Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
-
Natesan Thirumalaivasan
, Deepak Verma
, Ramyakrishna Pothu
, Ahmed Bahgat Radwan
Abstract
The landscape of prosthodontics and dental implantology is undergoing a transformative evolution, driven by remarkable advancements in materials science. This review explores the pivotal role of multifaceted materials, ceramics, polymers, metal alloys, and composites in revolutionizing dental restorative procedures. These materials are not only enhancing the mechanical properties and biocompatibility of dental prostheses and implants but also elevating aesthetic outcomes to meet patient expectations. Our discussion highlights how traditional materials like titanium (Ti) and cobalt-chromium (Co–Cr), alongside newer innovations such as zirconia and polymer-based composites, contribute to the restoration and enhancement of oral functions. Furthermore, this article delves into the integration of cutting-edge technologies such as 3D printing and computer-aided design/manufacturing, which synergize with these advanced materials to tailor dental solutions to individual patient needs, thereby improving both functional outcomes and patient satisfaction. As the field progresses, we anticipate future innovations to focus on increasing the sustainability of materials used, refining their properties through nanotechnology, and further personalizing dental care through digital workflows, setting a new standard in the interdisciplinary approach of modern dentistry.
Abbreviations
- 3D
-
three dimensional
- ABS
-
acrylonitrile butadiene styrene
- AP
-
adaptation process
- APW
-
assembled prosthetic works
- Au
-
gold
- CAD
-
computer-aided design
- CAL
-
clinical attachment level
- CAM
-
computer-aided manufacturing
- CM
-
collagen membrane
- Co
-
cobalt
- Co–Cr
-
cobalt–chromium
- CoM
-
casting over metal
- CPS
-
sterile saline
- Cr
-
chromium
- DC
-
direct construction
- DD
-
decontamination
- DRCs
-
dental resin composites
- ERL
-
Er:YAG laser
- NBM
-
natural bone mineral
- Ni
-
nickel
- Ni–Cr
-
nickel–chromium
- PC
-
polycarbonate
- PCL
-
poly(e-caprolactone)
- PDMS
-
polydimethylsiloxane
- PE
-
polyethylene
- PEd
-
partial edentulism
- PEEK
-
polyetheretherketone
- PEG
-
polyethylene glycol
- PGA
-
polyglycolic acid
- PLA
-
polylactic acid
- PME
-
permucosal healing element
- PMMA
-
polymethyl methacrylate
- PP
-
polypropylene
- PU
-
polyurethane
- RD
-
refractory duplicate
- RPD
-
removable partial dentures
- SiN
-
silicon nitride
- Ti
-
titanium
- Ti6Al4V
-
vanadium
- Ti–Al–V
-
titanium–aluminum–vanadium
- Ti-m
-
titanium
- Ti-p
-
etched titanium
- Y-TZP
-
tetragonal zirconia polycrystal
- ZrO2
-
zirconium dioxide
1 Introduction
Prosthodontics and dental implantology stand at the forefront of modern dentistry, offering patients innovative solutions for tooth replacement and oral rehabilitation. Over the past few decades, these fields have witnessed remarkable progress, largely propelled by advancements in materials science and technology. Among the key drivers of these advancements are the multifunctional materials that have emerged as critical components in the development of dental prostheses and implants [1,2]. This article explores innovative materials utilized in prosthodontics and dental implantology in depth, emphasizing their important role in transforming restorative dentistry. It underlines how these materials, which combine aesthetics, strength, and utility, are critical to the advancement and transformational impact of dental difficulties such as missing or broken teeth [3,4]. Continuous progress in materials science and technology contributes considerably to the creation of effective and better dental restorations and implants.
Structure of a dental implant: Dental implants differ from crowns in that they are a permanent solution, whereas crowns can be either temporary or permanent. Temporary crowns serve as placeholders until the implant has successfully integrated with the bone, after which a permanent crown is installed [5]. Dental implants are categorized based on their surgical phases, with the main types being one-stage and two-stage implants. In one-stage implants, the implant fixture is inserted in such a way that the implant’s prosthetic post protrudes into the oral cavity [6]. These implants are often supported by interimplant splints to mitigate the impact of excessive loading forces during the healing phase. After surgery, a permucosal healing element (PME) is attached to the implant body, which is typically positioned slightly above the bone crest [7]. Soft tissue is then arranged around the PME. Two-stage implants utilize a multicomponent system (osseointegration, Figure 1). The first stage involves embedding the implant body in the bone and covering it entirely with a mucoperiosteal flap. The fixture then undergoes a healing period with the bone through a process known as osseointegration [8–10]. Approximately 6 weeks later, the second stage is performed. During this stage, the implant fixture is exposed, allowing for the placement of the prosthetic component onto the implant.

Representation of dental prosthesis and implants. (a) Structure of a dental implant in comparison with a normal tooth, (b) elements of dental implant/name of the components or parts of dental implanting materials, and (c) steps involved in the dental implants.
The restoration of oral function and aesthetics following tooth loss or damage is a paramount goal in dentistry. Prosthodontics, a specialized branch of dentistry, focuses on the art and science of designing and fabricating dental prostheses that mimic the form and function of natural teeth [11]. Dental implantology, on the other hand, has revolutionized the field by offering a more permanent and stable solution for tooth replacement through the use of dental implants surgically anchored in the jawbone [12]. These remarkable advancements not only have enhanced the quality of life for countless individuals but have also opened new horizons for dental professionals. In this era of precision and patient-centric care, it is imperative to understand the dynamic interplay between materials and clinical outcomes. The foundation of this review lies in unraveling the fundamental requirements of materials used in prosthodontics and dental implantology, underscoring the nonnegotiable attributes of biocompatibility, mechanical robustness, and long-term stability [13,14,15]. As we navigate through the intricate terrain of multifunctional materials, encompassing ceramics, polymers, metals, and composite materials, their unique properties and applications will come into focus, offering insights into their suitability for various dental restorations, from crowns and bridges to dentures and implant-supported prostheses.
At the heart of these transformative developments are the materials utilized in prosthodontics and dental implantology. These materials play a multifaceted role, serving as the building blocks for dental restorations and implants, and influencing their aesthetics, durability, and functionality. The selection of appropriate materials is crucial, as it directly impacts the success of treatment outcomes, patient satisfaction, and long-term oral health [14]. Moreover, we will explore the ever-expanding realm of esthetic materials, exemplified by tooth-colored ceramics and resin-based composites [16], which play an instrumental role in achieving natural-looking dental restorations. The fusion of cutting-edge technologies, such as 3D printing and digital dentistry, with these multifaceted materials promises to redefine the boundaries of customization in dental prostheses and implants [17,18].
The demand for dental prostheses and implants that seamlessly blend with the natural dentition has propelled extensive research and innovation in materials science. Today, a plethora of materials, ranging from traditional metals and ceramics to cutting-edge polymers and composites, offer a wide array of options for clinicians and patients alike. These materials are meticulously engineered to exhibit properties such as biocompatibility, mechanical strength, esthetic appeal, and resistance to wear and degradation [19,20]. Moreover, we will explore the ever-expanding realm of esthetic materials, exemplified by tooth-colored ceramics and resin-based composites, which play an instrumental role in achieving natural-looking dental restorations. The fusion of cutting-edge technologies, such as 3D printing and digital dentistry, with these multifaceted materials promises to redefine the boundaries of customization in dental prostheses and implants. In this review article, we embark on a journey to explore the multifunctional materials that have transformed the landscape of prosthodontics and dental implantology. We will delve into the fundamental requirements of these materials, examine their unique properties, and elucidate their applications in various dental scenarios, including crowns, bridges, dentures, and implant-supported restorations. Furthermore, we will investigate the evolving role of emerging technologies, such as 3D printing and digital dentistry, in conjunction with these materials, and assess their impact on the field. As we navigate this multifaceted world of dental materials, we will also delve into the biomechanical aspects, emphasizing the importance of mechanical properties and load-bearing capabilities, as well as the role of bioactive and antibacterial materials in promoting the successful integration of implants and preventing complications [21,22].
Ultimately, this review article aims to underscore the indispensable role that multifunctional materials play in elevating the domains of prosthodontics and dental implantology. Through their integration with innovative techniques and technologies, these materials have redefined the standards of dental restorations and implant procedures, offering patients more durable, esthetically pleasing, and functional solutions for tooth replacement and oral rehabilitation [23,24]. In this rapidly evolving landscape, a deeper understanding of these materials becomes essential for dental professionals striving to provide the highest level of care and satisfaction to their patients. Our journey will not only traverse the aesthetic aspects but also delve into the biomechanical considerations that shape the success of dental implants and prostheses. We will illuminate the critical importance of materials’ mechanical properties, load-bearing capabilities, and resistance to fatigue, providing a comprehensive understanding of their role in the clinical context. Furthermore, we will explore the fascinating world of bioactive and antibacterial materials, uncovering their role in promoting osseointegration and safeguarding against peri-implantitis, thereby contributing to the long-term success of dental implants. In conclusion, this review article serves as an enlightening compass, guiding us through the multifaceted landscape of materials science as it converges with prosthodontics and dental implantology. The amalgamation of advanced materials, coupled with innovative techniques and technologies, has ushered in an era of heightened expectations, where patients can anticipate more enduring, aesthetically pleasing, and functionally superior solutions for tooth replacement and oral rehabilitation.
2 Historical overview
Prosthodontics and dental implantology are specialized branches of dentistry dedicated to the restoration and replacement of missing or damaged teeth [25,26,27]. Over the years, these fields have witnessed remarkable advancements in materials and techniques (Figure 2). These innovations aim to enhance patient satisfaction, improve esthetic outcomes, and bolster the mechanical properties of dental restorations and implants. As a result, a new category of materials, known as multifunctional materials, has emerged. These materials are specifically designed for use in prosthodontics and dental implantology, offering a diverse range of properties and functions to cater to the complex needs of patients in these disciplines. This review explores the historical development and current state of multifunctional materials in prosthodontics and dental implantology, shedding light on their pivotal role in modern dental practice.
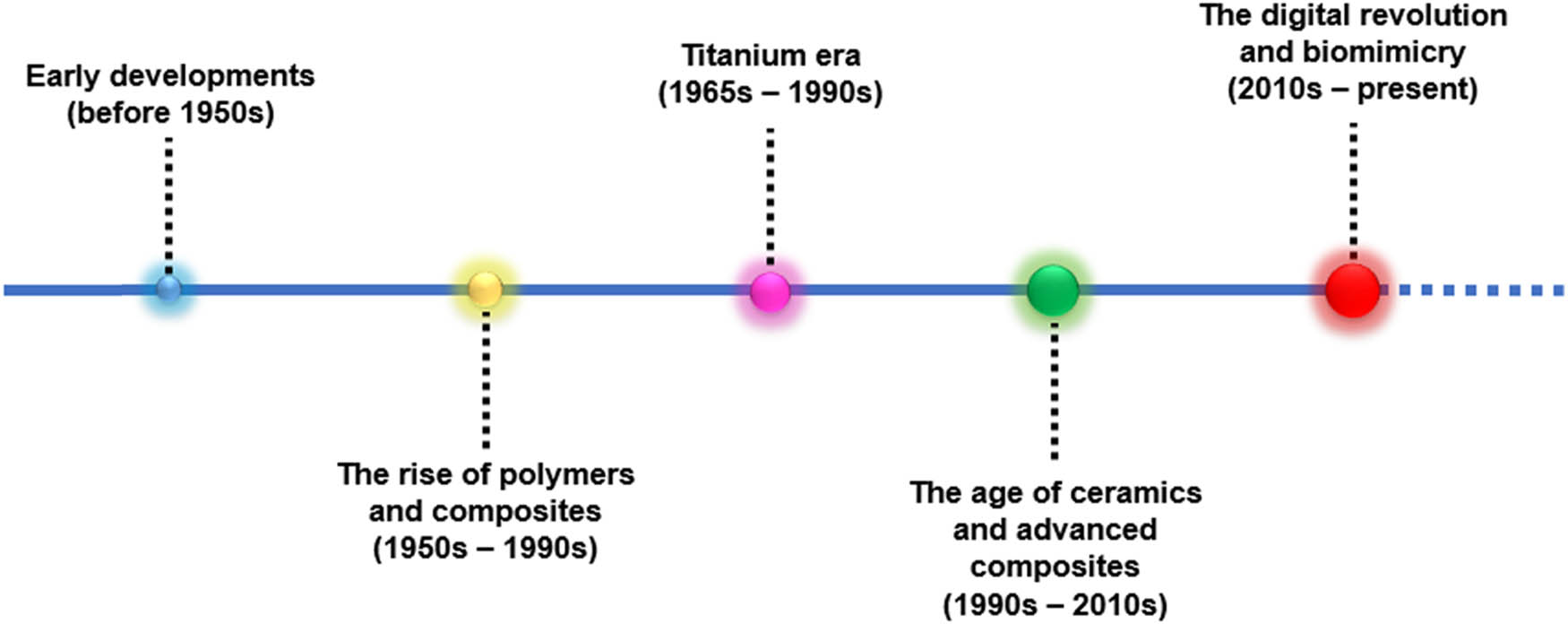
Various materials employed in dental prosthesis and implant techniques over the years.
2.1 Early developments (before 1950s)
In ancient Egypt around 1500 BCE, dental prosthetics were crafted using gold (Au) wires that were affixed to teeth. Occasionally, teeth from human donors were utilized as replacements for missing ones. Fast forward to Ancient Rome in the first century CE, Au continued to be a popular material for dental works, especially for crowns and bridges. Interestingly, the Etruscans of that era employed a mix of animal teeth, bone, and Au to create dental bridges. Au, due to its malleability, resistance to corrosion, and biocompatibility, was one of the earliest materials used in the realm of dentistry [28]. However, despite these beneficial properties, its premium cost and limited strength rendered it less suitable for certain dental applications. By the eighteenth century, porcelain emerged as a choice material, offering esthetically pleasing results for crowns and other fixed prostheses. Nevertheless, its brittleness posed challenges in its application [29].
2.2 Rise of polymers and composites (1950s–1980s)
In the seventeenth century, dentures saw the introduction of materials like ivory and, subsequently, porcelain for their fabrication. However, these materials were not optimal, presenting aesthetic shortcomings along with issues of fit and retention. The following century, the eighteenth century, witnessed the evolution of denture technology with the advent of the first porcelain dentures, which were intricately held together using Au wires or springs. By the nineteenth century, a significant shift in materials occurred when vulcanite rubber, an early variant of hard rubber, emerged [30]. This material quickly gained preference over ivory for denture bases, mainly due to its enhanced comfort and cost-effectiveness. The mid-twentieth century brought another advancement with the introduction of polymethyl methacrylate (PMMA) during the 1940s and 1950s [31]. PMMA rapidly became the preferred material for dentures, given its easy processing and satisfactory aesthetic qualities. Moreover, by the late 1950s and into the 1960s, composite resins, consisting of organic polymer matrices combined with inorganic fillers, grew in popularity [32]. This was attributable to their superior mechanical properties and enhanced esthetic appeal.
2.3 Titanium (Ti) era (1965–1990s)
In the seventeenth century through the early 1900s, metals like cobalt–chromium (Co–Cr) alloys gained popularity for the fabrication of removable partial dentures (RPD). However, the landscape of dentistry underwent a significant transformation in the 1950s when Per-Ingvar Brånemark introduced Ti [33]. Renowned for its biocompatible properties, Ti set a new benchmark in dental implants, heralding a revolution in implantology. Further advancements were seen in the late twentieth century with the introduction of ceramics, such as zirconia and alumina. These materials offered an optimal combination of strength and aesthetics, particularly for crowns and fixed prostheses. A groundbreaking discovery that further shaped the realm of dental implantology was “osseointegration” [34,35]. This term, coined by Per-Ingvar Brånemark in the 1960s, refers to the bone’s unique ability to bond directly with Ti without forming a fibrous layer [35]. Owing to its exceptional biocompatibility, strength, and the proven benefits of osseointegration, Ti and its alloys swiftly became the Au standard for dental implant materials.
2.4 Age of ceramics and advanced composites (1990s–2010s)
Bioactive materials, including bioactive glasses (BAGs) and ceramics, have been introduced to bond with living tissues. These materials have the unique ability to release therapeutic ions that assist in tissue regeneration. Additionally, there has been significant advancement in the realm of polymeric materials. High-performance polymers have been developed that show improved aesthetic properties along with the increased resilience [36]. One of the key breakthroughs in ensuring the success of implants has been the introduction of surface modification techniques [37]. Processes like acid etching, sandblasting, and laser treatments have been utilized to bolster the osseointegration of implants, ensuring a tighter bond with the surrounding bone [38]. Zirconia has emerged as a prominent material in the dental industry, thanks to its natural white hue and impressive strength [39]. As an alternative to traditional metal, zirconia is now frequently used in prosthodontic frameworks and dental implants. Meanwhile, glass ceramics such as lithium disilicate (LD) have seen a surge in popularity [40]. Their unique blend of strength and esthetics makes them highly sought after. Finally, to enhance the durability and mechanical properties of resin-based materials, fiber-reinforced composites have been introduced.
2.5 Digital revolution and biomimicry (2010s–present)
In recent times, there have been notable advancements in dental technology, as highlighted in Table 1. One of the major breakthroughs in this field has been the widespread use of computer-aided design (CAD) and computer-aided manufacturing (CAM) technology, which has become increasingly common [41]. This has enabled the precise fabrication of dental prosthetics. Concurrently, there has been a surge in the application of nanotechnology in the dental field. Nanofilled and nanohybrid composites have been developed for restorations, boasting superior mechanical properties and enhanced aesthetics [42]. Another transformative innovation has been the adoption of 3D printing technologies [43]. These are now frequently employed in the creation of dental prostheses, surgical guides, and even in the bioprinting of tissues. Additionally, the development of smart materials, particularly those with the ability to change properties in response to external stimuli like shape-memory alloys, has opened up new avenues in dentistry [44]. The precision and speed of dental restorations have been further improved with the broader adoption of CAD/CAM systems, which provide a range of material options. Finally, there is a growing interest in bioactive and smart materials. The dental industry is exploring materials capable of stimulating tissue regeneration and those with self-healing properties [45]. Moreover, the integration of nanosized particles and fibers into dental materials not only boosts mechanical properties and aesthetics but also introduces potential antibacterial properties [46].
Succinct summary of various multifunctional materials commonly used in prosthodontics and dental implantology
| S. no | Materials | Applications | Limitations | Advantages | Mechanical/physical properties | Biocompatibility | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ti (titanium) | Primary material for dental implants due to its excellent biocompatibility, strength, and ability to osseointegrate | Higher cost compared to other metals | Excellent strength and biocompatibility | High tensile strength and ductility | Highly biocompatible with human tissues, rarely causes allergic reactions | [47,48,49,50,51] |
| Used in making crowns, bridges, and dentures when combined with other prosthetic materials | Can be difficult to adjust or repair once placed | High corrosion resistance | Low density | ||||
| Ability to osseointegrate well with bone | Corrosion resistant | ||||||
| Common in substructures or frameworks of prosthetic devices due to its durability and lightweight nature | |||||||
| Frequently utilized in orthodontic appliances for its nontoxic and corrosion-resistant properties | |||||||
| 2 | Co–Cr alloys | Extensively used in making RPD due to their high corrosion resistance and mechanical strength | Potential for allergic reactions due to nickel content (in some alloys) | High mechanical strength | High elastic modulus | Generally biocompatible, but some individuals may react to nickel content | [52,53,54,55,56] |
| Excellent wear resistance | Good fatigue strength | ||||||
| Utilized for framework fabrication in fixed and removable prostheses | More difficult to polish than some other materials | Cost-effective compared to other high-end materials like titanium | Corrosion resistant | ||||
| Sometimes employed in full-cast crowns and bridges for their excellent wear resistance | |||||||
| Preferred in cases where strong, thin frameworks are needed, maximizing patient comfort | |||||||
| 3 | Ni–Cr alloy | Commonly used in fixed prosthodontics, particularly for porcelain-fused-to-metal crowns and bridges | Contains nickel, which can cause allergic reactions | Cost-effective | Moderate strength | Less biocompatible compared to titanium and Co–Cr alloys, potential for allergic reactions due to nickel | [57,58] |
| Good mechanical properties | High thermal conductivity | ||||||
| Valued for their thermal expansion compatibility with ceramics | Lower corrosion resistance compared to titanium and cobalt–chromium alloys | Thermal expansion compatibility with ceramics | Good resistance to thermal and mechanical fatigue | ||||
| 4 | Ceramics | Widely utilized for crowns, bridges, veneers, and inlays due to their aesthetic appeal and biocompatibility | Brittle nature can lead to fractures | Excellent aesthetic qualities | High hardness | Excellent biocompatibility, minimal risk of allergic reactions | [59,60] |
| Employed in prosthetics for their resemblance to natural tooth enamel | Higher cost than some metal alloys | High Biocompatibility | Low thermal conductivity | ||||
| Used in all-ceramic restorations for their translucency and color stability | Technique sensitive, requiring precise handling | Do not corrode | Good wear resistance | ||||
| 5 | Zirconia and alumina ceramics | Zirconia is popular for its high strength and aesthetic quality, making it suitable for crowns, bridges, and implant abutments | Higher cost than traditional ceramics | Exceptional strength and durability | Zirconia: High fracture toughness and flexural strength | Very high biocompatibility with negligible inflammatory response | [61,62] |
| Alumina ceramics are used for their translucency and strength, though less frequently than zirconia | Zirconia may require more tooth reduction during preparation | High aesthetic appeal with excellent color matching | Alumina: High hardness and stiffness | ||||
| Both materials are utilized for their excellent wear resistance and minimal abrasiveness to opposing natural teeth | Low wear to opposing teeth | ||||||
| 6 | Polymers | Employed in the fabrication of dentures, particularly the denture bases, due to their adaptability and comfort | Less durable than metal or ceramic materials, can wear out faster | Variable; generally lower strength compared to ceramics or metals but improved with reinforcement | Generally biocompatible; specifics depend on the polymer type | Denture bases, temporary prosthetics, orthodontic appliances | [63,64,65,66,67] |
| Used in temporary prosthetics and as part of certain orthodontic appliances | |||||||
| Recent advancements include reinforced polymers for increased strength in load bearing |
2.6 Future outlook and challenges
In terms of the future outlook and challenges in the field, there is a significant moment toward advancements in research. The primary aim is to develop materials that excel in biocompatibility, aesthetics, durability, and multifunctionality. The incorporation of cutting-edge smart materials, combined with the latest biotechnologies and AI-driven diagnostic and design tools, promises to usher in a new era of personalized and effective dental treatments. The field of prosthodontics and dental implantology has experienced a remarkable evolution, transitioning from the early days when Au and porcelain were predominantly used to the modern era dominated by smart materials, nanotechnology, and 3D printing. This journey underscores the relentless drive for innovation aimed at enhancing patient care. As material science and biotechnology push their boundaries further, the future is set to witness even more ground-breaking advancements. This progress, marked by the shift from materials like Au and ivory to contemporary smart materials, highlights the interdisciplinary nature of research that merges dentistry with material science. Such a fusion is paving the way for exhilarating innovations that will undoubtedly reshape patient-centric care in the coming years.
3 Material categories
Various types of materials have been utilized for dental implants, including metallic and metallic-alloy, ceramics, composites, and polymeric materials, among others. In the initial stages, metallic implants were predominantly used, as indicated in Figure 3.

Materials employed in dental prosthesis and implants.
3.1 Metal- and metallic alloy-based implants
3.1.1 Ti and its alloys in dental applications
In the ever-evolving realm of dentistry, materials play an indispensable role in determining the success of treatments, especially in restorative procedures and dental implants. Two significant materials, namely, Ti and its alloys, and Co–Cr alloys, have historically marked their territory in this domain due to their unparalleled properties. This article seeks to provide a brief overview of these materials, emphasizing their advantages, limitations, and potential future directions in dental applications. The multifaceted world of dentistry is perpetually in flux, with continuous advancements and innovations driving it forward. Central to this progression is the role of materials, serving as the very foundation upon which dental procedures are built. Their choice directly influences the outcomes, longevity, and overall efficacy of treatments, especially when it comes to restorative procedures and dental implants. Delving into this expansive material landscape, two metallic substances distinctly stand out due to their unique attributes: Ti and its alloys, along with Co–Cr alloys. This exposition offers a succinct insight into these materials, delineating their virtues, inherent challenges, and their potential trajectory in future dental applications.
3.1.1.1 Ti poisoning
Titanium implants are highly favored in dental applications due to their superior mechanical attributes and exceptional biocompatibility. However, there is a concern regarding the propensity of these implants to release titanium ions, which could pose localized and systemic risks. This ion emission has the potential to precipitate implant failure or exert deleterious effects on human physiological systems. It also discusses the possible health risks – such as inflammation, allergic reactions, and neurological disorders – that could result from titanium poisoning [68]. Challenges associated with titanium-based dental implants may stem from the release of titanium and titanium alloy particulates and ions into adjacent tissues due to corrosion and wear of the implant. This release can provoke inflammatory responses, culminating in osteolysis and potentially leading to the failure of the implant integration. Systemic deposition of these titanium ions and particles can incite adverse responses in distal tissues, exemplified by conditions such as yellow nail syndrome. In addition, hypersensitivity reactions may precipitate allergies and compromise implant functionality [69].
Titanium dioxide nanoparticles (TiO2 NPs) have been associated with cognitive deficits, evidenced by diminished expression of key blood–brain barrier transcripts in hippocampal tissues extracted from subjects following exposure protocols. This reduction corroborates with the paracellular permeability observed in our in vitro models. Entry of titanium particles into brain-mimetic endothelial cells is predominantly facilitated through caveolae-mediated endocytosis and macropinocytosis, instigating a pronounced proinflammatory cascade characterized by the upregulation of proinflammatory cytokines and proteins. This immune activation is partly regulated via the interleukin-1 receptor and interleukin-6 pathways. Moreover, elevated titanium concentrations have been detected in human tissues proximal to orthopedic implants, with both in vivo and in vitro analyses underscoring the potential neurotoxic implications of titanium exposure [70]. Titanium should not be thought of as an inert material because it can result in allergic reactions and is a potential cause of implant failure [71].
3.1.1.2 Mechanism of titanium toxicity
Titanium ions and titanium nanoparticles generate reactive oxygen species, which create cellular damage and inflammation surrounding tissues [72].
Titanium particles and ions can disrupt cellular signaling pathways, impairing cell growth and differentiation. This can cause cellular changes and contribute to the development of toxicity [73].
Increased concentrations of titanium ions and particles can cause necrosis or apoptosis in cells, which can cause direct damage and the release of proinflammatory mediators may result from this, leading to the inflammation and toxicity [74].
Ti and its alloys have revolutionized the field of dental applications, embodying the epitome of biocompatibility, strength, and resilience necessary for oral rehabilitations and prosthetics. Ti’s inherent properties, such as corrosion resistance and an affinity for osseointegration, make it an invaluable material in dentistry, primarily utilized in dental implants and prosthetic superstructures [75,76]. The light weight and remarkable biocompatibility of Ti allow for seamless integration into the bone structures, fostering stable and long-lasting dental restorations [77]. Various alloys of Ti have been developed and optimized to enhance specific mechanical properties, thus expanding the spectrum of dental applications. These alloys often amalgamate elements such as aluminum and vanadium, which bestow enhanced strength and durability, making them apt for the meticulous demands of the oral environment [78,79].
The adaptation of Ti and its alloys in dentistry is perpetually evolving, driven by incessant research and technological advancements. Innovative surface modifications and treatment processes have been introduced to improve the biological and mechanical interface between the dental implants and the surrounding bone, thus promoting better clinical outcomes [80]. The versatility of Ti allows it to be manipulated through contemporary fabrication techniques such as CAD/CAM, enabling precise customization and improving the overall efficacy and aesthetics of dental restorations [81]. Comprehensive studies and clinical trials underscore the significance of Ti in rendering reliable and effective solutions in restorative and implant dentistry. By capitalizing on Ti’s unique attributes and continual technological innovation, dental professionals can leverage this exceptional material to pave the way for advanced, sustainable, and patient-specific dental applications.
The role of Ti is indispensable in navigating the challenges encountered in dental rehabilitations, as it facilitates the confluence of functionality, durability, and biological harmony. In essence, Ti and its alloys have become the cornerstone in the realm of dental materials, holding a pivotal role in the pursuit of excellence in oral health and rehabilitation [82]. The literature vividly portrays the pivotal role of Ti, delineating its journey from being a novel material to an integral component in the odyssey of dental advancements. In conclusion, Ti and its alloys manifest as quintessential materials, shaping the future trajectory of innovative, resilient, and biocompatible solutions in dental applications.
3.1.2 Ti alloys: The Au standard in dental implants
Ti and its alloys have unequivocally become the Au standard in the realm of dental implants, revolutionizing the approach to mitigating tooth loss and revitalizing oral aesthetics and function. The inception of Ti as the cornerstone material for dental implants can be traced back to its exemplary biocompatibility, mechanical strength, and resistance to corrosion, characteristics that are instrumental in the realms of durability and long-term success. Diving into the heart of Ti’s biocompatibility, it becomes evident that this metallic element fosters an intimate relationship with human biology, particularly the bone tissue [83]. Ti implants remarkably encourage osseointegration, a natural process where the bone intimately binds with the implanted material, cultivating a robust foundation for the artificial tooth. This facilitates a symbiotic relationship where the implant not only replaces the lost tooth but also promotes the preservation and health of the jawbone, forestalling bone resorption and maintaining the structural integrity of the facial features.
Mechanical prowess is another accolade in Ti’s repository of attributes. The inherent strength and resilience of Ti and its alloys mimic the natural tenacity of a tooth root, ensuring that the implant can withstand the vicissitudes of masticatory pressures and diverse oral functionalities. This mechanical integrity is harmonized with a relative lightness, ensuring that the implant does not become a burdensome presence in the oral cavity but rather integrates seamlessly in terms of function and feel [84]. Corrosion resistance further elevates Ti’s stature in dental implantology. In the intricate environment of the mouth, where variables such as saliva, pH levels, and various biochemical interactions are constantly in flux, Ti demonstrates remarkable resilience. Its immunity to corrosive influences ensures that the integrity of the implant is steadfastly maintained, safeguarding both the aesthetics and the functional viability of the implanted structure over substantial periods.
3.1.2.1 Titanium–zirconium alloy (Straumann Roxolid)
Higher elongation and fatigue strength are two of the mechanical properties of titanium zirconium alloys containing 13–17% zirconium (TiZr1317) compared to pure titanium. Titanium and zirconium do not inhibit the growth of osteoblasts, which are necessary for osseointegration [85]. To meet the demands of dental implantologists, Straumann created Roxolid, which is 50% stronger than pure titanium [86]. The surface of TiZr1317 with a monophasic structure is topographically identical to that of pure titanium implants after sandblasting and acid etching [87]. Due to its exceptional mechanical qualities. Due to TiZr1317’s superior mechanical qualities, thin implants and implant components that can withstand high strains can be created, provided that the material exhibits a satisfactory level of biocompatibility comparable to that of pure titanium [88].
3.1.2.2 Titanium–aluminum–vanadium (Ti–Al–V)
Titanium forms alloys with various elements such as silver (Ag), aluminum (Al), argon (Ar), copper (Cu), iron (Fe), uranium (Ur), vanadium (V), and zinc (Zn). These alloys typically exist in three forms: alpha, beta, and alpha–beta (α-β) [89]. The specified morphologies are synthesized through the thermal treatment of pristine titanium in the presence of defined molarities of elements such as aluminum (Al) and vanadium (V), followed by the controlled cooling processes. These dopants act as phase-condition stabilizers. Specifically, aluminum is efficacious in stabilizing the alpha-phase microstructure of the alloy, concurrently augmenting its mechanical robustness while diminishing its overall mass. Conversely, vanadium serves to stabilize the beta-phase matrix. Incorporating either Al or V into the titanium matrix modifies the thermal boundaries at which the alpha to beta phase transformation occurs, thus facilitating the simultaneous presence of both phases within this modified thermal spectrum [90]. Desired forms can be obtained by quenching the alloy at room temperature. Heat treatment may be applied to increase the strength of these alloys. The most common alloys used for dental implants are of the alpha–beta variety, with the most common composition containing 6% Al and 4% V (Ti6Al4V).
Discussing the symphony of alloys in concert with titanium refines our understanding. Alloys such as Ti–Al–Va enhance the holistic performance of the implants. These concoctions of metallic synergy improve upon the already formidable characteristics of Ti, fine-tuning the balance between strength and weight and optimizing the adaptability and compatibility of the implants with the biological milieu. Exploring the innovative horizon, advancements in surface modifications and coatings of Ti implants further perpetuate their supremacy. Techniques such as sandblasting and acid etching enhance the surface topography of the implants, promoting improved cellular responses and accelerated osseointegration. Additionally, the exploration into nanotechnological enhancements and ceramic coatings broadens the spectrum of Ti’s applicability and performance, promising an evolving trajectory of excellence and adaptation to diverse patient needs and clinical scenarios.
Herrmann et al. investigated the microbial dynamics of commonly used implant/abutment materials at various biofilm maturity stages [91]. It highlights the complex relationship between surface texture and bacterial attachment. Examining a range of materials, from sand-blasted, acid-etched Ti to ZrO2 abutments, the study employs sophisticated methods such as reverse transcription-quantitative polymerase chain reaction and microarrays for an in-depth bacterial analysis. Interestingly, even though surface texture is a key factor in bacterial colonization, ZrO2 abutments, having a medium roughness, showed the most significant bacterial presence. This suggests other inherent material characteristics may impact bacterial growth. The consistent detection of periodontopathogens on every material, regardless of the observation period, emphasizes the importance of thorough postsurgical care. While the findings are informative, the study’s restricted sample size and its deviation from the clinical outcomes of bacterial colonization indicate the need for broader and varied future research.
These specimens were deliberately aligned at a precise, minimal distance, oriented toward the lingual surface of the incisors (highlighted by yellow arrows), to ensure the uninterrupted passage of saliva and to prevent the displacement of microbial biofilms. The schematic illustrates the strategic placement of materials as follows: zirconium dioxide (ZrO2) is allocated in the mesioincisal quadrant; pure, sand-blasted, acid-etched titanium (Ti-p) is deployed in the distoincisal quadrant; an alloy of titanium, 6% aluminum, and 4% vanadium with 1% trace elements (Ti6Al4V) is situated in the distocervical quadrant; and pure, mechanically processed titanium (Ti-m) is positioned in the mesiocervical quadrant.
Treatment of peri-implantitis was the subject of research by Schwarz et al. that compared two methods of surface debridement and decontamination [92]. This study compared the efficacy of an Er:YAG laser (ERL) with a more traditional method involving plastic curets, cotton pellets, and sterile saline (CPS). The comprehensive treatment plan included flap surgery, removal of granulation tissue, and implantoplasty, targeting both supra- and intra-bony defects. After a 6-month period, both the ERL and CPS methods [93] showed no substantial difference in their impact, achieving similar levels of bone fill as observed in radiographs. However, given the study’s short-term follow-up and the loss of two participants from a total of 32, the results require careful interpretation. While there were measurable differences in bleeding on probing and clinical attachment level values [94], these differences were not significant enough to favor one method over the other clinically. Consequently, the results mildly suggest a preference for the CPS method, considering its simplicity and cost-effectiveness, alongside comparable effectiveness to the ERL approach. Future studies with longer follow-up periods and larger sample sizes are necessary to gain more definitive insights.
The effectiveness of dental implants largely depends on their ability to integrate with bone (osseointegration) and their mechanical strength. The design details of implants, particularly their surface texture, play a key role in determining clinical success. Studies show that both commercially pure titanium and zirconia perform well yet implants with a roughened titanium surface generally achieve better osseointegration and greater longevity. On the other hand, zirconia implants are more prone to crack propagation, which can affect their resistance to wear and tear. In clinical settings where there are significant mechanical demands, rough titanium implants are often preferred because of their superior integration with bone. Despite this, zirconia implants still offer significant aesthetic benefits [95]. This research highlights the advantages of rough titanium implants, providing crucial insights for dental professionals and industry stakeholders.
In the broader schema of patient experiences and outcomes, titanium implants embody a narrative of reliability and quality of life improvement. The confidence instilled by a durable and naturally integrated implant enhances the psychological and social facets of the patient’s life, manifesting improvements that transcend the boundaries of oral health. Furthermore, the adaptability of titanium allows for a versatile range of applications, accommodating a multitude of dental rehabilitation needs, from single tooth replacements to comprehensive full-arch restorations. The environmental consciousness encapsulated in the choice of titanium and its alloys also reverberates with significance [96]. The sustainability of these materials, in terms of their long-lasting nature, minimizes the ecological footprint, embodying a conscientious approach to dental healthcare solutions. This echo of responsibility augments the ethical dimensions of titanium’s application in dental implants, reinforcing its position as an Au standard that resonates not only with technological prowess but also with a harmonious ethos of care, longevity, and respect for broader ecological considerations. In conclusion, titanium and its alloys have meticulously sculpted their legacy as the pinnacle in dental implant materials. Their orchestration of biocompatibility, mechanical strength, and corrosion resistance, along with a continual symphony of innovation and ethical resonance, underscores a narrative of excellence. This narrative is vibrantly alive, adapting and flourishing with each nuance of scientific advancement and each echo of human experience, reinforcing titanium’s unwavering stature as the Au standard in dental implants.
3.1.3 Co–Cr alloys: bridging strength and aesthetics
Co–Cr alloys have ascended to prominence within the dental sciences sector, serving as pivotal materials that harmonize robust mechanical attributes with aesthetic qualities. Historically, a diverse array of metals and alloys has been utilized for the fabrication of dental prostheses, including crowns and bridges, designed to mimic the functional and visual characteristics of natural dentition. The pursuit of an archetypal dental alloy, characterized by its exemplary mechanical strength, heightened biocompatibility, and superior aesthetic integration, has catalyzed the adoption and advancement of Co–Cr alloys in dental applications [97]. The selection of materials for dental prosthetics is paramount, as they must withstand the dynamically harsh conditions of the oral milieu, characterized by fluctuating temperatures, varying pH levels, and the substantial mechanical loads imparted during mastication. Co–Cr alloys are particularly notable in these rigorous environments due to their exceptional mechanical robustness and superior resistance to both wear and corrosion. Cobalt, an inherently strong and durable element, synergistically combines with chromium to forge alloys that manifest outstanding hardness and resilience. These properties are critical in coping with the considerable pressures and forces encountered within the oral cavity. Consequently, the enhanced mechanical stability of Co–Cr alloys contributes significantly to the longevity and reliability of dental restorations, rendering them an efficacious solution for dental prosthetic applications [98].
Aesthetics is another paramount concern in dental prosthetics [99]. People seek dental restorations that not only restore functionality but also blend seamlessly with their natural teeth, enhancing their appearance and confidence. Co–Cr alloys cater to this need by allowing for the creation of thin yet robust dental appliances, offering a more refined and less bulky appearance. The ability to fabricate thinner restorations also facilitates better gum health and easier cleaning, contributing to the overall aesthetic appeal and maintenance of the dental work. Moreover, Co–Cr alloys exhibit excellent biocompatibility, ensuring that they interact favorably with the biological tissues within the oral cavity. The absence of elements like nickel (Ni) [100], which could cause allergic reactions in some individuals, makes Co–Cr alloys safer and more comfortable for a broader spectrum of patients. This biocompatibility contributes to the overall success and acceptance of Co–Cr-based dental restorations, ensuring that patients can wear them with comfort and confidence over extended periods.
In Figure 4, oral rehabilitation for partial edentulism (PEd) involves the use of RPD or assembled prosthetic works (APWs). APWs consist of fixed (Pa) and removable (Pb) components, enhancing the ease of cleaning and chewing [101]. Typically, these prosthetics are crafted from Co–Cr alloys. To maintain research consistency, the design of the Pa framework was standardized across five Co–Cr alloys (0-A, 5-A, 10-A, 15-A, 16.4-A). The study examined three fabrication methods: refractory duplicate (RD, Pb), direct construction (DC, Pb−), and casting over metal (CoM, Pb+). Notably, the CoM method eliminated the need for Pa-Pb+ alignment and the adaptation process, resulting in a 91.7% improvement in component joining precision over RD and an 80.62% improvement over DC. The precision efficiency ranking of these methods is as follows: CoM, DC, and RD.
![Figure 4
Diagram of the transition from framework P (individual framework) to the series production of Pa frameworks by using the Ma framework wax model. Reproduced from the study of Uriciuc et al. [101], published by MDPI, 2021.](/document/doi/10.1515/ntrev-2025-0140/asset/graphic/j_ntrev-2025-0140_fig_004.jpg)
Diagram of the transition from framework P (individual framework) to the series production of Pa frameworks by using the Ma framework wax model. Reproduced from the study of Uriciuc et al. [101], published by MDPI, 2021.
Technological innovations have significantly contributed to the heightened utility of Co–Cr alloys within dental contexts. Contemporary fabrication methodologies, such as CAD/CAM, have transformed the production landscape for dental prosthetics, facilitating unprecedented precision and customization capabilities. These advancements permit the synthesis of Co–Cr alloy-based prosthetics that boast complex geometries and superior fit and finish, thereby enhancing both their aesthetic and functional attributes. In summation, Co–Cr alloys embody the requisite characteristics for advanced dental prosthetics, adeptly harmonizing robust mechanical properties with aesthetic finesse. Their outstanding durability, biocompatibility, and seamless integration with cutting-edge manufacturing technologies render them an essential component in the progressive development of dental restoration materials. Consequently, Co–Cr alloys epitomize the synthesis of scientific rigor and artistic precision in dental applications, marking a transformative phase in the materials and methodologies employed in dental prosthetics.
3.1.4 Co–Cr alloys in dental prosthetics
Co–Cr alloys have become indispensable in the field of dental prosthetics, merging functionality with biocompatibility to meet the diverse needs of patients and dental professionals alike. This composition of metals, primarily involving cobalt (Co) and chromium (Cr), is skillfully manipulated to create a variety of dental prosthetics, such as crowns, bridges, and partial dentures, due to their commendable mechanical properties, corrosion resistance, and affordability. Initially, the core tenet behind the utilization of Co–Cr alloys resides in their physical and mechanical properties. They possess a significant level of hardness and strength, which is instrumental in bearing the substantial masticatory forces exerted during chewing and biting. These alloys are resilient and offer exceptional durability and wear resistance, thereby ensuring the longevity of dental restorations. This is particularly crucial in the high-load bearing areas of the oral cavity, where the prosthetic materials are subjected to continuous stress and strain [102].
Co–Cr alloys are distinguished by their superior biocompatibility, an essential characteristic of materials used in dental prosthetics. They demonstrate exceptional affinity with the biological tissues of the oral cavity, ensuring minimal adverse biologic interactions and optimal tissue integration. The inherent corrosion resistance of Co–Cr alloys enhances their biocompatibility by reducing the release of metallic ions into the oral environment, which could potentially trigger toxic responses or hypersensitivity in susceptible individuals. These attributes render Co–Cr alloys a reliable choice for promoting oral health and safeguarding the integrity of dental tissues adjacent to prosthetic devices. Moreover, Co–Cr alloys excel in the aesthetic domain, a critical aspect of dental prosthetics. They facilitate the fabrication of slender, intricately detailed prosthetic structures that preserve a low profile while effectively restoring both the function and aesthetics of teeth. Furthermore, Co–Cr alloys can be skillfully employed as a framework over which ceramic or other visually appealing materials may be layered, thereby merging structural strength with aesthetic appeal. This combination ensures that the prosthetics not only meet functional requirements but also blend seamlessly with the natural dentition, enhancing the overall visual harmony.
In Figure 5, Co–Cr alloys, known for their robust mechanical properties, have transitioned from traditional casting to newer techniques like milling, laser melting, and presintered milling for dental and implant constructions [103]. This study explores these alloys’ hardness, yield strength, elastic modulus, and microstructure, categorized by manufacturing method, and examines the influence of heat treatment. Five Co–Cr alloys in various shapes are evaluated: cast, milled, laser melted, and presintered milled. Comparison is made with pure Ti grade 4 and Ti–6Al–4V (vanadium) ELI. Results show that laser-melted and presintered Co–Cr alloys have the highest mechanical properties and finer grain sizes. Ti–6Al–4V (vanadium) ELI exhibits superior hardness and yield strength over pure Ti grade 4. No significant differences are found after heat treatment. In summary, laser melting and presintered milling offer superior mechanical properties for Co–Cr alloys compared to casting and milling.
![Figure 5
The backscatter micrographs of heat-treated specimens: (a) Cast specimen with large grains, dendrites (darker gray), and white secondary phase particles (lighter gray), grain size >1 mm. (b) Milled specimen with secondary phase particles (white) along glide planes, possibly due to prior material deformation. (c) Laser melted specimens with ordered grain morphology typical for the process, grain size 20–100 mm, represented by channeling and/or strain contrast. (d) Presintered milled specimens showing many pores (black), grain size 10–100 mm, and twin boundaries with twinned grains (orange arrow) due to channeling contrast. Original magnification: ×1,000 ((a), (b), (d)), ×2,000 (c). Reproduced from the study of Kassapidou et al. [52], published by Elsevier, 2023.](/document/doi/10.1515/ntrev-2025-0140/asset/graphic/j_ntrev-2025-0140_fig_005.jpg)
The backscatter micrographs of heat-treated specimens: (a) Cast specimen with large grains, dendrites (darker gray), and white secondary phase particles (lighter gray), grain size >1 mm. (b) Milled specimen with secondary phase particles (white) along glide planes, possibly due to prior material deformation. (c) Laser melted specimens with ordered grain morphology typical for the process, grain size 20–100 mm, represented by channeling and/or strain contrast. (d) Presintered milled specimens showing many pores (black), grain size 10–100 mm, and twin boundaries with twinned grains (orange arrow) due to channeling contrast. Original magnification: ×1,000 ((a), (b), (d)), ×2,000 (c). Reproduced from the study of Kassapidou et al. [52], published by Elsevier, 2023.
Economic considerations further elevate the status of Co–Cr alloys in dental prosthetics. They offer a cost-effective alternative to other high-end materials such as Au and Ti, making dental restoration more accessible to a broader range of patients. This affordability does not compromise the quality and performance of the prosthetics, as Co–Cr alloys maintain a commendable standard of durability, functionality, and aesthetics. However, despite their numerous benefits, it is also essential to consider the challenges associated with the use of Co–Cr alloys. Allergic reactions, while rare, can still occur in some patients, necessitating careful consideration and patient history evaluation before opting for these materials [104]. In addition, the manipulation and casting of Co–Cr alloys require specialized knowledge and technical expertise, underscoring the need for dental professionals to be proficient in their use and handling to maximize their benefits and minimize potential complications.
In conclusion, Co–Cr alloys stand as a pillar in the landscape of dental prosthetics due to their multifaceted benefits ranging from exceptional mechanical properties to excellent biocompatibility. Their role in enhancing the durability, functionality, and aesthetic appeal of dental restorations is instrumental, providing a versatile and economical solution that caters to the diverse and dynamic needs of modern dentistry. Their thoughtful application, combined with a nuanced understanding of their properties and potential challenges, is crucial in leveraging their capabilities to foster optimal oral health outcomes in dental prosthetics.
3.1.5 Art of alloying: Enhancing utility with Ni–Cr alloy
The world of dental prosthetics has witnessed numerous advancements over the years, driven by a relentless quest for materials that combine strength, durability, and biocompatibility. In this intricate journey, the science of alloying emerges as an essential component. Alloying, the art of combining two or more metals to achieve enhanced properties, has led to the creation of a myriad of materials tailored to meet the diverse needs of various industries, including the medical and dental fields. Among these, the Ni–Cr alloy stands as a testament to the triumph of this science, particularly in the realm of dental prosthetics. Ni–Cr alloys are renowned for their outstanding mechanical properties, exemplary of the achievements possible through the meticulous manipulation of elements [105]. The amalgamation of Ni and Cr results in a material characterized by high tensile strength and a commendable level of hardness [106]. These attributes render it an excellent choice for dental prosthetics, where the need for a robust and durable material is not just preferred but is an absolute necessity. After all, the oral environment is a complex, dynamic system – a battlefield where materials are subjected to a variety of forces and elements, necessitating not only strength but resilience.
One of the defining characteristics of the Ni–Cr alloy is its extraordinary resistance to corrosion [107]. In the context of dental prosthetics, this is a golden attribute. The oral cavity is a highly corrosive environment, harboring a plethora of chemical substances, including acids and enzymes that can wage war against materials. Corrosion resistance is not just about maintaining the structural integrity of the prosthetic; it is intrinsically linked to the biocompatibility of the material. A corroded material can release ions into the oral environment, leading to toxic reactions and allergies [108]. Ni, when alloyed with Cr, exhibits an enhanced ability to withstand the corrosive influences of the oral environment. Cr, with its remarkable knack for forming a passive oxide layer on the surface of the alloy, acts as a shield. This invisible, yet potent, barrier is resistant to the corrosive substances found in the mouth, ensuring that the alloy remains unscathed, its structural integrity unimpaired, and its biocompatibility unchallenged.
The narrative surrounding Ni–Cr alloys in dental prosthetics encompasses a complex interplay of advanced materials science and biocompatibility issues. Nickel, recognized as a potent allergen, has elicited concerns from both dental professionals and patients. Although adverse reactions are uncommon, their occurrence necessitates a rigorous evaluation of the alloy’s use in clinical applications. This scenario epitomizes the delicate balance between leveraging the superior mechanical and corrosion-resistant properties of Ni–Cr alloys and mitigating allergenic risks, highlighting the critical role of tailored patient assessments and the relentless pursuit of alloy refinement. At the forefront of innovation within the domain of Ni–Cr alloys, research endeavors are robust, marked by a relentless drive to reduce the allergenic properties of nickel while enhancing the alloy’s intrinsic benefits. Advanced coating technologies, precision alloying techniques, and the investigation of novel substitute materials are at the cutting edge of strategies aiming to redefine Ni–Cr not merely as a viable option but as the premier choice for dental prosthetics. These scientific advancements underscore a broader commitment to optimizing material performance and patient safety in the field of dental materials science [109,110].
The evolution of Ni–Cr in dental prosthetics is a narrative of triumphant innovation, of the unyielding human spirit that seeks to conquer nature’s limitations. It is a journey characterized by the harmonious blend of art and science the art of alloying, where metals unite to form an entity greater than the sum of its parts, and the science of engineering, where every atom, every molecule, and every alloy is meticulously crafted to meet the stringent demands of the human body [111]. As we stand on the threshold of new discoveries, with technologies such as additive manufacturing and nanotechnology transforming the landscape, the story of Ni–Cr is still being written. Every challenge is an opportunity, and every limitation a stepping stone to new horizons. The art of alloying continues to evolve, not just in the quest for the perfect material but in the undying human aspiration for excellence – where science, art, and the human spirit unite to transform not just metals, but lives. In the world of dental prosthetics, Ni–Cr is not just an alloy; it is a symbol of human ingenuity, resilience, and the unyielding pursuit of perfection [111,112].
3.1.5.1 Fe–Cr–Ni-based alloys
Alloys made of stainless steel are utilized in implant and orthopedic devices. Stabilizer pins, ramus blades, ramus frames, and certain mucosal inserts are made of alloys based on iron [113]. Because nickel is a prominent constituent in this alloy, care must be taken while using it to preserve the passiviated (oxide) surface condition. The alloy is particularly susceptible to pitting corrosion. Patients with allergies should not use it. They are resistant to corrosion and have high galvanic potentials. Using titanium, cobalt, zirconium, or carbon implant biomaterials with it may cause galvanic coupling and biocorrosion.
3.1.6 Advanced coatings for enhanced biocompatibility and durability of magnesium implants
Magnesium alloys rank among the less toxic, one of the most biocompatible, and the most biodegradable biomaterials, thus making them strong candidates for use as orthopedic and dental implants. Nevertheless, their short maturation time in physiological samples questions their stability and efficiency. This is being addressed with advanced coating technologies which revolutionizes their use in medical and sports injury applications, improving their durability and functionality [114].
3.1.6.1 Innovative coating approaches
Current development concentrates on coating magnesium implants with reinforced nanocomposite nanoparticles, including magnetic nanoparticles, wollastonite, and hydroxyapatite (HAP). Moreover, this multifaceted coating approach not only protects the magnesium from premature degradation but also promotes bone cell attachment and proliferation, important for orthopedic healing and sports injury recovery. The use of the specialized combination form of these nanoparticles allows for their exploitation of tailored properties such as magnetic responsiveness, which can be advantageous for therapeutic applications [115].
The fabrication process of this membrane begins with careful and precise preparation of the magnesium alloy, and then on advanced coating such as electrophoretic deposition, plasma spraying, and soldering. The protective composites are layered systematically using these methods, which improve the interfacial bonding and crystallinity of the coatings. Fabrication to this level of precision results in robust, long-service life implants suitable for both athletic and medical applications [116].
3.1.6.2 Comprehensive characterization protocols
The advanced coatings are characterized by a combination of sophisticated techniques including electron microscopy and mechanical testing of coatings to reveal the nano-architecture and integrity of the coatings. Moreover, cytotoxicity and osteogenic assessment were performed to assess the implants’ ability to promote bone growth, without the corrosive risk, of incomparable consequences for their long-term in vivo effectiveness. The design process is exclusively dependent on finite element analysis (FEA) to simulate stress distribution and mechanical interactions between the implant and the device under physiological loads. This analysis then leads to refining implant designs to reduce common failure modes and enhance load-bearing capacity in the outline of the coating thickness and material composition [117].
At the same time, high rates of advancement in dental implantology employ FEA to find the optimal lengths and thread pitches of an implant. The validation of experimental assessments is carried out against this biomechanical modeling that feeds into design of dental implants that effectively disburse masticatory loads, providing longevity and safety of the implants. A synergistic approach combining FEA with experimental validations is beneficial to both orthopedic and dental implant designs by streamlining the development of new implants particularly designed for specific clinical needs. This approach pays off at once: it maximizes implant performance, and accelerates the path from concept to clinical, promising better patient outcomes in orthopedics and dentistry [118].
A change in the state of the art at the implant sciences level is the application of advanced coatings and innovative technologies in magnesium implant fabrication. In addition to broadening lifespans of implants, these improvements ensure integration and performance of implants in the body, establishing new milestones in the treatment and rehabilitation of patients in medical and athletic fields.
3.1.7 Aesthetic appeal through alloy fusion
In the dynamic world of dentistry, aesthetics often holds as much importance as functionality. As patients increasingly demand dental restorations that do not just serve their primary purpose but also look good, dental professionals are on a continual quest to find materials that meet both criteria. Among the myriad of options available, the fusion of Ni and Cr stands out. This alloy combination, often referred to as Ni–Cr, has proven to be both durable and aesthetically pleasing, making it a popular choice for various dental applications. The use of metals in dental restoration is not new. Au, for instance, has been used for centuries because of its durability and malleability. However, with the rising cost of Au and the demand for more cost-effective materials, dentists started exploring other metal alloys. By the twentieth century, the fusion of Ni and Cr emerged as a favorable choice. Its aesthetic silver-like appearance combined with its cost-effectiveness made it a compelling alternative to Au.
Ni–Cr alloys, commonly composed of approximately 80% nickel and 20% chromium – though variations in this ratio exist exhibit superior corrosion resistance, primarily attributable to the chromium content. Chromium contributes to the formation of a coherent oxide layer on the alloy’s surface upon exposure to oxygen. This oxide layer acts as a barrier, inhibiting oxidation and material degradation. The protective layer not only prolongs the functional lifespan of dental restorations but also preserves their aesthetic quality over extended periods. In addition, Ni–Cr alloys are noted for their high biocompatibility, a critical property in dental applications where materials are in persistent interaction with the oral environment. These materials must not elicit adverse biological responses or trigger allergic reactions. Through meticulous alloying and processing, Ni–Cr alloys minimize the likelihood of allergic responses or negative reactions in the majority of patients [119,120].
The aesthetic appeal of Ni–Cr is one of its standout features. Unlike some other metals that may discolor/decolorize or tarnish over time, Ni–Cr maintains its lustrous appearance [116]. Its natural silver-like hue closely resembles the color of natural teeth, especially when compared to the yellowish tint of Au. This makes it easier for dentists to achieve a natural look, especially when Ni–Cr is used in conjunction with porcelain or ceramic materials. Further, with advancements in dental technology, Ni–Cr can be manipulated to achieve varying degrees of translucency. This allows for even more accurate matching with the patient’s natural teeth, ensuring a seamless integration of the restoration into the oral environment.
Ni–Cr alloy finds application in various dental restorations, including crowns, bridges, and dentures. Crowns made of Ni–Cr alloy, often layered with porcelain, offer both strength and beauty. The underlying metal provides the necessary strength to withstand chewing forces, while the porcelain overlay imparts a lifelike appearance. Moreover, Ni–Cr’s ability to bond well with dental ceramics makes it an ideal choice for fixed dental bridges. These bridges are designed to replace missing teeth, and the aesthetic compatibility between Ni–Cr and ceramics ensures a natural-looking result. While Ni–Cr offers many advantages, it is essential to acknowledge its limitations. Some patients might have a Ni allergy, making it imperative for dental professionals to screen for such allergies before opting for Ni-Cr restorations. In addition, while Ni–Cr is resistant to corrosion, poor oral hygiene can still lead to issues like plaque accumulation around the restoration, which can compromise aesthetics over time.
In the domain of dental materials science, Ni–Cr alloys have emerged as a pivotal material due to their robust integration of mechanical strength, corrosion resistance, and aesthetic qualities. These properties render Ni–Cr alloys particularly advantageous for dental applications, aligning durability with visual harmony, thus garnering preference among dental professionals and patients. The intrinsic qualities of Ni–Cr, including its formidable structural integrity and excellent biocompatibility when alloyed, enable the creation of dental restorations that not only fulfill functional requisites but also aesthetically complement the patient’s dentition. Moreover, Ni–Cr alloys’ compatibility with advanced ceramic materials enhances their application in crafting prosthetic devices that are both efficacious and visually appealing. This synergy between metal and ceramic is instrumental in producing restorations that mimic the natural luster and translucency of tooth enamel, thereby elevating the overall aesthetic outcome.
In the broader context of dental technology, the strategic utilization of Ni–Cr alloys is integral to developing customized solutions that address individual patient profiles, particularly considering variability in oral biomechanics and potential metal sensitivities. Emphasizing a tailored approach in the deployment of these materials is crucial to optimizing therapeutic outcomes. Thus, the integration of Ni–Cr in dental restorative procedures not only marks a significant advancement in dental materials science but also enhances patient satisfaction through improved functional and aesthetic results, affirming its role as a transformative element in contemporary dental practice.
3.1.8 The revolution in dental aesthetics
The inclusion of Ni–Cr alloys in dentistry marked a turning point in the quest for natural-looking dental restorations. The fusion of this alloy with porcelain paved the way for dental work that was not only functional but also indistinguishable from natural teeth. As dental technologies continue to evolve, and patient expectations rise, materials like Ni–Cr will play an instrumental role in bridging the gap between functionality and aesthetics. In conclusion, the aesthetic appeal of Ni–Cr in dental applications has elevated the standard of dental restorations, offering a harmonious blend of strength, durability, and natural appearance. As dentistry continues its journey toward merging health, function, and beauty, the role of materials like Ni–Cr, with their intrinsic blend of science and art, becomes ever more critical.
3.2 Ceramics-based implants
Ceramic materials in the dental domain have emerged as a prominent solution, offering both aesthetic appeal and functional utility. Aesthetically, they mimic the natural appearance of teeth, blending seamlessly with the surrounding oral environment, thereby enhancing the patient’s smile and overall facial appearance. Functionally, they exhibit commendable strength, wear resistance, and biocompatibility, aligning with the body’s natural processes and tissues. Dental ceramics have demonstrated adaptability, catering to various applications, including crowns, bridges, veneers, inlays, and onlays [121,122]. Over the years, the evolution of dental ceramics has been influenced by advancements in material science and engineering. Traditional ceramics, although heralded for their aesthetic prowess, faced challenges regarding strength and durability. The introduction of contemporary ceramic materials, including zirconia and LD, has mitigated these challenges [123]. Zirconia, for instance, boasts remarkable strength and fracture resistance, making it a preferred choice for posterior restorations. Its ability to withstand masticatory forces without succumbing to cracks and wear is noteworthy.
LD, on the other hand, is celebrated for its optimal blend of strength and aesthetics [121]. Its translucent nature facilitates natural light transmission, bestowing a lifelike appearance to restorative works. In addition, the material’s resilient nature ensures longevity, even in environments subjected to intense mechanical forces. The process of crystallization augments its strength, rendering it a reliable solution for a myriad of dental applications. The incorporation of CAD/CAM technology in the fabrication of dental ceramics has revolutionized precision and customization. Dentists and technicians can design restorations with impeccable accuracy, ensuring a snug fit and optimal performance. The digital impressions and 3D modeling facilitate a streamlined workflow, reducing the turnaround time and enhancing patient comfort [124]. The precision intrinsic to this technology mitigates the risk of errors, ensuring that each restoration aligns with the specific contours and dimensions of the individual’s oral structure.
The biocompatibility of dental ceramics underscores their prominence in restorative dentistry. These materials exhibit an innate compatibility with the body’s tissues, reducing the risk of allergic reactions and sensitivities. Their inert nature curtails the likelihood of corrosion and degradation, promoting oral health and systemic wellness. Patients with metal sensitivities often gravitate toward ceramic restorations, finding solace in their hypoallergenic properties. Despite the accolades, dental ceramics are not immune to challenges. The material’s brittleness can be a concern, necessitating meticulous handling and installation. Advances in material science aim to augment the toughness and resilience of ceramics, integrating innovations like nano-ceramics, which amalgamate the benefits of composite materials and ceramics to offer enhanced performance.
Maintaining dental ceramics involves standard oral hygiene practices such as regular brushing, flossing, and professional cleanings, which are essential for the durability of ceramic restorations (Figure 6). The material’s inherent resistance to stains and discoloration further preserves its aesthetic quality over time. The future of dental ceramics is closely tied to continuous advancements in material science. Ongoing research into new ceramic formulations, powered by technological progress, heralds a future in which dental restorations are not only functional and visually pleasing but also enhance overall health. Innovations might include biointegration, tissue regeneration, and dynamic adaptation to the oral milieu, setting new standards for future dental ceramic materials. In summary, dental ceramics have established a significant presence in restorative dentistry through a seamless integration of beauty, functionality, and biocompatibility. Their development reflects the progress in material science and technology, with each breakthrough setting new standards for quality and performance [125]. As ongoing research opens new possibilities, dental ceramics are set to play a crucial role in the evolving field of restorative dentistry, offering solutions that go beyond repair to actively improve individual health. Ceramic-polymer hybrids combine the robustness of ceramics with the adaptability of polymers and are employed in various dental components like implant abutments and prostheses.
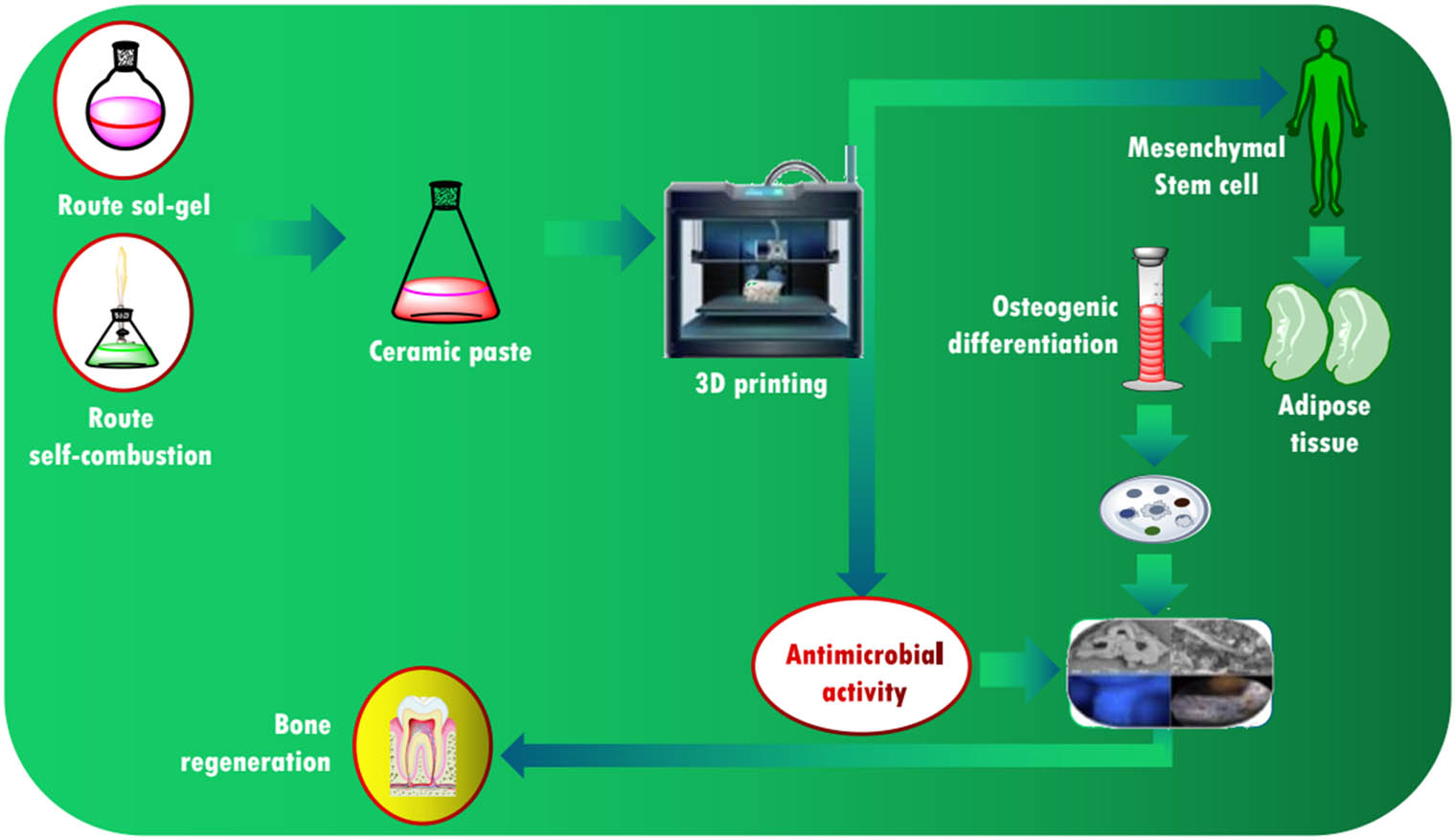
Effect of synthesis on the antimicrobial response of β-TCP/Mg with potential applications in the regeneration of dental tissue: 3D printing of ceramic paste in a β-TCP/Mg/bioglass system.
3.2.1 Zirconia and alumina ceramics
Zirconia and alumina ceramics have carved a significant niche in the dental domain, owing to their superior mechanical and aesthetic properties. Dentistry, a field that combines both the art of aesthetics and the robustness of mechanical engineering, has welcomed these materials as solutions for a myriad of applications, ranging from dental implants to crowns and bridges. Zirconia, scientifically known as zirconium dioxide (ZrO2), is renowned for its strength, durability, and biocompatibility. These characteristics make it an ideal choice for applications where strength is paramount, such as in the creation of dental implants and crowns. Zirconia’s color and translucency can be tailored to match the natural teeth, offering an aesthetic appeal that is often superior to other restorative materials.
Polymorphic zirconia structure is found in three different crystal forms: monoclinic (M), cubic (C), and tetragonal (T). At ambient temperature, zirconia takes on a monoclinic structure [126]. At 1,170°C, it transforms into a tetragonal phase, and at 2,370°C, it becomes a cubic phase. These phases are unstable at ambient temperature and fragment upon cooling. By adding CaO, MgO, and Y2O3 (Yttrium) to pure zirconia, the C-phase can be stabilized [127]. This produces a multiphase material known as partially stabilized zirconia, which combines the cubic, monoclinic, and tetragonal phases in the order of significance. Yttrium can be added to tetragonal zirconia polycrystals (TZP) at room temperature to produce a material that exclusively contains the tetragonal phase [128]. Yttria-stabilized titanium dioxide (TZP) exhibits favorable properties for biomedical applications, including low porosity, high density, strong bending, and compression strength.
Furthermore, zirconia has an edge due to its crack-resistant property and low thermal conductivity, ensuring both the comfort and safety of dental patients. The material’s ability to withstand wear and tear not only elevates its functional efficacy but also extends the longevity of the dental restorations. Enhanced by its capability to be milled to precise dimensions, zirconia has become a favorite for CAD/CAM systems in modern dentistry. On the other hand, alumina, or aluminum oxide ceramics brings their unique set of advantages to the table. Renowned for their excellent biocompatibility and hardness, alumina ceramics are resistant to wear and corrosion. Their inert nature makes them a suitable option for patients with allergies to metals, ensuring that adverse reactions are kept at bay. Although less translucent than zirconia, alumina is still capable of delivering satisfactory aesthetic outcomes, especially when high strength is a priority.
The advent of technology has further augmented the properties of these ceramic materials. Modifications and improvements in their microstructure have led to enhanced mechanical properties, including increased strength and toughness. For instance, the transformation toughened zirconia exhibits a metamorphosis in its crystal structure under stress, enhancing its toughness and resistance to crack propagation. In the realm of dentistry, the role of zirconia and alumina ceramics is not limited to their mechanical prowess. The aesthetic outcomes, a crucial aspect of dental restorations, are profoundly influenced by the optical properties of these materials. The ability to customize their color and translucency to impeccably mimic the natural dental structures has rendered these ceramics as preferred choices for restorations that are both functional and visually appealing.
Moreover, the integration of digital technology in dentistry, including intraoral scanners and CAD/CAM systems, has streamlined the utilization of zirconia and alumina. Dentists can now achieve precise fittings and optimal aesthetics with reduced chair-time, enhancing the patient experience. The digital workflow, from the diagnosis and treatment planning to the fabrication and placement of restorations, has been revolutionized, with zirconia and alumina ceramics at the forefront [129]. Their compatibility with various bonding systems, including adhesive cementation and mechanical retention, adds another feather to their cap. Zirconia, particularly, exhibits excellent bonding with resin cements, ensuring the stability and longevity of restorations. The ease of modification and adaptability of these materials have facilitated the development of restorative solutions tailored to individual patient’s needs and preferences.
Despite their myriad advantages, it is essential to consider the limitations and challenges associated with zirconia and alumina ceramics. Like any material, they are not devoid of drawbacks. Understanding these limitations, such as the potential for low-temperature degradation in zirconia and the less aesthetic appeal of alumina compared to zirconia, is pivotal for maximizing their clinical efficacy. In conclusion, zirconia and alumina ceramics have emerged as quintessential materials in contemporary dentistry. Balancing mechanical strength, biocompatibility, and aesthetics, they offer a comprehensive solution for a range of dental applications. Their evolution, propelled by technological advancements and extensive research, is a testament to the ongoing quest for excellence in delivering optimal oral health care [130]. As technology and research progress, we can anticipate further refinements and innovations in the application of zirconia and alumina ceramics in the dental domain, fostering a synergy of functionality, durability, and aesthetics.
3.2.2 Advantages of zirconia–alumina ceramics in dental domain
High strength and toughness: Zirconia, especially Y-TZP, demonstrates high strength and fracture toughness, making it suitable for load-bearing dental applications like crowns and bridges [131].
Biocompatibility: Both zirconia and alumina ceramics exhibit excellent biocompatibility, causing minimal inflammatory reactions when in contact with oral tissues.
Esthetics: Zirconia’s optical properties can be tailored to closely resemble that of natural teeth, making restorations almost indistinguishable.
Low wear: Zirconia and alumina are wear-resistant materials, which lead to less wear of the opposing natural tooth structure.
No metal infrastructure: There is no metal framework beneath, eliminating any possible metal allergies or grayish discoloration at the gum line.
Cementation flexibility: Zirconia restorations can be cemented using traditional cementation methods or adhesive bonding, providing flexibility for the dentist.
Low thermal conductivity: Zirconia and alumina have low thermal conductivity, which provides insulation against temperature changes, thus reducing tooth sensitivity.
Resistance to corrosion: Unlike metals, these ceramics resist corrosion in the oral environment, ensuring longevity and maintaining esthetics.
Digital workflow integration: Zirconia restorations can be easily integrated into digital dentistry workflows, from digital impressions to CAD/CAM fabrication.
Variety in translucency: Modern zirconia ceramics come in varying translucencies, from opaque for posterior teeth to highly translucent for anterior restorations.
3.2.3 Limitations of zirconia–alumina ceramics in dental domain
Brittleness: Like all ceramics, zirconia and alumina are inherently brittle. Though they have high strength, they lack the flexibility of metals.
Technique sensitivity: The success of zirconia restorations can be technique sensitive. Improper preparation or bonding procedures can result in restoration failure.
High stiffness: Zirconia’s stiffness, while providing strength, may lead to stress concentration and potential fracturing in certain situations.
Aging concerns: Zirconia, especially Y-TZP, may undergo low-temperature degradation over time in moist environments, which might reduce its mechanical properties [132].
Limited repairability: If a zirconia restoration chips or fractures after cementation, intraoral repair can be challenging.
Cost: The materials and technology associated with zirconia–alumina restorations tend to be more expensive than traditional materials.
Need for specialized equipment: Milling and sintering zirconia require specialized equipment, increasing setup costs for dental labs.
Wear of opposing dentition: Even though zirconia is wear resistant, if not polished adequately, it can cause wear on the opposing natural teeth.
Thickness requirement: Zirconia restorations require a certain thickness for optimal strength, which can necessitate more tooth reduction than some other materials.
Bonding challenges: Achieving a reliable bond between zirconia and the tooth or resin cements can be tricky and may require specific primers or treatments.
In conclusion, while zirconia–alumina ceramics offer several advantages in the dental domain, such as strength, esthetics, and biocompatibility, there are also limitations to consider. The choice of material should be based on the individual patient’s needs and the clinical situation.
3.3 Polymers
Polymer-based materials are integral to dentistry, offering diverse applications owing to their surface properties, mechanical strength, biocompatibility, ease of processing, and cost-effectiveness. Commonly utilized polymers in dentistry include PMMA, polyurethane, polyethylene, polycarbonate, polyetheretherketone (PEEK), polyethylene glycol, polydimethylsiloxane, polylactic acid (PLA), poly(e-caprolactone) (PCL), acrylonitrile butadiene styrene (ABS), and polypropylene. PUs (Figure 7) are widely employed in biomedical applications due to their versatility and biocompatibility. Urethane acrylate-based resins, suitable for digital light processing, exhibit excellent printability and mechanical properties, with the addition of components like poly (ethylene glycol) diacrylate and propylene glycol to adjust viscosity and mechanical attributes [133,134].

Chemical structure of polymers.
PCL (Figure 7, a biodegradable polyester known for its biocompatibility and in vivo stability, is synthesized through ring-opening polymerization of ε-caprolactone monomers. Its low melting point makes it suitable for printing techniques like fused deposition modeling (FDM) [135]. ABS (Figure 7), characterized by an amorphous structure, is derived from styrene, acrylonitrile, and polybutadiene, offering high rigidity and impact resistance [136]. Despite these qualities, PLA is preferred over ABS [135]. Techniques such as FDM and selective laser sintering are commonly used for 3D printing ABS. Modification of ABS with additives like silver nanoparticles has been explored to enhance its antibacterial properties when used in implants [136].
Polymers have become instrumental in the evolution of dental science, bringing about innovations that have not only enhanced the aesthetic aspects but also increased the functionality and durability of dental materials. Historically, dental procedures and materials were often uncomfortable, less durable, and not tailored for long-term use. However, with the advent of polymers, a renaissance in dental material science has emerged, providing solutions that are not only comfortable and aesthetically pleasing but also characterized by enhanced mechanical properties and longevity. One of the notable contributions of polymers in the dental domain is in the field of restorative dentistry. The application of polymer-based composites has revolutionized fillings, crowns, and bridges, offering a more natural appearance compared to the traditionally used amalgam or Au. These composites consist of a polymer matrix and filler particles that provide the desired mechanical and aesthetic properties. Biocompatibility is a cardinal attribute, ensuring that these materials are not reactive and are safe when implanted into the oral environment.
PEEK and PMMA are notable biocompatible polymers frequently employed in the dental domain. They have marked a significant innovation, propelling dentistry into an era where functionality, durability, and biocompatibility converge to offer optimal solutions for both clinicians and patients. Biodegradable polymers like PLA and polyglycolic acid (PGA), on the other hand, boast of their ability to be safely absorbed by the body, marking them as favorites in applications like guided tissue regeneration and sutures [137]. PEEK, renowned for its mechanical strength and stability, has found applications in dental implantology. This high-performance thermoplastic boasts excellent biocompatibility, resistance to chemical degradation, and an ability to be easily tailored or modified. It is the blend of these exceptional attributes that makes PEEK an attractive material for dental frameworks, offering an alternative to traditional materials like metal. The elasticity of the material, comparable to that of bone, provides a level of flexibility that assists in absorbing the forces exerted during chewing. This feature enhances the comfort for patients as illustrated in Figure 7.
In a similar stride, PMMA has been a cornerstone in prosthetic dentistry. It is characterized by its aesthetic appeal, mimicking the natural appearance of teeth and gums. PMMA’s ease of fabrication and repair makes it a popular choice for temporary prostheses, offering patients a functional and aesthetic solution while awaiting permanent restoration. Its biocompatible nature ensures minimal tissue irritation, aligning with the holistic objective of ensuring patient well-being. The narrative of biodegradable polymers like PLA and PGA is painted with the brushes of innovation and safety. PLA’s renewable sourcing from lactic acid is a nod toward sustainability. It boasts excellent biocompatibility and has been widely used in the fabrication of sutures and tissue engineering scaffolds. PGA complements this by offering superior mechanical strength, an attribute that has championed its use in high-strength suture materials [138]. Together, they epitomize the blend of natural degradation and mechanical strength, ensuring the temporary support of healing tissues before their natural dissolution, eliminating the need for surgical removal.
In the space of RPD, fiber-reinforced composites, particularly glass or carbon fiber-reinforced polymers, are revolutionary. They offer an ensemble of strength, durability, and aesthetics. Traditional materials like metals, though functionally adept, often fall short in aesthetic appeal. The incorporation of fiber-reinforced composites addresses this gap, offering patients a solution that is not just functional but also aesthetically pleasing. The reinforcement of polymers with fibers like glass or carbon enhances their mechanical properties, ensuring durability while maintaining a degree of flexibility for enhanced comfort [139]. The journey through the dental applications of these polymers underscores a narrative of innovation driven by the imperatives of biocompatibility, functionality, and aesthetics. The evolving landscape of dental materials is characterized by a dynamic interplay of these elements, each polymer carving its niche, yet collectively contributing to a broader narrative of enhanced patient care, safety, and well-being [140].
Dental material continues to embrace these polymers, the boundaries of possibility are expanded. Procedures become less invasive; materials, more compatible; and the convergence of function and aesthetics, more pronounced. In every grain of PLA, the echo of sustainability resounds; in every strand of fiber-reinforced composites, the narrative of strength intertwined with aesthetics is told; and in every application of PEEK and PMMA, the silent testimony of evolution, from the rudimentary to the complex, yet simplified solutions, is witnessed [141]. Each material is not just a substance but a story, an evolution, and a silent witness to the unfolding future of dentistry where materials, technology, and human touch converge to redefine the art of holistic oral care. Polymers in the dental domain embody a harmony of science and art – a blend of functional excellence and aesthetic mastery.
As dental science continues to evolve, the role of polymers is expected to expand, heralding innovations that promise enhanced quality of life, health, and well-being for patients around the world. The convergence of polymer science with technologies like artificial intelligence and nanotechnology is set to catalyze unprecedented innovations, setting the stage for a future where dental health is not just restored but optimized, where aesthetics and function unite in an intricate dance of precision, embodying the epitome of human ingenuity [142]. In conclusion, the incorporation of polymers in dental science symbolizes a journey of innovation and improvement. It underscores a future where the restoration, enhancement, and preservation of oral health are characterized by precision, comfort, and aesthetic splendor, marking a new chapter in a story where science and technology converge to enhance the human experience in profound ways. Each development in this field not only contributes to the enrichment of dental science but also underscores the limitless potential of human ingenuity when fueled by the ceaseless pursuit of excellence, innovation, and the betterment of the human condition.
3.3.1 Advantages and limitations
Biodegradable polymers, PEEK, PMMA, PLA, and PGA have been widely recognized in the field of dentistry for their unique sets of advantages and limitations. Each of these materials brings a distinctive set of properties that makes them suitable for various dental applications. PEEK is renowned for its impressive mechanical strength, biocompatibility, and stability, positioning it as a preferred material for dental implants and prosthetics. Its mechanical properties are comparable to that of bone, promoting osseointegration and ensuring stability of the implant over time. PEEK is also radiolucent, rendering it invisible on X-rays and beneficial for postoperative assessments. The material’s resistance to a wide range of chemicals ensures its stability in the corrosive environment of the mouth. However, PEEK is not without its limitations. The material’s color, a natural off-white, can sometimes be considered aesthetically unpleasing. Furthermore, the cost of PEEK can be a limiting factor, as it is relatively expensive to produce and process.
PMMA, on the other hand, has been a popular choice for dental prosthetics, especially dentures, due to its ease of fabrication, light weight, and aesthetics. Its translucency and ability to be tinted allows for the creation of natural-looking teeth and gums. Moreover, PMMA is relatively inexpensive, making it accessible to a wider patient population. Nevertheless, PMMA is often criticized for its lower mechanical strength compared to materials like PEEK. It can fracture under stress, leading to a need for frequent replacements. Furthermore, some patients might experience allergic reactions to the monomers released from the polymerized material.
Biodegradable polymers like PLA and PGA are gaining traction in dentistry for their eco-friendly and bioresorbable nature. PLA has been employed in the fabrication of screws, plates, and other fixation devices for its high tensile strength and modulus. It is renowned for reducing the need for a second surgery to remove the implant, as it naturally degrades in the body over time. However, the rate of degradation can be a limitation, as it is crucial to balance the degradation rate with the tissue healing rate to ensure structural support during the healing process. The material’s mechanical properties might also be inadequate for load-bearing applications.
PGA, similar to PLA, is appreciated for its biodegradability. It has a faster degradation rate, making it suitable for temporary implants that only need to provide support for a short period. The limitation, however, lies in its rapid loss of mechanical strength due to the quick degradation rate, which might not be suitable for all clinical applications. Both PLA and PGA also have limitations in terms of their thermal processing, which can sometimes affect their mechanical properties and degradation behavior. In the grand scheme of dental applications, the choice between PEEK, PMMA, PLA, and PGA is highly contingent on the specific application, the required mechanical properties, the aesthetic considerations, and the patient’s specific needs and constraints. PEEK is often chosen for its strength and biocompatibility, especially for long-term implants. PMMA is prevalent in prosthetics and dentures for its aesthetics and affordability. Meanwhile, biodegradable polymers like PLA and PGA are carving a niche for themselves for temporary implants and environmentally friendly options.
In conclusion, each of these materials is associated with a distinct combination of advantages and limitations. The evolution of dental materials is an ongoing journey, with continuous research aimed at optimizing the properties of existing materials like PEEK, PMMA, PLA, and PGA, and exploring new materials to overcome the present limitations. Balancing factors such as mechanical strength, biocompatibility, aesthetics, and cost will continue to be central in the advancement of dental materials to meet the diverse and evolving needs of patients and clinicians alike. The optimal material would offer a harmonious blend of strength, biocompatibility, aesthetics, and affordability, tailored to the specific requirements of each dental application. In the continuous quest for perfection, the collaboration between material scientists, engineers, and dental practitioners is pivotal, marrying theoretical knowledge with practical insights to innovate and refine the next generation of dental materials.
3.4 Composites and other materials for implants
3.4.1 Composites materials
3.4.1.1 Calcium phosphate (CaP) ceramics
In the field of implant dentistry, CaP-based materials are highly favored and commonly utilized. Among various biomaterials, CaPs stand out due to their excellent biocompatibility, strong osteoconductivity, ability to regenerate bone, and notable osteogenic properties [143,144]. In addition, the chemical composition and the structure of CaP ceramics closely resemble that of natural bone tissue. When combined with growth factors (GFs) or trace elements, CaPs can further enhance bone defect repair. Numerous CaP ceramics have been synthesized and applied in various dental applications, featuring calcium and phosphate as their main components. However, these ceramics possess diverse compositions and properties, which are outlined in Table 2.
Composition and characteristics of synthesized CaP ceramics
| Calcium phosphate | Chemical formula | Ca/P ratio | Solubility |
|---|---|---|---|
| Amorphous calcium phosphate (ACP) | Ca x H y (PO4) z ·nH 2 O (3 ≤ n ≤ 4.5) | 1.2–2.2 | High |
| Biphasic calcium phosphate (BCP) | Mixture of HAP and β-TCP | 1.5–1.67 | Dependent on HAP/TCP ratio |
| Calcium-deficient HAP (CDHA) | Ca9(HPO4)(PO4)5(OH) | 1.65–1.67 | Dependent on Ca/P ratio |
| HAP | Ca10(PO4)6(OH)2 | 1.67 | Low |
| Octocalcium phosphate (OCP) | Ca8(HPO4)2(PO4)4)·5H2O | 1.33 | Low |
| α-tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 1.5 | Moderate |
| β-tricalcium phosphate (β-TCP) | β-Ca3(PO4)2 | 1.5 | Moderate |
Among CaP materials, beta-tricalcium phosphate (β-TCP) and biphasic calcium phosphate (BCP) are widely recognized (Figure 7). These CaPs are available in various formulations and delivery modes, including coatings, granules, and blocks, tailored to different clinical needs. The different shapes and sizes of these CaPs are selected based on specific clinical scenarios. TCP has numerous polymorphs, including α, β, γ, and super α. However, only α and β phases are utilized as biomaterials [145], garnering significant attention in research since the early 1970s. Despite extensive studies, there remains ambiguity regarding this material. Resorbable TCP materials are preferred due to their ability to be gradually replaced by bone over time [146].
3.4.1.2 Application of TCP
Most materials containing TCP are osteoconductive, meaning that bone can grow into pores, channels, or pipelines on their surface [147]. One biocompatible substance that can be utilized to promote the growth of hard tissue is CaP [148,149]. It has been used in the following applications: capping agent [150], cleft palate [151], apical barrier [152], apexification [153], vertical bone defect [154], and implant coating. TCP is a CaP phase that can be reabsorbed and has certain advantageous qualities. It has also been demonstrated to support bone growth. However, because of its low mechanical strength and resistance to the propagation of cracks, it is challenging to sinter. Moreover, resorption of TCP occurs quickly and uncontrollably. The solubility of the TCP coating can vary, which could lead to an early coated implant failure [155].
3.4.1.3 Aluminum oxide
Alumina is frequently used for dental implants in medical application as it has better mechanical strength, high toughness, low density, and also corrosion resistance. The fact that alumina has strong ionic inter atomic bonding gives rise to its desirable material properties. Because alpha phase alumina is the stiffest and hardest of the oxide ceramics. The favorable material properties of alumina are a result of its strong ionic inter atomic bonding since among the oxide ceramics, alpha phase alumina is the stiffest and hardest [156].
3.4.1.4 HAP
HAP is the most extensively studied CaP, finds application in bulk form, as a coating and/or cements [157,158,159]. The lattice structure of HAP, an inorganic mineral, is (A10(BO4)6C2), where A, B, and C are specified by Ca, PO4, and OH. With a weight percentage of 18% phosphorus and 39.68% calcium, pure HAP has a Ca/P mole ratio of 1.67. The Ca/P ratio in certain commercial HAP products might be greater or lower than 1.67. The phase transition between calcium oxide (CaO) and TCP is indicated by the variation in the Ca/P ratio. More CaO than TCP can be observed in HAP with a Ca/P ratio greater than 1.67, and vice versa. This material can be categorized based on its porosity, phase, and processing method.
The human body contains HAP crystals in both the teeth and the bone. Dental implants have advanced significantly in recent years as a treatment for tooth decay and loss. The majority of the inorganic substance found in human teeth is a CaP that is connected to HAP. HAP’s characteristics and chemical composition are similar to those of the components found naturally in teeth. Moreover, the organic component of type-I collagen and the inorganic component of HAP make up the architecture of the teeth. These two elements combine to create a composite structure at the nanoscale, with the collagen network containing scattered nano-HAP. In addition to forming mineralized collagen, this composite is the starting point for biologically mineralized tissues, ranging from tendons and skin to hard mineralized tissues like teeth and bone. HAP exhibits exceptional biocompatibility, promoting osteoconduction and osseointegration. Due to these favorable properties, it is extensively favored as the biomaterial of choice in dentistry and orthopedic applications [159,160,161].
The manufacturing process and the basic components used in HAP implants affect the biological response, resulting in a range of product qualities. Furthermore, HAP alone cannot be employed as an implant in a load-bearing application due of its extremely brittle nature. Because uncoated metallic implants are not biocompatible with the surrounding tissue, they are also not appropriate. As a result, the use of biocompatible materials, such HAP, to metallic surfaces has emerged as a promising area in dentistry [162]. Considerable research has been done recently on the coating of HAP on load-bearing implants and used enormously. Mainly, dental implant based on HAP may offer an interlocking porous structure. This structure can function as the extracellular matrix, encouraging tissue regeneration and cellular development as they occur naturally. In addition, by encouraging a firm anchoring between the implant and the surrounding tissue without encouraging the formation of fibrous tissue, HAP can speed up the osseointegration process. When osseointegration is successful, the implant is preserved for an extended length of time, fully regaining functional ability.
The use of HAP in implants presents a number of issues or drawbacks, including the following. The intrinsic flaws and fine porosity of HAP used as implants have the potential to cause cracks. Moreover, there are situations when applying bulk HAP can result in a mismatch in the modulus of the implant and the bone. On the other hand, HAP always contains minute amounts of elements, like hydroxyl ions (OH−) and fluoride ions (F−), which results in a drop in solubility and an increase in crystallite size, both of which can boost apatite strength [163]. On the other hand, it has been observed that elements like chloride ions (Cl−) and phosphide ions (
3.4.2 Carbon and carbon-silicon based materials
Carbon and carbon-silicon based materials are often classified as ceramics because of their chemical inertness. These are also classified as metal free dental implants. These are good conductors of heat and electricity. Mainly, carbon and carbon-silicon-based materials are used as surface coatings in metallic and ceramic implants [164]. Their main disadvantages are (1) lack of mechanical strength, prone to scratching and (2) biodegradation may adversely affect tissue stability.
3.4.3 Inorganic compounds as filler materials
Several inorganic substances have shown promising outcomes in terms of remineralization and direct and indirect effects on biofilm formation. These include boron nitride, BAG, nanosized amorphous CaP, calcium fluoride (CaF2), and HAP. The chemicals’ effects differed based to their composition, the percentage or amount incorporated, and the intended clinical use. The remineralizing effects were shown as either indirect-like increasing the pH in the area around the material- or direct-like raising the mineral content of the dental tissue. To enhance the results of remineralization and the antibacterial action against the cariogenic biofilms, certain studies have documented combining inorganic remineralizing chemicals with other bioactive agents, such as quaternary ammonium compounds [165]. The more inert polymers have been combined with particulate or fibers of carbon, Al2O3, HAP, and glass ceramics. Some are porous, but others are constituted as solid-composite structural forms. Biodegradable polymers, such as polyvinyl alcohol polylactides or glycolides, cyanoacrylates, or other hydratable forms, have been combined with biodegradable CaPO4 particulate or fibers.
3.4.4 Fiber-based materials
3.4.4.1 Carbon fibers
A class of fiber known as “carbon fiber” is produced by pyrolyzing organic precursor fibers in an inert atmosphere, such as rayon, polyacrylonitrile. With a graphitic structure and strong, highly anisotropic crystallite covalent bonds for exceptionally large mechanical properties along the axis direction, carbon fiber has minimal mechanical properties in the transverse or perpendicular direction due to weak van der Waals forces between layers. Apart from their widely acknowledged structural and mechanical attributes, carbon fibers possess certain biocompatible qualities that have been established through clinical trials on animals and laboratory experiments [166]. Compared to compact bone, which has a density of 2.0 g/cm3, carbon fiber is lightweight at 1.6–2.2 g/cm3. High-strength, high-modulus carbon fibers with a flexible tiny diameter may be molded into intricately curved spaces with ease for a variety of applicable uses.
The main advantages of utilizing carbon fiber reinforcing in prostheses are improved impact strength and fatigue behavior. Carbon fiber-reinforced composites have very good mechanical qualities and perform better in terms of strength-to-weight ratio than other materials [167]. In addition to their noncorrosiveness, reduced density, increased stiffness, biocompatibility, chemical innervation, and increased stability, carbon fibers are also more economical.
3.4.5 Glass-based materials
3.4.5.1 Glass fibers (GFs)
GFs are small-diameter fibers made of glass based on silica that are extruded into thin strands. By varying the amount of raw materials, such as sand for silica, clay for alumina, calcite for calcium oxide, and colemanite for boron oxide, GF are created from melts and can be produced in a variety of compositions. Because silica or other materials are used in varying proportions, different types of GFs exhibit varied qualities, such as alkali resistance or excellent mechanical properties. GFs come in a variety of compositions, including A-, C-, D-, AR-, S-, and E-glass. Each type of GF has unique properties and applications, but they are all amorphous and made of a three-dimensional network of silica with randomly arranged oxygen and other atoms.
GFs are manufactured using high-alkali glass compositions containing approximately 25% lime and soda. Despite their vulnerability to water and alkaline substances, they are favored for their cost-effectiveness and ease of production, making them a popular choice in the plastics industry for filler material. When exposed to acidic environments, C-GFs excel due to their remarkable resistance to corrosion, although their strength is somewhat limited. On the other hand, D-GFs find utility in electronic boards as reinforcement due to their favorable electrical properties, despite lacking robust chemical resistance and strength. S-GFs stand out for their corrosion resistance, high modulus of elasticity, and low dielectric permittivity. However, their production complexity renders them costly compared to other types. AR-GFs boast a high melting point, offering exceptional impact strength and resistance to crack propagation thanks to their zirconium content. However, this attribute also restricts their applications. E-GFs, constituting half of the GF industry, are widely preferred due to their superior electrical insulation, high water resistance, and affordability [168,169]. Nevertheless, the presence of volatile fluorine and boron oxide compounds poses environmental contamination risks and can compromise the chemical consistency of the glass.
Only E- and S-GFs have been utilized in dentistry out of all of these varieties. There are numerous GF-reinforced dental products on the market, including PMMA-impregnated E-GF-reinforced composite, pre-impregnated S-GF-reinforced composite, and pre-impregnated E-GF-reinforced composite. Mainly, these GFs are enclosed into resin matrix to produce GF-reinforced composites (GF-RCs). Fine, thin GFs are chemically attached to a polymerized monomer matrix via silane coupling agents to form GF-RCs [170]. The idea of the fiber fillers’ reinforcing action is based on how stress is transferred from the polymer to the fibers and how each fiber contributes to stopping the spread of cracks.
GFs are used in a variety of industries, including dentistry, electrical boards, plastics manufacturing, radar housing, and engineering. They are used in the production of various dental goods, including orthodontic fixed retainers, endodontic post systems, and fixed partial dentures. In addition to providing acceptable aesthetics, GF-RC dental materials are also noncorrosive, highly durable, free of metal, hypoallergenic, suitable for chairside handling, biocompatible, and can be customized to meet the unique needs of a variety of dental applications. Glass ceramics are still used in dentistry; they come in a variety of compositions, including LD and mica-based dental glass that is sold commercially.
3.4.5.2 Mica-based implant material
Mica-based glass ceramics have better mechanical strength, are more stable, biocompatible, and have a color that is similar to teeth [171]. It is simple to make and can be applied with varied geometric shapes to meet the needs of diverse patient types. Laminated veneers used for posterior inlays and anterior crowns adhere to glass ceramics [172,173,174].
3.4.5.3 LD
LD base glass ceramics are another family of ceramic materials that have been widely used for producing monolithic single crowns. Since 1962, glass-ceramics based on leucite (KAlSi2O6) were developed as a porcelain composition containing leucite that could be fired directly onto common dental alloys [175]. LD (Li2O5Si2) is a glassy ceramic with a flexural strength of 400 MPa and good translucency, making it suitable for both anterior and posterior uses. Press ceramics have been available for nearly 25 years and are now available in the form of pressable multicolored ingots for highly aesthetic monolithic restorations.
Aesthetic dental materials known as LD glass ceramics (LDGC) are produced by a solid-state reaction from SiO2-Li2O-Al2O3-K2O-ZrO2-P2O5 glass systems. LDGCs are the oldest known technology for dental restorations and are renowned for their exceptional robustness. About 70% of the components of LDGC are needle-shaped Li2Si2O5 crystals contained in a matrix mostly made up of SiO2, Li2O, Al2O3, K2O, P2O5, and various oxide replacements. Li2Si2O5 crystals reinforced in the glassy matrix have diameters of a few microns, and their interlocking structure gives them a high strength (360–400 MPa). LDGCs are excellent materials for dental implants because of their improved translucency, chemical endurance, and cosmetic appeal.
Although LDGCs are weaker than normal yttria-stabilized polycrystals, they mimic natural teeth extremely well in terms of translucency and color. As a result, LDGCs are now being used extensively in ceramic restoration for veneers, inlays, onlays, and anterior/posterior crowns. Furthermore, 97.4 and 94.8% of monolithic LDGCs can live for up to 5 and 8 years after treatment, respectively, demonstrating the exceptional survival rates of these cells. As these numbers demonstrate, the success rates are likewise very good: 91.1% after 5 years and 69.8% after ten [176,177]. The goal of recent advancements in LDGCs is to increase their wear resistance and mechanical strength through improvements to their synthesis control, processing techniques, compositions, and heat treatments.
3.4.6 Silicon nitride (SiN)
SiN is an emerging material for dental implant applications. Due to its exceptional mechanical, physical, thermal, structural, and biological qualities, silicon nitride has showed promise for use in medical and dental implants. Among the qualities that are most impressive and clinically significant are the antibacterial surface and the ability of surface changes. In comparison to titanium implants, SiN dental implants have shown mechanical characteristics that approximate bone tissues, lowering stress shielding. Three polymorphic structures of silicon nitride exist: a cubic (γ-Si3N4) high-pressure/high-temperature modification with spinel structure, and two hexagonal α- and β-Si3N4 modifications. The structures of hexagonal silicon nitride are represented in Figure 8 as somewhat distorted SiN4 tetrahedra that share corners to form deformed hexagonal rings stacked in layers with ABAB (β-Si3N4) and ABCD (α-Si3N4) stacking sequences.
![Figure 8
Structures of hexagonal β- and trigonal α-silicon nitride. Arrangement of Si and N atoms in hexagonal β- and α-silicon nitride shown projected down the c axis onto the AB plane [178]. With permission from Wiley-VCH. Black circles: silicon atoms, white circles: nitrogen atoms.](/document/doi/10.1515/ntrev-2025-0140/asset/graphic/j_ntrev-2025-0140_fig_008.jpg)
Structures of hexagonal β- and trigonal α-silicon nitride. Arrangement of Si and N atoms in hexagonal β- and α-silicon nitride shown projected down the c axis onto the AB plane [178]. With permission from Wiley-VCH. Black circles: silicon atoms, white circles: nitrogen atoms.
3.4.7 Dental resin composites (DRCs)
DRCs are widely used materials for caries restoration. DRCs are tooth-colored restorations that are used to replace tooth structure that has decayed. The principal advantage over traditional dental amalgam is its aesthetic appearance. A resin-based matrix, like bisphenol A-glycidyl methacrylate, plus an inorganic filler, like silica, make up a typical composite resin. The composite’s mechanical properties, wear resistance, and translucency are all enhanced by the filler.
Restoration failures persist despite the development of diverse DRC types with varying properties. The failure of composite restorations has been attributed mostly to bulk fracture and subsequent caries. Numerous fillers with specialized purposes have been introduced and investigated to address these issues. Fillers that release ions, such Ag+, Ca2+, and F−, have been utilized to reduce secondary caries, while fillers with specific morphologies, including whisker, fiber, and nanotube, have been used to improve the mechanical qualities of DRCs. The functionality and longevity of DRCs are enhanced by these fillers [179].
3.5 Functional coatings and surface modified techniques in dental implants
The implant surface is covered with a coating different functions (Figure 9). There are various materials used for coating functionalized with various components, which has enhanced the osseointegration process and decreasing the biointegration.

Illustration of osseointegration and biointegration process in dental implanting, and diagrammatic illustration of various types of surface coating of dental implants.
The field of oral restoration makes extensive use of dental implants; however, issues like marginal bone resorption, peri-implantitis, and failed osseointegration continue to cause implant failures in clinical settings, limiting both patient satisfaction and implant success rates. Once dental implants have undergone osseointegration – the process of creating an interface between the implant’s surface and the bone – they can be used as support for either fixed or removable dental prosthesis. Osseointegration of dental implants is a necessary condition for the long-term outcome. Dental implants may not remain stable over time for biological (peri-implantitis, which occurs 20% of the time due to bacterial infections or microbial plaque) and mechanical reasons (stress shielding, which can lead to osteopenia and clenching-bruxism) reasons.
Numerous techniques and materials have been documented; functionalized coatings and/or surface-modified dental implants are two of them that may help solve the issues (Figure 10). To expedite the osseointegration processes and avoid peri-implantitis, new functionalized materials are developed to replace natural teeth with dental implants. A cascade mechanism that starts with the interactions between the dental implant’s surfaces and the blood and connective tissue initiates osteointegration. The optimal surface of implants has been linked more and more to among those designs in an ideal process of osseointegration. This surface design creates a safe side to prevent most oral bacteria and even have a sterilizing effect [179].

Different types of customized functional surface coatings for dental implants.
Dental implants can be made from a variety of materials, including metals, polymers, and ceramics; however, each material has its own set of drawbacks. For instance, metals have high elastic modulus and corrosion issues; polymers have poor strength and wear characteristics because of water absorption; and ceramics have brittleness and hardness issues. In case of metal alloys, the commonly used Ti alloy Ti-6 aluminum-4 vanadium offers superior mechanical properties and remarkable biocompatibility when compared to commercially available CpTi. The primary risk with employing these alloys is their potential for harmful effects due to the release of aluminum and vanadium.
Ti and its alloys are associated with osteopenia (through metal leaching or metal poisoning), a harmful disorder affecting bone. Implants loosen and fail as a result of an uneven transmission of loads and bone atrophy brought on by the notable variation in Young’s modulus of elasticity. Various surface modifications have been used to address these issues and enhance the interaction between the implant and the bone. Many surface modifications and methods are using Ti and its alloy-based implants, including sandblasting, acid etching, anodization, plasma spraying, and laser radiation, have been investigated recently to address these issues and enhance the interface between implant and bone. This has led to the development of a few commercially available surface-modified dental implants for clinical use.
Coated Ti implants with CaP nanoparticles (nCaP/BP) loaded with bisphosphonate (BP) and assessed the osteogenic potential of the resultant implants in comparison to the uncoated implants in vivo. The in vivo findings under osteoporotic conditions showed that the nCaP/BP-coated implants increased the percentage of total bone area (% BA) and bone-to-implant contact (% BIC) by 1.2 and 5.3 times, respectively, in comparison to noncoated ones; in contrast, these values increased by 1.6 and 3.7-fold, respectively, under healthy conditions [180]. Chitosan loaded with tetracycline or chlorhexidine digluconate was applied as a coating agent for titanium implants. The antimicrobial efficacy of the coated implant with tetracycline was evaluated, demonstrating significant suppression (95–99.9%) of Actinobacillus actinomycetemcomitans and Staphylococcus epidermidis growth without cytotoxic effects on human osteoblasts and fibroblasts cells over a 7-day period. Conversely, the coated implant with chlorhexidine digluconate exhibited antimicrobial activity (56–99.5%) for one to 2 days but demonstrated cytotoxicity to human osteoblasts and fibroblast cells on the first day of elution. Additionally, the coated implants achieved tetracycline and chlorhexidine digluconate release rates of 89 and 100% within 7 and 2 days, respectively [181].
The capacity of surface-modified PEEK implants containing nanocrystalline HAP to osseointegrate in vivo in rabbits from New Zealand. Histomorphometry data showed that the test implants increased the % BA and % BIC by 18.8 and 13.5%, respectively, in comparison to the uncoated control implants [179]. Moreover, the surface characteristics and therapeutic benefits of the implants are enhanced by coating them with pharmacological materials (such as BPs, antibiotics, antimicrobial peptides, and biomolecules).
Current research has looked at surface modifications including covering dental implants with various materials and biomolecules to achieve particular goals. Coatings containing HAP, magnesium, GFs, extracellular matrix proteins, carbon compounds, soft-tissue integrators, etc., that improve the osseointegration process. Recent years have seen the application and reporting of bioactive nano coatings (in the formulation – long-term controlled release) containing antibiotics, immunomodulators, polysaccharides, antimicrobial peptides, etc., some of these have even been commercialized.
Numerous academics have worked to advance implant surface engineering to improve a number of physiological reactions, including osteoblast attachment, proliferation, differentiation, matrix synthesis, and calcification in the peri-implant alveolar bone, in order to maximize osseointegration related to titanium implants. Nowadays, zirconia implants – whose white surfaces are seen as more aesthetically pleasing than titanium – have drawn a lot of attention. To encourage osseointegration, non-metallic surfaces need specific modification techniques.
Dental implants’ surface bioactivity can be increased by coating them with bioactive substances including HAP and CaPs. This enhances the interaction between the implant and the bone. CaP (Ca2+ and
In general, improving the osseointegration and promoting quicker healing can be accomplished by adjusting the implant surface’s characteristics, such as its chemical composition, free surface energy, and roughness. Furthermore, a hotspot for implantology research is micro-nano structural alteration of the implant surface, which may improve the hydrophilicity and bone conductivity of the implant and lower stress conduction. Furthermore, a variety of surface coating techniques that aim to improve the biological activity of implant surfaces are emerging quickly. These techniques are mostly related to the interdisciplinary domains of materials science and biology. In certain preclinical studies, active molecules grafting onto the implant surface is the most representative and promising modification technique that may lessen foreign body response and enhance osseointegration.
3.6 Applications and advances in prosthodontics and implantology
Prosthodontics and implantology, integral to dentistry, focus on replacing and restoring lost or damaged teeth. These fields have evolved significantly due to advancements in materials science, with metallic alloys, ceramics, and polymers playing prominent roles. Titanium and its alloys, renowned for strength, durability, and biocompatibility, are favored for dental implants due to osseointegration capabilities, providing stable, long-lasting solutions. Co–Cr alloys offer corrosion resistance and strength, making them ideal for dentures, crowns, and bridges. Ceramics, such as zirconia, provide aesthetic and functional benefits, mimicking natural tooth color and offering biocompatibility and strength comparable to metals [183,184]. They are commonly used in crowns, bridges, and veneers, although concerns about brittleness and repair complexity persist.
Polymers are crucial in the ecosystem of restorative materials due to their versatility and adaptability. They find extensive use in various applications, such as denture fabrication with acrylic resins. Their ability to be manipulated for desired aesthetics makes them attractive to patients seeking visually pleasing outcomes. Bio-compatible polymers like PEEK are gaining popularity for their lightweight nature and compatibility with imaging modalities. In implantology, polymers complement other materials like titanium implants paired with ceramic crowns, blending osseointegration capabilities with aesthetic appeal [182,183]. They serve supplementary roles in temporary prostheses and in combination with metals and ceramics to enhance comfort, adaptability, and aesthetics. The unique properties of each material contribute to tailored solutions for individual patients based on their needs, medical histories, and aesthetic preferences.
Innovations in material science drive prosthodontics and implantology to new heights, emphasizing biocompatibility to ensure seamless integration and minimize allergic reactions. Techniques like CAD/CAM and 3D printing enable intricate, patient-specific designs, merging form and function. The synergy of metallic alloys, ceramics, and polymers promises personalized, efficient, and aesthetically pleasing solutions, with ongoing research tackling material challenges [185,186,187,188,189,190]. Future efforts aim to strengthen ceramics, lighten metallic implants, and enhance polymer durability, ensuring comprehensive oral restoration. As technology and materials converge, the future holds unprecedented innovation, with biocompatible materials, advanced fabrication, and AI integration shaping patient-centric solutions. Metallic alloys, ceramics, and polymers remain central, evolving to offer holistic options in oral rehabilitation. Their diverse characteristics ensure versatility to meet diverse needs, while ongoing advancements promise even greater capabilities in the future.
4 Conclusions
The realm of prosthodontics and dental implantology stands on the precipice of a transformative era, driven by continuous innovations in material sciences and digital technologies. Our review has chronicled the evolution and integration of advanced materials such as ceramics, polymers, metals, and composites, which have collectively elevated the standards of dental prostheses and implants. The convergence of aesthetics with material function has not only enhanced the quality of life for patients but has also broadened the scope for dental professionals to provide superior, customized care. Looking forward, the future of dental materials is poised to harness the potential of bioactive and antibacterial substances that promote osseointegration and prevent peri-implantitis. The integration of 3D printing and digital dentistry techniques promises further customization and precision in dental restorations, reducing turnaround times and improving patient outcomes. Moreover, the emerging field of nano-dentistry could revolutionize material properties at the molecular level, offering unprecedented improvements in mechanical strength, durability, and biocompatibility.
As we navigate these advancements, it is essential to consider the sustainable development of dental materials. The exploration of eco-friendly manufacturing processes and the recycling of dental materials will be crucial for reducing the environmental impact of dental practices. Additionally, the advent of AI and machine learning offers potential predictive insights into material performance and patient-specific outcomes, ensuring optimal material choice and design. In conclusion, the continuous evolution of materials in prosthodontics and dental implantology not only promises enhanced functional outcomes and aesthetic satisfaction but also heralds a new age of personalized and precision dentistry. Embracing these advancements will require ongoing collaboration between researchers, clinicians, and industry stakeholders to ensure that the benefits of these technologies are realized across all facets of dental care.
Acknowledgments
This work was supported by Qatar University through a National Capacity Building Program Grant (NCBP) [QUCPCAM-22/24-463]. Statements made herein are solely the responsibility of the authors.
-
Funding information: Open Access funding was provided by the Qatar National Library.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Montero J. A review of the major prosthetic factors influencing the prognosis of implant prosthodontics. J Clin Med. 2021;10(4):816.10.3390/jcm10040816Search in Google Scholar PubMed PubMed Central
[2] Rupp F, Liang L, Geis-Gerstorfer J, Scheideler L, Hüttig F. Surface characteristics of dental implants: A review. Dent Mater. 2018;34(1):40–57.10.1016/j.dental.2017.09.007Search in Google Scholar PubMed
[3] Cherian JM, Samuel S, Sabu AM, Thomas AM, Injety RJ. Dental implants in growing patients: A quality assessment of systematic reviews. J Oral Biol Craniofac Res. 2023;13(5):610–5.10.1016/j.jobcr.2023.07.004Search in Google Scholar PubMed PubMed Central
[4] Osman RB, Swain MV. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials. 2015;8(3):932–58.10.3390/ma8030932Search in Google Scholar PubMed PubMed Central
[5] Mardiana A, Nardiatmo SPS, Haryo HM, Wahab W, Ahmad MH. A chairside immediate provisional crown for single dental implantation in aesthetic area: A case report. Med Clin Pract. 2020;3:100106.10.1016/j.mcpsp.2020.100106Search in Google Scholar
[6] D’Amico C, Bocchieri S, Sambataro S, Surace G, Stumpo C, Fiorillo L. Occlusal load considerations in implant-supported fixed restorations. Prosthesis. 2020;2(4):252–65.10.3390/prosthesis2040023Search in Google Scholar
[7] Chen L-J, Hao H, Li Y-M, Ting L, Guo X-P, Wang R-F. Finite element analysis of stress at implant–bone interface of dental implants with different structures. Trans Nonferrous Met Soc China. 2011;21(7):1602–10.10.1016/S1003-6326(11)60903-5Search in Google Scholar
[8] Pandey C, Rokaya D, Bhattarai BP. Contemporary concepts in osseointegration of dental implants: a review. Biomed Res Int. 2022;2022(1):6170452.10.1155/2022/6170452Search in Google Scholar PubMed PubMed Central
[9] Khan SN, Ramachandran M, Kumar SS, Krishnan V, Sundaram R. Osseointegration and more–A review of literature. Ind J Dent. 2012;3(2):72–6.10.1016/j.ijd.2012.03.012Search in Google Scholar
[10] Barfeie A, Wilson J, Rees J. Implant surface characteristics and their effect on osseointegration. Br Dent J. 2015;218(5):E9.10.1038/sj.bdj.2015.171Search in Google Scholar PubMed
[11] Carlsson GE. Critical review of some dogmas in prosthodontics. J Prosthodont Res. 2009;53(1):3–10.10.1016/j.jpor.2008.08.003Search in Google Scholar PubMed
[12] Hong DGK, Oh JH. Recent advances in dental implants. Maxillofac Plast Reconstr Surg. 2017;39:1–10.10.1186/s40902-017-0132-2Search in Google Scholar PubMed PubMed Central
[13] Rasouli R, Barhoum A, Uludag H. A review of nanostructured surfaces and materials for dental implants: surface coating, patterning and functionalization for improved performance. Biomater Sci. 2018;6(6):1312–38.10.1039/C8BM00021BSearch in Google Scholar PubMed
[14] Hossain N, Mobarak MH, Islam MA, Hossain A, Al Mahmud MZ, Rayhan MT, et al. Recent development of dental implant materials, synthesis process, and failure–a review. Results Chem. 2023;6:101136.10.1016/j.rechem.2023.101136Search in Google Scholar
[15] Abou Neel EA, Chrzanowski W, Salih VM, Kim H-W, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;42(8):915–28.10.1016/j.jdent.2014.05.008Search in Google Scholar PubMed
[16] Attin T, Wegehaupt FJ. Impact of erosive conditions on tooth-colored restorative materials. Dent Mater. 2014;30(1):43–9.10.1016/j.dental.2013.07.017Search in Google Scholar PubMed
[17] Turkyilmaz I, Wilkins GN. 3D printing in dentistry–exploring the new horizons. J Dent Sci. 2021;16(3):1037.10.1016/j.jds.2021.04.004Search in Google Scholar PubMed PubMed Central
[18] Tian Y, Chen C, Xu X, Wang J, Hou X, Li K, et al. A review of 3D printing in dentistry: Technologies, affecting factors, and applications. Scanning. 2021;2021(1):9950131.10.1155/2021/9950131Search in Google Scholar PubMed PubMed Central
[19] Marin E. History of dental biomaterials: biocompatibility, durability and still open challenges. Herit Sci. 2023;11(1):207.10.1186/s40494-023-01046-8Search in Google Scholar
[20] Mallineni SK, Nuvvula S, Matinlinna JP, Yiu CK, King NM. Biocompatibility of various dental materials in contemporary dentistry: a narrative insight. J Investig Clin Dent. 2013;4(1):9–19.10.1111/j.2041-1626.2012.00140.xSearch in Google Scholar PubMed
[21] Rexhepi I, Santilli M, D’Addazio G, Tafuri G, Manciocchi E, Caputi S, et al. Clinical applications and mechanical properties of cad-cam materials in restorative and prosthetic dentistry: A systematic review. J Funct Biomater. 2023;14(8):431.10.3390/jfb14080431Search in Google Scholar PubMed PubMed Central
[22] Michael FM, Khalid M, Walvekar R, Ratnam CT, Ramarad S, Siddiqui H, et al. Effect of nanofillers on the physico-mechanical properties of load bearing bone implants. Mater Sci Eng C. 2016;67:792–806.10.1016/j.msec.2016.05.037Search in Google Scholar PubMed
[23] Mosaddad SA, Abdollahi Namanloo R, Ghodsi R, Salimi Y, Taghva M, Naeimi Darestani M. Oral rehabilitation with dental implants in patients with systemic sclerosis: a systematic review. Immun Inflamm Dis. 2023;11(3):e812.10.1002/iid3.812Search in Google Scholar PubMed PubMed Central
[24] Al-Quran FA, Al-Ghalayini RF, Al-Zu’bi BN. Single-tooth replacement: factors affecting different prosthetic treatment modalities. BMC Oral Health. 2011;11:1–7.10.1186/1472-6831-11-34Search in Google Scholar PubMed PubMed Central
[25] Boyce RA. Prosthodontic principles in dental implantology: Adjustments in a coronavirus disease-19 pandemic-battered economy. Dent Clin. 2021;65(1):135–65.10.1016/j.cden.2020.09.011Search in Google Scholar PubMed PubMed Central
[26] Saeed F, Muhammad N, Khan AS, Sharif F, Rahim A, Ahmad P, et al. Prosthodontics dental materials: From conventional to unconventional. Mater Sci Eng: C. 2020;106:110167.10.1016/j.msec.2019.110167Search in Google Scholar PubMed
[27] De Kok IJ, Duqum IS, Katz LH, Cooper LF. Management of implant/prosthodontic complications. Dent Clin. 2019;63(2):217–31.10.1016/j.cden.2018.11.004Search in Google Scholar PubMed
[28] Knosp H, Holliday RJ, Corti CW. Gold in dentistry: alloys, uses and performance. Gold Bull. 2003;36(3):93–102.10.1007/BF03215496Search in Google Scholar
[29] Kandavalli SR, Wang Q, Ebrahimi M, Gode C, Djavanroodi F, Attarilar S, et al. A brief review on the evolution of metallic dental implants: history, design, and application. Front Mater. 2021;8:646383.10.3389/fmats.2021.646383Search in Google Scholar
[30] Critchley E, Pemberton MN. Latex and synthetic rubber glove usage in UK general dental practice: changing trends. Heliyon. 2020;6(5):03889.10.1016/j.heliyon.2020.e03889Search in Google Scholar PubMed PubMed Central
[31] Zafar MS. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers. 2020;12(10):2299.10.3390/polym12102299Search in Google Scholar PubMed PubMed Central
[32] Krishnakumar S, Senthilvelan T. Polymer composites in dentistry and orthopedic applications-a review. Mater Today: Proc. 2021;46:9707–13.10.1016/j.matpr.2020.08.463Search in Google Scholar
[33] Hoque ME, Showva N-N, Ahmed M, Rashid AB, Sadique SE, El-Bialy T, et al. Titanium and titanium alloys in dentistry: Current trends, recent developments, and future prospects. Heliyon. 2022;8(11):11300.10.1016/j.heliyon.2022.e11300Search in Google Scholar PubMed PubMed Central
[34] Walter N, Stich T, Docheva D, Alt V, Rupp M. Evolution of implants and advancements for osseointegration: A narrative review. Injury. 2022;53:S69–73.10.1016/j.injury.2022.05.057Search in Google Scholar PubMed
[35] Alghamdi HS. Methods to improve osseointegration of dental implants in low quality (type-IV) bone: an overview. J Funct Biomater. 2018;9(1):7.10.3390/jfb9010007Search in Google Scholar PubMed PubMed Central
[36] Ramburrun P, Pringle NA, Dube A, Adam RZ, D’Souza S, Aucamp M. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials. 2021;14(12):3167.10.3390/ma14123167Search in Google Scholar PubMed PubMed Central
[37] Sotova C, Yanushevich O, Kriheli N, Grigoriev S, Evdokimov V, Kramar O, et al. Dental implants: Modern materials and methods of their surface modification. Materials. 2023;16(23):7383.10.3390/ma16237383Search in Google Scholar PubMed PubMed Central
[38] Zhu G, Wang G, Li JJ. Advances in implant surface modifications to improve osseointegration. Mater Adv. 2021;2(21):6901–27.10.1039/D1MA00675DSearch in Google Scholar
[39] Apratim A, Eachempati P, Salian KKK, Singh V, Chhabra S, Shah S. Zirconia in dental implantology: A review. J Int Soc Prev Community Dent. 2015;5(3):147–56.10.4103/2231-0762.158014Search in Google Scholar PubMed PubMed Central
[40] Zarone F, Di Mauro MI, Ausiello P, Ruggiero G, Sorrentino R. Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health. 2019;19:1–14.10.1186/s12903-019-0838-xSearch in Google Scholar PubMed PubMed Central
[41] Sajjad A. Computer-assisted design/computer-assisted manufacturing systems: A revolution in restorative dentistry. J Indian Prosthodont Soc. 2016;16(1):96–9.10.4103/0972-4052.164905Search in Google Scholar PubMed PubMed Central
[42] Glowacka-Sobotta A, Ziental D, Czarczynska-Goslinska B, Michalak M, Wysocki M, Güzel E, et al. Nanotechnology for dentistry: prospects and applications. Nanomaterials. 2023;13(14):2130.10.3390/nano13142130Search in Google Scholar PubMed PubMed Central
[43] Rezaie F, Farshbaf M, Dahri M, Masjedi M, Maleki R, Amini F, et al. 3D printing of dental prostheses: Current and emerging applications. J Compos Sci. 2023;7(2):80.10.3390/jcs7020080Search in Google Scholar PubMed PubMed Central
[44] Balasubramanian M, Srimath R, Vignesh L, Rajesh S, editors. Application of shape memory alloys in engineering–A review. Journal of Physics: Conference Series. IOP Publishing; 2021.10.1088/1742-6596/2054/1/012078Search in Google Scholar
[45] Wang L-H, Gao S-Z, Bai X-L, Chen Z-L, Yang F. An up-to-date overview of dental tissue regeneration using dental origin mesenchymal stem cells: challenges and road ahead. Front Bioeng Biotechnol. 2022;10:855396.10.3389/fbioe.2022.855396Search in Google Scholar PubMed PubMed Central
[46] Featherstone JD. Dental restorative materials containing quaternary ammonium compounds have sustained antibacterial action. J Phys J Am Dent Assoc. 2022;153(12):1114–20.10.1016/j.adaj.2022.09.006Search in Google Scholar PubMed
[47] Wally ZJ, Van Grunsven W, Claeyssens F, Goodall R, Reilly GC. Porous titanium for dental implant applications. Metals. 2015;5(4):1902–20.10.3390/met5041902Search in Google Scholar
[48] Nicholson JW. Titanium alloys for dental implants: A review. Prosthesis. 2020;2(2):11.10.3390/prosthesis2020011Search in Google Scholar
[49] Gosavi S, Gosavi S, Alla R. Titanium: A miracle metal in dentistry Trends Biomater. Artif Organs. 2013;27(1):42–6.Search in Google Scholar
[50] Thirumalaivasan N. Letter to the Editor “assessing the performance of virus-mimic nanoparticles against other nano-drug delivery systems in efficiency, biocompatibility, and patient results”. Oral Oncol Rep. 2024;10:100436.10.1016/j.oor.2024.100436Search in Google Scholar
[51] Mansoor A, Khurshid Z, Khan MT, Mansoor E, Butt FA, Jamal A, et al. Medical and dental applications of titania nanoparticles: an overview. Nanomaterials. 2022;12(20):3670.10.3390/nano12203670Search in Google Scholar PubMed PubMed Central
[52] Kassapidou M, Stenport VF, Johansson CB, Syverud M, Johansson PH, Börjesson J, et al. Cobalt chromium alloys in fixed prosthodontics: Investigations of mechanical properties and microstructure. J Prosthet Dent. 2023;130(2):255.e1.10.1016/j.prosdent.2023.05.005Search in Google Scholar PubMed
[53] Vaicelyte A, Janssen C, Le Borgne M, Grosgogeat B. Cobalt–Chromium dental alloys: Metal exposures, toxicological risks, CMR classification, and EU regulatory framework. Crystals. 2020;10(12):1151.10.3390/cryst10121151Search in Google Scholar
[54] Majerič D, Lazić V, Majerič P, Marković A, Rudolf R. Investigation of CoCr dental alloy: example from a casting workflow standpoint. Crystals. 2021;11(8):849.10.3390/cryst11080849Search in Google Scholar
[55] Porojan S, Bîrdeanu M, Savencu C, Porojan L, editors. Structural and morphological approach of Co–Cr dental alloys processed by alternative manufacturing technologies. Journal of Physics: Conference Series. IOP Publishing; 2017.10.1088/1742-6596/885/1/012005Search in Google Scholar
[56] Ramirez-Ledesma AL, Lopez HF, Juarez-Islas JA. Evaluation of chill cast Co–Cr alloys for biomedical applications. Metals. 2016;6(8):188.10.3390/met6080188Search in Google Scholar
[57] Reclaru L, Unger R, Kirkpatrick C, Susz C, Eschler P-Y, Zuercher M-H, et al. Ni–Cr based dental alloys; Ni release, corrosion and biological evaluation. Mater Sci Eng C. 2012;32(6):1452–60.10.1016/j.msec.2012.04.025Search in Google Scholar PubMed
[58] Czepułkowska W, Wołowiec-Korecka E, editors. Analysis of the connection of a nickel-chromium alloy with dental ceramics after aluminum oxide abrasive blasting. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2020.10.1088/1757-899X/743/1/012043Search in Google Scholar
[59] Jiang Y, Zhang C, Xu J, Xu J, Zhan XQ, Ma N, et al. An overview of dental glass–ceramics: From material design to the manufacturing process. Int J Ceram Eng Sci. 2024;6:e10224.10.1002/ces2.10224Search in Google Scholar
[60] Benalcázar-Jalkh EB, Bergamo ET, Campos TM, Coelho PG, Sailer I, Yamaguchi S, et al. A narrative review on polycrystalline ceramics for dental applications and proposed update of a classification system. Materials. 2023;16(24):7541.10.3390/ma16247541Search in Google Scholar PubMed PubMed Central
[61] Khanlar LN, Salazar Rios A, Tahmaseb A, Zandinejad A. Additive manufacturing of zirconia ceramic and its application in clinical dentistry: A review. Dent J. 2021;9(9):104.10.3390/dj9090104Search in Google Scholar PubMed PubMed Central
[62] Burger W, Kiefer G. Alumina, zirconia and their composite ceramics with properties tailored for medical applications. J Compos Sci. 2021;5(11):306.10.3390/jcs5110306Search in Google Scholar
[63] Ali S, Alam BF, Rehman SU, Ahmad S, Iqbal K, Farooq I. Global research on dental polymers and their application: A bibliometric analysis and knowledge mapping. Saudi Dent J. 2023;35(2):197–205.10.1016/j.sdentj.2023.01.005Search in Google Scholar PubMed PubMed Central
[64] Xu X, He L, Zhu B, Li J, Li J. Advances in polymeric materials for dental applications. Polym Chem. 2017;8(5):807–23.10.1039/C6PY01957ASearch in Google Scholar
[65] Charasseangpaisarn T, Wiwatwarrapan C, Thunyakitpisal P, Srimaneepong V. Development of poly (methyl methacrylate)/poly (lactic acid) blend as sustainable biomaterial for dental applications. Sci Rep. 2023;13(1):16904.10.1038/s41598-023-44150-2Search in Google Scholar PubMed PubMed Central
[66] Imazato S, Ma S, Chen J-h, Xu HH. Therapeutic polymers for dental adhesives: loading resins with bio-active components. Dent Mater. 2014;30(1):97–104.10.1016/j.dental.2013.06.003Search in Google Scholar PubMed PubMed Central
[67] Xue J, Wang J, Feng D, Huang H, Wang M. Application of antimicrobial polymers in the development of dental resin composite. Molecules. 2020;25(20):4738.10.3390/molecules25204738Search in Google Scholar PubMed PubMed Central
[68] Kim KT, Eo MY, Nguyen TTH, Kim SM. General review of titanium toxicity. Int J Implant Dent. 2019;5:1–12.10.1186/s40729-019-0162-xSearch in Google Scholar PubMed PubMed Central
[69] Messous R, Henriques B, Bousbaa H, Silva FS, Teughels W, Souza JC. Cytotoxic effects of submicron-and nano-scale titanium debris released from dental implants: An integrative review. Clin Oral Investig. 2021;25:1627–40.10.1007/s00784-021-03785-zSearch in Google Scholar PubMed
[70] Shelly S, Zaltsman SL, Ben-Gal O, Dayan A, Ganmore I, Shemesh C, et al. Potential neurotoxicity of titanium implants: Prospective, in-vivo and in-vitro study. Biomaterials. 2021;276:121039.10.1016/j.biomaterials.2021.121039Search in Google Scholar PubMed
[71] Poli PP, de Miranda FV, Polo TOB, Santiago Júnior JF, Lima Neto TJ, Rios BR, et al. Titanium allergy caused by dental implants: A systematic literature review and case report. Materials. 2021;14(18):5239.10.3390/ma14185239Search in Google Scholar PubMed PubMed Central
[72] Kaur M, Singh K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater Sci Eng C. 2019;102:844–62.10.1016/j.msec.2019.04.064Search in Google Scholar PubMed
[73] Das K, Bose S, Bandyopadhyay A, Karandikar B, Gibbins BL. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. J Biomed Mater Res Part B: Appl Biomater. 2008;87(2):455–60.10.1002/jbm.b.31125Search in Google Scholar PubMed
[74] Liu N, Tang M. Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J Appl Toxicol. 2020;40(1):16–36.10.1002/jat.3817Search in Google Scholar PubMed
[75] Prasad S, Ehrensberger M, Gibson MP, Kim H, Monaco Jr EA. Biomaterial properties of titanium in dentistry. J Oral Biosci. 2015;57(4):192–9.10.1016/j.job.2015.08.001Search in Google Scholar
[76] Sotniczuk A, Garbacz H. Nanostructured bulk titanium with enhanced properties – strategies and prospects for dental applications. Adv Eng Mater. 2021;23(4):2000909.10.1002/adem.202000909Search in Google Scholar
[77] Ma N, Liu S, Liu W, Xie L, Wei D, Wang L, et al. Research progress of titanium-based high entropy alloy: methods, properties, and applications. Front Bioeng Biotechnol. 2020;8:603522.10.3389/fbioe.2020.603522Search in Google Scholar PubMed PubMed Central
[78] Noumbissi S, Scarano A, Gupta S. A literature review study on atomic ions dissolution of titanium and its alloys in implant dentistry. Materials. 2019;12(3):368.10.3390/ma12030368Search in Google Scholar PubMed PubMed Central
[79] Willis J, Li S, Crean SJ, Barrak FN. Is titanium alloy Ti‐6Al‐4 V cytotoxic to gingival fibroblasts – A systematic review. Clin Exp Dent Res. 2021;7(6):1037–44.10.1002/cre2.444Search in Google Scholar PubMed PubMed Central
[80] Cáceres D, Munuera C, Ocal C, Jiménez JA, Gutiérrez A, López MF. Nanomechanical properties of surface-modified titanium alloys for biomedical applications. Acta Biomater. 2008;4(5):1545–52.10.1016/j.actbio.2008.04.009Search in Google Scholar PubMed
[81] Papadiochou S, Pissiotis AL. Marginal adaptation and CAD-CAM technology: A systematic review of restorative material and fabrication techniques. J Prosthet Dent. 2018;119(4):545–51.10.1016/j.prosdent.2017.07.001Search in Google Scholar PubMed
[82] Hanawa T. Titanium–tissue interface reaction and its control with surface treatment. Front Bioeng Biotechnol. 2019;7:170.10.3389/fbioe.2019.00170Search in Google Scholar PubMed PubMed Central
[83] Hanawa T. Biocompatibility of titanium from the viewpoint of its surface. Sci Technol Adv Mater. 2022;23(1):457–72.10.1080/14686996.2022.2106156Search in Google Scholar PubMed PubMed Central
[84] Wang Z-M, Jia Y-F, Zhang X-C, Fu Y, Zhang C-C, Tu S-T. Effects of different mechanical surface enhancement techniques on surface integrity and fatigue properties of Ti-6Al-4V: a review. Crit Rev Solid State Mater Sci. 2019;44(6):445–69.10.1080/10408436.2018.1492368Search in Google Scholar
[85] Bosshardt DD, Chappuis V, Buser D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol. 20002017;73(1):22–40.10.1111/prd.12179Search in Google Scholar PubMed
[86] Chiapasco M, Casentini P, Zaniboni M, Corsi E, Anello T. Titanium–zirconium alloy narrow‐diameter implants (S traumann R oxolid®) for the rehabilitation of horizontally deficient edentulous ridges: prospective study on 18 consecutive patients. Clin Oral Implant Res. 2012;23(10):1136–41.10.1111/j.1600-0501.2011.02296.xSearch in Google Scholar PubMed
[87] Anchieta RB, Baldassarri M, Guastaldi F, Tovar N, Janal MN, Gottlow J, et al. Mechanical property assessment of bone healing around a titanium–zirconium alloy dental implant. Clin Implant Dent Relat Res. 2014;16(6):913–9.10.1111/cid.12061Search in Google Scholar PubMed
[88] Gottlow J, Dard M, Kjellson F, Obrecht M, Sennerby L. Evaluation of a new titanium‐zirconium dental implant: a biomechanical and histological comparative study in the mini pig. Clin Implant Dent Relat Res. 2012;14(4):538–45.10.1111/j.1708-8208.2010.00289.xSearch in Google Scholar PubMed
[89] Semiatin S. An overview of the thermomechanical processing of α/β titanium alloys: current status and future research opportunities. Metall Mater Trans A. 2020;51(6):2593–625.10.1007/s11661-020-05625-3Search in Google Scholar
[90] Ji X, Yu H, Guo B, Jiang F, Fu D, Teng J, et al. Post-dynamic α to β phase transformation and reverse transformation of Ti-5Al-3V alloy after hot deformation in two phase region. Mater Des. 2020;188:108466.10.1016/j.matdes.2019.108466Search in Google Scholar
[91] Herrmann H, Kern JS, Kern T, Lautensack J, Conrads G, Wolfart S. Early and mature biofilm on four different dental implant materials: an in vivo human study. Clin Oral Implant Res. 2020;31(11):1094–104.10.1111/clr.13656Search in Google Scholar PubMed
[92] Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri‐implantitis: a randomized controlled clinical study. J Clin Periodontol. 2011;38(3):276–84.10.1111/j.1600-051X.2010.01690.xSearch in Google Scholar PubMed
[93] Schwarz F, John G, Mainusch S, Sahm N, Becker J. Combined surgical therapy of peri‐implantitis evaluating two methods of surface debridement and decontamination. A two‐year clinical follow up report. J Clin Periodontol. 2012;39(8):789–97.10.1111/j.1600-051X.2012.01867.xSearch in Google Scholar PubMed
[94] van der Velden U. On the reliability of clinical attachment level measurements. J Clin Periodontol. 2022;49(11):1229.10.1111/jcpe.13702Search in Google Scholar PubMed PubMed Central
[95] Aragoneses J, Valverde NL, Fernandez-Dominguez M, Mena-Alvarez J, Rodriguez C, Gil J, et al. Relevant aspects of titanium and zirconia dental implants for their fatigue and osseointegration behaviors. Materials. 2022;15(11):4036.10.3390/ma15114036Search in Google Scholar PubMed PubMed Central
[96] Zhang Q, Zheng M, Huang Y, Kunte HJ, Wang X, Liu Y, et al. Long term corrosion estimation of carbon steel, titanium and its alloy in backfill material of compacted bentonite for nuclear waste repository. Sci Rep. 2019;9(1):3195.10.1038/s41598-019-39751-9Search in Google Scholar PubMed PubMed Central
[97] Viderščak D, Schauperl Z, Šolić S, Ćatić A, Godec M, Kocijan A, et al. Additively manufactured commercial Co–Cr dental alloys: comparison of microstructure and mechanical properties. Materials. 2021;14(23):7350.10.3390/ma14237350Search in Google Scholar PubMed PubMed Central
[98] Zhou Y, Li N, Yan J, Zeng Q. Comparative analysis of the microstructures and mechanical properties of Co–Cr dental alloys fabricated by different methods. J Prosthet Dent. 2018;120(4):617–23.10.1016/j.prosdent.2017.11.015Search in Google Scholar PubMed
[99] Manipal S, Mohan CA, Kumar DL, Cholan PK, Ahmed A, Adusumilli P. The importance of dental aesthetics among dental students assessment of knowledge. J Int Soc Prev Community Dent. 2014;4(1):48–51.10.4103/2231-0762.131266Search in Google Scholar PubMed PubMed Central
[100] Saito M, Arakaki R, Yamada A, Tsunematsu T, Kudo Y, Ishimaru N. Molecular mechanisms of nickel allergy. Int J Mol Sci. 2016;17(2):202.10.3390/ijms17020202Search in Google Scholar PubMed PubMed Central
[101] Uriciuc WA, Boșca AB, Babtan AM, Feurden CN, Ionel A, Vermeșan H, et al. Optimization of the manufacturing process by molding cobalt-chrome alloys in assembled dental frameworks. Prosthesis. 2021;3(3):245–60.10.3390/prosthesis3030024Search in Google Scholar
[102] Bojko Ł, Ryniewicz AM, Ryniewicz W. Strength tests of alloys for fixed structures in dental prosthetics. Materials. 2022;15(10):3497.10.3390/ma15103497Search in Google Scholar PubMed PubMed Central
[103] Nowak J, Steinberg K, Sokołowski J, Bociong K. Influence of various class cleaning agents for prosthesis on Co–Cr alloy surface. Open Chem. 2022;20(1):958–69.10.1515/chem-2022-0201Search in Google Scholar
[104] Odaira T, Xu S, Hirata K, Xu X, Omori T, Ueki K, et al. Flexible and tough superelastic Co–Cr alloys for biomedical applications. Adv Mater. 2022;34(27):2202305.10.1002/adma.202202305Search in Google Scholar PubMed
[105] Bauer J, Costa JF, Carvalho CN, Grande RHM, Loguercio AD, Reis A. Characterization of two Ni–Cr dental alloys and the influence of casting mode on mechanical properties. J Prosthodont Res. 2012;56(4):264–71.10.1016/j.jpor.2012.02.004Search in Google Scholar PubMed
[106] Saha S, Roy S. Metallic dental implants wear mechanisms, materials, and manufacturing processes: a literature review. Materials. 2022;16(1):161.10.3390/ma16010161Search in Google Scholar PubMed PubMed Central
[107] Majeed MN, Yousif QA, Bedair MA. Study of the corrosion of nickel–chromium alloy in an acidic solution protected by nickel nanoparticles. ACS Omega. 2022;7(34):29850–7.10.1021/acsomega.2c02679Search in Google Scholar PubMed PubMed Central
[108] Leban MB, Kosec T, Finšgar M. Corrosion characterization and ion release in SLM-manufactured and wrought Ti6Al4V alloy in an oral environment. Corros Sci. 2022;209:110716.10.1016/j.corsci.2022.110716Search in Google Scholar
[109] Barrios-Muriel J, Romero-Sánchez F, Alonso-Sánchez FJ, Salgado DR. Advances in orthotic and prosthetic manufacturing: a technology review. Materials. 2020;13(2):295.10.3390/ma13020295Search in Google Scholar PubMed PubMed Central
[110] Tobin EJ. Recent coating developments for combination devices in orthopedic and dental applications: A literature review. Adv Drug Deliv Rev. 2017;112:88–100.10.1016/j.addr.2017.01.007Search in Google Scholar PubMed
[111] Achitei DC, Baltatu MS, Vizureanu P, Sandu AV, Benchea M, Istrate B. Ni–Cr alloys assessment for dental implants suitability. Appl Sci. 2022;12(24):12814.10.3390/app122412814Search in Google Scholar
[112] Turdean GL, Craciun A, Popa D, Constantiniuc M. Study of electrochemical corrosion of biocompatible Co–Cr and Ni–Cr dental alloys in artificial saliva. Influence of pH of the solution. Mater Chem Phys. 2019;233:390–8.10.1016/j.matchemphys.2019.05.041Search in Google Scholar
[113] Porcayo-Calderon J, Casales-Diaz M, Salinas-Bravo V, Martinez-Gomez L. Corrosion performance of Fe‐Cr‐Ni alloys in artificial Saliva and Mouthwash solution. Bioinorg Chem Appl. 2015;2015(1):930802.10.1155/2015/930802Search in Google Scholar PubMed PubMed Central
[114] Xing F, Li S, Yin D, Xie J, Rommens PM, Xiang Z, et al. Recent progress in Mg-based alloys as a novel bioabsorbable biomaterials for orthopedic applications. J Magnes Alloy. 2022;10(6):1428–56.10.1016/j.jma.2022.02.013Search in Google Scholar
[115] Wang F, Semirumi D, He A, Pan Z, Alizadeh A. Physical, mechanical characterization, and artificial neural network modeling of biodegradable composite scaffold for biomedical applications. Eng Appl Artif Intell. 2024;136:108889.10.1016/j.engappai.2024.108889Search in Google Scholar
[116] Tripathi S, Raheem A, Dash M, Kumar P, Elsebahy A, Singh H, et al. Surface engineering of orthopedic implants for better clinical adoption. J Mater Chem B. 2024;12(44):11302–35.10.1039/D4TB01563KSearch in Google Scholar PubMed
[117] Alaneme KK, Kareem SA, Olajide JL, Sadiku RE, Bodunrin MO. Computational biomechanical and biodegradation integrity assessment of Mg-based biomedical devices for cardiovascular and orthopedic applications: A review. Int J Light Mater Manuf. 2022;5(2):251–66.10.1016/j.ijlmm.2022.02.003Search in Google Scholar
[118] Calazans Neto JV, Celles CA, de Andrade CS, Afonso CR, Nagay BE, Barão VA. Recent advances and prospects in β-type titanium alloys for dental implants applications. ACS Biomater Sci Eng. 2024;10(10):6029–60.10.1021/acsbiomaterials.4c00963Search in Google Scholar PubMed PubMed Central
[119] Hildebrand HF, Veron C, Martin P. Nickel, chromium, cobalt dental alloys and allergic reactions: an overview. Biomaterials. 1989;10(8):545–8.10.1016/0142-9612(89)90060-4Search in Google Scholar PubMed
[120] Arakelyan M, Spagnuolo G, Iaculli F, Dikopova N, Antoshin A, Timashev P, et al. Minimization of adverse effects associated with dental alloys. Materials. 2022;15(21):7476.10.3390/ma15217476Search in Google Scholar PubMed PubMed Central
[121] Sassi W, Boubaker H, Bahar S, Othman M, Ghorbal A, Zrelli R, et al. A challenge to succeed the electroplating of nanocomposite Ni–Cr alloy onto porous substrate under ultrasonic waves and from a continuous flow titanium nanofluids. J Alloy Compd. 2020;828:154437.10.1016/j.jallcom.2020.154437Search in Google Scholar
[122] Yun C-S, Hanawa T, Hong M-H, Min BK, Kwon T-Y. Biocompatibility of Ni–Cr alloys, with the same composition, prepared by two new digital manufacturing techniques. Mater Lett. 2021;305:130761.10.1016/j.matlet.2021.130761Search in Google Scholar
[123] Fu L, Engqvist H, Xia W. Glass–ceramics in dentistry: A review. Materials. 2020;13(5):1049.10.3390/ma13051049Search in Google Scholar PubMed PubMed Central
[124] Galante R, Figueiredo-Pina CG, Serro AP. Additive manufacturing of ceramics for dental applications: A review. Dent Mater. 2019;35(6):825–46.10.1016/j.dental.2019.02.026Search in Google Scholar PubMed
[125] Sarna-Boś K, Skic K, Sobieszczański J, Boguta P, Chałas R. Contemporary approach to the porosity of dental materials and methods of its measurement. Int J Mol Sci. 2021;22(16):8903.10.3390/ijms22168903Search in Google Scholar PubMed PubMed Central
[126] Špehar D, Jakovac M. Clinical evaluation of reduced-thickness monolithic lithium-disilicate crowns: One-year follow-up results. Processes. 2021;9(12):2119.10.3390/pr9122119Search in Google Scholar
[127] Mahrous A, Schneider GB. Enhancing student learning of removable prosthodontics using the latest advancements in virtual 3D modeling. J Prosthodont. 2019;28(4):471–2.10.1111/jopr.13044Search in Google Scholar PubMed
[128] Kaushik A, Gahletia S, Garg RK, Sharma P, Chhabra D, Yadav M, eds. Advanced 3D body scanning techniques and its clinical applications. 2022 International Conference on Computational Modelling, Simulation and Optimization (ICCMSO). IEEE; 2022.10.1109/ICCMSO58359.2022.00074Search in Google Scholar
[129] Möller B, Terheyden H, Açil Y, Purcz N, Hertrampf K, Tabakov A, et al. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: an in vivo and in vitro study. Int J Oral Maxillofac Surg. 2012;41(5):638–45.10.1016/j.ijom.2012.02.004Search in Google Scholar PubMed
[130] Sharma A, Bharti PS, Kaushik A, Punia U, Garg RK, Yadav M, et al. 3D Printable titanium alloys and their properties in biomedical applications: state of the art. Mechanical properties and characterization of additively manufactured materials. CRC Press; 2023. p. 107–20.10.1201/9781003430186-8Search in Google Scholar
[131] Kozlovskiy AL, Alin M, Borgekov DB. Study of polymorphic transformation processes and their influence in polycrystalline ZrO2 ceramics upon irradiation with heavy ions. Ceramics. 2023;6(1):686–706.10.3390/ceramics6010042Search in Google Scholar
[132] Zhang F, Spies BC, Vleugels J, Reveron H, Wesemann C, Müller W-D, et al. High-translucent yttria-stabilized zirconia ceramics are wear-resistant and antagonist-friendly. Dent Mater. 2019;35(12):1776–90.10.1016/j.dental.2019.10.009Search in Google Scholar PubMed
[133] Yoshida H, Matsui K, Ikuhara Y. Low‐temperature superplasticity in nanocrystalline tetragonal zirconia polycrystal (TZP). J Am Ceram Soc. 2012;95(5):1701–8.10.1111/j.1551-2916.2012.05150.xSearch in Google Scholar
[134] Spitznagel F, Boldt J, Gierthmuehlen P. CAD/CAM ceramic restorative materials for natural teeth. J Dent Res. 2018;97(10):1082–91.10.1177/0022034518779759Search in Google Scholar PubMed
[135] Rusu L-C, Ardelean LC, Jitariu A-A, Miu CA, Streian CG. An insight into the structural diversity and clinical applicability of polyurethanes in biomedicine. Polymers. 2020;12(5):1197.10.3390/polym12051197Search in Google Scholar PubMed PubMed Central
[136] Tigmeanu CV, Ardelean LC, Rusu L-C, Negrutiu M-L. Additive manufactured polymers in dentistry, current state-of-the-art and future perspectives-a review. Polymers. 2022;14(17):3658.10.3390/polym14173658Search in Google Scholar PubMed PubMed Central
[137] Tzeng J-J, Yang T-S, Lee W-F, Chen H, Chang H-M. Mechanical properties and biocompatibility of urethane acrylate-based 3D-printed denture base resin. Polymers. 2021;13(5):822.10.3390/polym13050822Search in Google Scholar PubMed PubMed Central
[138] Deng Y, Li J, He Z, Hong J, Bao J. Urethane acrylate‐based photosensitive resin for three‐dimensional printing of stereolithographic elastomer. J Appl Polym Sci. 2020;137(42):49294.10.1002/app.49294Search in Google Scholar
[139] Chen H, Lee S-Y, Lin Y-M. Synthesis and formulation of PCL-based urethane acrylates for DLP 3D printers. Polymers. 2020;12(7):1500.10.3390/polym12071500Search in Google Scholar PubMed PubMed Central
[140] Khorsandi D, Fahimipour A, Abasian P, Saber SS, Seyedi M, Ghanavati S, et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2021;122:26–49.10.1016/j.actbio.2020.12.044Search in Google Scholar PubMed
[141] Ziąbka M, Dziadek M, Pielichowska K. Surface and structural properties of medical acrylonitrile butadiene styrene modified with silver nanoparticles. Polymers. 2020;12(1):197.10.3390/polym12010197Search in Google Scholar PubMed PubMed Central
[142] Shrivastava SP, Dable R, Raj AN, Mutneja P, Srivastava SB, Haque M. Comparison of mechanical properties of PEEK and PMMA: An in vitro study. J Contemp Dent Pract. 2021;22:179–83.10.5005/jp-journals-10024-3077Search in Google Scholar
[143] Kaushik A, Garg RK. Searching the optimal parameters of a 3D scanner in surface reconstruction of a dental model using central composite design coupled with metaheuristic algorithms. Int J Interact Des Manuf. 2023;18:7401–11.10.1007/s12008-023-01587-zSearch in Google Scholar
[144] McBride AK, Turek SL, Zaghi AE, Burke KA. Mechanical behavior of hybrid glass/steel fiber reinforced epoxy composites. Polymers. 2017;9(4):151.10.3390/polym9040151Search in Google Scholar PubMed PubMed Central
[145] Joyce K, Fabra GT, Bozkurt Y, Pandit A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct Target. 2021;6(1):122.10.1038/s41392-021-00512-8Search in Google Scholar PubMed PubMed Central
[146] Alavi SE, Gholami M, Shahmabadi HE, Reher P. Resorbable GBR scaffolds in oral and maxillofacial tissue engineering: design, fabrication, and applications. J Clin Med. 2023;12(22):6962.10.3390/jcm12226962Search in Google Scholar PubMed PubMed Central
[147] Wang F, Harindintwali JD, Yuan Z, Wang M, Wang F, Li S, et al. Technologies and perspectives for achieving carbon neutrality. Innov. 2021;2(4):100180.10.1016/j.xinn.2021.100180Search in Google Scholar PubMed PubMed Central
[148] Xie C, Lu H, Li W, Chen F-M, Zhao Y-M. The use of calcium phosphate-based biomaterials in implant dentistry. J Mater Sci Mater Med. 2012;23:853–62.10.1007/s10856-011-4535-9Search in Google Scholar PubMed
[149] Cuylear DL, Elghazali NA, Kapila SD, Desai TA. Calcium phosphate delivery systems for regeneration and biomineralization of mineralized tissues of the craniofacial complex. Mol Pharm. 2023;20(2):810–28.10.1021/acs.molpharmaceut.2c00652Search in Google Scholar PubMed PubMed Central
[150] Al-Sanabani JS, Madfa AA, Al-Sanabani FA. Application of calcium phosphate materials in dentistry. Int J Biomater. 2013;2013(1):876132.10.1155/2013/876132Search in Google Scholar PubMed PubMed Central
[151] Drevet R, Fauré J, Benhayoune H. Bioactive calcium phosphate coatings for bone implant applications: a review. Coatings. 2023;13(6):1091.10.3390/coatings13061091Search in Google Scholar
[152] Boone II ME, Kafrawy A. Pulp reaction to a tricalcium phosphate ceramic capping agent. Oral Surg Oral Med Oral Pathol. 1979;47(4):369–71.10.1016/0030-4220(79)90262-7Search in Google Scholar PubMed
[153] Harbert H. Generic tricalcium phosphate plugs: an adjunct in endodontics. J Endod. 1991;17(3):131–4.10.1016/S0099-2399(06)81746-2Search in Google Scholar PubMed
[154] Mors W, Kaminski E. Osteogenic replacement of tricalcium phosphate ceramic implants in the dog palate. Arch Oral Biol. 1975;20(5–6):365–IN5.10.1016/0003-9969(75)90028-XSearch in Google Scholar
[155] Dorozhkin SV. Calcium orthophosphates as bioceramics: state of the art. J Funct Biomater. 2010;1(1):22–107.10.3390/jfb1010022Search in Google Scholar PubMed PubMed Central
[156] Jeong J, Kim JH, Shim JH, Hwang NS, Heo CY. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23(1):4.10.1186/s40824-018-0149-3Search in Google Scholar PubMed PubMed Central
[157] Von Arx T, Schenk RK, Buser D, Von Arx T, Cochran DL, Hermann JS, et al. Lateral ridge augmentation using different bone fillers and barrier membrane application: a histologic and histomorphometric pilot study in the canine mandible. Biomater Res. 2001;12(3):260–9.10.1034/j.1600-0501.2001.012003260.xSearch in Google Scholar PubMed
[158] Kalita SJ, Bhatt HA, Dhamne A. MgO–Na2O–P2O5‐based sintering additives for tricalcium phosphate bioceramics. J Am Ceram Soc. 2006;89(3):875–81.10.1111/j.1551-2916.2005.00854.xSearch in Google Scholar
[159] Al-Sabri FAA. Application of calcium phosphate materials in dentistry: a review. Tham Univ J Nat Appl Sci. 2011;4(1):1–19.10.59167/tujnas.v4i4.1287Search in Google Scholar
[160] Karunakaran D, Jeyachandran A, Venkatachalapathy V. Analysis of mechanical properties of Al2O3 coated dental implants. IJERT. 2015;4(9):373–5.10.17577/IJERTV4IS090395Search in Google Scholar
[161] Jaffe WL, Scott DF. Current concepts review-total hip arthroplasty with hydroxyapatite-coated prostheses. JBJS. 1996;78(12):1918–34.10.2106/00004623-199612000-00018Search in Google Scholar PubMed
[162] Yang JZ, Sultana R, Hu XZ, Ichim P. Novel layered hydroxyapatite/tri‐calcium phosphate–zirconia scaffold composite with high bending strength for load‐bearing bone implant application. Int J Appl Ceram Technol. 2014;11(1):22–30.10.1111/ijac.12024Search in Google Scholar
[163] Sakkers R, Dalmeyer R, Brand R, Rozing P, Van Blitterswijk C. Assessment of bioactivity for orthopedic coatings in a gap‐healing model. J Biomed Mater Res. 1997;36(2):265–73.10.1002/(SICI)1097-4636(199708)36:2<265::AID-JBM16>3.0.CO;2-FSearch in Google Scholar
[164] Thandavamoorthy R, Devarajan Y. Study on the characteristics of Napier grass fibre reinforced porcelain filler particulates poly lactic acid matrix biocomposite. J Reinf Plast Compos. 2024;07316844241253912.10.1177/07316844241253912Search in Google Scholar
[165] Mendelson BC, Jacobson SR, Lavoipierre AM, Huggins RJ. The fate of porous hydroxyapatite granules used in facial skeletal augmentation. Aesth Plast Surg. 2010;34:455–61.10.1007/s00266-010-9473-2Search in Google Scholar
[166] Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res. 1998;13(1):94–117.10.1557/JMR.1998.0015Search in Google Scholar
[167] Siddiqui HA, Pickering KL, Mucalo MR. A review on the use of hydroxyapatite-carbonaceous structure composites in bone replacement materials for strengthening purposes. Materials. 2018;11(10):1813.10.3390/ma11101813Search in Google Scholar
[168] Li X, Lv Z, Zhu H. Carbon/silicon heterojunction solar cells: state of the art and prospects. Adv Mater. 2015;27(42):6549–74.10.1002/adma.201502999Search in Google Scholar
[169] Bin-Jardan LI, Almadani DI, Almutairi LS, Almoabid HA, Alessa MA, Almulhim KS, et al. Inorganic compounds as remineralizing fillers in dental restorative materials: narrative review. Int J Mol Sci. 2023;24(9):8295.10.3390/ijms24098295Search in Google Scholar
[170] Siraparapu YD, Bassa S, Sanasi PD. A review on recent applications of biomaterials. Int J Sci Res. 2013;1:70–5.Search in Google Scholar
[171] Petersen R. Carbon fiber biocompatibility for implants. Fibers. 2016;4(1):1.10.3390/fib4010001Search in Google Scholar
[172] Chen H, Wang P, Pan J, Lawi AS, Zhu Y. Effect of alkali-resistant glass fiber and silica fume on mechanical and shrinkage properties of cement-based mortars. Constr Build Mater. 2021;307:125054.10.1016/j.conbuildmat.2021.125054Search in Google Scholar
[173] Ahmad J, González-Lezcano RA, Majdi A, Ben Kahla N, Deifalla AF, El-Shorbagy MA. Glass fibers reinforced concrete: Overview on mechanical, durability and microstructure analysis. Materials. 2022;15(15):5111.10.3390/ma15155111Search in Google Scholar PubMed PubMed Central
[174] Raja T, Devarajan Y. Study on the characteristics of novel fiber extracted from Foxtail palm fruits–A steps towards the sustainable development. Mater Lett. 2024;373:137145.10.1016/j.matlet.2024.137145Search in Google Scholar
[175] Xiang Q, Liu Y, Sheng X, Dan X. Preparation of mica-based glass-ceramics with needle-like fluorapatite. Dent Mater. 2007;23(2):251–8.10.1016/j.dental.2006.10.008Search in Google Scholar PubMed
[176] El-Meliegy E, van Noort R, El-Meliegy E, van Noort R. Machinable mica dental glass-ceramics. Glass Technol. 2012;193–208.10.1007/978-1-4614-1228-1_11Search in Google Scholar
[177] Tidehag P, Shen Z. Digital dentistry calls the change of ceramics and ceramic processes. Adv Appl Ceram. 2019;118(1–2):83–90.10.1080/17436753.2018.1511337Search in Google Scholar
[178] Lambert H, Durand J-C, Jacquot B, Fages M. Dental biomaterials for chairside CAD/CAM: State of the art. J Adv Prosthodont. 2017;9(6):486.10.4047/jap.2017.9.6.486Search in Google Scholar PubMed PubMed Central
[179] Heimann RB. Classic and advanced ceramics: from fundamentals to applications. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA; 2010.10.1002/9783527630172Search in Google Scholar
[180] Wang Y, Zhu M, Zhu X. Functional fillers for dental resin composites. Acta Biomater. 2021;122:50–65.10.1016/j.actbio.2020.12.001Search in Google Scholar PubMed
[181] Alavi SE, Panah N, Page F, Gholami M, Dastfal A, Sharma LA, et al. Hydrogel-based therapeutic coatings for dental implants. Eur Polym J. 2022;181:111652.10.1016/j.eurpolymj.2022.111652Search in Google Scholar
[182] David P, Gopathy V, Ravichandran M, Mohanavel V, Barve A, Raja T, et al. Fabrication and development of high temperature resisted bronze composites using 3D printed gate pattern through stir casting route. Therm Sci Eng Prog. 2024;53:102710.10.1016/j.tsep.2024.102710Search in Google Scholar
[183] Norowski PA, Courtney HS, Babu J, Haggard WO, Bumgardner JD. Chitosan coatings deliver antimicrobials from titanium implants: a preliminary study. Implant Dent. 2011;20(1):56–67.10.1097/ID.0b013e3182087ac4Search in Google Scholar PubMed
[184] Shekwa W. An investigation of antimicrobial compounds for dental health care from the leaf and stem extracts of carissa bispinosa (L.) desf ex brenan (apocynaceae). University of Limpopo; 2022.Search in Google Scholar
[185] Swain S, Patra A, Dwibedy P, Guru BMP, Rautray TR. Electrochemical synthesis of ceramics for biomedical applications. Advanced ceramic coatings for biomedical applications. Elsevier series in advanced ceramic materials; 2023. p. 87–110.10.1016/B978-0-323-99626-6.00007-XSearch in Google Scholar
[186] Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants–a review. Prog Mater Sci. 2009;54(3):397–425.10.1016/j.pmatsci.2008.06.004Search in Google Scholar
[187] Ribeiro AM, Flores-Sahagun TH, Paredes RC. A perspective on molybdenum biocompatibility and antimicrobial activity for applications in implants. J Mater Sci. 2016;51:2806–16.10.1007/s10853-015-9664-ySearch in Google Scholar
[188] Najeeb S, Zafar MS, Khurshid Z, Siddiqui F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J Prosthodont Res. 2016;60(1):12–9.10.1016/j.jpor.2015.10.001Search in Google Scholar PubMed
[189] Alqurashi H, Khurshid Z, Syed AUY, Habib SR, Rokaya D, Zafar MS. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J Adv Res. 2021;28:87–95.10.1016/j.jare.2020.09.004Search in Google Scholar PubMed PubMed Central
[190] Ligon SC, Liska R, Stampfl J, Gurr M, Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev. 2017;117(15):10212–90.10.1021/acs.chemrev.7b00074Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Development and performance evaluation of green aluminium metal matrix composites reinforced with graphene nanopowder and marble dust
- Morphological, physical, thermal, and mechanical properties of carbon nanotubes reinforced arrowroot starch composites
- Influence of the graphene oxide nanosheet on tensile behavior and failure characteristics of the cement composites after high-temperature treatment
- Central composite design modeling in optimizing heat transfer rate in the dissipative and reactive dynamics of viscoplastic nanomaterials deploying Joule and heat generation aspects
- Double diffusion of nano-enhanced phase change materials in connected porous channels: A hybrid ISPH-XGBoost approach
- Synergistic impacts of Thompson–Troian slip, Stefan blowing, and nonuniform heat generation on Casson nanofluid dynamics through a porous medium
- Optimization of abrasive water jet machining parameters for basalt fiber/SiO2 nanofiller reinforced composites
- Enhancing aesthetic durability of Zisha teapots via TiO2 nanoparticle surface modification: A study on self-cleaning, antimicrobial, and mechanical properties
- Nanocellulose solution based on iron(iii) sodium tartrate complexes
- Combating multidrug-resistant infections: Gold nanoparticles–chitosan–papain-integrated dual-action nanoplatform for enhanced antibacterial activity
- Novel royal jelly-mediated green synthesis of selenium nanoparticles and their multifunctional biological activities
- Direct bandgap transition for emission in GeSn nanowires
- Synthesis of ZnO nanoparticles with different morphologies using a microwave-based method and their antimicrobial activity
- Numerical investigation of convective heat and mass transfer in a trapezoidal cavity filled with ternary hybrid nanofluid and a central obstacle
- Halloysite nanotube enhanced polyurethane nanocomposites for advanced electroinsulating applications
- Low molar mass ionic liquid’s modified carbon nanotubes and its role in PVDF crystalline stress generation
- Green synthesis of polydopamine-functionalized silver nanoparticles conjugated with Ceftazidime: in silico and experimental approach for combating antibiotic-resistant bacteria and reducing toxicity
- Evaluating the influence of graphene nano powder inclusion on mechanical, vibrational and water absorption behaviour of ramie/abaca hybrid composites
- Dynamic-behavior of Casson-type hybrid nanofluids due to a stretching sheet under the coupled impacts of boundary slip and reaction-diffusion processes
- Influence of polyvinyl alcohol on the physicochemical and self-sensing properties of nano carbon black reinforced cement mortar
- Advanced machine learning approaches for predicting compressive and flexural strength of carbon nanotube–reinforced cement composites: a comparative study and model interpretability analysis
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Advances in the design and manipulation of self-assembling peptide and protein nanostructures for biomedical applications
- Photocatalytic pervious concrete systems: from classic photocatalysis to luminescent photocatalysis
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”
Articles in the same Issue
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Development and performance evaluation of green aluminium metal matrix composites reinforced with graphene nanopowder and marble dust
- Morphological, physical, thermal, and mechanical properties of carbon nanotubes reinforced arrowroot starch composites
- Influence of the graphene oxide nanosheet on tensile behavior and failure characteristics of the cement composites after high-temperature treatment
- Central composite design modeling in optimizing heat transfer rate in the dissipative and reactive dynamics of viscoplastic nanomaterials deploying Joule and heat generation aspects
- Double diffusion of nano-enhanced phase change materials in connected porous channels: A hybrid ISPH-XGBoost approach
- Synergistic impacts of Thompson–Troian slip, Stefan blowing, and nonuniform heat generation on Casson nanofluid dynamics through a porous medium
- Optimization of abrasive water jet machining parameters for basalt fiber/SiO2 nanofiller reinforced composites
- Enhancing aesthetic durability of Zisha teapots via TiO2 nanoparticle surface modification: A study on self-cleaning, antimicrobial, and mechanical properties
- Nanocellulose solution based on iron(iii) sodium tartrate complexes
- Combating multidrug-resistant infections: Gold nanoparticles–chitosan–papain-integrated dual-action nanoplatform for enhanced antibacterial activity
- Novel royal jelly-mediated green synthesis of selenium nanoparticles and their multifunctional biological activities
- Direct bandgap transition for emission in GeSn nanowires
- Synthesis of ZnO nanoparticles with different morphologies using a microwave-based method and their antimicrobial activity
- Numerical investigation of convective heat and mass transfer in a trapezoidal cavity filled with ternary hybrid nanofluid and a central obstacle
- Halloysite nanotube enhanced polyurethane nanocomposites for advanced electroinsulating applications
- Low molar mass ionic liquid’s modified carbon nanotubes and its role in PVDF crystalline stress generation
- Green synthesis of polydopamine-functionalized silver nanoparticles conjugated with Ceftazidime: in silico and experimental approach for combating antibiotic-resistant bacteria and reducing toxicity
- Evaluating the influence of graphene nano powder inclusion on mechanical, vibrational and water absorption behaviour of ramie/abaca hybrid composites
- Dynamic-behavior of Casson-type hybrid nanofluids due to a stretching sheet under the coupled impacts of boundary slip and reaction-diffusion processes
- Influence of polyvinyl alcohol on the physicochemical and self-sensing properties of nano carbon black reinforced cement mortar
- Advanced machine learning approaches for predicting compressive and flexural strength of carbon nanotube–reinforced cement composites: a comparative study and model interpretability analysis
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Advances in the design and manipulation of self-assembling peptide and protein nanostructures for biomedical applications
- Photocatalytic pervious concrete systems: from classic photocatalysis to luminescent photocatalysis
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”

