Abstract
Overuse of polymer products has led to severe environmental problems, which are threatening survival of creatures on earth. It is urgent to tackle enormous polymer wastes with proper cycling methods. Pyrolysis of polymers into high-value chemicals and fuels is displaying great potential to address the white pollution issue. In this study, we focus on chemical recycling of polystyrene, an important polymer in our everyday life, into valuable chemicals through simple pyrolysis strategy under nitrogen protection. It is found that yield of liquid product from polystyrene pyrolysis achieves as high as 76.24%, and there exists single component in the liquid product, which has been identified as styrene through hydrogen nuclear magnetic resonance spectra. Moreover, we propose monomer dissociation mechanism to explain the pyrolysis process of polystyrene based on the structure of polystyrene and experimental results.
1 Introduction
Large-scale application of synthetic polymer products has greatly accelerated social development and improved everyday life of human society in past decades (1,2,3). But excessive production of polymer products has given rise to severe environmental problems nowadays (4,5,6). The situation can be even worse in foreseen future because of the absence of effective tackling methods for waste polymers (7,8,9). Millions of tons of waste polymers produce every year, but are disposed inappropriately just by landfilling and incineration, which will lead to water, soil, and air contaminations (10,11). Even if functional polymers with novel properties for industrial applications are emerging one after another, effective disposal methods for waste polymers have not been put forward up to date (12,13,14). Since white pollutions have enormous negative influences on living of creatures on this planet, it is crucial to develop simple but effective strategy to realize chemical recycling of waste polymers without secondary pollutions.
Polymers mainly consist of long chains with repeated units in the structure and are usually chemically stable without any possibility to degrade into fragments by regular disposal methods (15,16,17). It seems to be an interesting strategy to pyrolyze waste polymers into valuable chemicals and fuels at high temperature under inert atmosphere (18,19,20). Polystyrene has become a common polymer that is utilized normally in everyday life, and there exist some companies for tackling polystyrene products. However, mainstream tackling method for waste polystyrene products is to recycle and reuse them. They collect waste polystyrene products and then clean them with solvents. After collecting and cleaning, these products are melted under high temperature and made into second-time products. But these recycled products always show deteriorated physical and chemical properties and are used in low-level scenarios. The pyrolysis method in this study can recycle waste polystyrene products without cleaning process, but directly obtain high-value styrene monomers. Large-scale applications at plant level have not been realized because of high energy cost, even if some companies have made some attempts previously.
In this study, we investigate pyrolysis methodology of waste polymers and take typical polystyrene as an example. Pyrolysis properties of polystyrene have been first studied by thermogravimetric analysis (TGA) technique and it is found that its optimal pyrolysis temperature should be 420°C under nitrogen atmosphere. The liquid product is the main product of polystyrene pyrolysis with a high mass-ratio yield of 76.24%, which consists a single component with high percentage of 73%. According to the gas chromatography (GC) and nuclear magnetic resonance results, the single component has been identified as styrene monomers. In addition, a monomer dissociation mechanism has been put forward to address the pyrolysis process of polystyrene under inert atmosphere. This work provides a typical demonstration of chemical recycling method for waste polymers.
2 Experimental section
2.1 Materials
Polystyrene was obtained from Millipore Sigma company. Deuterated chloroform was bought from Sinopharm Chemical Reagent. Nitrogen (N2) was purchased from Praxair company.
2.2 Pyrolysis process of polystyrene
First, nitrogen was pumped into the pyrolysis system to remove air for 0.5 h. Second, 5 g polystyrene was put into crucible and continued to pump nitrogen for 10 min. Third, the pyrolysis system started to heat up to 420°C at heating rate of 5°C min−1 under nitrogen atmosphere. Finally, gas, liquid, and solid products were obtained after persistent pyrolysis at 420°C under nitrogen protection for 2 h.
2.3 Characterization
TGA of polystyrene was carried out on TA-Q50 instrument. GC characterization of liquid products was made on CP3800 instrument. Hydrogen nuclear magnetic resonance (HNMR) analysis of liquid products was made on Bruker 400M NMR instrument.
3 Results and discussion
3.1 Chemical structure and pyrolysis process of polystyrene
In order to verify feasibility of pyrolysis method for chemical recycling of waste polymers, we utilized polystyrene, a common polymer in our everyday life, as a typical demonstration. Since the glass-transition temperature of polystyrene is as high as 100°C, polystyrene can be made into single-use containers for hot water and foods (21). Chemical structure of polystyrene is shown in Figure 1a, and its other information including formula, density, melting point, and solubility are also provided. In addition, schematic for the setup of polystyrene pyrolysis has been presented in Figure 1b. The pyrolysis process was protected by inert atmosphere (nitrogen in this work), whose flow rate was controlled by the flow meter in middle between gas container and pyrolysis reactor. A condenser with ice/water bath was set up after pyrolysis reactor for collection of liquid products from polystyrene pyrolysis. Gas phase products were taken out with nitrogen from the pyrolysis system, while solid phase products retained in the reactor.

(a) Chemical structure of polystyrene. (b) Schematic for the setup of polystyrene pyrolysis.
3.2 Thermogravimetric analysis and pyrolysis products of polystyrene
To determine the optimal reactive conditions for pyrolysis of polystyrene materials, TGA of polystyrene has been carried out with heating rate of 5°C min−1 under nitrogen atmosphere. TGA technique is actually the simulation of polystyrene pyrolysis at very low massloading of sample and can directly provide valuable informations for practical pyrolysis with large massloadings (22,23). TGA result of polystyrene is presented in Figure 2a, which indicates that polystyrene starts to degrade at 376°C and then achieves its highest degradation rate at the temperature of 420°C. Based on pyrolysis results of polystyrene from TGA results, we employed the pyrolysis temperature of 420°C with heating rate of 5°C min−1 under nitrogen atmosphere. Differential scanning calorimetry (DSC) can be utilized for detection of pyrolysis temperature, but its detection limit is below 300°C. Pyrolysis products of polystyrene mainly consist of three phases, including gas, liquid, and solid ones. The yield distribution of three products is displayed in Figure 2b, and the mass-ratio yields of gas, liquid, and solid products are 10.75%, 76.24%, and 13.01%, respectively. Liquid product is the main pyrolysis product, and thus is valuable for subsequent ingredient identification. It is possible to reduce pyrolysis temperature with catalysts, such as noble nanometals, transition metal oxides, and zeolites.

(a) Thermogravimetric analysis of polystyrene. (b) Yield distribution of various products from pyrolysis of polystyrene.
3.3 Gas chromatography spectra of liquid product from polystyrene pyrolysis
Since liquid product consists main pyrolysis products of polystyrene, it is essential to clarify the ingredient distribution of liquid product. To further identify the ingredient distribution of liquid product, GC characterization was adopted for analysis because of its high accuracy and good separating property for organic liquid samples (24,25). According to the GC spectra of liquid product in Figure 3, it is found that there exists a single component in liquid phase with a mass-ratio content as high as 73%. This result indicates that polystyrene materials can be directly converted into liquid products with single component through simple pyrolysis, which has great potential for applications in chemical industry. And it is an important task to identify the chemical structure of single component, which can be realized through subsequent separation and purification processes with organic methods. This pyrolysis method can also be employed for other polymers, including polyethylene and polypropylene. But product distributions are different from polystyrene because of different pyrolysis mechanisms, which are determined by specific chemical structures.

Gas chromatography spectra of liquid product from pyrolysis of polystyrene.
3.4 Structure identification of single component in liquid product
The single component indicated by GC spectra can be separated and purified by distillation method under N2 protection, and then identified with the aid of HNMR characterization. The HNMR spectra are shown in Figure 4a, which displays typical peaks which are the same with that of styrene monomers. The chemical shifts of different hydrogen atoms are analyzed and presented in the inset of Figure 4a. After analysis and comparison with standard spectra of styrene, it can be concluded that the single component in liquid product is styrene, which means that polystyrene intends to dissociate into styrene monomers under pyrolysis processes. It is worth mentioning that the recycled styrene has practical economic benefit and can be further utilized as valuable chemicals. In order to explain pyrolysis process of polystyrene, we propose a monomer dissociation mechanism as shown in Figure 4b. The polystyrene intends to degrade at the weak chemical bonds between α hydrogen of one styrene unit and β hydrogen of next styrene unit. This is mainly resulted from the high stereo-hindrance effect of benzene rings in polystyrene structures, and thus it is very difficult to fracture between α and β hydrogens in one styrene unit, finally leading to high pyrolysis selectivity of liquid products. This method shows potential for practical applications, but high energy cost should be alleviated for cost-effectiveness.
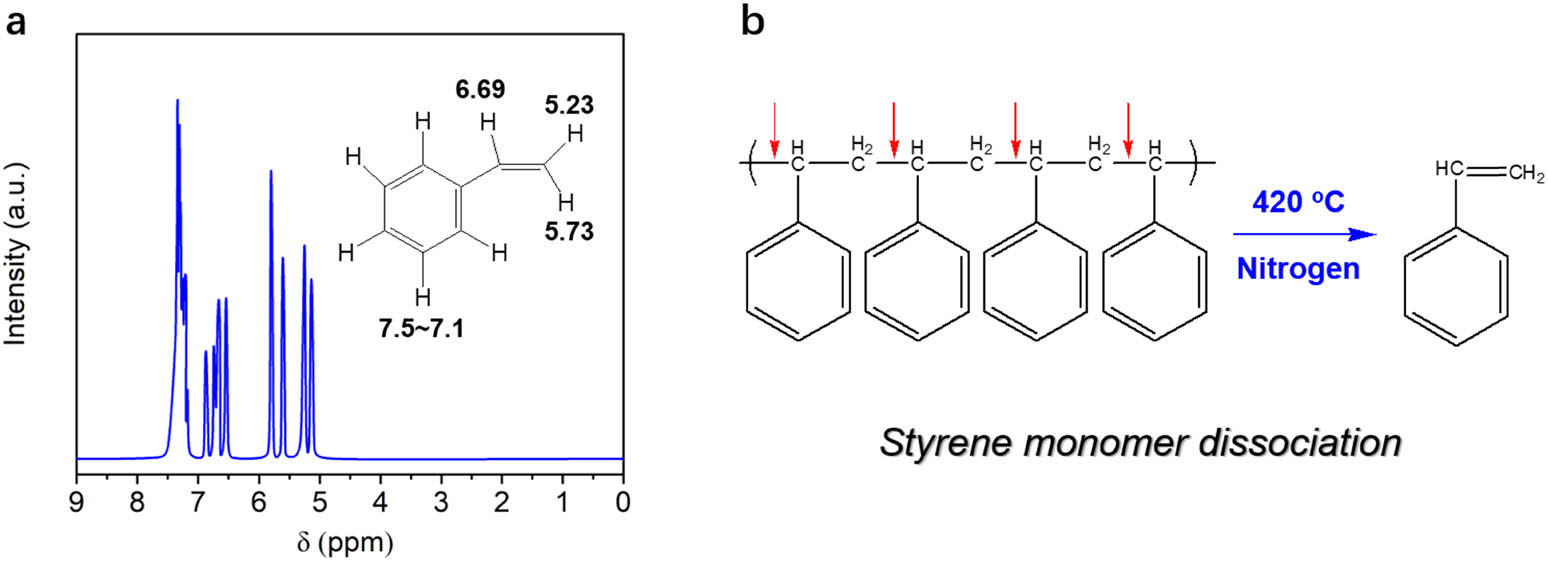
(a) HNMR spectra of the single component in liquid product from pyrolysis of polystyrene. (b) Styrene monomer dissociation mechanism of polystyrene pyrolysis.
4 Conclusion
In this study, simple pyrolysis has been reported as an effective method for chemical recycling of polystyrene, which is a common polymer in human society. The optimal degradation temperature was investigated to be 420°C through TGA analysis. The pyrolysis products mainly consisted of three phases, including gas (10.75%), liquid (76.24%), and solid (13.01%) ones. We found that there existed single component (73%) in liquid product from polystyrene pyrolysis under nitrogen atmosphere, which was obtained from the analysis result of GC spectra. It is clarified that the single component is styrene monomer identified from HNMR spectra of liquid product. Furthermore, we proposed a dissociation mechanism for explanation of polystyrene degradation at inert atmosphere under high temperature. The polystyrene chains prefer to degrade into monomers during pyrolysis processes owing to stereo-hindrance effect of benzene rings in the structure. The general research strategy is believed to be extended to other polymers, such as polyethylene, polydimethylsiloxane, and polyvinyl alcohol. We hope this study will shed light on chemical recycling of waste polymers.
-
Funding information: This work was supported by the Earth Engineering Center and Center for Advanced Materials for Energy and Environment at Columbia University.
-
Author contributions: Chao Lu: writing – original draft, methodology, formal analysis; Hang Xiao: formal analysis, visualization; Xi Chen: writing – review and editing, project administration, resources.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
(1) Gong J, Chen X, Tang T. Recent progress in controlled carbonization of (waste) polymers. Prog Polym Sci. 2019;94:1–32. 10.1016/j.progpolymsci.2019.04.001.Search in Google Scholar
(2) Okan M, Aydin HM, Barsbay M. Current approaches to waste polymer utilization and minimization: a review. J Chem Tech Biotech. 2019;94(1):8–21. 10.1002/jctb.5778.Search in Google Scholar
(3) Shen M, Cao H, Robertson ML. Hydrolysis and solvolysis as benign routes for the end-of-life management of thermoset polymer waste. Annu Rev Chem Biomol. 2020;11(1):183–201. 10.1146/annurev-chembioeng-120919-012253.Search in Google Scholar PubMed
(4) Saleem J, Adil Riaz M, Gordon M. Oil sorbents from plastic wastes and polymers: a review. J Hazard Mater. 2018;341:424–37. 10.1016/j.jhazmat.2017.07.072.Search in Google Scholar PubMed
(5) Singh N, Singh R, Ahuja IPS. Recycling of polymer waste with SiC/Al2O3 reinforcement for rapid tooling applications. Mater Tod Commun. 2018;15:124–7. 10.1016/j.mtcomm.2018.02.008.Search in Google Scholar
(6) Schmaltz E, Melvin EC, Diana Z, Gunady EF, Rittschof D, Somarelli JA, et al. Plastic pollution solutions: emerging technologies to prevent and collectmarineplastic pollution. Environ Int. 2020;144:106067. 10.1016/j.envint.2020.106067.Search in Google Scholar PubMed
(7) Agarwal V, Halli P, Helin S, Tesfaye F, Lundström M. Electrohydraulic fragmentation of aluminum and polymer fractions from waste pharmaceutical blisters. ACS Sustain Chem Eng. 2020;8(10):4137–45. 10.1021/acssuschemeng.9b06810.Search in Google Scholar
(8) Mumbach GD, Bolzan A, Machado RAF. A closed-loop process design for recycling expanded polystyrene waste by dissolution and polymerization. Polymer. 2020;209:122940. 10.1016/j.polymer.2020.122940.Search in Google Scholar
(9) Lloyd EM, Lopez Hernandez H, Feinberg EC, Yourdkhani M, Zen EK, Mejia EB, et al. Fully recyclable metastable polymers and composites. Chem Mater. 2019;31(2):398–406. 10.1021/acs.chemmater.8b03585.Search in Google Scholar
(10) Valerio O, Muthuraj R, Codou A. Strategies for polymer to polymer recycling from waste: current trends and opportunities for improving the circular economy of polymers in South America. Curr Opin Chem Eng. 2020;25:100381. 10.1016/j.cogsc.2020.100381.Search in Google Scholar
(11) Datta J, Kopczyńska P. From polymer waste to potential main industrial products: actual state of recycling and recovering. Crit Rev Env Sci Tec. 2016;46(10):905–46. 10.1080/10643389.2016.1180227.Search in Google Scholar
(12) Farinha CB, de Brito J, Veiga R. Assessment of glass fibre reinforced polymer waste reuse as filler in mortars. J Clean Prod. 2019;210:1579–94. 10.1016/j.jclepro.2018.11.080.Search in Google Scholar
(13) Lu C, Chen X. All-temperature flexible supercapacitors enabled by antifreezing and thermally stable hydrogel electrolyte. Nano Lett. 2020;20(3):1907–14. 10.1021/acs.nanolett.9b05148.Search in Google Scholar PubMed
(14) Hatti-Kaul R, Nilsson LJ, Zhang B, Rehnberg N, Lundmark S. Designing biobased recyclable polymers for plastics. Trends Biotechnol. 2020;38(1):50–67. 10.1016/j.tibtech.2019.04.011.Search in Google Scholar PubMed
(15) Zhang K, Monteiro MJ, Jia Z. Stable organic radical polymers: synthesis and applications. Poly Chem. 2016;7(36):5589–614. 10.1039/C6PY00996D.Search in Google Scholar
(16) Lu C, Chen X. In situ synthesized PEO/NBR composite ionogels for high-performance all-solid-state supercapacitors. Chem Commun. 2019;55(58):8470–3. 10.1039/c9cc03401c.Search in Google Scholar PubMed
(17) Borsodi N, Szentes A, Miskolczi N, Wu C, Liu X. Carbon nanotubes synthetized from gaseous products of waste polymer pyrolysis and their application. J Anal Appl Pyrolysis. 2016;120:304–13. 10.1016/j.jaap.2016.05.018.Search in Google Scholar
(18) Skvorčinskienė R, Striūgas N, Navakas R, Paulauskas R, Zakarauskas K, Vorotinskienė L. Thermal analysis of waste fishing nets for polymer recovery. Waste Biomass Valori. 2019;10(12):3735–44. 10.1007/s12649-019-00803-w.Search in Google Scholar
(19) Horvat N, Ng FT. Tertiary polymer recycling: study of polyethylene thermolysis as a first step to synthetic diesel fuel. Fuel. 1999;78(4):459–70. 10.1016/S0016-2361(98)00158-6.Search in Google Scholar
(20) Gringolts ML, Dement’ev KI, Kadiev KM, Maksimov AL, Finkel’shtein ES. Chemical Conversion of Polymer Wastes into Motor Fuels and Petrochemical Raw Materials (A Review). Petrol Chem. 2020;60(7):751–61. 10.1134/S0965544120070051.Search in Google Scholar
(21) Ho BT, Roberts TK, Lucas S. An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach. Crit Rev Biotechnol. 2018;38(2):308–20. 10.1080/07388551.2017.1355293.Search in Google Scholar PubMed
(22) Lu C, Wang D, Zhao J, Han S, Chen W. A continuous carbon nitride polyhedron assembly for high-performance flexible supercapacitors. Adv Funct Mater. 2017;27(8):1606219.10.1002/adfm.201606219Search in Google Scholar
(23) Anca-Couce A, Tsekos C, Retschitzegger S, Zimbardi F, Funke A, Banks S, et al. Biomass pyrolysis TGA assessment with an international round robin. Fuel. 2020;276:118002. 10.1016/j.fuel.2020.118002.Search in Google Scholar
(24) Nolvachai Y, Kulsing C, Marriott PJ. Multidimensional gas chromatography in food analysis. Trends Anal Chem. 2017;96:124–37. 10.1016/j.trac.2017.05.001.Search in Google Scholar
(25) Venkatasubramanian A, Sauer VTK, Roy SK, Xia M, Wishart DS, Hiebert WK. Nano-optomechanical systems for gas chromatography. Nano Lett. 2016;16(11):6975–81. 10.1021/acs.nanolett.6b03066.Search in Google Scholar PubMed
© 2021 Chao Lu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Research on the mechanism of gel accelerator on gel transition of PAN solution by rheology and dynamic light scattering
- Gel point determination of gellan biopolymer gel from DC electrical conductivity
- Composite of polylactic acid and microcellulose from kombucha membranes
- Synthesis of highly branched water-soluble polyester and its surface sizing agent strengthening mechanism
- Fabrication and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) modified with nano-montmorillonite biocomposite
- Fabrication of N-halamine polyurethane films with excellent antibacterial properties
- Formulation and optimization of gastroretentive bilayer tablets of calcium carbonate using D-optimal mixture design
- Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging
- Evaluation of physicochemical properties of film-based alginate for food packing applications
- Electrically conductive and light-weight branched polylactic acid-based carbon nanotube foams
- Structuring of hydroxy-terminated polydimethylsiloxane filled by fumed silica
- Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering
- Influence of composite structure design on the ablation performance of ethylene propylene diene monomer composites
- MOFs/PVA hybrid membranes with enhanced mechanical and ion-conductive properties
- Improvement of the electromechanical properties of thermoplastic polyurethane composite by ionic liquid modified multiwall carbon nanotubes
- Natural rubber latex/MXene foam with robust and multifunctional properties
- Rheological properties of two high polymers suspended in an abrasive slurry jet
- Two-step polyaniline loading in polyelectrolyte complex membranes for improved pseudo-capacitor electrodes
- Preparation and application of carbon and hollow TiO2 microspheres by microwave heating at a low temperature
- Properties of a bovine collagen type I membrane for guided bone regeneration applications
- Fabrication and characterization of thermoresponsive composite carriers: PNIPAAm-grafted glass spheres
- Effect of talc and diatomite on compatible, morphological, and mechanical behavior of PLA/PBAT blends
- Multifunctional graphene nanofiller in flame retarded polybutadiene/chloroprene/carbon black composites
- Strain-dependent wicking behavior of cotton/lycra elastic woven fabric for sportswear
- Enhanced dielectric properties and breakdown strength of polymer/carbon nanotube composites by coating an SrTiO3 layer
- Analysis of effect of modification of silica and carbon black co-filled rubber composite on mechanical properties
- Polytriazole resins toughened by an azide-terminated polyhedral oligomeric silsesquioxane (OADTP)
- Phosphine oxide for reducing flammability of ethylene-vinyl-acetate copolymer
- Study on preparation and properties of bentonite-modified epoxy sheet molding compound
- Polyhedral oligomeric silsesquioxane (POSS)-modified phenolic resin: Synthesis and anti-oxidation properties
- Study on structure and properties of natural indigo spun-dyed viscose fiber
- Biodegradable thermoplastic copolyester elastomers: Methyl branched PBAmT
- Investigations of polyethylene of raised temperature resistance service performance using autoclave test under sour medium conditions
- Investigation of corrosion and thermal behavior of PU–PDMS-coated AISI 316L
- Modification of sodium bicarbonate and its effect on foaming behavior of polypropylene
- Effect of coupling agents on the olive pomace-filled polypropylene composite
- High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide
- Removal of methylene blue in water by electrospun PAN/β-CD nanofibre membrane
- Theoretical and experimental studies on the fabrication of cylindrical-electrode-assisted solution blowing spinning nanofibers
- Influence of l-quebrachitol on the properties of centrifuged natural rubber
- Ultrasonic-modified montmorillonite uniting ethylene glycol diglycidyl ether to reinforce protein-based composite films
- Experimental study on the dissolution of supercritical CO2 in PS under different agitators
- Experimental research on the performance of the thermal-reflective coatings with liquid silicone rubber for pavement applications
- Study on controlling nicotine release from snus by the SIPN membranes
- Catalase biosensor based on the PAni/cMWCNT support for peroxide sensing
- Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol
- Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid
- Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride
- Polypropylene/graphene nanoplatelets nanocomposites with high conductivity via solid-state shear mixing
- Mechanical properties of fiber-reinforced asphalt concrete: Finite element simulation and experimental study
- Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery
- Preparation and characterizations of antibacterial–antioxidant film from soy protein isolate incorporated with mangosteen peel extract
- Preparation and adsorption properties of Ni(ii) ion-imprinted polymers based on synthesized novel functional monomer
- Rare-earth doped radioluminescent hydrogel as a potential phantom material for 3D gel dosimeter
- Effects of cryogenic treatment and interface modifications of basalt fibre on the mechanical properties of hybrid fibre-reinforced composites
- Stable super-hydrophobic and comfort PDMS-coated polyester fabric
- Impact of a nanomixture of carbon black and clay on the mechanical properties of a series of irradiated natural rubber/butyl rubber blend
- Preparation and characterization of a novel composite membrane of natural silk fiber/nano-hydroxyapatite/chitosan for guided bone tissue regeneration
- Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride
- A new method for plugging the dominant seepage channel after polymer flooding and its mechanism: Fracturing–seepage–plugging
- Analysis of the rheological property and crystallization behavior of polylactic acid (Ingeo™ Biopolymer 4032D) at different process temperatures
- Hybrid green organic/inorganic filler polypropylene composites: Morphological study and mechanical performance investigations
- In situ polymerization of PEDOT:PSS films based on EMI-TFSI and the analysis of electrochromic performance
- Effect of laser irradiation on morphology and dielectric properties of quartz fiber reinforced epoxy resin composite
- The optimization of Carreau model and rheological behavior of alumina/linear low-density polyethylene composites with different alumina content and diameter
- Properties of polyurethane foam with fourth-generation blowing agent
- Hydrophobicity and corrosion resistance of waterborne fluorinated acrylate/silica nanocomposite coatings
- Investigation on in situ silica dispersed in natural rubber latex matrix combined with spray sputtering technology
- The degradable time evaluation of degradable polymer film in agriculture based on polyethylene film experiments
- Improving mechanical and water vapor barrier properties of the parylene C film by UV-curable polyurethane acrylate coating
- Thermal conductivity of silicone elastomer with a porous alumina continuum
- Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate
- Combining good dispersion with tailored charge trapping in nanodielectrics by hybrid functionalization of silica
- Thermosensitive hydrogel for in situ-controlled methotrexate delivery
- Analysis of the aging mechanism and life evaluation of elastomers in simulated proton exchange membrane fuel cell environments
- The crystallization and mechanical properties of poly(4-methyl-1-pentene) hard elastic film with different melt draw ratios
- Review Articles
- Aromatic polyamide nonporous membranes for gas separation application
- Optical elements from 3D printed polymers
- Evidence for bicomponent fibers: A review
- Mapping the scientific research on the ionizing radiation impacts on polymers (1975–2019)
- Recent advances in compatibility and toughness of poly(lactic acid)/poly(butylene succinate) blends
- Topical Issue: (Micro)plastics pollution - Knowns and unknows (Guest Editor: João Pinto da Costa)
- Simple pyrolysis of polystyrene into valuable chemicals
- Topical Issue: Recent advances of chitosan- and cellulose-based materials: From production to application (Guest Editor: Marc Delgado-Aguilar)
- In situ photo-crosslinking hydrogel with rapid healing, antibacterial, and hemostatic activities
- A novel CT contrast agent for intestinal-targeted imaging through rectal administration
- Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review
- Towards the use of acrylic acid graft-copolymerized plant biofiber in sustainable fortified composites: Manufacturing and characterization
Articles in the same Issue
- Research Articles
- Research on the mechanism of gel accelerator on gel transition of PAN solution by rheology and dynamic light scattering
- Gel point determination of gellan biopolymer gel from DC electrical conductivity
- Composite of polylactic acid and microcellulose from kombucha membranes
- Synthesis of highly branched water-soluble polyester and its surface sizing agent strengthening mechanism
- Fabrication and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) modified with nano-montmorillonite biocomposite
- Fabrication of N-halamine polyurethane films with excellent antibacterial properties
- Formulation and optimization of gastroretentive bilayer tablets of calcium carbonate using D-optimal mixture design
- Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging
- Evaluation of physicochemical properties of film-based alginate for food packing applications
- Electrically conductive and light-weight branched polylactic acid-based carbon nanotube foams
- Structuring of hydroxy-terminated polydimethylsiloxane filled by fumed silica
- Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering
- Influence of composite structure design on the ablation performance of ethylene propylene diene monomer composites
- MOFs/PVA hybrid membranes with enhanced mechanical and ion-conductive properties
- Improvement of the electromechanical properties of thermoplastic polyurethane composite by ionic liquid modified multiwall carbon nanotubes
- Natural rubber latex/MXene foam with robust and multifunctional properties
- Rheological properties of two high polymers suspended in an abrasive slurry jet
- Two-step polyaniline loading in polyelectrolyte complex membranes for improved pseudo-capacitor electrodes
- Preparation and application of carbon and hollow TiO2 microspheres by microwave heating at a low temperature
- Properties of a bovine collagen type I membrane for guided bone regeneration applications
- Fabrication and characterization of thermoresponsive composite carriers: PNIPAAm-grafted glass spheres
- Effect of talc and diatomite on compatible, morphological, and mechanical behavior of PLA/PBAT blends
- Multifunctional graphene nanofiller in flame retarded polybutadiene/chloroprene/carbon black composites
- Strain-dependent wicking behavior of cotton/lycra elastic woven fabric for sportswear
- Enhanced dielectric properties and breakdown strength of polymer/carbon nanotube composites by coating an SrTiO3 layer
- Analysis of effect of modification of silica and carbon black co-filled rubber composite on mechanical properties
- Polytriazole resins toughened by an azide-terminated polyhedral oligomeric silsesquioxane (OADTP)
- Phosphine oxide for reducing flammability of ethylene-vinyl-acetate copolymer
- Study on preparation and properties of bentonite-modified epoxy sheet molding compound
- Polyhedral oligomeric silsesquioxane (POSS)-modified phenolic resin: Synthesis and anti-oxidation properties
- Study on structure and properties of natural indigo spun-dyed viscose fiber
- Biodegradable thermoplastic copolyester elastomers: Methyl branched PBAmT
- Investigations of polyethylene of raised temperature resistance service performance using autoclave test under sour medium conditions
- Investigation of corrosion and thermal behavior of PU–PDMS-coated AISI 316L
- Modification of sodium bicarbonate and its effect on foaming behavior of polypropylene
- Effect of coupling agents on the olive pomace-filled polypropylene composite
- High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide
- Removal of methylene blue in water by electrospun PAN/β-CD nanofibre membrane
- Theoretical and experimental studies on the fabrication of cylindrical-electrode-assisted solution blowing spinning nanofibers
- Influence of l-quebrachitol on the properties of centrifuged natural rubber
- Ultrasonic-modified montmorillonite uniting ethylene glycol diglycidyl ether to reinforce protein-based composite films
- Experimental study on the dissolution of supercritical CO2 in PS under different agitators
- Experimental research on the performance of the thermal-reflective coatings with liquid silicone rubber for pavement applications
- Study on controlling nicotine release from snus by the SIPN membranes
- Catalase biosensor based on the PAni/cMWCNT support for peroxide sensing
- Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol
- Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid
- Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride
- Polypropylene/graphene nanoplatelets nanocomposites with high conductivity via solid-state shear mixing
- Mechanical properties of fiber-reinforced asphalt concrete: Finite element simulation and experimental study
- Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery
- Preparation and characterizations of antibacterial–antioxidant film from soy protein isolate incorporated with mangosteen peel extract
- Preparation and adsorption properties of Ni(ii) ion-imprinted polymers based on synthesized novel functional monomer
- Rare-earth doped radioluminescent hydrogel as a potential phantom material for 3D gel dosimeter
- Effects of cryogenic treatment and interface modifications of basalt fibre on the mechanical properties of hybrid fibre-reinforced composites
- Stable super-hydrophobic and comfort PDMS-coated polyester fabric
- Impact of a nanomixture of carbon black and clay on the mechanical properties of a series of irradiated natural rubber/butyl rubber blend
- Preparation and characterization of a novel composite membrane of natural silk fiber/nano-hydroxyapatite/chitosan for guided bone tissue regeneration
- Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride
- A new method for plugging the dominant seepage channel after polymer flooding and its mechanism: Fracturing–seepage–plugging
- Analysis of the rheological property and crystallization behavior of polylactic acid (Ingeo™ Biopolymer 4032D) at different process temperatures
- Hybrid green organic/inorganic filler polypropylene composites: Morphological study and mechanical performance investigations
- In situ polymerization of PEDOT:PSS films based on EMI-TFSI and the analysis of electrochromic performance
- Effect of laser irradiation on morphology and dielectric properties of quartz fiber reinforced epoxy resin composite
- The optimization of Carreau model and rheological behavior of alumina/linear low-density polyethylene composites with different alumina content and diameter
- Properties of polyurethane foam with fourth-generation blowing agent
- Hydrophobicity and corrosion resistance of waterborne fluorinated acrylate/silica nanocomposite coatings
- Investigation on in situ silica dispersed in natural rubber latex matrix combined with spray sputtering technology
- The degradable time evaluation of degradable polymer film in agriculture based on polyethylene film experiments
- Improving mechanical and water vapor barrier properties of the parylene C film by UV-curable polyurethane acrylate coating
- Thermal conductivity of silicone elastomer with a porous alumina continuum
- Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate
- Combining good dispersion with tailored charge trapping in nanodielectrics by hybrid functionalization of silica
- Thermosensitive hydrogel for in situ-controlled methotrexate delivery
- Analysis of the aging mechanism and life evaluation of elastomers in simulated proton exchange membrane fuel cell environments
- The crystallization and mechanical properties of poly(4-methyl-1-pentene) hard elastic film with different melt draw ratios
- Review Articles
- Aromatic polyamide nonporous membranes for gas separation application
- Optical elements from 3D printed polymers
- Evidence for bicomponent fibers: A review
- Mapping the scientific research on the ionizing radiation impacts on polymers (1975–2019)
- Recent advances in compatibility and toughness of poly(lactic acid)/poly(butylene succinate) blends
- Topical Issue: (Micro)plastics pollution - Knowns and unknows (Guest Editor: João Pinto da Costa)
- Simple pyrolysis of polystyrene into valuable chemicals
- Topical Issue: Recent advances of chitosan- and cellulose-based materials: From production to application (Guest Editor: Marc Delgado-Aguilar)
- In situ photo-crosslinking hydrogel with rapid healing, antibacterial, and hemostatic activities
- A novel CT contrast agent for intestinal-targeted imaging through rectal administration
- Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review
- Towards the use of acrylic acid graft-copolymerized plant biofiber in sustainable fortified composites: Manufacturing and characterization

