Abstract
C22H18N2O3, monoclinic, C2/c (no. 15), a = 35.4037(10) Å, b = 6.0294(2) Å, c = 20.1702(7) Å, β = 122.239(4)°, V = 3641.8(2) Å3, Z = 8, Rgt(F) = 0.0482, wRref(F2) = 0.1406, T = 296(2) K.
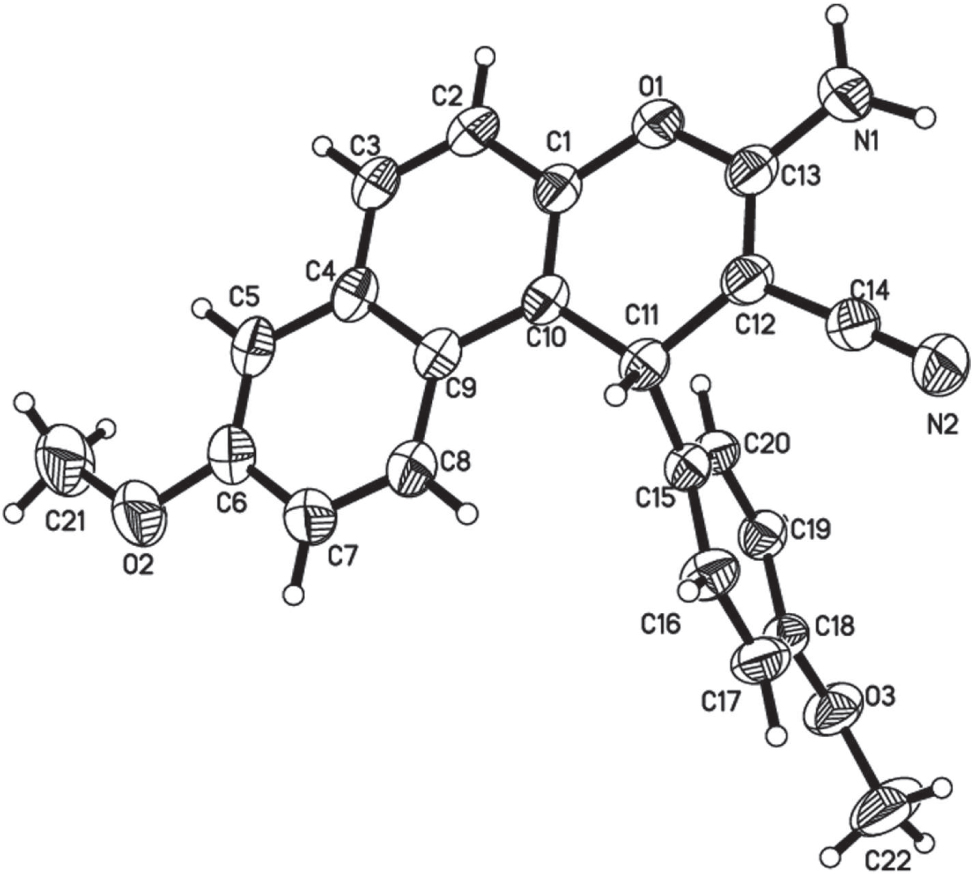
The title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.33 × 0.26 × 0.15 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 7.1 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 133.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 15808, 3215, 0.051 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2112 |
| N(param)refined: | 254 |
| Programs: | SHELX [19], Bruker programs [20] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.39568(5) | 0.4510(3) | 0.35450(9) | 0.0520(4) |
| O2 | 0.18840(6) | 0.2801(4) | 0.38573(13) | 0.0857(7) |
| O3 | 0.45879(6) | 0.6747(3) | 0.77610(9) | 0.0675(5) |
| N1 | 0.45598(8) | 0.6474(4) | 0.38627(15) | 0.0655(7) |
| N2 | 0.45686(7) | 1.1159(4) | 0.49854(13) | 0.0604(6) |
| C1 | 0.35758(7) | 0.4052(4) | 0.35625(12) | 0.0442(5) |
| C2 | 0.33393(7) | 0.2191(4) | 0.31141(13) | 0.0509(6) |
| H2A | 0.3439 | 0.1396 | 0.2829 | 0.061* |
| C3 | 0.29653(7) | 0.1542(4) | 0.30926(13) | 0.0515(6) |
| H3A | 0.2808 | 0.0266 | 0.2798 | 0.062* |
| C4 | 0.28068(7) | 0.2727(4) | 0.34997(12) | 0.0464(5) |
| C5 | 0.24123(7) | 0.2065(4) | 0.34635(14) | 0.0559(6) |
| H5A | 0.2256 | 0.0779 | 0.3175 | 0.067* |
| C6 | 0.22572(8) | 0.3270(5) | 0.38428(15) | 0.0604(7) |
| C7 | 0.24831(8) | 0.5201(5) | 0.42562(16) | 0.0666(7) |
| H7A | 0.2368 | 0.6054 | 0.4506 | 0.080* |
| C8 | 0.28637(8) | 0.5860(4) | 0.43024(15) | 0.0593(7) |
| H8A | 0.3012 | 0.7163 | 0.4588 | 0.071* |
| C9 | 0.30428(7) | 0.4645(4) | 0.39334(12) | 0.0447(5) |
| C10 | 0.34458(7) | 0.5288(4) | 0.39763(12) | 0.0427(5) |
| C11 | 0.37116(7) | 0.7288(4) | 0.44528(12) | 0.0438(5) |
| H11A | 0.3508 | 0.8603 | 0.4259 | 0.053* |
| C12 | 0.40867(7) | 0.7727(4) | 0.43146(12) | 0.0459(5) |
| C13 | 0.42061(7) | 0.6313(4) | 0.39378(13) | 0.0478(6) |
| C14 | 0.43557(7) | 0.9628(4) | 0.46799(13) | 0.0470(5) |
| C15 | 0.39082(7) | 0.7074(4) | 0.53316(12) | 0.0411(5) |
| C16 | 0.38758(8) | 0.8802(4) | 0.57454(13) | 0.0536(6) |
| H16A | 0.3698 | 1.0055 | 0.5469 | 0.064* |
| C17 | 0.40960(8) | 0.8754(4) | 0.65561(14) | 0.0570(6) |
| H17A | 0.4070 | 0.9966 | 0.6831 | 0.068* |
| C18 | 0.43500(7) | 0.6955(4) | 0.69573(12) | 0.0482(6) |
| C19 | 0.43809(7) | 0.5169(4) | 0.65544(13) | 0.0478(6) |
| H19A | 0.4552 | 0.3901 | 0.6832 | 0.057* |
| C20 | 0.41615(7) | 0.5245(4) | 0.57464(12) | 0.0448(5) |
| H20A | 0.4185 | 0.4026 | 0.5472 | 0.054* |
| C21 | 0.16506(11) | 0.0831(6) | 0.3491(2) | 0.1030(13) |
| H21A | 0.1418 | 0.0585 | 0.3607 | 0.154* |
| H21B | 0.1859 | −0.0422 | 0.3687 | 0.154* |
| H21C | 0.1512 | 0.0962 | 0.2922 | 0.154* |
| C22 | 0.46633(12) | 0.8713(6) | 0.82080(17) | 0.0942(11) |
| H22A | 0.4871 | 0.8389 | 0.8765 | 0.141* |
| H22B | 0.4379 | 0.9242 | 0.8124 | 0.141* |
| H22C | 0.4792 | 0.9858 | 0.8041 | 0.141* |
| H2N1 | 0.4776(9) | 0.720(4) | 0.4173(16) | 0.063(8)* |
| H1N1 | 0.4586(10) | 0.546(6) | 0.3549(19) | 0.097(11)* |
Source of material
A mixture of 6-methoxy-2-naphthol (0.01 mol), 4-anisaldehyde (0.01 mol), and malononitrile (0.01 mol), in absolute ethanol (30 mL) in the presence of piperidine (0.5 mL) was heated under microwave irradiation conditions for 2 min at 140 °C. The formed solid product was collected by filtration, washed with methanol and recrystallized from ethanol to give the title compound as yellow crystals; yield 88%; M.p.: 495–496 K (Lit. M.p.: 493–494 K [1]).
Experimental details
The methyl groups were idealized and refined using rigid groups allowed to rotate about the C—C bond (AFIX 137 option of the SHELX program [13]). The Uiso values of the hydrogen atoms of methyl groups were set to 1.5Ueq(C) and the Uiso values of all other hydrogen atoms were set to 1.2Ueq(C, N).
Discussion
Heterocyclic compounds containing oxygen are of interest due to their properties as new drugs. Literature reveals that chromenes and benzochromenes are an important chemical synthon, associated with a broad range of pharmacological activities such as antimicrobial [2], [3], [4], [5], anticancer agent [6] hypolipidemic [7], antioxidant [8, 9] , analgesic [10], antileishmanial [11, 12] , vascular-disrupting activity [13], estrogenic anticoagulant and antispasmolytic [14], blood platelet antiaggregating [15] effects and activities. As part of our programmed aim to develop new methodologies for the preparation of 1H-benzo[f]chromene derivatives [16], [17], [18], we have synthesized the title compound under microwave irradiation conditions.
In the title compound, the asymmetric unit of the title compound contains one independent molecule. The molecules are packed in the crystal structure via two strong classical intermolecular hydrogen bonds, N1—H2N1⋯N2i and N1—H1N1⋯O3ii. The H⋯A distances are 2.25(3) and 2.08(4) Å, respectively and the angles are 172(3) and 175(3)°, respectively. Symmetry codes: (i) −x + 1, −y + 2, −z + 1; (ii) x, −y + 1, z−1/2. All bond lengths and angles are in the expected ranges.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
References
1 Eid, F. A.; Abd El-Wahab, A. H. F.; Khafagy, M. M.; El-Hag Ali, G. A. M.: Synthesis and antimicrobial evaluation of naphtho[2,1-b]pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1, 2, 4]-triazolo[1,5-c]pyrimidine derivatives. Acta Pharm. 54 (2004) 13–26.Suche in Google Scholar
2 Singh, G.; Sharma, A.; Kaur, H.; Ishar, M.: Chromanyl-isoxazolidines as antibacterial agents: synthesis, biological evaluation, quantitative structure activity relationship, and molecular docking studies. Chem. Biol. Drug. Des. (2016) 87 213–223.10.1111/cbdd.12653Suche in Google Scholar PubMed
3 Vala, N. D.; Jardosh, H. H.; Patel, M. P.: 5-PS-TBD triggered general protocol for the synthesis of 4H-chromenes, pyrano[4,3-b]pyran and pyrano[3,2-c]chromene derivatives of 1H-pyrazole and their biological activities. Chin. Chem. Lett. 27 (2016) 168–172.10.1002/chin.201622150Suche in Google Scholar
4 Bingi, C.; Emmadi, N. R.; Chennapuram, M.; Poornachandra, Y.; Kumar, C. G.; Nanubolu, J. B.; Atmakur, K.: One-pot catalyst free synthesis of novel kojic acid tagged 2-aryl/alkyl substituted-4H-chromenes and evaluation of their antimicrobial and anti-biofilm. Bioorg. Med. Chem. Lett. 25 (2015) 1915–1919.10.1016/j.bmcl.2015.03.034Suche in Google Scholar PubMed
5 Killander, D.; Sterner, O.: Synthesis of the bioactive benzochromenes pulchrol and pulchral metabolites. Eur. J. Org. Chem. 8 (2014) 1594–1596.10.1002/ejoc.201301792Suche in Google Scholar
6 Reddy, B. V. S.; Divya, B.; Swaina, M.; Rao, T. P.; Yadav, J. S.; Vishnu Vardhan, M. V.: A domino Knoevenagel hetero-Diels-Alder reaction for the synthesis of polycyclic chromene derivatives and evaluation of their cytotoxicity. Bioorg. Med. Chem. Lett. 22 (2012) 1995–1999.10.1016/j.bmcl.2012.01.033Suche in Google Scholar PubMed
7 Sashidhara, K. V.; Kumar, M.; Modukuri, R. K.; Srivastava, A.; Puri, A.: Discovery and synthesis of novel substituted benzocoumarins as orally active lipid modulating agents. Bioorg. Med. Chem. Lett. 21 (2011) 6709–6713.10.1016/j.bmcl.2011.09.053Suche in Google Scholar PubMed
8 Fadda, A. A.; Berghot, M. A.; Amer, F. A.; Badawy, D. S.; Bayoumy, N. M.: Synthesis and antioxidant and antitumor activity of novel pyridine, chromene, thiophene and thiazole derivatives. Arch. Pharm. Chem. 345 (2012) 378–385.10.1002/ardp.201100335Suche in Google Scholar PubMed
9 Nareshkumar, J.; Jiayi, X.; Ramesh, M. K.; Fuyong, D.; Guo, J. Z.; Emmanuel, P.: Identification and structure-activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J. Med. Chem. 52 (2009) 7544–7569.10.1021/jm900146eSuche in Google Scholar PubMed
10 El-Sayed, A. T.; Ibrahim, M.: A. Synthesis and antimicrobial aActivity of chromone-linked-2-pyridone fused with 1,2,4-triazoles, 1,2,4-triazines and 1,2,4-triazepines ring systems. J. Braz. Chem. 21 (2010) 1007–1016.10.1590/S0103-50532010000600010Suche in Google Scholar
11 Foroumadi, A.; Emami, S.; Sorkhi, M.; Nakhjiri, M.; Nazarian, Z.; Heydar, S.; Ardestani, S.; Poorrajab, F.; Shafiee, A.: Chromene-based synthetic chalcones as potent antileishmani-al agents: synthesis and biological activity. Chem. Biol. Drug. Des. 75 (2010) 590–596.10.1111/j.1747-0285.2010.00959.xSuche in Google Scholar PubMed
12 Tanaka, J. C. A.; Da Silva, C. C.; Ferreira, I. C. P.; Machado, G. M. C.; Leon, L. L.; De Oliveira, A. J. B.: Antileishmanial activity of indole alkaloids from aspidospermaramiflorum. Phytomedicine 14 (2007) 377–380.10.1016/j.phymed.2006.09.002Suche in Google Scholar PubMed
13 Kasibhatla, S.; Gourdeau, H.; Meerovitch, K.; Drewe, J.; Reddy, S.; Qiu, L.; Zhang, H.; Bergeron, F.; Bouffard, D.; Yang, Q.; Herich, J.; Lamothe, S.; Cai, S. X.; Tseng, B.: Discovery and mechanism of action of a novel series of apoptosis in ducers with potential vascular targeting activity. Mol. Cancer Ther. 3 (2004) 1365–1373.10.1158/1535-7163.1365.3.11Suche in Google Scholar
14 Nareshkumar, J.; Jiayi, X.; Ramesh, M. K.; Fuyong, D.; Guo, J. Z.; Emmanuel, P.: Identification and structure-activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J. Med. Chem. 52 (2009) 7544–7569.10.1021/jm900146eSuche in Google Scholar PubMed
15 Lee, K.-S.; Khil, L.-Y.; Chae, S.-H.; Kim, D.; Lee, B.-H.; Hwang, G.-S.; Moon, C.-H.; Chang, T.-S.; Moon, C.-K.: Effects of DK-002, a synthesized (6aS,cis)-9,10-dimethoxy-7,11b-dihydro-indeno[2,1-c]chromene-3,6a-diol, on platelet activity. Life Sci. 78 (2006) 1091–1097.10.1016/j.lfs.2005.06.017Suche in Google Scholar PubMed
16 El-Agrody, A. M.; Al-Omar, M. A.; Amr, A. E. G. E.; Ng, S. W.; Tiekink, E. R. T.: 3-Amino-1-(4-fluorophenyl)-8-methoxy-1H-benzo[f]chromene-2-carbonitrile. Acta Crystallogr. E69 (2013) o476–o477.10.1107/S160053681300545XSuche in Google Scholar PubMed PubMed Central
17 Amr, A. E. G. E.; El-Agrody, A. M.; Al-Omar, M. A.; Amr, A. E. G. E.; Ng, S. W.; Tiekink, E. R. T.: 3-Amino-1-(4-fluorophenyl)-7-methoxy-1H-benzo[f]chromene-2-carbonitrile. Acta Crystallogr. E69 (2013) o478–o479.10.1107/S1600536813005473Suche in Google Scholar PubMed PubMed Central
18 El-Agrody, A. M.; Amr, A. E. G. E.; Sabry, N. M.; Al-Omar, M. A.; Ghabbour, H. A.: Crystal structure of 3-amino-9-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2. Z. Kristallogr. NCS 231 (2016) 1193–1195.10.1515/ncrs-2016-0128Suche in Google Scholar
19 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
20 Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2009).Suche in Google Scholar
©2017 Ahmed M. Fouda et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)manganese(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2) C42H40Cl2MnN6O10S2
- The crystal structure of 1,2-bis[2-methyl-5-(3-cyanophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H16F6N2S2
- Crystal structure of the first characterized polymeric copper and sodium complex diaqua-(tris-acetato-κO,O′)(μ2-acetato-κO′′)dinatrium copper(II) monohydrate, C8H18CuNa2O11
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-3-yl-methylene)aniline, C18H22N2
- Crystal structure of methyl 2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22N2O5
- Crystal structure of bis(1,3-bis(diphenylphosphino)propane-κ2P,P′)silver(I) trifluorosulfonate–methanol (1:0.5), [Ag(C27H26P2)]SO3CF3⋅0.5CH3OH
- Crystal structure of μ-1,4-bis(diphenylphosphine)butane-2,9-dimethyl-1,10-phenanthroline-κ2N:N′-bis(cyano-κC)dicopper(I)]-water, C58H56Cu2N6O2P2
- Crystal structure of N2,N6-bis(1-hydrazinyl-2-methyl-1-oxopropan-2-yl) pyridine-2,6-dicarboxamide, C15H23N7O4
- Crystal structure of 1,3-dimethyl-2-phenyl-1H-perimidin-3-ium iodide, C19H17IN2
- Crystal structure of diaqua-(2,2′-(butane-1,4-diyl)-bis(5-carboxy-1H-imidazole-4-carboxylato)-κ4O,O′,N,N′)cadmium(II) monohydrate, C14H18O11N4Cd
- Crystal structure of ethyl (E)-3-(cyclopropylamino)-2-(2,4-dichloro-5-fluorobenzoyl) acrylate, C15H14Cl2FNO3
- Crystal structure of bis-(1-(4-chlorophenyl)-3-phenyl-4-thenoyl-1H-pyrazol-5-ol-κ2O,O′)-(N,N-dimethylformamide)zinc(II), C43H31Cl2N5O5S2Zn
- Crystal structure of tetrakis(μ3-2-(N-(2-hydroxyethyl)amino)ethoxo)-tetrachloro-tetra-cobalt(II) methanol solvate, C17H44Cl4Co4N4O9
- Crystal structure of 3-amino-1-(4-bromophenyl)-9-methoxy-1H-benzo[f]chromene-2-carbonitrile, C21H15BrN2O2
- Crystal structure of 2-amino-4-(4-isopropyl-phenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2-c]chromene, C22H18N2O3
- Crystal structure of 3-amino-8-methoxy-1-(4-methoxy phenyl)-1H-benzo[f]chromene-2-carbonitrile, C22H18N2O3
- Crystal structure of monoaqua-[6,6′-((1E,1′E)-(1,2-phenylene bis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-nitrophenolato-κ4N,N′,O,O′)]zinc(II), C20H12Br2N4O7Zn
- Crystal structure of 11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one – acetonitril (1/2), C33H41N3O2
- Crystal structure of tert-butyl (phenylsulfinyl)carbamate, C11H15NO3S
- Crystal structure of catena-poly{diaqua-bis(3-(1H-1,2,4-triazol-1-yl)benzoato-κ2O:N)copper(II)} monohydrate, C18H18CuN6O7
- Crystal structure of tetraaqua-bis(3-(4H-1,2,4-triazol-4-yl)benzoato-κN)cobalt(II), C18H20CoN6O8
- The crystal structure of 4-bromo-N-cyclopropyl-2,5-difluorobenzenesulfonamide, C9H8BrF2NO2S
- Crystal structure of ([3,3′-bipyridine]-6,6′-dicarboxylato-κ2O:O′)-bis(1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) dihydrate, C37H35Ag2N5O6
- The crystal structure of (4-(1H-1,2,4-triazol-1-yl)benzoato-κN)-[4-(1H-1,2,4-triazol-1-yl)benzoic acid-κN]silver(I), C18H13AgN6O4
- Crystal structure of 3-(4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one, C17H16O2
- Crystal structure of bis(2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-methoxyphenolato-κ2O,N)copper(II), C36H38CuN4O6
- Crystal structure of poly[1,2-bis(1,2,4-triazol-4-yl)ethane-κ2N:N′]silver(I) bromate monohydrate]silver(I), C6H10AgBrN6O4
- The crystal structure of 2,3,5-triphenyl-2,3-dihydro-1H-tetrazol-1-ium 2,3-dioxoindoline-5-sulfonate, C27H19N5O5S
- Crystal structure of 3-(2-amino-1,3-selenazol-4-yl)-2H-chromen-2-one – dimethylformamide (1/1), C15H15N3O3Se
- Crystal structure of diethyl 3,3′-(diazene-1,2-diyl)(E)-dibenzoate, C18H18N2O4
- Crystal structure of cis-bis((1H-benzimidazol-2-yl)methanol-κN,O)-bis(isothiocyanato-κN)nickel(II), C18H16N6NiO2S2
- Crystal structure of bis(2-(2′-hydroxy-5′-methoxyphenyl)-1H-benzimidazole)boron – tetrahydrofuran (1/1), C36H37N4O6B
- Crystal structure of poly[aqua-bis(nitrato-κ2O,O′)-(μ3-1,3-benzimidazol-3-ium-1,3-diacetato-κ4O,O′:O′′:O′′′)dysprosium(III)], C11H11DyN4O11
- Crystal structure of n-butyl-tris(dicyclo-hexylamido)hafnium(IV), C40H75HfN3
- Crystal structure of (E)-1-[1-(3-chloro-4-fluoro-phenyl)ethylidene]-2-(2,4-dinitrophenyl)hydrazine, C28H20Cl2F2N8O8
- The crystal structure of ethyl 4-((2-hydroxybenzyl)amino)benzoate, a Schiff base, C16H17NO3
- Crystal structure of 3-(2-(4-isobutylphenyl)propanoyl)-1-methylimidazolidine-2,4-dione, C17H22N2O3
- Crystal structure of poly[(μ3-2-(pyrazin-2-ylthio)acetato-κ3N:O:S)silver(I)], C6H5AgN2O2S
- Crystal structure of (E)-1-(3-((E)-((2-hydroxynaphthalen-1-yl)methylene)amino)phenyl)ethanone O-benzyl oxime, C26H22N2O2
- Crystal structure of (E)-2,3-dihydroxybenzaldehyde O-(2-((((E)-1-(2,5-dihydroxyphenyl)ethylidene)amino)oxy)ethyl) oxime monohydrate, C17H20N2O7
- Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4
- Crystal stucture of 4-((10H-phenothiazin-10-yl)methyl)-2,6-di-tert-butylphenol, C27H31NOS
- The crystal structure of ethyl 1-(4-nitrophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C13H10F3N3O4
- Crystal structure of catena-poly[di-(μ3-oxido-κ3O:O:O)-tetraoxido-(μ2-5′-(pyrazin-2-yl)-1H,2′H-3,3′-bi(1,2,4-triazole)-κ2N:N′)dimolybdenum(VI)], C8H6Mo2N8O6
- Crystal structure of (3,6-dioxocyclohexa-1,4-diene-1,4-bis(olato)-κ4O,O′:O′′,O′′′)-bis(tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′))-dizinc(II) bis(hexafluorophosphate(V)), C42H38F12N8O4P2Zn2
- Crystal structure of catena-poly[hexakis(μ2-2-acetylphenolato-κ3O:O,O′)trimanganese(II)], C48H42Mn3O12
- The crystal structure of 2,2-difluoro-4-(trifluoromethyl)-2,5-dihydro-[1,3,2]dioxaborinino[5,4-c]chromen-3-ium-2-uide, C11H6BF5O3
- Crystal structure of methyl (E)-2-(4-(diethylamino)-2-hydroxybenzylidene)hydrazine-1-carboxylate, C13H19N3O3
- The crystal structure of tert-butyl 2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate, C10H15N3O2
- Crystal structure of 1,1-bis(η5-adamantylcyclopentadienyl)-3-phenyl-2-trimethylsilyl-2,3-dihydroisotitanazole, C42H55NSiTi
- Crystal structure of 2-(4-(2-(4-benzylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C28H29N3O2
- Important impurity of Flupirtine – a single crystal study on ethyl (6-amino-5-((ethoxycarbonyl)amino)pyridin-2-yl)(4-fluorobenzyl)carbamate, C18H21FN4O4
- Crystal structure of ethyl 3-(4-methoxyphenyl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-pyrazole-5-carboxylate, C22H22N2O5
- Crystal structure of 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate methanol solvate, C37H35N1O10
- The crystal structure of the inner salt of 2-[(aminoiminomethyl)amino]ethylcarbamic acid [systematic name: (2-((diaminomethylene)ammonio)ethyl)carbamate], C4H10N4O2
- Crystal structure of (8-hydroxy-5-nitroquinolinium) perchlorate – 8-hydroxy-5-nitroquinoline (1/1), C18H13ClN4O10
- The crystal structure of (5-methyl-1,2,4-oxadiazol-3-yl)ferrocene, C13H12FeN2O
- Crystal structure of methyl 1-(2-(fluorosulfonyl)ethyl)-2-oxocyclopentanecarboxylate, C9H13FO5S
- Crystal structure of the triclinic modification of 1-methyl-4-nitroimidzole, C4H5N3O2
- Corrigendum
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)manganese(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2) C42H40Cl2MnN6O10S2
- The crystal structure of 1,2-bis[2-methyl-5-(3-cyanophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H16F6N2S2
- Crystal structure of the first characterized polymeric copper and sodium complex diaqua-(tris-acetato-κO,O′)(μ2-acetato-κO′′)dinatrium copper(II) monohydrate, C8H18CuNa2O11
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-3-yl-methylene)aniline, C18H22N2
- Crystal structure of methyl 2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22N2O5
- Crystal structure of bis(1,3-bis(diphenylphosphino)propane-κ2P,P′)silver(I) trifluorosulfonate–methanol (1:0.5), [Ag(C27H26P2)]SO3CF3⋅0.5CH3OH
- Crystal structure of μ-1,4-bis(diphenylphosphine)butane-2,9-dimethyl-1,10-phenanthroline-κ2N:N′-bis(cyano-κC)dicopper(I)]-water, C58H56Cu2N6O2P2
- Crystal structure of N2,N6-bis(1-hydrazinyl-2-methyl-1-oxopropan-2-yl) pyridine-2,6-dicarboxamide, C15H23N7O4
- Crystal structure of 1,3-dimethyl-2-phenyl-1H-perimidin-3-ium iodide, C19H17IN2
- Crystal structure of diaqua-(2,2′-(butane-1,4-diyl)-bis(5-carboxy-1H-imidazole-4-carboxylato)-κ4O,O′,N,N′)cadmium(II) monohydrate, C14H18O11N4Cd
- Crystal structure of ethyl (E)-3-(cyclopropylamino)-2-(2,4-dichloro-5-fluorobenzoyl) acrylate, C15H14Cl2FNO3
- Crystal structure of bis-(1-(4-chlorophenyl)-3-phenyl-4-thenoyl-1H-pyrazol-5-ol-κ2O,O′)-(N,N-dimethylformamide)zinc(II), C43H31Cl2N5O5S2Zn
- Crystal structure of tetrakis(μ3-2-(N-(2-hydroxyethyl)amino)ethoxo)-tetrachloro-tetra-cobalt(II) methanol solvate, C17H44Cl4Co4N4O9
- Crystal structure of 3-amino-1-(4-bromophenyl)-9-methoxy-1H-benzo[f]chromene-2-carbonitrile, C21H15BrN2O2
- Crystal structure of 2-amino-4-(4-isopropyl-phenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2-c]chromene, C22H18N2O3
- Crystal structure of 3-amino-8-methoxy-1-(4-methoxy phenyl)-1H-benzo[f]chromene-2-carbonitrile, C22H18N2O3
- Crystal structure of monoaqua-[6,6′-((1E,1′E)-(1,2-phenylene bis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-nitrophenolato-κ4N,N′,O,O′)]zinc(II), C20H12Br2N4O7Zn
- Crystal structure of 11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one – acetonitril (1/2), C33H41N3O2
- Crystal structure of tert-butyl (phenylsulfinyl)carbamate, C11H15NO3S
- Crystal structure of catena-poly{diaqua-bis(3-(1H-1,2,4-triazol-1-yl)benzoato-κ2O:N)copper(II)} monohydrate, C18H18CuN6O7
- Crystal structure of tetraaqua-bis(3-(4H-1,2,4-triazol-4-yl)benzoato-κN)cobalt(II), C18H20CoN6O8
- The crystal structure of 4-bromo-N-cyclopropyl-2,5-difluorobenzenesulfonamide, C9H8BrF2NO2S
- Crystal structure of ([3,3′-bipyridine]-6,6′-dicarboxylato-κ2O:O′)-bis(1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) dihydrate, C37H35Ag2N5O6
- The crystal structure of (4-(1H-1,2,4-triazol-1-yl)benzoato-κN)-[4-(1H-1,2,4-triazol-1-yl)benzoic acid-κN]silver(I), C18H13AgN6O4
- Crystal structure of 3-(4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one, C17H16O2
- Crystal structure of bis(2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-methoxyphenolato-κ2O,N)copper(II), C36H38CuN4O6
- Crystal structure of poly[1,2-bis(1,2,4-triazol-4-yl)ethane-κ2N:N′]silver(I) bromate monohydrate]silver(I), C6H10AgBrN6O4
- The crystal structure of 2,3,5-triphenyl-2,3-dihydro-1H-tetrazol-1-ium 2,3-dioxoindoline-5-sulfonate, C27H19N5O5S
- Crystal structure of 3-(2-amino-1,3-selenazol-4-yl)-2H-chromen-2-one – dimethylformamide (1/1), C15H15N3O3Se
- Crystal structure of diethyl 3,3′-(diazene-1,2-diyl)(E)-dibenzoate, C18H18N2O4
- Crystal structure of cis-bis((1H-benzimidazol-2-yl)methanol-κN,O)-bis(isothiocyanato-κN)nickel(II), C18H16N6NiO2S2
- Crystal structure of bis(2-(2′-hydroxy-5′-methoxyphenyl)-1H-benzimidazole)boron – tetrahydrofuran (1/1), C36H37N4O6B
- Crystal structure of poly[aqua-bis(nitrato-κ2O,O′)-(μ3-1,3-benzimidazol-3-ium-1,3-diacetato-κ4O,O′:O′′:O′′′)dysprosium(III)], C11H11DyN4O11
- Crystal structure of n-butyl-tris(dicyclo-hexylamido)hafnium(IV), C40H75HfN3
- Crystal structure of (E)-1-[1-(3-chloro-4-fluoro-phenyl)ethylidene]-2-(2,4-dinitrophenyl)hydrazine, C28H20Cl2F2N8O8
- The crystal structure of ethyl 4-((2-hydroxybenzyl)amino)benzoate, a Schiff base, C16H17NO3
- Crystal structure of 3-(2-(4-isobutylphenyl)propanoyl)-1-methylimidazolidine-2,4-dione, C17H22N2O3
- Crystal structure of poly[(μ3-2-(pyrazin-2-ylthio)acetato-κ3N:O:S)silver(I)], C6H5AgN2O2S
- Crystal structure of (E)-1-(3-((E)-((2-hydroxynaphthalen-1-yl)methylene)amino)phenyl)ethanone O-benzyl oxime, C26H22N2O2
- Crystal structure of (E)-2,3-dihydroxybenzaldehyde O-(2-((((E)-1-(2,5-dihydroxyphenyl)ethylidene)amino)oxy)ethyl) oxime monohydrate, C17H20N2O7
- Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4
- Crystal stucture of 4-((10H-phenothiazin-10-yl)methyl)-2,6-di-tert-butylphenol, C27H31NOS
- The crystal structure of ethyl 1-(4-nitrophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C13H10F3N3O4
- Crystal structure of catena-poly[di-(μ3-oxido-κ3O:O:O)-tetraoxido-(μ2-5′-(pyrazin-2-yl)-1H,2′H-3,3′-bi(1,2,4-triazole)-κ2N:N′)dimolybdenum(VI)], C8H6Mo2N8O6
- Crystal structure of (3,6-dioxocyclohexa-1,4-diene-1,4-bis(olato)-κ4O,O′:O′′,O′′′)-bis(tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′))-dizinc(II) bis(hexafluorophosphate(V)), C42H38F12N8O4P2Zn2
- Crystal structure of catena-poly[hexakis(μ2-2-acetylphenolato-κ3O:O,O′)trimanganese(II)], C48H42Mn3O12
- The crystal structure of 2,2-difluoro-4-(trifluoromethyl)-2,5-dihydro-[1,3,2]dioxaborinino[5,4-c]chromen-3-ium-2-uide, C11H6BF5O3
- Crystal structure of methyl (E)-2-(4-(diethylamino)-2-hydroxybenzylidene)hydrazine-1-carboxylate, C13H19N3O3
- The crystal structure of tert-butyl 2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate, C10H15N3O2
- Crystal structure of 1,1-bis(η5-adamantylcyclopentadienyl)-3-phenyl-2-trimethylsilyl-2,3-dihydroisotitanazole, C42H55NSiTi
- Crystal structure of 2-(4-(2-(4-benzylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C28H29N3O2
- Important impurity of Flupirtine – a single crystal study on ethyl (6-amino-5-((ethoxycarbonyl)amino)pyridin-2-yl)(4-fluorobenzyl)carbamate, C18H21FN4O4
- Crystal structure of ethyl 3-(4-methoxyphenyl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-pyrazole-5-carboxylate, C22H22N2O5
- Crystal structure of 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate methanol solvate, C37H35N1O10
- The crystal structure of the inner salt of 2-[(aminoiminomethyl)amino]ethylcarbamic acid [systematic name: (2-((diaminomethylene)ammonio)ethyl)carbamate], C4H10N4O2
- Crystal structure of (8-hydroxy-5-nitroquinolinium) perchlorate – 8-hydroxy-5-nitroquinoline (1/1), C18H13ClN4O10
- The crystal structure of (5-methyl-1,2,4-oxadiazol-3-yl)ferrocene, C13H12FeN2O
- Crystal structure of methyl 1-(2-(fluorosulfonyl)ethyl)-2-oxocyclopentanecarboxylate, C9H13FO5S
- Crystal structure of the triclinic modification of 1-methyl-4-nitroimidzole, C4H5N3O2
- Corrigendum
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O

