Abstract
In this paper, konjac oligoglucomannan (KOGM) was obtained with a hydrolysis rate of 56.24% by controlling the hydrolysis conditions. KOGM was passed through a 0.2 kDa dialysis bag, a 3 kDa ultrafiltration tube, and a 5 kDa ultrafiltration tube, creating samples with molecular weights of 0.2–3 kDa (IV), 3–5 kDa (III), and >5 kDa (II), respectively. The in vitro antioxidant activities of the KOGM samples were tested by measuring their removal effects on ˙OH,
1 Introduction
Konjac is a perennial herbaceous plant belonging to the genus Amorphophallus, Araceae family. Its main active component is konjac glucomannan (KGM), a water-soluble natural polysaccharide [1]. KGM is generally considered to be formed by linking glucose and mannose in a ratio of 1:1.69 or 1.4:1 through β-1,4 glycosidic bonds; at the C3 position of the mannose group in the main chain, there are branched chains connected by β-1,3 bonds. With a molecular weight of 200–20,000 kDa, KGM has an acetyl group at the C6 position for every 19 sugar units in the main chain [2,3].
The application of KGM is bottlenecked by its large molecular weight, high viscosity, and low solubility. Therefore, much attention has been paid to the degradation of KGM into konjac oligoglucomannan (KOGM), a functional oligosaccharide consisting of 2–10 monosaccharide units. KOGM can be obtained by degradation through enzymatic hydrolysis, chemical method, and physical method [4]. Studies have shown that KOGM can enhance immunity, lower blood lipids and blood sugar, and slow down the aging process [5,6,7,8,9,10].
Recently, it has been learned that KOGMs with different molecular weights differ in structure and biological effect. Ma et al. [11] demonstrated that the increasing molecular weight of KOGM pushes up many physico–chemical properties: corn starch solubility, pseudoplasticity, viscoelasticity, gelatinization temperature, gelatinization time, and aging inhibition. Peng et al. [12] explored the gel properties of KOGMs with different molecular weights and proved that the gel properties of KOGM are best at a molecular weight of 650–700 kDa. Gao et al. [13] examined the sulfonated KOGMs with different molecular weights and revealed that KOGM with a molecular weight of 10–30 kDa has the best anticoagulant, antitumor, and antibacterial effects. Li et al. [14] found that KOGM has an optimal hypoglycemic effect when its molecular weight falls within a range of 30–80 kDa. However, there are very few reports on the oxidation performance of KOGMs with different molecular weights. It is meaningful to prepare KOGMs with different molecular weights and to explore their resistance to oxidation.
In this paper, konjac gum is hydrolyzed with β-mannanase. By controlling the hydrolysis conditions, KOGM samples were obtained with a hydrolysis rate of 56.24%. Then, the KOGM samples with different molecular weights were separated through dialysis and centrifugal ultrafiltration. The composition, in vivo antioxidant activity [15,16,17,18], and in vitro antioxidant activity of these samples were evaluated in detail.
2 Materials and methods
2.1 Materials
KGM (purity: 95%) was purchased from Wuhan Qingjiang Konjac Products Co., Ltd. β-Mannanase (activity ≥3,145 U/g) was purchased from Mianyang Habio Bioengineering Co., Ltd. Mice (50% males, 50% females; weight: 20 ± 2 g) were purchased from Shaanxi Medical Laboratory Animal Center. Malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX) detection kits were purchased from Shanghai Jianglai Biotechnology Co., Ltd. The dialysis bags were purchased from Shanghai Qiaoxing Trading Co., Ltd. The other reagents were all of analytical grade and produced domestically.
2.2 Methods
2.2.1 Preparation of KOGMs with different molecular weights
First, 5 g of konjac powder was evenly dispersed into 100 mL of 0.2 mol/L acetate buffer to obtain a 5% (w/v) solution. The solution was mixed thoroughly with β-mannanase (dosage: 30–150 U/g) to initiate the hydrolysis. The mixture reacted for 1–5 h in a water bath at pH of 3.0–7.0 and temperature of 30–70°C. After that, the mixture was taken out and boiled for 10 min to inactivate the enzyme and stop the hydrolysis. Then, the reaction solution was centrifuged at 4,000 rpm to remove the unreacted KGM. The supernatant was taken for a 0.2 h dialysis in a 0.2 kDa dialysis bag to remove monosaccharides and small molecule salts from the sample, creating sample KOGM I. Next, KOGM I was passed through a 5 kDa ultrafiltration tube, producing sample KOGM II with a molecular weight greater than 5 kDa and a dialysate with a molecular weight of less than 5 kDa. The dialysate was further filtered through a 3 kDa ultrafiltration tube, producing sample KOGM III with a molecular weight of 3–5 kDa and sample KOGM IV with a molecular weight of less than 3 kDa. The samples were freeze-dried for further use.
2.2.2 Determination of KGM hydrolysis rate
The content of reducing sugar was determined by the dinitrosalicylic acid colorimetric method [19]. The KGM hydrolysis rate was calculated using the equation (1):
where M1 and M2 are the total contents of reducing sugar before and after hydrolysis, respectively, and M is the content of the KGM.
2.2.3 Orthogonal test
To achieve the best degradation effect, it is important to optimize parameters for enzymatic degradation. Assuming all other factors as constants, the commonly used single-factor test examines only one factor, without considering the interaction between parameters. Hence, this test approach cannot judge whether multiple parameters are optimal. As a result, the orthogonal test was selected to determine the optimal parameters.
According to the orthogonal test design, the KGM hydrolysis rate was taken as the index to be evaluated. Then, four parameters of enzymatic hydrolysis were optimized, including time (A), temperature (B), pH (C), and enzyme dosage (D). Each factor was tested at three levels (Table 1). The results were subjected to orthogonal analysis to optimize the preparation conditions for the KOGM.
Factors and levels of the orthogonal test
| Parameter | Level | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| (A) Time (h) | 1 | 2 | 3 |
| (B) Temperature (°C) | 45 | 50 | 55 |
| (C) pH | 4.5 | 5 | 5.5 |
| (D) Enzyme dosage (U/g) | 50 | 90 | 130 |
2.2.4 Determination of number-average molecular weight (Mn) of KOGM [20]
The Mn of KOGM was measured by quantifying the hydrolysate composition through high-performance liquid chromatography. The parameters were configured as follows: chromatographic column, Shodex NH2P-50 4E (4.6 mm × 250 mm); mobile phase, water:acetonitrile = 65:35; flow rate, 1 mL/min; column temperature, 35°C; injection volume, 15 µL; and detector, refractive index detector.
2.2.5 Determination of in vitro antioxidant activity
2.2.5.1 ˙OH removal capacity [21]
The ˙OH removal capacity of KOGM was tested by the Fenton’s reagent method. First, 150 µL of the KOGM samples with different contents was placed in separate test tubes. Then, 300 µL of 1.8 mmol/L ferrous sulfate solution, 200 µL of 1.8 mmol/L sodium salicylate–ethanol solution, and 25 µL of 0.03% hydrogen peroxide were added in turn into each test tube. The mixture in each tube was shaken well and reacted for 30 min at 37°C. The absorbance of each solution was measured at the wavelength of 510 nm. Vitamin C (Vc) and distilled water were selected as the positive control and the blank control, respectively. The ˙OH removal rate was calculated using the equation (2):
where A0, A1, and A2 are the absorbances of the blank control, the sample groups and positive control, and 200 µL of ferrous sulfate solution as the reagent blank.
2.2.5.2 DPPH˙ removal capacity [8]
First, 0.2 mL of KOGM with different hydrolysis rates was mixed with 4 mL of 120 µmol/L DPPH˙ solution in separate test tubes. The mixture in each test tube was shaken well and placed in darkness for 30 min. Taking absolute ethanol as the blank, the absorbance of the solution in each test tube was measured at 548 nm. Vc and distilled water were selected as the positive control and the blank control, respectively. The DPPH˙ removal rate was calculated using the equation (2), where A2 is changed to the absorbance measured by replacing the DPPH˙ solution with a 95% ethanol solution as the reagent blank.
2.2.5.3 O 2 −
The removal effect of KOGM on
where A0 and A1 are the absorbances of the blank control and the sample groups and positive control, respectively.
2.2.6 Determination of in vivo antioxidant activity [23]
Fifty days before the experiment, 50 mice were fed basic diet for 5 days and were randomly divided into a normal control group (A), three KOGM experimental groups (B1, B2, and B3), and a positive control group (C). In each group, the number of male mice was equal to the number of female mice.
The normal control group was fed with 0.25 mL of normal saline every day; the three KOGM groups were fed with 80 mg/kg (i.e., 0.25 mL 6.4 mg/mL) KOGM II, KOGM III, and KOGM IV, respectively, every day. The positive control group was fed with 50 mg/kg Vc every day.
The mice were free to eat and drink water in the 2-week-long experiment. In the end, the mice were fasted for 12 h. The eyeballs were removed for blood collection, using heparin as an anticoagulant. The mice were killed through cervical dislocation. Then, the livers were taken out and made into 10% liver tissue homogenate. Finally, the SOD activity, GSH-PX activity, and MDA content were measured.
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals.
2.2.7 Statistical analysis
All experiments were performed in triplicate (n = 3) unless otherwise specified. Except that the orthogonal experiment was expressed by the mean, the others were expressed as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) was performed using SPSS version 19, and Duncan’s multiple range test was performed to determine the statistical differences among different samples. Differences at P < 0.05 were considered significant, and P < 0.01 was considered extremely significant.
3 Results
3.1 Results of single-factor tests
3.1.1 Effect of time on KGM hydrolysis rate
The effect of time on the hydrolysis rate was tested at a temperature of 50°C, pH 5, and an enzyme dosage of 90 U/g.
As shown in Figure 1, the hydrolysis rate gradually increased with time. The hydrolysis increased rapidly before 2 h, especially in the first 0.5 h of the reaction (P < 0.01). After 2 h, the hydrolysis rate increased slowly (P < 0.05). The trend basically conforms to the kinetic features of the enzymatic reaction. In the beginning, the high concentration of substrate promotes the reaction in the direction of product formation; with the lapse of time, the hydrolysis rate stabilizes due to enzyme inactivation and product inhibition [24].
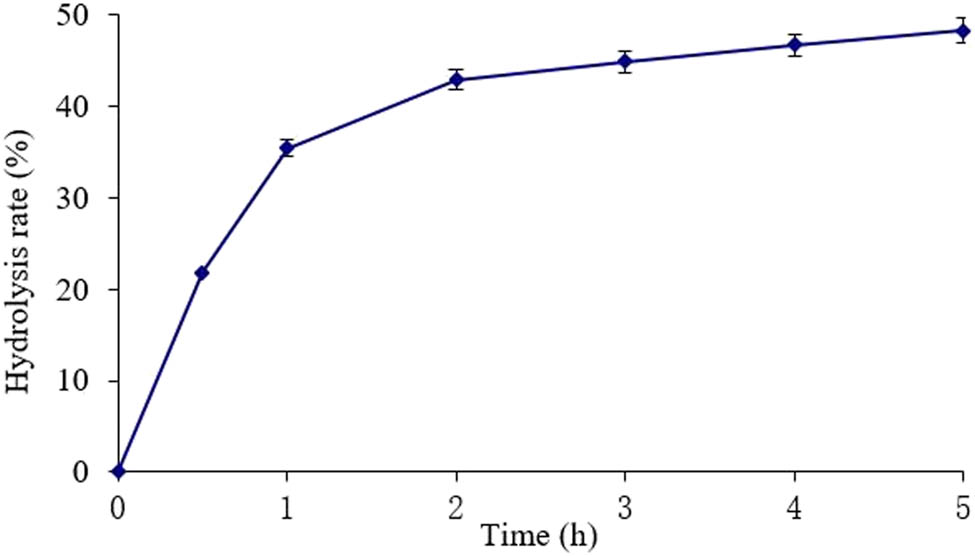
Effect of time on KGM hydrolysis rate.
3.1.2 Effect of temperature on KGM hydrolysis rate
The effect of temperature on the hydrolysis rate was tested at time 2 h, pH 5, and an enzyme dosage of 90 U/g.
As shown in Figure 2, the hydrolysis rate increased (P < 0.05) with temperature before reaching 50°C. This is because the enzymatic hydrolysis of konjac gum requires a certain activation energy. Meanwhile, high temperature makes konjac gum more soluble and KGM less viscous, thereby promoting the mass transfer of the reaction system. The hydrolysis rate peaked at 50°C. However, the enzyme became less stable and active at higher temperatures, causing a reduction in the hydrolysis rate.

Effect of temperature on KGM hydrolysis rate.
3.1.3 Effect of pH on KGM hydrolysis rate
The effect of pH on the hydrolysis rate was tested at a time of 2 h, a temperature of 50°C, and an enzyme dosage of 90 U/g.
As shown in Figure 3, β-mannanase exhibited a relatively good catalytic effect under acidic conditions. The optimal pH for enzymatic hydrolysis is 5.0. When the pH was below or above 5.0, the catalytic activity of the enzyme was greatly reduced. The main reason is that the excessively high or low pH affects the dissociation state of the active groups of the enzyme, reducing the chance for the enzyme to bind to the substrate [25].
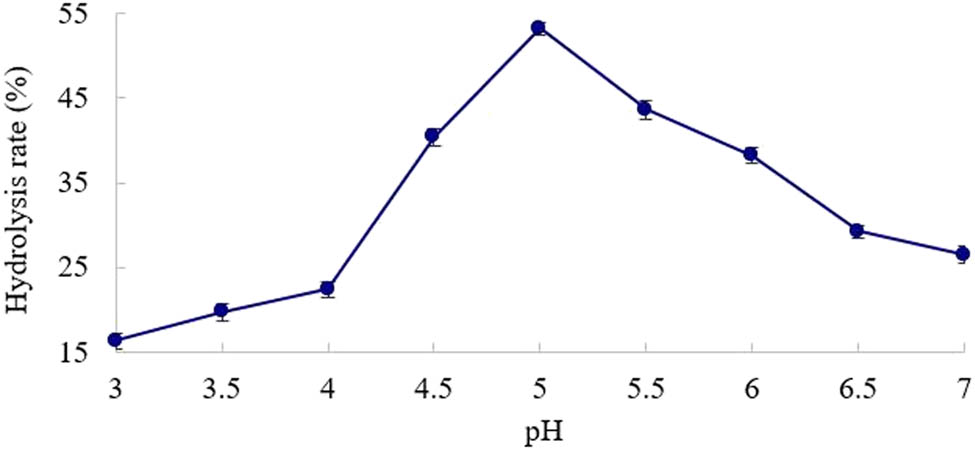
Effect of pH on KGM hydrolysis rate.
3.1.4 Effect of enzyme dosage on KGM hydrolysis rate
The effect of enzyme dosage on the hydrolysis rate was tested at a time of 2 h, a temperature of 50°C, and pH 5.
As shown in Figure 4, the hydrolysis rate initially increased with the enzyme dosage (P < 0.05), because the enzymatic reaction is insufficient at the low enzyme dosage. The hydrolysis rate reached the maximum when the enzyme dosage reached 90 U/g substrate. The hydrolysis rate did not increase, despite a further increase in enzyme dosage; there was no more substrate left for the extra enzyme molecules to bind with.

Effect of enzyme dosage on KGM hydrolysis rate.
3.2 Results of the orthogonal test
The effect of each parameter on the KGM hydrolysis rate was revealed by the above results of single-factor tests. On this basis, time (A), temperature (B), pH (C), and enzyme dosage (D) were selected for an L9(34) orthogonal test of four factors and three levels. The results of the orthogonal test are listed in Table 2, where K is the sum of the levels under each factor and R = (Kmax − Kmin)/3 is the influence range of each factor.
Results of the orthogonal test
| Experimental no | (A) Time (h) | (B) Temperature (°C) | (C) pH | (D) Enzyme dosage (U/g) | Hydrolysis rate (%) |
|---|---|---|---|---|---|
| 1 | 1 | 45 | 4.5 | 50 | 45.87 |
| 2 | 1 | 50 | 5 | 90 | 53.45 |
| 3 | 1 | 55 | 5.5 | 130 | 49.36 |
| 4 | 2 | 45 | 5 | 130 | 56.48 |
| 5 | 2 | 50 | 5.5 | 50 | 49.71 |
| 6 | 2 | 55 | 4.5 | 90 | 42.87 |
| 7 | 3 | 45 | 5.5 | 90 | 39.16 |
| 8 | 3 | 50 | 4.5 | 130 | 43.09 |
| 9 | 3 | 55 | 5 | 50 | 31.02 |
| K1 | 148.68 | 141.51 | 131.83 | 126.60 | |
| K2 | 149.06 | 146.25 | 140.95 | 135.48 | |
| K3 | 113.27 | 123.25 | 138.23 | 148.93 | |
| R | 11.93 | 7.67 | 3.04 | 7.44 |
Based on the R values in Table 2, the four factors were ranked as A > B > D > C in descending order of the effect on hydrolysis rate. According to ANOVA, the optimal parameter combination was A2B2C2D3: a time of 2 h, a temperature of 50°C, pH 5, and an enzyme dosage of 130 U/g.
3.3 Mn values of KOGMs with different molecular weights
Under the optimal parameter combination, the hydrolysis rate of konjac powder was 56.24%. After enzymolysis, each konjac powder solution was subjected to ultrafiltration and then Mn was measured (Table 3). The result (Mn = 1,328) shows that the molecular weight of konjac powder decreased after enzymolysis. The Mn of KOGM IV, which was obtained through 3 kDa centrifugal ultrafiltration, was 773. Hence, KOGM samples with different molecular weights can be acquired efficiently and quickly from KGM through β-mannanase hydrolysis and ultrafiltration.
Measured Mn values of the samples
| Sample | Mn |
|---|---|
| KGM | 64,530 |
| KOGM I | 1,328 |
| KOGM II | 1,352 |
| KOGM III | 1,176 |
| KOGM IV | 773 |
3.4 In vitro antioxidant activities of KOGMs with different molecular weights
3.4.1 ˙OH removal capacity
The ˙OH, as the most active radical, causes serious damage to adjacent biomolecules [26]. As shown in Figure 5, when their concentrations increased within 0–10 mg/mL, the ˙OH removal effects of the KOGMs with different molecular weights and Vc were on the rise. The strongest ˙OH removal effect belongs to KOGM IV (molecular weight: 200–3,000 Da); the ˙OH removal effect of 10 mg/mL KOGM IV was comparable to that of 1 mg/mL Vc. The weakest ˙OH removal effect belongs to KOGM II. The smaller the molecular weight, the better the ˙OH removal effects of the KOGM. The test results of KOGM IV were fitted by the least squares (LS) method, producing a one-variable quadratic polynomial: y = 0.3554x2 + 0.5407x + 27.24 (R² = 0.987). Thus, the IC50 of KOGM IV for the ˙OH was 7.2779 mg/mL.

˙OH removal effects of the KOGMs with different molecular weights and Vc.
3.4.2 DPPH˙ removal capacity
DPPH˙ is a stable lipophilic free radical, which has been widely used to evaluate the antioxidant activity of food and pharmaceutical raw materials [27]. As shown in Figure 6, when their concentrations increased within 0–5 mg/mL, the DPPH˙ removal effects of the KOGMs with different molecular weights and Vc were on the rise. The smaller the molecular weight, the better the removal effect. However, DPPH˙ was not removed at a low KOGM concentration (<1 mg/mL), whereas 87.2% of the DPPH˙ were removed when the Vc concentration was 0.25 mg/mL. Hence, KOGM showed a much weaker DPPH˙ removal effect than Vc at low concentrations. By contrast, 5 mg/mL KOGM IV (molecular weight: 200–3,000 Da) removed 71.8% of the DPPH˙ about 82.34% of the removal effect of the Vc. Therefore, KOGM has a good removal effect of DPPH˙, especially at low molecular weight. The test results of KOGM IV were fitted by the LS method, producing a one-variable quadratic polynomial: y = −1.1857x2 + 23.674x − 18.64 (R² = 0.987). Thus, the IC50 of KOGM IV for the DPPH˙ was 3.5199 mg/mL.
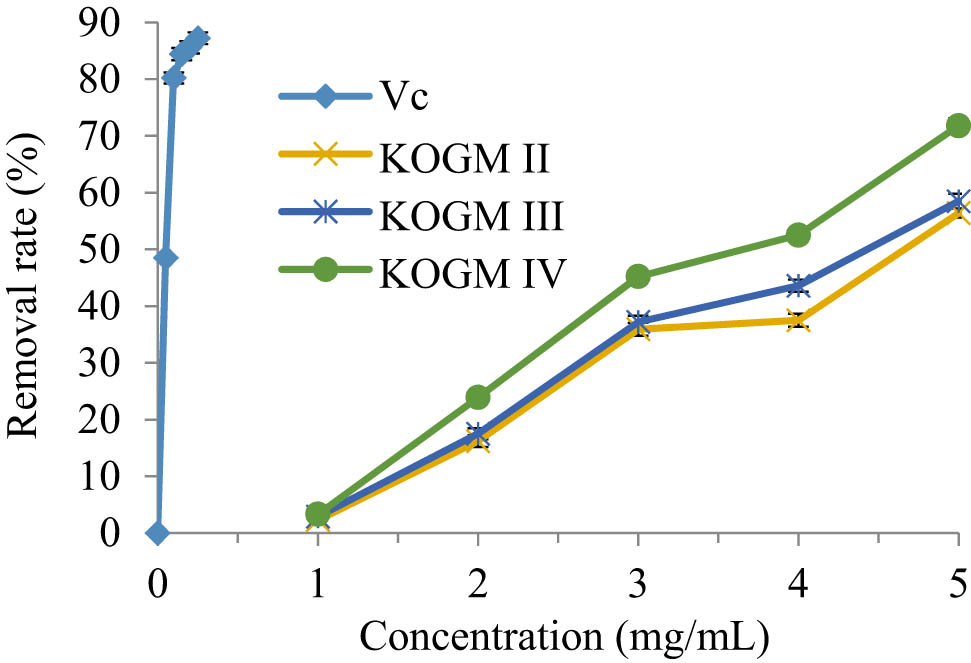
DPPH˙ removal effects of the KOGMs with different molecular weights and Vc.
3.4.3 O 2 −
Despite its inactive nature,

3.5 In vivo antioxidant activities of KOGMs with different molecular weights
The SOD and GSH-PX are the most important antioxidant enzymes in the body. They can remove free radicals on time and protect the body from oxidative damage. The MDA is a lipid peroxide produced under the imbalance between the oxidation system and antioxidant system of the body. The growth in the MDA will damage the cells. The MDA has often been used to measure the lipid peroxidation damage [29].
As shown in Table 4, the KOGM groups and positive control group were much higher than the normal control group in terms of the SOD and GSH-PX activities in the liver tissues, and lower than the latter in terms of the MDA content. Compared with the normal group, the KOGM IV group and the positive control group were the only two groups with very low MDA contents in the liver tissues; the KOGM III group and the positive control group were very high in terms of the SOD activity in the liver tissues, whereas KOGM IV had an extremely high SOD activity, about 1.22 times than that of the positive control group; the KOGM IV group and the positive control group were extremely high in terms of the GSH-PX activity in the liver tissues, whereas the other KOGM groups were very high in that respect.
Influence of molecular weight of KOGM on the MDA, SOD, and GSH-PX levels in the liver tissues of mice
| Groups | MDA (nmol mg prot−1) | SOD (U mg prot−1) | GSH-PX (U mg prot−1) |
|---|---|---|---|
| Normal group (A) | 21.45 ± 2.61 | 53.67 ± 12.89 | 152.45 ± 14.67 |
| KOGM II group (B1) | 20.82 ± 3.13 | 51.98 ± 11.17 | 238.98 ± 25.49* |
| KOGM III group (B2) | 19.13 ± 2.87 | 66.59 ± 12.49* | 256.32 ± 18.02* |
| KOGM IV group (B3) | 18.26 ± 3.39* | 70.98 ± 12.56** | 349.22 ± 35.08** |
| Positive control group (C) | 16.59 ± 3.11** | 58.16 ± 14.51* | 336.96 ± 25.56** |
Note: * P < 0.05 and ** P < 0.01 compared to the normal group.
4 Discussion
Although there have been many reports about the preparation of KOGM by degradation of KGM, most of them are studies on the degradation process by chemical, physical, and biological methods. There are few studies on the controllable degradation of KGM and the preparation of KOGMs with different molecular weights, and the molecular weight has a great impact on the efficiency of KOGM. In this paper, KGM was hydrolyzed by β-mannanase to prepare KOGM, and the best preparation process was obtained through the study of various factors of enzymatic hydrolysis. At the same time, through the orthogonal experimental analysis of various factors, it was found that the time of enzymatic hydrolysis had a significant impact on the enzymatic hydrolysis, so the controllable degradation of KGM could be realized by controlling the hydrolysis time. At the same time, three kinds of KOGMs with different molecular weights were obtained by centrifugation, dialysis, and ultrafiltration, which is the basis for the follow-up study of the biological function of KOGMs with different molecular weights and further exploration of the preparation of KOGMs with high oxidation resistance.
The metabolism of the human body produces a large number of free radicals. The free radicals of the body are in the dynamic balance of continuous generation and elimination. Once the free radicals in the body are produced too much or the body’s clearance ability is insufficient, the balance will be destroyed, which will cause adverse reactions such as aging and cancer [30]. In this study, three kinds of KOGMs with different molecular weights were tested for antioxidation in vitro and in vivo. In vitro experiments showed that KOGM had the ability to scavenge free radicals, and its scavenging ability was positively correlated with the concentration and negatively correlated with the molecular weight of KOGM. This is because the high concentration and low molecular weight of KOGM molecular chain contain more reduced hemiacetal hydroxyl, which can make the highly oxidized hydroxyl free reduction [31]; it has strong hydrogen supply capacity and can remove DPPH˙ with proton free radical; in addition, it contains high content of reducing ketone, which plays an antioxidant role by donating hydrogen atoms to destroy the free radical chain [32].
In human body, SOD can reduce
This study showed that KGM can be degraded in a controlled way and KOGMs with different molecular weights can be prepared by the enzymatic method. The antioxidative effect of KOGM was verified by experiments in vitro and in vivo, and the relationship between antioxidant and molecular weight was obtained, which lays a foundation for the preparation of high antioxidant KOGM and edible health products in future.
5 Conclusion and prospect
In this paper, the KOGM samples were prepared by hydrolyzing konjac powder for 2 h at a temperature of 50°C, pH 5, and a β-mannanase dosage of 130 U/g. The samples were subjected to ultrafiltration, creating three kinds of KOGMs with different molecular weights. Through in vitro and in vivo tests, KOGM was proved to have certain antioxidant activity, which was negatively correlated with the molecular weight; the antioxidant activity of KOGM was weaker than that of the Vc. Our research shows that the enzymatic method can successfully prepare the KOGMs with different molecular weights and that KOGM with a low molecular weight is a natural antioxidant.
However, the composition of KOGMs with a low molecular weight is still very complex and requires further study in order to elucidate a specific antioxidant mechanism. At the same time, it is not enough to verify the efficacy only in mice, and the use of the relevant knowledge and equipment in the field of clinical medicine is needed for further study to verify its antioxidant effect.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Chua M, Baldwin TC, Hocking TJ, Chan K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J Ethnopharmacol. 2010;128(2):268–78.10.1016/j.jep.2010.01.021Suche in Google Scholar
[2] Katsuraya K, Okuyama K, Hatanaka K, Oshima R, Sato T, Matsuzaki K. Constitution of konjac glucomannan: chemical analysis and 13C NMR spectroscopy. Carbohydr Polym. 2003;53(2):183–9.10.1016/S0144-8617(03)00039-0Suche in Google Scholar
[3] Zhang C, Yang FQ. Konjac glucomannan, a promising polysaccharide for OCDDS. Carbohydr Polym. 2014;104:175–81.10.1016/j.carbpol.2013.12.081Suche in Google Scholar PubMed
[4] Liu HL, Jiang ZM, Liu YG, Liu X, Chen Y, Zhang S. The preparation and physiological function of functional oligosaccharides and its application. China Food Addit. 2015;2015(12):158–66.Suche in Google Scholar
[5] Chen M, Wu Y, Yan Q, Zhao J, Feng L, He M, et al. Effects of dietary konjac oligosaccharide supplementation on serum immune parameters and intestinal immunity of Schizothorax prenanti. Fish Sci. 2019;85(1):157–65.10.1007/s12562-018-1252-zSuche in Google Scholar
[6] Cheng MJ, Han F, Li XD, Liu Y. Therapeutic effects of konjac mannan oligosaccharides for hyperlipidemia rat. Military Med J South China. 2017;31(2):71–5.Suche in Google Scholar
[7] Hoving LR, Katiraei S, Heijink M, Pronk A, van der Wee‐Pals L, Streefland T, et al. Dietary mannan oligosaccharides modulate gut microbiota, increase fecal bile acid excretion, and decrease plasma cholesterol and atherosclerosis development. Mol Nutr Food Res. 2018;62(10):1700942.10.1002/mnfr.201700942Suche in Google Scholar PubMed PubMed Central
[8] Liu J, Xu Q, Zhang J, Zhou X, Lyu F, Zhao P, et al. Preparation, composition analysis and antioxidant activities of konjac oligo-glucomannan. Carbohydr Polym. 2015;130:398–404.10.1016/j.carbpol.2015.05.025Suche in Google Scholar PubMed
[9] Zeng Y, Zhang J, Zhang Y, Men Y, Zhang B, Sun Y. Prebiotic, immunomodulating, and antifatigue effects of konjac oligosaccharide. J Food Sci. 2018;83(12):3110–7.10.1111/1750-3841.14376Suche in Google Scholar PubMed
[10] Zheng C, Li F, Hao Z, Liu T. Effects of adding mannan oligosaccharides on digestibility and metabolism of nutrients, ruminal fermentation parameters, immunity, and antioxidant capacity of sheep. J Anim Sci. 2018;96(1):284–92.10.1093/jas/skx040Suche in Google Scholar PubMed PubMed Central
[11] Ma S, Zhu P, Wang M, Wang F, Wang N. Effect of konjac glucomannan with different molecular weights on physicochemical properties of corn starch. Food Hydrocoll. 2019;96:663–70.10.1016/j.foodhyd.2019.06.014Suche in Google Scholar
[12] Peng SH, Wen CR, Yao MN, Lin HM, Jian WJ. Study on the gel properties of konjac glucomannan of different molecular weight. J Southwest Univ (Nat Sci Ed). 2010;32(11):151–7.Suche in Google Scholar
[13] Gao SJ, Hou ZW, Wu CD, Guo JM. Preparation of sulfonated konjac glucomannan of different molecular weight and study on their biological activity. Chem Bioeng. 2007;9:33–6.Suche in Google Scholar
[14] Li C, Wang Y, He W, Xie B. Studies on the antidiabetic effect of konjac glucomannan with different molecular chains on experimental diabetes mice. J Chin Med Mater. 2004;27(2):110–3.Suche in Google Scholar
[15] Rezig L, Sadaa M, Trabelsi N, Tammar S, Limam H, Bettaieb Rebey I, et al. Chemical composition, antioxidant and antimicrobial activities of Aloysia Triphylla L. essential oils and methanolic extract. Italian J Food Sci. 2019;31(3):556–72.Suche in Google Scholar
[16] Fredotović Ž, Puizina J. Edible allium species: chemical composition, biological activity and health effects. Italian J Food Sci. 2018;31(1):19–39.Suche in Google Scholar
[17] Misir G, Koral S. Effects of ultrasound treatment on biochemical, structural, functional properties and antioxidant activity of protein hydrolysate of rainbow trout (Onchorhynchus mykiss) by-products. Italian J Food Sci. 2018;31(2):205–23.Suche in Google Scholar
[18] Shin D, Chae KS, Choi HR, Lee SJ, Gim SW, Kwon GT, et al. Bioactive and pharmacokinetic characteristics of pre-matured black raspberry, Rubus Occidentalis. Italian J Food Sci. 2018;30(3):428–39.Suche in Google Scholar
[19] Chen J, Liu D, Shi B, Wang H, Cheng Y, Zhang W. Optimization of hydrolysis conditions for the production of glucomanno-oligosaccharides from konjac using β-mannanase by response surface methodology. Carbohydr Polym. 2013;93(1):81–8.10.1016/j.carbpol.2012.05.037Suche in Google Scholar PubMed
[20] Chen Z, Wang S, Shang L, Zhou P, Li J, Li B. An efficient and simple approach for the controlled preparation of partially degraded konjac glucomannan. Food Hydrocoll. 2020;108:106017.10.1016/j.foodhyd.2020.106017Suche in Google Scholar
[21] Yin JY, Ma LY, Siu KC, Wu JY. Effects of ultrasonication on the conformational, microstructural, and antioxidant properties of konjac glucomannan. Appl Sci. 2019;9(3):461.10.3390/app9030461Suche in Google Scholar
[22] Gao J, Zhang T, Jin ZY, Xu XM, Wang JH, Zha XQ, et al. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015;169:430–8.10.1016/j.foodchem.2014.08.016Suche in Google Scholar
[23] Li Y, Lü F, Xu Y, Zhou H. Effect of functional oligosaccharides from ulva lactuca on immunomodulatory and antioxidant activity in mice. J Chin Inst Food Sci Technol. 2018;18(12):32–8.Suche in Google Scholar
[24] Su RX, Qi W, He ZM. Molecular weight distribution and kinetic model for enzymatic hydrolysis of water-soluble polysaccharides. J Chem Eng Chin Universities. 2006;20(4):565.Suche in Google Scholar
[25] Garcia T, Sanchez N, Martinez M, Aracil J. Enzymatic synthesis of fatty esters: part I. Kinetic approach. Enzyme Microb Technol. 1999;25(7):584–90.10.1016/S0141-0229(99)00082-4Suche in Google Scholar
[26] Jaberian H, Piri K, Nazari J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013;136(1):237–44.10.1016/j.foodchem.2012.07.084Suche in Google Scholar PubMed
[27] Letelier ME, Molina-Berríos A, Cortés-Troncoso J, Jara-Sandoval J, Holst M, Palma K, et al. DPPH and oxygen free radicals as pro-oxidant of biomolecules. Toxicol vitro. 2008;22(2):279–86.10.1016/j.tiv.2007.08.002Suche in Google Scholar PubMed
[28] Fu WY, Xu LH, Zhang YY, Lam PKS. Regulatory effect of reactive oxygen species on apoptosis induced by chemicals. Chin J Pharmacol Toxicol. 2002;16(6):464–70.Suche in Google Scholar
[29] Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X, et al. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromolecules. 2005;37(4):195–9.10.1016/j.ijbiomac.2005.10.008Suche in Google Scholar PubMed
[30] Zeng W, Shi B. Common methods of antioxidant activity evaluation for natural products. Chem Ind Eng Prog. 2013;32(6):1205–13.Suche in Google Scholar
[31] Qin CQ, Chi WL, Shu HB. Research development of chitosan bioactivities in vivo. J Xiaogan Univ. 2004;2004(6):5–9.Suche in Google Scholar
[32] Yuan H, Song J, Zhang W, Li X, Li N, Gao X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorg Med Chem Lett. 2006;16(5):1329–34.10.1016/j.bmcl.2005.11.057Suche in Google Scholar PubMed
[33] Dong WB, Liu D, Yang J, Xu XM. The seperation of flavonoids and polyphenols from eucommia ulmoides leaves and their antioxidation in vivo. Appl Mech Mater. 2012;108:146–51.10.4028/www.scientific.net/AMM.108.146Suche in Google Scholar
© 2020 Weidong Yang, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”
Artikel in diesem Heft
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”

