Abstract
The antifungal activity of Trichoderma hamatum K01 to control Colletotrichum gloeosporioides C01 was recorded. Pathogenicity of C. gloeosporioides C01 on citrus leaves was confirmed by the Koch postulate. T. hamatum K01 inhibited the colony growth and conidia production of C. gloeosporioides C01 by 70.55 and 79.07%, respectively. Crude extracts from T. hamatum K01 expressed an antifungal activity against C. gloeosporioides C01. The crude TK01-MeOH showed the most potent inhibitory effect on the colony growth and conidia production at the ED50 values of 273 and 355 ppm, respectively. Nanofiber TK01M gave the highest inhibition on the colony growth and conidia production at the ED50 values of 13 and 3 ppm, respectively. Control mechanism was related to the major pyrone metabolite group (6-pentyl-2H-pyran-2-one), organic compounds (benzoic acid, hexadecane, tetracosane), fatty acids (palmitic acid, linoleic acid, tetradecanoic acid, pentadecanoic acid, hexadecenoic acid, ethyl ester, linoleic acid ethyl ester, and ethyl oleate), and sorbicillin from GC-MS analysis, which were produced by T. hamatum K01. It is reported for the first time to isolate secondary metabolite pyrone (6-pentyl-2H-pyran-2-one) from T. hamatum K01, and the first report of nanofibers constructed from T. hamatum K01 against C. gloeosporioides.
1 Introduction
Citrus (Citrus reticulata) is a small and evergreen shrub crop belonging to Rutaceae. This crop grows in the tropical and subtropical climates and in the temperate regions [1]. Citrus fruits are considered economically essential crops due to their valuable component and health-related benefits (e.g., soluble and insoluble fibers) [2]. Citrus cultivation is also one of the major agricultural livelihoods of small-scale farmers; hence, policies that support citrus farmers help to increase employment opportunities and income [3]. Thailand produced 1.2 million tons of citrus fruits on 0.1 million ha, which was reported by Food and Agricultural Organization of the United Nations [4]. However, disease infestation greatly affects citrus production, particularly by Colletotrichum gloeosporioides, one of the known pathogenic species worldwide causing anthracnose of citrus fruit [5]. This anthracnose disease shows the dieback of branches and affects the postharvest condition of crops [6]. Moreover, it compromises the quality and quantity of citrus fruits, thereby limiting production and negatively impacting fruit export and marketability [7].
Chemical fungicides have always been extensively used to control this disease in orchards. This practice is harmful to the environment as it accumulates toxic substances. Moreover, in the long run, chemical fungicides loose effectiveness as the plant pathogen develops pesticide resistance [8]. Latest agricultural technologies such as the use of microorganisms as biocontrol agents or as potential sources of metabolites have been considered an alternative to suppress plant pathogens and reduce the wide-ranging use of chemical fungicides. Trichoderma fungus has been reported as the common biological control agent against fungal diseases [9]. Trichoderma species have also been documented to control various plant pathogens such as Sclerotinia sclerotiorum, Rhizoctonia solani, Verticillium dahliae, Phytophthora nicotianae, and Phytophthora cinnamomi [10].
Trichoderma species produce bioactive compounds such as trichotoxin A, trichotoxin B, trichodecenins, trichorovins, and trichocellins. These compounds showed fungal activities against phytopathogens, Gram-positive bacteria, and some viruses [11]. In addition, many other secondary metabolites such as volatile, nonvolatile terpenes, pyrone, siderophores, nitrogen-containing compounds, peptaibols, and nonribosomal peptides are also produced from T. harzianum, T. asperellum, T. longibrachiatum, T. citrinoviride, and T. virens [11,12,13,14]. Kumar et al. [15] reported that T. harzianum and T. viride produced volatile and nonvolatile organic compounds against seed-borne pathogens (C. gloeosporioides, Alternaria alternata, Fusarium oxysporum, Curvularia lunata, and Rhizoctonia solani). Trichoderma hamatum combined with Pseudomonas aeruginosa BJ10–86 expressed a fungal activity against collar rot disease of chili pepper caused by P. capsici, and it can also stimulate seed germination of chili pepper [16].
Therefore, the research findings aimed to evaluate the antifungal activity of T. hamatum K01 to control C. gloeosporioides causing citrus anthracnose, and to test natural product nanofiber constructed from T. hamatum K01 to inhibit citrus anthracnose pathogen.
2 Materials and methods
2.1 Source of fungal isolates
For the pathogenic fungus, disease samples were collected from anthracnose lesions on leaves of the citrus plant. Tissue transplanting technique was performed to isolate Colletotrichum spp. Symptoms of anthracnose disease on leaves were cleaned with running tap water. Plant tissues were cut into 2 mm pieces at lesions in the advanced margin between healthy and diseased parts. These pieces were then soaked for 5 min in a 10% sodium hypochlorite solution for surface disinfection and washed three times with sterilized distilled water. The tissue pieces were left dried with sterilized tissue paper for 1 min, then placed into water agar (WA), and incubated at room temperature (27–30°C) for 2–3 days. Hyphal tips from advanced margin tissue in WA were cut and transferred to PDA plates to obtain a pure culture. T. hamatum K01 was used as the antagonistic fungus, obtained from the collection of Prof. Dr Kasem Soytong, Department of Plant Production Technology, Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang (KMITL), Bangkok, Thailand.
2.2 Characterization and identification of pathogenic fungus and antagonistic fungus
2.2.1 Morphological characterization
The isolates were identified based on morphological characterizations. Initially, the isolate was cultured in PDA and stored at room temperature (27–30°C) for 7 days. Morphological characterization was performed by observing the general characteristics and structures. The colony characteristics, size, and shape of spores were recorded under a compound microscope and scanning electron microscopy (SEM).
2.2.2 Molecular identification
The isolated fungi were identified by molecular technique using the cetyltrimethylammonium bromide (CTAB) protocol for deoxyribonucleic acid (DNA) extraction following the methods of Tongon and Soytong [17]. Initially, fungal isolates were separately cultured in potato dextrose broth (PDB) for 7 days at room temperature (27–30°C). The biomass was collected and ground in a pestle with liquid nitrogen. Cell extraction was carried out in a tube containing 1–2 g of ground powder, and the phenolic compound was separated by 2 µL of β-mercaptoethanol and 700 µL of 2× CTAB buffer. The tube was heated in a water bath at 65°C for 1 h. The 24:1 volume of chloroform to isoamyl alcohol was added to the tube, then centrifuged at 14,000 rpm for 5 min. Two microliters of RNase were transferred to the upper aqueous layer in a sterile tube for 30 min at 37°C, then mixed with 50 µL of 10% CTAB. The solution was centrifuged at 14,000 rpm for 5 min. The DNA was precipitated by isopropanol and then centrifuged at 14,000 rpm for 20 min at 4°C. DNA pellets were cleaned with 70% and continued by 95% of ethanol. The DNA was dried in the incubator at 37°C before being dissolved in 20–30 µL with TE buffer.
Then, polymerase chain reaction (PCR) was used to amplify the DNA template of each fungus using the modified PCR program from Grunwald et al. [18]. A 25 µL master mix of PCR was prepared to contain 14.3 µL of water NA, 2.5 µL Tag buffer, 0.2 µL of Tag DNA, 4 µL of dNTP, 1 µL of DNA, 1 µL of ITS1 (5′-TCCGTAGGT-GAACCTGCGG-3′), and 1 µL of ITS4 (5′-TCCTCCGCT-TATTGATATGC-3′). The ITS region was subjected to a PCR procedure that included an initial denaturation at 95°C for 5 min, followed by 30 cycles of 94°C for 1 min, 52°C for 1 min, 72°C for 50(s), and a final extension of 72°C and stored at −20°C until the PCR product was used. The PCR products were electrophoresed in a 1% agarose gel to check the DNA bands. The sequences were recognized as species by utilizing Basic Local Alignment Search Tools to align the sequences from National Center for Biotechnology Information (NCBI). The phylogenetic tree was constructed using the MEGA-X software Tamura-Nei model, bootstrap, and the maximum likelihood approach was generated with 1,000 replications using the same MEGA-X software [19]. The fungal isolates were confirmed as T. hamatum K01 and C. gloeosporioides and were used in further experiments.
2.3 Pathogenicity test
Pathogenicity test was performed using the detached leaves method to fulfill Koch’s postulate. C. gloeosporioides isolate C01 was subcultured in PDA. Then, citrus leaves were collected from healthy citrus trees and surface disinfected with 75% ethyl alcohol. Detached leaves were wounded using a sterilized needle. The culture agar plugs of the pathogen were cut at 0.5 cm diameter, then inoculated onto the wounded leaves. Noninoculated ones were treated with agar plugs that served as the control. All treatments were incubated in a moist chamber at room temperature (27–30°C) for 7 days. The experiment was conducted with four replications arranged in a completely randomized design (CRD). Disease incidence was classified as follows: 1 = usual green and nonaggressive, 2 = pale green and low aggressive, 3 = pale brown and medium aggressive, and 4 = dark brown and highly aggressive [20].
2.4 Identification of secondary metabolite from T. hamatum K01 by gas chromatography/mass spectrometry (GC/MS)
Secondary metabolites from T. hamatum K01 were prepared from crude methanol (TK01-MeOH) by GC/MS. In detail, crude TK01-MeOH was diluted in methanol and analysed by an Agillen 6890 N gas chromatograph with an Agilent 5973 mass detector equipped with an HP-5 silica capillary column (30 m 0.25 mm ID, 0.25 εL film thickness). The oven temperature program consisted of an initial 50°C for 3 min, and the temperature was increased with the heating rate by 10°C/min to 200°C held for 3 min, then further temperature was increased at 15°C/min to 260°C, which was held for 20 min. Helium was used as the carrier gas at a flow rate of 1 mL/min. MS analysis was carried out over a detection range of 30–500 amu. The sample was prepared (1/1) g/mL of crude TK01-MeOH/methanol, and the sample of 0.2 µL was injected with a split ratio of 50:1. The injector and detector were maintained at the respective temperatures of 250 and 270°C. Individual compounds were identified by MS, and their identity was confirmed by comparison of Kovat’s retention index with reference to a homologous series of n-alkanes. Percentage composition was determined based on GC peak area and retention time as calculated by a Shimadzu CR6A data processor.
2.5 Evaluation of antagonistic fungus T. hamatum K01 against C. gloeosporioides C01
2.5.1 Dual culture assay
To compare the inhibition activity of T. hamatum K01 against C. gloeosporioides, a dual culture assay was performed. The antagonistic fungus and pathogenic isolate were separately cultured in PDA at room temperature (27–30°C) for 7 days. Agar plugs of the pathogen (0.5 cm2) were cut and placed into 9 cm PDA medium plates at 1.5 cm on one side, while the antagonistic fungus was placed at 1.5 cm on the opposite side. Each agar plug of the antagonistic fungus and the pathogenic isolate was cultured in PDA alone and served as the control. These agar plugs were incubated for 10 days at room temperature until the nontreated control were fully grown in plates. The experiment was arranged in a CRD with four replicates. The following data were collected: colony growth diameter (cm) and the number of conidia produced using a hemocytometer. The percentage of inhibition in the colony diameter and conidia produced was calculated using equations (1) and (2), adapted from the study of Song et al. [21], as shown below. Data were analyzed using analysis of variance (ANOVA), and treatment means were compared with Duncan’s multiple range test (DMRT) at p < 0.05 and p < 0.01.
2.5.2 Crude extract test in vitro
For the determination of in vitro antifungal activities of T. hamatum K01 against C. gloeosporioides C01, crude extract tests were conducted using three different partitioned extracts (hexane, ethyl acetate, and methanol) from T. hamatum K01. The extraction method was performed following the protocol described by Kanokmedhakul et al. [22]. T. hamatum K01 was grown in PDB and incubated for 45 days at room temperature (27–30°C). The biomass was collected from PDB medium and filtrated through cheesecloth to yield fresh weight biomass and air-dried for 7 days to yield dry biomass. The dried biomass was grounded, and sequencing was carried out by soaking in hexane, ethyl acetate, and methanol as solvents in 1:1 v/v for 3 days. These solvents were evaporated through a rotary vacuum to yield crude hexane, crude ethyl acetate, and crude methanol. Each crude extract was named crude TK01-hexane, TK01-EtOAc, and TK01-MeOH. Each crude extract was weighed for each concentration using an electrical balance, dissolved in 2% dimethyl sulfoxide (DMSO), and mixed with PDA medium before autoclaving at 121°C 15 l bs/inch2 for 30 min. The isolated pathogen, C. gloeosporioides, was separately cultured in PDA for 7 days. The agar plugs of the pathogen were cut at 0.5 cm2 using a sterilized cork borer, transferred to the middle-tested plates in each concentration, and incubated at room temperature (27–30°C) until the pathogen in control entirely grew in the plates. The experiment was carried out using a 3 × 6 factorial CRD with four replications. Crude extracts represented factor A, and factor B was represented by different concentrations of 0, 10, 50, 100, 500, and 1,000 ppm (µg/mL). Data on colony growth (cm) and conidia production were collected. The percentage of inhibition in both the colony diameter and conidia was calculated using equations (3) and (4). Treatment means were compared using DMRT at p < 0.05 and p < 0.01. Effective doses (ED50) were calculated using probit analysis.
2.5.3 Nanofiber from T. hamatum K01 against C. gloeosporioides C01
Nanofiber from T. hamatum K01 was evaluated to inhibit the growth of C. gloeosporioides C01, causing anthracnose disease of citrus. The nanofiber was constructed from crude hexane, ethyl acetate, and methanol from T. hamatum K01 following the method by Dar and Soytong [23]. Each crude extract was separately incorporated into the polylactic acid-based nanofiber through electrospinning to yield nano-TK01H, nano-TK01E, and nano-TK01M, respectively. Each nanofiber was characterized, and its size was measured using SEM. The experiment was arranged using a two-factor factorial experiment in CRD with four replicates. Factor A was the test nanofiber, while factor B was the concentration with the following levels: 0, 3, 5, 10, and 15 ppm (µg/mL). Each nanofiber was dissolved in 2% DMSO and mixed to the PDA medium before autoclaving at 121°C, 15 lbs/inch2 for 30 min. The culture of the pathogen was cut at the peripheral colony with a sterilized cork borer (0.5 cm in diameter). Agar plugs of the pathogen were transferred to the middle of 5 cm Petri dishes and PDA mixed with the concentrations of each nanofiber. All plates were incubated at room temperature (27–30°C) until the pathogen in control fully grew in plates. The pathogen from each treatment was observed for abnormal spores or conidia under a compound microscope.
2.6 Statistical data analysis
Data collections were measured regarding colony growth and counted number of conidia production using a hemacytometer. The data were statistically analyzed using ANOVA. The effective dose (ED50) was calculated by probit analysis using SPSS statistical. Treatment means were compared by using DMRT at p < 0.05 and p < 0.01. The percentage of inhibition in both the colony diameter and conidia production was calculated using equations (3) and (4).
3 Results
3.1 Morphological characteristics of the pathogen and antagonistic fungus
The isolated C. gloeosporioides C01 was morphologically characterized. Characterization results showed that C. gloeosporioides C01 isolates fully grew in PDA plates at 7 days by about 9 cm. Colonies on PDA were cottony white at the beginning and turned to dark gray from the center to the whole PDA plate and appeared purple color when observed upside down. The fungus produced conidiophores and dark brown setae of about 25–130 × 3–5 µm. One-cell conidium was also observed as ovoid and slightly carve of about 10–15 µm in length and 5–7 in width (Figure 1 and Table 1). Meanwhile, T. hamatum K01 was morphologically identified. It showed that T. hamatum K01 grew fully in 9 cm petri dishes within 3 days. The colony pattern was thin white on the surface to brown when it was older and yellow pigment on the reverse side. It was pure yellow when observed it upside down. Observation under a light compound microscope and SEM showed that the culture was smooth-walled whip-like, hyphal elongations from 2.8 to 5.7 µm with an average of 4.4 µm diameter. It constructs apices roughened conidiophore from 3.2 to 18.3 µm with an average of 11.3 µm diameter with granules and produces phialides conidia 2.7–4.1 µm with an average of 3.27 µm in width, and 3.8–5.7 µm with an average of 4.63 µm in length (Figure 2 and Table 1).
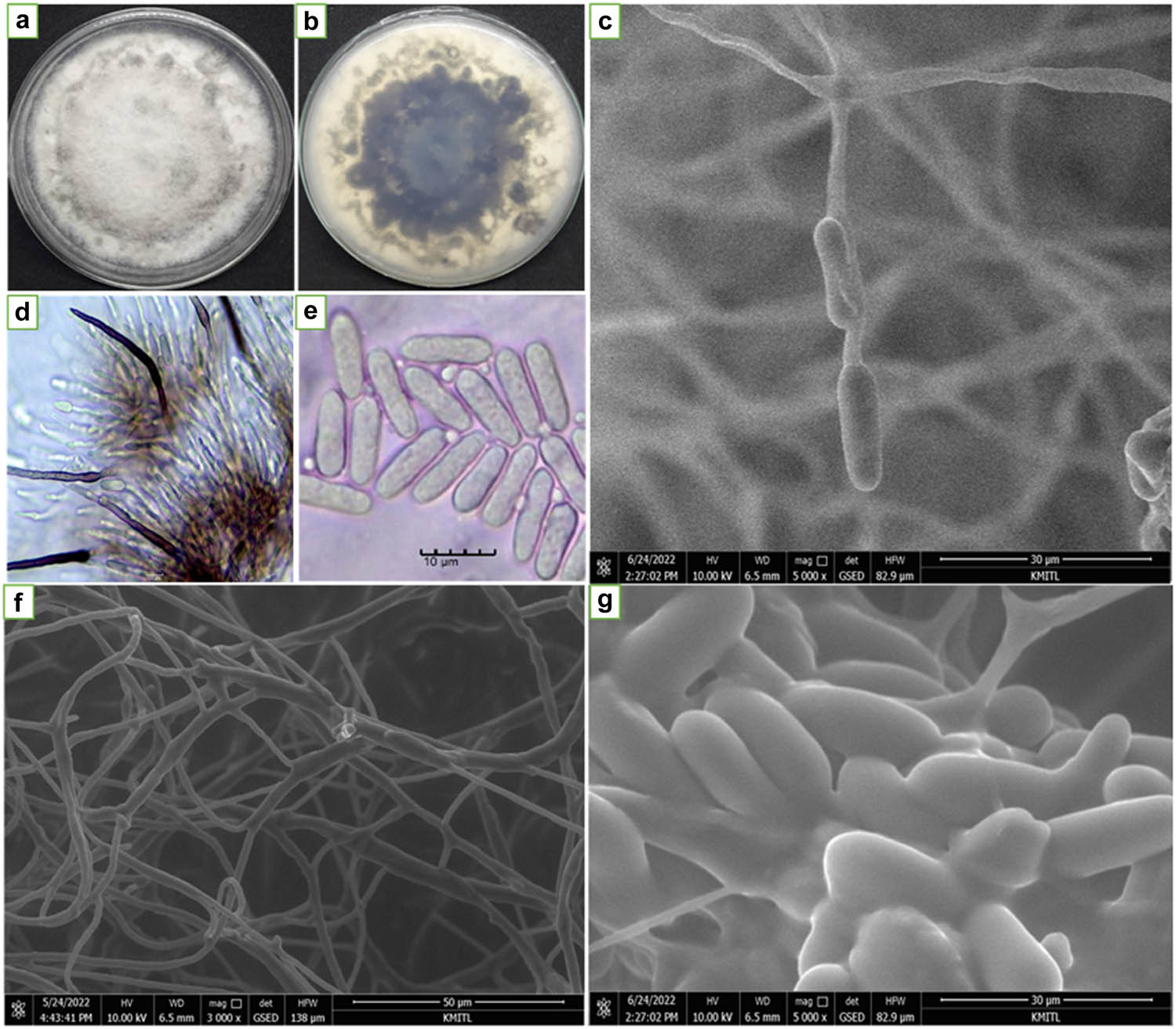
Colony growth patterns and the general characteristic of C. gloeosporioides C01. (a) Colony pattern on PDA; (b) colony pattern on PDA upside down; (c and d) setae conidiophores; (e and g) conidia and (f) hyphal. Parts d and e were observed under light compound microscope (scale bar 10 µm, 40× magnification) and c, f, and g were observed using SEM.
General characteristic of C. gloeosporioides C01 and T. hamatum K01
| Fungi | Structures | Maximum (µm) | Minimum (µm) | Mean (µm) |
|---|---|---|---|---|
| C. gloeosporioides C01 | Setaea (length) | 195.1 | 52 | 98.53 |
| Setaea (width) | 7.9 | 4.2 | 6.4 | |
| Conidiab (length) | 17 | 8 | 12.5 | |
| Conidiab (width) | 7 | 5.5 | 6.25 | |
| Hyphaea (diameter) | 12 | 2.7 | 5.8 | |
| T. hamatum K01 | Phialides conidiab (length) | 5.7 | 3.8 | 4.63 |
| Phialides conidiab (width) | 4.1 | 2.7 | 3.27 | |
| Chlamydosporea (diameter) | 18.3 | 3.2 | 11.3 | |
| Hyphaeb (diameter) | 5.7 | 2.8 | 4.4 |
aData were collected from 30 separate of each structure. bData were collected from 50 separate of each structure.
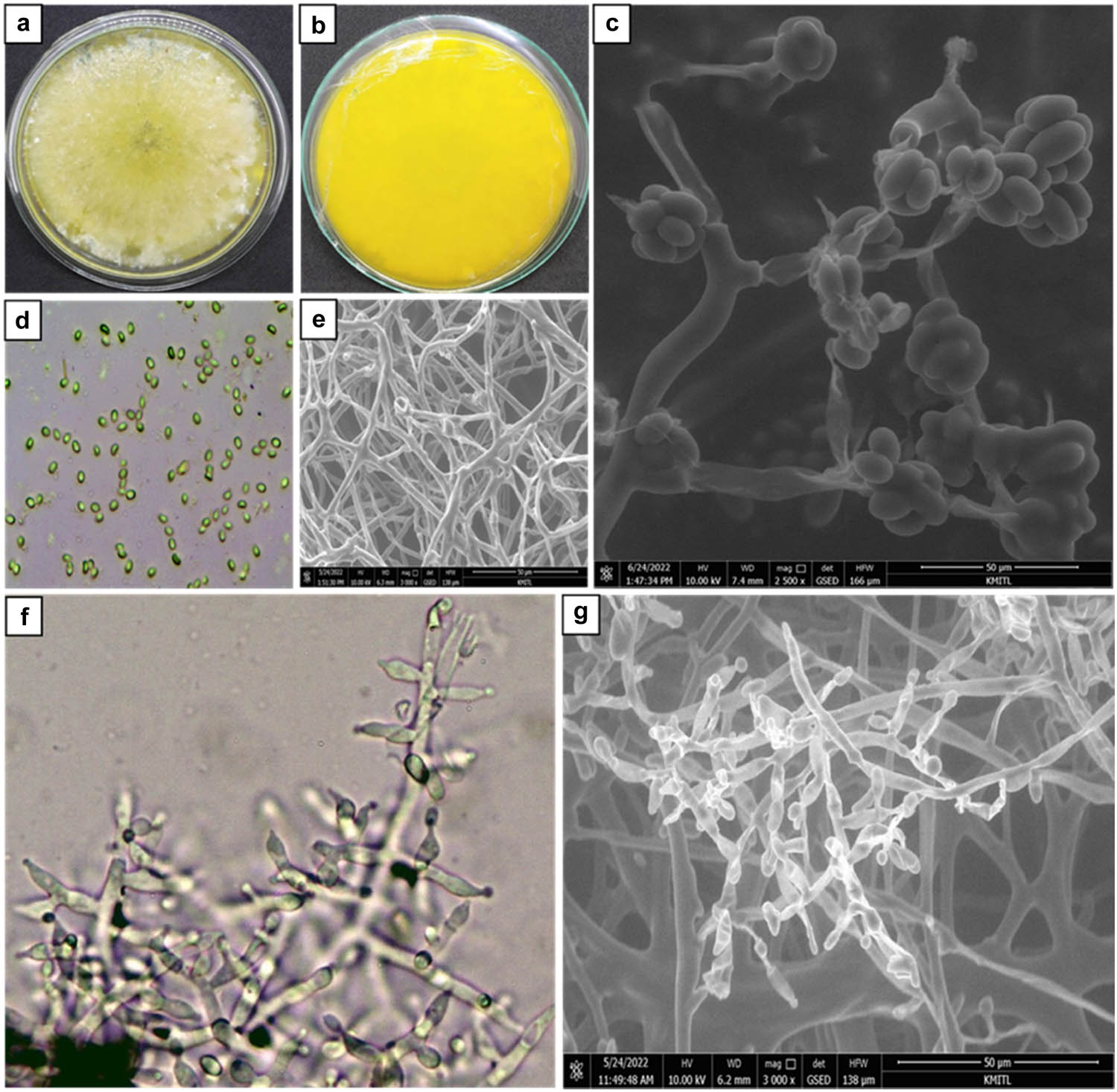
Morphological characterization of T. hamatum K01; (a) colony growth on PDA media; (b) colony growth on PDA media observed upside to down; (c and d) conidia; (e) hyphal; and (f and g) apices roughened conidiophore. (d) and (f) were observed under light compound microscope and (c), (e), and (g) using SEM.
3.2 Molecular identification of the pathogen and antagonistic fungus
Molecular identification was made to confirm the species level of pathogenic isolate and antagonistic fungus. The pathogenic isolate was confirmed as C. gloeosporioides C01. It was in the same clade with KM519999 C. gloeosporioides isolate LL5, KY033215 C. gloeosporioides isolate p1-1, MT993622 C. gloeosporioides isolate s12, HQ264182 C. gloeosporioides isolate Cg-224, MT919170 C. gloeosporioides isolate ESP15, and KY930619. KY930619 with Sordaria fimicola was considered the outgroup (Figure 3). Meanwhile, T. hamatum TK01 is closely related to AB737864 T. hamatum isolate FKI-6011, MW750434 T. hamatum isolate GL19, OL439486 T. hamatum strain Th23, MW763159 T. hamatum isolate TISC-3, MN880214 T. hamatum strain JAHLH2, and KX424842 T. hamatum isolate NGL1, with EF025942 C. gloeosporioides Cm 89 being compared as outgroup (Figure 4).
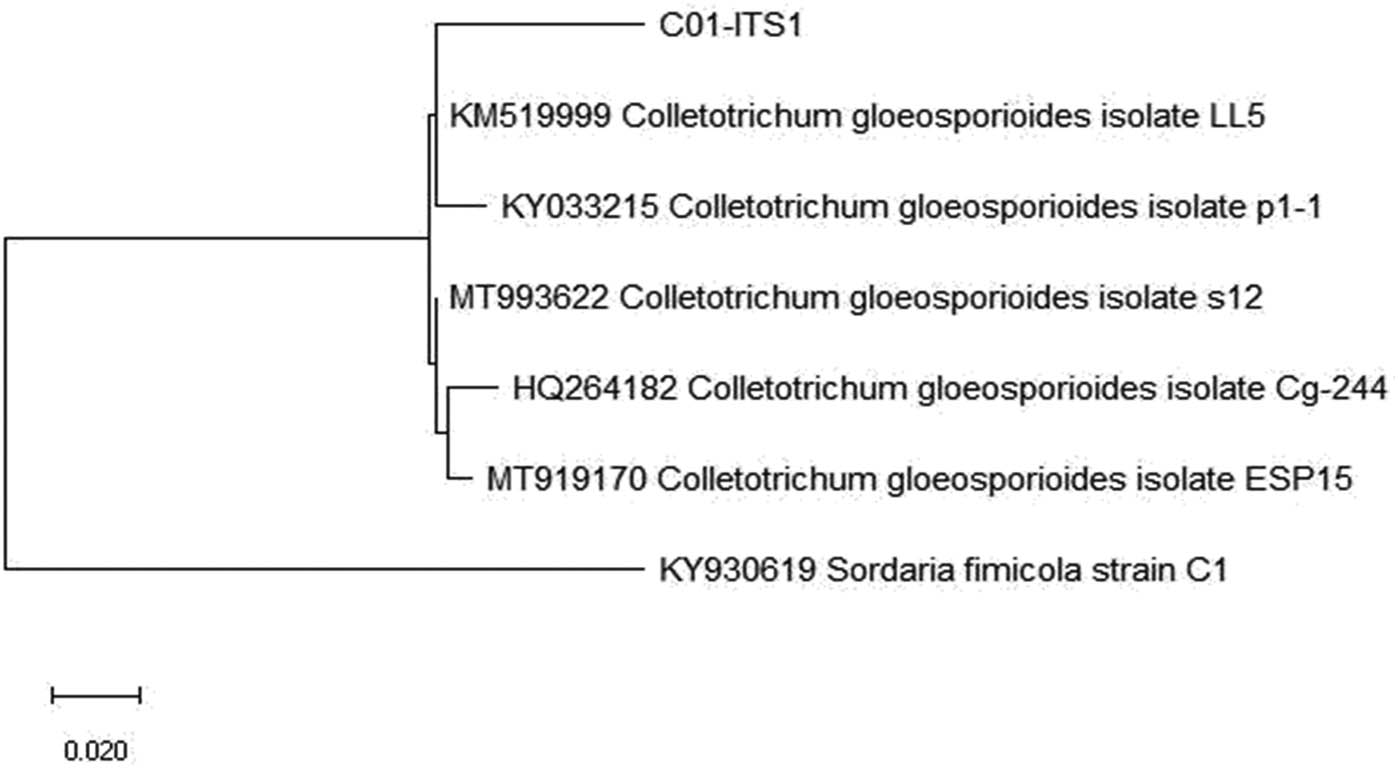
Phylogenetic tree of C. gloeosporioides C01 from GenBank with 99.54% bootstrap value constructed after the distance-based analysis of the universal primer ITS1, ITS4, and 5.8S region of the rDNA.
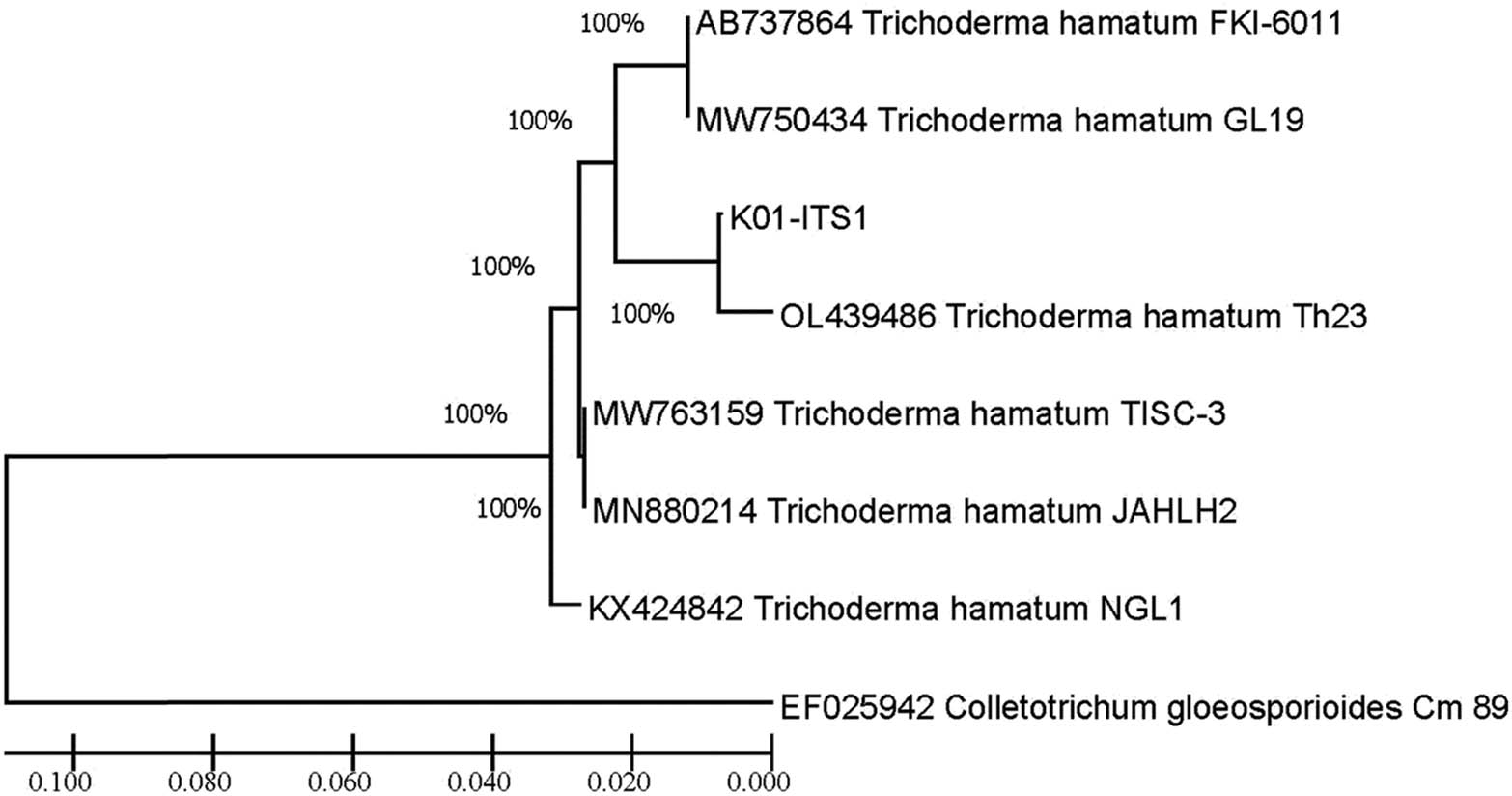
Phylogenetic tree of T. hamatum K01 from GenBank with 100% bootstrap value constructed after the distance-based analysis of the universal primer ITS1, ITS4, and 5.8S region of the rDNA.
3.3 Pathogenicity test
C. gloeosporioides C01 was proved to be the pathogenic isolate on citrus leaves using the detached leaves technique in a moist chamber. The results showed that C. gloeosporioides caused anthracnose on citrus leaves after 7 days of inoculation. The symptoms of detached leaves changed to dark brown lesions around agar plugs from 1.5 to 2.75 cm in diameter and expanded larger lesions to the whole detached leaves (Figure 5a), which was evaluated as level three (3 = pale brown and medium aggressive). In addition, it was clearly seen that C. gloeosporioides invaded to citrus compared to noninoculated control with no symptoms and remained healthy, which was evaluated as usual green and nonaggressive (Figure 5b).

Pathogenicity of C. gloeosporioides C01 on citrus leaves using the detached method. (a) The detached leaves of citrus inoculated by C. gloeosporioides C01; (b) noninoculated control.
3.4 Identification of constituents from T. hamatum K01
Active compounds from T. hamatum K01 were identified using GC-MS analysis. The result showed that 34 compounds were detected with 97.37% of total crude MeOH and found to contain polyhydric alcohol (20.35%), organic compound (3.88%), hydrocarbon (5.45%), alpha-phenylacetamide (4.64%), fatty acid (61.86%), miscellaneous (0.99%), and unknown (0.19%), as shown in Table 2.
Constituents detected from T. hamatum K01 using GC/MS analysis
| No. | Class | Constituents | % | RT (min) |
|---|---|---|---|---|
| 1 | Polyhydric alcohol | Glycerin | 17.83 | 7.26 |
| 2 | 1,2,3-Propannetriol | 2.35 | 8.5 | |
| 3 | Mannitol | 0.17 | 18.56 | |
| 4 | Organic compound | Oxirane | 0.77 | 9.61 |
| 5 | Benzoic acid | 0.31 | 10.83 | |
| 6 | Phenol | 0.13 | 14.25 | |
| 7 | 2-Methyl-1-(m-methoxybenzyl)-1-pyrrolinium | 0.3 | 14.63 | |
| 8 | Hexadecane | 0.33 | 15.26 | |
| 10 | 1,2-Azaborolidine, 1-(1,1-dimethylethyl) | 0.68 | 16.5 | |
| 11 | 6-Pentyl-2H-Pyran-2-one | 1.14 | 16.61 | |
| 12 | Tetracosane | 0.22 | 17.5 | |
| 13 | Hydrocarbon | Benzene | 4.1 | 10.89 |
| 14 | 5,5-Diphenyl-2-cyclohexenone | 0.34 | 23.1 | |
| 15 | Exo-(5,6-dihydro 3,8-phenanthrolino) | 1.01 | 24.6 | |
| 16 | Alpha-phenylacetamide | Benzene acetamide | 3.36 | 12.86 |
| 17 | (R)-5,6-Dihydro-6-pentyl-2H-pyran-2-one | 1.29 | 13.9 | |
| 18 | Fatty acid | 4-Methyl-2,6-di-tert butylphenol | 0.34 | 14.31 |
| 19 | Tetradecanoic acid | 0.27 | 16.68 | |
| 20 | Palmitic acid | 0.57 | 17.82 | |
| 21 | Tetradecanoic acid, 12-methyl- | 0.41 | 18.23 | |
| 22 | Pentadecanoic acid | 15.5 | 19.84 | |
| 23 | Hexadecenoic acid, ethyl ester | 12.33 | 20.38 | |
| 24 | Hexadecenoic acid, 14-methyl-, ethyl ester | 0.22 | 20.89 | |
| 25 | 9,12-Octadecadienoic acid (z, z)-methyl ester | 6.26 | 21.79 | |
| 26 | 9-Octadecadienoic acid (z)-methyl ester | 4.55 | 21.86 | |
| 27 | Octadecadienoic acid | 3.06 | 22.14 | |
| 28 | Linoleic acid | 4.51 | 22.23 | |
| 29 | 9-Octadecadienoic acid, (E)-trans-delta | 8.25 | 22.29 | |
| 30 | Linoleic acid ethyl ester | 3.65 | 22.53 | |
| 31 | Ethyl oleate | 1.37 | 22.59 | |
| 32 | Octadecadienoic acid, ethyl ester | 0.57 | 22.83 | |
| 33 | Miscellaneous | Sorbicillin | 0.99 | 20.9 |
| 34 | Unknown | Naphtho[2,1-b] furan | 0.19 | 21.5 |
| Polyhydric alcohol | 20.35% | |||
| Organic compound | 3.88% | |||
| Hydrocarbon | 5.45% | |||
| Alpha-phenylacetamide | 4.65% | |||
| Fatty acid | 61.86% | |||
| Miscellaneous | 0.99% | |||
| Unknown | 0.19% | |||
| Total | 97.37% | |||
RT – retention of time.
3.5 Evaluation of antagonistic fungus against C. gloeosporioides C01
3.5.1 Dual culture test
T. hamatum K01 was used as a biological control agent against C. gloeosporioides C01, a causal agent of citrus anthracnose. The edge active growing mycelia of C. gloeosporioides (0.5 cm diameter) was paired on the individual of 9 cm diameter PDA medium plates with antagonistic fungus T. hamatum K01 at 1.5 cm apart. T. hamatum K01 expressed control mechanisms by reducing colony growth and conidia production of C. gloeosporioides by 70.55 and 79.07%, respectively (Table 3). Furthermore, it was observed that T. hamatum K01’s growth almost covered the pathogen colony and caused abnormal conidia, as shown in Figure 6. It was demonstrated that T. hamatum K01 significantly inhibited C. gloeosporioides in the dual culture test.
Effect of antagonistic fungus, T. hamatum K01 against C. gloeosporioides C01 using dual culture method
| Treatments | Colony diameter (cm) | Colony inhibition (%) | Number of conidia (× 106) | Conidia inhibition (%) |
|---|---|---|---|---|
| Control | 9.00a | 0.00 | 58.55a | 0.00 |
| Dual culture | 2.65b | 70.55 | 12.25b | 79.07 |
| CV% | 2.64 | — | 27.13 | — |
Treatment means were averaged of four replications. Means followed by common letters are not the same letters. It was not significantly different by DMRT at p <0.01.
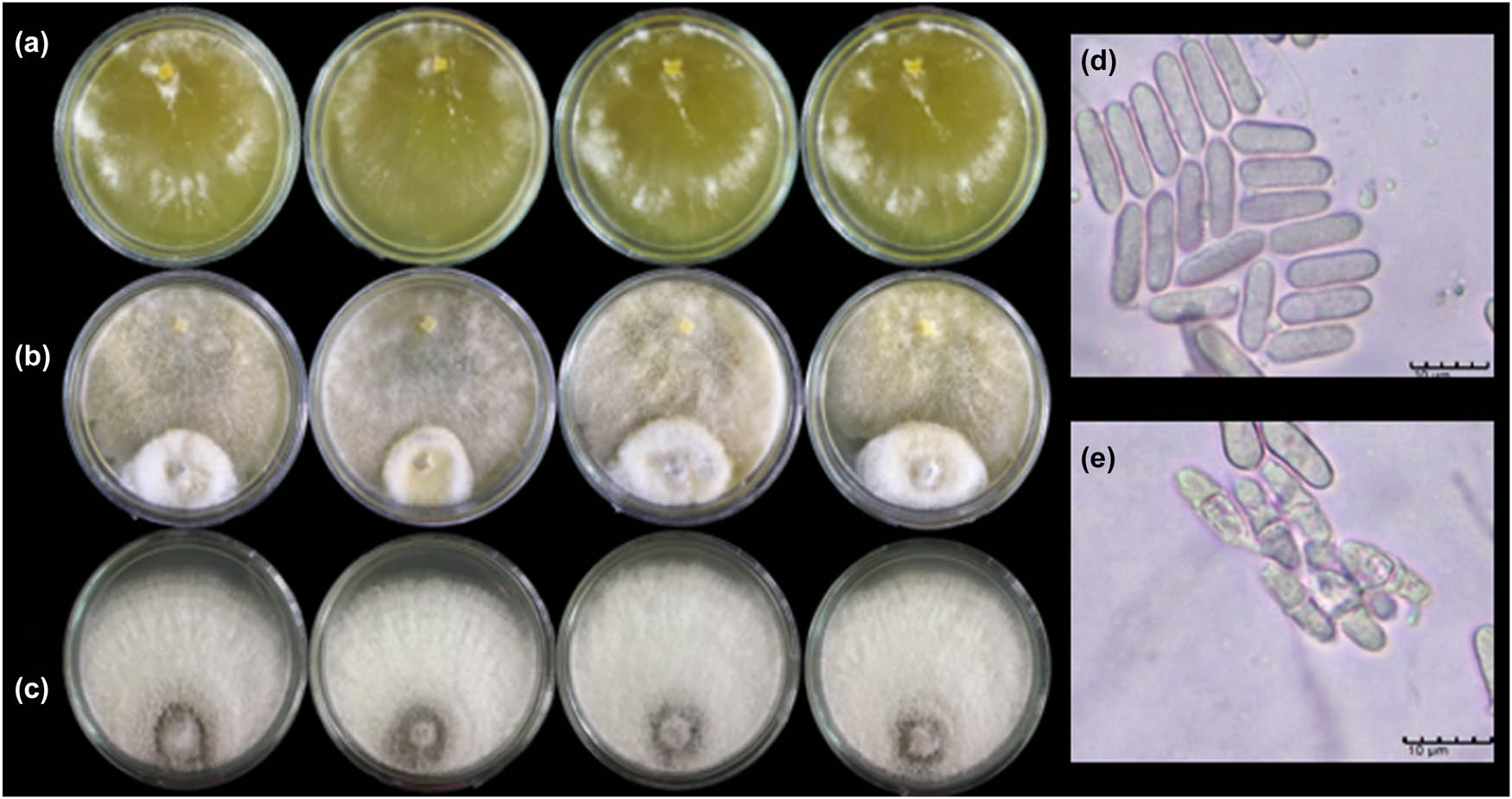
Dual culture test of T. hamatum K01 against C. gloeosporioides C01. (a) T. hamatum K01; (b) dual culture plates of T. hamatum K01 versus C. gloeosporioides C01; (c) C. gloeosporioides C01; (d) normal conidia produce; and (e) abnormal conidia produce of C. gloeosporioides C01 caused by T. hamatum K01.
3.5.2 Bioactivity of T. hamatum K01 against C. gloeosporioides C01 in vitro
The ED50 value of crude TK01-EtOAc was 607 ppm to inhibit conidia production. Moreover, ED50 values of crude TK01-MeOH for colony inhibition and conidia production were 273 and 355 ppm, respectively. It can be demonstrated that metabolite crude extract TK01-MeOH from T. hamatum K01 gave the most effective inhibitory activities against C. gloeosporioides. It caused abnormal conidia production of C. gloeosporioides C01, as shown in Figure 7 (Table 4).

Crude extracts derived from T. hamatum K01 against C. gloeosporioides C01. (a) Crude TK01-hexane; (b) crude TK01-EtOAc; (c) crude TK01-MeOH and the effect of crude extracts derived from T. hamatum K01 on conidia production of C. gloeosporioides; (d) normal conidia; (e) abnormal conidia caused by crude TK01-hexane; (f) abnormal conidia caused by crude TK01-EtOAc; and (g) abnormal conidia caused by crude TK01-MeOH.
Crude extracts derived from T. hamatum K01 against C. gloeosporioides C01 causing citrus anthracnose diseases
| Crude extract | Concentration ppm (µg/mL) | Colony diameter (cm) | Growth inhibition (%) | ED50 (ppm) | Conidia production (106) | Inhibition (%) | ED50 (ppm) |
|---|---|---|---|---|---|---|---|
| TK01-hexane | 0 | 5.00a | — | — | 86.75a | — | — |
| 10 | 4.65b | 7 | 76.00a | 12.34 | |||
| 50 | 4.32c | 13.6 | 62.45b | 27.97 | |||
| 100 | 3.82d | 23.6 | 60.15b | 30.62 | |||
| 500 | 3.35e | 33 | 52.20bc | 38.63 | |||
| 1,000 | 3.12e | 37.6 | 46.85c | 45.96 | |||
| TK01-EtOAc | 0 | 5.00a | — | — | 86.75a | — | 607 |
| 10 | 4.67b | 6.6 | 79.90ab | 7.84 | |||
| 50 | 3.82c | 23.6 | 76.15ab | 12.16 | |||
| 100 | 3.62c | 27.6 | 67.65b | 21.97 | |||
| 500 | 3.00d | 40 | 49.00c | 43.48 | |||
| 1,000 | 2.77e | 44.6 | 31.80d | 63.32 | |||
| TK01-MeOH | 0 | 5.00a | — | 273 | 86.70a | — | 355 |
| 10 | 4.52b | 9.6 | 73.60ab | 15.1 | |||
| 50 | 4.22c | 15.6 | 71.25b | 17.82 | |||
| 100 | 3.37d | 32.6 | 64.20b | 25.95 | |||
| 500 | 2.17e | 56.6 | 42.85c | 50.57 | |||
| 1,000 | 1.07f | 78.6 | 23.25d | 73.21 | |||
| CV (%) | 4.69 | 14.67 |
The average of four replications, the means followed by the common letters are not significantly different by DMRT at p < 0.01.
3.5.3 Testing nanofiber from T. hamatum K01 against C. gloeosporioides C01
Nanofiber from T. hamatum K01 was characterized and its size was measured using an SEM, as shown in Figure 8 and Table 5. Its ability at low concentrations (3–15 ppm) to control C. gloeosporioides C01 was tested. The result revealed that nano-TK01M at 15 ppm showed the highest antifungal activity toward C. gloeosporioides C01, which inhibited colony growth and conidia production of 59.37 and 100%, respectively. Moreover, the ED50 values of nano-TK01M to inhibit colony growth and conidia production were 13 and 3 ppm, respectively. The ED50 values of nano-TK01E were 28 and 9 ppm, respectively. The ED50 values of nano-TK01H were 33 and 15 ppm, respectively (Table 6). Moreover, nanofiber not only inhibited the colony, but also degraded the conidia, caused abnormal conidia.
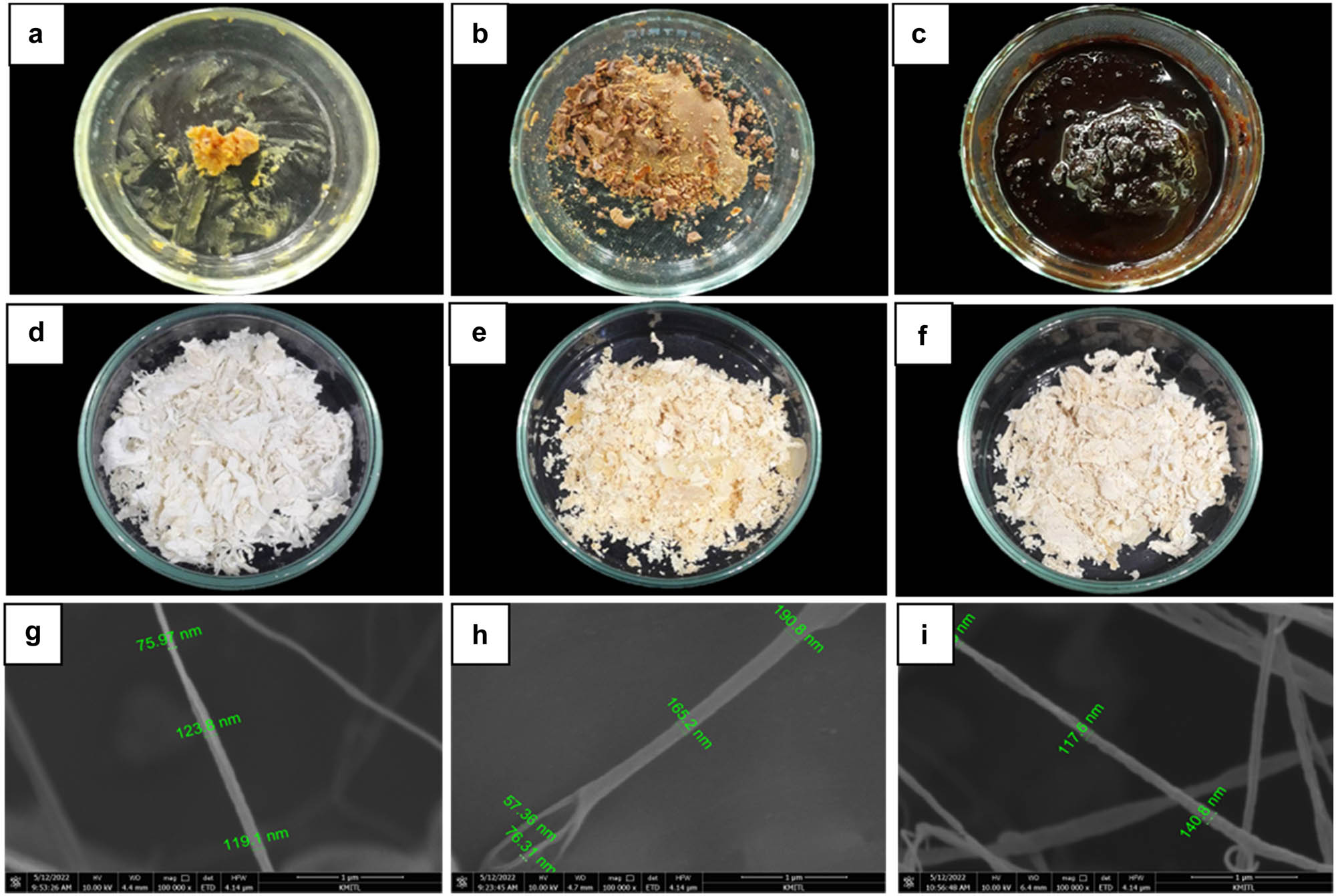
Characteristic of crude extract and nanoparticle from T. hamatum K01; (a) crude TK01-hexane; (b) crude TK01-EtOAc; (c) crude TK01-MeOH; (d) nano-TK01H; (e) nano-TK01E; (f) nano-TK01M; (g), (h), and (i) size of each nanoparticle was measured using SEM.
Measurement of the size (diameter) of nanofibers from T. hamatum K01 using SEM
| Nanoparticles | Mean (nm) | Max (nm) | Min (nm) |
|---|---|---|---|
| Nano-TK01H | 122 | 168 | 75 |
| Nano-TK01E | 187 | 242 | 57 |
| Nano-TK01M | 136 | 256 | 90 |
Data were collected from 20 samples by measuring the size (diameter) of each nanofiber.
Testing nanofibers from T. hamatum K01 against C. gloeosporioides C01
| Nanoparticles | Concentration (ppm) | Colony growth (cm) | Growth inhibition (%) | ED50 (ppm) | Conidia production (105) | Inhibition (%) | ED50 (ppm) |
|---|---|---|---|---|---|---|---|
| Nano-TK01H | 0 | 4.90a | — | 33 | 216.25a | — | 15 |
| 3 | 4.06b | 17.04f | 162.75ab | 19.93d | |||
| 5 | 3.82bc | 21.89f | 153.50ab | 24.74de | |||
| 10 | 3.70cd | 24.49ef | 141.00ab | 31.35cd | |||
| 15 | 3.38de | 30.76de | 84.24bc | 58.59bc | |||
| Nano-TK01E | 0 | 4.90a | — | 28 | 216.25a | — | 9 |
| 3 | 3.81bc | 22.16f | 154.00ab | 23.91d | |||
| 5 | 3.41de | 30.29de | 125bc | 36.06cd | |||
| 10 | 3.32ef | 32.10cde | 81.75bc | 56.72bc | |||
| 15 | 3.05fgh | 37.72bcd | 53.50cd | 74.21bc | |||
| Nano-TK01M | 0 | 4.90a | — | 13 | 216.25a | — | 3 |
| 3 | 3.28efg | 32.81cd | 137.25ab | 37.20c | |||
| 5 | 2.98gh | 39.00bc | 49.50cd | 75.45ab | |||
| 10 | 2.82h | 42.33b | 0.00d | 100a | |||
| 15 | 1.98i | 59.37a | 0.00d | 100a | |||
| CV% | 4.46 | 14.49 | 31.91 | 40.39 |
The average of four replications, the means followed by the common letters are not significantly different by DMRT at p < 0.01.
4 Discussion
C. gloeosporioides C01 was isolated from the citrus leaf anthracnose using tissue transplanting techniques. Morphological characterization showed that C. gloeosporioides produces septate mycelia, hyaline, one-cell conidium, ovoid, and slightly carve. These characteristics are similar to the reports of Ajay Kumar [24], who characterized C. gloeosporioides. Meanwhile, molecular identification results showed that C. gloeosporioides, the pathogenic isolate, was closely related to the clade of C. gloeosporioides with GenBank accession numbers KM519999, KY033215, MT993622, MT919170, and KY930619 supported by 99.54% bootstrap value and similar to the findings of Hassan et al. [25]. For molecular identification of antagonistic fungus, T. hamatum K01 was confirmed to be in the same clade as the sequence of T. hamatum with GenBank accession numbers AB737864, MW750434, OL439486, MN880214, MW763159, and KX424842. Furthermore, identification was recorded with a 100% bootstrap value constructed after the distance-based analysis of the universal primer ITS1, ITS4, and 5.8S region of the rDNA, consistent with the study of Abdelkhalek et al. [26].
The pathogenicity test proved C. gloeosporioides C01 as the causal agent of citrus anthracnose disease. The second infected leaf in Figure 5 shows foliar damage, but the leaf can still be seen as green, which is different from the other replicas that are all brown and damaged. This could be explained by the fact that the leaf samples were collected from the same plant but at different ages, with older leaves showing more aggressive symptoms than younger leaves. This finding is supported by the study by Liu et al. [27], who found that Colletotrichum spp. symptoms develop faster on older leaves than on younger leaves in the same crop using the same detection method. However, the antifungal activities of T. hamatum K01 proved to be antagonized C. gloeosporioides C01. In dual culture test, T. hamatum K01 inhibited colony growth and conidia production by 70.55 and 79.77%, respectively. Such finding is confirmed by Mulatu et al. [28] that T. hamatum AU23 effectively suppressed the colony growth of F. xylarioides (coffee wilt disease) by 76.9%. Similarly, Shovan et al. [29] reported that T. harzianum inhibited the colony growth of Colletotrichum dematium causing soybean anthracnose of 89.44% [30]. Other biological control agent, Emericella nidulans, is reported by Talubnak and Soytong [31] to inhibit the colony growth and conidia production of C. gloeosporioides causing anthracnose of Vanilla planifolia by 49.44 and 75.31%, respectively. The control mechanisms of Trichoderma species are involved in mycoparasitism and antibiosis [32].
In this study, crude metabolites of T. hamatum K01 inhibited the colony growth and conidia production of C. gloeosporioides. Crude TK01-MeOH significantly inhibited the colony and conidia production by 78 and 73% with the ED50 of 273 and 355 ppm.
The previous study reported that antifungal activities of ethyl acetate crude extract derived from T. hamatum PC02 inhibited conidia production against C. gloeosporioides by 67.73% [33]. Similarly, methanol and ethyl acetate extracts from Trichoderma koningiopsis VM115, Trichoderma longibrachiatum VM99, Trichoderma brevicompactum VM102, and Trichoderma asperellum showed a significant antifungal activity toward Pyricularia oryzae causing rice blast disease, which conidia germination was completely inhibited at 250 µg/mL [34]. Sour et al. [35] found that crude hexane, ethyl acetate, and methanol derived from Chaetomium cupreum expressed a fungal activity against C. gloeosporioides causing anthracnose disease on orchids, with the ED50 values of 794, 624, and 879 µg/mL, respectively.
The highlight of the investigation was nanofibers constructed from T. hamatum K01 that can be used at lower concentrations than the crude extracts to suppress C. gloeosporioides C01. Nano-TK01M inhibited the colony growth and conidia production of C. gloeosporioides C01 at the ED50 values of 13 and 3 ppm, respectively. ED50 values of nano-TK01E were 28 and 9 ppm, respectively, and those of nano-TK01H were 33 and 15 ppm, respectively. Similarly, the silver nanoparticle from T. hamatum gave the highest inhibition zone of four soil-borne pathogenic fungi, namely, Fusarium spp., F. solani, F. semitectum, F. oxysporum, and F. roseum [36]. This study revealed that the antifungal effect was related to the major organic compounds (benzoic acid, hexadecane, 6-pentyle-2H-pyran-2-one, tetracosane), alpha-phenylacetamide ((R)-5,6-dihydro-6-pentyl-2H-pyran-2-one), fatty acid (palmitic acid, linoleic acid, tetradecanoic acid, pentadecanoic acid, hexadecenoic acid, ethyl ester, linoleic acid ethyl ester, and ethyl oleate), and sorbicillin, which was found to be produced from T. hamatum K01 that implies antibiosis control mechanism against C. gloeosporioides C01. This research finding was confirmed by the study of Reino et al. [11], who reported that pyrone name (6-pentyle-2H-pyran-2-one) and sorbicillin metabolites produced from Trichoderma species exhibited a strong antifungal activity against Rhizoctonia solani and Fusarium oxysporum f. sp. lycopersici. Similarly, pyrone antibiotic (6-pentyl-α-pyrone) produced from T. koningii was also reported to inhibit Rhizoctonia root rot pathogen [37]. Active metabolite ((R)-5,6-dihydro-6-pentyl-2H-pyran-2-one) from Trichoderma spp. significantly controlled sapstain fungi of Pinus radiata sapwood caused by Sphaeropsis sapinea (syn. Diplodia pinea) [38] and Penicillium species [39]. Trichoderma species are known to produce metabolite pyrone (6-pentyle-2H-pyran-2-one), (R)-5,6-dihydro-6-pentyl-2H-pyran-2-one); sorbicillin acts as active metabolite against pathogenic organisms [11,37,40] and fatty acid (palmitic acid, linoleic acid, hexadecenoic acid ethyl ester) was detected from Trichoderma sp. EFI671 acts as bioactive compound to inhibit several plant pathogens [41].
5 Conclusion
Research on the control mechanism of T. hamatum K01 against pathogenic fungus T. hamatum K01 produced major secondary metabolites, pyrone, organic compound, fatty acid, and sorbicillin, which exhibited the best antimicrobial potential in the suppression of C. gloeosporioides C01 causing anthracnose of citrus. T. hamatum K01 showed promising effects in controlling plant pathogen, which could be a viable alternative for plant disease protection, and nontoxic to human and the environment.
Acknowledgments
We would like to acknowledge PhD KMITL Scholarship 2019, King Mongkut’s Institute of Technology Ladkrabang (KMITL), Ladkrabang, Bangkok 10520, Thailand, which offers the research project to be successfully done.
-
Funding information: The study was finically supported with sources of PhD KMITL Scholarship 2019, King Mongkut’s Institute of Technology Ladkrabang (KMITL), Ladkrabang, Bangkok 10520.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
[1] Abobatta WF. Nutritional benefits of citrus fruits. J Am J Biomed Sci Res. 2019;3(1):303–6. 10.34297/AJBSR.2019.03.000681.Suche in Google Scholar
[2] Pragasam SJ, Rasool M. Dietary component p-coumaric acid suppresses monosodium urate crystal-induced inflammation in rats. Inflamm Res. 2013;62(5):489–98. 10.1007/s00011-013-0602-7.Suche in Google Scholar PubMed
[3] Tran Nguyen TT, Le HH, Ho TMH, Dogot T, Burny P, Bui TN, et al. Efficiency analysis of the progress of orange farms in Tuyen Quang Province, Vietnam towards sustainable development. Sustainability. 2020;12(8):3170. 10.3390/su12083170.Suche in Google Scholar
[4] Hung PM, Wattanachai P, Kasem S, Poeaim S. Efficacy of Chaetomium species as biological control agents against Phytophthora nicotianae root rot in citrus. Mycobiology. 2015;43(3):288–96. 10.5941/MYCO.2015.43.3.288.Suche in Google Scholar PubMed PubMed Central
[5] Phoulivong S, Cai L, Chen H, McKenzie EHC, Abdelsalam K, Chukeatirote E, et al. Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers. 2010;44(3):33–43. 10.1007/s13225-010-0046-0.Suche in Google Scholar
[6] Wang W, de Silva DD, Moslemi A, Edwards J, Ades PK, Crous PW, et al. Colletotrichum species causing anthracnose of citrus in Australia. J Fungi. 2021;7(1):47. 10.3390/jof7010047.Suche in Google Scholar PubMed PubMed Central
[7] Phoulivong S, McKenzie E, Hyde K. Cross infection of Colletotrichum species; a case study with tropical fruits. Curr Res Environ Appl Mycol. 2012;2(2):99–111. 10.5943/cream/2/2/2.Suche in Google Scholar
[8] Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol. 2014;7(4):133–41. 10.1007/s12154-014-0113-1.Suche in Google Scholar PubMed PubMed Central
[9] Akhtar MS, Siddiqui ZA. Biocontrol of a root-rot disease complex of chickpea by Glomus intraradices, Rhizobium sp. and Pseudomonas straita. J Crop Prot. 2008;27(5):410–7. 10.1016/j.cropro.2007.07.009.Suche in Google Scholar
[10] Aleandri MP, Chilosi G, Bruni N, Tomassini A, Vettraino AM, Vannini A. Use of nursery potting mixes amended with local Trichoderma strains with multiple complementary mechanisms to control soil-borne diseases. J Crop Prot. 2015;67(3):269–78. 10.1016/j.cropro.2014.10.023.Suche in Google Scholar
[11] Reino J, Guerrero R, Hernández-Galán R, Collado I. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem Rev. 2008;7(1):89–123. 10.1007/s11101-006-9032-2.Suche in Google Scholar
[12] Zeilinger S, Gruber S, Bansal R, Mukherjee P. Secondary metabolism in Trichoderma – Chemistry meets genomics. Fungal Biol Rev. 2016;30(1):74–90. 10.1016/j.fbr.2016.05.001.Suche in Google Scholar
[13] Mukherjee P, Horwitz B, Kenerley C. Secondary metabolism in Trichoderma – A genomic perspective. Microbiology. 2012;158(1):35–45. 10.1099/mic.0.053629-0.Suche in Google Scholar PubMed
[14] Contreras-Cornejo H, Macías-Rodríguez L, Del-Val E, Larsen J. Ecological functions of Trichoderma spp. And their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol Ecol. 2016;92(4):36. 10.1093/femsec/fiw036.Suche in Google Scholar PubMed
[15] Kumar S, Chandra R, Behera L, Keswani C, Sansinenea E. Dual Trichoderma consortium mediated elevation of systemic defense response against early blight in potato. Eur J Plant Pathol. 2022;162(3):681–96. 10.1007/s10658-021-02431-4.Suche in Google Scholar
[16] Chemeltorit P, Mutaqin K, Widodo W. Combining Trichoderma hamatum THSW13 and Pseudomonas aeruginosa BJ10–86: A synergistic chili pepper seed treatment for Phytophthora capsici infested soil. Eur J Plant Pathol. 2016;147(1):157–66. 10.1007/s10658-016-0988-5.Suche in Google Scholar
[17] Tongon R, Soytong K. Nanoparticles Derived from Active Metabolites of Chaetomium cupreum CC3003 against Phytophthora Rot of Durian. Int J Agric Biol. 2022;27(1):19–27. 10.17957/IJAB/15.1894.Suche in Google Scholar
[18] Grunwald NJ, Martin FN, Larsen MM, Sullivan CM, Press CM, Coffey MD, et al. Phytophthora-ID.org: A sequence-based Phytophthora identification tool. Plant Dis. 2011;95(3):337–42. 10.1094/PDIS-08-10-0609.Suche in Google Scholar PubMed
[19] Tamura K, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–9. 10.1093/molbev/msm092.Suche in Google Scholar PubMed
[20] Kean S, Soytong K, To-Anun C. Application of biological fungicides to control citrus root rot under field condition in Cambodia. Int J Agric Technol . 2010;6(2):219–30.Suche in Google Scholar
[21] Song J, Soytong K, Kanokmedhakul S, Kanokmedhakul K, Poeaim S. Antifungal activity of microbial nanoparticles derived from Chaetomium spp. against Magnaporthe oryzae causing rice blast. Plant Prot Sci. 2020;56(3):180–90. 10.17221/41/2019-PPS.Suche in Google Scholar
[22] Kanokmedhakul S, Kanokmedhakul K, Nasomjai P, Louangsysouphanh S, Soytong K, Isobe M, et al. Antifungal Azaphilones from the fungus Chaetomium cupreum cc3003. J Nat Prod. 2006;69(6):891–5. 10.1021/np060051v.Suche in Google Scholar PubMed
[23] Dar J, Soytong K. Construction and characterization of copolymer nanomaterials loaded with bioactive compounds from Chaetomium species. Int J Agric Technol. 2014;10(4):823–31.Suche in Google Scholar
[24] Ajay Kumar G. Colletotrichum gloeosporioides: Biology, pathogenicity and management in India. J Plant Pathol. 2014;2(2):2. 10.4172/2329-955X.1000125.Suche in Google Scholar
[25] Hassan O, Jeon J, Chang T, Shin J, Oh N, Lee Y. Molecular and morphological characterization of Colletotrichum species in the Colletotrichum gloeosporioides complex associated with persimmon anthracnose in South Korea. Plant Dis. 2018;102(5):1015–24. 10.1094/PDIS-10-17-1564-RE.Suche in Google Scholar PubMed
[26] Abdelkhalek A, Al-Askar AA, Arishi AA, Behiry SI. Trichoderma hamatum strain Th23 promotes tomato growth and induces systemic resistance against tobacco mosaic virus. J Fungi. 2022;8(3):228. 10.3390/jof8030228.Suche in Google Scholar PubMed PubMed Central
[27] Liu G, Kennedy R, Greenshields DL, Peng G, Forseille L, Selvaraj G, et al. Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol Plant Microbe Interact. 2007;20(10):1308–19. 10.1094/MPMI-20-10-1308.Suche in Google Scholar PubMed
[28] Mulatu A, Megersa N, Abena T, Kanagarajan S, Liu Q, Tenkegna TA, et al. Biodiversity of the genus Trichoderma in the rhizosphere of coffee (Coffea arabica) plants in Ethiopia and their potential use in biocontrol of coffee wilt disease. Crop. 2022;2(2):120–41. 10.3390/crops2020010.Suche in Google Scholar
[29] Shovan L, Bhuiyan K, Begum JA, Pervez Z. In vitro Control of Colletotrichum dematium causing anthracnose of soybean by fungicides, plant extracts and Trichoderma harzianum. J Sustainable Crop Prod. 2008;3(3):10–7.Suche in Google Scholar
[30] Keswani C, Singh SP, Singh H. A superstar in biocontrol enterprise: Trichoderma spp. Biotech Today. 2013;3(2):27–30. 10.5958/2322-0996.2014.00005.2.Suche in Google Scholar
[31] Talubnak C, Soytong K. Biological control of vanilla anthracnose using Emericella nidulans. Int J Agric Technol. 2010;6(1):47–55.Suche in Google Scholar
[32] Blaszczyk L, Siwulski M, Sobieralski K, Lisiecka J, Jedryczka M. Trichoderma spp.–application and prospects for use in organic farming and industry. J Plant Prot Res. 2014;54(4):309–17. 10.2478/jppr-2014-0047.Suche in Google Scholar
[33] Soytong K, Srinon W, Rattanacherdchai K, Kanokmedhakul S, Kanokmedhakul K. Application of antagonistic fungi to control anthracnose disease of grape. Int J Agric Technol. 2005;1(1):33–41.Suche in Google Scholar
[34] Leylaie S, Zafari D. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front Microbiol. 2018;9(2):1484. 10.3389/fmicb.2018.01484.Suche in Google Scholar PubMed PubMed Central
[35] Sour V, Phonpho S, Soytong K. Antifungal activities of endophytic fungi isolated from orchids against Colletotrichum gloeosporioides caused anthracnose in orchids. Int J Agric Technol . 2015;11(8):1949–61.Suche in Google Scholar
[36] El-Wakil DA. Antifungal activity of silver nanoparticles by Trichoderma species: Synthesis, characterization and biological evaluation. Egypt J Phytopathol. 2020;48(1):71–80. 10.21608/EJP.2020.49395.1015.Suche in Google Scholar
[37] Khan RAA, Najeeb S, Hussain S, Xie B, Li Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms. 2020;8(6):817–123. 10.3390/microorganisms8060817.Suche in Google Scholar PubMed PubMed Central
[38] Vanneste JL, Farrell RL, Holland PT. Biological control of sapstain fungi with natural products and biological control agents: A review of the work carried out in New Zealand. Mycol Res. 2002;106(2):228–32. 10.1017}S0953756201005020.Suche in Google Scholar
[39] Daguerre Y, Edel-Hermann V, Steinberg C. Fungal genes and metabolites associated with the biocontrol of soil-borne plant pathogenic fungi. Fungal Metab. 2017;106:33–104. 10.1007/978-3-319-25001-4_27.Suche in Google Scholar
[40] Ngo MT, Van Nguyen M, Han JW, Park MS, Kim H, Choi GJ. In vitro and in vivo antifungal activity of sorbicillinoids produced by Trichoderma longibrachiatum. J Fungi. 2021;7(6):428–104. 10.3390/jof7060428.Suche in Google Scholar PubMed PubMed Central
[41] Kaushik N, Díaz CE, Chhipa H, Julio LF, Andrés MF, and González-Coloma AM. Chemical composition of an aphid antifeedant extract from an endophytic fungus, Trichoderma sp. EFI671. Microorganisms. 2020;8(3):420. 10.3390/microorganisms8030420.Suche in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer
Artikel in diesem Heft
- Regular Articles
- The impact of COVID-19 pandemic on business risks and potato commercial model
- Effects of potato (Solanum tuberosum L.)–Mucuna pruriens intercropping pattern on the agronomic performances of potato and the soil physicochemical properties of the western highlands of Cameroon
- Machine learning-based prediction of total phenolic and flavonoid in horticultural products
- Revamping agricultural sector and its implications on output and employment generation: Evidence from Nigeria
- Does product certification matter? A review of mechanism to influence customer loyalty in the poultry feed industry
- Farmer regeneration and knowledge co-creation in the sustainability of coconut agribusiness in Gorontalo, Indonesia
- Lablab purpureus: Analysis of landraces cultivation and distribution, farming systems, and some climatic trends in production areas in Tanzania
- The effects of carrot (Daucus carota L.) waste juice on the performances of native chicken in North Sulawesi, Indonesia
- Properties of potassium dihydrogen phosphate and its effects on plants and soil
- Factors influencing the role and performance of independent agricultural extension workers in supporting agricultural extension
- The fate of probiotic species applied in intensive grow-out ponds in rearing water and intestinal tracts of white shrimp, Litopenaeus vannamei
- Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo
- Diallel analysis of length and shape of rice using Hayman and Griffing method
- Physicochemical and microbiological characteristics of various stem bark extracts of Hopea beccariana Burck potential as natural preservatives of coconut sap
- Correlation between descriptive and group type traits in the system of cow’s linear classification of Ukrainian Brown dairy breed
- Meta-analysis of the influence of the substitution of maize with cassava on performance indices of broiler chickens
- Bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium MA115 and its potential use as a seafood biopreservative
- Meta-analysis of the benefits of dietary Saccharomyces cerevisiae intervention on milk yield and component characteristics in lactating small ruminants
- Growth promotion potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon
- Prioritizing IoT adoption strategies in millennial farming: An analytical network process approach
- Soil fertility and pomelo yield influenced by soil conservation practices
- Soil macrofauna under laying hens’ grazed fields in two different agroecosystems in Portugal
- Factors affecting household carbohydrate food consumption in Central Java: Before and during the COVID-19 pandemic
- Properties of paper coated with Prunus serotina (Ehrh.) extract formulation
- Fertiliser cost prediction in European Union farms: Machine-learning approaches through artificial neural networks
- Molecular and phenotypic markers for pyramiding multiple traits in rice
- Natural product nanofibers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides
- Role of actors in promoting sustainable peatland management in Kubu Raya Regency, West Kalimantan, Indonesia
- Small-scale coffee farmers’ perception of climate-adapted attributes in participatory coffee breeding: A case study of Gayo Highland, Aceh, Indonesia
- Optimization of extraction using surface response methodology and quantification of cannabinoids in female inflorescences of marijuana (Cannabis sativa L.) at three altitudinal floors of Peru
- Production factors, technical, and economic efficiency of soybean (Glycine max L. Merr.) farming in Indonesia
- Economic performance of smallholder soya bean production in Kwara State, Nigeria
- Indonesian rice farmers’ perceptions of different sources of information and their effect on farmer capability
- Feed preference, body condition scoring, and growth performance of Dohne Merino ram fed varying levels of fossil shell flour
- Assessing the determinant factors of risk strategy adoption to mitigate various risks: An experience from smallholder rubber farmers in West Kalimantan Province, Indonesia
- Analysis of trade potential and factors influencing chili export in Indonesia
- Grade-C kenaf fiber (poor quality) as an alternative material for textile crafts
- Technical efficiency changes of rice farming in the favorable irrigated areas of Indonesia
- Palm oil cluster resilience to enhance indigenous welfare by innovative ability to address land conflicts: Evidence of disaster hierarchy
- Factors determining cassava farmers’ accessibility to loan sources: Evidence from Lampung, Indonesia
- Tailoring business models for small-medium food enterprises in Eastern Africa can drive the commercialization and utilization of vitamin A rich orange-fleshed sweet potato puree
- Revitalizing sub-optimal drylands: Exploring the role of biofertilizers
- Effects of salt stress on growth of Quercus ilex L. seedlings
- Design and fabrication of a fish feed mixing cum pelleting machine for small-medium scale aquaculture industry
- Indicators of swamp buffalo business sustainability using partial least squares structural equation modelling
- Effect of arbuscular mycorrhizal fungi on early growth, root colonization, and chlorophyll content of North Maluku nutmeg cultivars
- How intergenerational farmers negotiate their identity in the era of Agriculture 4.0: A multiple-case study in Indonesia
- Responses of broiler chickens to incremental levels of water deprivation: Growth performance, carcass characteristics, and relative organ weights
- The improvement of horticultural villages sustainability in Central Java Province, Indonesia
- Effect of short-term grazing exclusion on herbage species composition, dry matter productivity, and chemical composition of subtropical grasslands
- Analysis of beef market integration between consumer and producer regions in Indonesia
- Analysing the sustainability of swamp buffalo (Bubalus bubalis carabauesis) farming as a protein source and germplasm
- Toxicity of Calophyllum soulattri, Piper aduncum, Sesamum indicum and their potential mixture for control Spodoptera frugiperda
- Consumption profile of organic fruits and vegetables by a Portuguese consumer’s sample
- Phenotypic characterisation of indigenous chicken in the central zone of Tanzania
- Diversity and structure of bacterial communities in saline and non-saline rice fields in Cilacap Regency, Indonesia
- Isolation and screening of lactic acid bacteria producing anti-Edwardsiella from the gastrointestinal tract of wild catfish (Clarias gariepinus) for probiotic candidates
- Effects of land use and slope position on selected soil physicochemical properties in Tekorsh Sub-Watershed, East Gojjam Zone, Ethiopia
- Design of smart farming communication and web interface using MQTT and Node.js
- Assessment of bread wheat (Triticum aestivum L.) seed quality accessed through different seed sources in northwest Ethiopia
- Estimation of water consumption and productivity for wheat using remote sensing and SEBAL model: A case study from central clay plain Ecosystem in Sudan
- Agronomic performance, seed chemical composition, and bioactive components of selected Indonesian soybean genotypes (Glycine max [L.] Merr.)
- The role of halal requirements, health-environmental factors, and domestic interest in food miles of apple fruit
- Subsidized fertilizer management in the rice production centers of South Sulawesi, Indonesia: Bridging the gap between policy and practice
- Factors affecting consumers’ loyalty and purchase decisions on honey products: An emerging market perspective
- Inclusive rice seed business: Performance and sustainability
- Design guidelines for sustainable utilization of agricultural appropriate technology: Enhancing human factors and user experience
- Effect of integrate water shortage and soil conditioners on water productivity, growth, and yield of Red Globe grapevines grown in sandy soil
- Synergic effect of Arbuscular mycorrhizal fungi and potassium fertilizer improves biomass-related characteristics of cocoa seedlings to enhance their drought resilience and field survival
- Control measure of sweet potato weevil (Cylas formicarius Fab.) (Coleoptera: Curculionidae) in endemic land of entisol type using mulch and entomopathogenic fungus Beauveria bassiana
- In vitro and in silico study for plant growth promotion potential of indigenous Ochrobactrum ciceri and Bacillus australimaris
- Effects of repeated replanting on yield, dry matter, starch, and protein content in different potato (Solanum tuberosum L.) genotypes
- Review Articles
- Nutritional and chemical composition of black velvet tamarind (Dialium guineense Willd) and its influence on animal production: A review
- Black pepper (Piper nigrum Lam) as a natural feed additive and source of beneficial nutrients and phytochemicals in chicken nutrition
- The long-crowing chickens in Indonesia: A review
- A transformative poultry feed system: The impact of insects as an alternative and transformative poultry-based diet in sub-Saharan Africa
- Short Communication
- Profiling of carbonyl compounds in fresh cabbage with chemometric analysis for the development of freshness assessment method
- Special Issue of The 4th International Conference on Food Science and Engineering (ICFSE) 2022 - Part I
- Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy
- Special Issue on FCEM - International Web Conference on Food Choice & Eating Motivation - Part I
- Traditional agri-food products and sustainability – A fruitful relationship for the development of rural areas in Portugal
- Consumers’ attitudes toward refrigerated ready-to-eat meat and dairy foods
- Breakfast habits and knowledge: Study involving participants from Brazil and Portugal
- Food determinants and motivation factors impact on consumer behavior in Lebanon
- Comparison of three wine routes’ realities in Central Portugal
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Environmentally friendly bioameliorant to increase soil fertility and rice (Oryza sativa) production
- Enhancing the ability of rice to adapt and grow under saline stress using selected halotolerant rhizobacterial nitrogen fixer

