All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
-
Haiyan Pei
, Yingliang Liu
, Dongfang Wang
Abstract
Non-conjugated multi-functionalized all-optical photorefractive (PR) polymers were designed and synthesized via ring-opening metathesis polymerization (ROMP) of two monomers using Grubbs II catalyst as an initiator. The polymers were characterized by infrared (IR) spectrum, gel permeation chromatography (GPC), UV-visible spectrum (UV-Vis), differential scanning calorimetry (DSC), thermogravimetry (TG) and so on. The number-average molar mass (Mn) of the polymers is no less than 8.5 × 104 while their glass transition temperatures of 52°C and 45°C are close to the room temperature, which is helpful to the enhancement of PR orientation and the fabrication of PR devices. The polymers have good thermal stability and great solubility in tetrahydrofuran (THF). Without any plasticizer, the transparent optical films can be prepared by solution-casting with THF solution of polymers. Their PR properties at 633 nm were evaluated by two beam coupling (TBC) experiments under zero electric field without prepoling. It is shown that the single-component polymer has obvious all-optical PR behavior.
1 Introduction
Photorefractive (PR) polymeric materials, including fully functional polymers and polymer composites, have been studied for years owing to their potential applications in phase conjugation, high-density optical data storage, neural network simulations, image processing and so on (1,2,3,4,5,6). Compared to polymer composites, fully functional PR polymers have more advantages because all the functional bodies with PR effect are covalently bonded on the polymers, which eliminates the problems of phase separation and crystallization, so that the potential devices have better stability and longer warranty period (1,7,8,9,10). However, fully functionalized PR polymers are so far reported to be almost having a main-chain conjugated structure being prepared with complicated synthetic processes, which are not beneficial to the potential application (7,8). In the past few years, organic amorphous PR molecular materials have also been developed. Their good PR performance and simple synthesis method can provide theoretical basis for the molecular design of full-functional/multi-functional NCMC polymer (11,12,13,14). However, owing to the fact that the synthesis of chromophore functional monomers is not easy, the number of full-functional PR polymers with NCMC structure is relatively limited (9). In other words, due to the large volume of monomers and the side effects of functional groups, the direct polymerization of functional monomers usually presents a low molecular weight polymer, which is not conducive to the film-forming performance (15,16,17,18,19). In order to solve this problem, we focus on ring-opening metathesis polymerization (ROMP). The progress in ROMP of cycloalkene derivatives provides a good way for the synthesis of norbornene functional monomers. While, it is rarely used in the preparation of functionalized PR polymers (20,21,22,23,24,25,26). In addition, all-optical PR materials have become another important research topic due to the convenient signal-writing without the application of external electrical field (27,28,29). Therefore, it is an interesting subject to synthesize all-optical multi-functional PR polymers with well-defined structure and high molecular weight by simple ring-opening metathesis polymerization.
In our previous work (26), we have designed norbornene functional monomers with azobenzene chromophores and the polymers were synthesized by ROMP using Grubbs I catalyst. However, due to the high glass transition temperature of the polymer, the plasticizers need to be added to reduce the glass transition temperature when the PR devices were prepared. In addition, the photosensitizer C60 was also added, which is not conducive to future application. Therefore, it is our goal to design and synthesize the polymers with glass transition temperature close to room temperature while their PR properties can be displayed without any photosensitizer. In this paper, two norbornene monomers, with/without methine chromophore, were first designed and synthesized via Vilsmeier and Knoevenagel reactions. Then, the fully functional PR polymers with NCMC structure were synthesized by ROMP using Grubbs II catalyst, in which the long alkyl spacer between the functional chromophore and methine group was expected to ensure the low glass temperature and free rotation of PR chromophore. The synthesized polymers were characterized by infrared (IR) spectra, UV-vis spectra, gel permeation chromatography (GPC), differential scanning calorimetry (DSC), thermogravimetry (TG) and so on. As a result, the single-component fully functionalized PR polymers with low Tg and high molecular weight were presented. In the measurement process of TBC carried out at 633 nm, it was found that the polymers were able to take on an all-optical PR performance at room temperature and zero electrical field without any plasticizer, any photosensitizer and prepoling, which provide the convenient precondition for practical PR application.
2 Experimental
2.1 Materials
Grubbs II catalyst, 5-norbornene-2-formyl chloride, 6-chlorohexanol and 9-bromo-1-nonanol, respectively, were purchased, respectively, from Sigma-Aldrich, Puyang Huicheng Chemical Co. Ltd, Shanghai Bangcheng Chemical Co. Ltd and Beijing Isomersyn Technology Co. Ltd. Other analytical or chemical purity chemicals were also obtained from Chinese reagent companies and used directly. Before using, tetrahydrofuran (THF) needs to be dried with sodium using benzophenone as indicator, and dichloromethane (CH2Cl2) should be purified by calcium hydride distillation under N2.
2.2 Characterization
The melting point was measured by an X-5A melting point tester. The 1H-NMR spectra were recorded by Bruker DRX-400 NMR spectrometer with CDCl3 as solvent. IR spectra were determined on a Nicolet-460 FTIR spectrometer. Elemental analysis was performed on the EA 1110 CHNS-O instrument. The GPC measurement calibrated using polystyrene standards was measured with THF as eluent at 40°C by a Waters M515 instrument. Shimadzu 3010 UV-visible spectrometer was used to determine the UV-vis spectra with THF as solvent. Under a nitrogen atmosphere, the DSC measurement was performed on a TA-100 DSC with a heating rate of 10°C/min. TG analysis was carried on a Netzsch-209 TGA system with a heating rate of 10°C/min.
2.3 Synthesis
The synthetic route of monomers and polymers is shown in Scheme 1. Compounds 1 and 6 were prepared according to our previously reported synthetic procedures (15,26).

Synthetic route of PR polymers.
2.3.1 Preparation of compound 2 (30)
Compound 1 (9 g) and acetic anhydride (70 mL) were added to a single-necked flask. The solution was heated to reflux for 1 h under stirring. After cooling, the solution was poured into a large amount of ice water and extracted with chloroform. After that, the resulting solution was dried overnight with anhydrous magnesium sulfate. The filtrate was filtered by suction. The solvent was removed by rotary evaporator to obtain light yellow solid with a yield of 90%. 1H-NMR (CDCl3, TMS, δ): 8.0 (d, J = 7.6 Hz, 2H), 7.4 (m, 2H), 7.3 (d, J = 8.4 Hz, 2H), 7.2 (m, 2H), 4.3 (t, 2H, J = 7.2 Hz, NCH2), 4.0 (t, J = 6.4 Hz, 2H, OCH2), 2.0 (d, 3H, J = 4.4 Hz, COCH3), 1.8–1.9 (t, J = 7.6 Hz, 2H), 1.5–1.6 (t, J = 7.2 Hz, 2H), 1.3–1.4 (m, 4H).
2.3.2 Preparation of compound 3 (30)
The flask containing compound 2 (4.0 g, 0.014 mol) was placed in an ice water bath and then DMF (20 mL) was added to dissolve it. After stirring for 10 min, POCl3 (2.4 mL) was slowly added to the solution. The solution was stirred at 100°C for 3 h and then cooled to room temperature. The resulting yellow solution was poured into ice water and neutralized with diluted KOH solution to pH = 6–8. Then, the obtained solution was extracted with chloroform and dried by anhydrous magnesium sulfate. After removing the solvent, the residue was purified by silica gel column chromatography with chloroform as eluent, and the yellow solid was obtained in 53% yield. 1H-NMR (CDCl3, TMS, δ): 10.0 (s, 1H, aldehyde proton), 8.6 (s, 1H), 8.1 (d, J = 7.6 Hz, 1H), 8.0 (m, 1H), 7.5 (m, 1H), 7.4 (d, J = 8.4 Hz, 2H), 7.3 (m, 1H), 4.3 (t, J = 7.2 Hz, 2H, NCH2), 4.0 (t, J = 6.8 Hz, 2H, OCH2), 2.0 (d, J = 4.4 Hz, 3H, COCH3), 1.9 (t, J = 7.2 Hz, 2H), 1.5–1.6 (t, J = 6.8 Hz, 2H), 1.2–1.4 (t, J = 7.2 Hz, 4H).
2.3.3 Preparation of compound 4 (30)
Ethanol (100 mL) was added to compound 3 (4.0 g, 4 mmol), stirred for 10 min and then KOH (4.0 g, 70 mmol) was added. Then, the obtained solution was cooled after heating to reflux for 3 h. Half of the solvent was removed before the mixture was poured into the water to obtain the sediment. The sediment was filtered and fully cleaned with water. After drying under vacuum, the yellow solid was obtained by recrystallization with ethanol, and the yield was 30%. 1H-NMR (CDCl3, TMS, δ): 10.0 (s, 1H, aldehyde proton), 8.6 (s, 1H), 8.1 (d, J = 7.6 Hz, 1H), 8.0 (m, 1H), 7.5 (d, J = 8.0 Hz, 1H), 7.4 (m, 2H), 7.3 (m, 1H), 4.3 (t, J = 7.2 Hz, 2H, NCH2), 3.6 (t, J = 6.4 Hz, 2H, OCH2), 1.8–1.3 (m, 8H).
2.4 Preparation of compound 5 (30)
Compound 4 (2 g, 6.4 mmol) and 4-nitrophenylacetic acid (1.2 g, 7.44 mmol) were dissolved with anhydrous ethanol. Then, piperidine (1.2 mL) was added to the solution under stirring drop by drop. The mixed solution was heated to reflux for 2 h and then cooled. The residue was poured into the ethanol to obtain the sediment. The sediment was filtered and recrystallized with anhydrous ethanol. Then, the orange solid was obtained and the yield was 58%. 1H-NMR (CDCl3, TMS, δ): 7.3–8.3 (m, 12H), 4.4 (t, J = 7.6 Hz, 2H, OCH2), 3.4 (t, J = 6.8 Hz, 2H, NCH2), 1.3–1.8 (m, 8H).
2.4.1 Preparation of M1 (31)
Compound 5 (9 g, 20 mmol) was dissolved in purified dry dichloromethane (30 mL). At room temperature, triethylamine (2.4 g, 24 mmol) was added with stirring. Then, 5-norbornene-2-formyl chloride (6.6 g, 20 mmol) was added slowly and reacted at room temperature for 4 h. The solvent was removed and the crude product was purified by silica gel column chromatography with CH2Cl2 as eluent. The yellow solid compound M1 with the yield of 99% was finally obtained by recrystallization from n-hexane. Mp = 133.4–134.5°C. 1H-NMR (CDCl3, TMS, δ): 8.7 (s, 1H), 8.1–8.4 (m, 2H), 8.1–8.2 (d, J = 7.6 Hz, 2H), 7.8–7.9 (d, J = 6.4 Hz, 3H), 7.2–7.6 (m, 4H), 6.0–6.2 (m, 2H, –CH═), 4.3–4.4 (t, J = 6.8 Hz, 2H, NCH2), 4.0 (m, 2H). 2.2–3.0 (m, 3H, CH), 1.0–2.0 (m, 12H, CH2). 13C-NMR (δ, CDCl3): 25.8, 26.9, 28.5, 29.2, 30.3, 41.6, 42.5, 43.4, 45.7, 46.6, 49.7, 64.3, 99.0, 104.6, 109.2, 109.4, 118.4, 120.2, 120.8, 123.4, 124.0, 126.0, 127.7, 135.7, 137.8, 138.1, 140.9, 141.4, 146.5, 147.1, 176.4. Elemental analysis, calcd: C, 75.11; H, 5.94; N, 7.51. Found: C, 74.85; H, 6.02; N, 7.42.
2.4.2 Preparation of M2 (31)
Compound M2 was prepared by a similar procedure to M1 and obtained as a buff viscous matter in the yield of 70%. 1H-NMR (CDCl3, TMS, δ): 8.1 (d, J = 7.6 Hz, 1H), 7.2–7.5 (m, 7H), 6.2 (m, 2H), 4.3 (t, 2H, J = 7.2 Hz), 4.0 (m, 2H), 2.9–3.2 (m, 3H), 1.2–2.2 (m, 10H). 13C-NMR (δ, CDCl3): 32.4, 34.5, 68.8, 71.1, 73.3, 76.8, 79.0, 83.6, 86.4, 88.8, 90.5, 100.5, 131.7, 132.1, 132.4, 144.6, 144.8, 159.5, 162.8, 163.4, 165.8, 183.2. Elemental analysis, calcd: C, 81.08; H, 8.21; N, 3.26. Found: C, 79.61; H, 7.905; N, 3.077.
2.4.3 Polymer synthesis (31)
Polymers P1 and P2 were synthesized according to the same procedure as follows: the air in the two polymerization reaction tubes was removed by vacuum pump, and the air in the tube was replaced by argon. After repeated operation for three times, the monomer (total molar amount: 1 mmol) was dissolved in dried dichloromethane under the protection of nitrogen. Then, the dichloromethane solution (2 mL) of Grubbs II catalyst (2 µmol) was injected into the mixture by a microinjector after the reaction was performed at room temperature for 12 h. The target polymer was obtained by precipitation with methanol, filtration and vacuum drying.
Polymer P1: elemental analysis, found: C, 77.80; H, 7.02; N, 5.01.
Polymer P2: elemental analysis, found: C, 77.10; H, 6.77; N, 5.28.
2.5 PR device fabrication and TBC measurement
The PR device fabrication and TBC measurement were similar to our previous work (26). However, compared with the previous work, there is an obvious difference. In this work, the samples for PR measurement were fabricated by single-component polymers without any plasticizer and photosensitizer.
3 Results and discussion
3.1 Synthesis of monomers and polymers
The synthetic route of monomers and polymers is shown in Scheme 1. The synthesis of compounds 1 and 6 is carried out according to the previously reported procedures (15,26). In the preparation process of monomers, many more efforts have been paid to the synthesis of compound 4 owing to the existence of two reaction sites, in which the terminal hydroxyl group can react with 5-norbornene-2-formyl chloride yielding norbornene monomer for ROMP and the reaction of aldehyde group in the 3-position of carbazole ring will yield C═C by Knoevenagel condensation reaction with the compounds bearing active hydrogen, by which electron acceptor substituent is introduced into carbazole ring by Knoevenagel condensation reaction with aldehyde group in the 3-position. Therefore, the multi-functional monomer M1 is prepared by the reaction of 5-norbornene-2-formyl chloride with compound 5, which is achieved by the reaction of compound 4 with 4-nitrophenylacetic acid. The successful achievement of M1 is proven by 1H-NMR, 13C-NMR, IR spectra and elemental analysis. Its chemical structure is in accordance with Scheme 1. Polymers P1 and P2 are synthesized by the ROMP copolymerization of the monomers M1 and M2 utilizing Grubbs II catalyst on the basis of the adjusted course in the references (25,26). The ratio of monomer to initiator ([M]/[I]) is set to be 500:1. After 12 h of reaction at room temperature, the polymerization was terminated with an excess of ethyl vinyl ether. After the reaction is carried out for 12 h at room temperature, the polymerization is terminated with excess ethyl vinyl ether. Polymers P1 and P2 are obtained through precipitation from methanol in the high yield of ∼95%. As listed in Table 1, two polymers have a higher Mn of 1.5 × 105 and 8.5 × 104 than our previous work using Grubbs I catalyst, which is less than 2.3 × 104. Their molecular weight distribution index is 1.44 and 2.13, respectively. All the GPC data are listed in Table 1. The broad proton signals of 1H-NMR in Figure 1 are the typical characteristics of the polymers.
GPC data of the norbornene polymers*
| Polymer | Mn (×104) | Mw (×104) | Polydispersity |
|---|---|---|---|
| P1 | 15.4 | 22.2 | 1.44 |
| P2 | 8.5 | 18.1 | 2.13 |
- *
Calibrated with polystyrene standard.
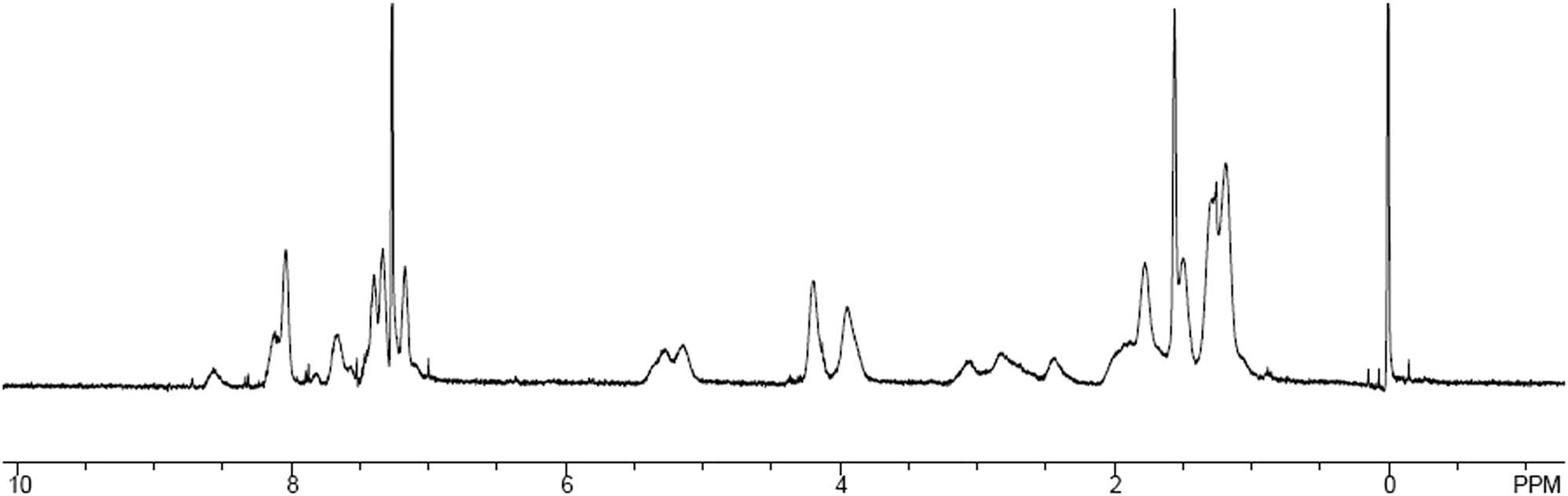
1H-NMR spectrum of polymer P1.
The solubility is an important requirement for organic PR composites, because the samples for PR measurement were generally fabricated by a simple solution-casting. Herein, at room temperature, the solubility of the polymer was tested in a common solvent such as THF, CH3Cl and CH2Cl2 using a 10 mg/mL polymer solutions, as listed in Table 2. The results showed that two polymers have an excellent solubility in common solvents, indicating that the optical films for PR measurement can be made by solution-casting.
Polymer solubility at room temperature*
| Polymer | THF | CH3Cl | CH2Cl2 |
|---|---|---|---|
| P1 | + | + | + |
| P2 | + | + | + |
- *
Tested with a solution concentration of 10 mg/mL; solubility: +, soluble; −, insoluble; ±, partially soluble.
3.2 Thermal stability
The thermal stability of polymers P1 and P2 was investigated under nitrogen atmosphere at a heating rate of 10°C/min. The TG and differential thermogravimetric (DTG) curves are shown in Figure 2. The TG curves of the polymers began to incline at the beginning of temperature rise, that is to say, the chromophore containing nitrile group on the polymers was slowly removed. As we all know, the molecules with large polarity are more likely to be activated and decomposed by external energy than those with small polarity. Evidently, Polymers P1 and P2 have two weight-loss processes derived from the decomposition of different groups. All the TG and DTG data are listed in Table 3.
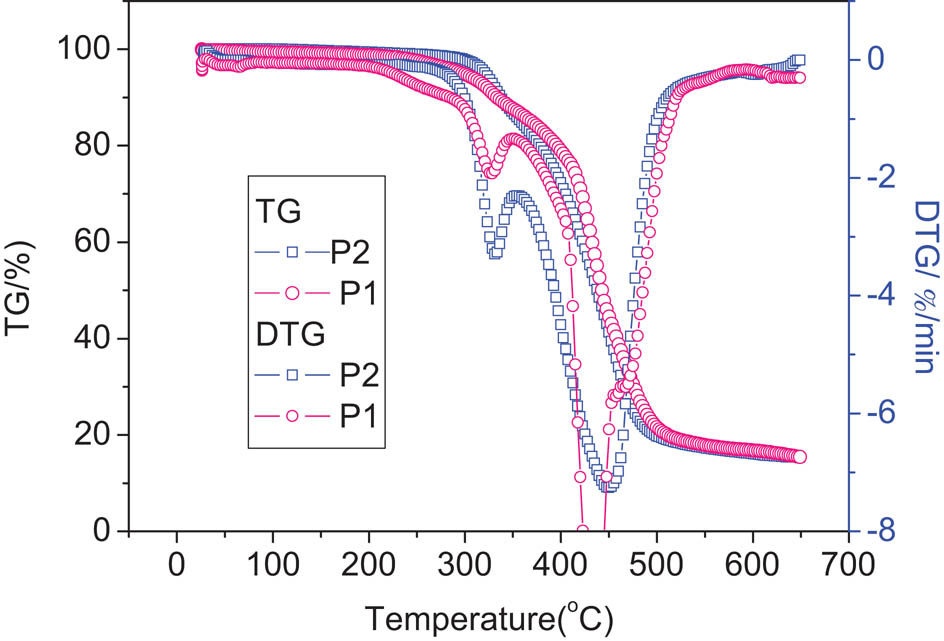
TG and DTG curves of polymers P1 and P2.
Thermal analytic data of the polymers
| Polymer | Tg (°C) | Tonset (°C) | Td (°C) | Tp (°C) |
|---|---|---|---|---|
| P1 | 45 | 253 | 302 | 328, 437 |
| P2 | 52 | 296 | 325 | 330, 450 |
The glass transition temperature (Tg) is a very important parameter for the study of the polymers with nonlinear optical effects (32). The DSC curves of two polymers are illustrated in Figure 3 and Tg of the polymers are listed in Table 3. Before the test, the samples were heated to 200°C at a heating rate of 10°C/min, then cooled to −30°C rapidly, so that both the polymer samples had the same thermal history. It can be seen from the DSC curve that both polymers have no melting and recrystallization peaks, but only a single glass transition, indicating that the synthesized polymers are amorphous polymers. The amorphous structure of PR polymers is favorable to the formation of a uniform and transparent film, which normally leads to low optical loss in transmission and a consequent enhancement of their photorefraction (18). The Tg of P2 is 52°C, which is higher than 45°C of P1 due to the increase in volume and polarity of rigid side chain through the introduction of methine group. Their Tg values are lower compared with our previous work, in which the polymers have a higher Tg value from 75°C to 153°C (26). The low Tg value facilitates PR measurement and device fabrication at room temperature and the PR devices are able to be fabricated without the help of any plasticizer.
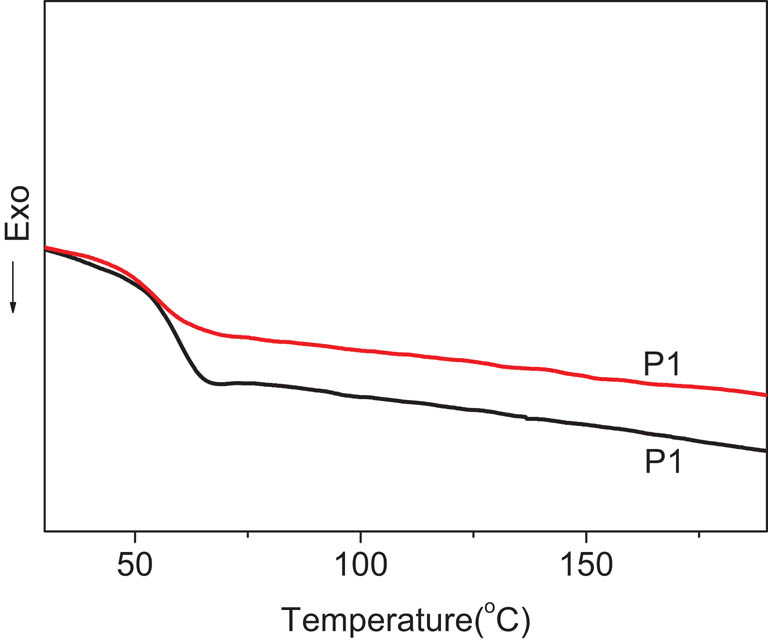
DSC curves of polymers P1 and P2.
3.3 UV-vis absorption
The UV-vis spectra of the monomers and polymers are shown in Figure 4. All the monomers and polymers except for M2 have a similar absorption in the visible region from 350 to 500 nm, which is assigned to the charge transfer from carbazole to methine chromophore. It is obvious that the absorption maximum of the polymers is almost located at the same position as M1. Additionally, the relationship between the absorbance and the solution concentration at the absorption maximum is measured using the THF solutions of P1 and P2 with different concentrations, as plotted in Figure 5. Obviously, the polymer solutions at different concentrations have the same absorption maxima. This point shows that no another intermolecular interaction is produced with the increase in solution concentration. And that, with the change in concentration, there was no significant blue shift or red shift in the position of maximum absorption. The molar absorption coefficient at the absorption maximum is calculated by the formula: ε = A/bc. Here, A is the absorbance value; b is the cuvette thickness; c is the solution concentration of repeating units. The results suggest that the molar absorption coefficients of P1 and P2 are 1.47 × 104 L/mol cm and 1.99 × 105 L/mol cm, respectively.
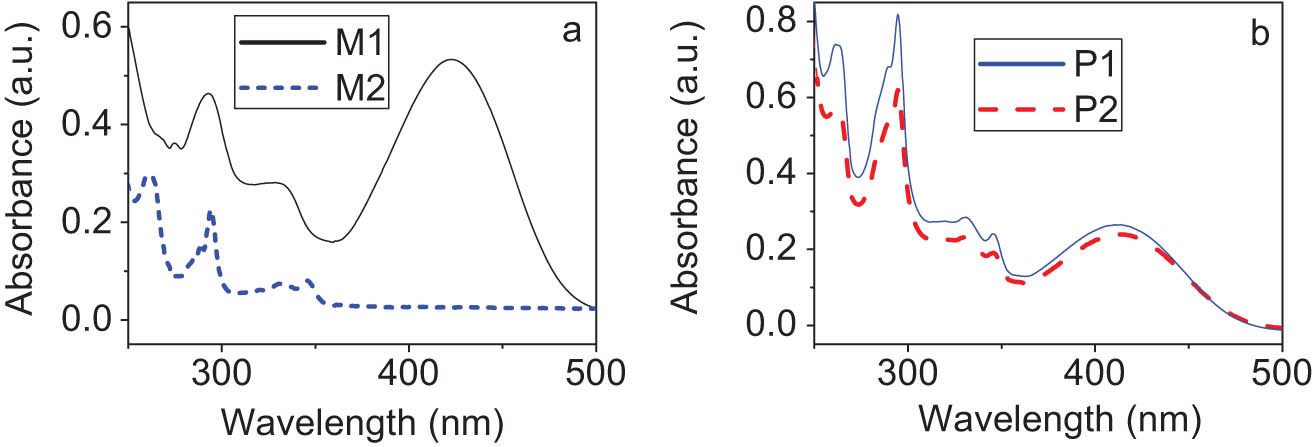
UV-vis absorption spectra of monomers (a) and polymers (b) in THF.
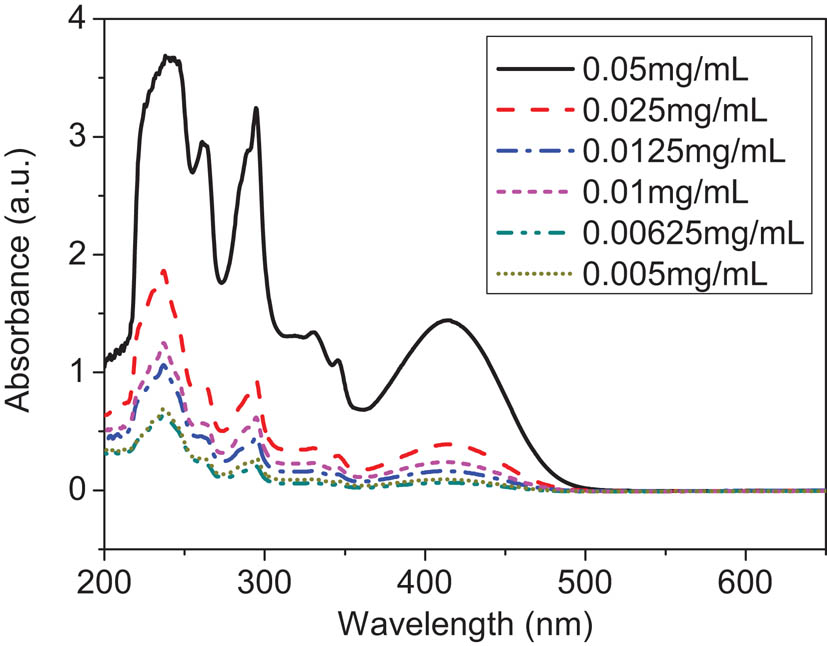
UV-vis absorption spectra of polymer P2 in THF at different concentrations.
3.4 All-optical PR property
The PR effect can only be confirmed by holographic optical technology. Two wave coupling (TBC) experiment is the most commonly used holographic optical technology to evaluate the PR properties of the materials (1). The all-optical PR effect is measured by TBC experiments at zero electric field using the pure polymer film without prepoling and any plasticizer. The temperature is a very important parameter in the measurement of PR effect, because it will affect the whole process of PR effect, including the generation, transport, capture of charge and the orientation of chromophore in the electric field (1,32). Generally speaking, when the operating temperature is near Tg, the PR dynamic performance is controlled by the chromophore orientation rate (1), so the best operating temperature should be near Tg. If the PR test is carried out at room temperature, the material design should consider making its Tg close to room temperature (32) in order to obtain higher PR properties. In other words, the chromophore reorientation would be facilitated when Tg is close to the experimental temperature, which is the room temperature in our experiments, resulting in the more excellent orientation enhancement effect. Because the Tg value (45°C) of P2 is closer to room temperature than P1 (52°C) as shown in Table 3, Polymer P2 takes on a higher coupling coefficient Γ of 26 than 10 cm−1 of Polymer P1. Notably, the photoassisted trans–cis–trans isomerization of methine group results in the vibration of TBC signal, as shown in Figure 6. Finally, the PR NCMC polymers, which bear an evident all-optical PR property without prepoling and any plasticizer, are achieved. This kind of single-component PR device will make the practical PR application more easy, safe and convenient.
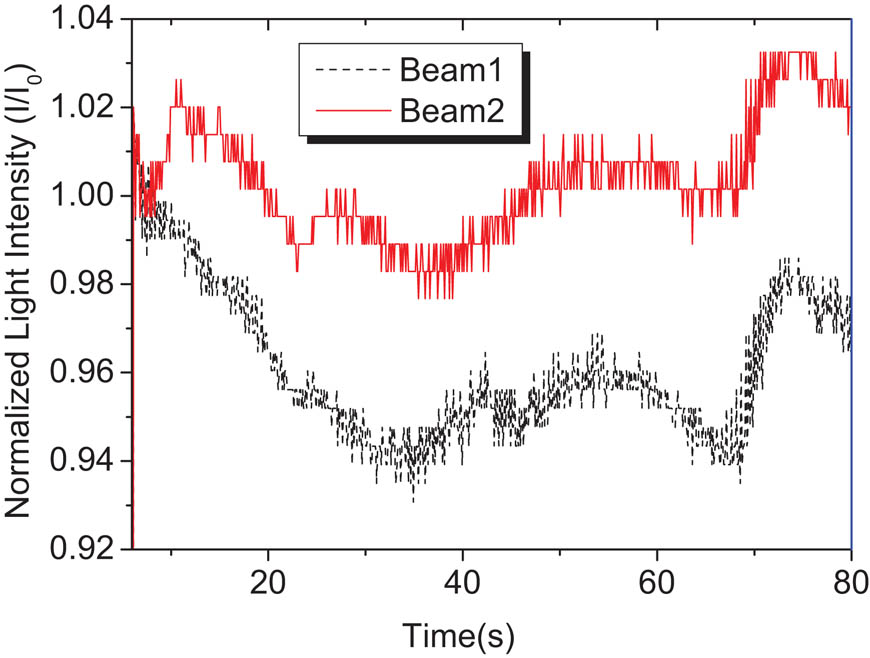
TBC signal of pure polymer P2 at zero electric field.
4 Conclusions
Non-conjugated multi-functionalized all-optical PR polymers are presented by ROMP using Grubbs II catalyst as an initiator. The polymers have a higher number-average molecular weight (Mn) than 8.5 × 104 and the Tg of 52°C and 45°C close to the room temperature, which is advantageous to the PR orientation enhancement. The good thermal stability and excellent solubility in THF allow us to fabricate the optically transparent films through solution-casting without any plasticizer. The evident all-optical PR performance at 633 nm is achieved at zero electrical field from the single-component polymers, affording more easy, safe and convenient PR practical application.
Acknowledgments
The authors greatly acknowledge the financial supports from the National Natural Science Foundation of China (NSFC, No. U1304212, 51603236) and the Development Foundation for Distinguished Junior Researchers at Zhengzhou University (No. 1421320043).
References
(1) Ostroverkhova O, Moerner WE. Organic photorefractives: mechanisms, materials, and applications. Chem Rev. 2004;104(7):3267–314.10.1021/cr960055cSearch in Google Scholar
(2) Day D, Gu M, Smallridge A. Rewritable 3D bit optical data storage in a PMMA-based photorefractive polymer. Adv Mater. 2001;13(12–13):1005–7.10.1002/1521-4095(200107)13:12/13<1005::AID-ADMA1005>3.0.CO;2-7Search in Google Scholar
(3) Tay S, Blanche PA, Voorakaranam R, Voorakaranam P, Tunc AV, Lin W, et al. An updatable holographic three-dimensional display. Nature. 2008;451:694–8.10.1038/nature06596Search in Google Scholar
(4) Xu SG, Fang C, Wu YZ, Wu WB, Guo Q, Zeng J, et al. Photorefractive hyper-structured molecular glasses constructed by calix[4]resorcinarene core and carbazole-based methine nonlinear optical chromophore. Dyes Pigm. 2017;142:8–16.10.1016/j.dyepig.2017.03.001Search in Google Scholar
(5) Marder SR, Kippelen B, Jen AKY, Peyghambarian N. Design and synthesis of chromophores and polymers for electro-optic and photorefractive applications. Nature. 1997;388:845–51.10.1038/42190Search in Google Scholar
(6) Talarico M, Golemme A. Optical control of orientational bistability in photorefractive liquid crystals. Nat Mater. 2006;5:185–8.10.1038/nmat1578Search in Google Scholar
(7) You W, Cao SK, Hou ZJ, Yu LP. Fully Functionalized Photorefractive Polymer with Infrared Sensitivity Based on Novel Chromophores. Macromolecules. 2003;36(19):7014–9.10.1021/ma034587oSearch in Google Scholar
(8) Zong WS, Wang S, Li J, Wang JT, Li MM, Liu YL, et al. An all-optical photorefractive miktoarm star polymer synthesized via a combination of RAFT polymerization and click reaction. React Funct Polym. 2019;143:104321–9.10.1016/j.reactfunctpolym.2019.104321Search in Google Scholar
(9) Zong WS, Wang LX, Guo Q, Li J, Wu WB, Liu YL, et al. A calix[4]resorcinarene-based hyper-structured molecule bearing disperse red 1 as the chromophore with enhanced photorefractive performance under non-electric field. Dyes Pigm. 2019;160:579–86.10.1016/j.dyepig.2018.08.050Search in Google Scholar
(10) Chen YW, He YK, Wang F, Chen HY, Gong QH. Synthesis and characterization of bi-functional photorefractive polymers. Polymer. 2001;42:1101–7.10.1016/S0032-3861(00)00467-5Search in Google Scholar
(11) Shi J, Huang MM, Xin YR, Chen ZJ, Gong QH, Xu SG, et al. Synthesis and characterization of a series of carbazole-based monolithic photorefractive molecules. Mater Lett. 2005;59:2199–203.10.1016/j.matlet.2005.02.066Search in Google Scholar
(12) Zhang L, Shi J, Jiang ZW, Huang MM, Chen ZJ, Gong QH, et al. Photorefractive performance of hyperstructured cyclotriphosphazene molecular glasses containing carbazole moieties. Adv Funct Mater. 2008;18:362–8.10.1002/adfm.200700257Search in Google Scholar
(13) Zhang L, Xu SG, Yang Z, Cao SK. Photorefractive effect in triphenylamine-based monolithic molecular glasses with low Tg. Mater Chem Phys. 2011;126:804–10.10.1016/j.matchemphys.2010.12.038Search in Google Scholar
(14) Shi J, Jiang ZW, Cao SK. Synthesis of carbazole-based photorefractive polymers via post-azo-coupling reaction. React Funct Polym. 2004;59:87–91.10.1016/j.reactfunctpolym.2003.12.010Search in Google Scholar
(15) Zhang L, Huang MM, Jiang ZW, Yang Z, Chen ZJ, Gong QH, et al. A carbazole-based photorefractive polyphosphazene prepared via post-azo-coupling reaction. React Funct Polym. 2006;66:1404–10.10.1016/j.reactfunctpolym.2006.04.003Search in Google Scholar
(16) Shi J, Jiang ZW, Cao SK. A postfunctionalization strategy to develop carbazole-based photorefractive polymers with low Tg. React Funct Polym. 2005;62:223–230.10.1016/j.reactfunctpolym.2004.11.006Search in Google Scholar
(17) Shi J, Huang MM, Chen ZJ, Gong QH, Cao SK. Carbazole-based azo group-containing single component polymer exhibiting photorefractive performance. J Mater Sci. 2004;39:3783–5.10.1023/B:JMSC.0000030738.49806.8dSearch in Google Scholar
(18) Zhang L, Shi J, Huang MM, Jiang ZW, Yang Z, Chen ZJ, et al. Photorefractive properties of polyphosphazenes containing carbazole-based multifunctional chromophores. Polymer. 2008;49:2107–14.10.1016/j.polymer.2007.09.038Search in Google Scholar
(19) Hawker CJ, Wooley KL. The convergence of synthetic organic and polymer chemistries. Science. 2005;309:1200–5.10.1126/science.1109778Search in Google Scholar PubMed
(20) Nuyken O, Pask S. Ring-opening polymerization—an introductory review. Polymers. 2013;5:361–403.10.3390/polym5020361Search in Google Scholar
(21) Biswas S, Belfield K, Das R, Ghosh S, Hebard A. Superparamagnetic nanocomposites templated with pyrazole-containing diblock copolymers. Polymers. 2012;4:1211–25.10.3390/polym4021211Search in Google Scholar
(22) Suriboot J, Bazzi H, Bergbreiter D. Supported catalysts useful in ring-closing metathesis, cross metathesis, and ring-opening metathesis polymerization. Polymers. 2016;8:1401–23.10.3390/polym8040140Search in Google Scholar
(23) Cui J, Yang JX, Li YG, Li YS. Synthesis of high performance cyclic olefin polymers (COPs) with ester group via ring-opening metathesis polymerization. Polymers. 2015;7:1389–409.10.3390/polym7081389Search in Google Scholar
(24) Liaw DJ, Wang KL, Lee KR, Lai JY. Ring-opening metathesis polymerization of new norbornene-based monomers containing various chromophores. J Polym Sci Part A: Polym Chem. 2006;45:3022–31.10.1002/pola.22056Search in Google Scholar
(25) Grubbs RH. Olefin-metathesis catalysts for the preparation of molecules and materials (Nobel Lecture 2005). Adv Synth Catal. 2007;349:34–40.10.1002/adsc.200600523Search in Google Scholar
(26) Pei HY, Li W, Liu YL, Wang DF, Wang J, Shi J, et al. Ring-opening metathesis polymerization of norbornene derivatives for multifunctionalized all-optical photorefractive polymers with a non-conjugated main chain. Polymer. 2012;53:138–44.10.1016/j.polymer.2011.11.018Search in Google Scholar
(27) Andrzej M, Jaroslaw M, Piotr P, Marcin Z. Photorefractive-like all-optical switching in nematic-photoconducting polymer liquid crystal cell. Mol Cryst Liq Cryst. 2008;489:119–34.10.1080/15421400802219718Search in Google Scholar
(28) Lee WK, Chan TS. Photorefractive hologram writing with high modulation depth in photovoltaic media under different boundary conditions. Opt Commun. 2008;281:5884–8.10.1016/j.optcom.2008.08.015Search in Google Scholar
(29) Liu YL, Pei HY, Zhang L, Shi J, Cao SK. Advances in organic all-optical photorefractive materials. Macromol Symp. 2012;317–318:227–39.10.1002/masy.201200006Search in Google Scholar
(30) Kim DW, Moon H, Park SY, Hong S. Synthesis of photoconducting nonlinear optical side-chain polymers containing carbazole derivatives. React Funct Polym. 1999;42:73–86.10.1016/S1381-5148(98)00062-5Search in Google Scholar
(31) Liaw DJ, Wang KL, Lee KR, Lai JY. Ring-opening metathesis polymerization of new norbornene-based monomers containing various chromophores. J Polym Sci. 2007;45:3022–31.10.1002/pola.22056Search in Google Scholar
(32) Ribierre JC, Cheval G, Huber F, et al. Direct comparison of mechanical and electro-optic responses of a low Tg photorefractive doped polymer. J Appl Phys. 2002;91:1710–2.10.1063/1.1428788Search in Google Scholar
© 2020 Haiyan Pei et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery


