Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
-
Jie Li
and Dalong Zhu
Abstract
Diabetes mellitus with atherosclerosis (AS) adds to the social burden. This study aimed to investigate whether advanced glycation end product (AGE) levels were correlated with inflammation and carotid AS (CAS) in type 2 diabetes mellitus (T2DM) patients. A total of 50 elderly T2DM patients and 50 age-matched senior healthy subjects were recruited in this study. T2DM patients were classified into two groups based on the intima–media thickness (IMT) of the carotid artery from color Doppler ultrasonography. Patients with IMT > 1 mm were classified into the T2DM + CAS group (n = 28), and patients with IMT < 1 mm were assigned as the T2DM + non-atherosclerosis (NAS) group (n = 22). The plasma levels of AGEs, receptor for AGE (RAGE), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) of all subjects were measured by enzyme-linked immunosorbent assay. The T-lymphocyte subsets were analyzed by a flow detector. T2DM + CAS patients showed significantly higher concentrations of AGEs, RAGE, TNF-α, and IFN-γ in the peripheral blood. The highest levels of CD4+ T cells were observed in the T2DM + CAS group. The AGE level was positively correlated with the concentrations of RAGE, TNF-α, IFN-γ, and CD4+. In summary, the results showed that the levels of AGEs may be correlated with the inflammatory status in T2DM patients with CAS.
1 Introduction
Diabetes mellitus is a metabolic disorder characterized by the impaired response to insulin and decreased insulin secretion in the body, resulting in chronic hyperglycemia [1]. Type 2 diabetes mellitus (T2DM) is the most common type of diabetes with a global prevalence of 350 million people in 2014 [2]. The number of T2DM patients is expected to increase significantly in the coming years, posing serious health and economic challenges. T2DM is associated with many long-term complications in the heart, blood vessels, kidney, nerves, and eyes. About 4.9 million people die of diabetes each year, in which around 50% is due to cardiovascular complications [3]. Atherosclerosis (AS) is a common complication of T2DM. Early detection and intervention of AS are of vital significance in the comprehensive management of T2DM. Carotid artery (CA) is the most easily involved blood vessel in AS; and its location is relatively superficial, but the degree and nature of carotid AS (CAS) can reflect the severity of the lesion. AS is actually a chronic inflammatory disease, in which various inflammatory and immune responses are involved. It has been reported that patients with diabetes presented larger necrotic cores in their coronary arteries and enhanced inflammation involved with macrophages and T-lymphocytes compared to patients without diabetes [4]. The intima–media thickness (IMT) of CA, defined as the distance from the leading edge of the media–adventitia interface to the leading edge of the lumen–intima interface, has been widely used as an indicator of the level of AS development [5,6].
Increased serum level of advanced glycation end products (AGEs) has been reported in T2DM patients with AS, which suggests its association with the development of vascular complications [7]. AGEs can interact with the receptor for AGEs (RAGEs) to induce inflammation [8,9]. Late glycosylation can induce glycosylation of proteins related to lipid metabolism, leading to lipid function damage and lipid metabolism disorder and eventually vascular complications in diabetes [10,11]. There are different degrees of lymphocyte infiltration in human atherosclerotic plaques. The T cells also play an important role in AS at the early stage, especially the clonal selection and expansion of their subsets [12]. Lymphocyte subsets mainly participate in the formation of AS by secreting cytokines [13,14].
To further understand how AGEs accelerate diabetic AS, we investigated the plasma level of AGEs, RAGE, T-lymphocyte subsets, and inflammatory cytokines including tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) in the peripheral blood of elderly patients with type 2 diabetes. This study was undertaken to observe the effects of AGEs on T-lymphocyte-secreting inflammatory cytokines and to provide new insights for the prevention and treatment of complications of type 2 diabetes.
2 Materials and methods
2.1 Participants
Fifty patients with T2DM (n = 50, male/female: 21/29) were recruited in this study. T2DM was diagnosed based on 1999 World Health Organization criteria: fasting plasma glucose ≥7.0 mmol/L or 2-h plasma glucose ≥11.1 mmol/L. The inclusion criteria were (1) newly diagnosed T2DM patients, from January to August 2018; (2) consistent diet and/or treatment plans for 2 weeks; (3) age between 60 and 70 years; (4) body mass index between 19 and 35 kg/m2; and (5) no symptoms of diabetic ketoacidosis observed in the past 6 months. Patients were excluded according to the following criteria: (1) type 1 diabetes mellitus; (2) clinical signs of acute and/or chronic infection; tumor, hematologic diseases, liver disease, renal dysfunction, cardiovascular, and cerebrovascular diseases; and (3) history of smoking. Fifty healthy elderly participants were also included in this study (n = 50; male/female: 26/24). All healthy subjects showed normal fasting plasma glucose (<6.1 mmol/L), normal 2-h plasma glucose (<7.8 mmol/L), and had no sign of CAS. All the clinical parameters of the recruited subjects are shown in Table A1.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the Ethics Committee of Clinical Research.
2.2 CA ultrasonography
Color Doppler ultrasonography was performed to measure the IMT of CA by using an ultrasound scanner (HP5500; GE, USA) with a linear transducer of 5–10 MHz frequency. Patients were examined at a supine position. The B-mode gray scale images of the common CA, the bulb, the internal carotids, and the external carotids were recorded. The IMT measures the thickness of the two layers of the artery wall, tunica intima, and tunica media. The normal IMT of CA as evaluated by B-mode imaging is less than 1.0 mm. IMT at or above 1 mm is considered to be associated with AS. Based on the results from CA ultrasonography, 50 diabetes patients were divided into two subgroups: (1) T2DM + NAS group (n = 22; male/female: 10/12) with IMT < 1.0 mm and (2) T2DM + CAS group (n = 28; male/female: 11/17) with IMT ≥ 1.0 mm.
2.3 Enzyme-linked immunosorbent assay (ELISA)
Peripheral blood was taken from all subjects following 8 h of fasting. The levels of AGEs, RAGE, TNF-α, and IFN-γ in the peripheral blood were measured by AGEs, RAGE, TNF-α, and IFN-γ ELISA kits (Uscn life, USA), respectively, according to the manufacturer’s protocol.
2.4 T-lymphocyte subset analysis
Fasting peripheral whole blood was collected between 7:00 and 9:00 am. Heparinized peripheral whole blood (400 µL) was diluted into 400 µL RPMI1640 medium, added with 42 µL 1 µg/mL of phorbol myristate acetate, 33 µL 50 µg/mL of ionomycin, and 13.6 µL 0.1 mg/mL monensin (Sigma, Saint Louis, USA) and incubated at 37°C/5% CO2 for 4.5 h. Then the peripheral blood mononuclear cells (PBMCs) were separated by high-speed refrigerated centrifuge (Eppendorf, Germany). For staining, 100 µL of PBMCs was incubated with PerCP-Cy5.5 conjugated antihuman CD4 monoclonal antibody (clone: OKT4, Cat.: 85-45-0048-42) and antihuman CD25 monoclonal antibodies (clone: BC96, Cat.: 85-17-0259-42), separately. Each lymphocyte subset was collected by a flow cytometer (BD, USA), and the data were analyzed by FlowJo.
2.5 Statistical analysis
Software SPSS (Version 21.0) was used for statistical analysis. All data are reported as mean ± standard deviation and all data are normally distributed. Significant difference between the mean values of two groups was calculated by t test. One-way analysis of variance was used when comparison was among more than two groups. Correlation between AGEs and other clinical indicators was determined by age-adjusted partial correlation coefficient analysis. p < 0.05 was considered to be statistically significant.
3 Results
3.1 T2DM patients with CAS showed significantly higher IMT and elevated plasma levels of AGEs and RAGE
The IMT of CA in the healthy subjects was 0.68 ± 0.16 mm, which was not significantly different from that in the T2DM + NAS group (0.76 ± 0.20 mm). However, in the T2DM + CAS group, their average IMT (1.22 ± 0.21 mm) was significantly higher compared to that in the healthy subjects and the T2DM + NAS group (Figure 1). Plasma levels of AGEs in healthy subjects, T2DM + NAS group, and T2DM + CAS group were 32.85 ± 15.26, 53.47 ± 15.39, and 66.71 ± 16.36 ng/mL, respectively (Figure 2a). Healthy subjects showed significantly lower level of AGEs compared to the other two groups. Plasma level of RAGE was also significantly lower in the control group (0.31 ± 0.11 ng/mL) than that in the T2DM + NAS (0.47 ± 0.17 ng/mL) and T2DM + CAS groups (0.59 ± 0.21 ng/mL; Figure 2b).
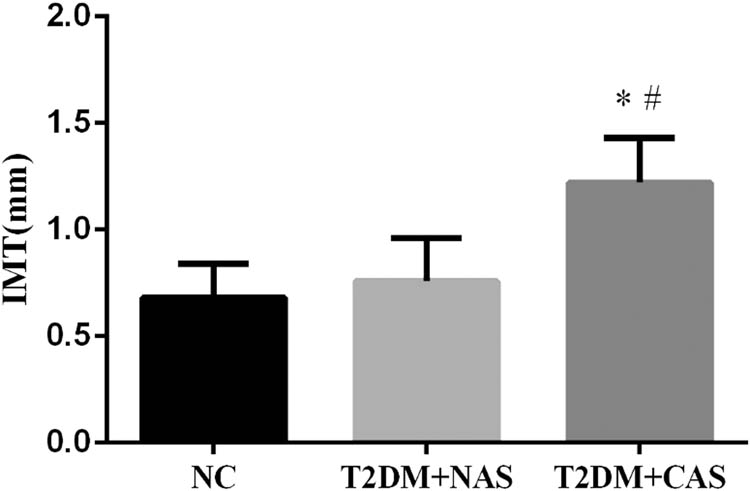
IMT of CA in healthy subjects, T2DM + NAS group, and T2DM + CAS group. The IMT of CA was examined by color Doppler ultrasonography. The IMT value of less than 1.0 mm was considered as normal. * indicates statistical significance (p < 0.05) compared to healthy subjects (NC); # indicates statistical significance (p < 0.05) compared to T2DM + NAS group.
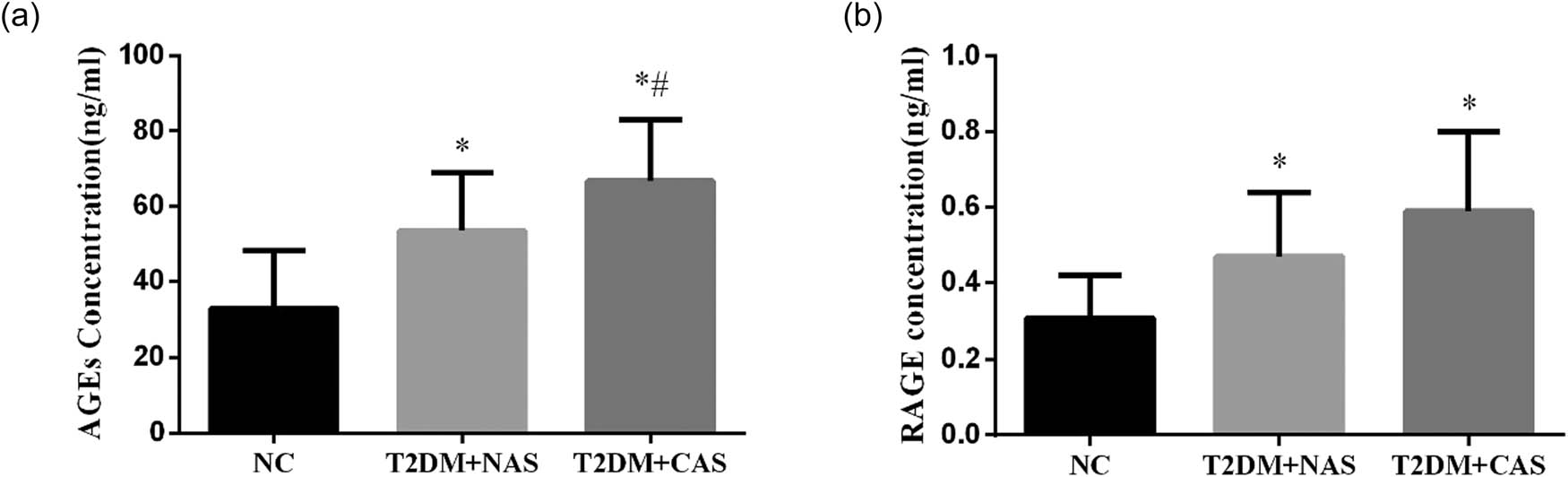
Plasma levels of AGEs and RAGE in healthy subjects, T2DM + NAS group, and T2DM + CAS group. Expression levels of AGEs (a) and RAGE (b) in peripheral blood were tested by ELISA in all three groups. * indicates statistical significance (p < 0.05) compared to healthy subjects (NC); # indicates statistical significance (p < 0.05) compared to the T2DM + NAS group.
3.2 Increased expression levels of TNF-α and IFN-γ were observed in T2DM patients with and without CAS
The expressions of inflammatory cytokines including TNF-α and IFN-γ were significantly higher in the T2DM + NAS and T2DM + CAS groups than those in the control group. The plasma levels of TNF-α were 7.82 ± 1.92, 8.94 ± 2.05, and 14.79 ± 3.15 pg/mL in healthy subjects, T2DM + NAS group, and T2DM + CAS group, respectively (Figure 3a). Meanwhile, the levels of IFN-γ were 1.40 ± 0.53, 1.61 ± 0.51, and 2.11 ± 0.37 pg/mL in the respective three groups (Figure 3b).
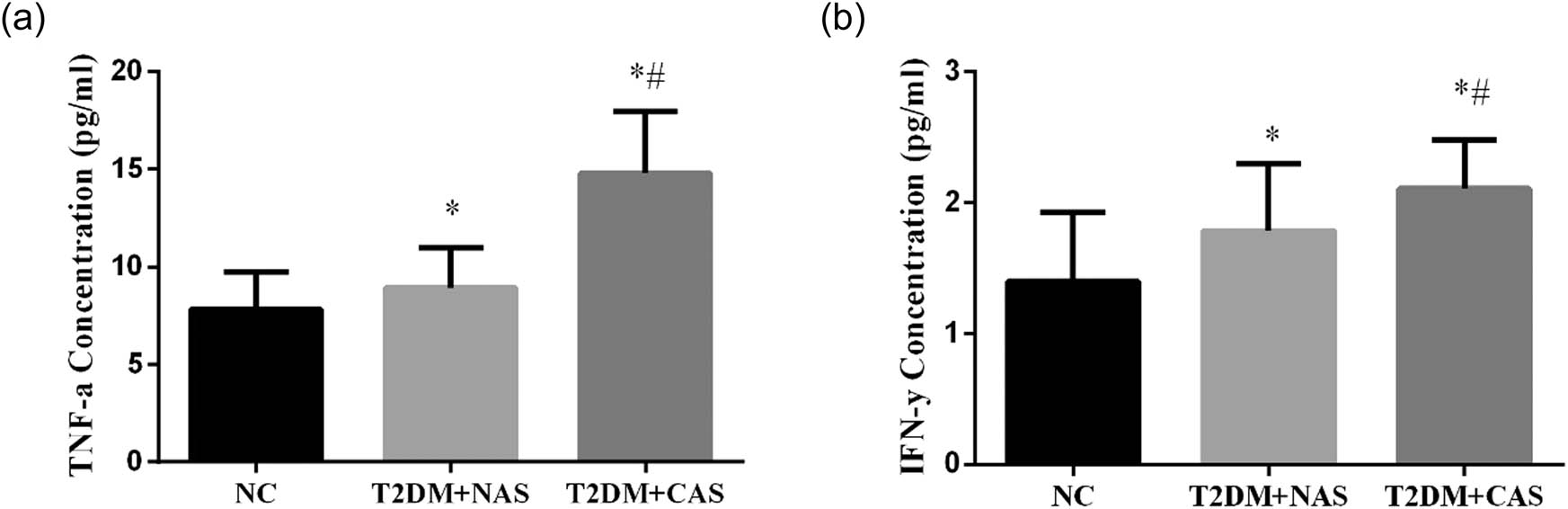
Expressions levels of TNF-α and IFN-γ in healthy subjects, T2DM + NAS group, and T2DM + CAS group. Expression levels of TNF-α (a) and IFN-γ (b) in peripheral blood were tested by ELISA in all three groups. * indicates statistical significance (p < 0.05) compared to healthy subjects (NC); # indicates statistical significance (p < 0.05) compared to the T2DM + NAS group.
3.3 The number of CD4+ T cells was increased in T2DM + CAS patients
Both the T2DM + NAS and T2DM + CAS groups had a significantly larger amount of CD4+ T cells compared to healthy subjects. Moreover, the number of CD4+ T cells in T2DM + NAS patients was significantly higher than that in the T2DM + CAS patients ( p < 0.05). The ratio of CD4+/CD8+ was also significantly lower in the healthy groups. In contrast, T2DM + CAS showed the least amount of CD8+ T cells among these groups (Table 1).
Determination of T-cell subsets in peripheral blood
| Grouping | CD4+ (%) | CD8+ (%) | CD4+/CD8+ (%) | CD4+ CD25+ Treg (%) |
|---|---|---|---|---|
| Control | 20.58 ± 1.46 | 26.34 ± 1.99 | 0.88 ± 0.12 | 4.10 ± 0.61 |
| T2DM + NAS | 23.49 ± 2.11a | 24.16 ± 2.23a | 0.97 ± 0.15a | 3.77 ± 0.35a |
| T2DM + CAS | 31.37 ± 2.49a,b | 23.05 ± 2.51a | 1.35 ± 0.24a,b | 3.36 ± 0.34a,b |
- a
p < 0.05 compared with the normal control group.
- b
p < 0.05 compared with the T2DM + NAS group.
3.4 Correlation analysis of AGEs and other clinical indicators
Correlation analysis was performed and the results showed that there was a positive correlation between AGEs and RAGE (Figure 4a), TNF-α (Figure 4b), IFN-γ (Figure 4c), and CD4+ (Figure 4d;P < 0.05). However, it was negatively correlated with CD8+ (Figure 4e) and CD4+ CD25+ (Figure 4f and Table 2).
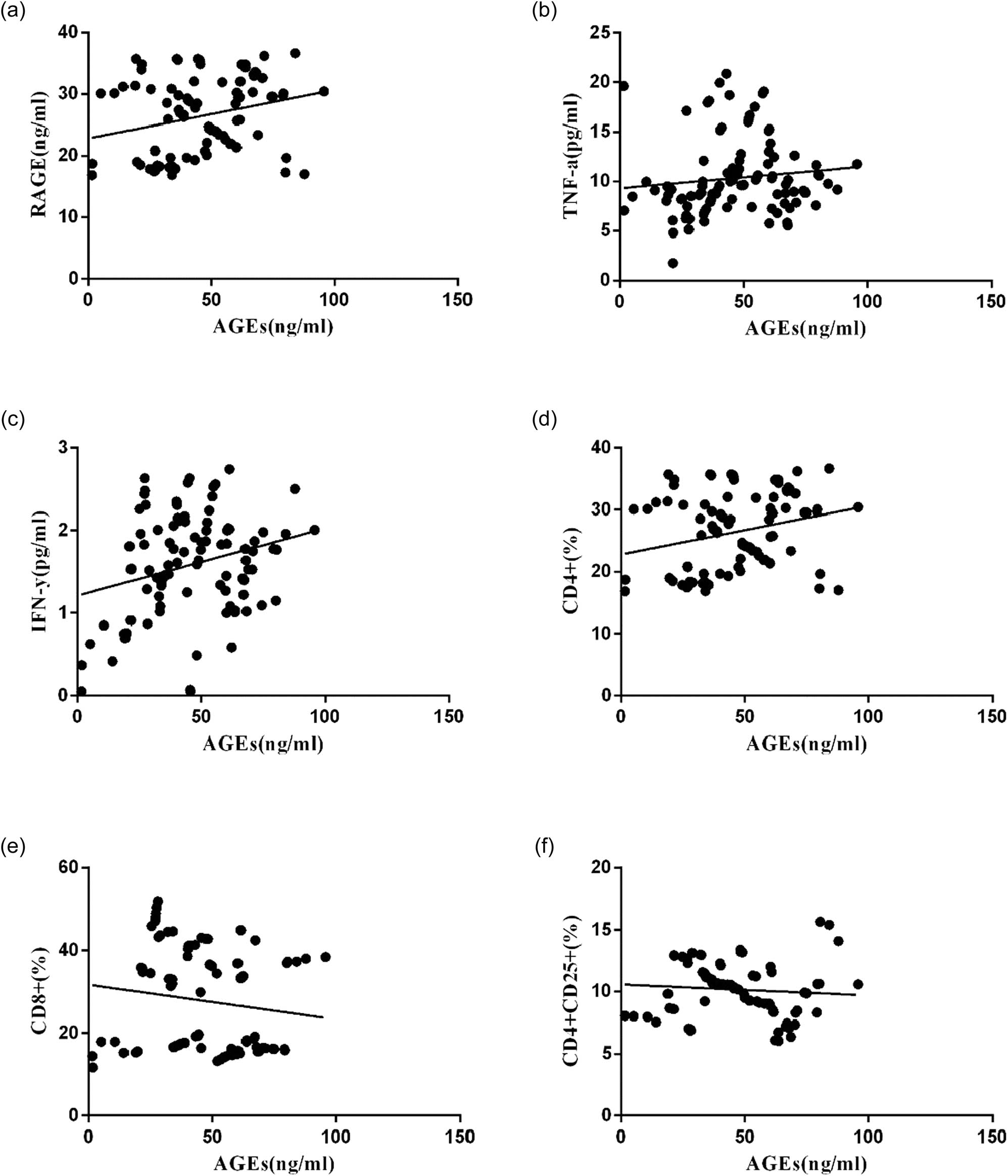
Scatter plots of linear correlation between peripheral blood AGE level and other clinical indicators. Pearson’s correlation coefficient analysis was used to calculate the correlation between peripheral blood AGEs level and RAGE (a), TNF-α (b), IFN-γ (c), CD4+ (d), CD8+ (e), and CD4+ CD25+ (f).
Correlation analysis of AGEs and other clinical indicators in three groups
| Index | Partial correlation analysis | |
|---|---|---|
| r | p | |
| RAGE (ng/ml) | 0.4388 | 0.0074 |
| TNF-α (pg/ml) | 0.2461 | 0.0189 |
| IFN-γ (pg/ml) | 0.1745 | 0.0301 |
| CD4+ (%) | 0.2503 | 0.0065 |
| CD8+ (%) | −0.1566 | 0.0797 |
| CD4+ CD25+ (%) | −0.1743 | 0.0612 |
4 Discussion
AS is a common vascular complication in T2DM [15]. IMT has been widely used as a noninvasive indicator of AS at subclinical stage [16]. Our lab performed color Doppler ultrasonography to measure the IMT of CA in senior T2DM patients. The diagnostic criterion of AS is IMT ≥ 1 mm. Based on the results of IMT, we classified 50 T2DM patients into the T2DM + CAS group (n = 22) with IMT > 1 mm and the T2DM + NAS group (n = 28) with IMT < 1 mm.
AGEs can be rapidly cleaned up from blood [17], and the levels of AGEs were low in normal physiological conditions. However, aging or diabetes with high sugar content can accelerate the saccharification process, leading the body to produce AGEs spontaneously. When the generation rate is higher than the degradation rate, it will lead to accumulation of AGEs in the body [18]. AGEs interact with their RAGE to initiate intracellular signaling pathways involving the change in vascular structure and then accelerate the progression of AS [19]. RAGE is a multiligand receptor that belongs to the immunoglobulin superfamily, often seen on the surface of smooth muscle cells, macrophages, T-lymphocytes, glomerular podocytes, and neurons [20]. Most studies focused on the effect of AGE-RAGE on reactive oxygen species generation in the process of AS. However, whether AGEs-RAGE interaction causes AS by affecting T lymphocytes remains unknown.
AS is characterized by chronic inflammation. Numerous neutrophils, mononuclear macrophages, and T lymphocytes are found in plaques at various stages of AS, so the relationship between AS and innate immune pattern recognition has been of concern for a long time. Recent studies suggest that AGEs are a molecular pattern related to damage in the body, and RAGE is a pattern recognition receptor. After being recognized by RAGE, AGEs can activate the immune response. From the perspective of immune mechanism, the research on the mechanism of AGE-RAGE reaction promoting the occurrence and development of diabetic AS is a hot spot.
Previously, it has been found that T cells express the receptor of AGEs on the surface, and RAGE with a molecular weight of 50–60 kd is the main one. Imani et al. labeled AGEs with I125 to observe the binding ability of peripheral lymphocyte surface receptors [21]. It was found that the ability of lymphocyte-binding AGEs significantly increased after 48 h of pre-stimulation of peripheral blood lymphocytes by phytohemagglutinin. CD4+ T cells increased from 34.2% to 92%, while CD8+ T cells increased from 58.5% to 90%, and it was found that peripheral blood lymphocytes expressed IFN-γ up to ten times higher. This suggests that AGE is one of the factors affecting immune disorders of T cells and plays a role in vascular complications of diabetes mellitus.
The major class of T lymphocytes present in AS is CD4+, which can differentiate into Th1 and Th2 cells based on the local milieu of cytokines. The Th1 lineage may be the key regulator of lymphocytic influence in the development of AS [22]. Both Th1 cells and CD8+ T cells can secrete inflammatory cytokines IFN-γ and TNF-α. We found significantly higher levels of AGEs, RAGE, IFN-γ, and TNF-α in the T2DM + CAS group compared with the T2DM + NAS group and controls. It suggested that the increased concentration of AGEs and RAGE in peripheral blood was correlated with the occurrence and development of AS in T2DM. Meanwhile, T2DM + CAS patients also showed imbalanced T cells and decreased Treg cells. Pearson analysis revealed a positive correlation between AGEs and RAGE, CD4+, IFN-γ, and TNF-α, suggesting that combining AGEs and RAGE activates the immune response in the body and promotes the secretion of inflammatory factors IFN-γ and TNF-α. Weiser et al. showed that IFN-γ synergized with other cytokines to elevate the expression of the adhesion molecules, vascular cell adhesion molecule-1 in brain endothelial cells [23]. IFN-γ also impaired the cellular cholesterol balance, by reducing the expression of cholesterol 27-hydroxylase, to facilitate the pathogenesis of AS [24]. Another potential mechanism of IFN-γ contributing to the atherosclerotic process was through a p53-dependent DNA damage pathway in cellular senescence [25]. TNF-α is also considered as a key factor involved in AS. Ridker et al. found that the plasma concentration of TNF-α is persistently increased in postmyocardial ischemia patients with higher risk of recurrent coronary events [26]. Treg cells were also reported to have a regulatory effect on the initiation and progression of AS [27]. In this study, senior T2DM + CAS patients showed a lower concentration of CD4+ CD25+ Treg in peripheral blood than that in the healthy subjects. Although no correlation was found between AGEs vs CD8+ and AGEs vs Treg, significantly decreased levels of CD8+ T cells and Treg cells were observed in T2DM + CAS patients. It suggests the involvement of CD8+ T cells and Treg cells in AS.
In this study, we would like to address several study limitations. First, the study was performed in a single center, and further studies may involve multicenter so as to increase the power of our current findings. Second, as using IMT as a predictor of cardiovascular risk has been controversial among studies [28], we should carefully interpret our findings and may employ more diagnostic tools to confirm the current findings. Third, conflicting results regarding the role of AGEs in the development of coronary artery disease have been reported [29]; further studies may increase the sample size to confirm our findings. Finally, the present study suggested a link between AGEs and the extent of inflammation; however, whether the changes in the inflammatory status contribute to the development of coronary artery disease in T2DM patients still require mechanistic studies.
In conclusion, the development of AS in T2DM is a complex process. In the present study, our results showed that the levels of AGEs may be correlated with the inflammatory status in T2DM patients with CAS. Our results may suggest a link between AGE levels and the extent of inflammation, which may contribute to the development of CAS in T2DM patients.
Acknowledgments
This study was supported by the Nanjing Medical University Science and Technology Development Fund Project (2017NJMUZD145) and the Nanjing Medical Science and Technology Development Project (YKK17269).
Appendix
Clinical parameters of the recruited subjects
| Parameters | NC | T2DM | P |
|---|---|---|---|
| Age (years) | 65.6 ± 3.1 | 64.7 ± 3.9 | >0.05 |
| BMI (kg/m2) | 24.3 ± 2.6 | 27.6 ± 3.2 | <0.05 |
| Triglycerides (mg/dL) | 111.7 ± 42.3 | 123.7 ± 35.6 | >0.05 |
| Total cholesterol (mg/dL) | 163.7 ± 32.3 | 156.5 ± 38.9 | >0.05 |
| HDL cholesterol (mg/dL) | 41.2 ± 9.9 | 44.1 ± 11.4 | >0.05 |
| LDL cholesterol (mg/dL) | 104.8 ± 28.9 | 94.9 ± 21.7 | >0.05 |
| Glycaemia (mg/dL) | 92.6 ± 10.2 | 151.4 ± 21.1 | <0.05 |
| HbA1c (%) | 6.1 ± 0.4 | 7.4 ± 0.7 | <0.05 |
Conflict of interest: The authors state no conflict of interest.
References
[1] Nawaz MS, Shah KU, Khan TM, Rehman AU, Rashid HU, Mahmood S, et al. Evaluation of current trends and recent development in insulin therapy for management of diabetes mellitus. Diabetes Metab Syndr. 2017 Dec;11(Suppl 2):S833–9.10.1016/j.dsx.2017.07.003Search in Google Scholar PubMed
[2] Oktay AA, Akturk HK, Jahangir E. Diabetes mellitus and hypertension: a dual threat. Curr Opin Cardiol. 2016 Jul;31(4):402–9.10.1097/HCO.0000000000000297Search in Google Scholar PubMed
[3] Prystupiuk OM. Biomarkers and risk factors of cardiovascular system disease in diabetes mellitus type 2. Lik Sprava. 2013 Jan–Feb;1:73–7.Search in Google Scholar
[4] Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):191–204.10.1161/ATVBAHA.116.306256Search in Google Scholar PubMed PubMed Central
[5] Pleskovic A, Letonja MS, Vujkovac AC, Nikolajevic Starcevic J, Gazdikova K, Caprnda M, et al. C-reactive protein as a marker of progression of carotid atherosclerosis in subjects with type 2 diabetes mellitus. Vasa. 2017 May;46(3):187–92.10.1024/0301-1526/a000614Search in Google Scholar PubMed
[6] Willeit P, Thompson SG, Agewall S, Bergstrom G, Bickel H, Catapano AL, et al. Inflammatory markers and extent and progression of early atherosclerosis: meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur J Prev Cardiol. 2016 Jan;23(2):194–205.10.1177/2047487314560664Search in Google Scholar PubMed PubMed Central
[7] Bansal S, Chawla D, Banerjee BD, Madhu SV, Tripathi AK. Association of RAGE gene polymorphism with circulating AGEs level and paraoxonase activity in relation to macro-vascular complications in Indian type 2 diabetes mellitus patients. Gene. 2013 Sep 10;526(2):325–30.10.1016/j.gene.2013.05.013Search in Google Scholar PubMed
[8] Chen Y, Wu Y, Gan X, Liu K, Lv X, Shen H, et al. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J Ethnopharmacol. 2016 Dec 24;194:850–60.10.1016/j.jep.2016.10.079Search in Google Scholar PubMed
[9] Xing Y, Ji Q, Li X, Ming J, Zhang N, Zha D, et al. Asiaticoside protects cochlear hair cells from high glucose-induced oxidative stress via suppressing AGEs/RAGE/NF-kappaB pathway. Biomed Pharmacother. 2017 Feb;86:531–6.10.1016/j.biopha.2016.12.025Search in Google Scholar PubMed
[10] Wu T, Chen X, Wang Y, Xiao H, Peng Y, Lin L, et al. Aortic plaque-targeted andrographolide delivery with oxidation-sensitive micelle effectively treats atherosclerosis via simultaneous ROS capture and anti-inflammation. Nanomedicine. 2018 Oct;14(7):2215–26.10.1016/j.nano.2018.06.010Search in Google Scholar PubMed
[11] Yamagishi SI, Sotokawauchi A, Matsui T. Pathological role of advanced glycation end products (AGEs) and their receptor axis in atrial fibrillation. Mini Rev Med Chem. 2019;19(13):1040–8.10.2174/1389557519666190311140737Search in Google Scholar PubMed
[12] de Boer OJ, Becker AE, van der Wal AC. T lymphocytes in atherogenesis-functional aspects and antigenic repertoire. Cardiovasc Res. 2003 Oct 15;60(1):78–86.10.1016/S0008-6363(03)00341-9Search in Google Scholar
[13] Chen S, Crother TR, Arditi M. Emerging role of IL-17 in atherosclerosis. J Innate Immun. 2010;2(4):325–33.10.1159/000314626Search in Google Scholar PubMed PubMed Central
[14] Kassem KM, Ali M, Rhaleb NE. Interleukin 4: its role in hypertension, atherosclerosis, valvular, and nonvalvular cardiovascular diseases. J Cardiovasc Pharmacol Ther. 2019 Aug 11;25:1074248419868699.10.1177/1074248419868699Search in Google Scholar PubMed PubMed Central
[15] Kaneko R, Sawada S, Tokita A, Honkura R, Tamura N, Kodama S, et al. Serum cystatin C level is associated with carotid arterial wall elasticity in subjects with type 2 diabetes mellitus: a potential marker of early-stage atherosclerosis. Diabetes Res Clin Pract. 2018 May;139:43–51.10.1016/j.diabres.2018.02.003Search in Google Scholar PubMed
[16] Jiang F, Wang J, Zhang R, Chen M, Peng D, Sun X, et al. Effects of active and passive smoking on the development of cardiovascular disease as assessed by a carotid intima-media thickness examination in patients with type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015 May;42(5):444–50.10.1111/1440-1681.12379Search in Google Scholar PubMed
[17] Xu H, Wang Z, Wang Y, Hu S, Liu N. Biodistribution and elimination study of fluorine-18 labeled Nepsilon-carboxymethyl-lysine following intragastric and intravenous administration. PLoS One. 2013;8(3):e57897.10.1371/journal.pone.0057897Search in Google Scholar PubMed PubMed Central
[18] Kurt A, Andican G, Siva ZO, Andican A, Burcak G. The effects of n-3 long-chain polyunsaturated fatty acid supplementation on AGEs and sRAGE in type 2 diabetes mellitus. J Physiol Biochem. 2016 Dec;72(4):679–87.10.1007/s13105-016-0506-4Search in Google Scholar PubMed
[19] Li Y, Liu S, Zhang Z, Xu Q, Xie F, Wang J, et al. RAGE mediates accelerated diabetic vein graft atherosclerosis induced by combined mechanical stress and AGEs via synergistic ERK activation. PLoS One. 2012;7(4):e35016.10.1371/journal.pone.0035016Search in Google Scholar PubMed PubMed Central
[20] Rasool M, Malik A, Butt TT, Ashraf MAB, Rasool R, Zahid A, et al. Implications of advanced oxidation protein products (AOPPs), advanced glycation end products (AGEs) and other biomarkers in the development of cardiovascular diseases. Saudi J Biol Sci. 2019 Feb;26(2):334–9.10.1016/j.sjbs.2018.08.024Search in Google Scholar PubMed PubMed Central
[21] Imani F, Horii Y, Suthanthiran M, Skolnik EY, Makita Z, Sharma V, et al. Advanced glycosylation endproduct-specific receptors on human and rat T-lymphocytes mediate synthesis of interferon gamma: role in tissue remodeling. J Exp Med. 1993 Dec 1;178(6):2165–72.10.1084/jem.178.6.2165Search in Google Scholar PubMed PubMed Central
[22] Charerntantanakul W, Fabros Jr D. Saponin Quil A up-regulates type I interferon-regulated gene and type I and II interferon expressions which are suppressed by porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2018 Jan;195:76–83.10.1016/j.vetimm.2017.11.009Search in Google Scholar
[23] Lopez P, Rodriguez-Carrio J, Martinez-Zapico A, Perez-Alvarez AI, Benavente L, Caminal-Montero L, et al. IgM anti-phosphorylcholine antibodies associate with senescent and IL-17 + T cells in SLE patients with a pro-inflammatory lipid profile. Rheumatol. 2019 Jul 13;59(2):407–17.10.1093/rheumatology/kez264Search in Google Scholar
[24] Cho JH, Kim MJ, Kim KJ, Kim JR. POZ/BTB and AT-hook-containing zinc finger protein 1 (PATZ1) inhibits endothelial cell senescence through a p53 dependent pathway. Cell Death Differ. 2012 Apr;19(4):703–12.10.1038/cdd.2011.142Search in Google Scholar
[25] Engelbertsen D, Autio A, Verwilligen RAF, Depuydt MAC, Newton G, Rattik S, et al. Increased lymphocyte activation and atherosclerosis in CD47-deficient mice. Sci Rep. 2019 Jul 23;9(1):10608.10.1038/s41598-019-46942-xSearch in Google Scholar
[26] Bash LD, White K, Patel MD, Liu J, Mavros P, Mahaffey KW. Cardiovascular risk factors and secondary events among acute and chronic stable myocardial infarction patients: findings from a managed care database. Cardiol Ther. 2019 Aug 20;8(2):329–43.10.1007/s40119-019-00147-5Search in Google Scholar
[27] Huang Y, Hu H, Liu L, Ye J, Wang Z, Que B, et al. Interleukin-12p35 deficiency reverses the Th1/Th2 imbalance, aggravates the Th17/Treg imbalance, and ameliorates atherosclerosis in ApoE-/- mice. Mediat Inflamm. 2019;2019:3152040.10.1155/2019/3152040Search in Google Scholar
[28] Naslund U, Lundgren A, Vanoli D, Norberg M. Is intima-media thickness a predictor for cardiovascular risk? – Authors’ reply. Lancet. 2019 Aug 3;394(10196):381.10.1016/S0140-6736(19)30343-5Search in Google Scholar
[29] Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018 Nov 23;24(1):59.10.1186/s10020-018-0060-3Search in Google Scholar PubMed PubMed Central
© 2020 Jie Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”
Articles in the same Issue
- Plant Sciences
- Dependence of the heterosis effect on genetic distance, determined using various molecular markers
- Plant Growth Promoting Rhizobacteria (PGPR) Regulated Phyto and Microbial Beneficial Protein Interactions
- Role of strigolactones: Signalling and crosstalk with other phytohormones
- An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants
- Functional divergence and adaptive selection of KNOX gene family in plants
- In silico identification of Capsicum type III polyketide synthase genes and expression patterns in Capsicum annuum
- In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.)
- CRISPR/Cas9 or prime editing? – It depends on…
- Study on the optimal antagonistic effect of a bacterial complex against Monilinia fructicola in peach
- Natural variation in stress response induced by low CO2 in Arabidopsis thaliana
- The complete mitogenome sequence of the coral lily (Lilium pumilum) and the Lanzhou lily (Lilium davidii) in China
- Ecology and Environmental Sciences
- Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol
- Analysis of ethanol dehydration using membrane separation processes
- Activity of Vip3Aa1 against Periplaneta americana
- Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation
- Spatiotemporal dynamics of terrestrial invertebrate assemblages in the riparian zone of the Wewe river, Ashanti region, Ghana
- Antifungal activity of selected volatile essential oils against Penicillium sp.
- Toxic effect of three imidazole ionic liquids on two terrestrial plants
- Biosurfactant production by a Bacillus megaterium strain
- Distribution and density of Lutraria rhynchaena Jonas, 1844 relate to sediment while reproduction shows multiple peaks per year in Cat Ba-Ha Long Bay, Vietnam
- Biomedical Sciences
- Treatment of Epilepsy Associated with Common Chromosomal Developmental Diseases
- A Mouse Model for Studying Stem Cell Effects on Regeneration of Hair Follicle Outer Root Sheaths
- Morphine modulates hippocampal neurogenesis and contextual memory extinction via miR-34c/Notch1 pathway in male ICR mice
- Composition, Anticholinesterase and Antipedicular Activities of Satureja capitata L. Volatile Oil
- Weight loss may be unrelated to dietary intake in the imiquimod-induced plaque psoriasis mice model
- Construction of recombinant lentiviral vector containing human stem cell leukemia gene and its expression in interstitial cells of cajal
- Knockdown of lncRNA KCNQ1OT1 inhibits glioma progression by regulating miR-338-3p/RRM2
- Protective effect of asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death
- Prevalence of dyslipidemia in Tibetan monks from Gansu Province, Northwest China
- Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/β-catenin signaling via regulating lncRNA PCAT6/ miR-326 axis
- MiR-542-3p suppresses neuroblastoma cell proliferation and invasion by downregulation of KDM1A and ZNF346
- Calcium Phosphate Cement Causes Nucleus Pulposus Cell Degeneration Through the ERK Signaling Pathway
- Human Dental Pulp Stem Cells Exhibit Osteogenic Differentiation Potential
- MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma
- Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating the microRNA-34a-5p/NOTCH1 signaling pathway
- Large Brunner’s gland adenoma of the duodenum for almost 10 years
- Neurotrophin-3 accelerates reendothelialization through inducing EPC mobilization and homing
- Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models
- FXYD6 overexpression in HBV-related hepatocellular carcinoma with cirrhosis
- Risk factors for elevated serum colorectal cancer markers in patients with type 2 diabetes mellitus
- Effect of hepatic sympathetic nerve removal on energy metabolism in an animal model of cognitive impairment and its relationship to Glut2 expression
- Progress in research on the role of fibrinogen in lung cancer
- Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients
- MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB
- Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia
- Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
- Effect of FibroScan test in antiviral therapy for HBV-infected patients with ALT <2 upper limit of normal
- LncRNA SNHG15 regulates osteosarcoma progression in vitro and in vivo via sponging miR-346 and regulating TRAF4 expression
- LINC00202 promotes retinoblastoma progression by regulating cell proliferation, apoptosis, and aerobic glycolysis through miR-204-5p/HMGCR axis
- Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats
- GeneXpert Technology for the diagnosis of HIV-associated tuberculosis: Is scale-up worth it?
- Circ_001569 regulates FLOT2 expression to promote the proliferation, migration, invasion and EMT of osteosarcoma cells through sponging miR-185-5p
- Lnc-PICSAR contributes to cisplatin resistance by miR-485-5p/REV3L axis in cutaneous squamous cell carcinoma
- BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells
- MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process
- Inhibition of lncRNA LINC00461/miR-216a/aquaporin 4 pathway suppresses cell proliferation, migration, invasion, and chemoresistance in glioma
- Upregulation of miR-150-5p alleviates LPS-induced inflammatory response and apoptosis of RAW264.7 macrophages by targeting Notch1
- Long non-coding RNA LINC00704 promotes cell proliferation, migration, and invasion in papillary thyroid carcinoma via miR-204-5p/HMGB1 axis
- Neuroanatomy of melanocortin-4 receptor pathway in the mouse brain
- Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages
- Influences of advanced glycosylation end products on the inner blood–retinal barrier in a co-culture cell model in vitro
- MiR-4328 inhibits proliferation, metastasis and induces apoptosis in keloid fibroblasts by targeting BCL2 expression
- Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients
- Long non-coding RNA SNHG3 accelerates progression in glioma by modulating miR-384/HDGF axis
- Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease
- PCR-detectable Candida DNA exists a short period in the blood of systemic candidiasis murine model
- CircHIPK3/miR-381-3p axis modulates proliferation, migration, and glycolysis of lung cancer cells by regulating the AKT/mTOR signaling pathway
- Reversine and herbal Xiang–Sha–Liu–Jun–Zi decoction ameliorate thioacetamide-induced hepatic injury by regulating the RelA/NF-κB/caspase signaling pathway
- Therapeutic effects of coronary granulocyte colony-stimulating factor on rats with chronic ischemic heart disease
- The effects of yam gruel on lowering fasted blood glucose in T2DM rats
- Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043-miR-31-KLF3 axis
- CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis
- Relationship of FTO gene variations with NAFLD risk in Chinese men
- The prognostic and predictive value of platelet parameters in diabetic and nondiabetic patients with sudden sensorineural hearing loss
- LncRNA SNHG15 contributes to doxorubicin resistance of osteosarcoma cells through targeting the miR-381-3p/GFRA1 axis
- miR-339-3p regulated acute pancreatitis induced by caerulein through targeting TNF receptor-associated factor 3 in AR42J cells
- LncRNA RP1-85F18.6 affects osteoblast cells by regulating the cell cycle
- MiR-203-3p inhibits the oxidative stress, inflammatory responses and apoptosis of mice podocytes induced by high glucose through regulating Sema3A expression
- MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells
- CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-κB pathway in rats with osteoarthritis
- Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension
- Long noncoding RNA FTX ameliorates hydrogen peroxide-induced cardiomyocyte injury by regulating the miR-150/KLF13 axis
- Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis
- CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells
- Decitabine shows anti-acute myeloid leukemia potential via regulating the miR-212-5p/CCNT2 axis
- Testosterone aggravates cerebral vascular injury by reducing plasma HDL levels
- Bioengineering and Biotechnology
- PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect
- The thickness of surface grafting layer on bio-materials directly mediates the immuno-reacitivity of macrophages in vitro
- Silver nanoparticles: synthesis, characterisation and biomedical applications
- Food Science
- Bread making potential of Triticum aestivum and Triticum spelta species
- Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations
- Effect of addition of dried potato pulp on selected quality characteristics of shortcrust pastry cookies
- Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities
- Animal Sciences
- Changes in the fecal microbiome of the Yangtze finless porpoise during a short-term therapeutic treatment
- Agriculture
- Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability
- Application of extrusion-cooking technology in hatchery waste management
- In-field screening for host plant resistance to Delia radicum and Brevicoryne brassicae within selected rapeseed cultivars and new interspecific hybrids
- Studying of the promotion mechanism of Bacillus subtilis QM3 on wheat seed germination based on β-amylase
- Rapid visual detection of FecB gene expression in sheep
- Effects of Bacillus megaterium on growth performance, serum biochemical parameters, antioxidant capacity, and immune function in suckling calves
- Effects of center pivot sprinkler fertigation on the yield of continuously cropped soybean
- Special Issue On New Approach To Obtain Bioactive Compounds And New Metabolites From Agro-Industrial By-Products
- Technological and antioxidant properties of proteins obtained from waste potato juice
- The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part I
- Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN
- The impedance analysis of small intestine fusion by pulse source
- Errata
- Erratum to “Diagnostic performance of serum CK-MB, TNF-α and hs-CRP in children with viral myocarditis”
- Erratum to “MYL6B drives the capabilities of proliferation, invasion, and migration in rectal adenocarcinoma through the EMT process”
- Erratum to “Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation”

