Abstract
To investigate the effects of growth hormone (GH) on pubertal obese male rats, a rat model of high-fat diet-induced obesity was established in juvenile male rats. The model rats were divided into the treatment group (GH) and the non-treatment group (physiological saline). After 4 weeks, we measured the levels of alanine transaminase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), estrogen (E2), testosterone (T), and insulin-like growth factor (IGF-1). The morphological changes of the liver and testis were assessed, and the expression of aromatase was detected. The levels of ALT, AST, TC, TG, LDL-C, E2, and IGF-1 in the treatment group were significantly lower than in the non-treated model rats (P < 0.001). The levels of HDL-C and T of GH-treated rats were significantly higher than those of the non-treatment group (P < 0.001). Compared with non-treated model rats, GH-treated model rats showed reduced liver steatosis, improved morphological structure of the testicular seminiferous tubules, and an increased number of spermatogenic cells. The treatment group also showed lower expression of aromatase in the liver and testis compared with the non-treatment group. GH partially protected pubertal male rats from obesity-induced lipid metabolic disorder and sexual retardation.
1 Introduction
Obesity is a disorder of energy balance characterized by the accumulation of excessive body fat. Specific lifestyle behaviors, such as excessive intake of high-fat and high-calorie foods and a lack of exercise, have led to an increased prevalence of obesity in children and adolescents. In the past decade, the prevalence of childhood obesity in the United States has continued to increase, and approximately one-fifth of children were obese in 2016 [1]. The rate of obesity in Chinese boys has increased from 9 to 15.4% between 2007 and 2016 and now ranks seventh in the world [2]. Obesity has been shown to affect lipid metabolism and increase the risk of metabolic syndrome [3]. Obesity also reduces testosterone levels, resulting in male secondary hypogonadism [4,5]. The incidence of obesity and sexual retardation has been increasing in male children, and how to properly treat these children has become an important clinical issue.
Growth hormone (GH), a protein hormone secreted by the adenohypophysis, stimulates tissue growth through anabolism. GH has currently been used in many clinical fields, such as short stature in children [6], GH deficiency in adults [7], and burn injury [8]. Previous studies have shown that GH not only induces lipolysis and improves lipid metabolism [9] but also improves the function of Leydig cells and sperm quality, thus playing an important role in the reproductive system [10]. However, few studies have reported the effects of GH on obese male adolescents with dyslipidemia and delayed sexual maturity. In this study, we investigated the effects of GH on lipid metabolism and sexual development in pubertal obese male rats with the aim of providing scientific support for the clinical treatment of delayed sexual maturity in obese male adolescents.
2 Materials and methods
2.1 Reagents
Recombinant human GH was purchased from GenSci (Changchun, China). Paraformaldehyde was obtained from Shanghai Shenggong (Shanghai, China). Phosphate buffered saline (PBS) powder was purchased from Zhongshan (Beijing, China). Anhydrous ethanol was obtained from the Pharmaceutical Group (Beijing, China). Hematoxylin staining solution was purchased from Jiancheng (Nanjing, China). Eosin staining solution was purchased from Biyuntian (Shanghai, China). Neutral resin was obtained from the Sinopharm Group (Beijing, China). The primary antibody against cytochrome P450 (rabbit origin) was purchased from Zhengneng (Chengdu, China). An horseradish peroxidase (HRP)-labeled secondary antibody (goat rabbit) was obtained from Abcam (Burlingame, CA, USA).
3 Establishment of the rat model of obesity
Specific pathogen-free grade male standard deviation (SD) rats (3 weeks old, weighing 50–55 g, n = 41) were purchased from Beijing Huafukang Biotechnology Co., Ltd, Beijing, China. All animals were housed in an environment with a temperature of 23–25°C and had free access to food. Rats assigned to the model group (n = 26) were fed a high-fat diet, while those in the control group (n = 15) were fed regular chow. At the age of 7 weeks, the body length, body weight, waist circumference, penis length, and testicular size of all rats were measured. Lee’s index was calculated using the following formula: Lee’s index =
The model was considered to be successfully established when Lee’s index was above the maximal value of the control group (mean ± SD) [12].
-
Ethical approval: The research related to animal use has complied with all the relevant national regulations and institutional policies for the care and use of animals and has been approved by the Ethics Committee of Liaocheng People’s Hospital (LPH-2019052).
4 Groups and treatment
At the age of 7 weeks, eight model rats were randomly divided into two groups (n = 4 per group): the treatment group and the non-treatment group. The treatment group received a daily subcutaneous injection of GH (3.0 IU/kg per day) for 4 weeks. The non-treatment group was subcutaneously injected with physiological saline by the same procedure. A control group of rats (regular chow-fed rats, n = 5) received a subcutaneous injection of physiological saline. All rats were fed regular chow during the treatment.
5 Measurements
Physical parameters, including body length, body weight, waist circumference, penis length, and testicular size, were monitored before dissection.
At 4 weeks following treatment, rats were anesthetized with 7% chloral hydrate (0.5 mL/100 g), and 10 mL of blood was collected via the abdominal aorta into a tube containing ethylene diamine tetra-acetic acid. After 2 h, the serum was collected and stored at −20°C. The levels of estrogen (E2), testosterone (T), and insulin-like growth factor (IGF-1) were measured using enzyme linked immunosorbent assay (ELISA) kits purchased from Beijing Likechuangxin Biotechnology Corporation (Beijing, China). The levels of alanine transaminase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were detected using ELISA kits obtained from the Nanjing Jiangcheng Bioengineering Institute (Nanjing, China).
6 Histopathological analysis
After blood collection from rats, the liver and testis were removed, washed with PBS, and fixed with 4% paraformaldehyde. The tissue was dehydrated, paraffin-embedded, and cut into 5-μm-thick sections. The sections were dewaxed to water and stained with H&E. The morphological changes of the liver and testis were observed under an optical microscope (400-fold magnification).
For immunohistochemical staining, tissue sections were dewaxed, blocked with goat serum, and incubated with primary antibody against aromatase (dilution ratio 1:100) overnight at 4°C. The sections were then incubated with HRP-labeled goat anti-rabbit antibody (dilution ratio: 1:50) at 37°C for 30 min. The sections were then observed under an optical microscope. The cytoplasm of the positively stained cells was yellow-brown. The integrated and average optical density of each slide was measured by Image-Pro Plus.
7 Statistical analysis
The data were analyzed using SPSS 23.0 software. The data that were normally distributed were expressed as mean ± standard deviation and compared using one-way analysis of variance followed by pairwise comparison using the least significant difference method. The skewed data were expressed as median (minimum, maximum) and compared by nonparametric test followed by pairwise comparison using the Kruskal-Wallis H rank-sum test. P < 0.05 indicated statistical significance.
8 Results
8.1 Comparison of physical parameters between model and control rats
To investigate the effects of GH on lipid metabolism and sexual development in pubertal obese male rats, we established a rat model of obesity. At 7 weeks of age, eight rats in each group were screened to determine whether the model was successfully established. The model group showed a significantly higher weight and the ratio of waist circumference to body length (W/H) compared with control rats (P < 0.05). The Lee’s index of the model group was also significantly higher than that of the control group (P < 0.05), indicating that the obesity model was successfully established. The penis length of model rats was significantly shorter than that of control animals (P < 0.05). However, no significant difference in the testicular volume was observed between the two groups (P > 0.05) (Table 1).
Comparison of physical parameters of model and control rats
| Model rats (n = 8) | Control rats (n = 8) | P-value | |
|---|---|---|---|
| Body weight (g) | 194.43 ± 15.48 | 175.74 ± 13.09 | 0.021 |
| W/H | 0.79 ± 0.03 | 0.63 ± 0.03 | <0.001 |
| Lee’s index | 315.08 ± 3.91 | 281.53 ± 5.15 | <0.001 |
| Penis length (mm) | 8.99 (8.48, 10.04) | 10.27 (9.78, 12.74) | 0.002 |
| Testicular volume (cm3) | 1.84 ± 0.26 | 2.08 ± 0.37 | 0.171 |
8.2 Comparison of physical parameters among control, non-GH-treated model, and GH-treated model groups
At 4 weeks after treatment, GH-treated rats showed significantly lower Lee’s index and W/H and increased penis length and testicular volume compared with the non-treatment group (P < 0.05). There was no significant difference in body weight between the treatment and non-treatment groups (P > 0.05). No significant differences were observed in Lee’s index, penis length, and testicular volume between the treatment group and the control animals (P > 0.05). However, the body weight and W/H of GH-treated rats were significantly higher than those of the control group (P < 0.05) (Table 2).
Comparison of physical parameters among control, non-GH-treated model, and GH-treated model groups
| Control group (n = 5) | Non-treatment group (n = 4) | Treatment group (n = 4) | |
|---|---|---|---|
| Body weight (g) | 272.26 ± 21.57 | 328.85 ± 16.44* | 330.43 ± 25.95* |
| W/H | 0.69 ± 0.03 | 0.87 ± 0.04* | 0.78 ± 0.01*^ |
| Lee’s index | 292.26 (278.79, 294.69) | 315.59 (300.79, 331.34)* | 286.10 (284.33, 287.90)^ |
| Penis length (mm) | 11.96 (10.46, 12.82) | 9.61 (9.06, 10.06)* | 11.60 (11.44, 11.82)^ |
| Testicular volume (cm3) | 3.50 ± 0.23 | 2.56 ± 0.19* | 3.62 ± 0.24^ |
*P < 0.05, compared with the control group; ^P < 0.05, compared with the non-treatment group.
8.3 Comparison of serological indices among control, non-GH-treated model, and GH-treated model groups
The ALT, AST, TC, TG, LDL-C, E2, and IGF-1 levels were significantly lower in the treatment group, while the levels of HDL-C and T were significantly higher in the treatment group compared with levels in the non-treatment group (P < 0.05). GH-treated model rats also showed significantly higher levels of ALT, AST, TC, TG, LDL-C, E2, and IGF-1, but lower levels of HDL-C and T compared with levels in the control group (P < 0.05) (Table 3).
Comparison of serological indices among control, non-GH-treated model, and GH-treated model groups
| Control group (n = 3) | Non-treatment group (n = 3) | Treatment group (n = 3) | |
|---|---|---|---|
| ALT (IU/L) | 33.80 ± 1.33 | 153.09 ± 1.75* | 82.66 ± 1.16*^ |
| AST (IU/L) | 27.47 ± 0.85 | 120.42 ± 0.63* | 53.71 ± 2.09*^ |
| TC (mmol/L) | 6.36 ± 0.10 | 28.70 ± 0.29* | 14.80 ± 0.37*^ |
| TG (mmol/L) | 2.57 ± 0.09 | 10.80 ± 0.14* | 5.86 ± 0.06*^ |
| HDL-C (mmol/L) | 2.76 ± 0.22 | 0.81 ± 0.04* | 1.88 ± 0.02*^ |
| LDL-C (mmol/L) | 2.93 ± 0.06 | 9.00 ± 0.04* | 4.90 ± 0.03*^ |
| E2 (ng/L) | 5.14 ± 0.08 | 20.81 ± 0.63* | 10.12 ± 0.23*^ |
| T (ng/mL) | 1.26 ± 0.01 | 0.22 ± 0.02* | 0.87 ± 0.02*^ |
| IGF-1 (μg/L) | 5.11 ± 0.10 | 12.45 ± 0.47* | 7.30 ± 0.22*^ |
*P < 0.05, compared with the control group; ^P < 0.05, compared with the non-treatment group.
8.4 Comparison of H&E staining of liver and testis among control, non-GH-treated model, and GH-treated model groups
H&E staining showed that the hepatocytes of control rats had normal morphological structure, abundant cytoplasm, and large and round nuclei located in the center of the cells (Figure 1a). The non-treatment group showed severe hepatic steatosis, with lipid droplets of different sizes in the cytoplasm and nuclei squeezed to one side (Figure 1b). However, the hepatocytes of GH-treated rats showed reduced fat vacuoles, improved morphological features, and better arrangement compared with non-treated animals (Figure 1c).
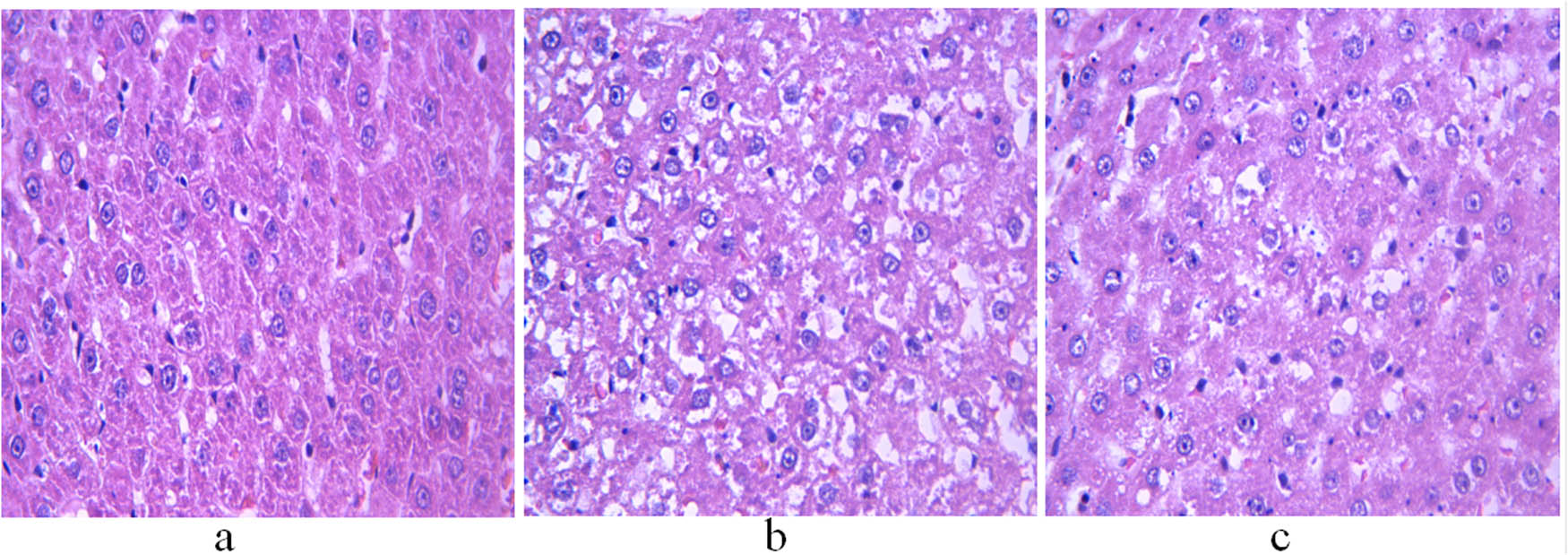
H&E staining of the liver of 11-week-old rats. (a) Control group; (b) non-treatment group; and (c) treatment group. Scale bar = 400 μm.
The seminiferous tubules of the control group had normal morphological structures. Spermatogenic cells were observed in the seminiferous epithelium. Spermatogenesis was observed near the lumen. Leydig cells were distributed singly or in groups between seminiferous tubules (Figure 2a). The non-treatment group showed atrophic seminiferous tubules and a reduced number of spermatogenic cells and Leydig cells. Some spermatogenic cells were necrotized and fell off, blocking the lumen (Figure 2b). Treatment with GH restored the morphological structure of the seminiferous tubules to a certain extent and increased the number of spermatogenic cells. In addition, the regeneration of spermatozoa was observed near the lumen, and Leydig cells were orderly distributed between seminiferous tubules (Figure 2c).
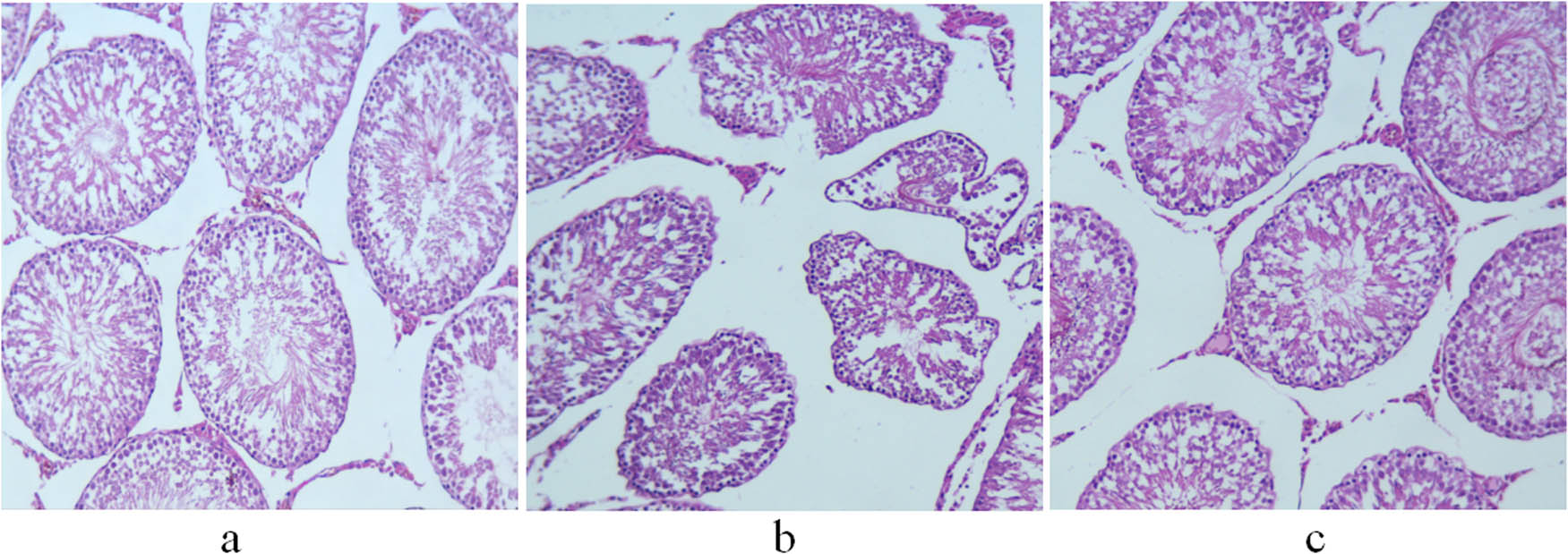
H&E staining of the testis of 11-week-old rats. (a) Control group; (b) non-treatment group; and (c) treatment group. Scale bar = 100 μm.
8.5 Comparison of immunohistochemical staining of aromatase in the liver and testis among control, non-GH-treated model, and GH-treated model groups
The integrated and average optical densities of aromatase-positive staining in the liver were significantly different among the three groups (P < 0.05). The integrated and average optical densities of the treatment group were increased compared with those of the controls (P < 0.05) but were significantly decreased compared with those of the non-treatment group (P < 0.05) (Figures 3 and 4).
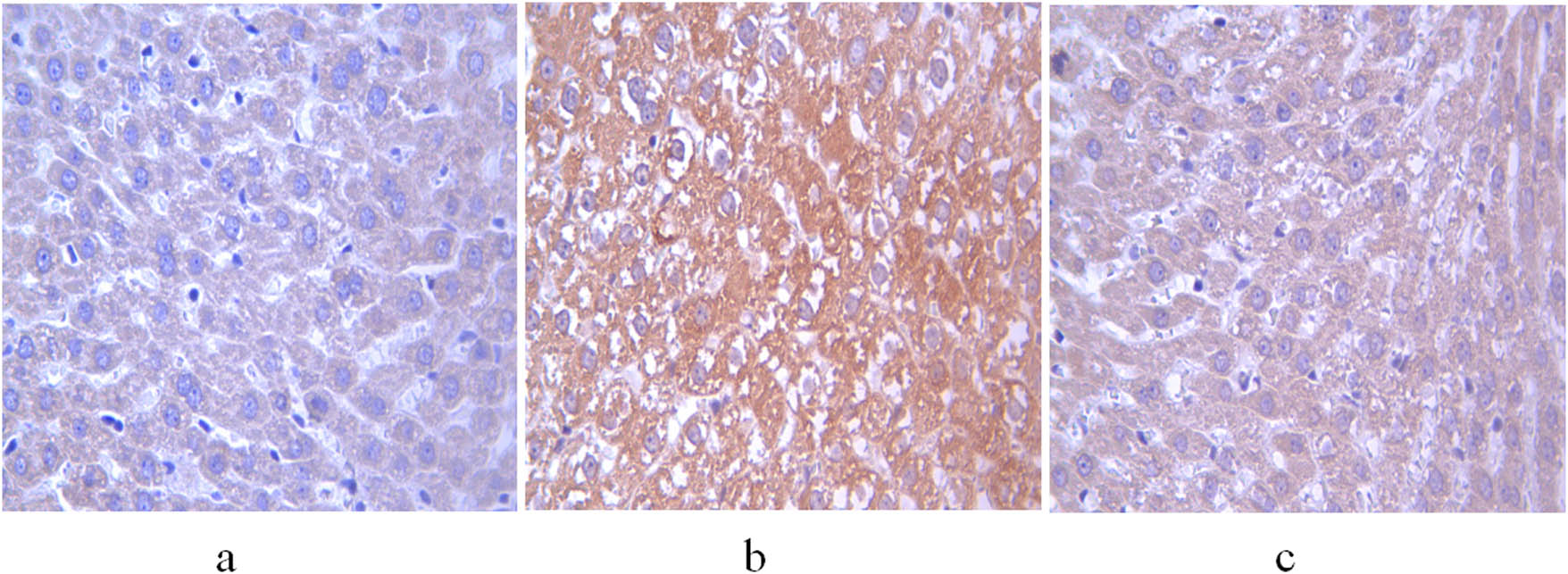
Expression of aromatase in the liver of 11-week-old rats. (a) Control group; (b) non-treatment group; and (c) treatment group. Scale bar = 400 μm.
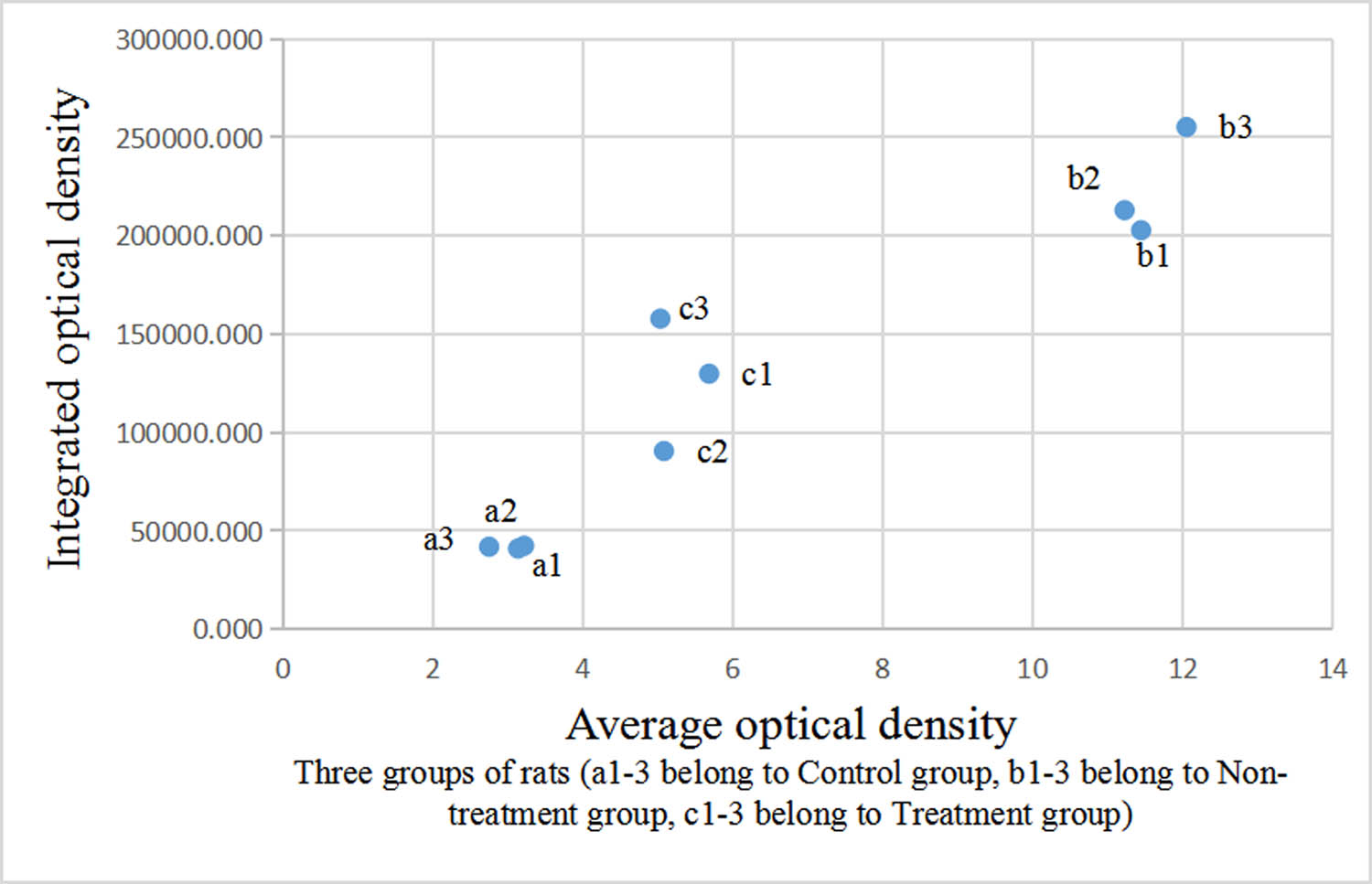
Comparison of optical density of aromatase-positive staining in the liver.
The integrated and average optical densities of aromatase-positive staining in the testis were also significantly different among the three groups (P < 0.05). The integrated and average optical densities of the treatment group were increased compared with those of the controls (P < 0.05) but were significantly decreased compared with those of the non-treatment group (P < 0.05) (Figures 5 and 6).
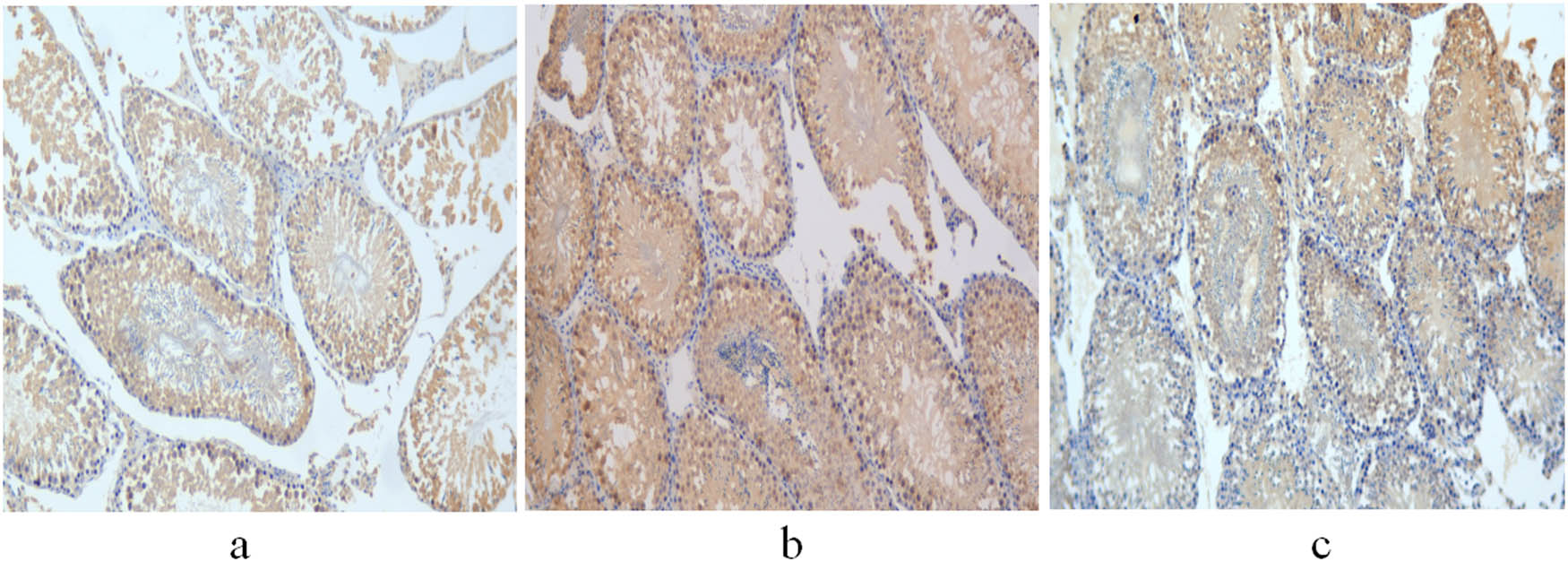
Expression of aromatase in the testis of 11-week-old rats. (a) Control group; (b) non-treatment group; and (c) treatment group. Scale bar = 100 μm.
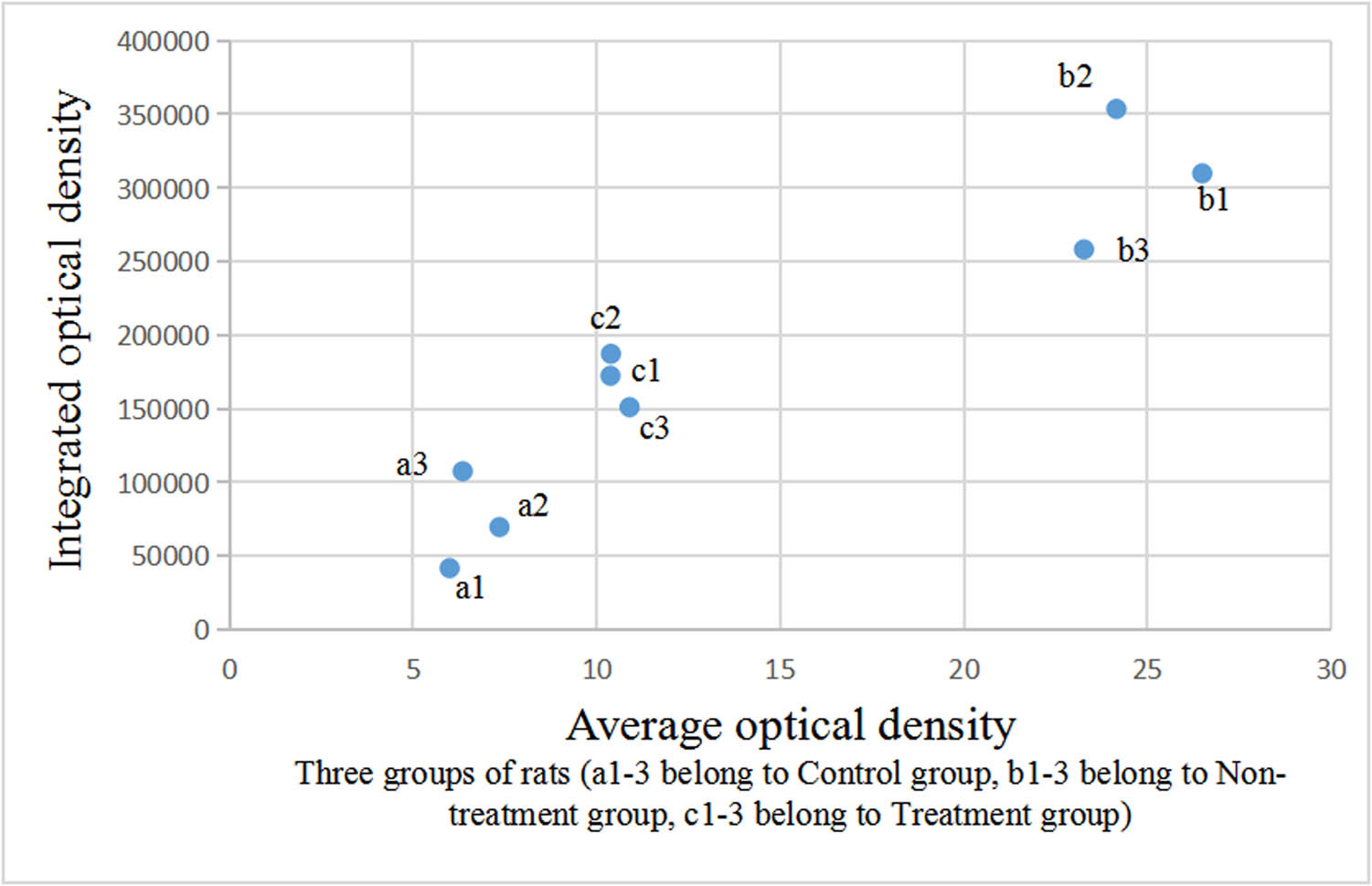
Comparison of optical density of aromatase-positive staining in the testis.
9 Discussion
A high-fat diet leads to an excessive accumulation of fat in the body. The mesenteric adipose tissues have the highest adipogenic activity; therefore, the continuous proliferation of abdominal adipocytes is a key contributor to abdominal obesity [13]. The W/H ratio is an important indicator of abdominal obesity [14]; a higher W/H value corresponds with a higher risk of metabolic disorders [15]. In this study, the body weight and W/H ratio of model rats were significantly higher than those of the control group at the age of 7 weeks, indicating that a high-fat diet before puberty led to abdominal obesity in rats. Continuous accumulation of fat increases the levels of circulating free fatty acids, contributing to the occurrence of hyperlipidemia, type 2 diabetes, and cardiovascular diseases [16]. The incidence of dyslipidemia in obese children is 28–46% [17]. Lipid spectrum disorders are usually manifested by increased TC, TG, and LDL and decreased HDL-C [17], which was in line with the blood lipid profiles of rats in the non-treatment group. In addition, excessive deposition of fat (mainly in the form of TG) in the liver may lead to adipokine imbalance, dysregulated apoptosis of hepatocytes, and enterogenic endotoxin invasion, resulting in nonalcoholic fatty liver disease and accompanied progressive liver damage [18]. At the age of 11 weeks, the non-treatment group showed significantly impaired liver function, with structural disorder and hepatocyte steatosis, suggesting that dietary management alone could not completely improve metabolic disorders in obese rats.
GH participates in lipid metabolism by binding to receptors on the surface of target cells; its receptors exist in a variety of tissues, but most receptors originate from the liver [19]. GH binding to its receptor results in the activation of multiple signaling pathways, leading to the regulation of the transcription of GH-responsive genes in the liver, and the interaction among these pathways has been shown to inhibit lipid synthesis and reduce the accumulation of TG [20]. GH also reduces the amount of fat by affecting the differentiation and proliferation of preadipocytes [21]. GH has a robust lipolytic effect on abdominal fat. Our results showed that the W/H of the treatment group was significantly decreased compared with that of the non-treatment group, which also confirms the above view. A previous study showed that GH treatment reduced visceral fat in obese adults without changing their body weight [21]. In our study, no significant difference in body weight was observed between the GH treatment and non-treatment groups. However, GH-treated rats showed a significantly decreased Lee’s index compared with the non-treatment group, which may be due to the linear growth-promoting effect of GH. Our results showed that the levels of TC, TG, and LDL-C were significantly lower in GH-treated rats, while the levels of HDL-C were higher than those in the non-treatment group. Evaluation by light microscope revealed that the hepatocytes in GH-treated rats were arranged in a more orderly way, the degree of steatosis was reduced, and the morphology and structure were restored to a certain extent. Although there were still differences compared with the control group, these findings indicated that dysregulated lipid metabolism in pubertal obese rats was ameliorated by GH treatment. With the decrease in lipid accumulation, the impaired liver function gradually recovered, which was directly manifested as the decrease of ALT and AST levels.
As the major anabolic mediator of the GH effect, IGF-1 depends on GH and regulates the secretion of GH through a negative feedback system [22]. Although IGF-1 is not involved in GH-induced lipolysis due to the absence of functional IGF-1 receptors in adipocytes [23], IGF-1 indirectly regulates lipolysis by affecting the structure and function of adipose tissues [20]. Our results showed that the levels of IGF-1 in model animals were higher than those in the control group; this may be because the stability and biological activity of IGF-1 are regulated by insulin-like growth factor binding protein-1 (IGFBP-1), a specific binding protein produced by the liver, as well as insulin in vivo. Adipose tissue as an endocrine organ is closely related to insulin sensitivity. Visceral fat accumulation induces the secretion of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-8, which reduces insulin sensitivity and leads to hyperinsulinemia and even insulin resistance [24]. Insulin levels were not detected in this study, but we speculate that obese rats might have elevated insulin levels compared with controls. Hyperinsulinemia not only reduces the levels of IGFBP-1 [22] but also induces the production of IGF-1. Low levels of IGFBP-1 enhance the activity of IGF-1 [25], eventually leading to the upregulation of IGF-1 and the feedback inhibition of GH. At the end of our experiment, the levels of IGF-1 in the treatment group were significantly lower than those in the non-treatment group, which may be explained by decreased insulin levels caused by the reduction of abdominal fat.
Puberty is mainly characterized by sexual maturity and accelerated growth, both of which are related to the activation of the hypothalamic–pituitary–gonadal (HPG) axis and its coordination with the GH-IGF-1 axis [26]. Obesity-induced hypogonadism in males is related to excessive expression of aromatase, a complex cytochrome P450 enzyme that catalyzes the conversion of testosterone into E2 in adipose tissues [27]. An accumulation of adipose tissue enhances the function of aromatase, resulting in the downregulation of testosterone and upregulation of E2. Elevated E2 levels inhibit the activity of the HPG axis, which further impedes the production of testosterone [28]. Consistent with these findings, the non-treatment group in our study showed significantly increased expression of aromatase, decreased testosterone levels, and increased E2 levels compared with the control group. Obesity inhibits the secretion of GH in vivo [29]. GH deficiency not only affects the activity of testosterone and its derivative dihydrotestosterone but also reduces the number of androgen receptors in the prepuce [30], which affects the development of the penis. In our study, the penis length of 7-week-old model rats was significantly smaller than that of the control group, but the difference in the testicular volume was not significant, which may be related to the slow development of the testis before puberty.
GH is essential for the maturation of the mammalian reproductive system because it not only regulates the production of steroids but it also plays an important role in spermatogenesis and gonadotropin secretion. GH binds to receptors in Leydig cells and promotes testosterone synthesis by inducing the transcription of the steroidogenic acute regulatory protein and 3β-hydroxysteroid dehydrogenase genes, while testosterone regulates spermatogenesis by acting synergistically with follicle-stimulating hormone on Sertoli cells [31]. Experimental studies showed that GH treatment significantly increased sperm activity in infertile men [32] and improved the number and morphology of germ cells [33]. In our study, the expressions of aromatase in the liver and testis of the treatment group were lower than those of the non-treatment group. The secretion of testosterone in GH-treated rats was increased, which promoted the development of the reproductive system. There was no difference in penis length and testis volume between the treatment and control groups. Light microscopy showed the improved morphological structure of testicular seminiferous tubules and spermatogenesis in the treatment group, indicating that GH improves testicular function in model rats. The effects of GH on the growth of the testis and penis are mostly indirectly mediated by IGF-1. GH induces the secretion of IGF-1 by Sertoli cells, which functions synergistically with luteinizing hormone to regulate the production of androgen by Leydig cells, thereby promoting the development of the reproductive system [31].
The clinical application of GH has been shown to improve adolescent abdominal obesity and lipid levels. Kamel et al. treated six severely obese boys (10–12 years old) with GH for 6 without dietary adjustment and found that the overall percentage of fat was decreased and glucose homeostasis was not negatively affected [34]. Another study treated 18 obese boys (8–16 years old) with GH treatment for 1 year and found that their body mass index standard deviation scores and the levels of LDL-C, TG, TC, and alanine aminotransferase were decreased [35]. In addition, 21 obese women (13–21 years old) were reported to have decreased TC levels after 6 months of GH treatment, and the changes in IGF-1 levels were negatively correlated with those in TC, TG, and very low-density lipoprotein [36].
In this study, the dosage of GH and the route of exposure were chosen based on previous studies in male rats. Oh et al. established a micropenis rat model and found that treatment with GH (2.5 mg/kg, alternate days) promoted the growth of the penis and testis, and maintained structural integrity [30]. Huh et al. treated prepubertal rats with different doses of GH (1 or 2 IU/kg per day) and found that GH promoted the synthesis of testosterone and induced the early onset of puberty in a dose-dependent manner [37]. Administration of peripubertal rats with GH at a dosage of 2–11 IU/kg per day also promoted their body growth [37]. Few studies have tested the toxicity of GH at a high dose, which is worthy of further study.
Puberty in male rats is from 6–10 weeks after birth [38]. In this study, we established a model of obesity in juvenile male rats at 7 weeks of age. GH treatment partially protected pubertal male rats from an obesity-induced metabolic disorder and sexual retardation. Some serological indices of GH-treated rats were still significantly different from those of the control group. Whether a longer treatment duration or the combination with other treatments, such as exercise intervention, would exert better therapeutic effects warrants further investigation.
10 Conclusion
Our study provides scientific support for the use of GH in treating lipid metabolism and delayed sexual maturity in pubertal obese male rats. The clinical use of GH warrants further exploration.
-
Funding information: Authors state no funding involved.
-
Author contributions: S.G.: investigation, data curation, writing - original draft; J.Z.: methodology, validation; G.L.: resources, supervision, writing - review and editing
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;141(3):e20173459.10.1542/9781610022781-prevalenceSearch in Google Scholar
[2] González-Álvarez MA, Lázaro-Alquézar A, Simón-Fernández MB. Global trends in child obesity: Are figures converging? Int J Env Res Public Health. 2020;17(24):9252.10.3390/ijerph17249252Search in Google Scholar PubMed PubMed Central
[3] Nehus E, Mitsnefes M. Childhood obesity and the metabolic syndrome. Pediatr Clin North Am. 2019;66(1):31–43.10.1016/j.pcl.2018.08.004Search in Google Scholar PubMed
[4] Crisóstomo L, Pereira SC, Monteiro MP, Raposo JF, Oliveira PF, Alves MG. Lifestyle, metabolic disorders and male hypogonadism - A one-way ticket? Mol Cell Endocrinol. 2020;516:110945.10.1016/j.mce.2020.110945Search in Google Scholar PubMed
[5] Mushannen T, Cortez P, Stanford FC, Singhal V. Obesity and Hypogonadism-A narrative review highlighting the need for high-quality data in adolescents. Child (Basel). 2019;6(5):63.10.3390/children6050063Search in Google Scholar PubMed PubMed Central
[6] Collett-Solberg PF, Ambler G, Backeljauw PF, Bidlingmaier M, Biller BMK, Boguszewski MCS, et al. Diagnosis, genetics, and therapy of short stature in children: A growth hormone research society international perspective. Horm Res Paediatr. 2019;92(1):1–14.10.1159/000502231Search in Google Scholar PubMed PubMed Central
[7] Ramos-Leví AM, Marazuela M. Treatment of adult growth hormone deficiency with human recombinant growth hormone: An update on current evidence and critical review of advantages and pitfalls. Endocrine. 2018;60(2):203–18.10.1007/s12020-017-1492-1Search in Google Scholar PubMed
[8] Chu ZG, Li Z, Wang AH, Ruan QF, Wu H, Ruan JJ, et al. Observation on clinical effects of recombinant human growth hormone on the treatment of children with severe burn. Zhonghua Shao Shang Za Zhi. 2018;34(8):522–5.Search in Google Scholar
[9] Kubo T, Furujo M, Takahashi K, Hyodo Y, Tsuchiya H, Hattori M, et al. Effects of growth hormone treatment on lipid profiles. Indian J Pediatr. 2018;85(4):261–5.10.1007/s12098-017-2509-8Search in Google Scholar PubMed
[10] Mora Rodríguez JA, Porchia LM, Camargo F, López-Bayghen E. The use of insulin-like growth factor 1 improved the parameters of the seminogram in a patient with severe oligoasthenoteratozoospermia. SAGE Open Med Case Rep. 2019;7:2050313x19834154.10.1177/2050313X19834154Search in Google Scholar PubMed PubMed Central
[11] Xue LJ, Han JQ, Zhou YC, Peng HY, Yin TF, Li KM, et al. Untargeted metabolomics characteristics of nonobese nonalcoholic fatty liver disease induced by high-temperature-processed feed in Sprague-Dawley rats. World J Gastroenterol. 2020;26(46):7299–311.10.3748/wjg.v26.i46.7299Search in Google Scholar PubMed PubMed Central
[12] Chen J, Yang Y, Zhang G, Yang Q, Liu Y. Detection and significance of zinc nutritional status in obese rats. Chin J Lab Diagnosis. 2015;19:1049–51.Search in Google Scholar
[13] Dhawan D, Sharma S. Abdominal obesity, adipokines and non-communicable diseases. J Steroid Biochem Mol Biol. 2020;203:105737.10.1016/j.jsbmb.2020.105737Search in Google Scholar PubMed PubMed Central
[14] Mitchell L, Bel-Serrat S, Heinen M, Mehegan J, Murrin C, O’Brien S, et al. Waist circumference-to-height ratio and body mass index for obesity classification in Irish children. Acta Paediatr. 2021;110(5):1541–7.10.1111/apa.15724Search in Google Scholar PubMed
[15] Vasquez F, Correa-Burrows P, Blanco E, Gahagan S, Burrows R. A waist-to-height ratio of 0.54 is a good predictor of metabolic syndrome in 16-year-old male and female adolescents. Pediatr Res. 2019;85(3):269–74.10.1038/s41390-018-0257-8Search in Google Scholar PubMed PubMed Central
[16] Henderson GC. Plasma free fatty acid concentration as a modifiable risk factor for metabolic disease. Nutrients. 2021;13(8):2590.10.3390/nu13082590Search in Google Scholar PubMed PubMed Central
[17] Calcaterra V, Verduci E, Pascuzzi MC, Magenes VC, Fiore G, Di Profio E, et al. Metabolic derangement in pediatric patient with obesity: The role of ketogenic diet as therapeutic tool. Nutrients. 2021;13(8):2805.10.3390/nu13082805Search in Google Scholar PubMed PubMed Central
[18] Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J Gastroenterol. 2018;24(27):2974–83.10.3748/wjg.v24.i27.2974Search in Google Scholar PubMed PubMed Central
[19] Frank SJ. Classical and novel GH receptor signaling pathways. Mol Cell Endocrinol. 2020;518:110999.10.1016/j.mce.2020.110999Search in Google Scholar PubMed PubMed Central
[20] Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol. 2020;16(3):135–46.10.1038/s41574-019-0280-9Search in Google Scholar PubMed PubMed Central
[21] Berryman DE, Henry B, Hjortebjerg R, List EO, Kopchick JJ. Developments in our understanding of the effects of growth hormone on white adipose tissue from mice: implications to the clinic. Expert Rev Endocrinol Metab. 2016;11(2):197–207.10.1586/17446651.2016.1147950Search in Google Scholar PubMed PubMed Central
[22] Caputo M, Pigni S, Agosti E, Daffara T, Ferrero A, Filigheddu N, et al. Regulation of GH and GH Signaling by Nutrients. Cells. 2021;10(6):1376.10.3390/cells10061376Search in Google Scholar PubMed PubMed Central
[23] Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. 2016;14:3.10.1186/s12967-015-0762-zSearch in Google Scholar PubMed PubMed Central
[24] de Mutsert R, Gast K, Widya R, de Koning E, Jazet I, Lamb H, et al. Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: The Netherlands epidemiology of obesity study. Metab Syndr Relat Disord. 2018;16(1):54–63.10.1089/met.2017.0128Search in Google Scholar PubMed
[25] LeRoith D, Holly JMP, Forbes BE. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol Metab. 2021;52:101245.10.1016/j.molmet.2021.101245Search in Google Scholar PubMed PubMed Central
[26] Hull KL, Harvey S. Growth hormone and reproduction: A review of endocrine and autocrine/paracrine interactions. Int J Endocrinol. 2014;2014:234014.10.1155/2014/234014Search in Google Scholar PubMed PubMed Central
[27] Colleluori G, Chen R, Turin CG, Vigevano F, Qualls C, Johnson B, et al. Aromatase inhibitors plus weight loss improves the hormonal profile of obese hypogonadal men without causing major side effects. Front Endocrinol (Lausanne). 2020;11:277.10.3389/fendo.2020.00277Search in Google Scholar PubMed PubMed Central
[28] De Lorenzo A, Noce A, Moriconi E, Rampello T, Marrone G, Di Daniele N, et al. MOSH Syndrome (Male Obesity Secondary Hypogonadism): Clinical assessment and possible therapeutic approaches. Nutrients. 2018;10(4):474.10.3390/nu10040474Search in Google Scholar PubMed PubMed Central
[29] Hjelholt A, Høgild M, Bak AM, Arlien-Søborg MC, Bæk A, Jessen N, et al. Growth hormone and obesity. Endocrinol Metab Clin North Am. 2020;49(2):239–50.10.1016/j.ecl.2020.02.009Search in Google Scholar PubMed
[30] Oh JK, Im YJ, Park K, Paick JS. Effects of combined growth hormone and testosterone treatments in a rat model of micropenis. Endocr Connect. 2018;7(11):1150–7.10.1530/EC-18-0200Search in Google Scholar PubMed PubMed Central
[31] Ipsa E, Cruzat VF, Kagize JN, Yovich JL, Keane KN. Growth Hormone and Insulin-Like Growth Factor Action in Reproductive Tissues. Front Endocrinol (Lausanne). 2019;10:777.10.3389/fendo.2019.00777Search in Google Scholar PubMed PubMed Central
[32] Khourdaji I, Lee H, Smith RP. Frontiers in hormone therapy for male infertility. Transl Androl Urol. 2018;7(Suppl 3):S353–66.10.21037/tau.2018.04.03Search in Google Scholar PubMed PubMed Central
[33] Bingol-Kologlu M, Bahadir GB, Vargun R, Ilkay H, Bagriacik EU, Yolbakan S, et al. Effects of local and sustained release of FGF, IGF, and GH on germ cells in unilateral undescended testis in rats. Urology. 2010;75(1):223–8.10.1016/j.urology.2009.04.017Search in Google Scholar PubMed
[34] Kamel A, Norgren S, Elimam A, Danielsson P, Marcus C. Effects of growth hormone treatment in obese prepubertal boys. J Clin Endocrinol Metab. 2000;85(4):1412–9.10.1210/jc.85.4.1412Search in Google Scholar
[35] Wu J, Zhao F, Zhang Y, Xue J, Kuang J, Jin Z, et al. Effect of one-year growth hormone therapy on cardiometabolic risk factors in boys with obesity. Biomed Res Int. 2020;2020:2308124.10.1155/2020/2308124Search in Google Scholar PubMed PubMed Central
[36] Slattery M, Bredella MA, Stanley T, Torriani M, Misra M. Effects of recombinant human growth hormone (rhGH) administration on body composition and cardiovascular risk factors in obese adolescent girls. Int J Pediatr Endocrinol. 2014;1:22.10.1186/1687-9856-2014-22Search in Google Scholar PubMed PubMed Central
[37] Huh K, Nah WH, Xu Y, Park MJ, Gye MC. Effects of recombinant human growth hormone on the onset of puberty, leydig cell differentiation, spermatogenesis and hypothalamic KISS1 expression in immature male rats. World J Mens Health. 2021;39(2):381–8.10.5534/wjmh.200152Search in Google Scholar PubMed PubMed Central
[38] Zhou Y, Xu Z, Su G. Laboratory animal science. Beijing: The Science Publishing Company; 2004.Search in Google Scholar
© 2022 Shujuan Guo et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Effects of direct oral anticoagulants dabigatran and rivaroxaban on the blood coagulation function in rabbits
- The mother of all battles: Viruses vs humans. Can humans avoid extinction in 50–100 years?
- Knockdown of G1P3 inhibits cell proliferation and enhances the cytotoxicity of dexamethasone in acute lymphoblastic leukemia
- LINC00665 regulates hepatocellular carcinoma by modulating mRNA via the m6A enzyme
- Association study of CLDN14 variations in patients with kidney stones
- Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook
- Regulation of miR-30b in cancer development, apoptosis, and drug resistance
- Informatic analysis of the pulmonary microecology in non-cystic fibrosis bronchiectasis at three different stages
- Swimming attenuates tumor growth in CT-26 tumor-bearing mice and suppresses angiogenesis by mediating the HIF-1α/VEGFA pathway
- Characterization of intestinal microbiota and serum metabolites in patients with mild hepatic encephalopathy
- Functional conservation and divergence in plant-specific GRF gene family revealed by sequences and expression analysis
- Application of the FLP/LoxP-FRT recombination system to switch the eGFP expression in a model prokaryote
- Biomedical evaluation of antioxidant properties of lamb meat enriched with iodine and selenium
- Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-κB pathway
- Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance
- Potential regulatory mechanism of TNF-α/TNFR1/ANXA1 in glioma cells and its role in glioma cell proliferation
- Effect of the genetic mutant G71R in uridine diphosphate-glucuronosyltransferase 1A1 on the conjugation of bilirubin
- Quercetin inhibits cytotoxicity of PC12 cells induced by amyloid-beta 25–35 via stimulating estrogen receptor α, activating ERK1/2, and inhibiting apoptosis
- Nutrition intervention in the management of novel coronavirus pneumonia patients
- circ-CFH promotes the development of HCC by regulating cell proliferation, apoptosis, migration, invasion, and glycolysis through the miR-377-3p/RNF38 axis
- Bmi-1 directly upregulates glucose transporter 1 in human gastric adenocarcinoma
- Lacunar infarction aggravates the cognitive deficit in the elderly with white matter lesion
- Hydroxysafflor yellow A improved retinopathy via Nrf2/HO-1 pathway in rats
- Comparison of axon extension: PTFE versus PLA formed by a 3D printer
- Elevated IL-35 level and iTr35 subset increase the bacterial burden and lung lesions in Mycobacterium tuberculosis-infected mice
- A case report of CAT gene and HNF1β gene variations in a patient with early-onset diabetes
- Study on the mechanism of inhibiting patulin production by fengycin
- SOX4 promotes high-glucose-induced inflammation and angiogenesis of retinal endothelial cells by activating NF-κB signaling pathway
- Relationship between blood clots and COVID-19 vaccines: A literature review
- Analysis of genetic characteristics of 436 children with dysplasia and detailed analysis of rare karyotype
- Bioinformatics network analyses of growth differentiation factor 11
- NR4A1 inhibits the epithelial–mesenchymal transition of hepatic stellate cells: Involvement of TGF-β–Smad2/3/4–ZEB signaling
- Expression of Zeb1 in the differentiation of mouse embryonic stem cell
- Study on the genetic damage caused by cadmium sulfide quantum dots in human lymphocytes
- Association between single-nucleotide polymorphisms of NKX2.5 and congenital heart disease in Chinese population: A meta-analysis
- Assessment of the anesthetic effect of modified pentothal sodium solution on Sprague-Dawley rats
- Genetic susceptibility to high myopia in Han Chinese population
- Potential biomarkers and molecular mechanisms in preeclampsia progression
- Silencing circular RNA-friend leukemia virus integration 1 restrained malignancy of CC cells and oxaliplatin resistance by disturbing dyskeratosis congenita 1
- Endostar plus pembrolizumab combined with a platinum-based dual chemotherapy regime for advanced pulmonary large-cell neuroendocrine carcinoma as a first-line treatment: A case report
- The significance of PAK4 in signaling and clinicopathology: A review
- Sorafenib inhibits ovarian cancer cell proliferation and mobility and induces radiosensitivity by targeting the tumor cell epithelial–mesenchymal transition
- Characterization of rabbit polyclonal antibody against camel recombinant nanobodies
- Active legumain promotes invasion and migration of neuroblastoma by regulating epithelial-mesenchymal transition
- Effect of cell receptors in the pathogenesis of osteoarthritis: Current insights
- MT-12 inhibits the proliferation of bladder cells in vitro and in vivo by enhancing autophagy through mitochondrial dysfunction
- Study of hsa_circRNA_000121 and hsa_circRNA_004183 in papillary thyroid microcarcinoma
- BuyangHuanwu Decoction attenuates cerebral vasospasm caused by subarachnoid hemorrhage in rats via PI3K/AKT/eNOS axis
- Effects of the interaction of Notch and TLR4 pathways on inflammation and heart function in septic heart
- Monosodium iodoacetate-induced subchondral bone microstructure and inflammatory changes in an animal model of osteoarthritis
- A rare presentation of type II Abernethy malformation and nephrotic syndrome: Case report and review
- Rapid death due to pulmonary epithelioid haemangioendothelioma in several weeks: A case report
- Hepatoprotective role of peroxisome proliferator-activated receptor-α in non-cancerous hepatic tissues following transcatheter arterial embolization
- Correlation between peripheral blood lymphocyte subpopulations and primary systemic lupus erythematosus
- A novel SLC8A1-ALK fusion in lung adenocarcinoma confers sensitivity to alectinib: A case report
- β-Hydroxybutyrate upregulates FGF21 expression through inhibition of histone deacetylases in hepatocytes
- Identification of metabolic genes for the prediction of prognosis and tumor microenvironment infiltration in early-stage non-small cell lung cancer
- BTBD10 inhibits glioma tumorigenesis by downregulating cyclin D1 and p-Akt
- Mucormycosis co-infection in COVID-19 patients: An update
- Metagenomic next-generation sequencing in diagnosing Pneumocystis jirovecii pneumonia: A case report
- Long non-coding RNA HOXB-AS1 is a prognostic marker and promotes hepatocellular carcinoma cells’ proliferation and invasion
- Preparation and evaluation of LA-PEG-SPION, a targeted MRI contrast agent for liver cancer
- Proteomic analysis of the liver regulating lipid metabolism in Chaohu ducks using two-dimensional electrophoresis
- Nasopharyngeal tuberculosis: A case report
- Characterization and evaluation of anti-Salmonella enteritidis activity of indigenous probiotic lactobacilli in mice
- Aberrant pulmonary immune response of obese mice to periodontal infection
- Bacteriospermia – A formidable player in male subfertility
- In silico and in vivo analysis of TIPE1 expression in diffuse large B cell lymphoma
- Effects of KCa channels on biological behavior of trophoblasts
- Interleukin-17A influences the vulnerability rather than the size of established atherosclerotic plaques in apolipoprotein E-deficient mice
- Multiple organ failure and death caused by Staphylococcus aureus hip infection: A case report
- Prognostic signature related to the immune environment of oral squamous cell carcinoma
- Primary and metastatic squamous cell carcinoma of the thyroid gland: Two case reports
- Neuroprotective effects of crocin and crocin-loaded niosomes against the paraquat-induced oxidative brain damage in rats
- Role of MMP-2 and CD147 in kidney fibrosis
- Geometric basis of action potential of skeletal muscle cells and neurons
- Babesia microti-induced fulminant sepsis in an immunocompromised host: A case report and the case-specific literature review
- Role of cerebellar cortex in associative learning and memory in guinea pigs
- Application of metagenomic next-generation sequencing technique for diagnosing a specific case of necrotizing meningoencephalitis caused by human herpesvirus 2
- Case report: Quadruple primary malignant neoplasms including esophageal, ureteral, and lung in an elderly male
- Long non-coding RNA NEAT1 promotes angiogenesis in hepatoma carcinoma via the miR-125a-5p/VEGF pathway
- Osteogenic differentiation of periodontal membrane stem cells in inflammatory environments
- Knockdown of SHMT2 enhances the sensitivity of gastric cancer cells to radiotherapy through the Wnt/β-catenin pathway
- Continuous renal replacement therapy combined with double filtration plasmapheresis in the treatment of severe lupus complicated by serious bacterial infections in children: A case report
- Simultaneous triple primary malignancies, including bladder cancer, lymphoma, and lung cancer, in an elderly male: A case report
- Preclinical immunogenicity assessment of a cell-based inactivated whole-virion H5N1 influenza vaccine
- One case of iodine-125 therapy – A new minimally invasive treatment of intrahepatic cholangiocarcinoma
- S1P promotes corneal trigeminal neuron differentiation and corneal nerve repair via upregulating nerve growth factor expression in a mouse model
- Early cancer detection by a targeted methylation assay of circulating tumor DNA in plasma
- Calcifying nanoparticles initiate the calcification process of mesenchymal stem cells in vitro through the activation of the TGF-β1/Smad signaling pathway and promote the decay of echinococcosis
- Evaluation of prognostic markers in patients infected with SARS-CoV-2
- N6-Methyladenosine-related alternative splicing events play a role in bladder cancer
- Characterization of the structural, oxidative, and immunological features of testis tissue from Zucker diabetic fatty rats
- Effects of glucose and osmotic pressure on the proliferation and cell cycle of human chorionic trophoblast cells
- Investigation of genotype diversity of 7,804 norovirus sequences in humans and animals of China
- Characteristics and karyotype analysis of a patient with turner syndrome complicated with multiple-site tumors: A case report
- Aggravated renal fibrosis is positively associated with the activation of HMGB1-TLR2/4 signaling in STZ-induced diabetic mice
- Distribution characteristics of SARS-CoV-2 IgM/IgG in false-positive results detected by chemiluminescent immunoassay
- SRPX2 attenuated oxygen–glucose deprivation and reperfusion-induced injury in cardiomyocytes via alleviating endoplasmic reticulum stress-induced apoptosis through targeting PI3K/Akt/mTOR axis
- Aquaporin-8 overexpression is involved in vascular structure and function changes in placentas of gestational diabetes mellitus patients
- Relationship between CRP gene polymorphisms and ischemic stroke risk: A systematic review and meta-analysis
- Effects of growth hormone on lipid metabolism and sexual development in pubertal obese male rats
- Cloning and identification of the CTLA-4IgV gene and functional application of vaccine in Xinjiang sheep
- Antitumor activity of RUNX3: Upregulation of E-cadherin and downregulation of the epithelial–mesenchymal transition in clear-cell renal cell carcinoma
- PHF8 promotes osteogenic differentiation of BMSCs in old rat with osteoporosis by regulating Wnt/β-catenin pathway
- A review of the current state of the computer-aided diagnosis (CAD) systems for breast cancer diagnosis
- Bilateral dacryoadenitis in adult-onset Still’s disease: A case report
- A novel association between Bmi-1 protein expression and the SUVmax obtained by 18F-FDG PET/CT in patients with gastric adenocarcinoma
- The role of erythrocytes and erythroid progenitor cells in tumors
- Relationship between platelet activation markers and spontaneous abortion: A meta-analysis
- Abnormal methylation caused by folic acid deficiency in neural tube defects
- Silencing TLR4 using an ultrasound-targeted microbubble destruction-based shRNA system reduces ischemia-induced seizures in hyperglycemic rats
- Plant Sciences
- Seasonal succession of bacterial communities in cultured Caulerpa lentillifera detected by high-throughput sequencing
- Cloning and prokaryotic expression of WRKY48 from Caragana intermedia
- Novel Brassica hybrids with different resistance to Leptosphaeria maculans reveal unbalanced rDNA signal patterns
- Application of exogenous auxin and gibberellin regulates the bolting of lettuce (Lactuca sativa L.)
- Phytoremediation of pollutants from wastewater: A concise review
- Genome-wide identification and characterization of NBS-encoding genes in the sweet potato wild ancestor Ipomoea trifida (H.B.K.)
- Alleviative effects of magnetic Fe3O4 nanoparticles on the physiological toxicity of 3-nitrophenol to rice (Oryza sativa L.) seedlings
- Selection and functional identification of Dof genes expressed in response to nitrogen in Populus simonii × Populus nigra
- Study on pecan seed germination influenced by seed endocarp
- Identification of active compounds in Ophiopogonis Radix from different geographical origins by UPLC-Q/TOF-MS combined with GC-MS approaches
- The entire chloroplast genome sequence of Asparagus cochinchinensis and genetic comparison to Asparagus species
- Genome-wide identification of MAPK family genes and their response to abiotic stresses in tea plant (Camellia sinensis)
- Selection and validation of reference genes for RT-qPCR analysis of different organs at various development stages in Caragana intermedia
- Cloning and expression analysis of SERK1 gene in Diospyros lotus
- Integrated metabolomic and transcriptomic profiling revealed coping mechanisms of the edible and medicinal homologous plant Plantago asiatica L. cadmium resistance
- A missense variant in NCF1 is associated with susceptibility to unexplained recurrent spontaneous abortion
- Assessment of drought tolerance indices in faba bean genotypes under different irrigation regimes
- The entire chloroplast genome sequence of Asparagus setaceus (Kunth) Jessop: Genome structure, gene composition, and phylogenetic analysis in Asparagaceae
- Food Science
- Dietary food additive monosodium glutamate with or without high-lipid diet induces spleen anomaly: A mechanistic approach on rat model
- Binge eating disorder during COVID-19
- Potential of honey against the onset of autoimmune diabetes and its associated nephropathy, pancreatitis, and retinopathy in type 1 diabetic animal model
- FTO gene expression in diet-induced obesity is downregulated by Solanum fruit supplementation
- Physical activity enhances fecal lactobacilli in rats chronically drinking sweetened cola beverage
- Supercritical CO2 extraction, chemical composition, and antioxidant effects of Coreopsis tinctoria Nutt. oleoresin
- Functional constituents of plant-based foods boost immunity against acute and chronic disorders
- Effect of selenium and methods of protein extraction on the proteomic profile of Saccharomyces yeast
- Microbial diversity of milk ghee in southern Gansu and its effect on the formation of ghee flavor compounds
- Ecology and Environmental Sciences
- Effects of heavy metals on bacterial community surrounding Bijiashan mining area located in northwest China
- Microorganism community composition analysis coupling with 15N tracer experiments reveals the nitrification rate and N2O emissions in low pH soils in Southern China
- Genetic diversity and population structure of Cinnamomum balansae Lecomte inferred by microsatellites
- Preliminary screening of microplastic contamination in different marine fish species of Taif market, Saudi Arabia
- Plant volatile organic compounds attractive to Lygus pratensis
- Effects of organic materials on soil bacterial community structure in long-term continuous cropping of tomato in greenhouse
- Effects of soil treated fungicide fluopimomide on tomato (Solanum lycopersicum L.) disease control and plant growth
- Prevalence of Yersinia pestis among rodents captured in a semi-arid tropical ecosystem of south-western Zimbabwe
- Effects of irrigation and nitrogen fertilization on mitigating salt-induced Na+ toxicity and sustaining sea rice growth
- Bioengineering and Biotechnology
- Poly-l-lysine-caused cell adhesion induces pyroptosis in THP-1 monocytes
- Development of alkaline phosphatase-scFv and its use for one-step enzyme-linked immunosorbent assay for His-tagged protein detection
- Development and validation of a predictive model for immune-related genes in patients with tongue squamous cell carcinoma
- Agriculture
- Effects of chemical-based fertilizer replacement with biochar-based fertilizer on albic soil nutrient content and maize yield
- Genome-wide identification and expression analysis of CPP-like gene family in Triticum aestivum L. under different hormone and stress conditions
- Agronomic and economic performance of mung bean (Vigna radiata L.) varieties in response to rates of blended NPS fertilizer in Kindo Koysha district, Southern Ethiopia
- Influence of furrow irrigation regime on the yield and water consumption indicators of winter wheat based on a multi-level fuzzy comprehensive evaluation
- Discovery of exercise-related genes and pathway analysis based on comparative genomes of Mongolian originated Abaga and Wushen horse
- Lessons from integrated seasonal forecast-crop modelling in Africa: A systematic review
- Evolution trend of soil fertility in tobacco-planting area of Chenzhou, Hunan Province, China
- Animal Sciences
- Morphological and molecular characterization of Tatera indica Hardwicke 1807 (Rodentia: Muridae) from Pothwar, Pakistan
- Research on meat quality of Qianhua Mutton Merino sheep and Small-tail Han sheep
- SI: A Scientific Memoir
- Suggestions on leading an academic research laboratory group
- My scientific genealogy and the Toronto ACDC Laboratory, 1988–2022
- Erratum
- Erratum to “Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study”
- Erratum to “A two-microRNA signature predicts the progression of male thyroid cancer”
- Retraction
- Retraction of “Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis”
Articles in the same Issue
- Biomedical Sciences
- Effects of direct oral anticoagulants dabigatran and rivaroxaban on the blood coagulation function in rabbits
- The mother of all battles: Viruses vs humans. Can humans avoid extinction in 50–100 years?
- Knockdown of G1P3 inhibits cell proliferation and enhances the cytotoxicity of dexamethasone in acute lymphoblastic leukemia
- LINC00665 regulates hepatocellular carcinoma by modulating mRNA via the m6A enzyme
- Association study of CLDN14 variations in patients with kidney stones
- Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook
- Regulation of miR-30b in cancer development, apoptosis, and drug resistance
- Informatic analysis of the pulmonary microecology in non-cystic fibrosis bronchiectasis at three different stages
- Swimming attenuates tumor growth in CT-26 tumor-bearing mice and suppresses angiogenesis by mediating the HIF-1α/VEGFA pathway
- Characterization of intestinal microbiota and serum metabolites in patients with mild hepatic encephalopathy
- Functional conservation and divergence in plant-specific GRF gene family revealed by sequences and expression analysis
- Application of the FLP/LoxP-FRT recombination system to switch the eGFP expression in a model prokaryote
- Biomedical evaluation of antioxidant properties of lamb meat enriched with iodine and selenium
- Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-κB pathway
- Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance
- Potential regulatory mechanism of TNF-α/TNFR1/ANXA1 in glioma cells and its role in glioma cell proliferation
- Effect of the genetic mutant G71R in uridine diphosphate-glucuronosyltransferase 1A1 on the conjugation of bilirubin
- Quercetin inhibits cytotoxicity of PC12 cells induced by amyloid-beta 25–35 via stimulating estrogen receptor α, activating ERK1/2, and inhibiting apoptosis
- Nutrition intervention in the management of novel coronavirus pneumonia patients
- circ-CFH promotes the development of HCC by regulating cell proliferation, apoptosis, migration, invasion, and glycolysis through the miR-377-3p/RNF38 axis
- Bmi-1 directly upregulates glucose transporter 1 in human gastric adenocarcinoma
- Lacunar infarction aggravates the cognitive deficit in the elderly with white matter lesion
- Hydroxysafflor yellow A improved retinopathy via Nrf2/HO-1 pathway in rats
- Comparison of axon extension: PTFE versus PLA formed by a 3D printer
- Elevated IL-35 level and iTr35 subset increase the bacterial burden and lung lesions in Mycobacterium tuberculosis-infected mice
- A case report of CAT gene and HNF1β gene variations in a patient with early-onset diabetes
- Study on the mechanism of inhibiting patulin production by fengycin
- SOX4 promotes high-glucose-induced inflammation and angiogenesis of retinal endothelial cells by activating NF-κB signaling pathway
- Relationship between blood clots and COVID-19 vaccines: A literature review
- Analysis of genetic characteristics of 436 children with dysplasia and detailed analysis of rare karyotype
- Bioinformatics network analyses of growth differentiation factor 11
- NR4A1 inhibits the epithelial–mesenchymal transition of hepatic stellate cells: Involvement of TGF-β–Smad2/3/4–ZEB signaling
- Expression of Zeb1 in the differentiation of mouse embryonic stem cell
- Study on the genetic damage caused by cadmium sulfide quantum dots in human lymphocytes
- Association between single-nucleotide polymorphisms of NKX2.5 and congenital heart disease in Chinese population: A meta-analysis
- Assessment of the anesthetic effect of modified pentothal sodium solution on Sprague-Dawley rats
- Genetic susceptibility to high myopia in Han Chinese population
- Potential biomarkers and molecular mechanisms in preeclampsia progression
- Silencing circular RNA-friend leukemia virus integration 1 restrained malignancy of CC cells and oxaliplatin resistance by disturbing dyskeratosis congenita 1
- Endostar plus pembrolizumab combined with a platinum-based dual chemotherapy regime for advanced pulmonary large-cell neuroendocrine carcinoma as a first-line treatment: A case report
- The significance of PAK4 in signaling and clinicopathology: A review
- Sorafenib inhibits ovarian cancer cell proliferation and mobility and induces radiosensitivity by targeting the tumor cell epithelial–mesenchymal transition
- Characterization of rabbit polyclonal antibody against camel recombinant nanobodies
- Active legumain promotes invasion and migration of neuroblastoma by regulating epithelial-mesenchymal transition
- Effect of cell receptors in the pathogenesis of osteoarthritis: Current insights
- MT-12 inhibits the proliferation of bladder cells in vitro and in vivo by enhancing autophagy through mitochondrial dysfunction
- Study of hsa_circRNA_000121 and hsa_circRNA_004183 in papillary thyroid microcarcinoma
- BuyangHuanwu Decoction attenuates cerebral vasospasm caused by subarachnoid hemorrhage in rats via PI3K/AKT/eNOS axis
- Effects of the interaction of Notch and TLR4 pathways on inflammation and heart function in septic heart
- Monosodium iodoacetate-induced subchondral bone microstructure and inflammatory changes in an animal model of osteoarthritis
- A rare presentation of type II Abernethy malformation and nephrotic syndrome: Case report and review
- Rapid death due to pulmonary epithelioid haemangioendothelioma in several weeks: A case report
- Hepatoprotective role of peroxisome proliferator-activated receptor-α in non-cancerous hepatic tissues following transcatheter arterial embolization
- Correlation between peripheral blood lymphocyte subpopulations and primary systemic lupus erythematosus
- A novel SLC8A1-ALK fusion in lung adenocarcinoma confers sensitivity to alectinib: A case report
- β-Hydroxybutyrate upregulates FGF21 expression through inhibition of histone deacetylases in hepatocytes
- Identification of metabolic genes for the prediction of prognosis and tumor microenvironment infiltration in early-stage non-small cell lung cancer
- BTBD10 inhibits glioma tumorigenesis by downregulating cyclin D1 and p-Akt
- Mucormycosis co-infection in COVID-19 patients: An update
- Metagenomic next-generation sequencing in diagnosing Pneumocystis jirovecii pneumonia: A case report
- Long non-coding RNA HOXB-AS1 is a prognostic marker and promotes hepatocellular carcinoma cells’ proliferation and invasion
- Preparation and evaluation of LA-PEG-SPION, a targeted MRI contrast agent for liver cancer
- Proteomic analysis of the liver regulating lipid metabolism in Chaohu ducks using two-dimensional electrophoresis
- Nasopharyngeal tuberculosis: A case report
- Characterization and evaluation of anti-Salmonella enteritidis activity of indigenous probiotic lactobacilli in mice
- Aberrant pulmonary immune response of obese mice to periodontal infection
- Bacteriospermia – A formidable player in male subfertility
- In silico and in vivo analysis of TIPE1 expression in diffuse large B cell lymphoma
- Effects of KCa channels on biological behavior of trophoblasts
- Interleukin-17A influences the vulnerability rather than the size of established atherosclerotic plaques in apolipoprotein E-deficient mice
- Multiple organ failure and death caused by Staphylococcus aureus hip infection: A case report
- Prognostic signature related to the immune environment of oral squamous cell carcinoma
- Primary and metastatic squamous cell carcinoma of the thyroid gland: Two case reports
- Neuroprotective effects of crocin and crocin-loaded niosomes against the paraquat-induced oxidative brain damage in rats
- Role of MMP-2 and CD147 in kidney fibrosis
- Geometric basis of action potential of skeletal muscle cells and neurons
- Babesia microti-induced fulminant sepsis in an immunocompromised host: A case report and the case-specific literature review
- Role of cerebellar cortex in associative learning and memory in guinea pigs
- Application of metagenomic next-generation sequencing technique for diagnosing a specific case of necrotizing meningoencephalitis caused by human herpesvirus 2
- Case report: Quadruple primary malignant neoplasms including esophageal, ureteral, and lung in an elderly male
- Long non-coding RNA NEAT1 promotes angiogenesis in hepatoma carcinoma via the miR-125a-5p/VEGF pathway
- Osteogenic differentiation of periodontal membrane stem cells in inflammatory environments
- Knockdown of SHMT2 enhances the sensitivity of gastric cancer cells to radiotherapy through the Wnt/β-catenin pathway
- Continuous renal replacement therapy combined with double filtration plasmapheresis in the treatment of severe lupus complicated by serious bacterial infections in children: A case report
- Simultaneous triple primary malignancies, including bladder cancer, lymphoma, and lung cancer, in an elderly male: A case report
- Preclinical immunogenicity assessment of a cell-based inactivated whole-virion H5N1 influenza vaccine
- One case of iodine-125 therapy – A new minimally invasive treatment of intrahepatic cholangiocarcinoma
- S1P promotes corneal trigeminal neuron differentiation and corneal nerve repair via upregulating nerve growth factor expression in a mouse model
- Early cancer detection by a targeted methylation assay of circulating tumor DNA in plasma
- Calcifying nanoparticles initiate the calcification process of mesenchymal stem cells in vitro through the activation of the TGF-β1/Smad signaling pathway and promote the decay of echinococcosis
- Evaluation of prognostic markers in patients infected with SARS-CoV-2
- N6-Methyladenosine-related alternative splicing events play a role in bladder cancer
- Characterization of the structural, oxidative, and immunological features of testis tissue from Zucker diabetic fatty rats
- Effects of glucose and osmotic pressure on the proliferation and cell cycle of human chorionic trophoblast cells
- Investigation of genotype diversity of 7,804 norovirus sequences in humans and animals of China
- Characteristics and karyotype analysis of a patient with turner syndrome complicated with multiple-site tumors: A case report
- Aggravated renal fibrosis is positively associated with the activation of HMGB1-TLR2/4 signaling in STZ-induced diabetic mice
- Distribution characteristics of SARS-CoV-2 IgM/IgG in false-positive results detected by chemiluminescent immunoassay
- SRPX2 attenuated oxygen–glucose deprivation and reperfusion-induced injury in cardiomyocytes via alleviating endoplasmic reticulum stress-induced apoptosis through targeting PI3K/Akt/mTOR axis
- Aquaporin-8 overexpression is involved in vascular structure and function changes in placentas of gestational diabetes mellitus patients
- Relationship between CRP gene polymorphisms and ischemic stroke risk: A systematic review and meta-analysis
- Effects of growth hormone on lipid metabolism and sexual development in pubertal obese male rats
- Cloning and identification of the CTLA-4IgV gene and functional application of vaccine in Xinjiang sheep
- Antitumor activity of RUNX3: Upregulation of E-cadherin and downregulation of the epithelial–mesenchymal transition in clear-cell renal cell carcinoma
- PHF8 promotes osteogenic differentiation of BMSCs in old rat with osteoporosis by regulating Wnt/β-catenin pathway
- A review of the current state of the computer-aided diagnosis (CAD) systems for breast cancer diagnosis
- Bilateral dacryoadenitis in adult-onset Still’s disease: A case report
- A novel association between Bmi-1 protein expression and the SUVmax obtained by 18F-FDG PET/CT in patients with gastric adenocarcinoma
- The role of erythrocytes and erythroid progenitor cells in tumors
- Relationship between platelet activation markers and spontaneous abortion: A meta-analysis
- Abnormal methylation caused by folic acid deficiency in neural tube defects
- Silencing TLR4 using an ultrasound-targeted microbubble destruction-based shRNA system reduces ischemia-induced seizures in hyperglycemic rats
- Plant Sciences
- Seasonal succession of bacterial communities in cultured Caulerpa lentillifera detected by high-throughput sequencing
- Cloning and prokaryotic expression of WRKY48 from Caragana intermedia
- Novel Brassica hybrids with different resistance to Leptosphaeria maculans reveal unbalanced rDNA signal patterns
- Application of exogenous auxin and gibberellin regulates the bolting of lettuce (Lactuca sativa L.)
- Phytoremediation of pollutants from wastewater: A concise review
- Genome-wide identification and characterization of NBS-encoding genes in the sweet potato wild ancestor Ipomoea trifida (H.B.K.)
- Alleviative effects of magnetic Fe3O4 nanoparticles on the physiological toxicity of 3-nitrophenol to rice (Oryza sativa L.) seedlings
- Selection and functional identification of Dof genes expressed in response to nitrogen in Populus simonii × Populus nigra
- Study on pecan seed germination influenced by seed endocarp
- Identification of active compounds in Ophiopogonis Radix from different geographical origins by UPLC-Q/TOF-MS combined with GC-MS approaches
- The entire chloroplast genome sequence of Asparagus cochinchinensis and genetic comparison to Asparagus species
- Genome-wide identification of MAPK family genes and their response to abiotic stresses in tea plant (Camellia sinensis)
- Selection and validation of reference genes for RT-qPCR analysis of different organs at various development stages in Caragana intermedia
- Cloning and expression analysis of SERK1 gene in Diospyros lotus
- Integrated metabolomic and transcriptomic profiling revealed coping mechanisms of the edible and medicinal homologous plant Plantago asiatica L. cadmium resistance
- A missense variant in NCF1 is associated with susceptibility to unexplained recurrent spontaneous abortion
- Assessment of drought tolerance indices in faba bean genotypes under different irrigation regimes
- The entire chloroplast genome sequence of Asparagus setaceus (Kunth) Jessop: Genome structure, gene composition, and phylogenetic analysis in Asparagaceae
- Food Science
- Dietary food additive monosodium glutamate with or without high-lipid diet induces spleen anomaly: A mechanistic approach on rat model
- Binge eating disorder during COVID-19
- Potential of honey against the onset of autoimmune diabetes and its associated nephropathy, pancreatitis, and retinopathy in type 1 diabetic animal model
- FTO gene expression in diet-induced obesity is downregulated by Solanum fruit supplementation
- Physical activity enhances fecal lactobacilli in rats chronically drinking sweetened cola beverage
- Supercritical CO2 extraction, chemical composition, and antioxidant effects of Coreopsis tinctoria Nutt. oleoresin
- Functional constituents of plant-based foods boost immunity against acute and chronic disorders
- Effect of selenium and methods of protein extraction on the proteomic profile of Saccharomyces yeast
- Microbial diversity of milk ghee in southern Gansu and its effect on the formation of ghee flavor compounds
- Ecology and Environmental Sciences
- Effects of heavy metals on bacterial community surrounding Bijiashan mining area located in northwest China
- Microorganism community composition analysis coupling with 15N tracer experiments reveals the nitrification rate and N2O emissions in low pH soils in Southern China
- Genetic diversity and population structure of Cinnamomum balansae Lecomte inferred by microsatellites
- Preliminary screening of microplastic contamination in different marine fish species of Taif market, Saudi Arabia
- Plant volatile organic compounds attractive to Lygus pratensis
- Effects of organic materials on soil bacterial community structure in long-term continuous cropping of tomato in greenhouse
- Effects of soil treated fungicide fluopimomide on tomato (Solanum lycopersicum L.) disease control and plant growth
- Prevalence of Yersinia pestis among rodents captured in a semi-arid tropical ecosystem of south-western Zimbabwe
- Effects of irrigation and nitrogen fertilization on mitigating salt-induced Na+ toxicity and sustaining sea rice growth
- Bioengineering and Biotechnology
- Poly-l-lysine-caused cell adhesion induces pyroptosis in THP-1 monocytes
- Development of alkaline phosphatase-scFv and its use for one-step enzyme-linked immunosorbent assay for His-tagged protein detection
- Development and validation of a predictive model for immune-related genes in patients with tongue squamous cell carcinoma
- Agriculture
- Effects of chemical-based fertilizer replacement with biochar-based fertilizer on albic soil nutrient content and maize yield
- Genome-wide identification and expression analysis of CPP-like gene family in Triticum aestivum L. under different hormone and stress conditions
- Agronomic and economic performance of mung bean (Vigna radiata L.) varieties in response to rates of blended NPS fertilizer in Kindo Koysha district, Southern Ethiopia
- Influence of furrow irrigation regime on the yield and water consumption indicators of winter wheat based on a multi-level fuzzy comprehensive evaluation
- Discovery of exercise-related genes and pathway analysis based on comparative genomes of Mongolian originated Abaga and Wushen horse
- Lessons from integrated seasonal forecast-crop modelling in Africa: A systematic review
- Evolution trend of soil fertility in tobacco-planting area of Chenzhou, Hunan Province, China
- Animal Sciences
- Morphological and molecular characterization of Tatera indica Hardwicke 1807 (Rodentia: Muridae) from Pothwar, Pakistan
- Research on meat quality of Qianhua Mutton Merino sheep and Small-tail Han sheep
- SI: A Scientific Memoir
- Suggestions on leading an academic research laboratory group
- My scientific genealogy and the Toronto ACDC Laboratory, 1988–2022
- Erratum
- Erratum to “Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study”
- Erratum to “A two-microRNA signature predicts the progression of male thyroid cancer”
- Retraction
- Retraction of “Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis”

