Abstract
The research on facile, low-cost, and green process for the uniform dispersion of graphene nanoplatelets (GNPs) into polymer matrix has always been a considerable challenge in practical applications. The Van der Waals interaction between graphene layers can easily cause aggregation of the nanofillers. Here, we propose a new method to solve this problem by involving solid-state shear mixing to obtain a well-dispersed nanocomposite. The comprehensive properties of nanocomposite, including antistatic properties, mechanical properties, and thermal stability, can be significantly enhanced by this method. The surface resistivity of the nanocomposite can be up to 2.4 × 107 Ω sq−1 under 1 wt% content of GNPs, which is significantly better than the value obtained by conventional melting compounding and meets the required standard of less than 3 × 108 Ω sq−1 for actual application antistatic materials. The impact strength of the nanocomposite increased by 120.8% when compared with neat PP. At the same time, the heat distortion temperature and initial decomposition temperature of the nanocomposite with only 0.5 wt% content of GNPs are improved by 11.7°C and 110°C, respectively. In addition, GNPs is a heterogeneous nucleating agent that leads PP to emerge β crystal form. This study provides an effective and practical reference for the broad-scale industrial preparation of polymer-based graphene nanocomposites.
Graphical abstract
Polypropylene/GNP nanocomposites with enhanced antistatic performance, impact strength, and thermostability via solid-state shear mixing processed.

1 Introduction
Graphene is a two-dimensional material with single-atom thickness connected by sp2-hybridized carbon atoms (1,2). Owing to the extremely high specific surface area, carrier mobility, thermal conductivity, and excellent mechanical strength, it is considered to be an ideal nanofiller for strengthening polymer and has also received considerable attention in the field of nanocomposites (3,4,5,6,7,8,9,10,11). However, same as other added nanofillers, the dispersion of graphene nanoplatelets (GNPs) is the main problem in the preparation of composite materials (12,13,14,15), and this directly affects the performance of composites.
The dispersion of GNPs is primarily determined by the preparation method of the composites (15,16,17,18). The common methods mainly include in situ polymerization (17), solution compounding (16,18), and melt compounding (15). In situ polymerization is the best preparation method with homogeneous dispersion at present, but the polymerization reaction is easily affected by the reaction conditions and side reactions (17), so it is difficult to control. The solution compounding requires the use of large amounts of organic solvents during the processing, which is not suitable for environmental protection and industrial production. As for melt compounding, GNP is mainly dispersed in the PP melt by mechanical equipment, it is an economical, environment-friendly, efficient method and is most suitable for industrialized manufacture (13,14,19). However, due to the inevitable agglomeration phenomenon of GNP in the processing, it cannot achieve uniform dispersion. Therefore, avoiding GNP agglomeration, dispersing it in the polymer matrix effectively, and reducing the use of organic solvents remain a major challenge in achieving green industrial preparation.
When the matrix material is a non-polar polymer such as polyolefin, it is also necessary to consider the adverse effects of the poor interface compatibility between graphene and the matrix on the uniform dispersion of GNPs in the matrix (13,14,20). Polypropylene (PP) is one of the most common polyolefins, which has been widely used due to its low cost, easy processing and molding, and balanced mechanical properties (8,21,22,23,24). To promote the dispersion of GNPs in PP matrix, adding compatibilizer or functional modification of graphene in the preparation process are two commonly used methods. For example, using PP-g-MA (25) as a compatibilizer has been found to improve the compatibility between GNP and the PP matrix, to promote the dispersion of graphene in the PP matrix. In addition, surface functionalization of graphene or its derivatives is also an alternative method to improve its dispersion in the matrix. Graphene oxide (GO), a class of graphene derivative, is rich in functional groups, which can be used for modification. It has been reported to improve the interaction between GO and PP matrix by grafting isocyanates (18), silane coupling agents (14), and polymer chains (13) at the functional sites to prevent agglomeration and effectively promote dispersion. Nevertheless, the volatilization and post-treatment of a large number of organic solvents used in the graphene modification process are likely to cause safety and environmental problems, and the cost is also relatively high. Not only that, the conductivity and mechanical properties of graphene after oxidation and reduction will be also lost to varying degrees (26). Therefore, it is significant to explore a green, simple, and low-cost method for preparing PP/GNP nanocomposites. It is reported that the mechanical shearing force provided by solid-state shear pulverization (SSSP) can overcome the above-mentioned problems and achieve uniform dispersion of nano-fillers such as lignocellulose nanofibers (27) and carbon nanotubes (28) in the PP matrix. Therefore, solid-state shear mixing may be a practical means for dispersing GNPs in the PP matrix.
In this work, a simple and environmental-friendly strategy of solid-state shear mixing was adopted to prepare PP/GNP nanocomposites with uniform dispersion. At the same time, the antistatic properties, mechanical properties, and thermal stability of the nanocomposites have also been improved to a certain extent. Besides, the relationship between the structure and performance of the nanocomposites and their related mechanisms were also discussed. This research will provide an effective and convenient way for large-scale industrial polymer-based graphene nanocomposites production.
2 Experimental
2.1 Materials
PP powder (PP-H, GD, 040) with melt flow rate (MFR) of 4 ± 2.0 g/10 min was purchased from Maoming Petrochemical Co., Ltd. (China). N-Methyl-2-pyrrolidone (NMP) was purchased from J & K Chemical Co., Ltd. (China). Graphene nanoplatelets with an oxygen content of 1 wt% were purchased from Qinhuangdao Zhongke Hanqi Technology Co., Ltd. (China). All chemicals were used directly without purification.
2.2 Synthesis method of PP/GNP nanocomposites
Nanocomposites with different GNP contents were prepared. First, the PP powder, GNP, and a small amount of NMP were manually mixed together by stirring. The blend was further mixed with torque rheometer (Haake polylab OS) at 90°C with a torque of 15–20 N m for 1 h to achieve uniform dispersion of GNPs under the shearing force (Scheme 1). The sheared product was then dried in a vacuum oven at 50°C for 12 h to remove NMP thoroughly. A total of six samples were prepared and named as shown in Table 1.

Schematic illustration of preparation of PP/GNP nanocomposites.
Name and composition of samples
| Sample name | PP (g) | GNP (g) | NMP (g) | Content of GNP (wt%) |
|---|---|---|---|---|
| PP | 100 | 0.0 | 0.0 | 0.0 |
| PP/G0.5 | 99.5 | 0.5 | 10.0 | 0.5 |
| PP/G1.0 | 99.0 | 1.0 | 10.0 | 1.0 |
| PP/G1.5 | 98.5 | 1.5 | 10.0 | 1.5 |
| PP/G2.5 | 97.5 | 2.5 | 10.0 | 2.5 |
| PP/G3.5 | 96.5 | 3.5 | 10.0 | 3.5 |
*Note: G is short for GNP.
To further disperse the GNPs in the PP matrix to facilitate storage and transportation, the above mixture was melt-mixed in a twin-screw extruder at 195°C and a screw speed of 90 rpm. Dumbbell-shaped test samples were injection molded with injection molding machine (Haake MiniJet Pro) at 205°C with a pressure of 70 MPa maintained for 15 s. The square plate samples (100 × 100 × 2 mm) were prepared by the hot press at 175°C and 15 MPa for 30 min.
2.3 Characterization
Section-cross morphology of the nanocomposites and surface morphology of GNPs were observed by scanning electron microscope SEM (S-4800, Hitachi) at 10 kV acceleration voltage. The samples were broken brittlely in liquid nitrogen and subjected to gold sputtering treatment.
The dimensions of pure GNP and its products after solid shear mixing were analyzed by AFM (Multimode 8, Bruker) in which the samples (0.005 wt% suspensions of ethanol) were deposited on neat mica to dry.
The crystal structure was analyzed by X-ray diffractometer XRD (D8 focus, Bruker) with a Cu Kα radiation source (λ = 0.154 nm), and the 2θ scan range was from 5° to 80° with the scanning speed of 0.1 s/step.
Differential scanning calorimetric (DSC) analysis was done by NETZSCH 200-F3 under N2 atmosphere. Samples were heated from 30°C to 200°C and held at that temperature for 2 min to eliminate the thermal history. Then, the temperature of each sample was reduced to 30°C and kept constant for 2 min. The samples were later re-heated to 200°C while recording the cooling curve and the reheating curve. The mass of each sample was about 10 mg, and the heating and cooling rates were 10°C/min in all situations (16).
The electrical resistivity was measured by a megohmmeter (Model 800, ACL) at 25°C. Each sample was measured five times at different positions, and the measurement results were averaged.
Tensile test was carried out according to GB/T 1040-2006 with universal mechanics tester (5966, Instron) at a crosshead moving speed of 25 mm/min. Five specimens of each group were tested in parallel to obtain the mean value. The impact strength of samples (type V notch and 80 × 10 × 4 mm) was carried out according to ISO 179-1:2000 with a material testing machine (XJ-300). Five specimens of each group were tested in parallel to obtain the mean value.
Heat distortion temperature (HDT) was observed by heat distortion tester (HDV/V-3116, JJ-Test) according to GB/T1634.2-2004. Each sample was heated from 20°C at a rate of 2°C/min until the test specimen deflected 0.34 mm. The applied stress of the sample was 0.45 MPa, and the test was carried out in silicone oil. Three specimens of each group were tested in parallel to obtain the mean value.
Thermogravimetry of samples was examined by TGA (Q 50) under N2 atmosphere. The 3–5 mg sample used was heated at 10°C/min from 30°C to 700°C (13).
3 Results and discussion
3.1 Morphology of PP/GNP nanocomposites
The dispersion of nanofillers in the matrix affects almost all properties of composites. The dispersion efficiency was evaluated by SEM analysis. As shown in Figure 1a, the surface of neat PP is relatively smooth. Original GNPs are stacked on each other in Figure 1b and i of the AFM image; it is found that its radial size and thickness are about 1–2 μm and 35 nm, respectively. As shown in Figure 1c and d, flake-like GNP is well dispersed in the entire PP matrix without aggregation almost at lower content such as 0.5 and 1 wt%. Compared with Figure 1b, it is clear that the shear stress of the solid-state mixing during the preparation is beneficial for the dispersion of GNPs. However, GNPs cannot be sufficiently dispersed in the PP matrix by twin-screw compounding alone (Figure A1 in Appendix). From Figure 1j, it is found that the thickness of GNPs in the product (1.0 wt%) after solid-state shear mixing is about 35 nm, which is roughly the same as the thickness of the original GNPs. EDS was used to further analyze the dispersal of GNPs in the nanocomposites (Figure 1g and h) revealing the distribution of carbon and oxygen. Oxygen is evenly distributed on the entire sample surface, because the oxygen only comes from GNPs (oxygen content is about 1 wt%), which indicates that GNP is dispersed in the composite equally. However, due to the Van der Waals interaction between graphene layers, it is easy to cause agglomeration when GNP content is too high. As shown in Figure 1e, although GNP has good overall dispersibility under a content of 3.5 wt%, it partially agglomerated, weakening the dispersion effect.

SEM images of neat PP (a), GNP (b), PP/G0.5 (c), PP/G1.0 (d), and PP/G3.5 (e). High magnification SEM images of PP/G1.0 (f). EDS mapping of C and O of PP/G1.0 (g and h). AFM images and height profile along the white line of GNP (i) and PP/G1.0 (j).
Since PP is a semi-crystalline polymer, the crystalline behavior of PP including crystal type and crystallinity significantly affects the mechanical properties of the nanocomposites (29). The XRD patterns of PP and PP/GNP nanocomposites of different contents of GNP are shown in Figure 2. For neat PP, the peaks at approximately 14.2°, 17.0°, 18.7°, 21.9°, and 25.6° corresponding to the (110), (040), (130), (041), and (060) crystal plane, respectively, of α crystal forms of PP (19,30). For nanocomposites, a new peak corresponding to the (300) plane of β crystal form is exhibited at about 2θ = 16.2° (19). This indicates that the addition of GNP changes the crystal form of PP, and GNP is a heterogeneous nucleating agent of β crystal form beneficial to enhancing higher impact strength and toughness of nanocomposites (30), and it will be described later.

XRD patterns of neat PP and PP/GNP nanocomposites.
According to the method proposed by Turner-Jones (19,31), the relative content of β crystal form K β * was calculated using Eq. 1:
where I is the intensity of the diffraction peaks of α (110), α (040), α (130), α (041), α (060), and β (300) crystal plane. As listed in Table 2, K β * of the samples increases with the improvement of GNP contents until the GNP content reaches 1.0 wt%. Owing to a small increase of GNP when it has low content can provide more nucleation sites so that induces more β crystal form. When the GNP content is too high, the nucleation is saturated, that is, not enough PP polymer chains can be oriented and arranged along all of the graphene (30), so K β * basically remains unchanged.
The relative content of β crystal form of samples
| Samples | PP | PP/G0.5 | PP/G1.0 | PP/G1.5 | PP/G2.5 | PP/G3.5 |
|---|---|---|---|---|---|---|
| K β * (%) | 0 | 22.3 | 28.2 | 30.2 | 30.3 | 29.5 |
3.2 Crystallization behavior of PP/GNP nanocomposites
DSC was used to explore the effect of the addition of GNP on the crystallization behavior of the PP matrix. Figure 3a and b is the melting curve and non-isothermal crystallization curve, respectively. The DSC test parameters of neat PP and PP/GNP nanocomposites including melting enthalpy ΔH m, crystallization enthalpy ΔH c, melting point T m, crystallization temperature T c, and crystallinity X c are summarized in Table 3. The crystallinity X c was calculated using Eq. 2:
where ΔH 0 is the melting enthalpy of 100% crystalline PP and F PP is the mass fraction of PP in nanocomposites (16).

Melting (a) and crystallization (b) thermograms of neat PP and PP/GNP nanocomposites.
DSC test parameters of neat PP and PP/GNP nanocomposites
| Samples | ΔH c (J/g) | ΔH m (J/g) | T m (°C) | T c (°C) | X c (%) |
|---|---|---|---|---|---|
| PP | −83.51 | 80.17 | 173.2 | 118.6 | 38.4 |
| PP/G0.5 | −80.32 | 81.36 | 175.1 | 128.0 | 39.1 |
| PP/G1.0 | −75.16 | 79.41 | 170.7 | 127.4 | 38.4 |
| PP/G1.5 | −83.80 | 81.05 | 175.5 | 128.9 | 38.6 |
| PP/G2.5 | −75.77 | 74.95 | 170.8 | 127.0 | 36.8 |
| PP/G3.5 | −79.52 | 78.30 | 175.2 | 128.6 | 38.8 |
It can be seen from melting thermogram (Figure 3a) that the addition of GNP has no obvious effect on T m of the nanocomposites when compared with neat PP. From Figure 3b, it is obviously demonstrated that the T c increases with the increase of GNP content, that is, the crystallization of PP starts at a higher temperature. This indicates that the crystallization of PP is facilitated by GNP. This is because the surface of GNP serves as sites for PP crystallization, and GNP acts as a heterogeneous nucleating agent. Although GNP has been shown to promote the crystallinity of PP and induce the formation of uncommon crystals such as β crystal form (Figure 2 and Table 2), it has little impact on the X c of PP (Table 3). This indicates that the change in mechanical properties of nanocomposites is due to the transform of crystal form and the enhancement of nanofiller GNP, rather than by changing the crystallinity of the matrix.
3.3 Antistatic performance of PP/GNP nanocomposites
Surface resistivity is one of the commonly used indicators to characterize the antistatic performance of materials (32). The test results displayed in Figure 4 show that the increase in GNP content results in a downward trend of surface resistivity of the nanocomposites. This indicates that the nanofiller GNP can improve the conductive capacity of nanocomposites. The surface resistivity of the composite was 5.4 × 1011 Ω sq−1 at 0.5 wt% GNP content. The surface resistivity of the nanocomposite can be up to 2.4 × 107 Ω sq−1 under 1 wt% content of GNP, which meets the requirement that is less than 3.0 × 108 Ω sq−1 for practical application of antistatic materials (32,33). However, as shown in Figure A2, the surface resistivity and volume resistivity of the composites prepared by twin-screw compounding under the same GNP content are relatively large (more than 1012), which illustrates the necessity and superiority of the solid-state shear mixing preparation method. Besides, it can be concluded that the conductive percolation threshold is in the range of 1 wt%. This is due to the formation of conductive paths by increasing GNP content in the nanocomposites (34). Through the EDS mapping of Figure 1g and h, it is observed clearly that the oxygen is uniformly dispersed in the PP matrix and touched each other, illustrating that the GNP forms a uniform and continuous conductive paths inside the matrix so that the surface resistivity of the nanocomposites drops sharply. The conductive percolation threshold of the composites prepared by twin-screw compounding is between 1.5 and 2.5 wt% (Figure A2). It is worth noting that the percolation threshold value of 1 wt% obtained by this preparation method can be matched with the value obtained by mixing solutions commonly used in laboratories (35,36). However, this instrument avoids the cost, post-treatment, and environmental pollution caused by a large amount of organic solvent. Hence, it displays a broader industrial application prospect. The conductivity obtained by different methods will be compared later (Table 6).

Surface resistivity (a) and volume resistivity (b) of PP/GNP nanocomposites.
3.4 Mechanical properties of PP/GNP nanocomposites
Mechanical properties are critical to the practical application of antistatic materials (32). The impact strength of nanocomposites with different GNP contents is shown in Figure 5. The impact strength of the nanocomposites reaches a maximum value of 10.6 kJ m−2 at 1.0 wt% content of GNP. The impact strength of the nanocomposite increased by 120.8% when compared with neat PP (4.8 kJ m−2), demonstrating the significant toughening ability of GNP. This is because it acts as a nucleating agent for PP to produce β crystal form with higher impact strength and toughness as seen in the XRD patterns (Figure 2) (30). In addition, owing to the high aspect ratio and high mechanical strength of GNP and its uniform dispersion, it withstands part of the stress and hinders the spread and extension of cracks in the matrix. However, when the content of GNP is higher, it is impossible to avoid aggregation that generates stress concentration and weakens the enhancement effect under the applied stress (14). But until the content is increased to 3.5 wt%, the impact strength of the nanocomposites is still greater than that of neat PP. However, from Figure A3, the impact strength of the composites prepared by two-screw compounding decreases with the increase of GNP content, this is because of its poor dispersion effect, which can lead to defects in GNP. Hence, it shows the necessity and superiority of the preparation method of solid-state shear mixing.
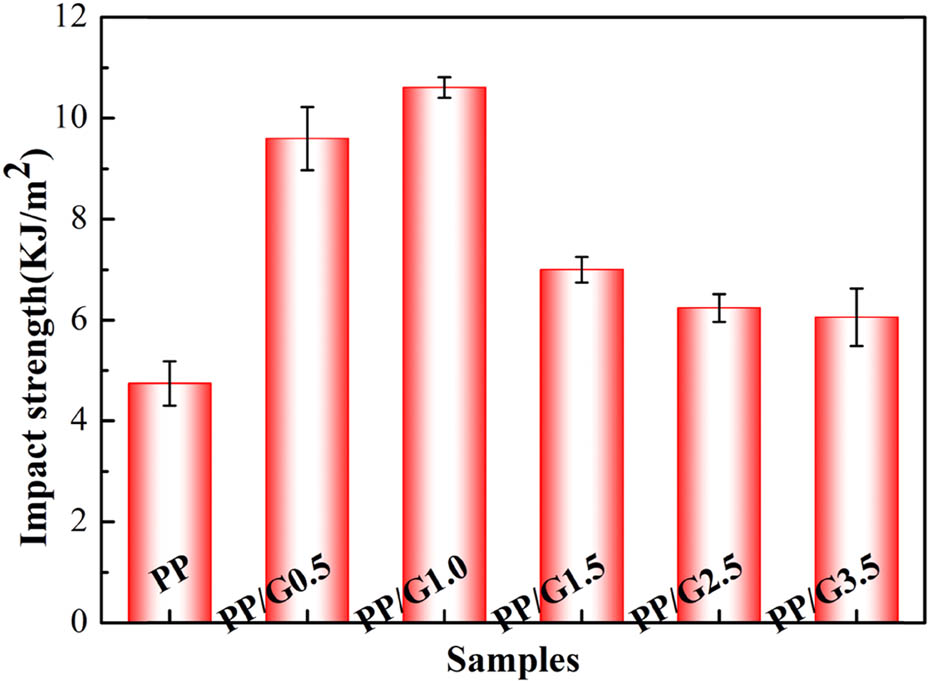
Impact strength of neat PP and PP/GNP nanocomposites.
The tensile performance test results of neat PP and its nanocomposites are listed in Table 4. The rigidity of nanocomposites is more strong than neat PP. Even with GNP content as low as 0.5 wt%, the yield strength and Young’s modulus of the nanocomposite are 37.3 and 1,184 MPa, which is an improvement of 13.7% and 25.3%, respectively, when compared with neat PP. This is due to the high mechanical strength of nanofiller GNP, its uniform dispersion, and powerful interfacial interaction between GNP and PP matrix (13,14,32). Besides, the molecular arrangement of PP changes greatly during the stretching process, especially near the yield point. The move and orientation of polymer chains are hindered by GNP under applied stress (32). The barrier effect of GNP becomes stronger with the increases of GNP content so that the nanocomposites show stronger rigidity. In addition to the above factors, the large decrease of the elongation at break of nanocomposites is also related to the Van der Waals interaction between graphene layers, which is very easy to form aggregation at high levels of GNP content. Combined with the SEM images (Figure 1e), it is found that partial agglomerations are formed by GNP under high content of 3.5 wt%, which brings structural defects to the nanocomposite, resulting in stress concentration so that elongation at break reduces to 29.9% (29). Nevertheless, as shown in Table A2, the yield strength and Young’s modulus of the PP/GNP composites prepared by two-screw compounding cannot be effectively enhanced. This is due to its poor dispersion effect, which is also proved by the SEM images (Figure A1).
Tensile mechanical properties of neat PP and PP/GNP nanocomposites
| Samples | Yield strength (MPa) | Young’s modulus (MPa) | Elongation at break (%) |
|---|---|---|---|
| PP | 32.8 ± 0.9 | 945.3 ± 52.5 | 1004.5 ± 33.7 |
| PP/G0.5 | 37.3 ± 0.4 | 1184.3 ± 11.0 | 534.8 ± 21.4 |
| PP/G1.0 | 39.1 ± 0.1 | 1207.2 ± 16.4 | 554.0 ± 10.1 |
| PP/G1.5 | 41.6 ± 0.4 | 1432.2 ± 41.3 | 145.1 ± 30.2 |
| PP/G2.5 | 42.1 ± 0.7 | 1618.8 ± 52.7 | 153.5 ± 27.7 |
| PP/G3.5 | 42.6 ± 0.3 | 1669.9 ± 24.2 | 29.9 ± 0.5 |
3.5 Thermal stability of PP/GNP nanocomposites
The heat distortion temperature (HDT) represents the maximum temperature that a material can withstand under normal mechanical behavior (13). As shown in Figure 6, the HDT of nanocomposites improves with the increase of GNP content. For the content as low as 0.5 wt%, the HDT of the nanocomposites is 95.9°C, which is a significant increase of 11.7°C when compared with 84.2°C of neat PP. This proves that a small amount of GNP can extend the temperature range of the nanocomposites, and it is a benefit to actual application value. This is due to many positive factors, such as the inherent high rigidity of GNP, its uniform dispersion, and the strong interfacial interaction between GNP and PP matrix (37).

Heat distortion temperature of neat PP and PP/GNP nanocomposites.
Nanocomposites also need steady thermal stability to cope with different usage scenarios. Figure 7a and b is the TGA curve and the differential thermogravimetric (DTG) curve of samples. Through Figure 7a, it is clearly found that the thermal stability of nanocomposites is better than that of neat PP, and thermal stability of the nanocomposites increased with an increase of GNP load. The initial decomposition temperature T in (the temperature when the sample mass loss is 5%), the maximum weight loss rate temperature T max, the maximum weight loss rate MLRmax, and the decomposition temperature range ΔT dr are summarized in Table 5. For the content as low as 0.5 wt%, the T in and T max of the composite are 426°C and 458°C, which are 110°C and 36°C higher than that of neat PP. It proves that a small amount of GNP improves the thermal stability of nanocomposites. The improvement of the thermal stability is mainly caused by several positive factors of nanofiller GNP. It leads to a tortuous mass transfer barrier preventing the evaporation of volatile degradation products (11). In the meantime, its huge surface area can absorb free radicals generated by the thermal decomposition of PP (38). ΔT dr is narrowed by increasing the GNP content. This may be due to the high thermal conductivity of GNP and the strong interfacial interaction between it and matrix (13).

TGA (a) and DTG (b) curves of neat PP and PP/GNP nanocomposites under N2 atmosphere.
TGA test parameters of neat PP and PP/GNP nanocomposites
| Samples | T in (°C) | T max (°C) | MLRmax (%/°C) | ΔT dr (°C) |
|---|---|---|---|---|
| PP | 316 | 422 | 0.02 | 222 |
| PP/G0.5 | 426 | 458 | 0.03 | 118 |
| PP/G1.0 | 430 | 460 | 0.04 | 89 |
| PP/G1.5 | 438 | 461 | 0.04 | 100 |
| PP/G2.5 | 438 | 461 | 0.04 | 90 |
| PP/G3.5 | 439 | 465 | 0.03 | 90 |
Finally, we compared the preparation processing with other methods (Table 6). Compared with melt compounding, the electrical conductivity of nanocomposites prepared by solid shear mixing with the same GNP addition (1.0 wt%) is obviously superior (14,35,39,41). And the electrical conductivity acquired by this preparation method is comparable to that obtained by in situ polymerization and solution mixing, which are recognized by researchers as a good dispersive preparation method of graphene (17,40). But compared with both of them, our preparation method has the advantages of high production efficiency and environmental friendliness. In addition, the improved efficiency of this preparation method on impact strength, yield strength, and Young’s modulus is higher than that of other methods, showing excellent comprehensive enhancement effect. These are all due to the admirable dispersion of GNP caused by the shear stress of solid-state mixing during the preparation process.
Properties of PP/GNP nanocomposites by different processing
| Processing | Contents of GNP (wt%) | The characteristics of processing | σ (S/m) | Modulus increase (%) | Yield strength increase (%) | Impact strength increase (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Melt compounding | 1 | Poor dispersion effect | 10−8 | 13.3 | 9.3 | N/A | (39) |
| In situ polymerization | 0.96 | Complex production equipment and low efficiency | 10−8 | N/A | N/A | N/A | (17) |
| Solution compounding | 1 | Need a lot of organic solvents | 10−7 | N/A | N/A | N/A | (40) |
| Melt compounding | 1 | Poor dispersion effect | 10−20 | N/A | N/A | N/A | (35) |
| Melt compounding | 1 | Poor dispersion effect | 10−13 | N/A | N/A | N/A | (41) |
| Melt compounding | 1 | Poor dispersion effect | 10−13 | 19.3 | 10.6 | N/A | (42) |
| Melt compounding | 1 | Poor dispersion effect | N/A | N/A | 12.5 | 33.3 | (14) |
| Melt compounding | 2.17 | Poor dispersion effect | N/A | N/A | N/A | 16.7 | (29) |
| Melt compounding | 1 | Poor dispersion effect | N/A | 22.8 | 7.2 | N/A | (43) |
| solid-state shear pulverization | 0.81 | Complex production equipment | 10−10 | 62.6 | 48.1 | −51.6 | (44) |
| Our work | 1 | Uniform and high efficiency | 10 −8 | 19.2 | 27.7 | 120.8 |
4 Conclusion
An industrially applicable preparation method for PP/GNP nanocomposites has been developed. The characterization results showed that GNP was uniform dispersed in the PP/GNP nanocomposites. Additionally, the antistatic and mechanical properties of the nanocomposites were improved significantly. Just adding 1.0 wt% of GNP, it made the surface resistivity of the nanocomposite was 2.4 × 107 Ω sq−1, which met the required standard of less than 3.0 × 108 Ω sq−1 for actual application antistatic materials and much better than the value obtained by common conventional melting compounding. Besides, compared with solution compounding and in situ polymerization, our preparation method has the advantages of high production efficiency and environmental friendliness. This good dispersibility of GNP in the nanocomposite also induced PP to generate β crystal form with higher impact strength without changing its crystallinity. The impact strength of the nanocomposites increased by 120.8% compared with neat PP while the yield strength and Young’s modulus also improved to varying degrees simultaneously. Finally, the addition of a small amount (0.5 wt%) of GNP greatly improved the thermal stability (HDT and T in) of the nanocomposites by 11.7°C and 110°C, respectively. Therefore, this study has shown the methods to obtain high-performance PP/GNP nanocomposites with the multi-functional antistatic application for industrial production.
-
Funding information: This study was supported by the National Natural Science Foundation of China (No. 51772306 and 51472253).
-
Author contributions: Xiaoliang Zhao: investigation, methodology, data curation, data treatment, writing – original draft; Dayong Huang: methodology, investigation, writing – review and editing; Chinomso M. Ewulonu: writing – review and editing; Min Wu: methodology, writing – review and editing, supervision, resources; Chao Wang: writing – review and editing, resources; Yong Huang: methodology, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
Appendix
A1 Synthesis method
Nanocomposites with the same GNP content were prepared by two-screw compounding. First, the PP powder, GNP were manually mixed together by stirring. The blend was further mixed with a twin-screw extruder at 195°C with a screw speed of 90 rpm. A total of six samples were prepared and named as listed in Table A1.

SEM images of T-PP/0.5 (a), T-PP/1.0 (b), T-PP/3.5 (c). It can be observed that large-scale GNPs are stacked together, and there are some holes in the cross-section, which indicates that the GNP dispersion effect is poor, and a satisfactory dispersion effect cannot be obtained through two-screw compounding. Compared with Figure 1, this further illustrates the necessity and superiority of solid-state shear mixing.

Surface resistivity (a) and volume resistivity (b) of composites prepared by two-screw compounding.

Impact strength of neat PP and composites prepared by two-screw compounding.
Name and composition of samples
| Sample name | PP (g) | GNP (g) | Content of GNP (wt%) |
|---|---|---|---|
| PP | 100 | 0.0 | 0.0 |
| T-PP/G0.5 | 99.5 | 0.5 | 0.5 |
| T-PP/G1.0 | 99.0 | 1.0 | 1.0 |
| T-PP/G1.5 | 98.5 | 1.5 | 1.5 |
| T-PP/G2.5 | 97.5 | 2.5 | 2.5 |
| T-PP/G3.5 | 96.5 | 3.5 | 3.5 |
*Note: T is short for two-screw compounding, G is short for GNP.
Tensile mechanical properties of neat PP and composites prepared by two-screw compounding
| Samples | Yield strength (MPa) | Young’s modulus (MPa) | Elongation at break (%) |
|---|---|---|---|
| PP | 32.8 ± 0.9 | 945.3 ± 52.5 | 1004.5 ± 33.7 |
| T-PP/G0.5 | 34.2 ± 1.6 | 1129.0 ± 57.9 | 554.5 ± 139.2 |
| T-PP/G1.0 | 34.1 ± 0.6 | 1136.9 ± 38.4 | 370.4 ± 118.0 |
| T-PP/G1.5 | 32.6 ± 0.7 | 1110.0 ± 40.9 | 58.9 ± 5.7 |
| T-PP/G2.5 | 34.3 ± 0.9 | 1196.3 ± 59.2 | 78.9 ± 78.5 |
| T-PP/G3.5 | 33.5 ± 0.7 | 1175.1 ± 34.5 | 39.8 ± 17.9 |
The molecular weight and molecular weight distribution of samples
| Samples | M n (g/mol) | M w (g/mol) | PD |
|---|---|---|---|
| Neat PP | 60000 | 246000 | 4.1 |
| Neat PP after solid-state shear mixing | 57000 | 275000 | 4.8 |
| PP/G1.0 | 58000 | 265000 | 4.6 |
Note: M n is short for number average molecular weight, M w is short for weight average molecular weight, PD = M w/M n, which is short for polydispersity coefficient.
References
(1) Allen MJ , Tung VC , Kaner RB . Honeycomb carbon: a review of graphene. Chem Rev. 2010;110(1):132–45. 10.1021/cr900070d.Search in Google Scholar PubMed
(2) Geim AK , Novoselov KS . The rise of graphene. Nat Mater. 2007;6(3):183–91. 10.1038/nmat1849.Search in Google Scholar PubMed
(3) Liu X , Shao XY , Fang GB , He HF , Wan ZG . Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites. E-Polymers. 2017;17(1):3–14. 10.1515/epoly-2016-0094.Search in Google Scholar
(4) Mo YF , Yang CL , Xing YF , Wang MS , Ma XG . Nonbond interactions between graphene nanosheets and polymers: a computational study. E-Polymers. 2014;14(3):169–76. 10.1515/epoly-2013-0090.Search in Google Scholar
(5) Novoselov KS , Fal’ko VI , Colombo L , Gellert PR , Schwab MG , Kim K . A roadmap for graphene. Nature. 2012;490(7419):192–200. 10.1038/nature11458.Search in Google Scholar PubMed
(6) Kim H , Abdala AA , Macosko CW . Graphene/polymer nanocomposites. Macromolecules. 2010;43(16):6515–30. 10.1021/ma100572e.Search in Google Scholar
(7) Gong T , Peng SP , Bao RY , Yang W , Xie BH , Yang MB . Low percolation threshold and balanced electrical and mechanical performances in polypropylene/carbon black composites with a continuous segregated structure. Compos Part B Eng. 2016;99:348–57. 10.1016/j.compositesb.2016.06.031.Search in Google Scholar
(8) Xu ZW , He SH , Zhang JJ , Huang SJ , Chen AF , Fu XL , et al. Relationship between the structure and thermal properties of polypropylene/graphene nanoplatelets composites for different platelet-sizes. Compos Sci Technol. 2019;183:107826. 10.1016/j.compscitech.2019.107826.Search in Google Scholar
(9) Zheng W , Chen WG , Zhao Q , Ren SX , Fu YQ . Interfacial structures and mechanisms for strengthening and enhanced conductivity of graphene/epoxy nanocomposites. Polymer. 2019;163:171–7. 10.1016/j.polymer.2018.12.055.Search in Google Scholar
(10) Gao WW , Zhao NF , Yu T , Xi JB , Mao AR , Yuan MQ , et al. High-efficiency electromagnetic interference shielding realized in nacre-mimetic graphene/polymer composite with extremely low graphene loading. Carbon. 2020;157:570–7. 10.1016/j.carbon.2019.10.051.Search in Google Scholar
(11) Sun T , Luo W , Luo YF , Wang Y , Zhou ST , Liang M , et al. Self-reinforced polypropylene/graphene composite with segregated structures to achieve balanced electrical and mechanical properties. Ind Eng Chem Res. 2020;59(24):11206–18. 10.1021/acs.iecr.0c00825.Search in Google Scholar
(12) Chipara M , Jones B , Chipara DM , Li JH , Lozano K , Valloppilly S , et al. On orientation memory in high density polyethylene - carbon nanofibers composites. E-Polymers. 2017;17(4):303–10. 10.1515/epoly-2016-0286.Search in Google Scholar
(13) Yuan BH , Bao CL , Song L , Hong NN , Liew KM , Hu Y . Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem Eng J. 2014;237:411–20. 10.1016/j.cej.2013.10.030.Search in Google Scholar
(14) Bian J , Wang ZJ , Lin HL , Zhou X , Xiao WQ , Zhao XW . Thermal and mechanical properties of polypropylene nanocomposites reinforced with nano-SiO2 functionalized graphene oxide. Compos Part A Appl S. 2017;97:120–7. 10.1016/j.compositesa.2017.01.002.Search in Google Scholar
(15) Wang B , Peng D , Lv RH , Na B , Liu HS , Yu Z . Generic melt compounding strategy using reactive graphene towards high performance polyethylene/graphene nanocomposites. Compos Sci Technol. 2019;177:1–9. 10.1016/j.compscitech.2019.04.013.Search in Google Scholar
(16) Bafana AP , Yan XR , Wei X , Patel M , Guo ZH , Wei SY , et al. Polypropylene nanocomposites reinforced with low weight percent graphene nanoplatelets. Compos Part B Eng. 2017;109:101–7. 10.1016/j.compositesb.2016.10.048.Search in Google Scholar
(17) Huang Y , Qin Y , Zhou Y , Niu H , Yu ZZ , Dong JY . Polypropylene/graphene oxide nanocomposites prepared by in situ Ziegler-Natta polymerization. Chem Mater. 2010;22(13):4096–102. 10.1021/cm100998e.Search in Google Scholar
(18) Sengupta R , Bhattacharya M , Bandyopadhyay S , Bhowmick AK . A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog Mater Sci. 2011;36(5):638–70. 10.1016/j.progpolymsci.2010.11.003.Search in Google Scholar
(19) Jun YS , Um JG , Jiang GP , Lui G , Yu AP . Ultra-large sized graphene nano-platelets (GnPs) incorporated polypropylene (PP)/GnPs composites engineered by melt compounding and its thermal, mechanical, and electrical properties. Compos Part B Eng. 2018;133:218–25. 10.1016/j.compositesb.2017.09.028.Search in Google Scholar
(20) Yuan BH , Sheng HB , Mu XW , Song L , Tai QL , Shi YQ , et al. Enhanced flame retardancy of polypropylene by melamine-modified graphene oxide. J Mater Sci. 2015;50(16):5389–401. 10.1007/s10853-015-9083-0.Search in Google Scholar
(21) An YJ , Wang SH , Li R , Shi DZ , Gao YX , Song L . Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene. E-Polymers. 2019;19(1):32–9. 10.1515/epoly-2019-0005.Search in Google Scholar
(22) Alkan U , Karabul Y , Bulgurcuoglu AE , Kilic M , Ozdemir ZG , Icelli O . Polypropylene/basalt thick film composites: structural, mechanical and dielectric properties. E-Polymers. 2017;17(5):417–25. 10.1515/epoly-2017-0035.Search in Google Scholar
(23) Liu HH , Gu SL , Cao H , Li X , Li YJ . A dense packing structure constructed by flake and spherical graphite: Simultaneously enhanced in-plane and through-plane thermal conductivity of polypropylene/graphite composites. Compos Commun. 2020;19:25–9. 10.1016/j.coco.2020.02.007.Search in Google Scholar
(24) He SH , Zhang JJ , Xiao XT , Hong XM , Lai YJ . Investigation of the conductive network formation of polypropylene/graphene nanoplatelets composites for different platelet sizes. J Mater Sci. 2017;52(22):13103–19. 10.1007/s10853-017-1413-y.Search in Google Scholar
(25) Sheshmani S , Ashori A , Fashapoyeh MA . Wood plastic composite using graphene nanoplatelets. Int J Biol Macromol. 2013;58:1–6. 10.1016/j.ijbiomac.2013.03.047.Search in Google Scholar PubMed
(26) Zhang JJ , He SH , Lv PR , Chen YQ . Processing-morphology-property relationships of polypropylene-graphene nanoplatelets nanocomposites. J Appl Polym Sci. 2017;134(8):44486. 10.1002/app.44486.Search in Google Scholar
(27) Iwamoto S , Yamamoto S , Lee SH , Endo T . Solid-state shear pulverization as effective treatment for dispersing lignocellulose nanofibers in polypropylene composites. Cellulose. 2014;21(3):1573–80. 10.1007/s10570-014-0195-5.Search in Google Scholar
(28) Masuda J , Torkelson JM . Dispersion and major property enhancements in polymer/multiwall carbon nanotube nanocomposites via solid-state shear pulverization followed by melt mixing. Macromolecules. 2008;41(16):5974–7. 10.1021/ma801321j.Search in Google Scholar
(29) Kalaitzidou K , Fukushima H , Drzal LT . Mechanical properties and morphological characterization of exfoliated graphite-polypropylene nanocomposites. Compos Part A Appl S. 2007;38(7):1675–82. 10.1016/j.compositesa.2007.02.003.Search in Google Scholar
(30) Kalaitzidou K , Fukushima H , Askeland P , Drzal LT . The nucleating effect of exfoliated graphite nanoplatelets and their influence on the crystal structure and electrical conductivity of polypropylene nanocomposites. J Mater Sci. 2008;43(8):2895–907. 10.1007/s10853-007-1876-3.Search in Google Scholar
(31) Jones AT , Aizlewood JM , Beckett DR . Crystalline forms of isotactic polypropylene. Makromol Chem. 1964;75:134–58.10.1002/macp.1964.020750113Search in Google Scholar
(32) Wang H , Xie GY , Fang MH , Ying Z , Tong Y , Zeng Y . Electrical and mechanical properties of antistatic PVC films containing multi-layer graphene. Compos Part B Eng. 2015;79:444–50. 10.1016/j.compositesb.2015.05.011.Search in Google Scholar
(33) Wei ZB , Zhao Y , Wang C , Kuga S , Huang Y , Wu M . Antistatic PVC-graphene composite through plasticizer-mediated exfoliation of graphite. Chinese J Polym Sci. 2018;36(12):1361–7. 10.1007/s10118-018-2160-5.Search in Google Scholar
(34) Ajorloo M , Fasihi M , Khoramishad H . The role of nanofiller size and polymer chain configuration on the properties of polypropylene/graphite nanoplates composites. J Taiwan Inst Chem Eng. 2020;108:82–91. 10.1016/j.jtice.2019.12.010.Search in Google Scholar
(35) Gkourmpis T , Gaska K , Tranchida D , Gitsas A , Muller C , Matic A , et al. Melt-mixed 3D hierarchical graphene/polypropylene nanocomposites with low electrical percolation threshold. Nanomaterials. 2019;9(12):1766. 10.3390/nano9121766.Search in Google Scholar PubMed PubMed Central
(36) Marsden AJ , Papageorgiou DG , Valles C , Liscio A , Palermo V , Bissett MA , et al. Electrical percolation in graphene-polymer composites. 2d Mater. 2018;5(3):032003. 10.1088/2053-1583/aac055.Search in Google Scholar
(37) Zhang YF , Lin XF , Hu H . Combined effect of chemically compound graphene oxide-calcium pimelate on crystallization behavior, morphology and mechanical properties of isotactic polypropylene. Polym Adv Technol. 2020;31(10):2301–11. 10.1002/pat.4950.Search in Google Scholar
(38) Wang X , Kalali EN , Wan JT , Wang DY . Carbon-family materials for flame retardant polymeric materials. Prog Mater Sci. 2017;69:22–46. 10.1016/j.progpolymsci.2017.02.001.Search in Google Scholar
(39) Li CQ , Zha JW , Long HQ , Wang SJ , Zhang DL , Dang ZM . Mechanical and dielectric properties of graphene incorporated polypropylene nanocomposites using polypropylene-graft-maleic anhydride as a compatibilizer. Compos Sci Technol. 2017;153:111–8. 10.1016/j.compscitech.2017.10.015.Search in Google Scholar
(40) Lan Y , Liu H , Cao XH , Zhao SG , Dai K , Yan XR , et al. Electrically conductive thermoplastic polyurethane/polypropylene nanocomposites with selectively distributed graphene. Polymer. 2016;97:11–9. 10.1016/j.polymer.2016.05.017.Search in Google Scholar
(41) Nisar M , Bernd MDS , da Silva LCP , Geshev J , Basso NRD , Galland GB . Polypropylene nanocomposites with electrical and magnetic properties. J Appl Polym Sci. 2018;135(42). 10.1002/app.46820.Search in Google Scholar
(42) Huang CL , Lou CW , Liu CF , Huang CH , Song XM , Lin J-H . Polypropylene/graphene and polypropylene/carbon fiber conductive composites: mechanical, crystallization and electromagnetic properties. Appl Sci. 2015;5(4):1196–210. 10.3390/app5041196.Search in Google Scholar
(43) Gaska K , Manika GC , Gkourmpis T , Tranchida D , Gitsas A , Kadar R . Mechanical behavior of melt-mixed 3D hierarchical graphene/polypropylene nanocomposites. Polymers. 2020;12(6):1309. 10.3390/polym12061309.Search in Google Scholar PubMed PubMed Central
(44) Wakabayashi K , Brunner PJ , Masuda J , Hewlett SA , Torkelson JM . Polypropylene-graphite nanocomposites made by solid-state shear pulverization: effects of significantly exfoliated, unmodified graphite content on physical, mechanical and electrical properties. Polymer. 2010;51(23):5525–31. 10.1016/j.polymer.2010.09.007.Search in Google Scholar
© 2021 Xiaoliang Zhao et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Research on the mechanism of gel accelerator on gel transition of PAN solution by rheology and dynamic light scattering
- Gel point determination of gellan biopolymer gel from DC electrical conductivity
- Composite of polylactic acid and microcellulose from kombucha membranes
- Synthesis of highly branched water-soluble polyester and its surface sizing agent strengthening mechanism
- Fabrication and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) modified with nano-montmorillonite biocomposite
- Fabrication of N-halamine polyurethane films with excellent antibacterial properties
- Formulation and optimization of gastroretentive bilayer tablets of calcium carbonate using D-optimal mixture design
- Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging
- Evaluation of physicochemical properties of film-based alginate for food packing applications
- Electrically conductive and light-weight branched polylactic acid-based carbon nanotube foams
- Structuring of hydroxy-terminated polydimethylsiloxane filled by fumed silica
- Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering
- Influence of composite structure design on the ablation performance of ethylene propylene diene monomer composites
- MOFs/PVA hybrid membranes with enhanced mechanical and ion-conductive properties
- Improvement of the electromechanical properties of thermoplastic polyurethane composite by ionic liquid modified multiwall carbon nanotubes
- Natural rubber latex/MXene foam with robust and multifunctional properties
- Rheological properties of two high polymers suspended in an abrasive slurry jet
- Two-step polyaniline loading in polyelectrolyte complex membranes for improved pseudo-capacitor electrodes
- Preparation and application of carbon and hollow TiO2 microspheres by microwave heating at a low temperature
- Properties of a bovine collagen type I membrane for guided bone regeneration applications
- Fabrication and characterization of thermoresponsive composite carriers: PNIPAAm-grafted glass spheres
- Effect of talc and diatomite on compatible, morphological, and mechanical behavior of PLA/PBAT blends
- Multifunctional graphene nanofiller in flame retarded polybutadiene/chloroprene/carbon black composites
- Strain-dependent wicking behavior of cotton/lycra elastic woven fabric for sportswear
- Enhanced dielectric properties and breakdown strength of polymer/carbon nanotube composites by coating an SrTiO3 layer
- Analysis of effect of modification of silica and carbon black co-filled rubber composite on mechanical properties
- Polytriazole resins toughened by an azide-terminated polyhedral oligomeric silsesquioxane (OADTP)
- Phosphine oxide for reducing flammability of ethylene-vinyl-acetate copolymer
- Study on preparation and properties of bentonite-modified epoxy sheet molding compound
- Polyhedral oligomeric silsesquioxane (POSS)-modified phenolic resin: Synthesis and anti-oxidation properties
- Study on structure and properties of natural indigo spun-dyed viscose fiber
- Biodegradable thermoplastic copolyester elastomers: Methyl branched PBAmT
- Investigations of polyethylene of raised temperature resistance service performance using autoclave test under sour medium conditions
- Investigation of corrosion and thermal behavior of PU–PDMS-coated AISI 316L
- Modification of sodium bicarbonate and its effect on foaming behavior of polypropylene
- Effect of coupling agents on the olive pomace-filled polypropylene composite
- High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide
- Removal of methylene blue in water by electrospun PAN/β-CD nanofibre membrane
- Theoretical and experimental studies on the fabrication of cylindrical-electrode-assisted solution blowing spinning nanofibers
- Influence of l-quebrachitol on the properties of centrifuged natural rubber
- Ultrasonic-modified montmorillonite uniting ethylene glycol diglycidyl ether to reinforce protein-based composite films
- Experimental study on the dissolution of supercritical CO2 in PS under different agitators
- Experimental research on the performance of the thermal-reflective coatings with liquid silicone rubber for pavement applications
- Study on controlling nicotine release from snus by the SIPN membranes
- Catalase biosensor based on the PAni/cMWCNT support for peroxide sensing
- Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol
- Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid
- Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride
- Polypropylene/graphene nanoplatelets nanocomposites with high conductivity via solid-state shear mixing
- Mechanical properties of fiber-reinforced asphalt concrete: Finite element simulation and experimental study
- Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery
- Preparation and characterizations of antibacterial–antioxidant film from soy protein isolate incorporated with mangosteen peel extract
- Preparation and adsorption properties of Ni(ii) ion-imprinted polymers based on synthesized novel functional monomer
- Rare-earth doped radioluminescent hydrogel as a potential phantom material for 3D gel dosimeter
- Effects of cryogenic treatment and interface modifications of basalt fibre on the mechanical properties of hybrid fibre-reinforced composites
- Stable super-hydrophobic and comfort PDMS-coated polyester fabric
- Impact of a nanomixture of carbon black and clay on the mechanical properties of a series of irradiated natural rubber/butyl rubber blend
- Preparation and characterization of a novel composite membrane of natural silk fiber/nano-hydroxyapatite/chitosan for guided bone tissue regeneration
- Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride
- A new method for plugging the dominant seepage channel after polymer flooding and its mechanism: Fracturing–seepage–plugging
- Analysis of the rheological property and crystallization behavior of polylactic acid (Ingeo™ Biopolymer 4032D) at different process temperatures
- Hybrid green organic/inorganic filler polypropylene composites: Morphological study and mechanical performance investigations
- In situ polymerization of PEDOT:PSS films based on EMI-TFSI and the analysis of electrochromic performance
- Effect of laser irradiation on morphology and dielectric properties of quartz fiber reinforced epoxy resin composite
- The optimization of Carreau model and rheological behavior of alumina/linear low-density polyethylene composites with different alumina content and diameter
- Properties of polyurethane foam with fourth-generation blowing agent
- Hydrophobicity and corrosion resistance of waterborne fluorinated acrylate/silica nanocomposite coatings
- Investigation on in situ silica dispersed in natural rubber latex matrix combined with spray sputtering technology
- The degradable time evaluation of degradable polymer film in agriculture based on polyethylene film experiments
- Improving mechanical and water vapor barrier properties of the parylene C film by UV-curable polyurethane acrylate coating
- Thermal conductivity of silicone elastomer with a porous alumina continuum
- Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate
- Combining good dispersion with tailored charge trapping in nanodielectrics by hybrid functionalization of silica
- Thermosensitive hydrogel for in situ-controlled methotrexate delivery
- Analysis of the aging mechanism and life evaluation of elastomers in simulated proton exchange membrane fuel cell environments
- The crystallization and mechanical properties of poly(4-methyl-1-pentene) hard elastic film with different melt draw ratios
- Review Articles
- Aromatic polyamide nonporous membranes for gas separation application
- Optical elements from 3D printed polymers
- Evidence for bicomponent fibers: A review
- Mapping the scientific research on the ionizing radiation impacts on polymers (1975–2019)
- Recent advances in compatibility and toughness of poly(lactic acid)/poly(butylene succinate) blends
- Topical Issue: (Micro)plastics pollution - Knowns and unknows (Guest Editor: João Pinto da Costa)
- Simple pyrolysis of polystyrene into valuable chemicals
- Topical Issue: Recent advances of chitosan- and cellulose-based materials: From production to application (Guest Editor: Marc Delgado-Aguilar)
- In situ photo-crosslinking hydrogel with rapid healing, antibacterial, and hemostatic activities
- A novel CT contrast agent for intestinal-targeted imaging through rectal administration
- Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review
- Towards the use of acrylic acid graft-copolymerized plant biofiber in sustainable fortified composites: Manufacturing and characterization
Articles in the same Issue
- Research Articles
- Research on the mechanism of gel accelerator on gel transition of PAN solution by rheology and dynamic light scattering
- Gel point determination of gellan biopolymer gel from DC electrical conductivity
- Composite of polylactic acid and microcellulose from kombucha membranes
- Synthesis of highly branched water-soluble polyester and its surface sizing agent strengthening mechanism
- Fabrication and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) modified with nano-montmorillonite biocomposite
- Fabrication of N-halamine polyurethane films with excellent antibacterial properties
- Formulation and optimization of gastroretentive bilayer tablets of calcium carbonate using D-optimal mixture design
- Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging
- Evaluation of physicochemical properties of film-based alginate for food packing applications
- Electrically conductive and light-weight branched polylactic acid-based carbon nanotube foams
- Structuring of hydroxy-terminated polydimethylsiloxane filled by fumed silica
- Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering
- Influence of composite structure design on the ablation performance of ethylene propylene diene monomer composites
- MOFs/PVA hybrid membranes with enhanced mechanical and ion-conductive properties
- Improvement of the electromechanical properties of thermoplastic polyurethane composite by ionic liquid modified multiwall carbon nanotubes
- Natural rubber latex/MXene foam with robust and multifunctional properties
- Rheological properties of two high polymers suspended in an abrasive slurry jet
- Two-step polyaniline loading in polyelectrolyte complex membranes for improved pseudo-capacitor electrodes
- Preparation and application of carbon and hollow TiO2 microspheres by microwave heating at a low temperature
- Properties of a bovine collagen type I membrane for guided bone regeneration applications
- Fabrication and characterization of thermoresponsive composite carriers: PNIPAAm-grafted glass spheres
- Effect of talc and diatomite on compatible, morphological, and mechanical behavior of PLA/PBAT blends
- Multifunctional graphene nanofiller in flame retarded polybutadiene/chloroprene/carbon black composites
- Strain-dependent wicking behavior of cotton/lycra elastic woven fabric for sportswear
- Enhanced dielectric properties and breakdown strength of polymer/carbon nanotube composites by coating an SrTiO3 layer
- Analysis of effect of modification of silica and carbon black co-filled rubber composite on mechanical properties
- Polytriazole resins toughened by an azide-terminated polyhedral oligomeric silsesquioxane (OADTP)
- Phosphine oxide for reducing flammability of ethylene-vinyl-acetate copolymer
- Study on preparation and properties of bentonite-modified epoxy sheet molding compound
- Polyhedral oligomeric silsesquioxane (POSS)-modified phenolic resin: Synthesis and anti-oxidation properties
- Study on structure and properties of natural indigo spun-dyed viscose fiber
- Biodegradable thermoplastic copolyester elastomers: Methyl branched PBAmT
- Investigations of polyethylene of raised temperature resistance service performance using autoclave test under sour medium conditions
- Investigation of corrosion and thermal behavior of PU–PDMS-coated AISI 316L
- Modification of sodium bicarbonate and its effect on foaming behavior of polypropylene
- Effect of coupling agents on the olive pomace-filled polypropylene composite
- High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide
- Removal of methylene blue in water by electrospun PAN/β-CD nanofibre membrane
- Theoretical and experimental studies on the fabrication of cylindrical-electrode-assisted solution blowing spinning nanofibers
- Influence of l-quebrachitol on the properties of centrifuged natural rubber
- Ultrasonic-modified montmorillonite uniting ethylene glycol diglycidyl ether to reinforce protein-based composite films
- Experimental study on the dissolution of supercritical CO2 in PS under different agitators
- Experimental research on the performance of the thermal-reflective coatings with liquid silicone rubber for pavement applications
- Study on controlling nicotine release from snus by the SIPN membranes
- Catalase biosensor based on the PAni/cMWCNT support for peroxide sensing
- Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol
- Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid
- Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride
- Polypropylene/graphene nanoplatelets nanocomposites with high conductivity via solid-state shear mixing
- Mechanical properties of fiber-reinforced asphalt concrete: Finite element simulation and experimental study
- Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery
- Preparation and characterizations of antibacterial–antioxidant film from soy protein isolate incorporated with mangosteen peel extract
- Preparation and adsorption properties of Ni(ii) ion-imprinted polymers based on synthesized novel functional monomer
- Rare-earth doped radioluminescent hydrogel as a potential phantom material for 3D gel dosimeter
- Effects of cryogenic treatment and interface modifications of basalt fibre on the mechanical properties of hybrid fibre-reinforced composites
- Stable super-hydrophobic and comfort PDMS-coated polyester fabric
- Impact of a nanomixture of carbon black and clay on the mechanical properties of a series of irradiated natural rubber/butyl rubber blend
- Preparation and characterization of a novel composite membrane of natural silk fiber/nano-hydroxyapatite/chitosan for guided bone tissue regeneration
- Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride
- A new method for plugging the dominant seepage channel after polymer flooding and its mechanism: Fracturing–seepage–plugging
- Analysis of the rheological property and crystallization behavior of polylactic acid (Ingeo™ Biopolymer 4032D) at different process temperatures
- Hybrid green organic/inorganic filler polypropylene composites: Morphological study and mechanical performance investigations
- In situ polymerization of PEDOT:PSS films based on EMI-TFSI and the analysis of electrochromic performance
- Effect of laser irradiation on morphology and dielectric properties of quartz fiber reinforced epoxy resin composite
- The optimization of Carreau model and rheological behavior of alumina/linear low-density polyethylene composites with different alumina content and diameter
- Properties of polyurethane foam with fourth-generation blowing agent
- Hydrophobicity and corrosion resistance of waterborne fluorinated acrylate/silica nanocomposite coatings
- Investigation on in situ silica dispersed in natural rubber latex matrix combined with spray sputtering technology
- The degradable time evaluation of degradable polymer film in agriculture based on polyethylene film experiments
- Improving mechanical and water vapor barrier properties of the parylene C film by UV-curable polyurethane acrylate coating
- Thermal conductivity of silicone elastomer with a porous alumina continuum
- Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate
- Combining good dispersion with tailored charge trapping in nanodielectrics by hybrid functionalization of silica
- Thermosensitive hydrogel for in situ-controlled methotrexate delivery
- Analysis of the aging mechanism and life evaluation of elastomers in simulated proton exchange membrane fuel cell environments
- The crystallization and mechanical properties of poly(4-methyl-1-pentene) hard elastic film with different melt draw ratios
- Review Articles
- Aromatic polyamide nonporous membranes for gas separation application
- Optical elements from 3D printed polymers
- Evidence for bicomponent fibers: A review
- Mapping the scientific research on the ionizing radiation impacts on polymers (1975–2019)
- Recent advances in compatibility and toughness of poly(lactic acid)/poly(butylene succinate) blends
- Topical Issue: (Micro)plastics pollution - Knowns and unknows (Guest Editor: João Pinto da Costa)
- Simple pyrolysis of polystyrene into valuable chemicals
- Topical Issue: Recent advances of chitosan- and cellulose-based materials: From production to application (Guest Editor: Marc Delgado-Aguilar)
- In situ photo-crosslinking hydrogel with rapid healing, antibacterial, and hemostatic activities
- A novel CT contrast agent for intestinal-targeted imaging through rectal administration
- Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review
- Towards the use of acrylic acid graft-copolymerized plant biofiber in sustainable fortified composites: Manufacturing and characterization

