The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
-
Muhamad Amin
, Yoga Pramujisunu
Abstract
Introduction
Probiotics have been commonly practiced in commercial shrimp farms to increase pond production. However, these possibilities were based on the results of in vitro studies or laboratory in vivo trials. While studies on probiotic applications in commercial-scale farms are still rarely investigated, this study addresses the fate of probiotic species in ponds and the intestinal tract of white shrimps reared in an intensive aquaculture system.
Material and methods
Four commercial probiotic species (Lactobacillus plantarum, Lactobacillus fermentum, Bacillus subtilis, and Pseudomonas putida) were applied to the commercial shrimp ponds (@800 m2 area of high-density polyethene ponds) in the morning at a dose of 5 ppm once every 2 days in the first month, and once a week from second month onward. Then, the presence of the probiotic species was traced by collecting the rearing water and shrimp’s intestines on day 47 of culture to monitor their composition and abundance using high-throughput sequencing.
Results
None of the commercial probiotic species could be detected from both rearing water and shrimp intestinal tracts. These results suggest that the probiotic species had low viability and adaptability in the rearing pond as well as the shrimp intestines when applied on commercial-scale farms. These facts may explain the high variation in the yield among shrimp ponds in spite of having similar treatments.
Conclusion
Probiotic strains had low viability and adaptability in commercial farms. Thus, methods and strategies in probiotic application to commercial-scale shrimp farms should be evaluated and further developed to increase probiotic efficacy.
1 Introduction
Probiotics have been considered an eco-friendly approach to increasing the yield of aquaculture production through several mechanisms including maintaining water quality, growth performance, or survival rate of aquatic organisms [1]. For example, studies have confirmed that probiotic application has enabled us to significantly reduce antibiotic use in aquaculture industries and avoid the occurrence of antibiotic resistance genes of microbes [2]. Some probiotics have been documented to produce digestive enzymes such as protease, amylase, lipase, alginate lyase, and cellulase which help animal hosts to digest ingested diets [3]. Probiotic strains were documented to produce antimicrobial compounds active against bacterial pathogens [4]. Also, some probiotic species have the capacity to degrade and prevent the accumulation of aquaculture waste in culture ponds including solid organic waste or soluble toxic chemicals such as ammonia (NH3) or nitrite (NO2) [5,6,7].
Despite the benefits of the usage of probiotics in aquaculture, most of these studies were based on in vivo studies or in vivo laboratory trials and very small-scale rearing systems where environmental conditions were easily controlled. Some studies confirmed that the results of in vitro and in vivo studies are frequently uncorrelated. In a review by Toledo et al. [8], it was stated that many studies had inconsistent results concerning the efficacy of probiotic treatments on shrimp survival and growth performance by in vitro and in vivo studies. These inconsistent results were due to the fact that environmental conditions in commercial shrimp ponds could vary, fluctuate, and may be very difficult to control. According to a study by Huerta-Rábago et al. [9], it was reported that commercial probiotics consisting of Bacillus spp., Lactobacillus spp., and Saccharomyces spp. introduced to a commercial shrimp farm could not be detected in rearing water due to competition with native microflora in the rearing water, and different environmental conditions. The probiotics addition also had a significant effect on the specific growth rate or survival rate of white shrimp. Salinity for instance in marine aquaculture is very critical for the survival of some bacteria which were isolated from the terrestrial organism [10]. Do these various limitations pose a question of whether probiotic strains can survive and significantly contribute to the quality of rearing water, digestibility, or disease resistance as being reported by many in vitro or laboratory-scale studies?
To address this question, there is a need for a study that will trace the composition and abundance of commercial probiotic species applied in commercial shrimp farms (pond and the intestinal tract of white shrimps) in an intensive aquaculture system using a high-throughput sequencing.
2 Materials and methods
2.1 Sampling location, culture system, and probiotic application
A commercial shrimp farm located at the ordinate point, 113°01′14.7′′ E and 6°52′59.3′′ L were selected for the present study. White shrimp, Litopenaeus vannamei, was cultivated through an intensive system (275 indiv/m2 and applied with commercial probiotics) in high-density polyethylene ponds (@800 m2 area and a water depth of 120 cm). The pond consisted of three plots with an area of 800 m2 and a stocking population of 220,000 individuals. The commercial probiotics were Lactobacillus plantarum, L. fermentum, Bacillus subtilis, and Pseudomonas putida. The probiotic consortia was applied in the morning at a dose of 5 ppm once every 2 days. Siphoning of solid wastes (feces and uneaten feed) was carried out 1 day before the stocking of the fry and during the rearing period for as much as 2–5 days to adjust the age of the shrimp. Feeding of shrimps was done manually 1–5 times a day according to the shrimp sizes.
2.2 Collection of water and shrimps’ intestinal samples
Water sampling was carried out according to the protocol previously described by Gomes et al. [11] with a slight modification. Water samples were collected from six ponds using a long pole sampling device and a 20 mL sterile plastic cup. The collected water was stored in a 50 mL falcon tube which was previously filled with 30 mL of absolute ethanol for DNA preservation. Samples were kept on ice until processed in the laboratory within the next 8 h. The shrimp intestine was sampled as previously described by Amin et al. [12]. A total of 30 healthy shrimps showing no symptoms of the disease were collected from 3 shrimp ponds (10 shrimps per pond) on day 47. The length and weight of shrimps were measured individually using a ruler and balance. Thereafter, each shrimp was washed with sterile distilled water, followed by 76% alcohol and rewashed with sterile distilled water to remove exogenous bacterial contamination. Then, the intestinal tract of each shrimp was dissected aseptically and placed into a sterile 1.5 mL microcentrifuge tube containing RNAlaterTM (R0901, SIGMA) and stored at −20°C until DNA extraction.
-
Ethics approval: The conducted research is not related to either human or animal use.
2.3 Extraction and amplification of bacterial DNA
DNA from pond water and the intestinal tract of white shrimp was extracted using the DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. In brief, falcon tubes were decontaminated with 76% ethanol and washed with sterile distilled water. Thereafter, all the falcon tubes were centrifuged (3,220 × g, 10 min, 6°C) for DNA precipitation and the supernatant was discarded [11]. The precipitated pellet was mixed with a buffer contained in the PowerBead Tube (Qiagen, Hilden, Germany). Other steps were carried out based on the manufacturer’s protocol [13]. The DNA amplification process was carried out through several stages, namely, denaturation, annealing, and extension which are carried out in as many cycles as needed using a thermocycler. The stages started with predenaturation at a temperature of 94°C for 5 min, then the sample was heated in the annealing stage at 56°C for 1 min, and continued heating at the extension stage at 72°C for 1 min. For the extension stage, the sample was heated at 72°C for 7 min for 40 repetitions [14].
2.4 Sequencing and bioinformatic analysis
DNA samples with a concentration of 50 ng/µL were sent to Novogene Biological Information Technology Co. (Singapore) for sequencing and community analysis of microbiota in the digestive tract of white shrimps using Next Generation Sequencing (NGS, Illumina platform) based on 16S rRNA gene. Prior to sequencing, the hypervariable V3–V4 region of the 16S rRNA gene was amplified by polymerase chain reaction with primer pairs 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) [14]. The results of the bacterial sequences that have been obtained were then analyzed using the UPARSE software. Sequences with a similarity of 97% were designated as the same operational taxonomic unit (OTU). The taxonomic classification of each OTU representative sequence was carried out using the MOTHUR program through the SILVA database with a confidence level of 95%.
3 Results
3.1 Profiles of probiotic species in grow-out ponds
The results showed that the number of bacteria classified as Ordo Lactobacillales was quite abundance in the three ponds (Figure 1). A total of 4,704 bacterial sequences or 5% of total bacteria detected in pond 1 were assigned to Ordo Lactobacillales, of which 4,375 sequences (93%) were identified as genus Lactobacillus and belonged to 12 bacterial species Table 1. From pond 2, a total of 4,572 bacterial sequences (5% of the total identified bacteria in pond 2) were assigned to Ordo Lactobacillales, of which 3,795 sequences (83%) were classified as genus Lactobacillus and belonged to 12 bacterial species, Table 1. Both pond 1 and pond 2 appeared to be very similar in terms of Lactobacillales proportions (5%) and the number of Lactobacillus species (12 species). Five most dominant species in both ponds 1 and 2 were Lactobacillus aviarius followed by Lactobacillus sp. (OUT_39), Lactobacillus sp. (OUT_97), Lactobacillus sp. (OUT_157), and Lactobacillus salivarius (OUT_165). The only difference between the 2 ponds was that Lactobacillus iners (OTU_228) in pond 2 was more abundant than in pond 1, 64 and 4 for ponds 2 and 1, respectively. While the other 11 species were higher in pond 1.
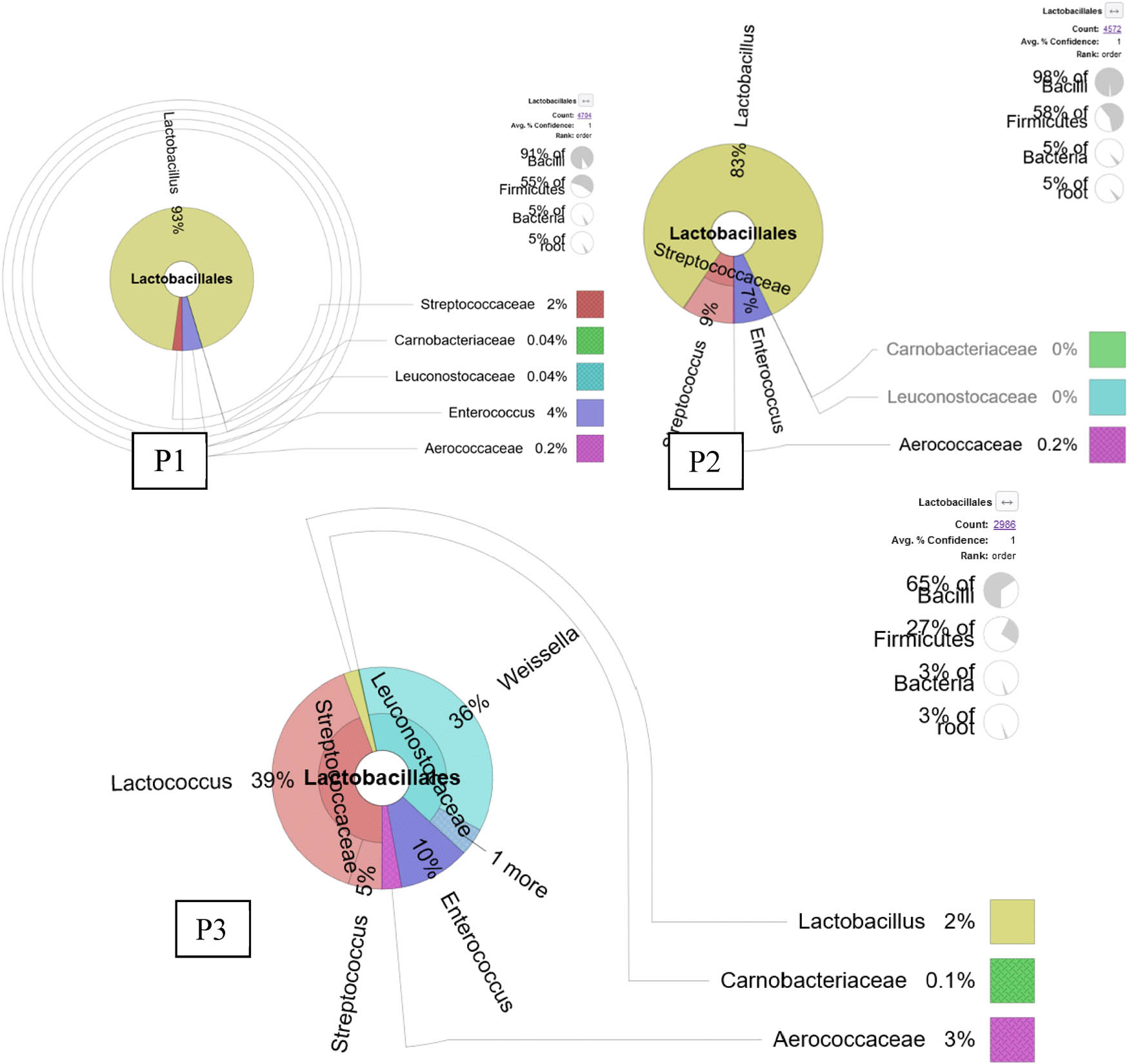
KRONA visually displays the analysis result of taxonomic annotation. Circles from inside to outside stand for different taxonomic ranks, and the area of the sector means a respective proportion of different OTU annotation results. The abundance of Lactobacillus spp. Was detected from the rearing water of commercial shrimp ponds on the day of culture (DOC) 47. P1 is pond 1, P2 is pond 2, and P3 is pond 3.
Bacterial species identified from rearing water of commercial shrimp ponds on the day of culture (DOC) 47
| Consensus lineage | Sequence numbers | OTU ID | ||
|---|---|---|---|---|
| P1 | P2 | P3 | ||
|
1,938 | 1,584 | — | OTU_19 |
|
1,116 | 914 | 2 | OTU_39 |
|
687 | 591 | — | OTU_97 |
|
386 | 456 | — | OTU_157 |
|
112 | 95 | 60 | OTU_165 |
|
91 | 86 | — | OTU_226 |
|
4 | 64 | — | OTU_288 |
|
6 | 4 | 3 | OTU_363 |
|
15 | 9 | — | OTU_514 |
|
13 | 4 | — | OTU_535 |
|
6 | 4 | — | OTU_548 |
|
8 | 2 | — | OTU_590 |
P1, P2, and P3 are shrimp pond 1, shrimp pond 2, and shrimp pond 3. OUT is operational taxonomic unit. “–” means not detected.
From pond 3, a total of 2,986 sequences or 3% of the total identified bacteria in pond 3 were assigned to Ordo Lactobacillales, of which 65 sequences (2% of Lactobacillales) were identified as genus Lactobacillus and belonged to three bacterial species which are Lactobacillus sp. (2 sequences), L. salivarius (60 sequences), and L. ruminis (3 sequences). However, none of the Lactobacillus species identified in the three ponds showed to be the introduced probiotic species which were L. plantarum and L. fermentum.
Member of genus Bacillus was not found in ponds 2 and 3, but was found only in pond 1, Figure 2. A total of 441 bacterial sequences or 0.4% of the total detected bacterial sequences were assigned to Ordo Bacillales. Of which 395 sequences or 99% were classified as Bacillus sp. (OTU_160).

KRONA visually displays the analysis result of taxonomic annotation. Circles from inside to outside stand for different taxonomic ranks, and the area of the sector means a respective proportion of different OTU annotation results. The abundance of Bacillus spp. Were detected from the rearing water of commercial shrimp ponds. P1, P2, and P3 are ponds 1, 2, and 3, respectively.
Other NGS results showed that Pseudomonas spp. were detected only from two ponds with very low abundance (Figure 3). A total of 39 bacterial sequences or 0.04% of total bacterial sequences detected from rearing water of pond 1 were assigned to Ordo Pseudomonadales, but none of them belonged to Pseudomonas spp. While in pond 2, 35 bacterial sequences were assigned to Ordo Pseudomonadales, and only one sequence was identified as Pseudomonas azotoformans. The highest abundance sequences of Ordo Pseudomonadales were detected from pond 3 which were 6,325 bacterial sequences, of which 303 sequences belonged to the genus Pseudomonas and were assigned to 3 species which were Pseudomonas psychrotolerans (213 sequences), Pseudomonas azotoformans (81 sequences), and Pseudomonas sp. (9 sequences) (Table 2). These results indicated that Pseudomonas putida which came from commercial probiotics had difficulties adapting and proliferating in the rearing water of shrimp ponds. Based on NGS results, the most abundant species was P. Psychrotolerans (213 sequences) followed by Pseudomonas azotoformans (81 sequences) and Pseudomonas sp. with 9 sequences.
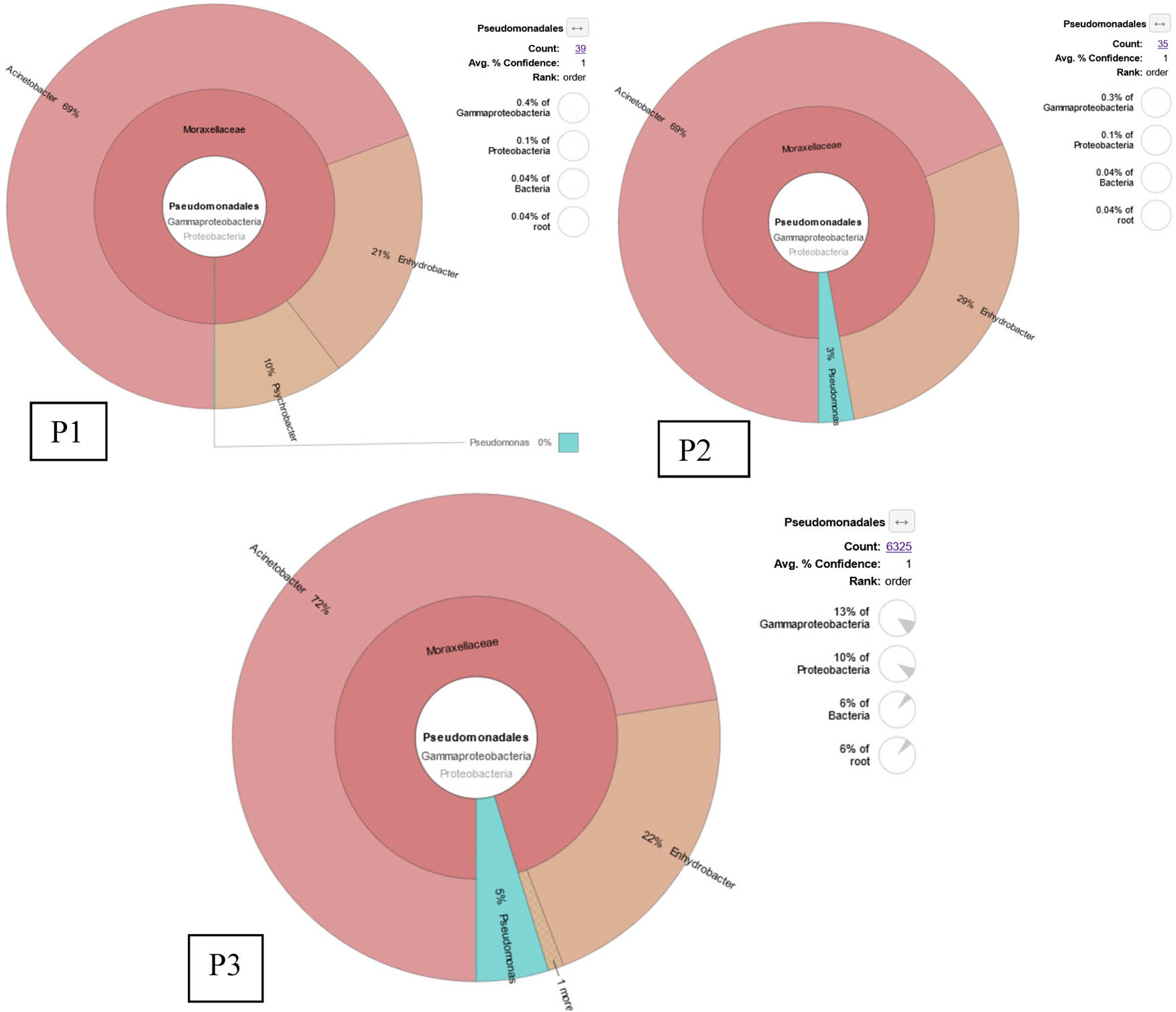
KRONA visually displays the analysis result of taxonomic annotation. Circles from inside to outside stand for different taxonomic ranks, and the area of the sector means a respective proportion of different OTU annotation results. The abundance of Pseudomonas spp. was detected from the rearing water of commercial shrimp ponds. P1 is pond 1, P2 is pond 2, and P3 is pond 3.
Bacterial species identified from rearing water of commercial shrimp ponds on DOC 47
| Consensus Lineage | Sequence numbers | #OTU ID | ||
|---|---|---|---|---|
| P1 | P2 | P3 | ||
| Pseudomonas psychrotolerans | — | — | 213 | OTU_145 |
| Pseudomonas azotoformans | — | 1 | 81 | OTU_266 |
| Pseudomonas sp. | — | — | 9 | OTU_600 |
P1, P2, and P3 are shrimp pond 1, shrimp pond 2, and shrimp pond 3. OTU is operational taxonomic unit. “–” means not detected.
3.2 Profiles of probiotic strains in intestinal tracts
3.2.1 Lactobacillus in shrimp intestines
From the shrimp intestines collected in pond 1, a total of 172 bacterial sequences or 0.2% of the total identified bacteria were assigned to Ordo Lactobacillales. Of these sequences, 90 sequences (52% of Lactobacillales) belonged to genus Streptococcus, 33 sequences (19% of Lactobacillales) belonged to genus Enterococcus, 17 sequences (10% of Lactobacillales) belonged to genus Lactobacillus, 9% (16 OTUs) belonged to genus Weisella, 5% (9 sequences) belonged to genus Lactococcus, and 4% (7 sequences) belonged to genus Leuconostoc (Figure 4). The 17 sequences of genus Lactobacillus were identified as 3 species which were L. ruminis (12 sequences), L. aviaries (4 sequences), and Lactobacillus sp. (1 sequence) (Table 3).

KRONA visually displays the analysis result of taxonomic annotation. Circles from inside to outside stand for different taxonomic ranks, and the area of the sector means a respective proportion of different OTU annotation results. Number and proportion of Lactobacillus spp. in the GI tract of white shrimps are depicted in the figure. Each figure represents sampling locations (P1 is pond 1, P2 is pond 2, and P3 is pond 3). Each figure consists of ten pooled shrimp intestines.
Lactobacillus identified from the gastrointestinal (GI) tracts of white shrimps reared in commercial shrimp ponds
| Consensus lineage | P1 | P2 | P3 | OTU ID |
|---|---|---|---|---|
| Lactobacillus aviarius | 4 | 60 | 685 | OTU_29 |
| Lactobacillus sp. | — | 97 | — | OTU_36 |
| Lactobacillus pentosus | — | 339 | — | OTU_51 |
| Lactobacillus reuteri | — | 287 | 132 | OTU_87 |
| Lactobacillus sp. | 1 | 157 | 22 | OTU_96 |
| Lactobacillus futsaii | — | 79 | 2 | OTU_172 |
| Lactobacillus salivarius | — | 46 | 9 | OTU_174 |
| Lactobacillus sp. | — | — | 84 | OTU_187 |
| Lactobacillus ruminis | 12 | 20 | 18 | OTU_216 |
| Lactobacillus saerimneri | — | 10 | — | OTU_532 |
| Lactobacillus agilis | — | 4 | 1 | OTU_534 |
| Lactobacillus acidipiscis | — | 5 | — | OTU_610 |
| Lactobacillus sp. | — | 469 | 1 | OTU_793 |
P1, P2, and P3 are shrimp pond 1, shrimp pond 2, and shrimp pond 3. OTU is operational taxonomic unit. “–” means not detected.
From the shrimp intestines collected in pond 2, a total of 1,669 bacterial sequences or 2% of the total identified bacteria were assigned to Ordo Lactobacillales. 1,569 sequences (94% of Lactobacillales) belonged to genus Lactobacillus, 84 sequences (5% of Lactobacillales) belonged to Streptococcus, 11 sequences (0.7% of Lactobacillales) belonged to Enterococcus, and one sequence belonged to Weisella (Figure 4). 1,569 Lactobacillus were identified as 12 species and the 3 top most abundant species were Lactobacillus sp. (469 sequences), followed by L. pentosus (339 sequences), and L. reuteri (287 sequences). While the lowest abundance species were L. agilis and L. acidipiscis with a single sequence each (Table 3).
Furthermore, a total of 1,265 bacterial sequences were assigned to Ordo Lactobacillales from the shrimp intestines collected from pond 3. Of these sequences, 945 sequences (75% of Lactobacillales) were identified as genus Lactobacillus. Lower taxonomic annotation indicated that the sequences were classified into 12 bacterial species. The 3 top most abundant species were Lactobacillus sp. (216 sequences), followed by L. pentosus (209 sequences) and L. reuteri (101 sequences) (Table 3).
3.2.2 Bacillus in shrimp intestines
From the shrimp intestines collected in pond 1, 48 sequences or 0.05% of the total identified bacteria were classified as Family Bacillaceae. Of the sequence, 18 sequences were identified as Bacillus badius, 24 sequences as Bacillus sp., and 6 sequences were identified as B. thermoamylovorans (Figure 5). While from the shrimp intestines collected in pond 2, 43 sequences or 0.05% of the total identified bacteria were assigned to Family Bacillaceae (Figure 5). Of these, 36 sequences (84% of Bacillaceae) belonged to genus Oceanobacillus. Six sequences (14% of Bacillaceae) belonged to genus Bacillus, and were identified as four species which were B. thermoamylovorans (2 sequences), B. badius (2 sequences), Bacillus coagulans (1 sequence), and Bacillus sp. (1 sequence) (Table 4).
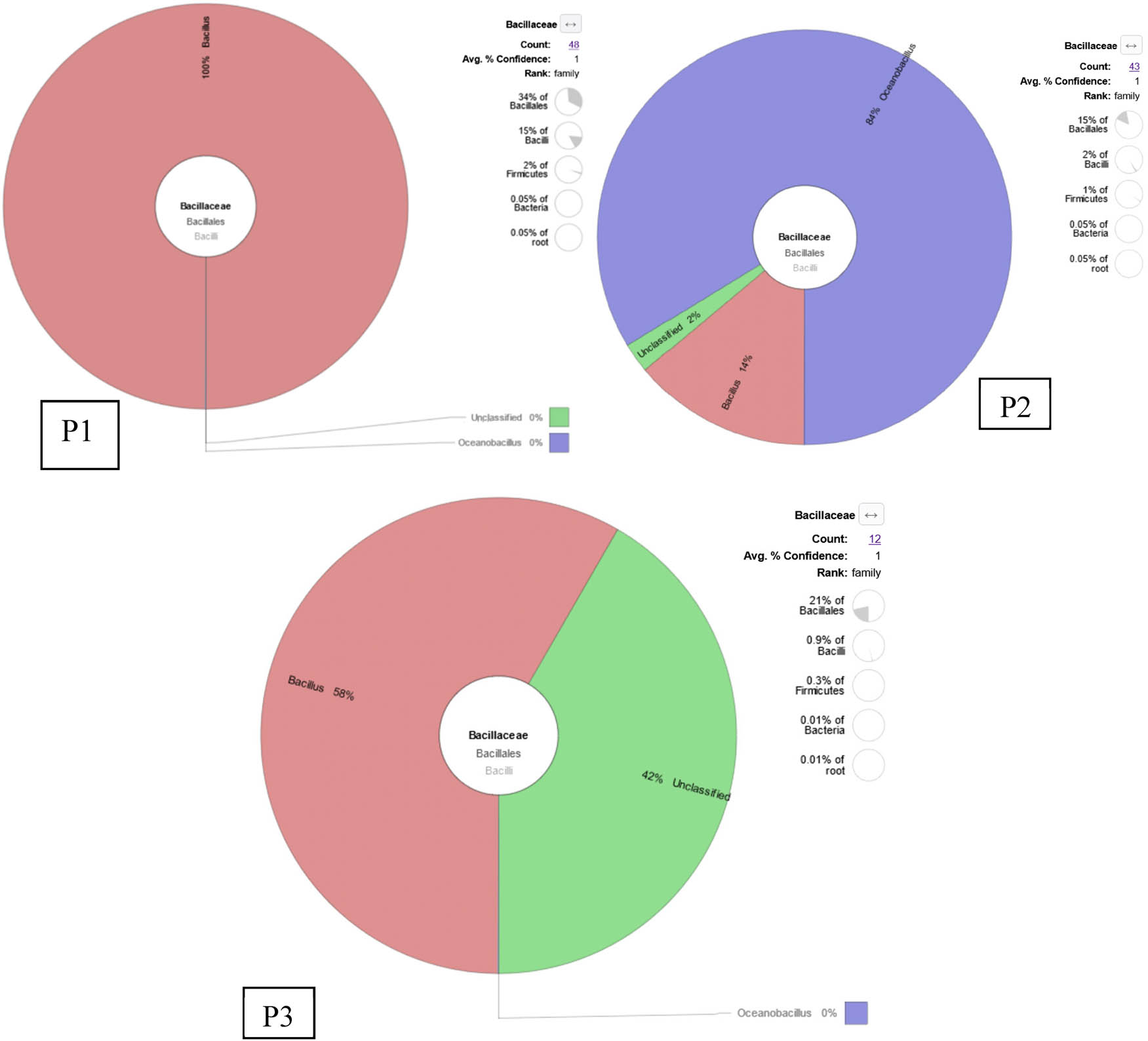
KRONA visually displays the analysis result of taxonomic annotation. Circles from inside to outside stand for different taxonomic ranks, and the area of the sector means the respective proportion of different OTU annotation results. Each figure represents sampling locations (P1 is pond 1, P2 is pond 2, and P3 is pond 3). Each figure consists of ten pooled shrimp intestines.
Bacterial species identified from the GI tracts of white shrimps reared in commercial shrimp ponds
| Consensus lineage | P1 | P2 | P3 | OTU ID |
|---|---|---|---|---|
| Bacillus badius | 18 | 2 | — | OTU_335 |
| Bacillus sp. | 24 | 1 | — | OTU_358 |
| Bacillus thermoamylovorans | 6 | 2 | 1 | OTU_365 |
| Bacillus coagulans | — | 1 | 2 | OTU_473 |
P1, P2, and P3 are shrimp pond 1, shrimp pond 2, and shrimp pond 3. OTU is operational taxonomic unit. “–” means not detected.
In addition, from the shrimp intestines collected in pond 3, 12 bacterial sequences or 0.01% of the total identified bacterial sequences were assigned into Family Bacillaceae (Figure 5). Of these, 7 sequences (58%) were identified as B. thermoamylovorans, while the other 5 sequences (42% of Bacillaceae) were “unclassified.”
3.2.3 Pseudomonas in shrimp intestines
Pseudomonas spp. also appeared to be in very low abundance in the intestinal tract of white shrimp reared in commercial ponds (Table 5 and Figure 6).
Genus Pseudomonas were identified from the GI tracts of white shrimps reared in commercial shrimp ponds
| Consensus lineage | P1 | P2 | P3 | OTU ID |
|---|---|---|---|---|
| Pseudomonas geniculata | — | 5 | — | OTU_623 |
| Pseudomonas sp. | 4 | 2 | — | OTU_395 |
P1, P2, and P3 are shrimp pond 1, shrimp pond 2, and shrimp pond 3. OTU is operational taxonomic unit. “–” means not detected.
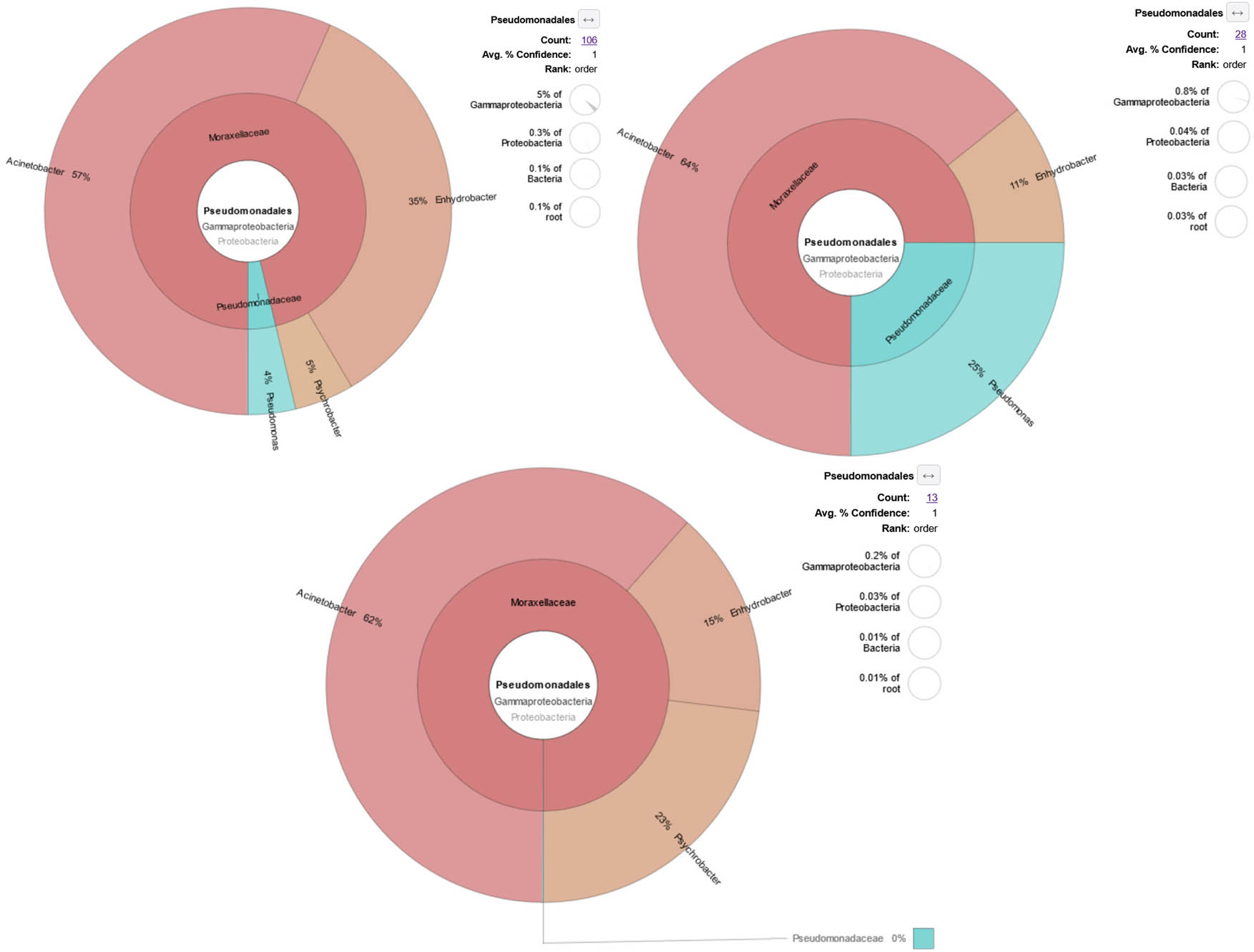
KRONA visually displays the analysis result of taxonomic annotation. Circles from inside to outside stand for different taxonomic ranks, and the area of the sector means the respective proportion of different OTU annotation results. The image depicts the number and proportion of Pseudomonas in the GI tract of white shrimps. Each figure represents sampling locations (P1 is pond 1, P2 is pond 2, and P3 is pond 3). Each figure consists of 10 pooled shrimp intestines.
In pond 1, a total of 106 sequences or 0.1% of the total identified bacterial sequences were assigned into Ordo Psedomonadales, of which only 4 sequences (4% of Pseudomonadales) were identified as Pseduomonas sp. While in pond 2, 28 sequences or 0.03% of total bacterial sequences were assigned to Ordo Pseudomonadales. Seven sequences (25% of Pseudomonadales) belonged to genus Pseudomonas, 5 sequences of P. geniculata, and 2 sequences of Pseudomonas sp. Furthermore, 13 sequences were assigned to Ordo Psedomonadales but none belonged to Pseudomonas spp. in pond 3.
4 Discussion
The application of probiotics has been considered the most eco-friendly method to boost aquaculture production through several mechanisms including maintaining water quality, improving growth rate, and enhancing disease resistance [1]. However, positive results of probiotic applications are mostly based on in vitro studies or small-scale in vivo trials in which all environmental conditions are easily managed and controlled. Meanwhile, the application of probiotics on large scales such as on commercial shrimp farms is still less investigated. Thus, questions such as does introduced probiotics could cope or compete with native bacteria and contribute to the culture of organisms in commercial farms remained to be answered. The present study traced and identified four commercial probiotic species (L. plantarum, L. fermentum, B. subtilis, and P. putida) which were applied in three commercial shrimp ponds. Lactobacillus and Bacillus are among the most frequently used microorganisms as probiotics in both terrestrial and aquatic cultured species [8]. Bacillus-based probiotics generally improved specific growth rate and feed conversion ratio through several mechanisms such as digestive enzyme secretion and production of many supplemental nutrients such as biotin, vitamin B12, fatty acids, essential amino acids, and other necessary growth factors [3]. Meanwhile, Lactobacillus-based probiotics have been reported to produce antimicrobial compounds to suppress bacterial pathogens [4].
The result of this study indicated that none of the four commercial probiotics was able to be detected in the shrimp ponds nor the intestinal tracts of white shrimps sampled on DOC 47. Each shrimp pond appeared to develop specific microbial communities in both rearing water and the shrimp intestines. Ponds 1 and 2, for instance, had 12 Lactobacillus species and the most dominant species was L. aviarius, but pond 3 had only two species of Lactobacillus and was dominated by L. salivarius. Similarly, the genus Bacillus which developed in rearing water was different from commercial bacillus. In addition, bacillus was only detected in pond 1, and none was detected in ponds 2 and 3. While three Pseudomonas were detected in pond 3 (P. psychrotolerans, P. azotoformans, and Pseudomonas sp.), only one species in pond 2 (P. azotoformans) and none were detected in pond 1. A similar result was reported by Huerta-Rábago et al. [9], where three commercial probiotics (Bacillus sp., Lactobacillus sp., and Saccharomyces sp.) introduced into white shrimp ponds at nursery stages could not be detected on DOC 7, 21, and 42. These results may suggest that the introduced probiotics were unable to cope with their new environments and failed to proliferate and grow in the target sites (the intestinal tracts of white shrimps or rearing water). There were several possibilities as to why the commercial bacteria were unable to survive based on previous studies. First, the probiotic species were isolated from significantly different environmental conditions and therefore had difficulty in adapting to the environmental condition in the shrimp ponds or intestines of shrimps. A large loss of viability has been frequently attributed to the high acid and bile salt concentrations in the stomach and intestines [15]. Conditions of rearing water that are different from conditions in culture including dissolved oxygen, pH, salinity, temperature, and nutrient sources will affect the growth rate of probiotic bacteria and total cell yields [1]. Another possibility is that native bacteria out-compete the introduced probiotics for the same organic substrate such as carbon [1]. This result might explain the inconsistent results concerning the efficacy of probiotic treatments on the survival and growth performance of white shrimps [8].
Since the introduced probiotics were not viable in the target sites, a question to be answered is “are these commercial probiotics able to contribute to the aquaculture species? According to Chauhan and Singh [16], probiotic viability is a very important factor in aquaculture species and serves as one of the prerequisites in screening probiotics for aquaculture. Less viable probiotics may not contribute well because the commercial probiotics are not viable in the target sites; thus, they may not contribute to shrimp farms. This might be the reason why studies reported that the probiotic application does not have a significant effect on the production yields. A study by Huerta-Rábago et al. [9] reported that the addition of commercial probiotics did not affect the dominant bacteria in both phyla and genus levels in rearing ponds. Similarly, a study by Arias-Moscoso et al. [17] also reported that the addition of commercial organic and ammonia-oxidizing bacteria does not have any significant effect on water quality or waste degradation in shrimp farms cultured with biofloc technology. All these facts suggest that methods and strategies in applying probiotics in aquaculture species should still be carefully restudied in order to increase their efficacy.
Other authors explained that probiotics may modify the balance of microbial communities in the target sites [18,19]. A study by Torpee et al. [20], for instance, reported that the introduction of probiotics suppresses opportunistic and/or pathogenic bacteria in the intestinal tract of white shrimp. Similarly, Vargas-Albores et al. [21] documented that probiotic strains keep the microbial balance of beneficial bacteria by suppressing the growth of vibrio. Then, what is the effect of probiotics in the present study on the microbial composition in general? The results of the present study showed that probiotic supplementation appeared not to change the structure of microbial compositions in the GITs of shrimps, indicated by no significant different in the top threbacterial phyla in both probiotic-treatment and the controls, which were Proteobacteria, Bacteroidetes and Planctomycetes. At the genus level, Rhodopirellula, Ketogulonicigenium, Ruegeria, and Sulfurimonas were the most dominant phyla regardless of probiotic treatment. The bacterial diversity (phyla and genera) in probiotic treatments was also very similar to the control. microbial compositions in the rearing water of the shrimp ponds largely varied in both rearing water and the intestine of white shrimps even though all sampling ponds had similar treatments. The large variation in the microbial composition of water ponds as well as intestinal tracts of white shrimps has been previously reported in many studies [12,22]. The present study indicated that the application of probiotics in commercial outdoor farms where environmental conditions are difficult to control seems not effective yet. This hypothesis has been supported by many studies which reported no probiotic effect of the productions [9,17]. Thus, more studies and investigations are still required to apply probiotics on commercial outdoor farms.
Acknowledging these issues, the probiotic application in commercial outdoor shrimp farms should be evaluated. More studies are still required in order to develop more effective strategies, especially in the commercial outdoor system. Applying probiotics directly as practiced in the present study should be avoided. Some factors such as time and frequency of administration, probiotic species, administration (encapsulation) method [23], and the supplementation of prebiotics to support the nutrient requirements for probiotic species should be considered. In terms of introducing time, probiotics may exert a better effect when introduced during early life [24]. Previous studies also explained stable gut microflora in the early life stages of white shrimp have not yet been established therefore a perfect time to introduce, stir and manipulate its microbial species. In addition, shrimp at larval and early post-larval stages have less developed immune systems which may exclude the introduced probiotics species [25]. Furthermore, probiotic species may also determine the viability of probiotics in outdoor commercial farms. Some studies revealed that several commercial probiotics were isolated from terrestrial which have very different environmental conditions such as salinity, nutrient availability, pH, or dissolved oxygen. These differences made such probiotic species difficult to adapt, grow, and proliferate in aquatic environments. Thus, it is highly recommended to isolate and develop native/indigenous probiotic strains from surrounding environments. The native/indigenous probiotics may more easily adapt and contribute to aquaculture production.
The concept of indigenous probiotics has been documented to be more effective in enhancing aquaculture productions and viability is the keyword behind the success. In addition, based on the present study results, Lactobacillus appeared to be good candidates in general both in rearing water and intestinal tracts since its availability seems better than both Bacillus and Pseudomonas. Our observation that probiotic treatment is less effective in earthen containers could be related to the difficulty of exerting control over variables (probiotic access, temperature, dose, farm hygiene, etc.). Lactobacillus is also a member of lactic acid bacteria whose members have generally regarded as safe status for probiotics [26]. The other approach is synbiotic, which is the application of probiotics and prebiotics at the same time. Prebiotics is a nutrient which is required by probiotics to grow and proliferate in target sites [27]. Few studies have recently reported the application of synbiotics in white shrimps [28]. These approaches should be investigated more to increase the effectiveness and efficacy of probiotics in commercial outdoor farms of white shrimps.
5 Conclusion
Four commercial probiotic species applied in the commercial grow-out shrimp ponds could not be detected from the rearing water or intestinal tracts of the white shrimps. These facts might answer why commercial ponds applying probiotics had high yield variations. The characteristics of probiotic species and environmental conditions in commercial outdoor farms may explain these results. Thus, more studies on selecting proper probiotic strains having good tolerance in a wide range of environmental conditions or strategies on how probiotics are applied in commercial outdoor farms should be done in future in order to increase the probiotic efficacy in white shrimp production.
Acknowledgments
The authors thank colleges at the Department of Aquaculture, Faculty of Fisheries and Marine Universitas Airlangga for all support and technical advice during the experiment.
-
Funding information: This study was also financially supported by The Ministry of Education, Culture, Science and Technology, the Republic of Indonesia through Fundamental Research with Grant no. 672/UN3/2022.
-
Author contributions: M.A1. – Conceptualization, methodology, software, validation, data collection, data analysis, writing original draft, funding acquisition, and submission; Y.P. – Methodology, investigation, data collection, data analysis, draft writing, and editing; M.L. – Data collection, writing original draft, review, editing, and approved the final manuscript; Y.C. – Data collection, draft writing, editing, data analysis, review, and editing; O.A.O. – Data collection, sequencing, data analysis, draft writing, editing, review, and approved the final manuscript; M.A2. – Experimental design, data collection, sequencing, data analysis, review, editing, and approved the final manuscript; A.P.W.N. – Conceptualization, data collection, data analysis, review, and approved the final manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Hlordzi V, Kuebutornye FK, Afriyie G, Abarike ED, Lu Y, Chi S, et al. The use of Bacillus species in maintenance of water quality in aquaculture: A review. Aquac Rep. 2020;18:100503.10.1016/j.aqrep.2020.100503Search in Google Scholar
[2] Pepi M, Focardi S. Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the mediterranean area. Int J Env Res Public Health. 2021;18(11):5723.10.3390/ijerph18115723Search in Google Scholar PubMed PubMed Central
[3] Amin M. Marine protease-producing bacterium and its potential use as an abalone probiont. Aquac Rep. 2018;12:30–5.10.1016/j.aqrep.2018.09.004Search in Google Scholar
[4] Amin M, Adams MB, Burke CM, Bolch CJ. Isolation and screening of lactic acid bacteria associated with the gastrointestinal tracts of abalone at various life stages for probiotic candidates. Aquac Rep. 2020;17:100378.10.1016/j.aqrep.2020.100378Search in Google Scholar
[5] Elsabagh M, Mohamed R, Moustafa EM, Hamza A, Farrag F, Decamp O, et al. Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquacult Nutr. 2018;24(6):1613–22.10.1111/anu.12797Search in Google Scholar
[6] Amin M, Agustono A, Prayugo P, Ali M, Hum NNMF. Comparison of total nutrient recovery in aquaponics and conventional aquaculture systems. Open Agric. 2021;6(1):682–8.10.1515/opag-2021-0032Search in Google Scholar
[7] Amin M, Agustono A, Ali M, Prayugo P, Hum NNMF. Apparent nutrient utilization and metabolic growth rate of Nile tilapia, Oreochromis niloticus, cultured in recirculating aquaculture and biofloc systems. Open Agric. 2022;7(1):445–54.10.1515/opag-2022-0109Search in Google Scholar
[8] Toledo A, Frizzo L, Signorini M, Bossier P, Arenal A. Impact of probiotics on growth performance and shrimp survival: A meta-analysis. Aquac. 2019;500:196–205.10.1016/j.aquaculture.2018.10.018Search in Google Scholar
[9] Huerta-Rábago JA, Martínez-Porchas M, Miranda-Baeza A, Nieves-Soto M, Rivas-Vega ME, Martínez-Córdova LR. Addition of commercial probiotic in a biofloc shrimp farm of Litopenaeus vannamei during the nursery phase: effect on bacterial diversity using massive sequencing 16S rRNA. Aquac. 2019;502:391–9.10.1016/j.aquaculture.2018.12.055Search in Google Scholar
[10] Yan M, Zhang X, Hu L, Huang X, Zhou Q, Zeng G, et al. Bacterial community dynamics during nursery rearing of pacific white shrimp (Litopenaeus vannamei) revealed via high-throughput sequencing. Indian J Microbiol. 2020;60(2):214–21.10.1007/s12088-019-00853-7Search in Google Scholar PubMed PubMed Central
[11] Gomes GB, Hutson KS, Domingos JA, Chung C, Hayward S, Miller TL, et al. Use of environmental DNA (eDNA) and water quality data to predict protozoan parasites outbreaks in fish farms. Aquac. 2017;479:467–73.10.1016/j.aquaculture.2017.06.021Search in Google Scholar
[12] Amin M, Kumala RRC, Mukti AT, Lamid M, Nindarwi DD. Metagenomic profiles of core and signature bacteria in the guts of white shrimp, Litopenaeus vannamei, with different growth rates. Aquac. 2022;550:737849.10.1016/j.aquaculture.2021.737849Search in Google Scholar
[13] Turner CR, Uy KL, Everhart RC. Fish environmental DNA is more concentrated in aquatic sediments than surface water. Biol Conserv. 2015;183:93–102.10.1016/j.biocon.2014.11.017Search in Google Scholar
[14] Gao S, Pan L, Huang F, Song M, Tian C, Zhang M. Metagenomic insights into the structure and function of intestinal microbiota of the farmed Pacific white shrimp (Litopenaeus vannamei). Aquac. 2019;499:109–18.10.1016/j.aquaculture.2018.09.026Search in Google Scholar
[15] Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J Control Rel. 2012;162(1):56–67.10.1016/j.jconrel.2012.06.003Search in Google Scholar PubMed
[16] Chauhan A, Singh R. Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis. 2019;77(2):99–113.10.1007/s13199-018-0580-1Search in Google Scholar
[17] Arias-Moscoso JL, Espinoza-Barrón LG, Miranda-Baeza A, Rivas-Vega ME, Nieves-Soto M. Effect of commercial probiotics addition in a biofloc shrimp farm during the nursery phase in zero water exchange. Aquac Rep. 2018;11:47–52.10.1016/j.aqrep.2018.06.001Search in Google Scholar
[18] Swain S, Hauzoukim SKG, Das SK, Roy A. Application of probiotics in aquaculture. J Pharm Innov. 2021;10(7):146–9.Search in Google Scholar
[19] Butt UD, Lin N, Akhter N, Siddiqui T, Li S, Wu B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021;114:263–81.10.1016/j.fsi.2021.05.003Search in Google Scholar PubMed
[20] Torpee S, Kantachote D, Rattanachuay P, Chiayvareesajja S, Tantirungkij M. Dietary supplementation with probiotic Rhodobacter sphaeroides SS15 extract to control acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus in cultivated white shrimp. J Invertebr Pathol. 2021;186:107585.10.1016/j.jip.2021.107585Search in Google Scholar PubMed
[21] Vargas-Albores F, Martínez-Córdova LR, Hernández-Mendoza A, Cicala F, Lago-Lestón A, Martínez-Porchas M. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquac. 2021;544:737050.10.1016/j.aquaculture.2021.737050Search in Google Scholar
[22] Zhou L, Qu Y, Qin JG, Chen L, Han F, Li E. Deep insight into bacterial community characterization and relationship in the pond water, sediment and the gut of shrimp (Penaeus japonicus). Aquac. 2021;539:736658.10.1016/j.aquaculture.2021.736658Search in Google Scholar
[23] Pato U, Ayu DF, Riftyan E, Restuhadi F, Pawenang WT, Firdaus R, et al. Cellulose Microfiber Encapsulated Probiotic: Viability, Acid and Bile Tolerance during Storage at Different Temperature. Emerg sci J. 2022;6(1):106–17.10.28991/ESJ-2022-06-01-08Search in Google Scholar
[24] Wang R, Guo Z, Tang Y, Kuang J, Duan Y, Lin H, et al. Effects on development and microbial community of shrimp Litopenaeus vannamei larvae with probiotics treatment. AMB Express. 2020;10(1):1–14.10.1186/s13568-020-01041-3Search in Google Scholar PubMed PubMed Central
[25] Kulkarni A, Krishnan S, Anand D, Kokkattunivarthil Uthaman S, Otta SK, Karunasagar I, et al. Immune responses and immunoprotection in crustaceans with special reference to shrimp. Rev Aquac. 2021;13(1):431–59.10.1111/raq.12482Search in Google Scholar
[26] García-Cano I, Rocha-Mendoza D, Kosmerl E, Zhang L, Jiménez-Flores R. Technically relevant enzymes and proteins produced by LAB suitable for industrial and biological activity. Appl Microbiol Biotechnol. 2020;104(4):1401–22.10.1007/s00253-019-10322-2Search in Google Scholar PubMed
[27] Wee W, Hamid NKA, Mat K, Khalif RIAR, Rusli ND, Rahman MM, et al. The effects of mixed prebiotics in aquaculture: A review. Aquac Fish. 2022, in press.10.1016/j.aaf.2022.02.005Search in Google Scholar
[28] Zhou L, Li H, Qin JG, Wang X, Chen L, Xu C, et al. Dietary prebiotic inulin benefits on growth performance, antioxidant capacity, immune response and intestinal microbiota in Pacific white shrimp (Litopenaeus vannamei) at low salinity. Aquaculture. 2020;518:734847.10.1016/j.aquaculture.2019.734847Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer
Articles in the same Issue
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer

