Swimming attenuates tumor growth in CT-26 tumor-bearing mice and suppresses angiogenesis by mediating the HIF-1α/VEGFA pathway
-
Jiapeng Li
and Aling Shen
Abstract
Low physical activity correlates with increased cancer risk in various cancer types, including colorectal cancer (CRC). However, the ways in which swimming can benefit CRC remain largely unknown. In this study, mice bearing tumors derived from CT-26 cells were randomly divided into the control and swimming groups. Mice in the swimming group were subjected to physical training (swimming) for 3 weeks. Compared with the control group, swimming clearly attenuated tumor volume and tumor weight in CT-26 tumor-bearing mice. RNA sequencing (RNA-seq) identified 715 upregulated and 629 downregulated transcripts (including VEGFA) in tumor tissues of mice in the swimming group. KEGG pathway analysis based on differentially expressed transcripts identified multiple enriched signaling pathways, including angiogenesis, hypoxia, and vascular endothelial growth factor (VEGF) pathways. Consistently, IHC analysis revealed that swimming significantly downregulated CD31, HIF-1α, VEGFA, and VEGFR2 protein expression in tumor tissues. In conclusion, swimming significantly attenuates tumor growth in CT-26 tumor-bearing mice by inhibiting tumor angiogenesis via the suppression of the HIF-1α/VEGFA pathway.
1 Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide, as more than 1.9 million new cases and 994,000 deaths were reported in 2020. Therefore, this disease ranks third in terms of incidence and second in mortality [1]. The development and progression of CRC are associated with multiple factors, including lifestyle, environmental factors, and genetic and epigenetic changes [2]. Despite the rapid development of various therapeutic strategies and combinations of these treatments, the outcomes of CRC patients remain unsatisfactory. Therefore, safer and less toxic therapeutic strategies are urgently needed.
Early evidence from observational studies has indicated that exercise exhibits a remarkable safety profile without obvious inherent toxicities and may even reduce the rate or severity of treatment-associated adverse events compared with other cancer therapeutics [3]. After the first preclinical evidence indicated that exercise inhibits tumor growth in a mouse model [4], a greater number of studies have demonstrated that exercise improves the quality of life in patients after surgery [5], improves chemotherapy efficacy [6], and is correlated with a reduced risk of recurrence and cancer-associated mortality [7]. Further investigation has indicated that exercise significantly attenuates tumor growth and metastasis, reduced serum levels of monocyte chemoattractant protein-1 (MCP-1), and decreased tumor hypoxia [8], and enhancement of intertumoral NK cell infiltration and activation [9] might be the essential underlying mechanisms. One study on CRC showed that increased physical activity after diagnosis was correlated with the reduction of cancer recurrence and mortality in stage III CRC patients [10] and reduction in the risk of CRC-specific and overall mortality in patients with stages I to III CRC [11]. However, the benefits and underlying mechanisms of exercise in patients with cancer, including CRC, require further exploration.
Multiple evidence suggested that low physical activity and high amounts of sedentary time correlate with increased cancer risk in various cancer types [12,13,14]. Previous systematic reviews and meta-analyses revealed that low physical activity and high amounts of sedentary time contribute to an increased risk of CRC [15,16]. By contrast, physical activity (including jogging and swimming) reduces CRC risk [17]. However, as a major aquatic exercise, the benefits of swimming and the underlying anti-CRC mechanisms remain largely unknown. Therefore, the current study was intended to assess the benefits of swimming with respect to tumor growth and to explore its underlying mechanisms against CRC.
2 Materials and methods
2.1 Materials
Fetal bovine serum (Cat. no. 1981614), RPMI-1640 (Cat. no. 1049101), 0.05% trypsin–EDTA (Cat. no. 293606), and penicillin-streptomycin were purchased from Thermo Fisher Scientific (Carlsbad, CA, USA). BD Matrigel (Cat. no. 354230) was obtained from BD Biosciences (San Jose, CA, USA). HIF-1α antibody (Cat. no. GTX127309) was provided by GeneTex Inc. (San Antonio, TX, USA). VEGFA (Cat no. ab1316), VEGFR2 (Cat no. ab2349), and CD31 (Cat no. ab28364) antibodies were purchased from Abcam (Cambridge Science Park, Cambridge, UK). Horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. Kit-0017) was obtained from Maixin Corp. (Fuzhou, Fujian, China). All other chemicals used were purchased from Solarbio Corp. (Beijing, China).
2.2 Cell culture
The CT-26 murine colon carcinoma cell line was purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin (100 U/mL) and streptomycin (100 µg/mL) at 37°C and 5% of CO2 in a humidified incubator. Cells were subcultured at 80–90% confluency.
2.3 Animals
Twenty male BALB/c mice (age, 4–6 weeks; weight, 20 ± 2 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). Mice were housed under specific pathogen-free conditions at 22–26°C and 60 ± 5% humidity with a 12 h dark/light cycle. Food and water were provided ad libitum throughout the experiment.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and were approved by the Committee of Fujian University of Traditional Chinese Medicine (No. 2019-030).
2.4 Construction of the mouse xenograft model and measurement of tumor volume
After the mice were fed adaptively for one week, CT-26 cells (1 × 106 cells/100 µL) in 100 µL of Matrigel (50%) were subcutaneously injected into the right flank area. The tumor volume was determined by measuring the major (L) and minor (W) diameters using an electronic vernier caliper and was calculated according to the following formula: tumor volume = L × W 2/2. To determine tumor growth, 20 tumor-bearing mice were randomly divided into the control group (n = 10) and the swimming group (n = 10) according to tumor volume three days after injection.
2.5 Exercise intervention program
In the pilot study, CT-26 tumor-bearing mice in the swimming group were subjected to physical training by swimming in water (30 ± 2°C) twice per day, 6 days per week. Initially, the mice typically swam voluntarily for approximately 10 min, after which they floated on the water and made intermittent swimming motions. The time was extended for 10 min each time until 30 min was reached. To induce mice to continuously swim for a longer duration, we used a stick to pull the water to drive them. During the experiment, the tumor volume and body weight were measured every three days. After 3 weeks, when the experiment ended, the mice were anesthetized with isoflurane and sacrificed. Tumor tissues were then removed and weighed.
2.6 RNA sequencing (RNA-Seq) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses
Tumor tissues from each group (n = 6) were randomly selected, and total RNA was extracted with TRIzol (Tiangen, Beijing, China). RNA integrity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and RNA concentration was measured using a Qubit RNA Assay Kit in a Qubit Fluorometer (Invitrogen, CA, USA). A total of 1 µg RNA per sample was used. Briefly, the NEBNext rRNA Depletion Kit was used to remove rRNA from the total RNA sample. The NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, Beijing, China) was used to construct the sequencing libraries according to the manufacturer’s instructions. Sequenced reads were trimmed for the adaptor sequence and then mapped to the hg38 whole genome using Hisat2 v2.0.5. Raw counts and fragments per kilobase million were calculated using StringTie v1.3.3. Differential expression analyses were performed using the limma package (cut-off > 2; P < 0.05). Differentially expressed transcripts (DETs) were identified using volcano plots and hierarchical clustering plots. KEGG pathway enrichment analysis was used to identify enriched signaling pathways represented among the DETs. The experiments were performed by CapitalBio (Beijing, China). The raw data were submitted to the Gene Expression Omnibus (GEO) (Submission No.: GSE 149405).
2.7 Immunohistochemistry
Immunohistochemistry was used to detect the expression of HIF-1α, VEGFA, VEGFR2, and CD31. Tumor tissues from each group were fixed in 4% paraformaldehyde (pH 7.4) for 24 h, processed, embedded in paraffin, and cut into 4 µm-thick sections. The slides containing tumor tissues were subjected to antigen retrieval and were then incubated with 3% hydrogen peroxide to block any endogenous peroxidase activity. After blocking nonspecific protein binding at 25°C for 10 min, the sections were incubated with primary antibodies against HIF-1α, VEGFA, VEGFR2, or CD31 (all diluted 1:200) at 4°C overnight. After the slides were washed in PBS, they were incubated with HRP-conjugated secondary antibody and then washed with PBS. The slides were then incubated with DAB chromogen, followed by counterstaining in diluted hematoxylin. After staining, images from each sample (five samples were randomly selected from each group) were obtained at ×400 magnification using a light microscope (LEICA: DM6000B, Wetzlar, Germany). Six fields of view were randomly selected for each slide, and the average percentage of positively stained cells in each field was counted using the true color multifunctional cell image analysis system Image-Pro Plus (Media Cybernetics, Rockville, MD, USA).
2.8 Statistical analysis
Data were presented as the mean ± SD for the indicated number of independently performed experiments. Statistical analysis was performed with Student’s t-test using SPSS24.0. Differences with P < 0.05 were considered statistically significant.
2.9 Data availability
The RNA-seq data associated with this article are available in the GEO repository [GEO Submission: GSE 149405].
3 Results
3.1 Swimming attenuates growth of CT-26 cell-derived tumors in vivo
To assess the benefits of exercise on tumor growth, CT-26 cells were transplanted into BALB/c mice, followed by quantitative daily swimming. Tumor volume was monitored and clearly indicated that it was decreased in the swimming group compared with the control group (Figure 1a; P < 0.05). Consistently, a significant decrease in the tumor weight was observed in the swimming group compared to that in the control group (Figure 1b; P < 0.05). Moreover, monitoring of body weights did not reveal any obvious differences between the control and swimming groups (Figure 1c; P > 0.05). These data indicate that swimming significantly attenuates the growth of CT-26 cell-derived tumors in vivo.
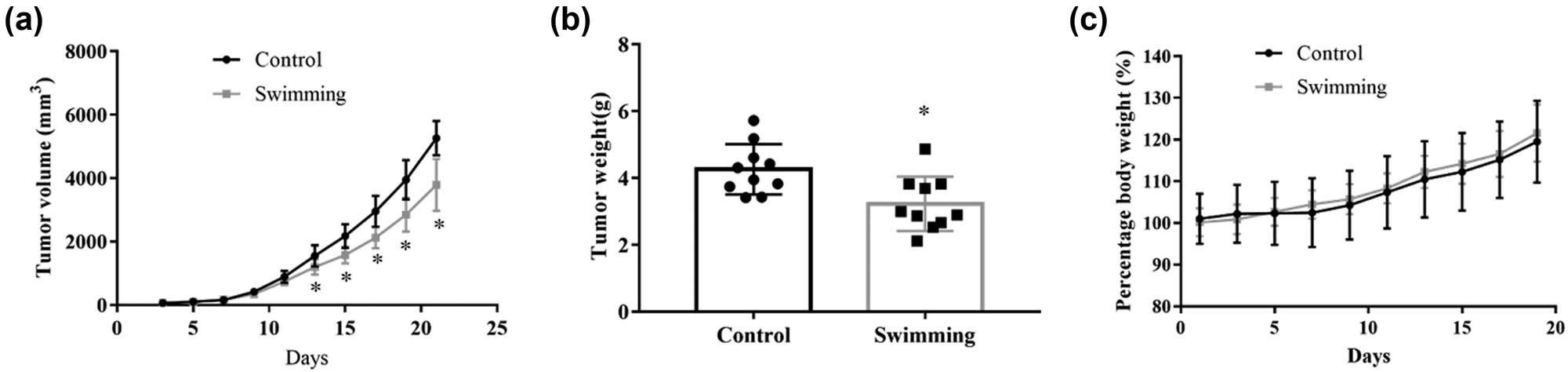
Effects of swimming on the growth of tumors derived from transplanted colorectal cancer cells in mice. (a) Tumor volume was monitored during the exercise period for 21 days. (b) Tumor weight was determined using an electronic scale at the end of the experiment. (c) The body weights of the mice were recorded during the experiment. Data are presented as the mean ± SD. * P < 0.05, vs control.
3.2 Swimming regulates the expression of multiple genes in CT-26 cell-derived tumors
To further explore the underlying mechanism by which swimming attenuates tumor growth, RNA-seq was performed to DETs in tumor tissues between the control and swimming groups. As shown in Figure 2a and b (GEO Submission: GSE149405), we found 715 upregulated and 629 downregulated transcripts in the swimming group compared with the control group. The expression level of multiple genes associated with tumor growth, such as STAT3, PDGFA, PLD2, and PIK3R2, showed a significant decrease (Table S1). Table 1 also shows the top 20 altered DETs. These data suggest that swimming attenuates tumor growth by targeting multiple genes.

Identification of DETs. RNA-Seq was performed to determine the DETs in tumor tissues between the control and swimming groups. The DETs are presented using volcano plots (a) and hierarchical clustering plots (b). Cut-off >2, P < 0.05.
Top 20 of different genes
| ID | log FC | P value | Symbol |
|---|---|---|---|
| ENSMUST00000168776 | 9.24465 | 0.000611 | 2-Sep |
| ENSMUST00000105265 | 8.316306 | 0.002433 | Cnot2 |
| ENSMUST00000107846 | 7.745867 | 3.62 × 10−5 | Clta |
| ENSMUST00000089776 | 7.327142 | 0.004677 | Cep152 |
| ENSMUST00000087582 | 7.324338726 | 0.010539031 | Hnrnpm |
| ENSMUST00000119398 | 7.302732096 | 0.021052217 | Fgfr1 |
| ENSMUST00000025841 | 7.094498 | 3.27 × 10−5 | Mus81 |
| ENSMUST00000145960 | 7.025383 | 7.73 × 10−8 | Ipo8 |
| ENSMUST00000169734 | 6.768333 | 0.015967 | Vps53 |
| ENSMUST00000117179 | 6.744676 | 0.016023 | Fgfr1 |
| ENSMUST00000178282 | 6.387904 | 0.003841 | Igha |
| ENSMUST00000150759 | 6.286162 | 0.001185 | Unk |
| ENSMUST00000203193 | 6.280975 | 0.01071 | 8-Mar |
| ENSMUST00000212205 | 6.187989 | 0.007475 | Wwp2 |
| ENSMUST00000107847 | 6.002754 | 0.017555 | Clta |
| ENSMUST00000106513 | 5.998047 | 0.029482 | Mknk1 |
| ENSMUST00000098080 | 5.950979 | 0.033919 | Dcun1d3 |
| ENSMUST00000124408 | 5.805759 | 0.008427 | Asph |
| ENSMUST00000084027 | 5.802834 | 0.043785 | Fgfr1 |
| ENSMUST00000172638 | 5.757515 | 0.00226 | Prdm5 |
| ENSMUST00000187609 | −8.64438 | 0.00018 | Nupr1 |
| ENSMUST00000173154 | −7.94081 | 8.17 × 10−5 | Exosc10 |
| ENSMUST00000154428 | −7.80568 | 0.000387 | Unc45a |
| ENSMUST00000206592 | −7.40941 | 9.95 × 10−6 | Stambp |
| ENSMUST00000079896 | −7.08613 | 0.000393 | Tmem192 |
| ENSMUST00000217929 | −6.8355 | 0.039698 | Epb41l2 |
| ENSMUST00000155905 | −6.82367 | 0.018103 | Tex10 |
| ENSMUST00000175778 | −6.81097 | 0.030131 | Sbf1 |
| ENSMUST00000098826 | −6.35686 | 0.001161 | Dlc1 |
| ENSMUST00000201575 | −6.31666 | 0.003582 | Ctbp1 |
| ENSMUST00000107209 | −6.23803 | 0.031545 | Gabpb2 |
| ENSMUST00000196204 | −6.23232 | 0.002753 | Gbp4 |
| ENSMUST00000105964 | −6.18801 | 0.002259 | Gmeb1 |
| ENSMUST00000160134 | −6.06014 | 0.006475 | Dab2 |
| ENSMUST00000212378 | −5.91456 | 1.21 × 10−5 | Rpl18a |
| ENSMUST00000132520 | −5.76178 | 0.001297 | Nadsyn1 |
| ENSMUST00000020681 | −5.49563 | 0.006015 | Slu7 |
| ENSMUST00000226740 | −5.44789 | 0.006064 | Gnl3 |
| ENSMUST00000156314 | −5.40884 | 0.001071 | Rnf20 |
| ENSMUST00000216055 | −5.371 | 0.000664 | Gm48362 |
3.3 Swimming suppresses angiogenesis in CT-26 cell-derived tumors
To further analyze the involved signaling pathway, enrichment analysis of pathways based on DETs was performed. As shown in Figure 3a, multiple signaling pathways were enriched, including the angiogenesis, hypoxia, and VEGF pathways. To verify the mechanism by which swimming regulates tumor angiogenesis, IHC was performed to detect the expression of CD31. Compared with the control group, swimming significantly reduced CD31 expression in tumor tissues (Figure 3b), which demonstrates the attenuation of tumor angiogenesis due to swimming.
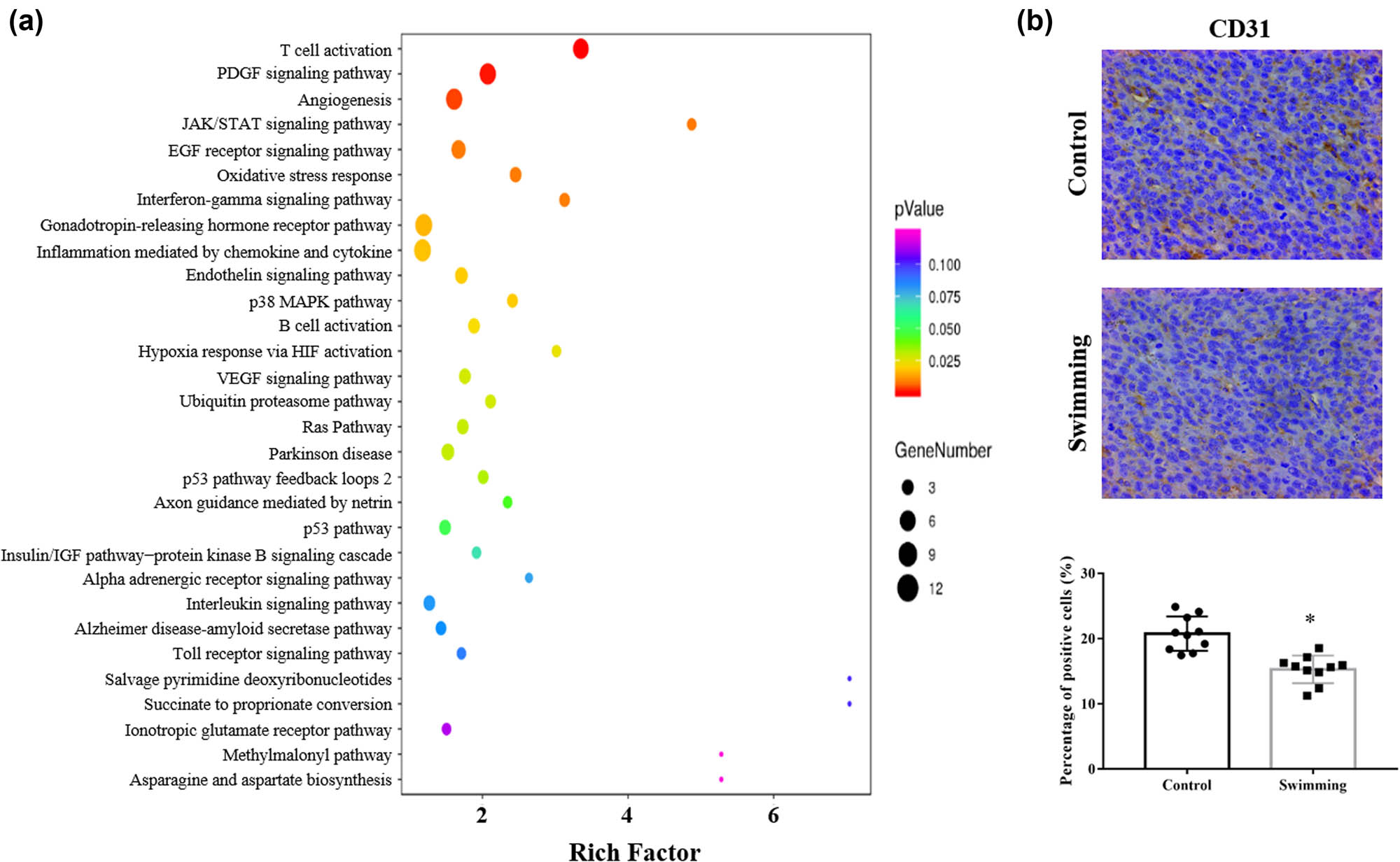
Effects of swimming on related signaling pathways and tumor angiogenesis. (a) KEGG pathway enrichment analysis based on DETs was used to identify related pathways. (b) IHC analysis was performed to determine CD31 expression in tumor tissues from mice in both the control and swimming groups; representative images were obtained at ×400 magnification. The average percentage of positively stained cells was counted using Image-Pro Plus. Data are presented as the mean ± SD. *P < 0.05, vs control.
3.4 Swimming inhibits the HIF-1α/VEGFA signaling pathway
Owing to the essential role of HIF-1α in tumor angiogenesis, we further detected the expression of HIF-1α. As shown in Figure 4, HIF-1α expression at the protein level was significantly decreased in the swimming group compared with the control group (P < 0.05). Furthermore, as essential downstream effectors of the HIF-1α pathway, VEGFA and VEGFR2 expressions were analyzed from RNA-Seq results and confirmed by IHC analysis. As shown in Figure 5a and b, the mRNA expression of VEGFA was significantly downregulated in tumor tissues of the swimming group (P < 0.05; vs control group), while the mRNA of VEGFR2 remained unchanged in tumor tissues between the control and swimming groups (P > 0.05). Moreover, expression of both VEGFA and VEGFR2 proteins was obviously decreased in tumor tissues of the swimming group (P < 0.05; vs control group). These results suggest that the suppression of the HIF-1α/VEGFA/VEGFR2 signaling pathway might be an important underlying mechanism by which swimming attenuates tumor growth in vivo.

Effect of swimming on HIF-1α expression in tumor tissues. IHC analysis was performed to determine HIF-1α expression in tumor tissues from mice in both the control and swimming groups; representative images were obtained at ×400 magnification. The average percentage of positively stained cells was counted using Image-Pro Plus. Data are presented as the mean ± SD. *P < 0.05, vs control.
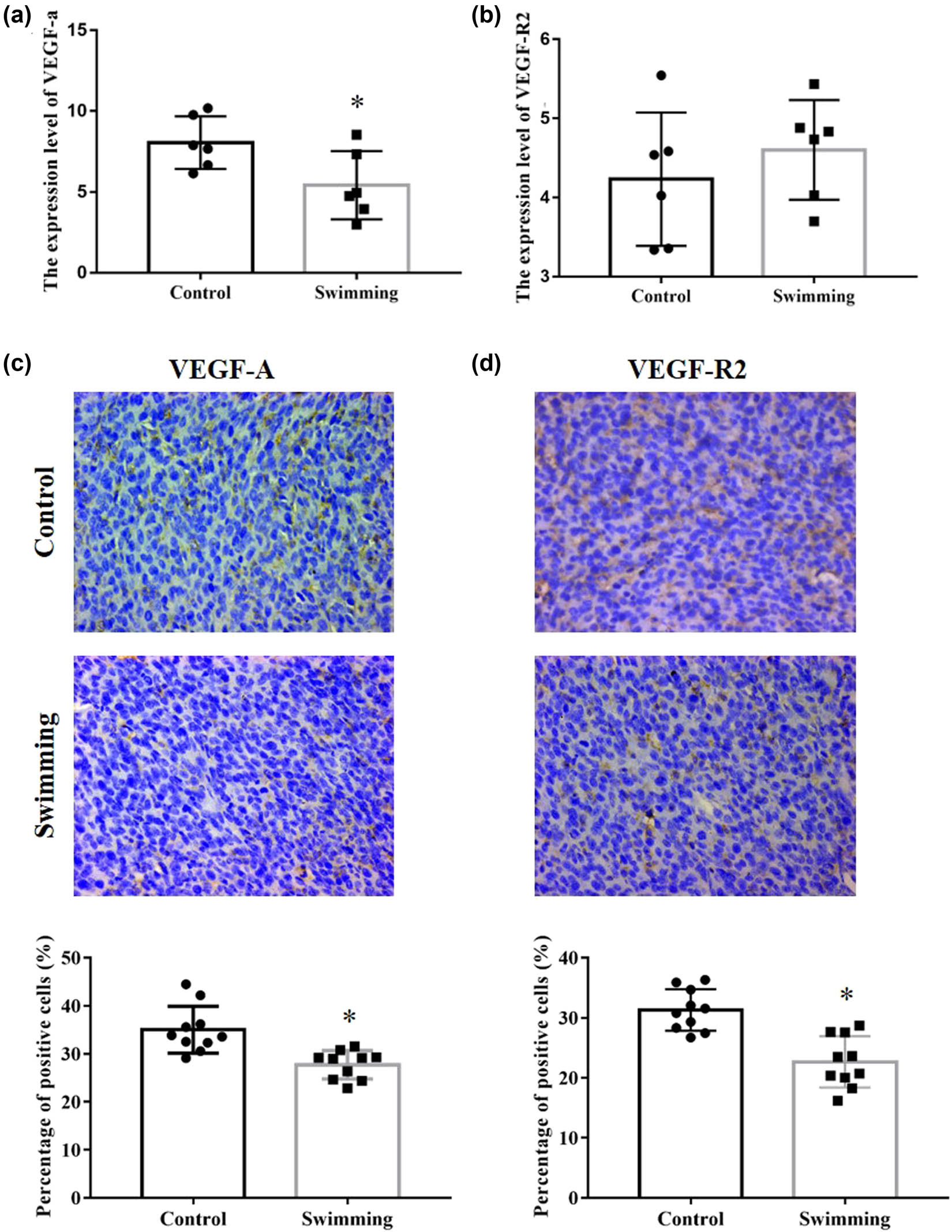
Effects of swimming on VEGFA and VEGFR2 expression in tumor tissues. The mRNA expression level of VEGFA (a) and VEGFR2 (b) in tumor tissues between control and swimming groups was analyzed by the RNA-Seq result. IHC analysis was performed to determine the expression of VEGFA (c) and VEGFR2 (d) in tumor tissues of mice in both the control and swimming groups; representative images were obtained at ×400 magnification. The average percentage of positively stained cells was counted using Image-Pro Plus. Data are presented as the mean ± SD. *P < 0.05, vs control.
4 Discussion
Increasing evidence has revealed that exercise results in multiple benefits, including suppression of tumor growth [4], improvements in quality of life [5], increased chemotherapy efficacy [6], and reductions in the risk of recurrence and cancer-associated mortality [7]. However, as a major form of aquatic exercise, the benefits of swimming in CRC remain largely unknown. In the current study, we demonstrated that swimming clearly attenuated the growth of CT-26 cell-derived tumors in vivo. Mechanistic studies identified 715 upregulated and 629 downregulated transcripts (including VEGFA) in tumor tissues derived from CT-26 cells in vivo after swimming. Further pathway analysis revealed significant enrichment of multiple pathways, including angiogenesis, hypoxia, and VEGF signaling pathways. Consistently, swimming also reduced the protein expression of CD31, HIF-1α, VEGFA, and VEGFR2, which suggests that the essential role of swimming is the suppression of tumor angiogenesis and that the inhibition of the HIF-1α/VEGFA/VEGFR2 axis might be an underlying mechanism by which swimming attenuates CRC tumor growth.
Previous studies in mice demonstrated that 30–60 min/day of swimming leads to a protective effect against cancer in mice [22,23]. Consistently, our current study revealed that swimming significantly alleviated tumor growth in CT-26 tumor-bearing mice. These studies indicated the benefit of swimming on tumors. However, owing to the contrary effects of the exercise of varying intensities in different diseases and states, including cancer [24] and inflammation [25], the benefit of different intensities of swimming on tumor growth should be further explored. Moreover, the effects of swimming on the quality of life and the underlying mechanism should be further investigated.
Although it has been reported that exercise protects against the development of certain cancers and lowers the risk of recurrence, the underlying mechanism by which swimming achieves this is largely unknown. To explore the complicated mechanism of swimming on CRC, identification of DETs using RNA-seq technology revealed that swimming led to 715 upregulated and 629 downregulated transcripts. Among these DETs, multiple genes, including STAT3 [18], PDGFA [19], PLD2 [20], and PIK3R2 [21], have been reported to be involved in tumor growth. Moreover, swimming altered the expression of some genes with less involvement or no known involvement in cancer, which should be further explored in future studies. To further investigate the involved signaling pathway, KEGG pathway enrichment analysis was used to identify the enriched signaling pathways based on DETs. Multiple signaling pathways, including the angiogenesis, hypoxia, and VEGF pathways were significantly enriched, which encouraged us to further explore the underlying mechanism by which swimming attenuates tumor angiogenesis and the HIF-1α/VEGFA pathway activation.
As a central mechanism for tumor growth, angiogenesis plays an essential role in tumor development and growth of human CRC [26,27]. Therefore, targeting tumor angiogenesis represents a novel strategy to combat CRC [28]. Previous studies revealed that exercise promotes vascular maturity [28], increases blood flow [29], and reduces the number of blood vessels [30,31]. Consistently, our current study demonstrated that swimming obviously downregulated CD31 expression, which suggests reduced microvessel density (MVD) and angiogenesis. Hypoxia is a common characteristic of solid tumors and plays an essential role in promoting tumor angiogenesis [32]. Under hypoxic conditions, the key mediator HIF-1α accumulates, dimerizes with HIF-1β, translocates into the nucleus, and binds to cis-acting hypoxia-response elements in target genes leading to increased transcription [33]. In our current study, RNA-Seq did not find the change of HIF-1A mRNA expression, while the determination of HIF-1α protein expression indicated that swimming clearly attenuated HIF-1α expression, which suggests the improvement effect of swimming on hypoxic conditions, consistent with previous studies [6,34]. These studies suggest that swimming might attenuate the activation of HIF-1α by increasing the protein expression of HIF-1α but did not affect its mRNA expression. However, the translocation of HIF-1α should be further explored in a future study.
Moreover, as a transcription factor, accumulated HIF-1α mediates the expression of more than 200 target genes, including VEGFA [35,36], which promotes endothelial cell proliferation and angiogenesis by binding to its receptor VEGFR2 [37]. As expected, swimming obviously reduced both the mRNA and protein expression of VEGFA and protein expression of VEGFR2, which might be one of the underlying mechanisms by which swimming suppresses tumor angiogenesis. However, the regulatory effects of swimming on hypoxia and angiogenesis should be further addressed, especially the distinction among different exercise intensities, times, and environments. Additionally, enriched signaling pathways other than HIF-1α/VEGFA should be investigated in tumor tissues after swimming.
In conclusion, swimming significantly attenuates the growth of CT-26 cell-derived tumors in vivo, reduces tumor angiogenesis, and downregulates the expression of HIF-1α, VEGFA, and its receptor VEGFR2. These studies suggest that swimming is a safe and viable intervention strategy for cancer patients. However, the effect of swimming on tumor vascular normalization, perfusion, oxygen transport, and anaerobic glucose metabolism, as well as its underlying mechanisms, should be further explored.
-
Funding information: This research was supported by an Educational research project for young and middle-aged teachers in Fujian Province, Natural Science Foundation of Fujian Province (2021J01951), The Scientific Research Foundation for the High-level Talents, Fujian University of Traditional Chinese Medicine (X2020004-talents).
-
Author contributions: A.S., J.P., and H.C. conceived and designed the experiments. J.L., Y.L., and A.S. conducted bioinformatics analyses. M.W., Y.C., X.C., and H.L. conducted data analysis. Y.L., Y.C., and Y.H. drew the images. A.S., J.P., Y.L., H.C., J.L. wrote and revised the manuscript. All authors read and approved the final version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The RNA-seq data associated with this article are available in the GEO repository [GEO Submission: GSE 149405].
References
[1] Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.10.3322/caac.21492Search in Google Scholar PubMed
[2] Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–60.e16.10.1053/j.gastro.2014.12.035Search in Google Scholar PubMed PubMed Central
[3] Christensen JF, Simonsen C, Hojman P. Exercise training in cancer control and treatment. Compr Physiol. 2018;9:165–205.10.1002/cphy.c180016Search in Google Scholar PubMed
[4] Sheng ZJ, Qin CJ, Wei CW, Miao LC, Hua ZG, Rui C, et al. The effect of aerobic exercise and Macrothele raven venom on tumor-bearing mice. Int J Sports Med. 2015;36:93–100.10.1055/s-0034-1385877Search in Google Scholar PubMed
[5] Hoffman AJ, Brintnall RA, von Eye A, Jones LW, Alderink G, Patzelt LH, et al. Home-based exercise: promising rehabilitation for symptom relief, improved functional status and quality of life for post-surgical lung cancer patients. J Thorac Dis. 2014;6:632–40.Search in Google Scholar
[6] Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015;107:djv040.10.1093/jnci/djv040Search in Google Scholar PubMed PubMed Central
[7] Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86.10.1001/jama.293.20.2479Search in Google Scholar PubMed
[8] Buss Linda A, Dachs, Gabi U. Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE−/− mice. J Appl Physiol. 2018;124:938–49.10.1152/japplphysiol.00738.2017Search in Google Scholar PubMed
[9] Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–62.10.1016/j.cmet.2016.01.011Search in Google Scholar PubMed
[10] Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41.10.1200/JCO.2006.06.0863Search in Google Scholar PubMed
[11] Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34.10.1200/JCO.2006.06.0855Search in Google Scholar PubMed
[12] Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–604.10.1016/j.ejca.2010.07.028Search in Google Scholar PubMed
[13] Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1548–61.10.1016/j.jsams.2012.11.808Search in Google Scholar
[14] Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–25.10.1001/jamainternmed.2016.1548Search in Google Scholar PubMed PubMed Central
[15] Byers T, Nestle M, McTiernan A, Doyle C, Currie-Williams A, Gansler T, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2002;52:92–119.10.3322/canjclin.52.2.92Search in Google Scholar PubMed
[16] Leitzmann M, Powers H, Anderson AS, Scoccianti C, Berrino F, Boutron-Ruault MC, et al. European code against cancer 4th edition: physical activity and cancer. Cancer Epidemiol. 2015;39(Suppl 1):S46–55.10.1016/j.canep.2015.03.009Search in Google Scholar PubMed
[17] Eaglehouse YL, Koh WP, Wang R, Aizhen J, Yuan JM, Butler LM. Physical activity, sedentary time, and risk of colorectal cancer: the Singapore Chinese Health Study. Eur J Cancer Prev. 2017;26:469–75.10.1097/CEJ.0000000000000369Search in Google Scholar PubMed PubMed Central
[18] Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (London, England). 2019;39:22.10.1186/s40880-019-0368-6Search in Google Scholar PubMed PubMed Central
[19] Zong S, Li W, Li H, Han S, Liu S, Shi Q, et al. Identification of hypoxia-regulated angiogenic genes in colorectal cancer. Biochem Biophys Res Commun. 2017;493:461–7.10.1016/j.bbrc.2017.08.169Search in Google Scholar PubMed
[20] Lee CS, Ghim J, Song P, Suh PG, Ryu SH. Loss of phospholipase D2 impairs VEGF-induced angiogenesis. BMB Rep. 2016;49:191–6.10.5483/BMBRep.2016.49.3.219Search in Google Scholar
[21] Azizi B, Ziaei A, Fuchsluger T, Schmedt T, Chen Y, Jurkunas UV. p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci. 2011;52:9291–7.10.1167/iovs.11-8312Search in Google Scholar PubMed PubMed Central
[22] Almeida PW, Gomes-Filho A, Ferreira AJ, Rodrigues CE, Dias-Peixoto MF, Russo RC, et al. Swim training suppresses tumor growth in mice. J Appl Physiol. 1985;2009(107):261–5.10.1152/japplphysiol.00249.2009Search in Google Scholar PubMed
[23] Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–96.10.1038/onc.2011.37Search in Google Scholar PubMed PubMed Central
[24] Zhang QB, Zhang BH, Zhang KZ, Meng XT, Jia QA, Zhang QB, et al. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene. 2016;35:4122–31.10.1038/onc.2015.484Search in Google Scholar PubMed
[25] Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, et al. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22:643–52.10.1111/j.1600-0838.2010.01288.xSearch in Google Scholar PubMed
[26] Li S, Shi X, Chen M, Xu N, Sun D, Bai R, et al. Angiogenin promotes colorectal cancer metastasis via tiRNA production. Int J Cancer. 2019;145:1395–1407.10.1002/ijc.32245Search in Google Scholar PubMed
[27] Chen H, Feng J, Zhang Y, Shen A, Chen Y, Lin J, et al. Pien Tze Huang inhibits hypoxia-induced angiogenesis via HIF-1 alpha/VEGF-A pathway in colorectal cancer. Evid Based Complement Alternat Med. 2015;2015:454279.10.1155/2015/454279Search in Google Scholar
[28] Cortes E, Lachowski D, Robinson B, Sarper M, Teppo JS, Thorpe SD, et al. Tamoxifen mechanically reprograms the tumor microenvironment via HIF-1A and reduces cancer cell survival. EMBO reports. 2019;20:e46557.10.15252/embr.201846557Search in Google Scholar PubMed PubMed Central
[29] McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 2014;106:dju036.10.1093/jnci/dju036Search in Google Scholar PubMed PubMed Central
[30] Isanejad A, Alizadeh AM, Amani Shalamzari S, Khodayari H, Khodayari S, Khori V, et al. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. 2016;151:30–40.10.1016/j.lfs.2016.02.090Search in Google Scholar PubMed
[31] Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol. 1985;2004(96):2249–56.10.1152/japplphysiol.01210.2003Search in Google Scholar PubMed PubMed Central
[32] Bao L, Chen Y, Lai HT, Wu SY, Wang JE, Hatanpaa KJ, et al. Methylation of hypoxia-inducible factor (HIF)-1alpha by G9a/GLP inhibits HIF-1 transcriptional activity and cell migration. Nucleic Acids Res. 2018;46:6576–91.10.1093/nar/gky449Search in Google Scholar PubMed PubMed Central
[33] Semenza GL. Life with oxygen. Science. 2007;318:62–4.10.1126/science.1147949Search in Google Scholar PubMed
[34] Wiggins JM, Opoku-Acheampong AB, Baumfalk DR, Siemann DW, Behnke BJ. Exercise and the tumor microenvironment: potential therapeutic implications. Exerc Sport Sci Rev. 2018;46:56–64.10.1249/JES.0000000000000137Search in Google Scholar PubMed
[35] Rzepakowska A, Zurek M, Grzybowski J, Pihowicz P, Gornicka B, Niemczyk K, et al. Microvascular density and hypoxia-inducible factor in intraepithelial vocal fold lesions. Eur Arch Otorhinolaryngol. 2019;276:1117–25.10.1007/s00405-019-05355-2Search in Google Scholar PubMed PubMed Central
[36] Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985). 2000;88:1474–80.10.1152/jappl.2000.88.4.1474Search in Google Scholar PubMed
[37] Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27.10.1111/joim.12019Search in Google Scholar PubMed
© 2022 Jiapeng Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Effects of direct oral anticoagulants dabigatran and rivaroxaban on the blood coagulation function in rabbits
- The mother of all battles: Viruses vs humans. Can humans avoid extinction in 50–100 years?
- Knockdown of G1P3 inhibits cell proliferation and enhances the cytotoxicity of dexamethasone in acute lymphoblastic leukemia
- LINC00665 regulates hepatocellular carcinoma by modulating mRNA via the m6A enzyme
- Association study of CLDN14 variations in patients with kidney stones
- Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook
- Regulation of miR-30b in cancer development, apoptosis, and drug resistance
- Informatic analysis of the pulmonary microecology in non-cystic fibrosis bronchiectasis at three different stages
- Swimming attenuates tumor growth in CT-26 tumor-bearing mice and suppresses angiogenesis by mediating the HIF-1α/VEGFA pathway
- Characterization of intestinal microbiota and serum metabolites in patients with mild hepatic encephalopathy
- Functional conservation and divergence in plant-specific GRF gene family revealed by sequences and expression analysis
- Application of the FLP/LoxP-FRT recombination system to switch the eGFP expression in a model prokaryote
- Biomedical evaluation of antioxidant properties of lamb meat enriched with iodine and selenium
- Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-κB pathway
- Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance
- Potential regulatory mechanism of TNF-α/TNFR1/ANXA1 in glioma cells and its role in glioma cell proliferation
- Effect of the genetic mutant G71R in uridine diphosphate-glucuronosyltransferase 1A1 on the conjugation of bilirubin
- Quercetin inhibits cytotoxicity of PC12 cells induced by amyloid-beta 25–35 via stimulating estrogen receptor α, activating ERK1/2, and inhibiting apoptosis
- Nutrition intervention in the management of novel coronavirus pneumonia patients
- circ-CFH promotes the development of HCC by regulating cell proliferation, apoptosis, migration, invasion, and glycolysis through the miR-377-3p/RNF38 axis
- Bmi-1 directly upregulates glucose transporter 1 in human gastric adenocarcinoma
- Lacunar infarction aggravates the cognitive deficit in the elderly with white matter lesion
- Hydroxysafflor yellow A improved retinopathy via Nrf2/HO-1 pathway in rats
- Comparison of axon extension: PTFE versus PLA formed by a 3D printer
- Elevated IL-35 level and iTr35 subset increase the bacterial burden and lung lesions in Mycobacterium tuberculosis-infected mice
- A case report of CAT gene and HNF1β gene variations in a patient with early-onset diabetes
- Study on the mechanism of inhibiting patulin production by fengycin
- SOX4 promotes high-glucose-induced inflammation and angiogenesis of retinal endothelial cells by activating NF-κB signaling pathway
- Relationship between blood clots and COVID-19 vaccines: A literature review
- Analysis of genetic characteristics of 436 children with dysplasia and detailed analysis of rare karyotype
- Bioinformatics network analyses of growth differentiation factor 11
- NR4A1 inhibits the epithelial–mesenchymal transition of hepatic stellate cells: Involvement of TGF-β–Smad2/3/4–ZEB signaling
- Expression of Zeb1 in the differentiation of mouse embryonic stem cell
- Study on the genetic damage caused by cadmium sulfide quantum dots in human lymphocytes
- Association between single-nucleotide polymorphisms of NKX2.5 and congenital heart disease in Chinese population: A meta-analysis
- Assessment of the anesthetic effect of modified pentothal sodium solution on Sprague-Dawley rats
- Genetic susceptibility to high myopia in Han Chinese population
- Potential biomarkers and molecular mechanisms in preeclampsia progression
- Silencing circular RNA-friend leukemia virus integration 1 restrained malignancy of CC cells and oxaliplatin resistance by disturbing dyskeratosis congenita 1
- Endostar plus pembrolizumab combined with a platinum-based dual chemotherapy regime for advanced pulmonary large-cell neuroendocrine carcinoma as a first-line treatment: A case report
- The significance of PAK4 in signaling and clinicopathology: A review
- Sorafenib inhibits ovarian cancer cell proliferation and mobility and induces radiosensitivity by targeting the tumor cell epithelial–mesenchymal transition
- Characterization of rabbit polyclonal antibody against camel recombinant nanobodies
- Active legumain promotes invasion and migration of neuroblastoma by regulating epithelial-mesenchymal transition
- Effect of cell receptors in the pathogenesis of osteoarthritis: Current insights
- MT-12 inhibits the proliferation of bladder cells in vitro and in vivo by enhancing autophagy through mitochondrial dysfunction
- Study of hsa_circRNA_000121 and hsa_circRNA_004183 in papillary thyroid microcarcinoma
- BuyangHuanwu Decoction attenuates cerebral vasospasm caused by subarachnoid hemorrhage in rats via PI3K/AKT/eNOS axis
- Effects of the interaction of Notch and TLR4 pathways on inflammation and heart function in septic heart
- Monosodium iodoacetate-induced subchondral bone microstructure and inflammatory changes in an animal model of osteoarthritis
- A rare presentation of type II Abernethy malformation and nephrotic syndrome: Case report and review
- Rapid death due to pulmonary epithelioid haemangioendothelioma in several weeks: A case report
- Hepatoprotective role of peroxisome proliferator-activated receptor-α in non-cancerous hepatic tissues following transcatheter arterial embolization
- Correlation between peripheral blood lymphocyte subpopulations and primary systemic lupus erythematosus
- A novel SLC8A1-ALK fusion in lung adenocarcinoma confers sensitivity to alectinib: A case report
- β-Hydroxybutyrate upregulates FGF21 expression through inhibition of histone deacetylases in hepatocytes
- Identification of metabolic genes for the prediction of prognosis and tumor microenvironment infiltration in early-stage non-small cell lung cancer
- BTBD10 inhibits glioma tumorigenesis by downregulating cyclin D1 and p-Akt
- Mucormycosis co-infection in COVID-19 patients: An update
- Metagenomic next-generation sequencing in diagnosing Pneumocystis jirovecii pneumonia: A case report
- Long non-coding RNA HOXB-AS1 is a prognostic marker and promotes hepatocellular carcinoma cells’ proliferation and invasion
- Preparation and evaluation of LA-PEG-SPION, a targeted MRI contrast agent for liver cancer
- Proteomic analysis of the liver regulating lipid metabolism in Chaohu ducks using two-dimensional electrophoresis
- Nasopharyngeal tuberculosis: A case report
- Characterization and evaluation of anti-Salmonella enteritidis activity of indigenous probiotic lactobacilli in mice
- Aberrant pulmonary immune response of obese mice to periodontal infection
- Bacteriospermia – A formidable player in male subfertility
- In silico and in vivo analysis of TIPE1 expression in diffuse large B cell lymphoma
- Effects of KCa channels on biological behavior of trophoblasts
- Interleukin-17A influences the vulnerability rather than the size of established atherosclerotic plaques in apolipoprotein E-deficient mice
- Multiple organ failure and death caused by Staphylococcus aureus hip infection: A case report
- Prognostic signature related to the immune environment of oral squamous cell carcinoma
- Primary and metastatic squamous cell carcinoma of the thyroid gland: Two case reports
- Neuroprotective effects of crocin and crocin-loaded niosomes against the paraquat-induced oxidative brain damage in rats
- Role of MMP-2 and CD147 in kidney fibrosis
- Geometric basis of action potential of skeletal muscle cells and neurons
- Babesia microti-induced fulminant sepsis in an immunocompromised host: A case report and the case-specific literature review
- Role of cerebellar cortex in associative learning and memory in guinea pigs
- Application of metagenomic next-generation sequencing technique for diagnosing a specific case of necrotizing meningoencephalitis caused by human herpesvirus 2
- Case report: Quadruple primary malignant neoplasms including esophageal, ureteral, and lung in an elderly male
- Long non-coding RNA NEAT1 promotes angiogenesis in hepatoma carcinoma via the miR-125a-5p/VEGF pathway
- Osteogenic differentiation of periodontal membrane stem cells in inflammatory environments
- Knockdown of SHMT2 enhances the sensitivity of gastric cancer cells to radiotherapy through the Wnt/β-catenin pathway
- Continuous renal replacement therapy combined with double filtration plasmapheresis in the treatment of severe lupus complicated by serious bacterial infections in children: A case report
- Simultaneous triple primary malignancies, including bladder cancer, lymphoma, and lung cancer, in an elderly male: A case report
- Preclinical immunogenicity assessment of a cell-based inactivated whole-virion H5N1 influenza vaccine
- One case of iodine-125 therapy – A new minimally invasive treatment of intrahepatic cholangiocarcinoma
- S1P promotes corneal trigeminal neuron differentiation and corneal nerve repair via upregulating nerve growth factor expression in a mouse model
- Early cancer detection by a targeted methylation assay of circulating tumor DNA in plasma
- Calcifying nanoparticles initiate the calcification process of mesenchymal stem cells in vitro through the activation of the TGF-β1/Smad signaling pathway and promote the decay of echinococcosis
- Evaluation of prognostic markers in patients infected with SARS-CoV-2
- N6-Methyladenosine-related alternative splicing events play a role in bladder cancer
- Characterization of the structural, oxidative, and immunological features of testis tissue from Zucker diabetic fatty rats
- Effects of glucose and osmotic pressure on the proliferation and cell cycle of human chorionic trophoblast cells
- Investigation of genotype diversity of 7,804 norovirus sequences in humans and animals of China
- Characteristics and karyotype analysis of a patient with turner syndrome complicated with multiple-site tumors: A case report
- Aggravated renal fibrosis is positively associated with the activation of HMGB1-TLR2/4 signaling in STZ-induced diabetic mice
- Distribution characteristics of SARS-CoV-2 IgM/IgG in false-positive results detected by chemiluminescent immunoassay
- SRPX2 attenuated oxygen–glucose deprivation and reperfusion-induced injury in cardiomyocytes via alleviating endoplasmic reticulum stress-induced apoptosis through targeting PI3K/Akt/mTOR axis
- Aquaporin-8 overexpression is involved in vascular structure and function changes in placentas of gestational diabetes mellitus patients
- Relationship between CRP gene polymorphisms and ischemic stroke risk: A systematic review and meta-analysis
- Effects of growth hormone on lipid metabolism and sexual development in pubertal obese male rats
- Cloning and identification of the CTLA-4IgV gene and functional application of vaccine in Xinjiang sheep
- Antitumor activity of RUNX3: Upregulation of E-cadherin and downregulation of the epithelial–mesenchymal transition in clear-cell renal cell carcinoma
- PHF8 promotes osteogenic differentiation of BMSCs in old rat with osteoporosis by regulating Wnt/β-catenin pathway
- A review of the current state of the computer-aided diagnosis (CAD) systems for breast cancer diagnosis
- Bilateral dacryoadenitis in adult-onset Still’s disease: A case report
- A novel association between Bmi-1 protein expression and the SUVmax obtained by 18F-FDG PET/CT in patients with gastric adenocarcinoma
- The role of erythrocytes and erythroid progenitor cells in tumors
- Relationship between platelet activation markers and spontaneous abortion: A meta-analysis
- Abnormal methylation caused by folic acid deficiency in neural tube defects
- Silencing TLR4 using an ultrasound-targeted microbubble destruction-based shRNA system reduces ischemia-induced seizures in hyperglycemic rats
- Plant Sciences
- Seasonal succession of bacterial communities in cultured Caulerpa lentillifera detected by high-throughput sequencing
- Cloning and prokaryotic expression of WRKY48 from Caragana intermedia
- Novel Brassica hybrids with different resistance to Leptosphaeria maculans reveal unbalanced rDNA signal patterns
- Application of exogenous auxin and gibberellin regulates the bolting of lettuce (Lactuca sativa L.)
- Phytoremediation of pollutants from wastewater: A concise review
- Genome-wide identification and characterization of NBS-encoding genes in the sweet potato wild ancestor Ipomoea trifida (H.B.K.)
- Alleviative effects of magnetic Fe3O4 nanoparticles on the physiological toxicity of 3-nitrophenol to rice (Oryza sativa L.) seedlings
- Selection and functional identification of Dof genes expressed in response to nitrogen in Populus simonii × Populus nigra
- Study on pecan seed germination influenced by seed endocarp
- Identification of active compounds in Ophiopogonis Radix from different geographical origins by UPLC-Q/TOF-MS combined with GC-MS approaches
- The entire chloroplast genome sequence of Asparagus cochinchinensis and genetic comparison to Asparagus species
- Genome-wide identification of MAPK family genes and their response to abiotic stresses in tea plant (Camellia sinensis)
- Selection and validation of reference genes for RT-qPCR analysis of different organs at various development stages in Caragana intermedia
- Cloning and expression analysis of SERK1 gene in Diospyros lotus
- Integrated metabolomic and transcriptomic profiling revealed coping mechanisms of the edible and medicinal homologous plant Plantago asiatica L. cadmium resistance
- A missense variant in NCF1 is associated with susceptibility to unexplained recurrent spontaneous abortion
- Assessment of drought tolerance indices in faba bean genotypes under different irrigation regimes
- The entire chloroplast genome sequence of Asparagus setaceus (Kunth) Jessop: Genome structure, gene composition, and phylogenetic analysis in Asparagaceae
- Food Science
- Dietary food additive monosodium glutamate with or without high-lipid diet induces spleen anomaly: A mechanistic approach on rat model
- Binge eating disorder during COVID-19
- Potential of honey against the onset of autoimmune diabetes and its associated nephropathy, pancreatitis, and retinopathy in type 1 diabetic animal model
- FTO gene expression in diet-induced obesity is downregulated by Solanum fruit supplementation
- Physical activity enhances fecal lactobacilli in rats chronically drinking sweetened cola beverage
- Supercritical CO2 extraction, chemical composition, and antioxidant effects of Coreopsis tinctoria Nutt. oleoresin
- Functional constituents of plant-based foods boost immunity against acute and chronic disorders
- Effect of selenium and methods of protein extraction on the proteomic profile of Saccharomyces yeast
- Microbial diversity of milk ghee in southern Gansu and its effect on the formation of ghee flavor compounds
- Ecology and Environmental Sciences
- Effects of heavy metals on bacterial community surrounding Bijiashan mining area located in northwest China
- Microorganism community composition analysis coupling with 15N tracer experiments reveals the nitrification rate and N2O emissions in low pH soils in Southern China
- Genetic diversity and population structure of Cinnamomum balansae Lecomte inferred by microsatellites
- Preliminary screening of microplastic contamination in different marine fish species of Taif market, Saudi Arabia
- Plant volatile organic compounds attractive to Lygus pratensis
- Effects of organic materials on soil bacterial community structure in long-term continuous cropping of tomato in greenhouse
- Effects of soil treated fungicide fluopimomide on tomato (Solanum lycopersicum L.) disease control and plant growth
- Prevalence of Yersinia pestis among rodents captured in a semi-arid tropical ecosystem of south-western Zimbabwe
- Effects of irrigation and nitrogen fertilization on mitigating salt-induced Na+ toxicity and sustaining sea rice growth
- Bioengineering and Biotechnology
- Poly-l-lysine-caused cell adhesion induces pyroptosis in THP-1 monocytes
- Development of alkaline phosphatase-scFv and its use for one-step enzyme-linked immunosorbent assay for His-tagged protein detection
- Development and validation of a predictive model for immune-related genes in patients with tongue squamous cell carcinoma
- Agriculture
- Effects of chemical-based fertilizer replacement with biochar-based fertilizer on albic soil nutrient content and maize yield
- Genome-wide identification and expression analysis of CPP-like gene family in Triticum aestivum L. under different hormone and stress conditions
- Agronomic and economic performance of mung bean (Vigna radiata L.) varieties in response to rates of blended NPS fertilizer in Kindo Koysha district, Southern Ethiopia
- Influence of furrow irrigation regime on the yield and water consumption indicators of winter wheat based on a multi-level fuzzy comprehensive evaluation
- Discovery of exercise-related genes and pathway analysis based on comparative genomes of Mongolian originated Abaga and Wushen horse
- Lessons from integrated seasonal forecast-crop modelling in Africa: A systematic review
- Evolution trend of soil fertility in tobacco-planting area of Chenzhou, Hunan Province, China
- Animal Sciences
- Morphological and molecular characterization of Tatera indica Hardwicke 1807 (Rodentia: Muridae) from Pothwar, Pakistan
- Research on meat quality of Qianhua Mutton Merino sheep and Small-tail Han sheep
- SI: A Scientific Memoir
- Suggestions on leading an academic research laboratory group
- My scientific genealogy and the Toronto ACDC Laboratory, 1988–2022
- Erratum
- Erratum to “Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study”
- Erratum to “A two-microRNA signature predicts the progression of male thyroid cancer”
- Retraction
- Retraction of “Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis”
Articles in the same Issue
- Biomedical Sciences
- Effects of direct oral anticoagulants dabigatran and rivaroxaban on the blood coagulation function in rabbits
- The mother of all battles: Viruses vs humans. Can humans avoid extinction in 50–100 years?
- Knockdown of G1P3 inhibits cell proliferation and enhances the cytotoxicity of dexamethasone in acute lymphoblastic leukemia
- LINC00665 regulates hepatocellular carcinoma by modulating mRNA via the m6A enzyme
- Association study of CLDN14 variations in patients with kidney stones
- Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook
- Regulation of miR-30b in cancer development, apoptosis, and drug resistance
- Informatic analysis of the pulmonary microecology in non-cystic fibrosis bronchiectasis at three different stages
- Swimming attenuates tumor growth in CT-26 tumor-bearing mice and suppresses angiogenesis by mediating the HIF-1α/VEGFA pathway
- Characterization of intestinal microbiota and serum metabolites in patients with mild hepatic encephalopathy
- Functional conservation and divergence in plant-specific GRF gene family revealed by sequences and expression analysis
- Application of the FLP/LoxP-FRT recombination system to switch the eGFP expression in a model prokaryote
- Biomedical evaluation of antioxidant properties of lamb meat enriched with iodine and selenium
- Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-κB pathway
- Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance
- Potential regulatory mechanism of TNF-α/TNFR1/ANXA1 in glioma cells and its role in glioma cell proliferation
- Effect of the genetic mutant G71R in uridine diphosphate-glucuronosyltransferase 1A1 on the conjugation of bilirubin
- Quercetin inhibits cytotoxicity of PC12 cells induced by amyloid-beta 25–35 via stimulating estrogen receptor α, activating ERK1/2, and inhibiting apoptosis
- Nutrition intervention in the management of novel coronavirus pneumonia patients
- circ-CFH promotes the development of HCC by regulating cell proliferation, apoptosis, migration, invasion, and glycolysis through the miR-377-3p/RNF38 axis
- Bmi-1 directly upregulates glucose transporter 1 in human gastric adenocarcinoma
- Lacunar infarction aggravates the cognitive deficit in the elderly with white matter lesion
- Hydroxysafflor yellow A improved retinopathy via Nrf2/HO-1 pathway in rats
- Comparison of axon extension: PTFE versus PLA formed by a 3D printer
- Elevated IL-35 level and iTr35 subset increase the bacterial burden and lung lesions in Mycobacterium tuberculosis-infected mice
- A case report of CAT gene and HNF1β gene variations in a patient with early-onset diabetes
- Study on the mechanism of inhibiting patulin production by fengycin
- SOX4 promotes high-glucose-induced inflammation and angiogenesis of retinal endothelial cells by activating NF-κB signaling pathway
- Relationship between blood clots and COVID-19 vaccines: A literature review
- Analysis of genetic characteristics of 436 children with dysplasia and detailed analysis of rare karyotype
- Bioinformatics network analyses of growth differentiation factor 11
- NR4A1 inhibits the epithelial–mesenchymal transition of hepatic stellate cells: Involvement of TGF-β–Smad2/3/4–ZEB signaling
- Expression of Zeb1 in the differentiation of mouse embryonic stem cell
- Study on the genetic damage caused by cadmium sulfide quantum dots in human lymphocytes
- Association between single-nucleotide polymorphisms of NKX2.5 and congenital heart disease in Chinese population: A meta-analysis
- Assessment of the anesthetic effect of modified pentothal sodium solution on Sprague-Dawley rats
- Genetic susceptibility to high myopia in Han Chinese population
- Potential biomarkers and molecular mechanisms in preeclampsia progression
- Silencing circular RNA-friend leukemia virus integration 1 restrained malignancy of CC cells and oxaliplatin resistance by disturbing dyskeratosis congenita 1
- Endostar plus pembrolizumab combined with a platinum-based dual chemotherapy regime for advanced pulmonary large-cell neuroendocrine carcinoma as a first-line treatment: A case report
- The significance of PAK4 in signaling and clinicopathology: A review
- Sorafenib inhibits ovarian cancer cell proliferation and mobility and induces radiosensitivity by targeting the tumor cell epithelial–mesenchymal transition
- Characterization of rabbit polyclonal antibody against camel recombinant nanobodies
- Active legumain promotes invasion and migration of neuroblastoma by regulating epithelial-mesenchymal transition
- Effect of cell receptors in the pathogenesis of osteoarthritis: Current insights
- MT-12 inhibits the proliferation of bladder cells in vitro and in vivo by enhancing autophagy through mitochondrial dysfunction
- Study of hsa_circRNA_000121 and hsa_circRNA_004183 in papillary thyroid microcarcinoma
- BuyangHuanwu Decoction attenuates cerebral vasospasm caused by subarachnoid hemorrhage in rats via PI3K/AKT/eNOS axis
- Effects of the interaction of Notch and TLR4 pathways on inflammation and heart function in septic heart
- Monosodium iodoacetate-induced subchondral bone microstructure and inflammatory changes in an animal model of osteoarthritis
- A rare presentation of type II Abernethy malformation and nephrotic syndrome: Case report and review
- Rapid death due to pulmonary epithelioid haemangioendothelioma in several weeks: A case report
- Hepatoprotective role of peroxisome proliferator-activated receptor-α in non-cancerous hepatic tissues following transcatheter arterial embolization
- Correlation between peripheral blood lymphocyte subpopulations and primary systemic lupus erythematosus
- A novel SLC8A1-ALK fusion in lung adenocarcinoma confers sensitivity to alectinib: A case report
- β-Hydroxybutyrate upregulates FGF21 expression through inhibition of histone deacetylases in hepatocytes
- Identification of metabolic genes for the prediction of prognosis and tumor microenvironment infiltration in early-stage non-small cell lung cancer
- BTBD10 inhibits glioma tumorigenesis by downregulating cyclin D1 and p-Akt
- Mucormycosis co-infection in COVID-19 patients: An update
- Metagenomic next-generation sequencing in diagnosing Pneumocystis jirovecii pneumonia: A case report
- Long non-coding RNA HOXB-AS1 is a prognostic marker and promotes hepatocellular carcinoma cells’ proliferation and invasion
- Preparation and evaluation of LA-PEG-SPION, a targeted MRI contrast agent for liver cancer
- Proteomic analysis of the liver regulating lipid metabolism in Chaohu ducks using two-dimensional electrophoresis
- Nasopharyngeal tuberculosis: A case report
- Characterization and evaluation of anti-Salmonella enteritidis activity of indigenous probiotic lactobacilli in mice
- Aberrant pulmonary immune response of obese mice to periodontal infection
- Bacteriospermia – A formidable player in male subfertility
- In silico and in vivo analysis of TIPE1 expression in diffuse large B cell lymphoma
- Effects of KCa channels on biological behavior of trophoblasts
- Interleukin-17A influences the vulnerability rather than the size of established atherosclerotic plaques in apolipoprotein E-deficient mice
- Multiple organ failure and death caused by Staphylococcus aureus hip infection: A case report
- Prognostic signature related to the immune environment of oral squamous cell carcinoma
- Primary and metastatic squamous cell carcinoma of the thyroid gland: Two case reports
- Neuroprotective effects of crocin and crocin-loaded niosomes against the paraquat-induced oxidative brain damage in rats
- Role of MMP-2 and CD147 in kidney fibrosis
- Geometric basis of action potential of skeletal muscle cells and neurons
- Babesia microti-induced fulminant sepsis in an immunocompromised host: A case report and the case-specific literature review
- Role of cerebellar cortex in associative learning and memory in guinea pigs
- Application of metagenomic next-generation sequencing technique for diagnosing a specific case of necrotizing meningoencephalitis caused by human herpesvirus 2
- Case report: Quadruple primary malignant neoplasms including esophageal, ureteral, and lung in an elderly male
- Long non-coding RNA NEAT1 promotes angiogenesis in hepatoma carcinoma via the miR-125a-5p/VEGF pathway
- Osteogenic differentiation of periodontal membrane stem cells in inflammatory environments
- Knockdown of SHMT2 enhances the sensitivity of gastric cancer cells to radiotherapy through the Wnt/β-catenin pathway
- Continuous renal replacement therapy combined with double filtration plasmapheresis in the treatment of severe lupus complicated by serious bacterial infections in children: A case report
- Simultaneous triple primary malignancies, including bladder cancer, lymphoma, and lung cancer, in an elderly male: A case report
- Preclinical immunogenicity assessment of a cell-based inactivated whole-virion H5N1 influenza vaccine
- One case of iodine-125 therapy – A new minimally invasive treatment of intrahepatic cholangiocarcinoma
- S1P promotes corneal trigeminal neuron differentiation and corneal nerve repair via upregulating nerve growth factor expression in a mouse model
- Early cancer detection by a targeted methylation assay of circulating tumor DNA in plasma
- Calcifying nanoparticles initiate the calcification process of mesenchymal stem cells in vitro through the activation of the TGF-β1/Smad signaling pathway and promote the decay of echinococcosis
- Evaluation of prognostic markers in patients infected with SARS-CoV-2
- N6-Methyladenosine-related alternative splicing events play a role in bladder cancer
- Characterization of the structural, oxidative, and immunological features of testis tissue from Zucker diabetic fatty rats
- Effects of glucose and osmotic pressure on the proliferation and cell cycle of human chorionic trophoblast cells
- Investigation of genotype diversity of 7,804 norovirus sequences in humans and animals of China
- Characteristics and karyotype analysis of a patient with turner syndrome complicated with multiple-site tumors: A case report
- Aggravated renal fibrosis is positively associated with the activation of HMGB1-TLR2/4 signaling in STZ-induced diabetic mice
- Distribution characteristics of SARS-CoV-2 IgM/IgG in false-positive results detected by chemiluminescent immunoassay
- SRPX2 attenuated oxygen–glucose deprivation and reperfusion-induced injury in cardiomyocytes via alleviating endoplasmic reticulum stress-induced apoptosis through targeting PI3K/Akt/mTOR axis
- Aquaporin-8 overexpression is involved in vascular structure and function changes in placentas of gestational diabetes mellitus patients
- Relationship between CRP gene polymorphisms and ischemic stroke risk: A systematic review and meta-analysis
- Effects of growth hormone on lipid metabolism and sexual development in pubertal obese male rats
- Cloning and identification of the CTLA-4IgV gene and functional application of vaccine in Xinjiang sheep
- Antitumor activity of RUNX3: Upregulation of E-cadherin and downregulation of the epithelial–mesenchymal transition in clear-cell renal cell carcinoma
- PHF8 promotes osteogenic differentiation of BMSCs in old rat with osteoporosis by regulating Wnt/β-catenin pathway
- A review of the current state of the computer-aided diagnosis (CAD) systems for breast cancer diagnosis
- Bilateral dacryoadenitis in adult-onset Still’s disease: A case report
- A novel association between Bmi-1 protein expression and the SUVmax obtained by 18F-FDG PET/CT in patients with gastric adenocarcinoma
- The role of erythrocytes and erythroid progenitor cells in tumors
- Relationship between platelet activation markers and spontaneous abortion: A meta-analysis
- Abnormal methylation caused by folic acid deficiency in neural tube defects
- Silencing TLR4 using an ultrasound-targeted microbubble destruction-based shRNA system reduces ischemia-induced seizures in hyperglycemic rats
- Plant Sciences
- Seasonal succession of bacterial communities in cultured Caulerpa lentillifera detected by high-throughput sequencing
- Cloning and prokaryotic expression of WRKY48 from Caragana intermedia
- Novel Brassica hybrids with different resistance to Leptosphaeria maculans reveal unbalanced rDNA signal patterns
- Application of exogenous auxin and gibberellin regulates the bolting of lettuce (Lactuca sativa L.)
- Phytoremediation of pollutants from wastewater: A concise review
- Genome-wide identification and characterization of NBS-encoding genes in the sweet potato wild ancestor Ipomoea trifida (H.B.K.)
- Alleviative effects of magnetic Fe3O4 nanoparticles on the physiological toxicity of 3-nitrophenol to rice (Oryza sativa L.) seedlings
- Selection and functional identification of Dof genes expressed in response to nitrogen in Populus simonii × Populus nigra
- Study on pecan seed germination influenced by seed endocarp
- Identification of active compounds in Ophiopogonis Radix from different geographical origins by UPLC-Q/TOF-MS combined with GC-MS approaches
- The entire chloroplast genome sequence of Asparagus cochinchinensis and genetic comparison to Asparagus species
- Genome-wide identification of MAPK family genes and their response to abiotic stresses in tea plant (Camellia sinensis)
- Selection and validation of reference genes for RT-qPCR analysis of different organs at various development stages in Caragana intermedia
- Cloning and expression analysis of SERK1 gene in Diospyros lotus
- Integrated metabolomic and transcriptomic profiling revealed coping mechanisms of the edible and medicinal homologous plant Plantago asiatica L. cadmium resistance
- A missense variant in NCF1 is associated with susceptibility to unexplained recurrent spontaneous abortion
- Assessment of drought tolerance indices in faba bean genotypes under different irrigation regimes
- The entire chloroplast genome sequence of Asparagus setaceus (Kunth) Jessop: Genome structure, gene composition, and phylogenetic analysis in Asparagaceae
- Food Science
- Dietary food additive monosodium glutamate with or without high-lipid diet induces spleen anomaly: A mechanistic approach on rat model
- Binge eating disorder during COVID-19
- Potential of honey against the onset of autoimmune diabetes and its associated nephropathy, pancreatitis, and retinopathy in type 1 diabetic animal model
- FTO gene expression in diet-induced obesity is downregulated by Solanum fruit supplementation
- Physical activity enhances fecal lactobacilli in rats chronically drinking sweetened cola beverage
- Supercritical CO2 extraction, chemical composition, and antioxidant effects of Coreopsis tinctoria Nutt. oleoresin
- Functional constituents of plant-based foods boost immunity against acute and chronic disorders
- Effect of selenium and methods of protein extraction on the proteomic profile of Saccharomyces yeast
- Microbial diversity of milk ghee in southern Gansu and its effect on the formation of ghee flavor compounds
- Ecology and Environmental Sciences
- Effects of heavy metals on bacterial community surrounding Bijiashan mining area located in northwest China
- Microorganism community composition analysis coupling with 15N tracer experiments reveals the nitrification rate and N2O emissions in low pH soils in Southern China
- Genetic diversity and population structure of Cinnamomum balansae Lecomte inferred by microsatellites
- Preliminary screening of microplastic contamination in different marine fish species of Taif market, Saudi Arabia
- Plant volatile organic compounds attractive to Lygus pratensis
- Effects of organic materials on soil bacterial community structure in long-term continuous cropping of tomato in greenhouse
- Effects of soil treated fungicide fluopimomide on tomato (Solanum lycopersicum L.) disease control and plant growth
- Prevalence of Yersinia pestis among rodents captured in a semi-arid tropical ecosystem of south-western Zimbabwe
- Effects of irrigation and nitrogen fertilization on mitigating salt-induced Na+ toxicity and sustaining sea rice growth
- Bioengineering and Biotechnology
- Poly-l-lysine-caused cell adhesion induces pyroptosis in THP-1 monocytes
- Development of alkaline phosphatase-scFv and its use for one-step enzyme-linked immunosorbent assay for His-tagged protein detection
- Development and validation of a predictive model for immune-related genes in patients with tongue squamous cell carcinoma
- Agriculture
- Effects of chemical-based fertilizer replacement with biochar-based fertilizer on albic soil nutrient content and maize yield
- Genome-wide identification and expression analysis of CPP-like gene family in Triticum aestivum L. under different hormone and stress conditions
- Agronomic and economic performance of mung bean (Vigna radiata L.) varieties in response to rates of blended NPS fertilizer in Kindo Koysha district, Southern Ethiopia
- Influence of furrow irrigation regime on the yield and water consumption indicators of winter wheat based on a multi-level fuzzy comprehensive evaluation
- Discovery of exercise-related genes and pathway analysis based on comparative genomes of Mongolian originated Abaga and Wushen horse
- Lessons from integrated seasonal forecast-crop modelling in Africa: A systematic review
- Evolution trend of soil fertility in tobacco-planting area of Chenzhou, Hunan Province, China
- Animal Sciences
- Morphological and molecular characterization of Tatera indica Hardwicke 1807 (Rodentia: Muridae) from Pothwar, Pakistan
- Research on meat quality of Qianhua Mutton Merino sheep and Small-tail Han sheep
- SI: A Scientific Memoir
- Suggestions on leading an academic research laboratory group
- My scientific genealogy and the Toronto ACDC Laboratory, 1988–2022
- Erratum
- Erratum to “Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study”
- Erratum to “A two-microRNA signature predicts the progression of male thyroid cancer”
- Retraction
- Retraction of “Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis”

