Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
-
Sheenam Sharma
Abstract
Background
The traditional plant Trigonella foenum-graecum L. has been used in the regulation of low blood glucose levels since ancient ages.
Objectives
Our research claimed on the potential of T. graecum L. leaf extract in treating hypertension in diabetes mellitus through various targets via molecular docking studies.
Methods
Diabetes-associated hypertension was induced in all rats, except the normal control group, using freshly prepared dexamethasone sodium (10 mg/kg) administered subcutaneously to overnight starved rats. Diabetic rats were orally administered chloroform and hydroethanolic extracts of T. graecum L. leaves at varying concentrations for a duration of 45 days. Biochemical, histopathological, and computational studies were investigated at the end of the treatment.
Result
The results of the study revealed that the hydroethanolic extract exhibited excellent protective activity against diabetes-associated hypertension. At both intervals, hydroethanolic and chloroform extracts exhibited significant (p <0.0001) reduction in systolic blood pressure levels. With respect to blood glucose levels, the chloroform (400 mg/kg) and hydroethanolic (200 mg/kg) extracts exhibited 45.7 and 47.3% reduction, respectively. High-performance liquid chromatography analysis confirmed the presence of quercetin in the serum samples of treated groups. Histopathological analysis revealed that groups treated with higher doses of extract showed cellular improvement and restoration of the normal morphological structure. Association of quercetin with PPAR-γ (peroxisome proliferator-activated receptor gamma) and ACE (angiotensin converting enzyme) gene was found to be positive for the treatment of diabetes-associated hypertension through computational analysis.
Conclusions
In conclusion, hydroethanolic and chloroform extracts of T. graecum L. leaves may provide novel options for the clinical management of type 2 diabetes-associated hypertension. The in silico study supports the therapeutic potential of its bioactive compounds, anticipating further pharmacological and clinical investigations.
Graphical Abstract
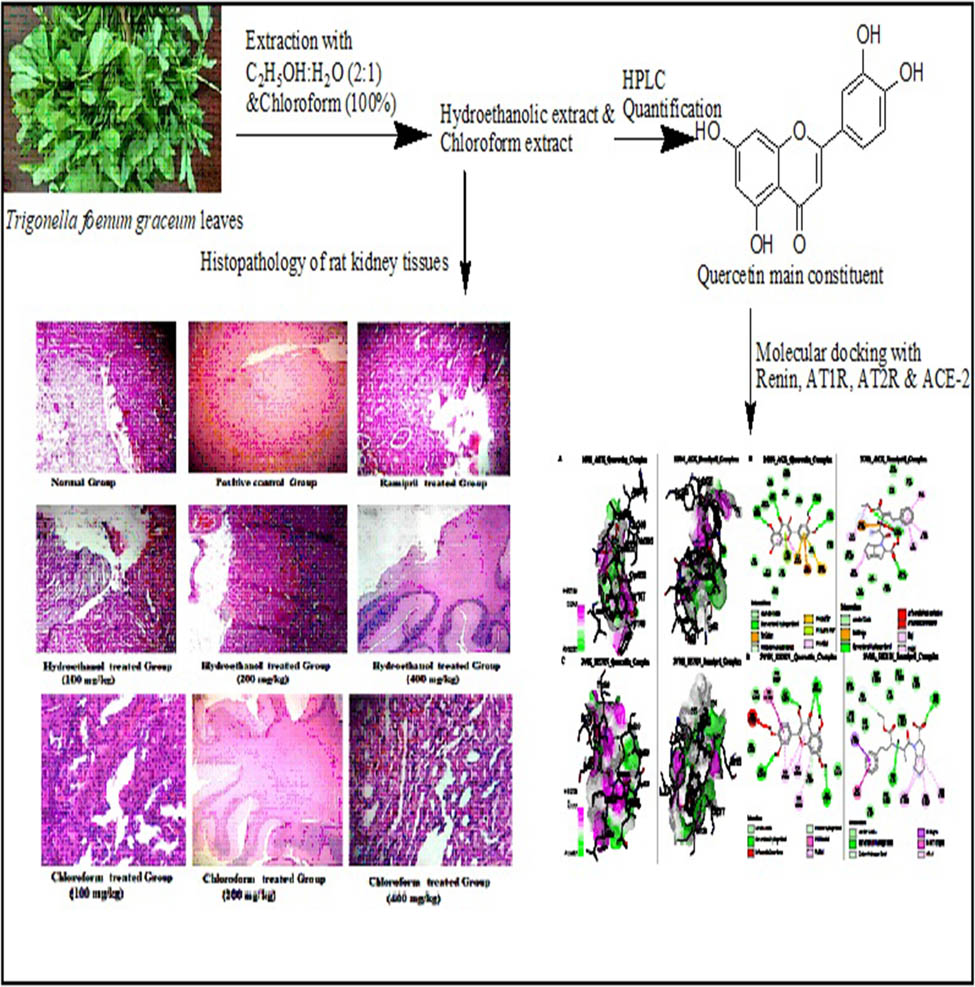
1 Introduction
A metabolic disorder is a non-communicable disease affecting the people of all age groups due to disruptions in normal glucose levels, insulin sensitivity, cardiovascular functions, cholesterol levels, and renal functions [1]. Diabetes mellitus is one of the metabolic disorders that pose a significant threat to human health globally and is characterized by an increase in blood glucose level due to a decrease in the insulin secretion or uptake of insulin by the cells related to the resistance mechanisms. The number of diabetic patients is rising more in low- and middle-income countries than in high-income countries. According to the International Diabetes Federation (IDF) Diabetes Atlas 11th Edition (2025), approximately 589 million adults between age 20 and 79 suffered from diabetes worldwide in 2024, representing about 1 in 9 adults, and the number is expected to rise to 853 million by 2050 [2]. Also, diabetes mellitus is associated with secondary complications such as diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy. Diabetes-associated high BP and hyperlipidemia is another disease cluster difficult to treat with single-drug therapy. The physicians have opted for multidrug therapy that further leads to adverse side effects [3]. Hence, there is a need to search for alternative sources of drugs that possess fewer or no side effects. In this context, medicinal herbs have attracted attention for the treatment of diabetes and associated complications due to less severe side effects. These natural products (NPs) obtained from marine plants, microorganisms, and animal sources have a history of developing new therapeutic agents. These NPs have unique structural characteristics such as a high number of sp3 carbon atoms and chiral centers that provide them the unique ability to interact with biological receptors, thereby exhibiting specific biological functions [4].
Trigonella foenum-graecum L., commonly referred to as fenugreek or methi, is an angiosperm plant classified under the Fabaceae family. Over the course of history, it has been employed in the Indian subcontinent for various purposes, including as a condiment and a medicinal remedy. It is commonly utilized as a traditional herb in Western countries and is composed of several aromatic compounds that give food their unique color and characteristics [5]. Fresh and desiccated leaves are utilized as ingredients in Indian cuisine, although fresh leaves are traditionally added into salads [6,7]. Researchers have reported that the seeds and leaves of T. graecum possess several medicinal properties including anti-inflammatory, anti-spasmodic, anticancer, astringent, carminative, emollient, expectorant, febrifuge, anti-obesity, anti-ulcer, skeletal muscle relaxant, and uterine tonic effects, as well as the ability to lower triglycerides in both humans and animals [8,9,10,11,12]. Several studies have reported its antidiabetic effects, including T. graecum L. seed powder improved lipid metabolism in type II diabetic patients with significant reduction in total cholesterol, triglyceride, and low-density lipoprotein (LDL) levels and an increase in the high-density lipoprotein (HDL) level [13]. Several mechanisms of antidiabetic effects of fenugreek include increased glucose transporter type 4 (GLUT-4) translocation and hexokinase activity, decreased fructose 1,6-bisphosphatase and glucose-6-phosphatase activities, inhibition of α-amylase, protection of β-cells, and increased insulin release [14]. The methanolic extract of fenugreek seeds was also reported to have antihypertensive effects in nephrectomized deoxycorticosterone acetate-salt-induced and fructose-induced hypertensive rats. Mechanism of action studies involved the serotonergic antagonistic effects [15]. In another study, fenugreek along with amlodipine, a calcium ion channel blocker, showed better control of blood pressure (BP) and improved response of amlodipine in hypertensive rat models [16]. An extensive array of phytochemicals, including coumarins (methyl coumarin, trimethyl coumarin, and trigocoumarin), alkaloids (fenugreekine, trigonelline, and choline), flavonoids (naringenin, kaempferol, isovitexin, quercetin, and luteolin), and phenolic compounds (catechin, vanillic acid, syringic acid, gentisic acid, and chlorogenic acid) are present in this plant [17,18]. Galactomannan is another main constituent of fenugreek reported for its antidiabetic effects [19]. All these compounds exhibit various pharmacological properties via multiple mechanisms. In recent years, in silico approaches have emerged as powerful tools in the early stages of drug discovery. Molecular docking, a computational technique that predicts the preferred orientation of a molecule when bound to a target receptor, is widely used to assess the binding affinity and interaction profiles of phytochemicals with key biological targets [20].
Additionally, ADMET (absorption, distribution, metabolism, excretion, and toxicity) prediction models help evaluate the pharmacokinetic and safety profiles of these molecules, reducing the risk of late-stage failures in drug development. Incorporating such computational tools alongside experimental evaluations provides a comprehensive understanding of a compound’s pharmacological potential and supports the rational design of novel therapeutic agents. Therefore, the integration of in silico techniques in this study enhances the reliability and efficiency of identifying effective anti-diabetic and anti-hypertensive compounds from T. graecum L.
In the present investigation, the study was undertaken to evaluate the pharmacological effects of hydroethanolic and chloroform extracts of T. graecum L. leaves in a diabetes-associated hypertension rat model. Further, a molecular docking study of identified compounds was also carried out on target receptors.
2 Materials and methods
2.1 Drugs and chemicals
Ramipril and dexamethasone sodium phosphate were gifted by M/s. Ind. Swift Pharmaceutical Ltd., Baddi, Himachal Pradesh, India. Ascorbic acid, catechin, thiobarbituric acid (TBA), and gallic acid were purchased from Sigma Chemical Co., USA. Biochemical kits for estimating glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides were purchased from Ecoline, Merck Ltd., India. A radioimmunoassay reagent for insulin determination was purchased from Stat Diagnostics (Linco Research Inc.), India.
2.2 Plant material and preparation of the extracts
T. graecum L. leaves were collected from the Government Botanical Garden at Khijrabad, Yamunanagar, Haryana, India, authenticated by NISCAR Consult, New Delhi, India (NISCAIR/RHMD/consult/-2322/102). Three kilograms of desiccated powdered leaves were extracted (Soxhlet extraction) with ethanol and water in a ratio of 2:1 and with chloroform (100%) for 72 h and filtered. The filtrates of chloroform and hydroethanolic extracts were concentrated on a rotary evaporator at 40°C under reduced pressure. The dried extracts were stored in the refrigerator at 4°C until further use.
2.3 Total bioactive content
2.3.1 Estimation of total phenolic content
We determined the total phenolic content using the Folin–Ciocalteau assay, following the technique reported earlier [21]. We used gallic acid as the reference. We diluted a 1.0 g/ml extract solution to 46 ml using distilled water. Folin–Ciocalteu reagent (1 ml) was added, rested for 3 min, followed by the addition of sodium carbonate (shaken for 180 min). At 760 nm, the absorbance was noted. The phenolic chemicals were calculated as gallic acid equivalents (GAEs).
2.3.2 High performance liquid chromatography with diode array detection (HPLC-DAD) quantitative analysis of plant extract
Chloroform and hydroethanolic extract solutions were prepared by mixing the respective extracts and solvents in a 1:10 ratio, shaking them for 60 s at 150 rpm for 12 h, filtering through Whatman filter paper no. 1, and then storing them at 4°C. HPLC analysis was performed on a JASCO HPLC system with a Cosmosil (NacalaiTesque, Inc., Japan) CN-MS (250 mm × 4.6 mm) column, an autosampler (AS-1555-10), and a photodiode array detector (MD910). The column temperature was maintained at 25°C. Samples were eluted using the mobile phase of ethanol:distilled water with gradient elution (95:5 to 0:100 v/v), adjusted to pH 3.5 at a flow rate of 1.0 ml/min, detected at 267 nm. Data acquisition and analysis were carried out using Borwin Integrator software, version 1.21, chromatography analysis software. The standard solution Quercetin (10 mg) was dissolved in 10 ml of methanol to prepare a stock solution of 1,000 µg/ml. A working standard solution was prepared by serial dilution of the standard stock solution. The sample injection volume taken was 10 μL.
2.4 In vitro antioxidant activity
2.4.1 Reducing power assay
Approximately 2.5 ml volume of different concentrations of extracts was combined with sodium phosphate buffer (2.5 ml) and potassium ferricyanide (2.5 ml, pH 6.6). After incubating at 50°C for 20 min, 2.5 ml of 10% trichloroacetic acid was added and centrifuged at 1,000 rpm for 8 min. The 5 ml aliquot was removed from the top layer of each solution, and to this 5 mL of deionized water and 1 ml of ferric chloride (0.1%) were added. The absorbance was measured at 700 nm in triplicate, and the effective concentration (EC50) value was computed using ascorbic acid as a standard reagent. An increase in absorbance indicates an increase in reducing power [22].
2.5 In vivo activity
2.5.1 Experimental animals and approval
Albino Wistar rats (180–200 g) were procured from an approved animal house at the National Institute of Pharmaceutical Education and Research (Mohali, Punjab, India). Animals were maintained at the approved animal house of M. M. College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana, Haryana, India. The animals were housed under standard temperature (24–28°C) and relative humidity (60–70%) conditions with a 12:12 light–dark cycle. The animals had free access to food pellets and water ad libitum. The experimental protocol (MMCP-IAEC-15/16) was approved by the Institutional Animal Ethics Committee (IAEC) constituted in accordance with the rules and guidelines of the Committee for the Purpose of Control and Supervision on Experimental Animals (CPCSEA), India.
2.5.2 Drugs and dosage
Extracts were administered orally for 45 days at 100, 200, and 400 mg/kg doses in 0.2 ml (2% w/v) carboxymethyl cellulose with 2.0% Tween 80. Ramipril (1 mg/kg/day) was also administered orally using oral gavage for 45 days. The dose of the drugs was selected to measure the dose-dependent effect. All rats except the normal control group received freshly prepared dexamethasone sodium 10 mg/kg subcutaneously to overnight starved rats in groups 2–9 to induce diabetes-associated hypertension.
2.5.3 Experimental design
Animals were divided into nine groups. Each group consisted of six animals. Group 1 was administered 2% w/v carboxymethyl cellulose with 2.0% Tween 80 and served as the normal control group. Group 2 received dexamethasone sodium 10 mg/kg subcutaneously and served as the positive control group. Group 3 was treated with ramipril 1 mg/kg/day and served as the standard group. Groups 4, 5, and 6 were treated once daily with 100, 200, and 400 mg/kg chloroform extract of T. graecum L., respectively. Groups 7, 8, and 9 were treated once daily with 100, 200, and 400 mg/kg hydroethanolic extract of T. graecum L., respectively. The prescribed treatment was for 45 days.
2.5.4 Biochemical estimations
Blood samples were collected on days 7, 14, and 21 (retro-orbital route) to measure glucose levels. At the end (45th day) of the experiment, the lipid profile and serum insulin were also measured [23].
2.5.5 Body weight measurement
Rats were weighed from each group at intervals of 0th, 7th, 14th, 21st, and 45th days on a standard electronic balance.
2.5.6 BP measurement
The BP was quantified non-invasively utilizing a tail-cuff sphygmomanometer (Kent Scientific) on the 15th and 30th days. Animals were acclimatized for 5 min, the initial four readings were discarded, and the mean of five subsequent readings was calculated [24].
2.5.7 Histopathological findings
At the end of the experiment, kidneys were isolated from each group and fixed in 10% formalin, processed, and stained with hematoxylin and eosin (H&E) for microscopic examination.
2.5.8 Statistical analysis
The mean ± standard deviation was used to express the data. One-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests were used to assess the statistical significance between mean values. Statistical significance was defined as p <0.05.
2.6 In silico studies
2.6.1 ADME/T studies
Quercetin and ramipril were assessed for their pharmacokinetic parameters and toxicity predictions via the ADMETlab 2.0 web interface [25].
2.6.2 Boiled egg plot
The boiled egg plot is a three-region Cartesian plane with yellow (yolk area), white, and gray sections. If the chemical of interest is found in the yolk area, it has a greater likelihood of crossing the blood–brain barrier (BBB). Placement in the white zone, on the other hand, indicates a higher likelihood of considerable intestinal absorption. Chemicals in the gray zone are more likely to be non-absorbent and non-penetrative. The Swiss ADME software was used for the generation of this plot for quercetin and ramipril [26].
2.6.3 Molecular docking
X-ray crystallographic structures of the essential target proteins of the renin–angiotensin system (RAS pathway), i.e., angiotensin-converting enzyme (ACE) (PDB ID: 1O86), renin (PDB ID: 2V0Z), angiotensin II type 1 receptor (AT1R) (PDB ID: 4ZUD), angiotensin II type 2 receptor (AT2R) (PDB ID: 7JNI), and angiotensin-converting enzyme 2 (ACE-2) (PDB ID: 8B9P), were obtained from the Protein Data Bank [27,28,29,30,31]. Molecular docking was performed using AMDock (Assisted Molecular Docking), a user-friendly graphical tool [32,33,34]. The receptors were prepared by taking out water molecules, other atoms, and attached ligands, then adding hydrogen atoms and adjusting the energy to make sure the shapes were correct and to reduce any overlaps. The 3D structures of quercetin and ramipril were obtained from the PubChem repository. The docked complexes were visualized using Discovery Studio to examine the binding modes and interactions between the ligands and receptors.
3 Results
3.1 Phytoconstituent analysis
Preliminary phytochemical studies found that the chloroform extract of T. graecum L. contained only flavonoids and alkaloids, whereas the hydroethanolic extract confirmed the presence of triterpenoids, flavonoids, alkaloids, saponins, tannins, and phenols.
3.2 Determination of total phenolic content
The total phenolic content was calculated using the linear equation y = mx + c, R 2 = 0.999, obtained from the calibration curve (Figure 1) of gallic acid, where y is the absorbance and x is the amount of gallic acid. It was found to be 253 µg/ml as GAEs.
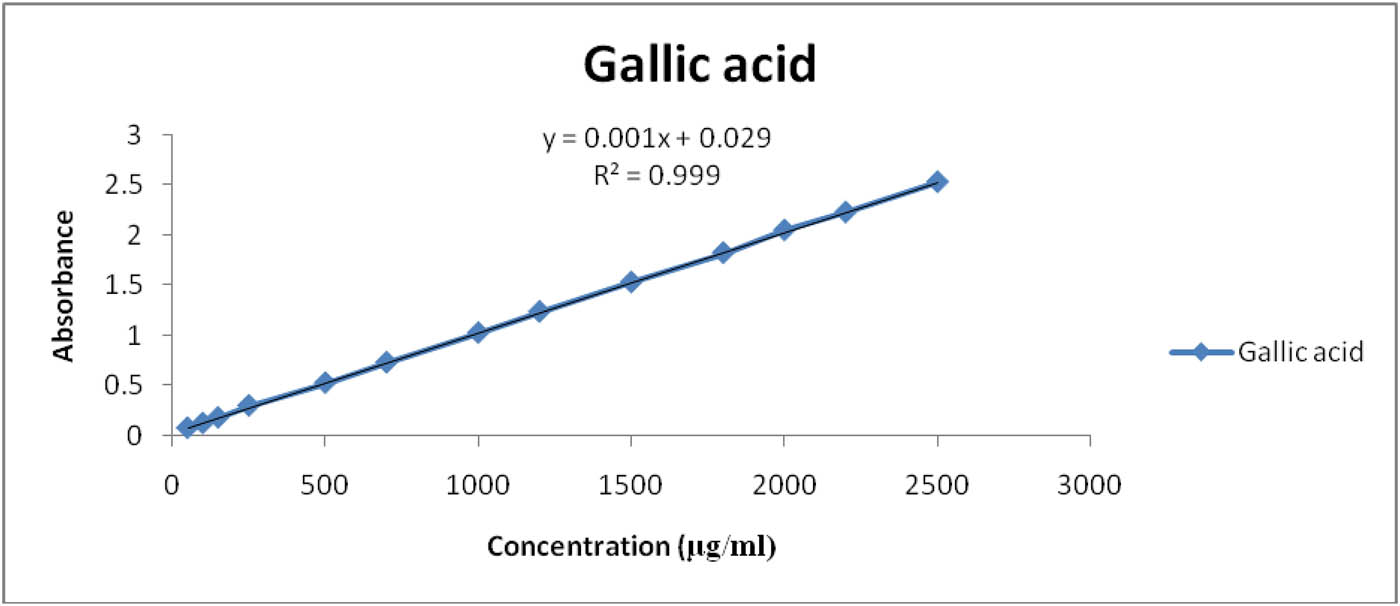
Estimation of total phenol content in the hydroethanolic extract of T. graceum L.
3.3 Reducing power assay
Reducing power is an indicator of the electron-donating ability of the compound used for testing the antioxidant or radical scavenging ability of the extracts. It is used to evaluate the ability of extracts to reduce Fe3+ to Fe2+. Figure 2a shows the extracts’ reducing power increasing with concentration. The reducing power of hydroethanolic extract (Figure 2b) was found to be 1.32 μg/ml compared to the standard compound at 1.686 μg/ml. The concentration-dependent percentage inhibition of hydroethanolic extracts ranged from 73.4 to 94.3% with an IC50 value of 53.63 μg/ml, while for chloroform extracts (Figure 2c), the percentage inhibition was from 82.4 to 94.3% with an IC50 value of 64.03 μg/ml.

(a) Reducing power assay and (b) DPPH assay of hydroethanolic and (c) chloroform extracts of T. graceum L.
3.4 HPLC quantitative analysis of extract
The presence of quercetin was detected in both the extracts with retention times of 5.6 and 5.8 s for chloroform and hydroethanolic extracts, respectively (Figure 3a and b), whereas it was 5.59 s for the standard (Figure 3d). In addition, the overlay spectra of quercetin and the extracts at the same retention time are illustrated in Figure 3c. Quercetin concentration was found to be higher in the chloroform extract than in the hydroethanolic extract.

HPLC chromatogram of the (a) chloroform extract and (b) hydroalcoholic extract. (c) Standard Quercetin at 360 nm. (d) Overlay spectra of T. graecum L.
3.5 Blood glucose parameter
Blood glucose levels were found to increase substantially on the 7th day in all groups, except for the normal control group, after dexamethasone administration. On the 14th day, the 400 mg/kg chloroform (27.85%) and hydroethanolic extract (28.48%)-treated group showed a maximum reduction in the glucose levels as compared to the positive control group, and it was statistically significant (p <0.001). The percentage reduction in blood glucose levels of treated groups was found to be between 36 and 40% on the 21st day. In comparison with both the extracts on the 45th day, the chloroform extract-treated group (47.3%) at 400 mg/kg and the hydroethanolic extract (45.7%)-treated group at 200 mg/kg showed better reduction capability in blood glucose levels as compared to the positive control group (Table 1).
Effect of hydroethanolic and chloroform extracts of T. gracecum L.on serum blood glucose level (mg/dl) at different time intervals
| Before treatment | After treatment | ||||
|---|---|---|---|---|---|
| Groups | 0th day | 7th day | 14th day | 21st day | 45th day |
| Normal control | 85.2 ± 5.2 | 82.9 ± 3.7 | 81.5 ± 6.9 (57.64%) | 86.89 ± 8.11 (58.20%) | 80.04 ± 5.7 (58.69%) |
| Positive (DM) | 81.5 ± 4.2 | 197.75 ± 28.9 | 192.4 ± 15.1 | 207.90 ± 24.5 | 193.8 ± 20.5 |
| Ramipril treated (1 mg/kg) | 83.9 ± 5.03 | 163.2 ± 18.6 | 154.16 ± 20#a (19.87%) | 132.8 ± 8.1#a (36.12%) | 128.6 ± 13.90#a (33.64%) |
| Hydroethanolic extract treated (100 mg/kg) | 83.21 ± 11.3 | 179.6 ± 23.66 | 162.3 ± 18.5 (15.64%) | 158.0 ± 14.01 (24%) | 152.3 ± 28.6#b (21.41%) |
| Hydroethanolic extract treated (200 mg/kg) | 86.85 ± 9.35 | 166.7 ± 22.7 | 146 ± 20.4#a (24.11%) | 130.55 ± 7.68#a (37.20%) | 105 ± 10.4#a (45.82%) |
| Hydroethanolic extract treated (400 mg/kg) | 84.4 ± 4.8 | 167.3 ± 20.1 | 137.6 ± 12.8#a (28.48%) | 126.4 ± 4.6#a (39.20%) | 108.61 ± 12.0#a (43.95%) |
| Chloroform extract treated (100 mg/kg) | 87.68 ± 4.8 | 183.2 ± 7.8 | 158.7 ± 14.7#c (17.51%) | 132.66 ± 11.1#a (36.19%) | 127.86 ± 10.2#a (34.05%) |
| Chloroform extract treated (200 mg/kg) | 86.71 ± 10.69 | 163.5 ± 28.7 | 146.7 ± 20.2#a (23,75%) | 130.3 ± 10.56#a (37.32%) | 122.05 ± 8.7#a (37.02%) |
| Chloroform extract treated (400 mg/kg) | 73.23 ± 8.16 | 169.0 ± 32.2 | 138.8 ± 16.4#a (27.85%) | 123.28 ± 7.7#a (40.70%) | 101.6 ± 16.7#a (47.57%) |
| Overall P value | ns | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| F | 37.83 | 364.87 | 244.5 | 268.1 | 238.3 |
Statistical analysis of data was carried out by one-way ANOVA followed by Tukey’s multiple range test. The values are mean ± standard deviation (SD) for each group (n = 6). P-value less than 0.05 was considered significant. # Positive control vs all groups, a p <0.0001, b p <0.01, and c p <0.05.
3.6 BP parameter
Administration of dexamethasone was noted to significantly increase the BP levels at the 7th day in all groups except for the normal control group (Figure 4). On day 15, two higher doses of hydroethanolic and chloroform extracts showed substantially significant (p < 0.001) reduction in systolic BP levels by 13.8 and 26.4 mm Hg and 14.8 and 26.5 mm Hg, respectively, as compared to the positive control group, whereas diastolic BP levels did not find a significant reduction in BP levels. On the 30th day, the reduction of systolic BP level was maintained for both different extracts (200 and 400 mg/kg), and in the diastolic BP level, only 400 mg/kg showed a less significant reduction as compared to the positive control group. Ramipril as a standard drug showed the highest significant reduction (p < 0.0001) in both BP levels as compared to the positive control group.

Effect of hydroethanolic and chloroform extracts of T. graecum L. on BP levels (mm Hg) after 15 and 30 day intervals.
3.7 Body weight measurement
All treated groups showed a slight reduction in body weight at all three intervals after the 7th day. Chloroform and hydroethanolic extracts at 400 mg/kg were shown to maintain the body weight from the 14th day onward till the 45th day. The ramipril-treated group showed significantly increased body weight at the 21st day as compared to the positive control group (Table 2).
Effect of hydroethanolic and chloroform extracts of T. gracecum L. on body weight at different intervals
| Body weight (g/week) | ||||||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | |||||
| Groups (n = 6) | 0th day | 7th day | 14th day | 21st day | 45th day | |
| 1. | Normal control | 165.6 ± 21.0 | 159.1 ± 15.9 | 159.3 ± 13.8 | 156.8 ± 16.8 | 161.1 ± 18.62 |
| 2. | Positive control (DM) | 170.5 ± 21.6 | 163.5 ± 15.0 | 156.6 ± 14.82 | 148.3 ± 12.56 | 137.1 ± 9.9 |
| 3. | Ramipril treated (1 mg/kg) | 174.6 ± 10.67 | 173.5 ± 7.3 | 176.1 ± 8.6 | 181.3 ± 14.36#a | 176.5 ± 12.35 |
| 4. | Hydroethanolic extract treated (100 mg/kg) | 171.6 ± 11.7 | 157.1 ± 17.7 | 136.5 ± 7.4 | 147.6 ± 7.3 | 146.8 ± 5.11 |
| 5. | Hydroethanolic extract treated (200 mg/kg) | 175.6 ± 27.2 | 154.3 ± 21.8 | 139.5 ± 19.2 | 140.8 ± 19.7 | 137.6 ± 20.0 |
| 6. | Hydroethanolic extract treated (400 mg/kg) | 169.1 ± 28.2 | 142.3 ± 10.5#c | 141.1 ± 8.2 | 139.1 ± 9.7 | 143 ± 8.2 |
| 7. | Chloroform treated (100 mg/kg) | 165.5 ± 8.6 | 153.8 ± 12.3 | 141.6 ± 10.0 | 140.3 ± 10.0 | 134.8 ± 6.8 |
| 8. | Chloroform extract treated (200 mg/kg) | 176 ± 15.7 | 150.5 ± 8.2 | 140.3 ± 6.7 | 135.3 ± 8.0 | 144 ± 4.8 |
| 9. | Chloroform extract treated (400 mg/kg) | 167.1 ± 13.6 | 152.1 ± 10.9 | 155 ± 10.9 | 158.1 ± 6.1 | 153.8 ± 9.9 |
| Overall p value | 0.967 | 0.039 | <0.0001 | <0.0001 | <0.0001 | |
| F value | 0.286 | 2.2 | 7.4 | 7.8 | 7.54 | |
Statistical analysis of data was carried out by one-way ANOVA, followed by Tukey’s multiple range test. The values are mean ± SD for each group (n = 6). P value less than 0.05 was considered significant. #Positive control vs all groups, a p <0.001, b p <0.01, and c p <0.05.
3.8 Biochemical parameters
Administration of dexamethasone leads to a significant increase in triglycerides and LDL, as well as a decrease in HDL and insulin levels in the positive control group (Table 3). Regarding lipid levels, at the 45th day, the hydroethanolic and chloroform extract-treated group showed non-significant HDL levels as compared to the ramipril-treated group. In the case of LDL and triglyceride levels, treatment with hydroethanolic extract and chloroform extract brought these altered parameters near the normal level. Insulin levels were significantly reduced with administration of 400 mg/kg chloroform-, hydroethanolic extract-, and ramipril-treated groups.
Effect of hydroethanolic and chloroform extracts of T. graecum L. on serum lipid profile and insulin level (mg/dl) at 45th day
| Groups | HDL-c | LDL-c | Triglycerides | Insulin |
|---|---|---|---|---|
| Normal control | 41.8 ± 4.6 | 90.8 ± 11.4 | 94.7 ± 8.8 | 17.6 ± 3.7 |
| Positive control (DM) | 20.9 ± 9.1 | 198.5 ± 10.1 | 210.9 ± 25.3 | 41.2 ± 6.5 |
| Ramipril treated (1 mg/kg) | 39.9 ± 9.4#b | 107.8 ± 28.0 #a | 217.9 ± 28.5 | 24.6 ± 6.2#a |
| Hydroethanolic extract treated (100 mg/kg) | 22.6 ± 6.3 | 128.0 ± 25.32#a | 160.3 ± 38.5 | 33.7 ± 6.6 |
| Hydroethanolic extract treated (200 mg/kg) | 26.3 ± 9.7¥ns | 121.2 ± 17.0# a | 152.6 ± 25.9#c | 30.8 ± 5.6 |
| Hydroethanolic extract treated (400 mg/kg) | 35.7 ± 7.8¥ns | 103.4 ± 9.9# a | 116.6 ± 19.9#a | 27.4 ± 7.1#b |
| Chloroform extract treated (100 mg/kg) | 21.0 ± 8.9 | 133.9 ± 29.6#a | 172.7 ± 32.4 | 35.4 ± 5.8 |
| Chloroform extract treated (200 mg/kg) | 22.5 ± 5.0 | 117.1 ± 17.8#b | 144.3 ± 43.8#c | 27.2 ± 7.0#c |
| Chloroform extract treated (400 mg/kg) | 29.3 ± 10.6¥ns | 106.1 ± 17.2# a | 109.2 ± 20.2#a | 21.0 ± 2.4#a |
| Overall p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| F value | 6.12 | 10.34 | 13.33 | 9.50 |
Statistical analysis of data was carried out by one-way ANOVA followed by Tukey’s multiple range test. The values are mean ± SD for each group (n = 6). P value less than 0.05 was considered significant. #Positive control vs all groups, a p <0.001, b p <0.01, and c p <0.05. ¥ Ramipril vs all groups. ns not significant.
3.9 Histopathological findings
The lengthy microvilli remained intact, and the proximal tubular cells exhibited typical cellular behavior in the normal control group (Figure 5). We observed that podocyte pedicles and interpedicular diaphragms possessed characteristic morphologies within the glomerulus. Rats that received diabetes treatment showed much worse damage to their kidney structures, including more cell buildup in the spaces between cells, scarring in the glomeruli, and swelling in the surrounding areas. We observed a noticeable improvement after administering two distinct concentrations of both chloroform and hydroethanolic extracts (200 and 400 mg/kg).

Histopathology of rat kidney tissues after treatment with different extracts of T. graecum L. In the normal group, the arrow represents the normal structure of glomeruli. In the positive control group, the arrow shows hemorrhage and tubular damage due to proteinuria; in the ramipril-treated group, the arrow shows mild inflammation with the normal structure of glomeruli; in other extract-treated groups, the arrow shows recovered glomeruli with some distorted one. RBCs are seen with hemorrhage in the hydroethanolic extract-treated group (100 mg/kg).
3.10 Analysis of quercetin in serum on the 45th day using HPLC-DAD
Quercetin was absent in the serum of the normal control group, as depicted in Figure 6. The retention time of the standard quercetin was found to be 10.49, indicating the presence of quercetin at a 93.22% level. The retention time of chloroform extract-treated group was on par with the hydroethanolic extract-treated group (400 mg/kg) on the 45th day: 10.74 (70.23% concentration) and 10.59 (95.27% concentration), respectively.

Estimation of quercetin in serum in different extract-treated groups. (a) Normal control. (b) Standard quercetin. (c) Chloroform extract showing quercetin. (d) Hydroethanolic extract showing quercetin.
3.11 In silico results
3.11.1 ADME/T Studies
The supplementary tables (Tables S1 and S2) provide a comprehensive overview of the predicted ADME/T properties, as well as their corresponding values and probabilities for the two compounds: ramipril and quercetin. ADME/T studies showed comparable absorptive properties for ramipril and quercetin. However, ramipril exhibited higher hepatotoxicity and AMES mutagenesis probabilities. Bioavailability radar suggested a better safety profile for quercetin (Figure S1). Boiled egg plot results indicated that neither drug could cross the BBB but had high GI absorption (Figure S2).
3.11.2 Docking results
We performed docking between proteins of the RAS pathway and both drugs (quercetin and ramipril) to evaluate the affinity of quercetin toward the RAS pathway. The affinity scores are presented in Table S3.
3.11.2.1 Interaction of quercetin and ramipril with ACE
The binding affinities of quercetin and ramipril toward ACE were found to be −7.42 and −6.25 kcal/mol, respectively, indicating that quercetin has a stronger affinity for ACE than ramipril. Figure 7 displays the 3D and 2D molecular interactions, respectively, of quercetin and ramipril with ACE (PDB ID: 1O86). Quercetin was observed to form a complex with ACE through conventional hydrogen bonding, pi–sulfur, pi–cation, and van der Waals interactions. The key amino acid residues involved in these interactions include Tyr146, Leu161, Glu349, and Arg348 (hydrogen bonding); Ser147 (pi–lone pair); Cys352 and Cys370 (pi–sulfur); Lys343 (pi–cation); and Phe512, His353, Glu143, Val351, Val350, Pro344, Gln160, Glu162, Thr150, Aal149, and Trp185 (van der Waals). In contrast, ramipril forms conventional hydrogen bonds, salt bridges, alkyl bonds, and van der Waals interactions with ACE. The parts of ACE that interact with ramipril include Tyr135 and Arg124 (which form hydrogen bonds); with Leu139, Tyr62, Ala89, Ala125, and Ile88 (which form alkyl bonds); with Glu123 (which forms salt bridges); and Leu122, Thr92, Asn85, Trp220, Ile204, Tyr200, and Ser517 (which have van der Waals interactions). These findings suggest that while both ligands bind to ACE through a variety of interactions, quercetin involves additional pi-interactions, while ramipril features alkyl and salt bridge interactions, which may contribute to its effectiveness as an ACE inhibitor.
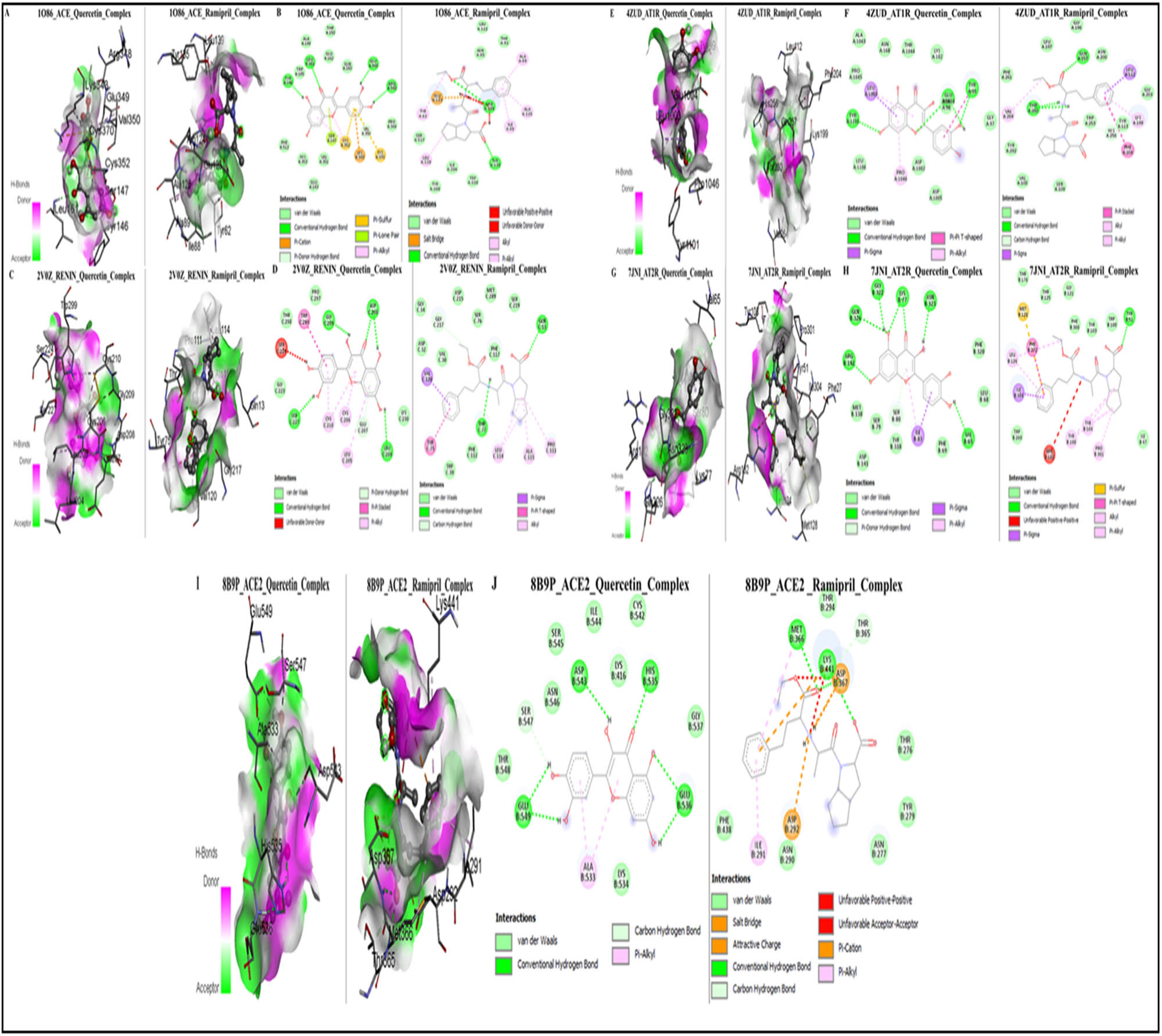
Illustration of the protein–ligand interaction of quercetin and ramipril with targets involved in the RAS pathway. (a) 3D structures of the ACE_quercetin and ACE_ramipril complexes; (b) 2D structures of ACE_quercetin and ACE_ramipril complexes; (c) 3D structures of renin_quercetin and renin_ramipril complexes; (d) 2D structures of renin_quercetin and renin_ramipril complexes; (e) 3D structures of AT1R_quercetin and AT1R_ramipril complexes; (f) 2D structures of AT1R_quercetin and AT1R_ramipril complexes; (g) 3D structures of AT2R_quercetin and AT2R_ramipril complexes; (h) 2D Structures of AT2R_quercetin and AT2R_ramipril complexes; (i) 3D structures of ACE2_quercetin and ACE2_ramipril complexes; (j) 2D structures of ACE2_quercetin and ACE2_ramipril complexes.
3.11.2.2 Interaction of quercetin and ramipril with renin
Quercetin binds to renin more strongly than ramipril, with binding affinities of −8.1 and −5.74 kcal/mol, respectively. Figure 7 shows the 3D and 2D ways that quercetin and ramipril interact with renin (PDB ID: 2V0Z). We found that renin and quercetin form a complex through conventional hydrogen bonding, pi–pi stacking, pi–alkyl, unfavorable donor–donor, and van der Waals interactions. The amino acid residues of renin involved in these interactions with quercetin were Gly209, Ser227, Asp208, and Leu204 (conventional hydrogen bonds); Trp299 (pi–pi stacking); Cys210, Cys206, and Leu205 (pi–alkyl); Ser224 (unfavorable donor–donor); and Lys230, Pro297, Thr298, and Gly223 (van der Waals). On the other hand, ramipril was observed to form conventional hydrogen bonds, pi–sigma, pi–pi T-shaped, carbon–hydrogen bonds, alkyl, and van der Waals interactions with renin. The amino acid residues of renin involved in interactions with ramipril were Thr77, Gln12 (conventional hydrogen bonding); Val120 (pi–sigma); Tyr75 (pi–pi T-shaped); Gly217 (carbon–hydrogen bonds); Leu114 and Aal115, and Pro111 (alkyl); and Gly34, Asp32, Val30, Asp215, Ser76, Phe117, Met289, Ser219, Phe112, and Trp39 (van der Waals).
3.11.2.3 Interaction of quercetin and ramipril with AT1R
The binding affinities of quercetin and ramipril toward AT1R were −6.2 and −6.43 kcal/mol, respectively, indicating that both compounds have comparable affinity toward AT1R, with quercetin showing a slightly better affinity than ramipril. Figure 7 shows the 3D and 2D images of how quercetin and ramipril interact with the AT1R (PDB ID: 4ZUD). Quercetin was found to form a complex with AT1R through conventional hydrogen bonds, pi–sigma, pi–alkyl, pi–pi T-shaped, and van der Waals interactions. The amino acid residues of AT1R involved in these interactions with quercetin were Glu1004, Tyr99, and Leu204 (conventional hydrogen bond); Leu1003 (pi–sigma); Ala1043, Pro1045, Asn168, Thr1044, Lys102, Asn98, Gly97, Asp1002, and Asp1005 (van der Waals); Tyr99 (pi–pi T-shaped); and Pro1046 and Leu1003 (pi–alkyl). In contrast, ramipril formed interactions via conventional hydrogen bonds, pi–sigma, pi–pi stacked, van der Waals, alkyl, and pi–alkyl interactions. The amino acid residues of renin interacting with ramipril were Phe261, Leu197, Gly196, Asn200, Gly203, Tyr113, Trp253, Ser109, Val108, and Tyr292 (van der Waals); Thr260 and Gln257 (conventional hydrogen bonds); Leu112 (pi–sigma); Phe204 (pi–pi stacked); Lys199 and Val264 (alkyl and pi–alkyl).
3.11.2.4 Interaction of quercetin and ramipril with AT2R
The binding affinities of quercetin and ramipril toward AT2R were −6.87 and −7.32 kcal/mol, respectively, indicating that quercetin has a strong affinity for AT2R, though slightly weaker than that of ramipril. Figure 7 shows the 3D and 2D ways that quercetin and ramipril interact with the AT2R (PDB ID: 7JNI). Quercetin was found to form a complex with AT2R through conventional hydrogen bonds, pi–sigma, pi–donor hydrogen bonds, and van der Waals interactions. The amino acids in AT2R that interacted with quercetin include Arg142, Gln326, Gly322, Lys77, Asn323, and Val65 (for conventional hydrogen bonds); Ile83 (for pi–sigma); Met138, Ser79, Tyr318, Asp141, Phe69, Phe320, and Leu68 (for van der Waals interactions); and Ser80 (for pi–donor hydrogen bonds). In contrast, ramipril formed interactions via conventional hydrogen bonds, pi–sulfur, pi–pi T-shaped, alkyl, pi–alkyl, pi–sigma, and unfavorable positive–positive interactions. The amino acid residues of renin interacting with ramipril were Thr178, Thr125, Gly121, Phe308, Tyr103, Trp100, Ilr47, and Trp269 (van der Waals); Met128 (pi–sulfur); Phe272 (pi–pi T-shaped); Leu124, Tyr108, Tyr104, and Pro301 (alkyl, pi–alkyl), and Ile304 (pi–sigma); Arg182 (unfavorable positive); and Tyr51 (conventional hydrogen bonds).
3.11.2.5 Interaction of quercetin and ramipril with ACE-2
The binding affinities of quercetin and ramipril for ACE2 were found to be −5.77 and −6.1 kcal/mol, respectively. This indicates that quercetin exhibits strong affinity for AT2R2, although slightly weaker than that of ramipril. Figure 6i and j shows how quercetin and ramipril interact with the ACE2 (PDB ID: 8B9P) in both 3D and 2D views. Quercetin was found to form a complex with ACE2 through conventional hydrogen bonds, pi–alkyl interactions, carbon–hydrogen bonds, and van der Waals forces. The specific ACE2 amino acid residues involved in interactions with quercetin were Asp543, His535, Glu536, and Glu549 (conventional hydrogen bonds); Thr548, Asn546, Ser545, Ile544, Lys416, Cys542, Gly537, and Lys534 (van der Waals interactions); Ala533 (pi–alkyl interaction); and Ser547 (carbon–hydrogen bond). In contrast, ramipril formed interactions with ACE2 via conventional hydrogen bonds, van der Waals forces, pi–alkyl interactions, pi–cation interactions, unfavorable positive–positive interactions, attractive charge interactions, and salt bridges. The ACE2 residues involved in ramipril interactions included Thr 294, Thr 276, TyrR 279, Asn 277, Asn 290, and Phe 438 (van der Waals); Met 366, Lys 441, and Asp 367 (conventional hydrogen bonds); Ile 291 (pi–alkyl); Asp 292, Asp 367, and Lys 441 (pi–cation, attractive charge, and salt bridge interactions); and Ile 291 (pi–alkyl).
Molecular docking studies revealed distinct binding profiles for quercetin and ramipril across various targets in the RAS. Quercetin showed stronger ability to attach to ACE and renin than ramipril, indicating it might work better to block these enzymes. However, ramipril, as a standard drug, showed slightly higher affinity for ACE2 and AT2R, where both compounds exhibited comparable binding. While quercetin created a greater range of connections, including π-based interactions, ramipril had more alkyl, salt bridge, and negative interactions between positive charges. These findings suggest that quercetin has potential as an alternative inhibitor, while ramipril, due to its targeted affinity for ACE2 and AT2R, remains a strong therapeutic agent.
4 Discussion
Metabolic disorders are metabolic syndromes associated with insulin resistance and cardiovascular risk factors, including myocardial infarction, heart failure, and hypertension [33]. Excess glucose in the bloodstream leads to hyperglycemia. Glucocorticoids can cause insulin resistance by blocking the liver’s ability to use glucose, which may lead to problems in making glycogen and higher amounts of free fatty acids. While the precise mechanisms at play remain elusive, insulin resistance potentially plays a role in the pathogenesis of hypertension, impaired glucose tolerance, and lipids [34]. ACE inhibitors stop the ACE in the RAS and kallikrein system, and they are known to be very effective for treating high BP [35]. Patients afflicted with diabetic nephropathy now benefit more from ACE inhibitors as a result of their well-documented safety. Nonetheless, certain extremely hazardous side effects are present in all allopathic medications and can be mitigated through the use of herbs [36].
T. graecum L., a traditional Indian herb, belongs to the Fabaceae family. This study explores T. graecum L. leaves for managing the symptoms of diabetes-induced hypertension [37]. In our investigation, both extracts confirmed the presence of flavonoids and alkaloids; however, glycosides, carbohydrates, proteins, and amino acids were only found in the chloroform extract. The chloroform extract also demonstrated antioxidant capability with an inhibitory concentration (IC50) value of 1.434 μg/ml, which is similar to ascorbic acid’s value of 1.686 μg/ml. Quercetin is an important flavonoid molecule that is present in T. graecum L. HPLC analysis detected the small concentration of quercetin in both the extracts, which is compared with the standard quercetin. The retention of quercetin was similar to the reported studies [38]. Antioxidant or free radical scavenging properties of T. graecum L. can be ascribed to the plant’s flavonoid (quercetin) and saponin contents. The protective effects of flavonoids in living systems are thought to come from their ability to move electrons, bind to metal catalysts, boost antioxidant enzymes, lower alpha-tocopherol radicals, and blocks the oxidation tadical system [39]. Our study revealed that both extracts led to a statistically significant (p < 0.0001) decrease in blood glucose levels persistently for 45 days. Furthermore, extracts at 400 mg/kg exhibited a reduction in LDL and triglyceride levels. Chloroform and hydroethanolic extracts at 400 mg/kg had HDL levels that were about the same as those in the group treated with ramipril. The insulin level returned to near normal after 45 days of treatment.
At doses of 200 and 400 mg/kg chloroform and hydroethanolic extracts, the systolic BP levels were measured on the 15th and 30th days, while the diastolic BP levels did not show any significant reduction at either interval. Another study reported that the methanolic extract showed similar reductions in BP levels. Histological exams showed that the shape and structure of kidneys in diabetic patients improved significantly in both groups that received the extract after treatment. Pharmacokinetic evaluation revealed traces of quercetin in the serum samples of the treated groups. This plant has a lot of important compounds, including quercetin, fenugreekine, nicotinic acid, sapogenins, phytic acid, scopoletin, trigonelline, diosgenin, gitogenin, neogitogenin, homoorientin, and saponaretin [40]. The likely process might turn on a specific receptor that works with tyrosine, which is found on the inside of the cell membrane. With the aid of ATP, it phosphorylates itself, modifies its conformation, and becomes activated via G-proteins. These processes result in the secretion of multiple second messengers, which subsequently stimulate protein kinases, allowing for Ca2+ influx and generating an effect comparable to insulin. It might also lower the production of phosphoenolpyruvate carboxykinase by depending on phosphoinositide 3-kinase (PI3K) and working like insulin by increasing PI3K and mitogen-activated protein kinase (MAP kinase). Trigonelline may enhance glucose uptake and insulin sensitivity via peroxisome proliferator activated receptor gamma (PPAR-γ) and GLUT-4 proteins. This upregulation of protein expressions has been linked to protective activity in diabetic nephropathy [13,41].
We investigated molecular receptor targets using an in silico molecular docking approach. PPARγ ligands have been studied for their potential effects on BP, and some research suggested that they may have a beneficial impact, particularly in models of hypertension [42]. PPARγ is a nuclear receptor that plays a key role in the regulation of various physiological processes, including glucose and lipid metabolism, inflammation, and vascular functions. Activation of PPARγ by its ligands can have systemic effects on these processes. Some proposed mechanisms by which PPARγ ligands influence BP include: (1) improvement of insulin sensitivity: PPARγ activation improves insulin sensitivity, and insulin resistance is often linked to hypertension and (2) anti-inflammatory activity [43].
Targeting PPARγ activation along with inhibiting the renin angiotensin-aldosterone system (RAAS) pathway may manage diabetes-associated hypertension [44]. Computational methods revealed that quercetin exhibited noteworthy high absorption, low BBB permeability, and a favorable toxicity profile. Docking studies unraveled high affinity of quercetin toward RAAS proteins, i.e., ACE, renin, AT1R, AT2R, and ACE-2. The RAAS pathway commences with the conversion of angiotensinogen to angiotensin I by renin, a rate-limiting step. Renin is a central hormone for modulation of BP and is the rate-limiting step in the RAAS. It cleaves angiotensinogen and releases angiotensin I, thus representing a viable target for managing hypertension [45]. This is evident by several effective clinically used renin inhibitors like enalkiren, remikiren, and aliskiren (Drug Bank n.d.). In the current study, ramipril was observed to bind with the S2 pocket and S3sp subpocket of renin [46]. Quercetin was observed to have high affinity toward renin and formed interactions with different amino acids. The interaction with Thr298 shows that quercetin attaches in the S1 pocket of renin, indicating that quercetin could influence how renin works [47]. Notably, ACE inhibitors like ramipril, lisinopril, captopril, etc., are effective in the clinical management of diabetic hypertension [48]. The S1 and S2 active sites of ACE are crucial for interaction with its inhibitors. These sites contain Glu162 and His353 amino acid residues. In fact, lisinopril, a well-established clinically used ACE inhibitor, binds with the S1 (Ala354 and Glu384) and S2 (His353) active sites of ACE. The analysis from the current study revealed that quercetin developed van der Waals interactions with Glu162 and His353 amino acid residues, suggesting that quercetin binds at the active site of ACE and engages the crucial amino acids required for inhibition of ACE activity. Moreover, the binding of quercetin specifically with His353, an amino acid that is involved in lisinopril-mediated ACE inhibition, confirms that quercetin has inhibitory activity on ACE [35]. Similarly, quercetin was identified to interact with ACE2; however, interactions of quercetin with those amino acids of ACE2 were not shown to interact with other ACE2 modulators in the literature. From this study we concluded that quercetin has more affinity and selectivity toward ACE than ACE2. Angiotensin II is a bioactive peptide that regulates the RAAS pathway. It binds to the AT-1 and AT-II receptors and creates vasoconstriction. AT-II connects with the Lys102 and Trp94–Gly97 parts of the AT-1 receptor, leading to effects on the heart and blood vessels, such as narrowing of the blood vessels. Lys102 and Trp94–Gly97 are present in the TMD3, ECL1, and WPFG motif regions of the AT-1R, respectively. Lys102 is crucial for the binding of AT-II with its AT1R, while the Trp94–Gly97 amino acids are crucial for AT1R receptor activation. In this study, quercetin interacts with Lys102 and Gly97, indicating that it can attach to important amino acids of AT-1R needed for activating the receptor. It also suggests that it can interfere with the binding of AT-II with AT1R, causing interruption in the RAAS pathway [49]. Quercetin was also observed to bind and form interactions with the amino acid residues of AT2R, suggesting it might disrupt AT-II binding and AT2-receptor activation. Moreover, the observations made in the current study state that quercetin interacts with AT2R amino acids; however, its interaction with the crucial amino acids of AT1R indicates its selective affinity toward AT1R. We observed that quercetin has the potential to modulate hypertension by interacting with the RAAS cascade through antagonism of renin, ACE, and AT1R, offering promising avenues for future therapeutic interventions. Additional research is required to gain a more profound understanding of the mechanisms underlying the hypoglycemic effects of this plant.
5 Conclusions
The study provides the usefulness of T. graecum L. in the treatment of diabetes-associated hypertension via pre-clinical studies. The chemical constituents present in the plant might be responsible for therapeutic effects of T. graecum L. in the treatment of diabetes-associated complications via multiple molecular mechanistic pathways by acting on distinct receptors, and an in silico study supported these results. A molecular mechanistic study of the plant extract may provide deeper understanding of the mechanisms related to diabetes-associated disorders. Further investigation is needed using clinical studies to validate its usefulness in treatment of this disorder.
Acknowledgments
The authors are grateful to management of MM(DU) for offering requisite technical help and the authors acknowledge the Ongoing Research Funding program (ORF-2025-981), King Saud University for funding this study.
-
Funding information: The authors of this study are highly obliged to the “Ongoing Research Funding” program number (ORF-2025-981), King Saud University, Riyadh, Saudi Arabia, for financial support for this research work.
-
Author contributions: Conceptualization: SS, AM, and SG; formal analysis: AM, SS, SR, KG, RC, NG, MHF, and BOA; resources & visualization: MI; writing – original draft preparation; NG, SR, SS, SD, AN, KG, and SB: writing – review and editing: SB, SG, SB. NAS, SD, and OIF. All authors read and approved the final manuscript.
-
Conflict of interest: The authors declare that there is no conflict of interest.
-
Ethical approval: The experimental protocol (MMCP-IAEC-15/16) was approved by the Institutional Animal Ethics Committee (IAEC) constituted in accordance with the rules and guidelines of the Committee for the Purpose of Control and Supervision on Experimental Animals (CPCSEA), India.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215–25.10.1177/1753944717711379Search in Google Scholar PubMed PubMed Central
[2] Chauhan S, Khatib MN, Ballal S, Bansal P, Bhopte K, Gaidhane AM, et al. The rising burden of diabetes and state-wise variations in India: insights from the global burden of disease study 1990–2021 and projections to 2031. Front Endocrinol (Lausanne). 2025;16:1505143.10.3389/fendo.2025.1505143Search in Google Scholar PubMed PubMed Central
[3] Kaliaperumal RD, Nandhini P, Mohan R, Nallamuthu P. Comparative efficacy and safety of Faricimab with other intravitreal anti-vascular endothelial growth factor in the treatment of Neovascular age related macular degeneration-A Systematic review and meta-analysis. Asian J Med Sci. 2025;16(5):136–44.10.71152/ajms.v16i5.4487Search in Google Scholar
[4] Bharate SB, Lindsley CW. Natural products driven medicinal chemistry. J Med Chem. 2024;67(23):20723–30.10.1021/acs.jmedchem.4c02736Search in Google Scholar PubMed
[5] Nagulapalli Venkata KC, Swaroop A, Bagchi D, Bishayee A. A small plant with big benefits: Fenugreek (Trigonella foenum‐graecum Linn.) for disease prevention and health promotion. Mol Nutr Food Res. 2017;61(6):1600950.10.1002/mnfr.201600950Search in Google Scholar PubMed
[6] Dhull SB, Kidwai MK, Noor R, Chawla P, Rose PK. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legum Sci. 2022;4(3):e129.10.1002/leg3.129Search in Google Scholar
[7] Visuvanathan T, Than LT, Stanslas J, Chew SY, Vellasamy S. Revisiting Trigonella foenum-graecum L.: pharmacology and therapeutic potentialities. Plants. 2022;11(11):1450.10.3390/plants11111450Search in Google Scholar PubMed PubMed Central
[8] Fatima H, Shahid M, Pruitt C, Pung MA, Mills PJ, Riaz M, et al. Chemical fingerprinting, antioxidant, and anti-inflammatory potential of hydroethanolic extract of Trigonella foenum-graecum. Antioxidants. 2022;11(2):364.10.3390/antiox11020364Search in Google Scholar PubMed PubMed Central
[9] Nagamma T, Konuri A, Bhat KM, Udupa PE, Nayak Y. Trigonella foenum-graecum L. seed extract modulates biochemical and histomorphological changes in therapeutic model of high-fat diet-fed ovariectomized rats. 3 Biotech. 2023;13(8):285.10.1007/s13205-023-03707-8Search in Google Scholar PubMed PubMed Central
[10] Liew FF, Visuvanathan T, Vellasamy S. Fenugreek (Trigonella foenum-graecum L.) modulates energy metabolism and anti-inflammatory response in obesity via combinatorial analysis. Nat Prod J. 2023;13(8):79–104.10.2174/2210315513666230309105835Search in Google Scholar
[11] Ghayur MN, Abdalla M, Khalid A, Ahmad S, Gilani AH. Trigonella foenum‐graecum methanolic extract on isolated smooth muscles and acetylcholinesterase enzyme: An in vitro and mechanistic in silico investigation. BioMed Res Int. 2022;1:4849464.10.1155/2022/4849464Search in Google Scholar PubMed PubMed Central
[12] Yan D, Yan Y, Ma RY, Chu JL, Mao XM, Li LL. Ameliorating effect of Trigonella foenum-graecum L.(fenugreek) extract tablet on exhaustive exercise-induced fatigue in rats by suppressing mitophagy in skeletal muscle. Eur Rev Med Pharmacol Sci. 2022;26:20.Search in Google Scholar
[13] Geberemeskel GA, Debebe YG, Nguse NA. Antidiabetic effect of fenugreek seed powder solution (Trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J Diab Res. 2019;1:8507453.10.1155/2019/8507453Search in Google Scholar PubMed PubMed Central
[14] Abou El-Soud NH, Khalil MY, Hussein JS, Oraby FS, Farrag AH. Antidiabetic effects of fenugreek alkaloid extract in streptozotocin induced hyperglycemic rats. J Appl Sci Res. 2007;3(10):1073–83.Search in Google Scholar
[15] Balaraman R, Dangwal S, Mohan M. Antihypertensive effect of Trigonella foenum-graecum seeds in experimentally induced hypertension in rats. Pharm Biol. 2006;44(8):568–75.Search in Google Scholar
[16] Alam MA, Bin Jardan YA, Raish M, Al-Mohizea AM, Ahad A, Al-Jenoobi FI. Effect of Nigella sativa and Fenugreek on the pharmacokinetics and pharmacodynamics of amlodipine in hypertensive rats. Cur Drug Metab. 2020;21(4):318–25.10.2174/1389200221666200514121501Search in Google Scholar PubMed
[17] Naika MB, Sathyanarayanan N, Sajeevan RS, Bhattacharyya T, Ghosh P, Iyer MS, et al. Exploring the medicinally important secondary metabolites landscape through the lens of transcriptome data in fenugreek (Trigonella foenum graecum L.). Sci Rep. 2022;12(1):13534.10.1038/s41598-022-17779-8Search in Google Scholar PubMed PubMed Central
[18] Singh P, Bajpai V, Gond V, Kumar A, Tadigoppula N, Kumar B. Determination of bioactive compounds of fenugreek (Trigonella foenum-graecum) seeds using LC-MS techniques. Methods Mol Biol. 2020;2107:377–93.10.1007/978-1-0716-0235-5_21Search in Google Scholar PubMed
[19] Anwar S, Desai S, Eidi M, Eidi A. Antidiabetic activities of fenugreek (Trigonella foenum-graecum) seeds. Nuts and Seeds in Health and Disease Prevention. London: Academic Press; 2011. p. 469–78.10.1016/B978-0-12-375688-6.10056-8Search in Google Scholar
[20] Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: A powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des. 2011;7(2):146–57.10.2174/157340911795677602Search in Google Scholar PubMed PubMed Central
[21] Lawag IL, Nolden ES, Schaper AA, Lim LY, Locher C. A modified folin-ciocalteu assay for the determination of total phenolics content in honey. Appl Sci. 2023;13(4):2135.10.3390/app13042135Search in Google Scholar
[22] Rahman MA, bin Imran T, Islam S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J Biol Sci. 2013;20(3):213–25.10.1016/j.sjbs.2012.11.007Search in Google Scholar PubMed PubMed Central
[23] Sirisha A, Gaur GS, Pal P, Bobby Z, Balakumar B, Pal GK. Effect of honey and insulin treatment on oxidative stress and nerve conduction in an experimental model of diabetic neuropathy Wistar rats. PLoS one. 2021;16(1):e0245395.10.1371/journal.pone.0245395Search in Google Scholar PubMed PubMed Central
[24] Gangwar A, Kumar P, Rawat A, Tiwari S. Noninvasive measurement of systolic blood pressure in rats: A novel technique. Ind J Pharmacol. 2014;46(3):351–2.10.4103/0253-7613.132207Search in Google Scholar PubMed PubMed Central
[25] Kausar MA, Anwar S, Eltayb WA, Kuddus M, Khaton F, El-Arabey AA, et al. MD simulation studies for selective phytochemical as potential inhibitors against major biological targets of Diabetic Nephropathy. Molecules. 2022;27(15):4980.10.3390/molecules27154980Search in Google Scholar PubMed PubMed Central
[26] Saghiri K, Daoud I, Melkemi N, Mesli F. QSAR study, molecular docking/dynamics simulations and ADME prediction of 2-phenyl-1H-indole derivatives as potential breast cancer inhibitors. Biointerface Res Appl Chem. 2022;13(2):154.10.33263/BRIAC132.154Search in Google Scholar
[27] Natesh R, Schwager SL, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme–lisinopril complex. Nature. 2003;421(6922):551–4.10.1038/nature01370Search in Google Scholar PubMed
[28] Rahuel J, Rasetti V, Maibaum J, Rüeger H, Göschke R, Cohen NC, et al. Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human renin. Chem & Biol. 2000;7(7):493–504.10.1016/S1074-5521(00)00134-4Search in Google Scholar
[29] Zhang H, Unal H, Desnoyer R, Han GW, Patel N, Katritch V, et al. Structural basis for ligand recognition and functional selectivity at angiotensin receptor. J Biol Chem. 2015;290(49):29127–39.10.1074/jbc.M115.689000Search in Google Scholar PubMed PubMed Central
[30] Perryman R, Renziehausen A, Shaye H, Kostagianni AD, Tsiailanis AD, Thorne T, et al. Inhibition of the angiotensin II type 2 receptor AT2R is a novel therapeutic strategy for glioblastoma. Proc Natl Acad Sci. 2022;119(32):e2116289119.10.1073/pnas.2116289119Search in Google Scholar PubMed PubMed Central
[31] Harman MA, Stanway SJ, Scott H, Demydchuk Y, Bezerra GA, Pellegrino S, et al. Structure-guided chemical optimization of bicyclic peptide (bicycle) inhibitors of angiotensin-converting enzyme-II. J Med Chem. 2023;66(14):9881–93.10.1021/acs.jmedchem.3c00710Search in Google Scholar PubMed
[32] Valdés-Tresanco MS, Valdés-Tresanco ME, Valiente PA, Moreno E. AMDock: A versatile graphical tool for assisting molecular docking with Autodock Vina and Autodock4. Biol Direct. 2020;15:1–2.10.1186/s13062-020-00267-2Search in Google Scholar PubMed PubMed Central
[33] Wang Z, Chen J, Zhu L, Jiao S, Chen Y, Sun Y. Metabolic disorders and risk of cardiovascular diseases: A two-sample mendelian randomization study. BMC Cardiovasc Dis. 2023;23(1):529.10.1186/s12872-023-03567-3Search in Google Scholar PubMed PubMed Central
[34] Beaupere C, Liboz A, Fève B, Blondeau B, Guillemain G. Molecular mechanisms of glucocorticoid-induced insulin resistance. Intern J Mol Sci. 2021;22(2):623.10.3390/ijms22020623Search in Google Scholar PubMed PubMed Central
[35] Herman LL, Padala SA, Ahmed I, Bashir K. Angiotensin-converting enzyme inhibitors (ACEI). In StatPearls. Orlando, Florida: StatPearls Publishing; 2025.Search in Google Scholar
[36] Cock IE. The safe usage of herbal medicines: Counter-indications, cross-reactivity and toxicity. Pharmacogn Communic. 2015;5(1):2–38.10.5530/pc.2015.1.2Search in Google Scholar
[37] Sarker DK, Ray P, Dutta AK, Rouf R, Uddin SJ. Antidiabetic potential of fenugreek (Trigonella foenum‐graecum): A magic herb for diabetes mellitus. Food Sci Nutr. 2024;12(10):7108–36.10.1002/fsn3.4440Search in Google Scholar PubMed PubMed Central
[38] Hossain MA, Nagooru MR. Biochemical profiling and total flavonoids contents of leaves crude extract of endemic medicinal plant Corydyline terminalis L. Kunth. Pharmacog J. 2011;3(24):25–30.10.5530/pj.2011.24.5Search in Google Scholar
[39] Yadav UC, Baquer NZ. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm Biol. 2014;52(2):243–54.10.3109/13880209.2013.826247Search in Google Scholar PubMed
[40] Xue WL, Li XS, Zhang J, Liu YH, Wang ZL, Zhang RJ. Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2007;16(Suppl 1):422–6.Search in Google Scholar
[41] Haxhiraj M, White K, Terry C. The role of fenugreek in the management of type 2 diabetes. Int J Mol Sci. 2024;25:6987.10.3390/ijms25136987Search in Google Scholar PubMed PubMed Central
[42] Mandal SK, Puri S, Kumar BK, Muzaffar-Ur-Rehman M, Sharma PK, Sankaranarayanan M, et al. Targeting lipid-sensing nuclear receptors PPAR (α, γ, β/δ): HTVS and molecular docking/dynamics analysis of pharmacological ligands as potential pan-PPAR agonists. Mol Diver. 2024;28(3):1423–38.10.1007/s11030-023-10666-ySearch in Google Scholar PubMed
[43] Botta M, Audano M, Sahebkar A, Sirtori CR, Mitro N, Ruscica M. PPAR agonists and metabolic syndrome: an established role? Int J Mol Sci. 2018;19(4):1197.10.3390/ijms19041197Search in Google Scholar PubMed PubMed Central
[44] Imenshahidi M, Roohbakhsh A, Hosseinzadeh H. Effects of telmisartan on metabolic syndrome components: A comprehensive review. Biomed Pharmacother. 2024;171:116169.10.1016/j.biopha.2024.116169Search in Google Scholar PubMed
[45] Ali M, Hassan M, Ansari SA, Alkahtani HM, Al-Rasheed LS, Ansari SA. Quercetin and kaempferol as multi-targeting antidiabetic agents against mouse model of chemically induced type 2 diabetes. Pharmaceuticals. 2024;17(6):757.10.3390/ph17060757Search in Google Scholar PubMed PubMed Central
[46] Rawendra RD, Aisha, Chen SH, Chang CI, Shih WL, Huang TC, et al. Isolation and characterization of a novel angiotensin-converting enzyme-inhibitory tripeptide from enzymatic hydrolysis of soft-shelled turtle (Pelodiscus sinensis) egg white: in vitro, in vivo, and in silico study. J Agr Food Chem. 2014;62(50):12178–85.10.1021/jf504734gSearch in Google Scholar PubMed
[47] Rathi V, Sagi SS, Yadav AK, Kumar M, Varshney R. Quercetin prophylaxis protects the kidneys by modulating the renin–angiotensin–aldosterone axis under acute hypobaric hypoxic stress. Sci Rep. 2024;14(1):7617.10.1038/s41598-024-58134-3Search in Google Scholar PubMed PubMed Central
[48] Zheng W, Tian E, Liu Z, Zhou C, Yang P, Tian K, et al. Small molecule angiotensin converting enzyme inhibitors: A medicinal chemistry perspective. Front Pharmacol. 2022;13:968104.10.3389/fphar.2022.968104Search in Google Scholar PubMed PubMed Central
[49] Balaraman R, Dangwal S, Mohan M. Antihypertensive effect of Trigonella foenum-greacum seeds in experimentally induced hypertension in rats. Pharm Biol. 2006;44(8):568–75.10.1080/13880200600896538Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- Synthesis and characterization of surfactants derived from phenolphthalein: In vivo and in silico studies of their antihyperlipidemic effect
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Analysis and study on volatile flavor compounds of three Yunnan cultivated cigars based on headspace-gas chromatography-ion mobility spectrometry
- Near-infrared IR780 dye-loaded poloxamer 407 micelles: Preparation and in vitro assessment of anticancer activity
- Study on the influence of the viscosity reducer solution on percolation capacity of thin oil in ultra-low permeability reservoir
- Detection method of Aristolochic acid I based on magnetic carrier Fe3O4 and gold nanoclusters
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Innovative synthesis of cobalt-based catalysts using ionic liquids and deep eutectic solvents: A minireview on electrocatalytic water splitting
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies


