettes and their aroma differences
- Cytotoxicity and molecular mechanisms of quercetin, gallic acid, and pinocembrin in Caco-2 cells: insights from cell viability assays, network pharmacology, and molecular docking
- Choline-based deep eutectic solvents for green extraction of oil from sour cherry seeds
- Green-synthesis of chromium (III) nanoparticles using garden fern and evaluation of its antibacterial and anticholinesterase activities
- Innovative functional mayonnaise formulations with watermelon seeds oil: evaluation of quality parameters and storage stability
- Molecular insights and biological evaluation of compounds isolated from Ferula oopoda against diabetes, advanced glycation end products and inflammation in diabetics
- Removal of cytotoxic tamoxifen from aqueous solutions using a geopolymer-based nepheline–cordierite adsorbent
- Unravelling the therapeutic effect of naturally occurring Bauhinia flavonoids against breast cancer: an integrated computational approach
- Characterization of organic arsenic residues in livestock and poultry meat and offal and consumption risks
- Synthesis and characterization of zinc sulfide nanoparticles and their genotoxic and cytotoxic effects on acute myeloid leukemia cells
- Activity of Coriandrum sativum methanolic leaf extracts against Eimeria papillata: a combined in vitro and in silico approach
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies

