Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
-
Godswill K. S. Kwashie
, Zippora Appiah-Kubi
Abstract
Drought is the most widespread threat to cocoa production. We assessed the combined effect of arbuscular mycorrhizal fungi (AMF) and potassium fertilizer on cocoa drought resilience and survival at the nursery to 2 years after transplanting to field. Nursery and field experiments were conducted at the FRNR farm (6°43N and 1°36W), Ghana, from 2020 to 2022. A 3 × 3 × 2 factorial experiment in a RCBD with three varieties of cocoa (CRG8914
1 Introduction
In West Africa, cocoa is planted on about six million hectares of land which provide about 70% of the total world production. It also represents about two-thirds of the livelihood of the majority of the smallholders’ farmers in Côte d’Ivoire and Ghana who are the leading producers (60%) in the world [1,2]. In the case of Ghana, over 850,000 households depend on the sector for their livelihood [3]. Given the importance of cocoa to smallholders’ livelihood, it could be a poverty trap if productivity and yield continue to decline or become unsustainable.
Land degradation caused by extreme weather conditions, particularly drought is a major constraint to cocoa seedlings’ survival, growth, and productivity [4,5,6]. Traditionally, cocoa was planted under thinned forest shade where it grows better within temperatures range of 18–31°C and annual rainfall between 1,500 and 2,000 mm. Low-precipitation periods (less than 100 mm/month) longer than three months affect the growth of cocoa [7]. Unfortunately, in recent times, there has been an increase in temperatures and rainfall has been erratic as a result of climate change [8]. Consequently, drought stress has become the most widespread environmental factor that negatively affects the productivity, yield, and sustainability of cocoa production in West Africa [4,5,6,9,10].
There are further predictions that between 2030 and 2050, there will be increased cocoa mortality, desiccation of flowers and fruit, and large portions of the major cocoa-growing areas in Ghana and Côte d’Ivoire will become unsuitable for cocoa production [6,11]. For instance, in Ghana, anecdotal estimates of in-field survival of cocoa seedlings implied that less than 30% actually survive the dry spells within the first 24 months after planting [10], since cocoa saplings are more sensitive to drought and will not recover if stressed beyond a critical level [5].
Under the current challenge, the use of drought-tolerant cocoa varieties or adoption of irrigation technology seems to be the most appropriate choice. However, breeding for drought-tolerant cocoa varieties is still at the infant stage and requires long period of time to validate results [4]. In addition, irrigation which is the predictable panacea has not been widely adopted in West Africa, where majority of the world’s cocoa is produced, due to practical limitations such as the remoteness of smallholder farms from water supplies and cost restrictions [5,12,13].
A possible approach which encompasses an integrated nutrient management could help farmers, especially if incorporated as part of the management practice. Among these strategies, there has been an increasing interest in beneficial soil microbes including arbuscular mycorrhizal fungi (AMF) and the use of potassium fertilizer which help to alleviate the detrimental effects of drought stress [14,15,16].
AMF are one of the most widespread symbiotic fungi colonizing the majority of agricultural plants. Besides the enhancement in plant nutrition, AMF have been considered as one of the most efficient practices to increase plant tolerance under water restrictions [17,18,19]. For example, Sepahvand et al. [20], Davison et al. [21], and Pons and Müller [22] reported that an important benefit of AMF colonization to the host plant under drought stress is a superior water allocation mediated by the fungal hyphal network, facilitating the colonized root access to water in a lower soil water potential. In addition, Rodriguez and Sanders [23] and Li et al. [24] opined that AMF represent a huge, but an unrealized resource for improving plants’ survival under drought condition. More importantly, the compatible combination of beneficial microbes such as AMF and other growth-promoting materials (e.g., potassium) to offer synergistic effects on plant tolerance to stressful environments including drought is also a bright perspective [18,25,26,27].
For instance, K helps in improving plants’ water uptake and conservation by increasing cell membrane stability, root growth, leaf area, and total dry mass under water-stressed (drought) conditions. In addition, maintaining sufficient K levels is critical for plant osmotic adjustment and for mitigating ROS damage as induced by drought stress [4,10].
In light of recent concerns that the majority of studies on this topic were conducted in greenhouses and that the factors affecting plant drought resistance under field conditions are not well understood [24], our study is innovative. In addition, Djan et al. [28] and Baligar and Fageria [29] suggested that cocoa varieties could show varied responses to drought stress in their early growth and survival. Thus, the use of bio-stimulants could be the alternative research approach to alleviate climate change-related stress on cocoa [4,26]. Therefore, this study aimed to provide an understanding of how AMF and potassium fertilizer affects the ability of cocoa seedlings at nursery to withstand drought conditions, and subsequently under field conditions 2 years after transplanting.
This represents an important opportunity for farmers to meet the global demand for cocoa beans in the confectionery industry and has implications for income of the majority (70%) of smallholder farmers who depend on cocoa as a source of livelihood in cocoa-growing regions of West Africa.
2 Materials and methods
2.1 Study area
The experiment was conducted at the FRNR farms (6°43N and longitude 1°36W (Figure 1), Department of Agroforestry at the Kwame Nkrumah University of Science and Technology, (KNUST), Ghana. The area falls within the moist semi-deciduous forest zone of Ghana and is characterized by a bimodal rainfall pattern, i.e., the major rainy season is from March to July, while the minor rainy season starts from August to November. The annual rainfall ranges between 1,250 and 1,500 mm with a mean annual temperature of 27°C and a mean annual humidity of 68% [30].
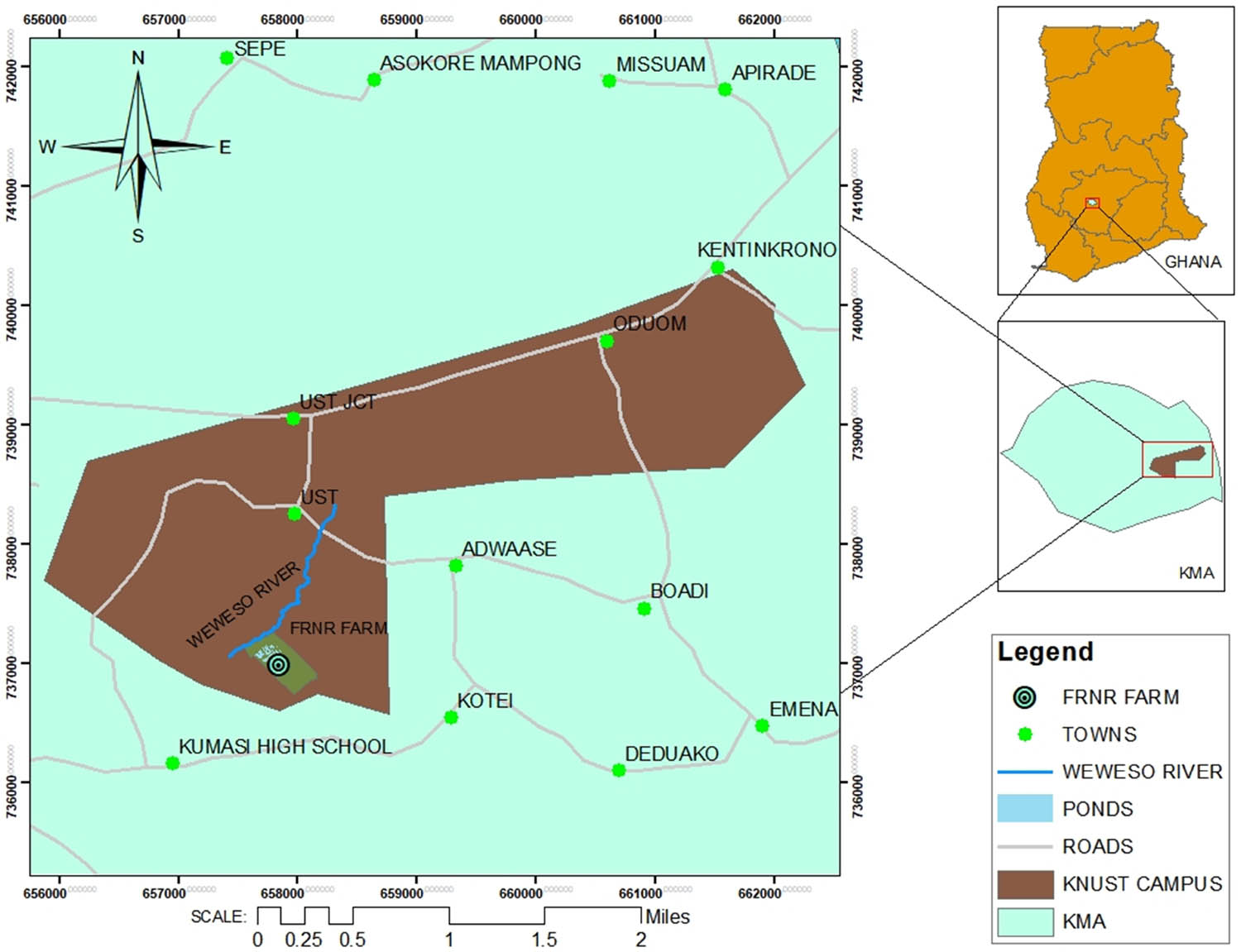
Map of Ghana showing the study area (FRNR demonstration farms).
The soils are classified as ferric acrisol with a sandy-loam textural class. The initial soil properties of the topsoil (0–20 cm) of the experimental site showed a pH of 5.8, organic carbon of 1.2 g kg−1, total nitrogen of 0.5 g kg−1, and organic matter of 2.07 g kg−1.
2.2 Experimental design
The experiment was conducted under nursery and field conditions from 2020 to 2022 cropping seasons. Under the nursery condition, a 3 × 3 × 2 factorial experiment was used with treatments arranged in a Randomized Complete Block Design with three replicates. The treatments consisted of three varieties of cocoa (CRG8914
At the field experiment, the treatments at the nursery were repeated in a Randomized Complete Block Design.
2.3 Extraction of AMF
The selection of specific arbuscular mycorrhizal taxa for a particular crop is known to be the best approach to improve crop growth and resilience to environmental stress such as drought [31,32].
Furthermore, the symbiotic effectiveness and adaptability of indigenous fungi are more dominant than non-native ones and could be more profitable [33]. Therefore, soil samples were collected (0–20 cm) from the rhizosphere of cocoa trees in five farms and examined for native AMF spores and structures using the Wet Sieving and Decantation methodology [34] and the Sucrose Centrifugation Technique [35].
In the extraction of the AMF using the Wet Sieving and Decanting Technique, the course soil samples were sieved through different mesh sizes (through 710 and 45 µm pore sieves with running water) to retain AMF spores and organic particles in fine soil. Ten grams (10 g) of fine soil was mixed with 100 ml of distilled water in a 500 ml conical flask. The soil mixture was shaken vigorously to free the AMF spores from soil and allowed to settle for 15–45 min, and the supernatant was decanted through standard sieves. The spores were then picked with the help of a pipette with the aid of a dissecting microscope.
In the second approach, the spores were further purified using the sucrose centrifugation technique which involves re-suspending the sieving in 40% sucrose solution, i.e., rinse standard sieves (after decanting the supernatant through standard sieves). The mixture was centrifuged at 1,750 rpm for 5 min and the supernatant was removed and poured into the sieves. The spores that hold on the sieves were carefully rinsed with tap water and the spores were collected using a pipette or needle with the aid of a dissecting microscope.
2.4 Preparation of AMF inoculum and inoculation of cocoa seedlings
AMF inoculum was prepared using the trap culture technique. Soil samples (0–20 cm depth) were taken from the rhizosphere of selected cocoa trees and mixed with solarized (at an average temperature of 40°C) soil. Trap culture was done using trap crops such as maize, cowpea, and rice planted for 3 months after which soil samples were examined for AMF spores and structures using the Wet Sieving and Decantation methodology [34] and the Sucrose Centrifugation Technique [35]. After 3 months of trap culture, the inoculum (potting soil) of about 5 g was dibbled (using a dibbling wooden stick) around the base of cocoa seedlings to infest the soil and colonize the roots.
2.5 Histochemical staining of cocoa seedlings
A histochemical staining of total AMF mycelium of cocoa seedlings was also conducted 5 months after inoculation to observe AMF structures in cocoa seedlings’ roots. With histochemical staining of total AMF mycelium in roots of cocoa seedlings, roots of cocoa seedlings were washed free of soil and cut into 1 cm long segments.
Two grams of root segments were weighed into Eppendorf tubes (2.0 mL) after which 2% (w/v) of KOH was sterilized in a pressure cooker for 15 min at 120°C or 1 h at 90°C in a water bath or oven sample.
Roots were then rinsed three times in water using a fine sieve or a mesh using forceps. Roots were covered with 2% (v/v) HCl for at least 30–45 min. The HCl was discarded and the roots were covered with 0.05% (w/v) trypan blue in lactoglycerol (1:1:1 lactic acid, glycerol, and water, for 15 min at 120°C in a pressure cooker for 15 min – 1 h at 90°C in water bath or oven). The tree roots were stained using a ratio of 5:1:1 lactoglycerol. Root samples were then placed into a Petri dish with 50% (v/v) glycerol to de-stain and viewed under a stereomicroscope.
2.6 Experimental procedure
At the nursery stage, 432 seeds of each cocoa variety were nursed in polybags filled with sterilized soil. At 6 days after sowing, 98% of the seeds germinated, and the seedlings’ growth was monitored for 4 months after which the different levels of the potassium (0, 2, and 4 g/plant) were applied to 216 selected seedlings of each variety.
The AMF inoculation of 216 seedlings of each variety was done 1 month after the application of the potassium based on the procedure of Wang et al. [36] and Pongpisutta et al. [37]. The other 216 seedlings were considered the non-AMF seedlings. Other nursery practices were observed according to the recommendation of the CRIG.
The seedlings were then subjected to drought stress (without watering or rainfall under a screen house condition) for 2 weeks and watered for another 2 weeks, this procedure was repeated during the 6-month growth period at the nursery before transplanting them (20 seedlings of each variety per replication) to the field in the month of June, 2020. Under both nursery and field conditions, early growth anatomical data (stem diameter, height, number of leaves) were taken but only below-ground biomass and above-ground biomass were taken at the nursery while the percentage survival was taken only under field conditions from 6 months till 24 months after transplanting.
2.7 Data analysis
The interactive effects of potassium and AMF on the various parameters of the three varieties were verified using a three-way analysis of variance after having checked the assumptions of normal distribution and the homogeneity of variance. The probability level of 5% was considered for the statistical significance; though where appropriate, p values less than 1% are stated. Mean separation was carried out using Tukey’s honestly significant difference procedure at 5% level of probability. Statistical analysis was performed using Statistix 8.0 software. Where shown, data are averages ± standard error (SE) of the three replicates.
3 Results
3.1 Interactive effect of AMF and K on the morphological growth characteristics of cocoa seedlings under nursery conditions
Six months after inoculation, the sole application of either K or AMF was not significantly different among the morphological characteristics of the cocoa varieties at the nursery. However, the interaction of AMF and K levels on the morphological characteristics varied (P < 0.05) among the cocoa varieties (Figure 2).
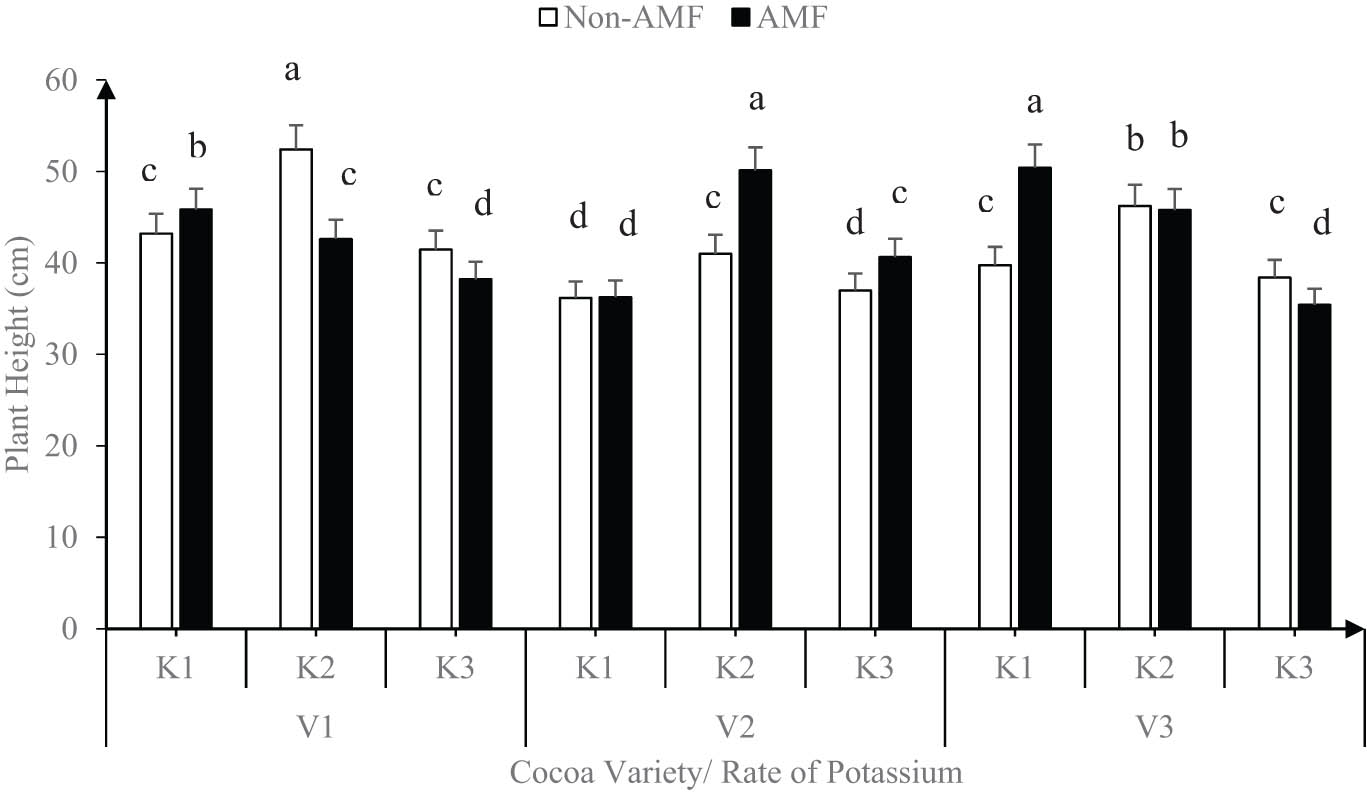
Effect of variety, AMF, and rate of potassium on plant height (error bars represent SEs).
The interaction of K2 and K1 with AMF in varieties 2 and 3 had the highest plant height (50.27 cm) but was similar to V1 applied with K2 without AMF. Generally, the average height (36.25 cm) of V2 and V3 applied with K1 and K3, respectively, with or without AMF was the lowest (Figure 2).
Similarly, with or without AMF, the number of leaves was not significantly different amount the cocoa varieties irrespective of the level of K applied (Figure 3). However, within each variety, the application of K at 2 g/plant (K2) with AMF had the highest number of leaves per plant. The lowest number of leaves was recorded in V3 either with or without AMF inoculation (Figure 3).
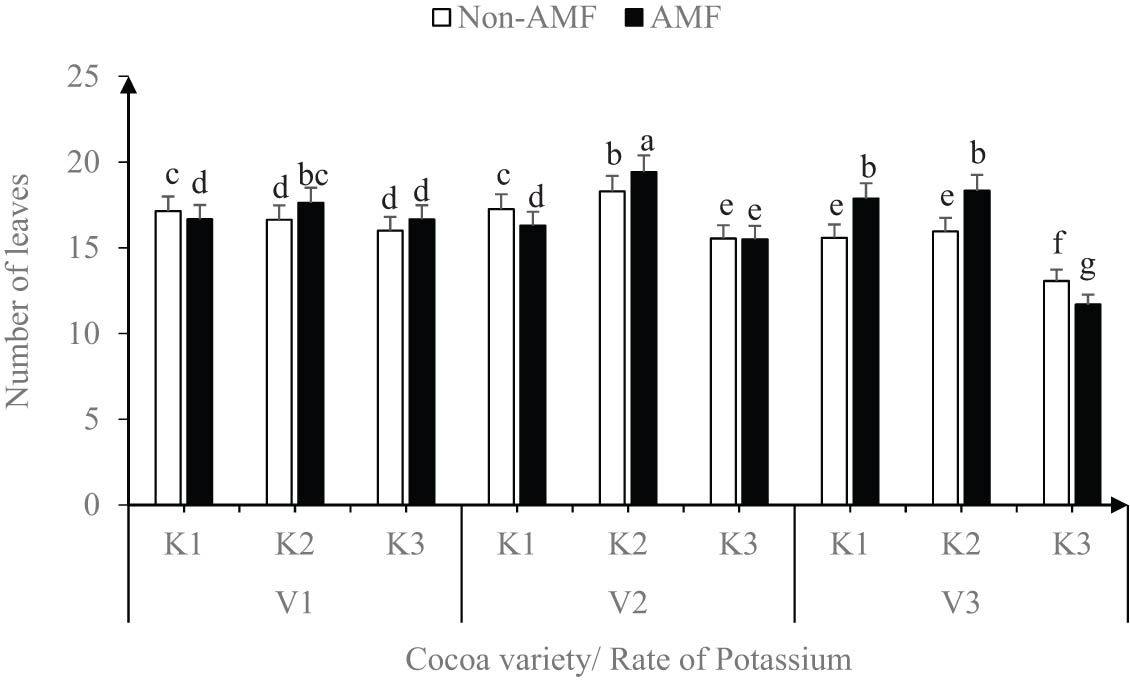
Effect of variety, AMF, and rate of potassium on number of leaves.
The stem diameter showed a similar pattern (Figure 4) of growth with the application of K at 2 g/plant (K2) with AMF recording the highest stem diameter of 8.7 mm compared to 8.1 mm in non-AMF inoculated cocoa seedlings. Among the varieties, only V3 showed a significant (P < 0.05) difference between AMF and non-AMF treatments. For instance, at V3, the interaction of AMF and K1 or K2 had a higher (P < 0.05) stem diameter (average of 8.9 mm) than non-AMF (average of 7.7 mm), but when the K level was increased to 4 g/plant (K3), the non-AMF recorded the highest stem diameter of 8.6 mm compared to 7.3 mm in the AMF seedlings (Figure 4).
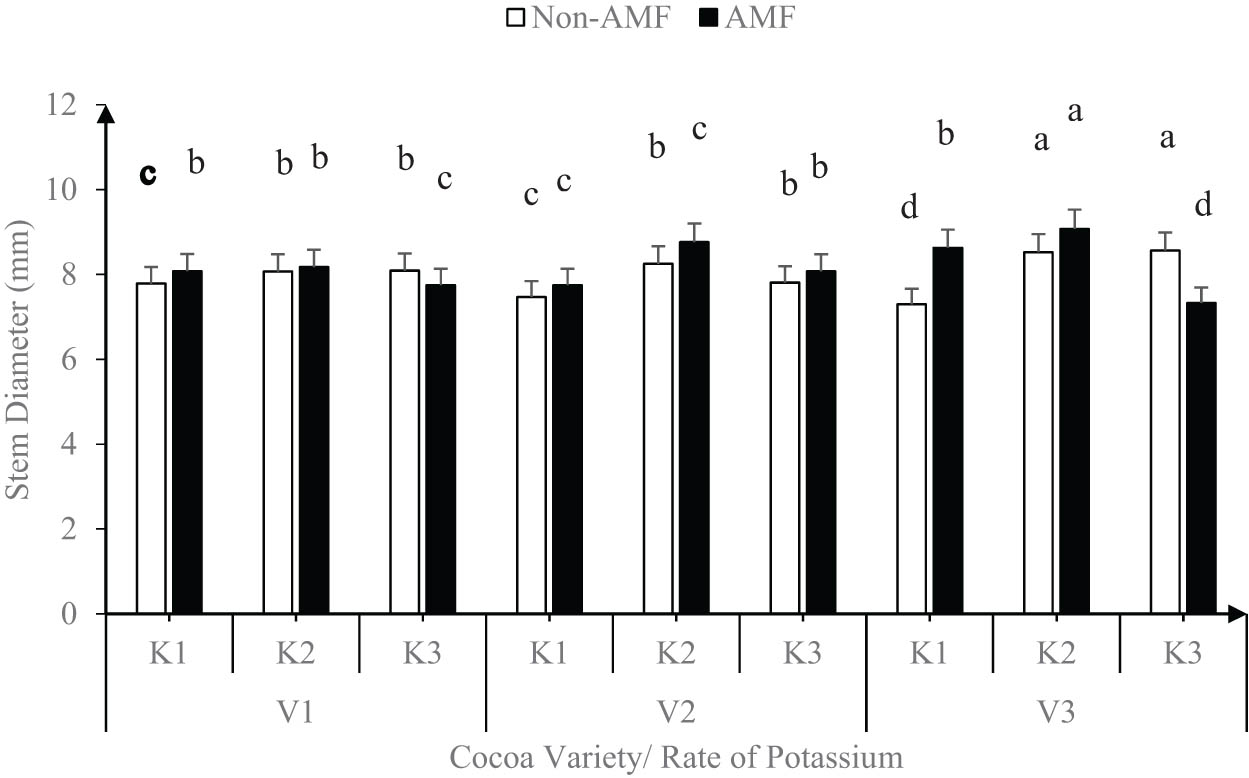
Effect of variety, AMF, and rate of potassium on stem diameter.
The interaction effect of K × AMF had a significant (P < 0.05) influence on both the below-ground and above-ground biomass of the cocoa varieties at 6 months after sampling in the nursery. Generally, sole treatments (K or AMF) had the lowest amount of above-ground biomass. For example, sole K at the different levels (K1, K2, and K3) had the lowest amount of above-ground biomass among the three varieties compared with the K levels combined with AMF (Table 1). A similar pattern was observed in the below-ground biomass except for K3 × non-AMF (2.06 g) which was significantly higher than K2 × AMF (1.89 g) in the V1 variety (Table 1).
Above-ground biomass and below-ground biomass of cocoa seedlings at the nursery stage, 6 months after treatments
| Above-ground biomass (g) | Below-ground biomass (g) | |||||
|---|---|---|---|---|---|---|
| Variety 1 | Variety 2 | Variety 3 | Variety 1 | Variety 2 | Variety 3 | |
| K1 × non-AMF | 3.75 ± 0.18 Ac | 3.23 ± 0.15 Bc | 3.60 ± 0.08 Ac | 1.16 ± 0.04 NSd | 1.28 ± 0.07 NSc | 1.14 ± 0.05 NSd |
| K2 × non-AMF | 3.22 ± 0.09 Ad | 2.54 ± 0.16 Bd | 2.39 ± 0.02 Bd | 1.40 ± 0.09 Ac | 1.26 ± 0.10 Ac | 1.15 ± 0.12 Bd |
| K3 × non-AMF | 3.58 ± 0.04 Acd | 2.80 ± 0.17 Bcd | 2.27 ± 0.05 Cd | 2.06 ± 0.06 Aab | 1.02 ± 0.08 Bd | 1.11 ± 0.03 Cd |
| K1 × AMF | 4.74 ± 0.10 NSb | 4.61 ± 0.11 NSa | 4.80 ± 0.13 NSa | 2.11 ± 0.02 Aa | 1.90 ± 0.12 Cb | 2.01 ± 0.17 ACb |
| K2 × AMF | 5.13 ± 0.17 Aa | 4.50 ± 0.26 Ba | 4.38 ± 0.7 Bb | 1.89 ± 0.04 Cb | 2.81 ± 0.10 Aa | 2.29 ± 0.03 Ba |
| K3 × AMF | 3.96 ± 0.30 Bc | 4.01 ± 0.12 Bb | 4.74 ± 0.13 Aa | 2.13 ± 0.09 Aa | 1.72 ± 0.06 Bb | 1.61 ± 0.09 Bc |
Means with different superscripts lowercase letters within the same columns are significantly different at P ≤ 0.05 while means with different uppercase letters within the same rows are significantly different at P ≤ 0.05. NS – not significant within rows. Variety 1 = CRG8914 × PA150; Variety 2 = AMAZ 15–15 × EQX78; and Variety 3 = PA150 × CRG 0314. K1 = 0 g/plant; K2 = 2 g/plant; and K3 = 4 g/plant.
Among the varieties, the treatment combination of K2 × AMF in V1 had the highest above-ground biomass (5.13 g) and this was significantly (P < 0.05) higher than V2 and V3 which were similar (Table 1). In addition, K3 × non-AMF in V3 had the lowest above-ground biomass (2.27 g) among the varieties and treatment combination. However, a different pattern was observed for the below-ground biomass among the three varieties, but not the treatment combinations. For instance, the same K2 × AMF but in V2 had the highest (P < 0.05) below-ground biomass, followed by V3 with the same treatment combinations while the treatment combination of K3 × non-AMF in V2 had the lowest below-ground biomass (1.02 g) among the varieties and treatment combinations (Table 1).
3.2 Effect of K and AMF on plant height and stem diameter of cocoa trees 2 years after transplanting to the field
The morphological growth characteristics (height and stem diameter) under field condition was significantly influenced by sole K2 and the interaction of the different K levels and AMF.
During the drought period (November–February) in both years (2021 and 2022), the interaction of K2 × AMF had the highest (P < 0.05) influence on the cocoa height followed by sole K2 (Figure 5). For instance, the average height for K2 × AMF interaction was 63 cm compared with 59.5 cm for sole K2. However, during the rainy season (May–July) sole K2 and K3 had the highest plant height and this was significantly different from any of the AMF combined treatments (Figure 5).
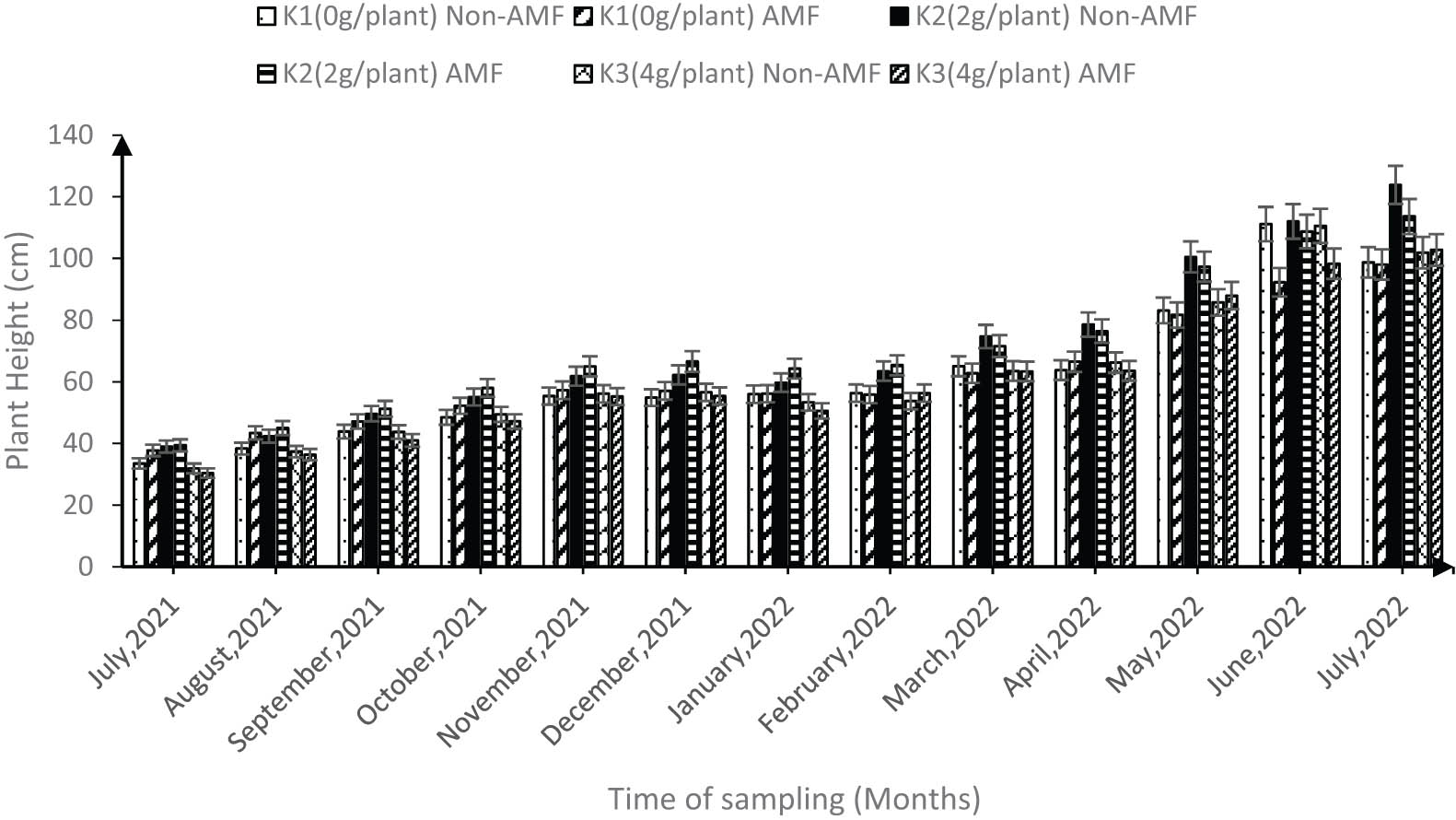
Synergic effect of K and AMF on the height of the cocoa plants under field conditions taken from July 2021 to July 2022 (error bars represent SEs).
However, the stem diameter did not show any significant difference between K at any level with AMF and sole K in both the dry and rainy seasons. For instance, from July 2022, the average stem diameter for K × AMF treatments was 17.13 mm while that of sole K was 18 mm, but the stem diameter significantly increased from the year 2021 to 2022 (Figure 6).
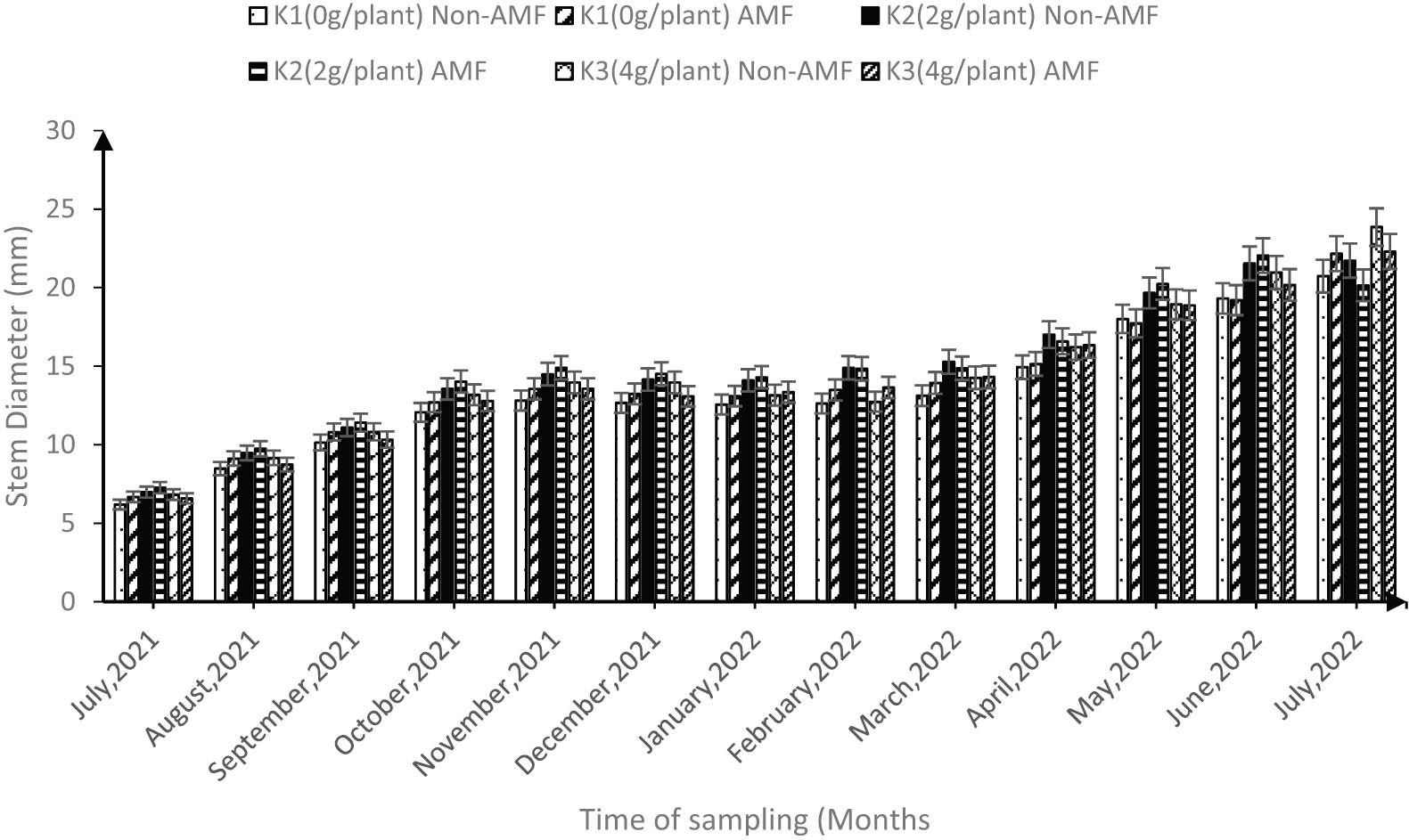
Synergic effect of AMF and K on morphological growth characteristics of cocoa trees after transplanting to the field from July 2021 to 2022 (error bars represent SEs).
3.3 Effect of K levels and their synergy with AMF on field survival of cocoa plants under different seasons at 2 years
The three cocoa varieties showed varied patterns of survival from 6 to 24 months after transplanting to the field. In addition, the treatment combinations had different influences on the cocoa varieties’ survival at the different seasons. Generally, K levels with AMF combinations recorded the lowest percent of mortality (Figure 7). For instance, at 6 months, which coincided with the rainy season (July 2021) V1 did not record any mortality while only V2 × K1 without AMF recorded 10% mortality. However, V3 had the highest mortality rate where V3 × K1 without AMF had 12% mortality followed by 10% for both V3 × K3 without AMF and V3 × K3 without AMF (Figure 7).
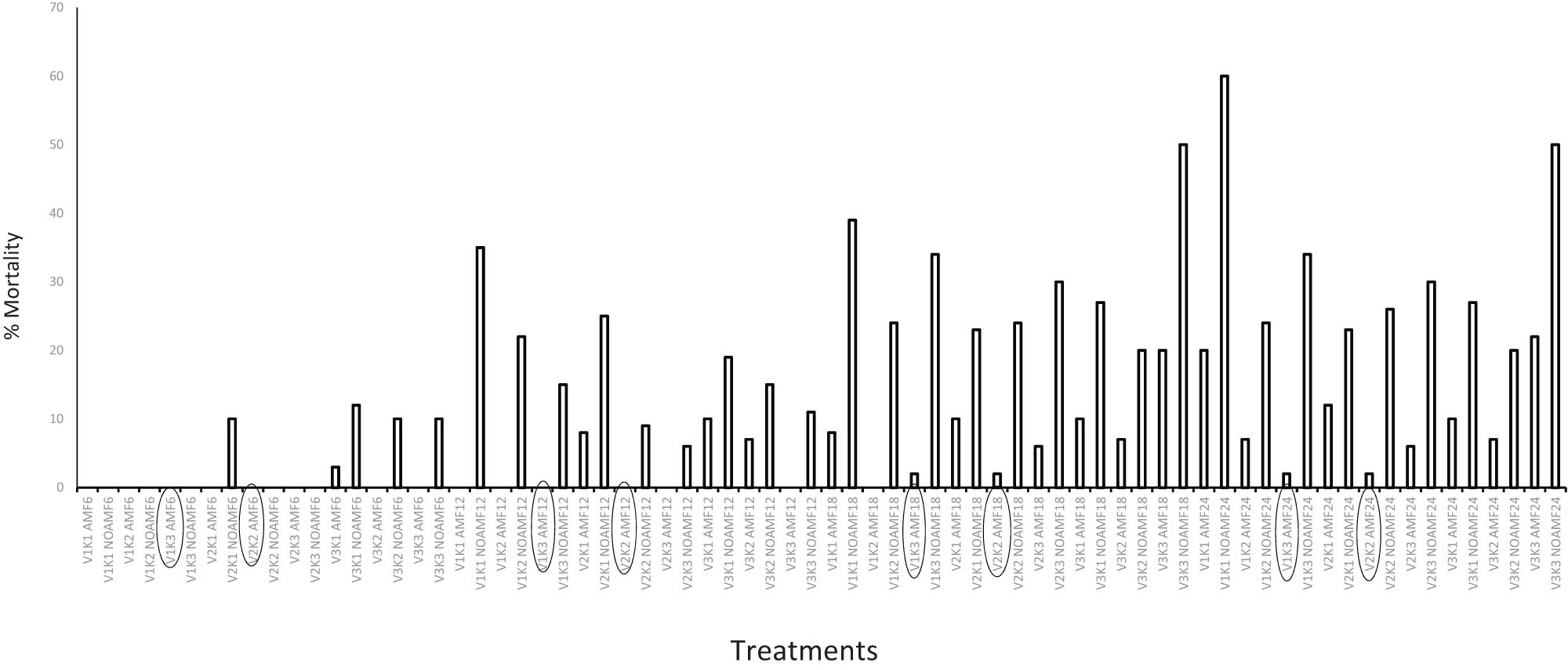
Mortality pattern of cocoa genotypes at different seasons (rainfall and drought) under field conditions over a period of 2 years. V1 – Variety 1 (CRG8914
The pattern changed at 12 months (during the dry season), in that V1 × K1, V2 × K1, and V1 × K3 all without AMF recorded the highest mortality rate of 35, 25, and 22%, respectively (Figure 7).
However, V1 × K1, V1 × K2, V1 × K3, V2 × K2, and V3 × K3 all in combination with AMF did not record any mortality at 12 months (Figure 7).
The pattern at 18 months was similar to 12 months, except that while V1 × K2 with AMF did not record any mortality V1 × K3 × AMF and V2 × K2 × AMF recorded 2% mortality each but the mortality for V3 × K3 without AMF and V1 × K1 without AMF increased to 50 and 39%, respectively (Figure 7).
At 24 months (during the dry season in January 2022), the mortality pattern showed that V1 × K1 without AMF and V3 × K3 without AMF continued to have the highest mortality rate of 60 and 50%, respectively.
Similarly, V1 × K3 × AMF and V2 × K2 × AMF still had the least (only 2% for each) mortality rate after 24 months of growth in the field (Figure 7).
4 Discussion
4.1 Synergic effect of AMF and K on cocoa early growth characteristics at the nursery stage
Drought is a worldwide eco-environmental problem with a greater threat to cocoa at the seedling stage, where it is more sensitive to drought and will not recover if stressed beyond a critical level [5,9]. To enhance plants’ characteristics for survival under drought, Kaba et al. [16] and Pons and Müller [22] suggested the application of potassium fertilizers and the introduction of AMF which has the potential to act as bio-stimulants. In addition, Nasim [38] and Droh et al. [39] indicated that the best-understood mechanism of AMF and potassium-mediated responses of plants under drought conditions involve their effects on plants’ morphological characteristics as these can affect their water relations. Therefore, we relied on the morphological responses of cocoa varieties to AMF inoculation and K application as indicators of drought resilience.
Our result showed that the three cocoa varieties had varied responses to both AMF inoculation and K application. However, the interaction of AMF and K levels improved on the morphological characteristics of the cocoa seedlings at the nursery compared with the non-AMF treatments or sole K applications in all the varieties (Figures 2–4). On average, V1 (CRG8914 × PA150) performed better in terms of plant height and number of leaves while V3 (PA150 × CRG 0314) performed better in terms of stem diameter at the nursery. For instance, the interaction of K2 and K1 with AMF in V2 and V3 had the highest plant height (average 50.27 cm), while the application of K at 2 g/plant (K2) with AMF had the highest number of leaves.
The stem diameter showed a similar pattern of growth with the application of K at 2 g/plant (K2) with AMF recording the highest stem diameter of 8.7 mm compared to 8.1 mm in non-AMF inoculated cocoa seedlings.
The performance of V1 and V3 can be attributed to the inherent genetic characteristics of PA150 parentage which exhibit higher biomass partitioning [10]. Plants of the same genus but different cultivars have been reported to response differently to both AMF inoculation and K application in tomato, wheat, and maize [22,40,41] and in cocoa and Pinus [16,42]. It is important to state that, we observed a promising performance of plants height in sole K application at 2 g/plant in V1 (Figure 2), which partly confirmed the assertion by Romheld and Kirkby [43] and Kaba et al. [16] on the potential of sole potassium application to enhance the growth characteristics of seedlings under drought conditions. However, we noticed that excess application of potassium inhibits the growth and root development of the cocoa seedlings. For example, K applied at 4 g/plant (K3) produced lower growth parameters and visual observation also showed that the K3 seedlings had symptoms of potassium toxicity which included leaf deformations such as chlorotic patches with burning of leaf and spotting along leaf margins as described by Xu et al. [44].
On dry weight biomass production, the synergic effect of AMF and K had a higher amount of both below-ground biomass and above-ground biomass in all the cocoa varieties at 6 months after sampling in the nursery compared to the sole K or AMF (Table 1). Despite the significant effect of AMF with the other levels of K, the synergy of K2 × AMF produced the highest above-ground biomass in V1 (5.13 g/plant) and below-ground biomass in V2 (2.81 g/plant). We can speculate that with optimum amount of K (e.g., 2 g/plant @ K2), AMF inoculated cocoa seedlings have the potential to produce larger amount of roots biomass and this could translate to their potential to explore greater soil for water and nutrients under drought conditions. This speculation is in light of recent findings by Usman et al. [45] and Fiorilli et al. [15] who indicated that varieties develop below-ground biomass as a strategy to avoid and tolerate drought conditions, including root biomass adjustments and anatomical alterations. It is important to mention that we did not find significant difference in both the above-ground biomass and below-ground biomass of the three cocoa varieties when AMF was applied as sole treatment while sole K treatment effects were significantly lower (Table 1). Our results vary with earlier findings that root and shoot biomass increased with sole AMF inoculation [46] and K application [10,16,39]. Our results further contrast with the recent findings of Abaid et al. [9] when they found that Lupinus albus plants inoculated with AMF had higher values for morphological parameters (e.g., stem, height, and dry weight) than non-mycorrhizal plants.
Therefore, our study at the nursery stage established that, in all the cocoa varieties, better morphological characteristics and below ground biomass are found in treatments with AMF combined with K, especially when applied at 2 g/plant (K2).
4.2 Growth characteristics and survival of the cocoa varieties 2 years after transplanting to the field
High seedling mortality during the establishment phase (6–24 months) of cocoa has become a critical limitation to sustainable cocoa farming. In Ghana, Anokye et al. [10] reported that anecdotal estimates of in-field survival of cocoa seedlings implied that less than 30% survive within the first 24 months after transplanting. Therefore, to mimic farmers’ practice, the cocoa seedlings were transplanted into the field after 6 months in the nursery. We observed that the morphological growth characteristics in the field was not influenced by the varieties. However, plant height under field condition was significantly influenced by the interaction of the different K levels and AMF, the highest was recorded when K2 was combined with AMF (Figure 5). During the dry season (November to February) in both 2021 and 2022, the interaction of K2 × AMF had the highest influence on the cocoa height, the average height for K2 × AMF interaction was 63 cm compared with 59.5 cm for sole K2. Querejeta et al. [47], Cuadros et al. [48], and Sendek et al. [49] found that the mycelium of AMF boosts root development by increasing root length, which improves the plant’s ability to absorb water and minerals from the soil and, as a result, increases plant growth parameters such as plant height under dry periods. Similarly, Kaba et al. [16] and Droh et al. [39] emphasized the significant role that K plays in enhancing the height of cocoa under drought conditions. Therefore, the impact observed in the synergic treatments proves that the benefits of K or AMF in enhancing plant height, especially during period of limited soil water, are greater when they are applied together. However, the scenario was different during the rainy season (May–July) where sole K2 and K3 had the highest plant height and stem diameter compared to any of the AMF combined treatments (Figures 5 and 6). For instance, in July 2022, the average stem diameter for any K × AMF treatments was 17.13 mm while that of sole K was 18 mm, suggesting that unlike the plant height under the dry period, the synergy did not have an effect on the stem diameter and plant height during the rainy season, suggesting that AMF could be less active when cocoa plants are not under drought stress. This observation is in line with previous studies [49,50,51] that reported that under optimal environmental conditions (e.g., high water and nutrient availability), interactions with AMF may be of lower importance or even lead to antagonistic relations. Yang et al. [52] further stressed that the above observation is particularly the case for plants characterized by expansive and fibrous root systems that depend less on the assistance of AMF.
Therefore, it may not be too surprising as cocoa has fibrous root systems that are active within the top 0–20 cm of the soil [53].
Most importantly to the smallholder cocoa farmer facing the threat of climate change, the three cocoa varieties varied in percentage mortality from 6 to 24 months after transplanting to the field (Figure 7). This mostly depended on the synergy of AMF and K and the season. Generally, any K level with AMF combinations recorded the lowest percent of mortality, with more greater survival rate recording during the dry season. For example, at 6 months (rainy season) V1 × K, with or without AMF did not record any mortality while V2 × K1 without AMF recorded 10% mortality. However, V3 had the highest mortality rate; for instance, V3 × K1 without AMF had 12% mortality followed by 10% for both V3 × K3 without AMF and V3 × K3 without AMF. The pattern changed at 12 months (drought season), where V1 × K1, V2 × K1, and V1 × K3 all without AMF recorded the highest mortality rate of 35%, 25%, and 22%, respectively (Figure 7). However, the synergy of AMF with V1 × K1, V1 × K2, V1 × K3, V2 × K2, and V3 × K3 did not record any mortality at 12 months (Figure 7). The pattern at 18 months was similar to 12 months, except that while V1 × K2 × AMF did not record any mortality V1 × K3 × AMF and V2 × K2 × AMF recorded 2% mortality each but the mortality for V3 × K3 without AMF and V1 × K1 without AMF increased to 50 and 39%, respectively (Figure 7). At 24 months (dry season in January 2022), where most cocoa trees are established on the field and beginning to fruit, we observed that the mortality pattern for V1 × K1 without AMF and V3 × K3 without AMF continued to have the highest mortality rate of 60 and 50%, respectively. Indicating that on average, over 55% of the seedling transplanted had died after 24 months. This average mortality (55%) confirmed the report by Anokye et al. [10] that less than 30% of cocoa seedlings transplanted to the field survive in Ghana after 24 months. However, both V1 × K3 × AMF and V2 × K2 × AMF still had the least mortality rate of 2% after 24 months of growth in the field (98% survival).
The differences in the mortality rate of the cocoa trees in the two seasons also suggest that under optimal environmental conditions (water and nutrient availability), K interactions with AMF may be of less importance to the survival of cocoa plants. This can be particularly the case for plants characterized by expansive and fibrous root systems such as cocoa [53] that depend less on the assistance of AMF [52]. In addition, the result of our study established that the synergic effect of V1 × K3 × AMF and V2 × K2 × AMF can help improve cocoa survival (about 98%) under field conditions (from 6 months to 2 years).
We also observed that the same treatment (the synergy of K2 × AMF) produced the highest above-ground biomass in V1 (5.13 g/plant) and below-ground biomass in V2 (2.81 g/plant).
This result can be attributed to the ability of AMF to enhance water and nutrient uptake [39,54] and increase drought tolerance [55] and also, the fact that adequate supply of potassium increases root growth and surface area for water uptake [43].
Therefore, our findings provide a possible approach to increasing cocoa survival under field conditions to 98% from the current less than 30% by using an integrated nutrient management approach of soil inoculation with AMF and K fertilization. This also has implications for cocoa production even under unfavorable climate variables (e.g., drought) that threaten sustainable cocoa production in cocoa growing regions of West Africa. In addition, our findings contribute to the achievement of the United Nations Sustainable Development Goal 13 (climate action).
5 Conclusion
We established that the synergic effect of K3 × AMF and K2 × AMF can help improve the survival (about 98%) of cocoa V1 and V2, respectively, under field condition (from 6 months to 2 years). However, we recommend that farmers adopt V2 (AMAZ 15–15 × EQX78) since less amount of K (2 g per plant) is required to apply with AMF compared to 4 g per plant in V1 (CRG8914 × PA150). But, where farmers cannot access V2, applying double amount of K (4gper plant) to V1 with AMF would produce the same effect.
In addition, the success of any AMF and K synergy on the field survival of cocoa is cultivar specific, depend on the season and the rate of K. AMF could be less effective in enhancing survival or even lead to antagonistic relations when the cocoa trees are not under drought stress. We also observed that any treatment combination that has the potential to improve the below-ground biomass of cocoa seedlings can have an impact on the survival rate under field condition, however, treatments with higher stem diameter did not necessarily translate to the long-term survival of the cocoa trees.
Our findings have three implications, first on cocoa and soil nutrients management, field establishment, survival, and yield of cocoa trees. The use of AMF with K applied at 2 g/plant (K2) to V2 represent a major solution to the current less than 30% survival under field conditions (from 6 to 24 months), as it can improve survival up to 98% after transplanting. Second, considering the current threat of drought caused by climate change on cocoa production in West Africa, and given the importance of cocoa to smallholder livelihoods, any approach to ensure cocoa survival and sustainable yield would increase farmers income and alleviate poverty.
Finally, our better understanding of how bio-stimulants such as AMF and potassium fertilizer affects the ability of cocoa plants to survive drought conditions represents an important opportunity for farmers to meet the global demand for cocoa beans in the confectionery industry.
Acknowledgments
We wish to thank DAAD, KNUST, and the Crops Research Institute of Ghana, Kumasi, for their financial and logistic support during the two years study of this project in Ghana. In addition, we are grateful to the Cocoa Research Institute of Ghana (CRIG) for supplying us with the cocoa varieties.
-
Funding information: This study was funded by the Deutscher Akademischer Austauschdienst (DAAD) under the Climate Research for Alumni and Postdocs in Africa, 2020 Grant [number 57516494], and the Office of Research Grant of the Kwame Nkrumah University of Science and Technology under the KNUST Research Fund (KReF) number KReF202021.
-
Author contributions: Conceived of or designed study: James S. Kaba and Akwasi Abunyewa. Performed research: Godswill K.S. Kwashie; James S. Kaba; Akwasi A. Abunyewa; Zippora Appiah-Kubi; Alberta Y. Asare; Ernest K. Agyei; and Hajara Muhammed. Analyzed data: Godswill K.S. Kwashie; James S. Kaba; and Akwasi A. Abunyewa. Contributed new methods or models: James S. Kaba; Akwasi Abunyewa; Zippora Appiah-Kubi; Hajara Muhammed; and Alberta Y. Asare. Wrote the paper: James S. Kaba; Akwasi Abunyewa; Zippora Appiah-Kubi; Alberta Y. Asare; Ernest K. Agyei; and Hajara Muhammed. All authors read and approved the final manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
[1] van Vliet JA, Slingerland MA, Waarts YR, Giller KE. A Living Income for Cocoa Producers in Côte d’Ivoire and Ghana? Front Sustain Food Syst. 2021 Oct;5:732831.10.3389/fsufs.2021.732831Search in Google Scholar
[2] Tyszler M, Bymolt R, Laven A. Demystifying the cocoa sector in Ghana and Côte d’Ivoire. Harv Dataverse. 2018;2:8–9.Search in Google Scholar
[3] COCOBOD. Cocoa: Cocoa is the mainstay of Ghana’s economy. https://www.cocobod.gh/pages/cocoa. Accessed on: 24 April 2021.Search in Google Scholar
[4] Seutra Kaba J, Abunyewa AA, Kugbe J, Kwashie GK, Owusu Ansah E, Andoh H. Arbuscular mycorrhizal fungi and potassium fertilizer as plant biostimulants and alternative research for enhancing plants adaptation to drought stress: Opportunities for enhancing drought tolerance in cocoa (Theobroma cacao L.). Sustain Environ. 2021 Jan;7(1):1963927.10.1080/27658511.2021.1963927Search in Google Scholar
[5] Lahive F, Hadley P, Daymond AJ. The physiological responses of cacao to the environment and the implications for climate change resilience. A review. Agron Sustain Dev. 2019 Feb;39:1–22.10.1007/s13593-018-0552-0Search in Google Scholar
[6] Schroth G, Läderach P, Martinez-Valle AI, Bunn C, Jassogne L. Vulnerability to climate change of cocoa in West Africa: Patterns, opportunities and limits to adaptation. Sci Total Environ. 2016 Jun;556:231–41.10.1016/j.scitotenv.2016.03.024Search in Google Scholar PubMed
[7] ICCO. Growing Cocoa: Origines of Cocoa and Its spread Around The world; 2021 Mar. https://www.icco.org/growing-cocoa/.Search in Google Scholar
[8] Kanwal S, Ilyas N, Batool N, Arshad M. Amelioration of drought stress in wheat by combined application of PGPR, compost, and mineral fertilizer. J Plant Nutr. 2017;40(9):1250–60.10.1080/01904167.2016.1263322Search in Google Scholar
[9] Abaid S, Bouiadjra Bachir SE, Toumi-Benali F, Dahlia F, Bouteldja R, Aggad H. Effect of mycorrhization and water stress on morphological parameters, concentration of phenolic compounds and antioxidant capacity of methanolic extract of Lupinus albus. Environ Exp Biol. 2021;19(1):23–31.10.22364/eeb.19.04Search in Google Scholar
[10] Anokye E, Lowor ST, Dogbatse JA, Padi FK. Potassium application positively modulates physiological responses of cocoa seedlings to drought stress. Agronomy. 2021;11:563. 10.3390/agronomy11030563.Search in Google Scholar
[11] Gateau-Rey L, Tanner EV, Rapidel B, Marelli JP, Royaert S. Climate change could threaten cocoa production: Effects of 2015-16 El Niño-related drought on cocoa agroforests in Bahia, Brazil. PLoS One. 2021;13(7):e0200454.10.1371/journal.pone.0200454Search in Google Scholar PubMed PubMed Central
[12] Carr MKV, Lockwood G. The water relations and irrigation requirements of cocoa (Theobroma Cacao L.) A Review. Exp Agric. 2011;47(4):653–76. 10.1017/S0014479711000421.Search in Google Scholar
[13] Friedman R, Hirons MA, Boyd E. Vulnerability of Ghanaian women cocoa farmers to climate change: A typology. Clim Dev. 2019;11(5):446–58. 10.1080/17565529.2018.1442806.Search in Google Scholar
[14] Faggioli VS, Covacevich F, Grilli G, Lorenzon C, Aimetta B, Sagadin M, et al. Environmental response of arbuscular mycorrhizal fungi under soybean cultivation at a regional scale. Mycorrhiza. 2022;32:425–38. 10.1007/s00572-022-01093-2.Search in Google Scholar PubMed
[15] Fiorilli V, Maghrebi M, Novero M, Votta C, Mazzarella T, Buffoni B, et al. Arbuscular mycorrhizal symbiosis differentially affects the nutritional status of two durum wheat genotypes under drought conditions. Plants. 2022;11(6):804.10.3390/plants11060804Search in Google Scholar PubMed PubMed Central
[16] Kaba JS, Asare AY, Andoh H, Kwashie GK, Abunyewa AA. Toward Sustainable Cocoa (Theobroma Cacao L) Production: The role of potassium fertilizer in cocoa seedlings drought recovery and survival. Int J Fruit Sci. 2022;22(1):618–27.10.1080/15538362.2022.2092932Search in Google Scholar
[17] Pauwels R, Graefe J, Bitterlich M. An arbuscular mycorrhizal fungus alters soil water retention and hydraulic conductivity in a soil texture specific way. Mycorrhiza. 2023;33(3):165–79. 10.1007/s00572-023-01106-8.Search in Google Scholar PubMed PubMed Central
[18] Posta K, Duc NH. Benefits of arbuscular mycorrhizal fungi application to crop production under water scarcity. Drought Detection and Solutions. UK: IntechOpen; 2020 Jan.10.5772/intechopen.86595Search in Google Scholar
[19] Abdellatif L, Lokuruge P, Hamel C. Axenic growth of the arbuscular mycorrhizal fungus Rhizophagus irregularis and growth stimulation by coculture with plant growth-promoting rhizobacteria. Mycorrhiza. 2019;29:591–8. 10.1007/s00572-019-00924-z.Search in Google Scholar PubMed
[20] Sepahvand T, Etemad V, Matinizadeh M, Shirvany A. Symbiosis of AMF with growth modulation and antioxidant capacity of Caucasian Hackberry (Celtis Caucasica L.) seedlings under drought stress. Cent Asian J Environ Sci Technol Innov. 2021;1:20–35.Search in Google Scholar
[21] Davison J, Öpik M, Zobel M, Vasar M, Metsis M, Moora M. Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. PLoS One. 2012;2012(7):e41938. 10.1371/journal.pone.00419.Search in Google Scholar
[22] Pons C, Müller C. Impacts of drought stress and mycorrhizal inoculation on the performance of two spring wheat cultivars. Plants. 2022;11(17):2187.10.3390/plants11172187Search in Google Scholar PubMed PubMed Central
[23] Rodriguez A, Sanders IR. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015;9(5):1053–61.10.1038/ismej.2014.207Search in Google Scholar PubMed PubMed Central
[24] Li J, Meng B, Chai H, Yang X, Song W, Li S, et al. Arbuscular Mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering antioxidant enzyme activities and photosynthesis. Front Plant Sci. 2019;10:499. 10.3389/fpls.2019.00499.Search in Google Scholar PubMed PubMed Central
[25] Duc NH, Mayer Z, Pék Z, Helyes L, Posta K. Combined inoculation of arbuscular mycorrhizal fungi, Pseudomonas fluorescens and Trichoderma spp. for enhancing defense enzymes and yield of three pepper cultivars. Appl Ecol Environ Res. 2017;15(3):1815–29. 10.15666/aeer/1503_18151829.Search in Google Scholar
[26] Giovannini L, Palla M, Agnolucci M, Avio L, Sbrana C, Turrini A, et al. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing Inocula. Agronomy. 2020;10(106):2–14. 10.3390/agronomy10010106.Search in Google Scholar
[27] Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella LR, Xu G, et al. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63(5):635–74. 10.1007/s11427-020-1683-x.Search in Google Scholar PubMed
[28] Djan E, Lowor ST, Dogbatse J, Owusu-Ansah F, Padi FK. A possible role for potassium in mediating cacao seedling responses to soil water stress. In: Proc 2017 Int Symposium Cocoa Res; 2018.Search in Google Scholar
[29] Baligar VC, Fageria NK. Influence of nitrogen forms and levels on the growth and nutrition of cacao. J Plant Nutr. 2017;40(5):709–18. 10.1080/01904167.2016.1262401.Search in Google Scholar
[30] Badu E, Kaba JS, Abunyewa AA, Dawoe KE, Olivia A, Barnes RV. Biochar and Inorganic Nitrogen Fertilizer Effects on Maize (Zea mays L) nitrogen use and yield in Moist Semi deciduous Forest zone of Ghana. J Plant Nutr. 2019;42(19):2407–22. 10.1080/01904167.2019.1659347.Search in Google Scholar
[31] Karlsen-Ayala E, Smith ME, Askey BC, Gazis R. Native ectomycorrhizal fungi from the endangered pine rocklands are superior symbionts to commercial inoculum for slash pine seedlings. Mycorrhiza. 2022;32:465–80. 10.1007/s00572-022-01092-3.Search in Google Scholar PubMed
[32] Deepika S, Kothamasi D. Plant hosts may influence arbuscular mycorrhizal fungal community composition in mangrove estuaries. Mycorrhiza. 2021;31:699–711. 10.1007/s00572-021-01049-y.Search in Google Scholar PubMed
[33] Van Geel M, De Beenhouwer M, Lievens B, Honnay O. Crop-specific and single species mycorrhizal inoculation is the best approach to improve crop growth in controlled environments. Agron Sustain Dev. 2016;36:37. 10.1007/s13593-016-0373-y.Search in Google Scholar
[34] Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc. 1963 Jun;46(2):235–44.10.1016/S0007-1536(63)80079-0Search in Google Scholar
[35] Ba D. Method for the recovery and quantitative estimation of propagules from soil. Methods Princ Mycorrhizal Res. 1982;2:29–36.Search in Google Scholar
[36] Wang Z, Liang J, Kuang Y, Li X, Chen H, Tang M, et al. Cultivation of arbuscular mycorrhizal Broussonetia papyrifera seedlings by planting the mycorrhizal nurse plant downwards. Mycorrhiza. 2022;32:203–12. 10.1007/s00572-022-01070-9.Search in Google Scholar PubMed
[37] Pongpisutta R, Rattanakreetakul C, Kaewgrajang T. Role of arbuscular mycorrhizal fungi (AMF) in cocoa (Theobroma cacao L.) seedlings growth. Khon Kaen Agr J. 2020;48(4):923–32. 10.14456/kaj.2020.84.Search in Google Scholar
[38] Nasim G. The role of arbuscular mycorrhizae in inducing resistance to drought and salinity stress in crops. In: Ashraf M, Ozturk M, Ahmad M, editors. Plant Adaptation and Phytoremediation. Dordrecht: Springer; 2010. p. 119–41.10.1007/978-90-481-9370-7_6Search in Google Scholar
[39] Droh G, Kouassi AB, Kouadjo ZCG, Zeze A, Nguetta AS, Sanders IR. Effects of two AMF on growth of cocoa seedlings (Theobroma cacao L.) I greenhouses. GJAR. 2016;3(3):157–64.Search in Google Scholar
[40] Duan HX, Luo CL, Li JY, Wang BZ, Naseer M, Xiong YC. Improvement of wheat productivity and soil quality by arbuscular mycorrhizal fungi is density- and moisture-dependent. Agron Sustain Dev. 2021;41:12.10.1007/s13593-020-00659-8Search in Google Scholar
[41] Elliott AJ, Daniell TJ, Cameron DD, Field KJ. A commercial arbuscular mycorrhizal inoculum increases root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. Plants People Planet. 2021;3:588–99.10.1002/ppp3.10094Search in Google Scholar PubMed PubMed Central
[42] Frank HER, Garcia K. Benefits provided by four ectomycorrhizal fungi to Pinus taeda under different external potassium availabilities. Mycorrhiza. 2021;31:755–66. 10.1007/s00572-021-01048-z.Search in Google Scholar PubMed
[43] Romheld V, Kirkby EA. Research on potassium in agriculture: Needs and prospects. Plant Soil. 2010;335(1–2):155–80. 10.1007/s11104-010-0520-1.Search in Google Scholar
[44] Xu X, Du X, Wang F, Sha J, Chen Q, Tian G, et al. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front Plant Sci. 2020;11:904. 10.3389/fpls.2020.00904.Search in Google Scholar PubMed PubMed Central
[45] Usman M, Ho-Plágaro T, Frank HER, Calvo-Polanco M, Gaillard I, Garcia K, et al. Mycorrhizal symbiosis for better adaptation of trees to abiotic stress caused by climate change in temperate and boreal forests. Front Glob Change. 2021;4:742392. 10.3389/ffgc.2021.742392.Search in Google Scholar
[46] Verbruggen E, Toby Kiers E. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol Appl. 2010 Sep;3(5–6):547–60.10.1111/j.1752-4571.2010.00145.xSearch in Google Scholar PubMed PubMed Central
[47] Querejeta J, Egerton-Warburton LM, Allen MF. Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia. 2003 Jan;134:55–64.10.1007/s00442-002-1078-2Search in Google Scholar PubMed
[48] Cuadros GA, Gomez R, Rodriguez NF. Symbiotic association of arbuscular mycorrhizal fungi and the root system of cocoa (Theobroma cacao L.) seedlings: effect of formononetin and phosphorus availability at soil level. Corpoica Cienc Tecnol Agropecu. 2011;12(1):77–85.10.21930/rcta.vol12_num1_art:217Search in Google Scholar
[49] Sendek A, Karakoç C, Wagg C, Domínguez-Begines J, do Couto GM, van der Heijden MG, et al. Drought modulates interactions between arbuscular mycorrhizal fungal diversity and barley genotype diversity. Sci Rep. 2019;9(1):1–15. 10.1038/s41598-019-45702-1.Search in Google Scholar PubMed PubMed Central
[50] Martin-Robles N, Lehmann A, Seco E, Aroca R, Rillig MC, Milla R. Impacts of domestication on the arbuscular mycorrhizal symbiosis of 27 crop species. N Phytol. 2018;218:322–34. 10.1111/nph.14962.Search in Google Scholar PubMed
[51] Gosling P, Mead A, Proctor M, Hammond JP, Bending GD. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol. 2013;198(2):546–56.10.1111/nph.12169Search in Google Scholar PubMed PubMed Central
[52] Yang Q, Zhao Z, Bai Z, Hou H, Yuan Y, Guo A, et al. Effects of mycorrhizae and water conditions on perennial ryegrass growth in rare earth tailings. RSC Adv. 2019;9(19):10881–8.10.1039/C8RA10442ESearch in Google Scholar
[53] Ribeiro MA, Da Silva JO, Aitken WM, Machado RC, Baligar VC. Nitrogen use efficiency in cacao genotypes. J Plant Nutr. 2008 Feb;31(2):239–49.10.1080/01904160701853720Search in Google Scholar
[54] Marschner H. Mineral nutrition of higher plants. Vol. 1995. 2nd edn. Germany: Institute of Plant Nutrition University of Hohenheim; 1995.Search in Google Scholar
[55] Davies Jr FT, Potter JR, Linuerman RG. Drought resistance of mycorrhizal pepper plants independent of leaf P concentration‐response in gas exchange and water relations. Physiol Plant. 1993 Jan;87(1):45–53.10.1111/j.1399-3054.1993.tb08789.xSearch in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer
Articles in the same Issue
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer

