Abstract
The bark of Hopea beccariana Burck is used to prevent damage to coconut sap. The purpose of this study was to determine the physicochemical and microbiological characteristics of various extracts of the bark of H. beccariana Burck as potential natural preservatives for coconut sap. The bark was extracted by maceration method for 24 h assisted by stirring using ethanol, methanol, n-hexane, and water at 60°C as solvent. The type of solvent used for extraction had a significant effect on the yield, total phenolic, total flavonoid, antioxidant activity, toxicity, and diameter of the inhibition zone against Lactobacillus plantarum. The highest yield was found in methanol extract at 22.34%, the highest total phenolic content was found in ethanol extract of 53.39 mg gallic acid equivalent/g extract, and the highest total flavonoid content was found in the aqueous extract at 60°C of 106.70 mg QE/g extract; all extracts have an IC50 value of 80.28–91.80 ppm (very strong antioxidant) and ascorbic acid of 5.78 ppm. Methanol extract is classified as very toxic with an LC50 of 38.21 ppm. The dominant compounds produced by gas chromatography–mass spectrometer were hexanedioic acid, bis(2-ethylhexyl) ester; ethyl oleate; 9-octadecenoic acid (Z)-, ethyl ester; and hexadecanoic acid, ethyl ester. The diameter of the inhibition zone for Saccharomyces cerevisiae was 13.50–14.72 mm, L. plantarum was 10.31–17.72 mm, and Leuconostoc mesenteroides was 13.25–18.06 mm. All extracts had minimum inhibitory concentration values of 2.5 mg/mL and minimum bactericidal concentration values of 2.5 mg/mL for n-hexane extract and 60°C water, while ethanol and methanol extracts had MBC values >50 mg/mL.
1 Introduction
Hopea beccariana Burck is a member of the Dipterocarpaceae family whose regional names are Cengal Pasir (Malay and Sarawak), Jangkang, Merawan, Merawan Batu (Malay), and Selangan Penak (Sabah) [1], while the local name of the plant in Mandor Village, Landak Regency, West Kalimantan Province-Indonesia, is Resak Jawai. The stem bark of H. beccariana Burck in West Kalimantan is used in the processing of coconut sugar, while other countries use the stem bark of Sacoglottis gabonensis and Alstonia boonei in Ikot Ekpene, Akwa Ibom State, Nigeria [2], Payorm (Shorea roxburghii G. Don) in Thailand [3], and Kiam wood (Cotylelobium lanceotatum Carih) in Thailand [4].
The bark of H. beccariana Burck, which is inserted into the coconut sap container during the sap-tapping process, aims to prevent the sap damage process caused by microbes while the sap is tapped. The presence of the bark of H. beccariana Burck added in the tapping process will produce juice that has good quality and can be processed into coconut sugar. It is suspected that the bark of H. beccariana Burck contains antimicrobial compounds, such as phenols and flavonoids. Based on existing references, the secondary metabolite content of plants from the Dipterocarpaceae family is very diverse and is believed to be an antimicrobial compound [5,6], such as phenols [7], oligostilbenoids (oligomer resveratrol) [8,9,10,11], flavonoids [12,13], and triterpenoid [14,15].
The active components in plants can be separated by extracting plant material using a solvent. The process of extracting active ingredients from plants has been carried out by several researchers [16,17,18,19,20]. Commonly used organic solvents to extract phytochemical content from plants are ethanol, methanol, n-hexane, and water. Methanol solvent was used to extract the phytochemistry compounds from the bark, fruit, and flowers of Crotalaria retusa L. [21]. Methanol and water solvents were used to determine the total phenol and flavonoid contents in the bark of Ricinodendron heudelotii [22]. Ethanol solvent was used to determine the total content of flavonoids and phenols in the leaves and roots of Euphorbia hirta [23]. Hot water was used to extract fresh Dendrobium sonia “Earsakul” orchids [24].
The stem bark of H. beccariana Burck extract obtained through the maceration process needs to be proven that the extract contains antimicrobial bioactive compounds, and then, the extract can be analyzed using gas chromatography–mass spectrometer (GC–MS) to see the secondary metabolite compounds. Several researchers have used GC–MS to determine the compound content of a plant [25,26,27,28]. For this reason, the role of secondary metabolite compounds that are antimicrobial and are believed to prevent the process of sap damage can be identified through the antimicrobial activity of the extracts obtained.
Currently, there is no scientific information related to the physicochemical characteristics of various types of H. beccariana Burck bark extract including yield, total phenol content, total flavonoid content, antioxidant activity based on the highest 50% inhibition concentration (IC50) value, toxicity based on lethality concentration 50% (LC50), bioactive compounds, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC). Therefore, in this study, it is necessary to extract the bark of H. beccariana Burck using various solvents of ethanol, methanol, n-hexane, and hot water. The purpose of this study was to determine the physicochemical and microbiological characteristics of various types of H. beccariana Burck extract, which have the potential as natural preservatives for coconut sap. Therefore, in this research, it is necessary to extract the bark of H. beccariana Burck using various solvents of ethanol, methanol, n-hexane, and hot water. The purpose of this study was to determine the physicochemical characteristics of various types of H. beccariana Burck extract, which have the potential as natural preservatives for coconut sap. It is hoped that the results of the study can provide information on the type of solvent used to extract the bark of H. beccariana Burck so that in the future the extract can be used to prevent the process of damage to coconut sap caused by microbes.
2 Materials and methods
2.1 Materials
The material used in this study was stem bark of H. beccariana Burck, obtained from Mandor Village, Mandor District, Landak Regency, West Kalimantan Province, Indonesia. The sampling location with the coordinate points is E 109°21′35.73″, N 00°18′26.50″ altitude of 41 m above sea level. The characteristics of the stem bark of H. beccariana Burck taken are the outer bark patterned with a mixed color of dark brown, gray, and green, while the inner bark is light brown, the age of the tree is ±20 years, and the height of the tree is ±12 m. The solvents used for the extraction process were ethanol (99.9%, J.T. Baker Avantor), methanol (99.8%, J.T. Baker Avantor), n-hexane (98.5%, J.T. Baker Avantor), and water.
2.2 Sample preparation
The stem bark of H. beccariana Burck is dried naturally using the sun until the moisture content reaches 9.70%. The bark was crushed with a disk mill (Type FFC-23 Qingdao Dahua Double Circle Machinery Co., LTD, China) into powder form [29] and then sieved through an 80 mesh sieve [30]. The dry powder obtained was packed in a plastic jar, tightly closed, and stored in a refrigerator at a low temperature (+4°C) until waiting for further use [21].
2.3 Extraction of H. beccariana Burck stem bark
The process of extracting the stem bark of H. beccariana Burck uses the maceration method and is modified from a combination of procedures carried out by [31,32,33]. Samples of H. beccariana Burck bark powder of 20 g mixed with 100 mL of solvent each (ethanol grade 99.8%, methanol grade 99.8%, n-hexane, and hot water temperature 60°C) were macerated while stirring using a magnetic stirrer at room temperature for 24 h. The solution is filtered with filter paper Whatman No. 42 in a vacuum. Solvents are removed using a rotary evaporator (Buchi Rotaevaporator R114, Switzerland) at 45°C for ethanol, methanol, and n-hexane solvents until 90% of the solvent is evaporated, while the water solvent is evaporated at a temperature of 60°C. The extract is stored at a temperature condition of +4°C until analysis.
2.4 Yield extract
The yield of the extract is the weight of the extracted material divided by the weight of the raw bark material multiplied by 100% [34].
2.5 Total phenolic content determination
The total phenolic content in the stem bark extract of H. beccariana Burck was determined by following the procedure from [35] using spectrophotometry by the Folin–Ciocalteu test method. The extract is made as much as 5 mg and aquadest are added until the volume reaches 5 mL in a measuring flask. The extract solution was pipetted 1 mL and added to 9 mL of distilled water in a volumetric flask (25 mL). The phenol reagent Folin–Ciocalteu has added as much as 1 mL, homogenized, and incubated for 5 min. A 7% solution of sodium carbonate (Na2CO3) (weighed as much as 3.5 g of Na2CO3 then dissolved with aquadest up to 50 mL) of 10 mL was added to the mixture. The volume of the solution is made up to 25 mL by adding aquadest. Gallic acid standard solutions were made in several concentrations, namely 20, 40, 60, 80, and 100 µg/mL, in the same way as the extract sample preparation described previously. Samples of the extract and standard solution of gallic acid were incubated for 90 min at room temperature. The absorbance value of the test sample and the standard solution was determined against the blank reagent at 550 nm with an ultraviolet (UV)/visible spectrophotometer. The total phenol content is expressed as mg of gallic acid equivalent (GAE)/g extract.
2.6 Total flavonoid content determination
The total flavonoid content of the stem bark of H. beccariana Burck extract was measured following the procedure of [35]. The total flavonoid content was measured by the aluminum chloride colorimetric test. The extract was made as much as 5 mg and added methanol until the volume reached 5 mL in a measuring flask. The extract solution was pipetted 1 mL and reacted with 4 mL of distilled water into a 10 mL volumetric flask. 0.3 mL of 5% sodium nitrite was added and left for 5 min, adding 0.3 mL of 10% aluminum chloride. After 5 min, 2 mL of 1 M sodium hydroxide was added to distilled water so that the volume becomes 10 mL. Standard solutions of quercetin were prepared at various concentrations (20, 40, 60, 80, and 100 µg/mL) in the same manner as previously described. Measuring the absorbance of the sample solution and standard solution based on the reagent blank at 510 nm using a UV/Visible spectrophotometer. The total flavonoid content in the extract was expressed as mg QE/g extract.
2.7 Antioxidant activity testing
Determination of the antioxidant activity of the bark extract of H. beccariana Burck using the DPPH (1,1-diphenyl-2-picrylhydrazyl) method following the procedure of [36,37] modified. The solution DPPH 0.4 mM is prepared by weighing DPPH 0.0158 g and dissolved with methanol p.a up to 100 mL in a measuring flask. A stock solution with a concentration of 1,000 ppm consisting of 50 mg of H. beccariana Burck stem bark extract dissolved in a measuring flask by adding up to 50 mL of methanol was made. On the absorption measurement of the blank DPPH solution, first pipetting 1 mL of 0.4 mM DPPH solution by adding methanol to 5 mL in the measuring flask. On the other hand, it prepares a series of extract solution concentrations from the stock that has been made (concentration of 1,000 ppm) for testing the free radical scavenging activity of DPPH with extract samples consisting of concentrations of 20, 40, 60, 80, 100, 120, and 140 ppm. Each concentration series plus 1 mL of DPPH 0.4 mM is then added methanol until the volume reaches 5 mL in the measuring flask. The solution of DPPH blanks and extract samples were homogenized and incubated for 30 min. Measure the uptake of the DPPH blank solution and extract the sample with UV–Vis spectrophotometry at a wavelength of 515 nm. As a comparison, ascorbic acid solutions were made with concentrations of 2, 4, 6, and 8 ppm, the work was the same as before.
The percentage of free radical scavenging is calculated by the formula:
and determination of IC50 value (50% inhibitory concentration) based on probit analysis from concentration log data with probit free radical scavenging percentage.
2.8 Toxicity (LC50) test of Hopea beccariaan Burck stem bark extract
Extract toxicity was carried out through the Brine Shrimp Lethality test (BSLT) [36]. Artemia salina L. eggs were incubated in a 1 L glass beaker filled with seawater and irradiated with a 40-W fluorescent lamp while being aerated for 48 h. The eggs hatch into nauplii and are ready to be tested. A sample stock solution with a concentration of 2,000 ppm (40 mg of bark extract in 20 mL of seawater) was made. Five sterile tubes were prepared, then add 1 mL of seawater and 10 nauplii into each tube, and sample solution from the stock solution of 2,000 ppm made into 10, 100, 500, and 1,000 ppm, while the control used 4 mL of seawater containing 10 nauplii without adding sample stock. The five tubes were incubated for 24 hours at room temperature. The mean percentage of nauplii mortality was plotted against the logarithm of the concentration of the bark extract of Hopea beccariaan Burck. The half maximal mortality concentration (LC50) was determined by calculating the antilogarithmic linear equation obtained from the curve of the relationship between the level of the bark extract and the mean percent mortality of Artemia nauplii.
2.9 Analysis of the content of bioactive compounds in extracts using GC–MS
GC–MS was used to identify the compounds present in the extract according to the procedure [38]. The analysis was carried out according to the specifications of the Shimadzu QP 2010 SE GC–MS equipment: column type is 5 ms, Restek Corp (30 m length). The condition of the injector is gas chromatography with a split ratio of 8.4, the sample flow rate is 21.8 mL/min, and the helium flow rate is 2.00 mL/min. The condition of the gas chromatographic column was that the initial column temperature was 60°C, the final column temperature was 290°C, the initial holding time was 5 min, the final holding time was 10 min, and the temperature rise rate was 6°C per minute. Mass spectroscopy conditions with interface temperature 250°C, electron ionization temperature 300°C, and detection range m/z at 45–500 m/z, with retention time (Rt) 40 min. The carrier gas used is helium with an amount of 45–500 atomic mass units (AMU). Compound identification and structure determination were based on a comparison of mass spectra and fragmentation profiles using published data using Wiley 229, NIST 12, and NIST 62 Library software.
2.10 Antimicrobial activity test using disk diffusion method
The antimicrobial activity test was carried out using the agar diffusion method using disk paper according to the procedure [39]. The test microbes used were the yeast Saccharomyces cerevisiae and the bacteria Lactobacillus plantarum and Leuconostoc mesenteroides. Cultures of S. cerevisiae were first grown on potato dextrose agar (PDA) media [40]. One inoculating loop of S. cerevisiae culture was inoculated on 5 mL of sterile PDA media and then incubated at 30°C for 48 h [41], while L. plantarum and L. mesenteroides were grown on de Man, Rogosa and Sharpe (MRS) broth media [42], and one loop of bacterial culture was inoculated on 5 mL of sterile MRS broth media and then incubated at 30°C for 24 h. Microbial suspension of 300 µL/Petri dish (for yeast) and 250 µL/Petri dish (for bacteria) on the agar surface by the spread plate method, so that each cup contains about 3 × 106 CFU. Disks (size 5 mm) were immersed in the bark extract of H. beccariana Burck at a concentration of 5% (250 µg/50 µL or 50 µg/µL) which was prepared from 1 mg of extract dissolved in 200 µL of sterile distilled water. The disks were placed on a medium that had been inoculated with yeast and bacteria aseptically, respectively. The positive control used fluconazole and streptomycin antibiotics each with a concentration of 250 ppm and the negative control used ethanol, methanol, n-hexane, and sterile distilled water as solvents. All plates were incubated at 30°C for 24 h. Observations were made on the diameter of the clear zone around the disk, which was recorded in millimeters (mm).
2.11 Determination of MIC and MBC
2.11.1 Preparation of growth media and microbial suspension
The yeast suspension of S. cerevisiae was grown on PDA media, while L. plantarum and L. mesenteroides were grown in MRS broth with a density of 106 CFU/mL. A suspension was prepared based on the turbidity of the McFarland 0.5 standard made from 9.95 mL of 1% sulfate acid solution and 0.05 mL of 1.175% barium chloride solution, which is equivalent to the density of yeast and bacteria 108 CFU/mL [43,44]. A tube containing a standard solution of 0.5 McFarland was prepared. Yeast and bacterial suspensions were prepared by taking 4–10 oses of PDA and MRS broth media that had been incubated for 24 h, putting them in a tube containing 0.9% natrium chloride and then homogenized. The yeast and bacterial suspensions were equalized for turbidity with a standard solution of 0.5 McFarland. The suspension that has been made is then diluted by pipetting 0.1 mL of yeast and bacterial suspension (108 CFU/mL) put into a sterile tube and added 9.9 mL of 0.9% natrium chloride solution so that the density of the yeast and test bacteria is 106 CFU/mL.
2.11.2 Determination of MIC
The determination of the MIC was carried out according to the modified study [42,45]. A series of concentrations of H. beccariana Burck bark extract 2.5, 5.0, 10, 30, and 50 mg/mL, which has been diluted with 1% CMC solvent, were made. Sterile test tubes were prepared according to the number of treatments. The test tubes were filled with 8.8 mL of PDA and 8.8 mL of sterile MRS broth, respectively. Each reaction tube was added with 1 mL of H. beccariana Burck extract according to the predetermined concentration and 200 µL of each culture of the yeast S. cerevisiae and L. plantarum and L. mesenteroides bacteria. All tubes were vortexed so that they were homogeneous. All reaction tubes were incubated for 24 h at 37°C. Determination of MIC based on the difference in optical density value after incubation minus before incubation resulted in the lowest negative concentration from the results of measuring using a spectrophotometer at a wavelength of 630 nm.
2.11.3 Determination of MBC
Determination of MBC refers to research [42] by first diluting the PDA and MRS broth and preparing five sterile Petri dishes. The bark extract of H. beccariana Burck from the concentration series was determined during the MIC test and also the control was put into a tube containing PDA and MRS broth. The tube was added to each suspension of yeast and bacteria, then homogenized and then poured into a sterile Petri dish, and waited for the media to solidify. After that, it was incubated at 37°C for 24 h. The incubation results can be seen by the presence or absence of colony growth on the media. The total bacterial colonies were calculated using the colony counter. Determination of MBC based on the absence of yeast and bacteria growth was not seen in the Petri dish.
2.12 Data analysis
The physicochemical and microbiological characteristics data were carried out in three replications and averaged by displaying the standard deviation (SD). The data obtained were analyzed by one-way analysis of variance (ANOVA) at a 5% level. If the treatment given has a significant effect, it is continued with the Tukey test at a 5% level. The SAS software version 9.4 was used to process ANOVA and Tukey test data, and Microsoft Excel 365 was used to collect and organize primary data.
3 Results
3.1 Physicochemical characteristics of Hopea beaccariana Burck bark extract
3.1.1 Yield extract of H. beccariana Burck stem bark
The results of the analysis of variance showed that the use of various types of solvents to extract the stem bark of H. beccariana Burck had a noticeable influence (α = 5%) on the yield. Figure 1 shows that ethanol and methanol have higher yields of 21.88 and 22.34%, respectively, compared to using n-hexane and water of 0.80 and 11.98%, respectively.
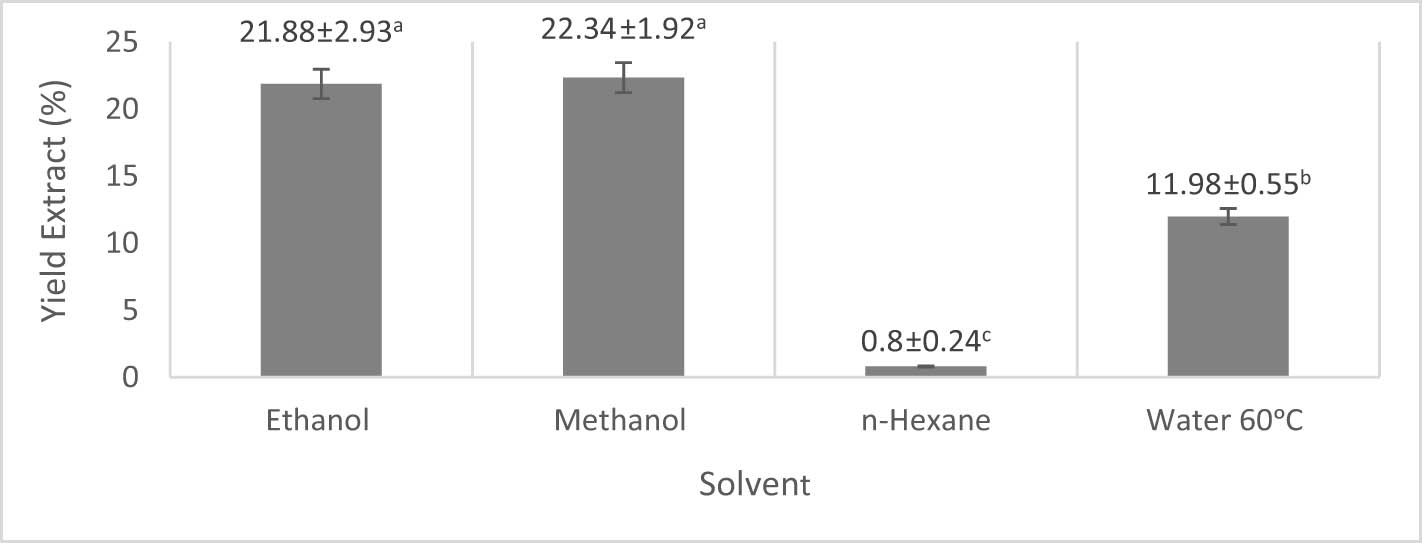
The yield of H. beccariana Burck stem bark extract from various solvents.
3.1.2 Total phenolic content of H. beccariana Burck stem bark extract
The results of the analysis of variance showed that the use of various types of solvents to extract the bark of H. beccariana Burck had a significant effect (α = 5%) on the total phenolic content. Table 1 shows that the ethanol extract of the bark of H. beccariana Burck has the highest total phenolic content (53.39 mg GAE/g) and is significantly different from other extracts, while the lowest total phenolic content was found in the n-hexane extract at 18.56 mg GAE/g.
Total phenolic content, total flavonoid, and antioxidant activity (IC50) of various types of H. beccariana Burck stem bark extract
| Solvent | Total phenolic content ± SD* (mg GAE/g extract) | Total flavonoid content ± SD* (mg QE/g extract) | Antioxidant activity, IC 50 ± SD* (ppm) |
|---|---|---|---|
| Ethanol | 53.39 ± 1.61a | 99.37 ± 6.78a | 80.28 ± 1.44b |
| Methanol | 30.47 ± 1.45b | 91.58 ± 9.90ab | 84.75 ± 1.77ab |
| n-Hexane | 18.56 ± 1.41c | 78.58 ± 3.30b | 81.83 ± 4.01b |
| Water 60°C | 33.94 ± 2.09b | 106.70 ± 0.99a | 91.80 ± 2.83a |
| Tukey α = 5% | 4.35 | 16.32 | 7.08 |
| Ascorbic acid | 5.78 | ||
*Values with different notations in the same column show a significant difference in the 5% Tukey test.
3.1.3 Total flavonoid content of H. beccariana Burck stem bark extract
The results of the analysis of variance showed that the use of various types of solvents to extract the bark of H. beccariana Burck had a significant effect (α = 5%) on the total flavonoid content. The extract was obtained by maceration process for 24 h at room temperature with three repetitions using solvents of ethanol, methanol, n-hexane, and hot water at 60°C and added with stirring using a magnetic stirrer, the total flavonoid content varied. Based on Table 1, the highest total flavonoid content was found in the bark extract of H. beccariana Burck using hot water at 60°C, which was 106.70 mg QE/g, while the lowest total flavonoid content was found in extracts using the n-hexane solvent of 78.58 mg QE/g.
3.1.4 Antioxidant activity (IC50) of H. beccariana Burck stem bark extract
The results of the analysis showed that the use of various types of solvents to extract the stem bark of H. beccariana Burck had a significant effect (α = 5%) on the IC50 value. Table 1 shows that all extracts of the bark of H. beccariana Burck, both ethanol, methanol, n-hexane, and water extracts are classified as having strong antioxidant activity with IC50 of 80.28, 84.75, 81.83, and 91.80 ppm, respectively, while the IC50 value of ascorbic acid as a comparison is 5.78 ppm.
3.1.5 Toxicity test of H. beccariana Burck stem bark extract
The results of the toxicity test using the brine shrimp lethality test (BSLT) method using Artemia salina L. sea shrimp larvae from various types of H. beccariana Burck bark extract are presented in Figure 2. The concentration of H. beccariana Burck stem bark extract used was up to 1,000 ppm in the toxicity test resulting that the methanol and water extracts being classified as very toxic with LC50 of 38.21 and 75.74 ppm, respectively.

Toxicity LC50 (ppm) of H. beccariana Burck stem bark extract from various solvents.
3.2 Content of bioactive compounds in H. beccariana Burck stem bark extract using GC–MS
3.2.1 H. beccariana Burck stem bark ethanol extract
The GC–MS chromatogram (Figure 3) shows 20 compounds identified from the ethanolic extract of the bark of H. beccariana Burck consisting of groups of alkaloids, alcohols, phenolics, aromatics, alkanes, alkenes, and fatty acid esters (Table 2).
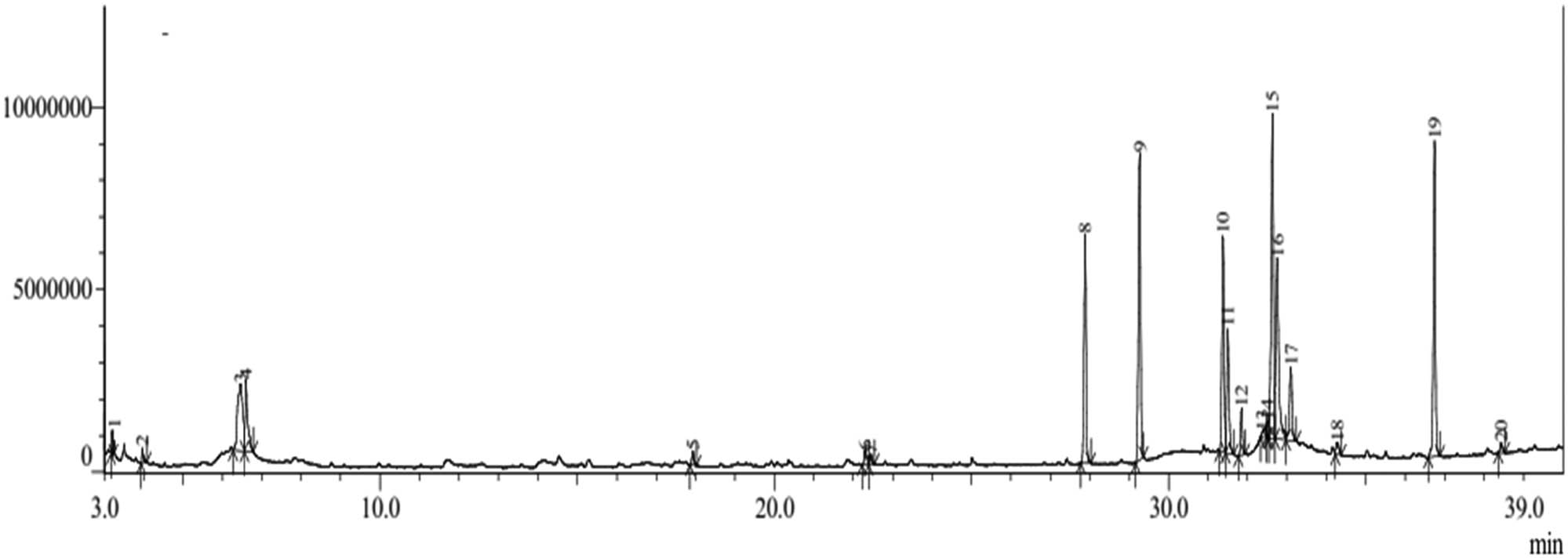
Chromatogram of GC–MS ethanol extract of H. beccariana Burck stem bark.
Identified compounds from ethanol extract of H. beccariana Burck stem bark based on GC–MS spectrum
| No. peak | Retention time (min) | Compound name | Formula molecule | Compound group | Area (%) |
|---|---|---|---|---|---|
| 1 | 3.201 | Pyrrolidine-.alpha.,.alpha.,.alpha.′,.alpha.′-D4 | C4H5D4N | Alkaloid | 0.42 |
| 2 | 3.960 | 2-Furanmethanol | C5H6O2 | Alcohol | 0.46 |
| 3 | 6.455 | Phenol | C6H6O | Phenol | 7.48 |
| 4 | 6.600 | 1-Hexanol, 2-ethyl | C8H18O | Alcohol | 3.68 |
| 5 | 17.924 | Pentadecane | C15H32 | Alkane | 0.60 |
| 6 | 22.296 | 9-Eicosene, (E) | C20H40 | Alkene | 0.85 |
| 7 | 22.430 | 1-Pentadecene | C15H30 | Alkene | 0.47 |
| 8 | 27.875 | Hexadecanoic acid, methyl ester | C17H34O2 | Fatty acid ester | 9.35 |
| 9 | 29.263 | Hexadecanoic acid, ethyl ester | C18H36O2 | Fatty acid ester | 13.42 |
| 10 | 31.381 | 9-Octadecenoic acid, methyl ester, (E)- | C19H36O2 | Fatty acid ester | 9.55 |
| 11 | 31.504 | 9-Octadecenoic acid, methyl ester, (E)- | C19H36O2 | Fatty acid ester | 5.96 |
| 12 | 31.854 | Octadecanoic acid, methyl ester | C19H38O2 | Fatty acid ester | 1.85 |
| 13 | 32.385 | Phenol, 4,4′-methylenebis- | C13H12O2 | Aromatic | 0.67 |
| 14 | 32.505 | 9,12-Octadecadienoic acid (Z, Z)-methyl ester | C19H34O2 | Fatty acid ester | 0.65 |
| 15 | 32.637 | Ethyl oleate | C20H38O2 | Fatty acid ester | 15.52 |
| 16 | 32.757 | Ethyl oleate | C20H38O2 | Fatty acid ester | 10.85 |
| 17 | 33.103 | Heptadecanoic acid, ethyl ester | C19H38O2 | Fatty acid ester | 3.70 |
| 18 | 34.276 | Ethyl linoleate | C20H36O2 | Fatty acid ester | 0.72 |
| 19 | 36.744 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | Fatty acid ester | 13.36 |
| 20 | 38.428 | Decanoic acid, 2-ethylhexyl ester | C18H36O2 | Fatty acid ester | 0.44 |
The group of fatty acid ester compounds was the largest identified. The percentage of the fatty acid ester compound group was 85.37 consisting of hexadecanoic acid, methyl ester (9.35%), hexadecanoic acid, ethyl ester (13.42%), 9-octadecenoic acid, methyl ester, (E)- (9.55%), 9-octadecenoic acid, methyl ester, (E)- (5.96%), octadecanoic acid, methyl ester (1.85%), ethyl oleate (15.52%), ethyl oleate (10.85%), heptadecanoic acid, ethyl ester (3.70%), ethyl linoleate (0.72%), hexanedioic acid, bis(2-ethylhexyl) ester (13.36%), and decanoic acid, 2-ethylhexyl ester (0.44%), The group of alkene compounds consists of 9-eicosene (E) (0.85%) and 1-pentadecene (0.47%). The group of alcohol compounds (4.14%) consisted of 2-furanmethanol (0.46%) and 1-hexanol, 2-ethyl (3.68%).
There was a group of compounds that identified only one compound, including groups of alkaloids, phenols, aromatics, and alkanes. The group of alkaloid compounds is pyrrolidine-.alpha.,.alpha.,.alpha.′,.alpha.′-D4 (0.42%). The group of phenolic compounds is phenol (7.48%). The group of aromatic compounds is phenol, 4,4′-methylenebis- (0.67%). The group of alkane compounds is pentadecane (0.60%).
3.2.2 H. beccariana Burck stem bark methanol extract
The GC–MS chromatogram (Figure 4) shows 20 compounds identified from the methanol extract of the bark of H. beccariana Burck consisting of groups of ketones, aldehydes, alcohols, phenolics, aromatics, alkanes, alkenes, and fatty acid esters (Table 3),
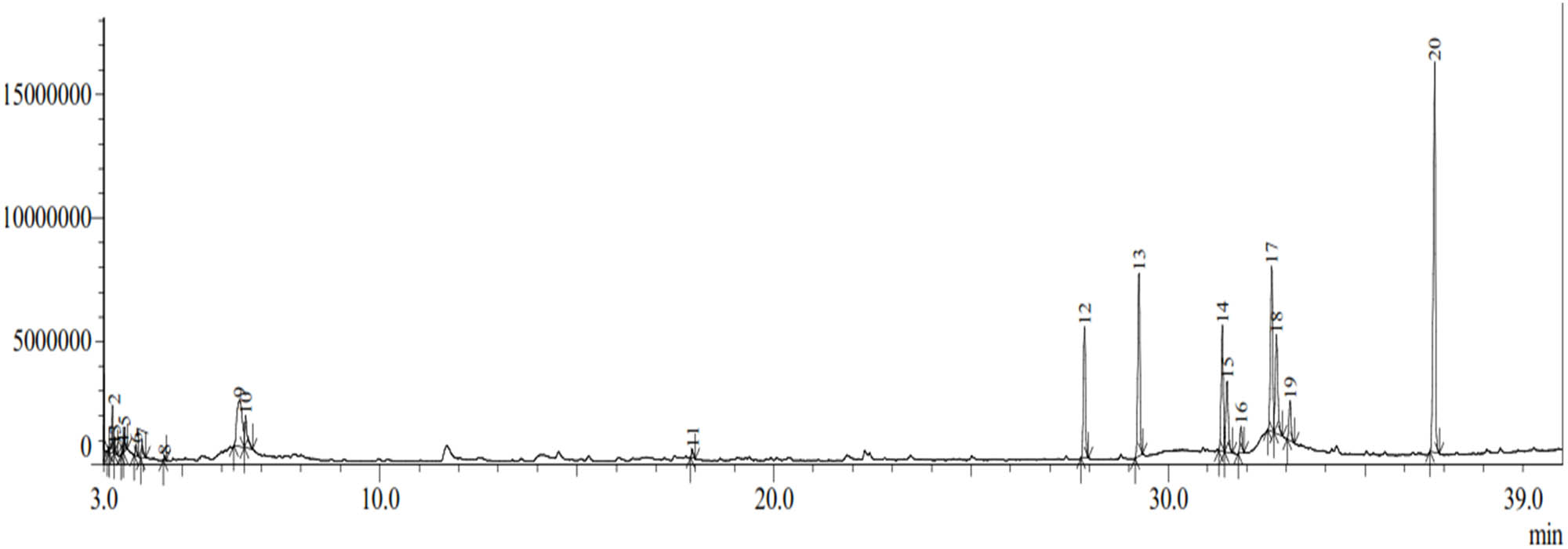
Chromatogram of GC–MS methanol extract of H. beccariana Burck stem bark.
Identified compounds from methanol extract of H. beccariana Burck stem bark based on GC–MS spectrum
| No. peak | Retention time (min) | Compound name | Formula molecule | Compound group | Area (%) |
|---|---|---|---|---|---|
| 1 | 3.121 | (2H)-Furan-3-one | C4H4O2 | Ketone | 0.15 |
| 2 | 3.223 | Ethane, 1,1′,1″-[methylidynetris(oxy)]tris | C7H16O3 | Alkane | 1.62 |
| 3 | 3.284 | Propanoic acid 2-hydroxy-ethyl ester | C5H10O3 | Fatty acid ester | 0.47 |
| 4 | 3.480 | 2.5-Furandione | C4H2O3 | Ketone | 0.27 |
| 5 | 3.531 | 2-Furancarboxaldehyde | C5H4O2 | Aldehyde | 1.06 |
| 6 | 3.822 | N-Methoxy formamide | C2H5NO2 | Aromatic | 0.40 |
| 7 | 3.975 | 2-Furanmethanol | C5H6O2 | Alcohol | 0.78 |
| 8 | 4.546 | Ethane, 1,1′,1″-[methylidynetris(oxy)]tris | C7H16O3 | Alkane | 0.15 |
| 9 | 6.454 | Phenol | C6H6O | Phenol | 8.07 |
| 10 | 6.602 | 1-Hexanol, 2-ethyl- | C8H18O | Alcohol | 2.26 |
| 11 | 17.924 | Pentadecane | C15H32 | Alkene | 0.48 |
| 12 | 27.875 | Hexadecanoic acid, methyl ester | C17H34O2 | Fatty acid ester | 8.71 |
| 13 | 29.254 | Hexadecanoic acid, ethyl ester | C18H36O2 | Fatty acid ester | 11.57 |
| 14 | 31.382 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | Fatty acid ester | 8.63 |
| 15 | 31.503 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | Fatty acid ester | 5.69 |
| 16 | 31.854 | Octadecanoic acid, methyl ester | C19H38O2 | Fatty acid ester | 1.68 |
| 17 | 32.634 | 9-Octadecenoic acid (Z)-, ethyl ester | C20H38O2 | Fatty acid ester | 11.52 |
| 18 | 32.755 | 9-Octadecenoic acid (Z)-, ethyl ester | C20H38O2 | Fatty acid ester | 7.93 |
| 19 | 33.103 | Heptadecanoic acid, ethyl ester | C19H38O2 | Fatty acid ester | 2.65 |
| 20 | 36.765 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | Fatty acid ester | 25.93 |
The group of fatty acid ester compounds was the largest identified (Table 3). The percentage of fatty acid ester compound group was 84.78 consisting of propanoic acid, 2-hydroxy-, ethyl ester (0.47%), hexadecanoic acid, methyl ester (8.71%), hexadecanoic acid, ethyl ester (11.57%), 9-octadecenoic acid (Z)-, methyl ester (8.63%), 9-octadecenoic acid (Z)-, methyl ester (5.69%), octadecanoic acid, methyl ester (1.68%), 9-octadecenoic acid (Z)-, ethyl ester (11.52%), 9-octadecenoic acid (Z)-, ethyl ester (7.93%), heptadecanoic acid, ethyl ester (2.65%), and hexanedioic acid, bis(2-ethylhexyl) ester (25.93%).
Only one or two groups of compounds were identified including groups of aldehydes, aromatics, phenols, alkenes, alcohols, ketones, and alkanes (Table 3). The aldehyde group is 2-furancarboxaldehyde (1.06%). The aromatic group is N-methoxy formamide (0.40%). The phenol group is phenol (8.07%). The alkene group is pentadecane (0.48%). The alcohol group included 2-furanmethanol (0.78%) and 1-hexanol, 2-ethyl- (2.26%). The ketone group of 0.42% consisted of (2H)-furan-3-one (0.15%) and 2,5-furandione (0.27%). The alkane group of 1.77% consisted of ethane, 1,1′,1″-[methylidynetris(oxy)]tris (1.62%), and ethane, 1,1′,1″-[methylidynetris(oxy)]tris (0.15%).
3.2.3 H. beccariana Burck stem bark n-hexane extract
The GC–MS chromatogram (Figure 5) shows 20 compounds identified from the n-hexane extract of the bark of H. beccariana Burck consisting of groups of phenolic compounds, alcohols, alkanes, alkenes, aromatics, and fatty acid esters (Table 4).
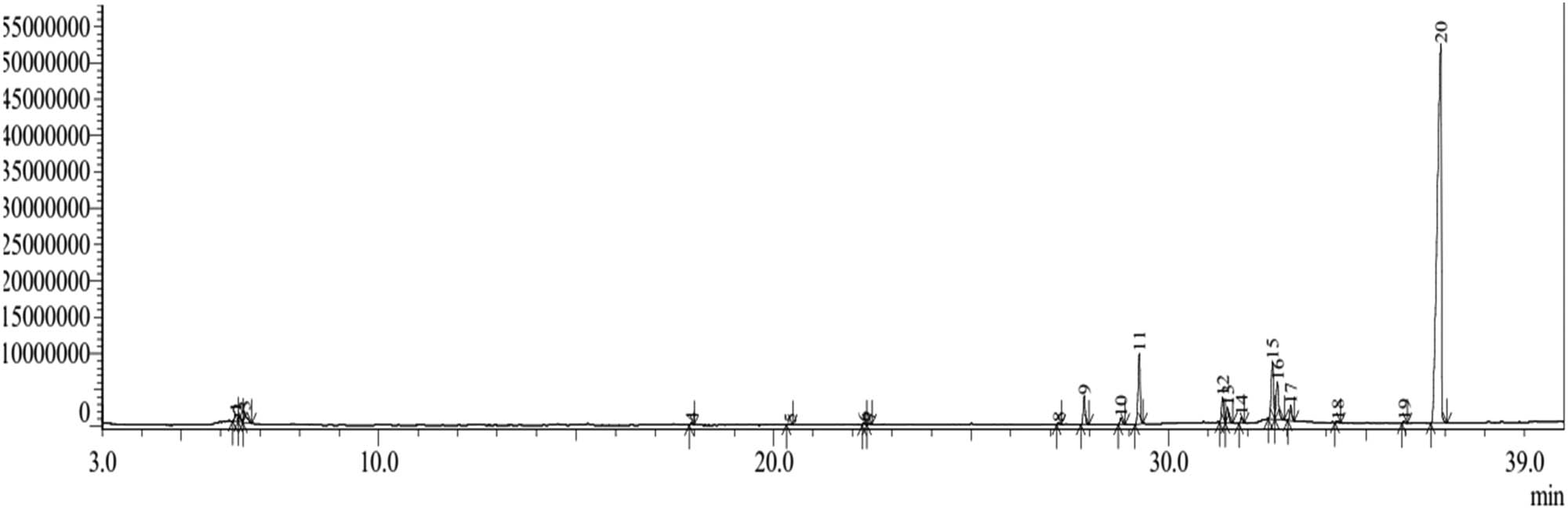
Chromatogram of GC–MS n-hexane extract of H. beccariana Burck stem bark.
Identified compounds from the n-hexane extract of H. beccariana Burck stem bark based on GC–MS spectrum
| No. peak | Retention time (min) | Compound name | Formula molecule | Compound group | Area (%) |
|---|---|---|---|---|---|
| 1 | 6.385 | Phenol | C6H6O | Phenol | 0.99 |
| 2 | 6.455 | Phenol | C6H6O | Phenol | 0.64 |
| 3 | 6.602 | 1-Hexanol, 2-ethyl- | C8H18O | Alcohol | 1.32 |
| 4 | 17.919 | Pentadecane | C15H32 | Alkane | 0.23 |
| 5 | 20.425 | Hexadecane | C16H34 | Alkane | 0.14 |
| 6 | 22.288 | 9-Octadecene, (E)- | C18H36 | Alkene | 0.20 |
| 7 | 22.428 | 1-Pentadecene | C15H30 | Fatty acid ester | 0.12 |
| 8 | 27.211 | Hexanedioic acid, diethyl ester | C10H18O4 | Fatty acid ester | 0.14 |
| 9 | 27.862 | Hexadecanoic acid, methyl ester | C17H34O2 | Fatty acid ester | 2.61 |
| 10 | 28.792 | Dibutyl phthalate | C16H22O4 | Aromatic | 0.63 |
| 11 | 29.256 | Hexadecanoic acid, ethyl ester | C18H36O2 | Fatty acid ester | 6.23 |
| 12 | 31.368 | 9-Octadecenoic acid, methyl ester, (E)- | C19H36O2 | Fatty acid ester | 2.48 |
| 13 | 31.493 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | Fatty acid ester | 1.72 |
| 14 | 31.846 | Octadecanoic acid, methyl ester | C19H38O2 | Fatty acid ester | 0.51 |
| 15 | 32.635 | Ethyl oleate | C20H38O2 | Fatty acid ester | 5.96 |
| 16 | 32.757 | 9-Octadecenoic acid (Z)-, ethyl ester | C20H38O2 | Fatty acid ester | 4.23 |
| 17 | 33.095 | Heptadecanoic acid, ethyl ester | C19H38O2 | Fatty acid ester | 1.38 |
| 18 | 34.280 | Ethyl linoleate | C20H36O2 | Fatty acid ester | 0.21 |
| 19 | 35.957 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | Fatty acid ester | 0.18 |
| 20 | 36.890 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | Fatty acid ester | 70.07 |
The group of fatty acid ester compounds was the largest identified (Table 4). The percentage of fatty acid ester compound group is 95.84% consisting of 1-pentadecene (0.12%), hexanedioic acid, diethyl ester (0.14%), hexadecanoic acid, methyl ester (2.61%), hexadecanoic acid, ethyl ester (6.23%), 9-octadecenoic acid, methyl ester, (E)- (2.48%), 9-octadecenoic acid (Z)-, methyl ester (1.72%), octadecanoic acid, methyl ester (0.51%), ethyl oleate (5.96%), 9-octadecenoic acid (Z)-, ethyl ester (4.23%), heptadecanoic acid, ethyl ester (1.38%), ethyl linoleate (0.21%), hexanedioic acid, bis(2-ethylhexyl) ester (0.18%), and Hexanedioic acid, bis(2-ethylhexyl) ester (70.07%).
Only one or two groups of compounds were identified (Table 4), including aromatic groups, alkenes, alcohols, phenols, and alkanes. The aromatic group is dibutyl phthalate (0.63%). The alcohol group is 1-hexanol, 2-ethyl- (1.32%). The alkene group is 9-octadecene, (E)- (0.20%). The phenol group (1.63%) consisted of phenol (0.99%) and phenol (0.64%). The alkane group (0.37%) consisted of pentadecane (0.23%) and hexadecane (0.14%).
3.2.4 Water 60°C extract stem bark of H. beccariana Burck
The GC–MS chromatogram (Figure 6) shows 20 compounds identified from the 60°C aqueous extracts of the bark of H. beccariana Burck consisting of groups of alkenes, aldehydes, fatty acids and fatty acid esters, ethers, steroids, and alcohols (Table 5).
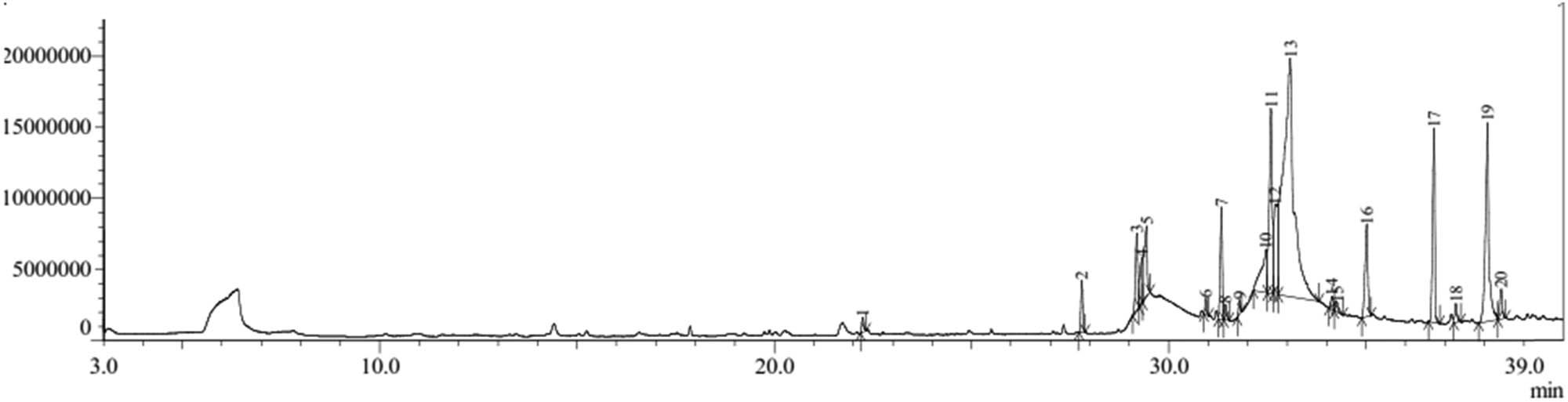
Chromatogram of GC–MS water 60°C extract of H. beccariana Burck stem bark.
Identified compounds from hot water 60°C extracts of H. beccariana Burck stem bark based on GC–MS spectrum
| No. peak | Retention time (min) | Compound name | Formula molecule | Compound group | Area (%) |
|---|---|---|---|---|---|
| 1 | 22.237 | 9-Octadecene, (E)- | C18H36 | Alkene | 0.48 |
| 2 | 27.796 | Hexadecanoic acid, methyl ester | C17H34O2 | Fatty acid ester | 1.52 |
| 3 | 29.188 | Hexadecanoic acid, ethyl ester | C18H36O2 | Fatty acid ester | 2.95 |
| 4 | 29.315 | 9-Octadecenal, (Z)- | C18H34O | Aldehyde | 1.89 |
| 5 | 29.431 | n-Hexadecanoic acid | C16H32O2 | Fatty acid | 3.80 |
| 6 | 30.936 | Oxirane, tetradecyl- | C16H32O | Ether | 0.51 |
| 7 | 31.328 | 9-Octadecenoic acid, methyl ester, (E)- | C19H36O2 | Fatty acid ester | 3.62 |
| 8 | 31.427 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | Fatty acid ester | 0.60 |
| 9 | 31.791 | Octadecanoic acid, methyl ester | C19H38O2 | Fatty acid ester | 0.40 |
| 10 | 32.455 | Ethyl linoleate | C20H36O2 | Fatty acid ester | 4.21 |
| 11 | 32.585 | 9-Octadecenoic acid (Z)-, ethyl ester | C20H38O2 | Fatty acid ester | 8.54 |
| 12 | 32.690 | Ethyl oleate | C20H38O2 | Fatty acid ester | 6.25 |
| 13 | 33.073 | Oleic acid | C18H34O2 | Fatty acid | 40.46 |
| 14 | 34.127 | 14-β-H-Pregna | C21H36 | Steroid | 0.47 |
| 15 | 34.260 | (R)-(−)-14-Methyl-8-hexadecyn-1-ol | C17H32O | Alcohol | 0.49 |
| 16 | 35.017 | Hexadecanoic acid, 2-hydroxy-1,3-propanediyl ester | C35H68O5 | Fatty acid ester | 4.31 |
| 17 | 36.726 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | Fatty acid ester | 6.76 |
| 18 | 37.276 | 9-Tetradecenal, (Z)- | C14H26O | Aldehyde | 0.69 |
| 19 | 38.076 | 9-Octadecenal, (Z)- | C18H34O | Aldehyde | 10.85 |
| 20 | 38.425 | Hexadecanoic acid, 2-hydroxy-1,3-propanediyl ester | C35H68O5 | Fatty acid ester | 1.19 |
The group of fatty acid compounds and fatty acid esters was the largest identified (Table 5). The percentage of fatty acid compounds and fatty acid esters is 84.61 consisting of hexadecanoic acid, methyl ester (1.52%), hexadecanoic acid, ethyl ester (2.95%), n-hexadecanoic acid (3.80%), 9-octadecenoic acid, methyl ester, (E)- (3.62%), 9-octadecenoic acid (Z)-, methyl ester (0.60%), octadecanoic acid, methyl ester (0.40%), ethyl linoleate (4.21%), 9-octadecenoic acid (Z)-, ethyl ester (8.54%), ethyl oleate (6.25%), oleic acid (40.46%), hexadecanoic acid, 2-hydroxy-1,3-propanediyl ester (4.31%), hexanedioic acid, bis(2-ethylhexyl) ester (6.76%), and hexadecanoic acid, 2-hydroxy-1,3-propanediyl ester (1.19%). The aldehyde group (3.40%) consisted of 9-octadecenal, (Z)- (1.89%), 9-tetradecenal, (Z)- (0.69%), and 9-octadecenal, (Z)- (10.85%).
The group of compounds identified only one compound, namely the ether group, steroids, alcohols, and alkenes (Table 5). The ether group is oxirane, tetradecyl- (0.51%). The steroid group was 14-β-H-pregna (0.27%). The alcohol group is (R)-(−)-14-methyl-8-hexadecyn-1-ol (0.49%), and the alkene group is 9-octadecene, (E)- (0.48%).
3.3 Microbiological characteristics of H. beccariana Burck stem bark extract
3.3.1 Inhibition zone diameter
The results of the analysis of variance showed that the use of various solvents to extract the bark of H. beccariana Burck had a significant effect (α = 5%) on L. plantarum, while for S. cerevisiae and L. mesenteroides it had an insignificant effect (α = 5%). The data on the diameter of the inhibition zone are presented in Table 6.
Diameter of inhibition zones of various types of H. beccariana Burck stem bark extract
| Extracts | Inhibition zone diameter (mm) ± SD | ||
|---|---|---|---|
| S. cerevisiae* | L. plantarum* | L. mesenteroides* | |
| Ethanol | 14.28 ± 3.29a | 17.72 ± 4.28a | 13.25 ± 3.44a |
| Methanol | 14.72 ± 0.92a | 15.67 ± 2.68ab | 15.22 ± 3.59a |
| n-Hexane | 13.50 ± 0.00a | 10.31 ± 1.63b | 13.83 ± 2.47a |
| Water 60°C | 14.56 ± 1.75a | 13.56 ± 1.86ab | 18.06 ± 8.22a |
| Tukey 5% | — | 7.34 | — |
*Values with different notations in the same column show significant differences in the 5% Tukey test.
The diameter of the fluconazole antibiotic inhibition zone (positive control) against the yeast S. cerevisiae was between 9.83 and 27.33 mm (Table 7), while the antimicrobial activity against the yeast S. cerevisiae in the negative control was only shown in ethanol and methanol solvents with the diameter of the inhibition zones 10.82 and 10.55 mm, respectively. Compared with the results of the study (Table 6), extracts of ethanol, methanol, n-hexane, and water at 60°C had the same inhibitory ability against yeast S. cerevisiae with inhibition zone diameters 14.28, 14.72, 13.50, and 14.56 mm, respectively. This means that the antimicrobial activity of the stem bark extract has the same ability as fluconazole antibiotics and is better than the negative control.
Inhibition zone diameter of positive control and negative control
| Kontrol | Jenis Ekstrak | Diameter zona penghambatan (mm) ± SD | ||
|---|---|---|---|---|
| S. cerevisiae | L. plantarum | L. mesenteroides | ||
| Positive control | ||||
| Fluconazole antibiotics | Ethanol | 12.33 ± 1.84 | nt | nt |
| Methanol | 27.33 ± 0.34 | nt | nt | |
| n-Hexane | 9.83 ± 0.60 | nt | nt | |
| Water 60°C | 10.28 ± 0.35 | nt | nt | |
| Streptomycin antibiotics | Ethanol | nt | 13.56 ± 4.88 | 11.36 ± 1.13 |
| Methanol | nt | 12.05 ± 0.25 | 11.25 ± 1.09 | |
| n-Hexane | nt | 11.34 ± 0.76 | 10.16 ± 0.17 | |
| Water 60°C | nt | 10.89 ± 0.63 | 18.42 ± 9.05 | |
| Negative control | Ethanol | 10.82 ± 0.02 | 10.39 ± 0.38 | 9.42 ± 0.51 |
| Methanol | 10.55 ± 0.15 | 8.51 ± 0.50 | 9.37 ± 0.38 | |
| n-Hexane | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Water 60°C | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
0.00 – does not show antimicrobial activity; nt – not tested.
The diameter of the zone of inhibition of streptomycin antibiotics (positive control) against L. plantarum bacteria ranged from 10.89 to 13.56 mm and L. mesenteroides bacteria between 10.16 and 18.42 mm. In the negative control, the antimicrobial activity against L. plantarum and L. mesenteroides bacteria was only shown in ethanol and methanol solvents with inhibition zone diameters 10.39, 8.51, 9.42, and 9.37 mm. respectively. Compared with the data shown in Table 6, the extract had an inhibitory zone diameter exceeding that of the positive control against L. plantarum and had the same strength against the positive control of L. mesenteroides and better than the negative control. This means that the resulting extract has antimicrobial compounds that can inhibit the growth of L. plantarum and L. mesenteroides bacteria.
3.3.2 MIC and MBC values
The concentrations of the tested H. beccariana Burck stem bark extract ranged from 2.5, 5.0, 10, 30, and 50 mg/mL. The data on the results of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests are shown in Table 8.
MIC and MBC of various types of stem bark extract of H. beccariana Burck against S. cerevisiae yeast and L. plantarum and L. mesenteroides bacteria
| Extract | Types of microbes | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|---|
| Ethanol | S. cerevisiae | 2.5 | >50 |
| L. plantarum | 2.5 | >50 | |
| L. mesenteroides | 2.5 | >50 | |
| Methanol | S. cerevisiae | 2.5 | >50 |
| L. plantarum | 2.5 | >50 | |
| L. mesenteroides | 2.5 | >50 | |
| n-Hexane | S. cerevisiae | 2.5 | >50 |
| L. plantarum | 2.5 | 2.5 | |
| L. mesenteroides | 2.5 | 2.5 | |
| Water 60°C | S. cerevisiae | 2.5 | 2.5 |
| L. plantarum | 2.5 | 2.5 | |
| L. mesenteroides | 2.5 | 2.5 |
Table 8 shows that the MIC values of all extracts with solvents of ethanol, methanol, n-hexane, and water were at the smallest concentration of 2.5 mg/mL for each microbe used. In the MBC test, especially the ethanol and methanol extracts with a concentration range of 2.5−50 mg/mL were still unable to kill the microbes tested for S. cerevisiae, L. plantarum, and L. mesenteroides. The n-hexane extract was unable to kill S. cerevisiae too; thus, they were requiring a concentration of >50 mg/mL to kill the test microbes used. The n-hexane extract was able to kill only L. plantarum and L. mesenteroides with a concentration of 2.5 mg/mL.
4 Discussion
4.1 Yield extract of H. beccariana Burck stem bark
The solvent used in the maceration process has a different degree of polarity, so the yield of the extract produced is also different. More polar solvents in the extraction process will produce more yields than using non-polar solvents. This is evidenced by [46] that extraction in highly polar solvents (ethanol and methanol) yields high extract yields compared to non-polar solvents such as n-hexane. Methanol solvent has easy properties to form hydrogen and water in plant tissue cells and can dissolve polar organic compounds [47]. Results were also reported by [48] that the best results were obtained when using ethanol as a solvent for the extraction of kinnow (Citrus reticulate L.) peel. [49] reported that the yield of n-hexane extract from Dillenia suffruticosa leaves was smaller (5.77%) compared to the yield of methanol extract (9.27%).
4.2 Total phenolic content of H. beccariana Burck stem bark extract
The use of n-hexane as a solvent resulted in lower total phenolic compared to ethanol, methanol, and water at 60°C. These results indicate that most of the phenolic compounds in the bark of Dipterocarpaceae are soluble in polar solvents. The extraction of phenolic compounds in plants is very suitable using polar solvents [50]. This study also used hot water at 60°C with a total phenolic content of 33.94 mg GAE/g extract. These results are the same as those of [51] that the total phenol content produced from a crude extract of Neem leaves using water as a solvent is higher than that of methanol and n-hexane. The release of hydrophilic phenolic compounds in plant cells will increase if given heat treatment [52].
Each H. beccariana Burck bark extract contains phenolic compounds that have the potential to prevent microbial damage to coconut sap. Phenolic compounds obtained from plant extracts can function as antimicrobials [7,53,54]. The phenolic extract obtained from honey functions as an antimicrobial and can inhibit the growth of gram-negative bacteria (Pseudomonas aeruginosa) and gram-positive bacteria (Enterococcus faecalis and Staphylococcus aureus) [55].
4.3 Total flavonoid content of H. beccariana Burck stem bark extract
The total flavonoid content obtained in the extract in this study was influenced by the type of solvent used for the extraction process. The levels of flavonoid compounds produced will vary because they are influenced by the source of the raw material or the type of plant, the type and condition of the solvent [56,57], and the type of extraction used [58,59].
The total flavonoid content (Table 2) produced using a solvent temperature of 60°C was higher than that of using ethanol, methanol, and n-hexane as solvents. Although statistically, the total levels of flavonoids produced by water solvents were not significantly different from those using ethanol and methanol solvents. The high level of total flavonoids is influenced by the water solvent, which is given heat treatment. However, the yield (Figure 1) and total phenol content (Table 1) were lower than ethanol and methanol without heat treatment. The results are in line with the research of [60] that the heat treatment given to the extraction process of Calophyllum inophyllum leaves using 80% methanol as a solvent at temperatures ranging from 30°C to a maximum temperature of 60°C increased the total flavonoid content but could increase the extraction causing the yield and total phenol content to be small. Water solvent is used in the extraction process and assisted by an increase in temperature; the total flavonoid content in the extract will increase [18].
In terms of the potential content of flavonoid compounds in each H. beccariana Burck bark extract. The extracted material can function as a natural ingredient to prevent the process of damage to coconut sap caused by microbes. Flavonoids from natural ingredients have the potential to affect antimicrobial activity [61,62,63]. The antimicrobial activity test shown in Table 6 stated that the stem bark extract of H. beccariana Burck has an inhibitory effect on the sap-destroying microbe, such as S. cerevisiae yeast and L. plantarum and L. mesenteroides bacteria. Some researchers state that flavonoid compounds from plants function as antimicrobials [12,13,64] and have antibacterial properties [65,66] because the hydroxyl group on the aromatic ring of flavonoids increases the activity of flavonoid compounds [67].
4.4 Antioxidant activity (IC50) of H. beccariana Burck stem bark extract
The antioxidant activity shown in Table 1 has an IC50 value that is almost the same as the ethanol extract of Eucheuma Cotoni [68], namely 90.10 ppm. The lower the IC50 measurement value, so the stronger the antioxidant activity. The strong antioxidant activity is reflected in the presence of phenolic and flavonoid compounds in the extract. The presence of total phenolic and flavonoid levels in plant extracts will indicate antioxidant activity [69,70]. The phenolic compounds in a plant material act as antioxidants because phenolic compounds have redox properties, have hydroxyl groups, and can inhibit free radicals [71]. The conformation of structural antioxidants is influenced by antioxidants in the extract and DPPH radicals, so the number of antioxidant hydroxyl groups in the extract will be the same as the number of reduced DPPH radical molecules [72]. Very high levels of flavonoid compounds correlated with having strong antioxidant activity in Hypericum perforatum extract [59].
4.5 Toxicity test of H. beccariana Burck stem bark extract
The division of toxicity test categories with the BSLT method was LC50 < 500 g/mL (1 g/mL = 1 ppm) classified as relatively toxic and LC50 < 100 g/mL classified as very toxic [73]. The LC50 value of <200 g/mL of the compounds tested by the BSLT method is classified as toxic compounds and has the potential to be used as an anticancer [74]. Compounds that have high toxicity activity have the potential for anticancer compounds [75]. Crude methanol extract of the bark and leaves of Diospyros mespiliformis was safe to use for 28 days for therapeutic purposes through acute and sub-chronic toxicity testing in rats [76]. However, it is necessary to do further research in vivo on the bark extract of H. beccariana Burck to determine the actual dose for safe use.
4.6 Content of bioactive compounds of H. beccariana Burck bark extract
4.6.1 H. beccariana Burck stem bark ethanol extract
The most dominant compounds found in the ethanol extract of the stem bark of H. beccariana Burck are classified as fatty acid ester compounds (Table 5) consisting of ethyl oleate (15.52 and 10.85%), hexadecanoic acid, ethyl ester (13.42%), and hexanedioic acid, bis(2-ethylhexyl)ester (13.36%). The group of fatty acid ester compounds includes antimicrobial compounds. Oleic compounds are fatty acids that can be produced by S. aureus with antimicrobial functions [77]. In addition to fatty acid ester compounds, phenolic compounds have the potential as antimicrobial compounds. The phenolic group bound to the fatty acid chain is antimicrobial and can inactivate gram-positive bacteria, such as Listeria innocua [78].
4.6.2 H. beccariana Burck stem bark methanol extract
The most dominant compounds found in the ethanol extract of the bark of H. beccariana Burck (Table 6) are hexanedioic acid, bis(2-ethylhexyl)ester (25.93%), hexadecanoic acid, ethyl ester (11.57%), and 9-octadecenoic acid (Z)-ethyl ester (11.57 and 7.93%). These compounds are classified as fatty acid ester compounds. Hexanedioic acid, bis(2-ethylhexyl)ester compounds were also identified in the methanol:water extract of Senegali gaumeri leaf, which has anthelmintic activity against eggs and larvae of Haemonchus contortus [79]. Phenol compounds were also identified in the methanol extract of about 8.07%. These compounds function as antimicrobials [80].
4.6.3 H. beccariana Burck stem bark n-hexane extract
The most dominant compounds found in the n-hexane extract of the bark of H. beccariana Burck (Table 7) were hexanedioic acid, bis(2-ethylhexyl)ester (70.07 and 0.18%) and hexadecanoic acid, ethyl ester (6.23 %). These compounds are classified as fatty acid ester compounds. Hexanedioic acid, bis(2-ethylhexyl)ester compound is found in the biosynthesis of Streptomyces sp. TN262 strain and has antimicrobial properties [81]. On the other hand, the GC–MS results also identified hexadecane compounds (0.14%), although the concentration of these compounds was small. Fatty acid compounds such as hexadecane compounds function as antimicrobials obtained from isolates of D-3 actinomycetes [82].
4.6.4 H. beccariana Burck stem bark water 60°C extract
The most dominant compounds found in the aqueous extract of the bark of H. beccariana Burck (Table 8) were 9-octadecenoic acid (Z)-ethyl ester (8.54%) and hexanedioic acid, bis(2-ethylhexyl)ester (6.76%). These compounds are classified as fatty acid ester compounds. The oleic acid compound identified was suspected to be antimicrobial. Oleic acid has antibacterial activity against S. aureus [83]. Hexanedioic acid, bis(2-ethylhexyl)ester is also present in the seed extract of Foeniculum vulgare Mill. functions as antimicrobial, anticancer, diuretic and anti-inflammatory [84] and is found in the wood extracts of Populus lasiocarpa and Populus tomentosa [85]. On the other hand, 14-β-H-pregna compounds were identified in the aqueous extract of the bark of H. beccariana Burck. The compound 14-β-H-pregna is a compound that has the prevention of diabetic retinopathy and this compound is found in the essential oils of the plants Scutellaria multicaulis and Scutellaria bornmuelleri [86].
4.7 Microbiological characteristics of H. beccariana Burck stem bark extract
4.7.1 Antimicrobial activity
The results shown in Table 6 that the bark extract of H. beccariana Burck used ethanol, methanol, n-hexane, and water at 60°C proved to be able to inhibit the growth of S. cerevisiae yeast and L. plantarum and L. mesenteroides bacteria. This is evident from the results shown in the GC–MS that each extract identified antimicrobial compounds with the largest group of compounds being fatty acid esters, followed by phenolic compounds. Groups of fatty acid compounds and fatty acid esters show their ability as antimicrobial compounds [87,88]. Phenol compounds can inhibit the growth of Lactobacillus bacteria [53]. Groups of compounds, such as hexanedioic acid, bis(2-ethylhexyl) ester, which are included in the group of fatty acid ester compounds, have antimicrobial properties. Hexanedioic acid, bis(2-ethylhexyl)ester compound is found in the methanol extract of Marine sponges (phylum Porifera), which functions as an antibacterial [89].
4.7.2 MIC and MBC values
Based on Table 8, the results of the MIC test were at 2.5 mg/mL against the S. cerevisiae yeast and L. plantarum and L. mesenteroides bacteria, which caused damage to coconut sap. These results indicate that the bark extract of H. beccariana Burck is still better than the study [42], which reported that propolis alcohol extract had an MIC at 50 mg/mL and propolis water extract had an MIC of 3.12–25 mg/mL of bacteria that cause spoilage in fish. The MIC value of the H. beccariana Burck extracts shown in Table 8 have an inhibitory concentration that is not much different from that of the methanol extract of the leaves of Bridelia micrantha (Hochst.) Baill had MICs against Streptococcus pyogenes, Salmonella typhi, and Candida albicans of 2.5, 1.25, and 2.5 mg/mL, respectively [90].
In the MBC test results, there were still extracts that were not able to kill the test microbes in the concentration range of 2.5–50 mg/mL. It is suspected that the bioactive components present in the extract have not been able to damage the cell membranes of yeasts and test bacteria, with the concentration of the extract given still inhibiting growth or being bacteriostatic and not yet capable of being bactericidal at the given concentration meaning that it has not been able to kill all the microbes present. An antibiotic is bacteriostatic if it only suppresses microbial growth and is bactericidal if it can kill microbes [91]. Extracts that have MBC test results of 2.5 mg/mL against yeast and bacteria mean that the extract has bioactive components that can damage the cell membranes of yeasts and bacteria with a small concentration of 2.5 mg/mL. The bioactivity of active compounds is produced due to the interaction between active components that are antagonistic so that antimicrobial properties are formed and can damage microbial cell membranes [92].
5 Conclusion
The use of various solvents for the maceration extraction process gave different results in terms of yield, total phenol content, total flavonoid content, antioxidant activity, and toxicity of Hopea beccarian Burck bark extract. Ethanol and methanol solvents can produce higher yields than using n-hexane and water at 60°C. The highest total phenol content was obtained in the use of ethanol as solvent, followed by water at 60°C, methanol, and n-hexane. The identified bioactive compounds using GC–MS are supporting materials to strengthen and provide inhibitory power against sap-destroying microbes. The use of water as a solvent resulted in a higher total flavonoid content for the bark extraction of H. beccariana Burck. On the other hand, water solvents are safe to use, environmentally friendly, inexpensive, affordable, and very suitable for reacting with other plant materials needed for the food and pharmaceutical fields. The extract can inhibit the growth of S. cerevisiae yeast and L. plantarum and L. mesenteroides bacteria, so it can be used as a natural preservative for coconut sap.
Acknowledgments
The authors would like to thank Tanjungpura University, Pontianak, and Sebelas Maret University, Surakarta, for their support.
-
Funding information: This research was funded by the Ministry of Education and Culture, Research and Technology of the Republic of Indonesia through a Doctoral Dissertation research grant.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
[1] MyBIS. Native plants Hopea beccariana (Merawan Batu). Malaysia Biodiversity Information System; 2020. [cited 2020 Nov 30]. p. 1–9. https://www.mybis.gov.my/dwc/438.Suche in Google Scholar
[2] Elijah AI, Ojimelukwe PC, Ekong US, Asamudo NU. Effect of Sacoglottis gabonensis and Alstonia boonei on the kinetics of Saccharomyces cerevisiae isolated from palm wine. Afr J Biotechnol. 2010;9(35):5730–4.Suche in Google Scholar
[3] Singsoong K, Benjapong W, Nitithamyong A, Tavichatwitayakul R, Karnpanit W, Moungsrichan N, et al. Preservatives used in coconut sap. Toxicol Lett. 2010;196(2010):S341–2. 10.1016/j.toxlet.2010.03.1079.Suche in Google Scholar
[4] Naknean P, Meenune M, Roudaut G. Characterization of palm sap harvested in Songkhla province. southern Thailand. Int Food Res J. 2010;17(4):977–86.Suche in Google Scholar
[5] Chung S-H, Lee S-K, Ji C-H, Park J-H. Vacuum packaged electromagnetic 2D scanning micromirror. Sens Actuators A Phys. 2019;290:147–55. 10.1016/j.sna.2019.03.021.Suche in Google Scholar
[6] Tan CC, Karim AA, Uthumporn U, Ghazali FC. Effect extraction temperature on the emulsifying properties of gelatin from black tilapia (Oreochromis mossambicus) skin. Food Hydrocoll. 2020;108:106024. 10.1016/j.foodhyd.2020.106024.Suche in Google Scholar
[7] Muhammad H, Qasim M, Ikram A, Versiani MA, Tahiri IA, Yasmeen K, et al. Antioxidant and antimicrobial activities of Ixora coccinea root and quantification of phenolic compounds using HPLC. South Afr J Bot. 2020;135:71–9. 10.1016/j.sajb.2020.08.012.Suche in Google Scholar
[8] Atun S, Achmad SA, Ghisalberti EL, Hakim EH, Makmur L, Syah YM. Oligostilbenoids from Vatica umbonata (Dipterocarpaceae). Biochem Syst Ecol. 2004;32(11):1051–3.10.1016/j.bse.2004.04.001Suche in Google Scholar
[9] Atun S, Achmad SA, Niwa M, Arianingrum R, Aznam N. Oligostilbenoids from Hopea Mengarawan (Dipterocarpaceae). Biochem Syst Ecol. 2006;34(8):642–4.10.1016/j.bse.2006.02.008Suche in Google Scholar
[10] Ito T, Hayashi K, Nishiguchi M, Hayashi T, Iinuma M. Resveratrol oligomer C-glucosides and anti-viral resveratrol tetramers isolated from the stem bark of Shorea uliginosa. Phytochem Lett. 2018;28(August):1–7. 10.1016/j.phytol.2018.07.026.Suche in Google Scholar
[11] Tanaka T, Ito T, Nakaya K, Iinuma M, Riswan S. Oligostilbenoids in stem bark of Vatica rassak. Phytochemistry. 2000;54(1):63–9.10.1016/S0031-9422(00)00026-1Suche in Google Scholar
[12] Phukhatmuen P, Meesakul P, Suthiphasilp V, Charoensup R, Maneerat T, Cheenpracha S, et al. Antidiabetic and antimicrobial flavonoids from the twigs and roots of Erythrina subumbrans (Hassk.) Merr. Heliyon. 2021;7(4):e06904. 10.1016/j.heliyon.2021.e06904.Suche in Google Scholar PubMed PubMed Central
[13] Yin L, Han H, Zheng X, Wang G, Li Y, Wang W. Flavonoids analysis and antioxidant, antimicrobial, and anti-inflammatory activities of crude and purified extracts from Veronicastrum latifolium. Ind Crop Prod. 2019;137(October 2018):652–61. 10.1016/j.indcrop.2019.04.007.Suche in Google Scholar
[14] Tonga JL, Kamdem MHK, Pagna JIM, Fonkui TY, Tata CM, Fotsing MCD, et al. Antibacterial activity of flavonoids and triterpenoids isolated from the stem bark and sap of Staudtia kamerunensis Warb. (Myristicaceae). Arab J Chem. 2022;15(10):104150. 10.1016/j.arabjc.2022.104150.Suche in Google Scholar
[15] Wei L, Zhang W, Yin L, Yan F, Xu Y, Chen F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron J Biotechnol. 2015;18(2):88–95. 10.1016/j.ejbt.2014.12.005.Suche in Google Scholar
[16] Kisa D, Kaya Z, İmamoğlu R, Genç N, Taslimi P, Taskin-Tok T. Assessment of antimicrobial and enzymes inhibition effects of Allium kastambulense with in silico studies: Analysis of its phenolic compounds and flavonoid contents. Arab J Chem. 2022;15(6);1–13. 10.1016/j.arabjc.2022.103810.Suche in Google Scholar
[17] Molina GA, González-Fuentes F, Loske AM, Fernández F, Estevez M. Shock wave-assisted extraction of phenolic acids and flavonoids from Eysenhardtia polystachya heartwood: A novel method and its comparison with conventional methodologies. Ultrason Sonochem. 2020;61(July 2019):104809. 10.1016/j.ultsonch.2019.104809.Suche in Google Scholar PubMed
[18] Guthrie F, Wang Y, Neeve N, Quek SY, Mohammadi K, Baroutian S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod Process. 2020;122:136–44. 10.1016/j.fbp.2020.05.002.Suche in Google Scholar
[19] Mehmood A, Ishaq M, Zhao L, Yaqoob S, Safdar B, Nadeem M, et al. Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoria ternatea L.). Ultrason Sonochem. 2019;51(September 2018):12–9. 10.1016/j.ultsonch.2018.10.013.Suche in Google Scholar PubMed
[20] Al-Matani SK, Al-Wahaibi RNS, Hossain MA. Total flavonoids content and antimicrobial activity of crude extract from leaves of Ficus sycomorus native to Sultanate of Oman. Karbala Int J Mod Sci. 2015;1(3):166–71. 10.1016/j.kijoms.2015.11.007.Suche in Google Scholar
[21] Sinan KI, Saftić L, Peršurić Ž, Pavelić SK, Etienne OK, Picot-Allain MCN, et al. A comparative study of the chemical composition, biological and multivariate analysis of Crotalaria retusa L. stem barks, fruits, and flowers obtained via different extraction protocols. South Afr J Bot. 2020;128:101–8.10.1016/j.sajb.2019.10.019Suche in Google Scholar
[22] Sut S, Dall’Acqua S, Bene K, di Marco SB, Sinan KI, Mahomoodally MF, et al. Ricinodendron heudelotii (Baill.) Heckel stem barks and seed extracts, a native food plant from Africa: Characterization by NMR and HPLC-DAD-ESI-MSn. Food Res Int. 2020;129:108877.10.1016/j.foodres.2019.108877Suche in Google Scholar PubMed
[23] Das K, Asdaq SMB, Khan MS, Amrutha S, Alamri A, Alhomrani M, et al. Phytochemical investigation and evaluation of in-vitro anti-inflammatory activity of Euphorbia hirta ethanol leaf and root extracts: A comparative study. J King Saud Univ - Sci. 2022;34(7):102261. 10.1016/j.jksus.2022.102261.Suche in Google Scholar
[24] Netramai S, Kijchavengkul T, Samsudin H, Lertsiri S. Data of microwave assisted extraction and conventional hot water extraction of Dendrobium sonia ‘Earsakul’ orchid flower. Data Br. 2020;31:105906.10.1016/j.dib.2020.105906Suche in Google Scholar PubMed PubMed Central
[25] Santos AS, Salgado HLC, Bonturi N, de Mello RFA, da Conceição LDKM, Miranda EA. Lipidomic Profile of Rhodotorula toruloides by GC/MS and Antioxidant Capacity of the Oil by DPPH and TLC-Plate Methods. South Afr J Chem. 2021;75:162–7.10.17159/0379-4350/2021/v75a20Suche in Google Scholar
[26] Baena-Pedroza A, Londoño-Giraldo LM, Taborda-Ocampo G. Volatilome study of the feijoa fruit [Acca sellowiana (O. Berg) Burret.] with headspace solid phase microextraction and gas chromatography coupled with mass spectrometry. Food Chem. 2020;328:127109.10.1016/j.foodchem.2020.127109Suche in Google Scholar PubMed
[27] Erwin E, Tonapa ZG, Alimuddin A. Toxicity assay of Baccaurea motleyana mull. arg. wood extracts (Rambai) and chemical compounds evaluation for the most active fraction. Res J Pharm Technol. 2020;13(11):5215–8. 10.5958/0974-360X.2020.00912.9.Suche in Google Scholar
[28] Adekoyeni O, Adegoke A, Ajayi F. Gc-ms analysis and identification of pharmacological Components of doum palm nuts. Niger J Sci Res. 2019;18(5):571–8. https://journal.abu.edu.ng/index.php/njsr/article/view/74/72.Suche in Google Scholar
[29] Baloglu MC, Llorent-Martínez EJ, Aumeeruddy MZ, Mahomoodally MF, Altunoglu YC, Ustaoglu B, et al. Multidirectional insights on Chrysophyllum perpulchrum leaves and stem bark extracts: HPLC-ESI-MS n profiles, antioxidant, enzyme inhibitory, antimicrobial and cytotoxic properties. Ind Crop Prod. 2019;134(April):33–42. 10.1016/j.indcrop.2019.03.066.Suche in Google Scholar
[30] Akbari S, Abdurahman NH, Yunus RM, Fayaz F. Microwave-assisted extraction of saponin, phenolic and flavonoid compounds from Trigonella foenum-graecum seed based on two level factorial design. J Appl Res Med Aromat Plants. 2019;14(July):100212. 10.1016/j.jarmap.2019.100212.Suche in Google Scholar
[31] Cheok CY, Chin NL, Yusof YA, Talib RA, Law CL. Optimization of total phenolic content extracted from Garcinia mangostana Linn. hull using response surface methodology versus artificial neural network. Ind Crop Prod. 2012;40(1):247–53. 10.1016/j.indcrop.2012.03.019.Suche in Google Scholar
[32] Harun Al Rashid M, Majumder S, Mandal V, Mandal SC, Thandavarayan RA. In search of suitable extraction technique for large scale commercial production of bioactive fraction for the treatment of diabetes: The case Diospyros melanoxylon Roxb. J Tradit Complement Med. 2019;9(2):106–18. 10.1016/j.jtcme.2017.11.003.Suche in Google Scholar PubMed PubMed Central
[33] Rajabi H, Jafari SM, Rajabzadeh G, Sarfarazi M, Sedaghati S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf A Physicochem Eng Asp. 2019;578(June):123644. 10.1016/j.colsurfa.2019.123644.Suche in Google Scholar
[34] Dirar AI, Alsaadi DHM, Wada M, Mohamed MA, Watanabe T, Devkota HP. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. South Afr J Bot. 2019;120:261–7. 10.1016/j.sajb.2018.07.003.Suche in Google Scholar
[35] Tambe VD, Bhambar RS. Estimation of total phenol, tannin, alkaloid and flavonoid in Hibiscus tiliaceus Linn. wood extracts. Res Rev J Pharmacogn Phytochem. 2014;2(4):41–7.Suche in Google Scholar
[36] Fadly D, Kusharto CM, Kustiyah L, Suptijah P, Muttalib YS. Bohari. In vitro study of antioxidant activity of carboxymethyl chitosan derived from silkworm (Bombyx mori L.) pupa against human plasma lipid peroxidation. Syst Rev Pharm. 2020;11(7):76–81.Suche in Google Scholar
[37] Dewi YSK, Lestari OA, Fadly D. Identification phytochemicals and antioxidant activities of various fractions of methanol extracts from bark of kulim tree (Scorodocarpus borneensis Becc.). Syst Rev Pharm. 2020;11(8):217–21. https://www.researchgate.net/publication/344271575_Identification_Phytochemicals_and_Antioxidant_Activities_of_Various_Fractions_of_Methanol_Extracts_from_Bark_of_Kulim_Tree_Scorodocarpus_borneensis_Becc.Suche in Google Scholar
[38] Fernandes A, Maharani R. Phytochemical and GC-MS analysis of oleoresin of Dipterocarpus gracilis blume: As a basic consideration for human remedy. Int J Pharm Sci Res. 2019;10(5):2224–9.Suche in Google Scholar
[39] Misra V, Solomon S, Mall AK, Prajapati CP, Ansari MI. Impact of chemical treatments on Leuconostoc bacteria from harvested stored cane/stale cane. Biotechnol Rep. 2020;27:e00501. 10.1016/j.btre.2020.e00501.Suche in Google Scholar PubMed PubMed Central
[40] Valder R, Nooralabettu KP. Microbial characteristics of freshly tapped Palmyra Palm (Borassus flabellifer) sap. Int J Sci Eng Res. 2018;9(1):347–53. https://www.ijser.org/researchpaper/Microbial-characteristics-of-freshly-tapped-Palmyra-Palm-Borassus-flabellifer-sap.pdf.Suche in Google Scholar
[41] Aung W, Watanabe Y, Hashinaga F. Isolation and phylogenetic analysis of two thermotolerant, fermentative yeast strains from liquid tapé ketan (Indonesian Rice Wine). Food Sci Technol Res. 2012;18(2):143–8.10.3136/fstr.18.143Suche in Google Scholar
[42] Kuley E, Kuscu MM, Durmus M, Ucar Y. Inhibitory activity of Co-microencapsulation of cell free supernatant from Lactobacillus plantarum with propolis extracts towards fish spoilage bacteria. LWT. 2021;146(April):111433. 10.1016/j.lwt.2021.111433.Suche in Google Scholar
[43] Oonmetta-aree J, Suzuki T, Gasaluck P, Eumkeb G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT. 2006;39(10):1214–20.10.1016/j.lwt.2005.06.015Suche in Google Scholar
[44] Shokri S, Shekarforoush SS, Hosseinzadeh S. Efficacy of low intensity ultrasound on fermentative activity intensification and growth kinetic of Leuconostoc mesenteroides. Chem Eng Process - Process Intensif. 2020;153:107955. 10.1016/j.cep.2020.107955.Suche in Google Scholar
[45] Liao S, Yang G, Ou Y, Huang S, Li B, Li A. Inhibitory impacts of essential oil (Zanthoxylum schinifolium Sieb. et Zucc) on the growth of Staphylococcus epidermidis. Food Biosci. 2022;49(April):101906. 10.1016/j.fbio.2022.101906.Suche in Google Scholar
[46] Nawaz H, Shad MA, Rehman N, Andaleeb H, Ullah N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz J Pharm Sci. 2020;56:e17129.10.1590/s2175-97902019000417129Suche in Google Scholar
[47] Minsas S, Nurdiansyah SI, Prayitno DI, Sofiana MSJ, Kalija TA, Fadly D, et al. Screening of bioactive compounds and antioxidant activity of ale-ale shellfish (Meretrix meretrix) crude extracts from West Kalimantan, Indonesia. Syst Rev Pharm. 2020;11(8):222–7. https://www.researchgate.net/publication/344271434_Screening_of_Bioactive_Compounds_and_Antioxidant_Activity_of_Ale-ale_Shellfish_Meretrix_meretrix_Crude_Extracts_from_West_Kalimantan_Indonesia.Suche in Google Scholar
[48] Safdar MN, Kausar T, Jabbar S, Mumtaz A, Ahad K, Saddozai AA. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal. 2017;25(3):488–500. 10.1016/j.jfda.2016.07.010.Suche in Google Scholar PubMed PubMed Central
[49] Dawood Shah M, Seelan Sathiya Seelan J, Iqbal M. Phytochemical investigation and antioxidant activities of methanol extract, methanol fractions and essential oil of Dillenia suffruticosa leaves. Arab J Chem. 2020;13(9):7170–82. 10.1016/j.arabjc.2020.07.022.Suche in Google Scholar
[50] Asante-Kwatia E, Adjei S, Jibira Y, Gyimah L, Adjei-Hinneh G, Amponsah IK, et al. Amphimas pterocarpoides harms.: An evaluation of flavonoid and phenolic contents, wound healing, anthelmintic and antioxidant activities of the leaves and stem bark. Heliyon. 2021;7(11):1–9. 10.1016/j.heliyon.2021.e08261.Suche in Google Scholar PubMed PubMed Central
[51] Khamis Al-Jadidi HS, Hossain MA. Studies on total phenolics, total flavonoids and antimicrobial activity from the leaves crude extracts of neem traditionally used for the treatment of cough and nausea. Beni-Suef Univ J Basic Appl Sci. 2015;4(2):93–8. 10.1016/j.bjbas.2015.05.001.Suche in Google Scholar
[52] Lou SN, Hsu YS, Ho CT. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J Food Drug Anal. 2014;22(3):290–5. 10.1016/j.jfda.2014.01.020.Suche in Google Scholar PubMed PubMed Central
[53] Zafar MS, Ullah R. Phenolic compound-derived natural antimicrobials are less effective in dental biofilm control compared to chlorhexidine. J Evid Based Dent Pract. 2021;21(2):101576. 10.1016/j.jebdp.2021.101576.Suche in Google Scholar PubMed
[54] Melguizo-Rodríguez L, Illescas-Montes R, Costela-Ruiz VJ, Ramos-Torrecillas J, de Luna-Bertos E, García-Martínez O, et al. Antimicrobial properties of olive oil phenolic compounds and their regenerative capacity towards fibroblast cells. J Tissue Viability. 2021;30(3):372–8.10.1016/j.jtv.2021.03.003Suche in Google Scholar PubMed
[55] Leyva-Jimenez FJ, Lozano-Sanchez J, Borras-Linares I, de la Luz Cadiz-Gurrea M, Mahmoodi-Khaledi E. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria. LWT. 2019;101:236–45. 10.1016/j.lwt.2018.11.015.Suche in Google Scholar
[56] Čukanović J, Tešević V, Jadranin M, Ljubojević M, Mladenović E, Kostić S. Horse chestnut (Aesculus hippocastanum L.) seed fatty acids, flavonoids and heavy metals plasticity to different urban environments. Biochem Syst Ecol. 2020;89:1–10. 10.1016/j.bse.2019.103980.Suche in Google Scholar
[57] Nurcholis W, Sya’bani Putri DN, Husnawati H, Aisyah SI, Priosoeryanto BP. Total flavonoid content and antioxidant activity of ethanol and ethyl acetate extracts from accessions of Amomum compactum fruits. Ann Agric Sci. 2021;66(1):58–62. 10.1016/j.aoas.2021.04.001.Suche in Google Scholar
[58] Oubihi A, Hosni H, Nounah I, Ettouil A, Harhar H, Alaoui K, et al. Phenolic content, antioxidant activity, anti-inflammatory potential and acute toxicity study of Thymus leptobotrys Murb. Extracts. Biochem Res Int. 2020;2020:8823209.10.1155/2020/8823209Suche in Google Scholar PubMed PubMed Central
[59] Seyrekoglu F, Temiz H, Eser F, Yildirim C. Comparison of the antioxidant activities and major constituents of three Hypericum species (H. perforatum, H. scabrum and H. origanifolium) from Turkey. South Afr J Bot. 2022;146:723–7. 10.1016/j.sajb.2021.12.012.Suche in Google Scholar
[60] Hapsari S, Yohed I, Kristianita RA, Jadid N, Aparamarta HW, Gunawan S. Phenolic and flavonoid compounds extraction from Calophyllum inophyllum leaves. Arab J Chem. 2022;15(3):103666. 10.1016/j.arabjc.2021.103666.Suche in Google Scholar
[61] Sani MSA, Bakar J, Azid A, Iqbal MJ. Chemometrics-based evaluation on the effect of sonication. contact time and solid-to-solvent ratio on total phenolics and flavonoids. free fatty acids and antibacterial potency of Carica papaya seed against S. enteritidis, B. cereus, V. vulnificus and P. mirabilis. Food Chem Adv. 2022;1(April):100033. 10.1016/j.focha.2022.100033.Suche in Google Scholar
[62] de Souza MC, de Souza Mesquita LM, Pena FL, Tamborlin L, da Silva LC, Viganó J, et al. Potential application for antimicrobial and antileukemic therapy of a flavonoid-rich fraction of Camellia sinensis. Food Chem Adv. 2022;1(May):100042. 10.1016/j.focha.2022.100042.Suche in Google Scholar
[63] Biharee A, Sharma A, Kumar A, Jaitak V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia. 2020;146(June):104720. 10.1016/j.fitote.2020.104720.Suche in Google Scholar PubMed
[64] Yuan G, Guan Y, Yi H, Lai S, Sun Y, Cao S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci Rep. 2021;11:10471.10.1038/s41598-021-90035-7Suche in Google Scholar PubMed PubMed Central
[65] Shamsudin NF, Ahmed QU, Mahmood S, Shah SAA, Khatib A, Mukhtar S, et al. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules. 2022;27(4):1149.10.3390/molecules27041149Suche in Google Scholar PubMed PubMed Central
[66] Wu SC, Yang ZQ, Liu F, Peng WJ, Qu SQ, Li Q, et al. Antibacterial effect and mode of action of flavonoids from licorice against methicillin-resistant Staphylococcus aureus. Front Microbiol. 2019;10:2489.10.3389/fmicb.2019.02489Suche in Google Scholar PubMed PubMed Central
[67] Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr Med Chem. 2015;22(1):132–49.10.2174/0929867321666140916113443Suche in Google Scholar PubMed
[68] Sofiana MSJ, Aritonang AB, Safitri I, Helena S, Nurdiansyah SI, Rikso R, et al. Proximate, phytochemicals, total phenolic content and antioxidant activity of ethanolic extract of Eucheuma spinosum seaweed. Syst Rev Pharm. 2020;11(8):228–32. https://www.researchgate.net/publication/344271674_Proximate_Phytochemicals_Total_Phenolic_Content_and_Antioxidant_Activity_of_Ethanolic_Extract_of_Eucheuma_spinosum_Seaweed.Suche in Google Scholar
[69] Mohamed Ahmed I, Al Juhaimi F, Osman M, Al Maiman S, Hassan A, Alqah H, et al. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT. 2020;131(C):109825. 10.1016/j.lwt.2020.109825.Suche in Google Scholar
[70] Subramanian R, Raj V, Manigandan K, Elangovan N. Antioxidant activity of hopeaphenol isolated from Shorea roxburghii stem bark extract. J Taibah Univ Sci. 2015;9(2):237–44. 10.1016/j.jtusci.2014.11.004.Suche in Google Scholar
[71] Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci. 2015;9(4):449–54. 10.1016/j.jtusci.2014.11.001.Suche in Google Scholar
[72] Li JE, Fan ST, Qiu ZH, Li C, Nie SP. Total flavonoid content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. cv. Jiangxiangru. LWT. 2015;64(2):1022–7. 10.1016/j.lwt.2015.07.033.Suche in Google Scholar
[73] Viol DI, Chagonda LS, Moyo SR, Mericli AH. Toxicity and antiviral activities of some medicinal plants used by traditional medical practitioners in Zimbabwe. Am J Plant Sci. 2016;7(11):1538–44. https://www.scirp.org/journal/paperinformation.aspx?paperid=69634.10.4236/ajps.2016.711145Suche in Google Scholar
[74] Perdana F, Eryanti Y, Zamri A. Synthesis and toxicity assessments some para-methoxy chalcones derivatives. Procedia Chem. 2015;16:129–33. https://www.sciencedirect.com/science/article/pii/S1876619615001886.10.1016/j.proche.2015.12.040Suche in Google Scholar
[75] Primahana G, Ernawati T, Dewi NLP, Dwiyatmi ID, Darmawan A, Hanafi M. Synthesis of 2-allylphenyl cinnamate and brine shrimp lethality test activity evaluation. Procedia Chem. 2015;16:694–9.10.1016/j.proche.2015.12.014Suche in Google Scholar
[76] Ebbo AA, Sani D, Suleiman MM, Ahmad A, Hassan AZ. Acute and sub-chronic toxicity evaluation of the crude methanolic extract of Diospyros mespiliformis hochst ex a. Dc (Ebenaceae) and its fractions. Toxicol Rep. 2020;7:1138–44. 10.1016/j.toxrep.2020.08.028.Suche in Google Scholar PubMed PubMed Central
[77] Subramanian C, Frank MW, Batte JL, Whaley SG, Rock CO. Oleate hydratase from Staphylococcus aureus protects against palmitoleic acid, the major antimicrobial fatty acid produced by mammalian skin. J Biol Chem. 2019;294(23):9285–94. 10.1074/jbc.RA119.008439.Suche in Google Scholar PubMed PubMed Central
[78] Huang K, Ashby R, Fan X, Moreau RA, Strahan GD, Nuñez A, et al. Phenolic fatty acid-based epoxy curing agent for antimicrobial epoxy polymers. Prog Org Coat. 2020;141(December 2019):105536. 10.1016/j.porgcoat.2019.105536.Suche in Google Scholar
[79] Castañeda-Ramírez GS, de Jesús Torres-Acosta JF, Sandoval-Castro CA, Borges-Argáez R, Cáceres-Farfán M, Mancilla-Montelongo G, et al. Bio-guided fractionation to identify Senegalia gaumeri leaf extract compounds with anthelmintic activity against Haemonchus contortus eggs and larvae. Vet Parasitol. 2019;270(May):13–9. 10.1016/j.vetpar.2019.05.001.Suche in Google Scholar PubMed
[80] Ahmad N, Anwar F, Zuo Y, Aslam F, Shahid M, Abbas A, et al. Wild olive fruits: phenolics profiling, antioxidants, antimicrobial, thrombolytic and haemolytic activities. Arab J Chem. 2022;15(12):104241. 10.1016/j.arabjc.2022.104241.Suche in Google Scholar
[81] Elleuch L, Shaaban M, Smaoui S, Mellouli L, Karray-Rebai I, Fourati-Ben Fguira L, et al. Bioactive secondary metabolites from a new terrestrial Streptomyces sp. TN262. Appl Biochem Biotechnol. 2010;162:579–93. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3128704/.10.1007/s12010-009-8808-4Suche in Google Scholar PubMed PubMed Central
[82] Ibnouf EO, Aldawsari MF, Ali Waggiallah H. Isolation and extraction of some compounds that act as antimicrobials from actinomycetes. Saudi J Biol Sci. 2022;29(8):103352. 10.1016/j.sjbs.2022.103352.Suche in Google Scholar PubMed PubMed Central
[83] Jumina J, Lavendi W, Singgih T, Triono S, Steven Kurniawan Y, Koketsu M. Preparation of monoacylglycerol derivatives from indonesian edible oil and their antimicrobial assay against Staphylococcus aureus and Escherichia coli. Sci Rep. 2019;9(1):1–8. 10.1038/s41598-019-47373-4.Suche in Google Scholar PubMed PubMed Central
[84] Hussein HJ, Hadi MY, Hameed IH. Study of chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography-mass spectrometry. J Pharmacogn Phyther. 2016;8(3):60–89.10.5897/JPP2015.0372Suche in Google Scholar
[85] Peng W, Li D, Zhang M, Ge S, Mo B, Li S, et al. Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J Biol Sci. 2017;24(2):399–404.10.1016/j.sjbs.2015.10.026Suche in Google Scholar PubMed PubMed Central
[86] Zahra G, Khadijeh B, Hossein D, Ali S. Essential oil composition of two Scutellaria species from Iran. J Tradit Chin Med Sci. 2019;6(3):244–53. 10.1016/j.jtcms.2019.07.003.Suche in Google Scholar
[87] Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu MJ. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz J Microbiol. 2007;38(4):739–42.10.1590/S1517-83822007000400028Suche in Google Scholar
[88] Nengroo ZR, Ahmad A, Tantary A, Ganie AS, Shah ZU. Design and synthesis of fatty acid-derived 4-methoxybenzylamides as antimicrobial agents. Heliyon. 2021;7(4):e06842.10.1016/j.heliyon.2021.e06842Suche in Google Scholar PubMed PubMed Central
[89] Kamaruding NA, Ismail N, Sokry N. Identification of antibacterial activity with bioactive compounds from selected marine sponges. Pharmacogn J. 2020;12(3):493–502.10.5530/pj.2020.12.76Suche in Google Scholar
[90] Asumang P, Boakye YD, Agana TA, Yakubu J, Entsie P, Akanwariwiak WG, et al. Antimicrobial, antioxidant and wound healing activities of methanol leaf extract of Bridelia micrantha (Hochst.) Baill. Sci Afr. 2021;14:e00980. 10.1016/j.sciaf.2021.e00980.Suche in Google Scholar
[91] Bernatová S, Samek O, Pilát Z, Šerý M, Ježek J, Jákl P, et al. Following the mechanisms of bacteriostatic versus bactericidal action using raman spectroscopy. Molecules. 2013;18:13188–99.10.3390/molecules181113188Suche in Google Scholar PubMed PubMed Central
[92] Jiang T, Liao W, Charcosset C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res Int. 2020;132:109035. 10.1016/j.foodres.2020.109035.Suche in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer
Artikel in diesem Heft
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer

