Abstract
6,7-Bis(hexylthio)-2-[(2-hydroxyethyl)thio]-3-methylthio-tetrathiafulvalene (TTF-2) is coupled with thiophene-3-carboxylic acid and thiophene-3,4-dicarboxylic acid by Steglich esterification, respectively, to afford 2-((4′,5′-bis(hexylthio)-5-(methylthio)-[2,2′-bi(1,3-dithiolylidene)]-4-yl)thio)ethyl thiophene-3-carboxylate (TTF-Th) and bis(2-((4′,5′-bis(hexylthio)-5-(methylthio)-[2,2′-bi(1,3-dithiolylidene)]-4-yl)thio)ethyl)thiophene-3,4-di-carboxylate (DTTF-Th). Their structures were characterized by ESI-MS, 1H NMR, and elemental analysis. Electropolymerization of TTF-Th and DTTF-Th was conducted with 0.1 M n-Bu4NPF6. The results indicated that both assemblies could rapidly form polymers via electrochemical deposition. In addition, their electrochromic performances illustrated that the color of P(TTF-Th) could switch from orange-yellow to dark blue, while P(DTTF-Th) changed its color from orange in the neutral state to dark blue in the oxidation state. Moreover, the electrochromic performances of P(DTTF-Th) were better than P(TTF-Th) due to the introduction of one extra TTF unit.
1 Introduction
Among all electrochromic materials, conjugated conductive polymers have drawn a great deal of attention during the past few decades due to their structural controllability, high coloration efficiency (CE), fast response time, low cost, and various color changes (1,2). Polythiophene is one of the most common conducting electrochromic polymers, owing to the ease of electropolymerization, stable electrochromic performance, significant color contrast, and considerable conductivity (3).
Compared with inorganic materials, the main drawbacks of conducting electrochromic polymers are the stability of oxidized state and machinability (4). For example, the oxidized unsubstituted polythiophene is unstable in air because of its high oxidation potential. β-Substituted polythiophene could have much lower oxidation potential when electron donors were introduced (4). Thus, numerous novel poly(3-substituted thiophenes) and poly(3,4-sunbstituted thiophenes) have been synthetized and studied, among which poly(3,4-(ethylenedioxy)thiophene) exhibited some extraordinary properties (5,6,7,8). Also, the introduction of long alky chains could improve the solubility of polymer to enhance the machinability.
Tetrathiafulvalene (TTF) and its derivatives with unique electron structure can be reversibly oxidized to radical monocation and dication, which are both thermodynamically stable and aromatic. TTF, as an outstanding electron donor, has generated considerable interest in optoelectronic materials, such as fluorescence probe (9), dye-sensitive solar cell (10), and light-sensitive ambipolar (11). It has also been reported to participate in the construction of conducting polymers in backbone or side chain (12,13,14,15,16).
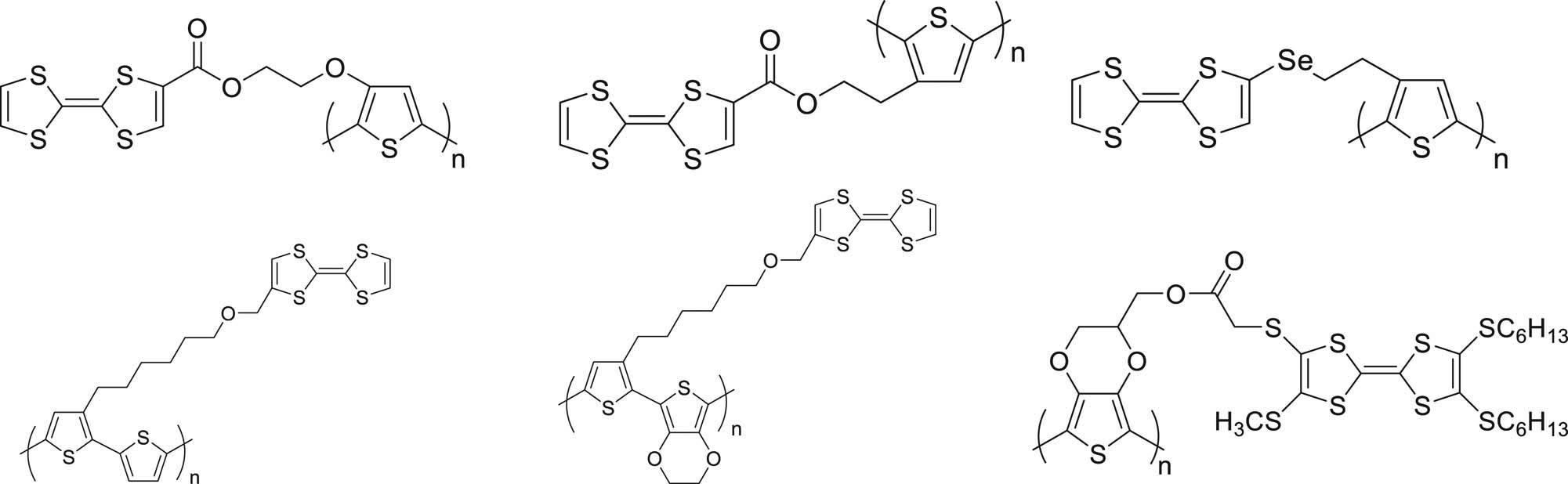
TTF–thiophene assembly and its polymer were first reported in 1991 by Bryce et al. (17). In the later decades, the electrochromic properties of TTF–thiophene polymers were studied (18,19). In our previous work, TTF derivatives with long alky chains had been developed to form various polymers (15,20,21,22,23), especially TTF–EDOT polymers. The patterns of TTF–thiophene polymers are shown in Figure 1.
We found that the intermolecular electron transfer between TTF and EDOT was not significant (23). Hence, TTF moiety was directly introduced to thiophene in this study. Moreover, we decided to attach different numbers of TTF units to thiophenes. By comparing the experimental results of different polymers, we can make a clear understanding of the influence of TTF numbers on electrochromic properties, which is the aim and the innovation of this study.
In this study, TTF and thiophenes were connected via Steglich esterification. The synthetic route is shown in Scheme 1. The assembly, used as monomers, was transformed into polymers via electropolymerization. The results indicated that the polymers possessed electrochromic performances when different potentials were applied to them. Moreover, performances vary because of the number of TTF units.
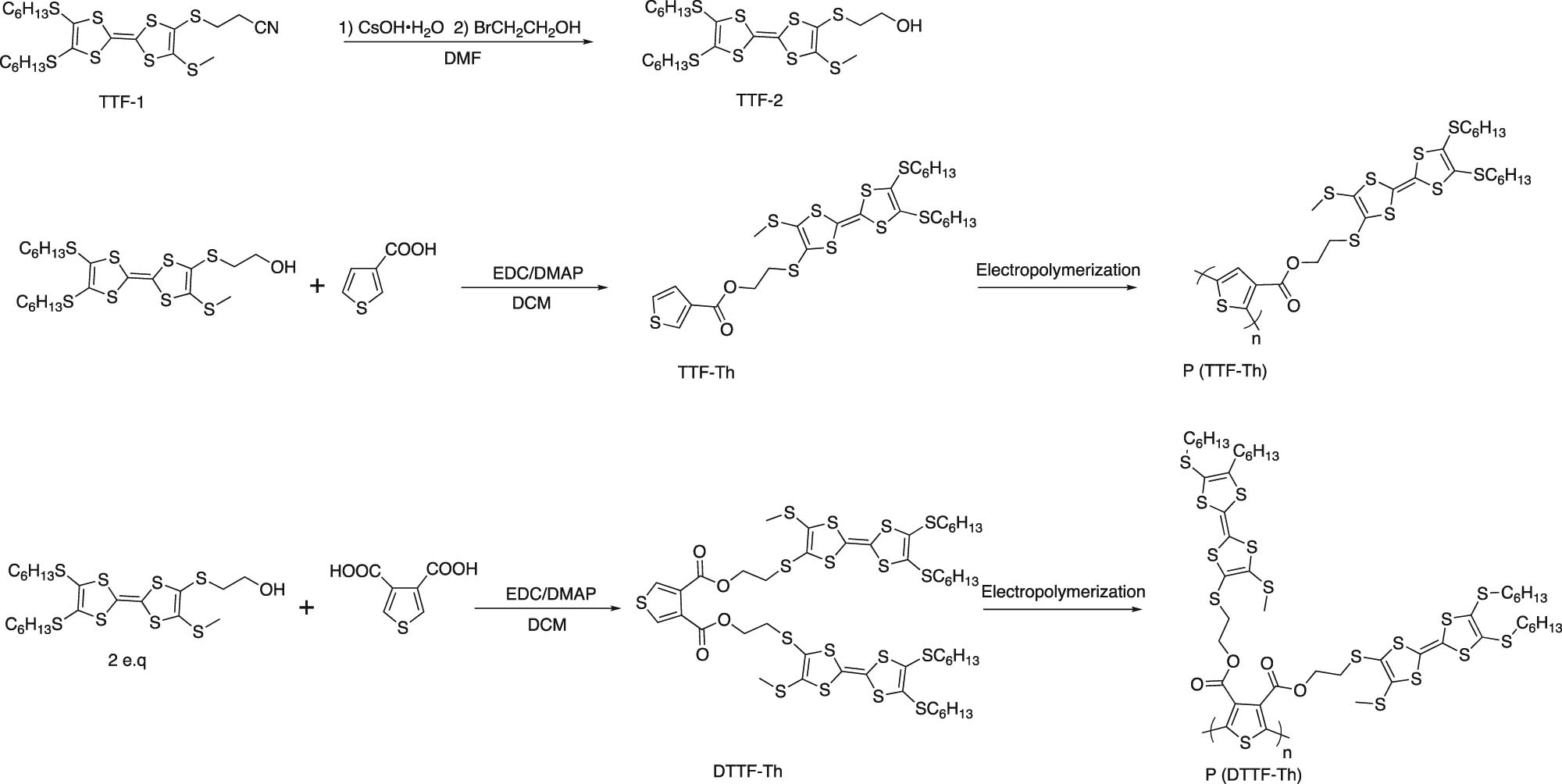
The synthetic route of P(TTF-Th) and P(DTTF-Th).
2 Experimental
2.1 Instruments and materials
1H NMR was recorded on a Bruker AVANCE 400 (Bruker BioSpin AG Co.); HRMS was obtained by a MAT 8430 spectrometer (ESI; Finnigan); UV-Vis was carried out with USB 2000 miniature UV-Vis spectrometer (Ocean Optics). Cyclic voltammetry and electropolymerization were performed in CHI 620E electrochemical workstation (Shanghai Chenhua Instruments Co. Ltd).
The synthesis of the precursor TTF-1 was performed based on the literature (24). All reagents and solvents were commercially available and provided by certified manufacturers. Dichloromethane (DCM) was dried by CaH2 and fresh-distilled accordingly; N,N-dimethylformamide (DMF) was dehydrated by 4 A molecular sieve.
2.2 Synthesis
2.2.1 TTF-2
TTF-1 (0.57 g, 1 mmol) was dissolved in freshly dried DMF (30 mL). A solution of CsOH (0.19 g, 1.1 mmol) in methanol (5 mL) was added after 10 min. The mixture was stirred under nitrogen for 30 min at room temperature. After bromoethanol (0.5 mL, 7.3 mmol) was added, the mixture was stirred for another 12 h before the completion of reaction. The reaction mixture was extracted by ethyl acetate (3 × 50 mL) and washed with DI water (3 × 50 mL). The organic layer was collected and concentrated by a vacuum evaporator. The residue was purified on silica column chromatography (petroleum ether [PE]:DCM = 2:1, v/v). A total of 0.412 g (73.8%) red powder was obtained. 1H NMR (CDCl3, 400 MHz, TMS, ppm): δ = 3.74 (t, J = 12.0 Hz, 2H, –CH2O), 2.95 (t, J = 12.0 Hz, 2H, –SCH2), 2.81 (t, J = 4.0 Hz, 4H, –SCH2), 2.47 (s, 3H, –SCH3), 1.60 (t, J = 16.0 Hz, 4H, –CH2), 1.42–1.26 (m, 12H, –C3H6), and 0.86 (t, J = 16.0 Hz, 6H, –CH3).
2.2.2 TTF-Th
Thiophene-3-carboylic acid (83 mg, 0.65 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC; 143 mg, 0.75 mmol), and 4-dimethylaminopyridine (DMAP; 92 mg, 0.75 mmol) were dissolved into freshly distilled DCM (20 mL). After stirring for 30 min at 40°C under nitrogen, TTF-2 (279 mg, 0.5 mmol) was added into the reaction mixture. After stirring for 24 h at RT under nitrogen, the reaction mixture was washed with 0.1 M hydrochloric acid (3 × 30 mL) and DI water (3 × 30 mL). The organic layer was collected and concentrated by a vacuum evaporator. The residue was purified on silica column chromatography (PE:DCM = 4:1, v/v). A total of 0.115 g (34.4%) orange powder was obtained. 1H NMR (CDCl3, 400 MHz, TMS, ppm): δ = 8.12 (s, 1H, thiophene), 7.51 (d, J = 4.0 Hz, 1H, thiophene), 7.30 (s, 1H, J = 4.0 Hz, thiophene), 3.54 (t, J = 12.0 Hz, 2H, –CH2CO); HRMS (ESI): [M + Na]+ calcd for: 691.0125; found: 691.0099.
2.2.3 DTTF-Th
Thiophene-3,4-dicarboxylic acid (43 mg, 0.25 mmol), EDC (143 mg, 0.75 mmol), and DMAP (92 mg, 0.75 mmol) were dissolved in freshly distilled DCM (20 mL). After stirring for 30 min at 40°C under nitrogen, TTF-2 (307 mg, 0.55 mmol) was added into the reaction mixture. After stirring for 24 h at RT under nitrogen, the reaction mixture was washed with 0.1 M hydrochloric acid (3 × 30 mL) and DI water (3 × 30 mL). The organic layer was collected and concentrated by a vacuum evaporator. The residue was purified on silica column chromatography (PE:DCM = 4:1, v/v). A total of 75 mg (24.0%) orange powder was obtained. 1H NMR (CDCl3, 400 MHz, TMS, ppm): δ = 7.91 (s, 2H, thiophene), 4.48 (t, J = 12.0 Hz, 4H, –CH2CO), and 3; HRMS (ESI): [M + Na]+ calcd for: 1275.0262; found: 1275.0268.
2.3 Electropolymeriazation
TTF-Th and DTTF-Th were electropolymerized by static-potential chronoamperometry. The initial potential and pace potential were 0 and 2.0 V, respectively. ITO glass was used as both the working and counter electrodes, and Ag/AgCl electrode was used as the reference electrode. The duration of polymerization was 600 s.
P(TTF-Th) was obtained by potentiostatic polymerization of TTF-Th. TTF-Th was dissolved in freshly distilled mixed solvent (acetonitrile [AN]:methylene chloride = 4:1, v/v). The concentration was 0.1 M; n-Bu4NPF6 (0.1 M) was used as the supporting electrolyte.
P(DTTF-Th) was afforded by potentiostatic polymerization of DTTF-Th. Other reaction conditions were the same as that used for P(TTF-Th).
2.4 Electrochromic analysis
The obtained polymer was dissolved in the electrolyte after electropolymerization. The solvent was removed by a vacuum evaporator. The solution of polymer in AN was sprayed on a piece of ITO glass. After being dried in air, the ITO glass was dipped into AN for another 24 h to remove extra n-Bu4NPF6. The film was obtained after being dried in air for 12 h. Figure 2 shows the films before and after immersion in AN.
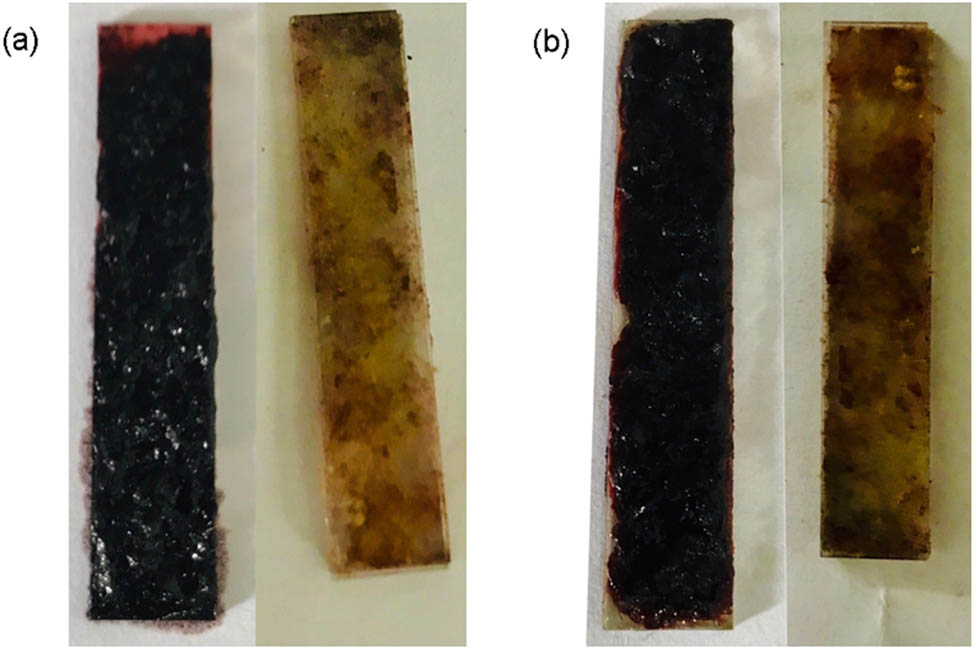
Digital picture of polymer films immersion in AN: (a) P(TTF-Th) and (b) P(DTTF-Th).
The prepared film was placed into a cuvette with 0.1 M n-Bu4NPF6/AN, which also worked as an electrolytic bath. ITO glass was used as the working electrode. Counter electrode was the platinum wire, and Ag/AgCl electrode was used as the reference electrode.
3 Results and discussion
3.1 Electrochemical and optical properties
The electrochemical redox behaviors of TTF-Th and DTTF-Th were examined by cyclic voltammetry at a potential scan rate of 100 mV s−1. The CV curves of TTF-2, TTF-Th, and DTTF-Th are shown in Figure 3, and their oxidation peak data are listed in Table 1. All three compounds exhibited two pairs of redox peaks. With the increase of the scan speed, the current of oxidation peaks enlarged. In the meantime, the peaks migrated due to the polarization of electrodes.
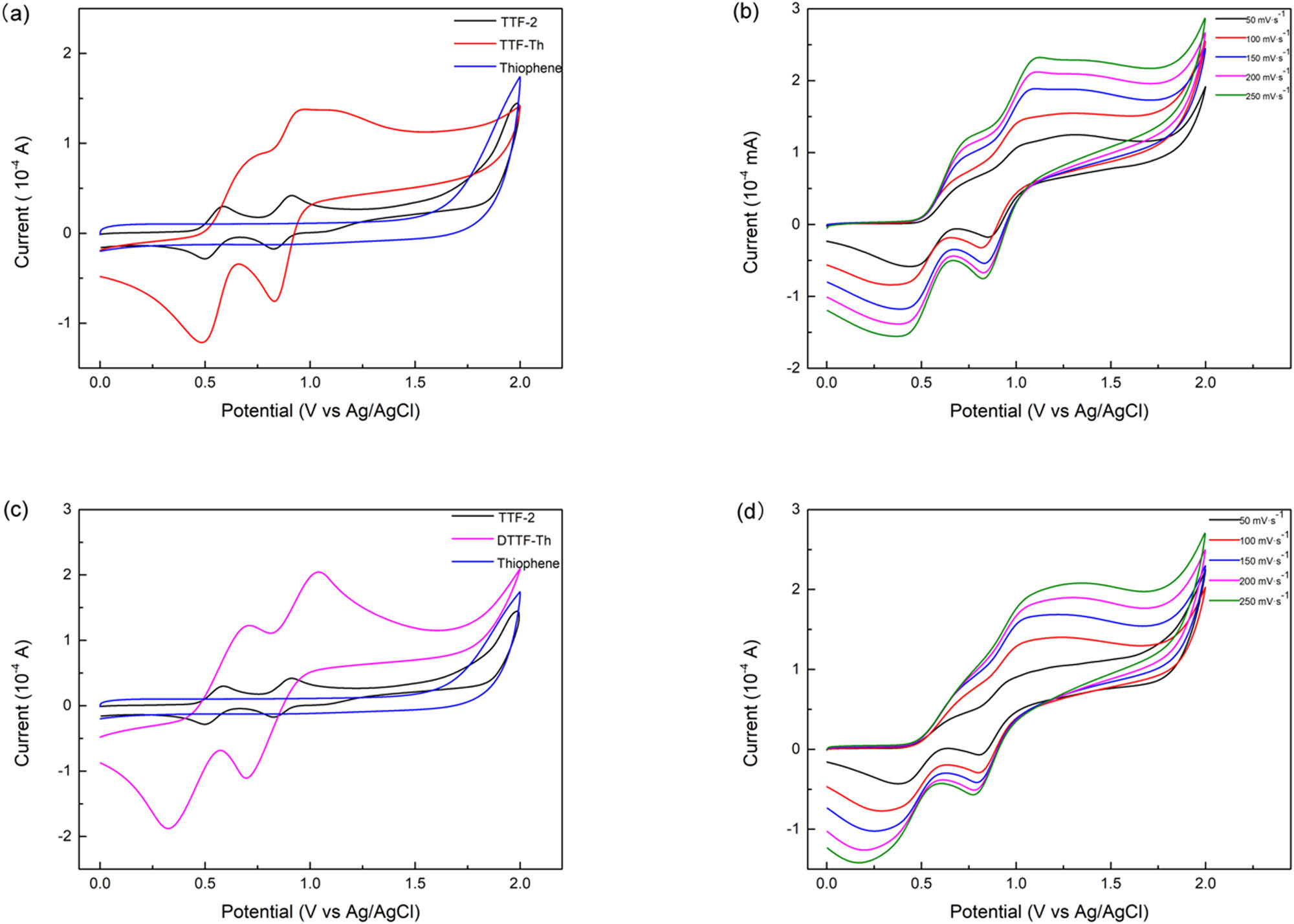
Cyclic voltammetry curves: (a) TTF-2, TTF-Th, and thiophene; (b) TTF-Th at different scan speeds; (c) TTF-2, DTTF-Th, and thiophene; and (d) DTTF-Th at different levels of scan speed.
Oxidation potentials and migration of TTF-2, TTF-Th, and DTTF-Th
| TTF-2 | 0.56 | 0.91 | ||
| TTF-Th | 0.71 | 0.96 | 150 | 50 |
| DTTF-Th | 0.71 | 1.03 | 150 | 120 |
Among these curves, TTF-2 showed two oxidation peaks at 0.56 and 0.91 V, which correspond to the successive oxidation of TTF unit into radial monocation TTF+·and dication TTF2+. TTF-Th and DTTF-Th displayed their
DTTF-Th was expected to exhibit four pairs of redox peaks since there are two TTF units in this molecule. However, only two pairs appeared at the actual curve, which showed that DTTF-Th is a parallel structure. There are two chemical equivalent TTF units in DTTF-Th.
The UV-Vis spectra of thiophene, TTF-2, TTF-Th, and DTTF-Th (1 × 10−5 M in DCM) are shown in Figure 4. The curve of thiophene shows a strong absorption peak at 211 nm, which refers to the characteristic absorption peak of π-conjugation thiophene ring. TTF-2 displayed a broad absorption band from 230 to 350 nm, with three fine peaks at 254, 311, and 342 nm, indicating the π–π* transition of the TTF unit. Also, the shoulder peak from 370 to 400 nm could be considered as the hydrogen bond interaction (18), which often can be observed in TTF alcohol and carboxylic acid derivatives. The absorption curve of TTF-Th looks like the combination of thiophene and TTF-2, which implies that TTF and thiophene are connected successfully. However, a 40 nm red shift of thiophene absorption peak could be observed, which indicated the intramolecular interaction between TTF and thiophene units. Like TTF-Th, the curve of DTTF-Th exhibited the similar tendency. A 50 nm red shift of thiophene absorption peak could also be observed, indicating stronger interaction between TTF and thiophene units. Compared with TTF-Th, the relative strength of absorption of TTF moiety was stronger because of the existence of two TTF units.
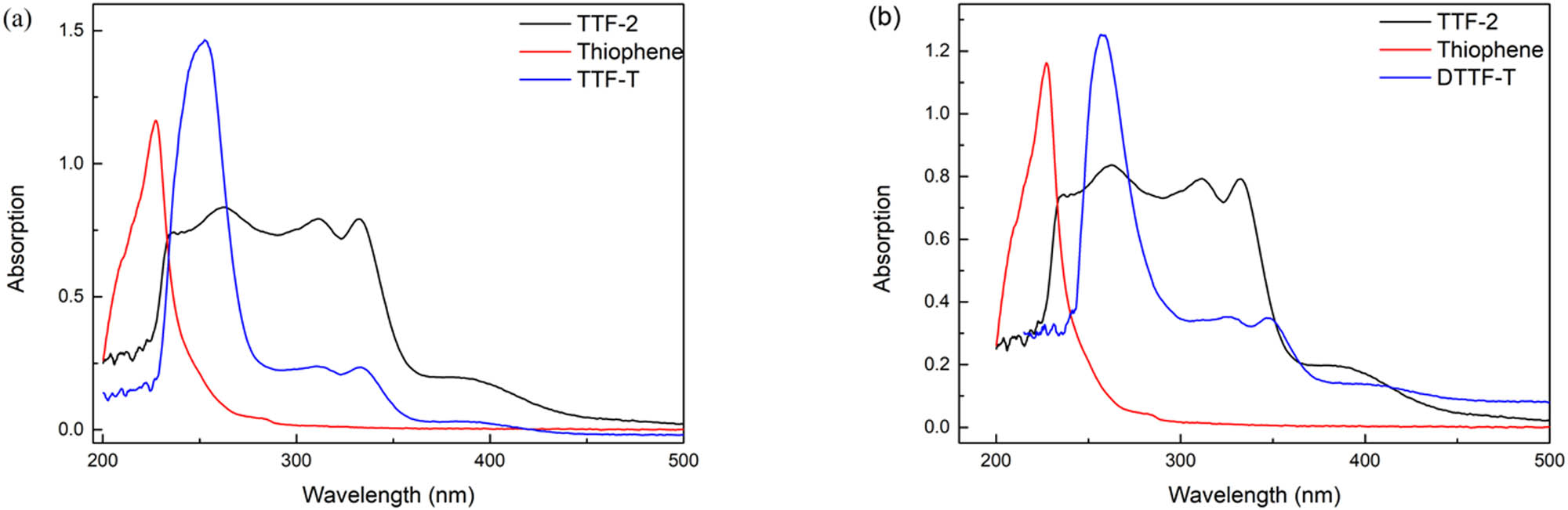
UV-Vis spectra: (a) TTF-2, TTF-Th, and thiophene; (b) TTF-2, DTTF-Th, and thiophene.
3.2 Electrochromic performances of the polymers
Figure 5 illustrates the electrochromic behaviors of P(TTF-Th). The wavelength range was from 350 to 850 nm because the ITO glass had influence on the absorption under 350 nm and the upper limit of detection of the instrument was 850 nm. The neutral polymer was orange with no obvious absorption between 350 and 850 nm. With the increase of the applied potential, the oxidation of the polymer film gradually happened at 0.8 V. An absorption band near 430 nm could be observed. The absorption band became stronger and stronger, which finally reached peak at 1.2 V. With increasing potential, the absorption band at 450 nm was weakened and disappeared, while a new and gradually enhanced absorption band appeared at 700 nm. The absorption maximum was observed at 1.5 V when the color of the polymer turned to blue.
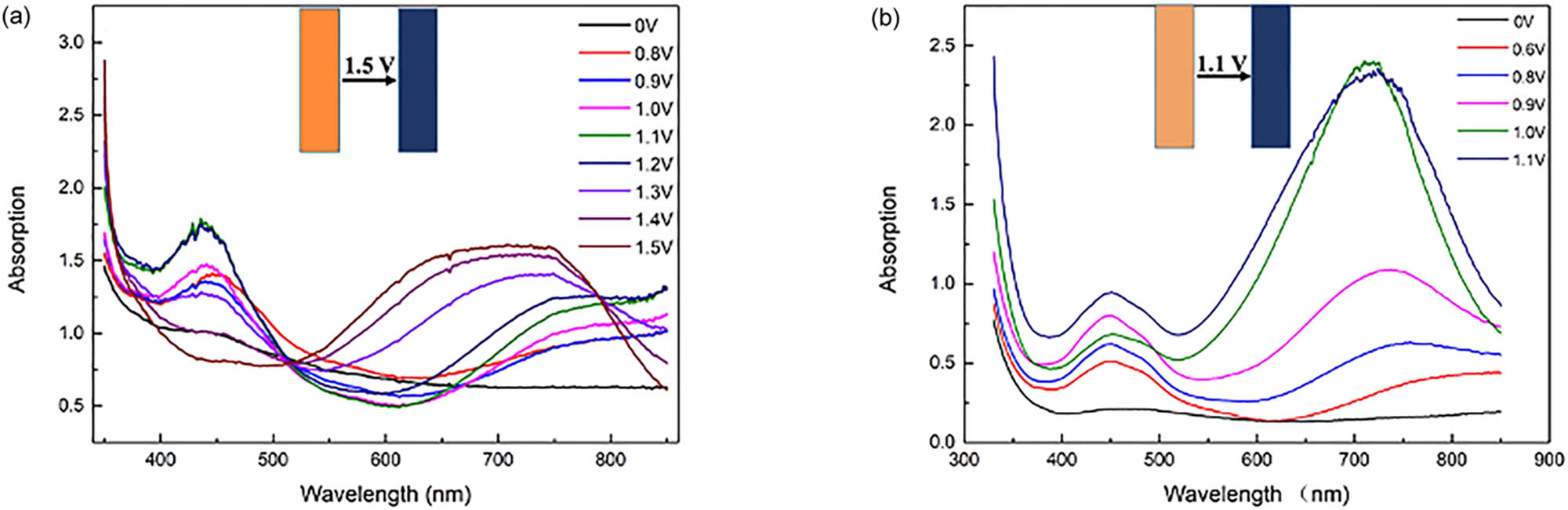
Electrochromic curves at increasing potentials: (a) P(TTF-Th) and (b) P(DTTF-Th).
The absorption band of P(TTF-Th) at 430 nm was considered as the absorption of oligothiophene, which was weakened due to further polymerization. The absorption band at 750 nm referred to the absorption of oxidized polymer.
Some unique electrochromic behaviors of P(DTTF-Th) were exhibited. Like P(TTF-Th), the absorption band at 450 nm appeared at a lower potential (0.6 V). With increasing potential, a new and gradually enhanced absorption band appeared at 700 nm, while the former 450 nm band was also enhanced. The absorption maximum was attained at 1.1 V, while the color of polymer turned to dark blue.
Compared with P(TTF-Th), P(DTTF-Th) was more symmetric. The absorption band of P(DTTF-Th) at 450 nm was considered as the absorption of radical cation of TTF moiety. Thus, the strength was enhanced with the increase of the applied potential. The absorption band at 750 nm also referred to the absorption of oxidized polymer. The lower response potential and the more obvious color change could be fairly attributed to the increase of TTF units.
The coloration response time of two polymers is shown in Figure 6. P(DTTF-Th) had quicker response time (2.7 s) than P (TTF-Th) (5.0 s). Optical contrast refers to the change of transmittance during coloration and bleed process. At 700 nm, the optical contrast of P(TTF-Th) could be easily calculated (16.56%). At 710 nm, P(TTF-Th) had more significant optical contrast (27.12%), which also shows the better electrochromic performance.
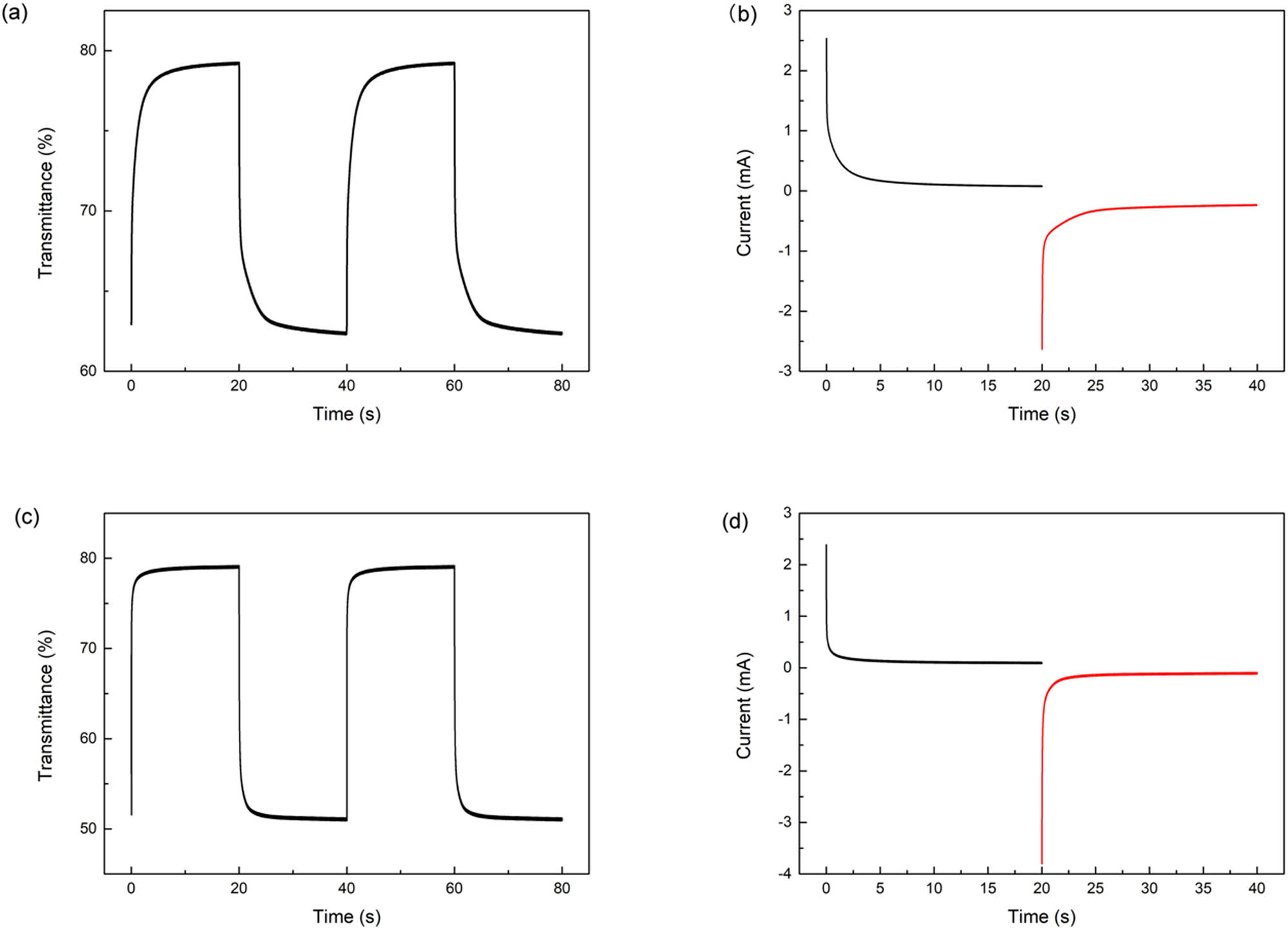
(a) The timing transmittance curves of P(TTF-Th); (b) the timing current curve of P(TTF-Th); (c) the timing transmittance curves of P(DTTF-Th); and (d) the timing current curve of P(DTTF-Th).
CE can be given by:
where ΔQ is the time integral of current on unit area. The integral can be obtained by timing current curves, and the area of electrode is provided by the manufacturer. Thus, the CE of P(TTF-Th) at 710 nm is 32.70 cm2 C−1. The value of P(DTTF-Th) is 59.31 cm2 C−1. All the electrochromic parameters are listed in Table 2.
Electrochromic performance parameters of two polymers
| Polymers | λmax (nm) | Tb (%) | Tc (%) | ΔT (%) | Response time (s) | CE (cm2 C−1) |
|---|---|---|---|---|---|---|
| P(TTF-Th) | 700 | 79.22 | 62.66 | 16.56 | 5.0 | 32.70 |
| P(DTTF-Th) | 710 | 79.03 | 51.91 | 27.12 | 2.7 | 59.31 |
3.3 Theoretical calculations
To better understand the electron and structure properties of two polymers, TTF-Th and DTTF-Th, were theoretically calculated by the hybrid density functional theory at the B3LYP/6-31G* level of theory. The geometries and the electron simulation of HOMO/LUMO orbitals are shown in Figure 7. It can be noted that the HOMO wave functions of TTF-Th were located at the TTF unit, the electron rich part, while the electron partly spreads to the thiophene moiety in the LUMO orbital. For DTTF-Th, the HOMO and LUMO wave functions were located on the TTF donor and thiophene acceptor moiety, respectively.
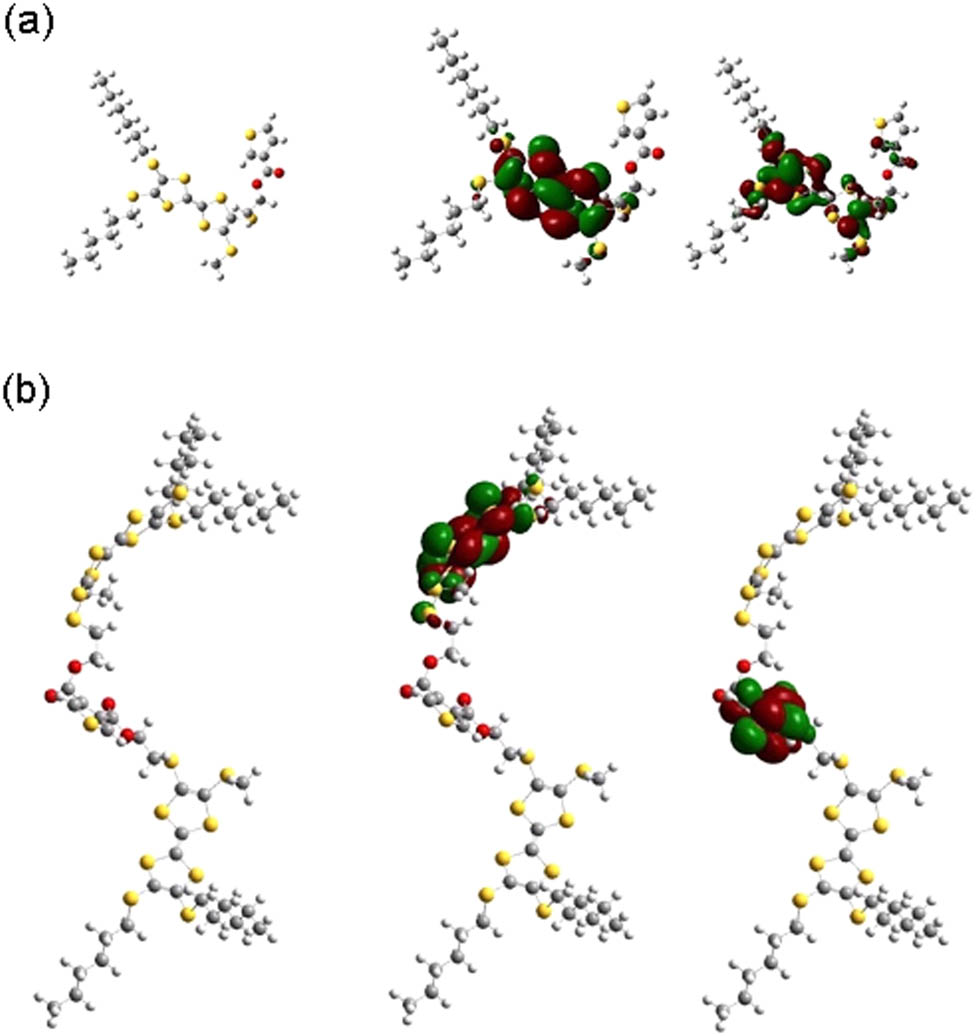
Geometries and HOMO/LUMO orbital simulations: (a) TTF-Th and (b) DTTF-Th.
In addition, the HOMO/LUMO orbital energies and theoretical band gaps of the polymers are listed in Table 3. The HUMO energy levels of TTF-Th and DTTF-Th were much lower than those of unsubstituted thiophene, and the values were close to the oxidation threshold (approximately −5.27 eV) (25), indicating that the synthesized polymers were resistant to oxidation in air, compared with unsubstituted polythiophene.
Theoretical calculated HOMO/LUMO energies and band gap of TTF-Th and DTTF-Th
| HOMO (eV) | LUMO (eV) | Eg (eV) | |
|---|---|---|---|
| Thiophene | −6.58 | −0.46 | −6.12 |
| TTF-Th | −5.09 | −1.34 | −3.75 |
| DTTF-Th | −5.02 | −1.80 | −3.22 |
4 Conclusions
Two novel TTF-thiophene assemblies, TTF-Th and DTTF-Th, were synthesized. The results of UV-Vis absorption spectra and redox showed that there was interaction between TTF and thiophene units in both TTF-Th and DTTF-Th. These two assemblies were electropolymerized to afford the corresponding polymers. P(DTTF-Th) exhibited better electrochromic properties, with lower starting and ending voltage, quicker response time, more significant optical contrast, and better CE. The number of TTF units does influence the electrochromic performances of polymers.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant No. 21576087).
Appendix
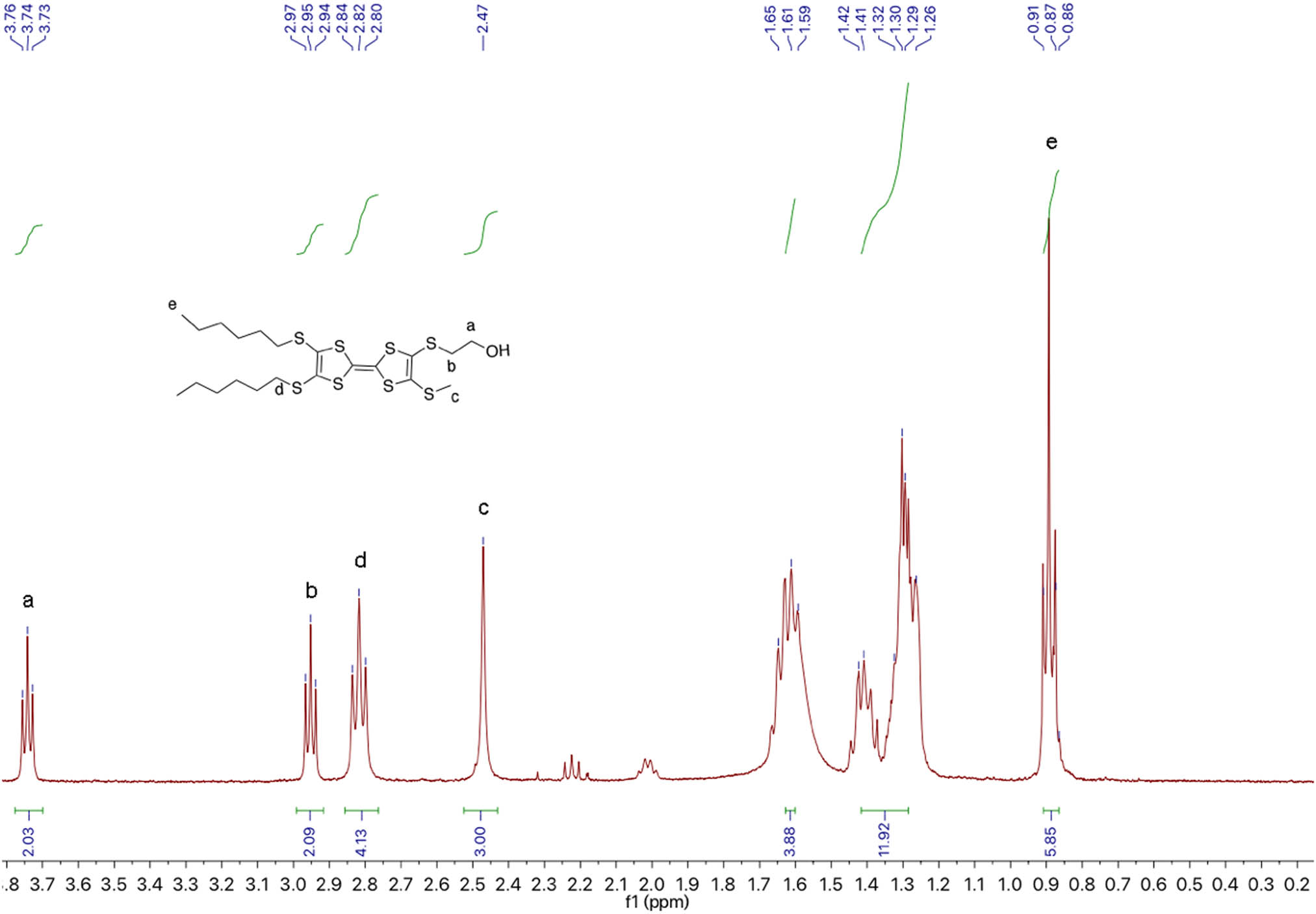
1H NMR of TTF-2.
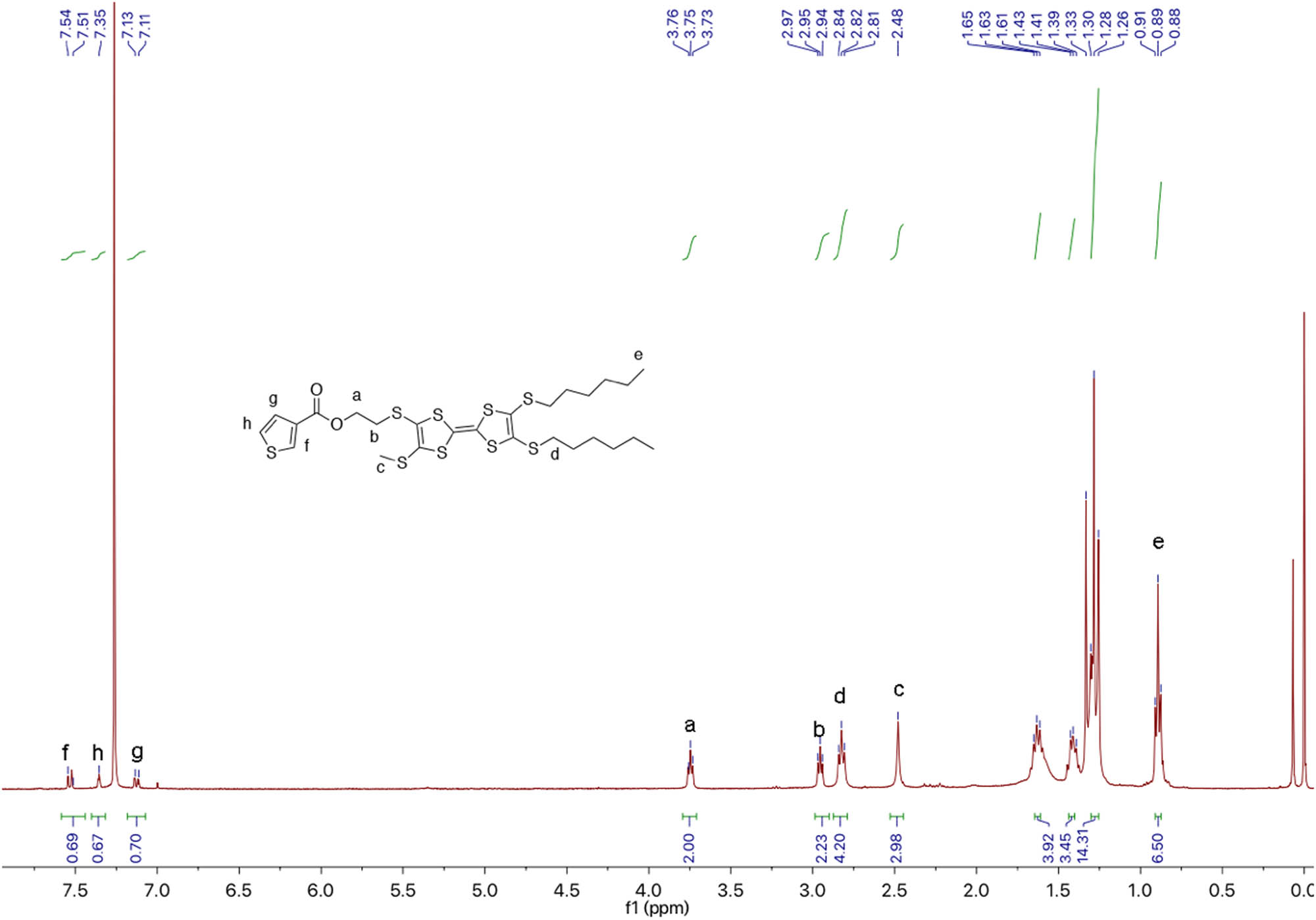
1H NMR of TTF-Th.
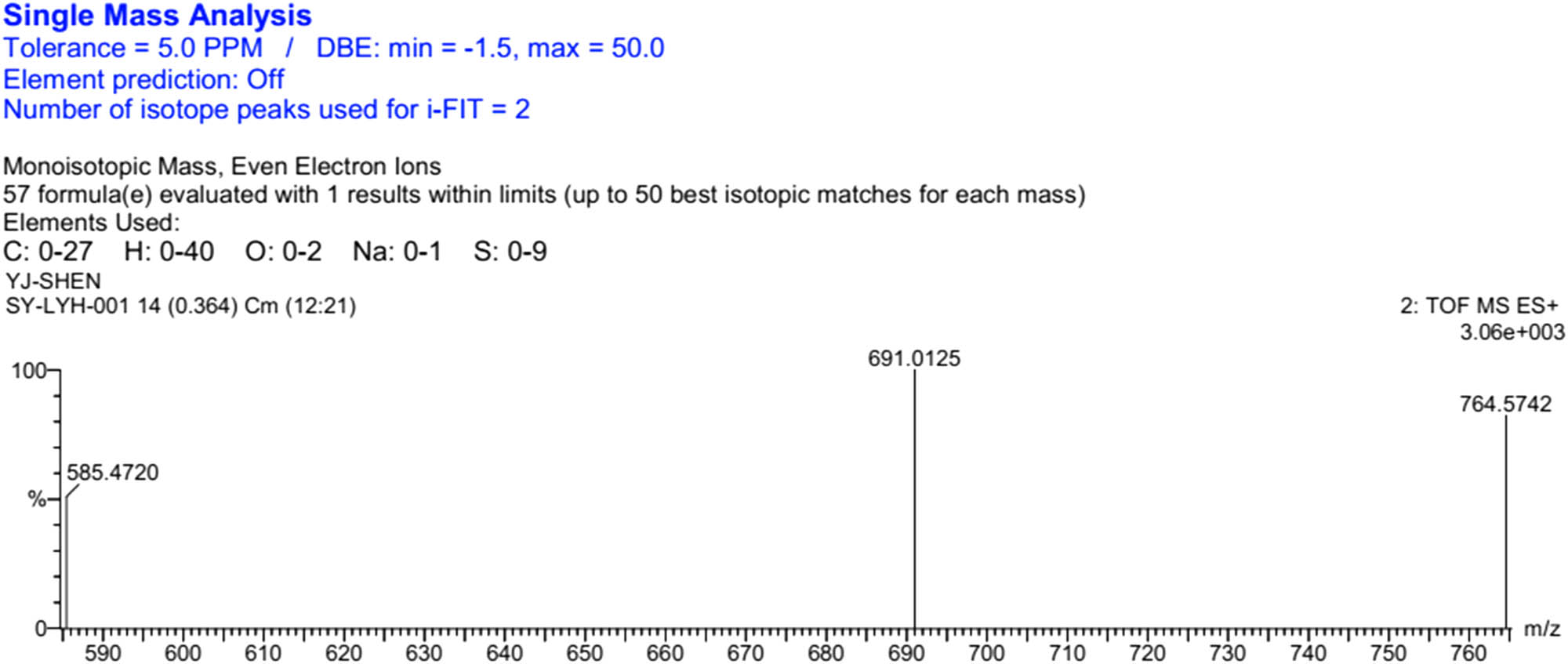
HRMS (ESI) of TTF-Th.
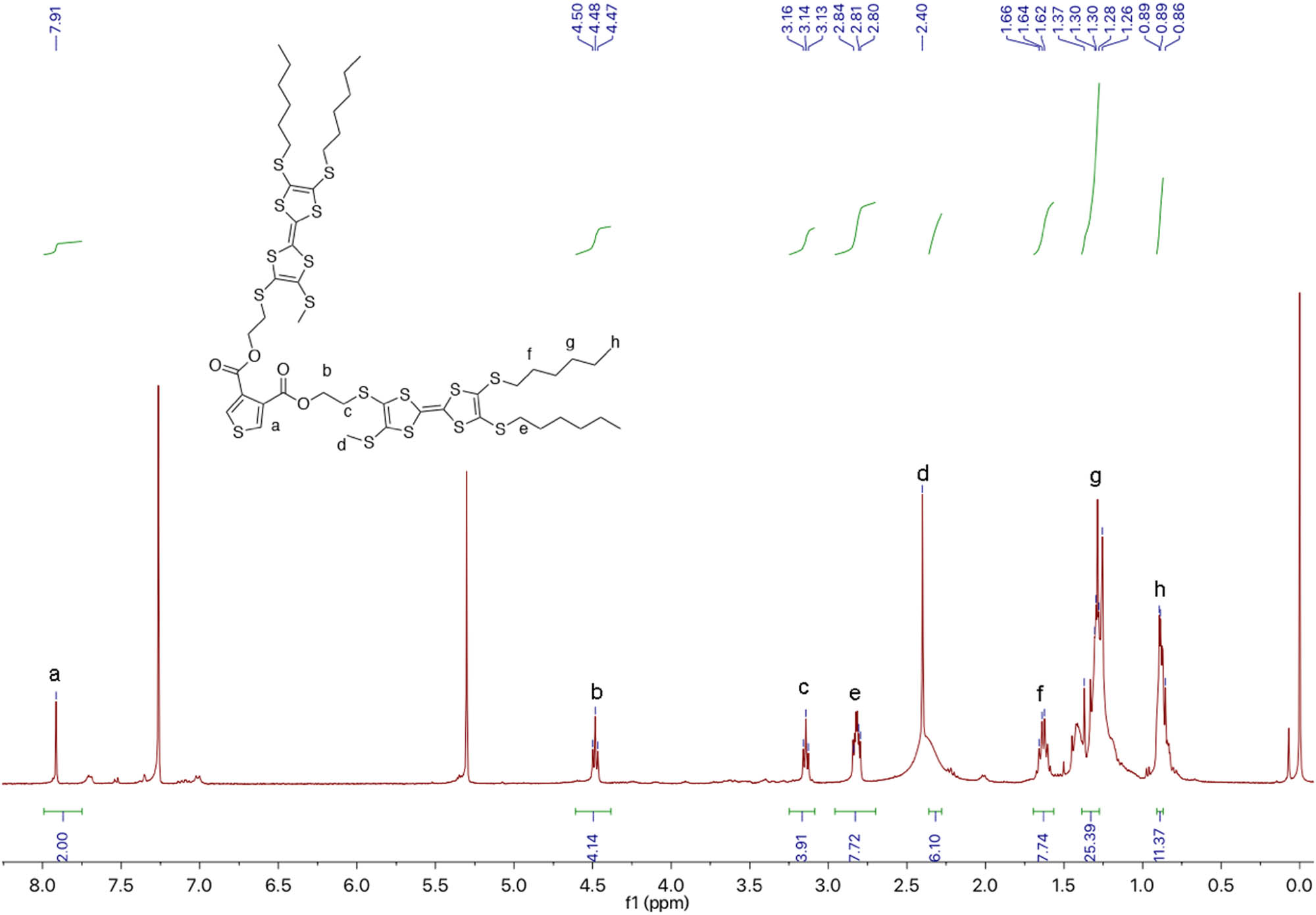
1H NMR of DTTF-Th.
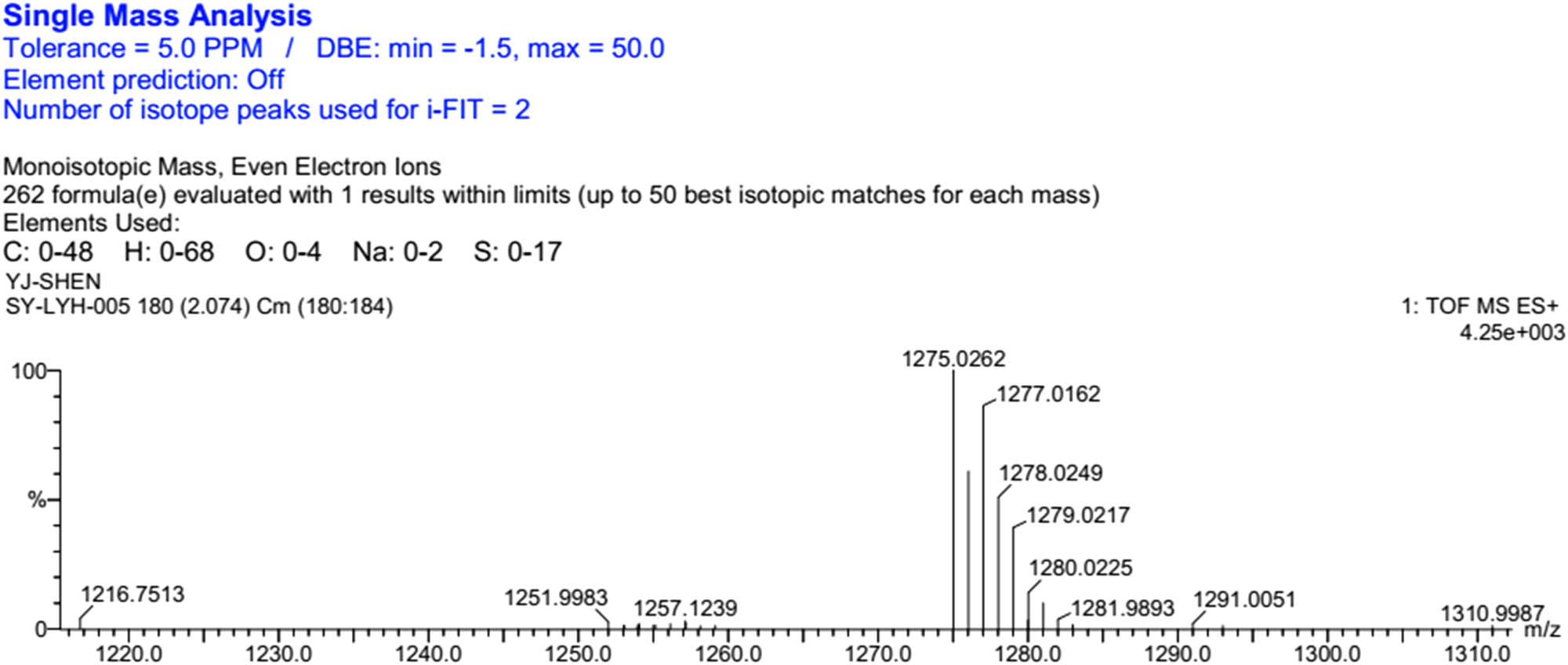
HRMS (ESI) of DTTF-Th.
References
(1) Hyodo K. Electrochromism of conducting polymers. Electrochim Acta. 1994;39(2):265–72. 10.1016/0013-4686(94)80062-6.Search in Google Scholar
(2) Sonmez G, Meng H, Wudl F. Organic polymeric electrochromic devices: polychromism with very high coloration efficiency. Chem Mater. 2004;16(4):574–80.10.1021/cm0301773Search in Google Scholar
(3) Roncali J. Conjugated poly(thiophenes): synthesis, functionalization, and applications. Chem Rev. 1992;92(4):711–38. 10.1021/cm0301773.Search in Google Scholar
(4) Mortimer RJ, Dyer AL, Reynolds JR. Electrochromic organic and polymeric materials for display applications. Displays. 2006;27(1):2–18. 10.1016/j.displa.2005.03.003.Search in Google Scholar
(5) Thompson BC, Kim Y, McCarley TD, Reynolds JR. Soluble narrow band gap and blue propylenedioxythiophene-cyanovinylene polymers as multifunctional materials for photovoltaic and electrochromic applications. J Am Chem Soc. 2006;128(39):12714–25. 10.1021/ja061274a.Search in Google Scholar PubMed
(6) Groenendaal L, Zotti G, Aubert P-H, Waybright SM, Reynolds JR. Electrochemistry of poly(3,4-alkylenedioxythiophene) derivatives. Adv Mater. 2003;15(11):855–79. 10.1002/adma.200300376.Search in Google Scholar
(7) Liu X, Hu Y, Shen L, Zhang G, Cao T, Xu J, et al. Novel copolymers based on PEO bridged thiophenes and 3,4-ethylenedioxythiophene: electrochemical, optical, and electrochromic properties. Electrochim Acta. 2018;288:52–60. 10.1016/j.electacta.2018.08.072.Search in Google Scholar
(8) Yao W, Shen L, Liu P, Xu J, Jiang Q, Liu G, et al. Electrochemical doping engineering tuning of the thermoelectric performance of a π-conjugated free-standing poly(thiophene-furan) thin-film. Mater Chem Front. 2020;4:597–604. 10.1039/C9QM00542K.Search in Google Scholar
(9) Wang W, Zhao N, Geng Y, Cui S, Hauser J, Decurtins S, et al. A highly sensitive TTF-functionalized probe for the determination of physiological thiols and its application in tumor cells. RSC Adv. 2014;4(62):32639–42. 10.1039/C4RA06455K.Search in Google Scholar
(10) Amacher A, Yi C, Yang J, Bircher MP, Fu Y, Cascella M, et al. A quinoxaline-fused tetrathiafulvalene-based sensitizer for efficient dye-sensitized solar cells. Chem. Commun. 2014;50(49):6540–2. 10.1039/C4CC02696A.Search in Google Scholar PubMed
(11) Pfattner R, Pavlica E, Jaggi M, Liu S, Decurtins S, Bratina G, et al. Photo-induced intramolecular charge transfer in an ambipolar field-effect transistor based on a π-conjugated donor–acceptor dyad. J Mater Chem C. 2013;1(25):3985. 10.1039/C3TC30442F.Search in Google Scholar
(12) Martínez JI, Abad E, Beltrán JI, Flores F, Ortega J. Barrier height formation in organic blends/metal interfaces: case of tetrathiafulvalene-tetracyanoquino-dimethane/Au(iii). J Chem Phys. 2013;139(21):214706. 10.1063/1.4836635.Search in Google Scholar
(13) Sinha J, Lee S, Kong H, Swift TW, Katz HE. Tetrathiafulvalene (TTF)-functionalized thiophene copolymerized with 3,3′-didodecylquaterthiophene: synthesis, TTF trapping activity, and response to trinitrotoluene. Macromolecules. 2013;46(3):708–17. 10.1021/ma3019365.Search in Google Scholar
(14) Ertas E, Bildirir H, Sahin O, Oksen I. Synthesis and properties of thiophene and dithiin functionalized tetrathiafulvalenes. Phosphorus Sulfur Silicon Relat Elem. 2013;188(12):1835. 10.1080/10426507.2013.788005.Search in Google Scholar
(15) Zhang L, Li M, Wang C, Wang Y, Shen Y. Electropolymerization and properties of 3,4-ethylenedioxythiophene backbone polymer with tetrathiafulvalene as pendant. J Appl Polym Sci. 2013;127(5):3356. 10.1002/app.37803.Search in Google Scholar
(16) Zhang L, Wu C, Wang C, Zuo H, Shen Y. 3,4-Ethylenedioxy-thiophene functionalizationed with tetrathiafulvalene: synthesis and selective esterification. J Heterocycl Chem. 2014;51(5):1277–81. 10.1002/jhet.1834.Search in Google Scholar
(17) Bryce MR, Chissel A, Gopal J, Kathirgamanathan P, Parker D. Towards highly oriented polythiophenes incorporating mesogenic or tetrathiafulvalene substituents. Synth Met. 1991;39(3):397–400. 10.1016/0379-6779(91)91766-4.Search in Google Scholar
(18) Huchet L, Akoudad S, Roncali J. Electrosynthesis of highly electroactive tetrathiafulvalene-derivatized polythiophenes. Adv Mater. 1998;10(7):541–5. 10.1002/(SICI)1521-4095(199805)10:7%3C541:AID-ADMA541%3E3.0.CO;2-1.Search in Google Scholar
(19) Huchet L, Akoudad S, Levillain E, Roncali J, Emge A, Bäuerle P. Spectroelectrochemistry of electrogenerated tetrathiafulvalene-derivatized poly (thiophenes): toward a rational design of organic conductors with mixed conduction. J Phys Chem B. 1998;102(40):7776–81. 10.1021/jp982593u.Search in Google Scholar
(20) Zuo H, Huang R, Zhang Q, Wang C, Shen Y. Synthesis and electrochromic performances of polymeric tetrathiafulvalene-bithiophene. Polym Bull. 2018;75(5):611–21. 10.1007/s00289-017-2056-5.Search in Google Scholar
(21) Liu Y, Wang C, Li M, Lai G, Shen Y. Synthesis and spectroscopic and electrochemical properties of TTF-derivatized polycarbazole. Macromolecules. 2008;41(6):2045–8. 10.1021/ma702035c.Search in Google Scholar
(22) Liu Y, Wang C, Li M, Lai G, Shen Y. Synthesis and properties of polysilanes with tetrathiafulvalene as pendant group. N J Chem. 2008;32(3):505–10. 10.1039/B713819A.Search in Google Scholar
(23) Huang R, Zou P, Zhang Q, Wang C, Shen Y. Intramolecular charge transfer between novel ProDOT-σ-TTF. Microelectron Eng. 2016;152:48–51. 10.1016/j.mee.2015.12.020.Search in Google Scholar
(24) Binet L, Fabre JM. Synthesis of new functionalized π-electron donors: primary hydroxy and primary amino multisulfur tetrathiafulvalenes. Synthesis. 1997;10:1179–84. 10.1055/s-1997-1321.Search in Google Scholar
(25) Sista P, Nguyen H, Murphy JW, Hao J, Dei DK, et al. Synthesis and electronic properties of semiconducting polymers containing benzodithiophene with alkyl phenylethynyl substituents. Macromolecules. 2010;43(19):8063–70. 10.1021/ma101709h.Search in Google Scholar
© 2020 Yuhao Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery