Abstract
The performance of polymer gel to plug a hydraulic fracture is greatly affected by its distribution patterns and gelling effect. In this study, the migration of a gel plugging agent in a fracture and its plugging after gelling were investigated by physical simulation experiments. In addition, the distribution patterns of the gel plugging agent and its plugging mechanism after gelling were investigated in detail. The results of this study revealed that the migration flowing behavior of the gel solution in a fracture can be divided into three streams: fracture flow, leak off flow, and matrix flow. Such behavior distributed the gel in three different patterns after gelling: gel clusters in the fracture, gel layer on the fracture surface, and dispersed gel lumps in the matrix pores-throats. Because of the leak off flow and the difference in components, the gel solution has apparent disproportional leak off–diffusion of components during its migration in a fracture, with less polymer molecules and loss of more cross-linking agent ions. The leak off of the cross-linking agent significantly deteriorates the gelling strength of the polymer gel, affecting its performance to plug a hydraulic fracture. The results also show that when the normalized concentration of the cross-linking agent ions in a fracture is less than 0.6, gel fails to plug the fracture effectively after gelling. When gelling was carried out by in situ cross-linking, polymer gel provided more satisfactory plugging performance than the gelling via ground pre-cross-linking.
1 Introduction
In recent years, the water shutoff methods of loose sandstone reservoirs using polymer gel as a most common plugging agent are applied to the chemical water shutoff of hydraulic fractures in low-permeability reservoirs (1). In the process of chemical water shutoff of a hydraulic fracture, the success of the water shutoff treatment is closely related to the gelling effect of the plugging agent in the fracture and is affected by its distribution patterns and degree of component leak off (2). Mazen et al. discussed the application of polymer microgels in conformance control (3). Ghodsieh et al. studied three sulfonated polyacrylamides and one hydrolyzed polyacrylamide and optimized several conditions including concentration for water shutoff (4). In hydraulic fracture water shutoff, the change in the concentration can be controlled by other conditions, such as filtration degree and filtration mode. During the gel treatment for fracture, the reduction in the gelant viscosity reflects the loss of the polymer and can greatly affect the gel formation. Therefore, keeping the gelant viscosity stationary during the gelant injection is really important. The common method for maintaining the viscosity of the gel is to improve the polymer concentration or inject the low-concentration polymer preflush. However, the increasing viscosity of the gelant in fracture still remains unclear (5). Unlike polymer molecules with high molecular weight and long molecular chain, the molecular weight of chromium ions is very small, and they can be lost easily during the gelant propagation and the shut-in period in fracture. Moreover, the presence of formation brine decreases the chromium concentration, resulting in extended gelation time, reduced gel strength, and finally making the gel treatment for fracture ineffective (6,7).
The distribution patterns of the plugging agent filled in a hydraulic fracture have not been investigated so far; however, a few studies reported the effect of plugging agent component leak off in a hydraulic fracture on the gelling effect and fracture plugging performance. Seright et al. (8) and Sydansk et al. (9) believed that the loss of a small amount of water from the plugging agent in a fracture would lead to dehydration, thus adversely affecting its gelling effect; in the case of high leak off degree, however, the concentration of plugging agent components would increase, indicating that the gelling strength of a plugging agent could be improved to plug the fracture. For conformance control, Ali et al. designed and optimized the flowing gel injection with both fracture and sandpack models. They considered the gel rheology, adsorption, swelling ratio, resistance factor, and residual resistance factor in their models (10). Junjian et al. studied the filtrate behavior and water shutoff performance of a certain gel in fractured media (11). Ganguly et al. reported that the gel plugging agent could cement the fracture surface during the gelling of the plugging agent, thus enhancing its fracture plugging performance (12). So far, the effect of the leak off degree of the plugging agent components on its gelling effect and plugging performance has not been characterized quantitatively. In this study, physical simulation experiments were carried out on the migration of the gel plugging agent in a fracture and its plugging after gelling, and the distribution patterns of the gel plugging agent and its plugging mechanism after gelling were investigated.
2 Materials and methods
2.1 Materials
The polymer used in the experiments is partially hydrolyzed polyacrylamide (HPAM) with a relative molecular weight of 12 million and a hydrolysis degree of 25%. The cross-linking agent used in the experiments is self-synthesized organic chromium cross-linking agent with a Cr3+ effective concentration of 5,500 mg/L. The polymer gel plugging agent system used in the experiments basically comprises 3,000 mg/L HPAM and 357.5 mg/L Cr3+. The water used in the experiments is simulated formation water prepared indoors with a total salinity of 12,000 mg/L.
The cross-linking agent used in the experiments is self-synthesized chromium(iii) acetate with a Cr3+ effective concentration of 5,500 mg/L, and its synthesis procedure is shown as follows: (1) add acetic acid (CH3COOH) into stirred deionized water, and the mass ratio of acid and water is 2:7; (2) add potassium dichromate (K2Cr2O7) into the above stirred solution, and the mass ratio of potassium dichromate and acetic acid is 1:2; (3) add sodium thiosulfate (Na2S2O3) into the above stirred solution, and the mass ratio of sodium thiosulfate and potassium dichromate is 4:1; (4) stir the solution continuously at 60°C for 4 h. Then the organic cross-linker chromium(iii) acetate is achieved. The structure of chromium(iii) acetate is shown in Scheme 1.
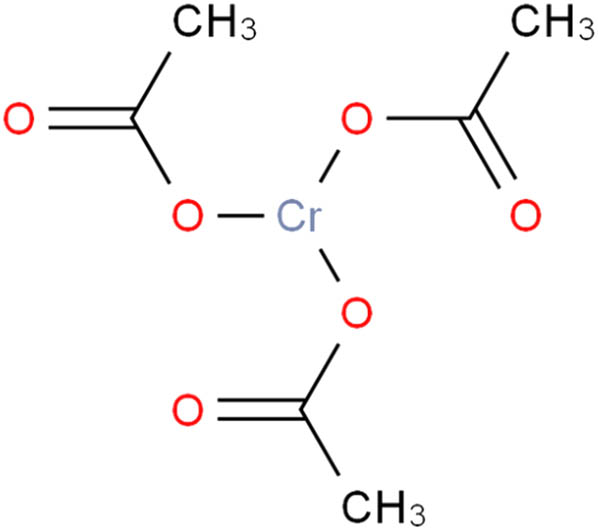
Structure of chromium(iii) acetate.
Two types of core were applied in the experiments: fractured cubical core and fractured cylindrical core. The former is 40 cm in length, 4.5 cm in width, and 4.5 cm in height, and the latter is 2.54 cm in diameter and 10 cm in length. Each core is provided with three sampling points evenly along its fracture and matrix. Fractures in the two types of cores are all uniform through fractures placed with proppants. The fracture widths range from 1 and 2 mm to 5 mm, and the matrix permeabilities range from 5 to 50 mD.
2.2 Methods
2.2.1 Determination method of Cr3+ concentration in gel
After the pretreatment of the gel plugging agent, the Cr3+ concentration in the gel was determined by spectrophotometry following the literature method (13).
2.2.2 Determination method of the gel component leak off in fracture
Figure 1 shows the numbers of sampling ports along the fracture and matrix of a fractured cubical core. Before the start of displacement, ports 1, 2, and 3 along the fracture were closed, and the back pressure at the fracture outlet was set. The polymer gel solution was injected into the fractured core at a constant rate of 0.5 mL/min, and then produced fluid was collected from the ports 4, 5, and 6 along the matrix. The viscosity of the produced fluid was determined, and the leak off concentration of the polymer was estimated. The Cr3+ concentration in the produced fluid was determined and normalized. CN is the ratio of the Cr3+ concentration in the produced fluid to that in the original solution. The leak off–diffusion degree of Cr3+ from the fracture into the matrix was estimated. The Cr3+ concentration in the produced fluid at the fracture outlet (End 4) was determined and normalized. The loss of Cr3+ in the fracture was estimated. The disproportional leak off degrees of the gel components (polymer molecules and cross-linking agent ions) were analyzed.
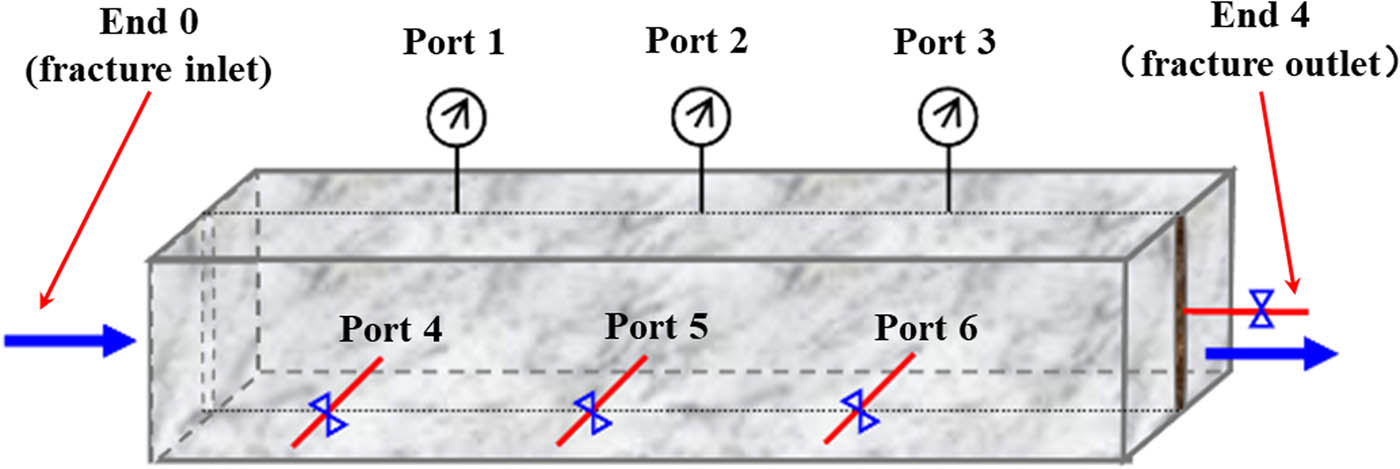
Numbers of sampling ports along the fracture and matrix of cubical core.
2.2.3 Evaluation indicators of the plugging performance of gel plugging agent in fracture
Based on the loss of the cross-linking agent (organic chromium) and its effect on fracture plugging, the following evaluation indicators for chromium ion loss and fracture plugging performance of polymer gel were established:
2.2.3.1 Evaluation indicators for the loss of Cr3+
The normalized Cr3+ concentration (CN) in the fluid sample displaced during injection was obtained by determining the Cr3+ concentration in the fluid sample collected from the fracture and matrix, and dividing that value by the Cr3+ concentration (357.5 mg/L) injected into the solution. The evaluation indicators for the loss of Cr3+ are listed in Table 1.
Evaluation indicators for the loss of Cr3+ in polymer gel
| Loss of Cr3+, CN | CN > 1 | CN = 1 | 0.9 ≤ CN < 1 | 0.7 ≤ CN < 0.9 | 0.5 ≤ CN < 0.7 | CN < 0.5 |
|---|---|---|---|---|---|---|
| Evaluation indicators | Negative loss | Zero loss | Low loss | Moderate loss | High loss | Very high loss |
2.2.3.2 Evaluation indicators for the fracture plugging performance of polymer gel
dP5 is defined as the ratio of the reverse water flooding differential pressure at different CN values (leak off degree) after gelling to the reverse water flooding differential pressure at CN = 1 (zero loss of Cr3+), i.e., dP5 is the normalized reverse water flooding pressure gradient of the polymer gel for fracture plugging when the loss of Cr3+ occurs, and this can be used to evaluate the effect of Cr3+ loss degree on the fracture plugging performance. The evaluation indicators are listed in Table 2.
Evaluation indicators for the fracture plugging performance of polymer gel
| Plugging performance, dP5 | dP5 > 1 | dP5 = 1 | 0.9 ≤ dP5 < 1 | 0.7 ≤ dP5 < 0.9 | 0.5 ≤ dP5 < 0.7 | dP5 < 0.5 |
|---|---|---|---|---|---|---|
| Evaluation indicators | Positive effect | No effect | Weak effect | Moderate effect | Strong effect | Very strong effect |
2.2.4 Microscopic visualization method of gel leak off depth
The polymer gel solution was dyed with methylene blue at a concentration of 5 mg/L. The polymer gel solution was injected into a fractured cylindrical core at a constant rate of 0.2 mL/min. The back pressure at the fracture outlet and the injection volume were experimentally determined. After completing solution injection, the core was taken out and cut open circumferentially with the circular section perpendicular to the fracture direction. The conditions of the fracture surface and surrounding matrix dyed by methylene blue as well as the distribution of the gel solution were observed using a handheld microscope (Anyty), and the microscopic images (with the magnification factors of 75, 150, and 500) were obtained. The leak off depth, defined as the depth of the matrix dyed with methylene blue, of the gel solution from the fracture into the matrix was measured by the Anyty processing software in the range 0–12 mm, with a measuring accuracy of 10 µm.
3 Migration and distribution patterns of polymer gel in fracture
After being injected into a fracture, gel solution first migrates along the fracture because of the big difference in the flow resistance between the fracture and matrix. However, during actual injection, a pressure difference between the fracture and matrix is created. Because of the concentration difference in the components, the solution in the fracture will also tend to migrate into matrix, and this phenomenon is known as the leak off behavior of gel solution. Therefore, the migration behavior of the gel solution in a fracture can be divided into three streams (as shown in Figure 2): (1) the flow of gel solution in the fracture, namely the fracture flow, which is generally the main stream; (2) the leak off flow from the fracture into the matrix under the pressure and concentration difference, which is the main stream where the leak off of plugging agent components occurs; (3) the flow of the lost components in the matrix parallel to the fracture flow, namely the matrix flow, which is generally a weak stream but more obvious in the presence of bypass flow of the gel solution or formation water.
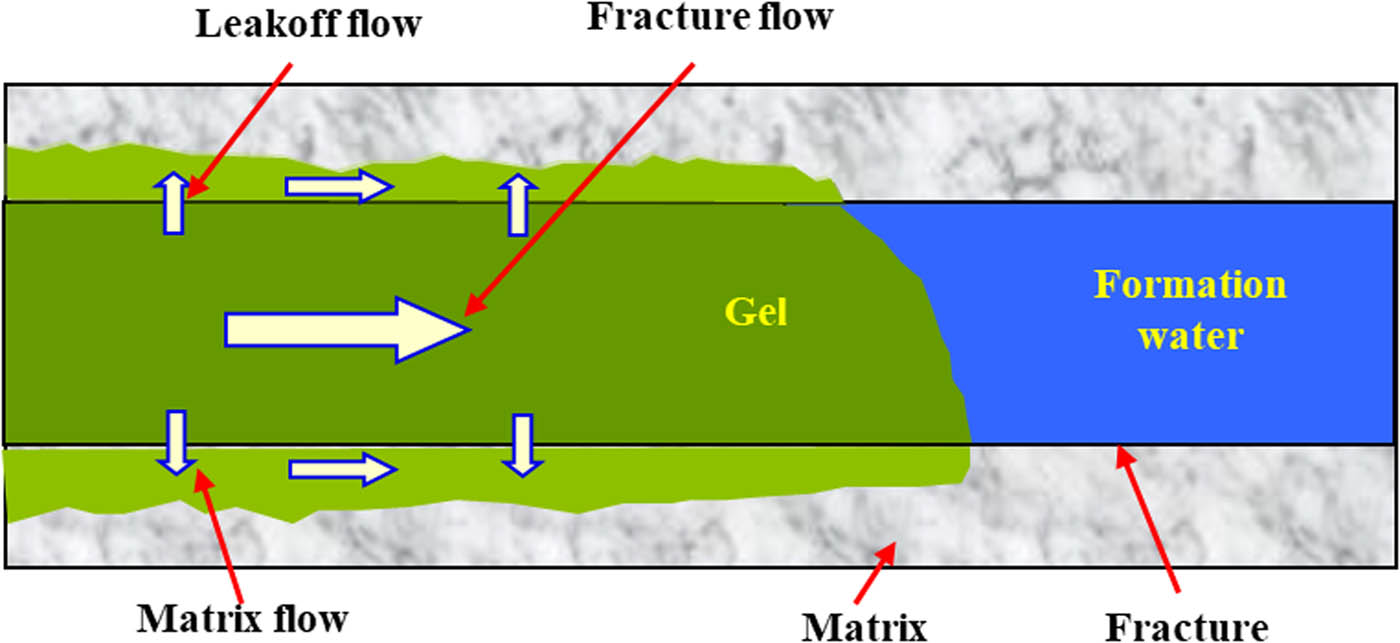
Migration flow of gel solution in the fracture and matrix.
The different migration behaviors of the plugging agent significantly affect the distribution patterns of the gel solution in a fracture and the surrounding matrix. Three main distribution patterns of the plugging agent after gelling were observed, corresponding to the three streams mentioned above, as shown in Figures 3 and 4.
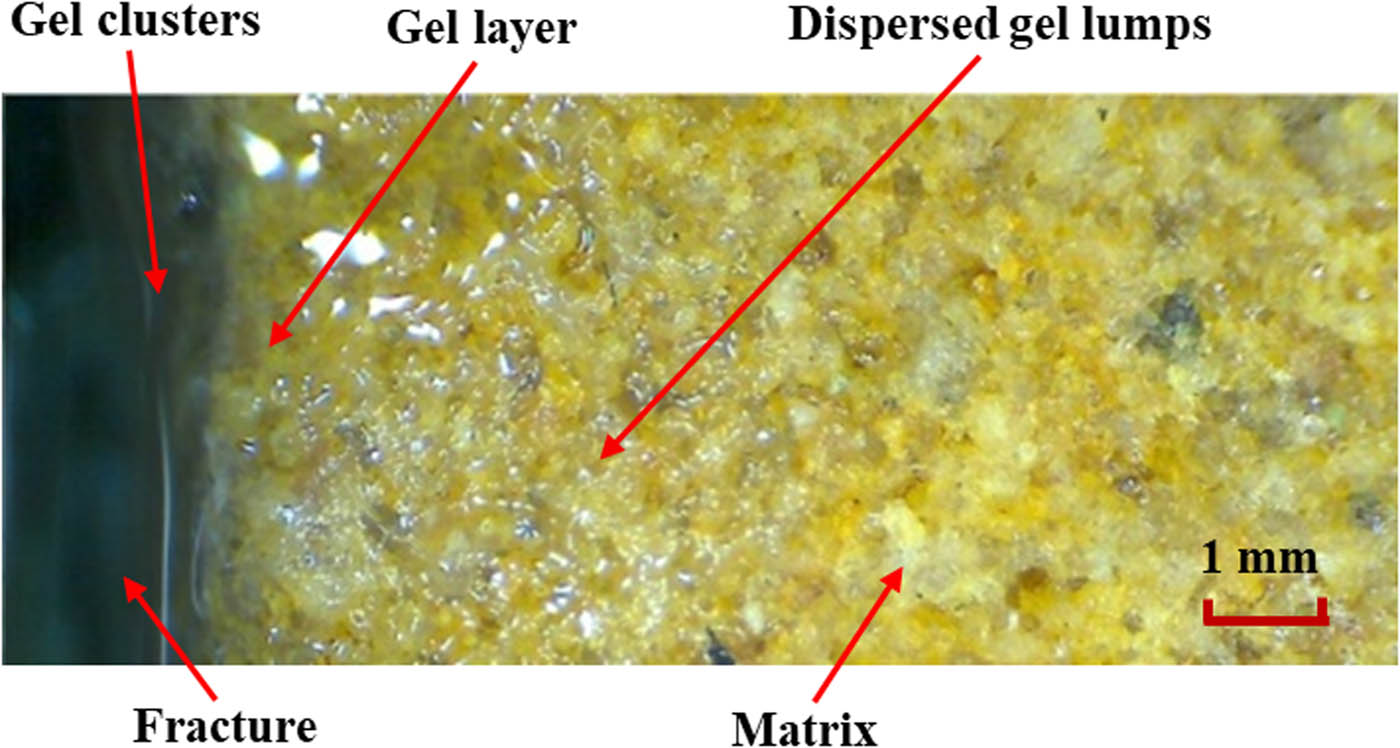
Overall distribution patterns of polymer gel in the fracture and matrix after gelling.
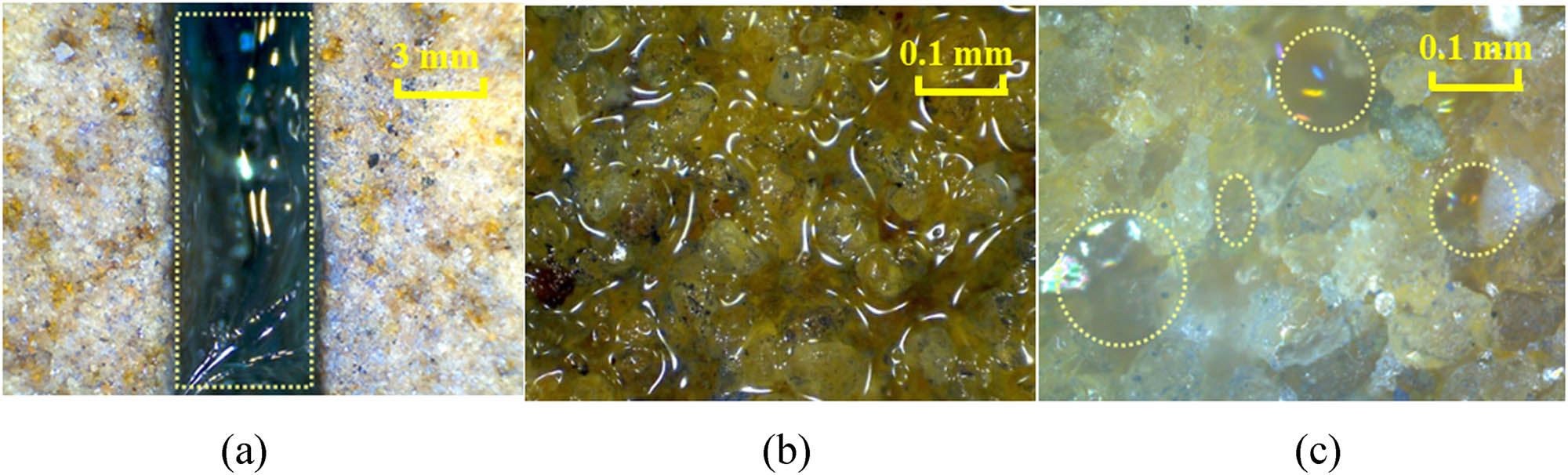
Occurrence states of polymer gel in the fracture and matrix after gelling. (a) Gel clusters, (b) gel layer, (c) dispersed lumps.
After the gelling of the gel solution, the fracture flow resides in the fracture and forms gel clusters, and is the main contribution of gel for fracture plugging. Therefore, the gelling effect of gel clusters directly affects the performance of the gel plugging agent. The gel layer transforms because of the polymer adsorption and aggregation layer built on the fracture surface and surrounding matrix during the leak off of the gel solution. The polymer molecules in the polymer adsorption and aggregation layer are tightly arranged, intertwined and adsorbed on the rock surface. Since some chromium ions might be bound in the leak off layer, partial cross-linking reaction will also occur to form a gel layer, whose main function is to enhance the cementing force between the gel clusters in the fracture and the fracture surface, in order to enhance its ability to reside in the fracture. In addition, the gel layer can also mitigate the bypass flow of injected water and formation water from the matrix into the fracture. The gel dispersoids in the matrix pores-throats are the reaction product of a small amount of polymer molecules and chromium ions migrating into the matrix pores. Because the polymer molecules entering the matrix are mainly concentrated in high-permeability pores, the main function of the dispersed gel colloids in the matrix is to block the high-permeability pore-throats.
4 Gelling and plugging mechanisms of gel plugging agent in fracture
A key factor for the polymer gel to effectively plug a hydraulic fracture is to achieve a high gelling strength in the fracture, while the most critical factor affecting the gelling strength is the concentration of components such as polymer and cross-linking agent (14). According to the literature data, polymer gel solution has disproportional leak off of components in a fracture. Under certain conditions, serious leak off–diffusion of chromium ions into matrix will occur, while polymer molecules will form a polymer leak off layer on the fracture surface and the shallow part of the matrix, as a result of which the component concentration of the polymer gel solution remained in the fracture, especially the change in the concentration of the cross-linking agent, will affect the gelling phenomenon. It is well-known that increasing the concentration of the polymer and the cross-linking agent can enhance the strength of the polymer gel solution after gelling; however, the concentrations of the polymer and cross-linking agent must be maintained within a reasonable range. If the polymer concentration is too high, incomplete gelling will occur, leading to imperfect fracture plugging in the presence of a large number of free polymers concurrently. However, when the cross-linking agent concentration is too high, the resulting gel will undergo dehydration with a large number of worm holes generated inside the gel, and its brittleness will also increase greatly, decreasing the plugging strength (15).
4.1 Gelling mechanism of gel plugging agent at different leak off degrees
Gross leak off of chromium ion concentration usually results in an excessively low concentration of chromium ion cross-linking agent in the gel solution remained in a fracture. Therefore, the reasonable concentration range of chromium ions in a fracture was experimentally investigated. A series of gel solutions with a polymer concentration of 3,000 mg/L were prepared, in which the concentrations of chromium ions gradually increased from 50 to 400 mg/L. After being mixed uniformly, all the solutions were placed in a thermostatic oven at 60°C for the same time for setting after 24 h, and their viscosities respectively were measured. The testing results are shown in Figure 5.
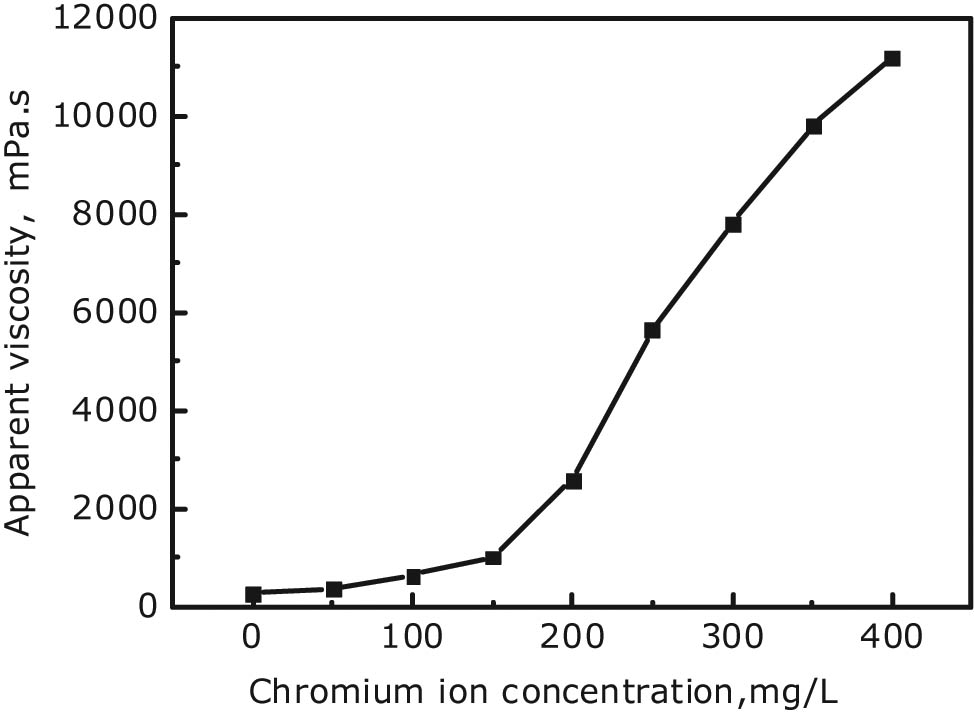
Curve of viscosity vs chromium ion concentration after the gelling of polymer gel.
The experimental results reveal that, when the chromium ion concentration was less than 150 mg/L, the viscosity of the gel after gelling gradually increased with the increase of chromium ion concentration. In general, however, the viscosity was low and increased slowly, mainly due to the incomplete cross-linking reaction between chromium ions and polyacrylamide molecules because of the insufficient concentration of chromium ions. The viscosity of the gel increased rapidly with increasing the chromium ion concentration exceeding 150 mg/L. If the viscosity of 5,000 mPa s is taken as the dividing point between high and low gelling strength, high gelling strength can be guaranteed only when the concentration of chromium ions reaches ≥245 mg/L. Figure 6 shows the physical gel after gelling with a polymer concentration of 3,000 mg/L and a chromium ion concentration of 357.5 mg/L (Figure 6a) and 200 mg/L (Figure 6b, dyed red). As can be seen from the tongues stuck out of the inverted bottles filled with gel, the gelling strength of the gel solution greatly decreases when the concentration of chromium ion is too low. Therefore, the excessive leak off–diffusion of chromium ions in a fracture should be prevented and controlled, to maintain their concentration within a reasonable range for ensuring the gelling effect and plugging strength.
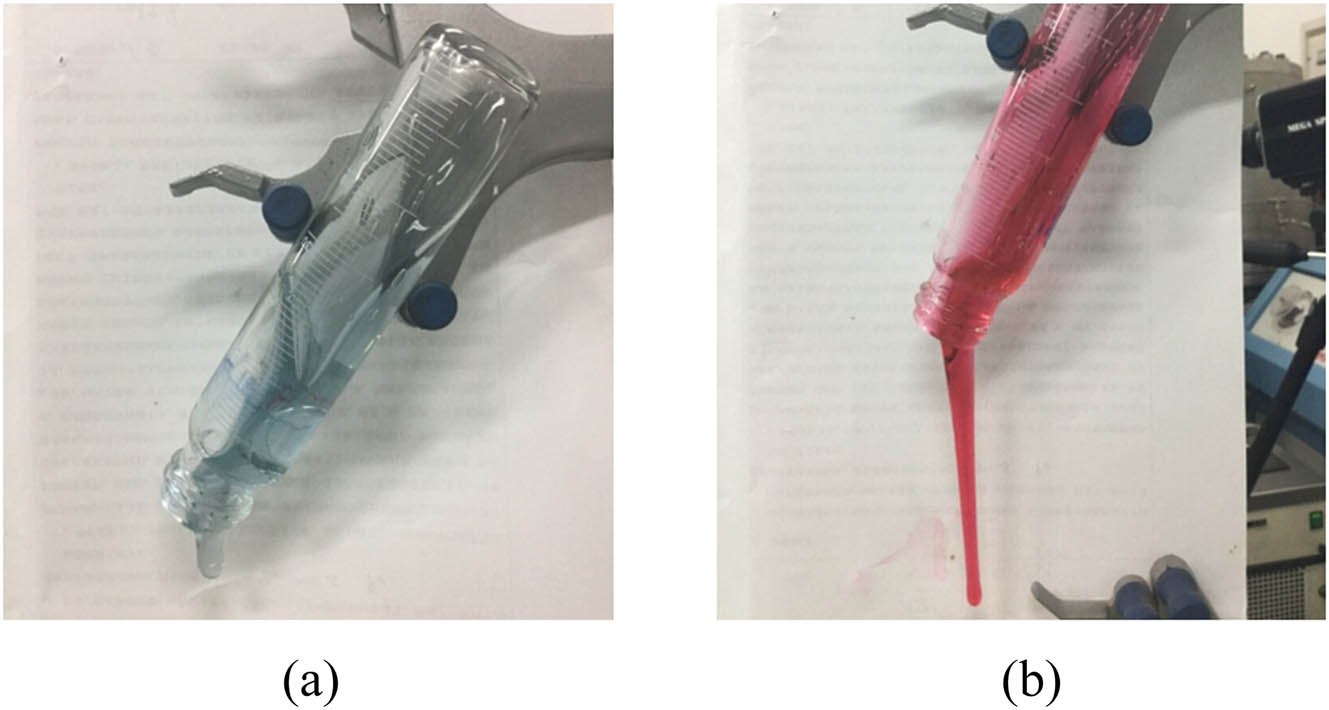
Effect of Cr3+ concentration on polymer gel. (a) Cr3+ concentration: 357.5 mg/L, (b) Cr3+ concentration: 200 mg/L.
In order to compressively study the effect of disproportional leak off of the gel components in a fracture on gelling strength, polymer gel solutions with the same component concentration (3,000 mg/L HPAM + 357.5 mg/L Cr3+) were dyed with methylene blue. Each solution was injected into a fractured cylindrical core with a fracture width of 2 mm and a matrix permeability of 50 mD. In order to have different leak off degrees of the gel solution, the back pressure at each fracture outlet was set at 0, 50, and 200 kPa. After the completion of injection, the injected solution was sealed and allowed to set at 60°C for 24 h for gelling. After gelling, the leak off situation of the gel at each fracture inlet was observed, as shown in Figure 7.
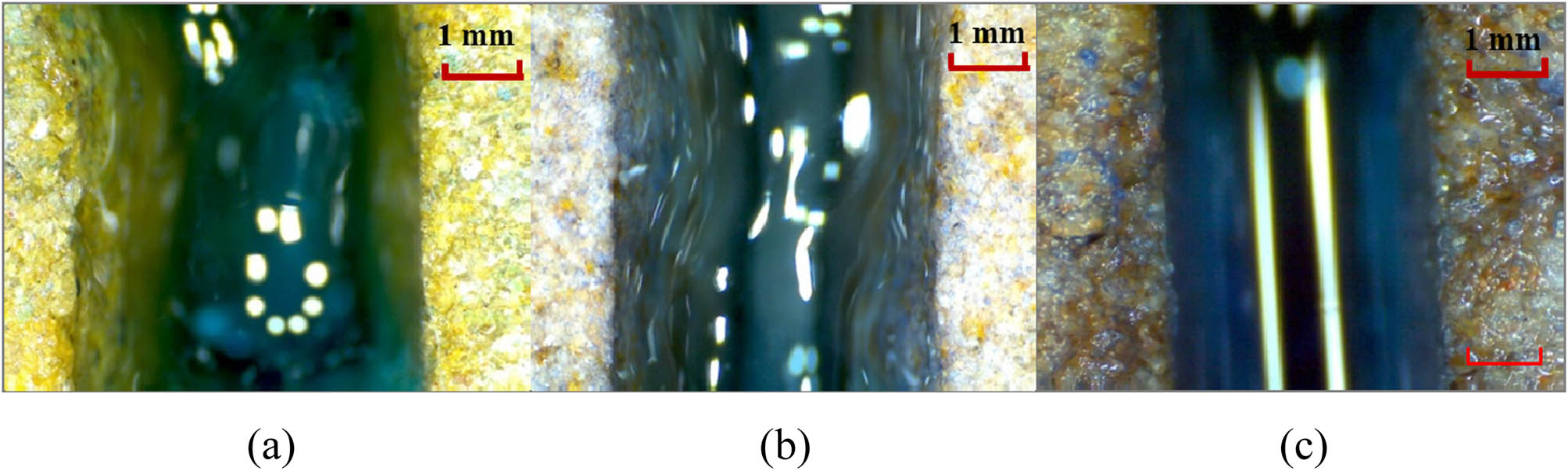
Gelling effect of polymer gel at different Cr3+ leak off degrees. (a) slight leak off, (b) moderate leak off, (c) gross leak off.
When the back pressure at the fracture outlet was 0 kPa, no obvious methylene blue dye was observed on the fracture surface and the surrounding matrix, indicating slight or very weak leak off of the polymer gel solution into the matrix. When the back pressure was 50 kPa, the amount of adsorbed methylene blue dye on the fracture surface was significant, and there was also dye phenomenon in the shallow part of the matrix. At this point, the leak off of gel solution into the matrix was moderate. When the back pressure was 100 kPa, both the fracture surface and the deep part of matrix were dyed by methylene blue. Under this condition, the leak off of gel solution worsened.
The gel gelled at different leak off degrees was removed from the fracture, and its viscosity was measured using a cone-and-plate viscometer. Meanwhile, the chromium ion concentration was measured by the Cr3+ determination method in the gel. The relationship between chromium ion concentration and the gelling strength (gel viscosity) at different leak off degrees was analyzed, and the experimental results are shown in Figure 8.
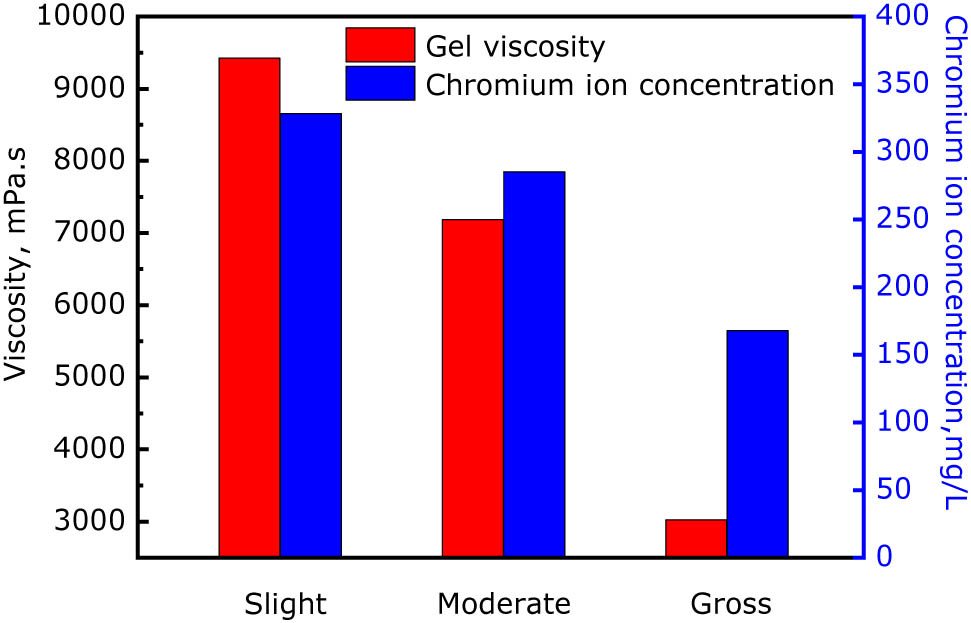
Relationship between gelling strength (gel viscosity) and chromium concentration at different leak off degrees.
As shown Figure 8, in the case of slight gel solution leak off in the fracture, the gel viscosity after gelling reached 9,425 mPa s, and the chromium ion concentration was 328.4 mg/L, i.e., only about 30 mg/L of chromium ions were lost, and had only little impact on the final gelling strength of the gel. In the case of moderate gel solution leak off into the matrix, the chromium ion concentration was only 265.3 mg/L; therefore, the gel viscosity after gelling decreased to 6,186 mPa s. In the case of gross solution gel leak off, the chromium ion concentration was only 167.8 mg/L and the gel viscosity was only 3,025 mPa s. These results also verify the conclusion that excessive leak off–diffusion of chromium ions into the matrix might affect the gelling effect of gel inside the fracture. Therefore, the leak off–diffusion of cross-linking agent ions must be controlled during the injection of the gel solution.
4.2 Plugging mechanism of gel plugging agent at different leak off degrees
The abovementioned analysis indicates that the main component leaked off from the gel solution is cross-linking agent ions, and therefore the leak off amount of the cross-linking agent can be used to represent the leak off degree of gel solution. Fractured cubical cores each with a fracture width of 2 mm and a matrix permeability of 50 mD were selected for the experiments. Polymer gel solutions of different volumes (3,000 mg/L HPAM + 357.5% Cr3+) were injected into the fractured cores at 0.5 mL/min with the back pressure at a fracture outlet of 100 kPa. The produced fluid was collected at the fracture outlet every 10 min, and the chromium ion concentration was tested and normalized. Figure 9 shows the curve of chromium ion concentration versus the volume of the gel solution injected.
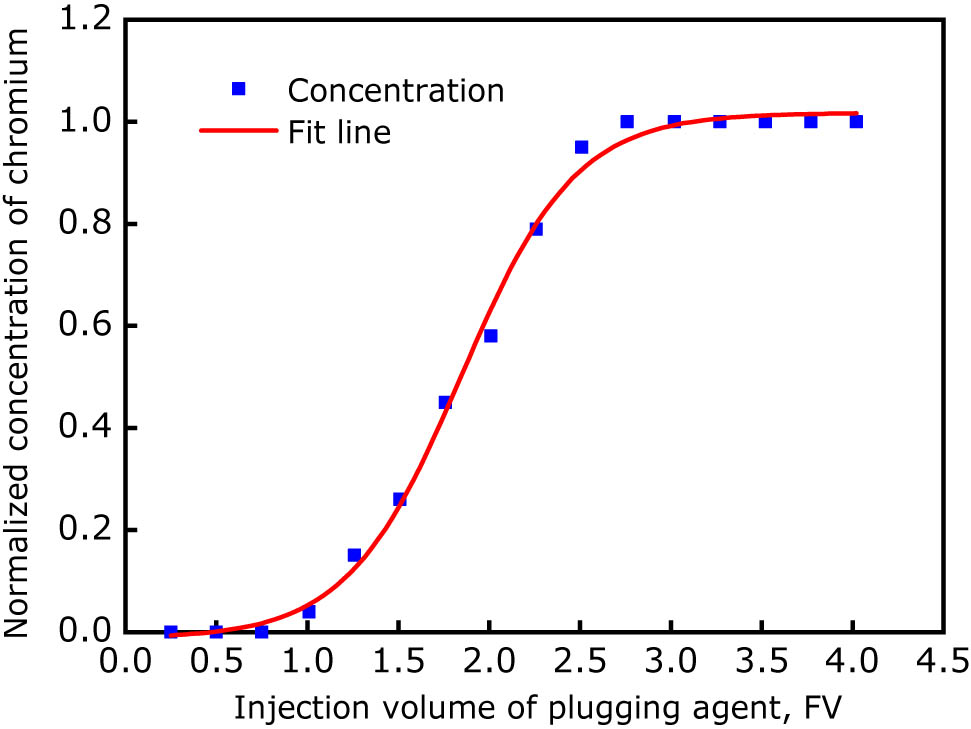
Curve of the chromium ion concentration in the produced fluid at fracture outlet vs the injection volume of the gel plugging agent.
Based on the relationship of the normalized concentration of chromium ions versus the injection volume of the gel solution, the injection volumes of the plugging agent corresponding to the set normalized concentrations of chromium ions (CN) were acquired, as listed in Table 3.
Injection volume of gel solution at different leak off degrees of cross-linking agent
| Item | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| CN | 1.0 | 0.9 | 0.8 | 0.6 | 0.4 |
| Injection volume of plugging agent, FV | 2.75 | 2.40 | 2.10 | 1.75 | 1.45 |
According to the correspondence between the normalized concentration (CN) of chromium ions and the injection volume of the plugging agent listed in the table, corresponding volume of the polymer gel solution was injected into five fractured cubical cores (wf = 2 mm, km = 50 mD), and then sealed for gelling. After completing the gelling process, reverse water flooding (vw = 0.5 mL/min) was conducted, and the water flooding pressure was recorded in real time. The experimental results are shown in Figure 10, indicating that, with the increase of the normalized concentration of chromium ions, the stable injection differential pressure for reverse water flooding gradually increases. When CN was equal to 0.4, the stable injection differential pressure was only 41 kPa, and the plugging performance was poor. When CN was equal to 0.8, the stable injection differential pressure was 205 kPa, and the plugging performance was obviously enhanced. When CN was equal to 1.0 there was no loss of chromium ions from the gel remained in the fracture, and the stable injection differential pressure reached 375 kPa. The experimental results reveal that the leak off of the cross-linking agent from the gel solution can significantly affect the performance of gel to plug the fracture, and is consistent with the conclusion of the previous studies indicating that the leak off of the cross-linking agent might reduce the gelling strength of the gel plugging agent.
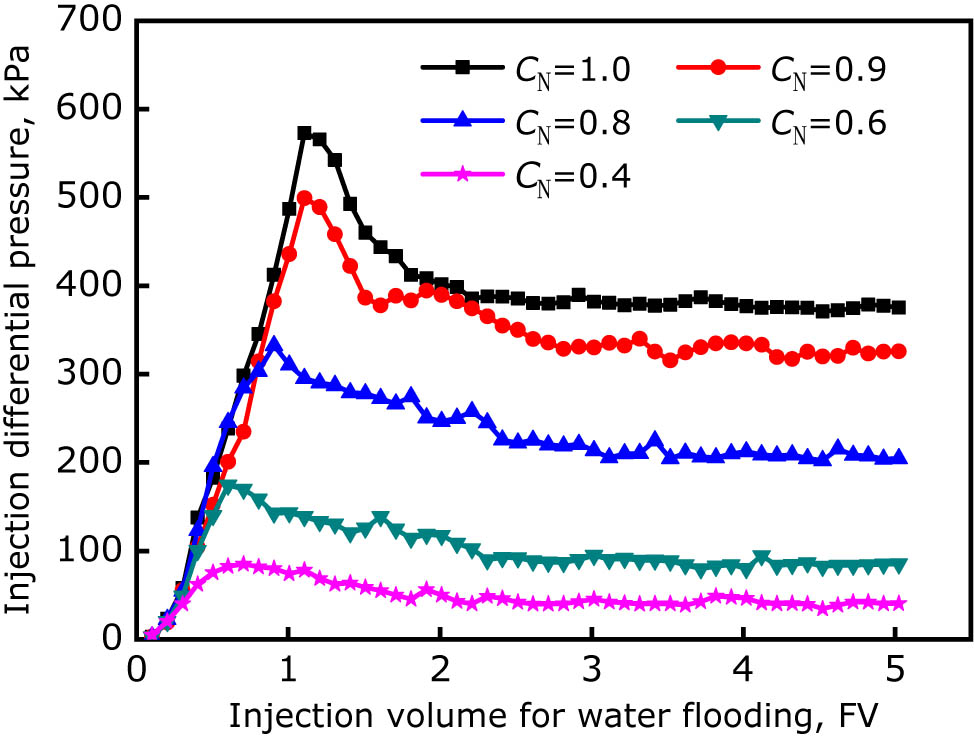
Reverse water flooding differential pressures for the gel plugging agent at different leak off degrees.
According to the performance evaluation indicators of the gel plugging agent to plug a fracture, the gel plugging performance (dP5) in the core with a fracture width of 2 mm and matrix permeability of 50 mD at different leak off degrees of chromium ions was calculated. In addition, the fracture plugging performance (dP5) of the gel plugging agent in the case of 1 and 5 mm fracture width was analyzed and calculated. The calculation results are shown in Tables 4–6.
Fracture plugging performance of polymer gel at different leak off degrees (Wf = 1 mm)
| Item | CN | Water flooding differential pressure (kPa) | dP5 | Effect degree |
|---|---|---|---|---|
| 1 | 1.0 | 434 | 1.00 | None |
| 2 | 0.9 | 400 | 0.92 | Weak |
| 3 | 0.8 | 308 | 0.71 | Moderate |
| 4 | 0.6 | 135 | 0.31 | Very strong |
| 5 | 0.4 | 78 | 0.18 | Very strong |
Fracture plugging performance of polymer gel at different leak off degrees (Wf = 2 mm)
| Item | CN | Water flooding differential pressure (kPa) | dP5 | Effect degree |
|---|---|---|---|---|
| 1 | 1.0 | 375 | 1.00 | None |
| 2 | 0.9 | 326 | 0.87 | Moderate |
| 3 | 0.8 | 205 | 0.64 | Strong |
| 4 | 0.6 | 85 | 0.23 | Very strong |
| 5 | 0.4 | 41 | 0.11 | Very strong |
Fracture plugging performance of polymer gel at different leak off degrees (Wf = 5 mm)
| Item | CN | Water flooding differential pressure (kPa) | dP5 | Effect degree |
|---|---|---|---|---|
| 1 | 1.0 | 3,320 | 1.00 | None |
| 2 | 0.9 | 282 | 0.85 | Moderate |
| 3 | 0.8 | 186 | 0.56 | Strong |
| 4 | 0.6 | 70 | 0.21 | Very strong |
| 5 | 0.4 | 36 | 0.11 | Very strong |
Tables 4–6 show that when the fracture width is 1 mm and the normalized concentration of chromium ions in the fracture is 0.9 and 0.8, the effect of chromium ion loss on the fracture plugging performance after gelling is weak and moderate, respectively; however, when the fracture width is 2 and 5 mm, the effect becomes moderate and strong, respectively. This indicates that under the same injection conditions, the gel plugging agent can easily plug a narrow fracture, but finds difficult to plug a wide fracture. According to the analysis on the distribution of the gel plugging agent in a fracture and its plugging mechanism in Section 2 of this study (migration and distribution patterns of polymer gel in a fracture), the main reason is that leak off amount of the gel solution in a narrow fracture is high enough to reach a balance state, and the gel has a strong capacity to reside in the fracture after gelling. The data show that when CN is ≤0.6, the fracture plugging performance of the gel after gelling is very poor with significant effect. Therefore, it is an important measurement indicator to control the leak off of the cross-linking agent in a fracture and ensure its effective concentration for gelling (16).
4.3 Plugging mechanisms of gel plugging agent with different gelling ways
The gelling of the polymer gel plugging agent is usually achieved by in situ cross-linking or ground pre-cross-linking. In the case of in situ cross-linking, the polymer and cross-linking agent are mixed together in a certain proportion and then injected into formation. At the formation temperature and pressure, the polymer and cross-linking agent cross-link to form gel. At present, in situ cross-linking is a commonly used way of gelling in oil fields (17). In the case of ground pre-cross-linking, the polymer and cross-linking agent are mixed together to a certain proportion, allowed to set still on the ground for a certain time, and then injected into formation after gelling (18). Compared to the in situ cross-linking, the plugging agent subjected to ground pre-cross-linking has a higher injection viscosity, and thus can mitigate or even prevent the leak off–diffusion of the gel components into matrix, but might significantly reduce its injectability. Moreover, the pre-cross-linked gel can undergo irreversible shear degradation during injection, which in turn would affect its plugging strength. In the case of in situ cross-linking, the gel solution has a higher injectability, a lower degree of shear degradation, and an inevitable leak off of the gel components (19).
In the experiment, two polymer gel solutions with the same component concentration were prepared. One gel solution was sealed and set still at 60°C for 24 h, and then injected into a fractured cubical core with a fracture width of 2 mm and a matrix permeability of 50 mD after complete gelling. The other gel solution was stirred at low speed at room temperature (25°C) to simulate the flowing process of the gel solution in the wellbore before reaching the target formation for 2 h and then injected into another fractured core with the same parameters. In the experiment, the injection volume of both gel solutions was 2.5 FV, and the pressure data during injection were acquired and plotted (as shown in Figure 11).
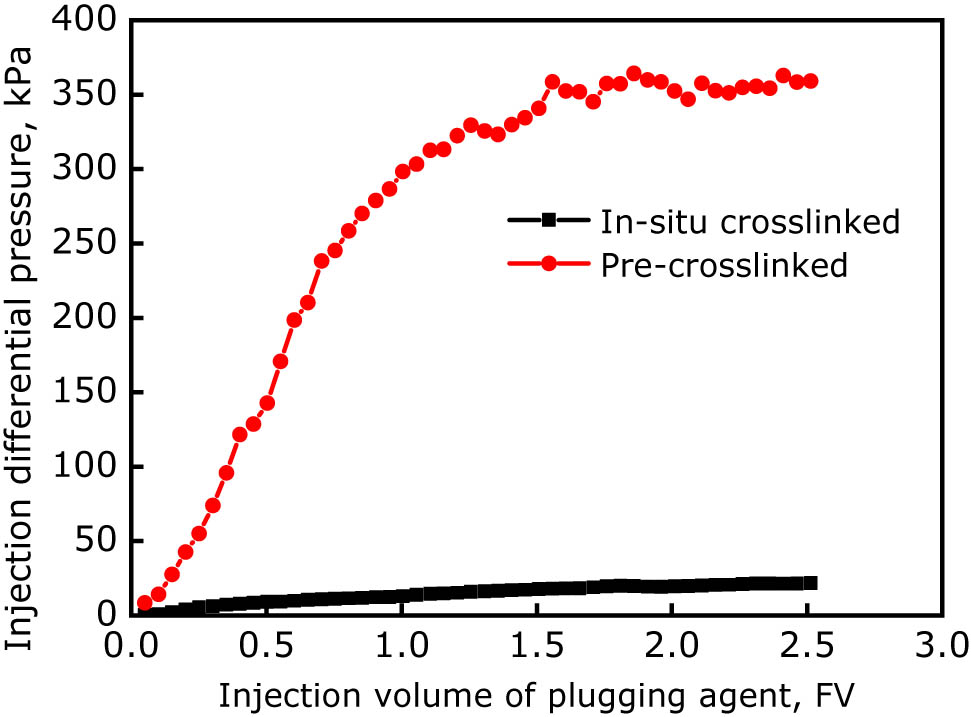
Injection differential pressures of gel solution in the case of in situ cross-linking and pre-cross-linking.
Figure 11 shows that, in the case of injecting in situ cross-linked gel solution, although the injection pressure kept on increasing with increasing injection volume, the increase in pressure was relatively small and only 22 kPa when the injection volume reached 2.5 FV, indicating high injectability of the in situ cross-linked gel solution. In contrast, the injection pressure of the pre-cross-linked gel increased rapidly at the initial stage because of its high viscosity, and then began to increase slowly and steadily after the injection volume exceeded 1.5 FV. Finally, the pressure stabilized at approximately 359 kPa, which was 15 times higher than that of the in situ cross-linked gel solution.
After completion of injection, the two cores were sealed and placed still at 60°C for 24 h. After the complete gelling of the in situ cross-linked gel, the cores were taken out for reverse water flooding. The pressure change during reverse water flooding is shown in Figure 12.
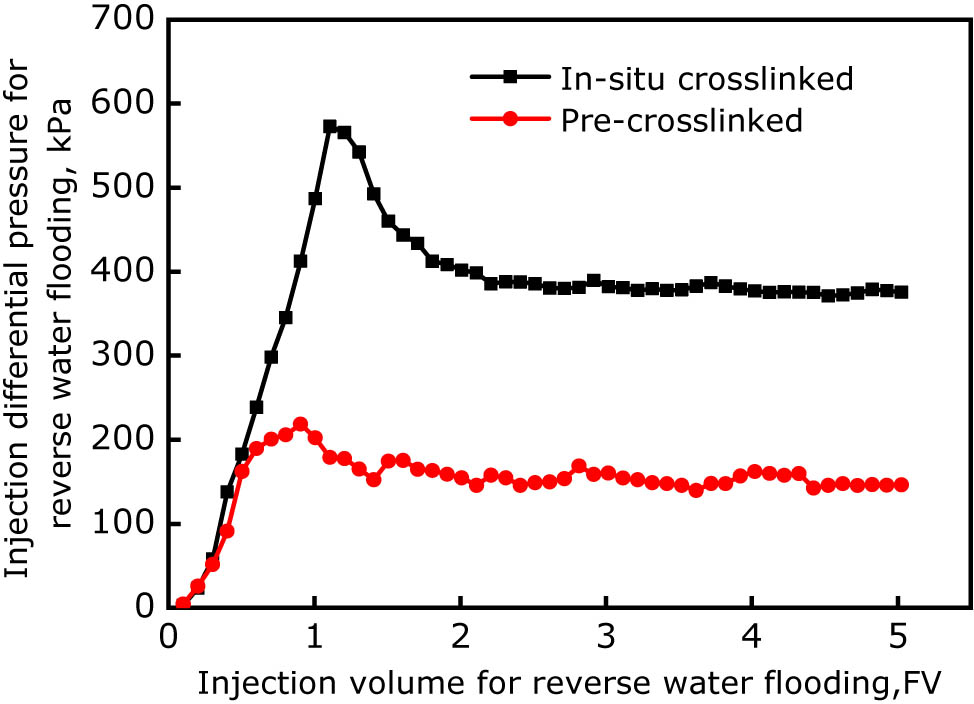
Differential pressures for reverse water flooding after gelling in the case of in situ cross-linking and pre-cross-linking.
At the initial stage of reverse water flooding, the pressure increased rapidly with increasing water injection volume. When the water injection exceeded a certain volume, the pressure decreased and eventually became stable. In the case of in situ cross-linked gel, a pressure peak was observed for reverse water flooding, indicating that the gel in the fracture was broken by the water injected. In the case of pre-cross-linked gel, the pressure peak was not obvious. Furthermore, no fluid was produced at the fracture outlet before the water flooding pressure reached the peak in the case of in situ cross-lined gel, and a small amount of gel and water appeared at the fracture outlet before the pressure reached the peak in the case of pre-cross-linked gel, indicating that some gel broke by water at that time. The above conclusions are also supported by the stable pressures for reverse water flooding obtained from the experimental data. When the reverse water flooding volume was 5 FV, the stable water flooding pressure of the in situ cross-linked gel was 375 kPa, and the plugging performance evaluation indicator (dP5) of the gel plugging agent was calculated as 1, while the stable water flooding pressure of the ground pre-cross-linked gel was only 146 kPa, and the calculated dP5 was 0.39. The experimental results show that the in situ cross-linked gel is better than the ground pre-cross-linked gel under the same conditions in terms of fracture plugging performance.
In order to further analyze the plugging performance of the in situ cross-linked gel and pre-cross-linked gel, the two gel solution systems were injected into a transparent round hollow pipe (with an inner diameter of 10 mm) under the same injection conditions, and then sealed for cementing. After that, reverse water flooding (the injected water was dyed blue with methylene blue) was conducted, and the breakthrough line of water flow (as shown in Figure 13) in the gel was observed. As shown in Figure 13a, the in situ cross-linked gel demonstrates a strong cementing ability with the pipe wall after gelling, and a flow line formed by the breakthrough of water along the gel center after the injection pressure reaching the peak. As the water flooding proceeds, the flow line would continue to scour the gel, causing the flow line diameter to expand. Reverse water flooding has a different water flow line in the case of pre-cross-linked gel. Figure 13b shows the formation of dispersed gel clusters in the pipe after the injection of pre-cross-linked gel and are weakly cemented onto the pipe wall. Under the water flooding, the gel is evenly driven out, and the flow line evenly extends along the whole pipe, deteriorating the plugging performance. The above phenomenon explains the inferior plugging performance of the pre-cross-linked gel compared to that of the in situ cross-linked gel.
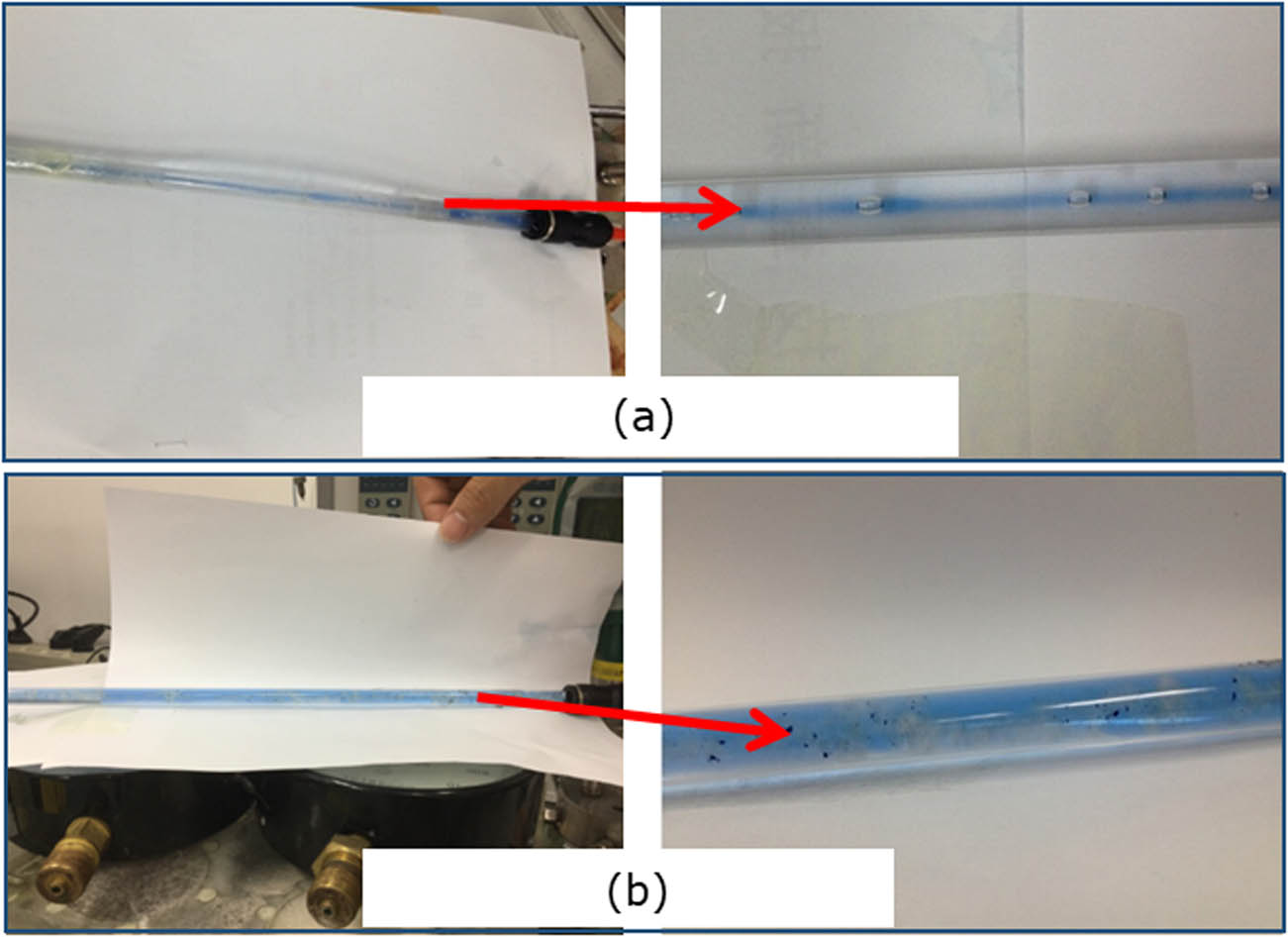
Reverse water flooding in transparent pipe in the case of different gel injection ways. (a) In situ crosslinked gel, (b) pre-crosslinked gel.
5 Conclusions
The conclusions of this study are as follows:
The migration flowing behavior of the polymer gel solution in a fracture can be divided into three types: fracture flow (flowing in the fracture), leak off flow (flowing from the fracture into the matrix), and matrix flow (flowing in the matrix). Such flowing behavior resulted in the different distribution patterns of the gel after gelling in the fracture and matrix.
Because of the leak off flow and the difference in the components, the gel solution has apparent disproportional leak off–diffusion of components during its migration in a fracture, with less polymer molecules and loss of more cross-linking agent ions. The leak off–diffusion of the cross-linking agent greatly reduces the gelling strength of the gel plugging agent. In the case of gross leak off of the cross-linking agent, the ion concentration decreased from 328.4 to 167.8 mg/L, and the drop in the gelling viscosity is one-third of that in the case of slight leak off.
The leak off degree of the gel components directly affects its performance to plug a fracture after gelling. The experimental results show that when the normalized concentration of the cross-linking agent ions after leak off is higher than 0.8, the effect of the leak off on the fracture plugging performance of gel is not significant; when the normalized concentration of the cross-linking agent ions is <0.6, gel fails to plug the fracture effectively after gelling. Therefore, it is necessary to reduce the leak off–diffusion degree of the cross-linking agent in a fracture and ensure its concentration within a reasonable range.
Compared to the gelling by ground pre-cross-linking, the in situ cross-linking for gelling resulted in the formation of a gel layer after the gelling of the polymer gel solution and can facilitate the better cementation between the gel and fracture surface, which in turn improves its fracture plugging performance.
Acknowledgments
This research was financially supported by the New EOR Technologies for High Water Cut Oilfields, the National Science and Technology Major Project (2016ZX05010-006).
References
(1) Chenglin G, Yahui L, Xiqun F, Changxi Z, Caili D, Guang Z. Study on rheology and microstructure of phenolic resin cross-linked nonionic polyacrylamide (NPAM) gel for profile control and water shutoff treatments. J Pet Sci Eng. 2018;169:546–52.10.1016/j.petrol.2018.06.016Suche in Google Scholar
(2) Xin C, Yiqiang L, ZheYu L, Jian Z, Cheng C, Ming M. Investigation on matching relationship and plugging mechanism of self-adaptive micro-gel (SMG) as a profile control and oil displacement agent. Powder Technol. 2020;364:774–84.10.1016/j.powtec.2020.02.027Suche in Google Scholar
(3) Mazen A, Chun H, Kamy S, Mojdeh D, Abdoljalil V. A critical review on use of polymer microgels for conformance control purposes. J Pet Sci Eng. 2014;122:741–53.10.1016/j.petrol.2014.06.034Suche in Google Scholar
(4) Ghodsieh M, Mohammad M, Feridun E, Dariush M, Amin D. An experimental investigation of polyacrylamide and sulfonated polyacrylamides based gels crosslinked with Cr(iii)-acetate for water shutoff in fractured oil reservoirs. J Disper Sci Technol. 2018;39(12):1780–9.10.1080/01932691.2018.1462712Suche in Google Scholar
(5) Hu J, Xinyu Y, Sanxi L, Peizhi Y, Jingchun Z. Nanocomposite gel of high-strength and degradability for temporary plugging in ultralow-pressure fracture reservoirs. Colloids Surf A. 2020;585:124108.10.1016/j.colsurfa.2019.124108Suche in Google Scholar
(6) Ding B, Dong M. Optimization of plugging high mobility zones in oil sands by injection of oil-in-water emulsion: experimental and modeling study. Fuel. 2019;257:116024.10.1016/j.fuel.2019.116024Suche in Google Scholar
(7) Yingrui B, Xiaosen S, Zengbao W, Xiutai Z. Experimental study of low molecular weight polymer/nanoparticle dispersed gel for water plugging in fractures. Colloids Surf A. 2018;551:95–107.10.1016/j.colsurfa.2018.04.067Suche in Google Scholar
(8) Seright RS. Washout of Cr(iii)–acetate-HPAM Gels from Fractures: International Symposium on Oilfield Chemistry. In: International Symposium on Oilfield Chemistry (5–7 February 2003), Houston, Texas; 2003. SPE-80200-MS.10.2118/80200-MSSuche in Google Scholar
(9) Sydansk RD, Xiong Y, Al-Dhafeeri AM, Schrader RJ, Seright RS. Characterization of partially formed polymer gels for application to fractured production wells for water-shutoff purposes. SPE Prod Facil. 2005;20(3):240–9.10.2118/89401-MSSuche in Google Scholar
(10) Goudarzi A, Zhang H, Varavei A, Taksaudom P, Hu Y, Delshad M, et al. A laboratory and simulation study of preformed particle gels for water conformance control. Fuel. 2015;140:502–13.10.1016/j.fuel.2014.09.081Suche in Google Scholar
(11) Junjian L, Chunming X, Yingrui B, Ruyi J, Falin W, Miao Z. Leak-off behavior and water shut-off performance of a polymer/chromium (Cr3+) gel in fractured media. J Cent South Univ. 2017;24:1418–29.10.1007/s11771-017-3546-1Suche in Google Scholar
(12) Ganguly S. Leak-off during placement of Cr(iii)-partially hydrolyzed polyacrylamide gelling solution in fractured porous media. Transp Porous Med. 2010;81(3):443–60.10.1007/s11242-009-9416-zSuche in Google Scholar
(13) Tang G, He S, Wang B. Simultaneous determination of chromium (vi) and chromium (iii) by ultraviolet-visible spectrophotometry with multivariate calibration. Chinese J Anal Chem. 1995;04:383–6.Suche in Google Scholar
(14) Guanghui L, Jijiang G, Guicai Z, Jinwei S. Viscosity characteristics of cationic polyacrylamide microsphere. J China Univ Pet. 2015;39(1):176–81.Suche in Google Scholar
(15) Yingrui B, Chunming X, Falin W, Junjian L, Yong S, Pingde L. Gelation study on a hydrophobically associating polymer/polyethylenimine gel system for water shut-off treatment. Energ Fuel. 2015;29(2):447–58.10.1021/ef502505kSuche in Google Scholar
(16) Imqam A, Wang Z, Bai B. The plugging performance of preformed particle gel to water flow through large opening void space conduits. J Pet Sci Eng. 2017;156:51–61.10.1016/j.petrol.2017.04.020Suche in Google Scholar
(17) Zhou Z, Abass H, Li X, Bearinger D, Frank W. Mechanisms of imbibition during hydraulic fracturing in shale formations. J Pet Sci Eng. 2016;125–32.10.1016/j.petrol.2016.01.021Suche in Google Scholar
(18) Lu X, Xie K, Cao B, Chen X. Study on gelling effect of Cr3+ polymer gel and its influence factors. J China Univ Pet. 2015;39(3):170–6.Suche in Google Scholar
(19) Standnes D, Skjevrak I. Literature review of implemented polymer field projects. J Pet Sci Eng. 2014;122:761–75.10.1016/j.petrol.2014.08.024Suche in Google Scholar
© 2020 Song Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Artikel in diesem Heft
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery


