Abstract
The aim of the present study was to investigate the ability of Bacillus megaterium IBBPo17 (GenBank KX499518) cells to produce biosurfactant when the growth was done in the presence of long-chain n-alkane n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose. B. megaterium IBBPo17 revealed a higher growth in the presence of n-hexadecane when the medium was supplemented with yeast extract, proteose peptone, or starch, compared with cellulose. Biosurfactant production was higher when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on yeast extract, proteose peptone, or starch supplemented medium, compared with biosurfactant produced on cellulose supplemented medium. A direct correlation between cell growth and biosurfactant production was observed. When the growth of B. megaterium IBBPo17 cells was higher, the decrease in pH values of the medium was higher too, and more amount of CO2 was released. Changes in cell morphology, aggregation of the cells in clusters, and biofilm formation were observed when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose. Due to its physiological abilities, this Gram-positive bacterium could be a promising candidate for the bioremediation of petroleum hydrocarbon polluted environments.
1 Introduction
Bacillus megaterium is a genus of Gram-positive, spore-forming, motile, mainly aerobic chemoheterotrophic bacterium belonging to the family Bacillaceae. Like other Bacillus strains, B. megaterium shows a wide range of physiological abilities, such as endospore formation and production of essential antibiotics, which allow the organism to grow and survive in widely diverse habitats (e.g., soil, seawater, sediment, petrochemical wastes) [1,2]. This nonpathogenic bacterium can grow in simple media on various carbon sources. Its ability to grow on various inexpensive carbon sources makes this bacterium an ideal industrial protein production host. Furthermore, B. megaterium strains, which usually carry multiple plasmids, are excellent hosts for gene expression [1]. The most important products occurring in B. megaterium are proteins like penicillin acylase, amylases, neutral protease, dehydrogenases, P-450 cytochrome monooxygenases, and vitamins with many biotechnological and industrial applications [1,2,3]. P-450 cytochrome monooxygenases are hemoproteins involved in a variety of reactions, including in the conversion of alkanes, terpenes, or aromatic compounds, and carbon source assimilation [3]. Several Bacillus species produce biosurfactants (also known as surface-active compounds), which are used in numerous industrial and environmental fields, including in petroleum industries and bioremediation of petroleum hydrocarbon polluted environments [4,5]. In the last few decades, there has been an increased scientific interest in the isolation of bacteria, which produce tensioactive molecules with excellent surfactant characteristics, low toxicity, and high emulsifying activity [6,7]. Biosurfactants are produced mainly by aerobic bacteria when they are grown in liquid media with several carbon sources. It is believed that biosurfactants are secreted into the culture medium to assist the growth of different bacterial strains by facilitating the transport and translocation of insoluble hydrophobic substrates (e.g., hydrocarbons, oils) across the cell membranes [8]. Most of the biosurfactants are produced from water-insoluble substrates, although many of them are obtained through soluble substrates (e.g., carbohydrates) or a combination of both [6,8]. Therefore, B. megaterium is an interesting microorganism due to its broad distribution in environments, biochemical versatility, protein secretion system, and its effectiveness as an industrial protein production strain [1].
The objective of the present study was to investigate the ability of B. megaterium strain IBBPo17 (GenBank KX499518) to produce biosurfactant when the growth was done in the presence of different nitrogen (i.e., yeast extract, proteose peptone) or carbon (i.e., starch, cellulose) sources. Long-chain n-alkane, such as n-hexadecane, with a high logarithm of the partition coefficient of the hydrocarbon in the octanol–water mixture (log POW value 9.15), was used as the hydrophobic carbon source in this study.
2 Materials and methods
2.1 Bacterial strain and culture conditions
Bacillus megaterium strain IBBPo17 (GenBank KX499518) was inoculated into a nutrient-rich LB (Luria–Bertani) medium. The flask was incubated for 24 h at 30°C on a rotary shaker (200 rpm). Bacterial cells were harvested by centrifugation, washed twice, and finally the cell pellets were resuspended (OD660 0.07) in basal medium (pH 7.2) containing: 0.1% K2HPO4, 0.1% KH2PO4, 1% NaCl, and 0.25% MgSO4, and supplemented with 0.5% nitrogen (i.e., yeast extract, proteose peptone) or 0.5% carbon (i.e., starch, cellulose) sources. Long-chain n-alkane, such as 5% (v/v) n-hexadecane, was finally added to the cell suspensions. The flasks were sealed and incubated for 72 h at 30°C on a rotary shaker (200 rpm).
Reagents used in this study were purchased from Merck (Darmstadt, Germany), Sigma-Aldrich (Saint-Quentin-Fallavier, France), or Bio-Rad Laboratories (Hercules, CA, USA).
2.2 Growth experiments
The cell growth was monitored by determining the optical density at 660 nm (OD660) using a SPECORD 200 UV-visible spectrophotometer (Analytik Jena, Jena, Germany). Briefly, the bacterial cells were harvested by centrifugation, washed twice, and the cell pellets were resuspended in basal medium, and then the OD660 was measured. The pH of cell-free culture broths was measured using a Hanna bench pH 213 meter (Woonsocket, Rhode Island, USA). The cell growth was also monitored by assessing the carbon dioxide (CO2) production, based on a method previously described by Darsa et al. [9]. Culture broths were titrated with 0.05 N NaOH, using 1% phenolphthalein as an indicator, and then the amount of carbon dioxide released (CO2 mg L−1) was calculated [9].
2.3 Scanning electron microscopy (SEM)
Cell pellets were fixed with 5% glutaraldehyde in 0.2 M potassium phosphate buffer for 24 h at room temperature and dehydrated in a graded series of ethanol for 30 min each (15%, 30%, 50%, 70%, 90%, and 100% ethanol). Gold-coated samples were examined using a JEOL JSM-6610LV scanning electron microscope (JEOL, Peabody, MA, USA) operating at 20 kV.
2.4 Enzyme production
Culture broths were spotted on wheat meal agar [10], starch agar [11], and carboxymethylcellulose agar [12] for protease, amylase, and cellulase production, respectively. The Petri plates were incubated for 24 h at 30°C for these qualitative analyses.
2.5 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Extracellular and whole-cell proteins were extracted from the cell-free culture broths and cell pellets, respectively, as previously described by Stancu [13]. Extracted proteins were dissolved in Laemmli buffer and denatured at 96°C for 10 min. Protein content was determined by measuring the optical density at 280 nm (OD280) using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). SDS-PAGE analyses were carried out using a Minigel-Twin system (Biometra, Göttingen, Germany). After electrophoretic separation on 12% polyacrylamide gel and staining with Coomassie brilliant blue [14], the protein profiles were analyzed.
2.6 Polymerase chain reaction (PCR) amplification and sequencing
Genomic DNA was extracted from the cell pellets with PureLink genomic kit (Invitrogen, Carlsbad, CA, USA) and used as a DNA template for the PCR amplification of alkane hydroxylase gene of Group III encoding alkB1 [15]. The PCR amplification of this gene was performed by using thermal Mastercycler pro S (Eppendorf, Hamburg, Germany) in a 50 µL reaction mixture containing: 0.5 µg genomic DNA, GoTaq G2 hot start polymerase (Promega, Madison, WI, USA), 5× GoTaq flexi buffer, MgCl2, dNTP mix, and ALK3-f/ALK3-r primers [15]. The PCR conditions included an initial denaturing step of 10 min at 94°C followed by 35 cycles of denaturing at 94°C for 1 min, annealing at 43°C for 30 s, extension at 72°C for 2 min, followed by a final extension of 10 min at 72°C. After electrophoretic separation on 1.5% TBE agarose gel [14] and staining with SYBR safe DNA gel stain (Invitrogen, Carlsbad, CA, USA), the PCR products were analyzed. The 330, 430, and 500 bp amplicons were sequenced with an ABI 3730XL at CeMIA SA (Larissa, Greece). The nucleotide sequences obtained were edited and aligned using the BioEdit software and compared with those available in the GenBank public database using the NCBI BLAST search algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.7 Biosurfactant production
Emulsification activity assay was used to quantify the biosurfactant in the cell-free culture broths. Briefly, n-hexadecane was added to cell-free culture broths (1:2, v/v), vortexed, and allowed to stand for 24 h, and then the emulsification index (E24) was determined [16].
Biosurfactant activity assay was also used to evaluate surfactant production by this bacterium. Cell-free culture broths were dropped onto the surface of Petri plates containing water and 0.01% Sudan black in n-hexadecane, and then spreading of the hydrocarbon film was observed when the biosurfactant was present in the cell-free culture broths [17].
Biosurfactant was extracted from the cell-free culture broths with chloroform–methanol (2:1, v/v), as previously described by Stancu [13]. Thin-layer chromatography (TLC) analyses were carried out using a CAMAG TLC system (Muttenz, Switzerland). Biosurfactant fractions were separated on TLC precoated silica gel 60 plates (Merck) using chloroform–methanol–water mixture (65:25:4, v/v/v) as mobile phase. After derivatization with iodine vapors to detect lipids or 0.2% orcinol in 53% sulfuric acid to detect sugars in the biosurfactant molecules, TLC plates were visualized and scanned under ultraviolet (UV) light (254 nm) and visible light (500 nm).
3 Results
3.1 Growth experiments
The growth of B. megaterium IBBPo17 cells in the presence of 5% n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose was monitored by measuring the optical density at 660 nm (OD660), pH, and CO2 released (Table 1). B. megaterium IBBPo17 exhibited a higher growth in the presence of n-hexadecane when the medium was supplemented with yeast extract, proteose peptone, or starch (OD660 between 1.19 and 1.26), compared with the growth on cellulose (OD660 0.83). Different bacteria can grow over a wide pH range, and each bacterial strain has its own tolerance level. The optimum pH for hydrocarbon biodegradation is between 6.0 and 8.0 [9]. The pH of the growth medium for B. megaterium IBBPo17 was adjusted to 7.2. Estimating the cellular growth by measuring the pH proved a decrease in pH values ranging from 6.87 and 7.05 when the medium was supplemented with yeast extract, proteose peptone, starch, or cellulose. The production of CO2 by B. megaterium IBBPo17 in the presence of n-hexadecane was higher when the growth medium was supplemented with yeast extract, proteose peptone, or starch (CO2 released between 660 and 880 mg L−1), compared with CO2 released on cellulose supplemented medium (440 mg L−1). The acquired results showed a direct correlation between the growth of the cells, pH, and CO2 production. When the growth of B. megaterium IBBPo17 cells was higher, the decrease in pH values of the culture medium was higher and more amount of CO2 was released.
Growth of B. megaterium IBBPo17 in the presence of n-hexadecane
| Cell growth | Medium with | |||
|---|---|---|---|---|
| Yeast extract | Proteose peptone | Starch | Cellulose | |
| OD660 (nm) | 1.26 | 1.21 | 1.19 | 0.83 |
| pH | 6.87 | 6.95 | 6.90 | 7.05 |
| CO2 (mg L−1) | 660 | 880 | 660 | 440 |
Cell growth was monitored by determining the OD660, pH, and CO2 released, and the values from the table represent the average of two separate determinations.
3.2 SEM
SEM studies of B. megaterium IBBPo17 grown in the presence of 5% n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose revealed changes in cell morphology. As observed, B. megaterium IBBPo17 grown on LB medium ranges between 1.6 and 3.8 µm in length [13]. The cell length of B. megaterium IBBPo17 grown in the presence of n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose ranges between 0.8 and 5.5 µm (Figure 1a–d). A decrease in cell size was observed when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on medium supplemented with yeast extract (approximate 0.7–1.0-fold), proteose peptone (0.6–0.7-fold), or starch (0.5–0.7-fold), as compared with the size of cells grown on LB medium. An increase in the cell size was observed in the presence of n-hexadecane when the medium was supplemented with cellulose (approximate 1.2–1.4-fold). Surface damages (i.e., rough outer surfaces, cell wall pores, leakage of internal contents) were observed when B. megaterium IBBPo17 cells were grown in the presence of n-hexadecane on medium supplemented with yeast extract, proteose peptone, or starch. No such surface damages were observed for bacterial cells grown in the presence of n-hexadecane on cellulose supplemented medium.
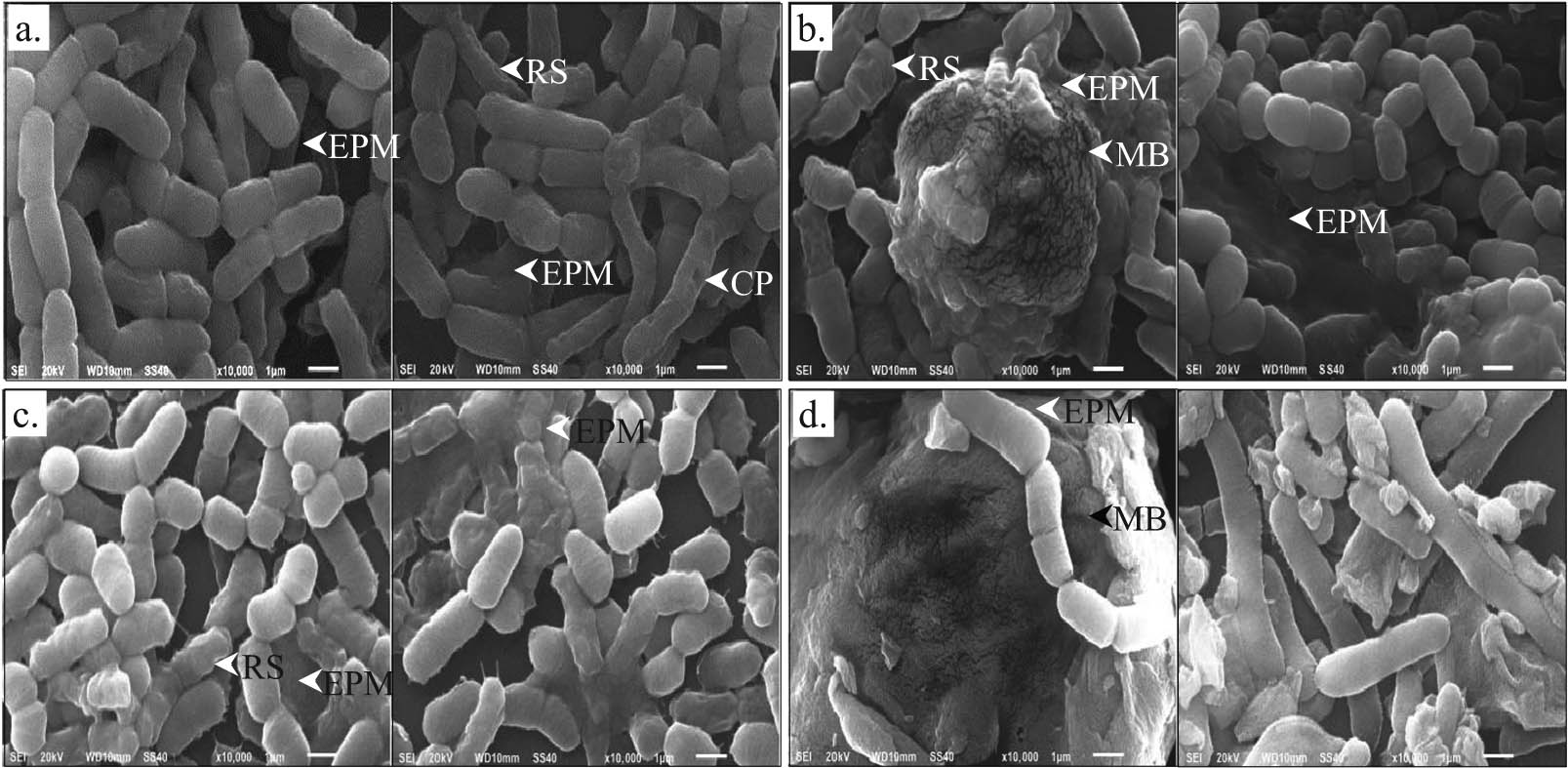
SEM studies of B. megaterium IBBPo17 grown in the presence of n-hexadecane. Medium with yeast extract (a), proteose peptone (b), starch (c), and cellulose (d); cell wall pores (CP), rough outer surfaces (RS), extracellular polymeric matrix (EPM), mature biofilm (MB).
B. megaterium IBBPo17 cells grown in the presence of n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose were surrounded by an extracellular polymeric matrix (EPM) (Figure 1a–d). The formation of cell clusters and mature biofilm (a three-dimensional structure) was also observed. The clustered bacterial cells were embedded in an additional EPM. The increase of EPM was observed when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on medium supplemented with proteose peptone, starch, or cellulose.
3.3 Enzyme production
B. megaterium IBBPo17 grown in the presence of 5% n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose produced protease, amylase, and cellulase (Figure 2a–d). No significant differences were observed in protease, amylase, and cellulase production when the growth medium for B. megaterium IBBPo17 cells was supplemented with yeast extract, proteose peptone, starch, or cellulose.

Enzyme production by B. megaterium IBBPo17 grown in the presence of n-hexadecane. Control (a), protease (b), amylase (c), and cellulase (d). Medium with yeast extract (1), proteose peptone (2), starch (3), and cellulose (4); confluent cell growth was observed.
3.4 SDS-PAGE
The protein profiles of B. megaterium IBBPo17 cells grown in the presence of 5% n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose were investigated by SDS-PAGE. B. megaterium IBBPo17 showed extracellular and whole-cell protein bands in the ranges of 16–150 kDa and 17–150 kDa, respectively (Figure 3a and b). Changes in the protein profiles of B. megaterium IBBPo17 cells were observed in the presence of n-hexadecane when the growth medium was supplemented with yeast extract, proteose peptone, starch, or cellulose. Extracellular proteins in the range of 32–150 kDa were produced in higher quantities by B. megaterium IBBPo17 grown in the presence of n-hexadecane on yeast extract, proteose peptone, or cellulose, as compared with those from cells grown on starch supplemented medium. Small alterations were also observed in the whole-cell protein profiles when B. megaterium IBBPo17 cells were grown in the presence of n-hexadecane on yeast extract, proteose peptone, starch, or cellulose supplemented medium.
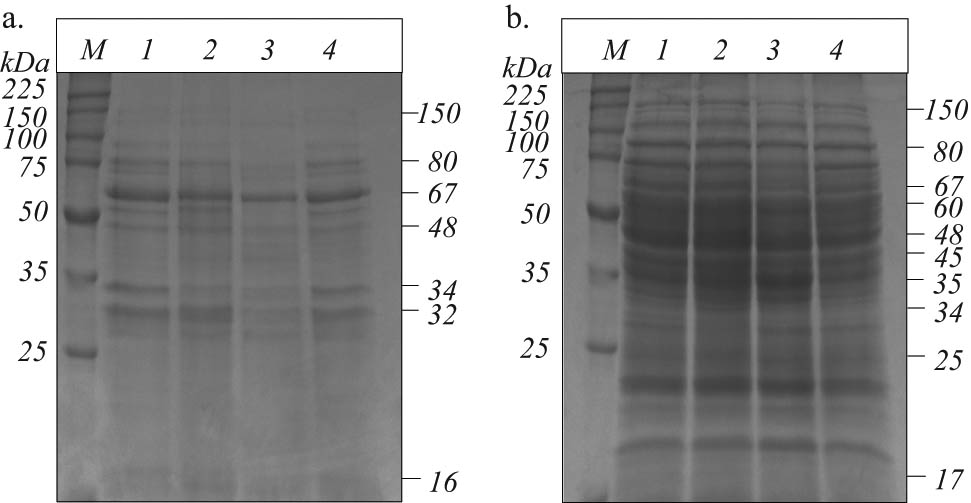
SDS-PAGE of extracellular (a) and whole-cell (b) proteins of B. megaterium IBBPo17 grown in the presence of n-hexadecane. Medium with yeast extract (1), proteose peptone (2), starch (3), and cellulose (4); broad range protein molecular weight marker, Promega (M).
3.5 PCR amplification and sequencing
Genomic DNA extracted from B. megaterium IBBPo17 cells grown in the presence of 5% n-hexadecane on basal medium supplemented with yeast extract, proteose peptone, starch, or cellulose was used as the template for PCR amplification of alkane hydroxylase gene of Group III encoding alkB1. As observed (Figure 4), the 330 bp expected amplicon for alkB1 gene was detected in lower quantities in DNA extracted from B. megaterium IBBPo17 cells grown in the presence of n-hexadecane on basal medium supplemented with yeast extract or proteose peptone, and in higher quantities in DNA extracted from cells grown on starch or cellulose supplemented medium. Furthermore, seven other distinct amplicons (i.e., 430 + 500 + 650 + 850 + 1,100 + 1,250 + 1,350 bp) were obtained when the ALK3-f/ALK3-r primers for alkB1 gene were used. The 330, 430, and 500 bp amplicons were subsequently sequenced using the amplification primers. The 330 bp fragment exhibited 88% sequence homology with alkane hydroxylase alkB2 gene of Pseudomonas aeruginosa PAK (AJ633616.1). The 430 bp fragment exhibited 88% sequence homology with peptidase M20 of B. megaterium (WP_098332943.1), and the 500 bp fragment showed 94% sequence homology with a hypothetical protein of B. megaterium (WP_074898083.1).

Detection of the alkB1 gene in B. megaterium IBBPo17 grown in the presence of n-hexadecane. Medium with yeast extract (1), proteose peptone (2), starch (3), and cellulose (4); 1 kb DNA ladder, Promega (M); the positions of 330, 430, and 500 bp fragments have been marked by arrows.
3.6 Biosurfactant production
The biosurfactant production by B. megaterium IBBPo17 cells grown in the presence of 5% n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose was further investigated in this study by the emulsification index (E24), biosurfactant activity, and TLC analyses. B. megaterium IBBPo17 exhibited the ability to produce biosurfactant with a good emulsification index (E24) when the growth was done in the presence of n-hexadecane on yeast extract (E24 50%), proteose peptone (E24 52%), starch (E24 48%), or cellulose (E24 45%) supplemented medium. Biosurfactant activity (quantified by the n-hexadecane spreading assay) was higher when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on yeast extract, proteose peptone, or starch supplemented medium (diameter 83 mm), compared with the activity of biosurfactant produced by the cells on cellulose (diameter 75 mm). As observed (Figure 5a–c), two fractions were detected by TLC analyses in the cell-free culture broths of B. megaterium IBBPo17 grown in the presence of n-hexadecane on yeast extract (retardation factor Rf 0.50, 0.55), starch (Rf 0.49, 0.53), or cellulose (Rf 0.49, 0.54), and four fractions when the growth medium was supplemented with proteose peptone (Rf 0.08, 0.12, 0.49, 0.53). All detected fractions (Rf 0.08, 0.12, 0.49–0.50, 0.53–0.55) revealed a positive reaction with iodine (light brown spots), indicating the presence of lipid moiety, and the fourth fraction (with Rf 0.53–0.55) revealed a positive reaction also with orcinol (dark red spot), indicating the presence of sugar moiety in the biosurfactant molecules.

TLC of biosurfactant produced by B. megaterium IBBPo17 grown in the presence of n-hexadecane. TLC plate visualized under UV light (a); TLC plate stained with iodine (b) or orcinol (c) and visualized under visible white light. Medium with yeast extract (1), proteose peptone (2), starch (3), and cellulose (4); sugar standards (SS), d-glucose (G), l-rhamnose (R), glycolipids (GL); the glycolipids have been marked by arrows.
4 Discussion
B. megaterium strain IBBPo17 (GenBank KX499518) was formerly isolated from an oily sludge sample by crude oil enrichment [13]. This Gram-positive bacterium, which possesses alkB1 and ndoM catabolic genes, was able to survive and grow in the presence of short-chain n-alkanes, such as n-decane, n-hexane, and cyclic solvents, such as cyclohexane, ethylbenzene, styrene, and toluene. As previously observed, B. megaterium IBBPo17 produced extracellular enzymes (protease, amylase, cellulase) and extracellular surfactant when the growth was done on nutrient-rich LB medium [13]. In the present study, we further investigated the ability of B. megaterium IBBPo17 to produce biosurfactant when the growth was done in the presence of 5% n-hexadecane on basal medium supplemented with different nitrogen (i.e., yeast extract, proteose peptone) or carbon (i.e., starch, cellulose) sources. The long-chain n-alkane, n-hexadecane, was used as the hydrophobic carbon source in order to evaluate the degradative potential of B. megaterium IBBPo17.
B. megaterium IBBPo17 revealed a higher growth in the presence of n-hexadecane when the medium was supplemented with yeast extract, proteose peptone, or starch, compared with the growth on cellulose. Nitrogen and carbon are the most important media components that act as essential stimulants for the growth of bacteria and also for the enzyme production [18]. Estimating the cellular growth by measuring the pH proved a decrease in pH values when the medium was supplemented with yeast extract, proteose peptone, starch, or cellulose, indicating the formation of organic acids (Table 1). Similar results were obtained by Darsa et al. [9] for another Gram-positive strain, B. subtilis, which was isolated from a soil sample collected from petrol bunks and workshops. The petrol utilization by B. subtilis induced changes in the optical density and pH values of the growth medium due to the production of organic acids. These acidic metabolic products are responsible for the decrease in pH of the growth medium [9].
The mineralization studies, which involve the measurements of total CO2 released, provide excellent information which confirms the active hydrocarbon degradation in polluted soils [19]. The production of CO2 by B. megaterium IBBPo17 in the presence of n-hexadecane was higher when the growth medium was supplemented with yeast extract, proteose peptone, or starch, compared with CO2 released on cellulose supplemented medium. Similarly, Darsa et al. [9] proved changes in the levels of CO2 released in the growth medium during petrol degradation by a B. subtilis strain.
Changes in cell morphology (e.g., cell sizes, cell surfaces) were observed when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose (Figure 1a–d). There are several reports in which the cell sizes are decreasing or increasing when bacteria are exposed to different stressful conditions [20,21,22]. The surface–volume ratio of the cells is the most important factor responsible for cell size changes. The reduction in the cell surface represents an effective mechanism of the cells to reduce the toxic effect of environmental stress factors by reducing the attachable surface in relation to the whole-cell volume [20]. Therefore, it was not surprising to observe cell size changes in B. megaterium IBBPo17 grown in the presence of n-hexadecane on yeast extract, proteose peptone, starch, or cellulose supplemented medium. Cell morphology alterations, which implicate modification in the cell membrane structure, alteration of the cell surface properties (e.g., permeability barrier, energy transducer), and metabolism, were reported in Gram-positive bacteria, including in different Bacillus strains, as a response to different environmental stresses (e.g., organic solvents). An increase in cell membrane permeability is considered the main reason for cell death [22].
Increase of EPM involved in biofilm formation was observed when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose (Figure 1a–d). Several Bacillus strains were able to form biofilms on different surfaces, and these bacteria are more resistant to environmental stress [23]. During biofilm development, cell morphological changes occur. Initially, the cells are short and motile, while in mature biofilm, the cells form long chains of nonmotile rods which adhere to each other and on the surfaces by secreting an EPM (mainly polysaccharides, proteins, and nucleic acids) [23].
No significant differences were observed in protease, amylase, and cellulase production when the growth medium for B. megaterium IBBPo17 cells was supplemented with yeast extract, proteose peptone, starch, or cellulose (Figure 2a–d). The production of extracellular enzymes is generally influenced by media components (e.g., nitrogen and carbon source) and several other factors (e.g., temperature, pH, aeration, incubation time). There is no defined medium for the best production of extracellular enzymes from different Bacillus strains [24,25,26]. Each strain has its unique conditions for maximum production of the extracellular enzymes, such as protease [18,24,27], amylase [28], and cellulase [25].
Changes in the protein profiles (including the 48, 67, and 80 kDa bands) of B. megaterium IBBPo17 cells were observed in the presence of n-hexadecane when the growth medium was supplemented with yeast extract, proteose peptone, starch, or cellulose (Figure 3a and b). Ahmetoglu et al. [26], Tiwari et al. [28], and Gaur and Tiwari [25] reported that the molecular weights of the proteases, amylase, and cellulase from different Bacillus strains (i.e., Bacillus sp. KG5, Bacillus tequilensis RG-01, Bacillus vallismortis RG-07) were 48, 67, and 80 kDa, respectively.
In this study, the alkB1 gene (330 bp amplicon) was detected in lower quantities in DNA extracted from B. megaterium IBBPo17 cells grown in the presence of n-hexadecane on basal medium supplemented with yeast extract or proteose peptone, and in higher quantities in DNA extracted from cells grown on starch or cellulose supplemented medium (Figure 4). Seven other distinct amplicons were also obtained by PCR when the primers for alkB1 gene were used.
B. megaterium IBBPo17 exhibited the ability to produce biosurfactant with a good emulsification index when the growth was done in the presence of n-hexadecane on yeast extract, proteose peptone, starch, or cellulose supplemented medium. A direct correlation between the growth of the cells and biosurfactant production was observed. Biosurfactant activity was higher when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on yeast extract, proteose peptone, or starch supplemented medium, compared with the activity of biosurfactant produced by the cells on cellulose. Two fractions were detected by TLC analyses in the cell-free culture broths of B. megaterium IBBPo17 grown in the presence of n-hexadecane on yeast extract, starch, or cellulose, and four fractions when the growth medium was supplemented with proteose peptone. All detected fractions showed a positive reaction with iodine, indicating the presence of lipid moiety, and one of the fractions showed a positive reaction also with orcinol, indicating the presence of sugar moiety in the biosurfactant molecules (Figure 5a–c). Based on the chemical composition, most of the biosurfactants produced by different Bacillus species were lipopeptides [5,7,16,29,30]. However, Thavasi et al. [31] reported that the biosurfactant produced by a B. megaterium strain was classified as a glycolipid with 28% carbohydrate and 70% lipid in its structure.
5 Conclusions
B. megaterium IBBPo17 revealed a higher growth in the presence of n-hexadecane when the medium was supplemented with yeast extract, proteose peptone, or starch, compared with the growth on cellulose. When the growth of B. megaterium IBBPo17 cells was higher, the decrease in pH values of the medium was higher, too, and more amount of CO2 was released. Changes in cell morphology and protein profiles were observed when B. megaterium IBBPo17 was grown in the presence of n-hexadecane on medium supplemented with yeast extract, proteose peptone, starch, or cellulose. A direct correlation between the growth of the cells and biosurfactant production was observed. Based on our data collected so far, B. megaterium IBBPo17 could be a promising candidate for the bioremediation of petroleum hydrocarbon polluted environments.
Acknowledgments
The study was funded by Project No. RO1567-IBB05/2019 from the Institute of Biology Bucharest of the Romanian Academy. The author is grateful to Ana Dinu, Alexandru Brînzan, and Gabriel Mihai Maria for their technical support.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Vary PS, Biedendieck R, Fuerch T, Meinhardt F, Rohde M, Deckwesr WD, et al. Bacillus megaterium-from simple soil bacterium to industrial protein production host. Appl Microbiol Biotechnol. 2007;76:957–67.10.1007/s00253-007-1089-3Search in Google Scholar PubMed
[2] Amin M, Rakhisi Z, Ahmady AZ. Isolation and identification of Bacillus species from soil and evaluation of their antibacterial properties. Avicenna J Clin Microb Infect. 2015;2:e23233.10.17795/ajcmi-23233Search in Google Scholar
[3] Brill E, Hannemann F, Zapp J, Brüning G, Jauch J, Bernhardt R. A new cytochrome P450 system from Bacillus megaterium DSM319 for the hydroxylation of 11-keto-β-boswellic acid (KBA). Appl Microbiol Biotechnol. 2014;98:1703–17.10.1007/s00253-013-5029-0Search in Google Scholar PubMed
[4] Kosaric N. Biosurfactants and their application for soil bioremediation. Food Technol Biotechnol. 2001;39:295–304.Search in Google Scholar
[5] Sifour M, Ouled-Haddar H, Aziz GM. Production of biosurfactants from two Bacillus species. Egypt J Aquat Res. 2005;31:142–8.Search in Google Scholar
[6] Silva RCFS, Almeida DG, Rufino RD, Luna JM, Santos VA, Sarubbo LA. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int J Mol Sci. 2014;15:12523–42.10.3390/ijms150712523Search in Google Scholar PubMed PubMed Central
[7] Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA. Biosurfactants: multifunctional biomolecules of the 21st Century. Int J Mol Sci. 2016;17:401. 10.3390/ijms17030401Search in Google Scholar PubMed PubMed Central
[8] Campos JM, Stamford TLM, Sarubbo LA, Luna JM, Rufino RD, Banat IM. Microbial biosurfactants as additives for food industries. Biotechnol Prog. 2013;29:1097–108.10.1002/btpr.1796Search in Google Scholar PubMed
[9] Darsa KV, Joseph Thatheyus A, Ramya D. Biodegradation of petroleum compound using the bacterium Bacillus subtilis. Sci Int. 2014;2:20–5.10.17311/sciintl.2014.20.25Search in Google Scholar
[10] Ogino H, Yasui K, Shiootani T, Ishihara T, Ishikawa H. Organic solvent-tolerant bacterium which secretes an organic solvent-stable proteolytic enzyme. Appl Env Microbiol. 1995;61:4258–62.10.1128/aem.61.12.4258-4262.1995Search in Google Scholar PubMed PubMed Central
[11] Vijayalakshmi A, Sushma K, Abha S, Chander P. Isolation and characterization of Bacillus subtilis KC3 for amylolytic activity. Int J Biosci Biochem Bioinf. 2012;2:336–41.10.7763/IJBBB.2012.V2.128Search in Google Scholar
[12] Trivedi N, Gupta V, Kumar M, Kumari P, Reddy CRK, Jha B. Solvent tolerant marine bacterium Bacillus aquimaris secreting organic solvent stable alkaline cellulase. Chemosphere. 2011;83:706–12.10.1016/j.chemosphere.2011.02.006Search in Google Scholar PubMed
[13] Stancu MM. Isolation and characterization of Bacillus megaterium IBBPo17 a solvent-tolerant bacterium. Waste Biomass Valori. 2019;10:3557–66.10.1007/s12649-018-0354-2Search in Google Scholar
[14] Sambrook J, Russel DW. Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001.Search in Google Scholar
[15] Kohno T, Sugimoto Y, Sei K, Mori K. Design of PCR primers and gene probes for general detection of alkane-degrading bacteria. Microbes Env. 2002;17:114–21.10.1264/jsme2.17.114Search in Google Scholar
[16] Nayarisseri A, Singh P, Singh SK. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation. 2018;14:304–14.10.6026/97320630014304Search in Google Scholar
[17] Jeon BY, Jung IL, Park DH. Mineralization of petroleum contaminated wastewater by co-culture of petroleum-degrading bacterial community and biosurfactant-producing bacterium. J Environ Protect. 2011;2:895–902.10.4236/jep.2011.27102Search in Google Scholar
[18] Sharma KM, Kumar R, Panwar S, Kumar A. Microbial alkaline proteases: optimization of production parameters and their properties. J Gen Eng Biotechnol. 2017;1:115–26.10.1016/j.jgeb.2017.02.001Search in Google Scholar
[19] Balba MT, Al-Awadhi N, Al-Daher R. Bioremediation of oil-contaminated soil: microbiological methods for feasibility assessment and field evaluation. J Microbiol Methods. 1998;32:155–64.10.1016/S0167-7012(98)00020-7Search in Google Scholar
[20] Neumann G, Veeranagouda Y, Karegoudar TB, Sahin O, Mausezahl I, Kabelitz N, et al. Cells of Pseudomonas putida and Enterobacter sp. adapt to toxic organic compounds by increasing their size. Extremophiles. 2005;9:163–8.10.1007/s00792-005-0431-xSearch in Google Scholar PubMed
[21] Veeranagouda Y, Karegoudar TB, Neumann G, Heipieper HJ. Enterobacter sp. VKGH12 growing with n-butanol as the sole carbon source and cells to which the alcohol is added as pure toxin show considerable differences in their adaptive responses. FEMS Microbiol Lett. 2006;254:48–54.10.1111/j.1574-6968.2005.00017.xSearch in Google Scholar PubMed
[22] Torres S, Pandey A, Castro GR. Organic solvent adaptation of Gram positive bacteria: applications and biotechnological potentials. Biotechnol Adv. 2011;29:442–52.10.1016/j.biotechadv.2011.04.002Search in Google Scholar PubMed
[23] Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol. 2013;11:157–68.10.1038/nrmicro2960Search in Google Scholar PubMed PubMed Central
[24] Priya JDA, Divakar K, Prabha MS, Selvam GP, Gautam P. Isolation, purification and characterisation of an organic solvent-tolerant Ca2+-dependent protease from Bacillus megaterium AU02. Appl Biochem Biotechnol. 2014;172:910–32.10.1007/s12010-013-0589-0Search in Google Scholar PubMed
[25] Gaur R, Tiwari S. Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol. 2015;15:19. 10.1186/s12896-015-0129-9.Search in Google Scholar PubMed PubMed Central
[26] Ahmetoglu N, Bekler FM, Acer O, Guven RG, Guve K. Production, purification and characterisation of thermostable metallo-protease from newly isolated Bacillus sp. KG5. Eurasia. J Biosci. 2015;9:1–11.Search in Google Scholar
[27] Anbu P. Characterization of solvent stable extracellular protease from Bacillus koreensis (BK-P21A). Int J Biol Mol. 2013;56:162–8.10.1016/j.ijbiomac.2013.02.014Search in Google Scholar PubMed
[28] Tiwari S, Shukla N, Mishra P, Gaur R. Enhanced production and characterization of a solvent stable amylase from solvent tolerant Bacillus tequilensis RG-01: thermostable and surfactant resistant. Sci World J. 2014;2014:972763. 10.1155/2014/972763.Search in Google Scholar PubMed PubMed Central
[29] Datta P, Tiwari P, Pandey LM. Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Bioresour Technol. 2018;270:439–48.10.1016/j.biortech.2018.09.047Search in Google Scholar PubMed
[30] Sharma S, Pandey LM. Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresour Technol. 2020;307:123261. 10.1016/j.biortech.2020.123261.Search in Google Scholar PubMed
[31] Thavasi R, Jayalakshmi S, Balasubramanian T, Banat IM. Production and characterization of a glycolipid biosurfactant from Bacillus megaterium using economically cheaper sources. World J Microbiol Biotechnol. 2008;24:917–25.10.1007/s11274-007-9609-ySearch in Google Scholar
© 2020 Mihaela Marilena Stancu, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”

