Abstract
A new piperonylic acid derivative (BPASD) was synthesized and evaluated as an organic nucleating agent for poly(l-lactic acid) (PLLA) via melt-crystallization; the other behaviors including cold-crystallization, melting process after crystallization, thermal stability in air atmosphere, and optical and mechanical properties of PLLA/BPASD samples were also investigated. The results of the melt-crystallization investigation showed that, in comparison to virgin PLLA, the BPASD could induce PLLA to crystallize in higher temperature region or at a faster cooling rate, suggesting that the BPASD as a heterogeneous nucleating agent could promote the crystallization of PLLA, but the melt-crystallization depended on the cooling rate, BPASD concentration, and the final melting temperature. With increasing of BPASD concentration, a shift to the lower temperature of cold-crystallization peak and decrease of crystallization enthalpy indicated that BPASD had an inhibition for the cold-crystallization of PLLA to some extent. The heating rate, crystallization temperature, the BPASD, and its concentration were critical factors to the melting process, and double-melting peaks appeared in heating were assigned to melting–recrystallization. Thermal decomposition behavior revealed that the addition of BPASD reduced the thermal stability of PLLA, but the interaction of PLLA and BPASD could prevent the decrease of the onset decomposition temperature. Further, the BPASD also decreased the light transmittance and elongation at the break of PLLA, but the tensile modulus and tensile strength of PLLA were enhanced.
1 Introduction
With a large-scale usage of fossil resources in the past three decades, the society has been suffering from the serious environmental pollution and the increasing shortage of resources. Under this circumstance, developing the environment-friendly materials from bio-renewable resources is very instructive to facilitate the social sustainable development. For the thermoplastic polymers, poly(l-lactic acid) (PLLA) as a typical representative of bio-based polymer has received more and more attentions due to its biodegradability, compostable, biocompatibility, and good processability (1), these features are also accelerating the application of PLLA in various fields such as automotive interiors (2,3), packaging (4,5), bone regeneration (6,7,8), drug release (9,10,11), etc. For instance, Fernandes et al. (12) developed porous PLLA–borosilicate bioactive glasses fiber mesh scaffolds. The relevant measurements showed that these PLLA–borosilicate bioactive glasses scaffolds presented a faster degradation rate with a constant release of inorganic species and not cytotoxic to cells, as well as the promoting ability to cell adhesion and proliferation.
Unfortunately, the poor crystallization ability of PLLA itself, resulting from the intrinsically low mobility of PLLA chains due to the hard and short repeating units and insufficient nucleators (13), leads to the product’s serious defects including poor heat resistance, low crystallinity, and long injection cycle (14,15,16), these defects not only increase the manufacturing cost, but also reduce the product quality, leading to a poor competitive with commodity plastics such as PP, PE, and PVC. Thus, replacing the traditional petroleum-based plastics through using PLLA must overcome the defect of the slow crystallization rate of PLLA. Up to now, the easiest and most feasible way to improve the crystallization of PLLA is still adding the heterogeneous nucleating agent (17,18), because the nucleating agent can reduce the surface free energy of nucleation to promote the polymer’s crystallization at high cooling rate. Many compounds are employed to evaluate its heterogeneous nucleation effect for PLLA (19,20,21); among these nucleating agents, talc (22), metal phosphonates (23), and amide derivatives (24) exhibited a better crystallization accelerating ability for PLLA. Pan et al. (25) reported that the only 1 wt% zinc phenylphosphonate, in comparison to the pure PLLA, could cause the crystallization half-time of PLLA to decrease from 28 to 0.63 min at 130℃, even upon the addition of 15 wt% zinc phenylphosphonate, the crystallization half-time of PLLA was only 0.33 min. Additionally, it is surprising that PLLA containing 0.02 wt% zinc phenylphosphonate could finish crystallization at cooling rate of 10℃/min. However, it is pity that the most of these nucleating agents can still not meet the industrialization requirements.
Overall, for organic nucleating agents, the heterogeneous nucleation ability for PLLA is slightly poorer than inorganic nucleating agents; however, organic nucleating agents exhibit better compatibility and designability. To overcome the aforementioned relatively poor nucleation ability, developing a large amount of organic nucleating agents to thoroughly reveal and confirm the effects of key structures and groups on the crystallization process of PLLA is very valuable, and based on this reason, many researchers gradually focused on the designing and synthesizing new nucleating agents with different structures (26,27,28). However, as far as the reported nucleating agents were concerned, the nucleation ability can still not meet the industrial requirements. Thus, it is also necessary to further develop the organic nucleating agent with new structures.
In this work, N,N′-sebacic bis(piperonylic acid) dihydrazide (BPASD) derived from piperonylic acid and sebacic dihydrazide was first used to demonstrate its role in crystallization process of PLLA. And the other performances including melting behavior, thermal stability, light transmittance, and mechanical properties of PLLA containing different BPASD concentration were also studied by differential scanning calorimeter (DSC), thermogravimetric analysis (TGA), transmittance instrument, and electronic tensile tester.
2 Experimental
2.1 Materials and reagents
4032D PLLA produced by Nature Works LLC of USA was purchased from Dongguan Luxin Plastic Co., Ltd., China, and the D content and Mw were 1.20% and 1.70 × 105 g/mol, respectively. All reagents of analytical purity were obtained from Chongqing Huanwei Chemical Company to synthesize BPASD, and these reagents included piperonylic acid, sebacic dihydrazide, N,N-dimethylformamide (DMF), triethylamine, and thionyl chloride.
2.2 Synthesis of BPASD
BPASD was synthesized according to the route as shown in Figure 1. First, the piperonylic acid chloride was obtained via acylation reaction of piperonylic acid. And then, the sebacic dihydrazide was dissolved in DMF using ultrasonic technology, followed by slowly adding the piperonylic acid chloride and triethylamine. The mixed solution was stirred at ice bath for 1.5 h and further stirred at 70℃ for 4 h. Finally, the reaction solution was poured into water after cooling to room temperature, and the following suspension was filtrated and washed using water for three times, the resulting white product was dried for 36 h under vacuum. Fourier transform infrared spectrometer (FT-IR) υ: 3265.6, 2922.3, 2908.7, 1693.7, 1647.4, 1603.2, 1581.0, 1504.6, 1490.3, 1469.3, 1443.5, 1338.2, 1314.1, 1286.5, 1263.0, 1172.7, 1117.4, 1037.9, 923.8, 878.5, 855.8, 811.9, 722.1 cm−1. 1H nuclear magnetic resonance (1H NMR) δ: ppm; 9.90 (s, 1H, NH), 9.84 (s, 1H, NH), 6.99–7.48 (m, 3H, Ar), 6.11 (s, 2H CH2), 2.15–2.19 (t, 2H, CH2), 1.54–1.55 (d, 2H, CH2), 1.27–1.30 (d, 4H, CH2).
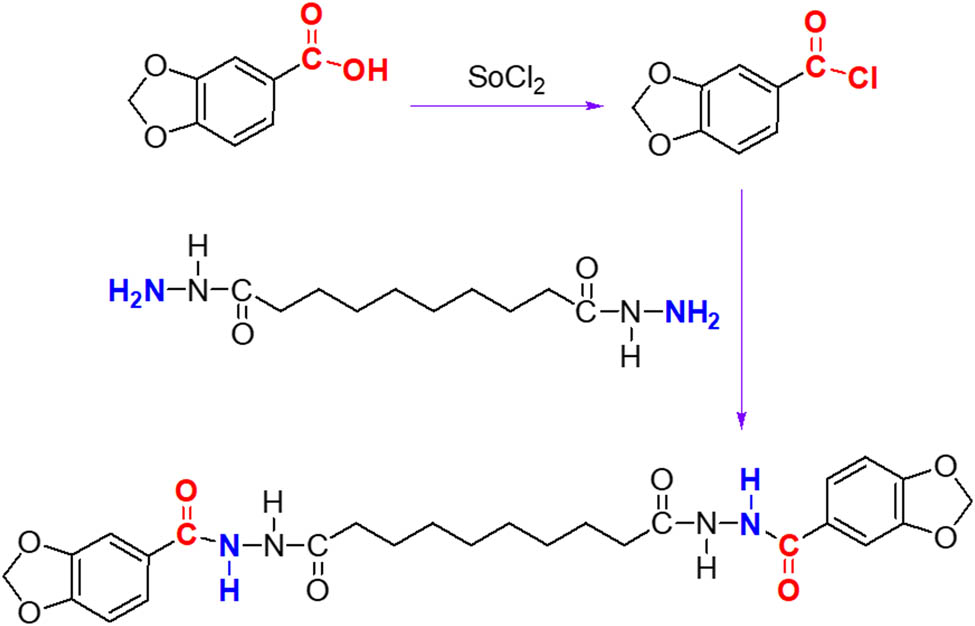
Synthetic route of BPASD.
2.3 Preparation of PLLA/BPASD sample
A torque rheometer was used to perform the blend of the dried PLLA and BPASD. The blending mass ratio of PLLA and BPASD was 99.5/0.5, 99/1, 98/2, 97/3, 95/5, respectively, and the relevant mixture was labeled as PLLA/0.5% BPASD, PLLA/1% BPASD, PLLA/2% BPASD, PLLA/3% BPASD, and PLLA/5% BPASD. And the other blending parameters were the melt-blending temperature of 180–200°C, the rotation speed of 32 rpm for 10 min, as well as the rotation speed of 64 rpm for 7 min. Finally, the mixture were hot pressed and cool pressed under 20 MPa to obtain the relevant testing samples.
2.4 Characterization and testing
The structural characterization of BPASD was performed using FT-IR (IS50) and 1H NMR (AVANCE 400 MHz). The FT-IR testing sample was prepared through KBr pellet, and the testing wavenumber was from 4,000 to 400 cm−1. The BPASD was dissolved using methyl sulfoxide before 1H NMR testing. The non-isothermal crystallization and melting processes of the virgin PLLA and PLLA/BPASD under different conditions were recorded by DSC (Q2000), and an indium standard was employed to calibrate the temperature and heat flow before testing, as well as the sample purge flow of the nitrogen was 50 mL/min during testing. The thermal decomposition behaviors of the virgin PLLA and PLLA/BPASD were tested by TGA (Q500) under the flowing air with 60 mL/min, and the testing temperature was from 40℃ to 650℃ at a heating rate of 5℃/min. A comparative study on the light transmittance of the virgin PLLA and PLLA/BPASD was carried out via A DR82 light transmittance meter, and a given sample was tested for five times to obtain the averaging light transmittance. The pneumatic-controlled impact shaping machine was used to prepare the tensile test specimens with dimensions of 25 mm × 4 mm × 0.5 mm, and the tensile tests of the virgin PLLA and BPASD-nucleated PLLA samples were performed on an electronic tensile tester (D&G DX-10000) at the speed of 1 mm/min.
3 Results and discussion
3.1 Melt-crystallization behavior
To evaluate BPASD’s nucleating role in accelerating the crystallization of PLLA, the melt-crystallization process of the virgin PLLA and all PLLA/BPASD samples from 190℃ at a cooling rate of 1℃/min was investigated using DSC (see Figure 2). As seen in Figure 2, for the virgin PLLA, the melt-crystallization peak can almost not be observed in DSC curve, indicating that the crystals do not form in cooling due to the poor crystallization ability of PLLA itself as reported (29,30,31), and this poor crystallization ability is further attributed to the fact that, upon cooling of 1℃/min, the PLLA itself can still not nucleate through the winding of chain segments. However, all PLLA samples containing BPASD exhibit the obvious and sharp melt-crystallization peak, as a result, the addition of BPASD promotes the crystallization of PLLA. That is, the BPASD plays a role of heterogeneous nucleating agent in accelerating the crystallization of PLLA. Meantime, Figure 2 also shows the influence of BPASD concentration on the melt-crystallization behavior of PLLA, and this effect can be classified into two types. When the BPASD concentration is 0.5 wt% to 2 wt%, the melt-crystallization peak shifts toward the higher temperature and becomes sharper, meaning that a larger amount of BPASD can cause the PLLA to crystallize at a higher temperature, and the BPASD concentration in PLLA matrix is unsaturated. When the loading of BPASD is 2 wt% to 5 wt%, the effect of BPASD concentration on the melt-crystallization peak is almost negligible, and the difference of both melt-crystallization peak temperature and melt-crystallization enthalpy are also very tiny, basically the 2 wt% BPASD is saturated concentration. And the melt-crystallization peak temperature of the PLLA/2% BPASD sample is 138.0℃, the melt-crystallization enthalpy is 50.8 J/g (the crystallinity is 55.7% according to the relevant equation (24)).

Melt-crystallization DSC curves of the virgin PLLA and all PLLA/BPASD samples.
Additionally, it is noted that the onset crystallization temperature (Toc) gradually increases with increasing of BPASD concentration, and the Toc of the PLLA/0.5% BPASD, PLLA/1% BPASD, PLLA/2% BPASD, PLLA/3% BPASD, and PLLA/5% BPASD is 136.0, 140.0, 141.1, 141.3 and 141.8℃, respectively. Compared with the other PLLA systems such as PLLA/BA (32), PLLA/MCB (33), PLLA/MgPA (34), etc., the PLLA/BPASD sample with the same mass ratio has the higher Toc, evidencing that BPASD possesses the better nucleation effect for PLLA. What is more, the crystallization temperature is often from glass transition temperature to 0.85 times of the melting temperature. Through Toc data analysis, it is also found that the Toc of all PLLA/BPASD are almost higher than the 0.85 times of the melting temperature (about 160–170℃, see melting behavior section), which further confirms the powerful crystallization promoting effect of BPASD for PLLA.
Figure 3 is the melt-crystallization DSC curves of all PLLA/BPASD samples from 190℃ at different cooling rates. It is clear that, with an increase in the cooling rate, the melt-crystallization peak shifts toward the lower temperature and become wider, the reason may be as follows: a higher cooling rate make PLLA have no enough time to form the regular molecular structure at set temperature, and the crystallization only occurs at following lower temperature. It is observed from Figure 3 that the only PLLA/0.5% BPASD sample has no discernible melt-crystallization peak, when the cooling rate is higher than 20℃/min; the other PLLA/BPASD samples exhibit the obvious melt-crystallization peak at all cooling rates, even when the cooling rate is 30℃/min, the melt-crystallization peak is still very obvious implying that the nucleating role of BPASD in crystallization of PLLA was quite effective again. This result is very instructive to industrial manufacture, because PLLA/BPASD can accelerate the crystallization upon fast cooling at 50℃/min.
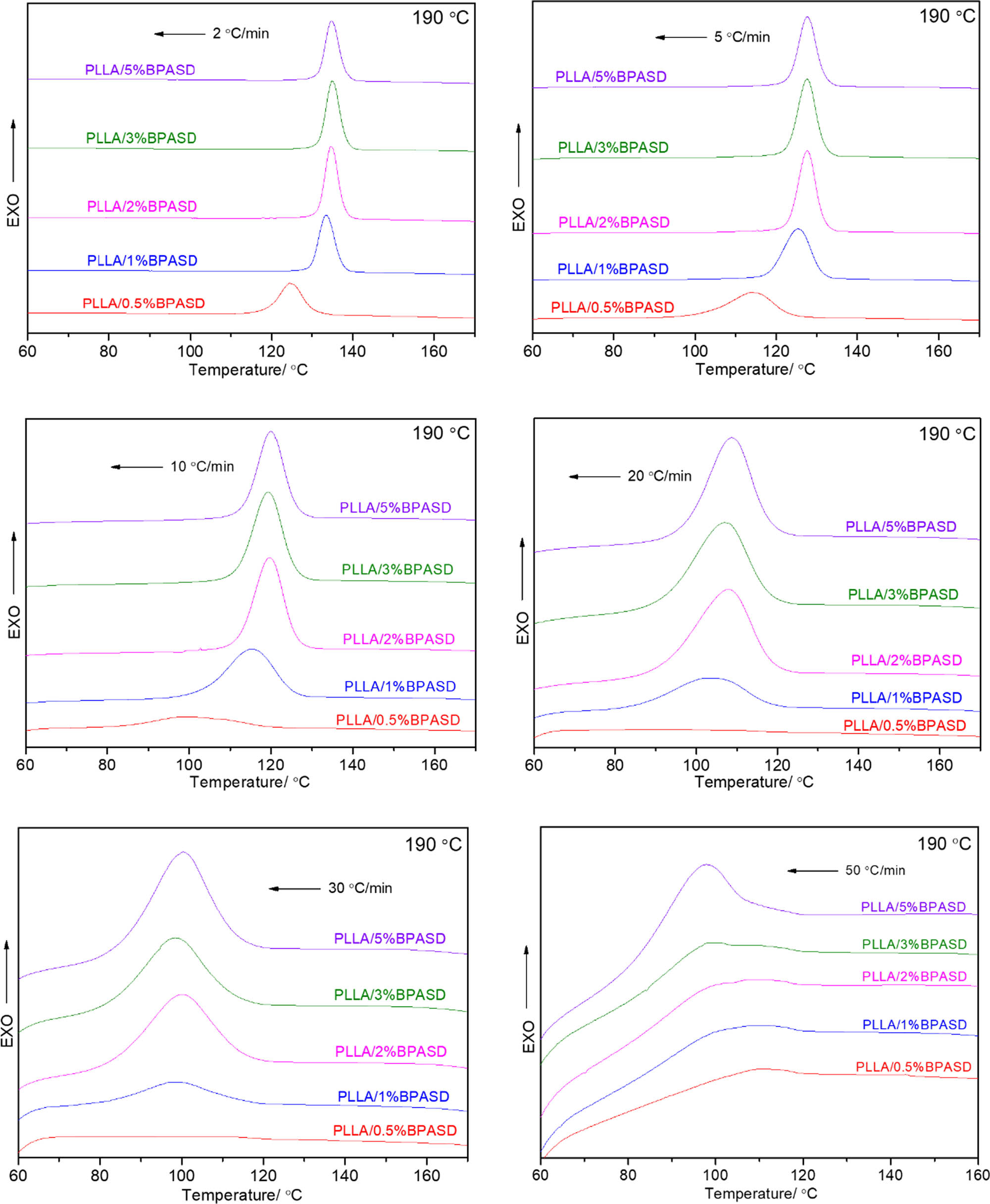
Melt-crystallization DSC curves of PLLA/BPASD samples from 190℃ at different cooling rates.
The surface and interface interaction of semi-crystalline polymer and nucleating agent is an important factor to polymer crystallization, and this interaction depends on the amount of the dissolved nucleating agent in matrix to some extent. Whereas the final melting temperature (Tf) directly determines the solubility of the nucleating agent in matrix, thus, as a speculation, the Tf must play an important role in crystallization of PLLA. Figure 4 is the melt-crystallization DSC curves of the PLLA/BPASD samples from the different melting temperatures at a cooling rate of 1℃/min. When the Tf is 180℃, apart from the PLLA/0.5% BPASD sample, the melt-crystallization peak temperature and Toc of the other PLLA/BPASD samples are higher comparing with those relevant data from the melt-crystallization from 190 to 200℃, especially the PLLA/5% BPASD sample has the highest melt-crystallization temperature of 139.2℃ and the largest Toc value of 142.4℃, indicating that the Tf of 180℃ is more beneficial to crystallize. The reason may be that, on one hand, a small amount of dissolved BPASD can promote the compatibility between PLLA and BPASD in the low temperature region; on the other hand, a larger amount of undissolved BPASD can supply more nucleation sites. As a result, the crystallization can occur at a higher temperature. However, when the Tf is 200℃, the PLLA/3% BPASD has the largest melt-crystallization enthalpy of 57.5 J/g, meaning that the crystallinity is 63.7%.
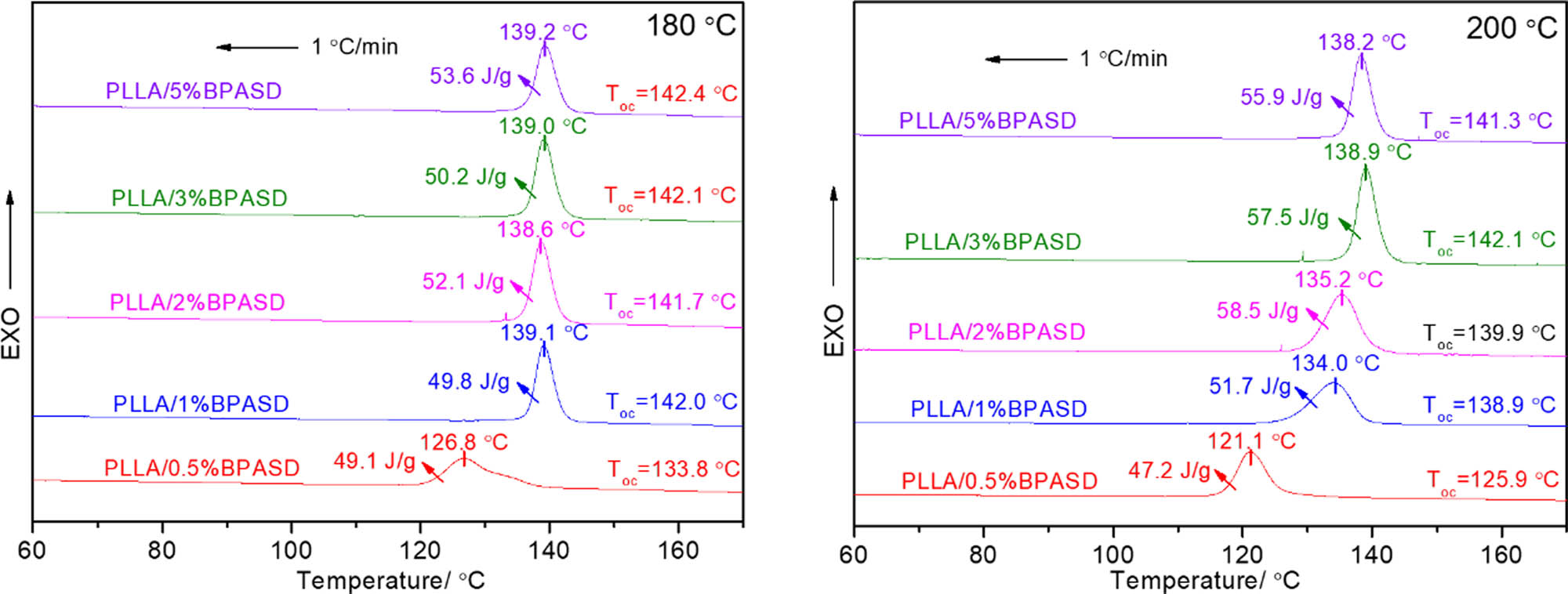
Melt-crystallization DSC curves of the PLLA/BPASD samples from the different melting temperatures.
3.2 Cold-crystallization behavior
The cold-crystallization process of PLLA/BPASD samples was further studied by DSC. Figure 5 displays the cold-crystallization DSC curves of the PLLA/BPASD samples from the room temperature at a heating rate of 1℃/min. As shown in Figure 5, the effects of BPASD concentration on the cold-crystallization are concluded into two types as the melt-crystallization process. When the BPASD concentration is 0.5 wt% to 1 wt%, the cold-crystallization peak slightly moves toward the higher temperature with increasing of BPASD concentration, but the cold-crystallization enthalpy decreases. In contrast, the cold-crystallization peak shifts to the lower temperature with increasing of BPASD concentration from 2 to 5 wt%, what is worse, the cold-crystallization enthalpy decreases from 24.6 to 18.5 J/g, indicating that less crystals are formed in heating, which results from that a larger amount of BPASD may have a more drastic inhibition for the motility of PLLA chains segment. In addition, through the comparative in crystallization enthalpy, it is a fact that the cold-crystallization enthalpy of a given PLLA/BPASD sample is less than 0.5 times of the melt-crystallization enthalpy, indicating that the crystallization temperature is very crucial, because the crystallization temperature is directly related to the motility of PLLA chains segment, that is, the lower the crystallization temperature is, the poorer the motility of PLLA chains segment is; as a result, the formation of the crystal becomes more difficult.
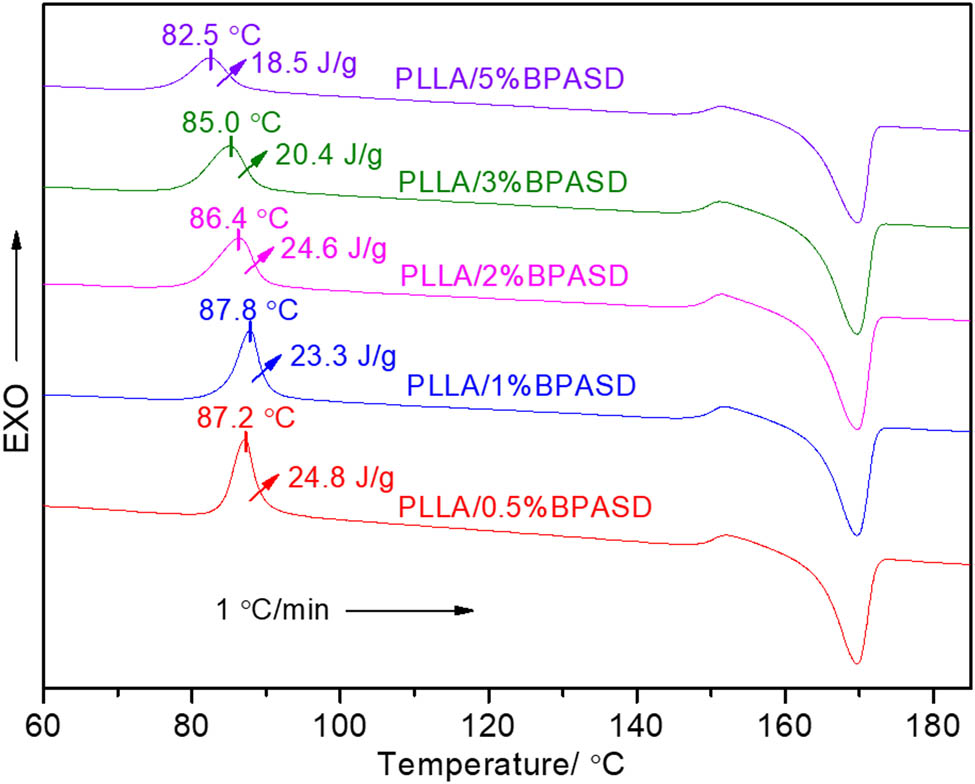
Cold-crystallization DSC curves of PLLA/BPASD samples.
In cold-crystallization section, we further investigate the effect of the heating rate on the cold-crystallization process of PLLA (see Figure 6). An increase in the heating rate leads to a shift to higher temperature of cold-crystallization peak because of the thermal inertia (35). However, the effect of BPASD concentration on the cold-crystallization process of PLLA at a given heating rate is consistent with that at other heating rates.
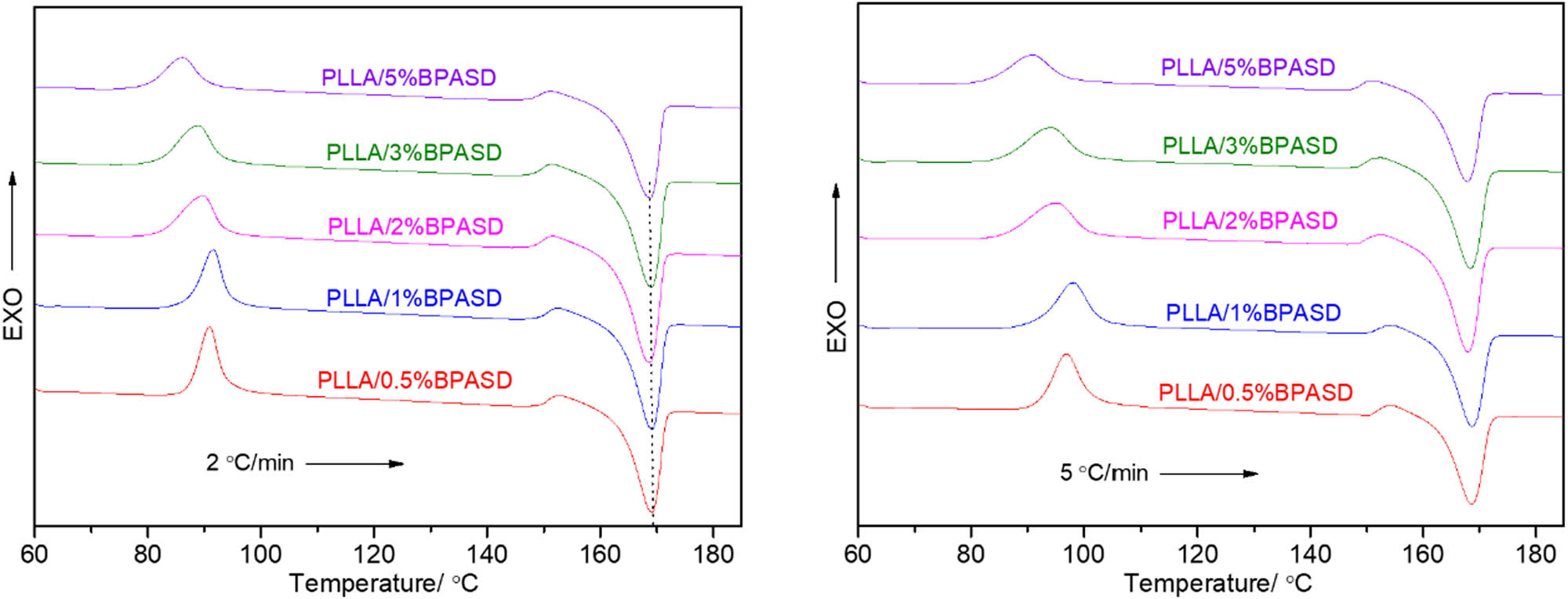
Cold-crystallization DSC curves of PLLA/BPASD samples at different heating rates.
3.3 Melting behavior
Non-isothermal crystallization behavior has confirmed the advanced nucleation ability of BPASD for PLLA; for the subsequent melting process, it is necessary to investigate this process to further reveal the nucleation role of BPASD. Figure 7 is the melting behavior of PLLA/BPASD samples at different heating rates after the melt-crystallization upon cooling of 1℃/min. The double melting peaks only appear in DSC curve of PLLA/0.5% BPASD sample, Moreover, the double melting peaks gradually degenerate into the single melting peak with an increase in the heating rate. First, the most of 0.5 wt% BPASD has served as heterogeneous nucleation sites for the crystallization of PLLA in cooling, which results in that the nucleation site from the BPASD is very little in heating; second, a larger heating rate is not beneficial to crystal growth. Thus, the formation of recrystallization in heating becomes more difficult with increasing of heating rate, representing that the higher-temperature melting peak gradually disappears. This result also indicates that the double-melting behavior in this study is assigned to melting–recrystallization (36). Additionally, it is observed that the melting temperature of PLLA/0.5% BPASD is obviously lower than that of other PLLA/BPASD sample, the probable reason is that the crystals of PLLA/0.5% BPASD sample, formed at a lower temperature in cooling, possess poorer perfect.

Melting behavior of PLLA/BPASD samples at different heating rates after melt-crystallization.
Figure 8 shows the melting behavior of PLLA/BPASD samples after isothermal crystallization for different time at set crystallization temperature. Because the crystallization temperatures located in the temperature range of 120–140℃ in melt-crystallization section, 120℃ (as a low crystallization temperature) and 140℃ (as a high crystallization temperature) are selected as the isothermal crystallization temperature. When the crystallization temperature is 120℃, the melting peak temperatures of PLLA/BPASD samples do almost not depend on the crystallization time, but an increase in the crystallization time can cause the weak high-temperature melting peak of PLLA with various BPASD contents from 2 to 5 wt% to disappear. Differing from the result from crystallization temperature of 120℃, all PLLA/BPASD samples only exhibit the single melting peak after isothermal crystallization at 140℃, furthermore, the melting peak shifts to the higher temperature with crystallization time, suggesting that a longer crystallization time can make the crystal become more perfect.
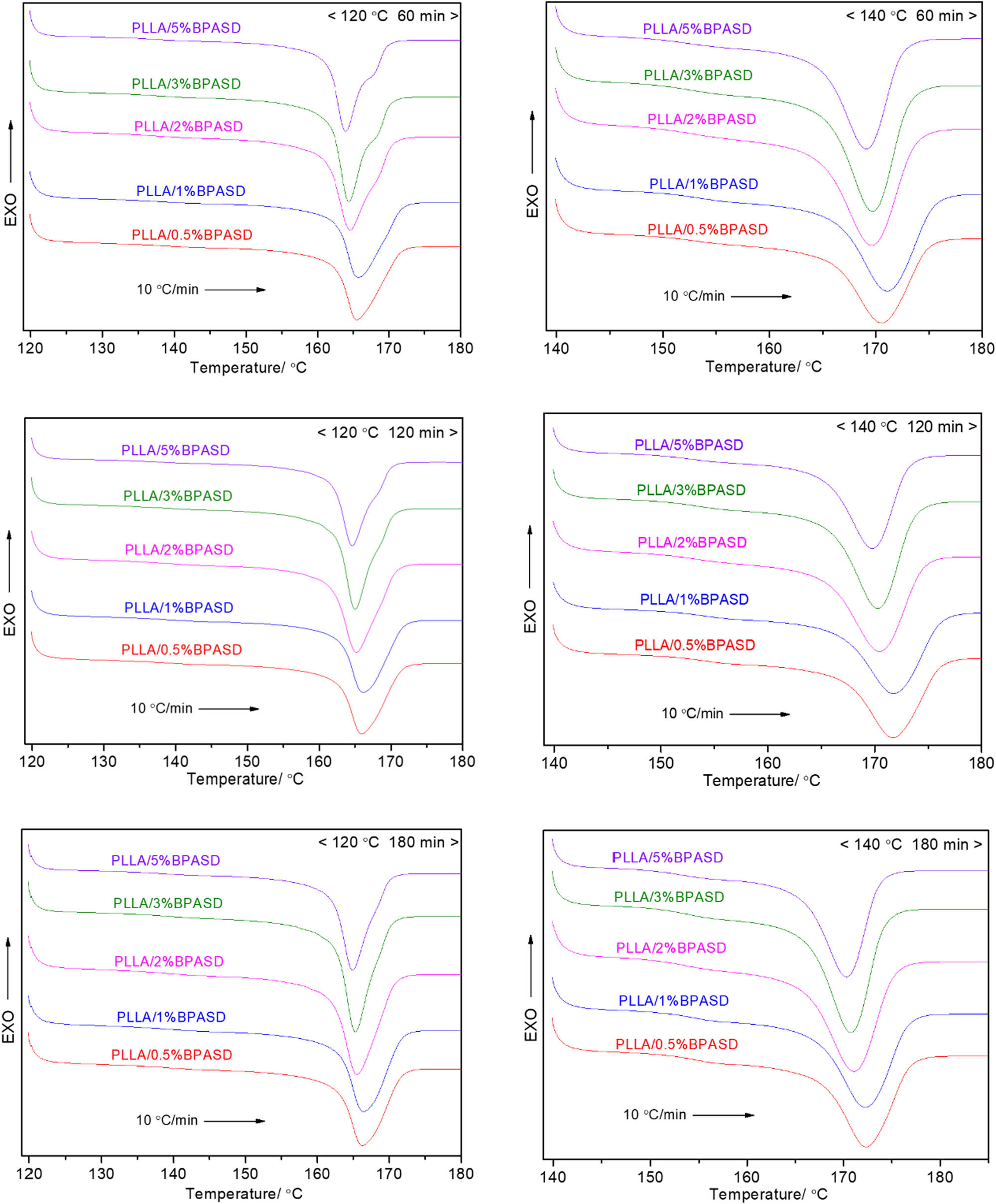
Melting behavior of PLLA/BPASD samples at a heating rate of 10℃/min after isothermal crystallization.
3.4 Thermal stability
In this study, we not only focused on the nucleation effect of BPASD for PLLA but also investigated the effect of BPASD on other performances of PLLA. The thermal decomposition processes of the virgin PLLA and PLLA/BPASD are shown in Figure 9. As it can be seen, all PLLA/BPASD samples as the virgin PLLA have only one thermal decomposition step; moreover, the thermal decomposition profile is very similar, indicating that the BPASD with loading of 0.5 wt% to 5 wt% cannot change the profile of PLLA. Meanwhile, this result also indicates that the PLLA and BPASD have the excellent compatibility to some extent. However, the BPASD significantly affects the onset decomposition temperature (Tod) of PLLA, and the Tod appeared at 341.3, 319.6, 316.7, 316.8, 308.5, and 321.1℃ for the virgin PLLA, PLLA/0.5% BPASD, PLLA/1% BPASD, PLLA/2% BPASD, PLLA/3% BPASD, and PLLA/5% BPASD. It is clear that the Tod of all PLLA/BPASD samples are lower than that of the virgin PLLA, meaning the addition of BPASD decreases the thermal stability of PLLA. However, it should be noted that the PLLA/5% BPASD sample has the maximal Tod value comparing with the other PLLA/BPASD samples, which is different from the effect of BPASD concentration from 0.5 to 3 wt% on the Tod, this resultant depends on the greater interaction of PLLA and BPASD.
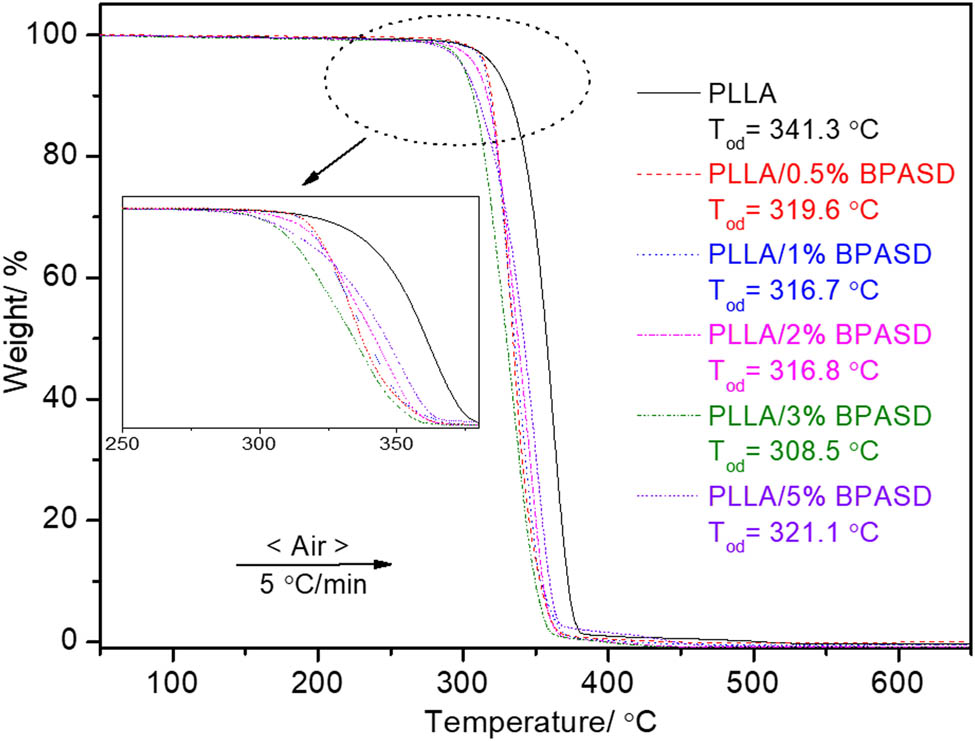
TGA curves of the virgin PLLA and PLLA/BPASD.
3.5 Optical and mechanical properties
Figure 10 is the light transmittance of the virgin PLLA and PLLA/BPASD. As shown in Figure 10, the effects of BPASD on the light transmittance of PLLA can be classified into three types, when the loading of BPASD is 0–0.5 wt%, the light transmittance only exhibits a very slight drop. And further increasing of BPASD concentration from 0.5 to 2 wt%, the light transmittance extremely decreases from 77.3% to 24.1%. However, when the BPASD concentration is higher than 2 wt%, the light transmittance exhibits a modest decrease, probable resulting from that the relevant PLLA/BPASD samples has almost saturated in color and luster, undoubtedly, more in-depth works need be performed to verify this speculation.
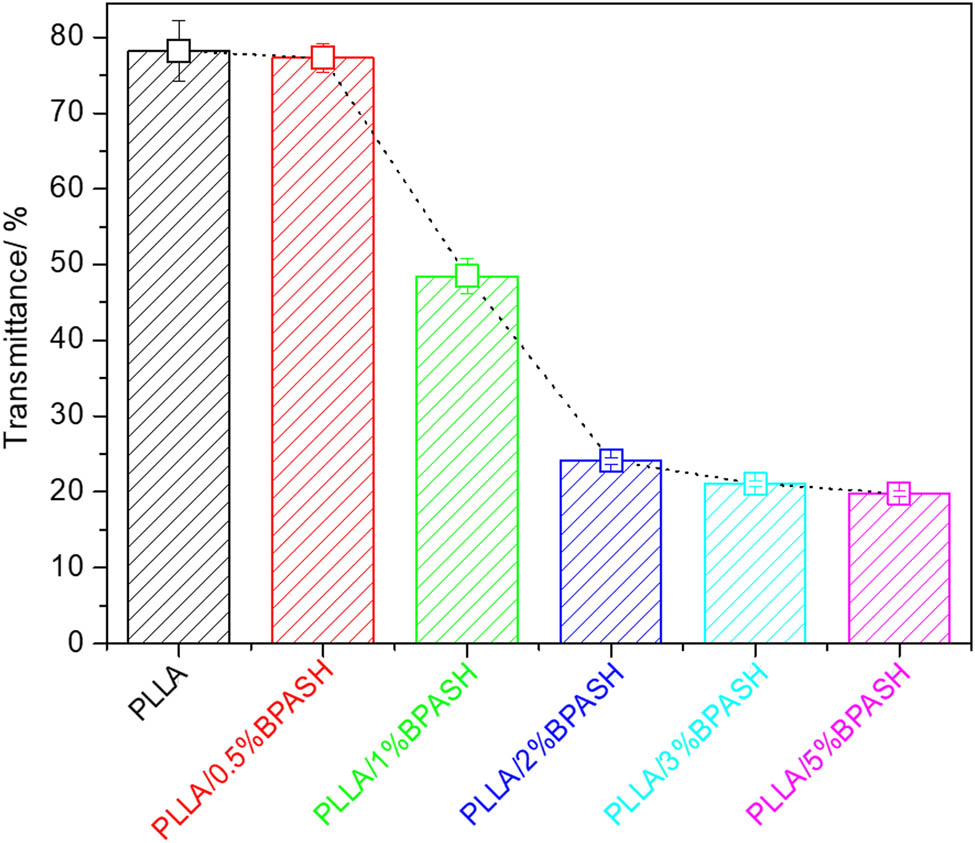
Light transmittance of the virgin PLLA and PLLA/BPASD.
The tensile modulus, tensile strength, and elongation at break of the virgin PLLA and BPASD-nucleated PLLA were listed in Table 1. As seen in Table 1, the effects of adding BPASH on the tensile modulus and tensile strength of PLLA are positive, that is, all PLLA/BPASH samples have the larger value of tensile modulus and tensile strength than the virgin PLLA because of the introduction of higher modulus BPASH, furthermore, among these PLLA/BPASH samples, PLLA/0.5% BPASH and PLLA/2% BPASH have the maximum tensile modulus (1677.6 MPa) and tensile strength (54.8 MPa), respectively. However, the elongation at break of PLLA almost steadily decreases from 6.9% to 3.6% with increasing BPASH concentration, indicating that the existence of BPASH causes the PLLA to become brittle, which is consistent with the influence of other nucleating agents on the elongation at break of PLLA (25,37).
The mechanical properties of the virgin PLLA and PLLA/PASH samples
| Sample | Tensile modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) |
|---|---|---|---|
| PLLA | 1536.6 ± 50.2 | 41.7 ± 2.2 | 6.9 ± 0.7 |
| PLLA/0.5% PASH | 1677.6 ± 46.1 | 48.8 ± 2.7 | 6.4 ± 0.4 |
| PLLA/1% PASH | 1541.2 ± 7.7 | 54.7 ± 1.7 | 4.7 ± 0.3 |
| PLLA/2% PASH | 1649.5 ± 26.5 | 54.8 ± 0.4 | 4.4 ± 0.3 |
| PLLA/3% PASH | 1642.8 ± 16.0 | 51.9 ± 1.3 | 3.8 ± 0.5 |
| PLLA/5% PASH | 1624.1 ± 21.8 | 51.8 ± 0.6 | 3.6 ± 0.1 |
4 Conclusion
In this study, the performances of PLLA modified by a new organic nucleator BPASD were investigated using DSC, TGA, transmittance instrument, and electronic tensile tester. The melt-crystallization behavior upon cooling of 1℃/min indicated that the BPASD could be acted as a good crystallization accelerator for promoting the fast nucleation of PLLA, and with an increase in the BPASD concentration, the melt-crystallization peak shifted to the higher temperature, exhibiting the better crystallization promoting ability; and the PLLA/5% BPASD sample had the highest Toc and melt-crystallization peak temperature. An increase in the cooling rate weakened the crystallization ability of PLLA/BPASD sample, but BPASD was able to accelerate the crystallization rate of PLLA even upon fast cooling at 50℃/min. The Tf was another key factor to affect the melt-crystallization process, and when Tf was 180℃, the crystallization could occur at a higher temperature. For the cold-crystallization process, when the BPASD concentration continuously increased, a shift to the lower temperature of cold-crystallization peak and decrease of enthalpy showed that BPASD had an inhibition for the cold-crystallization process of PLLA to some extent, but a higher heating rate could cause the cold-crystallization peak to move toward the higher temperature because of the thermal inertia. The melting behavior of PLLA/BPASD sample after crystallization further confirmed the nucleating role of BPASD for PLLA, and the difference in melting behavior depended on the heating rate, crystallization temperature, and BPASD concentration. Although the BPASD could not change the profile of PLLA, the addition of BPASD reduced the Tod of PLLA; on the other hand, the decrease of Tod may depend on the interaction of PLLA and BPASD. The tensile test showed that the introduction of BPASD could enhance the tensile modulus and tensile strength of PLLA, but the PLLA/BPASD samples became brittle, resulting in the continuous decrease of elongation at break.
Acknowledgements
This work was supported by National Natural Science Foundation of China (project number 51403027), Foundation of Chongqing Municipal Science and Technology Commission (cstc2017shmsA20021 and cstc2019jcyj-msxmX0876), Scientific and Technological Research Program of Chongqing Municipal Education Commission (project number KJQN201801319), and Foundation of Yongchuan District (project number Ycstc, 2018cc0801).
References
(1) Sun JH, Li L, Li J. Effects of furan-phosphamide derivative on flame retardancy and crystallization behaviors of poly(lactic acid). Chem Eng J. 2019;369:150–60.10.1016/j.cej.2019.03.036Search in Google Scholar
(2) Wu NJ, Yu JH, Lang WC, Ma XB, Yang Y. Flame retardancy and toughness of poly(lactic acid)/GNR/SiAHP composites. Polymers. 2019;11(7):1129.10.3390/polym11071129Search in Google Scholar PubMed PubMed Central
(3) Ghosh S, Krishnan S. Application of poly(lactic acid) fibres in automotive interior. Indian J Fibre Tex Res. 2007;32(1):119–21.Search in Google Scholar
(4) Li L, Bao RY, Gao T, Liu ZY, Xie BH, Yang MB, et al. Dopamine-induced functionalization of cellulose nanocrystals with polyethylene glycol towards poly(l-lactic acid) bionanocomposites for green packaging. Carbohydr Polym. 2019;203:275–84.10.1016/j.carbpol.2018.09.057Search in Google Scholar PubMed
(5) Wang L, Lee RE, Wang GL, Chu RKM, Zhao JC, Park CB. Use of stereocomplex crystallites for fully-biobased microcellular low-density poly(lactic acid) foams for green packaging. Chem Eng J. 2017;327:1151–1162.10.1016/j.cej.2017.07.024Search in Google Scholar
(6) Han J, Ma BJ, Liu HR, Wang T, Wang F, Xie CJ, et al. Hydroxyapatite nanowires modified polylactic acid membrane plays barrier/osteoinduction dual roles and promotes bone regeneration in a rat mandible defect model. J Biomed Mater Res A. 2018;106(12):3099–110.10.1002/jbm.a.36502Search in Google Scholar PubMed
(7) Ramesh S, Lungaro L, Tsikritsis D, Weflen E, Rivero IV, Elfick APD. Fabrication and evaluation of poly(lactic acid), chitosan, and tricalcium phosphate biocomposites for guided bone regeneration. J Appl Poly Sci. 2018;135(39):46692.10.1002/app.46692Search in Google Scholar
(8) Martin V, Ribeiro IA, Alves MM, Goncalves L, Claudio RA, Grenho L, et al. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanohydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mat Sci Eng C-Mater. 2019;101:15–26.10.1016/j.msec.2019.03.056Search in Google Scholar PubMed
(9) Yang FH, Niu XF, Gu XN, Xu CP, Wang W, Fan YB. Biodegradable magnesium-incorporated poly(l-lactic acid) microspheres for manipulation of drug release and alleviation of inflammatory response. ACS Appl Mater Interfaces. 2019;11(26):23546–57.10.1021/acsami.9b03766Search in Google Scholar PubMed
(10) Li SY, Guo SL. Optimal construction and pharmacokinetic study of CZ48-loaded poly(lactic acid) microbubbles for controlled drug delivery. Colloids Surf B Biointerfaces. 2019;178:269–75.10.1016/j.colsurfb.2019.02.047Search in Google Scholar PubMed
(11) Brzezinsk M, Socka M, Kost B. Microfluidics for producing polylactide nanoparticles and microparticles and their drug delivery application. Polym Int. 2019;68(6):997–1014.10.1002/pi.5753Search in Google Scholar
(12) Fernandes JS, Reis RL, Pires RA. Wetspun poly-l-(lactic acid)-borosilicate bioactive glass scaffolds for guided bone regeneration. Mat Sci Eng C-Mater. 2017;71:252–9.10.1016/j.msec.2016.10.007Search in Google Scholar PubMed
(13) Shen TF, Xu YS, Cai XX, Ma PM, Dong WF, Chen MQ. Enhanced crystallization kinetics of poly(lactide) with oxalamide compounds as nucleators: effect of spacer length between the oxalamide moieties. RSC Adv. 2016;6:48365.10.1039/C6RA04050KSearch in Google Scholar
(14) Yin HY, Wei XF, Bao RY, Dong QX, Liu ZY, Yang W, et al. Enhancing thermomechanical properties and heat distortion resistance of poly(l-lactide) with high crystallinity under high cooling rate. ACS Sustain Chem Eng. 2015;3:654–61.10.1021/sc500783sSearch in Google Scholar
(15) Li Y, Han CY, Yu YC, Xiao LG, Shao Y. Isothermal and nonisothermal cold crystallization kinetics of poly(l-lactide)/functionalized eggshell powder composites. J Therm Anal Calorim. 2018;131:2213–23.10.1007/s10973-017-6783-5Search in Google Scholar
(16) Liu JH, Cai JH, Tang XH, Weng YX, Wang M. Achieving highly crystalline rate and crystallinity in poly(l-lactide) via in situ melting reaction with diisocyanate and benzohydrazine to form nucleating agents. Polym Test. 2020;81:106216.10.1016/j.polymertesting.2019.106216Search in Google Scholar
(17) Fan YQ, Yu ZY, Cai YH, Yan SF, Chen XS, Yin JB. Crystallization behavior and crystallite morphology controlling of poly(l-lactic acid) by adding N,N′-bis(benzoyl) sebacic acid dihydrazide. Polym Int. 2013;62(4):647–57.10.1002/pi.4342Search in Google Scholar
(18) Xu XK, Zhen WJ, Bian SZ. Structure, performance and crystallization behavior of poly(lactic acid)/humic acid amide composites. Polymer Plast Tech Eng. 2018;57(18):1858–72.10.1080/03602559.2018.1434670Search in Google Scholar
(19) Li CL, Dou Q. Non-isothermal crystallization kinetics and spherulitic morphology of nucleated poly(lactic acid): effect of dilithium hexahydrophthalate as a novel nucleating agent. Thermochim Acta. 2014;594:31–38.10.1016/j.tca.2014.08.036Search in Google Scholar
(20) Gong XH, Pan L, Tang CY, Chen L, Li CQ, Wu CG, et al. Investigating the crystallization behavior of poly(lactic acid) using CdSe/ZnS quantum dots as heterogeneous nucleating agents. Compos Part B-Eng. 2016;91:103–10.10.1016/j.compositesb.2015.12.032Search in Google Scholar
(21) de Almeida JFM, da Silva ALN, Escocio VA, da Silva AHMDT, de Sousa AMF, Nascimento CR, et al. Rheological, mechanical and morphological behavior of polylactide/nano-sized calcium carbonate composites. Polym Bull. 2016;73(12):3531–45.10.1007/s00289-016-1656-9Search in Google Scholar
(22) Li Y, Han CY, Yu YC, Xiao LG, Shao Y. Effect of content and particle size of talc on nonisothermal melt crystallization behavior of poly(l-lactide). J Therm Anal Calorim. 2019;135(4):2049–58.10.1007/s10973-018-7365-xSearch in Google Scholar
(23) Wang SS, Han CY, Bian JJ, Han LJ, Wang XM, Dong LS. Morphology, crystallization and enzymatic hydrolysis of poly(l-lactide) nucleated using layered metal phosphonates. Polym Int. 2011;60:284–95.10.1002/pi.2947Search in Google Scholar
(24) Kawamoto N, Sakai A, Horikoshi T, Urushihara T, Tobita E. Nucleating agent for poly(l-lactic acid) – An optimization of chemical structure of hydrazide compound for advanced nucleation ability. J Appl Poly Sci. 2007;103(1):198–203.10.1002/app.25109Search in Google Scholar
(25) Pan PP, Liang ZC, Cao A, Inoue Y. Layered metal phosphonate reinforced poly(l-lactide) composites with a highly enhanced crystallization rate. ACS Appl Mater Interfaces. 2009;1(2):402–11.10.1021/am800106fSearch in Google Scholar PubMed
(26) Fan YQ, Yan SF, Yin JB. The relationship between solubility and nucleating effect of organic nucleating agent in poly(l-lactic acid). J Appl Poly Sci. 2019;136(7):46851.10.1002/app.46851Search in Google Scholar
(27) Ma PM, Xu YS, Shen TF, Dong WF, Chen MQ, Lemstra PJ. Tailoring the crystallization behavior of poly(l-lactide) with self-assembly-type oxalamide compounds as nucleators: 1. Effect of terminal configuration of the nucleators. Eur Polym J. 2015;70:400–11.10.1016/j.eurpolymj.2015.07.040Search in Google Scholar
(28) Cai YH, Tang Y, Zhao LS. Poly(l-lactic acid) with organic nucleating agent N,N,N′-tris(1H-benzotriazole) trimesinic acid acethydrazide: crystallization and melting behavior. J Appl Poly Sci. 2015;132(32):42402.10.1002/app.42402Search in Google Scholar
(29) Ye HM, Hou K, Zhou Q. Improve the Thermal and mechanical properties of poly(l-lactide) by forming nanocomposites with pristine vermiculite. Chinese J Polym Sci. 2016;34(1):1–12.10.1007/s10118-016-1724-5Search in Google Scholar
(30) Jin XZ, Yu X, Yang C, Qi XD, Lei YZ, Wang Y. Crystallization and hydrolytic degradation behaviors of poly(l-lactide) induced by carbon nanofibers with different surface modifications. Polym Degrad Stabil. 2019;170:109014.10.1016/j.polymdegradstab.2019.109014Search in Google Scholar
(31) Zhao LS, Cai YH. Investigating the physical properties of poly(l-lactic acid) modified using a aromatics succinic dihydrazide derivative. Polym Sci Ser A+. 2018;60(6):777–87.10.1134/S0965545X18070088Search in Google Scholar
(32) Cai YH, Zhao LS, Zhang YH. Role of N,N′-bis(1H-benzotriazole) adipic acid acethydrazide in crystallization nucleating effect and melting behavior of poly(l-lactic acid). J Polym Res. 2015;22:246.10.1007/s10965-015-0887-zSearch in Google Scholar
(33) Su ZZ, Guo WH, Liu YJ, Li QY, Wu CF. Non-isothermal crystallization kinetics of poly(lactic acid)/modified carbon black composite. Polym Bull. 2009;62:629–42.10.1007/s00289-009-0047-xSearch in Google Scholar
(34) Tian LL, Cai YH. Poly(l-lactic acid) modified by magnesium phenylmalonate: Thermal behavior, processing fluidity, and mechanical properties. Mater Sci-Medzg. 2018;24(1):81–87.10.5755/j01.ms.24.1.17947Search in Google Scholar
(35) Ratta V, Ayambem A, Mcgrath JE, Wilkes GL. Crystallization and multiple melting behavior of a new semicrystalline polyimide based on 1,3-bis(4-aminophenoxy)benzene (TPER) and 3,3′,4,4′-bisphenonetetracarboxylic dianhydride (BTDA). Polymer. 2001;42(14):6173–86.10.1016/S0032-3861(01)00010-6Search in Google Scholar
(36) Yasuniwa M, Tsubakihara S, Sugimoto Y, Nakafuku C. Thermal analysis of the double-melting behavior of poly(l-lactic acid). J Polym Sci Pol Phys. 2004;42(1):25–32.10.1002/polb.10674Search in Google Scholar
(37) Li Y, Xin SY, Bian YJ, Xu K, Han CY, Dong LS. The physical properties of poly(l-lactide) and functionalized eggshell powder composites. Int J Biol Macromol. 2016;85:63–73.10.1016/j.ijbiomac.2015.12.070Search in Google Scholar PubMed
© 2020 Li-Sha Zhao and Yan-Hua Cai, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery


