Abstract
Polymeric-based composites can contribute to enhancing the detection, stability, and performance of enzymatic biosensors, due to their high structural stability, conductivity, and biocompatibility. This work presents the fabrication of a nanocomposite of polyaniline (PAni)/gold nanoparticles (AuNP)/carboxylated multiwalled carbon nanotubes (cMWCNT) as functional support for covalently linked catalase (CAT) enzyme. PAni was electropolymerized on a screen-printed carbon electrode (SPCE) and decorated with AuNP to improve charge transfer properties. CAT was bonded through amide formation using the carboxylic groups of cMWCNT, resulting in PAni/AuNP/cMWCNT/CAT biosensor. The structural and electroactive characteristics of the nanocomposite were studied by SEM, FT-IR, and cyclic voltammetry. The optimal performance was achieved after CAT immobilization over PAni/AuNP/cMWCNT/nanocomposite, showing improved analytical features such as a fast amperometric response of 1.28 s, a wide detection range from 0.01 to 6.8 mM, a correlation coefficient (R 2) of 0.9921, a low detection limit of 2.34 µM, and an average recovery rate of 99.6% when evaluated in milk samples. Additionally, the bioelectrode showed excellent selectivity and retained bioactivity after 30 days of storage. Such remarkable performance proved the synergistic effects of both the high surface area of the cMWCNT and AuNP and the inherent PAni electroactivity, yielding direct electron transfer from CAT.
1 Introduction
Enzyme-based electrochemical biosensors have been the subject of extensive research due to their promising applications in food safety, clinical diagnosis, healthcare, and environmental monitoring (1). Compared with typical analytical techniques, biosensors offer advantages such as portability, low cost, and facility of use; however, they can suffer from poor stability and limited sensitivity that might affect the analytical performance (2). Commonly, enzymatic biosensors rely on carbon electrodes modified with high-performance nanomaterials like metal nanoparticles, graphene, or conductive polymers that allow enzyme linking and facilitates electron transfer between the enzyme redox center and the electrode. It is expected that the nanostructured support enhances the kinetics and chemical stability of the enzyme (3,4). The design of polymeric-based composites as enzymatic supports contributes to enhancing the detection, stability, and performance of biosensors because of their unique properties at the nanoscale such as high structural stability, conductivity, and biocompatibility (5). In this sense, polyaniline (PAni) has been incorporated in enzymatic biosensors due to its attractive features such as low cost, facile synthesis, conductivity, environmental stability, and biocompatibility, functioning as the transducer, mediator for electron transfer, or immobilization matrix (6). PAni, also, is an effective electroactive platform for enzymatic biosensor design due to its tunable redox properties, which facilitate the charge transfer process from the redox enzyme’s catalytic site to the polymeric structure (7). Furthermore, PAni is capable of immobilizing enzymes through different methods such as physical adsorption, covalent linking, or entrapping (8), resulting in an ideal component for developing electrochemical bioelectrodes.
Although the electrochemical properties of PAni are suitable for electrochemical detection, incorporation of electroactive entities into PAni, such as gold nanoparticles and carbon nanotubes, has been shown to allow synergistic effects on improved surface area and conductivity, improving the electrochemical performance of the platform with a faster response (7,9). Thus, to extend the PAni characteristics and tune its performance, composites based on PANi and AuNP have been studied, considering that the high surface to volume ratio of AuNP is highly beneficial for loading biomolecules (10). Besides, AuNP improves the electron transfer rate between the active centers of the immobilized enzyme and the electrode, resulting in enhanced performance (11). In addition, the combination of PAni with carbon-based components such as MWCNT provides advantages to the sensing systems such as chemical stability, enhanced mechanical properties, extensive surface area, and high conductivity (12).
Additionally, MWCNTs can act as an immobilization nano-matrix for covalent coupling through the induction of carboxylic acid groups and the reaction with the free amino groups on the enzyme (13). These modifications facilitate charge transfer and conductivity of the sensing platform (3,14), stabilizing the PAni and favoring retention of its inherent electrochemical activity, which provides more active nucleation sites, contributing, in conjunction, to improve biosensor performance (12).
Heme enzymes, including peroxidase, catalase, cytochrome c, hemoglobin, and myoglobin are involved in several fundamental biological processes. The redox chemistry of the heme iron as an active site and the biochemical diversity has led to the application as biological recognition elements in electrochemical biosensors for H2O2 and phenolic compounds (15).
Catalase, from the bovine liver (CAT, EC 1.11.1.6), is an essential oxidoreductase enzyme (16), with four equal subunits of 60 kDa molecular weight and a heme prosthetic group at its active site with metallic iron Fe(iii) (17). CAT is present in almost all aerobically respiring organisms, protecting cells from the toxic effects of hydrogen peroxide (H2O2) by decomposing it into oxygen and water without the formation of free radicals (18). According to Lonča and Fraaije (19), catalases are robust enzymes with unusually high catalytic rates and among the highest enzymatic rates. Therefore, CAT is used in diverse industrial applications as an effective H2O2 removal tool.
The fast, reliable, and accurate determination of H2O2 is of great interest given its substantial role in food, pharmaceutical, clinical, industrial, and environmental analyses; additionally, H2O2 is a biomarker of some cells and tumors (20). H2O2, also, is widely used as an antiseptic agent for preserving milk in the dairy industry. It inhibits microorganism growth and milk spoilage during the storage period. The use of H2O2 must not exceed 0.05% of the milk weight and is approved in the USA by the food and drug administration (FDA) for this function (21). The excess of residual H2O2 is harmful to humans; Hence, determining H2O2 in food and biological systems is very important in health and product pre-treatment (22). Therefore, novel devices and configurations for sensitive H2O2 detection are of significant attention like BiVO4/fluorine-doped tin oxide (23) and graphene oxide-poly(amidoamine) dendrimer-Fe2+/GCE (24). Also, some authors have designed H2O2 biosensors using CAT as the bioreceptor, linking the CAT with supports like chitosan, single-walled carbon nanotubes, and polyethyleneimine (18). Furthermore, CAT has excellent stability and could be an excellent choice for biosensor design, where shelf stability is important (25).
Because of the interesting properties of CAT as a biocatalyst and the efficient operation of PAni-cMWCNT-based immobilization supports, the development of an electroactive platform capable of both enzyme immobilization, and measure biological interaction between CAT and H2O2 by the electrochemical method is proposed. This work reports the construction of an amperometric bioelectrode departing from a screen-printed carbon electrode modified with electropolymerized PAni, which was subsequently optimized with AuNP and carboxylated MWCNT (cMWCNT). The PAni/AuNP/cMWCNT nanocomposite was applied as functional support for covalent immobilization of CAT used as a bioreceptor and exploited for quantifying H2O2 in PBS and milk samples. The assembled nanocomposite showed high retention of enzyme activity, resulting in improved analytical parameters as accuracy, sensitivity, and low limit of detection (LOD), as well as favorable long-term stability, which evidenced the beneficial synergistic effects of the assembled nanocomposite in the biosensor performance.
2 Materials and methods
2.1 Materials and reagents
Aniline, N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), chloroauric acid (HAuCl4), and CAT from bovine liver (EC 1.11.1.6, activity 5,000 units per mg) were obtained from Sigma-Aldrich. Sulfuric acid (H2SO4), nitric acid (HNO3), and hydrogen peroxide solution (30 wt% aqueous) were provided from Acros Organics. Phosphate buffer solution (PBS) (0.01 M, pH 7.4) was prepared from sodium chloride (NaCl), potassium chloride (KCl), and disodium hydrogen phosphate (Na2HPO4), and potassium dihydrogen phosphate (KH2PO4) was used as the supporting electrolyte. Whole-fat milk was bought from a local supermarket and analyzed without prior treatment, and the screen-printed carbon electrodes (SPCE) were obtained from Metrohm.
2.2 Characterization
Cyclic voltammetry (CV) and amperometry measurements were measured using an EmStat3+blue potentiostat, whereas electrochemical impedance spectroscopy (EIS) was performed using a Solartron 1260A potentiostat/galvanostat. Fourier-transform infrared spectroscopy (FTIR) was registered using Perkin-Elmer GX-FTIR equipment, and the morphology was analyzed with a JEOL 300-S scanning electron microscope (SEM). FTIR and SEM characterized each stage of electrode modification and enzyme immobilization.
2.3 Assembling of PAni/AuNP/cMWCNT
MWCNTs were obtained according to a previous procedure and subsequently carboxylated by acid treatment (26). Briefly, 50 mg of MWCNT was added in a solution of 10 mL of H2SO4 and HNO3 in a 3:1 volume ratio; the mixture was sonicated for 1 h to obtain a fine dispersion and then refluxed at 90°C for 1 h. The resulting carboxylated multiwalled carbon nanotubes (cMWCNTs) were filtered and washed until neutral pH was achieved and dried at 60°C for 12 h; finally, FTIR was used to verify carboxylation.
A solution of aniline (0.25 M) in H2SO4 (0.25 M) was used for electropolymerization onto the SPCE by CV, applying ten cycles at a potential from −0.5 to 1.0 V at a rate of 50 mV s−1. The resulting SPCE/PAni was immersed in a mixture of HAuCl4 (1 mM) in H2SO4 (0.25 M) to perform AuNP electrodeposition. The conditions were three cycles at a potential from −0.5 to 1 V and a rate of 20 mV s−1, obtaining the SPCE/PAni/AuNP. Then, 4 µL of cMWCNT (1 mg mL−1) were dropped cast on SPCE/PAni/AuNP and dried at room temperature. Finally, the modified surface was rinsed with distilled water to remove loose material, obtaining the SPCE/PAni/AuNP/cMWCNT functional support.
2.4 Catalase immobilization
CAT was covalently immobilized onto SPCE/PAni/AuNP/cMWCNT, using the EDC/NHS chemistry. First, the COOH groups of the cMWCNT were activated by casting 4 µL of a solution of EDC (20 mM) and NHS (20 mM) in PBS (0.01 M) over the electrode surface at room temperature. Finally, 5 µL of CAT (5 mg mL−1) were deposed on the modified electrode and allowed to dry at room temperature. The electrode was washed with PBS to remove unbound enzymes, obtaining the SPCE/PAni/AuNP/cMWCNT/CAT biosensor. Figure 1 illustrates the fabrication stages, which were characterized by FTIR, SEM, CV, and EIS.
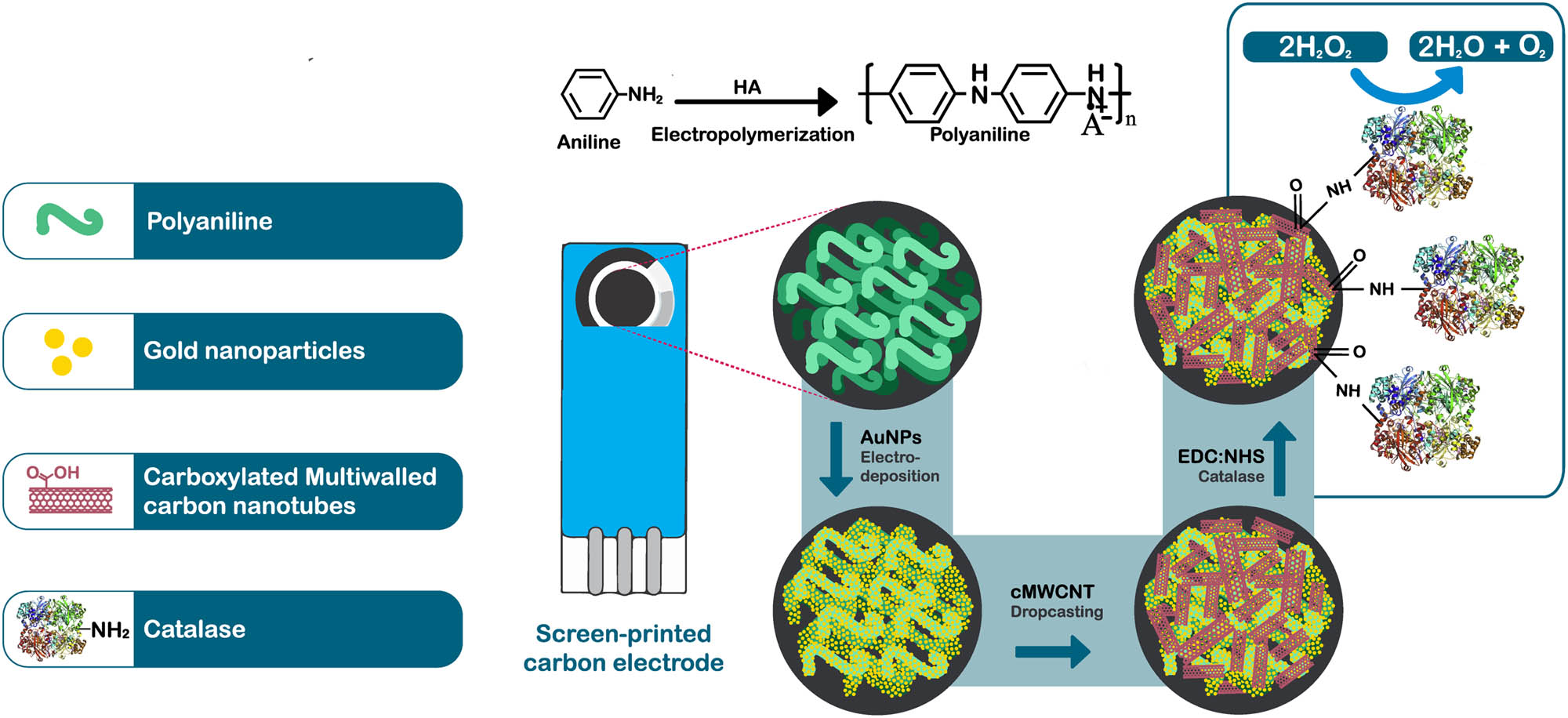
Scheme of step by step preparation of the SPCE/PAni/AuNP/cMWCNT/CAT biosensor.
2.5 Electrochemical measurements
The quantitative detection of H2O2 was performed using PBS (0.01 M) as the supporting electrolyte (pH 7.4) at 25°C. Before measurements, the PBS was deoxygenated by bubbling nitrogen for 10 min to protect the solution from oxygen. CV was evaluated with concentrations from 0.1 to 4 mM, using a potential from −1 to 1 V and a scan rate of 20 mV s− 1. The amperometric response of the modified electrode toward H2O2 was determined by applying a constant potential of −0.4 V in 25 mL of PBS at 0.01 M. During the measurements, the solution was kept under continuous magnetic stirring. After reaching a steady-state, a given amount of H2O2 solution was added to the PBS electrolyte and the change in current was recorded. Measurements were conducted by triplicate under the same conditions.
Finally, the proposed biosensor was used to determine H2O2 in spiked samples of whole milk purchased at a local supermarket. The milk samples were used as they came from the packing. Known concentrations of H2O2 were spiked using the whole milk as a matrix. The determination of H2O2 in the milk samples was carried out by amperometry.
3 Results and discussion
3.1 Morphology
SEM allowed corroborating the surface modifications at each stage of biosensor construction. Figure 2a portrays the electropolymerized PAni, showing fibrillar morphology with a diameter of approximately 200 nm and a few micrometers in length. Similar morphology of polyaniline was attributed to the electropolymerization process that promotes fiber formation (27). Figure 2b illustrates the second stage of modification with the PAni surface decorated by AuNP. The particles are embedded in the surface of the PAni fibers, showing good dispersion and spherical morphology of around 50 nm (inset). The third stage of fabrication includes the cMWCNT attached to the PAni/AuNP (Figure 2c), creating a porous network structure that is highly convenient for catalase immobilization. Figure 2d shows CAT immobilization on SPCE/PAni/AuNP/cMWCNT, with the enzyme forming a thin layer over the cMWCNT as seen in the inset. The change in morphology revealed a successful enzyme immobilization onto the support, in agreement with previous observations for CAT binding (28).
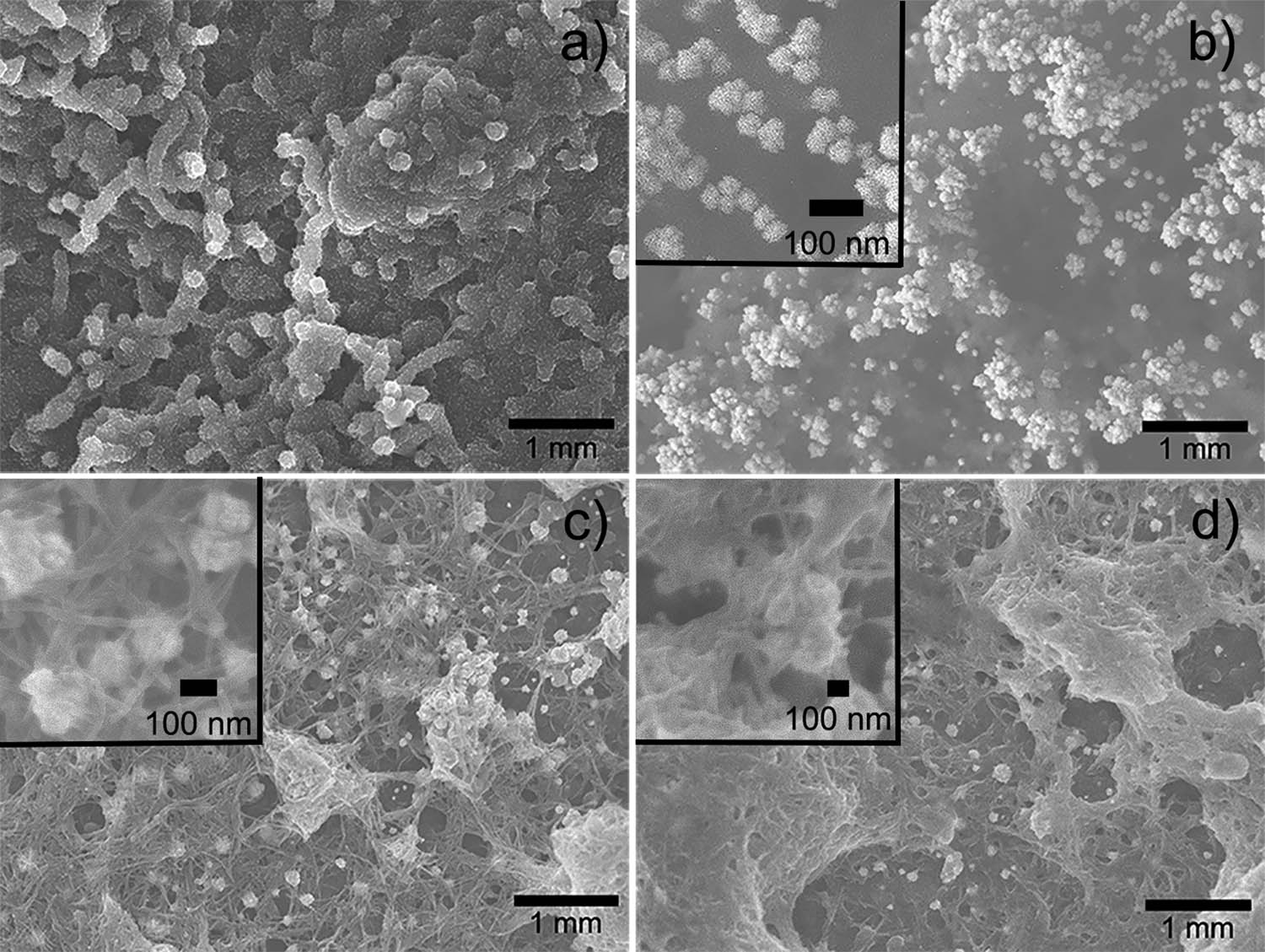
SEM images of (a) SPCE/PAni, (b) SPCE/PAni/AuNP, (c) SPCE/PAni/AuNP/cMWCNT, and (d) SPCE/PAni/AuNP/cMWCNT/CAT.
3.2 Functional group characterization
The reaction of the strong acids with the unstable carbon atoms of the MWCNT generated COOH groups (29). The spectrum of the cMWCNT (Figure A1 in Appendix) shows absorption bands at 3,298 and 1,727 cm−1, which correspond, respectively, to the stretching vibrations of the O–H and C═O bonds of the carboxyl group. The band at 1,627 cm−1 corresponds to the skeletal vibrations of the C═C bond of cMWCNT (30). Thus, the presence of these bands confirmed carbon nanotube’s carboxylation, which has been reported for equivalent treatments (29).
Figure 3a shows the spectrum of PAni/AuNP/cMWCNT showed the bands at 3,298, 1,727, and 1,627 cm−1, as described for the cMWCNT. Besides, a pair of bands at 1,542 and 1,463 cm−1, attributed, respectively, to the stretching vibrations of the C═C bond of the quinoid ring and the benzenoid ring, confirmed the PAni electropolymerization (31). The band at 1,370 cm−1 was related to the C–N stretching vibration in the neighborhood of a quinoid ring (32), suggesting that the π-bonded surface of the carbon nanotubes might interact actively with the conjugated structure of the PAni, primarily through the quinoid rings (33).
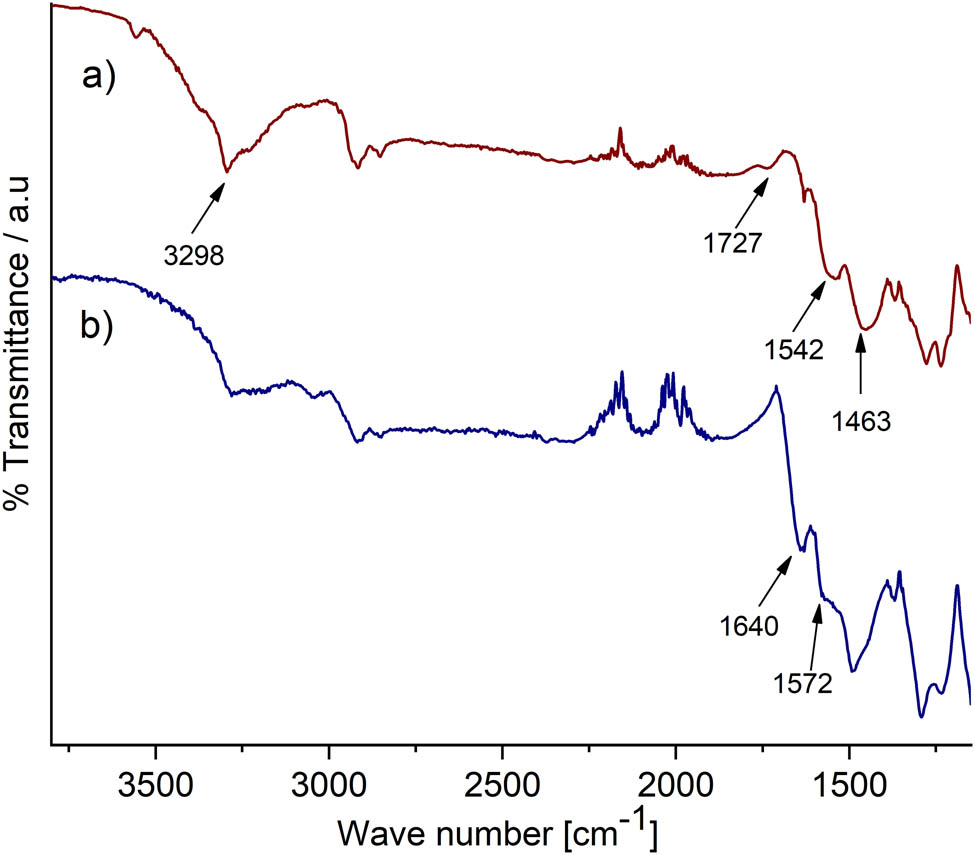
FTIR spectra of (a) SPCE/PAni/AuNP/cMWCNT, (b) SPCE/PAni/AuNP/cMWCNT/CAT.
Figure 3b illustrates the spectrum of PAni/AuNP/cMWCNT/CAT. As seen, peaks are present at 1,640 and 1,572 cm−1, these bands correspond on amide I and amide II bands, respectively (34). These peaks are located close to the amides bands of catalase (see Figure A2). According to Zuccarello et al. (15), the alterations of the relative intensities, positions, and shapes of the amide I and amide II bands of the enzyme indicate structural changes upon immobilization; Thus, these bands might be attributed to the formation of an amide bond between the NH2 groups of CAT and the COOH groups of cMWCNT. In addition, the band at 1,727 cm−1 practically disappeared, and the band at 3,298 cm−1 showed a considerable intensity decrease, which was also associated with the interaction between COOH and NH2 groups, confirming the covalent link of CAT and cMWCNT.
Similar behavior was reported by Chawla et al. (35) and Rawal et al. (36), where they correlated the covalent immobilization of the enzyme laccase on a modified electrode containing cMWCNT through the disappearance of the COOH band and the appearance of the amide bands.
3.3 Electrochemical characterization
The voltammogram of PAni, Figure 4a, showed the typical oxidation peaks due to the transition from leucoemeraldine to emeraldine and from emeraldine to pernigraniline (37), and only one cathodic peak was observed. This behavior may be attributed to the neutral pH and the saline phosphate buffer used as electrolyte support. According to Mažeikien et al. (38), the redox activity depends not only on the pH but also on the composition of the electrolyte solution. They found that the redox properties of sulfonated polyaniline were reduced in sodium salt solutions due to the exchange of hydrated sodium cations between the electrolyte solution and the polymer film during the electrochemical process, producing a less effective transition between the oxidation states of PAni. However, the electropolymerized PAni used in this work showed the ability to undergo an electrochemical redox process even in neutral pH, which is suitable for this application.
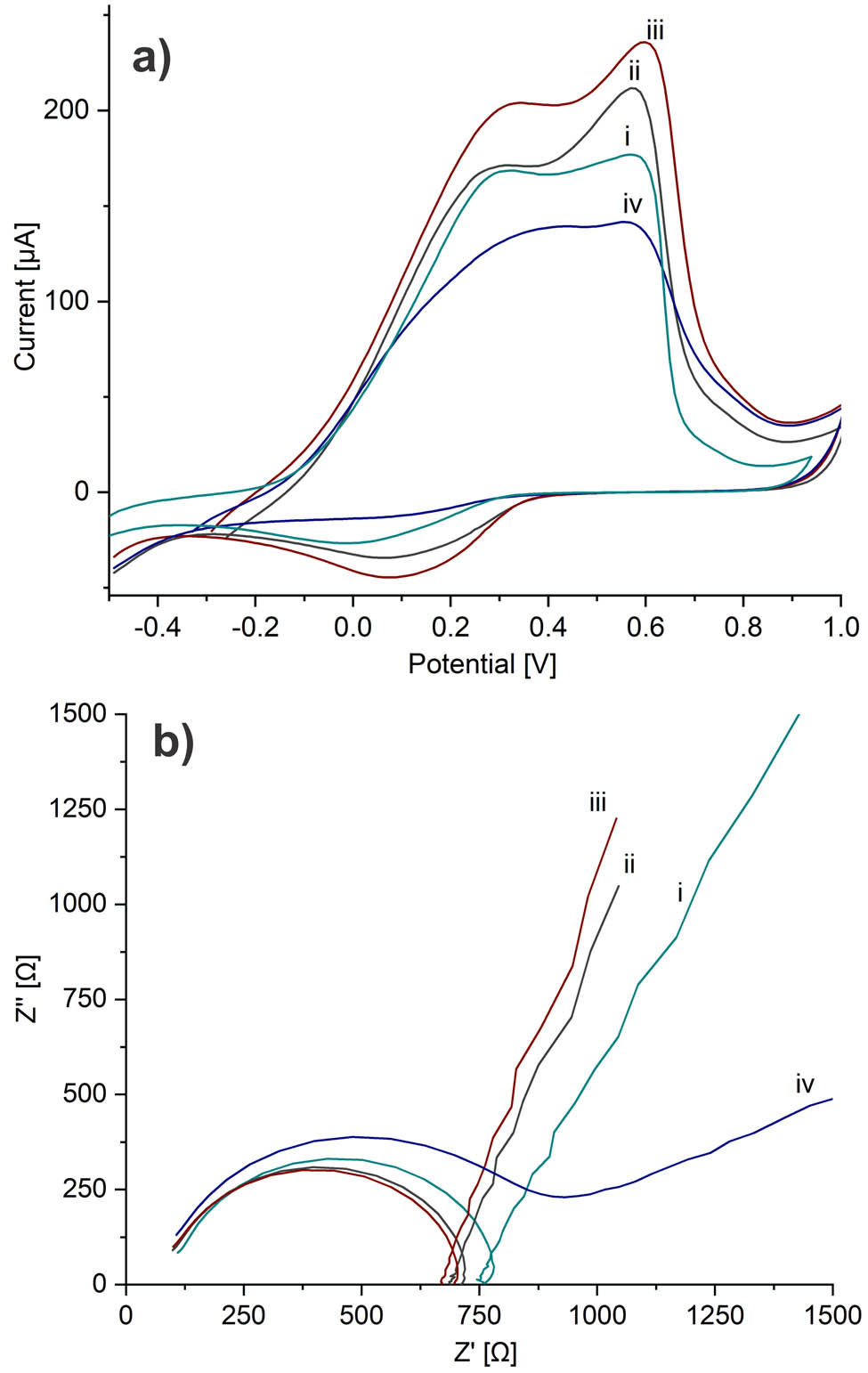
(a) Cyclic voltammetry and (b) electrochemical impedance spectroscopy of (i) SPCE/PAni, (ii) SPCE/PAni/AuNP, (iii) SPCE/PAni/AuNP/cMWCNT, and (iv) SPCE/PAni/AuNP/cMWCNT/CAT biosensor.
After the AuNP electrodeposition, the current intensity of the anodic peaks increased, suggesting the increase of the active surface of PAni and the formation of a more effective surface area as a charge transfer mediator (39). For PAni/AuNP/cMWCNT, the high surface area of cMWCNT and their tubular structure generated a more energetically favorable pathway for the charge transfer, improving the inherent PAni electroactivity (3). Finally, the biosensor showed a small decrease in the electroactive area, indicating a slower electron transfer at the electrode surface by the immobilized enzyme. This confirmed the enzyme binding over the PAni/AuNP/cMWCNT functional support while retaining the electroactivity of the system. Similar behavior displayed the insulating property of the protein layer (40).
Figure 4b illustrates the charge transfer at the electrode interface determined by EIS. The semicircle diameter at the high-frequency region represents the resistance charge transfer (R ct), caused by electrochemical reactions in contact with the interface between the electrode and the electrolyte, and the linear part of the low-frequency region corresponds to the diffusion process (41). Figure 4b shows the Nyquist plots of the electrodes at each modification stage. As seen, the R ct of PAni was more extensive than that of PAni/AuNP and PAni/AuNP/cMWCNT, suggesting that the electrode modified by AuNP and cMWCNT decreased the R ct and promoted the electron transfer between the electrolyte and the modified electrode. These results agreed with CV; consequently, the cMWCNT not only played the role of the functional nano-matrix for the enzyme immobilization but also improved the electroactivity and charge transfer capacity in the nanocomposite. The R ct of the biosensor increased in comparison with the previous stage due to the non-conductive nature of CAT. Another study attributed a similar behavior because the electrode surface suffers from blockage by the poorly enzyme conductivity (42).
3.4 Direct electron transference of catalase
Figure 5a displays the CV of CAT immobilized on PAni/AuNP/cMWCNT in PBS 0.01 M solution at a scan rate of 20 mV s−1. PAni/AuNP/cMWCNT/CAT exhibited a highly defined cathodic peak at −0.52 V and an anodic peak of lower intensity not well defined at −0.26 V, suggesting that the intrinsic electroactivity of PAni overlapped it. These redox peaks were attributed to the heme group FeIII/IV from the CAT enzyme (43). To corroborate the direct electron transfer from CAT to PAni/AuNP/cMWCNT, CAT was immobilized on the nanocomposite without PAni to verify the overlap of the anodic peak. The AuNP/cMWCNT/CAT revealed a cathodic peak, at −0.4 V and a well-defined anodic peak at −0.22 V (Figure 5a, inset), corroborating the overlapping. The cathodic peak intensity was considerably more prominent than the anodic peak in both voltammograms, attributed to the conformational changes of CAT due to the immobilization process (44). This behavior was comparable with recent observations with catalase, where the presence of the redox peaks of the heme group from catalase was taken as evidence of the direct electron transfer (45).
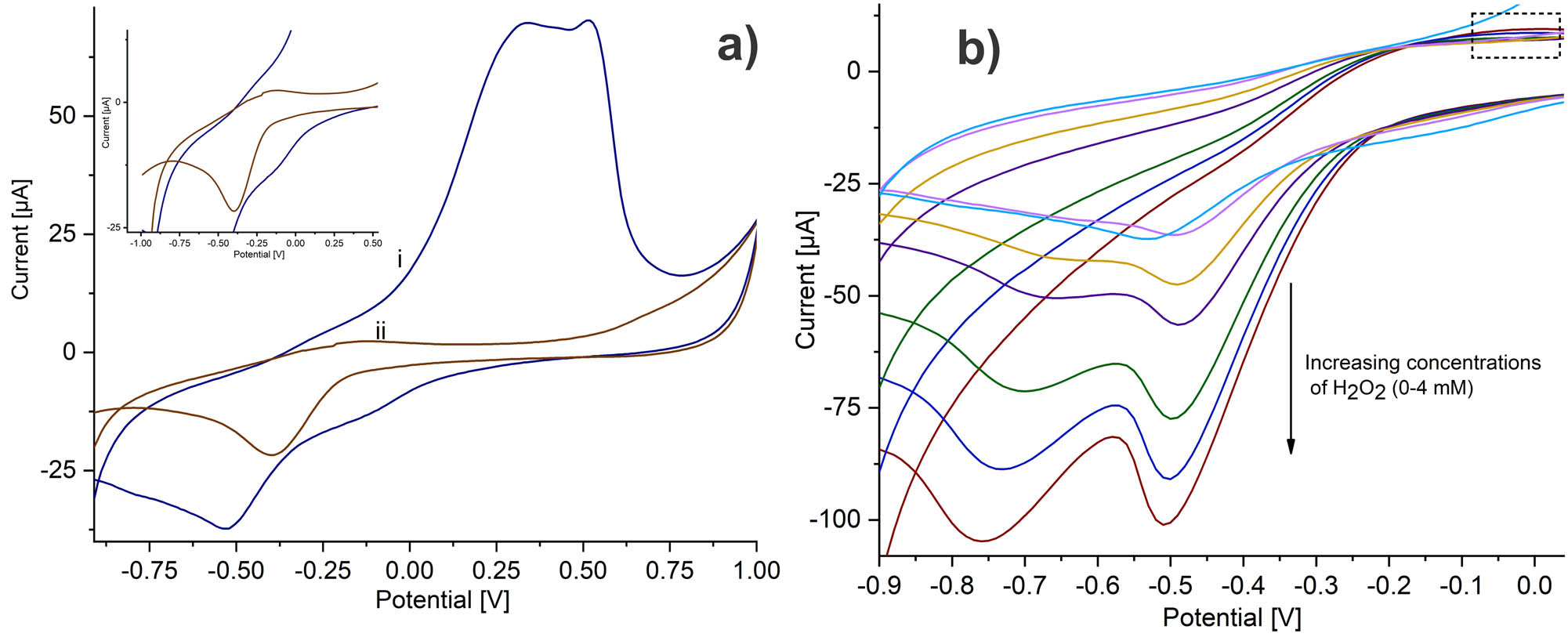
(a) Cyclic voltammetry of (i) SPCE/PAni/AuNP/cMWCNT/CAT and (ii) SPCE/AuNP/cMWCNT/CAT in PBS 0.01 M at 20 mV s−1. (b) Cyclic voltammetry for increasing concentrations of H2O2 in PBS 0.01 M, recorded using the SPCE/PAni/AuNP/cMWCNT/CAT biosensor at a scan rate of 20 mV s−1.
Thus, the nanocomposite properties, such as high surface area, improved electroactivity, conductivity, and functional sites for covalent linking, lead to good electron transfer communication between the center of CAT with the modified PAni/AuNP/cMWCNT electrode.
3.5 Electrocatalytic behavior of CAT-modified electrode toward H2O2
The catalytic activity of the nanocomposite for each step of modification toward H2O2 was evidenced by CV. Figure A3 shows the voltammograms of each electrode modification in the presence of 2 mM of H2O2. The modifications without CAT presented a broad reduction peak around −0.75 V, which is attributed to reducing O2 generated by the applied potential in the modified electrode at the H2O2 solution (46). While PAnI/AuNP/cMWCNT/CAT exhibited higher current intensity due to a cathodic peak at −0.4 V, such response is a probe of the enhanced catalytic activity from the anchored enzyme. Thus, incorporating the CAT in the nanocomposite led to an improved response towards H2O2 with less potential needed for the electron transfer process. Figure 5b displays that the cathodic peak at −0.49 V manifested a proportional increase in current as the H2O2 concentration raised. The CAT mechanism to H2O2 was proposed by Alfonso-Prieto et al. (47). According to this mechanism, CAT catalyzes the decomposition of H2O2 into water and oxygen (2H2O2 → 2H2O + O2). Equations 1 and 2 represent the enzymatic reaction of CAT to H2O2.
The active species responsible for oxidation and/or oxygenation reactions is a high-valent iron intermediate, oxoferryl porphyrin cation radical (Por·+-FeIV═O, Cpd I) obtained by the reaction with H2O2. Once Cpd I is formed (Eq. 1), it immediately reacts with a second molecule of H2O2, reducing Cpd I to regenerate CAT (Por-FeIII) with water and oxygen as by-products (Eq. 2). The reaction involves a two-electron redox process (48); therefore, the linked CAT over the support provided a fast direct electron transfer reaction between the heme group of CAT and the modified electrode surface and, consequently, an electrocatalytic activity toward H2O2.
It is worth saying that all components of the composite play a very important role in the electrochemical platform. The electrochemical characterization demonstrated that both cMWCNTs and AuNP helped to increase the active surface of PAni, improving the inherent PAni electroactivity. But also the incorporation of PAni on the nanocomposite is essential since it helps to maintain the direct electron transfer from the CAT (Figure A4). The nanocomposite without PAni presented a definition loss of the cathodic peak of CAT as the concentration of H2O2 increased. Whereas, with the presence of PAni, the cathodic peak of CAT remained defined. This behavior could be attributed to the biocompatible properties of PAni, which contribute to an optimal environment for enzyme performance. Hence, besides working as a transducer surface, the PAni could anchor some enzymes by physical adsorption or covalent linkage (6). Thus, a synergistic effect was formed in conjunction with the cMWCNTs and AuNP protecting the native structure of CAT and maintaining the accessibility of the catalytic sites and, therefore, facilitated the direct transfer of the CAT center to the electrode surface.
3.6 Amperometric determination of H2O2
According to the response observed in CV, the biosensor was evaluated for the detection of H2O2 by amperometry at −0.4 V. Figure A5 shows the amperometric response toward H2O2 at every stage of the nanocomposite assembly. After the modification of the SPCE with PAni and PAni/AuNP, a small current was recorded at the selected potential. However, PAni/AuNP/cMWCNT nanocomposite showed a clear improvement due to the electrocatalytic activity of cMWCNT toward H2O2. This step improved the electron transfer rate enabling the H2O2 reduction through electron transfer, which is the principle of non-enzymatic sensors (49). Indeed, some non-enzymatic amperometric sensors for H2O2 detection have been developed (50,51,52). Although these kinds of sensors show good electrochemical response and have the advantage of avoiding enzymatic susceptibility, they usually need a higher potential for H2O2 reduction.
With the immobilization of CAT, it was evident that the biosensor exhibited the best amperometric response for H2O2 detection. Furthermore, the incorporation of CAT into the nanocomposite led to an improved response toward H2O2 with less working potential needed for the electron transfer process. The electrode modified with PAni/AuNP/cMWCNT exhibits an enhanced catalytic activity toward H2O2 with the CAT loading, corroborating the results obtained by CV. The direct electron transfer between the heme group of CAT and the electrode surface significantly improved the device sensitivity because of the effective covalent linkage of CAT and the synergistic effects of the PAni/AuNP/cMWCNT support. Hence, CAT immobilization was critical in the biosensor assembly to enhance the amperometric response.
The biosensor capacity was evaluated by amperometry with successive additions of H2O2 at −0.4 V under optimized conditions. Figure 6a and b illustrates the i versus t curve and the calculated calibration curve (n = 3), respectively. The current as a function of H2O2 concentration showed a linear response of i to the increasing level of H2O2, with R 2 = 0.9921. The biosensor exhibited a linear range from 0.01 to 6.8 mM, a sensitivity of 58.8 µA mM−1 cm−2, and a LOD of 2.34 µM (LOD = 3.3 * σ/S, where σ and S are the standard deviation and sensitivity, respectively). Notably, the device showed a fast amperometric response after only 1.28 s.
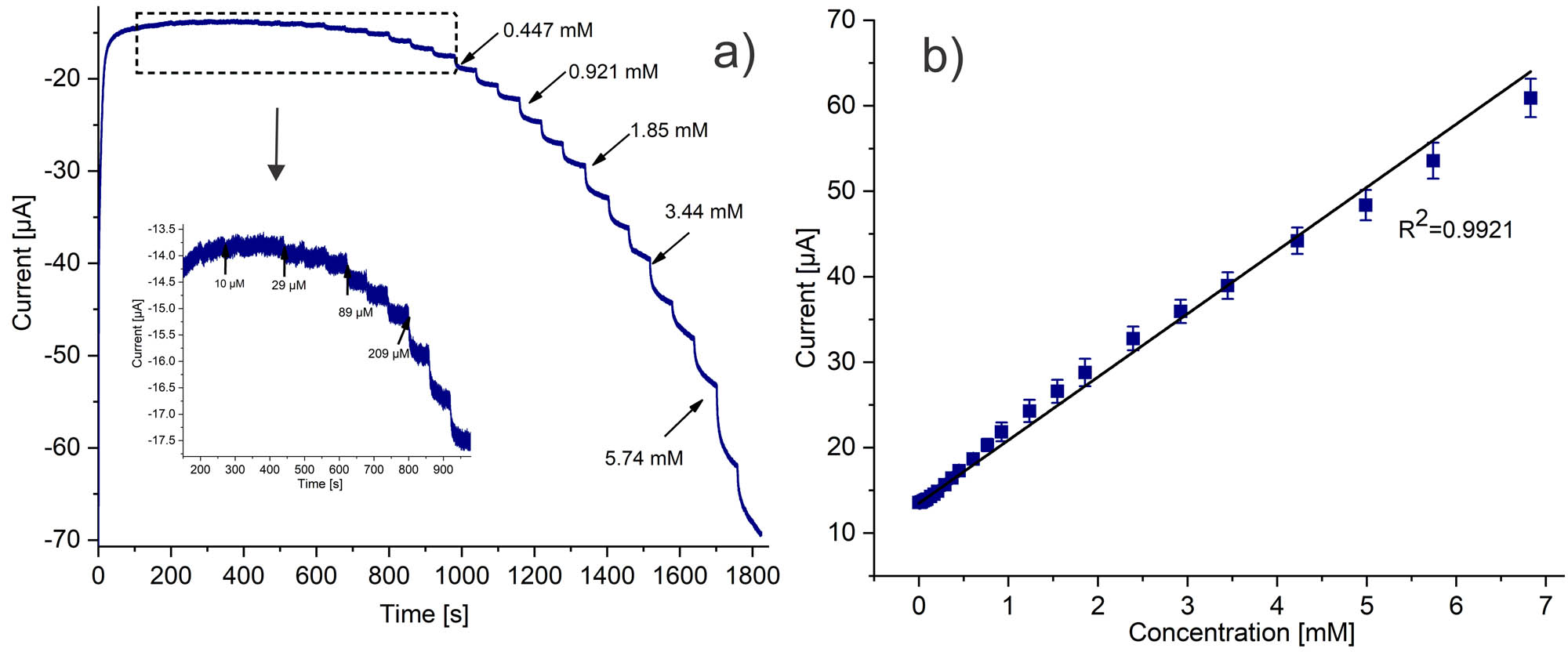
(a) Chronoamperometry response for successive H2O2 additions recorder using SPCE/PAni/AuNP/cMWCNT/CAT in PBS 0.01 M at −0.4 V. (b) Calibration curve.
Table 1 reports a comparison of electrocatalytic values of some electrochemical (bio)sensors for H2O2. The comparative data showed that the biosensor properties are comparable or even better concerning previously reported catalase biosensors, especially considering LOD. For instance, the biosensor based on CAT/Fe@G-MWCNTs showed a similar linear range, but higher LOD (45). In another report, CAT immobilized onto an inorganic hybrid micro-flower HMFs (Cu3(PO4)2)-modified GCE exhibited a wide linear concentration range but higher LOD (53). Similarly, the CAT/PNREthaline/Fe2O3NP/GCE biosensor showed a higher LOD and smaller linear range (54). It is worth saying that the notable performance of the PAni/AuNP/cMWCNT/CAT biosensor expressed the synergistic effects of both the high surface area of the cMWCNT and AuNP and the PAni inherent electroactivity.
Comparison of electroanalytical parameters of electrochemical (bio)sensors for H2O2 detection
| Electrode | LOD (µM) | Linear range (mM) | Sensitivity | k m (mM) | Reference |
|---|---|---|---|---|---|
| CAT/Fe@G-MWCNTs | 28.2 | 0.1–7 | 0.059 µA mM−1 cm−2 | 17.9 | (45) |
| PEI/CAT/MWCNT-NF/GCE | 1 | 0.01–5 | 30 μA mM−1 | 3 | (55) |
| CAT-BOX/GAD/MWCNTs/GCE | 54.4 | 0.05–0.99 | 21.34 µA mM−1 | 2.5 | (56) |
| CAT/MWCNT/Nafion/thionine | 8.7 | 0.01–0.1 | 6051.0 µA mM−1 cm−2 | — | (57) |
| CAT-HMFs/GCE | 50 | 1–10 | 3.471 µA mM−1 | — | (53) |
| Cat/PNREthaline/Fe2O3NP/GCE | 4.3 | 0.01–0.150 | 25.1 µA mM−1cm−2 | 0.13 | (54) |
| BiVO4/FTO | 8.5 | 0.05–1.5 | — | — | (23) |
| GO-PAMAM-Fe2+/GCE | 0.18 | 0.0005–2 | 2.71 μA μM−1cm−2 | — | (24) |
| HRP-NpAc-IL/MWCNT/GCE | 2.7 | 0.01–2.07 | 55.98 μA mM−1 cm−2 | — | (58) |
| HRP/AgNPs/PANI/G/GCE | 30 | 0.25–2.25 | 7.46 μA mM−1 | 5.03 | (59) |
| CAT/cMWCNT/AuNP/PAni/SPCE | 2.34 | 0.01–6.8 | 58.83 µA mM−1 cm−2 | 0.808 | This work |
3.7 Kinetic study
When the concentration of H2O2 increases, a response plateau appears (Figure A6(a)), which is characteristic of the typical Michaelis–Menten kinetic behavior (60). To study the enzyme-substrate kinetics, the apparent Michaelis–Menten constant (k m) was calculated from the linear dependence observed in Figure A6(b), using the electrochemical version of the Lineweaver–Burk, Eq. 3 (40):
where I ss is the steady-state current after the addition of H2O2, I max is the maximum current measured under saturated conditions, and C is the bulk concentration of the substrate. The analysis of the reciprocals of the steady-state current (1/I ss) versus H2O2 concentration (1/C) yields a k m of 0.808 mM. The low value of k m indicated, by comparing with other configurations with catalase (Table 1), that the CAT immobilized onto PAni/AuNP/cMWCNT retains bioactivity and high affinity toward H2O2.
3.8 Sensor stability and specificity
Common interfering substances, such as glucose, ascorbic acid, KCl, and NaCl, were tested for confirming sensor selectivity. Figure 7a shows no significant responses for the evaluated species but a repeatable current response after H2O2 injection, confirming high biosensor selectivity toward the analyte. The biosensor was kept at 4°C and assessed for 30 days to evaluate stability (Figure 7b). After storage, the biosensor retained almost 85% of the initial current. Such favorable long-term stability expressed the stable enzyme bonding with the cMWCNT at the electrode surface and the favorable biocompatible environment provided by the assembled nanocomposite.
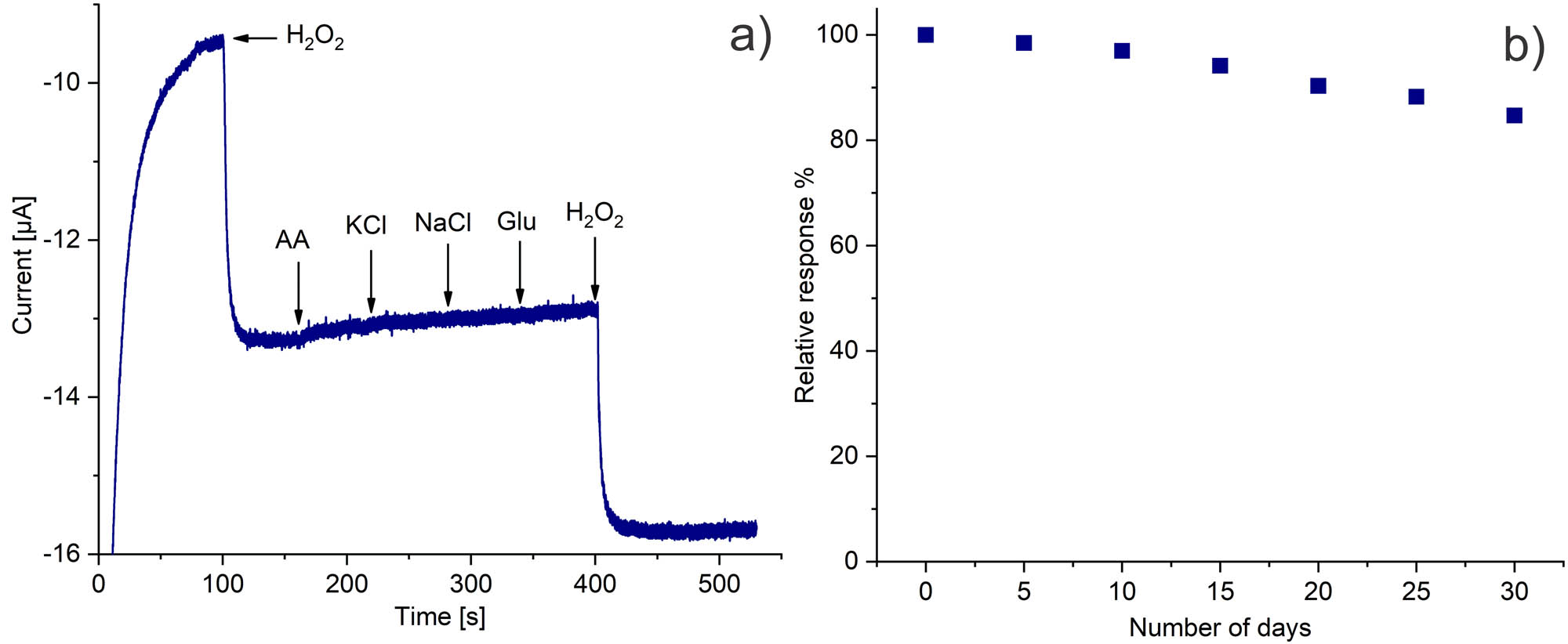
(a) Selectivity and (b) stability of the SPCE/PAni/AuNP/cMWCNT/CAT biosensor.
3.9 Determination of H2O2 in real samples
H2O2 is widely used as an antiseptic agent for preserving milk in the dairy industry. For practical purposes, the standard addition method was used to determine H2O2 concentrations in commercial milk (whole fat milk) at PAni/AuNP/cMWCNT/CAT by amperometric method. The standard addition method (regularly denoted as “spiking” the sample) is commonly used to determine the analyte concentration in complex samples containing other components that could interfere with the analyte signal, causing inaccuracy determination. The idea is to spike the sample and monitor the amperometric response change, assuming the response is only due to a change in analyte concentration (61). The analysis was carried out by spiking a known concentration of H2O2 in the milk samples without prior treatment and recording their recoveries. Before analysis, a blank was verified without the addition of H2O2 in the milk sample, which presented no signal. For every sample, three individual measurements were performed.
Table 2 shows the excellent recovery rates obtained, in the range of 92–104.8%. Besides, the H2O2 concentrations used, 0.2, 0.5, and 0.7 mM correspond to 0.00068%, 0.0017%, and 0.0023%, respectively; The use of H2O2 is approved in the USA by the FDA for treating milk, and it cannot exceed 0.05% of the milk weight (21). Therefore, in terms of detecting peroxide in milk for quality control, concentrations smaller than the minimum required were achieved.
Detection of hydrogen peroxide spiked in real milk samples
| Sample | Spiked (mM) | Found (mM) | Recovery (%) | RSDa (%) |
|---|---|---|---|---|
| Milk | 0.2 | 0.184 | 92 | 1.51 |
| 0.5 | 0.524 | 104.8 | 5.30 | |
| 0.7 | 0.715 | 102.1 | 6.03 |
- a
Triplicates were performed.
It should be noted that in this experiment, undiluted milk samples were used; the milk samples were used as they came from the packaging. This presents an advantage since the peroxide was detected in milk without a previous dilution step. Other similar biosensors have been studied to detect peroxide in milk samples; however, they diluted the milk in PBS to perform the detection (62,63,64). These results evidenced the biosensor suitability for the analysis of H2O2 in real samples, confirming its practical application for food quality control.
4 Conclusion
Electrochemical characterization demonstrated that both cMWCNT and AuNP facilitated the increase in the active surface of PAni, and PAni helped maintain direct electron transfer from CAT. The assembly of a PAni/AuNP/cMWCNT platform manifested a synergistic effect as functional support for catalase immobilization allowed retaining the catalytic activity and facilitated the direct electron transfer between the CAT and the modified electrode. The biosensor exhibited notable electrocatalytic activity toward H2O2 with high sensitivity, low LOD, wide linear range, excellent recovery with real milk samples, and favorable long-term stability. Such performance evidenced the catalytic activity of CAT due to the effective covalent linkage on the support. Therefore, the electrode, modified by the PAni/AuNP/cMWCNT nanocomposite, is functional as support for covalent immobilization of enzymes, with high performance, sensitivity, and selectivity.
Acknowledgment
We wish to thank CONACYT for the scholarship granted to Angélica Domínguez-Aragón (701394). Finally, we thank the Laboratorio Nacional de Nanotecnología (CIMAV) and Manuel Román, Claudia Hernández, Karla Campos, Martha Ochoa, and Paola Anchondo for their valuable collaboration during this research.
-
Funding information: This project was funded by the National Council for Science and Technology of Mexico (CONACYT) through the Project CB2016 288802-Q.
-
Author contributions: Angélica Domínguez-Aragón: investigation, methodology, formal analysis, writing – original draft; Rocio B. Domínguez: conceptualization, writing – review and editing; María del Rosario Peralta-Pérez: validation; Erasto Armando Zaragoza-Contreras: writing – review and editing, supervision, funding acquisition.
-
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix
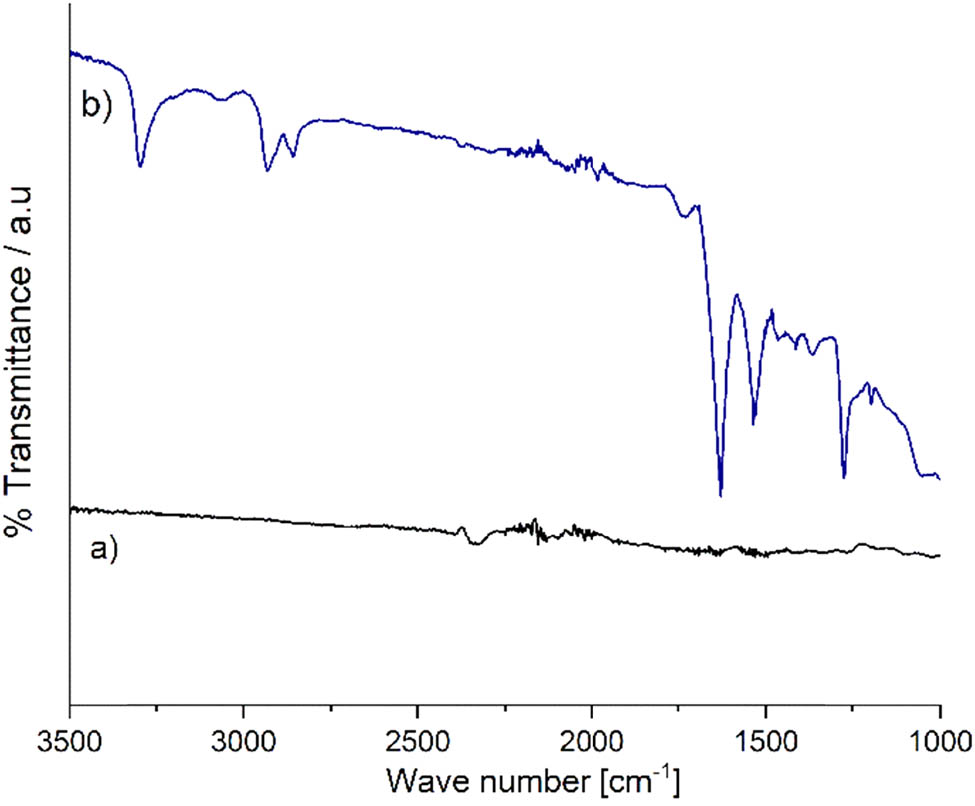
FTIR spectra of (a) MWCNT and (b) cMWCNT.
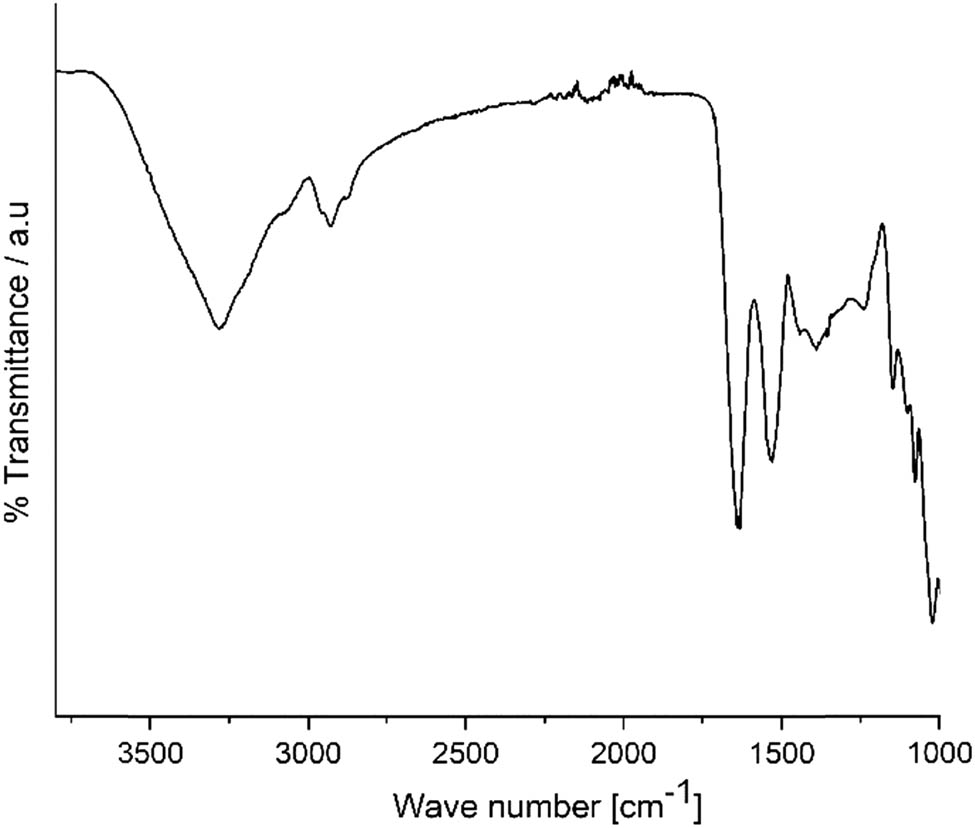
FTIR spectra of catalase.
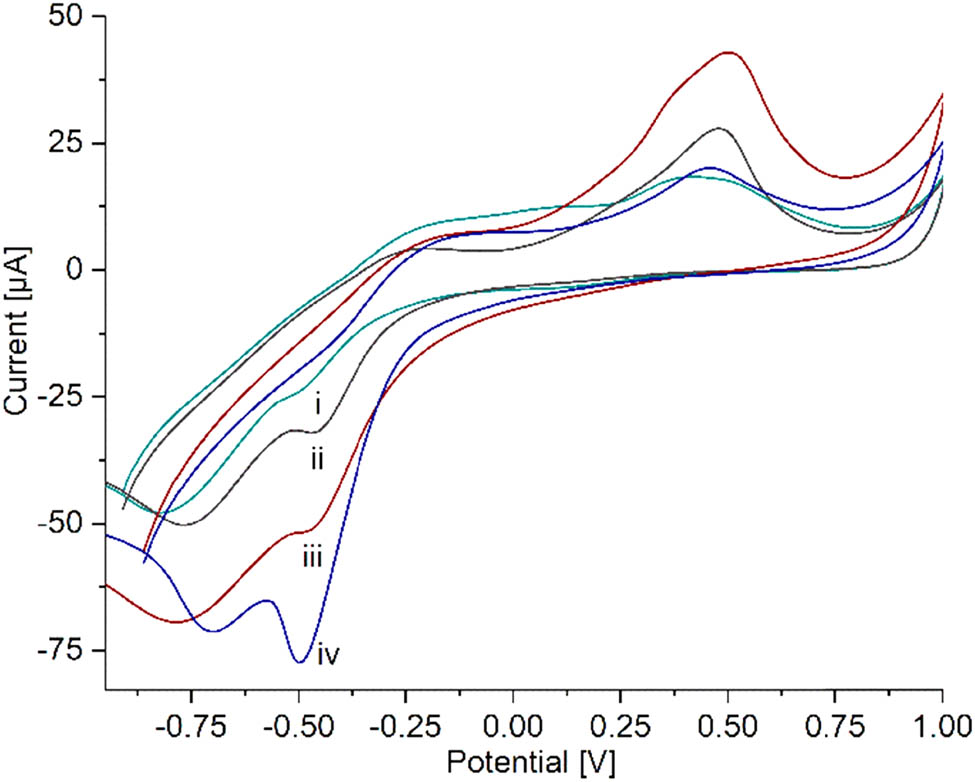
Cyclic voltammetry of (i) SPCE/PAni, (ii) SPCE/PAni/AuNP, (iii) SPCE/PAni/AuNP/cMWCNT, and (iv) SPCE/PAni/AuNP/cMWCNT/CAT biosensor in PBS 0.01 M in presence of 2 mM of H2O2 at 20 mV s−1.
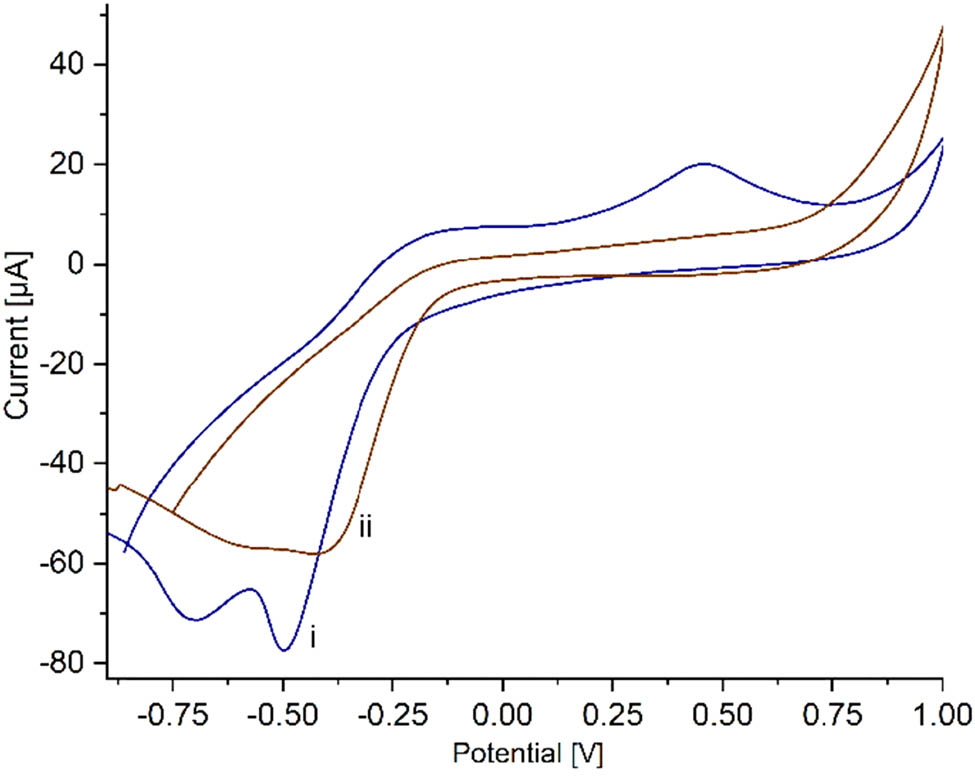
Cyclic voltammetry of (i) SPCE/PAni/AuNP/cMWCNT/CAT and (ii) SPCE/AuNP/cMWCNT/CAT in PBS 0.01 M in presence of H2O2 2 mM at 20 mV s−1.
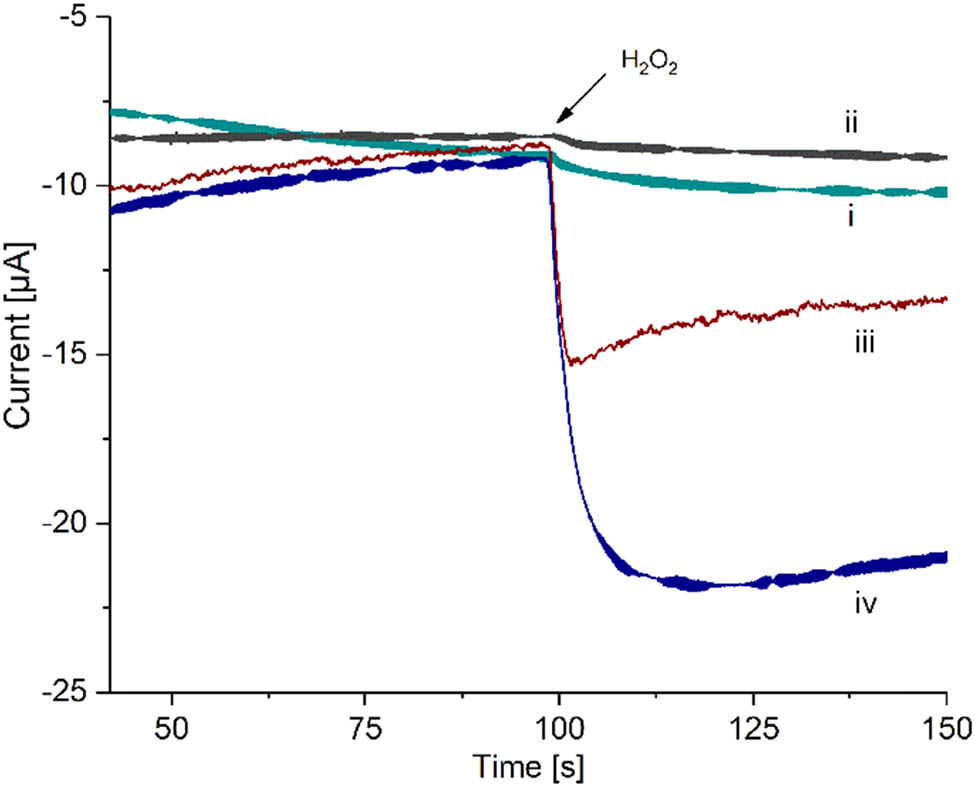
Amperometric response towards H2O2 addition on (i) SPCE/PAni, (ii) SPCE/PAni/AuNP, (iii) SPCE/PAni/AuNP/cMWCNT, and (iv) SPCE/PAni/AuNP/cMWCNT/CAT biosensor.
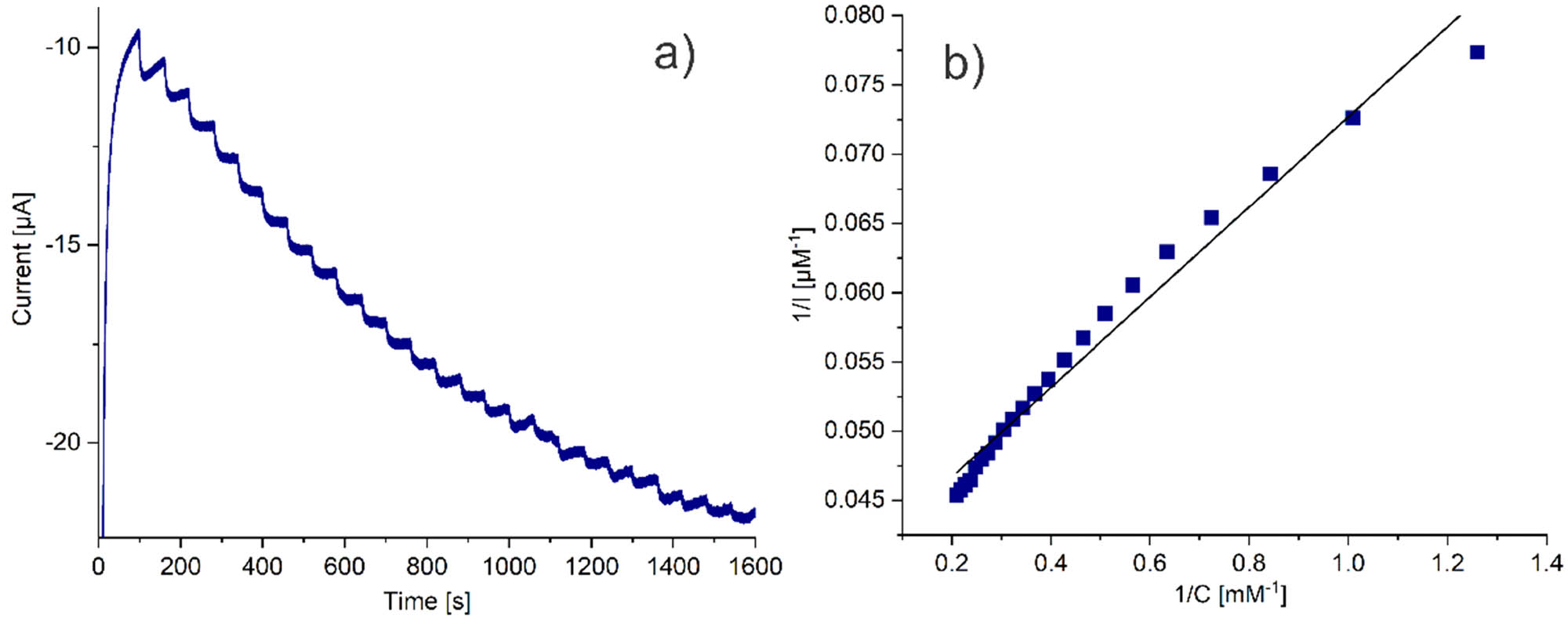
(a) Amperometric response of the SPCE/PAni/AuNP/cMWCNT/CAT biosensor towards successive addition of H2O2 and (b) Lineweaver–Burk plot of 1/I ss versus 1/C H2O2 for the determination of k m for the SPCE/PAni/AuNP/cMWCNT/CAT biosensor.
References
(1) Harrad LEL, Bourais I, Mohammadi H, Amine A. Recent advances in electrochemical biosensors based on enzyme inhibition for clinical and pharmaceutical applications. Sens (Switz). 2018;18(1):164. 10.3390/s18010164.Search in Google Scholar PubMed PubMed Central
(2) Guy OJ, Walker K-AD. Graphene functionalization for biosensor applications. Silicon Carbide Biotechnology. Swansea, United Kingdom: Elsevier; 2016. p. 85–141. 10.1016/B978-0-12-802993-0.00004-6.Search in Google Scholar
(3) Alim S, Vejayan J, Yusoff MM, Kafi AKM. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: a review. Biosens Bioelectron. 2018;121:125–36. 10.1016/j.bios.2018.08.051.Search in Google Scholar PubMed
(4) Idumah CI. Novel trends in conductive polymeric nanocomposites, and bionanocomposites. Synth Met. 2021;273:116674. 10.1016/j.synthmet.2020.116674.Search in Google Scholar
(5) Kumar H, Kumari N, Sharma R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: its issues and challenges. Env Impact Assess Rev. 2020;85:106438. 10.1016/j.eiar.2020.106438.Search in Google Scholar
(6) Lai J, Yi Y, Zhu P, Shen J, Wu K, Zhang L, et al. Polyaniline-based glucose biosensor: a review. J Electroanal Chem. 2016;782:138–53. 10.1016/J.JELECHEM.2016.10.033.Search in Google Scholar
(7) Malhotra B, Dhand C, Lakshminarayanan R, Dwivedi N, Mishra S, Solanki P, et al. Polyaniline-based biosensors. Nanobiosensors Dis Diagnosis. 2015;4:25. 10.2147/NDD.S64841.Search in Google Scholar
(8) Ghorbani Zamani F, Moulahoum H, Ak M, Odaci Demirkol D, Timur S. Current trends in the development of conducting polymers-based biosensors. TrAC Trends Anal Chem. 2019;118:264–76. 10.1016/j.trac.2019.05.031.Search in Google Scholar
(9) Kaur G, Kaur A, Kaur H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym Technol Mater. 2021;60(5):502–19. 10.1080/25740881.2020.1844233.Search in Google Scholar
(10) Zhang H, Liu R, Zheng J. Seed-mediated synthesis of polyaniline/Au nanocomposite and its application for a cholesterol biosensor. Synth Met. 2013;167:5–9. 10.1016/j.synthmet.2013.01.020.Search in Google Scholar
(11) Pu Z, Wang R, Wu J, Yu H, Xu K, Li D. A flexible electrochemical glucose sensor with composite nanostructured surface of the working electrode. Sens Actuators B Chem. 2016;230:801–9. 10.1016/j.snb.2016.02.115.Search in Google Scholar
(12) Oueiny C, Berlioz S, Perrin F-X. Carbon nanotube–polyaniline composites. Prog Polym Sci. 2014;39(4):707–48. 10.1016/j.progpolymsci.2013.08.009.Search in Google Scholar
(13) Bilal M, Anh Nguyen T, Iqbal HMN. Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization – a paradigm shift in biocatalyst design. Coord Chem Rev. 2020;422:213475. 10.1016/j.ccr.2020.213475.Search in Google Scholar
(14) Eguílaz M, Dalmasso PR, Rubianes MD, Gutierrez F, Rodríguez MC, Gallay PA, et al. Recent advances in the development of electrochemical hydrogen peroxide carbon nanotube–based (bio)sensors. Curr Opin Electrochem. 2019;14:157–65. 10.1016/j.coelec.2019.02.007.Search in Google Scholar
(15) Zuccarello L, Barbosa C, Todorovic S, Silveira CM. Electrocatalysis by Heme enzymes – applications in biosensing. Catalysts. 2021;11(2):218. 10.3390/catal11020218.Search in Google Scholar
(16) BRENDA:EC1.11.1.6. https://www.brenda-enzymes.org/enzyme.php?ecno=1.11.1.6; Published 2020.Search in Google Scholar
(17) Jiang H-J, Yang H, Akins DL. Direct electrochemistry and electrocatalysis of catalase immobilized on a SWNT-nanocomposite film. J Electroanal Chem. 2008;623(2):181–6. 10.1016/j.jelechem.2008.07.024.Search in Google Scholar
(18) Grigoras AG. Catalase immobilization – a review. Biochem Eng J. 2017;117:1–20. 10.1016/j.bej.2016.10.021.Search in Google Scholar
(19) Lončar N, Fraaije MW. Catalases as biocatalysts in technical applications: current state and perspectives. Appl Microbiol Biotechnol. 2015;99(8):3351–7. 10.1007/s00253-015-6512-6.Search in Google Scholar PubMed
(20) Meier J, Hofferber E M, Stapleton J A, Iverson N M. Hydrogen peroxide sensors for biomedical applications. Chemosensors. 2019;7(4):64. 10.3390/chemosensors7040064.Search in Google Scholar
(21) Abbas ME, Luo W, Zhu L, Zou J, Tang H. Fluorometric determination of hydrogen peroxide in milk by using a fenton reaction system. Food Chem. 2010;120(1):327–31. 10.1016/j.foodchem.2009.10.024.Search in Google Scholar
(22) Mollarasouli F, Kurbanoglu S, Asadpour-Zeynali K, Ozkan SA. Non-enzymatic monitoring of hydrogen peroxide using novel nanosensor based on CoFe2O4@CdSeQD magnetic nanocomposite and rifampicin mediator. Anal Bioanal Chem. 2020;412(21):5053–65. 10.1007/s00216-019-02306-y.Search in Google Scholar PubMed
(23) Yu Z, Lv S, Ren R, Cai G, Tang D. Photoelectrochemical sensing of hydrogen peroxide at zero working potential using a fluorine-doped tin oxide electrode modified with BiVO4 microrods. Microchim Acta. 2017;184(3):799–806. 10.1007/s00604-016-2071-5.Search in Google Scholar
(24) Tang J, Huang L, Cheng Y, Zhuang J, Li P, Tang D. Nonenzymatic sensing of hydrogen peroxide using a glassy carbon electrode modified with graphene oxide, a polyamidoamine dendrimer, and with polyaniline deposited by the Fenton reaction. Microchim Acta. 2018;185(12):569. 10.1007/s00604-018-3089-7.Search in Google Scholar PubMed
(25) Yang C, Denno ME, Pyakurel P, Venton BJ. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: a review. Anal Chim Acta. 2015;887:17–37. 10.1016/j.aca.2015.05.049.Search in Google Scholar PubMed PubMed Central
(26) Thi Mai Hoa L. Characterization of multi-walled carbon nanotubes functionalized by a mixture of HNO3/H2SO4. Diam Relat Mater. 2018;89:43–51. 10.1016/j.diamond.2018.08.008.Search in Google Scholar
(27) Rohom AB, Londhe PU, Mahapatra SK, Kulkarni SK, Chaure NB. Electropolymerization of polyaniline thin films. High Perform Polym. 2014;26(6):641–6. 10.1177/0954008314538081.Search in Google Scholar
(28) Liao L, Meng Y, Wang R, Jia B, Li P. Coupling and regulation of porous carriers using plasma and amination to improve the catalytic performance of glucose oxidase and catalase. Front Bioeng Biotechnol. 2019;7:426. 10.3389/fbioe.2019.00426.Search in Google Scholar PubMed PubMed Central
(29) Bora A, Mohan K, Pegu D, Gohain CB, Dolui SK. A room temperature methanol vapor sensor based on highly conducting carboxylated multi-walled carbon nanotube/polyaniline nanotube composite. Sens Actuators B Chem. 2017;253:977–86. 10.1016/j.snb.2017.07.023.Search in Google Scholar
(30) Peng S, Liu C, Fan X. Surface modification of graphene oxide by carboxyl-group: preparation, characterization, and application for proteins immobilization. Integr Ferroelectr. 2015;163(1):42–53. 10.1080/10584587.2015.1040328.Search in Google Scholar
(31) Ajeel KI, Kareem QS. Synthesis and characteristics of polyaniline (PANI) filled by graphene (PANI/GR) nano-films. J Phys Conf Ser. 2019;1234(1):012020. 10.1088/1742-6596/1234/1/012020.Search in Google Scholar
(32) Trchová M, Stejskal J. Polyaniline: the infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl Chem. 2011;83(10):1803–17. 10.1351/PAC-REP-10-02-01.Search in Google Scholar
(33) Zengin H, Zhou W, Jin J, Czerw R, Smith DW, Echegoyen L, et al. Carbon Nanotube Doped Polyaniline. Adv Mater. 2002;14(20):1480–3. 10.1002/1521-4095(20021016)14:20<1480:AID-ADMA1480>3.0.CO;2-O.Search in Google Scholar
(34) Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin (Shanghai). 2007;39(8):549–59. 10.1111/j.1745-7270.2007.00320.x.Search in Google Scholar
(35) Chawla S, Rawal R, Sharma S, Pundir CS. An amperometric biosensor based on laccase immobilized onto nickel nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode for determination of phenolic content in fruit juices. Biochem Eng J. 2012;68:76–84. 10.1016/j.bej.2012.07.008.Search in Google Scholar
(36) Rawal R, Chawla S, Devender, Pundir CS. An amperometric biosensor based on laccase immobilized onto Fe3O4NPs/cMWCNT/PANI/Au electrode for determination of phenolic content in tea leaves extract. Enzyme Microb Technol. 2012;51(4):179–85. 10.1016/j.enzmictec.2012.06.001.Search in Google Scholar
(37) Song E, Choi J-W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials. 2013;3(3):498–523. 10.3390/nano3030498.Search in Google Scholar
(38) Mažeikien R, Niaura G, Malinauskas A. Voltammetric study of the redox processes of self-doped sulfonated polyaniline. Synth Met. 2003;139(1):89–94. 10.1016/S0379-6779(03)00037-7.Search in Google Scholar
(39) Fusco G, Gallo F, Tortolini C, Bollella P, Ietto F, De Mico A, et al. AuNPs-functionalized PANABA-MWCNTs nanocomposite-based impedimetric immunosensor for 2,4-dichlorophenoxy acetic acid detection. Biosens Bioelectron. 2017;93(June 2016):52–6. 10.1016/j.bios.2016.10.016.Search in Google Scholar
(40) Mercante LA, Facure MHM, Sanfelice RC, Migliorini FL, Mattoso LHC, Correa DS. One-pot preparation of PEDOT:PSS-reduced graphene decorated with Au nanoparticles for enzymatic electrochemical sensing of H2O2. Appl Surf Sci. 2017;407:162–70. 10.1016/J.APSUSC.2017.02.156.Search in Google Scholar
(41) Yadav S, Kumar A, Pundir CS. Amperometric creatinine biosensor based on covalently coimmobilized enzymes onto carboxylated multiwalled carbon nanotubes/polyaniline composite film. Anal Biochem. 2011;419(2):277–83. 10.1016/j.ab.2011.07.032.Search in Google Scholar
(42) Aghamiri ZS, Mohsennia M, Rafiee-Pour H-A. Immobilization of cytochrome c on polyaniline/polypyrrole/carboxylated multi-walled carbon nanotube/glassy carbon electrode: biosensor fabrication. J Solid State Electrochem. 2019;23(7):2233–42. 10.1007/s10008-019-04300-x.Search in Google Scholar
(43) Shu Y, Chen J, Xu Q, Wei Z, Liu F, Lu R, et al. MoS2 nanosheet–Au nanorod hybrids for highly sensitive amperometric detection of H2O2 in living cells. J Mater Chem B. 2017;5(7):1446–53. 10.1039/C6TB02886A Search in Google Scholar
(44) Kowalewska B, Jakubow K. The impact of immobilization process on the electrochemical performance, bioactivity and conformation of glucose oxidase enzyme. Sens Actuators B Chem. 2017;238:852–61. 10.1016/j.snb.2016.07.138.Search in Google Scholar
(45) Soto D, Alzate M, Gallego J, Orozco J. Hybrid nanomaterial/catalase-modified electrode for hydrogen peroxide sensing. J Electroanal Chem. 2021;880:114826. 10.1016/j.jelechem.2020.114826.Search in Google Scholar
(46) Shimizu K, Sepunaru L, Compton RG. Innovative catalyst design for the oxygen reduction reaction for fuel cells. Chem Sci. 2016;7(5):3364–9. 10.1039/C6SC00139D.Search in Google Scholar PubMed PubMed Central
(47) Alfonso-Prieto M, Biarnés X, Vidossich P, Rovira C. The molecular mechanism of the catalase reaction. J Am Chem Soc. 2009;131(33):11751–61. 10.1021/ja9018572.Search in Google Scholar PubMed
(48) Seetharamaiah N, Seetharamaiah N, Pathappa N, Jose SM, Gurukar SS. Metal-ion co-ordination assembly based multilayer of one dimensional gold nanostructures and catalase as electrochemical sensor for the analysis of hydrogen peroxide. Sens Actuators B Chem. 2017;245:726–40. 10.1016/j.snb.2017.02.003.Search in Google Scholar
(49) Bai J, Sun C, Jiang X. Carbon dots-decorated multiwalled carbon nanotubes nanocomposites as a high-performance electrochemical sensor for detection of H2O2 in living cells. Anal Bioanal Chem. 2016;408(17):4705–14. 10.1007/s00216-016-9554-4.Search in Google Scholar PubMed
(50) Dong S, Xi J, Wu Y, Liu H, Fu C, Liu H, et al. High loading MnO2 nanowires on graphene paper: Facile electrochemical synthesis and use as flexible electrode for tracking hydrogen peroxide secretion in live cells. Anal Chim Acta. 2015;853:200–6. 10.1016/j.aca.2014.08.004.Search in Google Scholar PubMed
(51) Lee JH, Huynh-Nguyen B-C, Ko E, Kim JH, Seong GH. Fabrication of flexible, transparent silver nanowire electrodes for amperometric detection of hydrogen peroxide. Sens Actuators B Chem. 2016;224:789–97. 10.1016/j.snb.2015.11.006.Search in Google Scholar
(52) Aparicio-Martínez E, Ibarra A, Estrada-Moreno IA, Osuna V, Dominguez RB. Flexible electrochemical sensor based on laser scribed graphene/Ag nanoparticles for non-enzymatic hydrogen peroxide detection. Sens Actuators B Chem. 2019;301:127101. 10.1016/j.snb.2019.127101.Search in Google Scholar
(53) Zhang M, Chen M, Liu Y, Wang Y, Tang J. Catalase-inorganic hybrid microflowers modified glassy carbon electrode for amperometric detection of hydrogen peroxide. Mater Lett. 2019;243:9–12. 10.1016/j.matlet.2019.01.157.Search in Google Scholar
(54) da Silva W, Queiroz AC, Brett CMA. Nanostructured poly(phenazine)/Fe2O3 nanoparticle film modified electrodes formed by electropolymerization in ethaline – deep eutectic solvent. Microscopic and electrochemical characterization. Electrochim Acta. 2020;347:136284. 10.1016/j.electacta.2020.136284.Search in Google Scholar
(55) Vatsyayan P, Bordoloi S, Goswami P. Large catalase based bioelectrode for biosensor application. Biophys Chem. 2010;153(1):36–42. 10.1016/J.BPC.2010.10.002.Search in Google Scholar PubMed
(56) Dramińska S, Bilewicz R. Bienzymatic mediatorless sensing of total hydrogen peroxide with catalase and multi-copper enzyme co-adsorbed at carbon nanotube-modified electrodes. Sens Actuators B Chem. 2017;248:493–9. 10.1016/j.snb.2017.04.008.Search in Google Scholar
(57) Hashemnia S, Eskanari M. Preparation and electrochemical characterization of an enzyme electrode based on catalase immobilized onto a multiwall carbon nanotube-thionine film. J Chin Chem Soc. 2014;61(8):903–9. 10.1002/jccs.201300469.Search in Google Scholar
(58) Theyagarajan K, Elancheziyan M, Aayushi PS, Thenmozhi K. Facile strategy for immobilizing horseradish peroxidase on a novel acetate functionalized ionic liquid/MWCNT matrix for electrochemical biosensing. Int J Biol Macromol. 2020;163:358–65. 10.1016/j.ijbiomac.2020.07.005.Search in Google Scholar PubMed
(59) Li S, Xiong J, Shen J, Qin Y, Li J, Chu F, et al. A novel hydrogen peroxide sensor based on Ag nanoparticles decorated polyaniline/graphene composites. J Appl Polym Sci. 2015;132(37):800. 10.1002/app.42409.Search in Google Scholar
(60) Periasamy AP, Ho Y-H, Chen S-M. Multiwalled carbon nanotubes dispersed in carminic acid for the development of catalase based biosensor for selective amperometric determination of H2O2 and iodate. Biosens Bioelectron. 2011;29(1):151–8. 10.1016/j.bios.2011.08.010.Search in Google Scholar PubMed
(61) Saxberg BEH, Kowalski BR. Generalized standard addition method. Anal Chem. 1979;51(7):1031–8. 10.1021/ac50043a059.Search in Google Scholar
(62) Murphy M, Theyagarajan K, Thenmozhi K, Senthilkumar S. Quaternary ammonium based carboxyl functionalized ionic liquid for covalent immobilization of horseradish peroxidase and development of electrochemical hydrogen peroxide biosensor. Electroanalysis. 2020;32(11):2422–30. 10.1002/elan.202060240.Search in Google Scholar
(63) Thenmozhi K, Narayanan SS. Horseradish peroxidase and toluidine blue covalently immobilized leak-free sol-gel composite biosensor for hydrogen peroxide. Mater Sci Eng C. 2017;70:223–30. 10.1016/j.msec.2016.08.075.Search in Google Scholar PubMed
(64) Theyagarajan K, Elancheziyan M, Aayushi PS, Thenmozhi K. Facile strategy for immobilizing horseradish peroxidase on a novel acetate functionalized ionic liquid/MWCNT matrix for electrochemical biosensing. Int J Biol Macromol. 2020;163:358–65. 10.1016/j.ijbiomac.2020.07.005.Search in Google Scholar PubMed
© 2021 Angélica Domínguez-Aragón et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Research on the mechanism of gel accelerator on gel transition of PAN solution by rheology and dynamic light scattering
- Gel point determination of gellan biopolymer gel from DC electrical conductivity
- Composite of polylactic acid and microcellulose from kombucha membranes
- Synthesis of highly branched water-soluble polyester and its surface sizing agent strengthening mechanism
- Fabrication and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) modified with nano-montmorillonite biocomposite
- Fabrication of N-halamine polyurethane films with excellent antibacterial properties
- Formulation and optimization of gastroretentive bilayer tablets of calcium carbonate using D-optimal mixture design
- Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging
- Evaluation of physicochemical properties of film-based alginate for food packing applications
- Electrically conductive and light-weight branched polylactic acid-based carbon nanotube foams
- Structuring of hydroxy-terminated polydimethylsiloxane filled by fumed silica
- Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering
- Influence of composite structure design on the ablation performance of ethylene propylene diene monomer composites
- MOFs/PVA hybrid membranes with enhanced mechanical and ion-conductive properties
- Improvement of the electromechanical properties of thermoplastic polyurethane composite by ionic liquid modified multiwall carbon nanotubes
- Natural rubber latex/MXene foam with robust and multifunctional properties
- Rheological properties of two high polymers suspended in an abrasive slurry jet
- Two-step polyaniline loading in polyelectrolyte complex membranes for improved pseudo-capacitor electrodes
- Preparation and application of carbon and hollow TiO2 microspheres by microwave heating at a low temperature
- Properties of a bovine collagen type I membrane for guided bone regeneration applications
- Fabrication and characterization of thermoresponsive composite carriers: PNIPAAm-grafted glass spheres
- Effect of talc and diatomite on compatible, morphological, and mechanical behavior of PLA/PBAT blends
- Multifunctional graphene nanofiller in flame retarded polybutadiene/chloroprene/carbon black composites
- Strain-dependent wicking behavior of cotton/lycra elastic woven fabric for sportswear
- Enhanced dielectric properties and breakdown strength of polymer/carbon nanotube composites by coating an SrTiO3 layer
- Analysis of effect of modification of silica and carbon black co-filled rubber composite on mechanical properties
- Polytriazole resins toughened by an azide-terminated polyhedral oligomeric silsesquioxane (OADTP)
- Phosphine oxide for reducing flammability of ethylene-vinyl-acetate copolymer
- Study on preparation and properties of bentonite-modified epoxy sheet molding compound
- Polyhedral oligomeric silsesquioxane (POSS)-modified phenolic resin: Synthesis and anti-oxidation properties
- Study on structure and properties of natural indigo spun-dyed viscose fiber
- Biodegradable thermoplastic copolyester elastomers: Methyl branched PBAmT
- Investigations of polyethylene of raised temperature resistance service performance using autoclave test under sour medium conditions
- Investigation of corrosion and thermal behavior of PU–PDMS-coated AISI 316L
- Modification of sodium bicarbonate and its effect on foaming behavior of polypropylene
- Effect of coupling agents on the olive pomace-filled polypropylene composite
- High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide
- Removal of methylene blue in water by electrospun PAN/β-CD nanofibre membrane
- Theoretical and experimental studies on the fabrication of cylindrical-electrode-assisted solution blowing spinning nanofibers
- Influence of l-quebrachitol on the properties of centrifuged natural rubber
- Ultrasonic-modified montmorillonite uniting ethylene glycol diglycidyl ether to reinforce protein-based composite films
- Experimental study on the dissolution of supercritical CO2 in PS under different agitators
- Experimental research on the performance of the thermal-reflective coatings with liquid silicone rubber for pavement applications
- Study on controlling nicotine release from snus by the SIPN membranes
- Catalase biosensor based on the PAni/cMWCNT support for peroxide sensing
- Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol
- Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid
- Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride
- Polypropylene/graphene nanoplatelets nanocomposites with high conductivity via solid-state shear mixing
- Mechanical properties of fiber-reinforced asphalt concrete: Finite element simulation and experimental study
- Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery
- Preparation and characterizations of antibacterial–antioxidant film from soy protein isolate incorporated with mangosteen peel extract
- Preparation and adsorption properties of Ni(ii) ion-imprinted polymers based on synthesized novel functional monomer
- Rare-earth doped radioluminescent hydrogel as a potential phantom material for 3D gel dosimeter
- Effects of cryogenic treatment and interface modifications of basalt fibre on the mechanical properties of hybrid fibre-reinforced composites
- Stable super-hydrophobic and comfort PDMS-coated polyester fabric
- Impact of a nanomixture of carbon black and clay on the mechanical properties of a series of irradiated natural rubber/butyl rubber blend
- Preparation and characterization of a novel composite membrane of natural silk fiber/nano-hydroxyapatite/chitosan for guided bone tissue regeneration
- Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride
- A new method for plugging the dominant seepage channel after polymer flooding and its mechanism: Fracturing–seepage–plugging
- Analysis of the rheological property and crystallization behavior of polylactic acid (Ingeo™ Biopolymer 4032D) at different process temperatures
- Hybrid green organic/inorganic filler polypropylene composites: Morphological study and mechanical performance investigations
- In situ polymerization of PEDOT:PSS films based on EMI-TFSI and the analysis of electrochromic performance
- Effect of laser irradiation on morphology and dielectric properties of quartz fiber reinforced epoxy resin composite
- The optimization of Carreau model and rheological behavior of alumina/linear low-density polyethylene composites with different alumina content and diameter
- Properties of polyurethane foam with fourth-generation blowing agent
- Hydrophobicity and corrosion resistance of waterborne fluorinated acrylate/silica nanocomposite coatings
- Investigation on in situ silica dispersed in natural rubber latex matrix combined with spray sputtering technology
- The degradable time evaluation of degradable polymer film in agriculture based on polyethylene film experiments
- Improving mechanical and water vapor barrier properties of the parylene C film by UV-curable polyurethane acrylate coating
- Thermal conductivity of silicone elastomer with a porous alumina continuum
- Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate
- Combining good dispersion with tailored charge trapping in nanodielectrics by hybrid functionalization of silica
- Thermosensitive hydrogel for in situ-controlled methotrexate delivery
- Analysis of the aging mechanism and life evaluation of elastomers in simulated proton exchange membrane fuel cell environments
- The crystallization and mechanical properties of poly(4-methyl-1-pentene) hard elastic film with different melt draw ratios
- Review Articles
- Aromatic polyamide nonporous membranes for gas separation application
- Optical elements from 3D printed polymers
- Evidence for bicomponent fibers: A review
- Mapping the scientific research on the ionizing radiation impacts on polymers (1975–2019)
- Recent advances in compatibility and toughness of poly(lactic acid)/poly(butylene succinate) blends
- Topical Issue: (Micro)plastics pollution - Knowns and unknows (Guest Editor: João Pinto da Costa)
- Simple pyrolysis of polystyrene into valuable chemicals
- Topical Issue: Recent advances of chitosan- and cellulose-based materials: From production to application (Guest Editor: Marc Delgado-Aguilar)
- In situ photo-crosslinking hydrogel with rapid healing, antibacterial, and hemostatic activities
- A novel CT contrast agent for intestinal-targeted imaging through rectal administration
- Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review
- Towards the use of acrylic acid graft-copolymerized plant biofiber in sustainable fortified composites: Manufacturing and characterization
Articles in the same Issue
- Research Articles
- Research on the mechanism of gel accelerator on gel transition of PAN solution by rheology and dynamic light scattering
- Gel point determination of gellan biopolymer gel from DC electrical conductivity
- Composite of polylactic acid and microcellulose from kombucha membranes
- Synthesis of highly branched water-soluble polyester and its surface sizing agent strengthening mechanism
- Fabrication and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) modified with nano-montmorillonite biocomposite
- Fabrication of N-halamine polyurethane films with excellent antibacterial properties
- Formulation and optimization of gastroretentive bilayer tablets of calcium carbonate using D-optimal mixture design
- Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging
- Evaluation of physicochemical properties of film-based alginate for food packing applications
- Electrically conductive and light-weight branched polylactic acid-based carbon nanotube foams
- Structuring of hydroxy-terminated polydimethylsiloxane filled by fumed silica
- Surface functionalization of nanostructured Cu/Ag-deposited polypropylene fiber by magnetron sputtering
- Influence of composite structure design on the ablation performance of ethylene propylene diene monomer composites
- MOFs/PVA hybrid membranes with enhanced mechanical and ion-conductive properties
- Improvement of the electromechanical properties of thermoplastic polyurethane composite by ionic liquid modified multiwall carbon nanotubes
- Natural rubber latex/MXene foam with robust and multifunctional properties
- Rheological properties of two high polymers suspended in an abrasive slurry jet
- Two-step polyaniline loading in polyelectrolyte complex membranes for improved pseudo-capacitor electrodes
- Preparation and application of carbon and hollow TiO2 microspheres by microwave heating at a low temperature
- Properties of a bovine collagen type I membrane for guided bone regeneration applications
- Fabrication and characterization of thermoresponsive composite carriers: PNIPAAm-grafted glass spheres
- Effect of talc and diatomite on compatible, morphological, and mechanical behavior of PLA/PBAT blends
- Multifunctional graphene nanofiller in flame retarded polybutadiene/chloroprene/carbon black composites
- Strain-dependent wicking behavior of cotton/lycra elastic woven fabric for sportswear
- Enhanced dielectric properties and breakdown strength of polymer/carbon nanotube composites by coating an SrTiO3 layer
- Analysis of effect of modification of silica and carbon black co-filled rubber composite on mechanical properties
- Polytriazole resins toughened by an azide-terminated polyhedral oligomeric silsesquioxane (OADTP)
- Phosphine oxide for reducing flammability of ethylene-vinyl-acetate copolymer
- Study on preparation and properties of bentonite-modified epoxy sheet molding compound
- Polyhedral oligomeric silsesquioxane (POSS)-modified phenolic resin: Synthesis and anti-oxidation properties
- Study on structure and properties of natural indigo spun-dyed viscose fiber
- Biodegradable thermoplastic copolyester elastomers: Methyl branched PBAmT
- Investigations of polyethylene of raised temperature resistance service performance using autoclave test under sour medium conditions
- Investigation of corrosion and thermal behavior of PU–PDMS-coated AISI 316L
- Modification of sodium bicarbonate and its effect on foaming behavior of polypropylene
- Effect of coupling agents on the olive pomace-filled polypropylene composite
- High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide
- Removal of methylene blue in water by electrospun PAN/β-CD nanofibre membrane
- Theoretical and experimental studies on the fabrication of cylindrical-electrode-assisted solution blowing spinning nanofibers
- Influence of l-quebrachitol on the properties of centrifuged natural rubber
- Ultrasonic-modified montmorillonite uniting ethylene glycol diglycidyl ether to reinforce protein-based composite films
- Experimental study on the dissolution of supercritical CO2 in PS under different agitators
- Experimental research on the performance of the thermal-reflective coatings with liquid silicone rubber for pavement applications
- Study on controlling nicotine release from snus by the SIPN membranes
- Catalase biosensor based on the PAni/cMWCNT support for peroxide sensing
- Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol
- Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid
- Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride
- Polypropylene/graphene nanoplatelets nanocomposites with high conductivity via solid-state shear mixing
- Mechanical properties of fiber-reinforced asphalt concrete: Finite element simulation and experimental study
- Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery
- Preparation and characterizations of antibacterial–antioxidant film from soy protein isolate incorporated with mangosteen peel extract
- Preparation and adsorption properties of Ni(ii) ion-imprinted polymers based on synthesized novel functional monomer
- Rare-earth doped radioluminescent hydrogel as a potential phantom material for 3D gel dosimeter
- Effects of cryogenic treatment and interface modifications of basalt fibre on the mechanical properties of hybrid fibre-reinforced composites
- Stable super-hydrophobic and comfort PDMS-coated polyester fabric
- Impact of a nanomixture of carbon black and clay on the mechanical properties of a series of irradiated natural rubber/butyl rubber blend
- Preparation and characterization of a novel composite membrane of natural silk fiber/nano-hydroxyapatite/chitosan for guided bone tissue regeneration
- Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride
- A new method for plugging the dominant seepage channel after polymer flooding and its mechanism: Fracturing–seepage–plugging
- Analysis of the rheological property and crystallization behavior of polylactic acid (Ingeo™ Biopolymer 4032D) at different process temperatures
- Hybrid green organic/inorganic filler polypropylene composites: Morphological study and mechanical performance investigations
- In situ polymerization of PEDOT:PSS films based on EMI-TFSI and the analysis of electrochromic performance
- Effect of laser irradiation on morphology and dielectric properties of quartz fiber reinforced epoxy resin composite
- The optimization of Carreau model and rheological behavior of alumina/linear low-density polyethylene composites with different alumina content and diameter
- Properties of polyurethane foam with fourth-generation blowing agent
- Hydrophobicity and corrosion resistance of waterborne fluorinated acrylate/silica nanocomposite coatings
- Investigation on in situ silica dispersed in natural rubber latex matrix combined with spray sputtering technology
- The degradable time evaluation of degradable polymer film in agriculture based on polyethylene film experiments
- Improving mechanical and water vapor barrier properties of the parylene C film by UV-curable polyurethane acrylate coating
- Thermal conductivity of silicone elastomer with a porous alumina continuum
- Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate
- Combining good dispersion with tailored charge trapping in nanodielectrics by hybrid functionalization of silica
- Thermosensitive hydrogel for in situ-controlled methotrexate delivery
- Analysis of the aging mechanism and life evaluation of elastomers in simulated proton exchange membrane fuel cell environments
- The crystallization and mechanical properties of poly(4-methyl-1-pentene) hard elastic film with different melt draw ratios
- Review Articles
- Aromatic polyamide nonporous membranes for gas separation application
- Optical elements from 3D printed polymers
- Evidence for bicomponent fibers: A review
- Mapping the scientific research on the ionizing radiation impacts on polymers (1975–2019)
- Recent advances in compatibility and toughness of poly(lactic acid)/poly(butylene succinate) blends
- Topical Issue: (Micro)plastics pollution - Knowns and unknows (Guest Editor: João Pinto da Costa)
- Simple pyrolysis of polystyrene into valuable chemicals
- Topical Issue: Recent advances of chitosan- and cellulose-based materials: From production to application (Guest Editor: Marc Delgado-Aguilar)
- In situ photo-crosslinking hydrogel with rapid healing, antibacterial, and hemostatic activities
- A novel CT contrast agent for intestinal-targeted imaging through rectal administration
- Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review
- Towards the use of acrylic acid graft-copolymerized plant biofiber in sustainable fortified composites: Manufacturing and characterization

