Abstract
Spinal cord injury (SCI) is a serious damage to the spinal cord that can lead to life-long disability. It is classified by initial trauma and subsequent neuronal degeneration, marked by permanent impairment of brain function across the whole brain. This condition results in a progressive deterioration of cognitive function in patients and is frequently associated with psychological symptoms such as body’s movement (paralysis and autonomic dysreflexia), imposing a significant burden on both patients and their families. Nanomaterials such as antioxidant quantum dots (QDs) are an innovative approach, providing dual functionality in theranostics – concurrent therapeutic and diagnostic capacities in the biomedical domain, which can be utilized for disease prevention and therapy. This review thoroughly examines the potential of QDs to transform SCI care due to their inherent antioxidant characteristics, nanoscale accuracy, and capacity to reduce damage caused by reactive oxygen species. It underscores their function in safeguarding brain tissue, augmenting the viability and development of transplanted stem cells, and facilitating axonal regeneration. Moreover, their versatile use in imaging and real-time assessment of treatment results highlights their transformational potential. This study is significant as it connects developing nanotechnology with regenerative medicine for SCI, providing a comprehensive overview of present advances, problems, and future prospects. It examines pivotal concerns such QD toxicity, biocompatibility, and regulatory challenges, while investigating methods for enhancing formulations and incorporating QDs with combination medicines. This review offers a pathway for enhancing QD applications in neuroprotection and regeneration, with the intention of fostering multidisciplinary research and expediting clinical translation, so facilitating new therapies for SCI that enhance patient outcomes and quality of life.
Graphical abstract
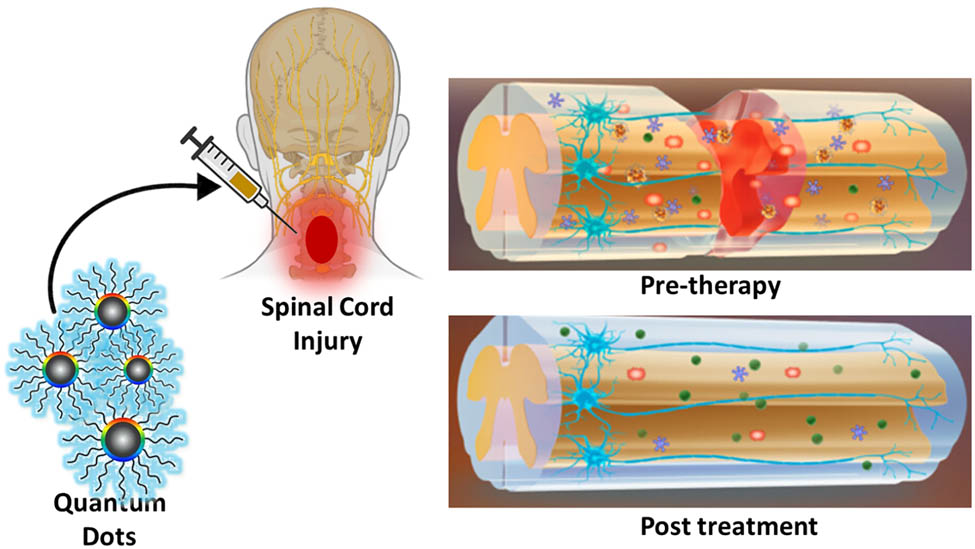
1 Introduction
Spinal cord injury (SCI) is a serious damage to the spinal cord that can lead to lifelong disability. The spinal cord is an intricate system of connections that transmits information and directives between the brain and the body. Numerous, different physiological routes inside the spinal cord facilitate the transfer of specialized information [1]. The corticospinal tract transmits motor function information, whereas the spinothalamic tract and posterior columns serve as the principal corporeal routes [2]. The posterior columns convey vibration, fine touch, and proprioception, while the spinothalamic tracts convey pain, warmth, and harsh trace. From the medulla oblongata, the spinal cord narrows to produce the conus medullaris, usually at vertebral level L2 [3,4,5]. The spinal cord is safeguarded by the meninges and the cervical, thoracic, and lumbar vertebrae [6]. The spinal cord has one anterior and two posterior spinal arteries, as well as radicular arteries located throughout the cord, including the artery of Adamkiewicz, which supplies the inferior two-thirds [7]. The venous drainage of the spinal cord occurs through a complicated system of valveless venous plexuses [8]. SCIs are classified into two categories: traumatic SCI (TSCI) and nontraumatic SCI. TSCIs result from a quick impact on the spine that causes cracks and dislocation of vertebrae. Traumatic spinal cord injury (TSCI) affects normal sensory, motor, and autonomic functioning, impacting a patient’s physical, psychological, and social wellbeing [9,10]. The management of SCI necessitates considerable healthcare resources and can impose a huge financial strain on patients, their families, and the society [11]. These costs are largely due to short-term intensive care and long-term subaltern concerns [12]. To enhance injury management, it is essential to measure the incidence and preponderance of SCIs to better comprehend occurrence rates and identify prevention strategies [13]. A growing concern is the cost-effective effects of SCI on healthcare workers and the system. Krueger et al. estimated that the lifetime economic load of SCI in Canada ranges from CAD$1.47 million for inadequate disability to CAD$3.03 million for total tetraplegia. Wound contaminations, displaced equipment, alternative readmissions, and long-term complications such as pressure ulcers, bladder and bowel dysfunction, neuropathic pain, and respirational disorders are included in these estimates. SCI costs Canada $2.67 billion annually, $1.57 billion directly and $1.10 billion indirectly. Hospitalizations ($0.17 billion, 6.5% of total costs), health care professional visits ($0.18 billion, 6.7%), equipment and home improvements ($0.31 billion, 11.6%), and assistant care ($0.87 billion, 32.7%) make up this total [13]. The Kingdom of Saudi Arabia (KSA) has one of the highest rates of SCIs worldwide [14,15]. According to the Global Burden of Disease report, traumatic injuries represent 22.6% of years of potential either in terms of disability or life lost in KSA [16]. Recent reports on Saudi male SCI patients reported 43.9% with cervical injury followed by 40.4% with thoracic injury and 3.5% with lumbar injury suffering with disability [15].
Modern neuroprotection and regeneration therapies for SCI focus on limiting tissue damage, preventing downstream injury cascades, and promoting neuronal regeneration. To control inflammation and prevent cellular damage, acute SCI treatments include corticosteroids, anti-inflammatory drugs, and surgical decompression [17]. Anti-inflammatory methylprednisolone is sometimes given within hours of injury to reduce inflammation and neuronal death. These medicines help manage initial damage, but their neuroprotective effects are often limited and come with serious adverse effects such as immunosuppression and metabolic issues [18,19]. Cell-based therapies have gained prominence, especially with mesenchymal stem cells (MSCs), neural progenitor cells, and Schwann cells (SCs). These cells can enhance neuronal viability, regulate immunological response, and facilitate axonal regeneration [20]. However, cell growth, host tissue integration, and immunological rejection can reduce their efficacy. Stem cell treatment may cause cancer or fibrosis [21]. In addition to cellular methods, biomaterial scaffolds are employed to offer structural support and directional cues for axonal regeneration [22]. These scaffolds, often composed of hydrogels or polymers, are engineered to replicate the extracellular matrix and can be infused with growth factors or pharmaceuticals to promote regeneration [23,24,25,26]. Despite promising in vitro results, material biocompatibility, degradation, and regulated release in the body’s complex milieu hamper clinical use of these scaffolds. Pharmaceutical treatments like neurotrophic factors and neuroprotective compounds have potential, but the blood-spinal cord barrier, targeted specificity, and short half-lives limit their usefulness [27]. The limits require new SCI treatment, with recent studies focusing on multifunctional, customized medications with neuroprotection and regeneration capabilities [28,29,30].
Through the development of more precise diagnostic tools, more targeted medication delivery systems, and innovative therapies, nanotechnology has revolutionized the medical field. Materials with unique properties at the nanoscale have great potential in the medical field, particularly in the fields of imaging, medication delivery, and regenerative medicine [31]. In the nanomaterial world, quantum dots (QDs) are promising due to their adaptable optical characteristics, photostability, broad absorption spectra, and emission patterns that vary with particle size [32]. The unique characteristics of QDs allow them to exceed traditional fluorescent dyes in bioimaging, providing vibrant and persistent images for cellular and molecular diagnostics [33]. QDs provide significant advantages in cancer diagnostics due to their unique optical properties and tunable surface chemistry. These nanoscale semiconductors can be engineered to specifically bind to tumor-associated biomarkers through surface modification with targeting ligands such as antibodies, peptides, or aptamers. Upon binding, QDs emit bright and stable fluorescence, allowing for highly sensitive and specific imaging of cancerous tissues. This targeted illumination facilitates early-stage detection, enables real-time tracking of tumor progression, and improves differentiation between malignant and healthy cells, ultimately contributing to more accurate diagnostics and personalized treatment strategies.
Furthermore, QDs are being engineered as multifunctional agents, integrating imaging and therapeutic capabilities in theranostic applications [34,35,36]. QDs continue to enhance nanomedicine, offering fascinating new potential for non-invasive, precisely focused medical interventions. Glioblastoma (GBM), a malignant central nervous system (CNS) tumor, is generally poorly excised after surgery due to its invasive proliferation and imprecise neuronal cell demarcation [37]. To overcome this limitation, a fluorescent 5-aminolevulinic acid was integrated with a spectroscopic probe utilized for GBM resection [38]. ZnCdSe/ZnS QDs were utilized in ultrasound-targeted microbubble destruction technology-assisted surgery [39]. Strong reactive oxygen species (ROS) activity, aggregation prevention, and toxicity reduction characterize SeQDS. Their small size allows them to cross the blood–brain barrier (BBB) quickly, and their accumulation in the brain reduces Alzheimer’s disease (AD) and improves cognitive and memory abilities [40]. A reduction in cell survival was observed when doxorubicin (DOX) was delivered using RGD-coupled graphene QDs, which are advantageous for fluorescence imaging and monitoring drug delivery [41]. Likewise, chlorotoxin-modified nanorods delivered DOX to cerebral tumors through the bloodstream, leading to significant inhibition of tumor proliferation [42]. Oxidative stress (OS) exacerbates SCI by facilitating free radical-induced cellular damage, hence impeding regeneration [43]. Effective neuroprotective techniques necessitate potent antioxidant molecules to mitigate this damage [44]. QDs, characterized by their significant tunability and robust antioxidant characteristics, emerge as viable options for SCI treatment [45,46,47]. Surface engineered QDs, particularly those with intrinsic antioxidant properties or those functionalized with ROS-scavenging ligands, can mitigate oxidative damage by neutralizing free radicals and restoring redox balance within the injured spinal cord microenvironment. Furthermore, their high surface area and adjustable surface chemistry enable the simultaneous delivery of antioxidant agents or genes that enhance endogenous antioxidant defense mechanisms. By mitigating OS, QDs may enhance neuronal and glial survival, impede apoptotic pathways, and foster a more conducive biochemical environment for axonal regeneration and functional recovery following SCI. Selenium-doped carbon quantum dots (Se‑CQDs) have demonstrated significant protective effects on astrocytes and PC12 cells against oxidative damage caused by H2O2 in vitro [48]. Additionally, the neuroprotective potential of Se-CQDs was examined in a model of contusion-induced TSCI. The findings indicated that Se-CQDs provided protection to the injured spinal cord by reducing inflammation, preventing the demyelination of nerve fibers, and inhibiting the apoptosis of neuronal cells. Ren et al. reported in comparison to large graphene oxide (GO) nanosheets, GO quantum dots (GOQDs) function as nanozymes that effectively reduce ROS and H2O2 in PC12 cells induced by 1-methyl-4-phenyl-pyridinium ion (MPP+) [45]. Furthermore, GOQDs demonstrate neuroprotective properties in a neuronal cell model by reducing apoptosis and α-synuclein levels. GOQDs effectively reduce ROS, apoptosis, and mitochondrial damage in zebrafish exposed to MPP+. Further, zebrafish pretreated with GOQDs exhibit enhanced locomotive activity and an increase in Nissl bodies within the brain, indicating that GOQDs effectively mitigate MPP+-induced neurotoxicity, unlike GO nanosheets. GOQDs play a role in mitigating neurotoxicity by enhancing amino acid metabolism, lowering tricarboxylic acid cycle activity, and reducing the activities of steroid biosynthesis, fatty acid biosynthesis, and galactose metabolic pathways, all of which are associated with antioxidation and neurotransmission. Furthermore, QDs can be used as therapeutic antioxidants and bioimaging agents, allowing real-time damage progression monitoring [49,50,51,52].
The emergence of artificial intelligence (AI) in the field of radiology where many promising tools are focused on the spine and spinal cord represents a paradigm shift in the diagnostic and prognosticating SCI, nevertheless their clinical utility remains uncertain [53]. The application of AI-enabled algorithms using machine learning (ML), deep learning, and convolutional neural networks approaches, which quickly determine and respond to SCI, have been created based on radiographs, computed tomography, and magnetic resonance imaging scans. Therefore, AI systems and research on spinal cord neural injury and restoration can mutually reinforce each other and drive medical innovation to analyze potential solutions [54]. More recently, Dietz et al., studied the potential for clinical integration of ML data in the patient population to improve diagnosis and prognostication of SCI [55]. Arslan and co-workers have recognized enhanced diagnosis of SCI on unconscious and uncooperative patients using dermatomal skin impendence analysis through artificial neural network [56]. In this study, classification methods of support vector machines and hierarchical cluster tree analysis showed improved diagnostic rates in injury and recovery. Interestingly, Tay et al. used ML to evaluate patients with SCI via diffusion tensor imaging [57]. Hence, we conclude that medical imaging will continue to garner significant application of ML for diagnosis and prognosis, and may offer particular benefit to management of SCI given its acuity, complexity variability, and multimodal treatment paradigms.
This multidisciplinary approach, combining advances in neuroscience, technology, and treatment, offers new perspectives and hopes for addressing SCI, a complex and challenging problem leading to a wide range of neurological illness. The aim of this review merges QDs antioxidant discoveries with SCI applications, a fresh approach that current research lacks. Our research on QDs provides a holistic view of SCI care and lays the groundwork for innovative, multifunctional therapeutics that exceed current therapy limitations. QDs’ therapeutic and diagnostic potential in spinal cord damage makes our review a significant, progressive neuroprotective scientific contribution.
1.1 QDs: Synthesis and properties
QDs are semiconductor nanocrystals exhibiting distinctive optical and electrical characteristics attributable to quantum confinement processes. These nanomaterials often measure between 2 and 10 nm in diameter, positioning them within a size range where their physical and chemical characteristics markedly diverge from those of bulk materials. The size tunability of QDs serves as a significant advantage in controlling their light-emission properties. This enables simultaneous excitation of various-sized QDs using a single light source, along with component-tunable broad spectral windows as shown in Figure 1 [58]. The elevated molar extinction coefficients of QDs contribute to the enhanced brightness of their fluorescence, in conjunction with a high quantum yield (QY) [59]. The elevated stability against photobleaching of QDs facilitates prolonged dynamic imaging [60]. The observed blinking of QDs is categorized into states of “dimmed” or “grey” (intermediate) intensities, ensuring the detection of a single dot event, such as the observation of an individual protein [61]. The distinctive size-dependent characteristics of QDs render them indispensable in domains such as optoelectronics, bioimaging, photovoltaics, and sensing technologies.
![Figure 1
The photophysical features of QDs made in biological or biomimetic systems. (a) Size-tunable fluorescence output and multiple QDs being excited by the same light source at the same time. (b) The QDs made up of various components have wide spectrum ranges that go from ultraviolet to infrared [58].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_001.jpg)
The photophysical features of QDs made in biological or biomimetic systems. (a) Size-tunable fluorescence output and multiple QDs being excited by the same light source at the same time. (b) The QDs made up of various components have wide spectrum ranges that go from ultraviolet to infrared [58].
Regarding the properties mentioned, QDs have established an excellent track record in the fields of medical and biological sciences. QDs are presently utilized as luminescent tools and labels in drug delivery and targeting, as well as in the sensing of DNA and oligonucleotides. They play a significant role in various scientific imaging methods, including molecular histopathology, flow cytometry-based identifications, disease identification, and biomedical imaging. Numerous studies have demonstrated the utility of QDs in clinical applications, such as sentinel lymph node visualization, micrometastasis detection, and photodynamic therapy (PDT) [62]. Furthermore, several applications have been suggested for QDs, such as carriers for drug delivery and light indicators for biological coding. Like other nanomaterials, there are concerns regarding the potential toxicity of QDs that need to be addressed prior to their clinical application [63].
Significant advancements have been achieved in novel synthesis pathways for QDs. The formation of QDs is primarily classified into top-down and bottom-up techniques, each utilizing different methodologies to attain the nanoscale size and specific features [64,65]. Top-down synthesis methods entail the disintegration of bulk materials into nanoscale particles via physical or mechanical procedures. These techniques are very effective for attaining accurate morphologies and structural configurations. Electron beam lithography (EBL) is a prevalent top-down method that employs a highly focused electron beam to intricately shape nanoscale objects with remarkable accuracy [66]. Despite its great efficacy, EBL is costly and time-consuming, constraining its scalability. A prevalent technique is mechanical milling, which reduces bulk materials to nanoparticles via high-energy grinding. This approach is economical and appropriate for large-scale manufacturing; nevertheless, it frequently yields particles with broad size dispersion and possible surface imperfections. Alternative top-down approaches, such as laser ablation and ion implantation, are utilized but frequently need advanced equipment and specialized knowledge. Conversely, bottom-up synthesis techniques entail the chemical or physical construction of QDs from atomic or molecule precursors. These approaches are preferred for their capacity to regulate the dimensions, shape, and surface characteristics of the QDs. Colloidal synthesis is the predominant method employed owing to its flexibility and accuracy. This technique entails high-temperature reactions utilizing surfactants or stabilizing chemicals to regulate particle development and inhibit aggregation. Colloidal synthesis is highly efficient for generating monodisperse QDs with adjustable optical and electrical characteristics. Additional notable bottom-up techniques encompass hydrothermal and solvothermal methods, which entail high-pressure, high-temperature reactions in either aqueous or organic solvents [67,68]. The methods employed are environmentally sustainable, scalable, and produce QDs with outstanding crystallinity. Table 1 represents a comprehensive classification of the synthesis methodologies for QDs, including their respective benefits and drawbacks. Bottom-up techniques, including hydrothermal, microwave-assisted, soft-template, and stepwise organic synthesis, provide benefits such as scalability, controllable particle dimensions, and doping potential. But they may be tedious, expensive, and occasionally susceptible to aggregation or need extensive purification. Top-down methods, such as oxidative/reductive cutting, electrochemical cutting, and pulsed laser ablation (PLA), are frequently more appropriate for large-scale production and expedited synthesis. Nonetheless, these methods may lead to inconsistent particle sizes, necessitate the use of aggressive chemicals or costly instruments, and are less favorable for doping methods. The synthesis of nanomaterials by microwave methods, a bottom-up approach offers benefits such as reduced reaction time, swift and uniform heating, and enhanced yield and purity. Phytic acid, which is rich in phosphorus, was combined with ethylenediamine in water and subjected to treatment in a domestic microwave oven for a duration of 8 min by Wang et al. [69]. Figure 2(a) illustrates the synthesis method employed for the phosphorus-containing carbon dots (CDs). The products acquired were subsequently purified through acetone extraction, resulting in green fluorescent CDs (as shown in Figure 2b) with a QY of 21.65%. Lower excitations showed two distinct peak emissions, whereas at higher excitations, a single peak emission was observed for these CDs. The authors demonstrated that a covalent linkage of the phosphorus functional groups to the graphite-like structure was evident in the CDs. Tang and coworkers demonstrated a microwave-assisted hydrothermal synthesis (Figure 2c) of graphene quantum dots (GQDs) derived from glucose, aiming to produce GQDs with diameters spanning from 2.9 to 3.9 nm. This study illustrated that the size of GQDs can be controlled within the range of 1.65–21 nm by adjusting the reaction time from 1 to 9 min. The GQDs exhibited excitation-dependent emission characteristics, with QYs calculated to range from 7 to 11%. The GQDs demonstrated the ability to convert blue light into white light, which was further confirmed by applying GQDs onto a blue-light emitting diode. The application of these GQDs enables the conversion of blue light into white light when they are applied to a blue-light-emitting diode.
Synthesis methods of QDs and their advantages and disadvantages
| Method | Synthesis route | Advantages | Disadvantages |
|---|---|---|---|
| Bottom-up | Hydrothermal | Simple and effective method; supports heteroatom doping and surface passivation | Time-consuming and costly; requires extensive purification; may produce minor aggregation; non-uniform sizes |
| Microwave assisted | Rapid reaction time; produces uniform particle sizes; enables doping with ease | Suitable for small-scale synthesis; requires intensive purification; limited industrial scalability | |
| Soft-template | Produces uniform particle sizes; straightforward purification; scalable for large production | Prone to particle aggregation | |
| Stepwise organic synthesis | Offers excellent control over structure, size, and photoluminescence (PL) properties | Complex and tedious processes; often stable only in organic solvents; limited scalability potential | |
| Top-down | Oxidative/reductive cutting | Utilizes readily available precursors; ideal for large-scale production | Non-uniform particle sizes; requires harsh chemicals and purification; less favorable for doping |
| Electrochemical Cutting | Rapid synthesis; produces uniform particle sizes with good crystallinity | Difficult to scale; costly raw materials; limited doping flexibility unless precursors are pre-doped | |
| PLA | Environmentally friendly; requires no harsh chemicals; straightforward purification | Requires costly equipment; less suited for heteroatom doping |
![Figure 2
(a) Schematic representation of the production of phosphorus-containing CDs. (b) Emission in the presence of natural light (on the left) and 365 nm UV radiation (on the right) [69]. (c) Synthesis of glucose-derived GQDs using microwave-assisted hydrothermal synthesis method.](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_002.jpg)
(a) Schematic representation of the production of phosphorus-containing CDs. (b) Emission in the presence of natural light (on the left) and 365 nm UV radiation (on the right) [69]. (c) Synthesis of glucose-derived GQDs using microwave-assisted hydrothermal synthesis method.
Non-metal QDs have gained significant attention as environmentally friendly and biocompatible alternatives to traditional metal-based QDs. Among these, CDs, GQDs, and polymer dots (PDs) have emerged as promising materials due to their unique optical, chemical, and structural properties [70,71,72]. CDs are zero-dimensional nanomaterials composed primarily of carbon, typically synthesized via hydrothermal, solvothermal, or microwave-assisted methods. These methods involve the carbonization of organic precursors such as citric acid, glucose, or chitosan [73,74,75,76]. CDs exhibit strong PL, high photostability, low toxicity, and excellent water dispersibility, making them suitable for biomedical imaging, sensing, and photocatalysis. The emission properties of CDs can be tuned by controlling the precursor composition, synthesis temperature, and surface functionalization. Notably, surface passivation with polymers or organic ligands can significantly enhance their QY and improve their stability. GQDs are small fragments of graphene sheets with lateral dimensions below 10 nm, often synthesized via top-down (e.g., oxidative cutting of GO) or bottom-up (e.g., carbonization of organic molecules) approaches [36,65]. GQDs possess unique sp2 carbon structures that provide excellent PL, superior chemical stability, and strong antioxidant properties [77]. Their tunable bandgap enables broad absorption across the visible to near-infrared spectrum. GQDs also demonstrate high biocompatibility, making them ideal candidates for bioimaging, drug delivery, and PDT [78]. Their fluorescence behavior is strongly influenced by surface functional groups, edge states, and quantum confinement effects. PDs are fluorescent polymeric nanoparticles that combine the advantages of organic dyes and QDs [79]. Typically synthesized through emulsion polymerization, nanoprecipitation, or self-assembly methods, PDs offer tunable fluorescence, high photostability, and low cytotoxicity. Their structure allows precise engineering of size, shape, and surface properties, making them versatile platforms for biosensing, imaging, and optoelectronic applications [80,81]. The emission properties of PDs can be tailored by modifying their polymer backbone or incorporating fluorophores with distinct electronic structures [82].
Their application in biological systems largely depends on their biocompatibility and colloidal stability, both of which are significantly influenced by the synthesis route employed [83]. The method of synthesis governs not only the size and surface chemistry of the QDs but also the presence of impurities, surface defects, and the nature of surface ligands all of which determine how QDs interact with biological environments.
Hydrothermal synthesis is a widely used method that involves the synthesis of QDs under high pressure and temperature in aqueous or non-aqueous solvents [84]. This technique often results in QDs with high crystallinity and fewer surface defects, which improves photostability and reduces the generation of ROS under illumination which is an important factor for biocompatibility. However, if not properly capped or functionalized, QDs synthesized via this route may aggregate in physiological media due to limited water dispersibility [85]. To improve biological stability, hydrophilic surface coatings such as polyethylene glycol, carboxylic acids, or zwitterionic ligands are often introduced.
Microwave-assisted techniques allow for rapid and uniform heating, resulting in highly monodisperse QDs with controlled sizes. The rapid reaction kinetics can limit the formation of impurities and reduce batch-to-batch variability. When paired with suitable precursors and stabilizing agents, QDs produced by this method exhibit enhanced colloidal stability and lower toxicity due to reduced defect densities [86]. Moreover, the ability to perform synthesis in aqueous media makes this approach more environmentally friendly and compatible with biological systems. Green or biosynthesis methods utilize natural reducing agents such as plant extracts, amino acids, or polysaccharides to produce QDs under mild conditions. These routes avoid toxic reagents and heavy metals, thereby enhancing the inherent biocompatibility of the resulting nanomaterials [58]. Furthermore, biomolecules used in synthesis often remain on the QDs surface as capping agents, improving water solubility and enabling interactions with biological targets. However, green synthesis may offer limited control over particle size and uniformity unless carefully optimized [87]. Traditional organometallic synthesis involves high-temperature reactions in organic solvents and typically produces high-quality QDs with excellent optical properties. However, these QDs are often capped with hydrophobic ligands like trioctylphosphine oxide, making them poorly dispersible in aqueous environments [88,89,90]. To render them biologically compatible, additional ligand exchange or encapsulation in amphiphilic polymers or liposomes is required [91]. These post-synthesis modifications are crucial to reduce cytotoxicity and improve in vivo stability [92]. Regardless of the initial synthesis method, surface functionalization plays a vital role in governing QD behavior in biological systems. Functionalization with biocompatible ligands not only enhances solubility and colloidal stability but also reduces protein adsorption and immune recognition [89].
1.2 Properties of QDs
Quantum confinement effects provide distinctive optical and electrical characteristics in QDs, providing them with several benefits over existing fluorophores, including organic dyes, fluorescent proteins, and lanthanide chelates [93]. Factors that significantly affect fluorophore behavior, and consequently their suitability for various applications, encompass the breadth of the excitation spectrum, the width of the emission spectrum, photostability, and the decay lifespan. Conventional dyes exhibit limited excitation spectra, necessitating illumination by light of a specific wavelength, which differs among individual dyes. QDs exhibit broad absorption spectra, enabling excitation across a wide range of wavelengths. This characteristic can be utilized to simultaneously excite multiple differently colored QDs using a single wavelength [94]. Traditional dyes exhibit broad emission spectra, indicating that the spectra of various dyes may significantly overlap. This restricts the quantity of fluorescent probes that can be utilized to label various biological molecules and be spectrally distinguished at the same time. Conversely, QDs have narrow emission spectra, which may be manipulated relatively easily by altering core size and composition, as well as by modifying surface coatings. They can be designed to emit light throughout a range of specific wavelengths, from ultraviolet (UV) to infrared (IR). The narrow emission and broad absorption spectra of QDs render them highly suitable for multiplexed imaging, where various colors and intensities are integrated to encode genes, proteins, and small-molecule libraries [95]. Due to their excellent photostability, they may facilitate the monitoring of prolonged interactions among multiple-labeled biological molecules within cells.
Photostability is an essential characteristic in several fluorescence applications, in which QDs provide a distinct advantage. In contrast to organic fluorophores that degrade after few minutes of light exposure, QDs exhibit remarkable stability, allowing for prolonged cycles of excitation and fluorescence for hours while maintaining high brightness and resistance to photobleaching. QDs have demonstrated greater photostability compared to several organic dyes [96,97], including Alexa488, which is noted as the most stable organic dye [98]. Chan and Nie reported semiconductor QDs with bright luminescence (zinc sulfide-capped cadmium selenide) that have been covalently attached to biomolecules for highly sensitive biological identification. In comparison to organic dyes such as rhodamine, these QDs demonstrate a 20 times greater brightness, a stability against photobleaching that is 100 times higher, and a spectral line width that is one-third narrower [97].
The characteristics of the PL of QDs are their size and excitation-dependent emission features. The study by Liu et al. stated the properties of polyethylenimine (PEI)-passivated carbon quantum dots, which exhibited stable multicolor luminescence dependent on the excitation wavelength [99]. The aqueous solution of these CDs exhibited blue, green, and red fluorescence when subjected to UV light excitation, blue, and green, respectively. The PL spectra of CD solution demonstrated a red-shifted emission characteristic, transitioning from 450 to 550 nm as the excitation wavelengths varied from 340 to 500 nm. This notable behavior was attributed to the size and surface state non-uniformity of the CDs passivated by PEI. Moreover, various studies have also indicated the presence of excitation wavelength independent CDs. Dong et al. documented the synthesis of nitrogen and sulfur co-doped CDs derived from citric acid and l-cysteine as precursors, which exhibited emission characteristics independent of excitation [100]. The authors elucidated that the PL of the CDs is dependent on surface states rather than shape, and that these surface states are homogeneous. In a prior study, similar excitation-independent emissions were achieved for nitrogen and zinc co-doped CDs [101].
The PL property has been observed to exhibit pH dependence. Pan et al. proposed hydrothermally prepared GQDs that exhibited strong emission under alkaline conditions, while in acidic environments, the PL emission was substantially quenched [102]. The PL intensity exhibited reversibility; specifically, when the pH of the GQD solution alternated between 12 and 1, the PL demonstrated a reversible change as shown in Figure 3a. It is crucial to observe that the pH of the solution affected only the PL intensity of GQDs, while the PL emission wavelength remained unchanged. Furthermore, the concentrations of the CDs or GQDs also affected their PL intensity or wavelength. The CDs synthesized from banana juice, as published by De and Karak, exhibited concentration-dependent photoluminescent characteristics [103]. The spectra presented in Figure 3b revealed that the PL intensity diminishes with rising concentrations of the CDs. The author elucidated that at low concentrations, the interaction among polar groups reduces, and at large concentrations, the abundance of polar functional groups tends to create agglomerates. Das et al. have observed a similar phenomenon for the nitrogen and sulfur co-doped CDs produced from kappa carrageenan and urea [104].
QDs are nanoscale semiconductor particles, often measuring 2–10 nm, exhibiting distinctive optical and electrical characteristics due to quantum confinement phenomena. Their dimensions directly affect their emission wavelength, with smaller QDs generating blue light and bigger ones emitting red [105]. QDs are composed of a crystalline semiconductor core, often fabricated from materials such as CdSe, PbS, or InP [106]. They may be categorized as core-only, core–shell (including a protective shell like ZnS to improve stability and efficiency), or alloyed QDs (incorporating mixed elements for customized features). QDs are typically spherical in morphology; however, they may also adopt morphologies such as rods or tetrapods, depending upon the synthesis conditions [107]. Surface functionalization with organic or inorganic ligands enhances solubility, stability, and versatility for applications in optoelectronics, photovoltaics, and biomedicine. On the other hand, CDs and GQDs are carbon-derived nanomaterials exhibiting exceptional optical, electrical, and structural characteristics, positioning them as sustainable substitutes for conventional semiconductor QDs. CDs are generally spherical nanoparticles, measuring less than 10 nm, characterized by amorphous or partially crystallized structures [108]. Their outstanding PL, minimal toxicity, and remarkable biocompatibility facilitate their use in bioimaging, sensors, and photocatalysis [109,110]. GQDs have significant quantum confinement and edge effects, providing adjustable fluorescence, elevated conductivity, and exceptional chemical stability [77]. Both CDs and GQDs are economical, eco-friendly, and exceptionally adaptable, with applications in energy storage, optoelectronics, antimicrobial agents, bioimaging, and environmental monitoring [111,112]. Table 2 summarizes recent advancements in CDs utilized as fluorescent probes for detecting neurological biomarkers in biological fluids. The table highlights key features such as synthesis methods, surface functionalization, target biomarkers, and detection limits, emphasizing the versatility and sensitivity of CDs in neurodiagnostic applications.
CDs utilized as fluorescent probes for the identification of neurological biomarkers in biological fluids
| Type of QD | Precursors | Excitation/emission wavelength (nm) | LOD | Neurological biofluids | Ref. |
|---|---|---|---|---|---|
| CDs-COOH functionalized | Tris (hydroxymethyl)aminomethane | 365/460 | 50 nM | Cerebral | [113] |
| N-doped CDs | Sodium citrate and tripolycyanamide | 345/440 | 5 nM | Human serum and rat brain microdialysate | [114] |
| Fe(+3)-doped CDs | Tris(hydroxymethyl)aminomethane | 365/445 | 9.1 nM | Rat brain microdialysate | [115] |
| CDs-antibody conjugate | Citric acid | 360/447 | 25 pg/mL | Human serum | [116] |
| N-doped GQDs | Citric acid and ammonia | 355/440 | 1.22 µM | Detection of tacrine | [117] |
| MIPs-CDs | Citric acid | 360/465 | 3 µM | — | [118] |
| Curcumin-GQDs | Citric acid | 370/500 | 12.4 pg/mL | Blood plasma (human) | [119] |
| Te-doped CDs | 2,7-Bis(phenylselanyl)-9H-fluoren-9-one | 380/440 | 8 pM | Brain of mild depression mice | [120] |
| S-doped CDs/AuNPs | Phenylamine-4-sulfonic acid | 365/465 | 0.23 µM | Ampoule, urine and serum human | [121] |
| CDs | Corn extract | 353/446 | 6.46 µM | Human cerebrospinal fluid and serum | [122] |
2 OS in SCI
Figure 4 offers a detailed examination of trauma-induced damage across multiple physiological systems, emphasizing SCI and associated OS processes [123,124,125]. The figure comprises two sections: the upper illustration presents a close-up of spinal cord trauma, illustrating the cellular origins of ROS following abrupt mechanical injury, whereas the lower section emphasizes the systemic complications and injury sites linked to severe bodily trauma [126]. The upper section depicts the key steps initiated by acute spinal injury, encompassing the compromise of cellular integrity and the ensuing production of ROS [127]. Mitochondria, depicted with indicators for ROS buildup, exhibit malfunction post-trauma and serve as significant sources of OS. Alongside mitochondrial ROS, immune cells, including neutrophils and macrophages, are attracted to the site of injury, where they secrete pro-inflammatory cytokines and ROS as components of the inflammatory response [128]. The ROS produced by mitochondrial failure and immune cell activity intensify cellular damage by mechanisms such as lipid peroxidation, protein oxidation, and DNA breakage, leading to neuronal cell death and scar tissue development that further impede recovery [129,130,131]. The lower section of the picture elaborates on the systemic consequences and prevalent trauma locations that often follow SCI. Traumatic brain injury and spinal cord damage frequently co-occur, resulting in various neurological consequences, including loss of consciousness, tension, and heightened sympathetic drive, which can exacerbate the total injury response [124,132,133,134,135]. Thoracic trauma, encompassing hypoxia and ischemia-reperfusion injury (IRI) [136], diminishes cardiac output and oxygen transport [137,138], hence impairing the tissue’s capacity to handle OS and aggravating neuronal injury [139]. Additional significant systemic effects encompass cognitive changes, psychomotor dysfunctions, infections resulting from soft tissue and muscular injuries [140], direct harm to organs like the spleen and liver, bone fractures, and hemorrhaging that may result in hypovolemic shock [141]. The trauma-induced “triad of death” (acidosis, coagulopathy, and hypothermia) is prominently featured, highlighting how these interrelated variables hinder recovery by impairing the body’s innate defense mechanisms.
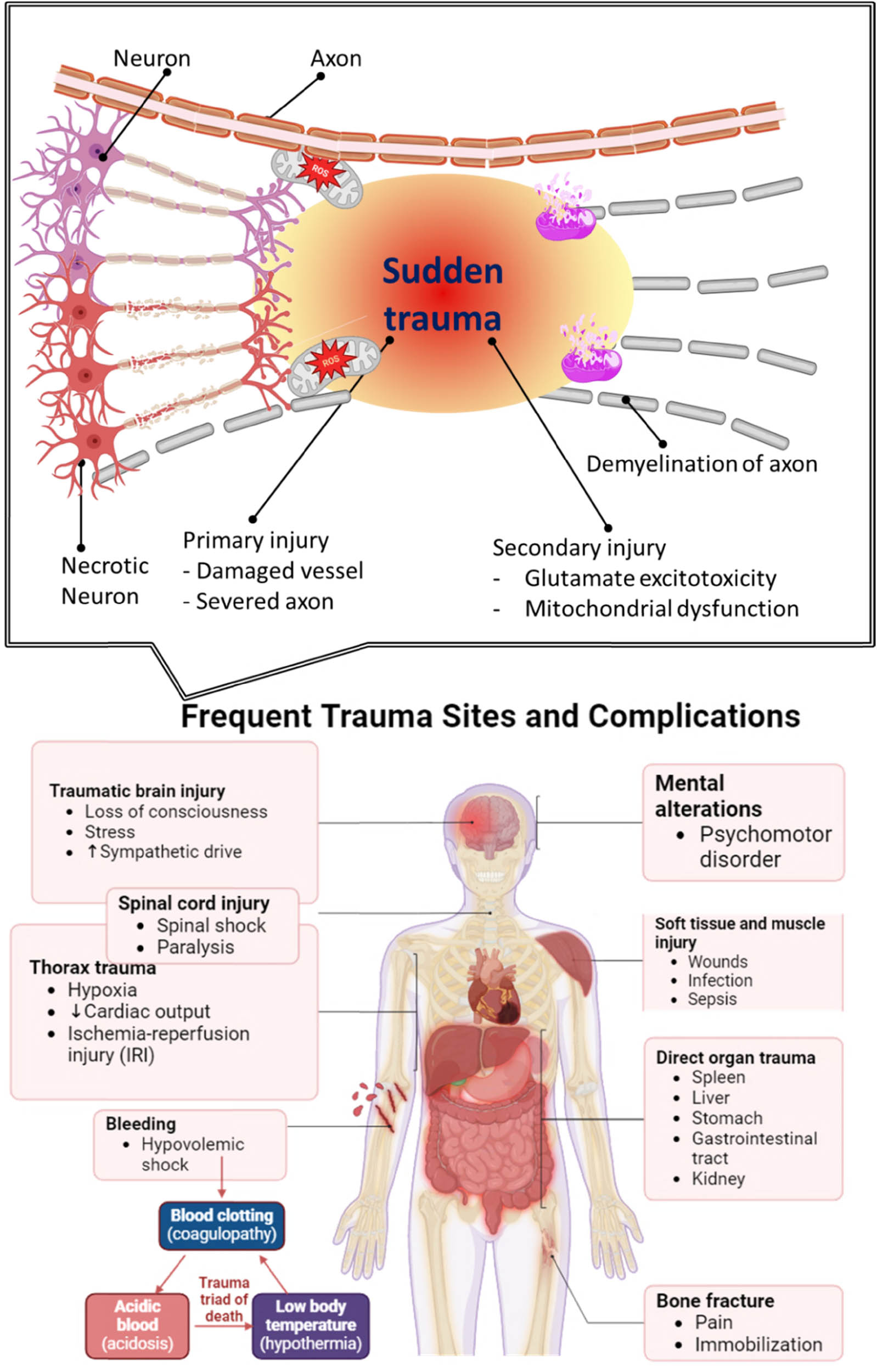
The impact of OS on neuronal cells and tissue in SCI.
Figure 5 delineates the intricate pathways of OS injury subsequent to SCI, showcasing a cascade of cellular and molecular disturbances that exacerbate secondary injury. Following SCI, the first trauma induces a microenvironmental disruption, initiating many metabolic processes that intensify OS and inflammation, all of which significantly contribute to the exacerbation of neuronal damage [142]. The illustration depicts many principal sources of ROS formation subsequent to SCI [143]. Neutrophil enzymatic processes are among the initial contributors, as neutrophils are swiftly drawn to the site of injury and emit ROS to eradicate injured cells and pathogens. Furthermore, hemoglobin and ferrous iron (Fe2⁺) are liberated during cellular lysis, engaging in Fenton reactions that generate extremely reactive hydroxyl radicals. Mitochondrial failure, a characteristic of SCI, concurrently results in excess calcium (Ca2⁺), disruption of the respiratory chain, and increased production of ROS [144]. This mitochondrial dysfunction not only produces ROS directly but also diminishes the cell’s capacity to regulate OS, hence intensifying ROS buildup. The endoplasmic reticulum (ER) experiences stress, leading to impaired protein folding and contributing to cellular malfunction, hence increasing OS [145]. The elevation of ROS triggers a detrimental cycle, illustrated by the positive feedback loop [146]. Increased ROS levels stimulate an inflammatory response, which then attracts more inflammatory cells, each contributing to increased ROS generation. This feedback loop exacerbates OS, sustaining cellular and tissue damage. The image illustrates that OS produces multiple detrimental effects downstream. ROS trigger lipid peroxidation, compromising cell membranes and enhancing their permeability, ultimately resulting in cell death [147]. ROS interact with proteins and nucleic acids, creating adducts that disrupt cellular function and facilitate neurodegeneration. Ultimately, these mechanisms lead to extensive neuronal apoptosis and autophagy, both of which undermine neural tissue integrity and impede recovery [148].

SCI-related OS damage mechanisms. Neutrophils, phagocytes, mitochondria, ER, and lysed red blood cells (Fe2+) generate excessive ROS after SCI. ROS emission beyond scavenging capacity causes OS. ROS promote lipid peroxidation, protein and DNA damage, and inflammatory response crosstalk, deteriorating SCI.
2.1 SCI OS hypothesis: Excess ROS generation
The production of excessive ROS is crucial in the secondary injury processes after SCI, greatly contributing to cellular and tissue damage. ROS are byproducts of normal cellular metabolism, mostly generated in mitochondria via oxidative phosphorylation [149]. Under physiological conditions, ROS levels are meticulously managed by antioxidant defense mechanisms [150]. Following SCI, there is a significant increase in ROS generation due to mitochondrial malfunction, infiltration of inflammatory cells, and the degradation of cellular structures, which surpasses the cellular antioxidant defenses. This ROS overload initiates a series of harmful events, resulting in neuronal death, tissue necrosis, and inflammation, which worsen the initial injury. In the context of SCI, ROS formation is predominantly heightened by OS processes, wherein excessive ROS compromise cellular membranes and organelles [151]. The mitochondria, due to their heightened sensitivity to stress, are a principal generator of ROS following injury. Spinal cord trauma disrupts mitochondrial activity, decreasing the electron transport chain (ETC) and increasing electron leakage, resulting in the formation of superoxide anions through reactions with oxygen [152]. Superoxide is then transformed into more reactive ROS, such as hydrogen peroxide and hydroxyl radicals, which target cellular lipids, proteins, and DNA. This oxidative damage undermines cellular integrity and facilitates apoptotic and necrotic cell death in neurons, oligodendrocytes, and astrocytes, resulting in additional functional and structural deterioration in the damaged spinal cord [153]. Moreover, ROS overload induces neuroinflammation by activating immune cells, including microglia and invading macrophages, which secrete pro-inflammatory cytokines and exacerbate ROS generation. The inflammatory response to SCI can be advantageous in many respects, as it eliminates debris and fosters a regenerative milieu; however, the overproduction of ROS by activated microglia and macrophages perpetuates a chronic inflammatory cycle that obstructs tissue regeneration. Pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and IL-1β, intensify the inflammatory response, establishing a detrimental milieu that obstructs axonal regeneration and remyelination [154]. This chronic inflammatory condition sustains a cycle of ROS production and oxidative harm, resulting in enduring functional impairments and ongoing pain in SCI patients [155].
The degradation of cellular and extracellular components caused by ROS overload further intensifies excitotoxicity, a phenomenon in which impaired neurons release excessive amounts of glutamate [156]. The increased glutamate concentration excessively activates N-methyl-d-aspartate receptors on neighboring neurons, resulting in intracellular calcium overload that subsequently enhances ROS generation and mitochondrial impairment [157]. The interaction between calcium and ROS intensifies cellular toxicity, leading to extensive neuronal death. Therapeutic approaches aimed at ROS and OS possesses considerable potential for alleviating SCI damage [158]. Antioxidants, including N-acetylcysteine (NAC), edaravone, and resveratrol, have demonstrated potential in experimental models by neutralizing ROS and diminishing lipid peroxidation. These drugs seek to restore the redox equilibrium, safeguard cellular integrity, and mitigate inflammation, thus maintaining neural functionality [159]. The intricacy of SCI pathology and the constraints of systemic antioxidant administration highlight the necessity for focused therapeutics that precisely regulates ROS levels within the spinal cord. Advancements in nanotechnology, including antioxidant-loaded nanoparticles, present exciting opportunities for precise control of ROS, thereby enhancing therapeutic outcomes in SCI patients [160]. The overproduction of ROS after SCI greatly contributes to subsequent damage mechanisms, such as neuronal death, inflammation, and excitotoxicity [161]. Mitigating excess ROS using specific antioxidant therapy and novel delivery mechanisms may enhance functional recovery and diminish long-term damage in SCI patients [162]. Nonetheless, additional study is required to comprehensively comprehend ROS dynamics and formulate effective, tailored therapies that may be used in clinical practice.
Liu et al. synthesized aldehyde-scavenging polypeptides (PAH)-curcumin conjugate nanoassemblies (PFCN) for neuroprotection in SCI by a straightforward in situ reaction-induced self-assembly method. Oxidative and acidic microenvironments in SCI may generate PAH and curcumin from synthesized PFCN [163]. PFCN mitigated neuroinflammation by eliminating harmful aldehydes and reactive nitrogen and oxygen species in neurons (Figure 6), controlling microglial M1/M2 polarization, and decreasing inflammation-related cytokines. In the contusive SCI rat model, intravenous PFCN was able to mitigate the detrimental spinal cord microenvironment, safeguard neurons, and enhance motor performance.
![Figure 6
Schematic representation of the preparation of PFCN for integrated treatment of SCI [163].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_006.jpg)
Schematic representation of the preparation of PFCN for integrated treatment of SCI [163].
Tauroursodeoxycholic acid (TUDCA) is a polar derivative of bile acid that has shown neuroprotective properties in various nervous illness models. Nonetheless, the impact and fundamental pathway of TUDCA on SCI remain inadequately clarified. This study seeks to examine the defensive benefits of TUDCA in the SCI mice model and the associated mechanisms involved [164]. TUDCA treatment may mitigate secondary injury and enhance functional recovery by diminishing OS, seditious reaction, and apoptosis resulting from primary injury, while also facilitating axon regeneration and remyelination, presenting a possible therapeutic option for human SCI recovery. From a molecular perspective, OS and inflammatory pathways are primary orchestrators of interconnected dysregulated pathways subsequent to SCI. It emphasizes the necessity of developing multitarget therapy for SCI sequelae. Polyphenols, as secondary metabolites generated from plants, possess the potential to serve as alternative physiological factors for the therapy of SCI. These secondary metabolites exhibited intonation consequences on neuronal OS, neuroinflammation, and aberrant extrinsic axonal tracts in the development and course of SCI. This review elucidates the significant significance of phenolic compounds as essential phytochemicals in modulating dysregulated OS and inflammatory signaling mediators, as well as extrinsic processes of axonal regeneration following SCI, based on preclinical and clinical investigations [165]. The activation of OS and apoptosis-induced cell death considerably contributes to the advancement of SCI. Current data indicate that maltol has natural antioxidative effects via inhibiting OS and apoptosis. Nonetheless, the substantial impact of maltol on SCI therapy is yet to be assessed. This work investigated maltol administration, which may induce Nrf2 expression and facilitate the retranslocation of Nrf2 from the cytosol to the nucleus, thereby inhibiting OS signaling and apoptosis-related neuronal cell death after SCI. Moreover, maltol administration augments PINK1/Parkin-mediated mitophagy in PC12 cells, promoting the restoration of mitochondrial functions [166]. The transplantation of bone marrow mesenchymal stem cells (BMSCs) has surfaced as a prospective therapy for SCI. The poor survival and differentiation rates of BMSCs in the spinal cord milieu considerably restrict their therapeutic efficacy. TUDCA, an active compound derived from bear bile, has shown neuroprotective, antioxidant, and antiapoptotic properties in SCI. The current work aims to investigate the potential advantages of merging TUDCA with BMSC transplantation in an animal model of SCI. The findings indicated that TUDCA markedly improved BMSC survivability while decreasing apoptosis and OS in both in vitro and in vivo settings. TUDCA expedited tissue regeneration and enhanced functional recovery post-BMSC implantation in SCI. The effects were mediated via the Nrf-2 signaling tract, as shown by the increase in Nrf-2, NQO-1, and HO-1 expression levels [167]. A thioketal-based, ROS-scavenging hydrogel was synthesized for the encapsulation of BMSCs, facilitating neurogenesis and axon re-formation by mitigating excessive ROS and reconstructing a regenerative milieu [168]. The hydrogel effectively encapsulated BMSCs and exhibited significant neuroprotection in vivo by diminishing endogenous ROS production, mitigating ROS-induced oxidative impairment, and downregulating inflammatory cytokines including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and TNF-α, thereby reducing cell apoptosis in spinal cord tissue. The BMSC-conjugated ROS-scavenging hydrogel decreased scar formation and promoted neurogenesis in spinal cord tissue, therefore significantly improving motor efficient regaining in SCI mice. To investigate the impact of photobiomodulation (PBM) on axon renewal and alterations in secretion from dorsal root ganglion (DRG) under OS after SCI, and to further examine how PBM-induced changes in DRG secretion influence macrophage polarization [169]. The PBM-DRG model was developed to conduct PBM on neurons subjected to OS in vitro. The outcome yielded energy of 4 J. Approximately 100 μM H2O2 was introduced to the culture medium to induce OS after SCI. A ROS test kit was applied to quantify ROS levels in the DRG. The neuronal survival rate was assessed by the CCK-8 assay, and axonal regeneration was examined via immunofluorescence techniques.
2.2 How QDs act as antioxidants?
QDs serve as effective antioxidants owing to their distinctive electrical configuration and adjustable surface characteristics, allowing them to neutralize ROS and avert oxidative harm. The antioxidant efficacy of QDs is mostly contingent upon their composition and surface changes, often accomplished by doping with elements such as sulfur, nitrogen, or cerium, which augment ROS scavenging capabilities [170]. These alterations allow QDs to emulate natural antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT), facilitating the conversion of superoxide radicals (
GQDs seem to be among the brightest antioxidants due to their suitable antioxidant properties, distinctive structure, superior cytocompatibility, and little toxicity. Nonetheless, the comparatively diminished antioxidant activity compared to inorganic semiconductor materials and the ambiguous antioxidant mechanism restricted their cellular applications. This article investigates the antioxidant process by examining the correlation between antioxidant behavior and oxygenated surface groups. The overall oxygen fraction was regulated by post-preparation reduction with NaBH4, while the specific types of oxygen functional groups were modified by free radicals during the synthesis of GQDs [171]. Hemmateenejad et al. developed a novel QD-based test to assess antioxidant and polyphenolic activity [172]. This experiment measures the inhibitory impact of antioxidant/polyphenolic substances on the UV-induced bleaching of l-cysteine-capped CdTe QDs. QDs demonstrated remarkable photostability in the absence of UV exposure, although they underwent fast bleaching when subjected to UV irradiation. The production of ROS during UV irradiation is likely the primary factor contributing to the photobleaching of QDs. The comparison of the photostability of QDs in buffer solution, both with and without sodium azide, a recognized quencher of singlet oxygen, corroborated the role of singlet oxygen in the photobleaching of QDs. The photobleaching impact caused by ROS may be mitigated by the presence of antioxidant or polyphenolic substances. We evaluated several antioxidant and polyphenolic substances, in addition to established antioxidants like trolox and four distinct varieties of tea. Chong et al. showed that GQDs may effectively scavenge various free radicals, therefore protecting cells from oxidative injury. Upon exposure to blue light, GQDs demonstrate considerable phototoxicity by elevating intracellular ROS levels and diminishing cell viability, due to the production of free radicals under light stimulation [173]. They also affirmed that the light-induced generation of ROS arises from the electron-hole pair and, crucially, demonstrate that singlet oxygen is produced by photoexcited GQDs via both energy-transfer and electron-transfer mechanisms (Figure 7). Furthermore, following light stimulation, GQDs enhance the oxidation of non-enzymatic antioxidants and facilitate lipid peroxidation, hence adding to the light induced-toxicity of GQDs. Our findings indicate that GQDs exhibit antioxidant and pro-oxidant properties, contingent upon light exposure, which will inform the protective application and advancement of significant anticancer and bactericidal uses for GQDs.
![Figure 7
GQDs scavenging various free radicals. ESR spectra of DMPO/˙OH adducts were acquired using 20 μM Fe2+, 50 mM DMPO, 20 μM H2O2, 10 mM PBS buffer (pH 7.27), and various GQD doses after 1 min of incubation. GQD concentration affects ˙OH-scavenging activity. After 1 min incubation, 25 mM BMPO, 10% DMSO, 2.5 mM KO2, 0.35 mM 18-crown-6, 10 mM PBS buffer (pH 7.27), and varied GQD doses yielded ESR spectra of BMPO/˙OOH adducts. GQD concentration affects
O
2
⋅
−
{\text{O}}_{2}^{\cdot -}
scavenging. (e) Samples with 0.1 mM DPPH˙, 10 mM PBS buffer (pH 7.27), and varied GQD doses were used to generate ESR spectra. Data were taken 5 min after incubation. (f) GQD concentration affects DPPH˙-scavenging [173].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_007.jpg)
GQDs scavenging various free radicals. ESR spectra of DMPO/˙OH adducts were acquired using 20 μM Fe2+, 50 mM DMPO, 20 μM H2O2, 10 mM PBS buffer (pH 7.27), and various GQD doses after 1 min of incubation. GQD concentration affects ˙OH-scavenging activity. After 1 min incubation, 25 mM BMPO, 10% DMSO, 2.5 mM KO2, 0.35 mM 18-crown-6, 10 mM PBS buffer (pH 7.27), and varied GQD doses yielded ESR spectra of BMPO/˙OOH adducts. GQD concentration affects
Recent investigations have shown that GQDs are anti- and pro-oxidant. Their efficiency is poor. We present chlorine-doped GQDs (Cl-GQDs) with variable Cl doping and enhanced anti- and pro-oxidant activity. Cl-GQDs had 7-fold and 3-fold greater scavenging and free radical-produced efficiency than undoped GQDs [174]. The production of ROS from GQDs under light irradiation was validated by ESR spectroscopy. TEMP was chosen as a spin trap for singlet oxygen, capable of selectively reacting with 1O2. Upon the entrapment of 1O2, a stable compound, 2,2,6,6-tetramethylpiperidine-1-oxyl, was generated, resulting in a distinctive ESR signal. Figure 8a demonstrates a pronounced ESR signal of TEMP in the Cl-GQDs-7.5V solution during irradiation. Furthermore, no ESR signal was seen in the control sample under dark conditions. The findings indicated that 1O2 might be produced by Cl-GQDs-7.5V under irradiation, consistent with prior studies. Upon photoexcitation, 1O2 was generated by energy transfer from the excited triplet state of the Cl-GQDs to the ground-state oxygen (Figure 8b). Furthermore, no
![Figure 8
(a) ESR spectra from Cl-GQDs-7.5V and TEMP samples with (black) or without (red) light. (b) 1O2 generating schematic [174].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_008.jpg)
(a) ESR spectra from Cl-GQDs-7.5V and TEMP samples with (black) or without (red) light. (b) 1O2 generating schematic [174].
Composite nanoparticles of naringenin-loaded β-cyclodextrin and CQDs were effectively synthesized. The findings indicated that the integration of CQDs not only augmented the antioxidant activities of nanoparticles but also boosted the encapsulation efficiency of naringenin. The creation of composite nanoparticles was validated by several characterization techniques. The zeta potential and Fourier transform infrared spectroscopy results demonstrated that electrostatic interactions and hydrogen bonding are the predominant factors in nanoparticle formation. The X-ray diffraction experiment indicated that the naringenin-β-CD-CQDs nanoparticles are in an amorphous state, contrasting with the crystalline states of naringenin, β-CD, and the naringenin-β-CD inclusion complex. Ultimately, tests of antioxidant activity against DPPH, ABTS+, and Fe2+ chelating demonstrated that the resultant composite nanoparticles exhibited superior antioxidant activity relative to their individual components [175].
Leveraging the antioxidant properties of QDs, their use in neuroprotection seems particularly advantageous, especially regarding neurodegenerative illnesses driven by OS and acute traumas like SCI. By neutralizing ROS and modifying cellular antioxidant pathways, QDs mitigate oxidative damage and contribute to the stabilization of neuronal cell settings. This decrease in OS inhibits subsequent consequences such as apoptosis, inflammation, and mitochondrial malfunction, all of which are essential for neuronal survival and function. Moreover, the capacity of QDs to selectively target and concentrate in brain tissues has further benefits, as they may provide localized antioxidant effects exactly where required. The inherent antioxidant characteristics of QDs provide neuroprotective advantages, indicating their potential as therapeutic agents in SCI, traumatic brain traumas, and chronic neurodegenerative disorders.
3 Role of antioxidant QDs in neuroprotection
Antioxidant QDs have emerged as viable options for neuroprotection in SCI owing to their distinctive features that mitigate OS, a significant factor in cellular damage associated with SCI [49]. OS, characterized by excessive ROS and inadequate antioxidant defense, induces lipid peroxidation, protein oxidation, and DNA damage, hence aggravating neuronal cell death, inflammation, and subsequent secondary injury [176]. Antioxidant QDs provide a precise method to reduce oxidative damage, perhaps interrupting this cycle and facilitating cellular healing and regeneration [177]. Doping QDs with sulfur, nitrogen, or cerium boosts their antioxidant ability, enabling these doped QDs to emulate natural enzymes like SOD and CAT, which neutralize superoxide anions and hydrogen peroxide, respectively [178]. The capacity to precisely adjust these characteristics via controlled synthesis is a notable benefit of QDs compared to conventional antioxidants, enabling the creation of highly targeted therapeutic agents for SCI therapy.
Upon arrival at the injury site, antioxidant QDs demonstrate ROS-scavenging capabilities that mitigate OS in neuronal and adjacent glial cells [177]. The decrease in ROS levels may safeguard mitochondrial function, inhibit apoptosis, and diminish inflammation. Research indicates that by mitigating oxidative damage, QDs may preserve cellular homeostasis and promote the survival of neuronal and glial cells, essential for functional recovery after SCI [179]. Furthermore, the antioxidant activities of QDs are augmented by their photostability, allowing for prolonged protective effects without the rapid destruction characteristic of most small-molecule antioxidants [180]. In addition to directly neutralizing ROS, antioxidant QDs affect cellular signaling pathways, especially those associated with inflammation and apoptosis, including the NF-κB and Nrf2 pathways [181]. NF-κB, a principal modulator of inflammation, is often increased after SCI and facilitates the secretion of pro-inflammatory cytokines, hence intensifying tissue damage. Antioxidant QDs may impede NF-κB activation, hence diminishing cytokine production and attenuating the inflammatory response [182]. Simultaneously, QDs stimulate the Nrf2 pathway, a principal regulator of antioxidant defenses, therefore enhancing cellular resistance to OS via the upregulation of endogenous antioxidant enzymes [183]. The combined mechanism of reducing inflammatory signals and enhancing antioxidant defenses makes QDs a versatile instrument in SCI treatment. Besides biochemical benefits, the diminutive size and extensive surface area of QDs enhance cellular absorption efficiency and enable possible conjugation with targeting ligands, hence facilitating targeted delivery to damage locations. Functionalizing QDs with chemicals that identify SCI-specific markers might provide a focused strategy, reducing off-target effects and enhancing neuroprotective effectiveness [184]. This trait is especially advantageous in neurodegenerative disorders such as SCI, when accuracy is essential to prevent damage to healthy, unaffected tissue [185]. The use of antioxidant QDs in neuroprotection remains nascent, with current research focusing on their biocompatibility and long-term safety. Although concerns about the toxicity of some classic QDs persist, new advancements in the production of non-toxic, biocompatible QDs have alleviated these dangers, facilitating their therapeutic use [186]. Future research aims to optimize QD formulations, enhance targeting mechanisms, and guarantee safe in vivo degradation. Due to their adaptability and diverse effects on OS and inflammation, antioxidant QDs provide substantial promise for improving neuroprotective techniques in SCI, providing optimism for increased recovery and functional restoration.
To discover new neuroprotective drugs, the pathophysiology and underlying molecular pathways must be examined. Numerous hypotheses have been proposed regarding the etiology of AD, including, but not limited to, the accumulation of amyloid beta (Aβ) neurotoxic plaques, hyper-phosphorylated tau protein, OS, overactive microglial cells leading to inflammation, and downregulation of cAMP response element-binding protein (CREB) [187]. AD is characterized by the activation of the amyloidogenic pathway, whereby the intracellular amyloid precursor protein is sequentially cleaved by β- and γ-secretases, resulting in the release of insoluble Aβ that accumulates and forms neurotoxic plaques. The deposition of Aβ activates microglial cells, triggers an inflammatory response, promotes the production of inflammatory mediators, and ultimately results in neuronal cell death [188]. Aβ has been shown to penetrate the mitochondrion, instigate OS, and generate ROS, resulting in mitochondrial malfunction, impaired ETC, dysregulated calcium homeostasis, and permanent cellular damage [189]. Aβ may also disrupt the production of CREB, a crucial protein for neural plasticity and memory formation and consolidation. CREB signaling also mitigates synapse loss induced by Aβ aggregation [190]. A different study sought to examine the molecular processes and neuroprotective properties of hyaluronic acid modified verapamil-loaded CQDs (VRH-loaded HA-CQDs) in an in vitro AD model caused by amyloid beta (Aβ) in SH-SY5Y and Neuro 2 a neuroblastoma cells [191]. Exposure to N-GQDs triggered ferroptosis in microglia via eliciting mitochondrial OS, but the ferroptotic effects elicited by A-GQDs were less pronounced under identical exposure conditions. This research will elucidate the mechanisms of GQDs-induced cellular damage across various forms of cell death and examine the impact of chemical modifications on GQDs’ toxicity [50]. The powerful environmental pesticide and weedicide paraquat is associated with neuromotor impairments and Parkinson’s disease (PD). We have assessed the neuroprotective function of citric acid-derived CQDs (Cit-CQDs) on paraquat-damaged human neuroblastoma-derived SH-SY5Y cell lines and on paraquat-exposed nematodes (Caenorhabditis elegans). Our observations indicate that Cit-CQDs may scavenge free radicals in vitro and reduce paraquat-induced ROS levels in SH-SY5Y cells. Moreover, Cit-CQDs safeguard the cell line against paraquat, which would otherwise induce cell death. Nematodes challenged with Cit-CQDs exhibit improved survival rates 72 h after paraquat exposure in comparison to controls (Figure 9). Paraquat destroys dopamine (DA) neurons, leading to impaired locomotor activity in worms [192].
![Figure 9
CQDs reduce paraquat-induced neuronal damage in vitro and in vivo [192].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_009.jpg)
CQDs reduce paraquat-induced neuronal damage in vitro and in vivo [192].
Prion-like amyloids self-template to generate toxic oligomers, protofibrils, and fibrils from their soluble monomers, a process associated with the initiation and progression of neurodegenerative diseases including AD, PD, Huntington’s, and systemic lysozyme amyloidosis. CQDs, derived from sodium citrate as a carbon precursor, were synthesized and described prior to evaluating their capacity to modulate amyloidogenic (fibril-forming) pathways. Hen-egg white lysozyme (HEWL) functioned as a model amyloidogenic protein [193]. A pulse-chase lysozyme fibril-forming experiment was established to investigate the influence of CQDs on the HEWL amyloid fibril formation, using ThT fluorescence as an indicator of mature fibril presence (Figure 10). The findings indicated that the Na–citrate-derived CQDs might interfere at various stages of the fibril-forming process by inhibiting the transformation of both monomeric and oligomeric HEWL intermediates into mature fibrils. Furthermore, the carbon nano material successfully dissolved oligomeric HEWL into monomeric HEWL and induced the disaggregation of mature HEWL fibrils.
![Figure 10
CQDs for the treatment of amyloid disorders [193].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_010.jpg)
CQDs for the treatment of amyloid disorders [193].
Another team of researchers presented a neuroprotective approach by encapsulating verapamil (VRH) into hyaluronic acid-modified CQDs and evaluating its efficacy against the free form in a rat model of AD generated by lipopolysaccharide (LPS). The experimental rats were categorized into seven groups: control, LPS, CQDs, early free VRH (FVRH), late FVRH, early verapamil CQDs (VCQDs), and late VCQDs [194]. The characterizations of VCQDs, the behavioral performance of the rats, histological and immunohistochemical alterations, certain AD hallmarks, OS biomarkers, neuro-affecting genes, and DNA fragmentation were assessed. VRH was effectively included into CQDs, as shown by the observed metrics. VRH demonstrated improvements in cognitive skills, alterations to brain architecture, reduced levels of Aβ and pTau, heightened antioxidant capacity, adjustable gene expression, and a reduction in DNA fragmentation. The administered treatment was more effective than the unbound medication. Furthermore, early intervention was superior than late intervention, corroborating the significance of the identified molecular targets in the progression of AD. VRH demonstrated several pathways in countering LPS-induced neurotoxicity via its anti-inflammatory and antioxidant attributes, therefore alleviating the characteristics of AD. GQDs and their nitrogen-doped counterparts exhibit excellent biocompatibility, as well as favorable optical and physicochemical features. GQDs have been thoroughly investigated due to several aspects, including their dimensions, surface charge, and interactions with other molecules present in biological environments [195]. This study succinctly clarifies the potential of electroactive GQDs and N-GQDs as neurotrophic agents. In vitro studies using the N2A cell line assessed the efficacy of GQDs and N-GQDs as neurotrophic agents, including fundamental assays such as the SRB test and neurite outgrowth assay. The findings derived from immunohistochemistry, confocal imaging investigations, and quantitative real-time PCR (qPCR) analyses confirmed those obtained from the neurite outgrowth experiment. TuJ1 serves as a neuritogenic marker in both the central and peripheral nervous systems during the first phases of neural development. Mature, post-mitotic neurons arise from neuronal differentiation, necessitating certain molecular and morphological changes in progenitors (Figure 11). The monoclonal antibody TuJ1 indicates that neuron-specific β-tubulin expression starts during neurogenesis.
![Figure 11
Representative confocal pictures of N2A cells after G1 and G2 treatments. TuJ1, stained in green and blue, indicates DAPI, which has marked the nucleus; all photos are at 60× magnification with a scale bar of 10 μm [195].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_011.jpg)
Representative confocal pictures of N2A cells after G1 and G2 treatments. TuJ1, stained in green and blue, indicates DAPI, which has marked the nucleus; all photos are at 60× magnification with a scale bar of 10 μm [195].
A separate research demonstrated the neuroprotective properties of CQDs in a human microglial cell model generated by LPS. LPS was observed to elicit cytotoxicity, generate ROS, and promote pro-inflammatory cytokines, specifically interleukin (IL)-1β, IL-6, and tumor necrosis factor-α, while concurrently downregulating enzymatic antioxidants, including nuclear factor-erythroid factor 2-related factor 2 (Nrf2), SOD, CAT, heme oxygenase (HO)-1, HO-2, and glutathione peroxidase. In contrast, CQDs treatment mitigated LPS-induced cytotoxicity, stimulated anti-inflammatory cytokines (IL-4, IL-10, and transforming growth factor β), and enhanced enzymatic antioxidants at both transcriptional and translational levels [196]. Jia et al. documented N-doped carbon dot nanozyme (CDzyme) exhibiting remarkable antioxidant properties for the treatment of depression via the modulation of redox homeostasis and gut flora [197]. The CDzymes synthesized using microwave-assisted rapid polymerization of histidine and glucose demonstrate enhanced biocompatibility. Leveraging their distinctive structure, CDzymes may provide enough electrons, hydrogen atoms, and protons for reduction processes, in addition to catalytic sites that emulate redox enzymes. These collaborative processes confer upon CDzymes a wide-ranging antioxidant capability to neutralize ROS and reactive nitrogen species (˙OH,
![Figure 12
(a) Study of community barplots, (b) study of community heatmaps at the genus level, and (c) pathways at KEGG Path Level 2. (d) Rank sum test for amino acid metabolic pathways; (e and f) KEGG module abundance analysis of amino acid metabolism across four groups. (*p < 0.05, **p < 0.01, ***p < 0.001 denotes a statistically significant difference) [197].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_012.jpg)
(a) Study of community barplots, (b) study of community heatmaps at the genus level, and (c) pathways at KEGG Path Level 2. (d) Rank sum test for amino acid metabolic pathways; (e and f) KEGG module abundance analysis of amino acid metabolism across four groups. (*p < 0.05, **p < 0.01, ***p < 0.001 denotes a statistically significant difference) [197].
Quercetin (Que) and p-phenylenediamine (p-PD) generated red-emitting CDs were manufactured using a one-step hydrothermal technique and intended as a new theranostic nano-agent for the multi-target therapy of AD. R-CD-75, with an improved formulation, demonstrated substantial suppression of Aβ aggregation and expedited depolymerization of mature Aβ fibrils (<4 h) at micromolar doses (2 and 5 μg/mL, respectively). Furthermore, R-CD-75 effectively scavenged ROS and exhibited enhanced red fluorescence imaging of Aβ plaques both in vitro and in vivo [199]. AD is a prevalent neurodegenerative disorder marked by advancing cognitive and physical decline. Neuroinflammation is associated with AD, and the misfolding and aggregation of amyloid protein in the brain induces an inflammatory milieu. Microglia is the primary agents of neuroinflammation, and their aberrant activation triggers the release of several inflammatory chemicals, facilitates neuronal death, and results in cognitive deficits [200]. This work used microglial membranes with caffeic acid-coupled CQDs to create an innovative biomimetic nanocapsule (CDs-CA-MGs) for AD therapy. The nasal delivery of CDs-CA-MGs may circumvent the BBB and directly address the location of inflammation. Following therapy with CDs-CA-MGs, AD mice exhibited less brain inflammation, reduced neuronal death, and markedly enhanced learning and memory capabilities. The aggregation of Aβ peptides and neurofibrillary tangles (NFTs) in the brain is a pivotal mechanism contributing to AD, which disrupts neuronal signaling and induces neurodegeneration. According to current knowledge, preventing the buildup of Aβ peptides and NFTs is essential in the therapy of ADs [201]. Recent study indicates that nanoparticles may enhance medicine delivery across the BBB effectively. GQDs have been shown to be excellent inhibitors of Aβ peptide aggregation. The diminutive dimensions of GQDs facilitate their effortless traversal of the BBB. Additionally, GQDs have fluorescent features that may facilitate the in vivo detection of Aβ content. In recent years, the low cytotoxicity and good biocompatibility of GQDs, in comparison to other carbon materials, provide an advantage in their eligibility for clinical research on AD.
3.1 QD-based modulation of inflammatory responses
The control of inflammatory responses by QDs and CDs is a potential approach for addressing SCI, where excessive inflammation aggravates tissue damage and hinders healing [202]. In SCI, initial mechanical damage triggers a series of subsequent injury processes, with inflammation being a pivotal factor [203]. This inflammatory response, although initially helpful, often turns harmful as it extends tissue damage, disturbs cellular homeostasis, and triggers apoptotic pathways, resulting in neuronal death. Nanomaterials, including QDs and CDs, provide distinct benefits as they may be tailored to target and modulate certain inflammatory pathways, providing targeted and regulated anti-inflammatory actions that may alleviate secondary harm [204].
QDs, particularly those doped or functionalized with heteroatoms, such as nitrogen, sulfur, or cerium, have demonstrated significant antioxidant potential in biological settings, including in vivo models. For example, Yang et al. synthesized cerium-doped CQDs that exhibited strong ROS-scavenging ability in a rat model of SCI. The treatment reduced malondialdehyde (MDA) levels and increased SOD activity, resulting in improved neuronal survival and reduced lesion volume [205]. In a separate study, Guo et al. reported that GQDs effectively attenuated OS in a transgenic mouse model of AD [51]. These QDs restored mitochondrial membrane potential, decreased intracellular ROS, and upregulated antioxidant enzymes such as CAT and glutathione peroxidase, ultimately improving cognitive function. Similarly, Luo et al. demonstrated that sulfur-doped carbon dots reduced ROS accumulation and inflammation in a murine model of IRI, contributing to decreased tissue necrosis and enhanced functional recovery [206].
QDs, due to their adjustable surface chemistry and functionalization potential, may engage with and influence critical inflammatory pathways in SCI. QDs may inhibit the activation of NF-κB, a pivotal regulator of inflammation that, when excessively active, facilitates the secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. By suppressing NF-κB, QDs may diminish the synthesis of these cytokines, hence lowering immune cell recruitment and mitigating tissue damage [207]. Furthermore, QDs may enhance anti-inflammatory mechanisms, including the activation of peroxisome proliferator-activated receptor gamma (PPAR-γ), which contributes to the resolution of inflammation by inhibiting pro-inflammatory gene expression. The simultaneous reduction of pro-inflammatory signaling and enhancement of anti-inflammatory pathways may stabilize the spinal tissue environment, therefore protecting neurons and glial cells against chronic inflammation. CDs are becoming recognized for their ability to influence inflammatory responses in SCI. CDs, often sourced from organic materials and infused with components such as nitrogen and sulfur, have intrinsic anti-inflammatory and antioxidant characteristics. CDs may eliminate ROS, so mitigating OS and indirectly attenuating inflammation, as ROS are principal activators of the NF-κB pathway [208]. This ROS-scavenging mechanism inhibits the subsequent activation of pro-inflammatory cytokines, hence diminishing cellular damage. Furthermore, CDs may influence macrophage polarization, a mechanism essential for SCI recovery. Macrophages occur in two primary states: the M1 (pro-inflammatory) and M2 (anti-inflammatory) phenotypes [209]. CDs have shown the capacity to transition macrophages from the M1 to the M2 phenotype, therefore facilitating tissue repair, enhancing cell viability, and mitigating neurotoxic inflammation. By enhancing M2 macrophage activity, CDs may provide a more conducive milieu for repair and regeneration in SCI.
In addition to these direct effects, QDs and CDs may be modified with targeting ligands, such as peptides or antibodies, to facilitate targeted distribution to spinal cord damage locations. This focused strategy improves their therapeutic effectiveness, enabling precise modulation of inflammatory responses without impacting adjacent healthy tissues. Functionalizing QDs or CDs with antibodies that identify inflammatory markers allows these nanomaterials to accurately target inflammation sites, hence enhancing the concentration of anti-inflammatory medicines at the damage location and reducing off-target effects. The targeting ability, together with their intrinsic anti-inflammatory and antioxidant properties, renders QDs and CDs very adaptable for SCI therapy [210]. The control of inflammation by QDs and CDs provides a comprehensive strategy for mitigating secondary damage in SCI. By inhibiting critical inflammatory pathways, altering macrophage morphologies, and scavenging ROS, these nanomaterials foster an environment conducive to brain repair and regeneration. With enhanced targeting capabilities and sustained biocompatibility, QDs and CDs possess significant promise as therapeutic agents for managing inflammation and facilitating healing in SCI.
Ayaz et al. examined the immunological response to CDs and assessed the impact of surface passivation agents on their immunomodulatory properties. Carbon dots passivated with poly(vinyl alcohol) had significant anti-inflammatory properties, whereas carbon dots passivated with alginate demonstrated pro-inflammatory effects. CDPEG exhibited modest anti-inflammatory properties, indicating its potential as an inert carrier in drug delivery research. The findings indicated that the kind of activated group present on the surface influenced the differential effects of CDs on the inflammatory capacity of macrophages by altering the production levels of pro-inflammatory cytokines TNFα and IL6 [211]. Tosic et al. showed the capacity of GQD to mitigate inflammatory CNS injury in the rat model of experimental autoimmune encephalomyelitis (EAE). Our findings demonstrate that GQD mitigates demyelination and clinical manifestations of EAE by disrupting the peripheral activation and proinflammatory activity of CNS-reactive Th1 cells, in addition to directly safeguarding CNS cells from immune-mediated damage [212]. A GelMA hydrogel infused with FTY720 CDs (FTY720-CDs@GelMA) was developed to facilitate the integration of NSCs for the purpose of filling the capsular cavity of SCIs by in situ injection, hence enhancing spinal cord regeneration (Schematic 1). The resulting hydrogel exhibits excellent biocompatibility and ROS scavenging properties, demonstrating significant protective effects on astrocytes and neural stem cells (NSCs) in vitro. Moreover, the implantation of FTY720-CDs@GelMA hydrogel enhanced neuronal differentiation of transplanted NSCs, reduced glial scar formation, and facilitated axonal regeneration and neural circuit restoration. The findings indicated that the integration of FTY720-CDs@GelMA hydrogel with NSCs significantly enhanced motor function recovery after SCI in rats, proposing a novel approach for comprehensive SCI therapy [213]. Yang et al. synthesized an amine-functionalized aspirin-derived carbon quantum dot (NACQD) with a nominal diameter of 6–13 nm. The NACQD has strong iron-binding and antioxidative properties. NACQD treatment, administered intrathecally, significantly reduced iron accumulation and OS in cerebral tissue, mitigated meningeal inflammation, and enhanced neurological recovery in a mouse model of intracerebral hemorrhage (ICH) (Figure 13). The intrathecal injection of NACQD serves as a viable therapeutic method to mitigate ICH harm, demonstrating its potential as a proof of concept [214].
![Figure 13
Protective function of NACQD in murine ICH models [214].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_013.jpg)
Protective function of NACQD in murine ICH models [214].
Following ICH, the excessive generation of ROS and iron ion accumulation are the primary contributors to subsequent injury. Eliminating surplus iron ions and ROS in the meningeal system may significantly mitigate subsequent damage after ICH. Tang et al. produced ginsenoside Rb1 CQDs (RBCQDs) using ginsenoside Rb1 and ethylenediamine by a hydrothermal process. RBCQDs demonstrate strong efficacy in scavenging ABTS+ free radicals and iron ions in solution. Following intrathecal injection, the distribution of RBCQDs is mostly confined to the subarachnoid region [215]. RBCQDs may neutralize ROS and bind iron ions in the meningeal system. RBCQD therapy markedly enhances blood circulation in the meningeal system, safeguarding degenerating neurons, ameliorating neurological function, and offering a novel therapeutic strategy for the clinical management of ICH.
3.2 Protection of neuronal cells and support of survival pathways
Antioxidant QDs have attracted considerable interest for their prospective neuroprotective effects in SCI models. The capacity of QDs to alleviate OS, a major factor in neuronal injury after SCI, renders those interesting candidates for enhancing neuroprotection and regeneration approaches. These nanomaterials, usually produced with doping elements like sulfur (S) and nitrogen (N) or integrated into polymeric matrices, have improved antioxidative properties and adjustable biocompatibility, thereby enabling their use in both in vitro and in vivo research. Understanding how different types of QDs correlate with specific biomedical strategies is crucial for advancing their clinical potential. Among the most widely studied are cadmium-based QDs, such as cadmium selenide (CdSe) and cadmium telluride (CdTe) [216]. These exhibit excellent optical properties, including high QY and narrow emission spectra, making them ideal for bioimaging, cell tracking, and diagnostic applications. However, their inherent cytotoxicity due to the release of heavy metal ions poses significant challenges for in vivo applications. To mitigate this, surface passivation with biocompatible polymers or encapsulation within inert shells like ZnS is often employed, although concerns about long-term safety persist [217]. In contrast, silicon quantum dots (Si QDs) offer superior biocompatibility, low toxicity, and biodegradability, making them attractive for drug delivery, PDT, and regenerative medicine. Their favorable pharmacokinetics and clearance profiles support their application in chronic disease models and long-term therapies [218]. Additionally, their tunable surface allows for conjugation with therapeutic molecules or targeting ligands, which is particularly advantageous in site-specific delivery systems for SCI and cancer therapy. Carbon-based QDs, including CQDs and GQDs, are increasingly explored due to their inherent antioxidant, anti-inflammatory, and neuroprotective properties. These QDs have shown significant promise in neurological applications, especially SCI, where secondary injury mechanisms such as OS and inflammation play a critical role [219]. CQDs can scavenge ROS, regulate inflammatory signaling, and support neuronal regeneration by facilitating neurotrophic factor delivery (e.g., brain-deducted neurotrophic factor [BDNF], nerve growth factor [NGF]). Their minimal cytotoxicity, tunable size, and surface functionalization make them ideal candidates for multifunctional nanocarriers in regenerative therapies. Furthermore, doped QDs, such as nitrogen, sulfur, phosphorus, or cerium-doped CQDs, exhibit enhanced therapeutic performance due to improved ROS-scavenging and enzyme-mimicking capabilities [220]. For example, cerium-doped QDs mimic CAT and SOD, enhancing their ability to mitigate oxidative damage in SCI and other neurodegenerative models [221]. These tailored QDs not only improve cellular viability but also modulate intracellular signaling pathways involved in apoptosis, autophagy, and neurogenesis. Other types, such as indium phosphide (InP) QDs, are emerging as less toxic alternatives to Cd-based QDs, with growing applications in bioimaging and targeted drug delivery [222]. Similarly, ZnO and Ag-doped QDs offer antimicrobial and anti-inflammatory benefits, which could be leveraged in SCI-associated infection management or wound healing. By aligning specific QD types with corresponding therapeutic functions whether imaging, ROS scavenging, neuroprotection, or drug delivery, researchers can better tailor nanomedicine platforms to disease-specific requirements [223]. A deeper understanding of these correlations not only enhances the translational potential of QDs but also aids in the rational design of future therapeutic systems targeting SCI and beyond.
In vitro models of SCI provide a regulated setting to assess the protective mechanisms and effectiveness of antioxidant QDs [224]. Neural cells, such as neurons and astrocytes, exposed to OS-related damage, are often used to evaluate the antioxidative capacity of QDs. Research indicates that QDs, including sulfur- and selenium-doped CDs, markedly decrease ROS levels in neural cells. These QDs mitigate free radicals via their many surface-active sites, such as hydroxyl, carboxyl, and amino groups, thereby inhibiting lipid peroxidation and preserving mitochondrial function. CQDs obtained from natural sources such as citric acid and nitrogen precursors have shown the capacity to maintain cell viability in models of OS-induced damage. They do this by regulating the activity of cellular antioxidant defense mechanisms, including SOD and CAT [225]. Furthermore, QDs functionalized with biomolecules, like neurotrophins or peptides, augment their selectivity for neuronal cells, promoting axonal expansion and synaptic repair. These data highlight the capability of QDs to mitigate the secondary damage phase of SCI, marked by inflammation and OS. The neuroprotective properties of antioxidant QDs have been confirmed in vivo using mouse models of SCI. These experiments often include the induction of SCI using contusion or compression methods, followed by the administration of QDs via localized injection or systemic distribution [226]. QDs have shown the ability to reduce neuronal death and maintain white matter integrity, thereby enhancing functional recovery. Selenium-doped QDs have shown significant effectiveness in decreasing OS indicators like MDA and increasing glutathione (GSH) levels in damaged spinal cord tissues [227]. The antioxidative effect is often enhanced by the control of inflammatory responses, since QDs diminish the activation of microglia and astrocytes, hence decreasing the production of pro-inflammatory cytokines like TNF-α and IL-6. Furthermore, the integration of QDs into hydrogels or alternative biomaterials facilitates sustained release, hence enhancing the duration of the therapeutic impact. Behavioral tests, such the Basso et al. locomotor rating scale [228], substantiate the effectiveness of QD-based therapies. Rodents administered antioxidant QDs have enhanced motor performance and decreased hindlimb paralysis relative to untreated controls. Histological research demonstrates less cavity development and increased neuronal survival, indicating strong neuroprotective effects [229]. Despite the great promise of antioxidant QDs in SCI treatment, several hurdles remain, including the optimization of biocompatibility, targeted distribution, and long-term safety. Integrating QDs with sophisticated delivery technologies, such as nanofiber scaffolds or injectable hydrogels, may enhance their therapeutic efficacy. Moreover, using these insights clinically requires thorough preclinical validation and the resolution of regulatory issues.
Regulating glial cell activation is essential for the treatment or prevention of neuroinflammation-related brain disorders. Ultrasmall 2D vanadium carbide QDs (V2C QDs) capable of traversing the BBB are synthesized, demonstrating remarkable anti-neuroinflammatory properties. The synthesized 2D V2C QDs, averaging 2.54 nm in size, exhibit commendable hydrophilicity, physiological stability, and proficient BBB permeability. The pharmacological impact of V2C QDs on inflammatory responses reveals intriguing outcomes in mitigating acquiring and storage deficits in BALB/c mice induced by LPS [230]. A separate study examined the possible neuroprotective properties of Ferulic acid against ischemia/repertory (I/R)-accelerated cerebral trauma both in vivo and in vitro, utilizing hematoxylin and eosin (H&E) and Nissl staining assays, flow cytometry, Hoechst 33258 staining, quantitative PCR, western blot analysis, and fluorescence microscopy [231]. Identifying the amyloid form that are critical promoters of the clinical hallmarks of AD is vital for comprehending subsequent processes, including tauopathies that facilitate neuroinflammation and result in neurological impairments. Chiang et al. documented the pattern and fabrication of a QDs imitative for greater orbicular oligomeric amyloid variety, serving as an endogenously fluorescent proxy for this cytotoxic amyloid fabrication to examine its role in eliciting inflammatory and stress response states in neuronal and glial cell types [232]. Figure 14 illustrates that the fomite and poly(ethylene glycol)-coated quantum dot treated cultures exhibit no discernible change in the amplitude of calcium transients, as quantified by the total fluorescence intensity normalized to the region of interest. Conversely, amyloid-beta quantum dots (ABQDs) elicit a much greater calcium response, detectable at the 200 s mark (Figure 14b–d) with the normalized intensiveness shown in Figure 14a. The elevated calcium inflow in QD-processed neuronal cells indicates excitotoxic stress in neurons, accompanied by observable neurite destruction.
![Figure 14
Calcium transients from neuroglial cultures in reaction to amyloid. (a) Plotted calcium transients from neuron-astrocyte co-cultures grounded with an equivalent volume dilution of vehicle in medium (b), 10 nM PEGQDs (c), or 10 nM ABQDs (d) followed by 30 mM KCl calcium induction [232].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_014.jpg)
Calcium transients from neuroglial cultures in reaction to amyloid. (a) Plotted calcium transients from neuron-astrocyte co-cultures grounded with an equivalent volume dilution of vehicle in medium (b), 10 nM PEGQDs (c), or 10 nM ABQDs (d) followed by 30 mM KCl calcium induction [232].
Wu et al. noted that CdTe QDs induced cell death and apoptosis in rat elementary cultured hippocampus neurons in a manner dependent on dosage, duration, and size. Neurons exposed to QD exhibited elevated levels of ROS and intracellular calcium, resulting in neuronal apoptosis and potential mortality, which may be entirely or partly mitigated by the antioxidant NAC. Future studies must examine the underlying processes by exploring the extrinsic and intrinsic channels via which CdTe QDs elicit neurotoxic effects [233].
Figure 15 illustrates the complex function of antioxidant QDs in alleviating secondary damage mechanisms linked to SCI. After SCI, six principal pathogenic processes are activated: OS, neuroinflammation, apoptosis, glial scar formation, poor angiogenesis, and neurodegeneration. Antioxidant QDs specifically target each route, halting the damage cascade and facilitating neuroprotection and regeneration. OS is an initial reaction to SCI, caused by excessive synthesis of ROS that surpasses cellular antioxidant defenses. This leads to significant harm to lipids, proteins, and DNA. The figure illustrates that QDs function as scavengers for ROS [234]. They emulate the function of antioxidant enzymes, including SOD and glutathione peroxidase (GPx), diminishing ROS levels and reinstating redox equilibrium. The heightened activity of endogenous antioxidants such as GSH enhances their protective effects, reducing oxidative damage at the molecular level [235]. Neuroinflammation, characterized by microglial activation and macrophage infiltration, ensues after OS and exacerbates secondary injury. Pro-inflammatory cytokines, including TNF-α and IL-6, are produced, perpetuating a feedback loop of inflammation. The graphic illustrates that QDs impede critical inflammatory pathways, notably NF-κB, thereby diminishing cytokine synthesis [236]. This inhibits the activation of inflammatory cells, reducing tissue damage and promoting a reparative environment. Apoptosis, or programmed cell death, significantly contributes to tissue loss after SCI. It is facilitated by both internal (mitochondrial) and extrinsic (death receptor) mechanisms, leading to the activation of caspases-3 and 9. The graphic demonstrates that QDs block apoptosis by obstructing caspases and activating the PI3K/Akt signaling pathway, hence enhancing cell survival. This intervention safeguards neurons and oligodendrocytes, sustaining the structural integrity of the spinal cord. The creation of glial scars, largely induced by reactive astrocytes, is identified as a significant impediment to axonal regeneration. The graphic illustrates that QDs influence astrocytic phenotypes, altering the equilibrium from neurotoxic A1 astrocytes to reparative A2 astrocytes [237]. This shift diminishes the inhibitory influence of the glial scar, promoting axonal regeneration and aiding brain repair. Impaired angiogenesis, resulting from vascular damage and hypoxia, is a significant concern in SCI. The figure illustrates that QDs facilitate angiogenesis by increasing vascular endothelial growth factor levels and fostering microvascular repair. The reestablishment of blood circulation and oxygen delivery facilitates tissue regeneration and mitigates the hypoxic conditions that impede healing. Ultimately, neurodegeneration, marked by axonal degradation and synaptic impairment, is a prolonged outcome of SCI [238]. The graphic highlights that QDs enhance neuroprotection by elevating the degrees of neurotrophic components, admitting BDNF and NGF. These elements facilitate axonal regeneration, synaptic reorganization, and neuronal viability, hence enhancing functional results. It clearly illustrates the pivotal function of QDs in targeting these pathways, with arrows connecting the SCI-induced degenerative processes to their corresponding QD-mediated therapies. This integrated architecture illustrates how QDs tackle several facets of secondary damage, offering a comprehensive therapy strategy. Their multifunctionality positions them as a revolutionary tool in the therapy of SCI, connecting oxidative damage, inflammation, cell death, and regeneration processes.
Diagram illustrating the pathways and mechanisms of the neuroprotective effects of antioxidant QDs in SCI models.
QDs have emerged as promising nanomaterials with significant potential in advancing neuroprotection and neuroregeneration, particularly in the context of SCI [180,239,240]. Their unique optical, electronic, and surface properties enable them to interact with neural cells in ways that can mitigate OS, promote cell survival, and enhance neuronal regeneration. As OS is a major contributor to secondary injury mechanisms in SCI, the antioxidant capabilities of QDs play a crucial role in protecting neurons from damage. One of the key neuroprotective mechanisms of QDs is their ability to scavenge ROS, which are excessively produced following SCI [149]. ROS accumulation can trigger lipid peroxidation, protein degradation, and DNA damage, ultimately leading to neuronal apoptosis and glial scarring. Antioxidant QDs, particularly those doped with elements such as selenium, cerium, or europium, have demonstrated potent ROS scavenging abilities. For instance, cerium oxide QDs exhibit regenerative redox properties, where their surface cerium ions (Ce³⁺/Ce⁴⁺) dynamically switch oxidation states to neutralize ROS [241]. Similarly, carbon-based QDs such as CDs and GQDs possess intrinsic antioxidant properties attributed to their abundant surface functional groups, including hydroxyl, carboxyl, and amino moieties [242]. These functional groups facilitate electron transfer processes that stabilize ROS, reducing OS-induced neuronal damage. In addition to their neuroprotective roles, QDs contribute to neuroregeneration by promoting cell proliferation, axonal growth, and synaptic repair. QDs can serve as carriers for neurotrophic factors such as BDNF, NGF, or glial cell line-derived neurotrophic factor [243]. By facilitating the sustained and localized release of these biomolecules, QDs create a microenvironment conducive to neuronal survival and axonal sprouting. Furthermore, surface-modified QDs can interact directly with cell surface receptors, activating intracellular signaling pathways that enhance neuronal growth. For example, QDs functionalized with peptides or bioactive ligands can stimulate the PI3K/Akt or MAPK/ERK pathways, which are crucial for promoting cell survival and inhibiting apoptosis. Moreover, QDs have shown potential in modulating the inflammatory response following SCI. The inflammatory cascade triggered by activated microglia and astrocytes often exacerbates neural damage. Studies have demonstrated that antioxidant QDs can suppress the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 while promoting the expression of anti-inflammatory mediators [244]. This immunomodulatory effect reduces glial scarring and creates a permissive environment for axonal regeneration [245]. Another key advantage of QDs in neuroregenerative applications is their potential for targeted delivery and bioimaging. Due to their tunable fluorescence and stable emission properties, QDs enable real-time tracking of neuronal repair processes. Functionalized QDs can selectively bind to injured neurons or regenerating axons, providing valuable insights into cellular dynamics during recovery [246]. While both SCI and neurodegenerative diseases share common secondary pathophysiological features such as OS, neuroinflammation, and mitochondrial dysfunction, it is critical to distinguish the underlying primary mechanisms that define each condition [247]. SCI is characterized by an acute mechanical trauma leading to immediate axonal disruption, vascular compromise, and subsequent formation of glial scars that impede axonal regeneration [30,248–250]. In contrast, neurodegenerative diseases such as Alzheimer’s and Parkinson’s are chronic progressive disorders driven by pathological protein aggregation, including amyloid-beta plaques, tau tangles, and α-synuclein accumulation, which result in gradual neuronal loss [251]. Although QDs may offer therapeutic advantages in contexts due to their ROS-scavenging, anti-inflammatory, and neuroregenerative properties, their application must be disease-specific, considering the distinct microenvironment and progression dynamics of each condition [252]. For instance, in SCI, QDs can be engineered to target the acute inflammatory milieu and promote axonal regrowth through neurotrophic factor delivery, whereas in AD, their utility may extend to imaging and modulating protein aggregation [253]. Therefore, a clear mechanistic understanding is crucial to guide the precise and effective application of QDs based involvements, ensuring they are specifically aligned with the unique pathophysiological features of SCI rather than being broadly applied within a generalized neurodegenerative context. Additionally, QDs integrated with responsive drug delivery systems allow for controlled release of therapeutic agents at the injury site, enhancing the precision and efficacy of neuroprotective treatments.
3.3 Advancements in regenerative medicine using antioxidant QDs
Improving stem cell treatment results in SCI with QDs demonstrating a significant advance in regenerative medicine. Stem cell treatment seeks to substitute impaired brain cells, facilitate axonal regeneration, and re-establish functional connection [254]. Nonetheless, obstacles such as diminished cell viability, suboptimal differentiation, and insufficient incorporation into host tissues limit its therapeutic efficacy. QDs may mitigate these constraints by providing multifunctional assistance to stem cell treatment via their distinctive optical, electrical, and antioxidant characteristics. QDs improve the viability of transplanted stem cells by reducing OS in the injury microenvironment [255]. ROS caused by SCI often result in diminished cell viability; nevertheless, QDs function as effective antioxidants, mitigating ROS and safeguarding transplanted cells from oxidative harm. This stabilization enhances stem cell viability and retention at the damage location. Moreover, QDs may alter the inflammatory milieu by suppressing pro-inflammatory pathways like NF-κB, diminishing cytokine-induced stem cell death, and establishing a more conducive habitat for cell engraftment [256]. Furthermore, QDs enhance the development and functional integration of stem cells via modulating cellular signaling pathways. QDs may facilitate the differentiation of stem cells into neurons and glial cells, essential for spinal cord regeneration, by releasing bioactive ions or delivering targeted stimulation. Their fluorescent characteristics allow for real-time monitoring of stem cell fate and distribution, aiding in the refinement of therapy procedures. Moreover, QDs may improve axonal guidance and synaptic connection by delivering bioelectrical signals, facilitating the functional integration of transplanted cells into the host brain network.
However, to support the feasibility of such integrated strategies, it is essential to understand and explain how QDs mechanistically interact with stem cells and influence their behavior both in vitro and in vivo. QDs can modulate stem cell fate through several mechanisms. Their surface functionalization enables the controlled release of bioactive molecules, such as growth factors and small-molecule drugs, which can direct stem cell differentiation toward specific cell types [257]. For example, carbon-based QDs functionalized with neurotrophic factors like BDNF or NGF have been shown to enhance neuronal differentiation of MSCs and NSCs. In one study, nitrogen-doped CQDs promoted neuronal lineage commitment of NSCs by modulating intracellular ROS levels and activating the PI3K/Akt pathway, a critical regulator of cell survival and differentiation [258]. This indicates that QDs can act as both carriers and bioactive modulators in a stem cell microenvironment. Moreover, QDs influence cell survival and engraftment, which are two critical factors for the success of stem cell therapy. OS at the injury site is a major barrier to stem cell survival. QDs with antioxidant properties, such as cerium-, sulfur-, or nitrogen-doped carbon dots, can neutralize ROS in the microenvironment and protect transplanted stem cells from apoptosis. In a recent in vivo study, cerium oxide QDs were co-administered with stem cells in a SCI model, significantly improving cell viability and functional recovery compared to stem cells alone. QDs also enhance the homing and tracking of transplanted cells [259]. Their tunable fluorescence properties allow for real-time, non-invasive imaging of stem cells post-transplantation. This capability enables precise monitoring of cell migration, integration, and survival over time, providing critical feedback for optimizing therapeutic protocols. For instance, CdSe/ZnS QDs have been successfully used to label MSCs without affecting their differentiation potential, allowing for in vivo tracking in animal models of neurodegeneration. Another avenue is QDs mediated gene delivery [260]. Functionalized QDs can deliver genetic material or siRNA to stem cells to promote specific phenotypic outcomes. For example, QDs conjugated with neural lineage-specific transcription factors or microRNAs have been used to steer stem cell differentiation toward neurons or oligodendrocytes, which are vital for remyelination and synaptic restoration in SCI repair [261]. Despite these promising attributes, QD–stem cell combinations must be carefully optimized for biocompatibility, long-term stability, and controlled release. The choice of QD type, surface chemistry, and dosage all critically influence stem cell responses. Non-toxic alternatives such as silicon QDs or carbon-based QDs are preferred over cadmium-based QDs due to lower cytotoxicity and better biodegradability.
Ma et al. investigated the novel use of DA-functionalized QDs to assess and alleviate neurotoxicity induced by DA quinone [262]. This finding is crucial for improving stem cell treatment results in SCI. The research emphasizes that DA-functionalized QDs may sensitively detect OS and neurotoxic consequences, offering real-time insights into cellular responses during stem cell therapies. The researchers used the optical features of these QDs to monitor the health of dopaminergic neurons and their oxidative states under neurotoxic environments. This meticulous monitoring may enhance the milieu for transplanted stem cells, assuring better survival, differentiation, and integration into the impaired brain networks. Moreover, the antioxidant characteristics of QDs might mitigate ROS, hence diminishing secondary injury in SCI. Within the framework of SCI, the combined role of sensitive detection and neuroprotection establishes DA-functionalized QDs as significant assets for improving the effectiveness of stem cell therapy (Figure 16). Their administration may enhance neuroprotection measures, diminish inflammation, and increase brain regeneration results, so advancing SCI therapy procedures. Another research highlighted the promise of combining nanotechnology with regenerative medicine to tackle intricate brain problems. Ensuring a conducive microenvironment is crucial for effective nerve regeneration. Cell-based treatment provides the potential for cell replacement and the enhancement of axonal development. Adipose tissue-derived mesenchymal stromal cells (Ad-MSC) are of interest due to their neuroregenerative and anti-inflammatory characteristics [263]. This research aimed to assess the impact of canine and murine Ad-MSC transplantation on the regeneration of the sciatic nerve.
![Figure 16
(a) Self-assembly and FL quenching/recovery of N-(3,4-dihydroxyphenethyl)-5-mercaptopentanamide (DAs) functionalized CdTe/ZnS QDs. Schematic of DA oxidation and Cys residue-DA quinone irreversible interaction and (b) DAs quinone-QD bright field pictures, FL micrographs, and merged images from different N-ethylmaleimide concentration [262].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_016.jpg)
(a) Self-assembly and FL quenching/recovery of N-(3,4-dihydroxyphenethyl)-5-mercaptopentanamide (DAs) functionalized CdTe/ZnS QDs. Schematic of DA oxidation and Cys residue-DA quinone irreversible interaction and (b) DAs quinone-QD bright field pictures, FL micrographs, and merged images from different N-ethylmaleimide concentration [262].
Agarwal et al. revealed that CdSe/ZnS core/shell QDs, surface-functionalized with a zwitterionic compact ligand, can transport a cell-penetrating lipopeptide to the developing chick embryo brain without observable damage [264]. The QD intensity was widespread over the brain, peaking between E8 and E11, with fluorescence concentrating in the choroid plexus before diminishing before hatching (E21/P0). No anomalies were identified in embryonic patterning or embryo survival, or in mRNA in situ hybridization. It was proposed that QDs may facilitate the identification and tracking of migrating NSCs, that the choroid plexus eliminates these injected QDs/nanoparticles from the brain post-E15, and that they can transport medicines and peptides to the developing brain. Maximum brain labeling was found at E8, during which QDs were widely and consistently dispersed throughout the whole brain, including the forebrain, midbrain, and hindbrain, creating different labeling patterns as shown by longitudinal brain sections (Figure 17).
E8 QD-peptide conjugate distribution. E8 embryonic chick brain QD-CL4-JB577 (red) distribution following spinal canal injection at E4. Representative coronal slices (40 μm) of the embryo’s forebrain (a), midbrain (b), and hindbrain (c) demonstrate similar QD distribution at 4 days post-injection (E8). A neuronal layer heavily labeled by QDs is indicated by the bracket in B. In panel B, the small white bracket indicates a neuronal layer that is heavily labeled with QDs.
CdSe/ZnS QDs may be conveyed from the olfactory tract to the brain by inhalation. Adult C57BL/6 mice were subjected to a 1 h nasal inhalation of aerosolized QDs, with nanoparticles identified 3 h post-exposure in the olfactory tract and olfactory bulb using several methods [265]. Subsequent to brief inhalation of solid QD nanoparticles, there is fast olfactory absorption and axonal conveyance to the brain/olfactory bulb, accompanied by the activation of microglial cells, indicating a pro-inflammatory response. Shen et al. synthesized 0D black phosphorus QDs modified with the antioxidant β-carotene and thoroughly examined them in SCs to clarify their potential for peripheral nerve regeneration [266]. In vitro studies revealed that BPQD@β-carotene exhibits little toxicity and excellent biocompatibility, promoting brain regeneration, angiogenesis, and the modulation of inflammation in stem cells. Additionally, the PI3K/Akt and Ras/ERK1/2 signaling pathways were active in SCs at the genetic, protein, and metabolite levels. The BPQD@β-carotene-embedded GelMA/PEGDA scaffold improved functional recovery by enhancing axon remyelination and regeneration, as well as increasing intraneural angiogenesis in peripheral nerve damage models including rats and beagle dogs.
4 Limitations, challenges, and future perspectives
The practical use of antioxidant QDs for SCI encounters considerable obstacles, mainly related to toxicity, stability, and biocompatibility. Although QDs have antioxidant characteristics, they often include heavy metals like cadmium or lead, raising concerns over long-term cytotoxicity and associated environmental risks. The deterioration of QDs may emit hazardous substances, restricting their safe use in vivo. Attaining stable non-toxic compositions while preserving their antioxidant effectiveness is a significant challenge. Stability is a significant problem; QDs must preserve their optical and structural integrity under physiological settings, including the OS characteristic of SCI. Strategies for functionalization to improve biocompatibility, like biopolymer coatings or the use of carbon-based QDs have shown potential but need thorough validation.
Regulatory obstacles further impede the clinical implementation of QDs. The licensing procedure for nanomaterials in biomedical applications is rigorous, requiring comprehensive analysis of their physicochemical characteristics, biodistribution, and long-term consequences. Thorough safety evaluations, including genotoxicity, immunogenicity, and pharmacokinetics, are required. The absence of defined testing techniques for nanomedicine creates inconsistency in safety assessments, extending the approval period. Ethical issues emerge, especially about the uncertain effects of QDs on human health and the environment. Interdisciplinary cooperation is crucial to tackle these difficulties. Improvements in eco-friendly synthesis techniques for generating biocompatible QDs and the establishment of durable coating processes may alleviate toxicity issues. Transparent regulatory frameworks designed for nanomedicine, in conjunction with preclinical investigations using humanized models, may expedite clinical translation. Notwithstanding these challenges, the capacity of antioxidant QDs to transform SCI therapy via neuroprotection and regeneration highlights the need of systematically addressing these concerns. Addressing these issues will be crucial in converting antioxidant QDs from a scientific breakthrough into a therapeutic reality for SCI.
Future investigations into antioxidant QDs for SCI should prioritize the optimization of formulations, the exploration of combination treatments, and the execution of extensive clinical studies. Enhancing QD formulations requires the creation of biocompatible, stable, and non-toxic variations specifically designed for biomedical purposes. Present efforts include using carbon-based or silicon-based QDs and implementing surface modifications with biopolymers or ligands to augment solubility, diminish toxicity, and increase targeted delivery. Modifications of their optical and antioxidant characteristics via dopants or hybrid materials may improve therapeutic effectiveness and mitigate stability issues in physiological environments. Combination treatments provide a viable approach to enhance the efficacy of antioxidant QDs in SCI therapy. Combining QDs with stem cell treatment, neuroprotective medicines, or anti-inflammatory medications may provide synergistic advantages by targeting numerous damage pathways concurrently. Co-delivery devices that integrate QDs with growth factors may enhance axonal regeneration while reducing oxidative damage. The dual role of QDs as both therapeutic agents and imaging instruments makes them excellent candidates for theranostic platforms, enabling real-time assessment of treatment results. Extensive clinical trials are essential for converting preclinical achievements into medicinal applications. Subsequent studies should focus on developing uniform methods for dosage, routes of administration, and assessments of long-term safety. Utilizing sophisticated preclinical models, such as humanized organoids or three-dimensional spinal cord constructions, may provide more precise insights into effectiveness and safety. Regulatory coordination is crucial to expedite permits and mitigate concerns about nanotoxicity, especially in relation to the environmental implications of QD manufacture and use. Progress in multidisciplinary research, including materials science, nanotechnology, and regenerative medicine, will be crucial in addressing existing difficulties. Through the enhancement of QD designs, the use of combination treatments, and the validation of safety via stringent trials, QDs has the potential to transform neuroprotection and regeneration approaches for SCI.
Despite the great potential of antioxidant QDs for neuroprotection and regeneration in SCI, a number of difficulties must be overcome before their clinical application. QDs’ biocompatibility and long-term safety remain a major concern, as degradation products and possible interactions with brain cells may cause cytotoxicity or inflammatory responses. The stability and controlled release of antioxidant properties must be optimized to ensure sustained therapeutic effects while minimizing off-target accumulation. A further limitation is the potential disruption of endogenous redox signaling pathways, which may compromise cellular homeostasis if not meticulously regulated. Moreover, it is essential to develop effective delivery strategies that enable targeted penetration of the blood-spinal cord barrier while reducing systemic exposure. Finally, the absence of standardized protocols for in vivo evaluation and limited large-animal studies hinder the validation of their efficacy in clinically relevant models. Addressing these limitations by enhanced surface modifications, superior nanocarrier systems, and thorough safety evaluations will be essential for the effective translation of antioxidant QDs into therapeutic applications for SCI.
To further advance the application of antioxidant QDs in SCI therapy, future research should focus on addressing the current limitations and exploring novel avenues for their use. One of the major challenges is the toxicity and biocompatibility of QDs, which must be optimized to ensure their safe use in vivo. Future studies should investigate the development of biocompatible surface coatings and the use of biodegradable QDs to minimize potential adverse effects. Additionally, improving the long-term safety profile of QDs through in-depth toxicological evaluations is critical for their clinical translation. Another promising direction involves enhancing the targeted delivery of QDs to specific sites within the spinal cord, utilizing strategies such as nanocarrier systems or ligand-receptor interactions. Moreover, combining QDs with other therapeutic modalities, such as stem cell therapy or gene delivery, could create synergistic effects, improving outcomes in SCI. Furthermore, exploring the use of QDs in advanced imaging techniques, such as real-time monitoring of injury progression and assessing treatment efficacy, could significantly enhance the diagnostic capabilities in SCI. By addressing these challenges and exploring these emerging applications, QDs have the potential to become a powerful tool for neuroprotection and spinal cord regeneration.
5 Conclusion
Antioxidant QDs have considerable promise for enhancing neuroprotection and regeneration after SCI. SCI is often marked by OS, inflammation, and secondary damage that intensify neuronal loss and impede recovery. Antioxidant QDs, with adjustable optical characteristics, superior biocompatibility (when tuned), and inherent antioxidant qualities, may successfully tackle these difficulties. QDs alleviate OS by neutralizing ROS, hence diminishing secondary neuronal injury. Their nanoscale dimensions and surface modifications provide targeted distribution to damage locations, enhancing therapeutic efficacy. Moreover, QDs may function as imaging agents, facilitating real-time observation of cellular responses and therapy effectiveness. The dual function of theranostics as both a therapeutic and diagnostic instrument is especially beneficial for SCI therapy, where accurate monitoring of damage development is essential. In the realm of neuroregeneration, QDs provide conducive environment for brain repair by safeguarding transplanted stem cells from oxidative harm, so enhancing their survival and differentiation. They may also be used with growth factors or other neuroprotective medicines to synergistically improve axonal regeneration and functional recovery. Carbon-based QDs have potential advantages owing to their reduced toxicity and enhanced biocompatibility relative to conventional metal-based QDs. Notwithstanding these benefits, obstacles persist in guaranteeing the long-term safety, stability, and therapeutic applicability of QD-based treatments. Enhancing QD formulations, minimizing possible cytotoxicity, and substantiating their efficiency via stringent preclinical and clinical trials are crucial for their effective utilization. Advancements in nanotechnology and regenerative medicine suggest that antioxidant QDs may transform SCI care by integrating neuroprotection with regenerative functions, hence facilitating greater functional recovery and quality of life for afflicted patients.
The use of nanotechnology in SCI research and therapy presents significant opportunity to tackle the intricacies of neuroprotection and regeneration. Nanomaterials, encompassing QDs, nanoparticles, and nanofibers, possess distinctive physicochemical qualities, including elevated surface area, adjustable functions, and superior biocompatibility. These qualities provide accurate targeting, real-time surveillance, and versatile therapy approaches customized to the complex pathophysiology of SCI. QDs have evolved as dual-purpose agents in theranostics, providing antioxidative protection against secondary damage while also allowing imaging to monitor cellular responses and treatment effects. Their potential encompasses the enhancement of the milieu for brain repair, the augmentation of viability and differentiation of transplanted stem cells, and the facilitation of axonal regeneration. Carbon-based nanoparticles have significant potential in addressing the safety concerns historically linked to nanotechnology in medicine, owing to their low toxicity and great stability. Nonetheless, the journey toward clinical implementation is replete with obstacles. Long-term safety, environmental issues, and regulatory obstacles must be resolved by comprehensive research, including standardized toxicity evaluations and ethical considerations. Collaboration among material scientists, doctors, and regulatory agencies is essential to surmount these challenges. Progress in scalable manufacturing techniques and environmentally benign nanomaterial synthesis may facilitate sustainable development and use. The use of nanotechnology into SCI therapy represents a significant transformation, advancing from traditional therapeutic methods to precision medicine. Utilizing the distinctive properties of nanoscale materials, researchers can advance neuroprotection, functional recovery, and enhance patient’s quality of life. The continuous integration of nanotechnology with regenerative medicine has significant potential to transform the understanding and treatment of SCI, establishing it as a fundamental element of future advancements in neural healing.
Acknowledgments
The authors acknowledge the King Salman Center for Disability Research for funding this work through Research Group no KSRG-2024-267.
-
Funding information: The authors extend their appreciation to the King Salman Center for Disability Research for funding this work through Research Group no KSRG-2024-267.
-
Author contributions: Rashid Ayub and Mohammad Tabish: collecting data and writing the original draft. Mohd Afzal: supervision, review and editing. Mohammad Tabish: visualization, Rashid Ayub: funding acquisition. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Laurer H, Maier B, El Saman A, Lehnert M, Wyen H, Marzi I. Distribution of spinal and associated injuries in multiple trauma patients. Eur J Trauma Emerg Surg. 2007;33:476–81.10.1007/s00068-007-7124-3Suche in Google Scholar PubMed
[2] Hasler RM, Exadaktylos AK, Bouamra O, Benneker LM, Clancy M, Sieber R, et al. Epidemiology and predictors of spinal injury in adult major trauma patients: European cohort study. Eur Spine J. 2011;20:2174–80.10.1007/s00586-011-1866-7Suche in Google Scholar PubMed PubMed Central
[3] Yadollahi M, Karajizadeh M, Bordbar N, Ghahramani Z. Incidence and pattern of traumatic spine injury in a single level I trauma center of southern Iran. Chin J Traumatol. 2023;26:199–203.10.1016/j.cjtee.2023.01.001Suche in Google Scholar PubMed PubMed Central
[4] De Morais DF, De Melo Neto JS, Spotti AR, Tognola WA. Predictors of clinical complications in patients with spinomedullary injury. Coluna/Columna. 2014;13:139–42.10.1590/S1808-18512014130200404Suche in Google Scholar
[5] Calenoff L, Chessare JW, Rogers L, Toerge J, Rosen JS. Multiple level spinal injuries: importance of early recognition. Am J Roentgenol. 1978;130:665–9.10.2214/ajr.130.4.665Suche in Google Scholar PubMed
[6] Anderson SD, Anderson DG, Vaccaro AR. Skeletal fracture demographics in spinal cord-injured patients. Arch Orthop Trauma Surg. 2004;124:193–6.10.1007/s00402-003-0605-xSuche in Google Scholar PubMed
[7] Taterra D, Skinningsrud B, Pękala PA, Hsieh WC, Cirocchi R, Walocha JA, et al. Artery of Adamkiewicz: a meta-analysis of anatomical characteristics. Neuroradiology. 2019;61:869–80.10.1007/s00234-019-02207-ySuche in Google Scholar PubMed PubMed Central
[8] Griessenauer CJ, Raborn J, Foreman P, Shoja MM, Loukas M, Tubbs RS. Venous drainage of the spine and spinal cord: a comprehensive review of its history, embryology, anatomy, physiology, and pathology. Clin Anat. 2015;28:75–87.10.1002/ca.22354Suche in Google Scholar PubMed
[9] Furlan JC, Noonan V, Singh A, Fehlings MG. Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma. 2011;28:1445–77.10.1089/neu.2009.1152Suche in Google Scholar PubMed PubMed Central
[10] Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2–12.10.1097/00007632-200112151-00002Suche in Google Scholar PubMed
[11] Krueger H, Noonan V, Trenaman L, Joshi P, Rivers C. The economic burden of traumatic spinal cord injury in Canada. Health Promot Chronic Dis Prev Can. 2013;33:113–22.10.24095/hpcdp.33.3.01Suche in Google Scholar
[12] Harvey C, Wilson S, Greene C, Berkowitz M, Stripling T. New estimates of the direct costs of traumatic spinal cord injuries: results of a nationwide survey. Spinal Cord. 1992;30:834–50.10.1038/sc.1992.160Suche in Google Scholar PubMed
[13] Munce SE, Wodchis WP, Guilcher SJ, Couris CM, Verrier M, Fung K, et al. Direct costs of adult traumatic spinal cord injury in Ontario. Spinal Cord. 2013;51:64–9.10.1038/sc.2012.81Suche in Google Scholar PubMed
[14] Memish ZA, Jaber S, Mokdad AH, AlMazroa MA, Murray CJ, Al Rabeeah AA, et al. Burden of disease, injuries, and risk factors in the Kingdom of Saudi Arabia, 1990–2010. Prev Chronic Dis. 2014;11:E169.10.5888/pcd11.140176Suche in Google Scholar PubMed PubMed Central
[15] Robert AA, Zamzami MM. Traumatic spinal cord injury in Saudi Arabia: a review of the literature. Pan Afr Med J. 2013;16:104.10.11604/pamj.2013.16.104.2902Suche in Google Scholar PubMed PubMed Central
[16] Gosselin RA, Spiegel DA, Coughlin R, Zirkle LG. Injuries: the neglected burden in developing countries. Bull World Health Organ. 2009;87:246–a.10.2471/BLT.08.052290Suche in Google Scholar
[17] Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–64.10.1016/j.spinee.2003.07.007Suche in Google Scholar PubMed
[18] Esen E, Taşar F, Akhan O. Determination of the anti-inflammatory effects of methylprednisolone on the sequelae of third molar surgery. J Oral Maxillofac Surg. 1999;57:1201–6.10.1016/S0278-2391(99)90486-XSuche in Google Scholar PubMed
[19] Feinberg SM, Feinberg AR, Pruzansky J, Fisherman EW. Methylprednisolone (medrol), a potent new anti-inflammatory steroid: therapeutic results in allergic diseases. J Am Med Assoc. 1957;165:1560–2.10.1001/jama.1957.72980300006009bSuche in Google Scholar PubMed
[20] Piekarska K, Dratwa M, Radwan P, Radwan M, Bogunia-Kubik K, Nowak I. Pro- and anti-inflammatory cytokines and growth factors in patients undergoing in vitro fertilization procedure treated with prednisone. Front Immunol. 2023;14:1250488.10.3389/fimmu.2023.1250488Suche in Google Scholar PubMed PubMed Central
[21] Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:1–20.10.1186/s13287-019-1212-2Suche in Google Scholar PubMed PubMed Central
[22] Hassanzadeh A, Rahman HS, Markov A, Endjun JJ, Zekiy AO, Chartrand MS, et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021;12:297.10.1186/s13287-021-02378-7Suche in Google Scholar PubMed PubMed Central
[23] Fernandez-Colino A, Iop L, Ferreira MSV, Mela P. Fibrosis in tissue engineering and regenerative medicine: treat or trigger? Adv Drug Deliv Rev. 2019;146:17–36.10.1016/j.addr.2019.07.007Suche in Google Scholar PubMed
[24] Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283.10.1155/2016/6940283Suche in Google Scholar PubMed PubMed Central
[25] Hejrati N, Wong R, Khazaei M, Fehlings MG. How can clinical safety and efficacy concerns in stem cell therapy for spinal cord injury be overcome? Expert Opin Biol Ther. 2023;23:883–99.10.1080/14712598.2023.2245321Suche in Google Scholar PubMed
[26] Ganguly S, Wulff D, Phan C-M, Jones LW, Tang XS. Injectable and 3D extrusion printable hydrophilic silicone-based hydrogels for controlled ocular delivery of ophthalmic drugs. ACS Appl Bio Mater. 2024;7:6286–96.10.1021/acsabm.4c00901Suche in Google Scholar PubMed
[27] Khodaverdi K, Bakhshi A, Mozafari M, Naghib SM. A review of chitosan-based nanocarriers as drug delivery systems for brain diseases: Critical challenges, outlooks and promises. Int J Biol Macromol. 2024;278:134962.10.1016/j.ijbiomac.2024.134962Suche in Google Scholar PubMed
[28] Spencer AP, Torrado M, Custódio B, Silva-Reis SC, Santos SD, Leiro V, et al. Breaking barriers: bioinspired strategies for targeted neuronal delivery to the central nervous system. Pharmaceutics. 2020;12:192.10.3390/pharmaceutics12020192Suche in Google Scholar PubMed PubMed Central
[29] Galindo AN, Rubio DAF, Hettiaratchi MH. Biomaterial strategies for regulating the neuroinflammatory response. Mater Adv. 2024;5:4025–54.10.1039/D3MA00736GSuche in Google Scholar
[30] Rocha LA, Silva D, Barata‐Antunes S, Cavaleiro H, Gomes ED, Silva NA, et al. Cell and tissue instructive materials for central nervous system repair. Adv Funct Mater. 2020;30:1909083.10.1002/adfm.201909083Suche in Google Scholar
[31] Zimmermann R, Vieira Alves Y, Sperling LE, Pranke P. Nanotechnology for the treatment of spinal cord injury. Tissue Eng Part B: Rev. 2021;27:353–65.10.1089/ten.teb.2020.0188Suche in Google Scholar PubMed
[32] Kubinová Š, Syková E. Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine. 2010;5:99–108.10.2217/nnm.09.93Suche in Google Scholar PubMed
[33] Song YH, Agrawal NK, Griffin JM, Schmidt CE. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv Drug Deliv Rev. 2019;148:38–59.10.1016/j.addr.2018.12.011Suche in Google Scholar PubMed PubMed Central
[34] Parameswaranpillai J, Das P, Ganguly S. Quantum dots and polymer nanocomposites: synthesis, chemistry, and applications. Abingdon (Oxfordshire), United Kingdom: CRC Press; 2022.10.1201/9781003266518Suche in Google Scholar
[35] Das P, Ganguly S, Marvi PK, Sherazee M, Tang X, Srinivasan S, et al. Carbon dots infused 3D printed cephalopod mimetic bactericidal and antioxidant hydrogel for uniaxial mechano‐fluorescent tactile sensor. Adv Mater. 2024;36:2409819.10.1002/adma.202409819Suche in Google Scholar PubMed PubMed Central
[36] Ganguly S, Das P, Banerjee S, Das NC. Advancement in science and technology of carbon dot-polymer hybrid composites: a review. Funct Compos Struct. 2019;1:022001.10.1088/2631-6331/ab0c80Suche in Google Scholar
[37] Reni M, Mazza E, Zanon S, Gatta G, Vecht CJ. Central nervous system gliomas. Crit Rev Oncol Hematol. 2017;113:213–34.10.1016/j.critrevonc.2017.03.021Suche in Google Scholar PubMed
[38] Wainwright JV, Endo T, Cooper JB, Tominaga T, Schmidt MH. The role of 5-aminolevulinic acid in spinal tumor surgery: a review. J Neurooncol. 2019;141:575–84.10.1007/s11060-018-03080-0Suche in Google Scholar PubMed PubMed Central
[39] Wu Q-L, Xu H-L, Xiong C, Lan Q-H, Fang M-L, Cai J-H, et al. c (RGDyk)-modified nanoparticles encapsulating quantum dots as a stable fluorescence probe for imaging-guided surgical resection of glioma under the auxiliary UTMD. Artif Cells Nanomed Biotechnol. 2020;48:143–58.10.1080/21691401.2019.1699821Suche in Google Scholar PubMed
[40] Garrudo FF, Chapman CA, Hoffman PR, Udangawa RW, Silva JC, Mikael PE, et al. Polyaniline-polycaprolactone blended nanofibers for neural cell culture. Eur Polym J. 2019;117:28–37.10.1016/j.eurpolymj.2019.04.048Suche in Google Scholar
[41] Dong J, Wang K, Sun L, Sun B, Yang M, Chen H, et al. Application of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug delivery. Sens Actuators B: Chem. 2018;256:616–23.10.1016/j.snb.2017.09.200Suche in Google Scholar
[42] Zhang W, Huang Z, Pu X, Chen X, Yin G, Wang L, et al. Fabrication of doxorubicin and chlorotoxin-linked Eu-Gd2O3 nanorods with dual-model imaging and targeted therapy of brain tumor. Chin Chem Lett. 2020;31:285–91.10.1016/j.cclet.2019.04.018Suche in Google Scholar
[43] Zhang T, Wang Y, Kong L, Xue Y, Tang M. Threshold dose of three types of quantum dots (QDs) induces oxidative stress triggers DNA damage and apoptosis in mouse fibroblast L929 cells. Int J Environ Res Public Health. 2015;12:13435–54.10.3390/ijerph121013435Suche in Google Scholar PubMed PubMed Central
[44] Li J, Li K, Shao D, Ding Y, Huang L, Zheng X. The synergistic antioxidant effect of polydopamine coating with amino-functionalized graphene quantum dots on osteoblast protection against oxidative stress. Appl Surf Sci. 2023;613:155950.10.1016/j.apsusc.2022.155950Suche in Google Scholar
[45] Ren C, Hu X, Zhou Q. Graphene oxide quantum dots reduce oxidative stress and inhibit neurotoxicity in vitro and in vivo through catalase‐like activity and metabolic regulation. Adv Sci. 2018;5:1700595.10.1002/advs.201700595Suche in Google Scholar PubMed PubMed Central
[46] Li K, Chen J, Bai S, Wen X, Song S, Yu Q, et al. Intracellular oxidative stress and cadmium ions release induce cytotoxicity of unmodified cadmium sulfide quantum dots. Toxicol Vitro. 2009;23:1007–13.10.1016/j.tiv.2009.06.020Suche in Google Scholar PubMed
[47] Gagné F, Auclair J, Turcotte P, Fournier M, Gagnon C, Sauvé S, et al. Ecotoxicity of CdTe quantum dots to freshwater mussels: impacts on immune system, oxidative stress and genotoxicity. Aquat Toxicol. 2008;86:333–40.10.1016/j.aquatox.2007.11.013Suche in Google Scholar PubMed
[48] Luo W, Wang Y, Lin F, Liu Y, Gu R, Liu W, et al. Selenium-doped carbon quantum dots efficiently ameliorate secondary spinal cord injury via scavenging reactive oxygen species. Int J Nanomed. 2020;15:10113–25.10.2147/IJN.S282985Suche in Google Scholar PubMed PubMed Central
[49] Fang Q, Tang M. Oxidative stress-induced neurotoxicity of quantum dots and influencing factors. Nanomedicine. 2024;19:1013–28.10.2217/nnm-2023-0326Suche in Google Scholar PubMed PubMed Central
[50] Wu T, Liang X, Liu X, Li Y, Wang Y, Kong L, et al. Induction of ferroptosis in response to graphene quantum dots through mitochondrial oxidative stress in microglia. Part Fibre Toxicol. 2020;17:30.10.1186/s12989-020-00363-1Suche in Google Scholar PubMed PubMed Central
[51] Guo X, Lie Q, Liu Y, Jia Z, Gong Y, Yuan X, et al. Multifunctional selenium quantum dots for the treatment of Alzheimer’s disease by reducing Aβ-neurotoxicity and oxidative stress and alleviate neuroinflammation. ACS Appl Mater Interfaces. 2021;13:30261–73.10.1021/acsami.1c00690Suche in Google Scholar PubMed
[52] Yadav P, Mimansa, Kailasam K, Shanavas A. Nontoxic metal-free visible light-responsive carbon nitride quantum dots cause oxidative stress and cancer-specific membrane damage. ACS Appl Bio Mater. 2022;5:1169–78.10.1021/acsabm.1c01219Suche in Google Scholar PubMed
[53] Gill SS, Ponniah HS, Giersztein S, Anantharaj RM, Namireddy SR, Killilea J, et al. The diagnostic and prognostic capability of artificial intelligence in spinal cord injury: a systematic review. Brain Spine. 2025;5:104208.10.1016/j.bas.2025.104208Suche in Google Scholar PubMed PubMed Central
[54] Tao G, Yang S, Xu J, Wang L, Yang B. Global research trends and hotspots of artificial intelligence research in spinal cord neural injury and restoration – a bibliometrics and visualization analysis. Front Neurol. 2024;15:1361235.10.3389/fneur.2024.1361235Suche in Google Scholar PubMed PubMed Central
[55] Dietz N, Jaganathan V, Alkin V, Mettille J, Boakye M, Drazin D. Machine learning in clinical diagnosis, prognostication, and management of acute traumatic spinal cord injury (SCI): A systematic review. J Clin Orthop Trauma. 2022;35:102046.10.1016/j.jcot.2022.102046Suche in Google Scholar PubMed PubMed Central
[56] Arslan YZ, Demirer RM, Palamar D, Ugur M, Karamehmetoglu SS. Comparison of the data classification approaches to diagnose spinal cord injury. Comput Math Methods Med. 2012;2012:803980.10.1155/2012/803980Suche in Google Scholar PubMed PubMed Central
[57] Tay B, Hyun JK, Oh S. A machine learning approach for specification of spinal cord injuries using fractional anisotropy values obtained from diffusion tensor images. Comput Math Methods Med. 2014;2014:276589.10.1155/2014/276589Suche in Google Scholar PubMed PubMed Central
[58] Zhou J, Yang Y, Zhang CY. Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem Rev. 2015;115:11669–717.10.1021/acs.chemrev.5b00049Suche in Google Scholar PubMed
[59] Wang L, Wang Y, Xu T, Liao H, Yao C, Liu Y, et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat Commun. 2014;5:5357.10.1038/ncomms6357Suche in Google Scholar PubMed
[60] Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5:763–75.10.1038/nmeth.1248Suche in Google Scholar PubMed
[61] Schmidt R, Krasselt C, Göhler C, von Borczyskowski C. The fluorescence intermittency for quantum dots is not power-law distributed: a luminescence intensity resolved approach. Acs Nano. 2014;8:3506–21.10.1021/nn406562aSuche in Google Scholar PubMed
[62] Wagner AM, Knipe JM, Orive G, Peppas NA. Quantum dots in biomedical applications. Acta Biomater. 2019;94:44–63.10.1016/j.actbio.2019.05.022Suche in Google Scholar PubMed PubMed Central
[63] Oh E, Liu R, Nel A, Gemill KB, Bilal M, Cohen Y, et al. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat Nanotechnol. 2016;11:479–86.10.1038/nnano.2015.338Suche in Google Scholar PubMed
[64] Sikiru S, Oladosu TL, Kolawole SY, Mubarak LA, Soleimani H, Afolabi LO, et al. Advance and prospect of carbon quantum dots synthesis for energy conversion and storage application: A comprehensive review. J Energy Storage. 2023;60:106556.10.1016/j.est.2022.106556Suche in Google Scholar
[65] Das P, Ganguly S, Banerjee S, Das NC. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res Chem Intermed. 2019;45:3823–53.10.1007/s11164-019-03823-2Suche in Google Scholar
[66] Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, et al. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 2012;7:1–14.10.1186/1556-276X-7-480Suche in Google Scholar PubMed PubMed Central
[67] Das P, Ganguly S, Agarwal T, Maity P, Ghosh S, Choudhary S, et al. Heteroatom doped blue luminescent carbon dots as a nano-probe for targeted cell labeling and anticancer drug delivery vehicle. Mater Chem Phys. 2019;237:121860.10.1016/j.matchemphys.2019.121860Suche in Google Scholar
[68] Das P, Ganguly S, Margel S, Gedanken A. Immobilization of heteroatom-doped carbon dots onto nonpolar plastics for antifogging, antioxidant, and food monitoring applications. Langmuir. 2021;37:3508–20.10.1021/acs.langmuir.1c00471Suche in Google Scholar PubMed
[69] Wang W, Li Y, Cheng L, Cao Z, Liu W. Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J Mater Chem B. 2014;2:46–8.10.1039/C3TB21370FSuche in Google Scholar PubMed
[70] Biranje A, Azmi N, Tiwari A, Chaskar A. Quantum dots based fluorescent probe for the selective detection of heavy metal ions. J Fluoresc. 2021;31:1241–50.10.1007/s10895-021-02755-8Suche in Google Scholar PubMed
[71] Ganguly S, Das P, Das S, Ghorai U, Bose M, Ghosh S, et al. Microwave assisted green synthesis of Zwitterionic photolumenescent N-doped carbon dots: An efficient ‘on-off’chemosensor for tracer Cr (+6) considering the inner filter effect and nano drug-delivery vector. Colloids Surf A: Physicochem Eng Asp. 2019;579:123604.10.1016/j.colsurfa.2019.123604Suche in Google Scholar
[72] Tabish M, Malik I, Alshahrani AM, Afzal M. Detection of an oxidative stress metabolite associated with neurodegenerative diseases: effect of heteroatom doped antioxidant carbon dots. RSC Adv. 2025;15:8354–66.10.1039/D5RA00274ESuche in Google Scholar PubMed PubMed Central
[73] Fahad Halim A, Poinern GEJ, Fawcett D, Sharma R, Surendran S. Biomass‐derived carbon nanomaterials: synthesis and applications in textile wastewater treatment, sensors, energy storage, and conversion technologies. CleanMat. 2024;2:4–58.10.1002/clem.15Suche in Google Scholar
[74] Dhoble SJ, Nande A, Kalyani NT, Tiwari A, Arof AK. Functional materials from carbon, inorganic, and organic sources: Methods and advances. United States: Woodhead Publishing; 2022.Suche in Google Scholar
[75] Ganguly S, Das P, Itzhaki E, Hadad E, Gedanken A, Margel S. Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl Mater Interfaces. 2020;12:51940–51.10.1021/acsami.0c14527Suche in Google Scholar PubMed
[76] Ganguly S, Margel S. Fluorescent quantum dots-based hydrogels: Synthesis, fabrication and multimodal biosensing. Talanta Open. 2023;8:100243.10.1016/j.talo.2023.100243Suche in Google Scholar
[77] Tian P, Tang L, Teng K, Lau S. Graphene quantum dots from chemistry to applications. Mater Today Chem. 2018;10:221–58.10.1016/j.mtchem.2018.09.007Suche in Google Scholar
[78] Bacon M, Bradley SJ, Nann T. Graphene quantum dots. Part Part Syst Charact. 2014;31:415–28.10.1002/ppsc.201300252Suche in Google Scholar
[79] Lu ZJ, Hu WJ, Qi XD, Sun DX, Wang Y, Yang JH. Selective localization of carbonized polymer dots in amorphous phase towards high breakdown strength and energy density of PVDF-based dielectric composites. Compos Part B: Eng. 2024;283:111627.10.1016/j.compositesb.2024.111627Suche in Google Scholar
[80] Wang B, Waterhouse GI, Yang B, Lu S. Advances in shell and core engineering of carbonized polymer dots for enhanced applications. Acc Chem Res. 2024;57:2928–39.10.1021/acs.accounts.4c00516Suche in Google Scholar PubMed
[81] Luppi BT, Primrose WL, Hudson ZM. Polymer dots with delayed fluorescence and tunable cellular uptake for photodynamic therapy and time‐gated imaging. Angew Chem Int Ed. 2024;63:e202400712.10.1002/anie.202400712Suche in Google Scholar PubMed
[82] Wang F, Zhou S, Zhang Y, Wang Y, Guo R, Xiao H, et al. Chiral phosphorescent carbonized polymer dots relayed light‐harvesting system for color‐tunable circularly polarized room temperature phosphorescence. Small. 2024;20:2306969.10.1002/smll.202306969Suche in Google Scholar PubMed
[83] Karami MH, Abdouss M. Recent advances of carbon quantum dots in tumor imaging. Nanomed J. 2024;11:13–35.Suche in Google Scholar
[84] Gulati S, Baul A, Amar A, Wadhwa R, Kumar S, Varma RS. Eco-friendly and sustainable pathways to photoluminescent carbon quantum dots (CQDs). Nanomaterials. 2023;13:554.10.3390/nano13030554Suche in Google Scholar PubMed PubMed Central
[85] Kortel M, Mansuriya BD, Vargas Santana N, Altintas Z. Graphene quantum dots as flourishing nanomaterials for bio-imaging, therapy development, and micro-supercapacitors. Micromachines. 2020;11:866.10.3390/mi11090866Suche in Google Scholar PubMed PubMed Central
[86] Zhu Y-J, Chen F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem Rev. 2014;114:6462–555.10.1021/cr400366sSuche in Google Scholar PubMed
[87] Ahmed SF, Mofijur M, Rafa N, Chowdhury AT, Chowdhury S, Nahrin M, et al. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ Res. 2022;204:111967.10.1016/j.envres.2021.111967Suche in Google Scholar PubMed
[88] Zhang Y, Clapp A. Overview of stabilizing ligands for biocompatible quantum dot nanocrystals. Sensors. 2011;11:11036–55.10.3390/s111211036Suche in Google Scholar PubMed PubMed Central
[89] Karakoti AS, Shukla R, Shanker R, Singh S. Surface functionalization of quantum dots for biological applications. Adv Colloid Interface Sci. 2015;215:28–45.10.1016/j.cis.2014.11.004Suche in Google Scholar PubMed
[90] Wenger WN, Bates FS, Aydil ES. Functionalization of cadmium selenide quantum dots with poly (ethylene glycol): ligand exchange, surface coverage, and dispersion stability. Langmuir. 2017;33:8239–45.10.1021/acs.langmuir.7b01924Suche in Google Scholar PubMed
[91] Mansur HS. Quantum dots and nanocomposites. Wiley Interdiscip Reviews: Nanomed Nanobiotechnol. 2010;2:113–29.10.1002/wnan.78Suche in Google Scholar PubMed
[92] Wang W, Zhang M, Pan Z, Biesold GM, Liang S, Rao H, et al. Colloidal inorganic ligand-capped nanocrystals: fundamentals, status, and insights into advanced functional nanodevices. Chem Rev. 2021;122:4091–162.10.1021/acs.chemrev.1c00478Suche in Google Scholar PubMed
[93] Wang F, Tan WB, Zhang Y, Fan X, Wang M. Luminescent nanomaterials for biological labelling. Nanotechnology. 2005;17:R1.10.1088/0957-4484/17/1/R01Suche in Google Scholar
[94] Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Opin Biotechnol. 2002;13:40–6.10.1016/S0958-1669(02)00282-3Suche in Google Scholar PubMed
[95] Gao X, Nie S. Quantum dot-encoded mesoporous beads with high brightness and uniformity: rapid readout using flow cytometry. Anal Chem. 2004;76:2406–10.10.1021/ac0354600Suche in Google Scholar PubMed
[96] Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6.10.1038/nbt764Suche in Google Scholar PubMed
[97] Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–8.10.1126/science.281.5385.2016Suche in Google Scholar PubMed
[98] Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, et al. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem. 1999;47:1179–88.10.1177/002215549904700910Suche in Google Scholar PubMed
[99] Liu C, Zhang P, Zhai X, Tian F, Li W, Yang J, et al. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials. 2012;33:3604–13.10.1016/j.biomaterials.2012.01.052Suche in Google Scholar PubMed
[100] Dong Y, Pang H, Yang HB, Guo C, Shao J, Chi Y, et al. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew Chem Int Ed. 2013;52:7800–4.10.1002/anie.201301114Suche in Google Scholar PubMed
[101] Wen Z-H, Yin X-B. Excitation-independent carbon dots, from photoluminescence mechanism to single-color application. RSC Adv. 2016;6:27829–35.10.1039/C5RA27172JSuche in Google Scholar
[102] Pan D, Guo L, Zhang J, Xi C, Xue Q, Huang H, et al. Cutting sp 2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J Mater Chem. 2012;22:3314–8.10.1039/c2jm16005fSuche in Google Scholar
[103] De B, Karak N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013;3:8286–90.10.1039/c3ra00088eSuche in Google Scholar
[104] Das P, Ganguly S, Mondal S, Bose M, Das AK, Banerjee S, et al. Heteroatom doped photoluminescent carbon dots for sensitive detection of acetone in human fluids. Sens Actuators B: Chem. 2018;266:583–93.10.1016/j.snb.2018.03.183Suche in Google Scholar
[105] García de Arquer FP, Talapin DV, Klimov VI, Arakawa Y, Bayer M, Sargent EH. Semiconductor quantum dots: Technological progress and future challenges. Science. 2021;373:eaaz8541.10.1126/science.aaz8541Suche in Google Scholar PubMed
[106] Gerion D, Pinaud F, Williams SC, Parak WJ, Zanchet D, Weiss S, et al. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J Phys Chem B. 2001;105:8861–71.10.1021/jp0105488Suche in Google Scholar
[107] Mokari T, Rothenberg E, Popov I, Costi R, Banin U. Selective growth of metal tips onto semiconductor quantum rods and tetrapods. Science. 2004;304:1787–90.10.1126/science.1097830Suche in Google Scholar PubMed
[108] Das P, Ganguly S, Bose M, Ray D, Ghosh S, Mondal S, et al. Surface quaternized nanosensor as a one-arrow-two-hawks approach for fluorescence turn “on–off–on” bifunctional sensing and antibacterial activity. N J Chem. 2019;43:6205–19.10.1039/C8NJ06308GSuche in Google Scholar
[109] Das P, Maruthapandi M, Saravanan A, Natan M, Jacobi G, Banin E, et al. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl Nano Mater. 2020;3:11777–90.10.1021/acsanm.0c02305Suche in Google Scholar
[110] Das P, Ganguly S, Mondal S, Ghorai UK, Maity PP, Choudhary S, et al. Dual doped biocompatible multicolor luminescent carbon dots for bio labeling, UV‐active marker and fluorescent polymer composite. Luminescence. 2018;33:1136–45.10.1002/bio.3520Suche in Google Scholar PubMed
[111] Maruthapandi M, Saravanan A, Das P, Luong JH, Gedanken A. Microbial inhibition and biosensing with multifunctional carbon dots: progress and perspectives. Biotechnol Adv. 2021;53:107843.10.1016/j.biotechadv.2021.107843Suche in Google Scholar PubMed
[112] Saravanan A, Das P, Maruthapandi M, Aryal S, Michaeli S, Mastai Y, et al. Heteroatom co-doping (N, NS, NB) on carbon dots and their antibacterial and antioxidant properties. Surf Interfaces. 2024;46:103857.10.1016/j.surfin.2024.103857Suche in Google Scholar
[113] Li L, Wang C, Liu K, Wang Y, Liu K, Lin Y. Hexagonal cobalt oxyhydroxide–carbon dots hybridized surface: high sensitive fluorescence turn-on probe for monitoring of ascorbic acid in rat brain following brain ischemia. Anal Chem. 2015;87:3404–11.10.1021/ac5046609Suche in Google Scholar PubMed
[114] Kong W, Wu D, Li G, Chen X, Gong P, Sun Z, et al. A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta. 2017;165:677–84.10.1016/j.talanta.2017.01.022Suche in Google Scholar PubMed
[115] Li L, Wang C, Luo J, Guo Q, Liu K, Liu K, et al. Fe3+-functionalized carbon quantum dots: a facile preparation strategy and detection for ascorbic acid in rat brain microdialysates. Talanta. 2015;144:1301–7.10.1016/j.talanta.2015.08.003Suche in Google Scholar PubMed
[116] Ma Y, Xu G, Wei F, Cen Y, Song Y, Ma Y, et al. Carbon dots based immunosorbent assay for the determination of GFAP in human serum. Nanotechnology. 2018;29:145501.10.1088/1361-6528/aaabeaSuche in Google Scholar PubMed
[117] Benítez-Martínez S, Caballero-Díaz E, Valcárcel M. Development of a biosensing system for tacrine based on nitrogen-doped graphene quantum dots and acetylcholinesterase. Analyst. 2016;141:2688–95.10.1039/C6AN00357ESuche in Google Scholar PubMed
[118] Jia Z, Luo Y, Wen H, Huang S, Du X, Xue W. A probe for fluorescence detection of the acetylcholinesterase activity based on molecularly imprinted polymers coated carbon dots. Chem Pharm Bull. 2019;67:795–800.10.1248/cpb.c18-00944Suche in Google Scholar PubMed
[119] Mars A, Hamami M, Bechnak L, Patra D, Raouafi N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal Chim Acta. 2018;1036:141–6.10.1016/j.aca.2018.06.075Suche in Google Scholar PubMed
[120] Zhang W, Wang R, Liu W, Wang X, Li P, Zhang W, et al. Te-containing carbon dots for fluorescence imaging of superoxide anion in mice during acute strenuous exercise or emotional changes. Chem Sci. 2018;9:721–7.10.1039/C7SC03878JSuche in Google Scholar PubMed PubMed Central
[121] Amiri M, Dadfarnia S, Shabani AMH, Sadjadi S. Non-enzymatic sensing of dopamine by localized surface plasmon resonance using carbon dots-functionalized gold nanoparticles. J Pharm Biomed Anal. 2019;172:223–9.10.1016/j.jpba.2019.04.037Suche in Google Scholar PubMed
[122] Sangubotla R, Kim J. A facile enzymatic approach for selective detection of γ-aminobutyric acid using corn-derived fluorescent carbon dots. Appl Surf Sci. 2019;490:61–9.10.1016/j.apsusc.2019.05.320Suche in Google Scholar
[123] Ortega MA, Fraile-Martinez O, García-Montero C, Haro S, Álvarez-Mon MÁ, De Leon-Oliva D, et al. A comprehensive look at the psychoneuroimmunoendocrinology of spinal cord injury and its progression: mechanisms and clinical opportunities. Mil Med Res. 2023;10:26.10.1186/s40779-023-00461-zSuche in Google Scholar PubMed PubMed Central
[124] Huang Z, Wang J, Li C, Zheng W, He J, Wu Z, et al. Application of natural antioxidants from traditional Chinese medicine in the treatment of spinal cord injury. Front Pharmacol. 2022;13:976757.10.3389/fphar.2022.976757Suche in Google Scholar PubMed PubMed Central
[125] Liu G, Deng B, Huo L, Jiang S, Fan X, Mo Y, et al. Temporal profiling and validation of oxidative stress-related genes in spinal cord injury. Brain Res Bull. 2023;205:110832.10.1016/j.brainresbull.2023.110832Suche in Google Scholar PubMed
[126] Lipinski MM, Wu J, Faden AI, Sarkar C. Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal. 2015;23:565–77.10.1089/ars.2015.6306Suche in Google Scholar PubMed PubMed Central
[127] Islam F, Bepary S, Nafady MH, Islam MR, Emran TB, Sultana S, et al. Polyphenols targeting oxidative stress in spinal cord injury: current status and future vision. Oxid Med Cell Longev. 2022;2022:8741787.10.1155/2022/8741787Suche in Google Scholar PubMed PubMed Central
[128] Zhang Z, Guth L, Steward O. Mechanisms of motor recovery after subtotal spinal cord injury: insights from the study of mice carrying a mutation (WldS) that delays cellular responses to injury. Exp Neurol. 1998;149:221–9.10.1006/exnr.1997.6717Suche in Google Scholar PubMed
[129] Barati E, Nikzad H, Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol Life Sci. 2020;77:93–113.10.1007/s00018-019-03253-8Suche in Google Scholar PubMed PubMed Central
[130] Ai G, Xiong M, Deng L, Zeng J, Xiao Q. Research progress on the inhibition of oxidative stress by teriparatide in spinal cord injury. Front Neurol. 2024;15:1358414.10.3389/fneur.2024.1358414Suche in Google Scholar PubMed PubMed Central
[131] Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. 2022;11:552.10.3390/cells11030552Suche in Google Scholar PubMed PubMed Central
[132] Mira RG, Lira M, Cerpa W. Traumatic brain injury: mechanisms of glial response. Front Physiol. 2021;12:740939.10.3389/fphys.2021.740939Suche in Google Scholar PubMed PubMed Central
[133] Ghaderi S, Rashno M, Brooshghalan SE, Salehi I, Sarihi A, Shahidi S, et al. P-coumaric acid reverses spatial cognitive decline in a rat model of traumatic brain injury: possible underlying mechanisms. J Funct Foods. 2024;120:106381.10.1016/j.jff.2024.106381Suche in Google Scholar
[134] Zhang Y, Zhang D, Jiao X, Yue X, Cai B, Lu S, et al. Uncovering the shared neuro-immune-related regulatory mechanisms between spinal cord injury and osteoarthritis. Heliyon. 2024;10:e30336.10.1016/j.heliyon.2024.e30336Suche in Google Scholar PubMed PubMed Central
[135] Liu C, Liu Y, Ma B, Zhou M, Zhao X, Fu X, et al. Mitochondrial regulatory mechanisms in spinal cord injury: A narrative review. Medicine. 2022;101:e31930.10.1097/MD.0000000000031930Suche in Google Scholar PubMed PubMed Central
[136] Cai Y, Xu H, Yan J, Zhang L, Lu Y. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury. Mol Med Rep. 2014;9:1542–50.10.3892/mmr.2014.2034Suche in Google Scholar PubMed
[137] Soares RO, Losada DM, Jordani MC, Évora P, Castro-e-Silva O. Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int J Mol Sci. 2019;20:5034.10.3390/ijms20205034Suche in Google Scholar PubMed PubMed Central
[138] Xu Z, Li Z. Experimental study on the role of Apelin-13 in alleviating spinal cord ischemia reperfusion injury through suppressing autophagy. Drug Des Dev Ther. 2020;14:1571–81.10.2147/DDDT.S241066Suche in Google Scholar PubMed PubMed Central
[139] Smuder AJ, Turner SM, Schuster CM, Morton AB, Hinkley JM, Fuller DD. Hyperbaric oxygen treatment following mid-cervical spinal cord injury preserves diaphragm muscle function. Int J Mol Sci. 2020;21:7219.10.3390/ijms21197219Suche in Google Scholar PubMed PubMed Central
[140] Wang Y, Pang Q-J, Liu J-T, Wu H-H, Tao D-Y. Down-regulated miR-448 relieves spinal cord ischemia/reperfusion injury by up-regulating SIRT1. Braz J Med Biol Res. 2018;51:e7319.10.1590/1414-431x20177319Suche in Google Scholar PubMed PubMed Central
[141] Yang H, Wang X, Wang Z, Wang G, Fu S, Li F, et al. Peripheral nerve injury induced by Japanese encephalitis virus in C57BL/6 mouse. J Virol. 2023;97:e01658-22.10.1128/jvi.01658-22Suche in Google Scholar PubMed PubMed Central
[142] Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci. 2004;1035:117–32.10.1196/annals.1332.008Suche in Google Scholar PubMed
[143] Agrawal K, Asthana S, Kumar D. Role of oxidative stress in metabolic reprogramming of brain cancer. Cancers. 2023;15:4920.10.3390/cancers15204920Suche in Google Scholar PubMed PubMed Central
[144] Deng F, Olvera-Vargas H, Zhou M, Qiu S, Sirés I, Brillas E. Critical review on the mechanisms of Fe2+ regeneration in the electro-Fenton process: fundamentals and boosting strategies. Chem Rev. 2023;123:4635–62.10.1021/acs.chemrev.2c00684Suche in Google Scholar PubMed
[145] Zeeshan HMA, Lee GH, Kim H-R, Chae H-J. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016;17:327.10.3390/ijms17030327Suche in Google Scholar PubMed PubMed Central
[146] Zhang X, Wan J, Mo F, Tang D, Xiao H, Li Z, et al. Targeting bone tumor and subcellular endoplasmic reticulum via near infrared II fluorescent polymer for photodynamic‐immunotherapy to break the step‐reduction delivery dilemma. Adv Sci. 2022;9:2201819.10.1002/advs.202201819Suche in Google Scholar PubMed PubMed Central
[147] Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li Y, et al. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97:1439–51.10.1007/s00204-023-03476-6Suche in Google Scholar PubMed
[148] Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021;22:4642.10.3390/ijms22094642Suche in Google Scholar PubMed PubMed Central
[149] Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50:264–74.10.1038/sc.2011.111Suche in Google Scholar PubMed
[150] Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23:134–47.10.1016/S0891-5849(96)00629-6Suche in Google Scholar
[151] Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–99.10.1016/j.bpobgyn.2010.10.016Suche in Google Scholar PubMed PubMed Central
[152] Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765:283–90.10.1016/S0006-8993(97)00573-8Suche in Google Scholar PubMed
[153] Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimer’s Dis. 2014;42:S125–52.10.3233/JAD-132738Suche in Google Scholar PubMed
[154] Weyers VS. Proinflammatory effects of the pHERV-W envelope protein on primary neonatal microglia of the Wistar rat related to multiple sclerosis. Dissertation. Düsseldorf. Heinrich-Heine-Universität; 2024.Suche in Google Scholar
[155] Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–95.10.1093/brain/awn109Suche in Google Scholar PubMed PubMed Central
[156] Balkhi HM, Gul T, Banday MZ, Haq E. Glutamate excitotoxicity: an insight into the mechanism. Int J Adv Res. 2014;2:361–73.Suche in Google Scholar
[157] Ma T, Cheng Q, Chen C, Luo Z, Feng D. Excessive activation of NMDA receptors in the pathogenesis of multiple peripheral organs via mitochondrial dysfunction, oxidative stress, and inflammation. SN Compr Clin Med. 2020;2:551–69.10.1007/s42399-020-00298-wSuche in Google Scholar
[158] Mira RG, Cerpa W. Building a bridge between NMDAR-mediated excitotoxicity and mitochondrial dysfunction in chronic and acute diseases. Cell Mol Neurobiol. 2021;41:1413–30.10.1007/s10571-020-00924-0Suche in Google Scholar PubMed PubMed Central
[159] Chinopoulos C, Adam‐Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology: novel aspects of an enduring theme. FEBS J. 2006;273:433–50.10.1111/j.1742-4658.2005.05103.xSuche in Google Scholar PubMed
[160] Wang J-F, Li Y, Song J-N, Pang H-G. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int. 2014;64:37–47.10.1016/j.neuint.2013.11.002Suche in Google Scholar PubMed
[161] Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:1–24.10.1186/s12974-019-1516-2Suche in Google Scholar PubMed PubMed Central
[162] Cascella R, Cecchi C. Calcium dyshomeostasis in Alzheimer’s disease pathogenesis. Int J Mol Sci. 2021;22:4914.10.3390/ijms22094914Suche in Google Scholar PubMed PubMed Central
[163] Liu Y, Lin F, Wu C, Liu W, Wang H, Xiao C, et al. In situ reaction-generated aldehyde-scavenging polypeptides-curcumin conjugate nanoassemblies for combined treatment of spinal cord injury. ACS Nano. 2024;18:7346–62.10.1021/acsnano.3c08662Suche in Google Scholar PubMed
[164] Hou Y, Luan J, Huang T, Deng T, Li X, Xiao Z, et al. Tauroursodeoxycholic acid alleviates secondary injury in spinal cord injury mice by reducing oxidative stress, apoptosis, and inflammatory response. J Neuroinflammation. 2021;18:1–13.10.1186/s12974-021-02248-2Suche in Google Scholar PubMed PubMed Central
[165] Fakhri S, Abbaszadeh F, Moradi SZ, Cao H, Khan H, Xiao J. Effects of polyphenols on oxidative stress, inflammation, and interconnected pathways during spinal cord injury. Oxid Med Cell Longev. 2022;2022:8100195.10.1155/2022/8100195Suche in Google Scholar PubMed PubMed Central
[166] Mao Y, Du J, Chen X, Al Mamun A, Cao L, Yang Y, et al. Maltol promotes mitophagy and inhibits oxidative stress via the Nrf2/PINK1/Parkin pathway after spinal cord injury. Oxid Med Cell Longev. 2022;2022:1337630.10.1155/2022/1337630Suche in Google Scholar PubMed PubMed Central
[167] Weng J, Wang L, Wang K, Su H, Luo D, Yang H, et al. Tauroursodeoxycholic acid inhibited apoptosis and oxidative stress in H2O2-induced BMSC death via modulating the Nrf-2 signaling pathway: the therapeutic implications in a rat model of spinal cord injury. Mol Neurobiol. 2024;61:3753–68.10.1007/s12035-023-03754-5Suche in Google Scholar PubMed PubMed Central
[168] Li Z, Zhao T, Ding J, Gu H, Wang Q, Wang Y, et al. A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury. Bioact Mater. 2023;19:550–68.10.1016/j.bioactmat.2022.04.029Suche in Google Scholar PubMed PubMed Central
[169] Zheng Q, Zhang J, Zuo X, Sun J, Liang Z, Hu X, et al. Photobiomodulation promotes neuronal axon regeneration after oxidative stress and induces a change in polarization from M1 to M2 in macrophages via stimulation of CCL2 in neurons: relevance to spinal cord injury. J Mol Neurosci. 2021;71:1290–300.10.1007/s12031-020-01756-9Suche in Google Scholar PubMed
[170] Das P, Ganguly S, Saha A, Noked M, Margel S, Gedanken A. Carbon-dots-initiated photopolymerization: an in situ synthetic approach for MXene/poly(norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl Mater Interfaces. 2021;13:31038–50.10.1021/acsami.1c08111Suche in Google Scholar PubMed
[171] Wang Y, Kong W, Wang L, Zhang JZ, Li Y, Liu X, et al. Optimizing oxygen functional groups in graphene quantum dots for improved antioxidant mechanism. Phys Chem Chem Phys. 2019;21:1336–43.10.1039/C8CP06768FSuche in Google Scholar PubMed
[172] Hemmateenejad B, Shamsipur M, Khosousi T, Shanehsaz M, Firuzi O. Antioxidant activity assay based on the inhibition of oxidation and photobleaching of L-cysteine-capped CdTe quantum dots. Analyst. 2012;137:4029–36.10.1039/c2an35588dSuche in Google Scholar PubMed
[173] Chong Y, Ge C, Fang G, Tian X, Ma X, Wen T, et al. Crossover between anti- and pro-oxidant activities of graphene quantum dots in the absence or presence of light. ACS Nano. 2016;10:8690–9.10.1021/acsnano.6b04061Suche in Google Scholar PubMed
[174] Wang L, Li Y, Wang Y, Kong W, Lu Q, Liu X, et al. Chlorine-doped graphene quantum dots with enhanced anti- and pro-oxidant properties. ACS Appl Mater Interfaces. 2019;11:21822–9.10.1021/acsami.9b03194Suche in Google Scholar PubMed
[175] Liang Y, Hou D, Ni Z, Cao M, Cai L. Preparation, characterization of naringenin, β-cyclodextrin and carbon quantum dot antioxidant nanocomposites. Food Chem. 2022;375:131646.10.1016/j.foodchem.2021.131646Suche in Google Scholar PubMed
[176] Xiao M, Pan Y, Tang S, Guo T, Yang L, Chen G. Carbon quantum dots amplify beneficial effects of EGCG against neural injuries by NLRP3 inflammasome after intracerebral hemorrhage. Int J Pharm. 2025;671:125281.10.1016/j.ijpharm.2025.125281Suche in Google Scholar PubMed
[177] Yan C, Wang Y, Ma Y, Liu H, Tang S, Li Y, et al. A multifunctional carbon quantum dot overcoming the BBB for modulating amyloid aggregation and scavenging reactive oxygen species. Mater Today Chem. 2024;40:102222.10.1016/j.mtchem.2024.102222Suche in Google Scholar
[178] Alamri OA, Qusti S, Balgoon M, Ageeli AA, Al-Marhaby F, Alosaimi AM, et al. The role of MoS2 QDs coated with DSPE-PEG-TPP in the protection of protein secondary structure of the brain tissues in an Alzheimer’s disease model. Int J Biol Macromol. 2024;255:128522.10.1016/j.ijbiomac.2023.128522Suche in Google Scholar PubMed
[179] Shen Y-J, Huang Y-C, Cheng Y-C. Advancements in antioxidant-based therapeutics for spinal cord injury: a critical review of strategies and combination approaches. Antioxidants. 2024;14:17.10.3390/antiox14010017Suche in Google Scholar PubMed PubMed Central
[180] He X, Zhu Y, Ma B, Xu X, Huang R, Cheng L, et al. Bioactive 2D nanomaterials for neural repair and regeneration. Adv Drug Deliv Rev. 2022;187:114379.10.1016/j.addr.2022.114379Suche in Google Scholar PubMed
[181] Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25:5474.10.3390/molecules25225474Suche in Google Scholar PubMed PubMed Central
[182] Surh Y-J, Na H-K. NF-κB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2008;2:313–7.10.1007/s12263-007-0063-0Suche in Google Scholar PubMed PubMed Central
[183] Bellezza I, Mierla AL, Minelli A. Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers. 2010;2:483–97.10.3390/cancers2020483Suche in Google Scholar PubMed PubMed Central
[184] Singh S, Singh TG. Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol. 2020;18:918–35.10.2174/1570159X18666200207120949Suche in Google Scholar PubMed PubMed Central
[185] Shen G, Jeong WS, Hu R, Kong ANT. Regulation of Nrf2, NF-κB, and AP-1 signaling pathways by chemopreventive agents. Antioxid Redox Signal. 2005;7:1648–63.10.1089/ars.2005.7.1648Suche in Google Scholar PubMed
[186] Rius-Pérez S, Pérez S, Martí-Andrés P, Monsalve M, Sastre J. Nuclear factor kappa B signaling complexes in acute inflammation. Antioxid Redox Signal. 2020;33:145–65.10.1089/ars.2019.7975Suche in Google Scholar PubMed
[187] Mosalam EM, Elberri AI, Sallam AS, Salem HR, Metwally EM, Abdallah MS, et al. Chronotherapeutic neuroprotective effect of verapamil against lipopolysaccharide-induced neuroinflammation in mice through modulation of calcium-dependent genes. Mol Med. 2022;28:139.10.1186/s10020-022-00564-8Suche in Google Scholar PubMed PubMed Central
[188] Thakur S, Dhapola R, Sarma P, Medhi B, Reddy DH. Neuroinflammation in Alzheimer’s disease: current progress in molecular signaling and therapeutics. Inflammation. 2023;46:1–17.10.1007/s10753-022-01721-1Suche in Google Scholar PubMed
[189] Sharma P, Srivastava P, Seth A, Tripathi PN, Banerjee AG, Shrivastava SK. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog Neurobiol. 2019;174:53–89.10.1016/j.pneurobio.2018.12.006Suche in Google Scholar PubMed
[190] Tropea MR, Gulisano W, Vacanti V, Arancio O, Puzzo D, Palmeri A. Nitric oxide/cGMP/CREB pathway and amyloid-beta crosstalk: from physiology to Alzheimer’s disease. Free Radic Biol Med. 2022;193:657–68.10.1016/j.freeradbiomed.2022.11.022Suche in Google Scholar PubMed
[191] Mosalam EM, Abdel-Bar HM, Elberri AI, Abdallah MS, Zidan AAA, Batakoushy HA, et al. Enhanced neuroprotective effect of verapamil-loaded hyaluronic acid modified carbon quantum dots in an in-vitro model of amyloid-induced Alzheimer’s disease. Int J Biol Macromol. 2024;275:133742.10.1016/j.ijbiomac.2024.133742Suche in Google Scholar PubMed
[192] Henriquez G, Ahlawat J, Fairman R, Narayan M. Citric acid-derived carbon quantum dots attenuate paraquat-induced neuronal compromise in vitro and in vivo. ACS Chem Neurosci. 2022;13:2399–409.10.1021/acschemneuro.2c00099Suche in Google Scholar PubMed
[193] Guerrero ED, Lopez-Velazquez AM, Ahlawat J, Narayan M. Carbon quantum dots for treatment of amyloid disorders. ACS Appl Nano Mater. 2021;4:2423–33.10.1021/acsanm.0c02792Suche in Google Scholar PubMed PubMed Central
[194] Mosalam EM, Elberri AI, Abdallah MS, Abdel-Bar HM, Zidan AAA, Batakoushy HA, et al. Mechanistic insights of neuroprotective efficacy of verapamil-loaded carbon quantum dots against LPS-induced neurotoxicity in rats. Int J Mol Sci. 2024;25:7790.10.3390/ijms25147790Suche in Google Scholar PubMed PubMed Central
[195] Raghavan A, Radhakrishnan M, Soren K, Wadnerkar P, Kumar A, Chakravarty S, et al. Biological evaluation of graphene quantum dots and nitrogen-doped graphene quantum dots as neurotrophic agents. ACS Appl Bio Mater. 2023;6:2237–47.10.1021/acsabm.3c00099Suche in Google Scholar PubMed
[196] Sanjay, Sharma A, Lee HJ. Honeyberry-derived carbon quantum dots ameliorate LPS-induced neuroinflammation and oxidative stress through Nrf2/HO-1 signalling in HMC3 cells. Artif Cells Nanomed Biotechnol. 2023;51:95–107.Suche in Google Scholar
[197] Jia H, Gong J, Hu Z, Wen T, Li C, Chen Y, et al. Antioxidant carbon dots nanozymes alleviate stress-induced depression by modulating gut microbiota. Langmuir. 2024;40:19739–50.10.1021/acs.langmuir.4c02481Suche in Google Scholar PubMed
[198] Yarandi SS, Peterson DA, Treisman GJ, Moran TH, Pasricha PJ. Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and diseases. J Neurogastroenterol Motil. 2016;22:201.10.5056/jnm15146Suche in Google Scholar PubMed PubMed Central
[199] Wei Z, Dong X, Sun Y. Quercetin-derived red emission carbon dots: A multifunctional theranostic nano-agent against Alzheimer’s β-amyloid fibrillogenesis. Colloids Surf B: Biointerfaces. 2024;238:113907.10.1016/j.colsurfb.2024.113907Suche in Google Scholar PubMed
[200] Hu Y, Cui J, Sun J, Liu X, Gao S, Mei X, et al. A novel biomimetic nanovesicle containing caffeic acid-coupled carbon quantum dots for the the treatment of Alzheimer’s disease via nasal administration. J Nanobiotechnol. 2024;22:642.10.1186/s12951-024-02912-8Suche in Google Scholar PubMed PubMed Central
[201] Ghosh S, Sachdeva B, Sachdeva P, Chaudhary V, Rani GM, Sinha JK. Graphene quantum dots as a potential diagnostic and therapeutic tool for the management of Alzheimer’s disease. Carbon Lett. 2022;32:1381–94.10.1007/s42823-022-00397-9Suche in Google Scholar
[202] Won JE, Park M, Hong S-H, Kim YS, Song H. Quantum dots as biocompatible small RNA nanocarriers modulating macrophage polarization to treat Asherman’s syndrome. npj Regener Med. 2025;10:15.10.1038/s41536-025-00403-4Suche in Google Scholar PubMed PubMed Central
[203] Yao Y, Wang Z, Huang X, Wei T, Liu N, Zou L, et al. Adverse outcome pathway-based strategies to mitigate Ag2Se quantum dot-induced neurotoxicity. ACS Nano. 2025;19:11029–48.10.1021/acsnano.4c16813Suche in Google Scholar PubMed
[204] Liu Y, Lin Z, Wang Y, Chen L, Wang Y, Luo C. Nanotechnology in inflammation: Cutting-edge advances in diagnostics, therapeutics and theranostics. Theranostics. 2024;14:2490.10.7150/thno.91394Suche in Google Scholar PubMed PubMed Central
[205] Yang W, Yue H, Lu G, Wang W, Deng Y, Ma G, et al. Advances in delivering oxidative modulators for disease therapy. Research. 2022;2022:9897464.10.34133/2022/9897464Suche in Google Scholar PubMed PubMed Central
[206] Luo D, Zhang H, An X, Zhao J, Feng C, Yin J, et al. Synergistic effects of sulfur-doped carbon dots/permanganate process for DCF degradation: Mechanism and pathways. J Hazard Mater. 2025;494:138567.10.1016/j.jhazmat.2025.138567Suche in Google Scholar PubMed
[207] Ding Y, Chen Q. The NF-κB pathway: a focus on inflammatory responses in spinal cord injury. Mol Neurobiol. 2023;60:5292–308.10.1007/s12035-023-03411-xSuche in Google Scholar PubMed
[208] Adeyi OE, Somade OT, Ajayi BO, James AS, Adeboye TR, Olufemi DA, et al. The anti-inflammatory effect of ferulic acid is via the modulation of NFκB-TNF-α-IL-6 and STAT1-PIAS1 signaling pathways in 2-methoxyethanol-induced testicular inflammation in rats. Phytomed Plus. 2023;3:100464.10.1016/j.phyplu.2023.100464Suche in Google Scholar
[209] Li D, Luo F, Guo T, Han S, Wang H, Lin Q. Targeting NF-κB pathway by dietary lignans in inflammation: Expanding roles of gut microbiota and metabolites. Crit Rev Food Sci Nutr. 2023;63:5967–83.10.1080/10408398.2022.2026871Suche in Google Scholar PubMed
[210] Chen Y, Ye X, Escames G, Lei W, Zhang X, Li M, et al. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023;28:51.10.1186/s11658-023-00462-9Suche in Google Scholar PubMed PubMed Central
[211] Ayaz F, Alas MO, Genc R. Differential immunomodulatory effect of carbon dots influenced by the type of surface passivation agent. Inflammation. 2020;43:777–83.10.1007/s10753-019-01165-0Suche in Google Scholar PubMed
[212] Tosic J, Stanojevic Z, Vidicevic S, Isakovic A, Ciric D, Martinovic T, et al. Graphene quantum dots inhibit T cell-mediated neuroinflammation in rats. Neuropharmacology. 2019;146:95–108.10.1016/j.neuropharm.2018.11.030Suche in Google Scholar PubMed
[213] Qi Z, Pan S, Yang X, Zhang R, Qin C, Yan H, et al. Injectable hydrogel loaded with CDs and FTY720 combined with neural stem cells for the treatment of spinal cord injury. Int J Nanomed. 2024;19:4081–101.10.2147/IJN.S448962Suche in Google Scholar PubMed PubMed Central
[214] Yang X, Tang X, Jia G, Wang Y, Yang L, Li Y, et al. Multifunctional carbon quantum dots: iron clearance and antioxidation for neuroprotection in intracerebral hemorrhage mice. ACS Appl Mater Interfaces. 2023;15:56820–33.10.1021/acsami.3c13580Suche in Google Scholar PubMed
[215] Tang X, Yang X, Yu Y, Wu M, Li Y, Zhang Z, et al. Carbon quantum dots of ginsenoside Rb1 for application in a mouse model of intracerebral Hemorrhage. J Nanobiotechnol. 2024;22:125.10.1186/s12951-024-02368-wSuche in Google Scholar PubMed PubMed Central
[216] Chen N, He Y, Su Y, Li X, Huang Q, Wang H, et al. The cytotoxicity of cadmium-based quantum dots. Biomaterials. 2012;33:1238–44.10.1016/j.biomaterials.2011.10.070Suche in Google Scholar PubMed
[217] Renuga V, Mohan CN, Jaabir MM, Prakash PA, Navaneethan M. Synthesis and surface passivation of CuInS2/MnS/ZnS core–multishell nanocrystals, their optical, structural, and morphological characterization, and their bioimaging applications. Ind Eng Chem Res. 2018;57:15703–21.10.1021/acs.iecr.8b03482Suche in Google Scholar
[218] Sakizadeh J, Cline JP, Snyder MA, Kiely CJ, McIntosh S. Biomineralization of nanocrystalline CdS/ZnS photocatalysts via controlled surface passivation for enhanced hydrogen evolution. ACS Appl Nano Mater. 2022;5:2293–304.10.1021/acsanm.1c03997Suche in Google Scholar
[219] Kays JC, Saeboe AM, Toufanian R, Kurant DE, Dennis AM. Shell-free copper indium sulfide quantum dots induce toxicity in vitro and in vivo. Nano Lett. 2020;20:1980–91.10.1021/acs.nanolett.9b05259Suche in Google Scholar PubMed PubMed Central
[220] Zhao S, Li C, Wu C, Hu J, Zhang Z, Lei B, et al. Effects of multifunctional cerium-doped carbon dots on photosynthetic capacity and nutritional quality of lettuce. Environ Sci: Nano. 2024;11:3137–49.10.1039/D4EN00374HSuche in Google Scholar
[221] Zhang M, Zhao L, Du F, Wu Y, Cai R, Xu L, et al. Facile synthesis of cerium-doped carbon quantum dots as a highly efficient antioxidant for free radical scavenging. Nanotechnology. 2019;30:325101.10.1088/1361-6528/ab12efSuche in Google Scholar PubMed
[222] Huang X, Tang M. Research advance on cell imaging and cytotoxicity of different types of quantum dots. J Appl Toxicol. 2021;41:342–61.10.1002/jat.4083Suche in Google Scholar PubMed
[223] Jagadeeswar V, Dhinesh V, Roopan SM, Samuel EJJ. Plant extract-mediated synthesis of Ag-doped ZnO: eco-friendly nanomaterial for environmental restoration, microbial inhibition, cell toxicity, antioxidant potential, and sensing. Colloid J. 2023;85:827–45.10.1134/S1061933X23600513Suche in Google Scholar
[224] Cheng P, Kuang F, Ju G. Aescin reduces oxidative stress and provides neuroprotection in experimental traumatic spinal cord injury. Free Radic Biol Med. 2016;99:405–17.10.1016/j.freeradbiomed.2016.09.002Suche in Google Scholar PubMed
[225] Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2018;44:36–49.10.1002/biof.1399Suche in Google Scholar PubMed
[226] Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21.10.1089/neu.1995.12.1Suche in Google Scholar PubMed
[227] Bai B, Qi S, Yang K, Yu X, Jian R, Zhang T, et al. Self‐assembly of selenium‐doped carbon quantum dots as antioxidants for hepatic ischemia‐reperfusion injury management. Small. 2023;19:2300217.10.1002/smll.202300217Suche in Google Scholar PubMed
[228] Song RB, Basso DM, da Costa RC, Fisher LC, Mo X, Moore SA. Adaptation of the Basso–Beattie–Bresnahan locomotor rating scale for use in a clinical model of spinal cord injury in dogs. J Neurosci Methods. 2016;268:117–24.10.1016/j.jneumeth.2016.04.023Suche in Google Scholar PubMed PubMed Central
[229] Xie D-M, Sun C, Tu Q, Li S, Zhang Y, Mei X, et al. Modified black phosphorus quantum dots promotes spinal cord injury repair by targeting the AKT signaling pathway. J Tissue Eng. 2023;14:20417314231180033.10.1177/20417314231180033Suche in Google Scholar PubMed PubMed Central
[230] He Z, Yang Q, Li X, Wang Z, Wen S, Dong MJ, et al. Vanadium carbide quantum dots exert efficient anti‐inflammatory effects in lipopolysaccharide‐induced BV2 microglia and mice. Small Sci. 2024;4:2300334.10.1002/smsc.202300334Suche in Google Scholar PubMed PubMed Central
[231] Ren Z, Zhang R, Li Y, Li Y, Yang Z, Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 2017;40:1444–56.10.3892/ijmm.2017.3127Suche in Google Scholar PubMed PubMed Central
[232] Chiang W, Urban JM, Yanchik-Slade F, Stout A, Hammond JM, Nilsson BL, et al. Hybrid amyloid quantum dot nano-bio assemblies to probe neuroinflammatory damage. ACS Chem Neurosci. 2024;15:3124–35.10.1021/acschemneuro.4c00183Suche in Google Scholar PubMed PubMed Central
[233] Wu T, He K, Zhan Q, Ang S, Ying J, Zhang S, et al. Partial protection of N-acetylcysteine against MPA-capped CdTe quantum dot-induced neurotoxicity in rat primary cultured hippocampal neurons. Toxicol Res. 2015;4:1613–22.10.1039/C5TX00127GSuche in Google Scholar
[234] Li Q, Shen X, Xing D. Carbon quantum dots as ROS-generator and-scavenger: A comprehensive review. Dye Pigm. 2023;208:110784.10.1016/j.dyepig.2022.110784Suche in Google Scholar
[235] Innocenzi P, Stagi L. Carbon dots as oxidant-antioxidant nanomaterials, understanding the structure-properties relationship. A critical review. Nano Today. 2023;50:101837.10.1016/j.nantod.2023.101837Suche in Google Scholar
[236] Sharma A, Choi H-K, Lee H-J. Carbon dots for the treatment of inflammatory diseases: An appraisal of in vitro and in vivo studies. Oxid Med Cell Longev. 2023;2023:3076119.10.1155/2023/3076119Suche in Google Scholar PubMed PubMed Central
[237] Park J-S, Rustamov N, Roh Y-S. The roles of NFR2-regulated oxidative stress and mitochondrial quality control in chronic liver diseases. Antioxidants. 2023;12:1928.10.3390/antiox12111928Suche in Google Scholar PubMed PubMed Central
[238] Kaur M, Aran KR. Unraveling the role of Nrf2 in dopaminergic neurons: a review of oxidative stress and mitochondrial dysfunction in Parkinson’s disease. Metab Brain Dis. 2025;40:123.10.1007/s11011-025-01552-7Suche in Google Scholar PubMed
[239] Perini G, Palmieri V, Ciasca G, De Spirito M, Papi M. Unravelling the potential of graphene quantum dots in biomedicine and neuroscience. Int J Mol Sci. 2020;21:3712.10.3390/ijms21103712Suche in Google Scholar PubMed PubMed Central
[240] Batool S, Nabipour H, Ramakrishna S, Mozafari M. Nanotechnology and quantum science enabled advances in neurological medical applications: diagnostics and treatments. Med Biol Eng Comput. 2022;60:3341–56.10.1007/s11517-022-02664-3Suche in Google Scholar PubMed
[241] Selvaraj S, Chauhan A, Radhakrishnan A, Rana G, Dutta V, Batoo KM, et al. Cerium oxide nanoparticles and their polymeric composites: advancements in biomedical applications. J Inorg Organomet Polym Mater. 2024;34:1–27.10.1007/s10904-024-03263-5Suche in Google Scholar
[242] Chen Y, Pang J, Ye L, Zhang Z, Lin S, Lin N, et al. Disorders of the central nervous system: Insights from Notch and Nrf2 signaling. Biomed Pharmacother. 2023;166:115383.10.1016/j.biopha.2023.115383Suche in Google Scholar PubMed
[243] Houldsworth A. Role of oxidative stress in neurodegenerative disorders: a review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024;6:1–12.10.1093/braincomms/fcad356Suche in Google Scholar PubMed PubMed Central
[244] Li D, Bai M, Guo Z, Cui Y, Mei X, Tian H, et al. Zinc regulates microglial polarization and inflammation through IKBα after spinal cord injury and promotes neuronal repair and motor function recovery in mice. Front Pharmacol. 2025;16:1510372.10.3389/fphar.2025.1510372Suche in Google Scholar PubMed PubMed Central
[245] Fornari Laurindo L, Aparecido Dias J, Cressoni Araújo A, Torres Pomini K, Machado Galhardi C, Rucco Penteado Detregiachi C, et al. Immunological dimensions of neuroinflammation and microglial activation: exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front Immunol. 2023;14:1305933.10.3389/fimmu.2023.1305933Suche in Google Scholar PubMed PubMed Central
[246] Lukacova N, Kisucka A, Kiss Bimbova K, Bacova M, Ileninova M, Kuruc T, et al. Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int J Mol Sci. 2021;22:13577.10.3390/ijms222413577Suche in Google Scholar PubMed PubMed Central
[247] Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Ther. 2020;37:113–39.10.1007/s12325-019-01148-5Suche in Google Scholar PubMed PubMed Central
[248] Luo J. TGF-β as a key modulator of astrocyte reactivity: disease relevance and therapeutic implications. Biomedicines. 2022;10:1206.10.3390/biomedicines10051206Suche in Google Scholar PubMed PubMed Central
[249] Ellen O, Ye S, Nheu D, Dass M, Pagnin M, Ozturk E, et al. The heterogeneous multiple sclerosis lesion: How can we assess and modify a degenerating lesion? Int J Mol Sci. 2023;24:11112.10.3390/ijms241311112Suche in Google Scholar PubMed PubMed Central
[250] Farooqui AA, Farooqui AA. Therapeutic importance of curcumin in neurological disorders other than Alzheimer disease. Therapeutic potentials of curcumin for Alzheimer disease. Cham, Switzerland: Springer; 2016. p. 297–334.10.1007/978-3-319-15889-1_8Suche in Google Scholar
[251] Krsek A, Baticic L. Nanotechnology-driven therapeutic innovations in neurodegenerative disorders: a focus on Alzheimer’s and Parkinson’s disease. Future Pharmacol. 2024;4:352–79.10.3390/futurepharmacol4020020Suche in Google Scholar
[252] Yadav S, Bukke SPN, Prajapati S, Singh AP, Chettupalli AK, Nicholas B. Nanobiosensors in neurodegenerative disease diagnosis: A promising pathway for early detection. Digit Health. 2025;11:20552076251342457.10.1177/20552076251342457Suche in Google Scholar PubMed PubMed Central
[253] Hassanzadeh-Khanmiri M, Moshari A, Kheradmand R, Haghgouei T, Homaei M, Charsouei S, et al. Nanomedicine: a cost-effective and powerful platform for managing neurodegenerative diseases. Metab Brain Dis. 2025;40:142.10.1007/s11011-025-01564-3Suche in Google Scholar PubMed
[254] Bahrampour Juybari K, Rizwan K, Faramarz S, Sadeghi A, Amirkhosravi A, Hadi Nematollahi M, et al. Carbon quantum dots as multi‐purpose nanomaterial in stem cell therapy. Chem Biodivers. 2023;20:e202200721.10.1002/cbdv.202200721Suche in Google Scholar PubMed
[255] Onoshima D, Yukawa H, Baba Y. Multifunctional quantum dots-based cancer diagnostics and stem cell therapeutics for regenerative medicine. Adv Drug Deliv Rev. 2015;95:2–14.10.1016/j.addr.2015.08.004Suche in Google Scholar PubMed
[256] Thamarai P, Karishma S, Kamalesh R, Shaji A, Saravanan A, Bibi S, et al. Current advancements in nanotechnology for stem cells. Int J Surg. 2024;110:7456–76.10.1097/JS9.0000000000002082Suche in Google Scholar PubMed PubMed Central
[257] Barreto da Silva T, Dias EA, Cardoso LMF, Gama JFG, Alves LA, Henriques-Pons A. Magnetic nanostructures and stem cells for regenerative medicine, application in liver diseases. Int J Mol Sci. 2023;24:9293.10.3390/ijms24119293Suche in Google Scholar PubMed PubMed Central
[258] Tsai I-T, Sun C-K. Stem cell therapy against ischemic heart disease. Int J Mol Sci. 2024;25:3778.10.3390/ijms25073778Suche in Google Scholar PubMed PubMed Central
[259] Wang Z, Chen T, Li X, Guo B, Liu P, Zhu Z, et al. Oxygen-releasing biomaterials for regenerative medicine. J Mater Chem B. 2023;11:7300–20.10.1039/D3TB00670KSuche in Google Scholar PubMed
[260] Yi DK, Nanda SS, Kim K, Selvan ST. Recent progress in nanotechnology for stem cell differentiation, labeling, tracking and therapy. J Mater Chem B. 2017;5:9429–51.10.1039/C7TB02532GSuche in Google Scholar
[261] Fernandes F, Kotharkar P, Chakravorty A, Kowshik M, Talukdar I. Nanocarrier mediated siRNA delivery targeting stem cell differentiation. Curr Stem Cell Res Ther. 2020;15:155–72.10.2174/1574888X14666191202095041Suche in Google Scholar PubMed
[262] Ma W, Liu H-T, Long Y-T. Monitoring dopamine quinone-induced dopaminergic neurotoxicity using dopamine functionalized quantum dots. ACS Appl Mater Interfaces. 2015;7:14352–8.10.1021/acsami.5b03044Suche in Google Scholar PubMed
[263] Sanchez DNR, Bertanha M, Fernandes TD, de Lima Resende LA, Deffune E, Amorim RM. Effects of canine and murine mesenchymal stromal cell transplantation on peripheral nerve regeneration. Int J Stem Cell. 2017;10:83.10.15283/ijsc16037Suche in Google Scholar PubMed PubMed Central
[264] Agarwal R, Domowicz MS, Schwartz NB, Henry J, Medintz I, Delehanty JB, et al. Delivery and tracking of quantum dot peptide bioconjugates in an intact developing avian brain. ACS Chem Neurosci. 2015;6:494–504.10.1021/acschemneuro.5b00022Suche in Google Scholar PubMed PubMed Central
[265] Hopkins LE, Patchin ES, Chiu PL, Brandenberger C, Smiley-Jewell S, Pinkerton KE. Nose-to-brain transport of aerosolised quantum dots following acute exposure. Nanotoxicology. 2014;8:885–93.10.3109/17435390.2013.842267Suche in Google Scholar PubMed PubMed Central
[266] Shen J, Sun Y, Liu X, Chai Y, Wang C, Xu J. Nerve regeneration potential of antioxidant-modified black phosphorus quantum dots in peripheral nerve injury. ACS Nano. 2024;18:23518–36.10.1021/acsnano.4c07285Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Development and performance evaluation of green aluminium metal matrix composites reinforced with graphene nanopowder and marble dust
- Morphological, physical, thermal, and mechanical properties of carbon nanotubes reinforced arrowroot starch composites
- Influence of the graphene oxide nanosheet on tensile behavior and failure characteristics of the cement composites after high-temperature treatment
- Central composite design modeling in optimizing heat transfer rate in the dissipative and reactive dynamics of viscoplastic nanomaterials deploying Joule and heat generation aspects
- Double diffusion of nano-enhanced phase change materials in connected porous channels: A hybrid ISPH-XGBoost approach
- Synergistic impacts of Thompson–Troian slip, Stefan blowing, and nonuniform heat generation on Casson nanofluid dynamics through a porous medium
- Optimization of abrasive water jet machining parameters for basalt fiber/SiO2 nanofiller reinforced composites
- Enhancing aesthetic durability of Zisha teapots via TiO2 nanoparticle surface modification: A study on self-cleaning, antimicrobial, and mechanical properties
- Nanocellulose solution based on iron(iii) sodium tartrate complexes
- Combating multidrug-resistant infections: Gold nanoparticles–chitosan–papain-integrated dual-action nanoplatform for enhanced antibacterial activity
- Novel royal jelly-mediated green synthesis of selenium nanoparticles and their multifunctional biological activities
- Direct bandgap transition for emission in GeSn nanowires
- Synthesis of ZnO nanoparticles with different morphologies using a microwave-based method and their antimicrobial activity
- Numerical investigation of convective heat and mass transfer in a trapezoidal cavity filled with ternary hybrid nanofluid and a central obstacle
- Halloysite nanotube enhanced polyurethane nanocomposites for advanced electroinsulating applications
- Low molar mass ionic liquid’s modified carbon nanotubes and its role in PVDF crystalline stress generation
- Green synthesis of polydopamine-functionalized silver nanoparticles conjugated with Ceftazidime: in silico and experimental approach for combating antibiotic-resistant bacteria and reducing toxicity
- Evaluating the influence of graphene nano powder inclusion on mechanical, vibrational and water absorption behaviour of ramie/abaca hybrid composites
- Dynamic-behavior of Casson-type hybrid nanofluids due to a stretching sheet under the coupled impacts of boundary slip and reaction-diffusion processes
- Influence of polyvinyl alcohol on the physicochemical and self-sensing properties of nano carbon black reinforced cement mortar
- Advanced machine learning approaches for predicting compressive and flexural strength of carbon nanotube–reinforced cement composites: a comparative study and model interpretability analysis
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Advances in the design and manipulation of self-assembling peptide and protein nanostructures for biomedical applications
- Photocatalytic pervious concrete systems: from classic photocatalysis to luminescent photocatalysis
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”
Artikel in diesem Heft
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Development and performance evaluation of green aluminium metal matrix composites reinforced with graphene nanopowder and marble dust
- Morphological, physical, thermal, and mechanical properties of carbon nanotubes reinforced arrowroot starch composites
- Influence of the graphene oxide nanosheet on tensile behavior and failure characteristics of the cement composites after high-temperature treatment
- Central composite design modeling in optimizing heat transfer rate in the dissipative and reactive dynamics of viscoplastic nanomaterials deploying Joule and heat generation aspects
- Double diffusion of nano-enhanced phase change materials in connected porous channels: A hybrid ISPH-XGBoost approach
- Synergistic impacts of Thompson–Troian slip, Stefan blowing, and nonuniform heat generation on Casson nanofluid dynamics through a porous medium
- Optimization of abrasive water jet machining parameters for basalt fiber/SiO2 nanofiller reinforced composites
- Enhancing aesthetic durability of Zisha teapots via TiO2 nanoparticle surface modification: A study on self-cleaning, antimicrobial, and mechanical properties
- Nanocellulose solution based on iron(iii) sodium tartrate complexes
- Combating multidrug-resistant infections: Gold nanoparticles–chitosan–papain-integrated dual-action nanoplatform for enhanced antibacterial activity
- Novel royal jelly-mediated green synthesis of selenium nanoparticles and their multifunctional biological activities
- Direct bandgap transition for emission in GeSn nanowires
- Synthesis of ZnO nanoparticles with different morphologies using a microwave-based method and their antimicrobial activity
- Numerical investigation of convective heat and mass transfer in a trapezoidal cavity filled with ternary hybrid nanofluid and a central obstacle
- Halloysite nanotube enhanced polyurethane nanocomposites for advanced electroinsulating applications
- Low molar mass ionic liquid’s modified carbon nanotubes and its role in PVDF crystalline stress generation
- Green synthesis of polydopamine-functionalized silver nanoparticles conjugated with Ceftazidime: in silico and experimental approach for combating antibiotic-resistant bacteria and reducing toxicity
- Evaluating the influence of graphene nano powder inclusion on mechanical, vibrational and water absorption behaviour of ramie/abaca hybrid composites
- Dynamic-behavior of Casson-type hybrid nanofluids due to a stretching sheet under the coupled impacts of boundary slip and reaction-diffusion processes
- Influence of polyvinyl alcohol on the physicochemical and self-sensing properties of nano carbon black reinforced cement mortar
- Advanced machine learning approaches for predicting compressive and flexural strength of carbon nanotube–reinforced cement composites: a comparative study and model interpretability analysis
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Advances in the design and manipulation of self-assembling peptide and protein nanostructures for biomedical applications
- Photocatalytic pervious concrete systems: from classic photocatalysis to luminescent photocatalysis
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”

![Figure 3
(a) pH-dependent PL spectra of the GQDs. The inset illustrates the relationship between PL intensity and pH, ranging from 12 to 1 [102]. (b) Change in photoluminescent intensity with varied concentrations [103].](/document/doi/10.1515/ntrev-2025-0211/asset/graphic/j_ntrev-2025-0211_fig_003.jpg)
