Abstract
A novel amphoteric polyacrylamide (PDAA) was prepared by inverse emulsion polymerization. The influence of its cationic degree and molecular weight on retention property was discussed. Then, the chemical structure and micromorphology of the obtained PDAA were characterized by Fourier infrared spectrum (FTIR), NMR hydrogen spectrum (1H NMR), gel permeation chromatography (GPC), and scanning electron microscope (SEM). Finally, the synergistic retention effects of polydimethyldiallylammonium chloride (PDADMAC), cationic starch (CS), cationic guar gum (CHPG), cationic chitosan (CTS), and polyamine (PA) on the novel PDAA were investigated. The results showed that the optimum cationic degree and molecular weight of PDAA were 25% and 4 million, respectively. The chemical structure of PDAA was confirmed by FTIR, 1H NMR, and GPC. SEM showed that the particle size of PDAA was between 150 and 600 nm, and the particles were very stable because no broken particles were found. In addition, most of the five cationic polymers have good synergistic retention effect on PDAA, and the order of synergistic effect was PDAA/PDADMAC > PDAA/CTS > PDAA/CS > PDAA/CHPG > PDAA/PA.
1 Introduction
Amphoteric polyacrylamide has both positive and negative charge groups, which has good water solubility. The anion group can protect the cation group and repel the “impurity anion” in the pulp system, so that the cation group will not be reacted or neutralized too early. Moreover, amphoteric polyacrylamide has the advantage of being suitable for a wide range of pH values (1,2). The surface of the paper pulp fiber is usually negatively charged and easy to adsorb the heterocations in the system, thus reducing the adsorption with the cation retention aid. In contrast, amphoteric polypropylene amide can preferentially adsorb heterocations in the system, so its retention effect is better than that of cationic polymer (3,4,5).
The polymer prepared by inverse emulsion polymerization has the advantages of fast dissolution and narrow molecular weight distribution (6) and avoids the defects of easy crosslinking and wide molecular weight distribution of products prepared by aqueous polymerization. Many excellent polymers have been prepared by inverse emulsion polymerization. For example, poly[N-(2-hydroxypropyl)methacrylamide] nanogels were synthesized via RAFT inverse miniemulsion polymerization (7). Cationic flocculant poly(AM-DMC)) (CPAM) and poly((methacrylic acid)-co-acrylamide) hydrogel nanoparticles crosslinked with methylenebisacrylamide have been prepared successfully via an inverse emulsion polymerization (8,9). This method can also be used to prepare paper retention aids (10), wastewater treatment agent (11,12,13), super absorbent material for paper (14), oil displacement agent (15), and other products (16,17).
The synergistic effect of retention aids in papermaking refers to that when several retention aids are used together in a certain way, their effect is stronger than the effects of using these additives alone (18). Retention aids and other polymer retention aids usually play a synergistic role when they are used together in the Wet end chemistry of papermaking. The synergistic mechanism mainly depends on the formation of more effective complex or in the form of mutual assistance. One of the retention aids can make up for the adverse effect of the other on the system, so as to show the synergistic effect. In this paper, a new amphoteric polyacrylamide (PDAA) was prepared by inverse emulsion polymerization and its synergistic retention with other cationic polymers was investigated.
2 Experimental
2.1 Materials
White oil, anhydrous ethanol, acetone, dehydrated sorbitan monooleate (Span80), dehydrated sorbitan monostearate (Tween20), acrylamide, acrylic acid, disodium ethylenediamine tetraacetate (EDTA), nonylphenol ethoxylate, ammonium persulfate, sodium bisulfite, and sodium hydroxide are all analytically pure and purchased from Beijing Chemical Reagent Company (Beijing, China). The waste paper deinking pulp with a beating degree of 38 °SR is self-made. Talcum powder is of industrial purity and purchased from Beijing Nanshan Xingwang Talcum Powder Factory (Beijing, China). Dimethyldiallylammonium chloride (DADMAC, 65% aqueous solution) used for the polymerization of PDAA and polydimethyldiallylammonium chloride (PDADMAC, 40% aqueous solution) used for compound cooperative retention with PDAA are purchased from Shandong Luyue Chemical Co., Ltd (Feicheng, China). Cationic starch (CS) is supplied by Mudanjiang spark Auxiliary Factory (Mudanjiang, China). Cationic guar gum (CHPG) is provided by Beijing Tianshi Chemical Co., Ltd (Beijing, China). Cationic chitosan (CTS) is purchased from Dongying Tianhua Biological Auxiliary Co., Ltd (Dongying, China). Polyamine (PA) is provided by Shanghai Hengli Water Treatment Materials Co., Ltd (Shanghai, China).
2.2 Methods
2.2.1 Preparation of sodium acrylate
About 100 g acrylic acid and 120 g water were added into a four-necked flask, which is then cooled to below 15℃ in an ice water bath and stirred. Sodium hydroxide solution with a concentration of 50% was slowly dropped into the four-port flasks until the pH reached 6.8–7.0. Finally, 40% sodium acrylate solution was obtained.
2.2.2 Preparation of novel amphoteric polyacrylamide by inverse emulsion
About 280 g white oil, 16 g Span80, and 4 g Tween20 were mixed and stirred for 10 min to obtain the oil phase. The water phase was prepared by mixing 43.7 g 40% sodium acrylate and 350 g water with acrylamide and DADMAC. The total weight of acrylamide and DADMAC was 332.5 g. To obtain PDAA emulsion with a cationic degree of 10%, 15%, 20%, 25%, and 30%, the weight of DADMAC was selected as 35, 52.5, 70, 87.5, and 105 g, respectively. A certain volume of 1 wt% ammonium persulfate and 3.5 mL of 1 wt% EDTA were added to the system successively and stirred for 5 min.
The water phase was slowly poured into the oil phase by a peristaltic pump. The mass ratio of the water phase to the oil phase is 7:3. The mixture was emulsified with a homogenizer at the speed of 6,000 rpm for 10 min. The emulsion was poured into a flask and charged with nitrogen for 1 h at a constant stirring speed of 300 rpm. The reaction was started by dropping 3.6 mL sodium bisulfite solution with a certain concentration within 1 h. After holding the temperature of 65℃ for 30 min, the PDAA emulsion product was obtained by adding 15 g nonylphenol polyoxyethylene to the system.
The molecular weight of PDAA was controlled by changing the volume of ammonium persulfate solution and the concentration of sodium bisulfite solution. The relevant values have been listed in Table 1.
Volume of ammonium persulfate solution, concentration of sodium bisulfite solution and the molecular weight of obtained PDAA
| Number | Volume of ammonium persulfate solution (mL) | Concentration of sodium bisulfite solution (wt%) | Molecular weight of obtained PDAA (million) |
|---|---|---|---|
| 1 | 3.5 | 3.0 | 2 |
| 2 | 2.8 | 2.6 | 3 |
| 3 | 2.45 | 2.4 | 3.5 |
| 4 | 2.1 | 2.2 | 4 |
| 5 | 1.75 | 2.0 | 4.5 |
2.2.3 Preparation of pulp
The waste cartons were torn up and soaked in normal temperature water for 48 h. The soaked pulp is beaten to 38 SR° by Wally beater at 2% concentration. The paper pulp is then concentrated with 80 mesh nylon cloth and sealed with a plastic bag for future use.
2.2.4 Preparation of handsheets
To make handsheet with a base weight of 60 g/m2, 1.6 g pulp, 0.28 g talcum powder, and 300 g water are mixed evenly. Polymer retention aid with the amount of 0, 0.01, 0.02, 0.03, 0.04, 0.05, and 0.06 wt% based on the weight of the pulp was separately added to the dispersed pulp and continuously stirred for 5 min. The polymer retention aid with a concentration of 500 mg/L was PDAA or a mixture of PDAA and another retention aid (PDADMAC, CTS, CS, CHPG, or PA) in the proportion of 1:0, 1:3, 1:1, 3:1, and 0:1, respectively. At last, the handsheet was formed on a sheet former and dried in a vacuum at 95℃.
2.2.5 Viscosity average molecular weight
The molecular weight of the obtained PDAA was determined by the viscosity method. The PDAA emulsion was precipitated with a mixture of ethanol and acetone with a volume ratio of 1:1. The precipitates were washed repeatedly with the mixture and then filtered and dried. According to GB/T 31246-2014 (19), the intrinsic viscosity (η) of cationic polymers was calculated using equations (1) and (2):
where η is intrinsic viscosity (mL/g), k = 4.75 × 10−3 is an empirical constant, and α = 0.80 is an empirical constant.
2.2.6 Retention rate of paper
The retention performance of polymers was evaluated by the retention rate of paper. The handsheets are dried to constant weight at 105℃, and the retention rate of paper is calculated by the following formula (equation (3)):
where m1 is the dry weight of the handsheets and m2 is the total weight of pulp and talcum powder used to prepare the handsheets.
2.2.7 Yield and monomer residue of PDAA
PDAA emulsion was precipitated, washed, and filtered with 1:1 acetone and ethanol solution and dried to constant weight in a vacuum drying chamber at 50℃. The yield of PDAA is calculated according to equation (4).
where m1 is the mass of the emulsion system before polymerization, c is the total concentration of the three monomers in the emulsion system, and m2 is the mass of PDAA after drying.
The residual acrylamide monomer in PDAA emulsion was extracted by ethanol and determined by high-performance liquid chromatography (HPLC).
2.3 Characterizations
2.3.1 Fourier transformed infrared spectroscopy (FT-IR) measurements
The chemical structure of PDAA was studied using Fourier transform infrared spectroscopy (FTIR) in Perkin Elmer RZX spectrometer using KBr technology, i.e., by making pellets of PDAA powder with KBr. FTIR spectra were recorded in a spectral range of 4,000–400 cm−1 with a resolution of 2 cm−1.
2.3.2 NMR hydrogen spectrum (1H-NMR)
The 1H-NMR spectrum of the dried PDAA sample was determined by a Bruker Avance NEO 600 (Bruker Company, Germany) in solvent of D2O at 500 MHz.
2.3.3 Gel permeation chromatography (GPC)
The molecular weight distribution of PDAA was determined by PL-GPC50 (agilent technologies, USA). Fourteen monodisperse polyethylene oxide (PEO) samples with molecular weights between 0.4 and 0.5 million are used as calibration standards.
2.3.4 Scanning electron microscopy (SEM)
The microstructure of PDAA was observed by scanning electron microscopy (SEM) (Hitachi S4800, Japan) at an accelerating voltage of 5.0 kV. Before imaging, the PDAA emulsion was dried under atmospheric conditions and then placed on a substrate with fixed conductive carbon tape and coated with platinum.
3 Results and discussion
3.1 Chemical structure and micromorphology of PDAA
The chemical structure of the obtained PDAA was analyzed by FTIR. It can be seen from Figure 1 that the broad peaks of 3354.17, 3194.65, and 1663.44 cm−1 are the characteristic absorption peaks of –C–NH2, –N–H and –C═O in –CONH2, while 2933.37 cm−1 is the expansion vibration absorption peak of –CH2, all of which indicates the presence of acrylamide monomers; 1318.06 and 1558.9 cm−1 (obscured by the peak of 1663.44 cm−1) are the characteristic absorption peaks of –COO–, indicating the presence of acrylic monomers; 1451.60 cm−1 is the absence of –CH3 in –N+(CH3)3 symmetrical bending vibration absorption peak, while 1414.54 cm−1 is N–CH3 stretching vibration peak, thus illustrating the existence of DADMAC monomer (21). In conclusion, it can be determined that PDAA has been successfully prepared by reverse emulsion polymerization.
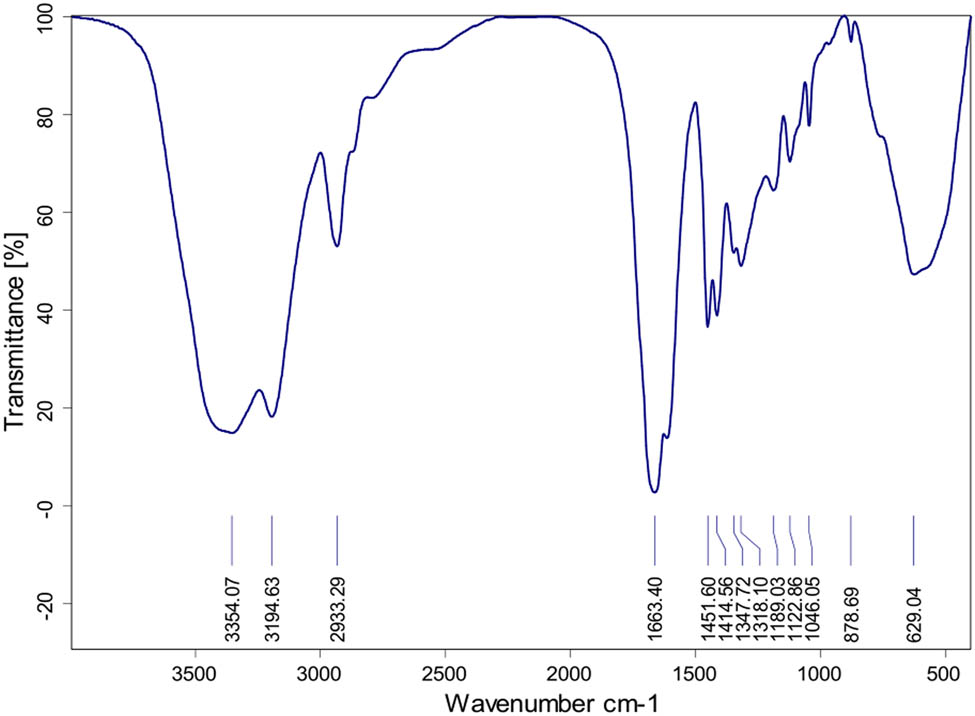
FT-IR spectrum of PDAA
The 1H NMR spectra of PDAA is shown in Figure 2. The signals at 3.81, 2.97, 2.15, and 1.70 ppm are attributed to the protons of the DADMAC unit (i.e,. c, d, b, and a marked in Figure 4) (22). Protons of the AM unit are evidenced by the asymmetric adsorption peaks at 1.57 and 2.15 ppm (i.e., e and f marked in Figure 4) (8). Bands at 1.70 and 2.15 ppm correspond to the protons of sodium acrylate unit (i.e., g and h marked in Figure 4). The signal at 1.11 and 3.65 ppm are due to the protons of ethanol (residual in polymer precipitation). The signal at 3.05 ppm maybe corresponds to the proton of emulsifiers. The results of FT-IR and 1H NMR demonstrated that PDAA copolymer was synthesized successfully.
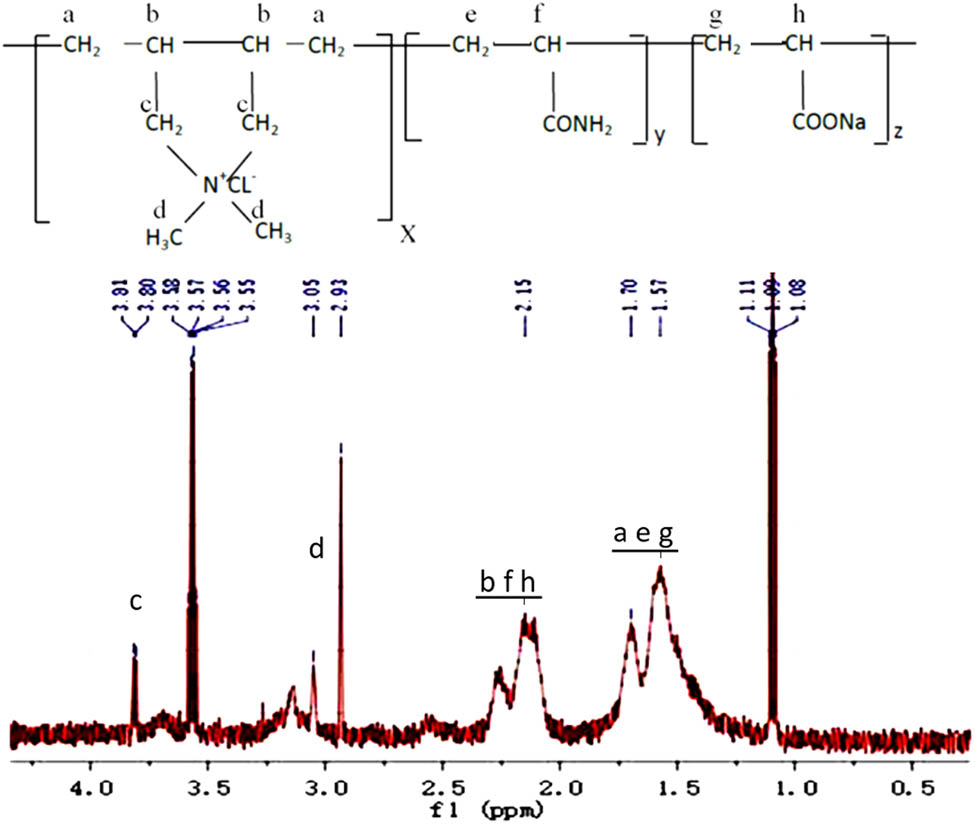
1H NMR spectra of PDAA.
The molecular weight distribution of PDAA determined by GPC is shown in Figure 3. It has been reported that the molecular weight distribution of the polymers prepared by inverse emulsion is about Mw/Mn = 1.35–1.55 while that of aqueous solution polymerization is Mw/Mn = 4.5–4.7 (8,23). It can be seen from Figure 5 that the molecular weight distribution of the PDAA product is very narrow, Mw/Mn = 1.12. This is lower than the usual emulsion prepared by inverse emulsion and is much lower than that prepared by aqueous solution polymerization. It is proved that the polymerization product prepared by inverse emulsion polymerization has the advantage of narrow molecular weight distribution.
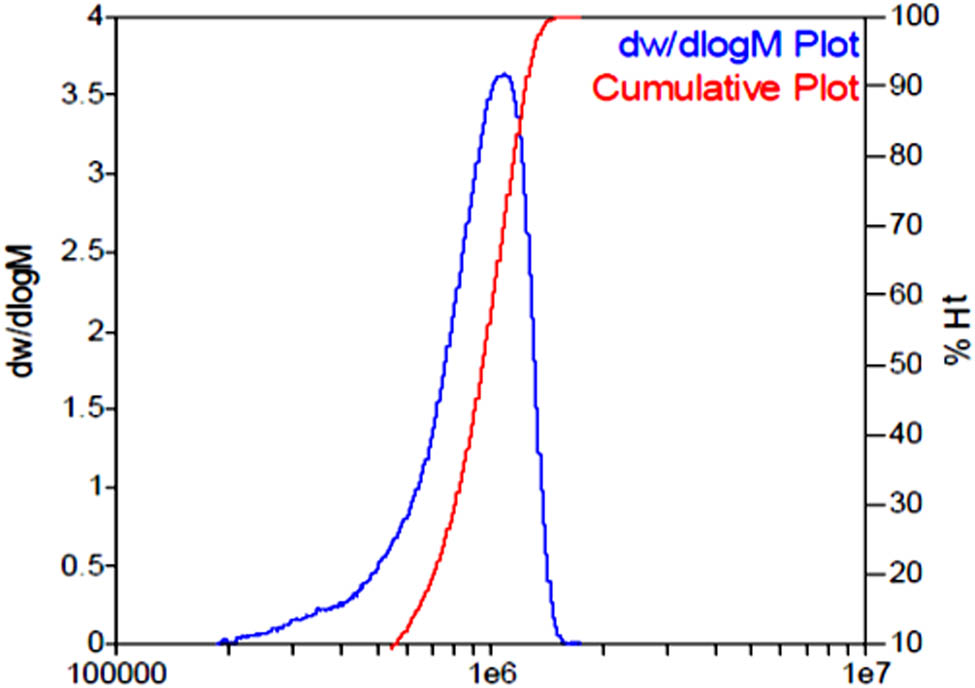
Molecular weight distribution of PDAA determined by GPC.
The micromorphology of PDAA was observed by SEM. Figure 4 shows the scanning electron microscopy (SEM) images of latex particles with a magnification of 10,000 times (a) and 25,000 times (b). It can be seen that the latex particles were regular spheres with a diameter of 150 to 600 nm, and no defective latex particles were found. This result indicates that the PDAA produced by the inverse emulsion system in this paper had good stability (24).
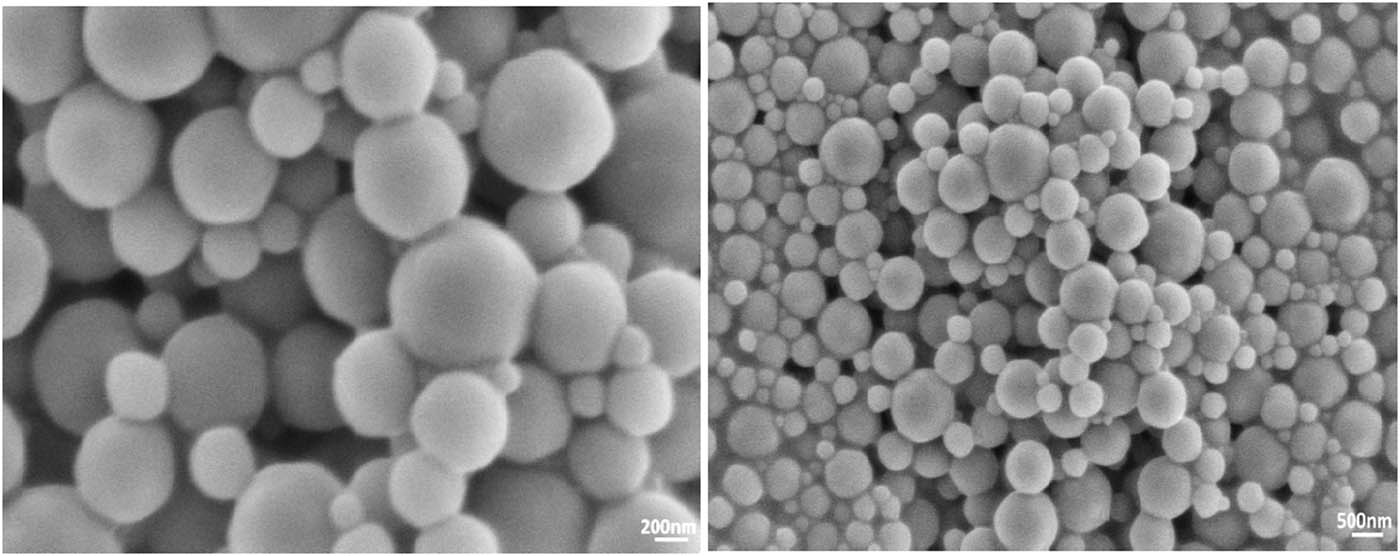
SEM images of PDAA at different magnification.
3.2 Effect of cationic degree of PDAA on the retention performance
The retention mechanism of the novel amphoteric polyacrylamide is that the anionic group first neutralizes the cationic waste in the paper material to reduce the electrostatic attraction resistance between the cationic group and the pulp fibers. The cationic group of retention aid adsorbs fibers and fine fibers in the paper pulp to form micro flocs, so that the fine pulp fibers are retained and the retention rate of paper material is improved (20). The proportion of cationic functional groups determines the electrostatic attraction of PDAA and fine pulp fibers. Thus, the retention properties of PDAA with 10%, 15%, 20%, 25%, and 30% cationic monomers for deinked pulp were studied.
As shown in Figure 5, the PDAA prepared with different content of cationic monomers all have good retention properties. The retention rate of paper increases with the increment of cationic monomer content in PDAA. When the cationic monomer content is more than 20%, the retention rate of paper increases slowly. The retention property of PDAA with 25% and 30% cationic monomers is similar. Therefore, to reduce the cost, the best content of cationic monomers in PDAA is selected as 25%.
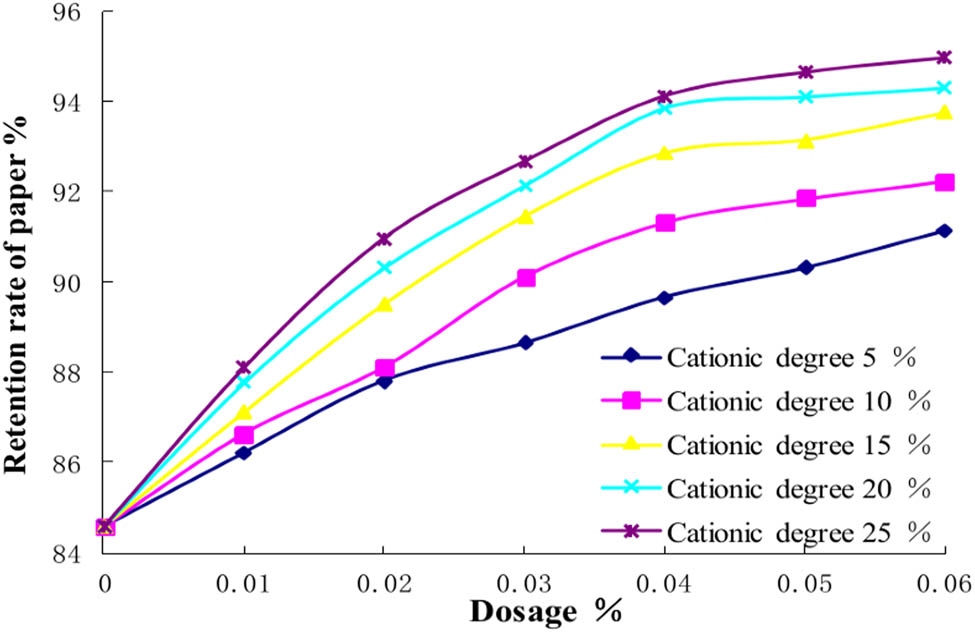
Effect of cationic degree on retention properties. The molecular weight of PDAA is 3 million.
3.3 Effect of molecular weight of PDAA on retention performance
It is reported that the molecular weight of the polymer is the main influencing factor of retention aid. In this paper, PDAA polymer emulsion particles with a molecular weight of 2–5 million, narrow molecular weight distribution and uniform particle size were easily obtained by inverse emulsion polymerization. The retention property of PDAA with molecular weight of 2, 3, 3.5, 4, and 4.5 million was studied when the addition amount was 0.01%, 0.02%, 0.03%, 0.04%, 0.05%, and 0.06%, respectively. The results are shown in Figure 6.
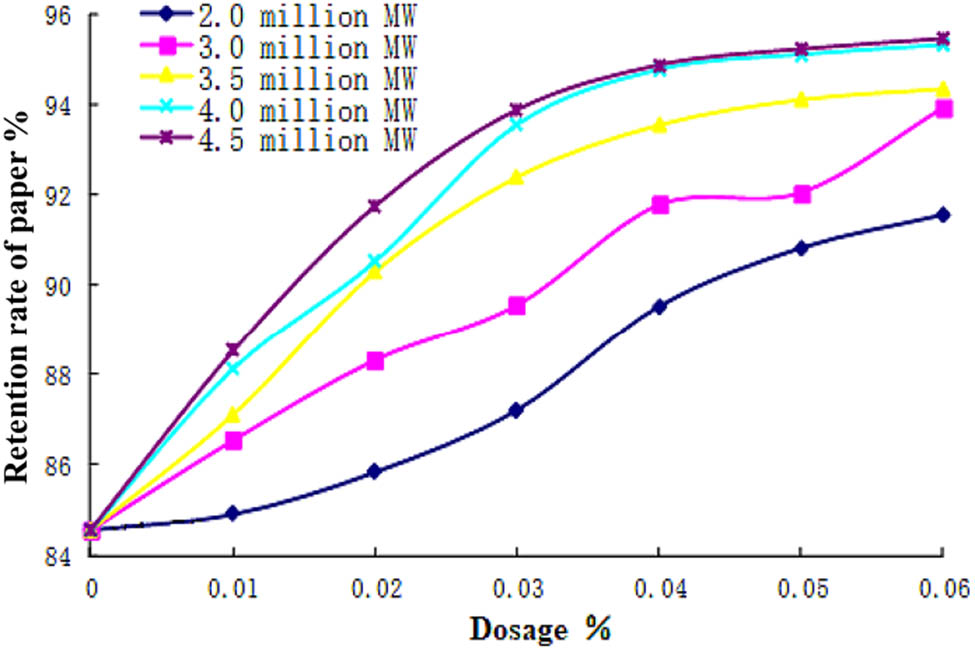
Effect of molecular weight on retention properties. The cationic degree of PDAA is 25 wt% based on the total weight of monomer.
It can be seen from Figure 2 that the retention capacity of PDAA increases with the increment of its molecular weight. The retention performance of products with a molecular weight of 2 and 3 million is poor, mainly due to the low molecular weight of the polymer, poor flocculation performance, and the formation of small flocs, which are not easy to retain. Therefore, with the increase of the dosage, the retention rate of paper material increases less. When the molecular weight is more than 4 million, the long molecular chain can form a larger flocculent, which makes a lot of small pulp fibers and filler flocculent remain in the paper, so that the retention rate of paper material can be improved rapidly. It can also be seen from Figure 2 that PDAA with a molecular weight of 4 and 4.5 million had similar paper retention when the addition amount increased to 0.04%. Because the polymer with small molecular weight is easier to prepare, 4 million is selected as the best molecular weight of PDAA. In addition, the PDAA has high yield and low residual monomer, which are 99.5% and 0.0252 wt%, respectively.
3.4 Synergistic retention of PDAA/PDADMAC
PDADMAC is widely used in the papermaking industry. It can be used as retention aid alone or as a retention aid system with anionic polymer or bentonite. PDAA/PDADMAC can be used together in a certain proportion, which can play a complementary role (25,26). PDADMAC can adsorb the anionic garbage inside the pulp, which enhances the adsorption of PDAA on the fine fibers and fibers, while the anionic group in PDAA can adsorb the cationic garbage in the pulp, thus removing the obstacles for the better interaction between PDADMAC and the fine fibers. A certain proportion of PDAA/PDADMAC can play a better role in synergistic retention. This paper discusses the retention performance of PDAA/PDADMAC with a mass ratio of 3:1, 1:1, and 1:3, respectively. The results are shown in Figure 7.
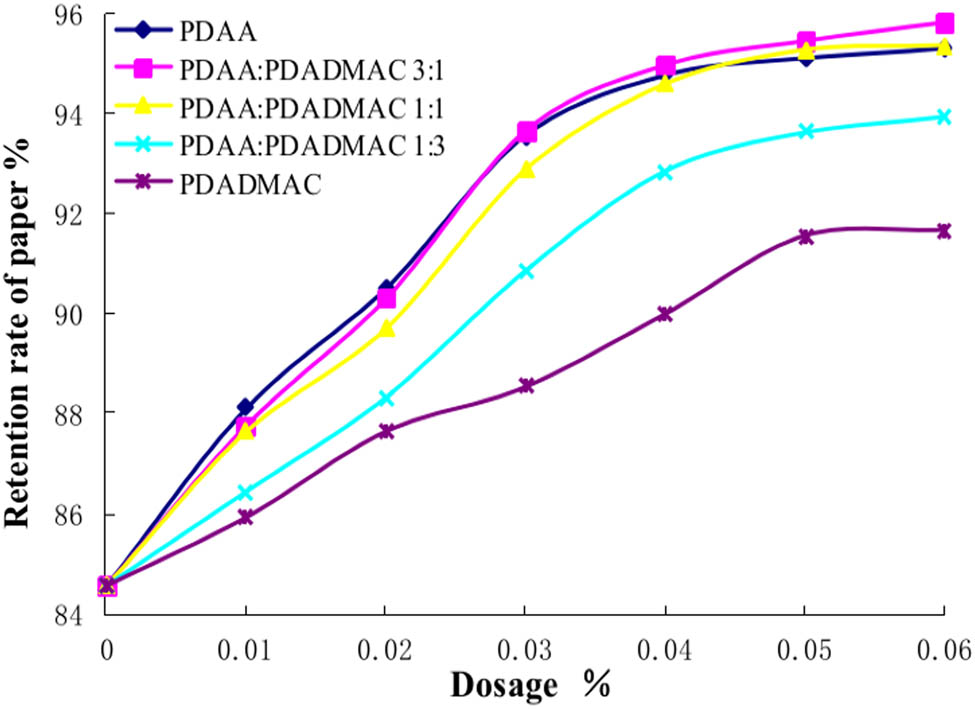
Synergistic retention of PDAA/PDADMAC. The molecular weight of PDAA is 4 million and the molecular weight of PDADMAC is 0.46 million.
It can be seen from Figure 7 that with the increase of the dosage of additives, the retention rate of paper material has a greater increase. When PDAA and PDADMAC were used in combination, the retention rate of paper was significantly higher than that of PDADMAC alone. The synergistic effect is more obvious with the increase of the proportion of PDAA. When the ratio of PDAA and PDADMAC is 1:1, the synergistic retention effect of PDAA and PDADMAC is similar to that of PDAA. When the proportion of PDAA and PDADMAC increased to 3:1, the synergistic retention effect of PDAA and PDADMAC was slightly better than that of PDAA. This shows that PDAA/PDADMAC has an ideal synergistic retention effect.
3.5 Synergistic retention of PDAA/CS
Cationic starch (CS) is an important derivative of starch ether. Because of its positive charge, it can be closely combined with the small pulp fibers and fillers with a negative charge. It is widely used in the wet end of papermaking. It can be used as reinforcement, retention aid, and filter aid to significantly improve the quality of paper, reduce consumption, and save cost (27). The addition of CS can increase the positive charge density of PDAA, to increase the adsorption of PDAA on fines and fillers (28). The synergistic retention mechanism of PDAA/CS is similar to that of PDAA/PDADMAC. The synergistic retention effect of PDAA/CS was studied by comparing the retention effects of adding PDAA, PDAA: CS 3:1, PDAA: CS 1:1, PDAA: CS 1:3, and CS, respectively. The results are shown in Figure 8.
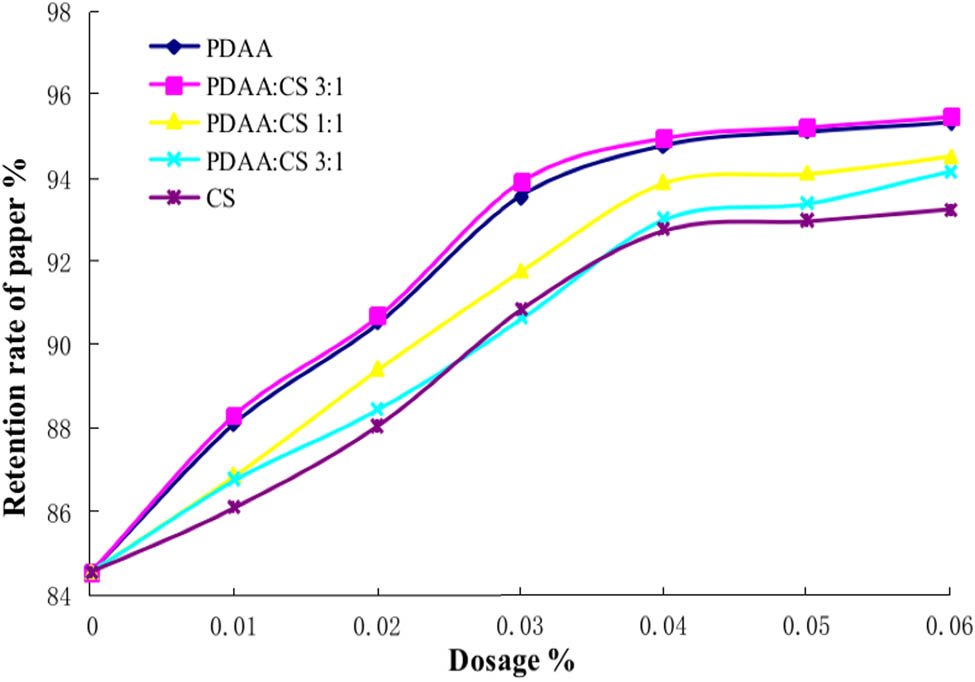
Synergistic retention of PDAA/CS. The molecular weight of PDAA is 4 million.
It can be seen from Figure 8 that the retention system of PDAA/CS composite has a better retention effect than CS. With the increase in the proportion of PDAA, the retention performance of the system increases gradually. When the ratio of PDAA:CS is 3:1, the retention performance is the best. It has been proved that the zeta potential of pulp is affected by the zeta potential of additives. The zeta negative potential of the pulp can be effectively reduced by adding a proper amount of cationic polymer, which results in flocculation. The reason for the synergistic effect of PDAA/CS is that on the one hand, the addition of CS reduces the zeta negative potential of paper and produces better flocculation, on the other hand, the addition of PDAA forms a large number of micro flocculation, which can play the best flocculation in a certain ratio, to play a better synergistic retention role.
3.6 Synergistic retention of PDAA/CHPG
Cationic guar gum (CHPG) is a kind of environmental friendly paper-making additive, which is made by modifying natural guar gum. It has a certain positive charge and is easy to adsorb with negatively charged pulp fibers and fillers. It can not only improve the retention rate and water filtering performance of paper but also maintain or improve the evenness of paper (29). CHPG can form a retention system with anionic, amphoteric polymer, or bentonite. In this paper, the retention performance of PDAA/CHPG in a certain proportion is discussed. The results are shown in Figure 9.
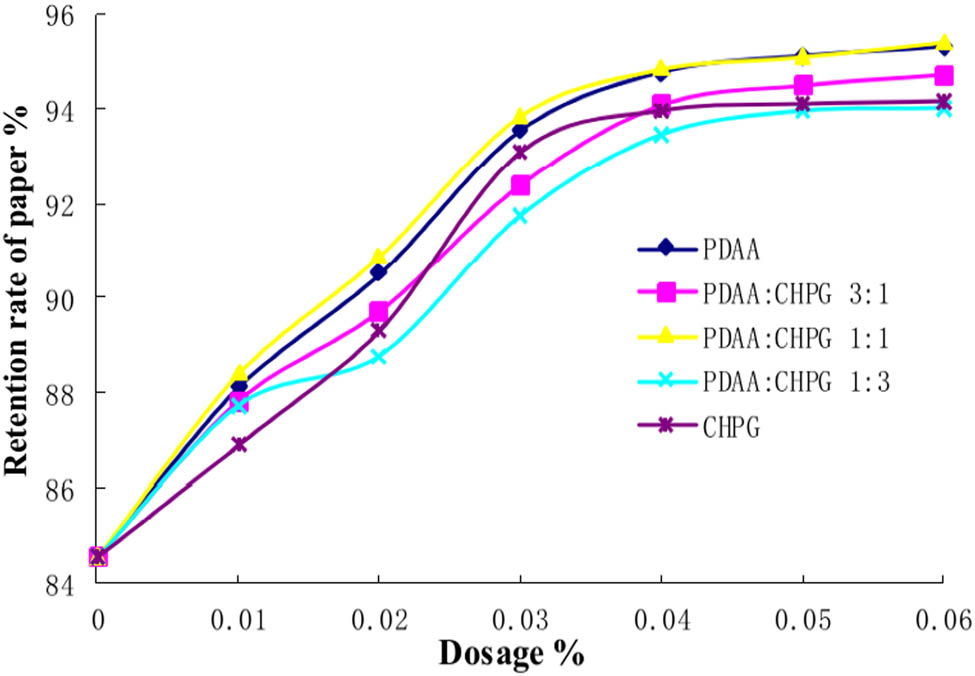
Synergistic retention of PDAA/CHPG. The molecular weight of PDAA is 4 million.
It can be seen from Figure 9 that the retention rate of paper increases with the increment of retention aid dosage in each group. With the increase in the amount of PDAA in PDAA/CHPG, the retention ability of the additives first increased and then decreased. When the ratio of PDAA to CHPG is 1:1, the retention effect of the system is the best, even better than PDAA. When the ratio of PDAA to CHPG is 1:3, the retention effect of the system is the worst, even worse than CHPG. The addition of a certain amount of CHPG can not only reduce the micro stickies in the deinked pulp of waste paper but also enhance the ability of PDAA to form micro flocs. Similarly, the addition of PDAA can eliminate the adverse effect of anionic waste in the pulp, and PDAA/CHPG can play an ideal role in synergistic retention. However, the CHPG in the ratio of PDAA/CHPG cannot be too large, the CHPG content will neutralize the negative charge of part of PDAA, reduce the adsorption capacity of PDAA with fine pulp fibers and fillers, while the retention ability will decrease.
3.7 Synergistic retention of PDAA/CTS
Cationic chitosan (CTS) is a kind of cationic polymer with retention property (30). The synergistic retention effect of PDAA and CTS was studied by using them in different proportions. The results are shown in Figure 10.
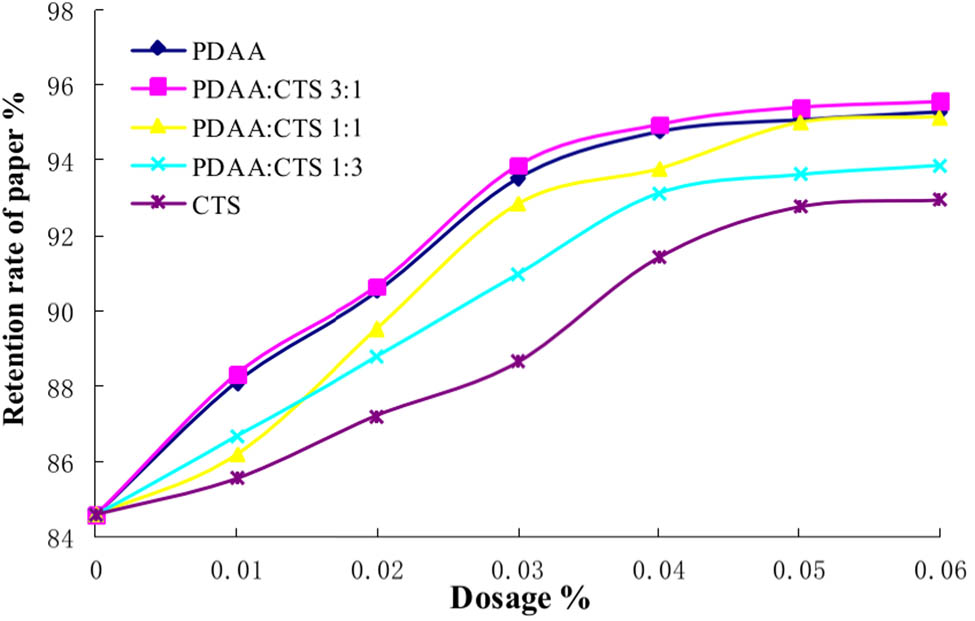
Synergistic retention of PDAA/CTS. The molecular weight of PDAA is 4 million.
It can be seen from Figure 10 that the retention rate of paper increases with the increase of retention system dosage. The order of retention performance is PDAA:CTS = 3:1, PDAA, PDAA:CTS = 1:1, PDAA:CTS = 1:3, CTS. It can be seen that with the increase in the proportion of PDAA in PDAA/CTS, the retention ability of the system is enhanced. The main reason may be that the retention performance of PDAA is better than that of CTS. In addition, PDAA and CTS also have a synergistic retention effect, because the retention performance of PDAA with CTS ratio of 3:1 is slightly better than that of PDAA alone.
3.8 Synergistic retention of PDAA/PA
Polyamine (PA) is a kind of water-soluble cationic polymer, which has the advantages of high positive charge density, good water solubility, easy control of molecular weight, high efficiency, nontoxicity, and low cost (31). Because of its strong positive charge and high molecular weight, PA cationic polymers can be used as retention aids in papermaking. The synergistic retention of PDAA/PA is discussed, and the results were shown in Figure 11.
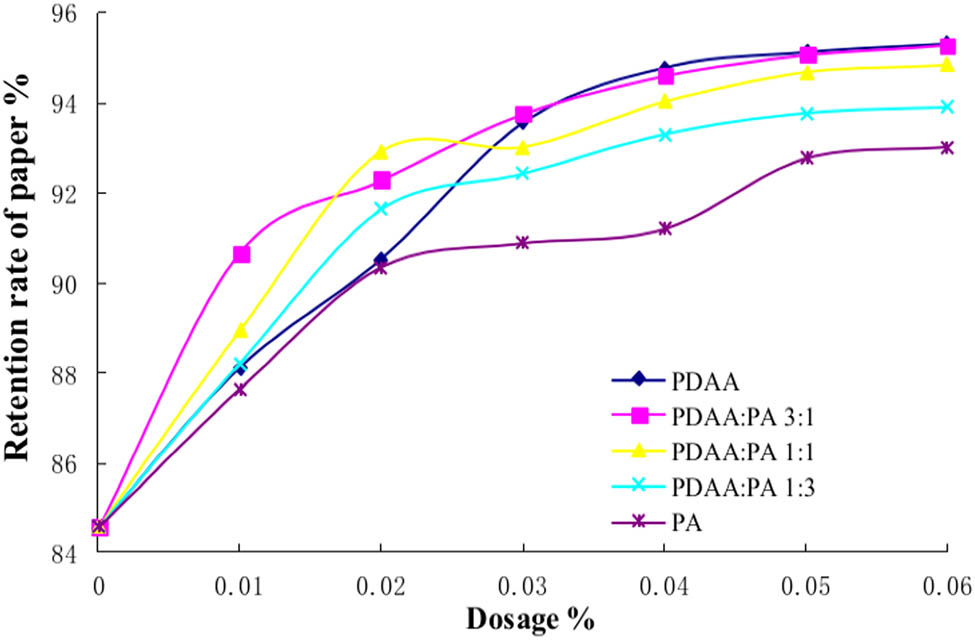
Synergistic retention of PDAA/PA. The molecular weight of PDAA is 3 million.
It can be seen from Figure 11 that the retention rate of paper increases gradually with the increase of the dosage of additives. With the increase of PDAA in the ratio of PDAA/PA, the retention performance of the retention system is gradually enhanced. The main reason may be that PA is a strong cationic polymer with relatively low molecular weight. The adsorption of fine pulp fibers is mainly through the charge adsorption mechanism to produce flocculation. The adsorption performance of fine pulp fibers and fillers is much worse than that of PDAA. Thus, the synergistic retention effect of PDAA/PA is not obvious.
4 Conclusion
The retention properties of novel amphoteric polyacrylamide, PDAA, with different cationic monomers and different molecular weight products were discussed. The results showed that the best cationic degree and molecular weights were 25% and 4 million, respectively.
The FT-IR and 1H NMR analysis confirmed that the product is a copolymer of cationic DADMAC monomer, sodium acrylate, and acrylamide. The GPC analysis indicated that the molecular weight distribution of the PDAA product is very narrow. The latex particles in the reverse emulsion are regular spheres with a diameter of 150 to 600 nm.
The synergistic retention effect of PDAA with five different cationic polymers was studied. The results show that the cationic polymer has a good synergistic retention effect with PDAA. The order of synergistic retention was PDAA/PDADMAC > PDAA/CTS > PDAA/CS > PDAA/CHPG > PDAA/PA.
Acknowledgments
Financial support for this work was received from the National Natural Science Foundation of China (31470605) and the State Forestry Administration of the People's Republic of China (2014-4-36).
References
(1) Singh RP, Pal S, Rana VK, Ghorai S. Amphoteric amylopectin: a novel polymeric flocculant. Carbohydr Polym. 2013;91(1):294–9.10.1016/j.carbpol.2012.08.024Search in Google Scholar PubMed
(2) Wang C, Wang X, Miao C, Wu Y. Preparation and properties of amphoteric polyacrylamide by seeded dispersion polymerization in ammonium sulfate solution. Polym Eng Sci. 2011;51(9):1742–8.10.1002/pen.21959Search in Google Scholar
(3) Qian L, Yanhao L, Yuanyuan B, Yan Y, Weitao H, Chun-Li Y. Optimizing the preparation conditions of amphoteric polyacrylamide as a strength additive for recycled paper. Nordic Pulp Pap Res J. 2018;33(2):297–308.10.1515/npprj-2018-3020Search in Google Scholar
(4) Wang PP, Zhu YY, Wang XY, Zhang XY, Zhu WY, Yao CL, et al. Application of amphoteric polyacrylamide solely or with the combination of cationic starch for paper strength improvement. Bioresources. 2018;13(4):7864–72.10.15376/biores.13.4.7864-7872Search in Google Scholar
(5) Yang Y, Zhang YT, Yao CL. Application effect of DMC/IA/AM amphoteric polyacrylamide in mixed recycled fibers. Zhongguo Zaozhi Xuebao/Transactions of China Pulp and Paper. 2015;30(1):15–9.Search in Google Scholar
(6) Tamsilian Y, Ramazani SAA, Shaban M, Ayatollahi S, Tomovska R. High molecular weight polyacrylamide nanoparticles prepared by inverse emulsion polymerization: reaction conditions-properties relationships. Colloid Polym Sci. 2016;294(3):513–25.10.1007/s00396-015-3803-5Search in Google Scholar
(7) Wutzel H, Richter FH, Li Y, Sheiko SS. Poly[N-(2-hydroxypropyl)methacrylamide] nanogels by RAFT polymerization in inverse emulsion. Polym Chem. 2014;5:1711–9.10.1039/C3PY01280HSearch in Google Scholar
(8) Cheng Z, Dong ZF, Su MJ, Zhang YH, Wang ZG, He PX. Synthesis of cationic polyacrylamide via inverse emulsion polymerization method for the application in water treatment. J Macromol Sci Part A. 2019;56(1):1–10.10.1080/10601325.2018.1547113Search in Google Scholar
(9) Zhong JX, Clegg JR, Ander EW, Peppas NA. Tunable poly((methacrylic acid)-co-acrylamide) nanoparticles through inverse emulsion polymerization. J Biomed Mater Res Part A. 2018;106(6):1677–86.10.1002/jbm.a.36371Search in Google Scholar PubMed PubMed Central
(10) Lu S, Liu R, Sun X. A study on the synthesis and application of an inverse emulsion of amphoteric polyacrylamide as a retention aid in papermaking. J Appl Polym Sci. 2002;84(2):343–50.10.1002/app.10340Search in Google Scholar
(11) Liu C, Hong B, Xu K, Zhang M, An H, Tan Y, et al. Synthesis and application of salt tolerance amphoteric hydrophobic associative flocculants. Polym Bull. 2014;71(12):3051–65.10.1007/s00289-014-1237-8Search in Google Scholar
(12) Yoon DH, Jang JW, Cheong IW. Synthesis of cationic polyacrylamide/silica nanocomposites from inverse emulsion polymerization and their flocculation property for papermaking. Colloids Surf A. 2012;411:18–23.10.1016/j.colsurfa.2012.06.036Search in Google Scholar
(13) Xie L, Shao ZQ, Wang HQ, Lv SY. Polymerization of acrylamide inverse microemulsion initiated directly by UV radiation. e-Polymers. 2011;80:1–9.10.1515/epoly.2011.11.1.874Search in Google Scholar
(14) Lim DW, Song KG, Yoon KJ, Ko SW. Synthesis of acrylic acid-based superabsorbent interpenetrated with sodium pva sulfate using inverse-emulsion polymerization. Eur Polym J. 2002;38(3):579–86.10.1016/S0014-3057(01)00164-1Search in Google Scholar
(15) Liu Z, Mendiratta S, Chen X, Zhang J, Li Y. Investigation of inverse emulsion assisted controlled release of polyacrylamides for enhanced oil recovery. RSC Adv. 2019;9:11968–77.10.1039/C9RA00465CSearch in Google Scholar
(16) Wormuth K. Superparamagnetic latex via inverse emulsion polymerization. J Colloid Interface Sci. 2001;241(2):366–77.10.1006/jcis.2001.7762Search in Google Scholar
(17) Zhong JX, Clegg JR, Ander EW, Peppas NA. Tunable poly((methacrylic acid)-co-acrylamide) nanoparticles through inverse emulsion polymerization. J Biomed Mater Res Part A. 2018;106(6):1677–86.10.1002/jbm.a.36371Search in Google Scholar PubMed PubMed Central
(18) Ariffin A, Razali MAA, Ahmad Z. PolyDADMAC and polyacrylamide as a hybrid flocculation system in the treatment of pulp and paper mills waste water. Chem Eng J. 2012;179:107–11.10.1016/j.cej.2011.10.067Search in Google Scholar
(19) GB/T 31246-2014, Technical conditions and test methods of cationic polyacrylamide as water treatment agent. Standardization Administration of China, Beijing, China.Search in Google Scholar
(20) Zhu YY, Jin ES, Song JL, Yao CL, Cheng Q. Progress in properties,synthesis and applications of amphoteric polyacrylamide. Chem Ind Eng Prog. 2015;34(3):758–66.Search in Google Scholar
(21) Jia Q, Song C, Li H, Zhang ZR, Liu HL, Yu YK, et al. Synthesis of strongly cationic hydrophobic polyquaternium flocculants to enhance removal of water-soluble dyes in wastewater. Res Chem Intermed. 2016;43(5):3395–13.10.1007/s11164-016-2832-0Search in Google Scholar
(22) Ma JY, Fu K, Fu X, Guan QQ, Ding L, Shi J, et al. Flocculation properties and kinetic investigation of polyacrylamide with different cationic monomer content for high turbid water purification. Sep Purif Technol. 2017;182:134–43.10.1016/j.seppur.2017.03.048Search in Google Scholar
(23) Liu Y, Lv CC, Ding J, Qian P, Yu Y, Ye S, et al. Characterization of a hybrid polyacrylamide and its flocculation properties in cyanide tailing suspensions. Water Sci Technol. 2017;76(9):1–12.10.2166/wst.2017.422Search in Google Scholar PubMed
(24) Zheng H, Sun Y, Zhu C, Guo J, Zhao C, Liao Y, et al. UV-initiated polymerization of hydrophobically associating cationic flocculants: synthesis, characterization, and dewatering properties. Chem Eng J. 2013;234:318–26.10.1016/j.cej.2013.08.098Search in Google Scholar
(25) Masumi T, Matsushita Y, Aoki D, Takama R, Saito K, Kuroda K, et al. Adsorption behavior of poly(dimethyl-diallylammonium chloride) on pulp fiber studied by cryo-time-of-flight secondary ion mass spectrometry and cryo-scanning electron microscopy. Appl Surf Sci. 2014;289:155–9.10.1016/j.apsusc.2013.10.125Search in Google Scholar
(26) Ravnjak D, Fuente E, Negro C, Blanco A. Flocculation of pulp fractions induced by fluorescently-labelled PDADMAC. Cellulose Chem Technol. 2006;40(1–2):77–85.Search in Google Scholar
(27) Wang JP, Yuan SJ, Wang Y, Yu HQ. Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties. Water Res. 2013;47(8):2643–8.10.1016/j.watres.2013.01.050Search in Google Scholar PubMed
(28) Sun YJ, Yang YL. Preparation of amphoteric polyacrylamide-grafted starch flocculant and its application. Adv Mater Res. 2013;815:427–31.10.4028/www.scientific.net/AMR.815.427Search in Google Scholar
(29) Xie W, Song Z, Liu Z, Qian X. Surface modification of pcc with guar gum using organic titanium ionic crosslinking agent and its application as papermaking filler. Carbohydr Polym. 2016;15:114–20.10.1016/j.carbpol.2016.05.010Search in Google Scholar PubMed
(30) Nicu R, Bobu E, Miranda R, Blanco A. Flocculation efficiency of chitosan for papermaking applications. BioResources. 2012;8(1):768–84.10.15376/biores.8.1.768-784Search in Google Scholar
(31) Gupta SK, Kumar NM, Misra R, Ansari FA, Dionysiou DD, Maity A, et al. Synthesis and performance evaluation of a new polymeric composite for the treatment of textile wastewater. Ind Eng Chem Res. 2015;16:1–28.10.1021/acs.iecr.5b03714Search in Google Scholar
© 2020 Kaiji Yang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery


