Abstract
Viral myocarditis (VMC) caused by coxsackievirus B3 (CVB3) infection is a life-threatening disease. The cardiac damage during VMC is not mainly due to the direct cytotoxic effect of the virus on cardiomyocytes but mostly involves the induction of immune responses. Integrin CD11b plays an important role in immune response, for instance, in the induction of Th17 cells. However, the role of CD11b in the pathogenesis of VMC remains largely unknown. In the present study, a mouse model of VMC was established by CVB3 infection and CD11b was knocked down in the VMC mice by transfection with siRNA-CD11b. The expression of CD11b and IL-17 in heart tissues, frequency of Th17 cells in spleen tissues and serum IL-17 levels were measured using quantitative RT-PCR, Western blot, immunohistochemistry, flow cytometry and ELISA. Results showed that CVB3 infection caused the pathological changes in heart tissues with the increases in the following indexes: expression of CD11b and IL-17 in heart tissues, frequency of Th17 cells in spleen tissues and serum IL-17 levels. The expression of CD11b was positively correlated with IL-17 expression in heart tissues. Depletion of CD11b attenuated the damage caused by CVB3 and decreased the frequency of Th17 cells in spleen tissues as well as in IL-17, IL-23 and STAT3 expression in heart tissues. In summary, our findings reveal that disruption of CD11b function reduced CVB3-induced myocarditis, suggesting that CD11b may be a novel therapeutic target for VMC.
1 Introduction
Viral myocarditis (VMC), which is primarily induced by coxsackievirus B (CVB), is a life-threatening disease. Massive replication of CVB and other viruses in cardiomyocytes triggers the strong host immune response characterized by the infiltration of immune cells and secretion of inflammatory factors, which result in myocardial damage [1,2]. Coxsackievirus B3 (CVB3) is a member of the positive-stranded RNA virus family, Picornaviridae, which has been reported to have recently caused 5–20% of cases of VMC [3,4,5]. Furthermore, CVB3 infection results in irreversible cytopathic effects at the cellular level and cardiac damage at the tissue level [4,6]. Th17 cells are a newly discovered group of CD4+ T-assisted cells. Studies have shown that Th17 cells mediate the tissue damage of a variety of autoimmune diseases [7,8]. Previous studies suggest that Th17 cell subsets and their effector IL-17 participate in the pathogenesis of VMC, because of the accumulation of Th17 cells throughout the course of VMC and the role of IL-17 in raising a variety of inflammatory factors, such as TNF-α, IL-1 and IL-6 [9,10,11].
Integrin is the main family of cell surface receptors formed by two subunits of α (120–185 kD) and β (90–110 kD). Integrin family proteins are classified according to the associated β-subunit: the β1 (CD29), β2 (CD18) and β3 (CD61). Members of the β2 (CD11/CD18) integrin family represent the most abundant integrins, which is designated by the different α-subunits such as lymphocyte function-associated antigen 1 (LFA-1; CD11a/CD18), Mac-1 (CD11b/CD18) and gp150/95 (CD11c/CD18). Integrin-mediated immune cell migration and cell-to-cell interactions are critical in immune response. β2 integrin (CD11/CD18) plays an important role in both immune response and inflammation. CD11b is widely expressed in a variety of immune cell subsets, such as dilated myocardial cells (DMCs), monocytes, macrophages, granulocytes and natural killer cells, and involved in many biological processes such as cell activation, cell chemotaxis, cytotoxicity and phagocytosis [12,13,14].
The regulatory effect of CD11b on Th17 cell functions has been reported by a few studies. In a mice model of autoimmune encephalomyelitis, CD11b+ myeloid suppressor cells (MDSCs) promoted the differentiation of naive CD4+ T-cell precursors to Th17 cells resulting in increased production of IL-17A [15]. This promoting effect of CD11b+ MDSCs was dependent on IL-1 receptor on CD4+ T cells. Selective depletion of MDSCs using gemcitabine attenuated the severity of autoimmune encephalomyelitis with reduced Th17 cells and inflammatory cytokines (IL-17A and IL-1β) in the lymphoid tissues and spinal cords [15]. However, in some antigen-presenting cells (e.g., dendritic cells, DMCs), CD11b+ likely plays an inhibitory role in CD4+ T activity and the differentiation to Th17 cells, because using the antibody against CD11b+ prevents the inhibition of Th17 differentiation by DMCs, while ligation of CD11b on DMCs constricted Th17 cell expansion within the CD4+ T cells [16,17,18]. These results suggest that the regulatory effect of CD11b on Th17 cells is probably related to the cells that express CD11b.
In this study, we aimed to investigate the potential roles of CD11b in CVB3-induced myocarditis. The expression of CD11b and IL-17 in heart tissues, frequency of Th17 cells in spleen tissues and serum IL-17 levels were determined in CVB3-infected mice. Moreover, this study knocked down CD11b in mice to determine whether CD11b was involved in the pathogenesis of CVB3-induced myocarditis.
2 Materials and methods
2.1 Animal treatments
In this study, 50 male BALB/c mice (aged 6- to 8-week-old) were purchased from the Animal Experiment Center of First Affiliated Hospital of Guangxi Medical University (Guangxi, China). The mice were randomly assigned to three groups as follows: control group (n = 20), CVB3 group (n = 20) and CVB3 + CD11b knockdown group (n = 20). In the last two groups, CVB3 infection was performed as described by Li et al. [19]. Briefly, CVB3 (Nancy strain; ATCC, Manassas, VA, USA) was maintained by passage in HeLa cells (CCL-2; ATCC). The viral titer was determined using a 50% tissue culture infectious dose (TCID50) assay on HeLa cell monolayers and calculated by the Reed–Muench method. Mice were infected with an intraperitoneal injection of 0.1 mL of phosphate-buffered saline (PBS; Thermo Fisher Scientific, Waltham, MA, USA) containing 103 TCID50 of the virus. In the CVB3 + CD11b knockdown group, mice were injected with siRNA-targeting CD11b (GenePharma, Shanghai, China) and EntransterTM-in vivo transfection reagent (Engreen Biosystem Co., Ltd., Beijing, China) from the vena caudalis, according to the method previously described [20], before CVB3 injection. Half the mice in each group underwent the following tests 1 week after the treatments; the remaining mice in each group underwent the following tests 2 weeks after the treatments.
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and has been approved by the National Institutes of Health’s Code of Ethics the Animal Use [21] and Ethics Committee of First Affiliated Hospital of Guangxi Medical University (Guangxi, China). Mice experiment was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, P. R. China, 1998).
2.2 Histopathology and myocarditis scoring
Heart tissues were collected from the mice 7 days after CVB3 infection. The tissues were fixed in 10% formalin and embedded in paraffin. Sections (5 µm thick) were cut and stained with hematoxylin and eosin (H&E). The extent of the myocardial lesions was quantified and scored for severity as follows: 0 = no inflammation; 1 = 1–5 distinct mononuclear inflammatory foci, with the involvement of 5% or less of the cross-sectional area; 2 = more than 5 distinct mononuclear inflammatory foci or the involvement of over 5% but not over 20% of the cross-sectional area; 3 = diffuse mononuclear inflammation involving over 20% of the area, without necrosis; and 4 = diffuse inflammation with necrosis. The analysis was performed in a double-blinded manner by a trained pathologist [22].
For immunohistochemistry (IHC) staining, the slides were routinely deparaffinized and hydrated. After inactivating endogenous peroxidase in 3% hydrogen peroxide, slides were then retrieved in citric acid buffer (pH 6.0) by microwave for 15 min. Slices were blocked in normal goat serum for 30 min at room temperature and incubated with anti-CD11b antibody (1:1,000; Abcam, Shanghai, China) overnight at 4°C. The slides were then washed with tris buffered saline plus tween (TBST) and incubated with appropriate secondary antibody for 2 h at 37°C. The sections were then washed with TBST and stained by using DAB Detection kit (Solarbio, Beijing, China). Finally, the sections were counterstained with hematoxylin.
2.3 Quantitative RT-PCR (qRT-PCR) analysis
Total RNA was extracted from the tissue and cell samples using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). Then 2 µg of the total RNA were reversely transcribed by using the SuperScript RT kit from Invitrogen. qRT-PCR was performed using the ABI PRISM7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with PowerUp™ SYBR® Green Master Mix (Thermo Fisher Scientific). The primer information is given in Table 1. The 2−ΔΔCt method was used to analyze the mRNA expression levels relative to that of the control (GAPDH) mRNA [23].
Primers used for PCR assay
| Gene name | Direction | Sequence (5′–3′) | Tm (°C) | Amplification size |
|---|---|---|---|---|
| IL-17 | Forward | TCCCACGAAATCCAGGATGC | 62 | 75 |
| Reverse | GGATGTTCAGGTTGACCATCAC | 60.9 | ||
| CD11b | Forward | GCCTTGACCTTATGTCATGGG | 60.4 | 185 |
| Reverse | CCTGTGCTGTAGTCGCACT | 61.6 | ||
| IL-23 | Forward | CTCAGGGACAACAGTCAGTTC | 60 | 119 |
| Reverse | ACAGGGCTATCAGGGAGCA | 62 | ||
| STAT-3 | Forward | CAGCAGCTTGACACACGGTA | 62.4 | 150 |
| Reverse | AAACACCAAAGTGGCATGTGA | 60.6 | ||
| GAPDH | Forward | GGAGCGAGATCCCTCCAAAAT | 61.6 | 197 |
| Reverse | GGCTGTTGTCATACTTCTCATGG | 60.9 |
2.4 Flow cytometry
The percentage of Th17 cells in CD4+ T cells was detected according to the method reported in the previous study [24]. Splenocytes were isolated and suspended in RPMI 1640 containing 10% fetal bovine serum, and red blood cells were lysed by incubation for 3 min in ACK lysis buffer (Tiangen, Shanghai, China). The cells were collected and resuspended at a density of 1.0 × 106 cells per mL. The cells were then stimulated for 4 h with 50 ng phorbol 12-myristate 13-acetate and 1 µg ionomycin per mL (Sigma-Aldrich, St. Louis, MO, USA), and cytokine secretion was blocked with 10 lg/mL brefeldin A (Sigma-Aldrich) at 37°C under 5% CO2 in a 24-well culture plate (Corning Costar, Corning, NY, USA) in RPMI 1640 medium supplemented with 100 U penicillin and 100 µg streptomycin per mL and 10% fetal bovine serum. Surface markers were stained with PE-labeled anti-mouse CD4 antibody (Biolegend). After the cells were washed, fixed, and permeabilized according to the manufacturer’s instructions (Biolegend, Beijing, China), they were stained intracellularly with an APC-conjugated anti-mouse IL-17A antibody (eBioscience, San Diego, CA, USA). After incubation at 4°C for 30 min, the samples were washed in staining buffer and measured by flow cytometry on a FACSCalibur cell sorter. The data were analyzed using CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
2.5 Western blot assay
Western blot was performed as previously described [25]. The heart tissues were homogenized on ice for 30 min in a RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitor. The lysates were heated at 95°C for 5 min and loaded on 10% gels (Bio-Rad, San Diego, CA, USA) for sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After electrophoretic separation, the proteins were transferred onto 0.2 µm nitrocellulose membranes (Amersham, Germany), blocked with 5% nonfat milk (in Tris-buffered saline [TBS] + 0.01% Tween) and incubated overnight at 4°C with the following primary antibodies: anti-CD11b (1:1,000; Abcam) and anti-GAPDH (1:1,000; Abcam). The horseradish peroxidase (HRP)-linked secondary antibodies (1:2,000; Abcam) and ECL kit (WBLUR0100; Millipore Corporation, Billerica, MA, USA) were further used to visualize the blots in the membrane. Proteins were finally visualized by an LAS-4000 mini system (Fujifilm, Japan). The intensity of protein bands was quantified with Quantity one software. The proteins of tissue expression were standardized to GAPDH levels.
2.6 ELISA
Serum IL-17A concentration was measured using ELISA (R&D Systems, Beijing, China), according to the manufacturer’s instructions. In brief, plates were coated with the diluted capture antibody (100 mL/well) and incubated overnight at 4°C. The serum samples were added to triplicate wells and incubated at 37°C for 2 h. After the samples were washed, the biotinylated detection antibody was added for 1 h, followed by 100 mL of streptavidin-conjugated HRP. TMB substrate (eBioscience) was added to each well. The absorbance was measured at 450 nm.
2.7 Statistical analysis
The software GraphPad 8.0 was used to analyze the data. All data were repeated as an independent experiment three times and presented as mean ± standard deviation(SD). Based on ANOVA, p < 0.05 was regarded as significantly different among the experimental groups.
3 Results
3.1 Increased CD11b expression during CVB3-induced mouse myocarditis
Male BALB/c mice were injected intraperitoneally with 103 TCID50 of CVB3 to construct an acute VMC model. Histological analysis showed higher myocarditis scores in cardiac tissues 1 and 2 week(s) after the CVB3 injection (p < 0.001; Figure 1a and b). The expression of CD11b was examined after CVB3 infection. As shown in Figure 1c and d, CD11b increased in the cardiac tissues in the first and second weeks (p < 0.05). These results indicated that CD11b expression increased in the cardiac tissues during CVB3-induced mouse myocarditis.
![Figure 1 Expression of CD11b was increased during CVB3-induced myocarditis in mice. (a) H&E staining of heart tissue sections was prepared at 1 and 2 week(s) postinfection. Cardiac inflammation was indicated by the arrows (magnification: ×200, scan bar: 50 µm). (b) The degree of the myocardial lesions was quantified and scored according to the method described in previous literature [22]. (c and d) CD11b expression in cardiac tissues at the first and second week(s) after the infection was examined by Western blotting. Data are shown as mean ± SD. *p < 0.05 and ***p < 0.001 compared with the control group.](/document/doi/10.1515/biol-2020-0085/asset/graphic/j_biol-2020-0085_fig_001.jpg)
Expression of CD11b was increased during CVB3-induced myocarditis in mice. (a) H&E staining of heart tissue sections was prepared at 1 and 2 week(s) postinfection. Cardiac inflammation was indicated by the arrows (magnification: ×200, scan bar: 50 µm). (b) The degree of the myocardial lesions was quantified and scored according to the method described in previous literature [22]. (c and d) CD11b expression in cardiac tissues at the first and second week(s) after the infection was examined by Western blotting. Data are shown as mean ± SD. *p < 0.05 and ***p < 0.001 compared with the control group.
3.2 Expression of CD11b and its correlations with the IL-17 expression
We analyzed the frequencies of Th17 cells in spleen as well as serum levels of the proinflammatory cytokines in CVB3-infected mice. Spleen tissues showed markedly increased frequencies of Th17 cells in mice after infection with CVB3 for 1 (p < 0.05) and 2 week(s) (p < 0.01; Figure 2a and b). CVB3 infection for 1 and 2 week(s) also caused the elevation of serum IL-17 level (Figure 2c; all p < 0.01). The levels of CD11b and IL-17 mRNA were significantly increased in cardiac tissues after infection with CVB3 for 1 and 2 week(s) (Figure 2d; all p < 0.05). As shown in Figure 2e, the mRNA of CD11b expression was significantly positively correlated with IL-17 mRNA expression in cardiac tissues after CVB3-infection for 1 (r = 0.5761, p < 0.01) and 2 week(s) (r = 0.6237, p < 0.01).
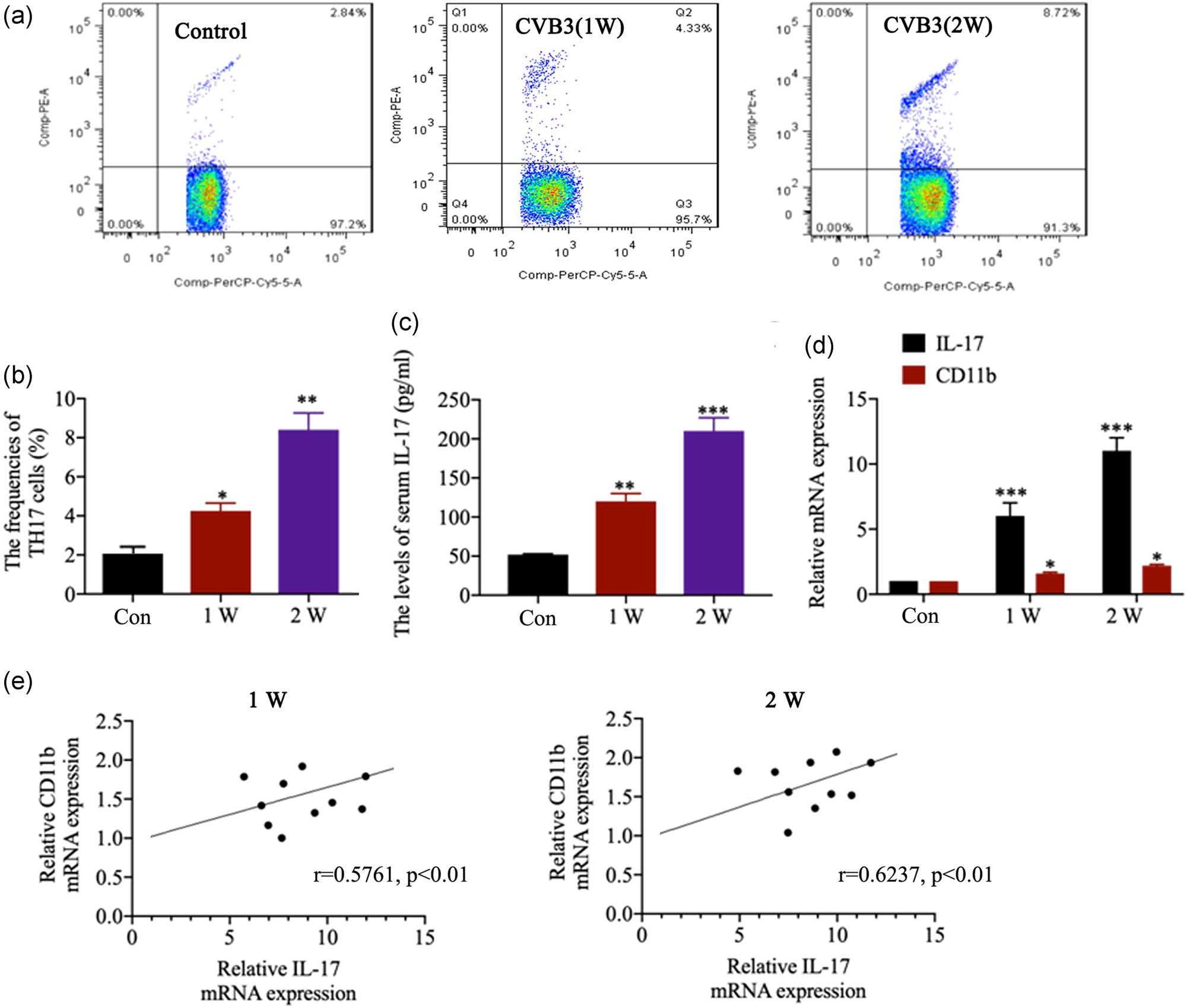
Expression of CD11b and its correlations with the IL-17 expression. (a and b) The frequencies of Th17 cells in CD4+ cells in spleen in control and CVB3 mice were analyzed by flow cytometry. (c) The serum IL-17 level in mice was evaluated by ELISA at the first and second week(s) after the infection. (d) The relative IL-17 and CD11b mRNA expression in heart tissues was evaluated by qRT-PCR after CVB3 infection for 1 and 2 weeks. (e) Correlation between CD11b and IL-17 mRNA expression in heart tissues. Data are shown as mean ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the control group.
3.3 CD11b deficiency ameliorated CVB3-induced myocarditis in mice
To determine the role of CD11b in CVB3-induced myocarditis, this study knocked down CD11b in mice. IHC analysis demonstrated that siRNA-CD11b inhibited the increase in CD11b in heart tissues in CVB3-infected mice (Figure 3a). As indicated by H&E staining, the pathological scores of heart sections in the CD11b-deficient group were lower or less than those in the CVB3 group (p < 0.05 in the first week and p < 0.01 in the second week; Figure 3b). Although the levels of serum IL-17 in the CVB3 groups increased dramatically (p < 0.001), CD11b knockdown decreased the levels of serum IL-17 compared to those in CVB3 groups (p < 0.05 or p < 0.01). These data indicate that CD11b deficiency ameliorated CVB3-induced myocarditis in mice.
![Figure 3 CD11b deficiency ameliorated CVB3-induced myocarditis in mice. Before CVB3 injection, mice were injected with siRNA-targeting CD11b and EntransterTM-in vivo transfection reagent from the vena caudalis to knock down CD11b in mice. (a) CD11b expression in heart tissues was assessed using IHC. CVB3: CVB3 injection, anti-CD11b: CD11b knock down. (b) H&E staining of heart tissue sections was prepared at 1 and 2 week(s) postinfection (magnification: ×200, scan bar: 50 µm). The degree of the myocardial lesions was quantified and scored according to the method described in previous literature [22]. (c) The serum IL-17 level in mice was evaluated by ELISA at the first and second week(s) after the infection. Data are shown as mean ± SD. **p < 0.01 and ***p < 0.001 compared with the control group, #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with the CVB3 group.](/document/doi/10.1515/biol-2020-0085/asset/graphic/j_biol-2020-0085_fig_003.jpg)
CD11b deficiency ameliorated CVB3-induced myocarditis in mice. Before CVB3 injection, mice were injected with siRNA-targeting CD11b and EntransterTM-in vivo transfection reagent from the vena caudalis to knock down CD11b in mice. (a) CD11b expression in heart tissues was assessed using IHC. CVB3: CVB3 injection, anti-CD11b: CD11b knock down. (b) H&E staining of heart tissue sections was prepared at 1 and 2 week(s) postinfection (magnification: ×200, scan bar: 50 µm). The degree of the myocardial lesions was quantified and scored according to the method described in previous literature [22]. (c) The serum IL-17 level in mice was evaluated by ELISA at the first and second week(s) after the infection. Data are shown as mean ± SD. **p < 0.01 and ***p < 0.001 compared with the control group, #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with the CVB3 group.
3.4 CD11b deficiency suppressed Th17 cell responses
As shown in Figure 4a and b, CVB3 mice showed markedly increased frequencies of Th17 cells in spleen tissues (p < 0.01), while CD11b depletion inhibited the increase in Th17 cells in CVB3 mice (p < 0.01). The levels of cardiac mRNA of the IL-17, IL-23 and STAT-3 expression were elevated significantly in CVB3-infected mice as compared to the control mice (p < 0.01 or p < 0.001; Figure 4c), while the IL-17, IL-23 and STAT-3 mRNA expression markedly reduced in the CD11b-deficient mice compared with the CVB3 mice, especially in the second week (p < 0.05 or p < 0.01). These data indicate that CD11b deficiency inhibited CVB3-induced Th17 cells and inflammation in mice.
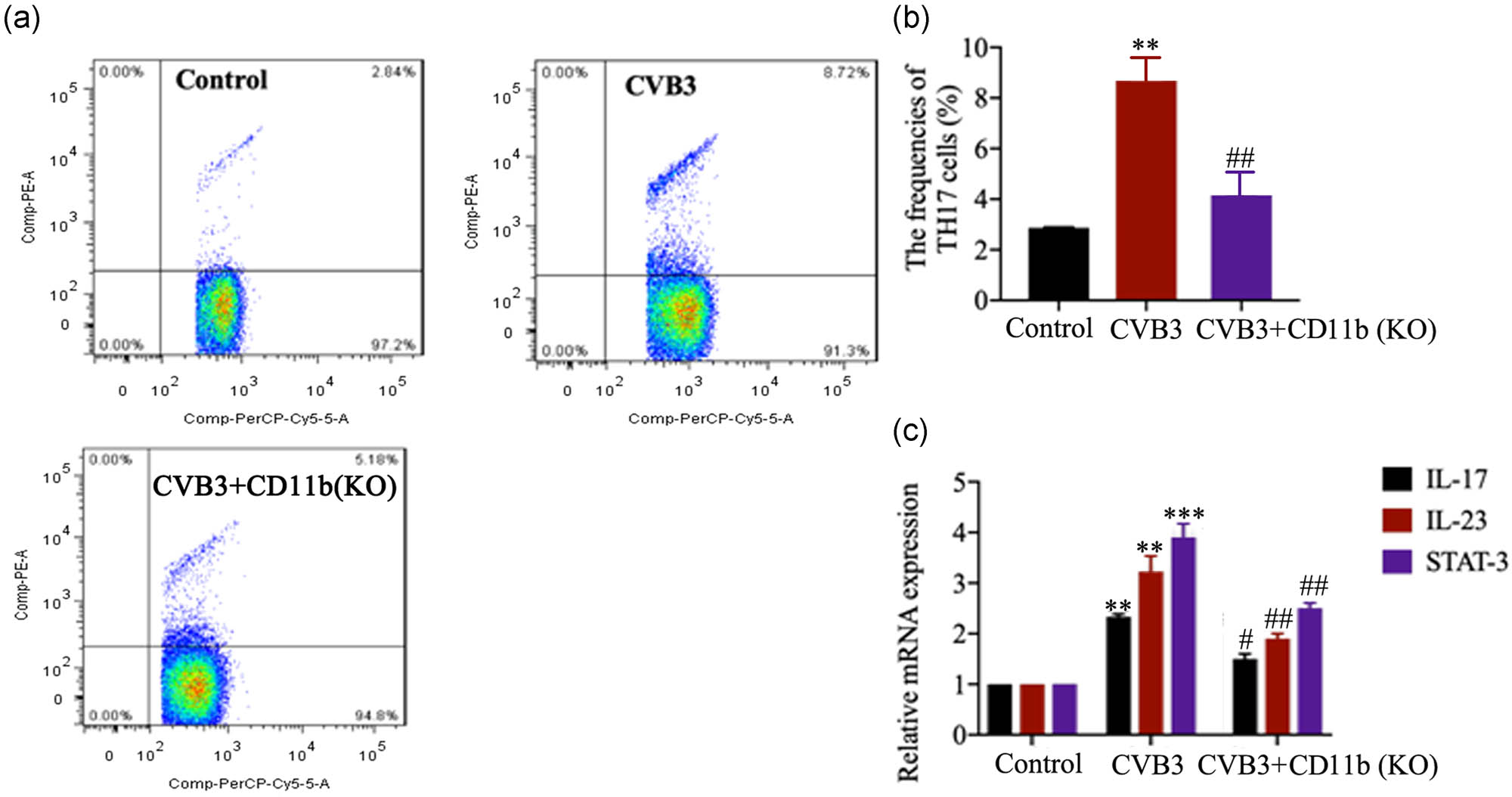
CD11b deficiency suppressed Th17 cell response. Before CVB3 injection, mice were injected with siRNA-targeting CD11b and EntransterTM-in vivo transfection reagent from the vena caudalis to knock down CD11b in mice. (a and b) The frequencies of Th17 cells in CD4+ cells in spleen in control and CVB3 mice were analyzed by flow cytometry 2 weeks after CVB3 injection. (c) The relative IL-17, IL-23 and STAT-3 mRNA expression in heart tissues was evaluated by qRT-PCR. Data are shown as mean ± SD. **p < 0.01 and ***p < 0.001 compared with the control group, #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with the CVB3 group.
4 Discussion
Understanding the pathogenesis of VMC is critical to improve the treatment strategies. The CVB3-infected mouse model summarizes many of the functional and pathological changes in the human VMC disease. In the myocardium of CVB3-infected mice, there is an inflammatory reaction characterized by the infiltration and expression of inflammatory mediators of lymphoid and myeloid cells which are believed to be responsible for the pathogenesis of VMC [26]. The present study showed that CVB3 infection caused the pathological change in the heart tissues of mice with the upregulation of CD11b. The use of siRNA-CD11b to block CD11b function significantly attenuated mouse CVB3-induced myocarditis with the reduction in proinflammatory factors and signaling molecules including IL-17, IL-23 and STAT-3. These data suggested that CD11b may be a therapeutic target for CVB3-induced VMC.
Numerous studies have suggested that cardiac damage during VMC is not mainly due to the direct cytotoxic effect of the virus on cardiomyocytes but mostly involves the induction of immune response [27]. Studies have identified that the differentiation of CD4+ cells to Th17 cells plays important role in VMC pathogenesis [9,10,11]. The accumulation of Th17 cells throughout the course of VMC and the release of IL-17 by Th17 cells caused the overproduction of a variety of inflammatory factors, such as TNF-α, IL-1 and IL-6, and consequent inflammatory damage of heart tissues. This study showed that the frequencies of Th17 cells in CD4+ cells in spleen tissues were dramatically increased after CVB3 infection. In addition, the serum IL-17 level and cardiac IL-17 mRNA expression levels were increased as well, suggesting the infiltration of spleen-derived Th17 cells to heart tissues. A recent study proposed that VMC pathology is driven by IL-17-producing CD4+ T cells because the severity of myocarditis in T-bet-knockout mice was associated with increased IL-17 expression in the heart [28].
Integrin CD11b plays an important role in inducing the adhesion of immune cells to endothelium. Although this study did not provide direct evidence that integrin CD11b facilitated the infiltration of Th17 cells to heart tissues, we found that CD11b expression was positively correlated with IL-17 expression in the heart tissues. Depletion of CD11b also decreased the frequencies of Th17 cells in CD4+ cells in spleen tissues, the serum IL-17 level and cardiac IL-17 mRNA expression levels. These data, at least, confirmed that the increase in IL-17 expression in heart tissues is associated with increased integrin CD11b expression. Integrin CD11b not only induced the adhesion of immune cells but also affected the differentiation of CD4+ cells to Th17 cells.
The regulatory effect on the differentiation of CD4+ cells to Th17 cells is largely dependent on the cells that express integrin CD11b, according to previous studies [15,16,17,18]. CD11b is widely expressed in diverse types of cells, such as myocardial cells, monocytes, macrophages, granulocytes and natural killer cells. However, in this study, it is unclear which type of CD11b-expressing cells drives the differentiation of CD4+ cells to Th17 cells. Further study should warrant to clarify this problem. In addition, although this study found increased CD11b expression in the cardiac tissues after CVB3 infection, the underlying molecular mechanism remains unclear. Therefore, an in-depth study on the mechanism is still necessary.
In conclusion, this study found that CD11b expression increased in the cardiac tissues in response to CVB3 infection; CD11b deficiency in turn attenuated CVB3-induced myocarditis in mice via inhibiting Th17 cell and the inflammation responses. Our findings suggest that CD11b might serve as a novel therapeutic treatment of VMC.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Leone O, Pieroni M, Rapezzi C, Olivotto I. The spectrum of myocarditis: from pathology to the clinics. Virchows Arch. 2019;475(3):279–301.10.1007/s00428-019-02615-8Search in Google Scholar PubMed
[2] Caforio ALP, Malipiero G, Marcolongo R, Iliceto S. Myocarditis: a clinical overview. Curr Cardiol Rep. 2017;19(7):63.10.1007/s11886-017-0870-xSearch in Google Scholar PubMed
[3] Rose NR. Viral myocarditis. Curr Opin Rheumatol. 2016;28(4):383–9.10.1097/BOR.0000000000000303Search in Google Scholar PubMed PubMed Central
[4] Fu Q, Gao L, Fu X, Meng Q, Lu Z. Scutellaria baicalensis inhibits Coxsackievirus B3-induced myocarditis via AKT and p38 pathways. J Microbiol Biotechnol. 2019;29(8):1230–9.10.4014/jmb.1904.04050Search in Google Scholar PubMed
[5] Müller I, Vogl T, Pappritz K, Miteva K, Savvatis K, Rohde D, et al. Pathogenic role of the damage-associated molecular patterns S100A8 and S100A9 in coxsackievirus B3-induced myocarditis. Circ Heart Fail. 2017;10(11):e004125.10.1161/CIRCHEARTFAILURE.117.004125Search in Google Scholar PubMed
[6] Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, et al. Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol. 2019;114(3):19.10.1007/s00395-019-0722-5Search in Google Scholar PubMed
[7] Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–97.10.1007/s00281-019-00733-8Search in Google Scholar PubMed
[8] Zhao M, Tan Y, Peng Q, Huang C, Guo Y, Liang G, et al. IL-6/STAT3 pathway induced deficiency of RFX1 contributes to Th17-dependent autoimmune diseases via epigenetic regulation. Nat Commun. 2018;9(1):583.10.1038/s41467-018-02890-0Search in Google Scholar PubMed PubMed Central
[9] Jin H, Guo X. Valproic acid ameliorates coxsackievirus-B3-induced viral myocarditis by modulating Th17/Treg imbalance. Virol J. 2016;13(1):168.10.1186/s12985-016-0626-zSearch in Google Scholar PubMed PubMed Central
[10] Wei B, Deng Y, Huang Y, Gao X, Wu W. IL-10-producing B cells attenuate cardiac inflammation by regulating Th1 and Th17 cells in acute viral myocarditis induced by coxsackie virus B3. Life Sci. 2019;235:116838.10.1016/j.lfs.2019.116838Search in Google Scholar PubMed
[11] Bracamonte-Baran W, Čiháková D. Cardiac autoimmunity: myocarditis. Adv Exp Med Biol. 2017;1003:187–221.10.1007/978-3-319-57613-8_10Search in Google Scholar
[12] Cruz FF, Borg ZD, Goodwin M, Coffey AL, Wagner DE, Rocco PR, et al. CD11b+ and Sca-1+ cells exert the main beneficial effects of systemically administered bone marrow-derived mononuclear cells in a murine model of mixed Th2/Th17 allergic airway inflammation. Stem Cell Transl Med. 2016;5(4):488–99.10.5966/sctm.2015-0141Search in Google Scholar
[13] Palmen MJ, Dijkstra CD, van der Ende MB, Peña AS, van Rees EP. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin Exp Immunol. 1995;101(2):351–6.10.1111/j.1365-2249.1995.tb08363.xSearch in Google Scholar
[14] Song P, Zhang J, Zhang Y, Shu Z, Xu P, He L, et al. Hepatic recruitment of CD11b+ Ly6C+ inflammatory monocytes promotes hepatic ischemia/reperfusion injury. Int J Mol Med. 2018;41(2):935–45.10.3892/ijmm.2017.3315Search in Google Scholar
[15] Yi H, Guo C, Yu X, Zuo D, Wang X. Mouse CD11b+ Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189(9):4295–304.10.4049/jimmunol.1200086Search in Google Scholar
[16] Varga G, Balkow S, Wild MK, Stadtbaeumer A, Krummen M, Rothoeft T, et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood. 2007;109(2):661–9.10.1182/blood-2005-12-023044Search in Google Scholar
[17] Ehirchiou D, Xiong Y, Xu G, Chen W, Shi Y, Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med. 2007;204(7):1519–24.10.1084/jem.20062292Search in Google Scholar
[18] Nowatzky J, Manches O, Khan SA, Godefroy E, Bhardwaj N. Modulation of human Th17 cell responses through complement receptor 3 (CD11 b/CD18) ligation on monocyte-derived dendritic cells. J Autoimmun. 2018;92:57–66.10.1016/j.jaut.2018.05.005Search in Google Scholar
[19] Li L, Li L, Xiao L, Shangguan J. Progranulin ameliorates coxsackievirus-B3-induced viral myocarditis by downregulating Th1 and Th17 cells. Exp Cell Res. 2018;367(2):241–50.10.1016/j.yexcr.2018.04.001Search in Google Scholar
[20] Yang K, Tang XD, Guo W, Xu XL, Ren TT, Ren CM, et al. BMPR2-pSMAD1/5 signaling pathway regulates RUNX2 expression and impacts the progression of dedifferentiated chondrosarcoma. Am J Cancer Res. 2016;6(6):1302–16.Search in Google Scholar
[21] Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10.10.1016/0304-3959(83)90201-4Search in Google Scholar
[22] Grabie N, Delfs MW, Westrich JR, Love VA, Stavrakis G, Ahmad F, et al. IL-12 is required for differentiation of pathogenic CD8 + T cell effectors that cause myocarditis. J Clin Investig. 2003;111(5):671–80.10.1172/JCI200316867Search in Google Scholar
[23] Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8.10.1006/meth.2001.1262Search in Google Scholar PubMed
[24] Namdari H, Izad M, Rezaei F, Amirghofran Z. Thymol as a reciprocal regulator of T cell differentiation: promotion of regulatory T cells and suppression of Th1/Th17 cells. Int Immunopharmacol. 2019;67:417–26.10.1016/j.intimp.2018.12.021Search in Google Scholar PubMed
[25] Jurisic V, Srdic-Rajic T, Konjevic G, Bogdanovic G, Colic M. TNF-α induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr Biol. 2011;239(3):115–22.10.1007/s00232-010-9309-7Search in Google Scholar PubMed
[26] Henar C, Pineda MA, Pilar MA, Susana G, Manuel F, Núria G. Inducible nitric oxide synthase and arginase expression in heart tissue during acute Trypanosoma cruzi infection in mice: arginase I is expressed in infiltrating CD68+ macrophages. J Infect Dis. 2008;197(12):1772–82.10.1086/529527Search in Google Scholar PubMed
[27] Kearney MT, Cotton JM, Richardson PJ, Shah AM. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgrad Med J. 2001;77(903):4–10.10.1136/pmj.77.903.4Search in Google Scholar PubMed PubMed Central
[28] Manu R, Nora M, Marty RR, Stephan D, Kurrer MO, Vukoslav K, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203(8):2009–19.10.1084/jem.20052222Search in Google Scholar PubMed PubMed Central
© 2020 Heng Wei et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”

