Abstract
Circular RNA sterile alpha motif domain containing 4A (circSAMD4A) was found to be differentially expressed in osteosarcoma and contributed to the tumorigenesis of osteosarcoma. However, the role of circSAMD4A in doxorubicin (DXR) resistance of osteosarcoma is yet to be elucidated. Levels of circSAMD4A, microRNA (miR)-218-5p and Krüppel-like factor 8 (KLF8) were detected using quantitative reverse transcription-polymerase chain reaction. Western blot was applied to detect the protein levels of KLF8, cyclin D1 and p21. Cell viability, cell cycle, migration and invasion were analyzed using Cell Counting Kit-8 assay, flow cytometry and transwell assay, respectively. The interaction between miR-218-5p and circSAMD4A or KLF8 was verified using dual-luciferase reporter assay or RNA immunoprecipitation assay. In vivo experiments were performed using murine xenograft models. CircSAMD4A and KLF8 were elevated in osteosarcoma, and knockdown of circSAMD4A or KLF8 sensitized osteosarcoma cells to DXR by mediating resistant cell viability, migration and invasion inhibition, and cell cycle arrest in vitro. miR-218-5p was decreased in osteosarcoma, and miR-218-5p inhibition enhanced DXR resistance. Besides, miR-218-5p was found to bind to circSAMD4A or KLF8, and subsequent rescue experiments indicated that miR-218-5p inhibition reversed the inhibitory effects of circSAMD4A silencing on DXR resistance, and silencing miR-218-5p enhanced DXR resistance by targeting KLF8 in osteosarcoma cells. Moreover, circSAMD4A could indirectly regulate KLF8 via miR-218-5p. Additionally, circSAMD4A knockdown enhanced the cytotoxicity of DXR in osteosarcoma in vivo via regulating miR-218-5p and KLF8. In all, circSAMD4A enhanced cell DXR resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis, suggesting a novel therapeutic target for therapy-resistant osteosarcoma.
1 Introduction
Osteosarcoma is the most frequent primary solid malignancy of bone, with a higher incidence in children and adolescents [1]. Standardized application of neoadjuvant chemotherapy plays key roles in the treatment of osteosarcoma, which significantly improves limb salvage and survival rates [2]. Doxorubicin (DXR) is one of the most active drugs for osteosarcoma treatment; however, DXR resistance gradually emerged in osteosarcoma patients, which limits the effects of the drug [3]. Thus, further investigations on the molecular mechanisms of DXR resistance are necessary to develop new targets to prevent DXR resistance.
Recent studies have suggested that non-coding RNAs (ncRNAs) and ncRNA-regulatory processes are involved in drug resistance in multiple types of cancers [4]. The ncRNAs account for greater than 90% of human RNAs and cannot encode proteins [5]. It has been documented that ncRNAs function as underlying players in multiple cellular processes, including cell cycle, differentiation, proliferation, metastasis, angiogenesis and oxidative stress [6,7,8]. Circular RNAs (circRNAs) are a new class of highly conserved ncRNAs forming a covalently closed continuous loop. Emerging evidence has identified the association between circRNAs and drug resistance in osteosarcoma [9,10]. Circular RNA sterile alpha motif domain containing 4A (circSAMD4A) is a newly identified circRNA, and Yanbin et al. found that circSAMD4A enhanced cell proliferation and the features of cancer stem cells in osteosarcoma by upregulating miR-1244-mediated MDM2 expression, suggesting the carcinogenic role of circSAMD4A in osteosarcoma [11]. However, the function of circSAMD4A in drug resistance in osteosarcoma remains unclear.
MicroRNAs (miRNAs) are well-documented small ncRNAs of approximately 22 nucleotides in length, which control specific gene expression programs by the regulation of post-transcriptional processes [12]. MiRNAs have been investigated to have vital functions in a variety of physiological and pathobiological processes, such as tumorigenesis and angiogenesis [13]. Besides, miRNAs also mediate chemoresistance of osteosarcoma, offering a new therapeutic target for osteosarcoma [14]. MiR-218-5p is a well-recognized tumor suppressor in various cancers [15,16], while the function of miR-218-5p in osteosarcoma is yet to be elucidated. Krüppel-like factor 8 (KLF8) is a protein encoded by KLF8 gene, which is a member of the KLF protein family. KLF8 has a significant role in regulating oncogenic transformation, cell cycle and epithelial to mesenchymal transition [17,18,19]. Recently, KLF8 was found to promote osteosarcoma carcinogenesis and progression [20,21]. Thus, we hypothesized that KLF8 might be associated with osteosarcoma chemoresistance.
In this study, we attempted to detect the functions of circSAMD4A, miR-218-5p and KLF8 in DXR resistance in osteosarcoma and explored whether there was a potential regulatory network among circSAMD4A, miR-218-5p and KLF8.
2 Materials and methods
2.1 Clinical samples
Tumor tissues and para-carcinoma tissues from 60 osteosarcoma patients who underwent surgical resection at Shaoxing Shangyu People’s Hospital were obtained and immediately stored at −80°C until use. All patients were treated preoperatively with DXR-based chemotherapy and were divided into the DXR-resistant group (treatment-resistant, N = 36) and the DXR-sensitive group (treatment-responsive, N = 24) depending on the sensitivity of osteosarcoma patients to DXR.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration and has been approved by the Ethics Committee of Shaoxing Shangyu People’s Hospital.
2.2 Cell culture
Human osteosarcoma cell lines HOS and U2OS and human osteoblast cell line hFOB1.19 were obtained from the Shanghai Academy of Life Science (Shanghai, China). HOS and U2OS cells were cultured in McCoy’s 5A medium (Gibco, Los Angeles, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and ampicillin and streptomycin. hFOB cells were grown in Dulbecco’s modified Eagle medium/F12 containing 10% FBS. All cells were incubated with 5% CO2 at 37°C.
DXR-resistant HOS (HOS/DXR) and U2OS (U2OS/DXR) cells were generated by continuously exposing parental HOS and U2OS cells to stepwise increasing doses of DXR (Sigma, San Francisco, CA, USA) over several months. DXR-resistant cells were cultured in the same media containing 1 µg/mL DXR at 37°C with 5% CO2 to retain their drug-resistant phenotype.
2.3 Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to conduct the extraction of total RNA by following the standard procedure. The synthesis of complementary DNAs (cDNAs) was performed using the PrimeScript RT reagent kit (Takara, Dalian, China), and then the synthesized cDNA template was amplified with SYBR Green I (Takara) on ABI7300. Fold changes were calculated by the 2−ΔΔCt method using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or U6 small nuclear RNA (U6) as the normalization control. The primers used were as follows: circSAMD4A: F 5′-TGAAGCCAGGAAACCTCGAC-3′, R 5′-GCCAGTCCTAGACCCAGGTA-3′; miR-218-5p: F 5′-AGCGAGATTTTCTGTTGTGCTT-3′, R 5′-GACGTTCCATGGTGCTTGAC-3′; KLF8: F 5′-GCTCACCGCAGAATCCATACA-3′, R 5′-GTGCACCGAAAAGGCTTGAT-3′; GAPDH: F 5′-CCCACATGGCCTCCAAGGAGTA-3′, R 5′-GTGTACATGGCAACTGTGAGGAGG-3′; U6: F 5′-GCTTCGGCAGCACATATACTAA-3′, R 5′-AACGCTTCACGAATTTGCGT-3′.
2.4 Cell transfection
The mimic and inhibitor of miR-218-5p (miR-218-5p mimic and anti-miR-218-5p) and their controls (miR-NC mimic and anti-NC) were obtained from RiboBio (Guangzhou, China). Small interfering RNA (siRNA) oligonucleotides targeting circSAMD4A (si-circSAMD4A; siRNA: 5′-AGCACAAGTACAAGAGGAAATdTdT-3′), siRNA oligonucleotides targeting KLF8 (si-KLF8; siRNA: 5′-UGAGUUUAUCCAUAUCGACCA-3′), siRNA oligonucleotides (si-NC), the scramble short hairpin RNA (shRNA) sequence (sh-NC) and shRNA targeting circSAMD4A (sh-circSAMD4A) were synthesized by Invitrogen. The transfection was conducted using Lipofectamine™ 2000 (Invitrogen) by following the instructions of the manufacturer.
2.5 Cell viability assay
Resistant cells transfected with the assigned vector for 48 h were seeded in 96-well plates (5,000 cells/well) overnight, and then they were exposed to increasing concentrations of DXR (0, 0.5, 1, 2, 4, 8 or 16 µg/mL), followed by incubation for another 48 h. Afterward, each well was incubated with Cell Counting Kit-8 (CCK-8) solution (10 µL/well; Beyotime, Shanghai, China) for about 2 h. Subsequently, the optical density was measured at 450 nm using a microplate reader, and the half-maximal inhibitory concentration (IC50) value was calculated for each cell line.
2.6 Cell cycle analysis
The transfected cells were harvested, and then the cells (1 × 105) were digested using trypsin to collect single-cell suspensions. After that, the cells were fixed with 75% ethanol for 4 h at 4°C, followed by incubation with propidium iodide (Cell Cycle Detection kit; BD Biosciences, San Jose, CA, USA). The percentage of cells in the G0/G1, S or G2/M phase was measured by flow cytometry with a FACS Calibur system (BD Bioscience).
2.7 Western blot
The extracted proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA), and then the membranes were incubated with primary antibodies against cyclin D1 (1:20,000; ab134175, Abcam, Cambridge, MA, USA), p21 (1:3,000; ab188224, Abcam), KLF8 (1:5,000; ab168527, Abcam) and horseradish peroxidase-conjugated secondary antibody (1:1,000; Sangon, Shanghai, China). Immunoreactive bands were visualized using an enhanced chemiluminescence kit (Beyotime) and normalized using GAPDH (1:10,000; ab8245, Abcam).
2.8 Cell migration and invasion analysis
Transwell chambers pre-coated with Matrigel (BD Biosciences) or uncoated were used to determine cell invasion or migration, respectively. The cells transfected with the assigned vector for 48 h were placed in the upper chambers with 200 µL of serum-free McCoy’s 5A medium, and the lower chambers were filled with 500 µL of McCoy’s 5A medium with FBS. After 24 h, the cells on the lower face of the membranes were fixed and stained, and counted using a microscope in five different fields.
2.9 Dual-luciferase reporter assay
MiR-218-5p in circSAMD4A or KLF8 3′-UTR wild-type (WT) and their mutated (MUT) sequences were separately cloned into pRL-TK luciferase plasmids (Promega, Shanghai, China). Then HOS/DXR and U2OS/DXR cells were co-transfected with 100 ng of constructed luciferase reporter plasmid and 40 nM miR-218-5p or miR-NC using Lipofectamine™ 2000 (Invitrogen). Finally, luciferase activity was detected using a dual luciferase assay kit (Promega).
2.10 RNA immunoprecipitation (RIP) assay
Resistant cells transfected with miR-218-5p mimic or miR-NC mimic were lysed using RIPA buffer, and then 100 µL of cell lysates was incubated with RIPA buffer containing magnetic beads conjugated with human anti-Argonaute2 (Ago2) antibody (Millipore) or normal mouse IgG (Millipore), followed by interaction with Proteinase K to digest the protein. Subsequently, immunoprecipitated RNA was extracted, and purified RNA was subjected to qRT-PCR analysis to examine the expression of circSAMD4A.
2.11 In vivo chemosensitivity assay
BALB/c nude mice (male, aged 3–5 weeks, N = 12) purchased from the National Laboratory Animal Center (Beijing, China) were divided into four groups with three mice in each group to establish mouse models. First, each mouse of two groups was subcutaneously injected with U2OS/DXR cells transfected with lentivirus-(lenti)-sh-NC, followed by treatment with PBS or DXR (3 mg/kg) every 3 days after 1 week of inoculation. Also, U2OS/DXR cells transfected with lenti-sh-circSAMD4A (sh-circSAMD4A) were subcutaneously injected into each mouse from the other two groups, followed by treatment with PBS or DXR (3 mg/kg) every 3 days after 1 week of inoculation. The volume of the tumor was calculated every week. At day 28, mice were killed, and tumor masses were weighed and collected for further molecular analysis.
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and has been approved by the Animal Research Committee of Shaoxing Shangyu People’s Hospital and implemented in line with the guidelines of the National Animal Care and Ethics Institution.
2.12 Statistical analysis
Data from thrice-repeated experiments were expressed as mean ± standard deviation and analyzed using GraphPad Prism 7 software. Statistical difference was detected using Student’s t-test or one-way analysis of variance followed by the Tukey post hoc test in different groups. P < 0.05 indicated statistical significance.
3 Results
3.1 Expression of circSAMD4A, miR-218-5p and KLF8 in DXR-resistant osteosarcoma tissues and cell lines
The levels of circSAMD4A, miR-218-5p and KLF8 were detected, and the results showed that relative to the non-tumor tissues and normal cell line hFOB, circSAMD4A (Figure 1a and b) and KLF8 (Figure 1i, j, m and n) were elevated, while miR-218-5p (Figure 1e and f) was decreased in osteosarcoma tissues and cell lines (HOS and U2OS). Importantly, osteosarcoma tissues were divided into the DXR-resistant group (treatment-resistant, N = 36) and the DXR-sensitive group (treatment-responsive, N = 24) depending on the sensitivity of osteosarcoma patients to DXR, and we found that circSAMD4A (Figure 1c) and KLF8 (Figure 1k and o) were notably higher, while miR-218-5p (Figure 1g) was lower in the treatment-resistant group than those in the treatment-responsive group. Similarly, in contrast with the parental osteosarcoma cell lines HOS and U2OS, circSAMD4A (Figure 1d) and KLF8 (Figure 1l and p) were also significantly increased and miR-218-5p (Figure 1h) was decreased in DXR-resistant cell lines HOS/DXR and U2OS/DXR. Additionally, we also discovered that miR-218-5p expression was negatively correlated with circSAMD4A (r = −0.7051, P < 0.0001; Figure 1q) and KLF8 (r = −0.8618, P < 0.0001; Figure 1r), and KLF8 expression was positively correlated with circSAMD4A in osteosarcoma tissues (r = 0.7564, P < 0.0001; Figure 1s). These data indicated that the dysregulation of circSAMD4A, miR-218-5p or KLF8 was associated with the DXR resistance, and there might be a connection among them in osteosarcoma.

The expression of circSAMD4A, miR-218-5p and KLF8 in DXR-resistant osteosarcoma tissues and cell lines. (a–p) Analysis of circSAMD4A, miR-218-5p and KLF8 expression levels in osteosarcoma tissues and matched non-tumor tissues (a, e, i and m), osteosarcoma cell lines (HOS and U2OS) and normal cell line hFOB (b, f, j and n), treatment-resistant group and treatment-responsive group (c, g, k and o) and DXR-resistant cell lines HOS/DXR and U2OS/DXR and their parental HOS and U2OS cells (d, h, l and p) using qRT-PCR or western blot. Each experiment was repeated three times, and the average was taken. (q–s) Correlation analysis between miR-218-5p and circSAMD4A or KLF8. *P < 0.05.
3.2 CircSAMD4A knockdown mitigates DXR resistance in osteosarcoma in vitro
The function of circSAMD4A in DXR resistance in osteosarcoma cells was analyzed in detail. HOS/DXR and U2OS/DXR cells were transfected with si-NC or si-circSAMD4A, and qRT-PCR analysis showed that si-circSAMD4A transfection significantly reduced the level of circSAMD4A in cells relative to the si-NC transfection (Figure 2a). Subsequently, CCK-8 assay exhibited that circSAMD4A knockdown combined with increasing doses of DXR (0, 0.5, 1, 2, 4, 8 or 16 µg/mL) gradually inhibited the viability of HOS/DXR and U2OS/DXR cells (Figure 2b); besides that, the IC50 values of HOS/DXR and U2OS/DXR cells for DXR in the circSAMD4A knockdown group were markedly lower than those in the cells of the si-NC group (Figure 2c). Meanwhile, we found that the number of HOS/DXR and U2OS/DXR cells in the S phase was decreased upon circSAMD4A silencing, while cells in the G0/G1 phase were accumulated, indicating cell cycle arrest (Figure 2d and e); also, the downregulation of cyclin D1 levels and upregulation of p21 levels induced by circSAMD4A knockdown in HOS/DXR and U2OS/DXR cells further suggested the cell cycle arrest (Figure 2f and g). In addition, transwell assay showed that the number of migrated and invaded HOS/DXR and U2OS/DXR cells was declined by circSAMD4A downregulation (Figure 2h and i). Taken together, circSAMD4A knockdown sensitized osteosarcoma cells to DXR by inhibiting resistant cell viability, cell cycle progression, migration and invasion.

CircSAMD4A knockdown mitigates DXR resistance in osteosarcoma in vitro. HOS/DXR and U2OS/DXR cells were transfected with si-NC or si-circSAMD4A. (a) qRT-PCR analysis of circSAMD4A expression in HOS/DXR and U2OS/DXR cells. (b) CCK-8 assay of resistant cell viability with increasing concentrations of DXR (0, 0.5, 1, 2, 4, 8 or 16 µg/mL). (c) CCK-8 assay of the IC50 values of resistant cells to DXR. (d and e) Flow cytometry analysis of the cell cycle of resistant cells. (f and g) Western blot analysis of cyclin D1 and p21 levels in resistant cells. (h and i) Transwell assay of the migration and invasion abilities of resistant cells. The experiments were performed three times. *P < 0.05.
3.3 CircSAMD4A is a sponge of miR-218-5p
Based on the above results, we knew that miR-218-5p expression was negatively correlated with circSAMD4A; thus, the potential relationship between them was investigated. According to the prediction of the StarBase program, we found that miR-218-5p might be a target of circSAMD4A (Figure 3a). To verify this prediction, a dual luciferase reporter assay was performed, and the results displayed that miR-218-5p overexpression significantly reduced the luciferase activity in HOS/DXR and U2OS/DXR cells transfected with WT-circSAMD4A, and there was no obvious change in cells transfected with MUT-circSAMD4A (Figure 3b). Additionally, RIP assay demonstrated that miR-218-5p upregulation elevated the enrichment of Ago2 on circSAMD4A both in HOS/DXR and U2OS/DXR cells (Figure 3c). Interestingly, we observed that circSAMD4A knockdown increased miR-218-5p expression in HOS/DXR and U2OS/DXR cells (Figure 3d). Collectively, circSAMD4A directly bound to miR-218-5p and negatively regulated its expression.

CircSAMD4A is a sponge of miR-218-5p. (a) The potential binding sites of circSAMD4A and miR-218-5p. (b) Dual-luciferase reporter assay in HOS/DXR and U2OS/DXR cells co-transfected with the reporter plasmid and the indicated miRNAs. (c) RIP analysis for the enrichment of Ago2 on circSAMD4A in HOS/DXR and U2OS/DXR cells. (d) qRT-PCR analysis of miR-218-5p expression in HOS/DXR and U2OS/DXR cells transfected with si-NC or si-circSAMD4A. The experiments were performed three times. *P < 0.05.
3.4 CircSAMD4A knockdown sensitizes osteosarcoma cells to DXR by binding to miR-218-5p
Whether miR-218-5p is involved in the action of circSAMD4A in DXR resistance in osteosarcoma cells was explored. First, HOS/DXR and U2OS/DXR cells were transfected with anti-miR-218-5p or anti-NC, and we found that miR-218-5p was significantly reduced by anti-miR-218-5p transfection compared to the anti-NC transfection (Figure 4a). After that, we found that miR-218-5p inhibition increased HOS/DXR and U2OS/DXR cell viability with 0.5–16 µg/mL DXR (Figure 4b) and upregulated the values of IC50 (Figure 4c). Flow cytometry analysis showed that miR-218-5p inhibition induced DXR-resistant cell cycle progression, reflected by the reduction of HOS/DXR and U2OS/DXR cells in the G0/G1 phase and elevation in the S phase (Figure 4d), as well as the increase of cyclin D1 and decrease of p21 in HOS/DXR and U2OS/DXR cells (Figure 4e and f). Meanwhile, the number of migrated and invaded HOS/DXR and U2OS/DXR cells was also increased by miR-218-5p inhibition (Figure 4g and h). Thus, all these results indicated that silencing of miR-218-5p promoted DXR resistance in osteosarcoma cells. Next, si-circSAMD4A + anti-NC or si-circSAMD4A + anti-miR-218-5p was transfected into HOS/DXR and U2OS/DXR cells. The results indicated that miR-218-5p inhibition reversed circSAMD4A knockdown-induced HOS/DXR and U2OS/DXR cell viability inhibition (Figure 4b and c), cell cycle arrest (Figure 4d–f), as well as migration and invasion suppression (Figure 4g and h). Altogether, circSAMD4A knockdown sensitized osteosarcoma cells to DXR by positively regulating miR-218-5p expression.

CircSAMD4A knockdown sensitizes osteosarcoma cells to DXR by binding to miR-218-5p. (a) qRT-PCR analysis of miR-218-5p expression in HOS/DXR and U2OS/DXR cells transfected with anti-miR-218-5p or anti-NC. (b–h) HOS/DXR and U2OS/DXR cells were transfected with anti-NC, anti-miR-218-5p, si-circSAMD4A + anti-NC or si-circSAMD4A + anti-miR-218-5p. (b) CCK-8 assay of resistant cell viability with increasing concentrations of DXR (0, 0.5, 1, 2, 4, 8 or 16 µg/mL). (c) CCK-8 assay of the IC50 values of HOS/DXR and U2OS/DXR cells to DXR. (d) Cell cycle analysis of resistant cells using flow cytometry. (e and f) Western blot analysis of cyclin D1 and p21 levels in resistant cells. (g and h) Migration and invasion analyses of resistant cells with transwell assay. All experiments were repeated three times independently. *P < 0.05.
3.5 KLF8 is a target of miR-218-5p
Considering the negative correlation between KLF8 and miR-218-5p, the regulatory relationship between them was evaluated. Through searching the TargetScan program, the putative binding sites of miR-218-5p on KLF8 were predicted (Figure 5a). Then a reduction of luciferase activity in HOS/DXR and U2OS/DXR cells co-transfected with WT-KLF8 and miR-218-5p mimic confirmed the direct interaction between KLF8 and miR-218-5p (Figure 5b). Also, RIP assay suggested that miR-218-5p overexpression increased the enrichment of Ago2 on KLF8 both in HOS/DXR and U2OS/DXR cells, further verifying that miR-218-5p targeted KLF8 (Figure 5c). Subsequently, we found that miR-218-5p inhibition upregulated the expression of KLF8 both at mRNA and protein levels (Figure 5d and e). Therefore, miR-218-5p targetedly repressed KLF8 expression.

KLF8 is a target of miR-218-5p. (a) Schematic representation of the predicted binding sites of miR-218-5p on KLF8. (b) Dual-luciferase reporter assay in HOS/DXR and U2OS/DXR cells co-transfected with the reporter plasmid and the indicated miRNAs. (c) RIP analysis for the enrichment of Ago2 on KLF8 in HOS/DXR and U2OS/DXR cells. (d and e) Analysis of KLF8 expression level in HOS/DXR and U2OS/DXR cells transfected with anti-NC or anti-miR-218-5p with qRT-PCR or western blot. The results are presented as the average of three independent experiments. *P < 0.05.
3.6 KLF8 knockdown attenuates the action of miR-218-5p in DXR resistance in osteosarcoma cells
We then studied whether the action of miR-218-5p in DXR resistance in osteosarcoma cells was mediated by KLF8. First, HOS/DXR and U2OS/DXR cells were transfected with si-KLF8 or si-NC, and KLF8 expression was notably reduced by si-KLF8 transfection as expected (Figure 6a and b). After that, CCK-8 assay showed that KLF8 knockdown combined with increasing doses of DXR (0, 0.5, 1, 2, 4, 8 or 16 µg/mL) suppressed HOS/DXR and U2OS/DXR cell viability (Figure 6c), and the IC50 values were also reduced by KLF8 silencing in HOS/DXR and U2OS/DXR cells (Figure 6d). In addition, KLF8 knockdown elevated the number of HOS/DXR and U2OS/DXR cells in the G0/G1 phase and reduced cells in the S phase, thereby inducing cell cycle arrest at G0/G1 (Figure 6e); moreover, the upregulation of p21 levels and downregulation of cyclin D1 levels induced by KLF8 knockdown also demonstrated the cell cycle arrest in HOS/DXR and U2OS/DXR cells (Figure 6f and g). Afterward, we found that KLF8 knockdown reduced the number of migrated and invaded HOS/DXR and U2OS/DXR cells (Figure 6h and i). Thus, KLF8 knockdown suppressed DXR resistance in osteosarcoma cells. Next, HOS/DXR and U2OS/DXR cells were transfected with anti-miR-218-5p + si-NC or anti-miR-218-5p + si-KLF8, and we discovered that KLF8 knockdown attenuated miR-218-5p inhibition-mediated DXR-resistant cell viability promotion (Figure 6c and d), cell cycle progression (Figure 6e–g) and cell migration and invasion enhancement (Figure 6h and i) in HOS/DXR and U2OS/DXR cells. These data suggested that miR-218-5p inhibition blocked the sensitivity of osteosarcoma cells to DXR by regulating KLF8.
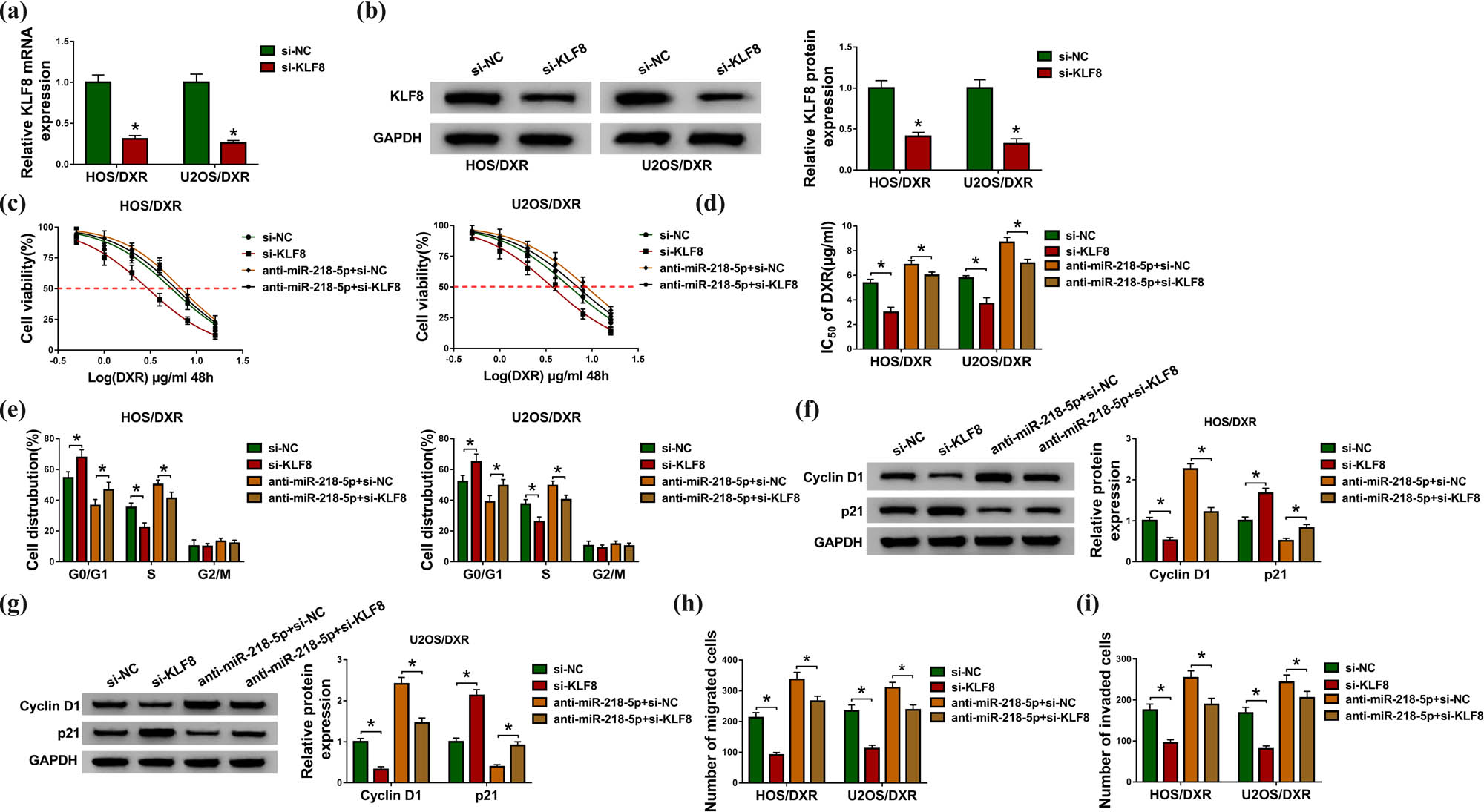
KLF8 knockdown suppresses the action of miR-218-5p in DXR resistance in osteosarcoma cells. (a and b) Analysis of KLF8 expression level in HOS/DXR and U2OS/DXR cells transfected with si-NC or si-KLF8 using qRT-PCR or western blot. (c–i) HOS/DXR and U2OS/DXR cells were transfected with si-NC, si-KLF8, anti-miR-218-5p + si-NC or anti-miR-218-5p + si-KLF8. (c) CCK-8 assay of resistant cell viability with increasing concentrations of DXR (0, 0.5, 1, 2, 4, 8 or 16 µg/mL). (d) CCK-8 assay of the IC50 values of resistant cells to DXR. (e) Flow cytometry analysis of the cell cycle of resistant cells. (f and g) Western blot analysis of cyclin D1 and p21 levels in resistant cells. (h and i) Measurement of the number of migrated and invaded resistant cells with transwell assay. All experiments were repeated three times independently. *P < 0.05.
3.7 CircSAMD4A positively regulates KLF8 via miR-218-5p
Given that KLF8 was positively correlated with circSAMD4A in osteosarcoma, we wanted to know whether circSAMD4A regulated KLF8 via miR-218-5p. Then we found that circSAMD4A knockdown reduced the expression level of KLF8 both at mRNA and protein levels, while this reduction was rescued following miR-218-5p inhibition in HOS/DXR and U2OS/DXR cells (Figure 7a and b). Therefore, we demonstrated that circSAMD4A positively regulated KLF8 via miR-218-5p in osteosarcoma cells.

CircSAMD4A positively regulates KLF8 via miR-218-5p. (a and b) Analysis of KLF8 expression level in HOS/DXR and U2OS/DXR cells transfected with si-NC, si-circSAMD4A, si-circSAMD4A + anti-NC or si-circSAMD4A + anti-miR-218-5p using qRT-PCR or western blot. Each experiment was repeated three times, and the average was taken. *P < 0.05.
3.8 CircSAMD4A knockdown enhances the cytotoxicity of DXR in osteosarcoma in vivo
The function of circSAMD4A in DXR-induced tumor growth suppression in vivo was investigated. The results suggested that circSAMD4A silencing accelerated DXR-induced suppression of tumor growth in vivo (Figure 8a and b). Afterward, molecular analysis exhibited that sh-circSAMD4A injection successfully induced the downregulation of circSAMD4A levels in the tumor masses (Figure 8c). Besides, circSAMD4A silencing increased miR-218-5p and decreased KLF8 expression in vivo (Figure 8d–f). Thus, we concluded that circSAMD4A knockdown promoted DXR-induced tumor suppression in osteosarcoma murine xenograft models by regulating miR-218-5p and KLF8 expression.

CircSAMD4A knockdown enhances the cytotoxicity of DXR in osteosarcoma in vivo. (a) Tumor volumes were calculated every week. (b) Tumor masses were collected and weighed on day 28. (c–e) qRT-PCR analysis of circSAMD4A, miR-218-5p and KLF8 expression in the tumor masses of each group. (f) Western blot analysis of KLF8 protein in tumor masses of each group. *P < 0.05.
4 Discussion
Osteosarcoma is a highly aggressive bone sarcoma, and with the advances in complete surgical resection and multi-agent chemotherapy, up to 70% of patients with localized limb tumors and high-grade osteosarcoma become long-term survivors [22]. Nevertheless, osteosarcoma patients with nonresectable, primary metastatic or relapse tumors still have poor prognosis [23]. The acquisition of drug resistance accounts for the majority of poor effects of chemotherapy in osteosarcoma. DXR is one of the most effective drugs for osteosarcoma standard chemotherapy, and a total of 40–45% of high-grade osteosarcoma patients are unresponsive or only partially responsive to DXR [24].
Tumor chemoresistance is a complex, multistep process hallmarked by a number of abnormal genes, proteins, ncRNAs and some related signal pathways [25]. Increasing research studies have indicated that circRNAs are critical mediators in DXR resistance in many cancers. For example, Liang et al. revealed that circKDM4C suppressed cell proliferation, metastasis and DXR resistance in breast cancer by regulating PBLD via miR-548p [26]. Shang’s team identified 49 circRNAs that were differentially expressed in DXR-resistant and sensitive THP-1 acute myeloid leukemia cell lines, and among them, circPAN3 knockdown could sensitize resistant THP-1 cells to DXR through the miR-153-5p/miR-183-5p-XIAP axis [27]. Importantly, Kun et al. demonstrated that circPVT1 knockdown restored DXR and cisplatin sensitivity of osteosarcoma cells via reducing ABCB1 expression [9]. Thus, targeting circRNAs may be a promising therapeutic strategy for DXR resistance. In this study, we found that circSAMD4A was elevated in osteosarcoma, especially in DXR-resistant cell lines and tissues. Then we demonstrated that circSAMD4A knockdown rescued the DXR sensitivity of DXR-resistant osteosarcoma cells by mediating the suppression of cell viability, migration and invasion, as well as the arrest of the cell cycle in vitro. Besides, murine xenograft models indicated that circSAMD4A silencing enhanced the cytotoxicity of DXR in osteosarcoma in vivo. Thus, circSAMD4A knockdown inhibited DXR resistance in osteosarcoma.
It has been reported that circRNAs contain diverse types and quantities of miRNA binding sites and can act as miRNA sponges, regulators of splicing and transcription and modifiers of parental gene expression, thereby they are implicated in a series of biological and pathological processes as well as drug resistance [28,29]. To verify whether circSAMD4A could act as a miRNA sponge, bioinformatics analysis was used. Among the predicted candidates, miR-218-5p was selected for further exploration owing to the anticancer effects of miR-218 in osteosarcoma [30,31,32]. As for miR-218-5p, it was reported to regulate tumor cells’ malignant biological behavior in a series of human cancers, such as non-small cell lung cancer and triple negative breast cancer [15,16]. In the present research, we first validated that circSAMD4A sequestered miR-218-5p through functioning as a miR-218-5p sponge and negatively regulated its expression. MiR-218-5p was decreased in DXR-resistant osteosarcoma tissues and cells, and miR-218-5p inhibition enhanced the viability, migration and invasion but induced cell cycle arrest in DXR-resistant osteosarcoma cells. More importantly, inhibition of miR-218-5p reversed the restoration of DXR sensitivity induced by circSAMD4A silencing in osteosarcoma.
Subsequently, we further identified the molecular targets of miR-218-5p using online software TargetScan. KLF8 harbored a putative complementary sequence for miR-218-5p. KLF8 is a dual transcription factor and can either suppress or activate the transcription of target genes, including cyclin D1, KLF4 and E-cadherin [33], which are related to tumor development in diverse cancer types including osteosarcoma [20,21]. Additionally, recent studies also exhibited that KLF8 contributed to chemoresistance in breast cancer [19], gastric cancer [33] and glioma [34]. In this study, we uncovered that KLF8 was elevated in DXR-resistant cell lines and tissues, and KLF8 knockdown promoted the cytotoxicity of DXR in osteosarcoma. Importantly, this study first verified that miR-218-5p directly targeted KLF8, and miR-218-5p regulated DXR resistance via KLF8 in osteosarcoma. In addition, we also revealed that circSAMD4A positively regulated KLF8 through acting as a sponge of miR-218-5p, and thus, a circSAMD4A/miR-218-5p/KLF8 network in osteosarcoma cells was identified.
In conclusion, this study demonstrated that knockdown of circSAMD4A or KLF8 and elevation of miR-218-5p restored the DXR sensitivity of osteosarcoma cells. Besides, we also found that the function of circSAMD4A in osteosarcoma was partially exerted via the miR-218-5p/KLF8 axis, which provided new insight into the mechanisms underlying the chemoresistance of osteosarcoma and potential therapeutic targets for osteosarcoma chemotherapy.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wittig JC, Bickels J, Priebat D, Jelinek J, Kellar-Graney K, Shmookler B, et al. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Family Physician. 2002;65:1123–37.Search in Google Scholar
[2] Anderson ME. Update on survival in osteosarcoma. Orthoped Clin. 2016;47:283–92.10.1016/j.ocl.2015.08.022Search in Google Scholar PubMed
[3] Hattinger CM, Fanelli M, Tavanti E, Vella S, Ferrari S, Picci P, et al. Advances in emerging drugs for osteosarcoma. Expert Opin Emerg Drugs. 2015;20:495–514.10.1517/14728214.2015.1051965Search in Google Scholar PubMed
[4] Ding B, Lou W, Xu L, Fan W. Non-coding RNA in drug resistance of hepatocellular carcinoma. Biosci Rep. 2018;38:BSR20180915.10.1042/BSR20180915Search in Google Scholar PubMed PubMed Central
[5] Consortium EP. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816.10.1038/nature05874Search in Google Scholar PubMed PubMed Central
[6] Panda AC, Abdelmohsen K, Gorospe M. SASP regulation by noncoding RNA. Mech Ageing Dev. 2017;168:37–43.10.1016/j.mad.2017.05.004Search in Google Scholar PubMed PubMed Central
[7] Hombach S, Kretz M. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol. 2016;937:3–17.10.1007/978-3-319-42059-2_1Search in Google Scholar PubMed
[8] Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–58.10.1093/nar/gkw027Search in Google Scholar PubMed PubMed Central
[9] Kun-Peng Z, Xiao-Long M, Chun-Lin Z. Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1. Int J Biol Sci. 2018;14(3):321–30.10.7150/ijbs.24360Search in Google Scholar PubMed PubMed Central
[10] Zhang H, Yan J, Lang X, Zhuang Y. Expression of circ_001569 is upregulated in osteosarcoma and promotes cell proliferation and cisplatin resistance by activating the Wnt/β-catenin signaling pathway. Oncol Lett. 2018;16:5856–62.10.3892/ol.2018.9410Search in Google Scholar PubMed PubMed Central
[11] Yanbin Z, Jing Z. CircSAMD4A accelerates cell proliferation of osteosarcoma by sponging miR-1244 and regulating MDM2 mRNA expression. Biochem Biophys Res Commun. 2019;516(1):102–11.10.1016/j.bbrc.2019.05.182Search in Google Scholar PubMed
[12] He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–31.10.1038/nrg1379Search in Google Scholar PubMed
[13] Kontomanolis E, Koukouli A, Liberis G, Stanulov H, Achouhan A, Pagkalos A. MiRNAs: regulators of human disease. Eur J Gynaecol Oncol. 2016;37:759–65.Search in Google Scholar
[14] Chen R, Wang G, Zheng Y, Hua Y, Cai Z. Drug resistance-related microRNAs in osteosarcoma: translating basic evidence into therapeutic strategies. J Cell Mol Med. 2019;23:2280–92.10.1111/jcmm.14064Search in Google Scholar PubMed PubMed Central
[15] Zhu K, Ding H, Wang W, Liao Z, Fu Z, Hong Y, et al. Tumor-suppressive miR-218-5p inhibits cancer cell proliferation and migration via EGFR in non-small cell lung cancer. Oncotarget. 2016;7:28075–85.10.18632/oncotarget.8576Search in Google Scholar PubMed PubMed Central
[16] Taipaleenmäki H, Farina NH, van Wijnen AJ, Stein JL, Hesse E, Stein GS, et al. Antagonizing miR-218-5p attenuates Wnt signaling and reduces metastatic bone disease of triple negative breast cancer cells. Oncotarget. 2016;7:79032.10.18632/oncotarget.12593Search in Google Scholar PubMed PubMed Central
[17] Zhang H, Liu L, Wang Y, Zhao G, Xie R, Liu C, et al. KLF8 involves in TGF-beta-induced EMT and promotes invasion and migration in gastric cancer cells. J Cancer Res Clin Oncol. 2013;139:1033–42.10.1007/s00432-012-1363-3Search in Google Scholar PubMed
[18] Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26:456–61.10.1038/sj.onc.1209796Search in Google Scholar PubMed
[19] Lu H, Hu L, Li T, Lahiri S, Shen C, Wason MS, et al. A novel role of Krüppel-like factor 8 in DNA repair in breast cancer cells. J Biol Chem. 2012;287:43720–9.10.1074/jbc.M112.418053Search in Google Scholar PubMed PubMed Central
[20] Sun Y, Cao L, Lin J, Yuan Y, Cao Z, Jia J. Upregulated miRNA-1236-3p in osteosarcoma inhibits cell proliferation and induces apoptosis via targeting KLF8. Eur Rev Med Pharmacol Sci. 2019;23:6053–61.Search in Google Scholar
[21] Lin F, Shen Z, Tang LN, Zheng SE, Sun YJ, Min DL, et al. KLF8 knockdown suppresses proliferation and invasion in human osteosarcoma cells. Mol Med Rep. 2014;9:1613–7.10.3892/mmr.2014.2027Search in Google Scholar PubMed PubMed Central
[22] Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13:357–68.10.2217/fon-2016-0261Search in Google Scholar PubMed
[23] Ritter J, Bielack S. Osteosarcoma. Ann Oncol. 2010;21:vii320–vii5.10.1093/annonc/mdq276Search in Google Scholar PubMed
[24] Buondonno I, Gazzano E, Jean SR, Audrito V, Kopecka J, Fanelli M, et al. Mitochondria-targeted doxorubicin: a new therapeutic strategy against doxorubicin-resistant osteosarcoma. Mol Cancer Therapeutics. 2016;15:2640–52.10.1158/1535-7163.MCT-16-0048Search in Google Scholar
[25] Zhang C-L, Zhu K-P, Ma X-L. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017;396:66–75.10.1016/j.canlet.2017.03.018Search in Google Scholar
[26] Liang Y, Song X, Li Y, Su P, Han D, Ma T, et al. circKDM4C suppresses tumor progression and attenuates doxorubicin resistance by regulating miR-548p/PBLD axis in breast cancer. Oncogene. 2019;38:6850–66.10.1038/s41388-019-0926-zSearch in Google Scholar
[27] Shang J, Chen W-M, Wang Z-H, Wei T-N, Chen Z-Z, Wu W-B. CircPAN3 mediates drug resistance in acute myeloid leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp Hematol. 2019;70:42–54.10.1016/j.exphem.2018.10.011Search in Google Scholar
[28] Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, et al. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141–8.10.1016/j.canlet.2015.06.003Search in Google Scholar
[29] Shao F, Huang M, Meng F, Huang Q. Circular RNA signature predicts gemcitabine resistance of pancreatic ductal adenocarcinoma. Front Pharmacology. 2018;9:584.10.3389/fphar.2018.00584Search in Google Scholar
[30] Wang DZ, Jing SF, Hao SB, Huang XY, Miao QT, Gao JF. MiR-218 promotes apoptosis of U2OS osteosarcoma cells through targeting BIRC5. Eur Rev Med Pharmacol Sci. 2018;22(20):6650–7.Search in Google Scholar
[31] Wang HT, Liu AG, Luo DS, Zhou ZN, Lin HG, Chen RZ, et al. miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells. Asian Pac J Tropical Med. 2014;7(12):1000–4.10.1016/S1995-7645(14)60176-0Search in Google Scholar
[32] Xuan C, Jin M, Gao Y, Xu S, Wang L, Wang Y, et al. miR-218 suppresses the proliferation of osteosarcoma through downregulation of E2F2. Oncol Lett. 2019;17(1):571–7.10.3892/ol.2018.9576Search in Google Scholar PubMed PubMed Central
[33] Zhang H, Sun L, Xiao X, Xie R, Liu C, Wang Y, et al. Krüppel-like factor 8 contributes to hypoxia-induced MDR in gastric cancer cells. Cancer Sci. 2014;105:1109–15.10.1111/cas.12483Search in Google Scholar PubMed PubMed Central
[34] Yu G, Wu F, Wang E. KLF8 promotes temozolomide resistance in glioma cells via β-catenin activation. Cell Physiol Biochem. 2016;38:1596–604.10.1159/000443100Search in Google Scholar PubMed
© 2020 Wei Wei et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”

