Abstract
Two benzimidazole-based conjugated polyelectrolytes (+)-PPBIPV and (-)-PPBIPV which have opposite charges on their side chains were synthesized via Heck coupling reaction and characterized by 1H-NMR, UV-vis and PL spectroscopy. These two polyelectrolytes are both consisted of benzimidazole derivatives and phenylenevinylene units. The absorption and emission spectra reveal that the polymers both have solvent-dependency and concentration-dependency, and they exhibit aggregation effect in aqueous solution. In the respect of ion detection, the aqueous solution of (+)-PPBIPV has excellent selectivity and sensitivity for Fe3+. Moreover, Pd2+ can almost completely quench the fluorescence of (+)-PPBIPV in methanol solution, and its quenching constant KSV is 5.93×104 M-1. For (-)-PPBIPV, Sn2+ can double the fluorescence intensity of its aqueous solution, while (-)-PPBIPV has good identification for Fe3+ in methanol with a KSV = 3.44×105 M-1. Hence, two polyelectrolytes have considerable potential to become effective fluorescent sensing materials for some specific metal ions. All of the stoichiometric relationships between metal ions and conjugated polyelectrolytes were calculated using Benesi-Hildebrand equation.
1 Introduction
Conjugated polyelectrolytes (CPEs) which are a kind of conjugated polymers with water soluble ionic groups have received considerable interests in the past decades. Their ionized side-chain groups make CPEs have excellent solubility in water and other polar solvents. CPEs are a versatile class of materials that have been applied in a variety of photoelectric devices including polymer light-emitting diodes (PLEDs) (1), photovoltaic cells (2) and chemical and biological sensors (3,4). In particular, the chemical structure of CPEs offers several advantages in sensor applications, such as the increasing sensitivity of detection and water solubility. Therefore, CPEs have been widely explored as optical probes for various targets, such as DNA (5), proteins (6), small molecules (7) and metal ions (7,8).
The conjugated polyelectrolytes can be classified into cationic and anionic types depending on the different charged groups introduced on the side chain. Specifically, few cationic conjugated polyelectrolytes were reported and almost all of them were not synthesized directly but prepared by quaternization after the polymerization. Since not all the side groups of the polymers are electrically charged during this process, fully charged cationic polyelectrolytes cannot be obtained by indirect method. In recent years, cationic CPEs have potential applications in biological imaging such as folate receptor (FR) over expressed cancer cells (9), selective recognition of mammalian cells (10) and anionic surfactants (11), monitoring the physical changes of lipid membrane (10). On the other hand, anionic CPEs have been widely applied in the biological and chemical analysis area with their excellent water solubility. Direct electron transfer over a longer working distance can be accomplished through the sulfonated conjugate polyelectrolyte, which has a strong signal in DNA detection (12). And a probe system as fluorescence turn-on assay of simple saccharides is based on three anionic conjugated polyelectrolytes and three quenched molecules substituted by boric acid (13). However, almost all the anionic CPEs are consisted of phenyl units (14), thiophene derivatives (15) and fluorene monomers (16), and there are few literatures focused on water soluble N-heterocyclic systems. This can be attributed to complexity of the synthesis and purification. Although the fluorescent sensing properties of conjugated polyelectrolytes in the field of biology and chemistry have been extensively studied, there are few reports on the detection of metal ions in both cationic and anionic conjugate polyelectrolytes.
Benzimidazole is a nitrogen-containing heterocyclic compound fused from an imidazole ring and a benzene ring. With chemical modification by using various specific chemical methods, derivatives of benzimidazole can interact with enzymes, receptors and metal ions in the living body (17). Therefore, they have a wide range of applications in medicine (18) and pesticides (19). In recent years, due to the structural characteristics of benzimidazole, its derivatives have also received extensive attention in the field of fluorescent sensors (20). The sp2 hybridized nitrogen atom on benzimidazole has a lone pair of electrons that can coordinate with metal ions, resulting in changes in optical properties to achieve the purpose of detection (21). Thus, there have been a number of reports about small molecule probes containing benzimidazole (22,23). Related studies on benzimidazole-containing conjugated polymers reported in the past have mainly focused on light-emitting diodes (24) and photovoltaic devices (25). However, few benzimidazole-based conjugated polymers have been utilized for fluorescent sensors, and they are basically oil soluble (26). Among these studies, most of the fluorescence sensing properties were investigated in non-aqueous system, such as tetrahydrofuran (27). Therefore, it is interesting to develop novel CPEs containing benzimidazole which can detect metal ions in the water effectively.
Herein, we have successfully designed and synthesized two novel conjugated polyelectrolytes cationic PPBIPV ((+)-PPBIPV) and anionic PPBPIV ((-)-PPBIPV), which were modified with quaternary ammonium salt and sulfonate in the side-chain, respectively. First, the synthesis and characterization of two monomers 5-bromo-2-(4-bromophenyl)-1-ethyl-1H-benzo[d]imidazole (monomer 1) and 5-bromo-2-(4-bromophenyl) -1-(3-sulfonylpropyl) -1H-benzo[d]imidazole (monomer 2) were accomplished. And then the two compounds were used as the copolymerization monomers to synthesize (+)-PPBIPV and (-)-PPBIPV via Heck coupling reaction with 1,4-Bis(3-(N,N,N-triethylammonium)-1-oxapropyl)-2, 5-divinyl-benzene dibromide ((+)-DVB) and 1,4-divinyl-2, 5-dibutyloxy-benzene (C4-DVB), respectively. Photophysics properties of (+)-PPBIPV and (-)-PPBIPV were studied with absorption and emission spectra by changing the kind of solvent. In the respect of metal sensing, we used fluorescence spectroscopy to investigate the metal ions response of (+)-PPBIPV and (-)-PPBIPV in aqueous solution and methanol solution, respectively. The fluorescence of these solutions is obviously affected with addition of specific metal ions, and these two polyelectrolytes which are oppositely charged exhibit totally different metal sensing properties, demonstrating their potential as metal ion probes. The results of this study can provide useful suggestions for the design and synthesis of novel conjugated polyelectrolytes materials in the area of fluorescence chemical sensor.
2 Experimental
2.1 Measurements
1H NMR spectra were recorded on a Bruker Avance II-400MHz spectrometer (Bruker, Karlsruhe, Germany) with tetramethylsilane as an internal reference. UV-vis absorption spectra were performed on an AnalytikJena Specord 200Plus UV-vis spectrophotometer (AnalytikJena, Jena, Germany). Photoluminescence (PL) spectra were measured by using a Hitachi F-4600 luminescence spectrophotometer (Hitachi, Tokyo, Japan). All spectra were measured at room temperature.
2.2 Materials
All of chemicals were purchased from Aladdin Industrial Corporation (Aladdin, Shanghai, China) and were used directly without further purification. 1,4-divinyl-2,5-dibutyloxy-benzene (C4-DVB) and 1,4-Bis(3-(N,N,N-triethylammonium)-1-oxapropyl)-2,5-divinyl-benzene dibromide ((+)-DVB) were synthesized according to the literature (28). 4-bromo-2-nitroaniline (1) and 4-bromobenzene-1,2-diamine (2) were prepared following the published procedures (29,30).
2.2.1 Synthesis of monomers
5-Bromo-2-(4-bromophenyl)-1H-benzo[d]imidazole (3)
4-bromobenzaldehyde (1.1 g, 6 mmol), sodium bisulfite (0.6 g, 6 mmol), and absolute ethanol (25 mL) were stirred at room temperature for 4 h. Then 4-bromobenzene-1,2-diamine(2) (1.1 g, 6 mmol) dissolved in DMF (15 mL) was added. The reaction mixture was subjected to reflux for 2 h. Subsequently, ethanol in the mixture was removed by rotary evaporation. The residue was dissolved in 20 mL ethyl acetate and washed with water. The organic phase was dried over anhydrous sodium sulfate, and then filtered. The solvent was removed by rotary evaporation and the crude product was purified by column chromatography (silica gel, petroleum ether/ethyl acetate: 4/1) to give a white solid. Yield: 1.30 g, 61.5%. 1H NMR (400 MHz, DMSO-d6, δ): 13.22 (s, 0.5H), 13.18 (s, 0.5H); 8.11 (d, J = 6.9 Hz, 2H); 7.90-7.68 (m, 3H); 7.63 (d, 0.5 H), 7.51 (d, 0.5 H); 7.36 (m, J = 9.4 Hz, 1H).
5-Bromo-2-(4-bromophenyl)-1-ethyl-1H-benzo[d]imidazole (monomer 1)
Under argon atmosphere, 5-bromo-2-(4-bromophenyl)-1H-benzo[d]imidazole(3) (1.5 g, 4 mmol) in DMF (15 mL) was stirred at 0°C and then sodium hydride (60%) (0.56 g, 14 mmol) was added. Subsequently, the mixture was stirred at room temperature for 2 h and then was heated to 40°C. Ethyl bromide (0.54 g, 5 mmol) was injected through a syringe in 15 min. The mixture was stirred at 80°C for 12 h and then water (15 mL) was added dropwise slowly. The organic phase was separated and the aqueous layer was extracted with dichloromethane. The combined organic layer was washed with water, dried over anhydrous sodium sulfate, filtered and the solvent was removed by rotary evaporation. The crude product was purified by column chromatography (silica gel, petroleum ether/ethyl acetate: 4/1) to give a white solid. Yield: 0.91 g, 63.8%. 1H NMR (400 MHz, CDCl3, δ): 7.97 (s, 0.5H), 7.64 (dd, J = 33.5, 8.3 Hz, 5H), 7.47-7.40 (m, 1H), 7.31 (d, J = 8.6 Hz, 0.5H); 4.26 (m, 2H); 1.47 (m, 3H).
5-Bromo-2-(4-bromophenyl) -1-(3-sulfonylpropyl) -1H-benzo [d]imidazole (monomer 2)
Under argon atmosphere, 5-bromo-2-(4-bromophenyl)-1H-benzo[d]imidazole(3) (0.7 g, 2 mmol) in DMF (10 mL) were stirred at 0°C and sodium hydride (60%) (0.32 g, 8 mmol) was added. Subsequently, the mixture was stirred at room temperature for 2 h and then was heated to 40°C. 1,3-Propanesultone (0.49 g, 4 mmol) dissolved in 5 mL DMF was added dropwise in 30 min. Subsequently, the reaction solution was stirred at 90°C for 12 h and cooled to room temperature. The mixture was poured into absolute ether (150 mL) and the precipitate was collected. The crude product was dried under vacuum at 60°C for 12 h and was purified by recrystallization from aqueous solution of potassium chloride to give a white solid. Finally this solid was dried under vacuum overnight again. Yield: 0.56 g, 56.4%. 1H NMR (400 MHz, DMSO-d6, δ): 8.04 (d, J = 1.7 Hz, 0.5H), 7.89 (d, J = 1.8 Hz, 0.5H); 7.82-7.62 (m, 5H); 7.42 (ddd, J = 22.8, 8.6, 1.8 Hz, 1H); 4.46 (dd, J = 15.0, 6.5 Hz, 2H); 2.39 (td, J = 7.1, 3.7 Hz, 2H); 2.0-1.93 (m, 2H).
2.2.2 Synthesis of polymers
(+)-PPBIPV
Under argon atmosphere, monomer 1 (0.29 g, 0.5 mmol), (+)-DVB (0.18 g, 0.5 mmol), palladium acetate (0.011 g, 0.05 mmol), tri(o-tolyl)phosphine (0.061 g, 0.2 mmol) and diisopropylamine (2 mL), toluene (1.5 mL) and DMSO (2 mL) was stirred in a two-neck flask at 100°C for 6 h. The reaction mixture was filtered and the filtrate was poured into the mixed solvent (ethyl ether/acetone/methanol: 8/6/1). The precipitate was filtered and then redissolved in DMSO (10 mL). The resulting solution was dialyzed against deionized water for 3 days using a 4kD MWCO cellulose membrane. Finally, the solvent was removed by rotary evaporation and the crude product was further dried under vacuum at 60°C for 48 h to give a yellow solid. Yield: 0.1128 g, 27.3%. 1H NMR (400 MHz, DMSO-d6, δ): 8.16-7.34 (m, 13H), 4.84-4.24 (m, 6H), 3.91 (s, 4H), 3.51 (s, 12H), 1.65-0.81 (m, 21H).
(-)-PPBIPV
Under argon atmosphere, the mixture solution of monomer 2 (0.25 g, 0.5 mmol), C4-DVB (0.14 g, 0.5 mmol), palladiumacetate (0.011 g, 0.05 mmol), tri(o-tolyl) phosphine (0.061 g, 0.2 mmol) and diisopropylamine (2.5 mL) and DMF (3 mL) was stirred at 100°C for 6 h. The reaction mixture was poured into methanol (20 mL) with dimethylglyoxime (0.158 g) and stirred for 10 min. The mixture solution was filtered and the filtrate was poured into the mixed solvent (ethyl ether/acetone: 1/1). The precipitate was filtered and then redissolved in DMSO (10 mL). The resulting solution was dialyzed against deionized water for 3 days using a 4kD MWCO cellulose membrane. Finally, the solvent was removed by rotary evaporation and the crude product was further dried under vacuum at 60°C for 48 h to give a yellow-brown solid. Yield: 0.1387 g, 43.4%. 1H NMR (400 MHz, DMSO-d6, δ): 8.13-6.87 (m, 13H), 4.55 (s, 2H), 4.12 (d, J = 37.1 Hz, 4H), 2.25-0.61 (m, 18H).
3 Results and discussion
3.1 Synthesis and characterization of monomers and polymers
The synthetic schemes for the monomers and the polymers are outlined in Scheme 1. During the formation of the imidazole ring, the position of the bromine substituent on the benzimidazole ring will be at the 4th or 5th position due to the different positions of oxidative dehydrogenation, and therefore compound 3 is a mixture. Of course, monomer 1 and monomer 2 derived from the nitrogen alkylation of compound 3 are also mixtures.
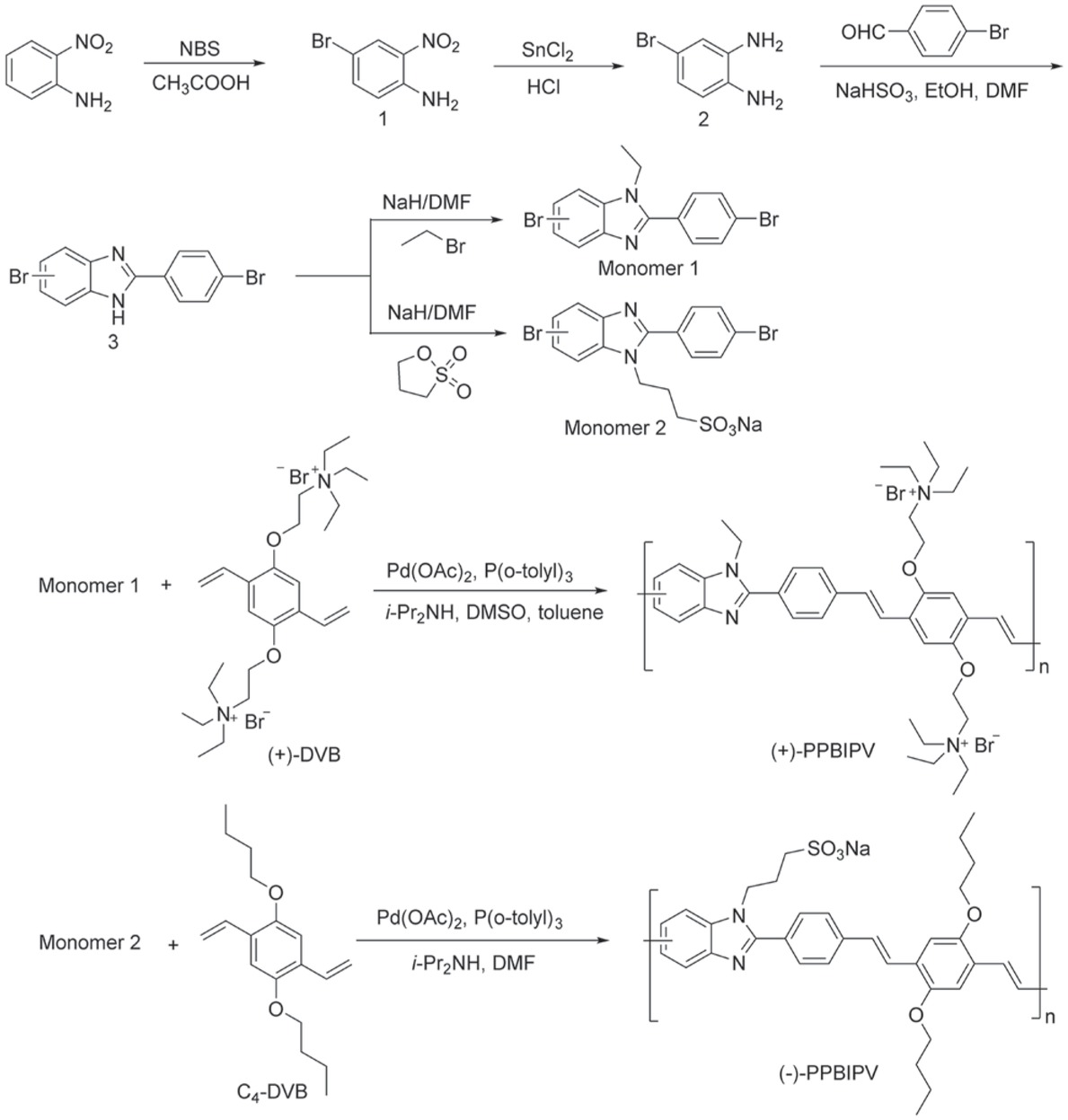
Synthetic routes of monomers and polymers.
The polymerization of the two polymers were both carried out via Heck coupling reaction using Pd(OAc)2 as catalyst and P(o-tolyl)3 as the corresponding ligand (31). Taking into account the presence of water soluble monomers and oil soluble monomers, we chose DMF that can dissolve most of the substances as solvent. During the procedure of the copolymerization, the conjugated length of (+)-PPBIPV and (-)-PPBIPV which was detected by the UV–vis spectrum was adjusted to be almost identical by controlling the polymerization time.
As shown in Figure 1, it can be seen that the characteristic peaks of polymer (+)-PPBIPV correspond well with the characteristic peaks of each monomer. Specifically, in the 1H NMR spectrum of (+)-PPBIPV, the presence of the peaks at 0.81-1.65 ppm is attributed to the characteristic peak of the methyl group in the alkyl side chain of monomer 1 and (+)-DVB; the peaks at 3.51 ppm and 3.91 ppm are all attributed to the hydrogen of N-CH2 on the quaternary ammonium group in (+)-DVB; the peaks at 4.24-4.84 ppm are respectively attributed to O-CH2 of (+)-DVB unit and N-CH2 of monomer 1 unit. Since the polymer is not a single pure material but a mixture of different molecular weights, a broad peak usually appears in the NMR spectrum of the polymer. Furthermore, the polymer is a compound with a large delocalized π bond, hydrogen atoms in the conjugated structure are often subjected to complex de-shielding effects, so the peaks at 7.34-8.16 ppm are the peaks of aromatic hydrogen atoms in the polymer backbone.
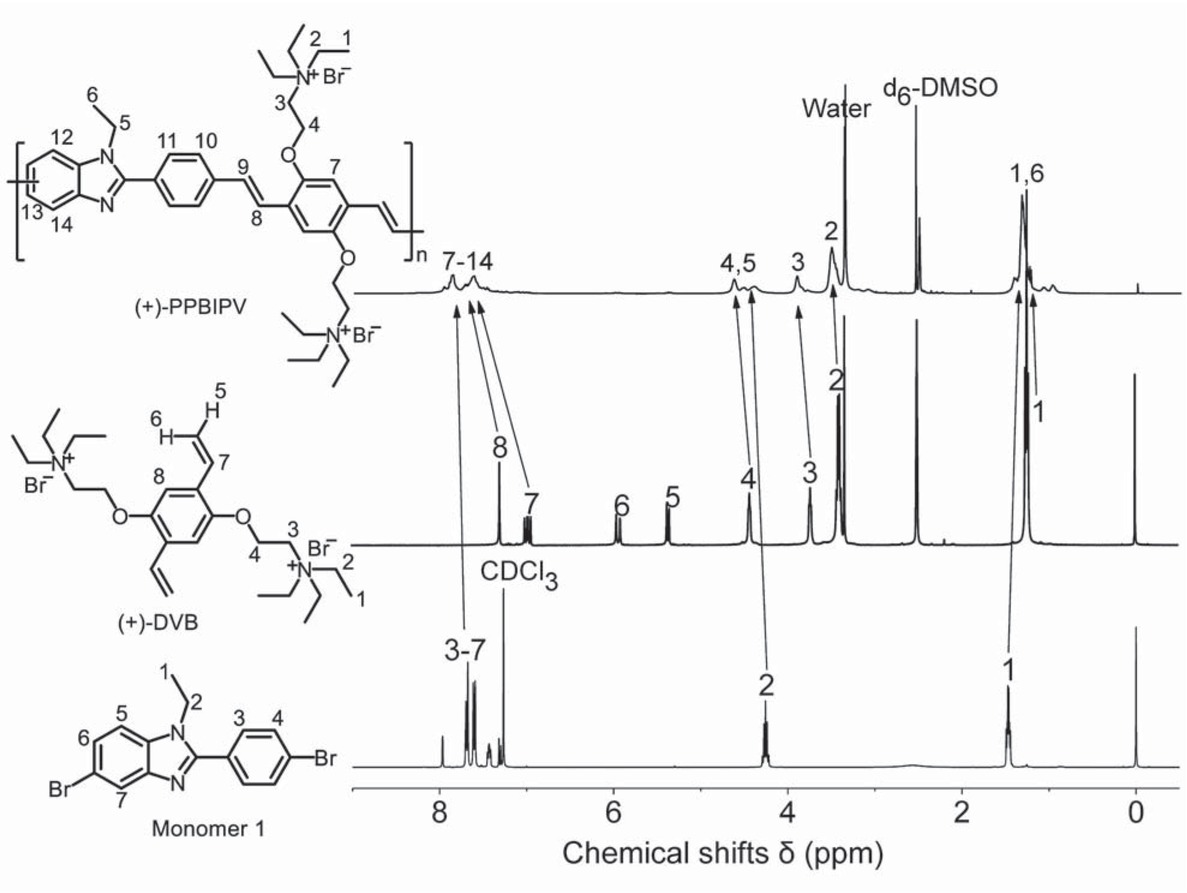
1H NMR spectra of monomer 1, (+)-DVB and (+)-PPBIPV.
Figure 2 shows the 1H NMR spectra of monomer 2, C4-DVB and (-)-PPBIPV. In the 1H NMR spectrum of (-)-PPBIPV, the presence of the peaks at 0.56-2.10 ppm is attributed to the signal peaks generated by hydrogen atoms not adjacent to oxygen and nitrogen atoms on the alkyl side chains, showing the monomer 2 and C4-DVB units have been introduced into the main chain of (-)-PPBIPV. These results can also be found in the spectrum of (+)-PPBIPV in Figure 1. Moreover, the hydrogen atoms adjacent to oxygen and nitrogen atoms in the alkyl side chain appear at 3.84-4.55 ppm, and the signals in the region of 6.97-8.09 ppm are due to the hydrogen atoms of benzene ring.
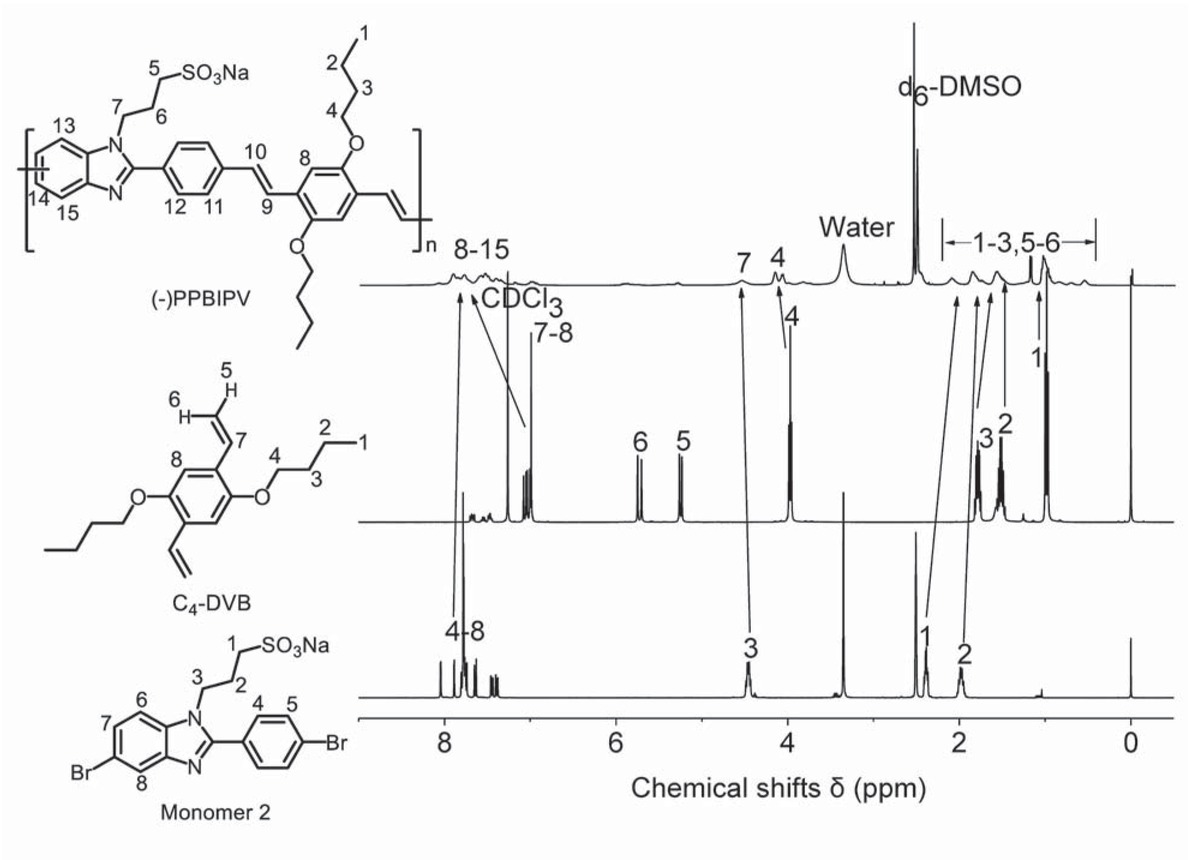
1H NMR spectra of monomer 2, C4-DVB and (-)-PPBIPV.
It is well-known that CPEs can aggregate in its solution and that aggregated polymers can be easily absorbed on the chromatographic column, so precise molecular weight information of CPEs is difficult to obtain from gel permeation chromatography (GPC). Another reason is that it is difficult to find a suitable solvent to conform to the measurement requests. Furthermore, owing to the aggregation phenomenon of CPEs, light scattering which is the common technique to measure the molecular weight as same as GPC is not available (32,33). Thus, the solutions of (+)-PPBIPV and (-)-PPBIPV were dialyzed by 4kD MWCO cellulose membrane, which ensured that the molecular weight of polymers were more than 4000 (at least eight repeating units). Although we can only get a general result in this way, but it is enough to prove that the polymerization of the two CPEs has been completed successfully and satisfied the requirement of photophysics and sensing measurement.
3.2 Basic photophysical properties of polymers
The absorption and emission data of monomer 1, monomer 2, (+)-DVB, C4-DVB, (+)-PPBIPV and (-)-PPBIPV are listed in Table 1. In the water, (+)-PPBIPV shows broad absorption band covered 300-500 nm visible light region and the absorption maximum at 415 nm. This phenomenon is associated with the increasing conjugate chain of the polymer, which decreases the energy from ground state to excited state. The fluorescence of (+)-PPBIPV is green in the solution, while the fluorescence of (+)-DVB and monomer 1 is purple, and its fluorescence intensity is significantly stronger than two monomers. In comparison with monomer 1, the PL emission maximum of (+)-PPBIPV at 501 nm showing red shifted 139 nm is attributed to its increasing conjugation degree. In addition, the maxima of absorption peak and fluorescence emission peak of (+)-PPBIPV are much larger than those of monomer 1 and (+)-DVB, which verifies the success of the polymerization.
Optical properties of (+)-DVB, C4-DVB, monomer 1, monomer 2, (+)-PPBIPV and (-)-PPBIPV.
| Monomers/Polymers | Solvent | UV-vis max (nm) | PL max (nm) | Stokes Shift (nm)/(cm-1) |
|---|---|---|---|---|
| (+)-DVB | Water | 331 | 387 | 56/178571 |
| C4-DVB | Dichloromethane | 346 | 398 | 52/192308 |
| Monomer 1 | Dichloromethane | 303 | 362 | 59/169492 |
| Monomer 2 | Water | 296 | 368 | 72/138889 |
| (+)-PPBIPV | Water | 415 | 501 | 86/116279 |
| Methanol | 403 | 470 | 67/149254 | |
| DMSO | 417 | 480 | 63/158730 | |
| (-)-PPBIPV | Water | 408 | 613 | 205/48780 |
| Methanol | 405 | 500 | 95/105263 | |
| DMSO | 417 | 495 | 78/128205 |
As same as (+)-PPBIPV, the UV-vis and PL maxima of (-)-PPBIPV both show large red shifts compared to its monomers. (-)-PPBIPV exhibits the absorption maximum at 408 nm and emission maximum at 613 nm in the water. There is almost no overlap between the ultraviolet absorption band and the fluorescence emission band of (-)-PPBIPV, and the Stokes shift reaches 205 nm, which can effectively prevent fluorescence self-absorption and exhibit the potential of fluorescence sensor materials.
The absorption and emission maxima of (+)-PPBIPV and (-)-PPBIPV are listed in Table 1, while the photos of conjugated polyelectrolytes in different solvents are shown in Figure 3. As shown in Figure 3a, the maximum absorption peak of (+)-PPBIPV is located in 403 nm in methanol. However, it exhibits a red shift in water and DMSO, which is located in 415 nm and 417 nm, respectively. Similarly, when the solvent is changed from methanol to DMSO or water, the emission maximum has been red shifted from 470 nm (in methanol) to 480 nm (in DMSO) or 501 nm (in water). In addition, the PL emission intensity of (+)-PPBIPV is significant in both methanol and DMSO, but weak in aqueous solution. In water, (+)-PPBIPV aggregates strongly by π-π stacking as same as other conjugated polyelectrolytes (34), which means that the conjugated layers of the polyelectrolytes are stacked mutually. This aggregation causes the conjugated polyelectrolytes to reach a higher degree of conjugation, making it easier for excitons to transfer between the backbones (35). Thus, the fluorescence quenching of (+)-PPBIPV in water may be attributed to energy dissipation caused by excitons transfer.
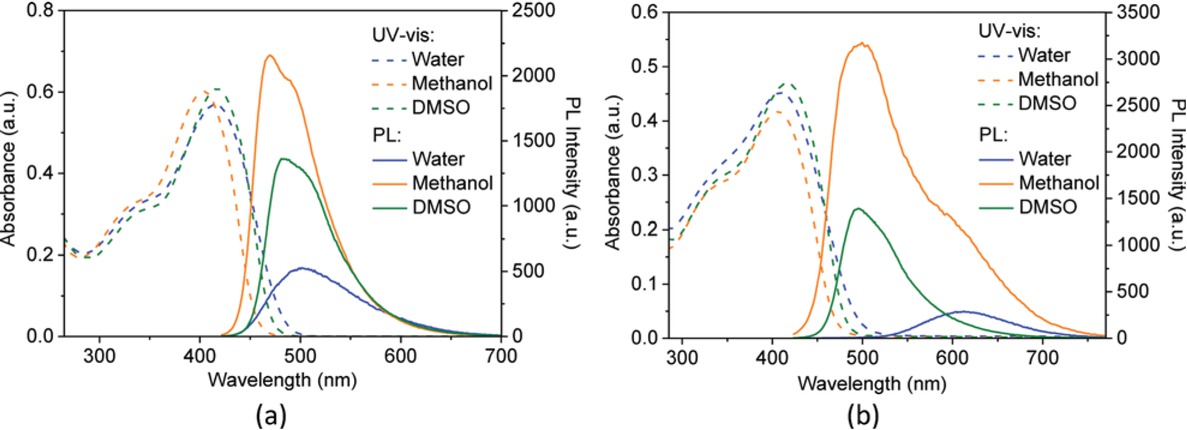
UV-vis and PL spectra of (+)-PPBIPV in water, methanol and DMSO (a); UV-vis and PL spectra of (-)-PPBIPV in water, methanol and DMSO (b).
Figure 3b shows that the maximum absorption peaks are located in 417 nm in DMSO, 408 nm in water, and 405 nm in methanol, respectively. In addition, the emission peak of (-)-PPBIPV is located in 500 nm in methanol, corresponding to the intrinsic emission (36). However, it is worth noting that the fluorescence of (-)-PPBIPV in aqueous solution is very weak, and the emission maximum has been red shifted from 408 nm to 613 nm, which is consistent with the reported anionic conjugated polyelectrolyte (36). In general, the peak at long wavelength corresponds to aggregation emission in water. The shape of emission peak and the fluorescence solvent dependence are also the same as other sulfonate-substituted conjugated polyelectrolytes (36,37). Compared to (+)-PPBIPV, the emission maximum of (-)-PPBIPV exhibits a greater red shift in water. This phenomenon can be attributed to the fact that (-)-PPBIPV has a stronger aggregation effect.
The fluorescence quantum yields (Φ) of (+)-PPBIPV and (-)-PPBIPV were measured by using quinine sulfate in 0.1 N sulfuric acid as standard (n = 1.336, Φ = 55%) according to the following equation (38):
where the subscript P and S denote to polymer solution and standard, respectively. Φ is the fluorescence quantum yield, F is the intensity of fluorescence, and n is the refractive index of the solvent.
The Φ of (+)-PPBIPV and (-)-PPBIPV in methanol are 33% and 46%, respectively, which are basically consistent to the reference (39). However, the Φ of benzimidazole-based conjugated polymers is nearly 80% in THF (24). As reference reported, conjugate polyelectrolytes have lower fluorescence quantum yields than the uncharged oil soluble conjugated polymers of the same main-chain units (39). This phenomenon can be attributed to the fact that (+)-PPBIPV and (-)-PPBIPV aggregate strongly in the solvent, resulting in relatively low fluorescence quantum yields. On the other hand, the benzimidazole units of (+)-PPBIPV and (-)-PPBIPV are fluorescent chromophores, so their fluorescence quantum yields are higher than other PPV-type conjugated polyelectrolytes (40).
3.3 Metal ions response of polymers
The fluorescence responses of (+)-PPBIPV and (-)-PPBIPV on various metal ions were investigated. The solutions of metal ion were prepared by PdCl2, FeCl3⋅6H2O, MgCl2⋅6H2O, SnCl2⋅2H2O, CoCl2⋅6H2O, ZnCl2, CuSO4⋅5H2O, MnSO4⋅H2O, NiCl2⋅6H2O, Cr(NO3)3⋅9H2O, FeSO4⋅7H2O, HgCl2 and CdCl2, respectively. All of the concentrations of polymers were 2×10-5 mol/L. In metal ion fluorescence sensing experiments (Figure 4 and Figure 7), when the metal ion is added to the polymer solution and the fluorescence intensity of the solution no longer changes, the ion concentration is called the limiting concentration.
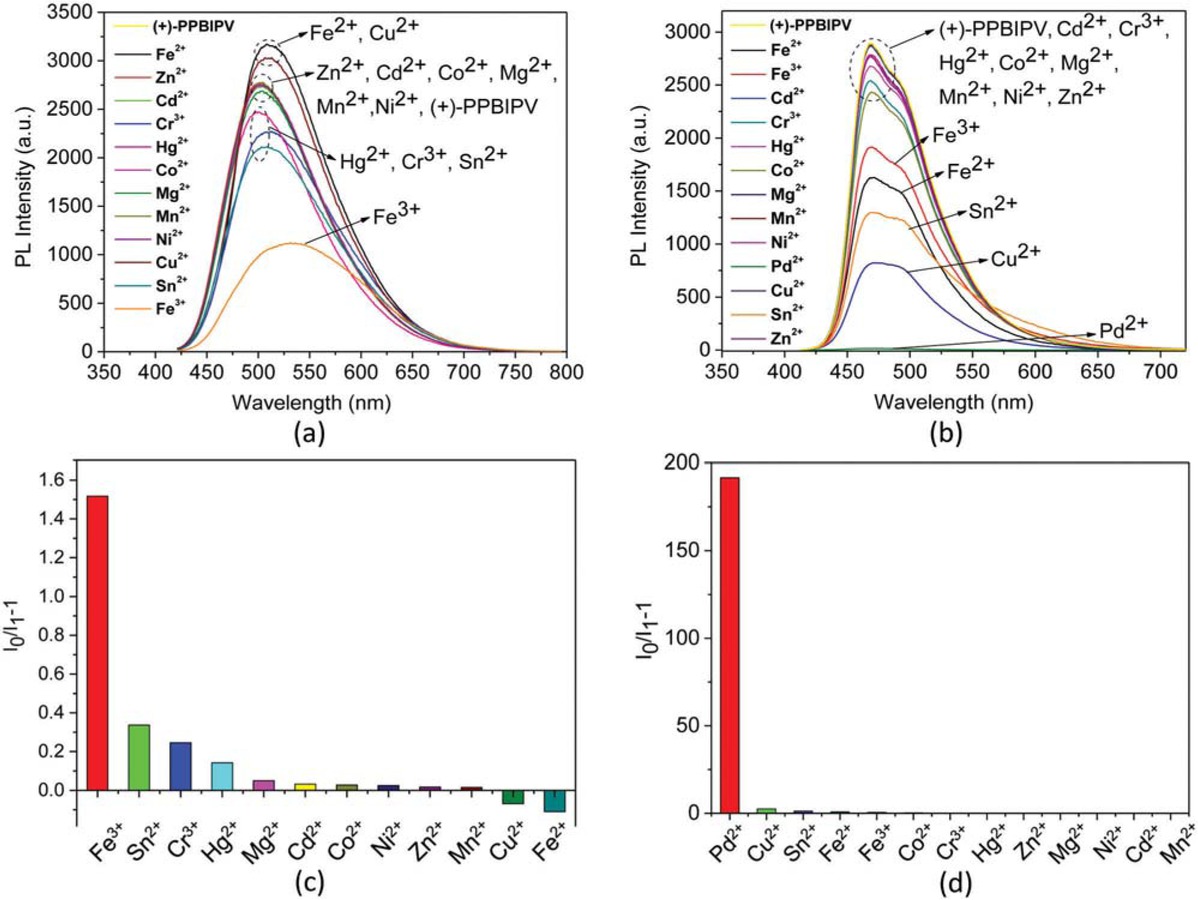
PL spectra of aqueous solution of (+)-PPBIPV with different metal ions (a); PL spectra of methanol solution of (+)-PPBIPV with different metal ions (b); PL quenching ratios (I0/I1-1) of aqueous solution of (+)-PPBIPV with different metal ions (c); PL quenching ratios (I0/I1-1) of methanol solution of (+)-PPBIPV with different metal ions (d).
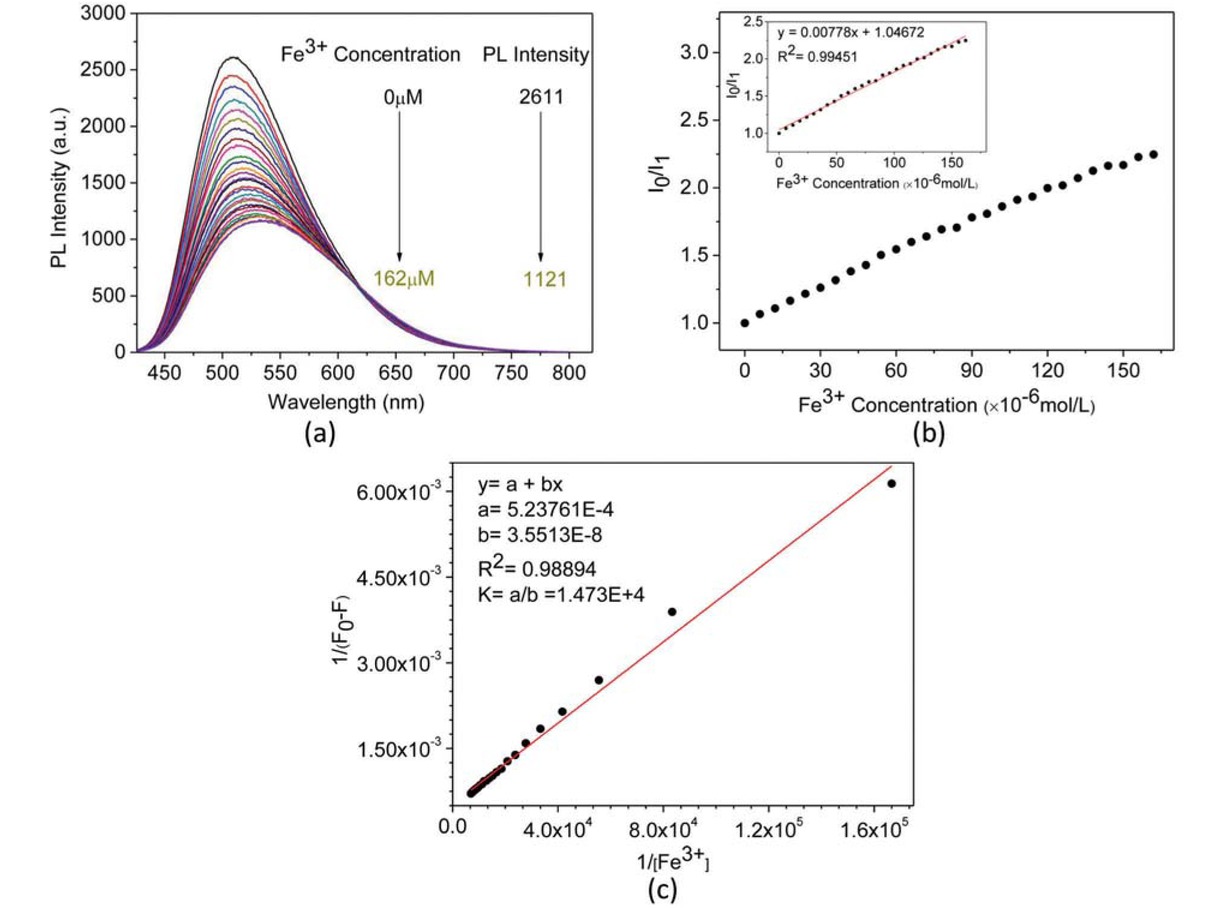
PL spectra of aqueous solution of (+)-PPBIPV with different concentrations of Fe3+ (a); PL titration curves of aqueous solution of (+)-PPBIPV with Fe3+ (b); The Benesi-Hildebrand plot of aqueous solution of (+)-PPBIPV with Fe3+ (c).
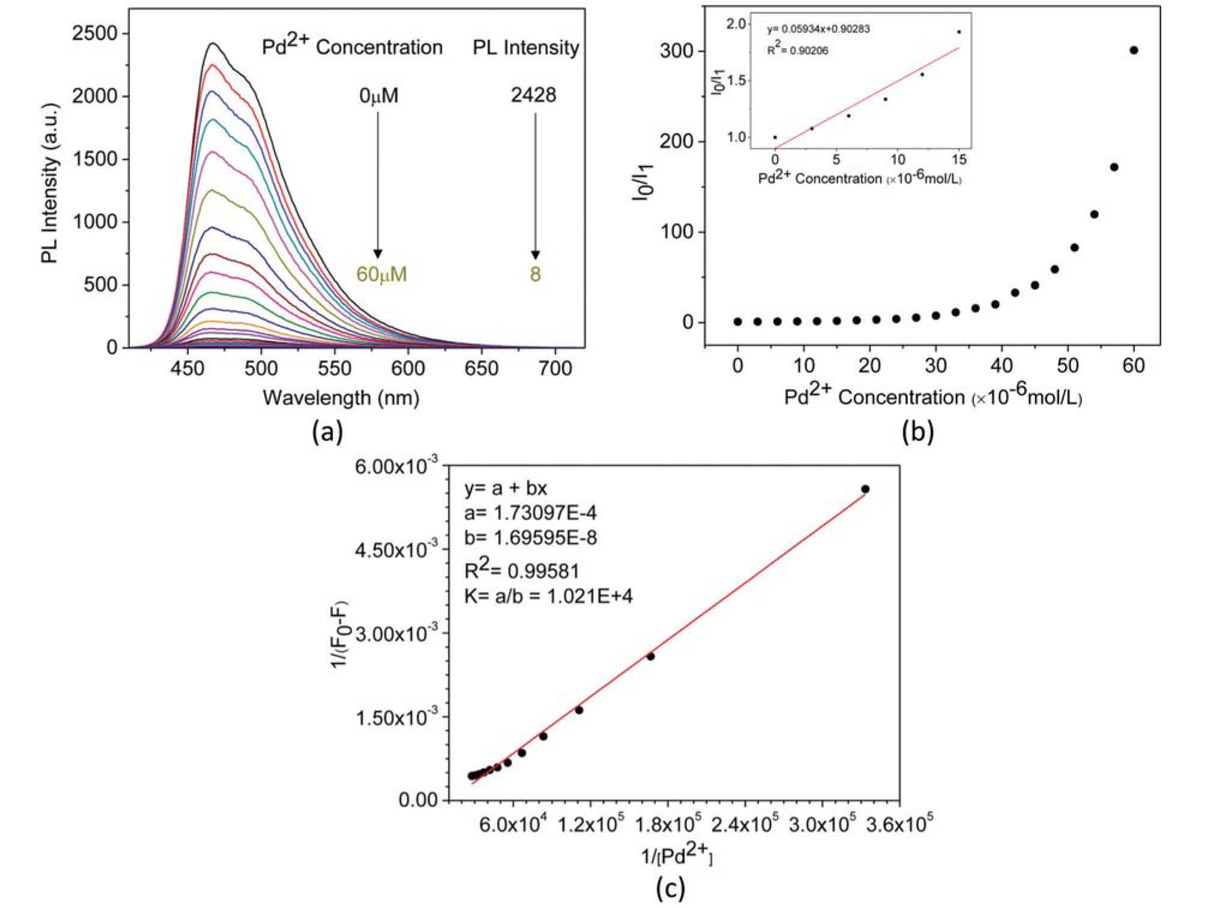
PL spectra of methanol solution of (+)-PPBIPV with different concentrations of Pd2+ (a); PL titration curves of methanol solution of (+)-PPBIPV with Pd2+ (b); The Benesi-Hildebrand plot of methanol solution of (+)-PPBIPV with Pd2+ (c).
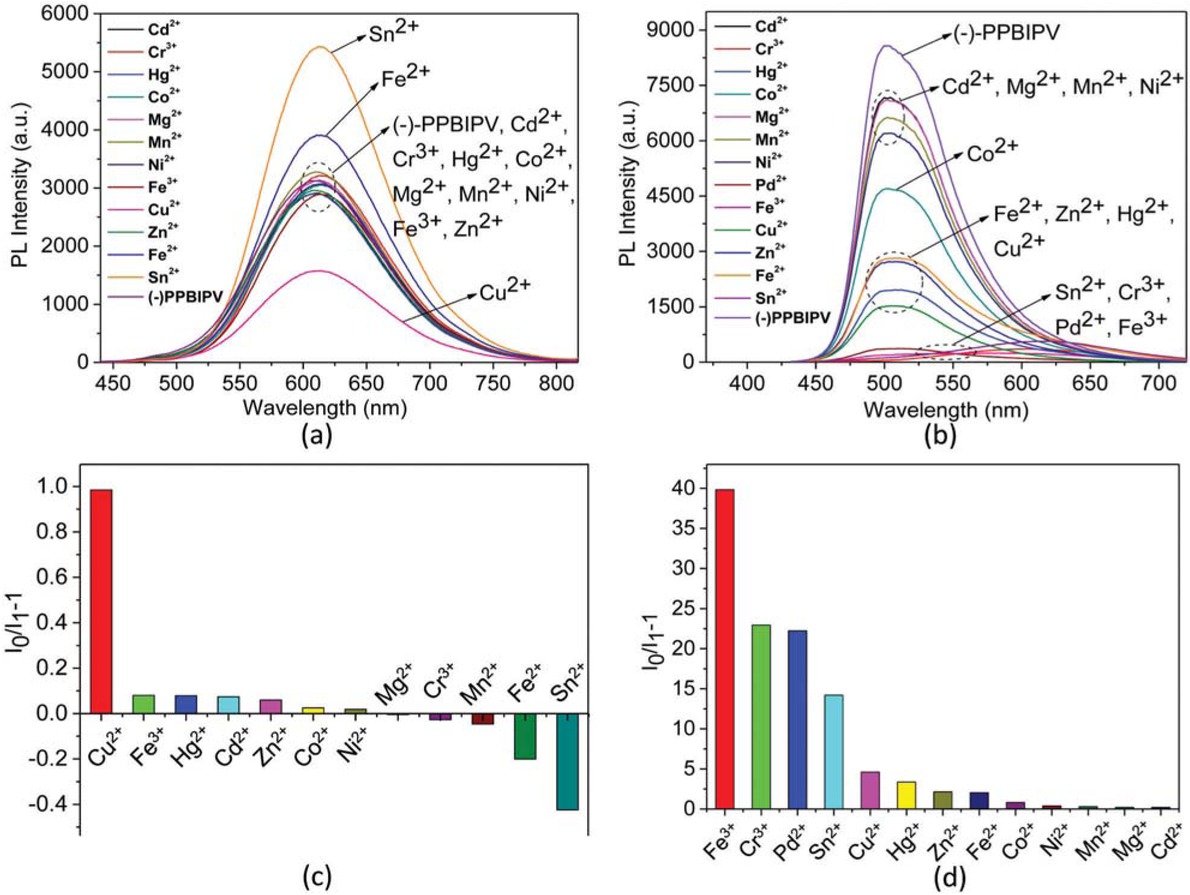
PL spectra of aqueous solution of (-)-PPBIPV with different metal ions (a); PL spectra of methanol solution of (-)-PPBIPV with different metal ions (b); PL quenching ratios (I0/I1-1) of aqueous solution of (-)-PPBIPV with different metal ions (c); PL quenching ratios (I0/I1-1) of methanol solution of (-)-PPBIPV with different metal ions (d).
3.4 Sensing properties of (+)-PPBIPV
Figure 4a shows the PL spectra of aqueous solution of (+)-PPBIPV with Fe2+, Zn2+, Cd2+, Cr3+, Hg2+, Co2+, Mg2+, Mn2+, Ni2+, Cu2+, Sn2+ and Fe3+. The PL max (nm) and maxima of fluorescence intensity of (+)-PPBIPV with different metal ions are listed in Table 2. It can be found that Fe3+, Sn2+, Cr3+ and Hg2+ can reduce the fluorescence of (+)-PPBIPV, but the decrease occurs most notably upon the addition of Fe3+. On the contrary, there is a small increase in fluorescence intensity of (+)-PPBIPV in the presence of Fe2+ and Cu2+. And other metal ions hardly lead to changes in PL spectrum of (+)-PPBIPV. The fluorescence-quenching ratios (I0/I1-1) of aqueous solution of (+)-PPBIPV in the presence of different metal ions are shown in Figure 4c. It should be mentioned that even if the fluorescence quenching ratios of Sn2+ and Cr3+ are 0.34 and 0.25, respectively, they are still small compared to 1.52 of Fe3+. These results show that (+)-PPBIPV has better fluorescent sensitivity to Fe3+ in aqueous solution.
PL max (nm) and maxima of fluorescence intensity of (+)-PPBIPV with different metal ions.
| (+)-PPBIPV in water | (+)-PPBIPV in methanol | ||||
|---|---|---|---|---|---|
| Metal ion | PL max (nm) | Fluorescence intensity (a.u.) | Metal ion | PL max (nm) | Fluorescence intensity (a.u.) |
| blank | 500 | 2822 | blank | 470 | 2889 |
| Cu2+ | 507 | 3030 | Cu2+ | 471 | 821 |
| Fe3+ | 532 | 1121 | Fe3+ | 470 | 1915 |
| Hg2+ | 500 | 2471 | Hg2+ | 468 | 2678 |
| Cd2+ | 504 | 2734 | Cd2+ | 469 | 2870 |
| Zn2+ | 502 | 2774 | Zn2+ | 469 | 2772 |
| Co2+ | 506 | 2746 | Co2+ | 469 | 2434 |
| Ni2+ | 501 | 2753 | Ni2+ | 469 | 2785 |
| Mg2+ | 503 | 2688 | Mg2+ | 468 | 2784 |
| Cr3+ | 508 | 2265 | Cr3+ | 469 | 2542 |
| Mn2+ | 504 | 2779 | Mn2+ | 470 | 2881 |
| Fe2+ | 508 | 3171 | Fe2+ | 472 | 1627 |
| Sn2+ | 503 | 2111 | Sn2+ | 471 | 1297 |
| Pd2+ | 473 | 15 | |||
The PL spectra of methanol solution of (+)-PPBIPV with various metal ions (Fe2+, Zn2+, Cd2+, Cr3+, Hg2+, Co2+, Mg2+, Mn2+, Ni2+, Cu2+, Sn2+, Fe3+ and Pd2+) were also investigated (Figure 4b). Pd2+ exhibits the greatest effect on the fluorescence of (+)-PPBIPV methanol solution, which almost completely quenches the fluorescence of the polymer with a quenching rate as high as 99%. Moreover, Cu2+, Sn2+, Fe2+ and Fe3+ can partially quench the solution fluorescence and their quenching rates are 72%, 55%, 44% and 34%, respectively. As shown in Figure 4d, the fluorescence quenching ratio of Pd2+ is much larger than other metal ions, indicating that (+)-PPBIPV has high selectivity and excellent sensitivity for Pd2+ in the methanol solution.
Figure 5a gives the fluorescence spectra of (+)-PPBIPV in water with different concentrations of Fe3+. As the concentration of Fe3+ increases, the fluorescence intensity of (+)-PPBIPV is quenched from 2611 to 1121. The fluorescence quenching efficiency of (+)-PPBIPV can be described by the Stern-Volmer equation (41): I0/I1 = 1 + KSV[Q], where I0 and I1 are the fluorescence intensities of the system in the absence and presence of Fe3+, respectively, KSV is the Stern-Volmer quenching constant, and [Q] is the Fe3+ concentration. At the concentrations of Fe3+ between 0 and 162 μM, a Stern-Volmer plot of the fluorescence intensity of (+)-PPBIPV versus the concentration of Fe3+ is linear with a KSV value of 7.78×103 M-1, as shown in Figure 5b. The I0/I1 versus [Fe3+] is a straight line, indicating that there is only a single quenching mechanism in this system. Investigating the fluorescence sensor based on benzimidazole, it is found that the quenching mechanism is generally forming a complex between the sp2 nitrogen atom and the metal ions (42). Therefore, the mechanism of fluorescence quenching of (+)-PPBIPV towards Fe3+ is static quenching in water. The stoichiometric relationship between Fe3+ and (+)-PPBIPV can be determined by the Benesi-Hildebrand expression based on fluorescence spectra (43): 1/(F0-F) = 1/(K(F0-Fmin)[Q]n) + 1/(F0-Fmin), where F0 and F are the fluorescence intensities in the absence and presence of Fe3+, respectively, Fmin is the minimum fluorescence intensity in the presence of Fe3+, and K is the association constant. The measured fluorescence intensity [1/(F0-F)] varies as a function of 1/[Fe3+] (n = 1) in a linear (R2 = 0.98894), indicating 1:1 stoichiometry between (+)-PPBIPV and Fe3+ (Figure 5c). Furthermore, the K obtained from the slope and intercept of the line is 1.47×104 M-1 for (+)-PPBIPV binding to Fe3+.
Subsequently, we investigated the ability of methanol solution (+)-PPBIPV to detect Pd2+. As Figure 6a shows, the fluorescence intensity of (+)-PPBIPV decreases regularly as the concentration of Pd2+ increases, from 0 to 60 μM. As shown in Figure 6b, a Stern-Volmer plot of the fluorescence intensity of (+)-PPBIPV versus the concentration of Pd2+ is a upward curve instead of a straight line, which indicates the fluorophore is quenched by both dynamic quenching and static quenching with Pd2+. On the other hand, when the concentration of Pd2+ is between 0 and 15 μM, a Stern-Volmer plot of the fluorescence intensity of (+)-PPBIPV versus the concentration of Pd2+ is linear with a KSV value of 5.93×104 M-1, indicating the mechanism of fluorescence quenching of methanol solution (+)-PPBIPV towards Pd2+ is static quenching in the low concentration region. Meanwhile, Benesi-Hildebrand plots of the fluorescence titration combined with (+)-PPBIPV and Pd2+ is yielded linear correlation coefficients of 0.99581, using the Benesi-Hildebrand equation of linear fitting based on 1:1 binding (n = 1) (Figure 6c). The strong correlation proves that there is 1:1 binding between (+)-PPBIPV and Pd2+, and the K is calculated as 1.02×104 M-1.
All of the measurement methods of (-)-PPBIPV are the same as (+)-PPBIPV. The PL max (nm) and maxima of fluorescence intensity of (-)-PPBIPV with different metal ions are listed in Table 3. As shown in Figures 7a and 7c, Cu2+ can quench the fluorescence of aqueous solution of (-)-PPBIPV by nearly 50%. Zn2+, Cd2+, Cr3+, Hg2+, Co2+, Mg2+, Mn2+, Ni2+ and Fe3+ exhibit little or no effect on the fluorescence intensity of the polymer. Both Fe2+ and Sn2+ can increase the fluorescence, but Sn2+ is more obviously, which doubles the fluorescence intensity of the polymer approximately.
PL max (nm) and maxima of fluorescence intensity of (-)-PPBIPV with different metal ions.
| (-)-PPBIPV in water | (-)-PPBIPV in methanol | ||||
|---|---|---|---|---|---|
| Metal ion | PL max (nm) | Fluorescence intensity (a.u.) | Metal ion | PL max (nm) | Fluorescence intensity (a.u.) |
| blank | 613 | 3126 | blank | 500 | 8576 |
| Cu2+ | 612 | 1575 | Cu2+ | 505 | 1529 |
| Fe3+ | 613 | 2894 | Fe3+ | 531 | 210 |
| Hg2+ | 610 | 2898 | Hg2+ | 508 | 1961 |
| Cd2+ | 611 | 2911 | Cd2+ | 500 | 7167 |
| Zn2+ | 609 | 2950 | Zn2+ | 504 | 2726 |
| Co2+ | 615 | 3050 | Co2+ | 502 | 4707 |
| Ni2+ | 613 | 3068 | Ni2+ | 501 | 6198 |
| Mg2+ | 613 | 3139 | Mg2+ | 502 | 7101 |
| Cr3+ | 614 | 3213 | Cr3+ | 603 | 358 |
| Mn2+ | 610 | 3277 | Mn2+ | 502 | 6635 |
| Fe2+ | 611 | 3911 | Fe2+ | 509 | 2819 |
| Sn2+ | 613 | 5430 | Sn2+ | 614 | 564 |
| Pd2+ | 510 | 369 | |||
Figure 7b shows the PL spectra of methanol solution of (-)-PPBIPV with different metal ions. Metal ions response of methanol solution of (-)-PPBIPV is more complicated than aqueous solution of (-)-PPBIPV. According to Figure 7d, the methanol solution of (-)-PPBIPV has certain selectivity for Fe3+, Pd2+, Cr3+ and Sn2+, especially Fe3+.
As Figure 8a shows, adding 60 μM Sn2+ results in 100% fluorescence enhancement of (-)-PPBIPV. Moreover, the Stern-Volmer plot of the fluorescence intensity of (-)-PPBIPV versus the concentration of Sn2+ is a downward curve, which can also be fitted as the equation approximatively, y = -0.01325x + 0.93436 (Figure 8b). In this system, the addition of Sn2+ increases the fluorescence intensity of (-)-PPBIPV, so the Benesi-Hildebrand equation takes this form (44): 1/(F-F0) = 1/(K(Fmax-F0)[Q]n) + 1/(Fmax-F0), where F0 and F are the fluorescence intensities in the absence and presence of Sn2+, respectively, Fmax is the maximum fluorescence intensity in the presence of Sn2+, and K is the association constant. The Benesi-Hildebrand equation fitting curves of the 1/(F-F0) to 1/[Sn2+] are shown in Figure 8c, yielded linear correlation coefficients of 0.99655. The result indicates 1:1 stoichiometry between (-)-PPBIPV and Sn2+, with a K value of 1.81×104 M-1.
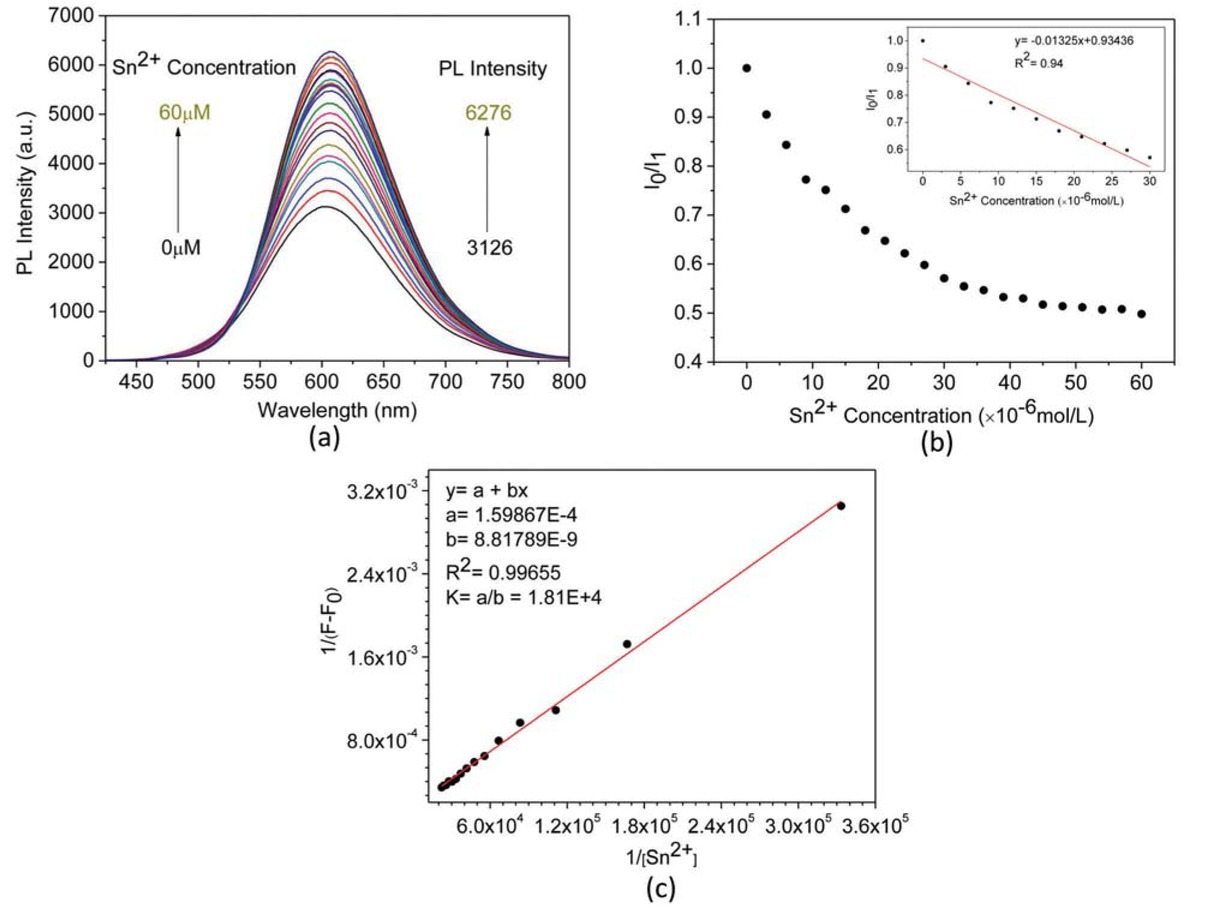
PL spectra of aqueous solution of (-)-PPBIPV with different concentration of Sn2+ (a); PL titration curves of aqueous solution of (-)-PPBIPV with Sn2+ (b); The Benesi-Hildebrand plot of aqueous solution of (-)-PPBIPV with Sn2+ (c).
Figure 9a shows the fluorescence spectra of the (-)-PPBIPV in methanol solution with the increasing concentration of Fe3+. Add 30 μM Fe3+, causing the fluorescence of (-)-PPBIPV to be quenched 97.5%. As shown in Figure 9b, a Stern-Volmer plot of the fluorescence intensity of (-)-PPBIPV versus the concentration of Fe3+ is a non-liner, which is similar to the Stern-Volmer plot of the methanol solution of (+)-PPBIPV with Pd2+. In the low concentration region, the mechanism of fluorescence quenching of methanol solution (-)-PPBIPV towards Fe3+ is static quenching with a KSV value of 3.44×105 M-1. From Figure 9c, the stoichiometric ratio of (-)-PPBIPV to Fe3+ in methanol solution is 1:1, and the association constant K is 5.39×104 M-1, which is much larger than the association constant of (+)-PPBIPV and Fe3+ in aqueous solution (K = 1.47×104 M-1). Except for the difference in solvent, the reason may be that the side chain of (-)-PPBIPV is a negatively charged sulfonic acid group, which has an electrostatic attraction with metal ions. This force causes a large amount of metal ions to aggregate around the polymer, so metal ions and polymer receptor units can be better combined.
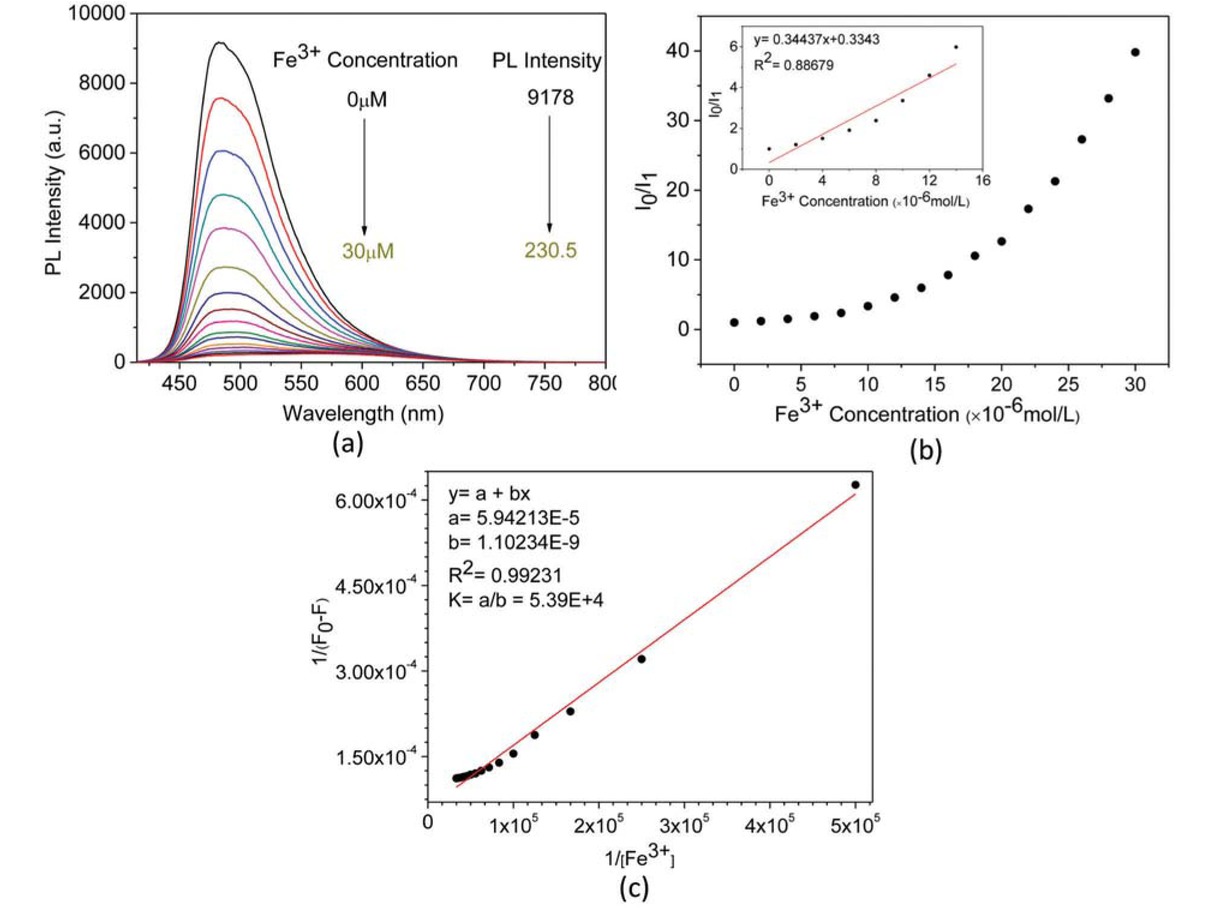
PL spectra of methanol solution of (-)-PPBIPV with different concentration of Fe3+ (a); PL titration curves of methanol solution of (-)-PPBIPV with Fe3+ (b); The Benesi-Hildebrand plot of methanol solution of (-)-PPBIPV with Fe3+ (c).
4 Conclusions
In summary, two novel benzimidazole-based conjugated polyelectrolytes (+)-PPBIPV and (-)-PPBIPV have been designed, synthesized and characterized successfully. In metal sensing aspects, it is demonstrated that Fe3+ can remarkably reduce the fluorescence of (+)-PPBIPV in aqueous solution. When the concentration of Fe3+ is in the range of 6-66 μM, Fe3+ can be detected by (+)-PPBIPV quantitatively. Furthermore, Pd2+ can quench almost all the fluorescence intensity of the polymer in methanol solution, of which KSV is 5.93×104 M-1, and the results show that there are both static quenching and dynamic quenching in this system. In the low concentration region, there is a single static quenching, and the stoichiometric ratio of (+)-PPBIPV and Pd2+ in methanol solution is 1:1. On the other hand, as for (-)-PPBIPV, Sn2+ can double the fluorescence of its aqueous solution, and the results shows that the stoichiometric relationship between (-)-PPBIPV and Sn2+ also is 1:1. Almost each ion can affect the fluorescence of (-)-PPBIPV in methanol solution, especially Fe3+, of which the fluorescence quenching efficiency is 97.5%. The reason for this phenomenon may be attributed to the complexation of metal ions with (-)-PPBIPV and the electrostatic attraction between positive metal ions and negative charges in side-chain of the polymer. By analyzing and comparing their response to metal ions, the influence of side chain electrification on the sensing properties of conjugated polymers can be further discussed. All results show that these two conjugated polyelectrolytes could potentially be used as effective fluorescent sensors for detecting metal ions in biological and environmental media.
Acknowledgements
This work is financially supported by the “Shuangzhi” Project of Sichuan Agricultural University (No. 00770105) and the National University Student Innovation Program (No. 201910626096).
References
1 Duan C., Wang L., Zhang K., Guan X., Huang F., Conjugated zwitterionic polyelectrolytes and their neutral precursor as electron injection layer for high-performance polymer light-emitting diodes. Adv. Mater., 2011, 23, 1665-1669.10.1002/adma.201004661Suche in Google Scholar PubMed
2 Seo J.H., Gutacker A., Sun Y., Wu H., Huang F., Cao Y., et al., Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J. Am. Chem. Soc., 2011, 133, 8416-8419.10.1021/ja2037673Suche in Google Scholar PubMed
3 Liang J., Li K., Liu B., Visual sensing with conjugated polyelectrolytes. Chem. Sci., 2013, 4, 1377.10.1039/c2sc21792aSuche in Google Scholar
4 You J., Park T., Kim J., Heo J.S., Kim H.S., Kim H.O., et al., Highly fluorescent conjugated polyelectrolyte for protein sensing and cell-compatible chemosensing applications. ACS Appl. Mater. Inter., 2014, 6, 3305-3311.10.1021/am500269tSuche in Google Scholar PubMed
5 Wang Y., Liu B., Mikhailovsky A., Bazan G.C., Conjugated polyelectrolyte-metal nanoparticle platforms for optically amplified DNA detection. Adv. Mater., 2010, 22, 656-659.10.1002/adma.200902675Suche in Google Scholar PubMed
6 Sun P., Lu X., Fan Q., Zhang Z., Song W., Li B., et al., Water-Soluble Iridium(III)-Containing Conjugated Polyelectrolytes with Weakened Energy Transfer Properties for Multicolor Protein Sensing Applications. Macromolecules, 2011, 44, 8763-8770.10.1021/ma201614zSuche in Google Scholar
7 Xia F., Zuo X., Yang R., Xiao Y., Kang D., Vallee-Belisle A., et al., Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc. Natl. Acad. Sci. USA, 2010, 107, 10837-10841.10.1073/pnas.1005632107Suche in Google Scholar PubMed PubMed Central
8 Kim B., Jung I.H., Kang M., Shim H.K., Woo H.Y., Cationic conjugated polyelectrolytes-triggered conformational change of molecular beacon aptamer for highly sensitive and selective potassium ion detection. J. Am. Chem. Soc., 2012, 134, 3133-3138.10.1021/ja210360vSuche in Google Scholar PubMed
9 Roy S., Gunukula A., Ghosh B., Chakraborty C., A folic acid-sensitive polyfluorene based “turn-off” fluorescence nanoprobe for folate receptor overexpressed cancer cell imaging. Sensor. Actuat. B-Chem., 2019, 291, 337-344.10.1016/j.snb.2019.04.029Suche in Google Scholar
10 Vazquez-Guillo R., Martinez-Tome M.J., Kahveci Z., Torres I., Falco A., Mallavia R., et al., Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe. Polymers, 2018, 10, 938-957.10.3390/polym10090938Suche in Google Scholar PubMed PubMed Central
11 Hussain S., Malik A.H., Iyer P.K., Highly precise detection, discrimination, and removal of anionic surfactants over the full pH range via cationic conjugated polymer: an efficient strategy to facilitate illicit-drug analysis. ACS Appl. Mater. Inter., 2015, 7, 3189-3198.10.1021/am507731tSuche in Google Scholar PubMed
12 Park S., Jeong J. E., Le V. S., Seo J., Yu B., Kim D.Y., et al., Enhanced Electron Transfer Mediated by Conjugated Polyelectrolyte and Its Application to Washing-Free DNA Detection. J. Am. Chem. Soc., 2018, 140, 2409-2412.10.1021/jacs.7b12382Suche in Google Scholar PubMed
13 Bojanowski N.M., Bender M., Seehafer K., Bunz U.H.F., Discrimination of Saccharides by a Simple Array. Chemistry, 2017, 23, 12253-12258.10.1002/chem.201700831Suche in Google Scholar PubMed
14 Park Y., Liu Z., Routh P.K., Kuo C.Y., Park Y.S., Tsai H., et al., DNA-assisted photoinduced charge transfer between a cationic poly(phenylene vinylene) and a cationic fullerene. Phys. Chem. Chem. Phys., 2015, 17, 15675-15678.10.1039/C5CP01309GSuche in Google Scholar
15 Jurin F.E., Buron C.C., Clément S., Mehdi A., Viau L., Lakard B., et al., Flexible and conductive multilayer films based on the assembly of PEDOT:PSS and water soluble polythiophenes. Org. Electron. , 2017, 46, 263-269.10.1016/j.orgel.2017.04.013Suche in Google Scholar
16 Huber R.C., Ferreira A.S., Thompson R., Kilbride D., Knutson N.S., Devi L.S., et al., Long-lived photoinduced polaron formation in conjugated polyelectrolyte-fullerene assemblies. Science, 2015, 348, 1340-1343.10.1126/science.aaa6850Suche in Google Scholar PubMed PubMed Central
17 Molina P., Tarraga A., Oton F., Imidazole derivatives: a comprehensive survey of their recognition properties. Org. Biomol. Chem., 2012, 10, 1711-1724.10.1039/c2ob06808gSuche in Google Scholar PubMed
18 Ajani O.O., Aderohunmu D.V., Ikpo C.O., Adedapo A.E., Olanrewaju I.O., Functionalized Benzimidazole Scaffolds: Privileged Heterocycle for Drug Design in Therapeutic Medicine. Arch. Pharm. Chem. Life Sci., 2016, 349, 475-506.10.1002/ardp.201500464Suche in Google Scholar PubMed
19 Ermler S., Scholze M., Kortenkamp A., Seven benzimidazole pesticides combined at sub-threshold levels induce micronuclei in vitro. Mutagenesis, 2013, 28, 417-426.10.1093/mutage/get019Suche in Google Scholar PubMed PubMed Central
20 Saluja P., Sharma H., Kaur N., Singh N., Jang D.O., Benzimidazole-based imine-linked chemosensor: chromogenic sensor for Mg2+ and fluorescent sensor for Cr3+ Tetrahedron, 2012, 68, 2289-2293.10.1016/j.tet.2012.01.047Suche in Google Scholar
21 Liu H., Zhang B., Tan C., Liu F., Cao J., Tan Y., et al., Simultaneous bioimaging recognition of Al3+ and Cu2+ in living-cell, and further detection of F- and S2- by a simple fluorogenic benzimidazole-based chemosensor. Talanta, 2016, 161, 309-319.10.1016/j.talanta.2016.08.061Suche in Google Scholar PubMed
22 Kim Y.S., Lee J.J., Lee S.Y., Jo T.G., Kim C., A highly sensitive benzimidazole-based chemosensor for the colorimetric detection of Fe(ii) and Fe(iii) and the fluorometric detection of Zn(ii) in aqueous media. RSC Adv., 2016, 6, 61505-61515.10.1039/C6RA10086DSuche in Google Scholar
23 Velmurugan K., Mathankumar S., Santoshkumar S., Amudha S., Nandhakumar R., Specific fluorescent sensing of aluminium using naphthalene benzimidazole derivative in aqueous media. Spectrochim. Acta A, 2015, 139, 119-123.10.1016/j.saa.2014.11.103Suche in Google Scholar PubMed
24 Zhu H., Tong H., Gong Y., Shao S., Deng C., Yuan W.Z., et al., Fluorene- and benzimidazole-based blue light-emitting copolymers: Synthesis, photophysical properties, and PLED applications. J. Polym. Sci. A1, 2012, 50, 2172-2181.10.1002/pola.25984Suche in Google Scholar
25 Shim J.Y., Lee B.H., Song S., Kim H., Kim J.A., Kim I., et al., Synthesis and properties of the conjugated polymers with indenoindene and benzimidazole units for organic photovoltaics. J. Polym. Sci. A1, 2013, 51, 241-249.10.1002/pola.26385Suche in Google Scholar
26 Xiang G., Lin S., Cui W., Wang L., Zhou L., Li L., et al., Metal complex of polymer with 2-(pyridin-2-yl)-1H-benzo[d]imidazole unit as a selectivity-tunable chemosensor for amino acids. Sensor. Actuat. B-Chem., 2013, 188, 540-547.10.1016/j.snb.2013.07.045Suche in Google Scholar
27 Xiang G., Cui W., Lin S., Wang L., Meier H., Li L., et al., A conjugated polymer with ethyl 2-(2-(pyridin-2-yl)-1H-benzo[d] imidazol-1-yl) acetate units as a novel fluorescent chemosensor for silver(I) detection. Sensor. Actuat. B-Chem., 2013, 186, 741-749.10.1016/j.snb.2013.06.061Suche in Google Scholar
28 Kou C., He X., Jiang X., Ni Y., Liu L., Huangfu C., et al., Novel isoindigo-based conjugated polyelectrolytes: Synthesis and fluorescence quenching behavior with water-soluble poly(p-phenylenevinylene)s. J. Polym. Sci. A1, 2015, 53, 2223-2237.10.1002/pola.27711Suche in Google Scholar
29 Gershon H., Clarke D.D., Gershon M., Preparation and Fungitoxieity of 3,6-Diehloroand 3,6-Dibromo-8-Quinolinols. Monatsh. Chem., 1994, 125, 723-730.10.1007/BF01277632Suche in Google Scholar
30 Wilson J.G., Hunt F.C., Iminodiacetic acid derivatives of benzimidazole. Synthesis of N-(Benzimidazol-2-ylmethyl) iminodiacetic acids. Aust. J. Chem., 1983, 36, 2317.10.1071/CH9832317Suche in Google Scholar
31 Cheng Y.-J., Luh T.-Y., Synthesizing optoelectronic heteroaromatic conjugated polymers by cross-coupling reactions. J. Organomet. Chem., 2004, 689, 4137-4148.10.1016/j.jorganchem.2004.08.011Suche in Google Scholar
32 Willis-Fox N., Gutacker A., Browne M.P., Khan A.R., Lyons M.E.G., Scherf U., et al., Selective recognition of biologically important anions using a diblock polyfluorene– polythiophene conjugated polyelectrolyte. Polym. Chem.-UK, 2017, 8, 7151-7159.10.1039/C7PY01478CSuche in Google Scholar
33 Schanze K., Pinto M., Conjugated Polyelectrolytes: Synthesis and Applications. Synthesis, 2002, 2002, 1293-1309.10.1055/s-2002-32541Suche in Google Scholar
34 Tan C., Atas E., Muller J.G., Pinto M.R., Kleiman V.D., Schanze K.S., Amplified quenching of a conjugated polyelectrolyte by cyanine dyes. J. Am. Chem. Soc., 2004, 126, 13685-13694.10.1021/ja046856bSuche in Google Scholar PubMed
35 Shen H., Kou C., He M., Yang H., Liu K., Synthesis and surfactochromicity of 1,4-diketopyrrolo[3,4-c]pyrrole(DPP)-based anionic conjugated polyelectrolytes. J. Polym. Sci. A1, 2014, 52, 739-751.10.1002/pola.27062Suche in Google Scholar
36 Liu K., Li Y., Yang M., Synthesis and solution properties of a meta-linked anionic poly(phenylene ethynylene) conjugated polyelectrolyte. e-Polymers, 2012, 12, 1-8.10.1515/epoly.2012.12.1.714Suche in Google Scholar
37 Tan C., Pinto M.R., Schanze K.S., Photophysics, aggregation and amplified quenching of a water-soluble poly(phenylene ethynylene). Chem. Commun., 2002, 446-447.10.1039/b109630cSuche in Google Scholar PubMed
38 Crosby G.A., Demas J.N., Measurement of photoluminescence quantum yields. Review. J. Phys. Chem., 1971, 75, 991-1024.10.1021/j100678a001Suche in Google Scholar
39 Fan Q.-L., Zhang G.-W., Lu X.-M., Chen Y., Huang Y.-Q., Zhou Y., et al., Cationic phenyl-substituted poly(p-phenylenevinylene) related copolymers with efficient photoluminescence and synthetically tunable emissive colors. Polymer, 2005, 46, 11165-11173.10.1016/j.polymer.2005.08.066Suche in Google Scholar
40 Treger J.S., Ma V.Y., Gao Y., Wang C.C., Wang H.L., Johal M.S., Tuning the optical properties of a water-soluble cationic poly(p-phenylenevinylene): surfactant complexation with a conjugated polyelectrolyte. J. Phys. Chem. B, 2008, 112, 760-763.10.1021/jp0767765Suche in Google Scholar PubMed
41 Long Y., Chen H., Wang H., Peng Z., Yang,Y., Zhang G., et al., Highly sensitive detection of nitroaromatic explosives using an electrospun nanofibrous sensor based on a novel fluorescent conjugated polymer. Anal. Chim. Acta, 2012, 744, 82-91.10.1016/j.aca.2012.07.028Suche in Google Scholar PubMed
42 Ceniceros-Gomez A.E., Ramos-Organillo A., Hernandez-Diaz J., Nieto-Martinez J., Contreras R., Castillo-Blum S.E., NMR study of the coordinating behavior of 2,6-bis(Benzimidazol-2’-yl) pyridine. Heteroatom Chem., 2000, 11, 392-398.10.1002/1098-1071(2000)11:6<392::AID-HC6>3.0.CO;2-GSuche in Google Scholar
43 Zhang Y., Wang G., Zhang J., Study on a highly selective fluorescent chemosensor for Fe3+ based on 1,3,4-oxadiazole and phosphonic acid. Sensor. Actuat. B-Chem., 2014, 200, 259-268.10.1016/j.snb.2014.04.050Suche in Google Scholar
44 Dhara A., Jana A., Guchhait N., Ghosh P., Kar S.K., Rhodamine-based molecular clips for highly selective recognition of Al3+ ions: synthesis, crystal structure and spectroscopic properties. New J. Chem., 2014, 38, 1627-1634.10.1039/C3NJ01447ASuche in Google Scholar
© 2020 Wei et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery
Artikel in diesem Heft
- Regular Articles
- The regulatory effects of the number of VP(N-vinylpyrrolidone) function groups on macrostructure and photochromic properties of polyoxometalates/copolymer hybrid films
- How the hindered amines affect the microstructure and mechanical properties of nitrile-butadiene rubber composites
- Novel benzimidazole-based conjugated polyelectrolytes: synthesis, solution photophysics and fluorescent sensing of metal ions
- Study on the variation of rock pore structure after polymer gel flooding
- Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking
- Investigation on degradation mechanism of polymer blockages in unconsolidated sandstone reservoirs
- Investigation on the effect of active-polymers with different functional groups for EOR
- Fabrication and characterization of hexadecyl acrylate cross-linked phase change microspheres
- Surface-induced phase transitions in thin films of dendrimer block copolymers
- ZnO-assisted coating of tetracalcium phosphate/ gelatin on the polyethylene terephthalate woven nets by atomic layer deposition
- Animal fat and glycerol bioconversion to polyhydroxyalkanoate by produced water bacteria
- Effect of microstructure on the properties of polystyrene microporous foaming material
- Synthesis of amphiphilic poly(ethylene glycol)-block-poly(methyl methacrylate) containing trityl ether acid cleavable junction group and its self-assembly into ordered nanoporous thin films
- On-demand optimize design of sound-absorbing porous material based on multi-population genetic algorithm
- Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface
- Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex
- Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material
- Preparation of novel amphoteric polyacrylamide and its synergistic retention with cationic polymers
- Effect of montmorillonite on PEBAX® 1074-based mixed matrix membranes to be used in humidifiers in proton exchange membrane fuel cells
- Insight on the effect of a piperonylic acid derivative on the crystallization process, melting behavior, thermal stability, optical and mechanical properties of poly(l-lactic acid)
- Lipase-catalyzed synthesis and post-polymerization modification of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters
- Dielectric, mechanical and thermal properties of all-organic PI/PSF composite films by in situ polymerization
- Morphological transition of amphiphilic block copolymer/PEGylated phospholipid complexes induced by the dynamic subtle balance interactions in the self-assembled aggregates
- Silica/polymer core–shell particles prepared via soap-free emulsion polymerization
- Antibacterial epoxy composites with addition of natural Artemisia annua waste
- Design and preparation of 3D printing intelligent poly N,N-dimethylacrylamide hydrogel actuators
- Multilayer-structured fibrous membrane with directional moisture transportability and thermal radiation for high-performance air filtration
- Reaction characteristics of polymer expansive jet impact on explosive reactive armour
- Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin
- Synthesis of aminated polystyrene and its self-assembly with nanoparticles at oil/water interface
- The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals
- Crystal transition and thermal behavior of Nylon 12
- All-optical non-conjugated multi-functionalized photorefractive polymers via ring-opening metathesis polymerization
- Fabrication of LDPE/PS interpolymer resin particles through a swelling suspension polymerization approach
- Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy
- Synthesis, electropolymerization, and electrochromic performances of two novel tetrathiafulvalene–thiophene assemblies
- Wetting behaviors of fluoroterpolymer fiber films
- Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff
- Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender
- Sulfonated poly(arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity
- Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery
- Effect of diacylhydrazine as chain extender on microphase separation and performance of energetic polyurethane elastomer
- Improved high-temperature damping performance of nitrile-butadiene rubber/phenolic resin composites by introducing different hindered amine molecules
- Rational synthesis of silicon into polyimide-derived hollow electrospun carbon nanofibers for enhanced lithium storage
- Synthesis, characterization and properties of phthalonitrile-etherified resole resin
- Highly thermally conductive boron nitride@UHMWPE composites with segregated structure
- Synthesis of high-temperature thermally expandable microcapsules and their effects on foaming quality and surface quality of foamed ABS materials
- Tribological and nanomechanical properties of a lignin-based biopolymer
- Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances
- Synthesis of a phosphoramidate flame retardant and its flame retardancy on cotton fabrics
- Preparation and characterization of thermoresponsive poly(N-isopropylacrylamide) copolymers with enhanced hydrophilicity
- Fabrication of flexible SiO2 nanofibrous yarn via a conjugate electrospinning process
- Silver-loaded carbon nanofibers for ammonia sensing
- Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection
- Solubility and diffusion coefficient of supercritical CO2 in polystyrene dynamic melt
- Curcumin-loaded polyvinyl butyral film with antibacterial activity
- Experimental-numerical studies of the effect of cell structure on the mechanical properties of polypropylene foams
- Experimental investigation on the three-dimensional flow field from a meltblowing slot die
- Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers
- Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate
- Seawater degradation of PLA accelerated by water-soluble PVA
- Review Article
- Mechanical properties and application analysis of spider silk bionic material
- Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties
- Structural design toward functional materials by electrospinning: A review
- Special Issue: XXXII National Congress of the Mexican Polymer Society
- Tailoring the morphology of poly(high internal phase emulsions) synthesized by using deep eutectic solvents
- Modification of Ceiba pentandra cellulose for drug release applications
- Redox initiation in semicontinuous polymerization to search for specific mechanical properties of copolymers
- pH-responsive polymer micelles for methotrexate delivery at tumor microenvironments
- Microwave-assisted synthesis of the lipase-catalyzed ring-opening copolymerization of ε-caprolactone and ω-pentadecanolactone: Thermal and FTIR characterization
- Rapid Communications
- Pilot-scale production of polylactic acid nanofibers by melt electrospinning
- Erratum
- Erratum to: Synthesis and characterization of new macromolecule systems for colon-specific drug delivery


