Abstract
The polyamide 12 (PA12) with different crystal forms is prepared with three crystallization paths. The crystal structures and corresponding thermal properties are systematically investigated. The results reveal that an α-form and a mixed (α + γ)-form of PA12 can be obtained by casting at 30°C and (40–80°C), respectively. Meanwhile, the γ-form of PA12 can be obtained by both casting at 90°C and slow melt cooling. However, the γ′-form is obtained only by melt quenching. Both the γ and γ′ forms of PA12 exhibit a single melting peak, whereas the α-form exhibits two melting peaks. The higher peak is attributed to the melting of γ-PA12, which originates from the melting–recrystallization of the α-PA12. It is found that the tensile properties of PA12 depend on the crystal forms. Both the γ and γ′-PA12 are strong and tough polymer materials, while α-PA12 is a strong but brittle polymer material.
1 Introduction
A class of linear polymers containing repeated polarity amide group (–NHCO–) in the main chain is called polyamide (PA) (1). Nylon 12 (PA12) is an important material with excellent physical properties such as toughness, abrasion resistance, and good oil resistance, which are attributed to the amide groups in the molecular and the hydrogen bonds between the adjacent molecular chains (2,3,4,5,6). The crystal form of PA12 is similar to those of other polyamides, which mainly contain four kinds of crystal forms: α, α′, γ, γ′ (7). The α-form can be obtained under specific conditions, such as high pressure or drawing (8,9). The α-form of PA12 can be produced by cooling above 500 MPa from the melt or by annealing the γ′-form at high pressure (above 500 MPa). The γ-form can be obtained by cooling slowly from the melt, annealing the α-form at high temperature (above 150°C), or annealing γ′-form above 110°C. Meanwhile, the structure of the γ′-form, similar to that of the γ-form, can be obtained by quenching from the melt (10), drawing the α-form above 70°C, or drawing the γ-form above 50°C.
The γ-form with a hexagonal structure is the most common one and can be easily obtained. It was reported that the γ-form showed only one strong reflection with the d-spacing at about 0.42 nm (11,12). The monoclinic structure has been assigned to the α-form, which exhibits two distinct peaks corresponding to d-spacing at about 0.37 and 0.44 nm (10). The γ′-form can be regarded as variant of the γ-form due to the similar structure possessed by both γ′-form and γ-form (13). It must be noted that, the thermal treatment under pressure can transform the γ′ phase into the α phase. On the contrary, the γ-form cannot be transformed into the α-form even under high pressure (14,15).
One of the main characteristics of Nylons is the strong interchain interaction that arises from the hydrogen bonding between amide groups (16,17). Long-alkane-segment Nylons have high CH2/amide ratios. The hydrogen bond structures of α and γ forms in PA12 are depicted in Figure 1. In the γ-form, the chains are aligned parallel and the full hydrogen bonding requires some twisting of the chains. The extended CH2 zigzags of each chain are in the plane of a molecular sheet, while the amide plane is twisted out of the polyethylene plane by about 60° (18). In the α-form, the molecular chains are antiparallel, and the full hydrogen bonding between extended chains can be easily attained (5,9).
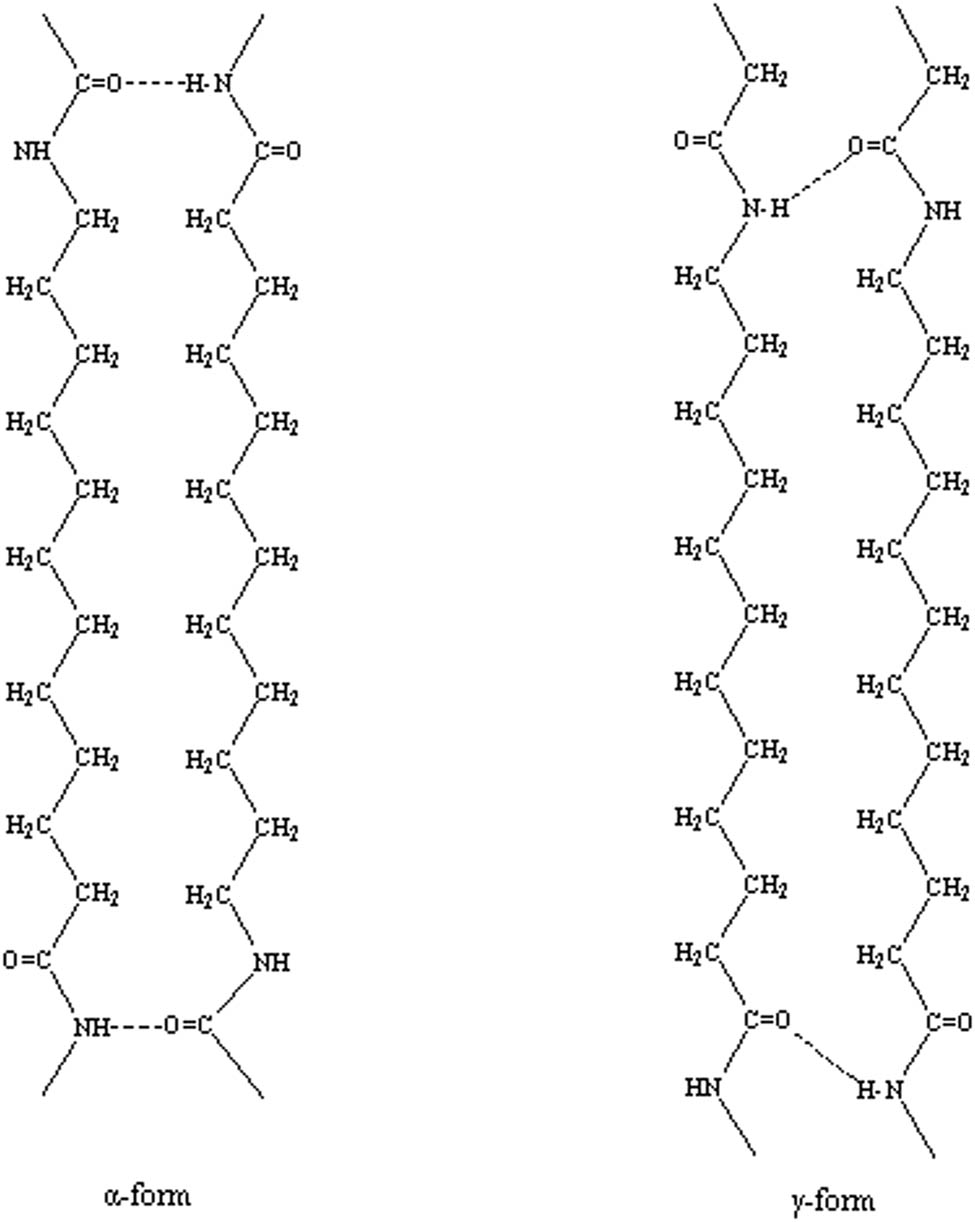
The hydrogen bond structures of α and γ forms in Nylon 12.
Although the crystal formation conditions of PA12 and the transformation between different crystal forms have been well researched, the crystal structure characteristics under the application environment of less than 100°C are still unclear. This report is dedicated to studying the crystal structure and thermal properties of PA12, which undergoes the isothermal crystallization in the range from room temperature to the temperature close to the melting point. The PA12 samples were prepared by three methods: solution casting at different temperatures, slow melt cooling, and melt quenching. The crystal and chemical structure, thermal properties, and mechanical properties of prepared PA12 samples were, respectively, characterized by wide-angle X-ray diffraction (WAXD), Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), and tensile tests, which may provide more theoretical support for the basic research and application of PA12.
2 Experimental
2.1 Materials and sample preparation
The PA12 pellets used in this work were purchased from Sigma-Aldrich and were dried at 80°C for 24 h in a vacuum oven to eliminate the moisture before processing.
The sample of solution casting was prepared by dissolving the PA12 in the phenol/ethanol (70/30) mixture. The concentration of solution was 40 mg/mL. In order to dissolve PA12 as soon as possible, an ultrasonic cleaner was used for 1 h. The casting temperature was selected at 30, 40, 50, 60, 80, and 90°C. The sample of melt cooling was obtained by cooling naturally from 220°C to room temperature. By contrast, the sample of melt quenching was prepared by cooling down rapidly in ice–water mixture from 220°C.
2.2 WAXD
The crystal structures were measured with a D8 DISCOVER diffractometer operated at 50 kV and 1,000 mA. The X-ray sources were nickel-filtered Cu Kα radiation (λ = 1.5418 Å). The WAXD data were collected from 3 to 32°. The specimens were fixed on the equipment with a hot stage and a hot-stretching stage.
2.3 DSC
The DSC (NETZSCH) thermograms of PA12 samples were measured from 5 to 220°C under nitrogen with a heating rate of 5°C/min.
2.4 FTIR
FTIR spectroscopy measurements were carried out with a Bruker TENSOR II FTIR spectrometer. The equipment was operated in transmission mode with 64 scans per sampling at a resolution of 4 cm−1 in the wavenumber range between 4,000 and 400 cm−1.
2.5 Tensile test
A dumbbell-shaped specimen was cut from the premade PA12 film. The tensile tests of tailored specimens were conducted on a UTM6104. All tests were carried out at room temperature with a speed of 10 mm/min according to GB/T 1040.2-2006.
3 Results and discussion
3.1 The different crystal forms and crystal transition analysis
The 2D-WAXD patterns and the corresponding 1D-WAXD curves of PA12 with three different crystallization processes are shown in Figures 2 and 3, respectively. The d-spacings of the three PA12 forms calculated from Figure 3 are summarized in Table 1.

The 2D-WAXD patterns of PA12 (a) α-form sample prepared by solution casting at 30°C, (b) γ-form sample prepared by melt cooling, and (c) γ′-form sample prepared by melt quenching.
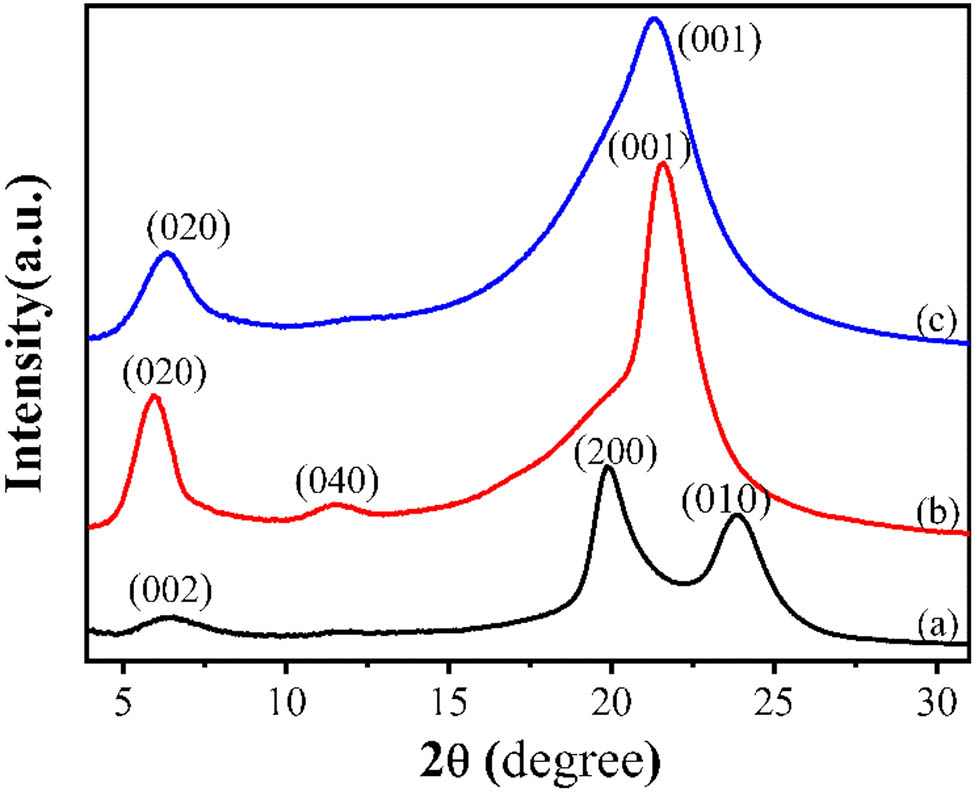
The WAXD patterns of PA12 of (a) α-form sample prepared by solution casting at 30°C, (b) γ-form sample prepared by melt cooling, and (c) γ′-form sample prepared by melt quenching.
The d-spacings of three different crystallization processes of PA12
| Form | Crystal plane | |||
|---|---|---|---|---|
| α-form | (002) plane | (200) plane | (010) plane | |
| d (nm) | 1.375 | 0.446 | 0.373 | |
| γ-form | (020) plane | (001) plane | ||
| d (nm) | 1.477 | 0.411 | ||
| γ′-form | (020) plane | (001) plane | ||
| d (nm) | 1.397 | 0.417 | ||
The sample obtained by solution casting at low temperature (30°C) shows a weak diffraction (002) at about 6.2° and two strong diffractions (200) and (010) at 20.2° and 23.1° (Figure 3a), corresponding to the triclinic α-form crystal (19). For the sample prepared by melt cooling (Figure 3b), two strong diffractions (020) and (001) and one weak diffraction (040) exist, belonging to the pseudohexagonal γ-form crystal. Two diffractions of (020) and (001) corresponding to the γ‘-form can be observed in the sample prepared by melt quenching (Figure 3c). It can be seen clearly that the diffraction peak (040) of γ-PA12 is more obvious than that of γ′-PA12. In addition, the diffraction ring of γ-form is sharper than that of γ′-form, indicating the lower crystallinity of γ′-form that may be caused by the rapid freezing of the molecular chain and imperfect crystallization during melt quenching.
The 2D-WAXD patterns and the corresponding 1D-WAXD curves of PA12 at different casting temperatures are, respectively, shown in Figures 4 and 5. With the casting temperature increasing, the (200) and (010) planes gradually become blurred, and two new diffraction peaks of (001) and (020) planes appear (Figure 4). This change can be clearly seen in Figure 5. Only α-form exists when the casting temperature is 30°C and the diffraction peaks from left to right are (002), (200), and (010), respectively. With the increase in casting temperature, the diffraction peaks (020) and (001) of γ-form appear and the intensity gradually increases, while the intensity of the diffraction peaks (200) and (010) of α-form gradually decreases, suggesting the existence of the mixed (α + γ)-form when the casting temperature is over 30°C. When the temperature increases to 80°C, a small amount of α-form still exists although the peaks are not visible, which is evidenced by the DSC results in Figure 7. At 90°C, only γ-form exists and the diffraction peaks from left to right are (020), (040), and (001), respectively. It can be concluded that it is difficult for the α-form to transform to the γ-form below the glass-transition temperature (Tg) of PA12 (around 50°C). However, this process can be accelerated by the segment motion beyond Tg.

The 2D-WAXD patterns of PA12 at different casting temperatures.
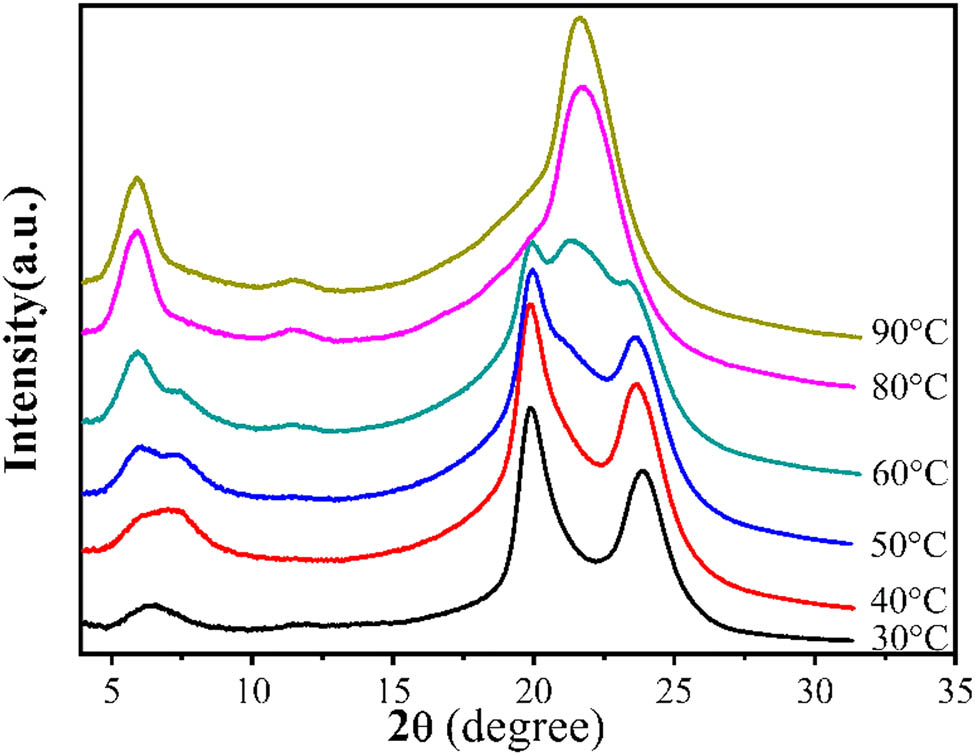
The WAXD patterns of PA12 at different casting temperatures.
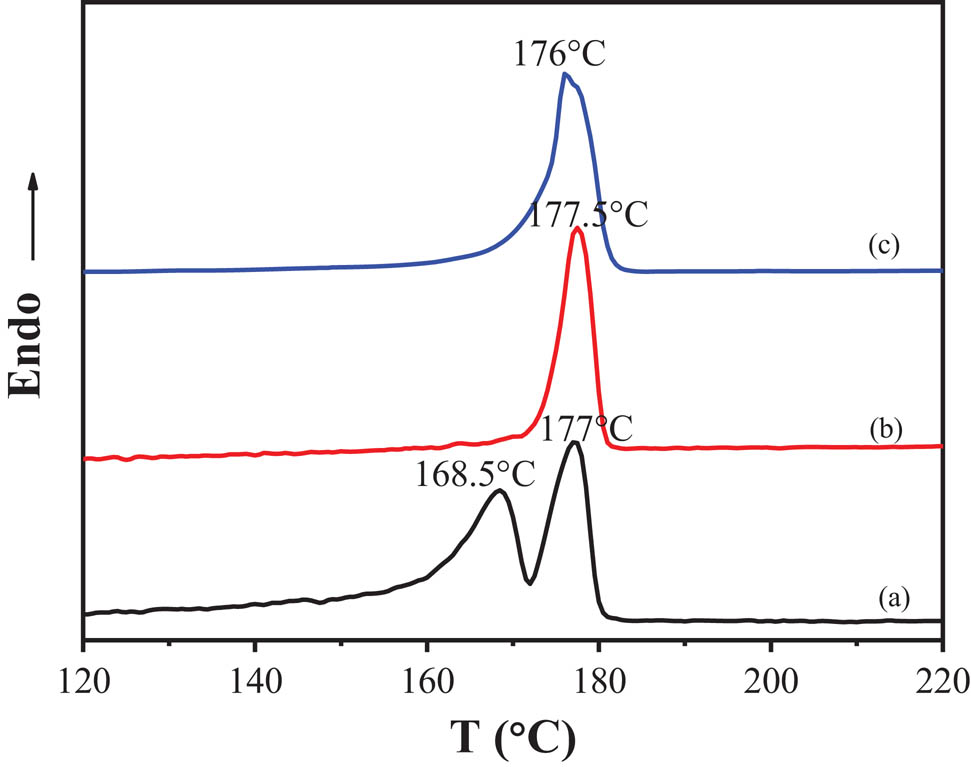
The DSC thermograms of PA12 (a) α-form sample prepared by solution casting at 30°C, (b) γ-form sample prepared by melt cooling, and (c) γ′-form sample prepared by melt quenching.
The phenomenon of crystalline evolution of PA12 casting at different temperatures suggests the influence of molecular motion on crystallization. Solution crystallization mainly includes the following three steps (20). First, the solvent enters the polymer by diffusion. Then, the intermolecular force is broken by the interaction between the solvent and polymer, and the mobility of the segment is improved. Finally, if the interaction between the polymer and the solvent is sufficiently strong, the polymer molecular chain may undergo rearrangement into the crystal state. It is supposed that the formation of α-form takes place when the temperature is below Tg (the glass-transition temperature of PA12 is around 50°C), and the rapid volatilization of solvent and high temperature above Tg are beneficial to the appearance of the more stable γ-form (21).
3.2 Thermal properties
The DSC curves of PA12 obtained from three different crystallization processes are shown in Figure 6. It can be observed that γ-form and γ′-form have only one melting endothermic peak. Interestingly, the α-form exhibits double melting endothermic peaks (Figure 6a), indicating the existence of either a mixed crystal structure of PA12 or a process related to melting–recrystallization during heating. It has been reported that α-form undergoes crystal transformation during the heating and melting process. After the α-form melts at 168.5°C, the molecular chain recombines and recrystallizes into the γ-form. Then the γ-form melts at 177°C, resulting in multiple melting behaviors (22). It can be calculated that the melting enthalpy of α-form, γ-form, and γ′-form is, respectively, 52.7, 51.6, and 43.4 J/g, and the crystallinity of those forms is 21.5%, 21.1%, and 17.7%, respectively. In conclusion, the α-form has a lower melting point than the γ-form and tends to transform into the γ-form during the heating process, which brings about the multiple melting behaviors in DSC.

The DSC thermograms of PA12 at different casting temperatures.
Figure 7 shows the DSC thermograms of PA12 that are cast at different temperatures. The ratio of the melting enthalpy of the first melting endothermic peak at different casting temperatures is shown in Table 2. It can be observed that there are two melting endotherms in α and (α + γ) forms, hereafter identified as peak I (left) and II (right). The area of peak I decreases with an increase in casting temperature, whereas that of peak II increases. There are still two melting endotherms when the casting temperature rises to 80°C, indicating that a small amount of α-form still exists. At the casting temperature of 90°C, there is only one melting endotherm (peak II), suggesting that only γ-form is left. The above experimental results confirm the supposition that the α-form crystallization of PA12 takes place predominantly when the casting temperature is below Tg, while the more stable γ-form tends to appear above Tg (23,24).
The ratio of enthalpy of fusion of the first melting endothermic peak of PA12 at different casting temperatures
| Casting temperature (°C) | 30 | 40 | 50 | 60 | 80 | 90 |
|---|---|---|---|---|---|---|
| The ratio of enthalpy of first melting endothermic peak (%) | 65.1 | 61.3 | 57.2 | 40.4 | 1.7 | 0 |
3.3 Fourier transform infrared (FTIR) spectroscopy
The FTIR absorption spectra of PA12 from three different crystallization processes are demonstrated in Figure 8. The hydrogen-bonded N–H stretching at 3,299 cm−1 in γ-PA12 and γ′-PA12 shifts to 3,315 cm−1 in α-PA12 form. The moderate amide B bond at 3,098 cm−1 in γ-PA12 and γ′-PA12 shifts to 3,092 cm−1 with weak intensity in α-PA12 (25). The amide II band (C–N stretching vibration plus CO–NH bending) appears at 1,561 cm−1 in γ-PA12, 1,557 cm−1 in γ′-PA12, and 1,545 cm−1 in α-PA12. The amide VI band (C═O out-of-plane bending) at 626 cm−1 in γ-PA12 and γ′-PA12 shifts to 577 cm−1 in α-PA12. The amide V band (N–H out-of-plane bending) at 680 cm−1 is observed in α-PA12 but not in the γ and γ′ forms (21,26,27).
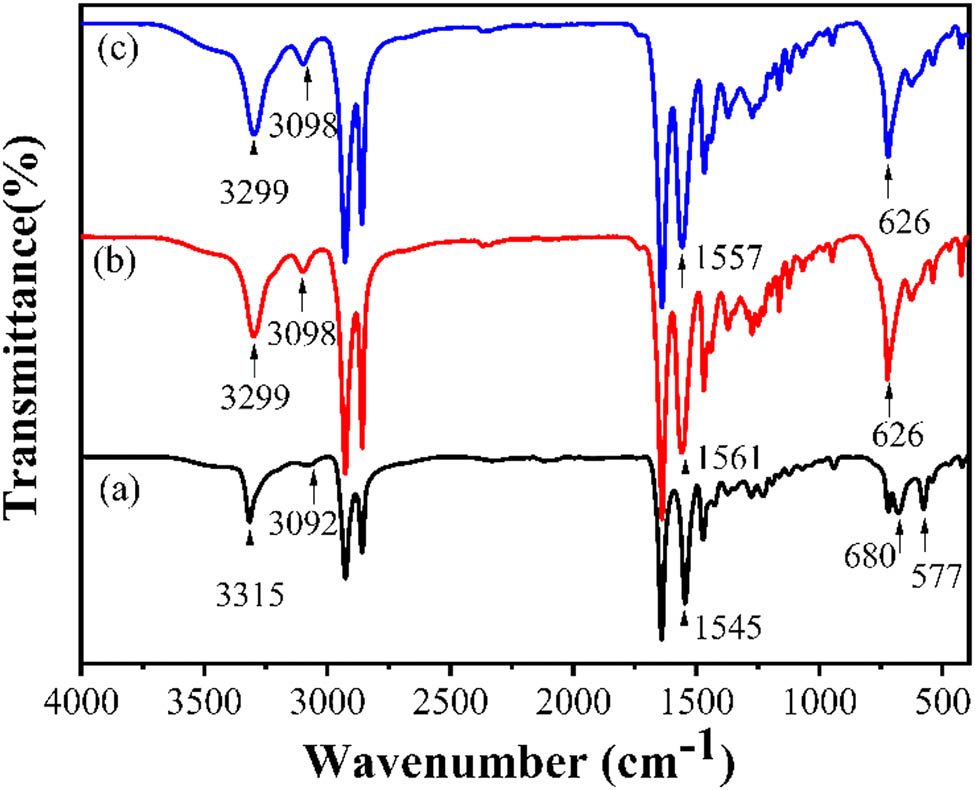
The FTIR diagrams of PA12 (a) α-form sample prepared by solution casting at 30°C, (b) γ-form sample prepared by melt cooling, and (c) γ′-form sample prepared by melt quenching.
The FTIR diagrams of PA12 at different casting temperatures are shown in Figure 9. The characteristic peaks have some deviation in the different crystal forms. The sample cast at 30°C shows the absorption bands at 680 and 577 cm−1, which are attributed to the α-form. On the contrary, the absorption band at 624 cm−1 belonging to the γ-form does not appear. The sample cast at 90°C shows the characteristic bands of the γ-form. Samples cast at temperatures from 40°C to 80°C show the mixed patterns of α and γ forms. The amide V band (N–H out-of-plane bending) at 680 cm−1 is observed in α and (α + γ) forms but not in the γ-form. The amide VI band (C═O out-of-plane bending) at 626 cm−1 in γ-PA12 shifts to 577 cm−1 in α-PA12, and the (α + γ)-form exhibits the amide VI band at 626 and 577 cm−1.

The FTIR diagrams of PA12 at different casting temperatures.
3.4 Mechanical properties
The mechanical properties of the three crystal forms of PA12 are shown in Table 3. The tensile strength of γ′-PA12 is 44.1 MPa, and the elongation at break is 446%. Moreover, the tensile strength of γ-PA12 is 63.9 MPa, and the elongation at break is 618%. The high tensile strength and large elongation at break mean that the γ-PA12 and γ′-PA12 are strong and tough polymer materials. However, the tensile strength and the elongation at break of α-PA12 are only separately 25.2 MPa and 18%, indicating that the α-PA12 is a strong but brittle polymer material. The mechanical properties of γ-PA12 are better than those of γ′-PA12, which are mainly attributed to the most stable γ-form obtained by cooling slowly from the melt, where the molecular chains are closely arranged and the crystallinity is higher. The result is consistent with the conclusion that the γ-form is the most stable crystal phase for PA12 (28).
The mechanical properties of three different forms of PA12
| Sample | Tensile strength (MPa) | Elastic modulus (MPa) | Elongation at break (%) |
|---|---|---|---|
| γ-PA12 | 63.9 | 411.8 | 618 |
| γ′-PA12 | 44.1 | 325.7 | 446 |
| α-PA12 | 25.2 | 308.5 | 18 |
4 Conclusions
PA12 samples with different crystal forms prepared by solution casting, slow melt cooling, and melt quenching were investigated by WAXD, DSC, FTIR, and tensile test. It is found that the γ-form crystal of PA12 can be obtained by both slow melt cooling and casting at 90°C, whereas the γ′-form crystal of PA12 belonging to a metastable structure of the γ-form can be obtained by melt quenching. The γ-form and (α + γ)-form crystals of PA12 are, respectively, obtained by casting at 30°C and higher temperatures (from 40°C to 80°C). The α-form of PA12 has a lower melting point than the γ-form and tends to transform into the γ-form on heating to the temperature near the melting point. The amide VI band (C═O bending) at 626 cm−1 in γ-PA12 and γ′-PA12 shifts to 577 cm−1 in α-PA12. The amide V band (N–H bending) at 680 cm−1 is observed in α-PA12 but not in γ and γ′ forms. The tensile strength, elastic modulus, and elongation at break of γ-PA12 and γ′-PA12 are much larger than those of α-PA12, in which the γ-PA12 and γ′-PA12 show strong and tough feature, whereas α-PA12 shows strong and brittle feature. It reveals that the different crystal forms of PA12 have different responses to the tensile properties. Therefore, it is possible to fabricate PA12 with advanced mechanical performance by tailoring the crystal forms.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant No. U1504527 and 21803060) and the National Key Research and Development Program of China Grant No. 2018YFD0400702.
References
(1) Kinoshita Y. An investigation of the structures of polyamide series. Macromol Chem Phys. 1959;33(1):1–20.10.1002/macp.1959.020330101Search in Google Scholar
(2) Marchildon K. Polyamides-still strong after seventy years. Macromol React Eng. 2011;5(1):22–54.10.1002/mren.201000017Search in Google Scholar
(3) Wang LH, Calleja FJB, Kanamoto T, Poter RS. The characterization and properties of Nylon 13,13. Polymer. 1993;34(22):4688–91.10.1016/0032-3861(93)90702-CSearch in Google Scholar
(4) Prieto A, Iribarren I, Muñoz-Guerra S. Structural studies of Nylon 13, 13. J Mater Sci. 1993;28(15):4059–62.10.1007/BF00351232Search in Google Scholar
(5) Jiang T, Liu M, Fu P, Wang YD, Fang YL, Zhao QX. Melting behavior, isothermal and nonisothermal crystallization kinetics of Nylon 1111. Polym Eng Sci. 2009;49(7):1366–74.10.1002/pen.21269Search in Google Scholar
(6) Cui X, Li W, Yan D. Investigation of odd–odd nylons based on undecanedioic acid. 2: Crystal structures. Polym Eng Sci. 2005;45(12):1673–9.10.1002/pen.20378Search in Google Scholar
(7) Mathias LJ, Johnson CG. Solid-state NMR investigation of Nylon 12. Macromolecules. 1991;24(23):6114–22.10.1021/ma00023a011Search in Google Scholar
(8) Ramesh C. Crystalline transitions in Nylon 12. Macromolecules. 1999;32(17):5704–6.10.1021/ma990494oSearch in Google Scholar
(9) Kamal T, Park SY, Park JH, Chang YW. Structural evolution of poly(ether-b-amide12) elastomers during the uniaxial stretching: An in situ wide-angle X-ray scattering study. Macromol Res. 2012;20(7):725–31.10.1007/s13233-012-0109-zSearch in Google Scholar
(10) Aharoni SM. n-Nylons: their synthesis, structure and properties. Chichester: John Wiley & Sons; 1997; Chapters 1.3 and 2.12.Search in Google Scholar
(11) Li L, Koch MHJ, de Jeu WH. Crystalline structure and morphology in Nylon-12: a small-and wide-angle X-ray scattering study. Macromolecules. 2003;36(5):1626–32.10.1021/ma025732lSearch in Google Scholar
(12) Wang D, Shao C, Zhao B, Zhao BJ, Bai LG, Wang X, Yan TZ. Deformation-induced phase transitions of polyamide 12 at different temperatures: an in situ wide-angle X-ray scattering study. Macromolecules. 2010;43(5):2406–12.10.1021/ma1000282Search in Google Scholar
(13) Murthy NS. Hydrogen bonding, mobility, and structural transitions in aliphatic polyamides. J Polym Sci Part B (Polym Phys). 2006;44(13):1763–82.10.1002/polb.20833Search in Google Scholar
(14) Hiramatsu N, Haraguchi K, Hirakawa S. Study of transformations among α, γ and γ′ forms in Nylon 12 by X-ray and DSC. Japanese J Appl Phys. 1983;22(2R):335.10.1143/JJAP.22.335Search in Google Scholar
(15) Hiramatsu N, Hashida S, Hirakawa S. Formation of α form Nylon 12 under high pressure. Japanese J Appl Phys. 1982;21(4R):651.10.1143/JJAP.21.651Search in Google Scholar
(16) Botta A, de Candia F, Palumbo R. Glass transition in aliphatic polyamides. J Appl Polym Sci. 1985;30(4):1669–77.10.1002/app.1985.070300432Search in Google Scholar
(17) Cannon CG. The infra-red spectra and molecular configurations of polyamides. Spectrochim Acta. 1960;16(3):302–19.10.1016/0371-1951(60)80092-6Search in Google Scholar
(18) Arimoto H, Ishibashi M, Hirai M, Chatani Y. Crystal structure of the γ-form of Nylon 6. J Polym Sci Part A Gen Pap. 1965;3(1):317–26.10.1002/pol.1965.100030132Search in Google Scholar
(19) Fernández CE, Bermúdez M, Versteegen RM, Meijer EW, Vancso GJ, Muñoz-Guerra S. An overview on 12-polyurethane: synthesis, structure and crystallization. Eur Polym J. 2010;46(11):2089–98.10.1016/j.eurpolymj.2010.09.018Search in Google Scholar
(20) Desai AB, Wilkes GL. Solvent-induced crystallization of polyethylene terephthalate. J Polym Sci Polym Symp. 2010;46(1):291–319.10.1002/polc.5070460123Search in Google Scholar
(21) Ishikawa T, Nagai S, Kasai N. Effect of casting conditions on polymorphism of Nylon-12. J Polym Sci Polym Phys Ed. 1980;18(2):291–9.10.1002/pol.1980.180180212Search in Google Scholar
(22) Ishikawa T, Nagai S, Kasai N. Thermal behavior of α Nylon-12. J Polym Sci Polym Phys Ed. 1980;18(6):1413–19.10.1002/pol.1980.180180619Search in Google Scholar
(23) Ishikawa T, Sugihara A, Hamada T, Nagai S, Yasuoka N, Kasai N. Change of fine texture of Nylon 12 by drawing. Nippon Kagaku Kaishi. 1973;9:1744–51.10.1246/nikkashi.1973.1744Search in Google Scholar
(24) Gordon GA. Glass transition in nylons. J Polym Sci Part A-2 Polym Phys. 1971;9(9):1693–702.10.1002/pol.1971.160090911Search in Google Scholar
(25) Miyazawa T. The characteristic band of secondary amides at 3100 cm−1. J Mol Spectrosc. 1960;4(1):168–72.10.1016/0022-2852(60)90076-XSearch in Google Scholar
(26) Simak VP. Spektroskopische untersuchungen der kristallinen modifikationen von polyamid-6. Appl Macromol Chem Phys. 1973;28(1):75–85.10.1002/apmc.1973.050280105Search in Google Scholar
(27) Abu-Isa I. α-γ transition in Nylon 6. J Polym Sci Part A-1 Polym Chem. 1971;9(1):199–216.10.1002/pol.1971.150090119Search in Google Scholar
(28) Gogolewski S, Czerntawska K, Gastorek M. Effect of annealing on thermal properties and crystalline structure of polyamides. Nylon 12 (polylaurolactam). Colloid Polym Sci. 1980;258(10):1130–6.10.1007/BF01382456Search in Google Scholar
© 2020 Ning Ma et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery


