Abstract
Bone regeneration after trauma, pathologic and surgical procedures is considered a major medical challenge. Due to limitations in using conventional approaches, cell based regenerative strategies may provide an alternative option to address such issues. In the current study, we sought to determine the osteogenic potential of dental pulp stem cells (DPSCs) isolated from impacted 3rd molars. DPSCs were isolated from human dental pulp tissue (n=6) using explant culture. Growth characteristics of DPSCs were determined using plating efficiency, and the number and time of population doublings. After characterization, DPSCs were induced to differentiate into osteoblasts and were assessed using polymerase chain reactions (PCR) and histological analysis. Results indicated that DPSCs can be isolated from impacted human third molars, and that DPSCs exhibited typical fibroblastic morphology and excellent proliferative potential. In addition, morphological changes, histological analysis and expression of lineage specific genes confirmed osteogenic differentiation of DPSCs. In conclusion, DPSCs isolated from impacted 3rd molars have high proliferative potential and ability to differentiate into osteoblasts.
1 Introduction
The refinement of bone imperfection and defects due to trauma, pathological conditions and surgical procedures is a significant challenge [1]. Currently, allografts, autologous bone grafts or alloplastic materials are used to overcome the above-mentioned defects. However, certain limitations such as inadequate quantity of grafting bones, deformity, donor site morbidity, poor biocompatibility, immunogenicity and compromised vascularity restrict their use for optimum clinical conditions [1, 2]. Cell-based tissue engineering strategies can offer unique therapeutic alternative approaches to address these issues. Isolation of stem cells (SCs) from human adult and deciduous teeth has been reported in the last decade [3]. Adult stem cell sources, such as dental pulp (a soft living tissue within teeth) is a source of MSC (mesenchymal stem cells) like cells and may be used for the repair of large bone defects in dentoalveolar and craniofacial regions [4, 5, 6, 7, 8]. It has been observed that mesenchymal stem cells (MSCs) can differentiate into bone-like cells when the appropriate environment is provided. As dental pulp stem cells (DPSCs) exhibit MSC like characteristics, we aimed to isolate human dental pulp stem cells (hDPSCs) from the impacted 3rd molar and induce them to differentiate into osteoblasts.
The use of DPSCs can be very cost-effective, less invasive, convenient, and safer with fewer complications and no ethical issues as compared with other MSCs sources such as bone marrow, peripheral blood, and umbilical cord blood etc. [9]. These cells can easily be cultivated and expanded for autologous, as well as allogenic medical use. Further, these cells can be cryopreserved, are immunoprivileged and also when grafted into allogenic tissues exhibit anti-inflammatory abilities. Highly proficient interaction with biomaterials makes it a favorable choice for bone engineering [10].
In oral, and maxillofacial surgery, the restoration of function, such as facial expression, occlusion and mastication is exquisitely complex and the unconventional possibilities of enhancing bone regeneration should be considered [11, 12, 13]. Since MSCs can be induced to differentiate into osteoblasts, the current study aims to evaluate the osteogenic potential of DPSCs which exhibit MSC-like characteristics and is a new unconventional source of stem cells. In addition, cryopreservation of DPSCs will allow use of these autologous cells at the time of care when needed [14, 15].
The present study is intended to assess the osteogenic potential of MSCs harvested from dental pulp of extracted impacted 3rd molar. DPSCs were isolated from the pulp using an explant culture method. Cells were characterized, and their proliferation was determined using parameters such as plating efficiency, population doubling time and population doublings. DPSCs were then induced to differentiate into osteoblasts and their differentiation was assessed using various parameters. Results indicated that dental pulp of 3rd molars can be used for isolation of stem cells that are highly proliferative and demonstrate osteogenic potential. This study will provide opportunity for the use of extracted teeth as an autologous cell source for patient care to restore bone defects.
2 Material and methods
This in vitro experimental study was conducted in the Tissue Engineering and Regenerative Medicine Laboratory, Department of Biomedical Sciences at King Edward Medical University/Mayo Hospital Lahore. Six samples of human healthy third molars were removed for orthodontic or prophylactic measures in the Oral and Maxillofacial Surgery Department of Mayo Hospital Lahore. Wisdom teeth with deep caries, periapical and periodontal disease, sclerosed pulp chamber or donors positive for HCV, HBV and HIV were excluded from study.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the Institutional Review Board (IRB) and AS&RB (Advance Studies and Research Board) at King Edward Medical University, Lahore. The ethical approval letter is #188/ RC/ KEMU.
2.1 Isolation and Culturing of DPSCs
Before extraction of healthy impacted 3rd molars, each subject was evaluated for systemic and oral infections. Immediately after extractions, the tooth was placed in phosphate buffer saline (PBS) supplemented with 1% penicillin (100U/ml) and streptomycin (100ug/ml). Before the extraction of pulp, molars were washed with ethanol (70%). An incision was made at the enamel and cementum junction using a cylindrical turbine bur. Pulp from tooth was removed with sterilized forceps or barbed brochae [6]. Pulp tissue was then washed with PBS and minced into 1–2 mm fragments. Tissue fragments were cultured in α-MEM (Minimum Essential Medium-Alpha, Caisson MEL07-500ML) supplemented with 1% non-essential amino acids (Caisson cat# 02161008), 1% penicillin/streptomycin solution (Capricorn PS-B, cat# CP13-1019), 1% L-glutamine (Hyclone cat# SH30034.01), 1% sodium pyruvate (Hyclone cat# SH30239.01) and 10% fetal bovine serum (FBS, Biochorm cat# 0878C). Six-well plates containing tissue pieces were incubated at 37°C, and 5% CO2 in humid environment. After 24 hours, the medium was replaced and cell cultures were observed daily. When a sufficient number of colonies was observed, tissue pieces were removed and fresh medium was added. When cultures reached 80–90% confluence, they were dissociated using trypsin-EDTA, counted and plated for subsequent experiments. DPSCs, like MSCs were defined by their fibroblastic morphology and plastic adherent properties [16].
2.2 Growth Characteristics of Dental Pulp Stem Cells
Growth characteristics of DPSCs were determined by using parameters such as plating efficiency (PE), number of population doublings (PD) and population doubling time (PDT).
2.3 Plating Efficiency (PE)
To determine plating efficiency, DPSCs at passage 1 were plated in low numbers as described by Choudhery et al [15]. Briefly, passage 1 DPSCs were trypsinized and counted using a hemocytometer. DPSCs were plated at a concentration of 40 cells per cm2 (1000 cells per 25 cm2 culture flasks). Complete culture medium was added, and cultures were incubated at 370C at 5% CO2 under humid conditions. After two weeks, absolute methanol was used to fix resultant colonies. Colonies of cells were then stained with 0.1% crystal violet dye, and counted. The following formula was used for measuring the plating efficiency [15]:
Plating Efficiency (P.E.) = (No. of colonies counted/ No. of cells initially plated) × 100
2.4 Population Doubling and Doubling Time
The number of population doublings was determined by passaging the cells serially at a 1:10 dilution. Initial and final cell numbers were determined at each passage for up to ten passages. Population doublings (PD) and population doubling time (PDT) was calculated using the following formulae [14, 15]:
Where, “cPDs” represents cumulative population doublings, and “No” represents cell number plated, “N” represents cell number harvested, “DT” is doubling time and “CT” is total time in culture.
2.5 Osteogenic differentiation of DPSCs
For in vitro osteogenic differentiation, passage two DPSCs at a concentration of 5×104 cells were plated in 6-well tissue culture plates with complete expansion culture media. When DPSCs cultures became 80%-90% confluent, the primary culture medium was replaced by osteogenic induction medium (Osteolife LM-0023, LOT NO 05007) supplemented with 1% penicillin/streptomycin solution (Capricorn cat# PS-B, Cat no CP13-1019). Osteogenic differentiation medium was replaced with fresh media after every 3-5 days and experiments were terminated after 21 days.
2.6 Assessment of Osteogenic DifferentiationVon Kossa staining
The assessment of DPSCs differentiation into osteoblasts was determined by “Von Kossa” staining [10]. Briefly, after 14 and 21 days of induction, the osteogenic differentiation medium was discarded, and cells were fixed with 10% formalin for 15 minutes. Cells were stained using a commercially available Von Kossa staining kit (MASTER TEC STAIN KITS lot No. AAEWD005) according to manufacturer instructions. Stained cultures were observed by bright field microscopy and images were captured.
2.7 Alizarin red staining
In addition to Von Kossa Staining, the osteogenic induced cultures were also stained with alizarin red S (Cat no 22889). Differentiated DPSCs were fixed with 10% formalin for 15 minutes and were stained with 2% Alizarin Red S for 15-30 minutes. Cultures were then thoroughly washed with deionized water and viewed under light microscope.
2.8 Polymerase chain reaction (PCR)
To evaluate the expression of lineage specific genes, polymerase chain reaction was performed. Briefly, RNA was isolated after day 14 and 21 of osteogenic induction by TRIZOL (Invitrogen) and cDNA was synthesized using reverse transcriptase (Wizscript cDNA synthesis kit). For PCR, WizPure™ PCR 2X Master Mix was used. Sequences for the primer pairs and their product lengths (bp) are given in Table 1 [10].
List of used primers and their sequences
| Sr # | Genes markers | PCR product (bp) | PCR primer set (5’-3’) |
|---|---|---|---|
| 1 | Beta actin | 137 | Forward primer: CGCATGGGTCAGAAGGATTC |
| Reverse primers: TAGAAGGTGTGGTGCCAGATTT | |||
| 2 | OCN | 229 | Forward primer: CTCACACTCCTCGCCCTATT |
| Reverse primer: CCTCCTGCTTGGACACAAA | |||
| 3 | RUNX | 250 | Forward primer: ACCTTGACCATAACCGTCTTCAC |
| Reverse primer: TCCCGAGGTCCATCTACTGTAAC |
The following PCR conditions were used; denaturation for 5 minutes at 95°C; followed by annealing for 30 seconds at 55°C and extension at 72°C. PCR products were visualized by agarose gel electrophoresis.
2.9 Data analysis
Statistical analysis was performed using GraphPad Prism 6 (Graphpad Software, Inc). Plating efficiency, population doublings and doubling time were presented as mean ± standard deviation.
3 Results
3.1 Isolation and Characterization of DPSCs
MSCs from human dental pulp were successfully isolated from the pulp of third molars from all samples (n=6). After 4-6 days in culture, cells started to grow out of the small tissue segments and attached to the plastic surface. After approximately two weeks of culture, adherent cells became 70%-80% confluent and formed a monolayer within three weeks as shown in Figure 1. MSCs were characterized by their plastic adherent properties and fibroblastic morphology.

OPG, extract tooth and explant culture. A) An OPG showing impacted tooth. B) Extracted tooth in PBS. C) Pieces of pulp. D) MSCs started coming out of small DP pieces at 6-10 days. DP-MSCs at approximately 2 weeks after culture E) Cells formed monolayer after 14-21 days. OPG: orthopentomogram, DP: Dental pulp; MSCs: Mesenchymal stem cells.
3.2 Growth Characteristics of Dental Pulp Stem Cells
Growth characteristics of DPSCs were defined by parameters such as plating efficiency, number and time of population doublings. Plating efficiency was performed to evaluate the clonal expansion capacity of DPSCs. DPSCs grew into colonies with different densities within two weeks (Figure 2A). DPSCs from all donors produced colonies of different sizes (Figure 2B, C), and exhibited high plating efficiency (5.35% ± 1.18) as demonstrated in Figure 2D.

DPSC derived colonies and plating efficiency. A) showing colonies (low and high density) derived from DPSCs after 14 days. Colonies were stained with crystal violet dye. B) Low density colony of DPSCs and C) showing high density colony of DPSCs. D) Shows plating efficiency of all samples with different ages (n=6).
When cultured DPSCs reached 80-90% confluence, they were trypsinized and serially passaged at 1:10 dilution to determine cumulative population doublings up to passage ten. DPSCs showed excellent proliferative potential and expanded rapidly in culture. The number of cPDs of DPSCs culture for ten passages was 24.38 ± 1.11 (Figure 3E). Similarly, the population doubling time of DPSCs was 1.95 ± 0.080 days as shown in figure 3F.

Proliferative potential of DPSCs. Passaging of DPSCs A) passage 1 B) passage 3 C) passage7 D) passage 10 E) cumulative population doublings of all six samples with different age groups from p0 to p 10 F) population doubling time of DPSCs was calculated for all six samples from P0- P10 , which was 1.95days ± 0.080, DP-MSCs shows an ideal PDT proving that these cells are easily expendable.
3.3 Osteogenic Differentiation of DPSCs
In osteogenic induction medium, the morphology of DPSC was changed from fibroblast to polygonal. After 21 days of induction, newly differentiated osteoblasts grew in multiple layers and stained positive for “Von Kossa” as well as “Alizarin red”, confirming the differentiation of DPSCs into osteoblasts. Positive RUNX2 expression was also observed at day 14 and 21, and significant up regulation of osteocalcin (OCN) was also observed after day 14 as shown in figure 4. RUNX2 transcription factor is known to appear at the initiation of osteogenic differentiation, and is considered the earliest and most precise marker for bone formation. The OCN protein marker is considered a late phase marker of osteogenic differentiation.
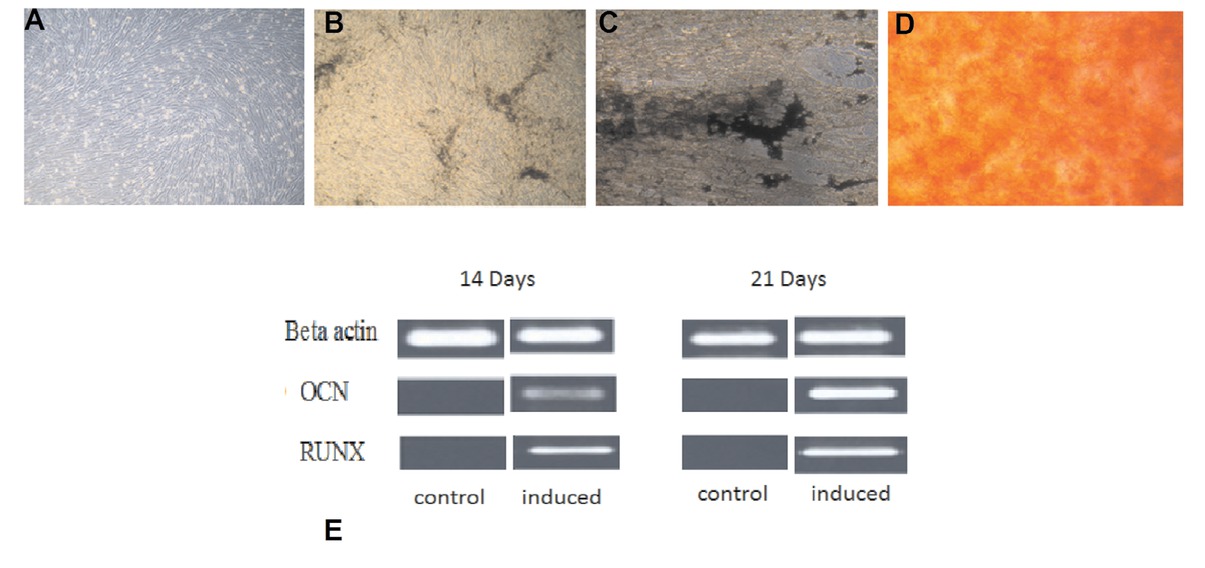
Differentiation potential of DPSCs. A) controlled, B, C) Von kossa staining at day 14 and 21 respectivly, confirming mineral deposition by newly formed osteoblasts D) alizarin red staining at day 21 E) showing positive results of RUNX2 and OCN at day 14 and 21 of osteogenic differentiation through polymerase chain reaction.
4 Discussion
The use of MSCs for regenerative medicine has garnered a significant consideration in recent years. Already, bone marrow stromal stem cells, adipose derived stem cells, cord blood and cord tissue have shown to be potent sources of MSCs [14, 15, 16, 17]. For the regeneration of bone, three components are required, namely; cells, scaffolds and growth factors [2]. Until now, BMSCs have been extensively exploited for osteogenic research and bone tissue restoration, but limited differentiation competence and proliferation potential makes it a less ideal source for MSCs [1, 2]. DPSCs possess a high proliferative potential and their source is readily available. Consequently, extracted wisdom teeth can serve as an ideal source of MSCs for tissue engineering. The development of the third molar teeth is a unique organogenesis event that occurs after the birth, being the last tooth to be developed at the age of six. Up until this point, prior to development initiation, embryonic tissue from the dental lamina remains dormant and undifferentiated with in the jawbones. Completion of crown formation occurs between the ages of 12-16 years and root development is completed at 18-25 years. These teeth can provide an optimal quality of dental pulp tissue with the presence of more cells and less fibers [4]. The main goal of this study was to isolate DPSCs from the dental pulp of healthy molars and differentiate these cells into osteoblasts. DPSCs can be isolated either by enzymatic digestion of pulp or by explant culture method [18, 20]. We selected explant culture method as it is inexpensive and gives a pure population of stem cells [21]. Within 3-4 days after primary culture, a certain number of cells (DPSCs) appeared in culture that resembled MSCs as defined by their spindle shape, fibroblastic morphology and plastic-adherent growth as documented previously [22, 23]. The International Society for Cellular Therapy (ISCT) defines the criteria for characterization of MSCs. According to this criteria, these cells exhibit positive expression of certain markers such as CD44, CD73, CD90, CD146 and CD166 while they are negative for hematopoietic lineage markers such as CD14, CD19, CD34 and CD45, as were exhibited by cells from other MSC sources [21, 22, 23]. Studies indicate that cells isolated by explant culture method are pure as compared to cells isolated by enzymatic digestion. DPSCs were isolated from the 3rd molars of donors of different ages. To evaluate growth characteristics, the cultures of DPSCs at 80%-90% were serially passaged up to passage 10. All resultant cultures of DPSCs showed high proliferative ability as indicated by their high number of population doublings (24.38 ±1.11) and low population doubling time (1.95 days ± 0.080). These results are in agreement with other similar studies by Huang et al [23], Suchánek J et al [24], Gronthos et al [22] and Kellner M et al [6]. DPSCs also exhibited high plating efficiency (5.35 ± 1.188), similar to the findings of Eslaminejad et al [25]. Stem cell numbers in dental pulp tissue when evaluated alongside other MSCs, i.e. bone marrow or adipose tissue, were shown to be less differentiated, bearing greater CFU-F as described by Gronthos et al [22] Huang et al [23] and El-Gendy et al [1]. The high proliferative potential of DPSCs indicate their potential use in applications such as tissue engineering and regenerative medicine.
Like MSCs from other sources, DPSC cells bear the capability to differentiate into multiple cell lineages under suitable in vitro conditions. In this in vitro experimental study, when DPSCs were cultured in media supplemented with dexamethasone, sodium β-glycerphosphate and L- ascorbic acid 2- phosphate for 21 days, they exhibited excellent differentiation into osteoblasts. We observed significant morphological changes from fibroblastic towards more plump and cuboidal shaped cells in induced cultures. In addition, newly differentiated osteoblasts formed organized ECM with calcium rich deposits in in vitro cultures that were detected by positive staining with “Von Kossa” and Alizarin Red. Results were similar to the findings documented by Graziano et al [26] and Kermani et al [13]. Expression of specific bone-forming gene markers was confirmed by PCR at day 14 and 21, to evaluate DPSC differentiation at the mRNA level. Over a period of 21 days, the transcription factor RUNX2 and osteocalcin (OCN) were up regulated in the monolayer of DPSCs under osteogenic conditions as normalized to control cultures of stem cell culture medium. The presence of RUNX2 was detected early during osteoblast differentiation, as an early osteogenesis marker at day 14, and was considered an important signal during osteoblast differentiation from DPSCs. RUNX2 also activates the expression of OCN which is a specific indicator of osteogenic differentiation. Previous, data indicated that OCN is produced only at the termination of osteogenic matrix maturation by matured osteoblasts, however, its presence can be detected as early as day 14 of osteogenic differentiation. These results were in line with other studies [27, 28, 29, 30].
Stem cell based bone tissue engineering holds a promising prospect in the treatment of medical and dental conditions [28, 29, 30]. Currently, in Pakistan, there is an increase in reported cases of trauma due to high-speed vehicle, bomb blast, violence, and cancers of the maxillofacial region. This study will help to lessen the resultant disabilities and promote the normalization of patients with better acceptance and functionality in the society. Excellent growth characteristics and osteogenic differentiation potential of human dental pulp stem cells in vitro suggests that DPSCs could be used for therapeutic purposes where restoration of bone is a challenge. Cryopreservation of autologous DPSCs would allow the banking of stem cells in anticipation with future therapies. In conclusion, the impacted 3rd molar is a rich and easily accessible source of DPSCs. Isolated DPSCs possess a high proliferative potential and ability to differentiate into osteoblasts and therefore could be used for regenerative medicine applications in oral and maxillofacial surgery.
Acknowledgments
The funding for this current study was provided by King Edward Medical University, Lahore, Pakistan. We are grateful to Prof Dr. Riaz Ahmed Warraich, for his unwavering support through this study.
Conflict of interest: Authors state no conflict of interest.
References
[1] El-Gendy R, Yang XB, Newby PJ, Boccaccini AR, Kirkham J. Osteogenic Differentiation of Human Dental Pulp Stromal cells on 45S5 BioglassR Based Scaffolds in Vitro and In Vivo. Tissue Eng. 2013;19(5, 6):707-1510.1089/ten.tea.2012.0112Search in Google Scholar
[2] Ito K, Yamada Y, Nakamura S, Ueda M. Osteogenic Potential of Effective Bone Engineering Using Dental Pulp Stem Cells, Bone Marrow Stem Cells and Periosteal Cells for Osseo integration of Dental Implants. Int J Oral Maxillofac Implants. 2011;26(5): 947-54.Search in Google Scholar
[3] Lizier NF, Kerkis A, Gomes CM, Hebling J, Oliveira CF, Caplan AI, et al. Scaling-up of dental pulp stem cells isolated from multiple niches. PLOS One. 2012;7(6):e39885. doi: 10.1371/journal.pone.0039885.10.1371/journal.pone.0039885Search in Google Scholar PubMed PubMed Central
[4] Vishwanath VR, Nadig RR, Nadig R, Prasanna JS, Karthik J, Pai VS. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: An in vitro study. J Conserv Dent. 2013;16 (5):423-8.10.4103/0972-0707.117509Search in Google Scholar PubMed PubMed Central
[5] Didilescu AC, Rusu MC, Nini G. Dental pulp as a stem cell reservoir. Rom J Morphol Embryol. 2013;54(3):473-8.Search in Google Scholar
[6] Kellner M, Steindorff MM, Strempel JF, Winkel A, Kuhnel MP, Stiesch M. Differences of isolated stem cells depend on donor age and the consequences for autologous tooth replacement. Arch Oral Biol. 2014;59(6):559-67.10.1016/j.archoralbio.2014.02.014Search in Google Scholar PubMed
[7] Ma L, Makino Y, Yamaza H, Akiyama K, Hoshino Y, Song G, et al. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS One. 2012;7(12): e51777. doi: 10.1371/journal. pone.0051777.10.1371/journal.pone.0051777Search in Google Scholar PubMed PubMed Central
[8] Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and application in regenerative medicine. J Tissue Eng Regen Med. 2015;9(11):1205-16.10.1002/term.1899Search in Google Scholar PubMed
[9] Shaikh RA. Therapeutic Potential of Stem Cells in Regenerative Dentistry; a Review of Literature. IDJSR. 2013;1(4):22-30.Search in Google Scholar
[10] Li JH, Liu DY, Zhang FM, Wang F, Zhang WK, Zhang ZT. Human dental pulp stem cell is a promising autologous seed cell for bone tissue engineering. Chin Med J. 2011;124(23):4022-28.Search in Google Scholar
[11] d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, et al. Human mendible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75-83.10.22203/eCM.v018a07Search in Google Scholar PubMed
[12] Huang CE, Hu FW, Yu CH, Tsai LL, Lee TH, Chou MY, et al. Concurrent expression of oct4 and nanog maintains mesenchymal stem-like property of human dental pulp cell. Int J MolSci. 2014;15(10):18623-39.10.3390/ijms151018623Search in Google Scholar PubMed PubMed Central
[13] Kermani S, Megat Abdul Wahab R, Zarina Zainol Abidin I, Zainal Ariffin Z, Senafi S, Hisham Zainal Ariffin S. Differentiation Capacity of Mouse Dental Pulp Stem Cell into Osteoblasts and Osteoclasts. Cell J. 2014;16(1):31-42.Search in Google Scholar
[14] Choudhery MS, Badowski M, Muise A, Harris DT. Utility of cryopreserved umbilical cord tissue for regenerative medicine. Current Stem Cell Research & Therapy. 2013;8(5):370:380.10.2174/1574888X11308050004Search in Google Scholar
[15] Choudhery MS, Badowski M, Muise A, Harris DT. Cryopreservation of whole adipose tissue for future use in regenerative medicine. Journal of Surgical Research. 2014; 187(1):24-35.10.1016/j.jss.2013.09.027Search in Google Scholar PubMed
[16] Mahmood R, Choudhery MS, Mehmood A, Khan SN, Riazuddin S. In vitro differentiation potential of human placenta derived cells into skin cells. Stem Cells International; 2015.10.1155/2015/841062Search in Google Scholar PubMed PubMed Central
[17] Fatima Q, Chaudhry N, Choudhery MS. Umbilical cord tissue derived mesenchymal stem cells can differentiate into skin cells. Open Life Sciences. 2018;13(1):544-552.10.1515/biol-2018-0065Search in Google Scholar PubMed PubMed Central
[18] Kim BC, Bae H, Kwon IK, Lee EJ, Park JH, Khademhosseini A, et al. Osteoblastic/Cementoblastic and Neural Differentiation of Dental Stem Cells and Their Applications to Tissue Engineering and Regenerative Medicine. Tissue Engineering Part B: Reviews. 2012;18(3):235–244.10.1089/ten.teb.2011.0642Search in Google Scholar
[19] Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13625-3010.1073/pnas.240309797Search in Google Scholar PubMed PubMed Central
[20] Ricordi C, Tzakis AG, Carroll PB, Zeng YJ, Rilo HL, Alejandro R, et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation. 1992;53(2):407-14.10.1097/00007890-199202010-00027Search in Google Scholar PubMed PubMed Central
[21] Choudhery MS, Badowski M, Muise A, Harris DT. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy. 2013;15(3):330-343.10.1016/j.jcyt.2012.11.010Search in Google Scholar PubMed
[22] Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Anti-aging protective effect of L-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell. 2018;54:105–113.10.1016/j.tice.2018.08.012Search in Google Scholar PubMed
[23] Fathi E, Farahzadi R, Valipour B, Sanaat D. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS One 2019;14(4):e0215678.10.1371/journal.pone.0215678Search in Google Scholar PubMed PubMed Central
[24] Suchánek J, Soukup T, Ivancaková R, Karbanová J, Hubková V, Pytlík R, et al. Human dental pulp stem cells--isolation and long term cultivation. Acta Medica (Hradec Kralove). 2007;50(3):195-201.10.14712/18059694.2017.82Search in Google Scholar
[25] Eslaminejad MB, Vahabi S, Shariati M, Nazarian H. In vitro Growth and Characterization of Stem Cells from Human Dental Pulp of Deciduous Versus Permanent Teeth. J Dent (Tehran). 2010;7(4):185-95.Search in Google Scholar
[26] Graziano A, d’Aquino R, Laino G, Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4(1):21-6.10.1007/s12015-008-9015-3Search in Google Scholar
[27] Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, et al. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013;353(1):65-78.10.1007/s00441-013-1630-xSearch in Google Scholar PubMed
[28] d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162-71.10.1038/sj.cdd.4402121Search in Google Scholar PubMed
[29] Wang Y, Yan M, Wang Z, Wu J, Wang Z, Zheng Y, et al. Dental pulp stem cells from traumatically exposed pulps exhibited an enhanced osteogenic potential and weakened odontogenic capacity. Arch Oral Biol. 2013; 58(11):1709-17.10.1016/j.archoralbio.2013.09.001Search in Google Scholar PubMed
[30] Bressan E, Ferroni L, Gardin C, Pinton P, Stellini E, Botticelli D, et al. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS One. 2012; 7(11):e49146.10.1371/journal.pone.0049146Search in Google Scholar PubMed PubMed Central
© 2020 Sadia Awais, et al. published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”

