Abstract
Insights into plant and bacterial associations, along with their genome mining, have paved the way for the improvement of the agriculture sector. Initially, 69 morphologically different bacterial strains were isolated from 6 different extreme environment samples. For in vitro screening of plant growth-promoting bacteria, auxin, hydrogen cyanide, and antibiotic production along with phosphate solubilization potential was estimated. Only 15 strains exhibited noteworthy production of plant growth-promoting compounds. Ochrobactrum ciceri CS-10 fostered Triticum aestivum and Zea mays seedling root growth remarkably (104.12 and 159%, respectively), while Bacillus australimaris TP-10 significantly increased the number of leaves in T. aestivum (166.66%) and Z. mays (133.33%) seedlings. These strains also boosted seedling biochemical traits, like indole acetic acid, peroxidase, and soluble protein content. Particularly, O. ciceri elevated peroxidase content greatly in T. aestivum (249.7%) and Z. mays (260.5%). Scanning electron micrographs of inoculated roots revealed the aggregation of cells at the roots of Z. mays, whereas single cells/micro-colonies were observed on T. aestivum roots. For in silico analysis, AntiSMASH was used for genome mining of the environmental Ochrobactrum sp. and B. australimaris reference genome. This genome mining unveiled diverse gene clusters encoding; terpenes, beta-lactones, acyl-amino-acids, aryl polyenes, lanthipeptide, and siderophores, etc. Two common biosynthetic gene clusters (terpenes and beta-lactones) were identified in these strains, which can act as plant growth promoters. This symbiotic plant–bacteria relationship has promising ecological and economic implications, offering avenues for beneficial applications.
1 Introduction
Plants inhabiting fields are not individual entities. They cohabit with a regulated and well-structured community of microbes which is the plant’s phytomicrobiome [1]. The phytomicrobiome comprises advantageous organisms like mycorrhizal fungi, nitrogen-fixing bacteria, biocontrol agents, and other plant growth-promoting rhizobacteria (PGPR), along with pathogenic microorganisms that are harmful to plant growth [2]. PGPR not only augment the growth and yield of crops but also aid in the control of plant pathogens [3].
There are several indirect methods by which bacteria can stimulate plant growth including nutrient cycling, disease suppression, induced systemic resistance, and release of phytohormones. Bacteria enable nutrient cycling by breaking down organic matter and releasing nutrients such as potassium, nitrogen, and phosphorus into the soil, which can then be absorbed by plants [4]. Some bacteria are capable of producing antibiotics or other compounds that inhibit the growth of pathogenic fungi and bacteria, thus, reducing the incidence of plant diseases [5]. Some bacteria can also induce systemic resistance in plants, making them more resistant to diseases and environmental stressors such as drought and extreme temperatures. PGPR can also catabolize stress signaling molecules, for instance, bacterial 1-aminocyclopropane-1-carboxylate (ACC) by producing ACC deaminase enzyme [6]. Various rhizobacteria play indirect roles as biological fertilizers and stimulants by producing different hormones such as gibberellins, cytokinin, ethylene, hydrogen cyanide (HCN), indole acetic acid (IAA), abscisic acid, and nitrogen fixation [7]. The most known PGPR belong to the phyla Bacteroidetes, Actinobacteria, Firmicutes, Cyanobacteria, and Proteobacteria, with Pseudomonas spp. and Bacillus spp. as their representative organisms [8]. Plants have developed the ability to endure severe environmental conditions, including high temperatures, drought, and salinity stress. There are some morphological and physiological adaptations to stress conditions that are fixed in the plant genome, but other than these inbuilt adaptations, some microbes also help plants to convey heat, drought, or salt tolerance [9].
Cereal grain is the most abundant food crop in the world. Some of the most common cereal grains include wheat, maize (also known as corn), rice, oats, barley, and rye. However, wheat, maize, and rice are the most widely consumed cereal grains, accounting for almost 90% of all cereal consumption globally [10]. These three grains are popular because they are relatively easy to grow, harvest, and process and have a long shelf life. These crops are attacked by various pathogens and insects, which can decrease their yield. In addition to traditional approaches such as pesticide use, plant-microbe interaction models are being explored as a means of improving crop health and productivity [11].
Extracellular polymeric substances (EPS) are secondary metabolites which are complex macromolecules secreted by many bacteria, and they play an essential role in bacterial colonization and biofilm formation. Biofilms are communities of bacteria that attach to surfaces, and they are formed when bacteria produce EPS, which acts as a glue to hold the bacteria together and attach them to a surface [12]. In the case of root colonization, EPS can promote bacterial attachment to the root surface and help to form a biofilm or microcolonies. These microcolonies facilitate the exchange of substrates between plants and bacteria [13].
Biosynthetic gene clusters (BGCs) refer to a group of two or more genes that are closely associated and work in conjunction to encode a biosynthetic pathway to produce a specific secondary metabolite [14]. Bacterial strains are usually focused on getting certain novel metabolites that are being secreted in large quantities. Contrastingly, a technique termed as one strain many compounds (OSMAC) approach is exploited to investigate a single bacterial strain at a deeper level to obtain the variety of compounds being produced by it [15]. The inability to culture bacterial strains in laboratories has resulted in certain unexplored BGCs in bacterial genomes. Thus, with the advancement in high throughput sequencing techniques, it has become easier to identify unexplored BGCs by culture-independent methods. Bioinformatics tools require various algorithms to dig in the sequenced dataset for the investigation of different domains, gene clusters, and regulatory sequences or to predict molecular structures of synthesized chemicals [16]. These bioinformatics tools can be accessed easily at the Secondary Metabolites Bioinformatics Portal (www.secondarymetabolites.org) which comprises a collection of databases and tools [17]. AntiSMASH is a mining tool that employs hidden Markov models to identify the secondary metabolite-producing gene clusters in the genomes of bacteria and fungi. This genome mining tool uses the curated reference data fingerprints of metagenomes and assembled genomes [18].
Several BGCs can produce metabolites that act as plant growth promoters. Examples of these metabolites include beta-lactones and terpenes. Beta-lactones are known to possess antifungal and antibacterial properties, which is a direct mechanism of plant growth promotion by preventing the plants from pathogens [19]. Terpenes are well-known for plant growth promotion by different direct and indirect mechanisms. It promotes root and shoot growth as well as maintains cell turgor by water preservation thus promoting plant growth. It also protects plants from pathogenic bacteria due to its antibacterial properties [20].
This study aimed to evaluate the bacterial isolates of extreme environments for their growth-promotion potential of T. aestivum and Z. mays seedlings as well as understanding the probable role of exopolysaccharides (EPS) in facilitating bacterial colonization of plant roots. Furthermore, the mining of reference genomes was intended to identify the BGCs that are directly or indirectly involved in plant growth promotion by bacterial isolates.
2 Materials and methods
2.1 Isolation of bacterial strains
Samples were collected from six different extreme environment sites in Pakistan including Tatta Pani hot spring (Kashmir), an agriculture farm, University of the Punjab (Lahore), the Soap Industry (Sheikhupura), Thar Desert (Sukkhur), Hunza Valley (Hunza), and Khewra Salt Mines (Khewra). Samples were collected aseptically and carried to the laboratory. Serial dilutions were made and plated on Luria-Bertani Agar for isolating the bacterial strains incubated at 37°C for 48 h to achieve the maximum number of strains.
2.2 Screening of bacterial strains for plant growth promoting characters
2.2.1 Antibacterial potential
The antibacterial potential of isolated strains was checked against Bacillus sp. (KC881030) and Escherichia coli (GM2163). This activity was estimated by agar well diffusion assay. Mueller–Hinton agar plates were swabbed with 24 h old culture and wells of 6 mm diameter were made in these plates. Isolates were grown in L-broth at 37°C for 24 h and then centrifuged to separate the cell debris from the supernatant. In respective wells, 50 µL of broth supernatant was poured and incubated at 37°C overnight. Ampicillin (10 µg/mL) was taken as a standard, and sterile broth was served as a negative control [21].
2.2.2 HCN production
For the estimation of HCN production potential, the method of Amna et al. was used with slight modifications [22]. Nutrient agar plates (supplemented with 4.4 g/L glycine) were streaked and a filter paper soaked in a solution of 0.5% picric acid and 2% Na2CO3 was placed over the plates. After the incubation period of 4 days at 37°C, the appearance of the orange-brown color on the filter paper was indicative of positive results.
2.2.3 Phosphate solubilization potential
Phosphate solubilizing bacteria were screened on a selective medium, i.e., Pikovskaya’s agar medium. The appearance of hollow zones surrounding bacterial growth, after incubation of 3–5 days, indicated the transformation of insoluble phosphate to soluble form, thus predicting the phosphate solubilization potential of bacteria [23].
2.2.4 IAA production potential
The IAA production potential of bacterial isolates was estimated by using the method of Amna et al. [22]. L-broth (supplemented with 0.1 g tryptophan) was inoculated with isolated bacterial strains and incubated for 7 days at optimum temperature. From this broth culture, 1 mL supernatant was mixed with 2 mL of Salkowski’s reagent and incubated in the dark for 30 min. For the estimation of IAA content, optical density (OD) was taken at 535 nm, and the concentration of IAA was calculated by using a standard curve.
2.2.5 ACC deaminase activity
For this assay, selected strains were cultured on tryptic soy agar then, these fresh cultures were used to inoculate tryptic soy broth (15 mL) and incubated on a shaking incubator at 37°C. The broth culture was centrifuged at 4°C for 10 min at 5,000g and the pellet was stored. This pellet was washed with Dworkin and Foster (DF) salts minimum medium twice and re-suspended in 15 mL DF salts minimum medium supplemented with 3 mM ACC. After 24 h of shaking incubation, the cell pellet was obtained by centrifuging the broth at 5,000g for 10 min and the pellet was washed two times using 5 mL of 0.1 M tris HCl solution and shifted to new eppendorf by dissolving in 0.1 M tris HCl and centrifuged at 10,000g for 1 min. To this pellet, 400 µL of 0.1 M tris HCl (8.0 pH) along with 20 µL toluene were added and vortexed for 30 s. This cell lysate was divided into 3 eppendorf (50 µL each). These replicates were labeled as A, B, and C. In A and B tubes, 5 µL of 0.5 M ACC was added while the C tube acted as a negative control (without ACC). A control tube was also prepared by adding 50 µL 0.1 M Tris HCl (pH 8.0) and 5 µL 0.5 M ACC. All tubes were vortexed for 5 s and incubated for 30 min at 30°C. In the next step, 500 µL 0.56 M HCl was added to the tubes, vortexed, and centrifuged at 10,000g for 5 min. A half milliliter of supernatant was shifted to a glass tube and 400 µL 0.56 M HCl and 150 µL 0.2% 2,4,dinitrophenylhydrazine (prepared in 2 N HCl) were added and left at 30°C for 30 min. In the last step, 1 mL of 2 N NaOH solution was added, vortexed, and absorbance was taken at 540 nm. The content of the tube changes its color from yellow to brown indicating positive results. The ACC deaminase concentration was determined with the help of a standard curve [22].
2.3 Plant microbe experiment
Plant-microbe interaction experiment of the selected strains was done by inoculating the seeds of T. aestivum and Z. mays in natural conditions. Garden soil with a loamy texture and pH of 8.2 was collected from the University of the Punjab. This soil was dried, sieved by a 2.2 mm sieve, and filled in the pots. These pots were placed in a completely randomized manner in a wirehouse. Seeds were purchased from Punjab Seeds Corporation, Lahore, Pakistan. These seeds were sterilized by soaking in 0.1% mercuric chloride solution for 15–20 min followed by repeated washing with autoclaved distilled water. Fresh bacterial broth cultures were centrifuged, and cells were harvested. These cells were re-suspended in autoclaved distilled water and the absorbance of all cultures was maintained at 0.5 to keep the number of bacterial cells constant (107–108 CFU/mL). Sterilized seeds were put in the bacterial suspension for 30 min. These bacterial-inoculated seeds were sown in the pots and allowed to germinate. After 14 days, seedlings were harvested to analyze their growth and biochemical parameters. For growth parameters, percentage germination, shoot and root length, and number of leaves and roots were determined, whereas for biochemical parameters, IAA production, peroxidase content, and total soluble protein content were analyzed [24].
2.4 Biochemical parameters of seedlings
IAA production potential, peroxidase content, and total soluble proteins in plants were estimated by using standard spectrophotometric methods [25].
2.4.1 IAA production potential
Frozen plant seedling was crushed with phosphate buffer saline (PBS) in a cold pestle and mortar. In 1 g of crushed plant, 2 mL of ethyl ether was poured, vortexed, and covered tightly. This tube was left overnight at 4°C. After centrifugation, 2 mL of NaHCO3 was added to the supernatant and the pellet was washed with 1 mL of ethyl ether. The supernatant of this tube was mixed with tube 1 and 1 mL of NaHCO3 was added. After shaking, the organic layer was shifted to another tube and the process was repeated twice. The pH of the mixture was adjusted to 3.0 with 6 N HCl and the inorganic layer was discarded after the addition of 1 mL of ethyl ether. Salkowski’s reagent (2 mL) was poured into the other layer and tubes were incubated at room temperature for 25–30 min. Blank was prepared by mixing 1 mL of ethyl ether with 2 mL of Salkowski’s reagent. Optical density was measured at 535 nm, and IAA was calculated in µg/g (micrograms per gram fresh weight of the plant) using a standard curve.
2.4.2 Peroxidase content
For extraction of peroxidase, 1 g of the plant was crushed with 4 mL of 0.1 M PBS (pH 7.0) and the supernatant was collected by centrifugation for 10 min at 14,000g. The supernatant was divided into two tubes (0.2 mL each) and mixed with 2.5 mL PBS. In the next step, 1% Guaiacol (0.2 mL) was added in 1 tube only and tubes were left for 15–20 min at room temperature. Following incubation, 1 mL of 0.3% H2O2 was dispensed in both tubes and their OD was observed at 470 nm. The blank tube was prepared by mixing 0.2 mL of distilled water, 2.5 mL of with PBS and 0.1 mL of 0.3% H2O2.
2.4.3 Soluble protein content
Folin’s mixture (2 mL) was mixed with 0.4 mL of plant supernatant (as extracted for peroxidase content) and incubated for 15 min at room temperature. In the following step, 0.2 mL of Folin–Ciocaltieu’s phenol reagent was added to the mixture and left for 45 min at room temperature. OD was noted at 750 nm, and concentration was estimated by standard curve.
2.5 Bacterial genome mining for BGCs
Reference genomes of Ochrobactrum sp. (GenBank GCA_002808625.1) and B. australimaris (GCA_001307105.1) were downloaded from NCBI Genome. The selected genomes were analyzed for BGCs by the AntiSMASH database, and the metabolic pathways of identified compounds were studied by Kyoto Encyclopedia of Genes and Genomes (KeGG) pathways [26].
2.6 Colonization of seedling roots by O. ciceri CS-10 and B. australimaris TP-10
Bacterial strains CS-10 and TP-10 were found to possess maximum growth promotion potential, thus the colonization of these strains on seedling roots was observed under a scanning electron microscope (SEM). Seedling roots were visualized by NanoSEM (FEI Nova 450) using built-in through lens detector and Everhart–Thornley detector [24].
3 Results
3.1 Isolation of bacterial strains
A total of 69 bacterial strains were isolated from 6 different sampling sites. Out of these, 25 strains were isolated from Tatta Pani hot spring, 14 from an agriculture farm, and 10 from the soap industry sampling site [27]. Whereas, from desert soil, Hunza valley soil, and salt mine soil samples, only 8, 7, and 5 isolates were obtained, respectively (Figure S1).
3.2 Screening of bacteria for plant growth-promoting characteristics
All the isolated bacterial strains were analyzed for their plant growth-promoting characteristics such as antibacterial activity, HCN production, and HCN production, phosphate solubilization and ACC deaminase activity.
3.2.1 Phosphate solubilization, HCN production, and antibacterial activity
The production of antimicrobial compounds is an indirect way of plant growth stimulation by inhibiting the attack of pathogens on plants [28]. In this study, only seven bacterial strains (10.14%) were able to inhibit the growth of the tested Bacillus sp. (Figure 1). Among these bacterial strains, TP-5 and TP-7 (isolates from the Tatta Pani region) exhibited inhibition potential for the Bacillus test strain, whereas from the desert soil sample, DS-2 and DS-3 inhibited the bacterial growth by showing an inhibition zone. SM-2 and SM-5 from the salt mine sample and HS-5 from the Hunza soil sample possessed antibacterial potential (Table S1).
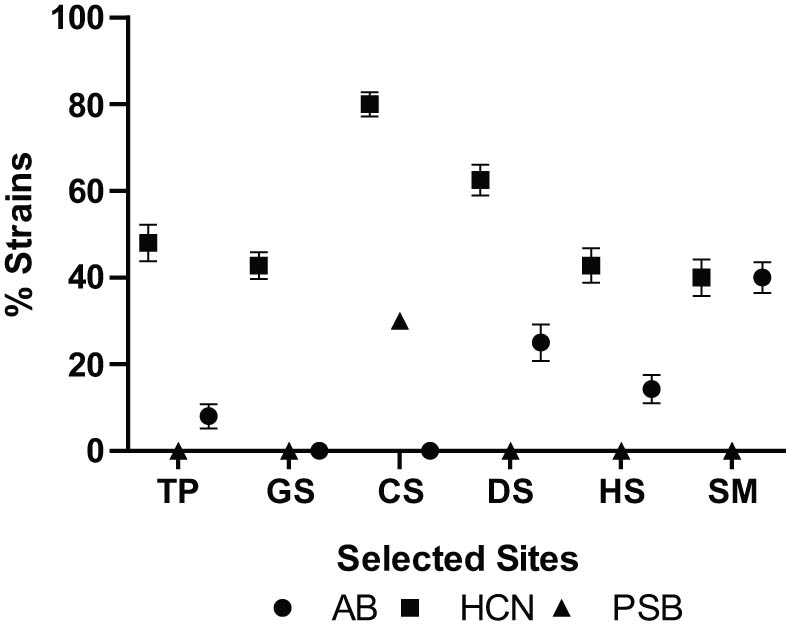
Bacterial strains (%) exhibiting antibacterial potential (AB), HCN production, and phosphate solubilization (PSB) in selected sites, i.e., Tatta Pani hot spring (TP), agriculture farm (GS), soap industry (CS), Thar desert (DS), Hunza valley (HS), and Khewra Salt mines (SM). The maximum percentage of bacterial strains possessing AB, HCN, and PSB potential was observed in SM (40%), DS (80%), and CS (30%) collection sites, respectively.
HCN production by bacteria is also a bio-control mechanism that helps to retard the growth of pathogens, thus promoting healthy plant growth [29]. HCN production was shown by 36 out of 69 strains, i.e., 52.17% of the total isolates (Figure 1). Concerning sample sites, a maximum number of HCN producers (80%) were identified in soap industry soil, i.e., eight out of ten. Desert soil samples also harbored HCN-producing bacteria. The ratio of HCN producers in this sample was 62.5%. In the Tatta Pani soil sample, 48% of strains were able to produce HCN while in the agriculture soil sample and Hunza soil sample, 42.8% of strains had the potential for HCN production. A minimum number of HCN-producing bacterial isolates (40%) was observed in the salt mine soil sample (Table S1).
Another major factor for enhanced plant growth is nutrient availability. Phosphate solubilizing bacteria can convert insoluble forms of phosphate into soluble forms, which enables plants to uptake the phosphate for growth and development. Plants use phosphorus to boost their growth [30]. Out of the total isolated 69 strains, only 3 strains, i.e., CS-2, CS-3, and CS-10 from soap industry soil had the potential to solubilize phosphorus from its insoluble form as indicated in Figure 1 (Table S1).
3.2.2 IAA production potential
IAA is the most produced phytohormone in bacteria. IAA fosters cell division, cell elongation, lateral root formation, and cell and tissue differentiation [31]. All isolated bacterial strains were screened for IAA production. These strains had IAA production potential in the range of 2–140 µg/mL. Among them, CS-10, GS-7, CS-6, CS-3, GS-1, DS-2, and CS-4 showed maximum IAA yields of 140.41, 134.56, 125.67, 119.37, 115.67, 109.74, and 103.81 µg/mL, respectively. Hunza Soil bacteria were found to have minimum or almost no IAA production as they could only produce IAA ranging from 1.96 to 9 µg/mL (Table S1).
3.2.3 ACC deaminase activity
ACC deaminase enzyme cleaves ACC (a precursor of ethylene), which otherwise causes hindrance in root elongation after the germination of seeds [32]. The plant growth-promoting characteristics of a total of 69 bacterial isolates were assessed, and it was observed that 15 strains had shown positive results for maximum characters. Thus, these selected strains were further targeted for their ACC deaminase activity. This activity was detected in all the strains ranging from 95 to 209.94 nmol α-Ketobutyrate/mg/h. The minimum activity was shown by TP-15 and CS-6 strains (95.66 and 99 nmol α-Ketobutyrate/mg/h, respectively). Whereas, the strains showing maximum activity were CS-10 (209.94), TP-10 (198.67), GS-17 (195.33), and DS-2 (195 nmol α-Ketobutyrate/mg/h). Other strains also had the potential to sequester ACC by ACC deaminase enzyme (Table S2).
3.3 Growth parameters of T. aestivum and Z. mays
3.3.1 Seed germination (%)
The effect of bacterial inoculations on seed germination was observed in T. aestivum, seed germination was promoted by most of the strains. As compared to control, pronounced increase in seed germination was shown by the GS-1 strain (76.46%) followed by a 70.58% increment in the seed germination by GS-17 and O. ciceri CS-10 strain (Figure 2). Other strains which significantly promoted seed germination in inoculated plants (as compared to control) included TP-4 (35.29%), TP-14 (35.29%), and CS-2 (41.46%) (Table S3). Seed germination of Z. mays was stimulated by the inoculation of most of the strains; however, few of them also showed a decrease in seed germination. After seed inoculation, a maximum increase in seed germination was observed by GS-17, B. australimaris TP-10, and O. ciceri CS-10, i.e., an increase of 30.76% over control (Figure 3).
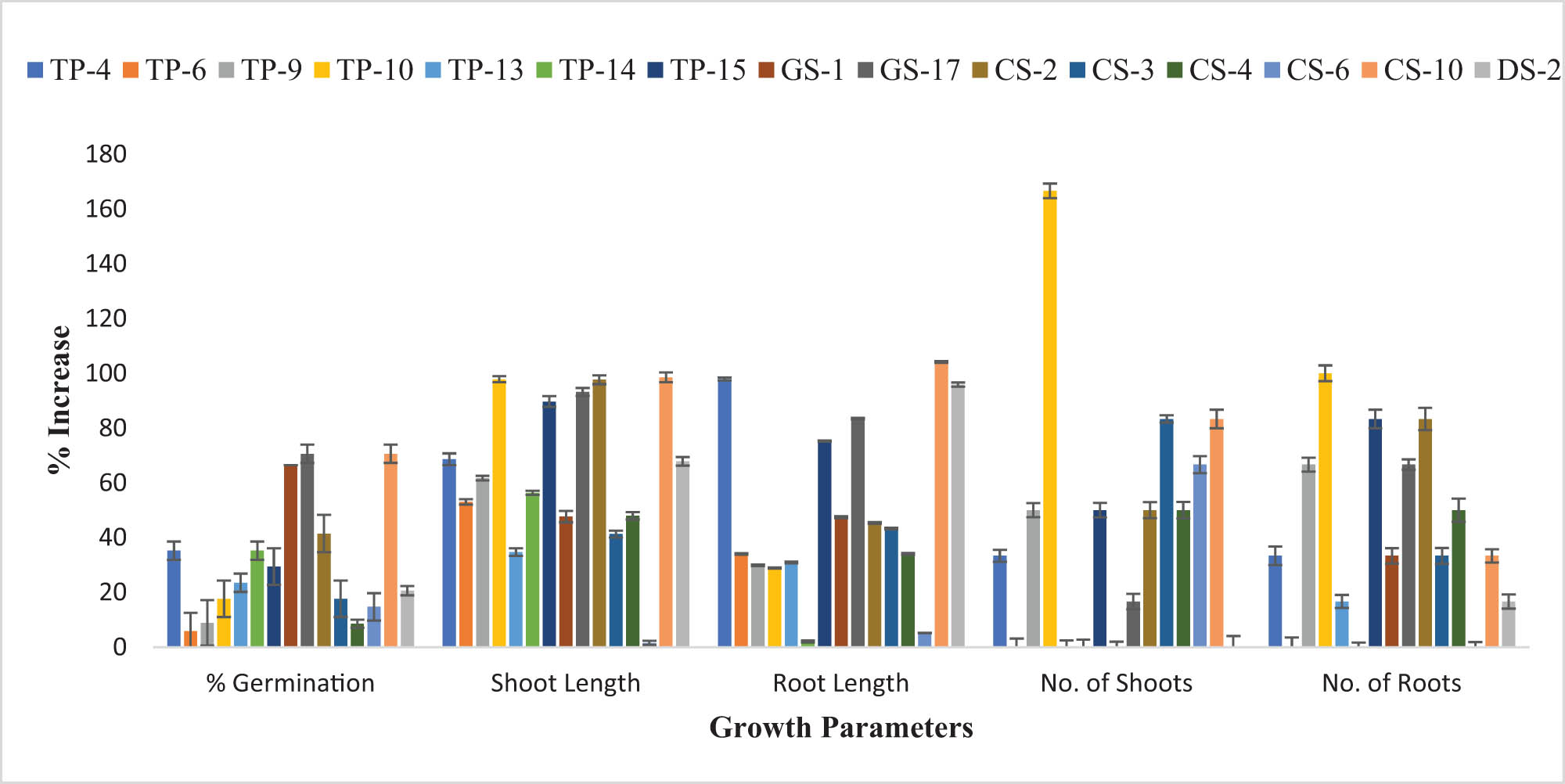
Improvement (%) in the growth parameters of T. aestivum after bacterial inoculation of seeds exhibiting that O. ciceri CS-10 strain enhanced the % germination, shoot length, and root length of plant seedlings significantly by 70.58, 98.49, and 104.12% as compared to the control, respectively. Whereas B. australimaris promoted the number of shoots (166.66%) and roots (100%).
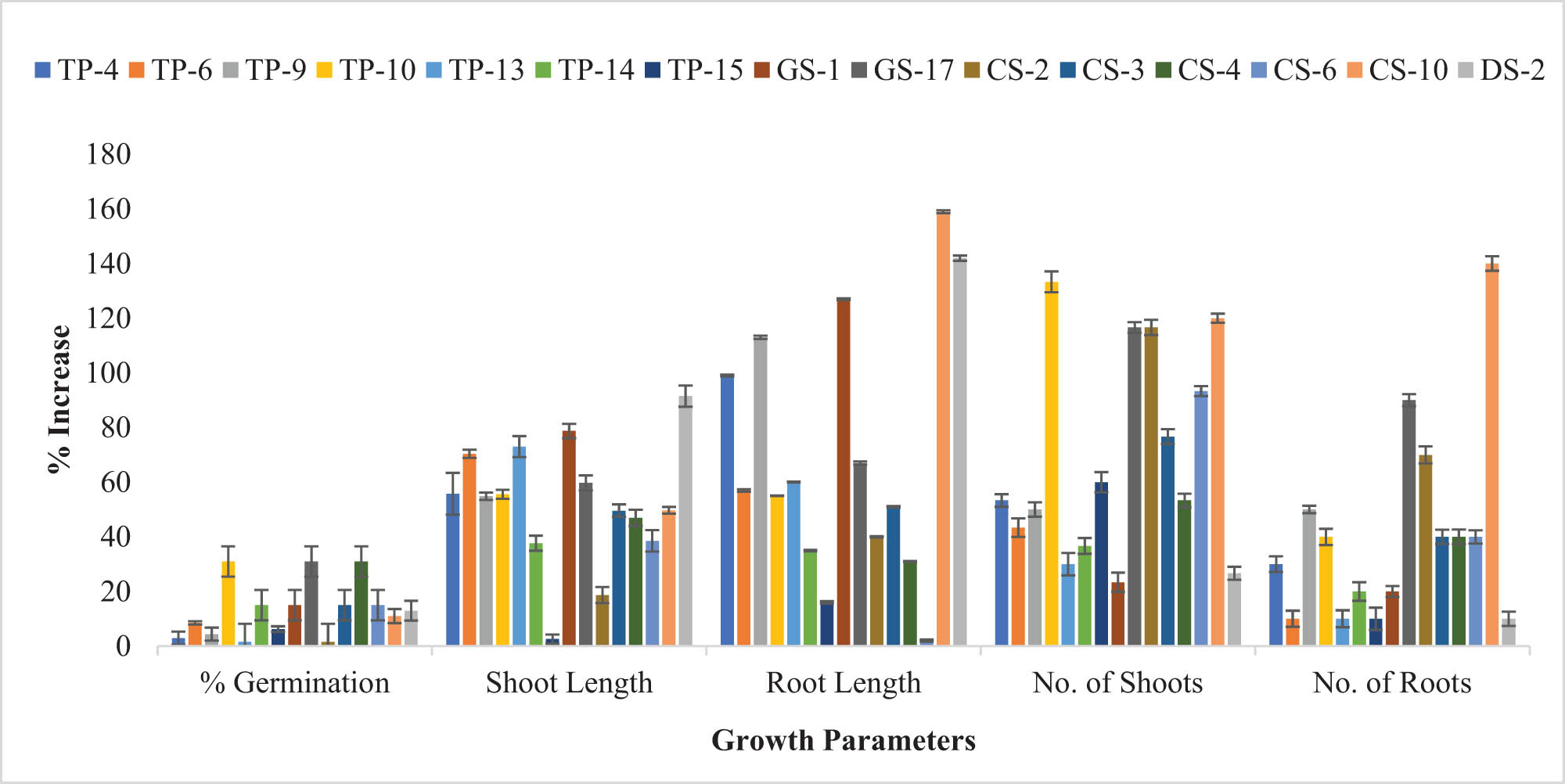
Improvement (%) in the growth parameters of Z. mays after bacterial inoculation of seeds showed that O. ciceri CS-10 strain had maximum potential to promote root length (159%) and number of roots (140%) while B. australimaris maximally increased the percentage germination (31%) and number of shoots (133.33%).
3.3.2 Shoot length (cm)
After bacterial inoculation, all the inoculated seeds showed a prominent increase in the shoot length of T. aestivum as compared to the control (Figure 2). Maximum increase in shoot length was observed by inoculation of O. ciceri CS-10 (98.49%), B. australimaris TP-10 (97.83%), GS-17 (93.21%), CS-2 (97.64%), and TP-15 (89.73%) when compared to the control. Other bacterial strains, i.e., TP-4, TP-6, TP-9, TP-14, GS-1, CS-3, CS-4, and DS-2 also augmented the shoot length (40–70%) (Table S3). Inoculation of seeds with selected strains promoted the growth of shoots in the Z. mays plant (Figure 3). DS-2 strain promoted the growth of shoots to 91.5% over the control. Other strains also showed significant increment in shoot length over control. The least increase in shoot length was shown by the TP-15 strain which improved the shoot length to 2.59% only as compared to the control (Table S3).
3.3.3 Root length (cm)
Maximum enhancement (104.12%) in the root length of T. aestivum was observed by O. ciceri CS-10 strain over control (Figure 2). Inoculation with other strains also showed a prominent increase in length ranging from 43 to 97% (Table S3). Inoculation of Z. mays with isolated plant growth-promoting bacterial strains showed significant (p < 0.05) potential to enhance the growth of roots (Figure 3). Among all selected strains, the maximum increment in root length was shown by the O. ciceri CS-10 strain (159.44%) and DS-2 (142.96%). Some other strains also showed a significant increase in root length, including GS-1 (127.06%), TP-9 (113.55%), and TP-4 (99.44%) (Table S3).
3.3.4 Number of leaves
The number of leaves in the T. aestivum seedlings was enhanced by the inoculation of isolated bacterial strains (Figure 2). B. australimaris TP-10 strain exhibited the maximum potential to increase the number of leaves (166.66%). After that, CS-10 and CS-3 enhanced the number of leaves by 83.33% as compared to the control (Table S4). In Z. mays, TP-10, CS-10, GS-17, and CS-2 showed a significant rise in the number of leaves by 133.33, 116.67, 116.67, and 120%, respectively (Figure 3). Other strains also promoted the number of leaves in bacterial-inoculated seedlings. In both plants, the B. australimaris TP-10 strain was observed to possess the prominent potential to increase the number of leaves.
3.3.5 Number of roots
Bacterial inoculation caused an increment in the number of roots in T. aestivum. The highest increase (100%) in the number of roots over the control was observed in the B. australimaris TP-10 strain. Bacterial strains TP-15 and CS-2 increased the number of roots by 83.33%, TP-9 and GS-17 by 66.67%, and by 50% in the case of CS-4 (Figure 2, Table S4). There was a pronounced increase in the number of roots of Z. mays inoculated with bacterial strains as compared to the control. O. ciceri CS-10 strain greatly increased (140%) the number of roots in plant seedlings. Bacterial strains GS-17 and CS-2 caused a significant increment in the number of roots over control by 90 and 70%, respectively.
3.4 Biochemical parameters of T. aestivum and Z. mays
The effect of bacterial inoculation also has a pronounced impact on biochemical parameters, i.e., IAA, soluble protein, and peroxidase content as given in Table S5.
3.4.1 IAA production Potential
In both T. aestivum and Z. mays seedlings, the maximum increase in IAA concentration, i.e., 139.17 and 147.56%, respectively, was observed in O. ciceri CS-10 strain inoculated plants as compared to control (Figure 4). Other strains also promoted the IAA production in plants, as compared to control, in the range of 45–118% for T. aestivum and 30–89% for Z. mays seedlings (Table S5). Some strains had shown less than 20% IAA augmentation potential in Z. mays seedlings. All treatments of T. aestivum and Z. mays significantly differed from control in their IAA content (Table S5).
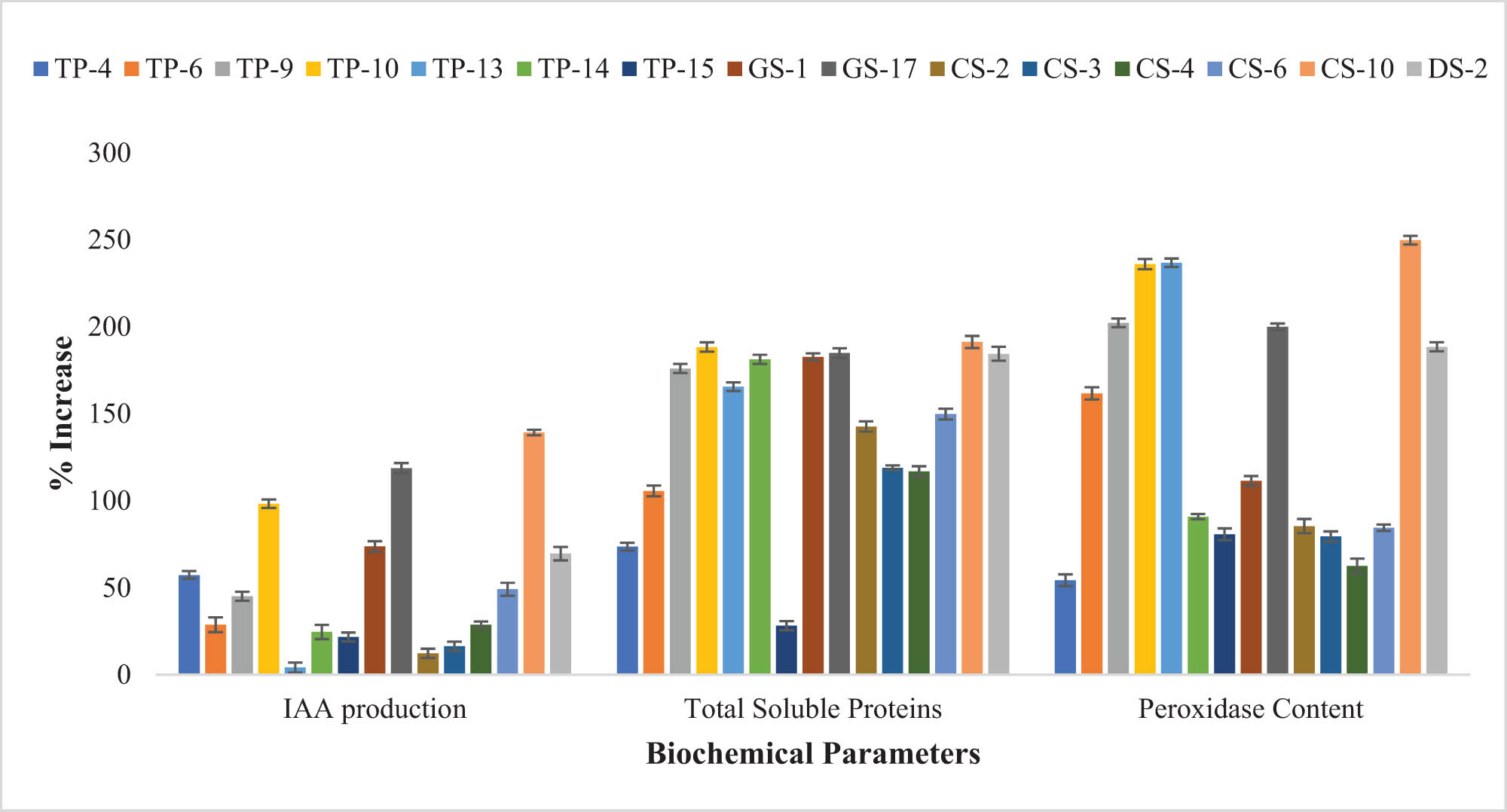
Improvement (%) in the biochemical parameters of T. aestivum after bacterial inoculation of seeds revealed that O. ciceri CS-10 strain enhanced the IAA production, total soluble proteins, and peroxidase content of seedlings as compared to the control.
3.4.2 Soluble protein content
Generally, a pronounced increase in soluble protein content was shown by each strain in T. aestivum and Z. mays (Figures 4 and 5). Maximum soluble protein content in T. aestivum (201.19%) and Z. mays (191.23%) was recorded in seedlings inoculated with O. ciceri CS-10, in comparison with the control. Inoculation of other bacterial strains also elevated the protein content in T. aestivum seedlings in the range of 50–173%, and in Z. mays seedlings ranging from 73 to 188%. The T. aestivum seedlings inoculated with the TP-4 strain and Z. mays seedling inoculated with TP-15 strain, showed the least rise in protein content, i.e., 10.35 and 28.18%, respectively (Table S5).
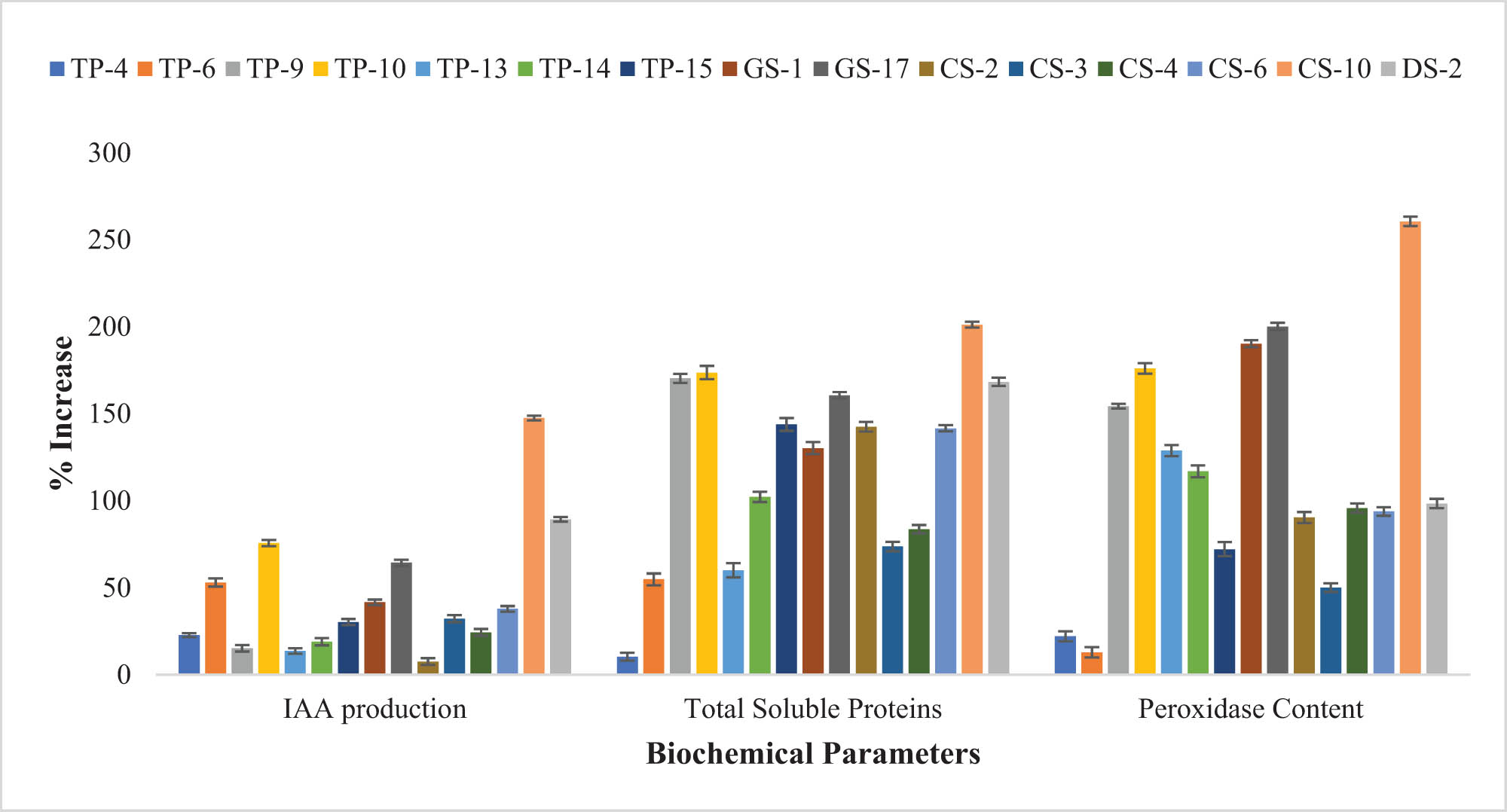
Improvement (%) in the biochemical parameters of Z. mays after bacterial inoculation of seeds revealed that O. ciceri CS-10 strain enhanced the IAA production, total soluble proteins, and peroxidase content of plant seedlings compared to the control.
3.4.3 Peroxidase content
Mostly, inoculation of selected strains caused a significant increase in the peroxidase content of seedlings (Figure 4). In both T. aestivum and Z. mays, a pronounced increase in peroxidase content as compared to control was observed by the inoculation of CS-10 strain, i.e., 249.725 and 260.51%, respectively. In T. aestivum, TP-9, TP-10, TP-13, GS-17, and CS-10 strains also showed >200% increase in peroxidase content. Some strains like DS-2 (188.47%), TP-6 (161.67%), and GS-1 (111.39%) also increased the plant peroxidase content of inoculated plants to a significant level (Table S5). In Z. mays, the inoculation of the GS-17 strain showed an increment of 200% over the control. Inoculation with the rest of the strains also caused increased peroxidase content (100–200%) in comparison to the control (Table S5).
3.5 Genome mining for BGCs of secondary metabolites
Plant growth promotion was shown by almost all the strains, but O. ciceri CS-10 (OP393897.1) and B. australimaris TP-10 (MZ489518.1) exhibited remarkable enhancement in all the studied growth and biochemical parameters. Thus, the reference genomes of these strains were used to analyze the secondary metabolite-producing BGCs in these bacteria with the help of antiSMASH.
3.5.1 BGCs of O. ciceri
Ochrobactrum ciceri reference genome (GenBank GCA_002808625.1) was analyzed for its secondary metabolite producing BGCs. A total of five secondary metabolite-producing gene clusters were present in the reference genome (Table S6). One of these clusters (region 1.1) encodes terpenes which is a carotenoid compound. Cluster BLAST of this gene cluster showed a good similarity index with clusters of Rhizobiales bacterium. In cluster 1 of O. ciceri reference genome, another region 1.2 encodes beta lactones. Acyl amino acids are encoded by region 4.10 and its cluster BLAST with the most similar known clusters revealed that this region has 25% similarity with ambactin that belongs to the non-ribosomal peptide (NRP) gene cluster. O. ciceri reference genome also had N-acetyl glutaminyl glutamine amide (NAGGN) gene cluster at 16.1 region. The last cluster found in this reference genome was 19.1 which encodes for aryl polyenes (Table 1).
BGCs in Ochrobactrum sp. and B. australimaris reference genomes identified by antiSMASH
| Region | Type | From | To | Most similar known cluster | Similarity (%) |
|---|---|---|---|---|---|
| Ochrobactrum sp. | |||||
| 2.1 | Acyl_amino_acids | 1 | 25,335 | Ambactin (NRP) | 25 |
| 4.1 | NAGGN | 53,772 | 74,621 | Hygromycin A (Saccharide) | 6 |
| 5.1 | Lanthipeptide-class-II | 98,886 | 121,741 | — | — |
| 7.1 | Beta-lactone | 348,473 | 376,297 | Fengycin (NRP) | 13 |
| 26.1 | Terpene | 104,869 | 125,702 | — | — |
| 26.2 | Aryl polyene | 131,791 | 172,972 | — | — |
| B. australimaris | |||||
| 1.1 | Terpene | 48,267 | 70,141 | — | — |
| 1.2 | T3PKS | 108,317 | 149,417 | — | — |
| 1.3 | Beta-lactone | 589,841 | 622,097 | Bottromycin (RiPP: Bottromycin) | 6 |
| 5.1 | Beta-lactone | 11,569 | 39,986 | Fengycin (NRP) | 53 |
| 11.1 | NRPS | 1 | 9,912 | Surfactin (NRP: Lipopeptide) | 8 |
| 20.1 | Other | 119,382 | 160,803 | Bacilysin (Other) | 85 |
| 20.2 | Lanthipeptide-class-II | 374,088 | 401,967 | Staphylococci C55α/β (RiPP: Lanthipeptide) | 70 |
| 20.3 | NRPS | 411,861 | 459,009 | Bacillibactin (NRP) | 53 |
| 22.1 | RRE-containing | 7,822 | 28,727 | — | — |
| 22.2 | Terpene, siderophore | 175,951 | 204,323 | Carotenoid (terpene) | 50 |
| 23.1 | NRPS | 119,675 | 163,877 | Surfactin (NRP: Lipopeptide) | 39 |
| 24.1 | NRPS | 126,552 | 154,543 | Lichenysin (NRP) | 50 |
3.5.2 BGCs of B. australimaris
AntiSMASH analysis of reference genome B. australimaris (GenBank GCA_001307105.1) for the identification of secondary metabolite gene clusters revealed the presence of 12 gene clusters in this genome (Table S6). In cluster 1, three regions were observed. Region 1.1 codes for terpenes, region 1.2 is known for type 3 polyketide synthases (T3PKS) while Region 1.3 encodes beta lactones. These three cluster regions showed a resemblance with regions of the genus Bacillus genomes. Beta-lactone cluster showed 6% similarity with other known clusters of ribosomally synthesized and post-translationally modified peptide (RiPP): bottromycins. RiPP cluster produces compounds that are synthesized by RNA and then these are modified post-translationally. Region 5.1 of this genome was also a beta lactone gene cluster, but it has maximum similarity (53%) with fengycin which is a NRP. It also showed the similarity of 50 and 30% genes with mycosubtilin (NRP + PKS) and plipastatin (NRP), respectively.
Region 11.1 encodes NRPS, and the cluster BLAST of this region revealed the most similar known cluster to be surfactin, an NRP: Lipopeptide (BGC0000433). It also showed a similarity of 14% genes with NRP lichenysin (BGC0000381). Cluster 20 of this bacterial genome consists of further 3 regions. Region 20.1 is a type of BGC that is known to encode compounds that do not fall into any particular category. Known cluster BLAST revealed the maximum similarity, i.e., 85% (BGC0001184) and 80% (BGC0000888) with bacilysin while 13% (BGC0000794) and 9% (BGC0000796) similarity with S- layer glycan belonging to saccharides. Region 20.2 was identified as a lanthipeptide cluster and its blast with other known clusters also had similarity with lanthipeptide. NRPS cluster was found at region 20.3 which had a similarity of 53% with genes of the bacillibactin cluster (BGC0000796). Whereas, BGC0001185 (bacillibactin cluster) showed 100% similarity. Maximum similarity (100%) of genes was also observed with the paenibactin gene cluster (BGC0000401). Region 22.1 encodes RRE-containing cluster which does not resemble any other known clusters whereas region 22.2 is known for encoding siderophores or terpenes that also have similarity with other known carotenoid clusters, i.e., terpenes. Regions 23.1 and 24.1 are the NRPS clusters that are most similar to surfactin and lichenysin, respectively (Table 1).
3.6 Common metabolic pathways in reference genomes
A comparison of BGC’s in the reference genome of O. ciceri and B. australimaris revealed that both genomes have terpene (Figure 6) and beta lactone synthesizing genes (Figure 7) in common. The metabolic pathways for these identified compounds were analyzed by KeGG pathways. Beta-lactone gene clusters are present in both strains. These metabolites are majorly involved in antibacterial activities. Analysis of the terpene metabolic pathway revealed that terpene can be synthesized by two pathways (i) mevalonate and (ii) non-mevalonate (MEP/DOXP pathway). Pathway analysis exhibited that both bacterial strains, Ochrobactrum sp. (MT180101) and Bacillus sp. (bsn5) produce terpenes by the mevalonate pathway. KeGG pathway analysis in reference sequence of Ochrobactrum sp. (MT180101) showed that enzymes involved in terpene synthesis include phosphate synthase and kinase, acyltransferase, cytidylyltransferase, diphosphate reductase, flavin prenyltransferase, polyprenyl synthetase, and isoprenyl transferase as shown in Figure 8a. Whereas enzymes for terpenoid biosynthesis in Bacillus sp. are also the same as the Ochrobactrum pathway, except phosphate reductoisomerase, geranyltranstransferase, heptaprenyl diphosphate synthase, and metalloprotease (Figure 8b).
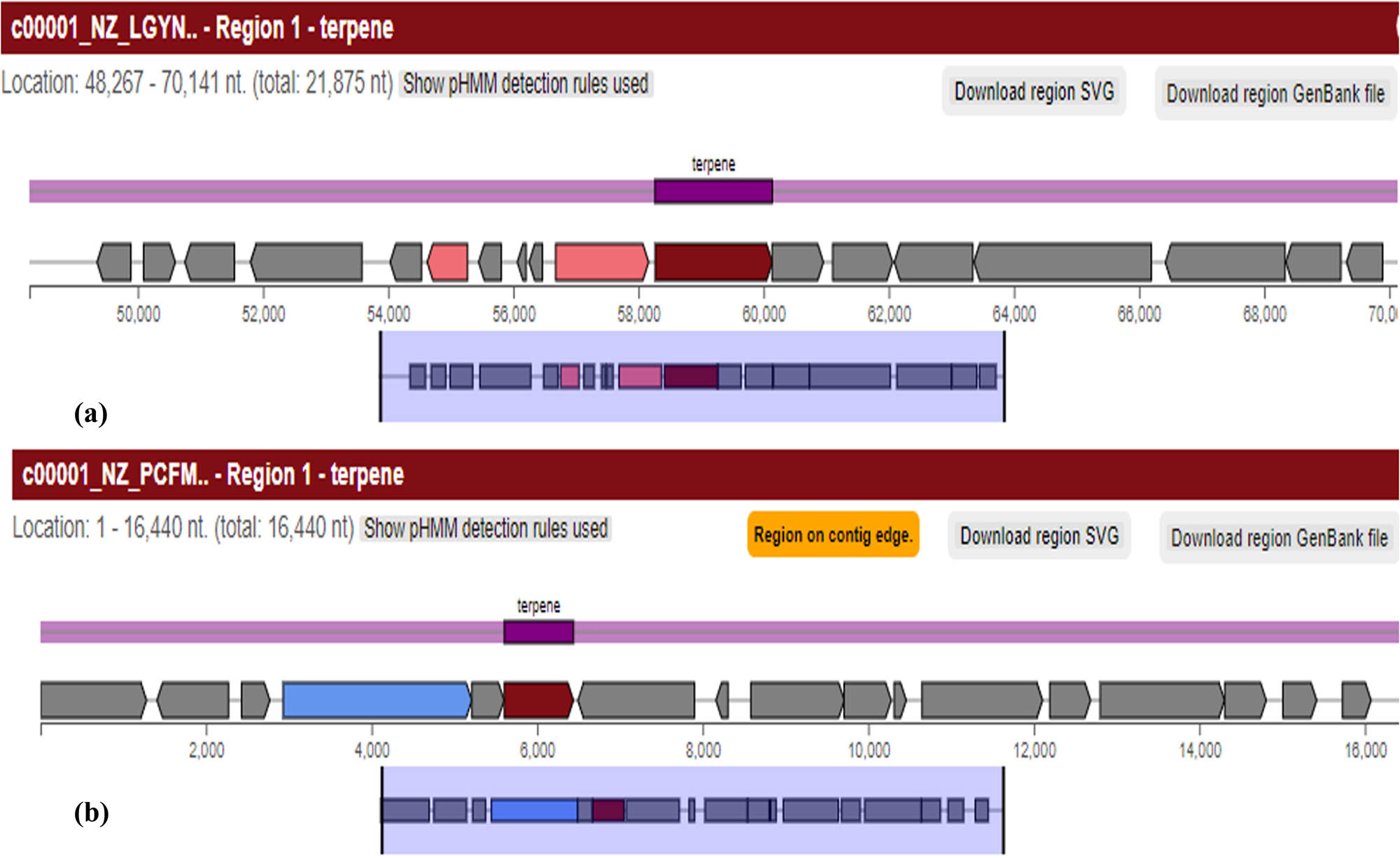
BGC of a terpene; (a) is depicting terpene synthesis region 1.1 in the B. australimaris reference genome. It is a 21,875 nt (from 48,267 to 70,141 nt) long gene; (b) is showing region 1.1 for terpene synthesis in the Ochrobactrum sp. reference genome but it is a 16,440 nt long gene that starts from 1 nt and ends at 16,440 nt.
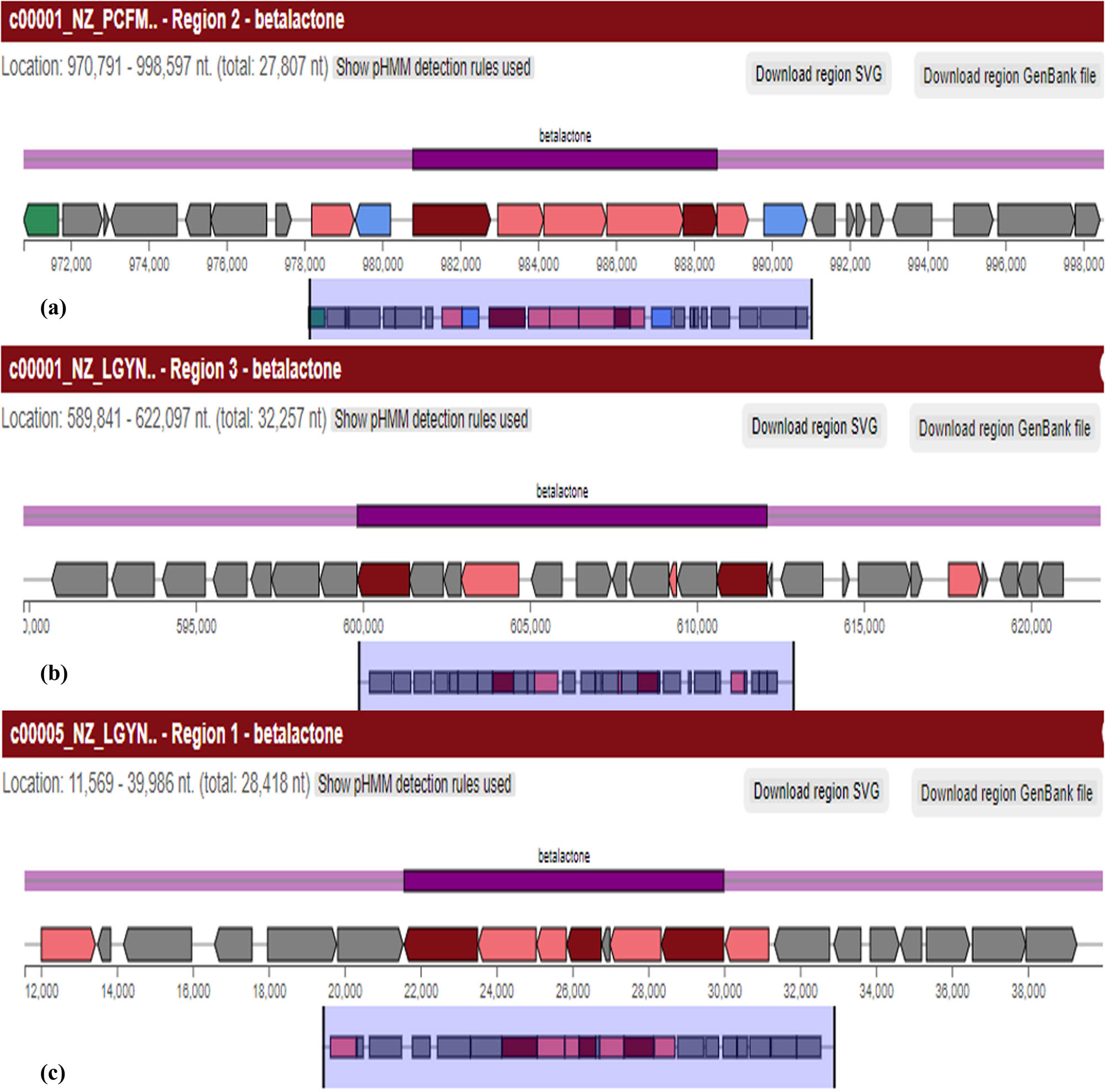
Comparison of BGC map of beta-lactone. (a) Shows beta-lactone synthesizing region 1.2, which is a 27,807 nt long gene, whereas (b) and (c) show the beta-lactone regions 1.3 (32,257 nt) and 5.1 (28,418 nt), respectively, in B. australimaris reference genome.
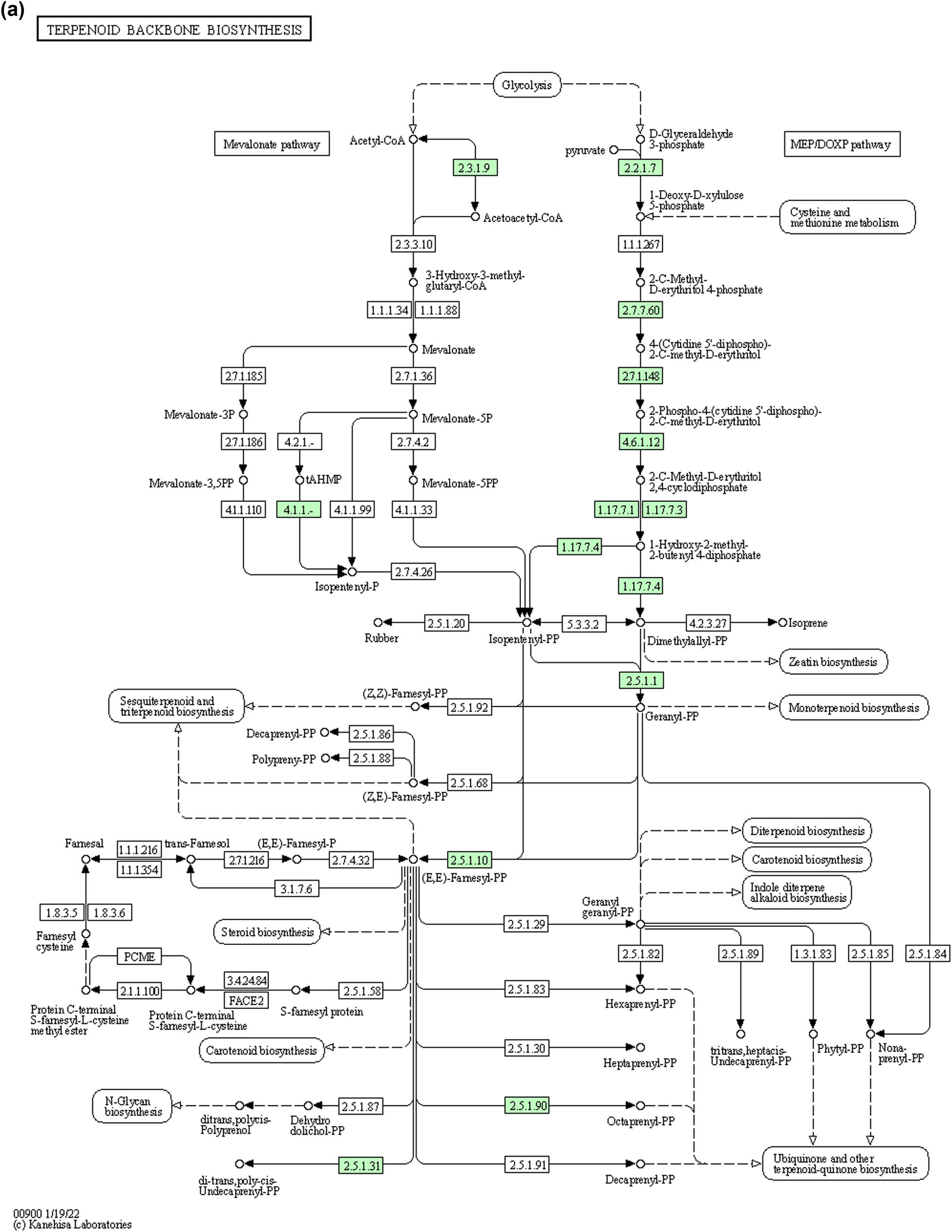
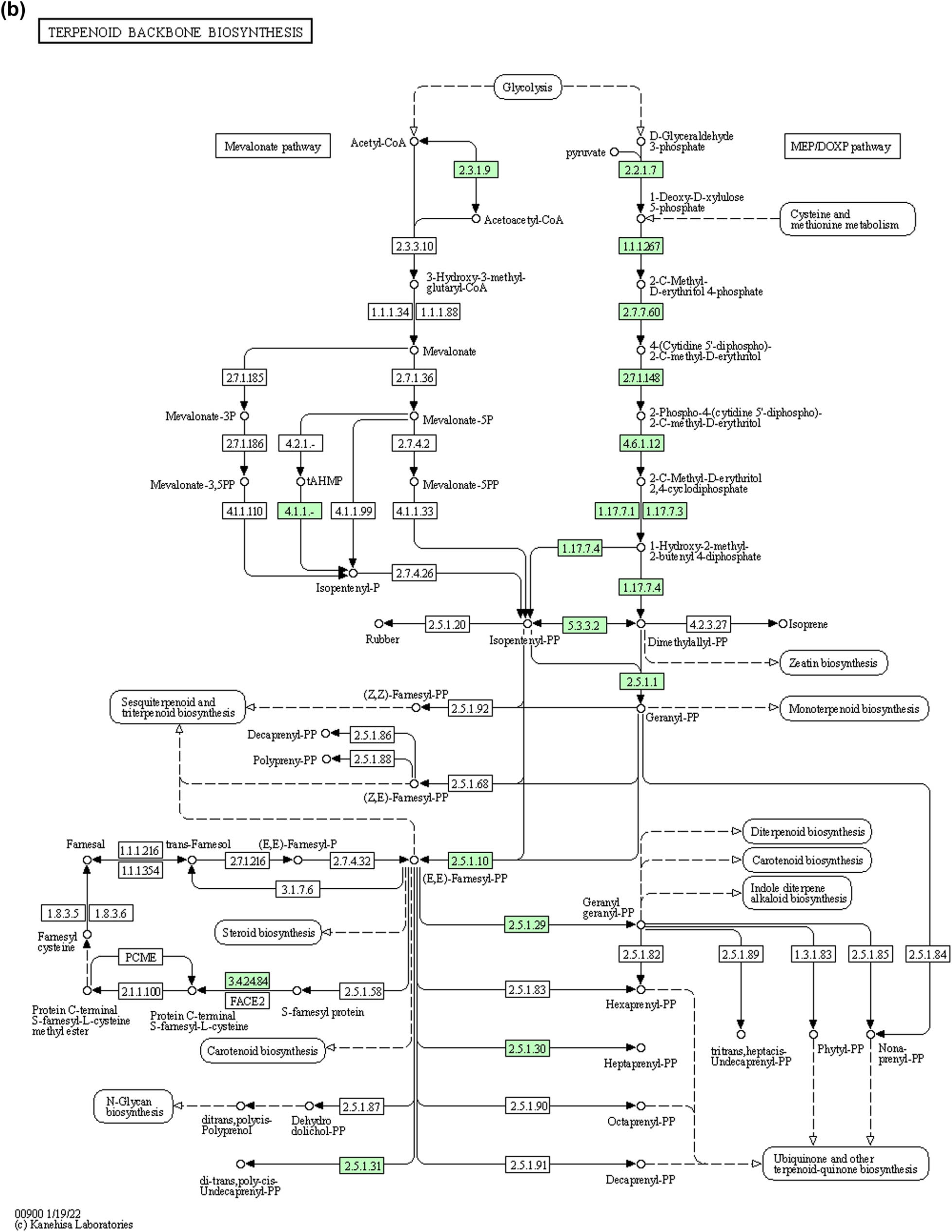
(a) Pathway of terpenoids backbone synthesis in Ochrobactrum sp. (MT180101) obtained by KeGG pathways. The enzymes involved in the synthesis of terpenes in Ochrobactrum sp. are indicated by green color. Where, 2.2.1.7: 1-deoxy-d-xylulose-5-phosphate-synthase, 2.2.1.7:1-deoxy-d-xylulose-5-phosphate synthase, 2.3.1.9: acetyl-CoA C-acyltransferase, 2.7.7.60: bifunctional 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase, 2.7.1.148: 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol kinase, 4.6.1.12: bifunctional 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase, 1.17.7.1, 1.17.7.3: flavodoxin-dependent (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase, 1.17.7.4: 4-hydroxy-3-methylbut-2-enyl diphosphate reductase, 2.5.1.129: UbiX family flavin prenyltransferase, 2.5.1.1, 2.5.1.10: polyprenyl synthetase family protein, 2.5.1.90: polyprenyl synthetase family protein, and 2.5.1.31: isoprenyl transferase. (b) Pathway of terpenoid backbone synthesis in Bacillus sp. (bsn5) obtained by KeGG pathways. The enzymes involved in the synthesis of terpenes in Bacillus sp. are indicated by green color. Where 2.2.1.7: 1-deoxy-d-xylulose-5-phosphate-synthase, 1.1.1. 267: 1-deoxy-d-xylulose 5-phosphate reductoisomerase, 2.3.1.9: acetyl-CoA C-acyltransferase, 2.7.7.60: bifunctional 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase, 2.7.1.148: 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol kinase, 4.6.1.12: bifunctional 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase, 1.17.7.1, 1.17.7.3: flavodoxin-dependent (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase, 1.17.7.4: 4-hydroxy-3-methylbut-2-enyl diphosphate reductase, 2.5.1.129: UbiX family flavin prenyltransferase, 2.5.1.1, 2.5.1.10: polyprenyl synthetase family protein, 2.5.1.29: geranyltranstransferase, 2.5.1.30: heptaprenyl diphosphate synthase component II and 3.4.24.84: putative membrane metalloprotease, and 2.5.1.31: isoprenyl transferase.
3.7 Colonization of seedling roots by O. ciceri CS-10 and B. australimaris TP-10
Interaction between plant growth-promoting bacterial strains (CS-10 and TP-10) and the root surface of T. aestivum and Z. mays seedlings was observed by SEM and prominent colonization at the surface of roots was observed. In silico analysis of reference genomes of B. australimaris and O. ciceri revealed that both strains possess gene clusters for terpenes and beta lactones. Terpenes are known to have the potential for attraction or repulsion of organisms. In SEM micrographs, bacteria were found to be attracted towards the roots of seedlings which may be attributed due to the release of terpenoids by these bacterial cells, and thus colonizing the bacterial cells on the root surface (Figure 9c–f). Beta-lactones are also known to act as signaling molecules and can interfere with the growth of other bacteria and fungi, thus limiting their growth. Consequently, it facilitates the desired bacterial species to flourish at a particular site. Hence, the prominent attachment of rod-shaped cells of O. ciceri CS-10 and B. australimaris TP-10 on the root surface may be due to beta-lactones produced by them (Figure 9c–f). Scanning electron micrographs also revealed that B. australimaris TP-10 preferred to attach as single cells on the wheat root surface (Figure 9e), whereas it formed aggregates of cells on the surface of roots of maize. The cells in these aggregates can be seen densely wrapped in mucilaginous compounds such as EPS which facilitated the aggregation or biofilm formation on the root surface (Figure 9f). However, the cells of O. ciceri CS-10 appeared as aggregates consisting of 2–3 cells forming micro-colonies on the surface of the roots of inoculated seedlings (Figure 9c–d). The root surface of CS-10 and TP-10 inoculated T. aestivum and Z. mays seedlings (Figure 9c–f) was uneven as compared to the roots of non-inoculated seedlings which appeared to possess smooth texture (Figure 9a and b). The root epidermis was damaged in the areas which were infested by O. ciceri CS-10, thus indicating their effective invasion strategy (Figure 9c–d). SEM micrographs also revealed enhanced colonization of maize root surface by both inoculated strains, O. ciceri CS-10, and B. australimaris TP-10, in comparison to the root surface of wheat seedlings (Figure 9c–f).
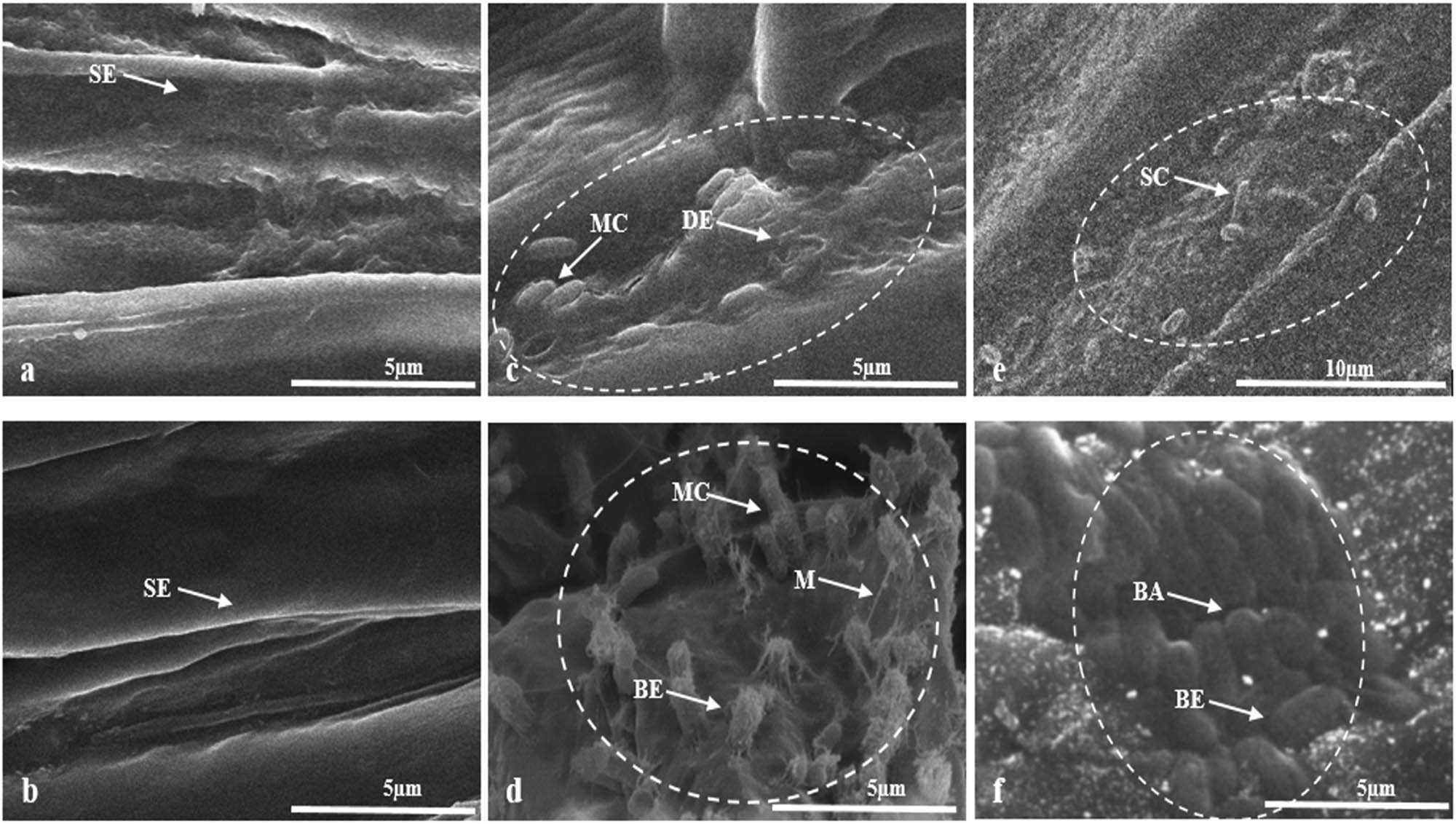
Scanning electron micrograph of wheat and corn seedlings after 2 weeks of pot treatment. Colonization of wheat plant roots by bacterial strains, i.e., O. ciceri (c) and B. australimaris (e) can be seen against control (a). Similarly, in corn plant roots (control = b), colonization of O. ciceri (d) and B. australimaris (f) is prominently evident. White circles indicate the areas of maximum bacterial colonization on seedling roots. The thread-like structures (M) indicated by arrows in d are demonstrating the production of mucilaginous compounds like EPS by bacterial strain, thus helping the bacterial cell to get attached to the root surface. (SE – smooth epidermis, MC – micro colony, DE – damaged epidermis, SC – single cell, M – mucilaginous compound, BE – bacteria entrapped in EPS, and BA – bacterial aggregation).
4 Discussion
Bacterial species are a vital component of soil as they are involved in a versatile range of biotic activities majorly associated with nutrient cycling and production of sustainable crops [33]. These bacterial species encourage the plant growth by secreting plant growth regulators, mobilizing soil nutrients, improving soil structure, biocontrol of phytopathogens, and bioremediating the contaminated soils by toxic heavy metals sequestration and deterioration of xenobiotic compounds, for instance, pesticides [34].
In the current study, the phyto-stimulatory effect of isolated bacterial strains on T. aestivum and Z. mays was observed under natural conditions. For this purpose, isolated strains were identified for their plant growth-promoting characteristics and were found to possess HCN, IAA, and ACC deaminase activity. These results comply with a study that indicated that bacteria aided in the growth of plants through phytohormone production, phosphate solubilization, nitrogen fixation, siderophores, and antibacterial compounds production [35]. ACC deaminase activity was also found in selected bacterial strains. Promotion of plant growth by ACC deaminase has also been reported previously by Herpell et al. According to the researchers, this ACC can act as growth-promoting enzyme by sequestering an ethylene precursor (ACC) which retards plant growth [36].
Inoculation with selected bacterial strains caused a significant rise in percentage germination of wheat plants higher than maize plants. This increment in T. aestivum and Z. mays percentage germination has also been highlighted by other researchers who suggest that when seeds are inoculated with plant growth promoting bacteria (PGPB), they tend to make nutrients available for the plant and may also release some phytohormones which beneficially influence the viability of seed as well as its rate of germination [37].
Other growth parameters including shoot and root length were also improved by these bacterial strains as compared to the control. The increment in shoot and root length is associated with the production of phytohormones by plant-associated bacteria for instance gibberellins, IAA, abscisic acid, and down-regulation of ethylene [38]. O. ciceri was observed to possess high IAA production as well as phosphate solubilization, in this study. Increased production of IAA and phosphate solubilization helps in the better development of plant growth parameters [39]. In compliance with this study, an increase in root number by plant growth-promoting bacteria such as Bacillus sp. has also been reported previously [40]. This increase is attributed to the fact that, with the increase in root number, the root system becomes more complex and gains volume which in turn helps the plant to uptake more nutrients and water from the surroundings [41].
Among the phytohormones that aid in the growth and development of plants, a very important one is auxin responsible for plant cell development. The most known auxin is IAA. Plant growth-promoting bacteria have the potential to produce IAA thus, promoting the growth of the plant [42]. IAA content was significantly increased in inoculated seedlings of T. aestivum and Z. mays. This finding is also justified by other studies that indicate a positive correlation between plant growth promotion and the release of IAA by bacteria [31].
Under stress conditions, increment in the soluble protein content of plants helps in the growth and development by making nitrogen available for plants [43]. In this study, T. aestivum and Z. mays seedlings were found to possess high soluble protein content after bacterial seed inoculations. This can be attributed to the fact that bacterial strains produce soluble proteins and make them available for plants to help in their growth and development. According to past studies, Bacillus species have been reported to enhance plant growth by producing proline, sugars, and amino acids [44]. Peroxidase content in plants is highly important for lowering the oxidative stress. The association of plant growth-promoting bacteria with plants results in the systematic induction of this enzyme within the plants. This enzyme participates in inducing pathogen resistance as well as reducing oxidative stress in plants [45]. In this study, peroxidase content was found to be very high in both T. aestivum and Z. mays plants. An increase in peroxidase content following bacterial inoculation in wheat plants has also been reported earlier [46].
Genome mining of bacteria provides insights into the identification of gene clusters associated with the production of secondary metabolites. This approach can unveil a wide array of potentially bioactive compounds that may be involved in plant-microbe interactions [47]. Reference genomes of O. ciceri and B. australimaris revealed the presence of various secondary metabolites producing gene clusters in them. Ochrobactrum sp. had five BGC’s including terpenes, beta lactones, acyl amino acids, NAGGN, and aryl polyenes. Plant growth regulation is affected by these secondary metabolites through direct or indirect methods. Terpenes are carotenoid compounds whereas beta lactones are reported to be classified as terpenes, polyketides, NRPs, or fatty acids that aim to up-regulate plant growth by indirect mechanisms such as antibacterial activity [48]. Other secondary metabolites produced by these bacteria include NAGGN which is known to up-regulate plant growth probably by osmoregulation [49]. Aryl polyenes can also help impart pigmentation to bacterial cells as well as these compounds protecting plants from oxidative stress. It is known to have a significant role in Gram-negative cell biology [50]. In the B. australimaris gene cluster, 5 out of 12 regions encode NRPS secondary metabolites. These NRP synthases produce NRPs that are known to possess various biological activities like antimicrobial and antiviral properties [51]. These antibacterial properties are indirectly linked to plant growth by bio-control activity [28]. As indicated by other researchers, terpene and siderophores-producing regions also prove to be beneficial for root growth stimulation [52].
Beta-lactones and terpenes gene clusters were found in both reference genomes. Plant growth promotion by beta-lactones has been reported previously. These compounds have been implicated in their antibacterial activity and enhancing plant response to external stresses [53]. Some other lactone compounds such as acyl homoserine lactones (AHL) are the inducer molecules for quorum sensing of gene expression. These AHL molecules aid plants in phosphate solubilization, nitrogen fixation, and bio-control activities [54]. Terpenes are also considered an important growth-promoting factor as it acts as carbon sources for mediating root growth in plants [55].
Bacteria colonize plant roots as part of a mutualistic relationship, known as plant-microbe interactions [56]. The interactions between the plant and bacteria are crucial for the plant’s growth and development, as bacteria can offer significant advantages to the plant including improved nutrient uptake, resistance to disease, and tolerance to stress [57]. In the current study, O. ciceri CS-10 and B. australimaris TP-10 were found to colonize the roots of wheat and maize plant seedlings. There are different mechanisms by which bacteria colonize the plant roots, but the most common one is by chemotaxis [58]. Specific chemicals released by the roots of plants attract bacteria, signaling the presence of a suitable environment for bacterial growth [59]. According to the in silico studies, bacterial strains used for seedling inoculation were found to possess genes for terpenes and beta-lactones biosynthesis in them. The association of bacterial strains with roots can be due to the release of terpenes which can play a role in facilitating bacterial colonization on plant roots. Some bacteria, particularly those that form mutualistic relationships with plants, produce terpenes that can help them to interact with the plant host and establish a stable colonization of the root system [60]. Bacteria produced beta-lactones and some terpenes that have been shown to prevent the growth of pathogenic fungi and other microorganisms that might otherwise outcompete the beneficial bacteria for nutrients and space on the surface of roots [60,61].
Bacterial cells were seen densely wrapped in EPS thus forming bacterial aggregates. This aggregation of cells is possible because, once the bacteria reach the root surface, they use various mechanisms to adhere to the root hairs, including the production of adhesins or the secretion of EPS that form a biofilm on the surface of roots. This biofilm provides a protective environment for the bacteria and allows them to interact with the plant cells [62]. The aggregation of bacterial cells due to the release of EPS is evident from another study that mentions that the secretion of root exudates including amino acids, EPS, and organic compounds is directly linked to improved plant growth and other microbial activities [63].
In scanning electron micrographs (Figure 9c and d), bacterial cells were seen to damage the root epidermis to colonize the root because the root epidermis acts as a physical barrier that prevents bacteria from penetrating the root [64]. Some bacteria secrete enzymes that can break down the cell walls of the epidermal cells, allowing them to penetrate the root [65]. Enhanced colonization of maize seedling roots can be attributed to the fact that maize has a relatively shallow root system with more lateral roots, while wheat has a deeper root system with fewer lateral roots [66]. This difference in root morphology can create different niches for bacterial colonization, with maize providing more opportunities for bacteria to attach and colonize. According to a study, Enterobacter C1D has been reported to enhance the growth of maize more than mung bean [67]. Maize has also been reported to produce certain exudates in the soil which makes a nutrient-rich environment for bacterial cells to survive [68]. According to a previous study, enhanced colonization of maize was observed by the Enterobacter FD17 strain which depicts the specific colonizing capacity of bacteria. The detailed mechanisms for the plant or species-specific colonization of bacterial cells are poorly understood and still need to be explored [69].
5 Conclusion
In conclusion, maize and wheat are economically important food crops. The current study highlighted the growth promotion potential of indigenous bacterial strains O. ciceri CS-10 and B. australimaris TP-10, which were isolated from the soap industry and Tatta Pani soil, respectively. These bacterial strains significantly boosted the growth and biochemical parameters of wheat and maize plant by enhancing the production of various phytohormones. In silico study of the O. ciceri and B. australimaris reference genomes suggested the presence of beta-lactone and terpene gene clusters in common. These biosynthetic compounds can be responsible for the attachment of bacterial cells to the plant roots. Maize plant roots were colonized more efficiently by the bacterial strains as compared to the wheat plant. Plant-microbe interaction is a low-input biotechnological system for the revitalization of the ecosystem. Thus, these bacterial strains can be beneficial for the agricultural sector.
Acknowledgments
University of the Punjab is highly acknowledged for its research facility. This research work is a part of PhD thesis of the first author Ms. Rimsha Dilshad.
-
Funding information: Higher Education Commission Pakistan provided funding for this research under HEC indigenous fellowship program (Phase 2 Batch 5) Pin No. 518-74646-2BS5-025.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
[1] Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013;37(5):634–63.10.1111/1574-6976.12028Search in Google Scholar PubMed
[2] Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1473. 10.3389/fpls.2018.01473.Search in Google Scholar PubMed PubMed Central
[3] Mishra P, Singh PP, Singh SK, Verma H. Sustainable agriculture and benefits of organic farming to special emphasis on PGPR. Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology. Elsevier; 2019. p. 75–87.10.1016/B978-0-12-817004-5.00005-1Search in Google Scholar
[4] Yadav AN, Kour D, Kaur T, Devi R, Yadav A, Dikilitas M, et al. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal Agric Biotechnol. 2021;33:102009.10.1016/j.bcab.2021.102009Search in Google Scholar
[5] Widnyana IK. PGPR (Plant Growth Promoting Rizobacteria) benefits in spurring germination, growth and increase the yield of tomato plants. In: Recent Advances in Tomato Breeding and Production. London, UK: IntechOpen; 2018.Search in Google Scholar
[6] Niu X, Song L, Xiao Y, Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front Microbiol. 2018;8:1–11. 10.3389/fmicb.2017.02580.Search in Google Scholar PubMed PubMed Central
[7] Kang SM, Khan AL, You YH, Kim JG, Kamran M, Lee IJ. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J Microbiol Biotechnol. 2014;24(1):106–12.10.4014/jmb.1304.04015Search in Google Scholar PubMed
[8] Meena M, Swapnil P, Divyanshu K, Kumar S, Tripathi YN, Zehra A, et al. PGPR‐mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J Basic Microbiol. 2020;60(10):828–61.10.1002/jobm.202000370Search in Google Scholar PubMed
[9] Bang C, Dagan T, Deines P, Dubilier N, Duschl WJ, Fraune S, et al. Metaorganisms in extreme environments: do microbes play a role in organismal adaptation. Zoology. 2018;127:1–19. 10.1016/j.zool.2018.02.004.Search in Google Scholar PubMed
[10] Stewart B, Lal R. Increasing world average yields of cereal crops: It’s all about water. Adv Agron. 2018;151:1–44.10.1016/bs.agron.2018.05.001Search in Google Scholar
[11] Basu S, Kumar G. Plant microbe interaction for changing endophytic colonization to improve plant productivity. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2021. p. 137–47. 10.1016/B978-0-444-64325-4.00012-2.Search in Google Scholar
[12] Mahto KU, Priyadarshanee M, Samantaray DP, Das S. Bacterial biofilm and extracellular polymeric substances in the treatment of environmental pollutants: Beyond the protective role in survivability. J Clean Prod. 2022;379(2):134759.10.1016/j.jclepro.2022.134759Search in Google Scholar
[13] Ajijah N, Fiodor A, Pandey AK, Rana A, Pranaw K. Plant growth-promoting bacteria (PGPB) with biofilm-forming ability: A multifaceted agent for sustainable agriculture. Diversity. 2023;15(1):112.10.3390/d15010112Search in Google Scholar
[14] Medema MH, Kottmann R, Yilmaz P, Cummings M, Biggins JB, Blin K, et al. Minimum information about a biosynthetic gene cluster. Nat Chem Biol. 2015;11(9):625–31.10.1038/nchembio.1890Search in Google Scholar PubMed PubMed Central
[15] Hemphill CFP, Sureechatchaiyan P, Kassack MU, Orfali RS, Lin W, Daletos G, et al. OSMAC approach leads to new fusarielin metabolites from Fusarium tricinctum. J Antibiot. 2017;70(6):726–32.10.1038/ja.2017.21Search in Google Scholar PubMed
[16] Foulston L. Genome mining and prospects for antibiotic discovery. Curr Opin Microbiol. 2019;51:1–8.10.1016/j.mib.2019.01.001Search in Google Scholar PubMed
[17] Meunier L, Tocquin P, Cornet L, Sirjacobs D, Leclère V, Pupin M, et al. Palantir: a springboard for the analysis of secondary metabolite gene clusters in large-scale genome mining projects. Bioinformatics. 2020;36(15):4345–7.10.1093/bioinformatics/btaa517Search in Google Scholar PubMed
[18] Dilshad R, Jamil N, Batool R. Biosynthetic gene clusters in bacteria: a review: bacterial secondary metabolites producing genes. Proc Pak Acad Sci: B. 2021;58(3):29–42.10.53560/PPASB(58-3)665Search in Google Scholar
[19] Habib N, Choudhry S. HPLC quantification of thymoquinone extracted from Nigella sativa L. (Ranunculaceae) seeds and antibacterial activity of its extracts against Bacillus species. Evid.-Based Complement. Alternat Med. 2021;2021:1–11.10.1155/2021/6645680Search in Google Scholar PubMed PubMed Central
[20] Ribeiro IDA, Bach E, Moreira FS, Müller AR, Rangel CP, Wilhelm CM, et al. Antifungal potential against Sclerotinia sclerotiorum (Lib.) de Bary and plant growth promoting abilities of Bacillus isolates from canola (Brassica napus L.) roots. Microbiol Res. 2021;248:126754.10.1016/j.micres.2021.126754Search in Google Scholar PubMed
[21] Jiang L, Seo J, Peng Y, Jeon D, Park SJ, Kim CY, et al. Genome insights into the plant growth-promoting bacterium Saccharibacillus brassicae ATSA2T. AMB Expr. 2023;13:9. 10.1186/s13568-023-01514-1.Search in Google Scholar PubMed PubMed Central
[22] Amna, ud DinB, SarfrazS, Xia Y, Kamran MA, Javed MT, et al. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol Environ Saf. 2019;183:109466. 10.1016/j.ecoenv.2019.109466.Search in Google Scholar PubMed
[23] Tariq MR, Shaheen F, Mustafa S, Sajid A, Fatima A, Shafiq M, et al. Phosphate solubilizing microorganisms isolated from medicinal plants improve growth of mint. PeerJ. 2022;10:e13782.10.7717/peerj.13782Search in Google Scholar PubMed PubMed Central
[24] Kalsoom A, Batool R, Jamil N. Beneficial rhizospheric associated traits of chromate resistant bacteria for remediation of Cr (VI) contaminated soil. Bioremediat J. 2022;2022:1–19.10.1080/10889868.2022.2054930Search in Google Scholar
[25] Batool R, Yrjälä K, Hasnain S. Alleviation of phyto-toxic effects of chromium by inoculation of chromium (VI) reducing Pseudomonas aeruginosa Rb-1 and Ochrobactrum intermedium Rb-2. Int J Agric Biol. 2015;17:21–30.Search in Google Scholar
[26] Sun YC, Sun P, Xue J, Du Y, Yan H, Wang LW, et al. Arthrobacter wenxiniae sp. nov., a novel plant growth-promoting rhizobacteria species harbouring a carotenoids biosynthetic gene cluster. Antonie Leeuwenhoek. 2022;115(3):353–64.10.1007/s10482-021-01701-9Search in Google Scholar PubMed
[27] Dilshad R, Batool R. Co-resistance of antibiotics and heavy metals in bacterial strains isolated from agriculture farm and soap industry. LGU J Life Sci. 2022;6(04):338–49.10.54692/lgujls.2022.0604233Search in Google Scholar
[28] Hassan SED. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res. 2017;8(6):687–95.10.1016/j.jare.2017.09.001Search in Google Scholar PubMed PubMed Central
[29] Tiwari S, Prasad V, Lata C. Bacillus: Plant growth promoting bacteria for sustainable agriculture and environment. New and future developments in microbial biotechnology and bioengineering. Elsevier; 2019. p. 43–55.10.1016/B978-0-444-64191-5.00003-1Search in Google Scholar
[30] Satyaprakash M, Nikitha T, Reddi E, Sadhana B, Vani SS. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int J Curr Microbiol Appl Sci. 2017;6(4):2133–44.10.20546/ijcmas.2017.604.251Search in Google Scholar
[31] Bhutani N, Maheshwari R, Negi M, Suneja P. Optimization of IAA production by endophytic Bacillus spp. from Vigna radiata for their potential use as plant growth promoters. Isr J Plant Sci. 2018;65(1–2):83–96. 10.1163/22238980-00001025.Search in Google Scholar
[32] Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase‐containing plant growth‐promoting rhizobacteria. Physiol Plant. 2003;118(1):10–5.10.1034/j.1399-3054.2003.00086.xSearch in Google Scholar PubMed
[33] Ahemad M, Khan M, Zaidi A, Wani P. Remediation of herbicides contaminated soil using microbes. Microbes Sustain Agric. 2009;261(5):1–84.Search in Google Scholar
[34] Ahemad M. Implications of bacterial resistance against heavy metals in bioremediation: a review. IIOAB J. 2012;3(3):39–46.Search in Google Scholar
[35] Guo DJ, Singh RK, Singh P, Li DP, Sharma A, Xing YX, et al. Complete genome sequence of Enterobacter roggenkampii ED5, a nitrogen fixing plant growth promoting endophytic bacterium with biocontrol and stress tolerance properties, isolated from sugarcane root. Front Microbiol. 2020;11:580081.10.3389/fmicb.2020.580081Search in Google Scholar PubMed PubMed Central
[36] Herpell JB, Alickovic A, Diallo B, Schindler F, Weckwerth W. Phyllosphere symbiont promotes plant growth through ACC deaminase production. ISME J. 2023;17:1267–77. 10.1038/s41396-023-01428-7.Search in Google Scholar PubMed PubMed Central
[37] De Souza NL, Rocha SS, Narezzi NT, Tiepo AN, de Oliveira ALM, Oliveira HC, et al. Differential impacts of plant growth-promoting bacteria (PGPB) on seeds of neotropical tree species with contrasting tolerance to shade. Trees. 2020;34(1):121–32.10.1007/s00468-019-01902-wSearch in Google Scholar
[38] Ambreetha S, Chinnadurai C, Marimuthu P, Balachandar D. Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere. 2018;5:57–66. 10.1016/j.rhisph.2017.12.001.Search in Google Scholar
[39] Rasul M, Yasmin S, Yahya M, Breitkreuz C, Tarkka M, Reitz T. The wheat growth-promoting traits of Ochrobactrum and Pantoea species, responsible for solubilization of different P sources, are ensured by genes encoding enzymes of multiple P-releasing pathways. Microbiol Res. 2021;246:126703.10.1016/j.micres.2021.126703Search in Google Scholar PubMed
[40] Irizarry I, White J. Application of bacteria from non‐cultivated plants to promote growth, alter root architecture and alleviate salt stress of cotton. J Appl Microbiol. 2017;122(4):1110–20.10.1111/jam.13414Search in Google Scholar PubMed
[41] Dal Cortivo C, Barion G, Visioli G, Mattarozzi M, Mosca G, Vamerali T. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric Ecosyst Environ. 2017;247:396–408.10.1016/j.agee.2017.07.006Search in Google Scholar
[42] Jeyanthi V, Kanimozhi S. Plant growth promoting rhizobacteria (PGPR)-prospective and mechanisms: A review. J Pure Appl Microbiol. 2018;12(2):733–49.10.22207/JPAM.12.2.34Search in Google Scholar
[43] Gupta P, Kumar V, Usmani Z, Rani R, Chandra A, Gupta VK. Implications of plant growth promoting Klebsiella sp. CPSB4 and Enterobacter sp. CPSB49 in luxuriant growth of tomato plants under chromium stress. Chemosphere. 2020;240:124944.10.1016/j.chemosphere.2019.124944Search in Google Scholar PubMed
[44] Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, Abd_Allah EF. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol. 2017;8. 10.3389/fmicb.2017.01887.Search in Google Scholar PubMed PubMed Central
[45] Maksimov I, Abizgil’dina R, Yusupova Z, Khajrullin R. Effect of Bacillus subtilis 26D on the hydrogen peroxide level and peroxidase activity in spring wheat plants. Agric Chem. 2010;55–60.Search in Google Scholar
[46] Minaeva O, Akimova E, Tereshchenko N, Zyubanova T, Apenysheva M, Kravets A. Effect of Pseudomonas bacteria on peroxidase activity in wheat plants when infected with Bipolaris sorokiniana. Russ J Plant Physiol. 2018;65(5):717–25.10.1134/S1021443718040052Search in Google Scholar
[47] Iqbal S, Qasim M, Rahman H, Khan N, Paracha RZ, Bhatti MF, et al. Genome mining, antimicrobial and plant growth-promoting potentials of halotolerant Bacillus paralicheniformis ES-1 isolated from salt mine. Mol Genet Genom. 2023;298(1):79–93.10.1007/s00438-022-01964-5Search in Google Scholar PubMed
[48] Robinson SL, Christenson JK, Wackett LP. Biosynthesis and chemical diversity of β-lactone natural products. Nat Prod Rep. 2019;36(3):458–75.10.1039/C8NP00052BSearch in Google Scholar PubMed
[49] Velásquez AC, Huguet-Tapia JC, He SY. Shared in planta population and transcriptomic features of nonpathogenic members of endophytic phyllosphere microbiota. Proc Natl Acad Sci (PNAS). 2022;119(14):e2114460119.10.1073/pnas.2114460119Search in Google Scholar PubMed PubMed Central
[50] Peter C, Marnix HM, Jan C, Kenji K, Wieland C, Laura B, et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell. 2014;158(2):412–21.10.1016/j.cell.2014.06.034Search in Google Scholar PubMed PubMed Central
[51] Agrawal S, Acharya D, Adholeya A, Barrow CJ, Deshmukh SK. Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential. Front Pharmacol. 2017;8:828. 10.3389/fphar.2017.00828.Search in Google Scholar PubMed PubMed Central
[52] Jiménez-Gómez A, Saati-Santamaría Z, Igual JM, Rivas R, Mateos PF, García-Fraile P. Genome insights into the novel species Microvirga brassicacearum, a rapeseed endophyte with biotechnological potential. Microorganisms. 2019;7(9):354.10.3390/microorganisms7090354Search in Google Scholar PubMed PubMed Central
[53] Adeleke BS, Ayangbenro AS, Babalola OO. Genomic analysis of endophytic Bacillus cereus T4S and its plant growth-promoting traits. Plants. 2021;10(9):1776. 10.3390/plants10091776.Search in Google Scholar PubMed PubMed Central
[54] Imran A, Saadalla MJA, Khan SU, Mirza MS, Malik KA, Hafeez FY. Ochrobactrum sp. Pv2Z2 exhibits multiple traits of plant growth promotion, biodegradation and N-acyl-homoserine-lactone quorum sensing. Ann Microbiol. 2014;64(4):1797–806.10.1007/s13213-014-0824-0Search in Google Scholar
[55] Junker RR, Tholl D. Volatile organic compound mediated interactions at the plant-microbe interface. J Chem Ecol. 2013;39(7):810–25.10.1007/s10886-013-0325-9Search in Google Scholar PubMed
[56] De Mandal S, Singh S, Hussain K, Hussain T. Plant–microbe association for mutual benefits for plant growth and soil health. In: Current Trends in Microbial Biotechnology for Sustainable Agriculture. Singapore: Springer; 2021. p. 95–121.10.1007/978-981-15-6949-4_5Search in Google Scholar
[57] Arif I, Batool M, Schenk PM. Plant microbiome engineering: expected benefits for improved crop growth and resilience. Trends Biotechnol. 2020;38(12):1385–96. 10.1016/j.tibtech.2020.04.015.Search in Google Scholar PubMed
[58] Feng H, Fu R, Hou X, Lv Y, Zhang N, Liu Y, et al. Chemotaxis of beneficial rhizobacteria to root exudates: The first step towards root–microbe rhizosphere interactions. Int J Mol Sci. 2021;22(13):6655.10.3390/ijms22136655Search in Google Scholar PubMed PubMed Central
[59] Xiang L, Harindintwali JD, Wang F, Redmile-Gordon M, Chang SX, Fu Y, et al. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ Sci Technol. 2022;56(23):16546–66.10.1021/acs.est.2c02976Search in Google Scholar PubMed PubMed Central
[60] Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;18(11):607–21.10.1038/s41579-020-0412-1Search in Google Scholar PubMed
[61] Yan B, Liu N, Liu M, Du X, Shang F, Huang Y. Soil actinobacteria tend to have neutral interactions with other co‐occurring microorganisms, especially under oligotrophic conditions. Environ Microbiol. 2021;23(8):4126–40.10.1111/1462-2920.15483Search in Google Scholar PubMed
[62] Santoyo G, Urtis-Flores CA, Loeza-Lara PD, Orozco-Mosqueda MDC, Glick BR. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology. 2021;10(6):475.10.3390/biology10060475Search in Google Scholar PubMed PubMed Central
[63] Kumari P, Meena M, Gupta P, Dubey MK, Nath G, Upadhyay R. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek). Biocatal Agric Biotechnol. 2018;16:163–71.10.1016/j.bcab.2018.07.030Search in Google Scholar
[64] Pharand B, Carisse O, Benhamou N. Cytological aspects of compost-mediated induced resistance against Fusarium crown and root rot in tomato. Phytopathology. 2002;92(4):424–38.10.1094/PHYTO.2002.92.4.424Search in Google Scholar PubMed
[65] Batool R, Yrjälä K, Hasnain S. Study on cellular changes and potential endotrophy of wheat roots due to colonization of chromium reducing bacteria. Int J Environ Sci Technol. 2015;12:3263–72. 10.1007/s13762-015-0757-6.Search in Google Scholar
[66] Lynch JP. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot. 2013;112(2):347–57.10.1093/aob/mcs293Search in Google Scholar PubMed PubMed Central
[67] Siddhi MV, Purvi J, Mugdha B, Archana G. Root exudates influence chemotaxis and colonization of diverse plant growth promoting rhizobacteria in the pigeon pea-maize intercropping system. Rhizosphere. 2021;18:100331.10.1016/j.rhisph.2021.100331Search in Google Scholar
[68] Susana BR, Germán A, Evelin C, Carolina P, Nicolás P, Marisa R. Root colonization and growth promotion of wheat and maize by Pseudomonas aurantiaca SR1. Soil Biol Biochem. 2009;41(9):1802–6.10.1016/j.soilbio.2008.10.009Search in Google Scholar
[69] Naveed M, Mitter B, Yousaf S, Pastar M, Afzal M, Sessitsch A. The endophyte Enterobacter sp. FD17: a maize growth enhancer selected based on rigorous testing of plant beneficial traits and colonization characteristics. Biol Fertil Soils. 2014;50:249–62.10.1007/s00374-013-0854-ySearch in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer
Articles in the same Issue
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer

