Abstract
A new type of phthalonitrile-etherified resole resin (PNR) was synthesized from resole resin and 4-nitrophthalonitrile. The differential scanning calorimetry results showed that the curing temperature of PNR is lower than that of phthalonitrile resin. Excellent thermal stability and bonding properties were obtained after curing at 220°C. TGA showed that in air, the temperature of 5% weight loss (T5%) of the cured PNR was 446°C, approximately 41°C higher than that of resole resin (RS), and the char yield at 800°C increased from 4% for RS to 33% for PNR. The shear strengths of PNR at room temperature and high temperature were increased by 8% and 133%, respectively, over those of RS, and after aging at 350°C for 2 h, these values were increased by 262% and 198%, respectively, over those of RS. Its excellent curing behavior, heat resistance and high bonding strength show that PNR can be used as a high temperature-resistant adhesive.
Graphical abstract
The introduction of nitrile groups improves the heat resistance of resole resin and also improves the adhesive properties of resole resin at high temperatures and its heat aging.
1 Introduction
Phenolic resins are traditional synthetic resins, which offer the advantages of low-smoke release as well as being flame retardant; weather, high temperature and humidity resistant; and inexpensive (1). However, they also suffer from high cross-linking density and toughness, and the presence of methylene and phenolic hydroxyl groups in the main chain makes it easy to oxidize, which affects its heat resistance and limits its applicability in modern high-tech industries (2,3). Phenolic resin can be categorized as novolac resin (NV) and resole resin (RS). RS can be cured by simple heating without a curing agent and is the traditional resin for adhesives. It has excellent bonding strength and weathering resistance (4). Many studies on the heat resistance of phenolic resin have been reported, including investigations of introduced silicon–boron heat-resistant structures (5,6), benzoxazines (7,8) and phenolic cyanate esters (9,10); phenolic resins modified with allyl groups and double bonds (11,12); propargyl phenolic resins (13,14); and phthalonitrile-modified NV (15,16,17,18,19).
Phthalonitrile resin is a new high-performance resin with excellent mechanical properties, low water absorption and outstanding high-temperature resistance (20). However, the curing reaction of phthalonitrile resin is very slow even at high temperature (20). Amines (20,21), metal salts (22,23) or phenolic compounds (24,25,26,27) can reduce the curing temperature. The heat resistance and mechanical properties of RS can be significantly improved by modification with nitrile groups. Moreover, phenols can promote the cross-linking and curing of nitrile groups (27). Recent studies have mainly focused on the nitrification of NV and achieved good modification results. There are few reports on the nitration of RS.
In this study, phthalonitrile-etherified resole resin (PNR) was prepared from 4-nitrophthalonitrile and RS. Its structure, curing behavior, thermal properties and bonding properties were studied.
2 Materials and methods
2.1 Materials
Phenol and 37% formaldehyde were purchased from Chemical Purity, Shanghai Guoyao Co., Ltd. 4-Nitrophthalonitrile was purchased from Shijiazhuang Aifa Chemical Technology Co., Ltd. N,N-Dimethylformamide (DMF) was purchased from Tianjin Guangfu Fine Chemical Research Institute. Anhydrous potassium carbonate, zinc oxide and butanone were purchased from Chemical Purity, Beijing Chemical Plant.
2.2 Preparation of resins
2.2.1 Preparation of RS
A total of 94 g of phenol was added to the three-necked flask, 1.6 g of zinc oxide was added, stirring was done, and the temperature was increased to 95°C to 100°C, and 154 g of formaldehyde 37% was slowly added dropwise. Continue stirring was done at this temperature for 3 h. After cooling to room temperature, the resin was washed with distilled water and then vacuumed to remove small molecules. When the resin gel time is between 120 and 300 s, butanone was added.
2.2.2 Preparation of PNR
First, according to the PNR chemical formula, 80 mL of dried DMF and 26 g of 4-nitrophthalonitrile were added to a 250 mL three-neck flask equipped with an agitator, thermometer and reflux condenser. After stirring until reagent dissolution, 30 g of RS was added, and the mixture was stirred to increase the temperature to 60°C. The reaction was carried out in nitrogen atmosphere. Then, 18 g of catalyst was added. Stirring was continued until all the solids had dissolved, and the contents were heated to 80°C and maintained at this temperature for 16 h. After the reaction was completed, the mixture was cooled to room temperature, and the reaction liquid was slowly poured into 1.5 L of cold water while stirring. The product was precipitated, neutralized by adding hydrochloric acid and then extracted with methyl ethyl ketone. Finally, the solvent was removed by distillation under reduced pressure to obtain yellowish brown PNR with a yield of 70–80%. The reaction route is shown in Figure 1.
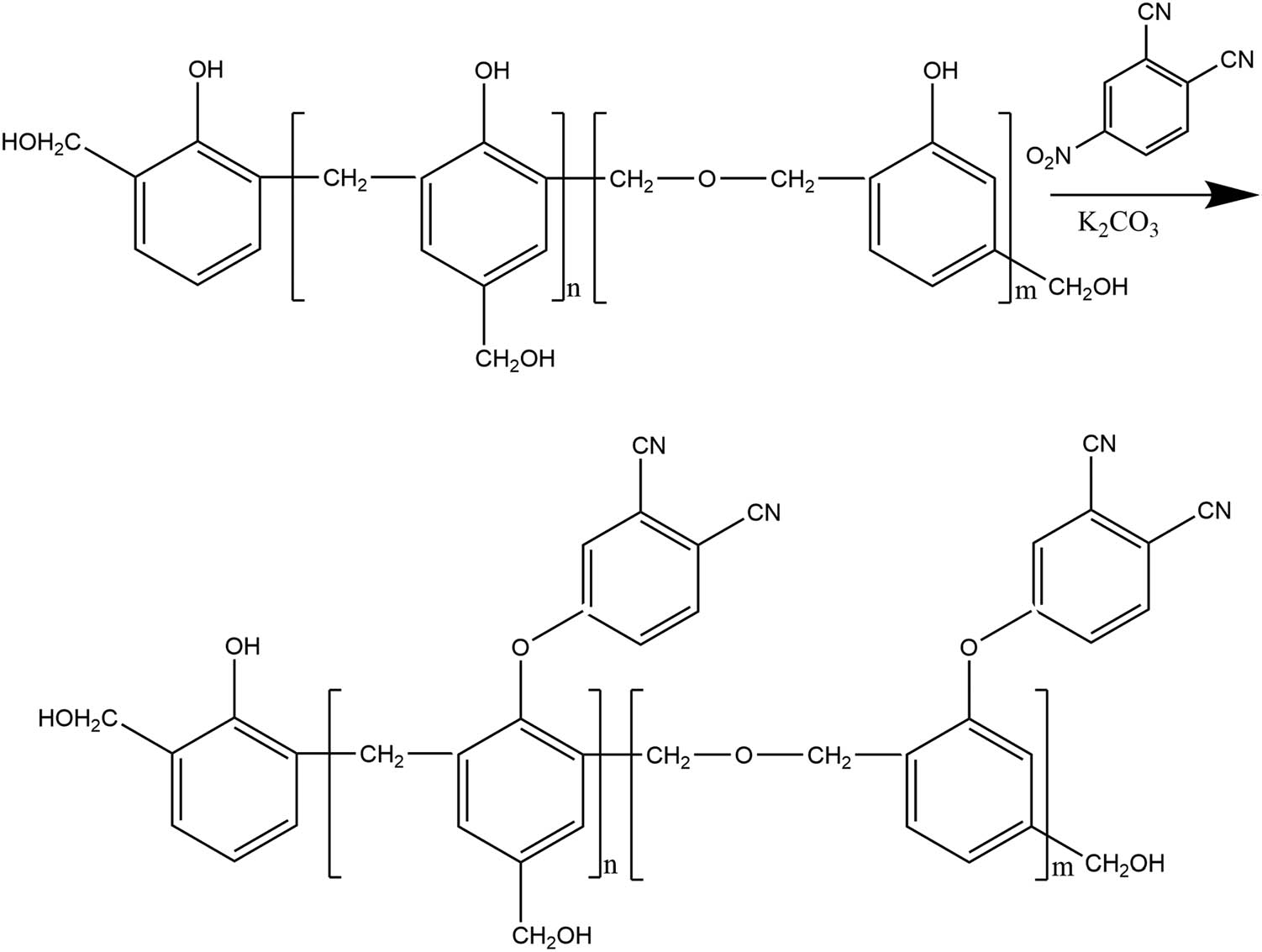
Synthesis of PNR.
2.3 Cure procedure
The PNR was cured by heating in an electric drying oven under air atmosphere at 180°C for 2 h, followed by 220°C for 4 h. The RS was cured by heating in an electric drying oven under air atmosphere at 150°C for 2 h, followed by 180°C for 4 h.
2.4 Characterization
The bond strength of PNR in steel-steel systems was measured by the lap shear method and compared with that of RS following GB/T 7124-2008. The strength was determined as the average value of five test samples. Specimen treatment: steel strips measuring 60 mm × 20 mm × 2 mm were used to test the shear strength. Mechanical polishing was performed with a no. 80 emery cloth, as the surface of the steel to be bonded needs to be smooth and uniform; then, the samples were washed and degreased with acetic acid ethyl acetate and dried at room temperature for 10–15 min. Gluing: a thin layer of resin was applied on the surface of the treated test piece, which was then left at room temperature for 10–15 min. Another thin layer of resin was applied, and the test piece was left at room temperature for 10–15 min. Finally, the test piece was placed in an oven at 80°C. After baking for 10–15 min, when the resin on the test piece could not be drawn or broken, the test piece was joined. The curing stresses ranged from 0.1 to 0.4 MPa, and the bonded test piece was thermally cured by heating in an electric drying oven under air atmosphere at 180°C for 2 h, followed by 220°C for 4 h. Fourier transform infrared (FT-IR) spectra were recorded from KBr discs with a Bruker VECTOR-22 spectrometer and the following parameters were noted: resolution, 4 cm−1; number of scans, 32; and scanning range, 4,000–600 cm−1. Proton nuclear magnetic resonance spectroscopy (1H-NMR) was performed with a Bruker Advance-400 NMR spectrometer with deuterated dimethyl sulfoxide-d6 as the solvent and tetramethylsilane as the internal standard. Differential scanning calorimetry (DSC) was carried out with a Perkin Elmer PYRIS-1 analyzer from 25 to 400°C at a heating rate of 10°C min−1 under nitrogen. Gel permeation chromatography (GPC) analysis was performed with a Shimadzu LC-20AD pump delivering tetrahydrofuran as the eluent and with polystyrene as the calibration standard. Dynamic mechanical analysis (DMA) was performed on a SEIKO SII Exstar DMA6100 in 3-point bending mode to measure the storage modulus and damping factor (tan δ) of the material in a nitrogen atmosphere from 20°C to 400°C at a heating rate of 5°C min−1 and a frequency of 1 Hz. Thermogravimetric analysis (TGA) was performed on a PerkinElmer Diamond calorimeter at a heating rate of 10°C min−1 under flowing nitrogen or air (flow rate 60 mL min−1). Rheological studies were performed from 40°C to 250°C at a heating rate of 5°C min−1 using a Bohlin rheometer (Gemini 200) with a 25 mm diameter parallel plate fixture. X-ray diffraction (XRD) was conducted with a SHIMADZU (XRD-7000) operating at 40 kV and 40 mA with Ni-filtered Cu Kα radiation (λ = 0.15406 nm) in the reflection mode. The 2θ was collected from 5° to 163° with the interval of 0.02° at a scanning speed of 4° min−1. The morphology of the surfaces of the resins was observed by SEM (JEOL JSM-IT300LV) operating at 20 kV.
3 Results and discussion
3.1 Characterization of the PNR
Figure 2 shows the FT-IR spectra of PNR and RS. As shown in Figure 2 (RS), the wide absorption band at 3,400 cm–1 corresponding to the hydroxyl was observed and the bending vibration band of the methylol group at 1,080 cm−1 was obvious. Compared to the spectrum of RS, the characteristic absorption band of phenolic hydroxyl groups at 3,400 cm−1 in the spectrum of PNR was less intense, and a characteristic absorption band for a cyano (CN) group at 2,230 cm−1 was present. Therefore, the CN bond was successfully introduced into the structure of RS. The bending vibration band of the methylol group at 1,080 cm−1 did not change significantly, indicating that there was no condensation between phenolic methylol groups. Compared with that of phthalonitrile novolac resin (27), there were no methylol groups in its structure.
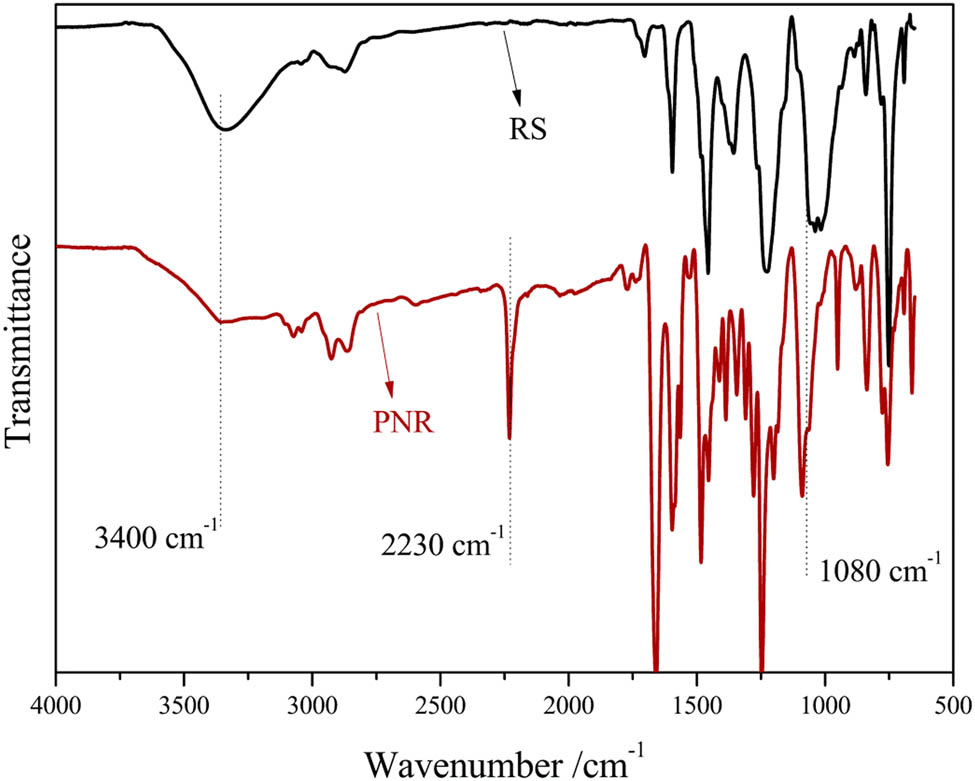
FT-IR spectra of PNR and RS.
As shown in Figure 3, the resonances at 6.6–7.1 ppm correspond to the benzene ring hydrogen in RS, while the resonances of benzyl hydrogen in PNR are located at 2.7–4.2 ppm. The resonances at 8.5–9.5 ppm, corresponding to the phenolic hydroxyl protons, were present in the spectrum of RS but absent in that of PNR, indicating phenol hydroxyl group reaction, and according to the H-NMR data, degree of phthalonitrile substitution was 69%. The new resonance at a chemical shift of 7.0–8.4 ppm in the PNR spectrum may correspond to the protons in phthalonitrile groups (16), and this result can confirm the occurrence of the etherification reaction of phthalonitrile and RS. The resonance of the methylol hydrogen at a chemical shift of 4.3–4.5 ppm did not change significantly, and there were no resonances at 4.6–4.8 ppm, corresponding to hydrogen on methylene ether moieties, indicating that RS itself did not undergo condensation reactions.
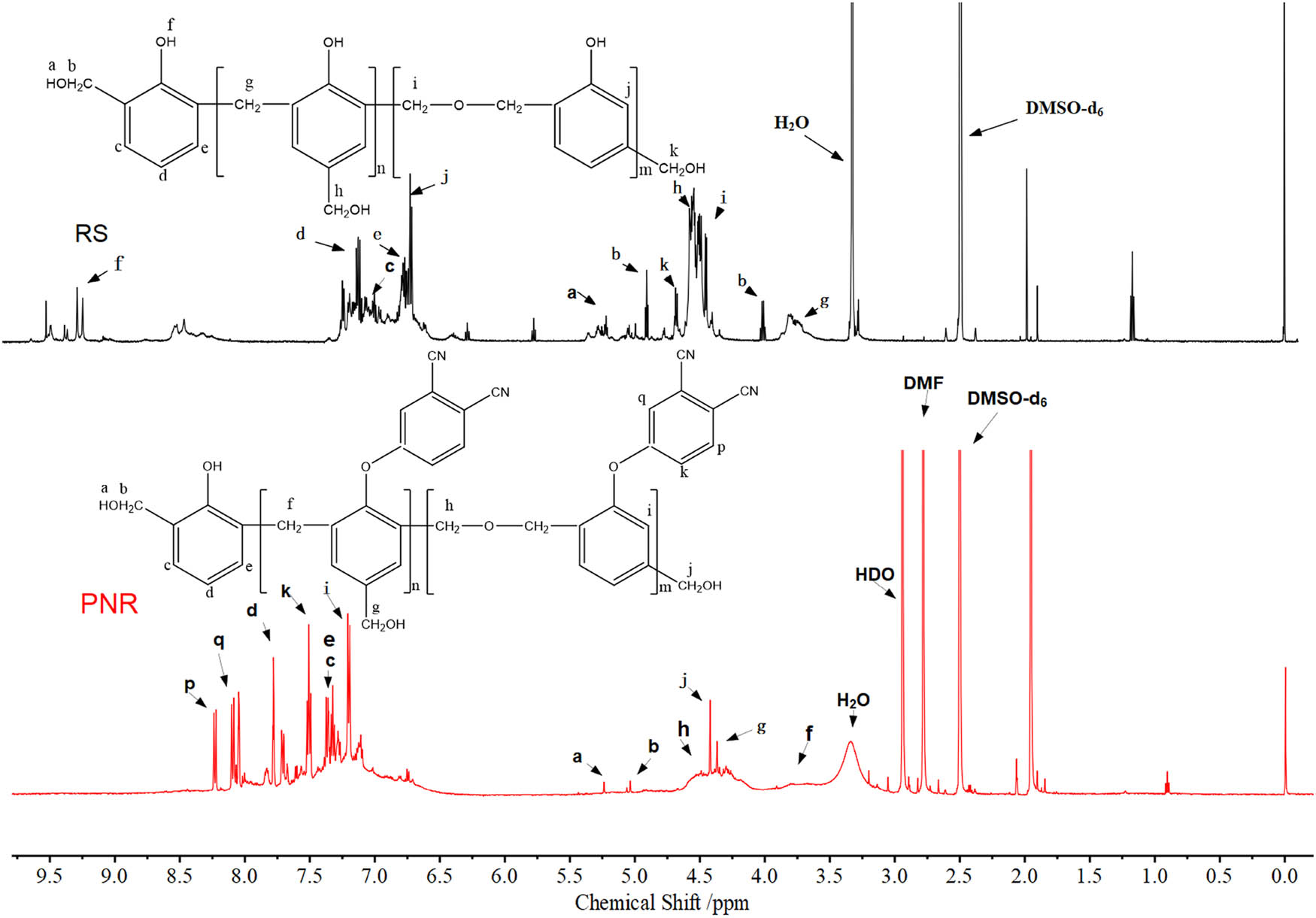
1H NMR spectra of PNR and RS.
The GPC chromatograms of PNR and RS are shown in Figure 4. The molecular weight characteristics of PNR and RS are presented in Tables 1 and 2. As shown in Figure 4, the GPC curves of RS and PNR had similar profiles, indicating that their molecular weight distribution characteristics are similar. The elution time of PNR is shorter than that of the RS, indicating that the molecular weight of PNR is higher, which was attributed to the etherification reaction between phthalonitrile and RS. As presented in Table 1, it could be seen that RS resin was a mixed polymer with a nonsingle structure, in which the number average molecular weight of the polymer whose main structure accounted for 66% was 1,437 g mol–1 with a polydispersity index of approximately 2.37. After modification, the number average molecular weight of 41.8% PNR was 3,449 g mol–1 with a polydispersity index of approximately 1.34. The number average molecular weight of phthalonitrile novolac resin ranges from 1,030 to 1,700 g mol–1 (27).
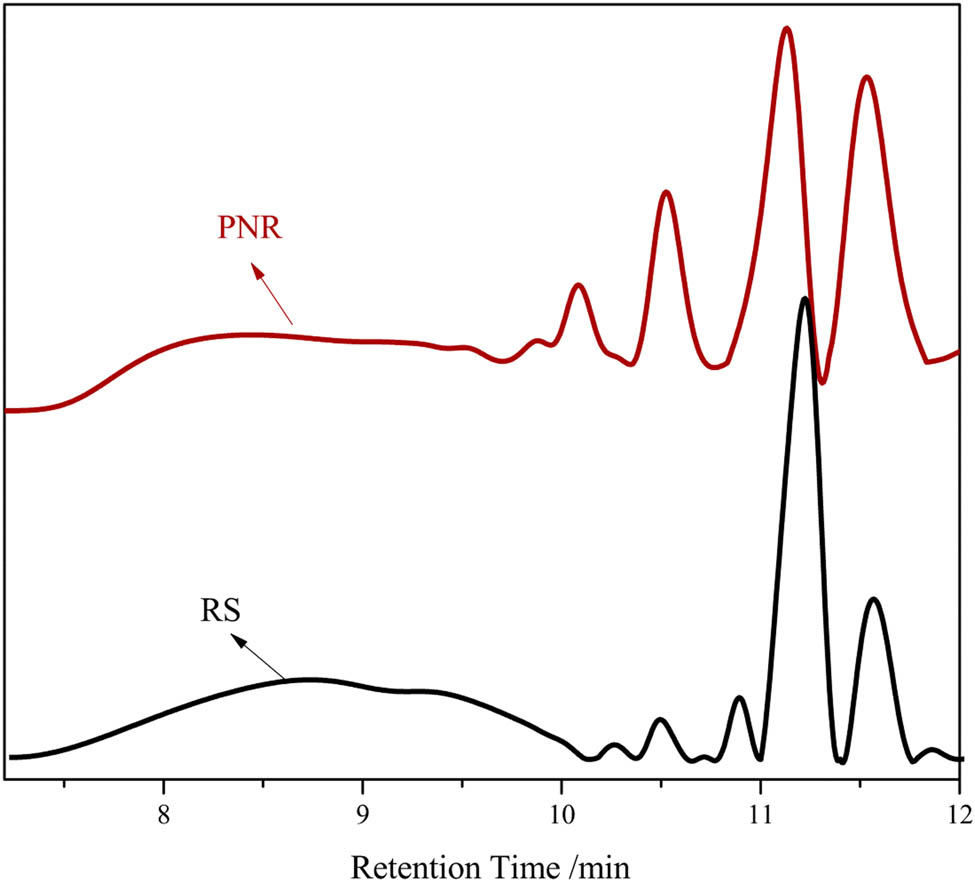
GPC results of PNR and RS.
GPC parameters of RS
| % | Mn | Mw | Mw/Mn | |
|---|---|---|---|---|
| I | 66.0 | 1,437 | 3,407 | 2.37 |
| II | 7.3 | 374 | 378 | 1.01 |
| III | 10.1 | 224 | 232 | 1.03 |
| IV | 5.6 | 131 | 133 | 1.01 |
| V | 11.0 | 79 | 81 | 1.02 |
GPC parameters of PNR
| % | Mn | Mw | Mw/Mn | |
|---|---|---|---|---|
| I | 41.8 | 3,449 | 4,638 | 1.34 |
| II | 10.6 | 1,225 | 1,251 | 1.02 |
| III | 6.8 | 800 | 809 | 1.01 |
| IV | 6.4 | 557 | 562 | 1.01 |
| V | 12.1 | 362 | 371 | 1.02 |
| VI | 14.1 | 200 | 204 | 1.02 |
| VII | 8.2 | 79 | 80 | 1.02 |
3.2 Curing behavior of the PNR
As shown in Figure 5, the thermal cure characteristics of PNR and RS were studied by the DSC analysis. The DSC curve of RS showed an exothermal peak between 150°C and 250°C with a peak value of 197°C. PNR produced several exothermic peaks between 150°C and 300°C with a peak temperature of 256°C. The curing reaction involves methylol condensation and nitrile polymerization, and the two shoulder peaks from 150°C to 200°C should correspond to the exothermic methylol self-condensation. Compared with those of phthalonitrile resins, the curing temperature of PNR was obviously lower, the curing time was significantly shortened, and the curing reaction was accelerated. These changes occurred because the phenolic hydroxyl groups in the PNR structure may promote the cyclization reaction, effectively increasing the content of heat-resistant heterocyclic rings in the cured PNR, as reported in the literature (28). In addition, compared with that of phthalonitrile novolac resin (PN) (16), the maximum exothermic curing temperature of PNR was lower by 28°C because PNR contains more methylol groups, which may accelerate the rate of curing of the nitrile groups.
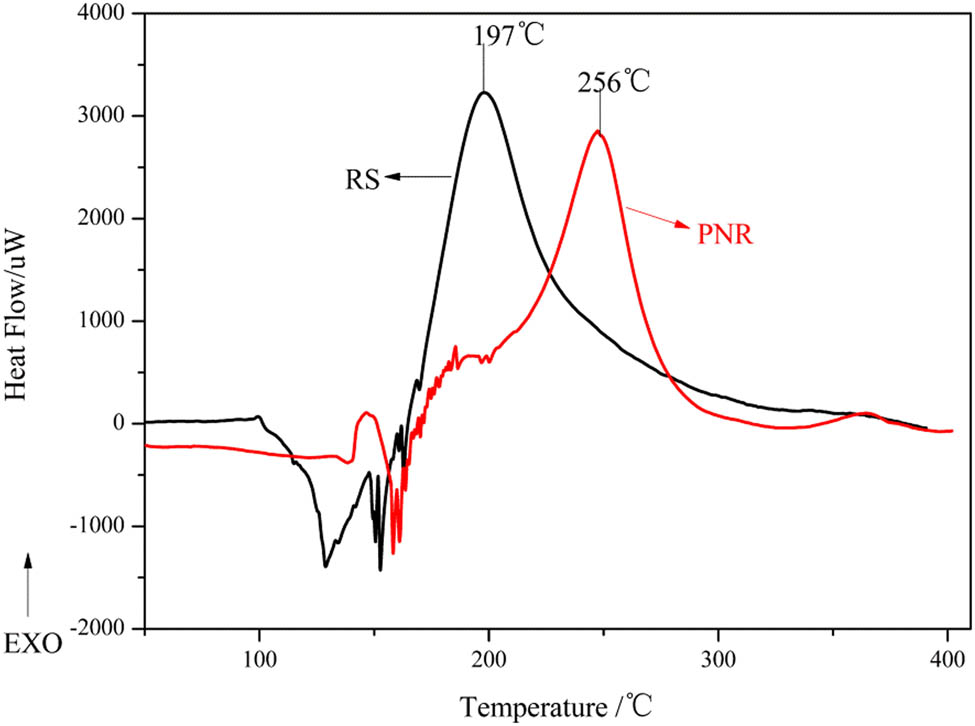
DSC results of PNR and RS.
The FT-IR spectra of PNR cured at different temperatures were acquired, and the results are shown in Figure 6. It can be seen from the FT-IR curve of PNR cured at 140°C that the peak of the characteristic absorption band of the methylol groups at 3,400 cm−1 had almost disappeared, indicating that the curing reaction of the methylol groups was almost complete. In addition, with the increasing curing temperature of the PNR, the characteristic peak of the CN groups at 2,230 cm−1 weakens, but the reaction is not complete. The peak heights of the characteristic absorption peaks of the nitrile groups cured at 220°C, 260°C and 300°C are similar. Therefore, the ideal curing process of PNR occurs at 220°C.
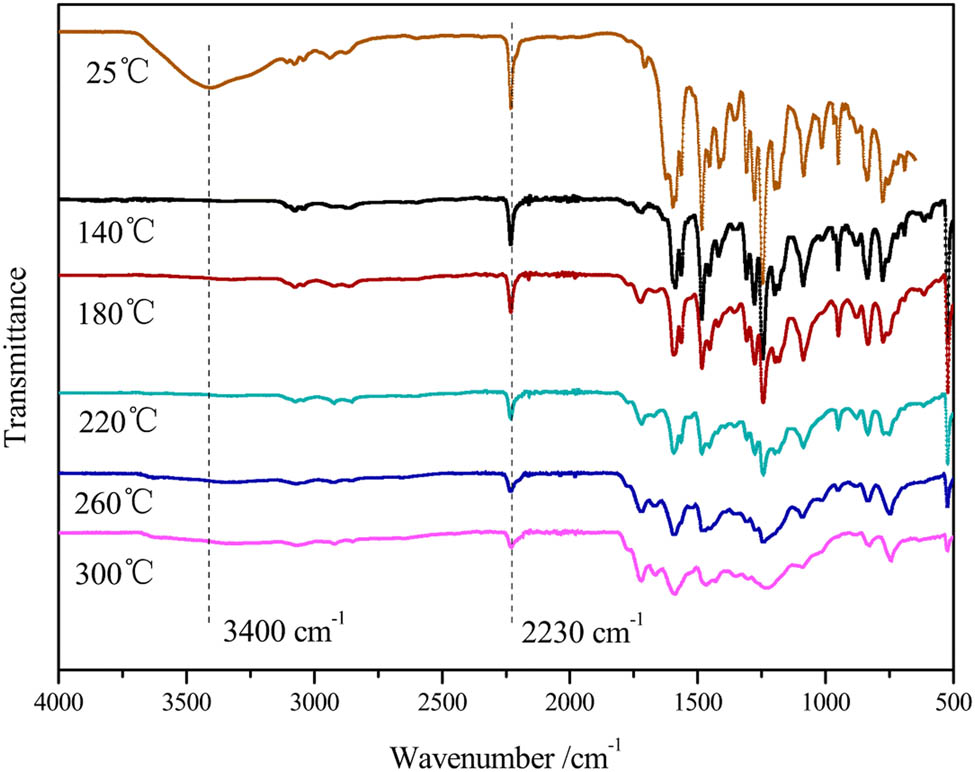
FT-IR spectra of PNR at different temperatures.
3.3 Thermal and thermal oxidation analysis
The thermal stability and thermal oxidation performance of the resin are important indexes for its application in a high-temperature environment. Thus, TGA of cured PNR and RS samples was performed in both air and nitrogen atmospheres, and the results are shown in Figure 7. Figure 7a shows that the temperature of the 5% weight loss (T5%) and char yield at 800°C of cured PNR in an air atmosphere were 446°C and 33%, respectively, which were approximately 41°C and 29% higher than those of cured RS. Furthermore, the T5% and char yield at 800°C of cured PNR in N2 atmosphere (Figure 7b) were 380°C and 76%, which were approximately 16°C and 16% higher than those of cured RS, which were 364°C and 60%, respectively. These results indicated that the introduction of phthalonitrile groups increased the content of heterocycles, which should be responsible for the high char yield of PNR (Table 3).
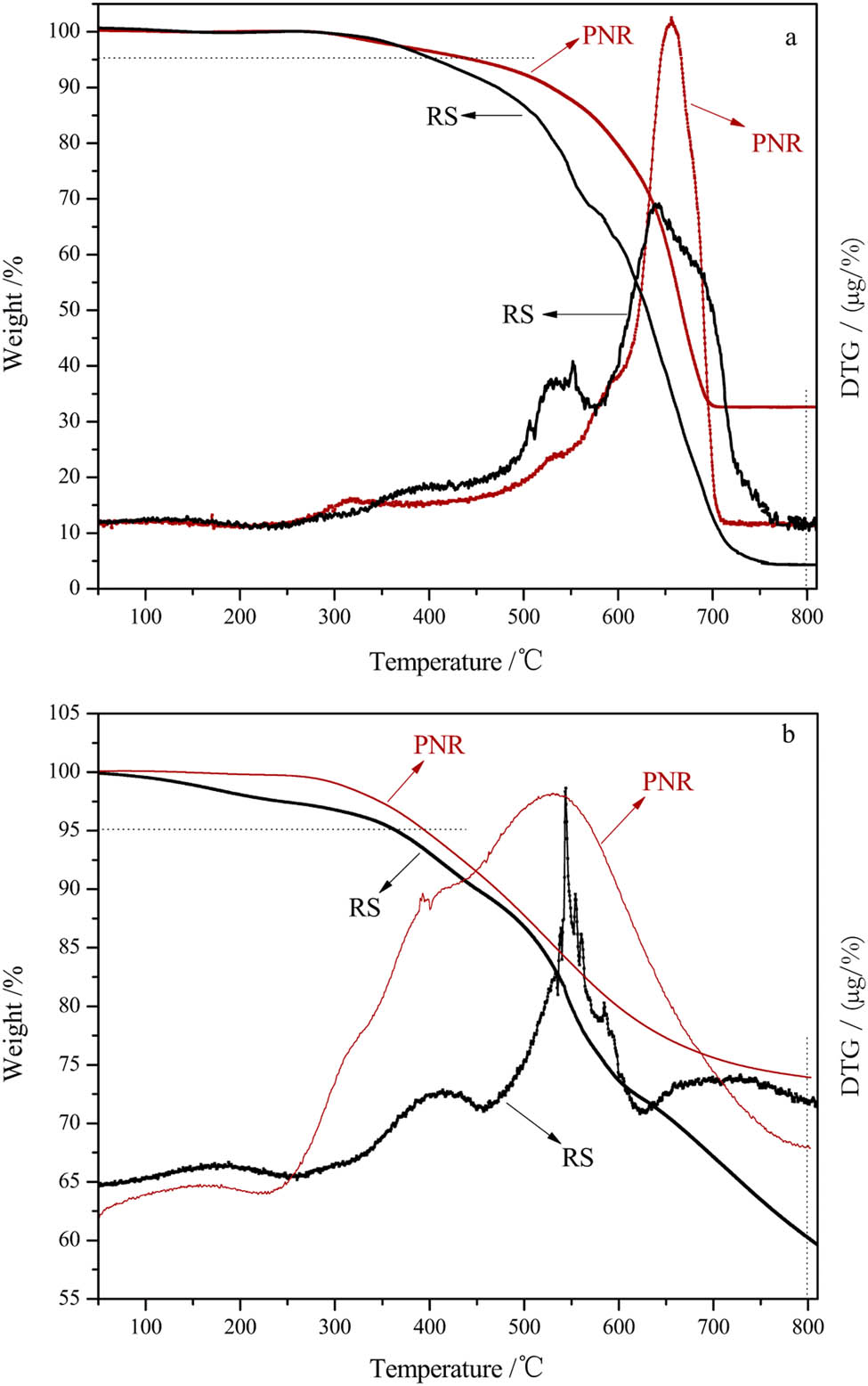
TGA curves of cured PNR and RS: (a) in air and (b) in N2.
TGA results of PNR and RS: (a) in air and (b) in N2
| Atmosphere | T5%/°C | T10%/°C | C800°C/% | |||
|---|---|---|---|---|---|---|
| PNR | RS | PNR | RS | PNR | RS | |
| Air | 446 | 405 | 529 | 472 | 33 | 4 |
| N2 | 380 | 364 | 463 | 449 | 76 | 60 |
T5%: the temperature of 5% weight loss.
T10%: the temperature of 10% weight loss.
C800°C: char yield at 800°C.
The dynamic mechanical behavior of the cured PNR and RS in terms of storage modulus (E′) and loss tangent (tan δ) is presented in Figure 8. The maximum of the tan δ curve represents the glass transition temperature (Tg), which is particularly an important parameter for polymers. The Tg of the PNR was 232°C, which was higher than the Tg of 197°C of the RS. This difference revealed that the cured PNR resin possessed much better thermal stability than the RS resin, which was due to the appearance of phthalocyanine and triazine rings with excellent heat resistance. However, the storage modulus of the cured PNR decreased dramatically at temperatures above 200°C, which might have been due to the high mobility of the molecular chains of the phenolic structures at high temperatures. Figure 8b shows that the PNR had a higher storage modulus and a higher modulus plateau (appearing after 250°C) than the RS, indicating that the PNR had undergone a secondary curing reaction, further improving its high-temperature performance.
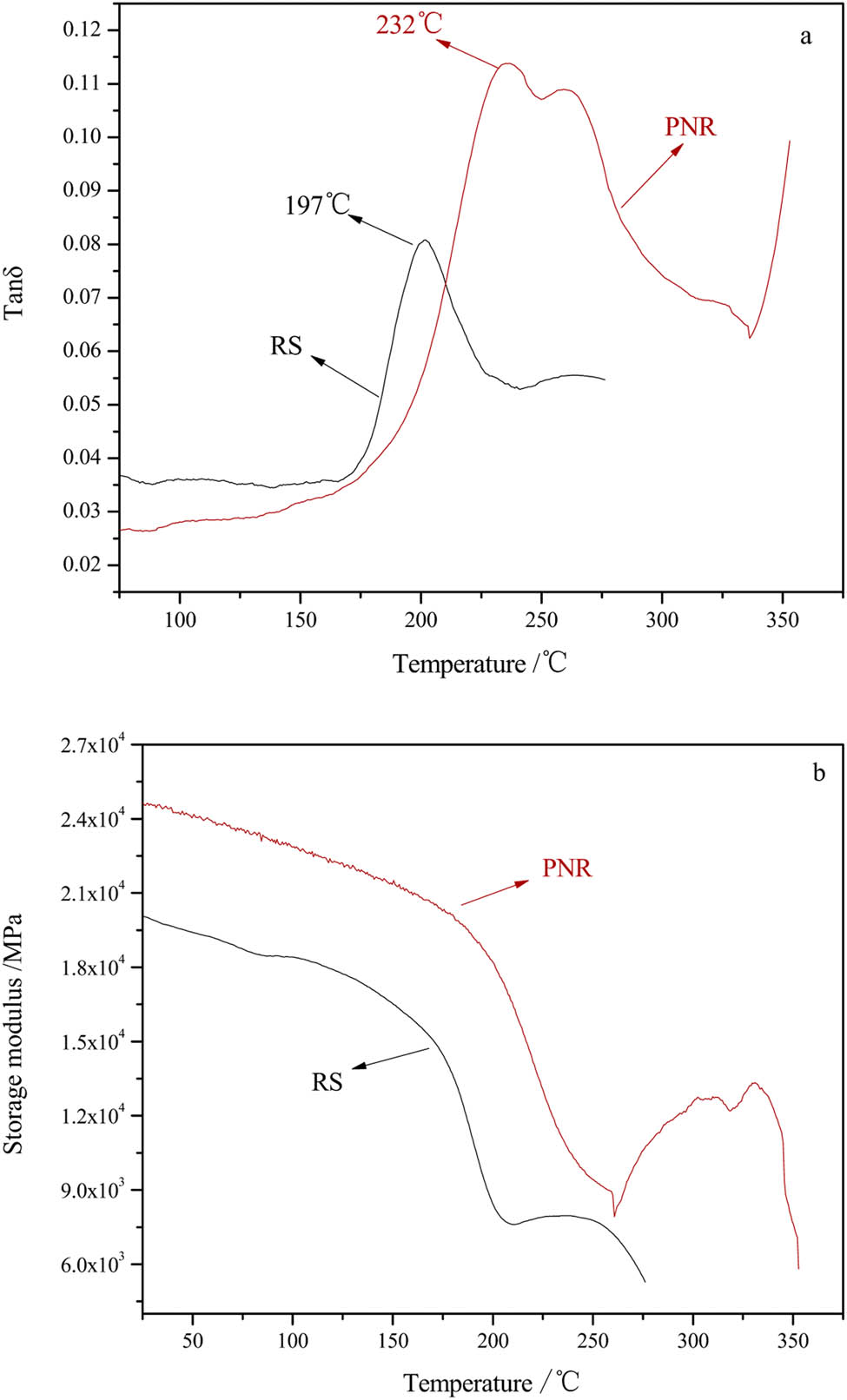
DMA curves of the PNR and RS: (a) tan δ and (b) storage modulus.
3.4 SEM of resins after thermal oxidation
Figure 9 shows the SEM images of the cured RS and PNR resins before and after aging. It could be seen from the figure that after aging of the cured RS resin at 350°C for 2 h, a large number of holes appeared on the surface of the resin due to high-temperature degradation, the resin degraded significantly. After aging at 350°C for 2 h, the surface of cured PNR resin produced fewer holes, indicating that the PNR resin degraded slowly at high temperature, and its heat aging resistance was better than RS resin.
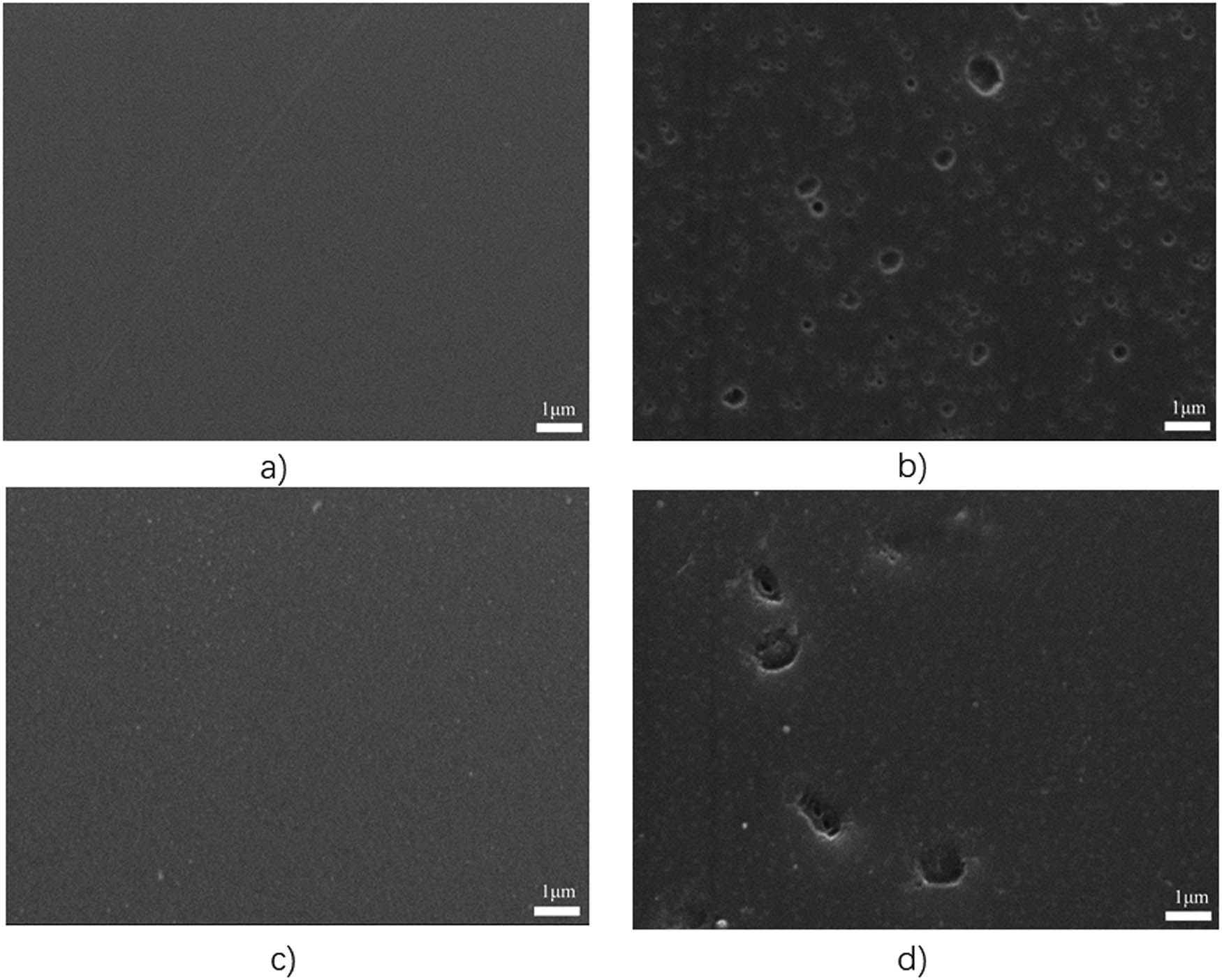
SEM images of cured PNR and RS: (a) cured RS, (b) cured RS/ageing at 350°C, (c) cured PNR and (d) cured PNR, aging at 350°C.
3.5 XRD of cured resins
Figure 10 shows the XRD of the cured PNR and RS resins. This figure also shows that the noncrystalline diffraction humps of the resin are 23.0° and 19.3°, respectively. The distance between atoms of the amorphous polymer could be calculated by the following equation:
where λ was the wavelength of the X-ray, 2θ was the diffraction angle, and R was the interatomic distance of the polymer. The atomic distance between the cured PNR and RS resin could be calculated as 0.482 and 0.574 nm. The closer the atomic distance was, the higher the heat resistance was (28). Therefore, the heat resistance of the cured PNR was better than that of RS resin.
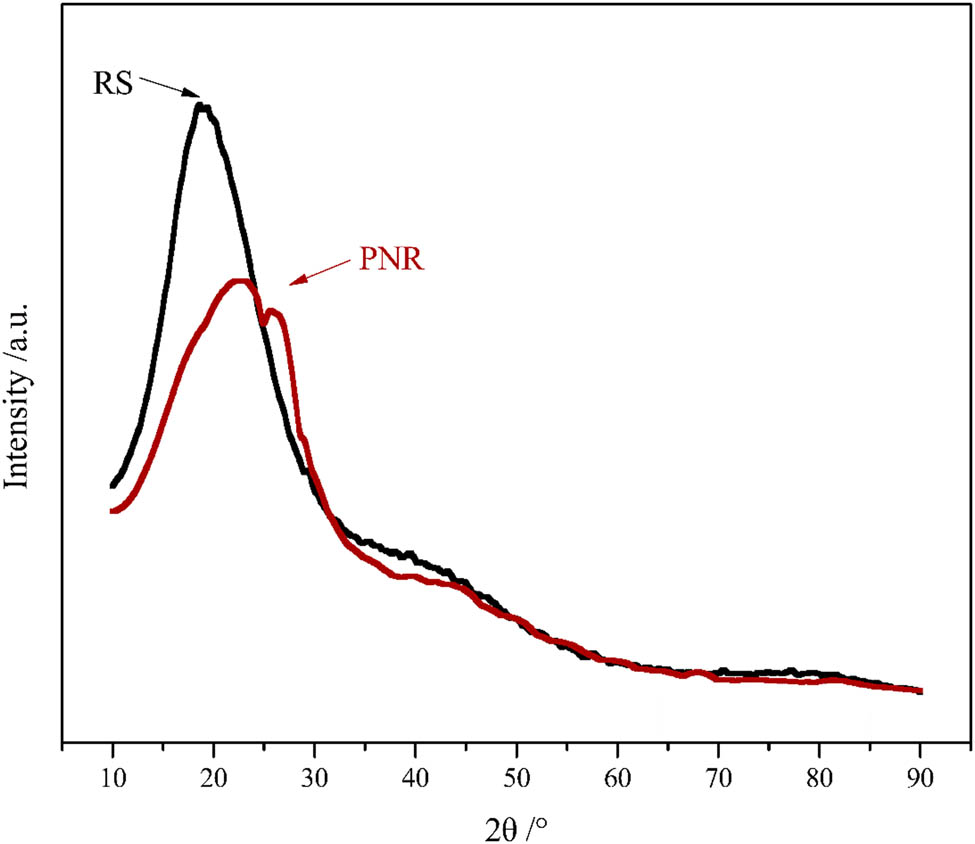
XRD patterns of cured PNR and RS.
3.6 Processability
The processability of the resin could be characterized by rheological properties. Therefore, the dynamic melt viscosity of the PNR was determined from 40°C to 250°C and compared with that measured for the RS. The results are presented in Figure 11. The viscosity–temperature curves of PNR and RS were similar. The viscosity of the PNR sharply decreased at 106°C, with the lowest value of 1.3 Pa s at 158°C, and then rapidly increased at 190°C. These results indicated that the RS possesses a narrow processing window of up to 49°C and that the PNR has a processing window of approximately 84°C, indicating that this resin has excellent processability.
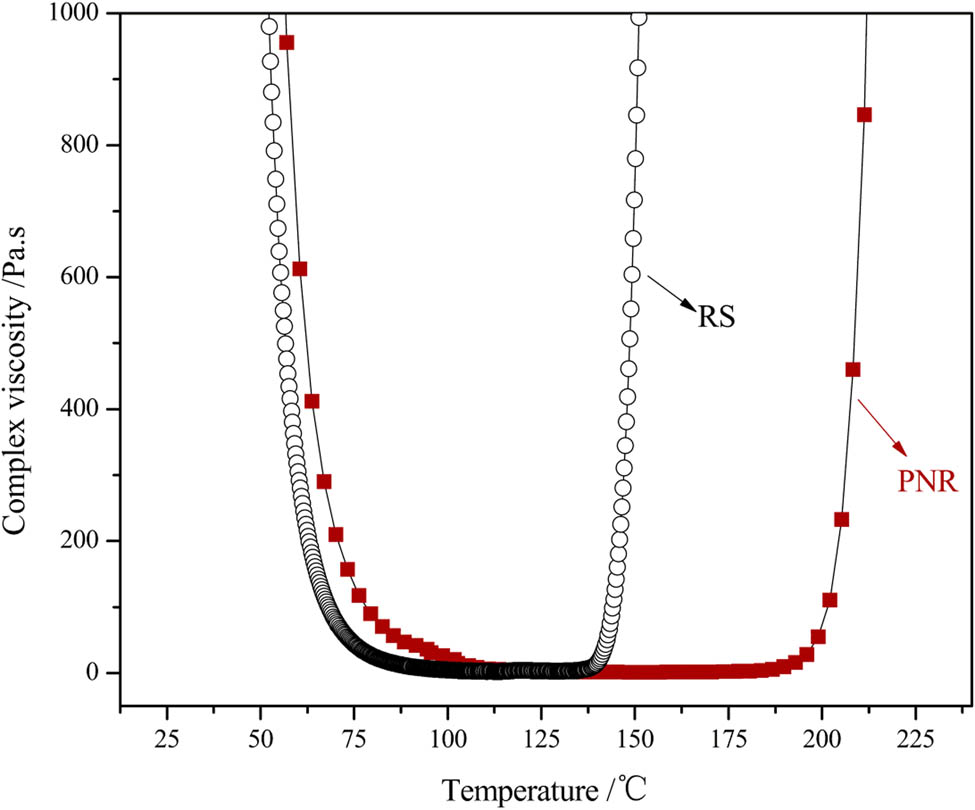
Complex viscosity variation with temperature for the PNR and RS.
3.7 Bonding properties of the PNR
The lap shear strength of the PNR and RS was studied by bonding steel to steel. Figure 12 shows the temperature dependence of the resin shear strength. The shear strength of the PNR and RS was almost the same at the room temperature and then decreased with the increasing temperature. The shear strength decreased after aging for 2 h at 350°C. It was likely that the degradation of methylene at high temperature reduced the cohesive force of the resin. However, the lap shear strength of the PNR decreased less than that of the RS, indicating that the PNR had better heat resistance than the RS. The lap shear strength of the PNR was only slightly affected by temperature. The lap shear strength of the PNR at 350°C was considerably increased (approximately 133%) compared to that of the RS because the cured PNR has a heat-resistant heterocyclic structure. It has been proven that the incorporation of nitrile groups is favorable for increasing high-temperature adhesion strength.
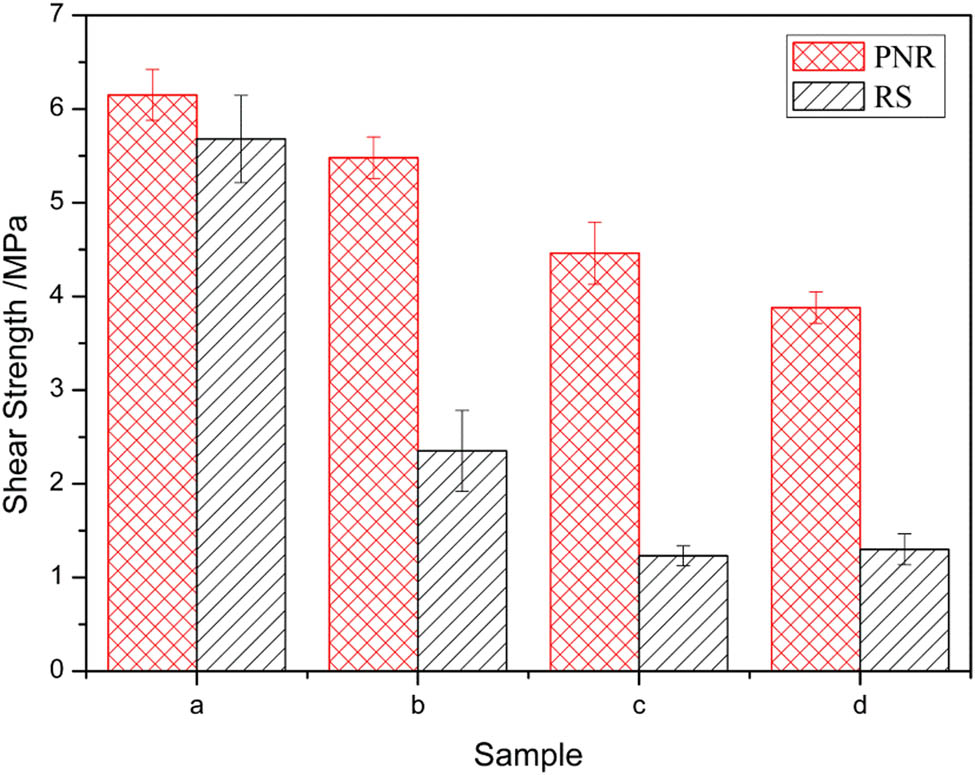
Shear strength of steel-steel bonds: (a) RT, (b) 350°C, (c) RT after 350°C/2 h and (d) 350°C after 350°C/2 h.
4 Conclusions
In this study, PNR was successfully synthesized and characterized. The synergistic effect among the phenolic hydroxyl groups, hydroxymethyl and nitrile groups significantly decreased the curing time and accelerated the curing reaction of the PNR compared with those of phthalonitrile resin. The PNR had the advantages of not only an excellent curing process and the low cost of RS but also the excellent thermal stability of the phthalonitrile resin. The adhesive properties of PNR at high temperature and its heat aging were significantly improved, indicating that this resin can be used as a matrix resin for high temperature-resistant adhesives and coatings.
References
(1) Gardziella A, Pilato L, Knop A. Phenolic resins: chemistry, applications, tandardization, safety and ecology. Berlin Heidelberg: Springer; 2000. 10.1007/978-3-662-04101-7.Search in Google Scholar
(2) Hirano K, Asami M. Phenolic resins – 100 years of progress and their future. React Funct Polym. 2013;73(2):256–69. 10.1016/j.reactfunctpolym.2012.07.003Search in Google Scholar
(3) Pilato L. Phenolic resins: a century of progress. Berlin Heidelberg: Springer. 2010. 10.1007/978-3-642-04714-5.Search in Google Scholar
(4) Zheng Y, Jiang Z, Sun Z, Ren H. Effect of microwave-assisted curing on bamboo glue strength: bonded by thermosetting phenolic resin. Construct Build Mater. 2014;68:320–5. 10.1016/j.conbuildmat.2014.07.014.Search in Google Scholar
(5) Li S, Chen F, Zhang B, Luo Z, Li H, Zhao T. Structure and improved thermal stability of phenolic resin containing silicon and boron elements. Polym Degrad Stabil. 2016;133:321–9. 10.1016/j.polymdegradstab.2016.07.020.Search in Google Scholar
(6) Zhang Y, Lee S, Yoonessi M, Liang K, Pittman CU. Phenolic resin–trisilanolphenyl polyhedral oligomeric silsesquioxane (POSS) hybrid nanocomposites: structure and properties. Polymer. 2006;47(9):2984–96. 10.1016/j.polymer.2006.03.005.Search in Google Scholar
(7) Kimura H, Matsumoto A, Ohtsuka K. New type of phenolic resin: curing reaction of phenol-novolac based benzoxazine with bisoxazoline or epoxy resin using latent curing agent and the properties of the cured resin. J Appl Polym Sci. 2009;112(3):1762–70. 10.1002/app.29301.Search in Google Scholar
(8) Xu MZ, Jia K, Liu XB. Effect of bisphenol-A on the structures and properties of phthalonitrile-based resin containing benzoxazine. Exp Polym Lett. 2015;9(6):567–81. 10.3144/expresspolymlett.2015.53.Search in Google Scholar
(9) Lu S-H, Zhou Z-W, Fang L, Liang G-Z, Wang J-L. Preparation and properties of cyanate ester modified by epoxy resin and phenolic resin. J Appl Polym Sci. 2007;103(5):3150–6. 10.1002/app.24617.Search in Google Scholar
(10) Tu Y, Yu R, Duan J, Hu L. Cyanate ester resin modified by phenolic resin containing diphenyl oxide segments with high molecular weight. Iran Polym J. 2016;25(10):863–73. 10.1007/s13726-016-0472-2.Search in Google Scholar
(11) Hao J, Rumin W, Shameel F, Shuirong Z. Properties and curing behavior of reactive blended allyl novolak with bismaleimide using dicumyl peroxide as a novel curing agent. J Appl Polym Sci. 2015;132(15):41829–41. 10.1002/app.41829.Search in Google Scholar
(12) Satheesh Chandran M, Sanil K, Sunitha K, Mathew D, Rao VL, Reghunadhan Nair CP. Alder-ene polymers derived from allyl aralkyl phenolic resin and bismaleimides: carbon fiber composites properties. Polym Adv Technol. 2016;27(8):984–92. 10.1002/pat.3758.Search in Google Scholar
(13) Wang M, Wei L, Zhao T. Addition-curable propargyl-containing novolac-type phenolic resin: its synthesis, characterization, cure, and thermal properties. J Appl Polym Sci. 2006;99(3):1010–7. 10.1002/app.22592.Search in Google Scholar
(14) Kalugin D, Luchinkin D, Nechausov S, Malakho A, Rogozin A, Garadja N. Novel propargyl-substituted azo-coupled phenolic resins. Polym Adv Technol. 2016;27(6):718–23. 10.1002/pat.3700.Search in Google Scholar
(15) Laskoski M, Dominguez DD, Keller TM. Synthesis and properties of aromatic ether phosphine oxide containing oligomeric phthalonitrile resins with improved oxidative stability. Polymer. 2007;48(21):6234–40. 10.1016/j.polymer.2007.08.028.Search in Google Scholar
(16) Zhang B, Luo Z, Zhou H, Liu F, Yu R, Pan Y, et al. Addition-curable phthalonitrile-functionalized novolac resin. High Perform Polym. 2012;24(5):398–404. 10.1177/0954008312440715.Search in Google Scholar
(17) Yang Y, Min Z, Yi L. A novel addition curable novolac bearing phthalonitrile groups: synthesis, characterization and thermal properties. Polym Bull. 2007;59(2):185–94. 10.1007/s00289-007-0765-x.Search in Google Scholar
(18) Li Z, Guo Y, Wang G, Xu S, Han Y, Liu X, et al. Preparation and characterization of a self-catalyzed fluorinated novolac-phthalonitrile resin. Polym Adv Technol. 2018;29(12):2936–42. doi: 10.1002/pat.4413.Search in Google Scholar
(19) Robert TM, Augustine D, Chandran M, Mathew S, Nair D, Graphene CPR. oxide induced fast curing of amino novolac phthalonitrile. RSC Adv. 2015;5(2):1198–204. 10.1039/c4ra08751h.Search in Google Scholar
(20) Devendra Kumar VC. Studies on crosslinking and thermal behavior of phthalonitrile end-capped imide monomer in presence of aromatic amines. J Appl Polym Sci. 2018;135(17):46151. 10.1002/app.46151.Search in Google Scholar
(21) Yang X, Zhang J, Lei Y, Zhong J, Liu X. Effect of different aromatic amines on the crosslinking behavior and thermal properties of phthalonitrile oligomer containing biphenyl ethernitrile. J Appl Polym Sci. 2011;121(4):2331–7. 10.1002/app.33949.Search in Google Scholar
(22) Ağırtaş MS, Gümüş İ, İzgi MS. Designing of new thermo stabile phthalocyanines: synthesis, characterization, and thermal studies. Synth React Inorg Met–Org Nano-Met Chem. 2012;42(9):1327–33. 10.1080/15533174.2012.680142.Search in Google Scholar
(23) Wu Z, Han J, Li N, Weng Z, Wang J, Jian X. Improving the curing process and thermal stability of phthalonitrile resin via novel mixed curing agents. Polym Int. 2017;66(6):876–81. 10.1002/pi.5328.Search in Google Scholar
(24) Dominguez DD, Keller TM. Properties of phthalonitrile monomer blends and thermosetting phthalonitrile copolymers. Polymer. 2007;48(1):91–7. 10.1016/j.polymer.2006.11.003.Search in Google Scholar
(25) Yang X, Li K, Xu M, Jia K, Liu X. Designing a low-temperature curable phenolic/benzoxazine-functionalized phthalonitrile copolymers for high performance composite laminates. J Polym Res. 2017;24(11):195–202. 10.1007/s10965-017-1360-y.Search in Google Scholar
(26) Hu Y, Weng ZH, Qi Y, Wang JY, Zhang SH, Liu C, et al. Self-curing triphenol A-based phthalonitrile resin precursor acts as a flexibilizer and curing agent for phthalonitrile resin. RSC Adv. 2018;8:32899–908. 10.1039/c8ra06926c.Search in Google Scholar PubMed PubMed Central
(27) Augustine D, Mathew D, Reghunadhan CP. Phenol-containing phthalonitrile polymers–synthesis, cure characteristics and laminate properties. Polym Int. 2013;62(7):1068–76, 10.1002/pi.4393.Search in Google Scholar
(28) Chen X, Shan S, Liu J, Qu X, Zhang Q. Synthesis and properties of high temperature phthalonitrile polymers based on o,m,p-dihydroxybenzene isomers. RSC Adv. 2015;5(98):80749–55. 10.1039/C5RA15321B.Search in Google Scholar
© 2020 Dayong Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Articles in the same Issue
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery


