Abstract
Diabetic nephropathy (DN) is one of the most common complications of diabetes mellitus. Increasing evidence suggests that microRNA-30c-5p (miR-30c-5p) participates in the pathogenesis of DN, but the mechanism has not been clearly understood. Therefore, this study aimed to investigate the biological role of miR-30c-5p in human DN progression in vitro. Compared with the controls, DN tissues and high glucose-induced HK-2 cells had significantly reduced miR-30c-5p levels, while ROCK2 expression was prominently elevated. Additionally, the miR-30c-5p mimic distinctly facilitated cell proliferation and blocked cell apoptosis and epithelial–mesenchymal transition (EMT). However, ROCK2 was a target gene of miR-30c-5p, and the effects of miR-30c-5p mimic on cell proliferation, apoptosis and EMT were reversed by ROCK2 upregulation in vitro. Furthermore, the pathogenesis of DN was regulated by the miR-30c-5p/ROCK2 axis via the PI3K/AKT pathway. MiR-30c-5p regulating cell proliferation, apoptosis and EMT through targeting ROCK2 via the PI3K/AKT pathway provides the novel potential target for clinical treatment of DN.
1 Introduction
Diabetic nephropathy (DN) is a major complication of diabetes mellitus, as well as the predominant cause of advanced renal disease [1]. Around 10–40% of patients with type 2 diabetes eventually develop DN in urban China [2]. However, the pathogenesis of DN is complex, influenced by multiple factors such as nonenzymatic saccharification, renal hemodynamic changes, hypertension, dyslipidemia, oxidative stress, protein kinase C activation, vasoactive substances and cytokines, as well as other genetic factors [3]. It seems that different types of kidney cells are sensitive to hyperglycemia in varying degrees [4]. Research on the pathogenesis of DN is particularly important to better understand the mechanism and thus develop more effective therapies.
MicroRNAs (miRNAs) have been shown to be a type of non-coding RNA with a length of 20–24 nucleotides (nts) [5]. MiRNAs are involved in a number of processes in the development of various diseases such as heart failure and cancer as well as diabetes [6,7,8]. Studies have reported that miRNAs are associated with some renal diseases. For example, miR-29c is a signature miRNA in high glucose (HG)-induced conditions, targeting sprouty homolog 1, and reduced expression of miR-29c prevents DN progression [9]. MiR-193a appears to induce focal segmental glomerulosclerosis by inhibiting the expression of Wilms’ tumor protein [10]. MiR-192 exerts its role in diabetic kidney glomeruli via inhibition of E-box repressors [11]. Moreover, miR-29s and miR-let-7s have been confirmed to function as key antifibrotic players in DN [12]. MiR-30c-5p in serum acts as a potential biomarker in multiple system atrophy [13]. Furthermore, miR-30c-5p has been revealed to be involved in reducing renal ischemia-reperfusion via macrophages [14]. In this study, the biological role of miR-30c-5p in DN was investigated using HG-induced HK-2 cells in vitro, which uncovered the possible mechanisms of miR-30c-5p’s effects on the progression of human DN.
Rho-associated coiled coil-containing protein kinase 2 (ROCK2) belongs to the Rho-associated kinase (ROCK) family. The ROCK family includes ROCK1 and ROCK2 members, and the high similarity of their amino acid sequences suggests that these two members perform many of the same functions [15]. Particularly, ROCK2 downregulation blocks HG-induced hyperpermeability in kidney glomerular endothelium [16]. Previous research indicates that ROCK2 is a critical regulator of axonal degeneration and neuronal death, as well as axonal regeneration in the central nervous system [17]. Thus, ROCK2 could be closely related to the development of multiple types of cancer, as well as diseases of the central nervous system and kidney dysfunction.
The underlying mechanism of ROCK2 in the progression of kidney diseases needs further exploration. Since phosphatidylinositol 3-kinase (PI3K) and protein kinase B (AKT) play vital roles in several cellular processes, including proliferation, apoptosis, migration and glucose metabolism [18,19], we hypothesized that the PI3K/AKT signaling pathway participates in the progression of DN.
Herein, we measured the level of miR-30c-5p and ROCK2 expression in DN tissues and in an HG-induced DN cell model. The regulatory mechanism of miR-30c-5p and ROCK2 in the pathogenesis of DN in vitro was investigated.
2 Materials and methods
2.1 Kidney tissue samples
Human DN tissue samples (n = 40) and the adjacent normal kidney tissues (n = 40) were donated by DN patients at Weihai Central Hospital. The DN patients included 18 males and 22 females, ranging from 40 to 65 years. All the patients were diagnosed and classified according to the World Health Organization diagnostic criteria for diabetes [20]. The DN patients underwent a negative urine protein test, with 30–300 mg/24 h urine albumin, indicating early renal damage. Moreover, the DN patients were diagnosed with the presence of specific nodular glomerulosclerosis of diabetes mellitus (the Kimmelstiel–Wilson lesion). We did not find other primary and secondary factors or complications causing renal damage.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration and has been approved by the Ethics Committee of Weihai Central Hospital.
2.2 Cell culture and treatment
Human kidney cells HK-2 were purchased from the Chinese Academy of Sciences Shanghai Cell Bank (Shanghai, China). HK-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Rockford, IL, USA) supplemented with 5.6 mmol/L glucose (Sigma, St. Louis, MO, USA), 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 100 U/mL penicillin and 100 µg/mL streptomycin, in an incubator with 5% CO2 at 37°C.
To establish the DN cell model, HK-2 cells were trypsinized, and 2 mL of cell suspension was added to each well of a six-well culture plate at a density of 1 × 106 cells/mL. Then, the cells were incubated in serum-free DMEM for 12 h until 70–80% confluence. HK-2 cells were treated with normal glucose (NG; final concentration of d-glucose 5.6 mmol/L), HG (final concentration of d-glucose 30 mmol/L) and high osmotic pressure control group (HO; 5.6 mmol/L d-glucose + 24.4 mmol/L d-mannitol).
2.3 Transient transfection
HK-2 cells were seeded in a six-well plate. When cells grew to 60–85% confluence, they were transfected with vectors or oligonucleotides using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Then, the HK-2 cells were cultured with NG, HG or HO after transfection for 6 h. The HK-2 cells in the mock group were treated with no sequences, and the HK-2 cells in the NC group were transfected with control miR-30c-5p. The vectors and oligonucleotides used were as follows: overexpression vector of ROCK2 (pcDNA-ROCK2), pcDNA 3.1 empty vector (pcDNA-control), small interfering RNA against ROCK2 (si-ROCK2), miR-30c-5p mimic (5′-UGUAAACAUCCUACACUCUCAGC-3′), miR-30c-5p inhibitor (5′-GCUGAGAGUGUAGGAUGUUUACU-3′) and miR-30c-5p negative control (miR-control, 5′-CUAACGCAUGCACAGUCGUACG-3′). The above sequences were synthesized by GenePharma (Shanghai, China).
2.4 Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissues and cells using TRIzol (Invitrogen) in accordance with the manufacturer s instructions. Subsequently, reverse transcription was conducted using an ALL-in-one miRNA reverse transcription kit (GeneCopoeia, Rockville, MD, USA) according to the manufacturer’s instructions. The obtained cDNA was temporarily maintained at −80°C or directly used. qPCR was carried out using a SYBR® Premix Ex TaqTM II Kit (TaKaRa, Dalian, China) and ABI 7500 PCR instrument (Applied Biosystems, Rockford, IL, USA). Finally, mRNA expression ratio was calculated using the 2−ΔΔCt method, and U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as the internal reference. The following primers were used: miR-30c-5p (forward: 5′-GCCGCTGTAAACATCCTACACT-3′ and reverse: 5′-GTGCAGGGTCCGAGGT-3′), ROCK2 (forward: 5′-GCCGCCGTTGCCATATTAAG-3′ and reverse: 5′-CTGGAGCTGGGGGCTTTTTA-3′), GAPDH (forward: 5′-GGTCACCAGGGCTGCTTTTA-3′ and reverse: 5′-TTCCCGTTCTCAGCCTTGAC-3′), and U6 (forward: 5′-CTCGCTTCGGCAGCACA-3′ and reverse: 5′-AACGCTTCACGAATTTGCGT-3′).
2.5 Western blot assay
HK-2 cells and tissues were harvested and lysed in RIPA lysis buffer (Millipore, Bedford, MA, USA) containing protease and phosphatase inhibitors. Then, total protein concentration was evaluated with a BCA Protein Assay Kit (Sangon Biotech, Shanghai, China), and loading buffer was used for modulating the volume of protein solution. Briefly, 20 µL of solution containing 30 µg of protein was added to each well of a 10% sodium dodecyl sulfate-polyacrylamide gel. The separated protein was electro-transferred onto polyvinylidene difluoride membranes (Millipore), which were then blocked with 5% bovine serum albumin (Sangon Biotech). Then, the membranes were incubated with primary antibodies: ROCK2 (1:1,000, ab71598; Abcam, Cambridge, MA, USA), E-cadherin (1:1,000, ab76055; Abcam), vimentin (1:1,000, ab8979; Abcam), α-smooth muscle actin (α-SMA; 1:1,000, ab108424; Abcam), snail1 (1:1,000, ab53519; Abcam), transforming growth factor β 1 (TGFB1; 1:1,000, ab92486; Abcam), PI3K (1:1,000, ab151549; Abcam), phosphorylated PI3K (p-PI3K; 1:1,000, ab182651; Abcam), AKT (1:1,000, ab64148; Abcam), phosphorylated AKT (p-AKT; 1:500, ab8933; Abcam) and GAPDH (1:1,000, ab8245; Abcam) overnight at 4°C. After washing, the membranes were incubated with a secondary antibody (1:5,000, ab205718 or ab205719; Abcam) for 1 h at room temperature. Subsequently, a Chemiluminescence Reagent Kit (Millipore) and a ChemiDoc MP Imaging System (Bio-Rad, Philadelphia, PA, USA) were used to visualize protein bands.
2.6 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
HK-2 cells were counted, and 5 × 103 cells were plated in a 96-well plate. After the cells were cultured for less than 24 h, they were transfected with vectors or oligonucleotides under the conditions of NG, HG or HO. Then, the cells were incubated for 24, 48, 72 and 96 h; the supernatant was discarded. MTT solution (20 µL) was added to a final concentration of 5 mg/mL and the cells were incubated for another 4 h at 37°C. Subsequently, 200 µL of DMSO was added into each well; formazan crystals were fully dissolved by oscillating the plate for 10 min. Finally, the optical density (OD) value in each well was measured at a wavelength of 490 nm using a Microplate Reader (Bio-Rad), and the OD of the blank well was regarded as zero.
2.7 Flow cytometry for analyzing cell apoptosis
An Annexin V-FITC Apoptosis Detection Kit (Beyotime, Shanghai, China) was used to detect cell apoptosis. HK-2 cells treated under different treatment conditions and in different transfected groups were washed twice with phosphate buffered saline on ice (Gibco), and then diluted with a 1× binding buffer to a final concentration of approximately 1 × 106 cells/mL. Subsequently, 5 µL of Annexin V-FITC and propidium iodide (PI) was added to the labeled cell tubes, and the cells were stained for 15 min at room temperature in the dark. Finally, cell apoptosis was detected using flow cytometer (BD Biosciences, San Jose, CA, USA).
2.8 Dual-luciferase reporter assay
TargetScan predicted that ROCK2 might be a target of miR-30c-5p. Thus, a dual-luciferase reporter assay was conducted to confirm the direct interaction between miR-30c-5p and ROCK2. Common fragments of the synthetic wild-type (ROCK2 3′-UTR WT) or mutant (ROCK2 3′-UTR MUT) of ROCK2 3′-UTR were introduced into a pGL3-Basic vector (Promega, Madison, WI, USA). Subsequently, the correctly sequenced ROCK2 3′-UTR WT and ROCK2 3′-UTR MUT were co-transfected into HK-2 cells, and the cells were collected after transfection for 48 h. Then, luciferase assay reagent and Renilla assay buffer were dissolved with Dual-Luciferase Reporter Assay System Kit reagent (Promega). Ten microliters of cell lysate were added to each well of an opaque 96-well plate before the measurement of the luciferase and Renilla activities. Meanwhile, the firefly activities, as the indicator of the reporter gene, were identified by Luciferase Assay Reagent II, and the internal reference of Renilla activity was measured using Stop&Glo Reagent. The reporter gene was described via counting the ratio of firefly activity/Renilla activity.
2.9 Statistical analysis
The data were analyzed using SPSS 19.0 software and presented as mean ± standard deviation of three independent experiments. Student’s t-test and one-way analysis of variance were employed to examine the difference of pair groups and multiple groups, respectively. Statistical significance was considered at P < 0.05.
3 Results
3.1 Level of miR-30c-5p was effectively decreased, whereas ROCK2 expression was significantly increased in DN tissues
In order to investigate the biological roles of miR-30c-5p and ROCK2 in DN development, the levels of miR-30c-5p and ROCK2 were analyzed by qRT-PCR. The results demonstrated that miR-30c-5p was greatly downregulated, while ROCK2 was notably upregulated in DN tissues (Figure 1a and b). Furthermore, the ROCK2 protein level was increased in the DN group (Figure 1c). Moreover, there was a negative linear correlation between miR-30c-5p level and ROCK2 expression (Figure 1d). In brief, the data suggested that miR-30c-5p and ROCK2 may play vital roles in the progression of DN.

Level of miR-30c-5p was effectively decreased, whereas ROCK2 expression was significantly increased in DN tissues. (a and b) qRT-PCR was carried out to analyze the miR-30c-5p level and the mRNA level of ROCK2 in DN tissues compared with the paired control. (c) Protein expression of ROCK2 was measured by a western blot assay. (d) Spearman’s correlation analysis showed an inverse correlation between miR-30c-5p and ROCK2 mRNA levels. *P < 0.05.
3.2 HG conditions induced ROCK2 but suppressed miR-30c-5p expression in HK-2 cells
To determine whether the expression of miR-30c-5p and ROCK2 in the HG-induced DN cell model was in line with the tissues, HK-2 cells were treated with NG, HG and HO. qRT-PCR results suggested that the level of miR-30c-5p was significantly reduced, while the ROCK2 mRNA level was increased in the HG group compared with that in NG and HO groups (Figure 2a and b). Western blot assay showed that the protein expression of mature ROCK2 was enhanced in the HG group (Figure 2c). These data show that the expression trend of miR-30c-5p and ROCK2 in HG-stimulated HK-2 cells is in line with that in DN tissues.
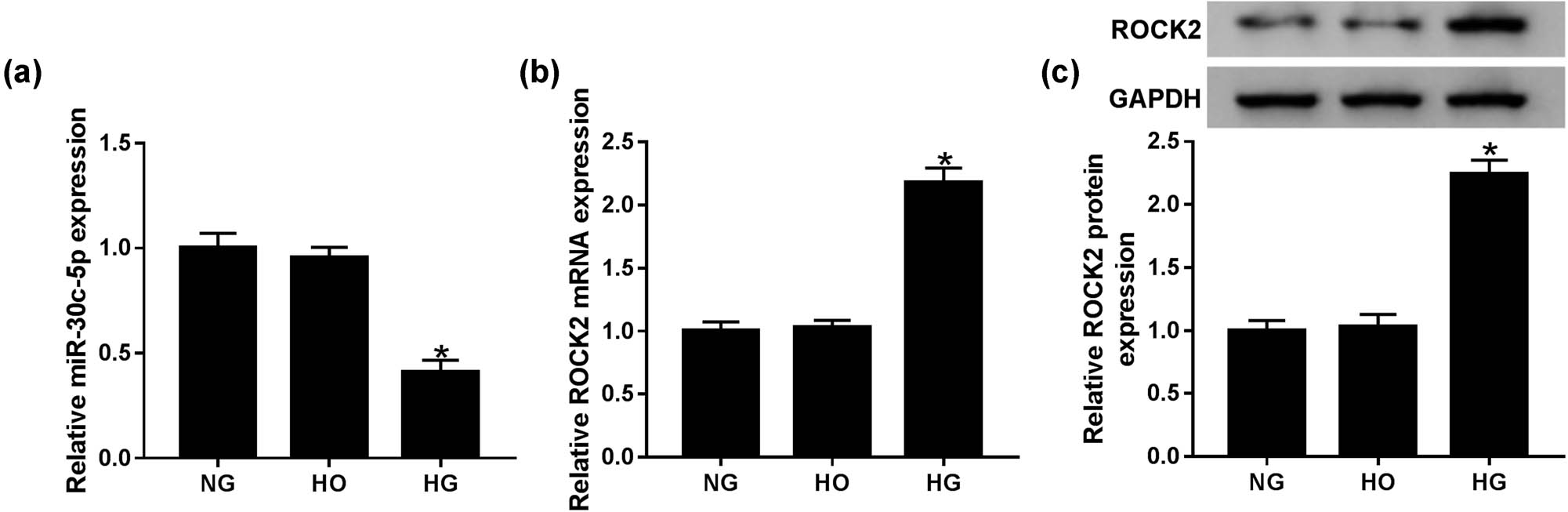
HG conditions in HK-2 cells induced ROCK2 expression but suppressed miR-30c-5p expression. (a and b) Levels of miR-30c-5p and ROCK2 were detected by qRT-PCR in HG-induced HK-2 cells. (c) Western blot assay was conducted to determine mature ROCK2 expression in vitro. NG: normal glucose (5.6 mmol/L d-glucose); HG: high glucose (30 mmol/L d-glucose); HO: high osmotic pressure solution (5.6 mmol/L d-glucose + 24.4 mmol/L d-mannitol). *P < 0.05.
3.3 MiR-30c-5p promoted cell proliferation and repressed cell apoptosis and epithelial-mesenchymal transition (EMT) in HG-induced HK-2 cells
Because of the low expression of miR-30c-5p in DN tissues, the assay aimed to investigate its potential biological role. An MTT assay was performed to detect cell proliferation of HK-2 cells under exposure to HG, and the result revealed that the proliferation of HK-2 cells in mock and NC groups was hindered with time, and there was no marked difference between these two groups. Simultaneously, the miR-30c-5p mimic dramatically promoted cell proliferation, whereas the miR-30c-5p inhibitor conspicuously restrained cell proliferation in HG-induced HK-2 cells (Figure 3a). As shown in Figure 3b, there was a distinct repression of cell apoptosis in HK-2 cells with the miR-30c-5p mimic, while the miR-30c-5p inhibitor prominently expedited the apoptosis rate in HK-2 cells. Furthermore, the boosted expression of E-cadherin, snail1 and TGFB1, as well as the inhibited levels of vimentin and α-SMA indicated that EMT was efficiently constrained by the miR-30c-5p mimic, while there was an opposite result caused by the miR-30c-5p inhibitor (Figure 3c). All evidence indicates that the miR-30c-5p mimic strikingly accelerates cell proliferation, while obviously impeding apoptosis and EMT in HG-stimulated HK-2 cells, while the miR-30c-5p inhibitor exerts an inverse effect.

MiR-30c-5p promoted cell proliferation and repressed cell apoptosis and EMT in HG-induced HK-2 cells. (a) MTT assay was performed to evaluate cell proliferation in the HG-induced DN cell model. (b) Cell apoptosis was examined by flow cytometry. (c) Levels of EMT-related proteins vimentin, E-cadherin, snail1, TGFB1 and α-SMA were identified via a western blot assay. *P < 0.05.
3.4 ROCK2 was a target gene of miR-30c-5p
TargetScan predicted the short binding sites shared by miR-30c-5p and ROCK2 (Figure 4a). Subsequently, a dual-luciferase reporter assay was conducted to confirm the interaction between miR-30c-5p and ROCK2, and the results uncovered that luciferase activity is efficiently inhibited by ROCK2 3′-UTR WT, while no evident difference was seen in the ROCK2 3′-UTR MUT group (Figure 4b). Additionally, qRT-PCR and western blot assays were performed to investigate the regulatory mechanism between miR-30c-5p and ROCK2, and the expression of ROCK2 was markedly suppressed by miR-30c-mimic but apparently induced by the miR-30c-5p inhibitor (Figure 4c and d). In short, these data suggest that ROCK2 is a direct downstream gene of miR-30c-5p.

ROCK2 is a target gene of miR-30c-5p. (a) TargetScan was used to predict the interaction between miR-30c-5p and ROCK2. (b) The relationship between miR-30c-5p and ROCK2 was confirmed by a dual-luciferase reporter assay. (c and d) The expression of ROCK2 was measured post-transfection with the miR-control, miR-30c-5p mimic and miR-30c-5p inhibitor, respectively. *P < 0.05.
3.5 ROCK2 inhibited cell proliferation and enhanced cell apoptosis and EMT in HG-induced HK-2 cells
To assess the therapeutic potential of ROCK2 in DN, si-ROCK2 and pcDNA-ROCK2 were transfected into HG-induced HK-2 cells. MTT assay results indicate that ROCK2 is an inhibitory factor in cell proliferation, and knockdown of ROCK2 evidently contributes to cell proliferation, as upregulation causes decreased cell proliferation in HG-induced HK-2 cells (Figure 5a). Moreover, cell apoptosis was dramatically repressed in the si-ROCK2 group, but apparently reinforced after transfection of pcDNA-ROCK2 in HG-treated HK-2 cells (Figure 5b). The EMT level was also determined through measuring the expression of vimentin, E-cadherin, α-SMA, snail1 and TGFB1, and the efficiently increased expression of E-cadherin, snail1 and TGFB1, and the conspicuously constrained expression of vimentin and α-SMA in the si-ROCK2 group suggest that knockdown of ROCK2 suppresses EMT in HG-induced HK-2 cells. The results of the pcDNA-ROCK2 group also support this conclusion (Figure 5c). These data reveal that knockdown of ROCK2 induces cell proliferation and suppresses apoptosis and EMT, whereas the function of ROCK2 overexpression in cell proliferation, apoptosis and EMT was opposite to that of ROCK2 silencing.

ROCK2 inhibited cell proliferation and enhanced cell apoptosis and EMT in HG-induced HK-2 cells. (a) Cell proliferation was analyzed by an MTT assay. (b) Flow cytometry was focused on HK-2 cell apoptosis. (c) The expression of EMT-related proteins including vimentin, E-cadherin, snail1, TGFB1 and α-SMA was assessed using a western blot assay. *P < 0.05.
3.6 MiR-30c-5p exerted its function by targeting ROCK2 in the HG-stimulated DN cell model
Due to the inverse functions of miR-30c-5p and ROCK2, miR-30c-5p mimic, miR-30c-5p mimic + vector and miR-30c-5p mimic + pcDNA-ROCK2 were transfected into HG-stimulated HK-2 cells, respectively. An MTT assay discovered that the promotion effect of miR-30c-5p mimic on cell proliferation was abrogated by pcDNA-ROCK2 (Figure 6a). Synchronously, cell apoptosis suppressed by miR-30c-5p was reversed via pcDNA-ROCK2 in vitro (Figure 6b). In addition, the inhibitory effect of miR-30c-5p mimic on EMT was restrained by overexpression of ROCK2 in HG-induced HK-2 cells (Figure 6c). In brief, the effects of miR-30c-5p mimic on cell proliferation, apoptosis and EMT were relieved by upregulation of ROCK2 in the HG-induced DN cell model.
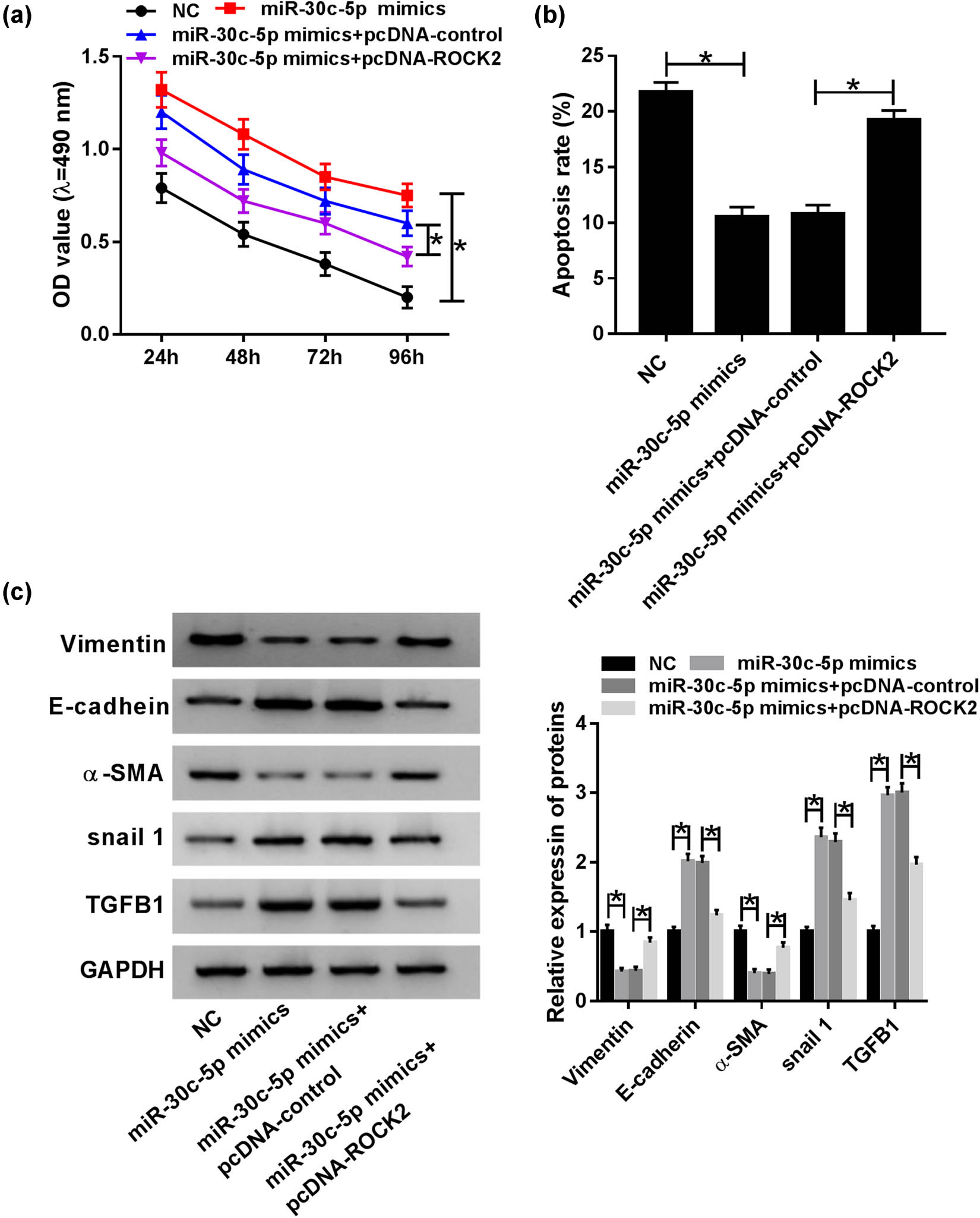
MiR-30c-5p exerted its function by targeting ROCK2 in the HG-stimulated DN cell model. (a) MTT assay was conducted to evaluate cell proliferation in vitro. (b) The impact of HG on cell apoptosis was analyzed via flow cytometry in HK-2 cells. (c) Western blot assay was used to determine mature protein expression of vimentin, E-cadherin, snail1, TGFB1 and α-SMA in HG-induced HK-2 cells. *P < 0.05.
3.7 The effects of miR-30c-5p and ROCK2 were exerted on DN progression via the PI3K/AKT signaling pathway
In order to investigate the regulatory mechanism of miR-30c-5p and ROCK2 in the progression of DN, we detected the expression of p-PI3K, PI3K, p-AKT and AKT after transfection with miR-30c-5p mimic, miR-30c-5p mimic + vector and miR-30c-5p mimic + pcDNA-ROCK2 in HK-2 cells treated with HG. The results showed that the expressions of both p-PI3K and p-AKT were remarkably decreased in the miR-30c-5p mimic group, and they were improved after co-transfection with miR-30c-5p and pcDNA-ROCK2 in vitro (Figure 7). This indicates that the roles of miR-30c-5p and ROCK2 are carried out via the PI3K/AKT signaling pathway.

Effects of miR-30c-5p and ROCK2 were exerted on DN progression via the PI3K/AKT signaling pathway. The expression of the PI3K/AKT pathway-related proteins including p-PI3K, PI3K, p-AKT and AKT was detected using a western blot assay and quantified via Image J software. *P < 0.05.
4 Discussion
It is well known that DN leads to end-stage kidney disease and increases all-cause mortality in diabetic patients [21]. According to the previous research, renal tubular damage is distinct in DN patients [22]. Here, HK-2 cells were treated with HG to cause damage, and the study found that the level of miR-30c-5p was obviously curbed in either HG-stimulated HK-2 cells or DN tissues. In contrast, ROCK2 expression was significantly augmented in the cell model and tissue samples. The aberrant expression of miR-30c-5p and ROCK2 showed their potential functions in the pathogenesis of human DN.
Emerging research indicates that miRNAs, such as miR-93 [23], miR-451 [24] and miR-23b [25], show aberrant expression in dysfunctional kidneys, including in DN. Understanding how miRNAs contribute to the progression of diseases is imminently important since several miRNAs are currently being explored as therapeutic agents for some diseases through early-stage clinical trials [26]. Moreover, miRNAs appear to be able to modify the expression of various target genes at transcription and post-transcription levels [27], and identification of classical or novel miRNA targets might provide unique therapeutic opportunities in different diseases. Based on the previous research examples, miR-27a is related to podocyte injury in DN via targeting Forkhead box protein O1 [28]. Over the past few decades, miR-30c has been proved to function as a tumor suppressor in colorectal carcinoma via regulating the target gene of B cell lymphoma 9 [29]. MiR-30c prevented diabetic cardiomyopathy by reducing the peroxisome proliferator-activated receptor alpha level [30]. Moreover, miR-30c-5p regulated neuropathic pain of rodents and was highly expressed in the spinal cord and dorsal root ganglia [31]. Furthermore, miR-30c-5p was associated with macrophage-mediated inflammation and pro-atherosclerosis signal pathways [32]. Increasing evidence reveals that EMT of glomerular endothelium is a modulation mechanism for potential cell dysfunction in endothelial injury [33]. In our study, miR-30c-5p was silent in DN tissue samples and HG-induced HK-2 cells. The miR-30c-5p mimic strikingly augmented cell proliferation, whereas it effectively hindered apoptosis and EMT in HG-induced DN cells. A previous study suggested that miR-29s and miR-let-7s exert antifibrotic effects on kidney fibrosis [12]. Consistently, miR-30c-5p has an antifibrotic effect through inhibiting EMT processes. This evidence suggested that miR-30c-5p participated in DN progression, and thus may be a novel target in clinical treatment of DN.
ROCK2 knockdown, which is regulated by miR-455-3p, suppressed renal fibrosis in DN [34], but it appears that the role of ROCK2 in the regulatory mechanism of diabetic heart disease is complex, referring to the phosphoinositide-dependent kinase-1/AKT signaling pathway [35]. Two members of the ROCK family have been associated with renal injury, e.g., a mouse model of unilateral ureteral obstruction nephropathy caused by tubulointerstitial fibrosis [36]. In this study, ROCK2 as the target factor was discovered to be directly targeted by miR-30c-5p. Furthermore, the promotion effect of the miR-30c-5p mimic on cell proliferation and inhibition effects on cell apoptosis and EMT were abolished by overexpression of ROCK2 in HG-stimulated HK-2 cells. Finally, the landmark proteins of the PI3K/AKT signaling pathway were also measured by a western blot assay, and the results demonstrated that miR-30c-5p and ROCK2 exerted roles at least partially via the PI3K/AKT signaling pathway in the progression of DN.
In summary, either the miR-30c-5p mimic or ROCK2 knockdown evidently induced cell proliferation, and notably restrained apoptosis and EMT in HG-stimulated HK-2 cells. Interestingly, the biological role of miR-30c-5p in cell proliferation, apoptosis and EMT was abrogated by overexpression of ROCK2 in vitro. Simultaneously, the miR-30c-5p/ROCK2 axis modulated the development of DN through the PI3K/AKT signaling pathway.
The level of miR-30c-5p was significantly curbed, while ROCK2 expression was effectively augmented in DN tissues. Moreover, HG-treated HK-2 cells were used to simulate a DN cell model in the study. Functionally, miR-30c-5p acted as a promoter of cell proliferation, while hindering cell apoptosis and EMT in HG-induced HK-2 cells. Additionally, the function of ROCK2 was opposite to that of miR-30c-5p in cell proliferation, apoptosis and EMT. Interestingly, ROCK2 was a target gene of miR-30c-5p and its overexpression abolished the effects of the miR-30c-5p mimic on cell proliferation, apoptosis and EMT in HG-stimulated HK-2 cells. Mechanically, the miR-30c-5p/ROCK2 axis regulated the progression of DN through the PI3K/AKT signaling pathway.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wu CC, Chen JS, Lu KC, Chen CC, Lin SH, Chu P, et al. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta. 2010;411:700–4.10.1016/j.cca.2010.01.036Search in Google Scholar PubMed
[2] Liu Z, Fu C, Wang W, Xu B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients – a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes. 2010;8:62.10.1186/1477-7525-8-62Search in Google Scholar PubMed PubMed Central
[3] Vikram A, Tripathi DN, Kumar A, Singh S. Oxidative stress and inflammation in diabetic complications. Int J Endocrinol. 2014;2014:679754.10.1155/2014/679754Search in Google Scholar PubMed PubMed Central
[4] Singh A, Friden V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, et al. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol. 2010;300:F40–8.10.1152/ajprenal.00103.2010Search in Google Scholar PubMed PubMed Central
[5] Hammond SM. An overview of microRNAs. Adv Drug Delivery Rev. 2015;87:3–14.10.1016/j.addr.2015.05.001Search in Google Scholar PubMed PubMed Central
[6] Grueter CE, Van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149:671–83.10.1016/j.cell.2012.03.029Search in Google Scholar PubMed PubMed Central
[7] van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–9.10.1126/science.1139089Search in Google Scholar PubMed
[8] Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–53.10.1038/nature10112Search in Google Scholar PubMed
[9] Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286:11837–48.10.1074/jbc.M110.194969Search in Google Scholar PubMed PubMed Central
[10] Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, et al. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med. 2013;19:481–7.10.1038/nm.3142Search in Google Scholar PubMed
[11] Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432–7.10.1073/pnas.0611192104Search in Google Scholar
[12] Srivastava SP, Goodwin JE, Kanasaki K, Koya D. Inhibition of angiotensin-converting enzyme ameliorates renal fibrosis by mitigating DPP-4 level and restoring antifibrotic microRNAs. Genes. 2020;11:211.10.3390/genes11020211Search in Google Scholar
[13] Vallelunga A, Iannitti T, Dati G, Capece S, Maugeri M, Tocci E, et al. Serum miR-30c-5p is a potential biomarker for multiple system atrophy. Mol Biol Rep. 2019;46:1661–6.10.1007/s11033-019-04614-zSearch in Google Scholar
[14] Zhang C, Yu S, Zheng B, Liu D, Wan F, Ma Y, et al. miR-30c-5p Reduces Renal Ischemia-Reperfusion Involving Macrophage. Med Sci Monit. 2019;25:4362–9.10.12659/MSM.914579Search in Google Scholar
[15] Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–93.10.1016/0014-5793(96)00811-3Search in Google Scholar
[16] Wang X, Zhao X, Feng T, Jin G, Li Z. Rutin prevents high glucose-induced renal glomerular endothelial hyperpermeability by inhibiting the ROS/Rhoa/ROCK signaling pathway. Planta Med. 2016;82:1252–7.10.1055/s-0042-110859Search in Google Scholar PubMed
[17] Koch J, Tönges L, Barski E, Michel U, Bähr M, Lingor P. ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis. 2014;5:e1225.10.1038/cddis.2014.191Search in Google Scholar PubMed PubMed Central
[18] Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405.10.1016/j.cell.2017.04.001Search in Google Scholar PubMed PubMed Central
[19] Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol. 2015;27:286–96.10.1016/j.smim.2015.08.001Search in Google Scholar PubMed PubMed Central
[20] Chi C, Loy SL, Chan SY, Choong C, Cai S, Soh SE, et al. Impact of adopting the 2013 World Health Organization criteria for diagnosis of gestational diabetes in a multi-ethnic Asian cohort: a prospective study. BMC Pregnancy Childbirth. 2018;18:69.10.1186/s12884-018-1707-3Search in Google Scholar PubMed PubMed Central
[21] He L, Qi Y, Rong X, Jiang J, Yang Q, Yamahara J, et al. The Ayurvedic medicine Salacia oblonga attenuates diabetic renal fibrosis in rats: suppression of angiotensin II/AT1 signaling. Evid Based Complement Alternat Med. 2011;2011:807451.10.1093/ecam/nep095Search in Google Scholar
[22] Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, et al. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complicat. 2003;17:11–5.10.1016/S1056-8727(02)00176-9Search in Google Scholar
[23] Badal SS, Wang Y, Long J, Corcoran DL, Chang BH, Truong LD, et al. miR-93 regulates Msk2-mediated chromatin remodelling in diabetic nephropathy. Nat Commun. 2016;7:12076.10.1038/ncomms12076Search in Google Scholar PubMed PubMed Central
[24] Sun Y, Peng R, Peng H, Liu H, Wen L, Wu T, et al. miR-451 suppresses the NF-kappaB-mediated proinflammatory molecules expression through inhibiting LMP7 in diabetic nephropathy. Mol Cell Endocrinol. 2016;433:75–86.10.1016/j.mce.2016.06.004Search in Google Scholar PubMed
[25] Zhao B, Li H, Liu J, Han P, Zhang C, Bai H, et al. MicroRNA-23b targets Ras GTPase-activating protein SH3 domain-binding protein 2 to alleviate fibrosis and albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2016;27:2597–608.10.1681/ASN.2015030300Search in Google Scholar PubMed PubMed Central
[26] Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discovery. 2013;12:847-65.10.1038/nrd4140Search in Google Scholar PubMed PubMed Central
[27] Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17:1712.10.3390/ijms17101712Search in Google Scholar PubMed PubMed Central
[28] Bai X, Geng J, Li X, Wan J, Liu J, Zhou Z, et al. Long noncoding RNA LINC01619 regulates microRNA-27a/forkhead box protein O1 and endoplasmic reticulum stress-mediated podocyte injury in diabetic nephropathy. Antioxid Redox Signal. 2018;29:355–76.10.1089/ars.2017.7278Search in Google Scholar PubMed
[29] Zhao D, Li M, Han J, Wang Y, Jiang L, Chang H. MiR-30c exerts tumor suppressive functions in colorectal carcinoma by directly targeting BCL9. Eur Rev Med Pharmacol Sci. 2019;23:3335–43.Search in Google Scholar
[30] Yin Z, Zhao Y, He M, Li H, Fan J, Nie X, et al. MiR-30c/PGC-1β protects against diabetic cardiomyopathy via PPARα. Cardiovasc Diabetol. 2019;18:7.10.1186/s12933-019-0811-7Search in Google Scholar PubMed PubMed Central
[31] Tramullas M, Francés R, de la Fuente R, Velategui S, Carcelén M, García R, et al. MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci Transl Med. 2018;10:eaao6299.10.1126/scitranslmed.aao6299Search in Google Scholar PubMed
[32] Ceolotto G, Giannella A, Albiero M, Kuppusamy M, Radu C, Simioni P, et al. miR-30c-5p regulates macrophage-mediated inflammation and pro-atherosclerosis pathways. Cardiovasc Res. 2017;113:1627–38.10.1093/cvr/cvx157Search in Google Scholar PubMed
[33] Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol. 2014;5:151.10.3389/fendo.2014.00151Search in Google Scholar PubMed PubMed Central
[34] Wu J, Liu J, Ding Y, Zhu M, Lu K, Zhou J, et al. MiR-455-3p suppresses renal fibrosis through repression of ROCK2 expression in diabetic nephropathy. Biochem Biophys Res Commun. 2018;503:977–83.10.1016/j.bbrc.2018.06.105Search in Google Scholar PubMed
[35] Lin G, Brownsey RW, MacLeod KM. Complex regulation of PKCβ2 and PDK-1/AKT by ROCK2 in diabetic heart. PLoS One. 2014;9:e86520.10.1371/journal.pone.0086520Search in Google Scholar PubMed PubMed Central
[36] Kanda T, Wakino S, Hayashi K, Homma K, Ozawa Y, Saruta T. Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int. 2003;64:2009–19.10.1046/j.1523-1755.2003.00300.xSearch in Google Scholar PubMed
© 2020 Lianshun Cui et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”

