Abstract
C22H18N2O3, orthorhombic, Pbca (no. 61), a = 14.8532(7) Å, b = 11.3579(6) Å, c = 22.1712(18) Å, V = 3740.3(4) Å3, Z = 8, Rgt(F) = 0.0596, wRref(F2) = 0.1653, T = 293(2) K.
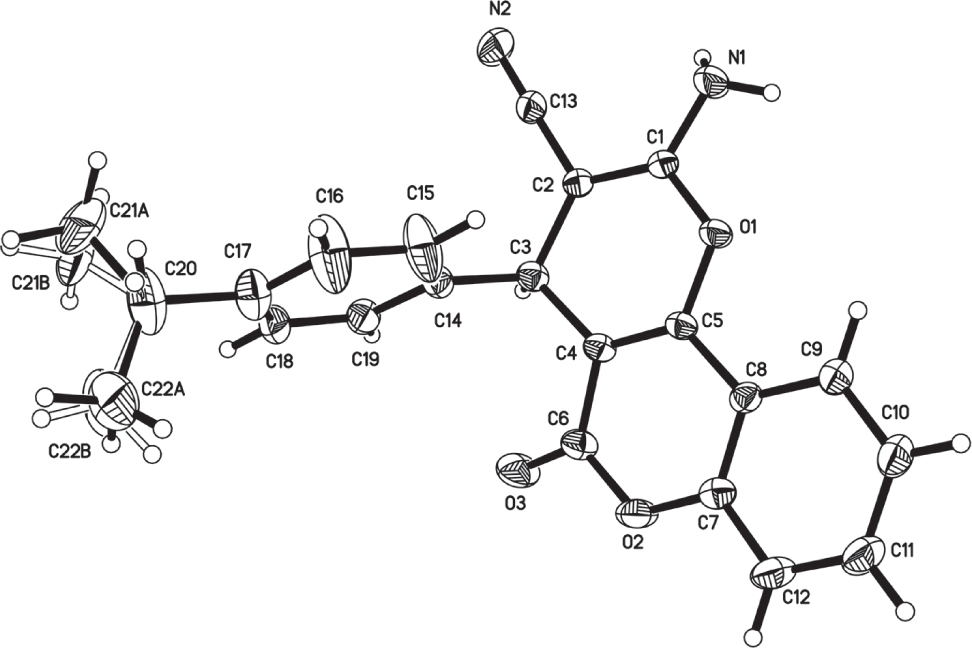
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.28 × 0.21 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.9 cm−1 |
| Diffractometer, scan mode: | Xcalibur, ω-scans |
| 2θmax, completeness: | 50°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 8867, 3299, 0.032 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2256 |
| N(param)refined: | 268 |
| Programs: | CrysAlisPRO [1], SHELX [2], OLEX2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.14089(17) | 0.6946(2) | 0.06000(9) | 0.0357(6) |
| C2 | 0.07861(17) | 0.7806(2) | 0.05626(8) | 0.0353(6) |
| C3 | −0.02137(16) | 0.7621(2) | 0.06168(10) | 0.0350(6) |
| H3 | −0.0502 | 0.7903 | 0.0246 | 0.042* |
| C4 | −0.03804(16) | 0.6312(2) | 0.06736(10) | 0.0333(6) |
| C5 | 0.02782(16) | 0.5523(2) | 0.07542(10) | 0.0317(6) |
| C6 | −0.12938(18) | 0.5891(2) | 0.06115(13) | 0.0422(7) |
| C7 | −0.07789(18) | 0.3941(2) | 0.08643(12) | 0.0405(6) |
| C8 | 0.01098(17) | 0.4295(2) | 0.08677(11) | 0.0336(6) |
| C9 | 0.07765(19) | 0.3466(2) | 0.09986(13) | 0.0435(7) |
| H9 | 0.1380 | 0.3684 | 0.1000 | 0.052* |
| C10 | 0.0534(2) | 0.2325(2) | 0.11244(14) | 0.0544(8) |
| H10 | 0.0975 | 0.1771 | 0.1213 | 0.065* |
| C11 | −0.0358(2) | 0.1995(3) | 0.11196(15) | 0.0594(9) |
| H11 | −0.0512 | 0.1220 | 0.1207 | 0.071* |
| C12 | −0.1023(2) | 0.2792(2) | 0.09881(15) | 0.0569(8) |
| H12 | −0.1625 | 0.2564 | 0.0982 | 0.068* |
| C13 | 0.11218(18) | 0.8972(2) | 0.04761(14) | 0.0449(7) |
| C14 | −0.06315(17) | 0.8269(2) | 0.11565(12) | 0.0379(6) |
| C15 | −0.0265(3) | 0.8203(3) | 0.17222(15) | 0.0836(13) |
| H15 | 0.0256 | 0.7763 | 0.1781 | 0.100* |
| C16 | −0.0653(3) | 0.8776(4) | 0.22075(17) | 0.1008(16) |
| H16 | −0.0385 | 0.8715 | 0.2585 | 0.121* |
| C17 | −0.1420(2) | 0.9429(3) | 0.21456(15) | 0.0606(9) |
| C18 | −0.1776(2) | 0.9513(3) | 0.15767(14) | 0.0520(8) |
| H18 | −0.2294 | 0.9958 | 0.1518 | 0.062* |
| C19 | −0.13839(18) | 0.8951(2) | 0.10859(13) | 0.0449(7) |
| H19 | −0.1636 | 0.9040 | 0.0705 | 0.054* |
| C20 | −0.1840(3) | 1.0070(3) | 0.26753(18) | 0.0892(13) |
| H20Aa | −0.1573 | 0.9899 | 0.3070 | 0.107* |
| H20b | −0.1347 | 1.0136 | 0.3025 | 0.107* |
| C21Ba | −0.2004(15) | 1.1356(8) | 0.2542(10) | 0.104(5) |
| H21Aa | −0.1482 | 1.1687 | 0.2352 | 0.157* |
| H21Ba | −0.2122 | 1.1767 | 0.2912 | 0.157* |
| H21Ca | −0.2513 | 1.1433 | 0.2279 | 0.157* |
| C22Ba | −0.2877(7) | 0.9925(18) | 0.2636(10) | 0.136(5) |
| H22Aa | −0.3110 | 1.0432 | 0.2326 | 0.205* |
| H22Ba | −0.3144 | 1.0131 | 0.3016 | 0.205* |
| H22Ca | −0.3021 | 0.9122 | 0.2541 | 0.205* |
| O1 | 0.11744(11) | 0.58008(14) | 0.07339(8) | 0.0412(5) |
| O2 | −0.14573(12) | 0.47180(16) | 0.07157(9) | 0.0518(6) |
| O3 | −0.19358(13) | 0.64855(18) | 0.04692(10) | 0.0608(6) |
| N1 | 0.23053(15) | 0.70270(19) | 0.05340(11) | 0.0536(7) |
| H1A | 0.2521 | 0.6343 | 0.0451 | 0.064* |
| H1B | 0.2418 | 0.7502 | 0.0241 | 0.064* |
| N2 | 0.14056(19) | 0.9894(2) | 0.04219(14) | 0.0663(8) |
| C21Ab | −0.1672(16) | 1.1355(9) | 0.2666(11) | 0.125(5) |
| H21Db | −0.1047 | 1.1503 | 0.2744 | 0.188* |
| H21Eb | −0.2032 | 1.1729 | 0.2970 | 0.188* |
| H21Fb | −0.1830 | 1.1665 | 0.2277 | 0.188* |
| C22Ab | −0.2624(9) | 0.9503(12) | 0.2942(6) | 0.096(4) |
| H22Db | −0.3119 | 0.9539 | 0.2664 | 0.145* |
| H22Eb | −0.2784 | 0.9901 | 0.3309 | 0.145* |
| H22Fb | −0.2486 | 0.8695 | 0.3029 | 0.145* |
aOccupancy: 0.48(2); bOccupancy: 0.52(2).
Source of material
A mixture of 4-isopropyl-benzaldehyde (10 mmol) and 4-hydroxycoumarin (20 mmol) was dissolved in 100 mL of ethanol [4]. A few drops of piperidine were added, and the mixture was stirred for 3 h at room temperature. After reaction completion as determined by TLC, water was added until precipitation occurred. After filtering, the precipitate was sequentially washed with ice-cooled water and ethanol and then dried under vacuum.
Experimental details
Hydrogen atoms were placed in calculated positions and were included in the refinement using the riding model approximation, with Uiso(H) set to 1.2Ueq(C,N) and Uiso(H) = 1.5Ueq(C) for methyl groups.
Discussion
Coumarins are an important component of numerous synthetic and natural products with widerange biological activities, including anticoagulant, insecticidal, anthelminthic, hypnotic, antifungal, phytoalexin, and HIV protease inhibition [5, 6] . Considering the importance of these compounds, several researchers focused on the synthesis of coumarin derivatives [7, 8] .
The crystal structure of the title compound contains one full molecule in the asymmetric unit. The isopropyl group is disorderd over two equally occupied positions. The pyran ring is basically planar (the Sigpln and Chisq parameters for defining least-squares planes in PLATON [9] are 0.057 and 2023.8, respectively). The amine group donates two H-bonds giving rise to a 2-D H-bonded pattern that extends along the a and b axes.
Acknowledgement
The author expresses his sincere gratitude for the financial support for this work by the Science and Technology Project of Xi’an City (No.CXY1531WL32) and the Educational Department Science Foundation of Shaanxi Province (No.15JK2154).
References
Agilent Technologies: CrysAlisPRO Software system, version 1.171.36.28, Agilent Technologies UK Ltd, Oxford, UK, 2013.Search in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Li, J.; Sui, Y.; Xin, J.-J.; Du, X.-L.; Li, J.-T.; Huo, H.-R.; Ma, H.; Wang, W.-H.; Zhou, H.-Y.; Zhan, H.-D.; Wang, Z.-J.; Li, C.; Sui, F.; Li, X.: Synthesis of biscoumarin and dihydropyran derivatives with promising antitumor and antibacterial activities. Bioorg. Med. Chem. Lett. 25 (2015) 5520–5523.10.1016/j.bmcl.2015.10.063Search in Google Scholar PubMed
Liu, G. M.; Xu, K.; Li, J.; Luo, Y. G.: 7,8-dithydroxycoumarins protect human neuroblastoma cells from Aβ- mediated neurotoxic damage via inhibiting JNK and p38MAPK pathways. Biomed. Res.-India 27 (2016) 591–595.Search in Google Scholar
Mei, L. L.; Wang, P.; Hu, X.: Pharmacokinetic study on the main active components of total coumarins of cnidii monnieri in rats. Lat. Am. J. Pharm. 32 (2013) 1139–1145.Search in Google Scholar
Ahmad, R. A.; Abdullah, S. H. A. S.; Serati-Nouri, H.; Aziz, R. A.: Antiproliferative activity of coumarin and cinnamon water extracts on human ovarian cancer cells. Lat. Am. J. Pharm. 33 (2014) 960–965.Search in Google Scholar
Zhu, Y.; Tai, B.; Xu, T. R.; Li, H. Y.: Synthesis and antitumor activity of a novel biscoumarin and its corresponding epoxydicoumarin. Lat. Am. J. Pharm. 34 (2015) 1905–1908.Search in Google Scholar
Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
©2017 Chang-Hu Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)manganese(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2) C42H40Cl2MnN6O10S2
- The crystal structure of 1,2-bis[2-methyl-5-(3-cyanophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H16F6N2S2
- Crystal structure of the first characterized polymeric copper and sodium complex diaqua-(tris-acetato-κO,O′)(μ2-acetato-κO′′)dinatrium copper(II) monohydrate, C8H18CuNa2O11
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-3-yl-methylene)aniline, C18H22N2
- Crystal structure of methyl 2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22N2O5
- Crystal structure of bis(1,3-bis(diphenylphosphino)propane-κ2P,P′)silver(I) trifluorosulfonate–methanol (1:0.5), [Ag(C27H26P2)]SO3CF3⋅0.5CH3OH
- Crystal structure of μ-1,4-bis(diphenylphosphine)butane-2,9-dimethyl-1,10-phenanthroline-κ2N:N′-bis(cyano-κC)dicopper(I)]-water, C58H56Cu2N6O2P2
- Crystal structure of N2,N6-bis(1-hydrazinyl-2-methyl-1-oxopropan-2-yl) pyridine-2,6-dicarboxamide, C15H23N7O4
- Crystal structure of 1,3-dimethyl-2-phenyl-1H-perimidin-3-ium iodide, C19H17IN2
- Crystal structure of diaqua-(2,2′-(butane-1,4-diyl)-bis(5-carboxy-1H-imidazole-4-carboxylato)-κ4O,O′,N,N′)cadmium(II) monohydrate, C14H18O11N4Cd
- Crystal structure of ethyl (E)-3-(cyclopropylamino)-2-(2,4-dichloro-5-fluorobenzoyl) acrylate, C15H14Cl2FNO3
- Crystal structure of bis-(1-(4-chlorophenyl)-3-phenyl-4-thenoyl-1H-pyrazol-5-ol-κ2O,O′)-(N,N-dimethylformamide)zinc(II), C43H31Cl2N5O5S2Zn
- Crystal structure of tetrakis(μ3-2-(N-(2-hydroxyethyl)amino)ethoxo)-tetrachloro-tetra-cobalt(II) methanol solvate, C17H44Cl4Co4N4O9
- Crystal structure of 3-amino-1-(4-bromophenyl)-9-methoxy-1H-benzo[f]chromene-2-carbonitrile, C21H15BrN2O2
- Crystal structure of 2-amino-4-(4-isopropyl-phenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2-c]chromene, C22H18N2O3
- Crystal structure of 3-amino-8-methoxy-1-(4-methoxy phenyl)-1H-benzo[f]chromene-2-carbonitrile, C22H18N2O3
- Crystal structure of monoaqua-[6,6′-((1E,1′E)-(1,2-phenylene bis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-nitrophenolato-κ4N,N′,O,O′)]zinc(II), C20H12Br2N4O7Zn
- Crystal structure of 11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one – acetonitril (1/2), C33H41N3O2
- Crystal structure of tert-butyl (phenylsulfinyl)carbamate, C11H15NO3S
- Crystal structure of catena-poly{diaqua-bis(3-(1H-1,2,4-triazol-1-yl)benzoato-κ2O:N)copper(II)} monohydrate, C18H18CuN6O7
- Crystal structure of tetraaqua-bis(3-(4H-1,2,4-triazol-4-yl)benzoato-κN)cobalt(II), C18H20CoN6O8
- The crystal structure of 4-bromo-N-cyclopropyl-2,5-difluorobenzenesulfonamide, C9H8BrF2NO2S
- Crystal structure of ([3,3′-bipyridine]-6,6′-dicarboxylato-κ2O:O′)-bis(1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) dihydrate, C37H35Ag2N5O6
- The crystal structure of (4-(1H-1,2,4-triazol-1-yl)benzoato-κN)-[4-(1H-1,2,4-triazol-1-yl)benzoic acid-κN]silver(I), C18H13AgN6O4
- Crystal structure of 3-(4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one, C17H16O2
- Crystal structure of bis(2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-methoxyphenolato-κ2O,N)copper(II), C36H38CuN4O6
- Crystal structure of poly[1,2-bis(1,2,4-triazol-4-yl)ethane-κ2N:N′]silver(I) bromate monohydrate]silver(I), C6H10AgBrN6O4
- The crystal structure of 2,3,5-triphenyl-2,3-dihydro-1H-tetrazol-1-ium 2,3-dioxoindoline-5-sulfonate, C27H19N5O5S
- Crystal structure of 3-(2-amino-1,3-selenazol-4-yl)-2H-chromen-2-one – dimethylformamide (1/1), C15H15N3O3Se
- Crystal structure of diethyl 3,3′-(diazene-1,2-diyl)(E)-dibenzoate, C18H18N2O4
- Crystal structure of cis-bis((1H-benzimidazol-2-yl)methanol-κN,O)-bis(isothiocyanato-κN)nickel(II), C18H16N6NiO2S2
- Crystal structure of bis(2-(2′-hydroxy-5′-methoxyphenyl)-1H-benzimidazole)boron – tetrahydrofuran (1/1), C36H37N4O6B
- Crystal structure of poly[aqua-bis(nitrato-κ2O,O′)-(μ3-1,3-benzimidazol-3-ium-1,3-diacetato-κ4O,O′:O′′:O′′′)dysprosium(III)], C11H11DyN4O11
- Crystal structure of n-butyl-tris(dicyclo-hexylamido)hafnium(IV), C40H75HfN3
- Crystal structure of (E)-1-[1-(3-chloro-4-fluoro-phenyl)ethylidene]-2-(2,4-dinitrophenyl)hydrazine, C28H20Cl2F2N8O8
- The crystal structure of ethyl 4-((2-hydroxybenzyl)amino)benzoate, a Schiff base, C16H17NO3
- Crystal structure of 3-(2-(4-isobutylphenyl)propanoyl)-1-methylimidazolidine-2,4-dione, C17H22N2O3
- Crystal structure of poly[(μ3-2-(pyrazin-2-ylthio)acetato-κ3N:O:S)silver(I)], C6H5AgN2O2S
- Crystal structure of (E)-1-(3-((E)-((2-hydroxynaphthalen-1-yl)methylene)amino)phenyl)ethanone O-benzyl oxime, C26H22N2O2
- Crystal structure of (E)-2,3-dihydroxybenzaldehyde O-(2-((((E)-1-(2,5-dihydroxyphenyl)ethylidene)amino)oxy)ethyl) oxime monohydrate, C17H20N2O7
- Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4
- Crystal stucture of 4-((10H-phenothiazin-10-yl)methyl)-2,6-di-tert-butylphenol, C27H31NOS
- The crystal structure of ethyl 1-(4-nitrophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C13H10F3N3O4
- Crystal structure of catena-poly[di-(μ3-oxido-κ3O:O:O)-tetraoxido-(μ2-5′-(pyrazin-2-yl)-1H,2′H-3,3′-bi(1,2,4-triazole)-κ2N:N′)dimolybdenum(VI)], C8H6Mo2N8O6
- Crystal structure of (3,6-dioxocyclohexa-1,4-diene-1,4-bis(olato)-κ4O,O′:O′′,O′′′)-bis(tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′))-dizinc(II) bis(hexafluorophosphate(V)), C42H38F12N8O4P2Zn2
- Crystal structure of catena-poly[hexakis(μ2-2-acetylphenolato-κ3O:O,O′)trimanganese(II)], C48H42Mn3O12
- The crystal structure of 2,2-difluoro-4-(trifluoromethyl)-2,5-dihydro-[1,3,2]dioxaborinino[5,4-c]chromen-3-ium-2-uide, C11H6BF5O3
- Crystal structure of methyl (E)-2-(4-(diethylamino)-2-hydroxybenzylidene)hydrazine-1-carboxylate, C13H19N3O3
- The crystal structure of tert-butyl 2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate, C10H15N3O2
- Crystal structure of 1,1-bis(η5-adamantylcyclopentadienyl)-3-phenyl-2-trimethylsilyl-2,3-dihydroisotitanazole, C42H55NSiTi
- Crystal structure of 2-(4-(2-(4-benzylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C28H29N3O2
- Important impurity of Flupirtine – a single crystal study on ethyl (6-amino-5-((ethoxycarbonyl)amino)pyridin-2-yl)(4-fluorobenzyl)carbamate, C18H21FN4O4
- Crystal structure of ethyl 3-(4-methoxyphenyl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-pyrazole-5-carboxylate, C22H22N2O5
- Crystal structure of 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate methanol solvate, C37H35N1O10
- The crystal structure of the inner salt of 2-[(aminoiminomethyl)amino]ethylcarbamic acid [systematic name: (2-((diaminomethylene)ammonio)ethyl)carbamate], C4H10N4O2
- Crystal structure of (8-hydroxy-5-nitroquinolinium) perchlorate – 8-hydroxy-5-nitroquinoline (1/1), C18H13ClN4O10
- The crystal structure of (5-methyl-1,2,4-oxadiazol-3-yl)ferrocene, C13H12FeN2O
- Crystal structure of methyl 1-(2-(fluorosulfonyl)ethyl)-2-oxocyclopentanecarboxylate, C9H13FO5S
- Crystal structure of the triclinic modification of 1-methyl-4-nitroimidzole, C4H5N3O2
- Corrigendum
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)manganese(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2) C42H40Cl2MnN6O10S2
- The crystal structure of 1,2-bis[2-methyl-5-(3-cyanophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H16F6N2S2
- Crystal structure of the first characterized polymeric copper and sodium complex diaqua-(tris-acetato-κO,O′)(μ2-acetato-κO′′)dinatrium copper(II) monohydrate, C8H18CuNa2O11
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-3-yl-methylene)aniline, C18H22N2
- Crystal structure of methyl 2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22N2O5
- Crystal structure of bis(1,3-bis(diphenylphosphino)propane-κ2P,P′)silver(I) trifluorosulfonate–methanol (1:0.5), [Ag(C27H26P2)]SO3CF3⋅0.5CH3OH
- Crystal structure of μ-1,4-bis(diphenylphosphine)butane-2,9-dimethyl-1,10-phenanthroline-κ2N:N′-bis(cyano-κC)dicopper(I)]-water, C58H56Cu2N6O2P2
- Crystal structure of N2,N6-bis(1-hydrazinyl-2-methyl-1-oxopropan-2-yl) pyridine-2,6-dicarboxamide, C15H23N7O4
- Crystal structure of 1,3-dimethyl-2-phenyl-1H-perimidin-3-ium iodide, C19H17IN2
- Crystal structure of diaqua-(2,2′-(butane-1,4-diyl)-bis(5-carboxy-1H-imidazole-4-carboxylato)-κ4O,O′,N,N′)cadmium(II) monohydrate, C14H18O11N4Cd
- Crystal structure of ethyl (E)-3-(cyclopropylamino)-2-(2,4-dichloro-5-fluorobenzoyl) acrylate, C15H14Cl2FNO3
- Crystal structure of bis-(1-(4-chlorophenyl)-3-phenyl-4-thenoyl-1H-pyrazol-5-ol-κ2O,O′)-(N,N-dimethylformamide)zinc(II), C43H31Cl2N5O5S2Zn
- Crystal structure of tetrakis(μ3-2-(N-(2-hydroxyethyl)amino)ethoxo)-tetrachloro-tetra-cobalt(II) methanol solvate, C17H44Cl4Co4N4O9
- Crystal structure of 3-amino-1-(4-bromophenyl)-9-methoxy-1H-benzo[f]chromene-2-carbonitrile, C21H15BrN2O2
- Crystal structure of 2-amino-4-(4-isopropyl-phenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2-c]chromene, C22H18N2O3
- Crystal structure of 3-amino-8-methoxy-1-(4-methoxy phenyl)-1H-benzo[f]chromene-2-carbonitrile, C22H18N2O3
- Crystal structure of monoaqua-[6,6′-((1E,1′E)-(1,2-phenylene bis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-nitrophenolato-κ4N,N′,O,O′)]zinc(II), C20H12Br2N4O7Zn
- Crystal structure of 11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one – acetonitril (1/2), C33H41N3O2
- Crystal structure of tert-butyl (phenylsulfinyl)carbamate, C11H15NO3S
- Crystal structure of catena-poly{diaqua-bis(3-(1H-1,2,4-triazol-1-yl)benzoato-κ2O:N)copper(II)} monohydrate, C18H18CuN6O7
- Crystal structure of tetraaqua-bis(3-(4H-1,2,4-triazol-4-yl)benzoato-κN)cobalt(II), C18H20CoN6O8
- The crystal structure of 4-bromo-N-cyclopropyl-2,5-difluorobenzenesulfonamide, C9H8BrF2NO2S
- Crystal structure of ([3,3′-bipyridine]-6,6′-dicarboxylato-κ2O:O′)-bis(1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) dihydrate, C37H35Ag2N5O6
- The crystal structure of (4-(1H-1,2,4-triazol-1-yl)benzoato-κN)-[4-(1H-1,2,4-triazol-1-yl)benzoic acid-κN]silver(I), C18H13AgN6O4
- Crystal structure of 3-(4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one, C17H16O2
- Crystal structure of bis(2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-methoxyphenolato-κ2O,N)copper(II), C36H38CuN4O6
- Crystal structure of poly[1,2-bis(1,2,4-triazol-4-yl)ethane-κ2N:N′]silver(I) bromate monohydrate]silver(I), C6H10AgBrN6O4
- The crystal structure of 2,3,5-triphenyl-2,3-dihydro-1H-tetrazol-1-ium 2,3-dioxoindoline-5-sulfonate, C27H19N5O5S
- Crystal structure of 3-(2-amino-1,3-selenazol-4-yl)-2H-chromen-2-one – dimethylformamide (1/1), C15H15N3O3Se
- Crystal structure of diethyl 3,3′-(diazene-1,2-diyl)(E)-dibenzoate, C18H18N2O4
- Crystal structure of cis-bis((1H-benzimidazol-2-yl)methanol-κN,O)-bis(isothiocyanato-κN)nickel(II), C18H16N6NiO2S2
- Crystal structure of bis(2-(2′-hydroxy-5′-methoxyphenyl)-1H-benzimidazole)boron – tetrahydrofuran (1/1), C36H37N4O6B
- Crystal structure of poly[aqua-bis(nitrato-κ2O,O′)-(μ3-1,3-benzimidazol-3-ium-1,3-diacetato-κ4O,O′:O′′:O′′′)dysprosium(III)], C11H11DyN4O11
- Crystal structure of n-butyl-tris(dicyclo-hexylamido)hafnium(IV), C40H75HfN3
- Crystal structure of (E)-1-[1-(3-chloro-4-fluoro-phenyl)ethylidene]-2-(2,4-dinitrophenyl)hydrazine, C28H20Cl2F2N8O8
- The crystal structure of ethyl 4-((2-hydroxybenzyl)amino)benzoate, a Schiff base, C16H17NO3
- Crystal structure of 3-(2-(4-isobutylphenyl)propanoyl)-1-methylimidazolidine-2,4-dione, C17H22N2O3
- Crystal structure of poly[(μ3-2-(pyrazin-2-ylthio)acetato-κ3N:O:S)silver(I)], C6H5AgN2O2S
- Crystal structure of (E)-1-(3-((E)-((2-hydroxynaphthalen-1-yl)methylene)amino)phenyl)ethanone O-benzyl oxime, C26H22N2O2
- Crystal structure of (E)-2,3-dihydroxybenzaldehyde O-(2-((((E)-1-(2,5-dihydroxyphenyl)ethylidene)amino)oxy)ethyl) oxime monohydrate, C17H20N2O7
- Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4
- Crystal stucture of 4-((10H-phenothiazin-10-yl)methyl)-2,6-di-tert-butylphenol, C27H31NOS
- The crystal structure of ethyl 1-(4-nitrophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C13H10F3N3O4
- Crystal structure of catena-poly[di-(μ3-oxido-κ3O:O:O)-tetraoxido-(μ2-5′-(pyrazin-2-yl)-1H,2′H-3,3′-bi(1,2,4-triazole)-κ2N:N′)dimolybdenum(VI)], C8H6Mo2N8O6
- Crystal structure of (3,6-dioxocyclohexa-1,4-diene-1,4-bis(olato)-κ4O,O′:O′′,O′′′)-bis(tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′))-dizinc(II) bis(hexafluorophosphate(V)), C42H38F12N8O4P2Zn2
- Crystal structure of catena-poly[hexakis(μ2-2-acetylphenolato-κ3O:O,O′)trimanganese(II)], C48H42Mn3O12
- The crystal structure of 2,2-difluoro-4-(trifluoromethyl)-2,5-dihydro-[1,3,2]dioxaborinino[5,4-c]chromen-3-ium-2-uide, C11H6BF5O3
- Crystal structure of methyl (E)-2-(4-(diethylamino)-2-hydroxybenzylidene)hydrazine-1-carboxylate, C13H19N3O3
- The crystal structure of tert-butyl 2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate, C10H15N3O2
- Crystal structure of 1,1-bis(η5-adamantylcyclopentadienyl)-3-phenyl-2-trimethylsilyl-2,3-dihydroisotitanazole, C42H55NSiTi
- Crystal structure of 2-(4-(2-(4-benzylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C28H29N3O2
- Important impurity of Flupirtine – a single crystal study on ethyl (6-amino-5-((ethoxycarbonyl)amino)pyridin-2-yl)(4-fluorobenzyl)carbamate, C18H21FN4O4
- Crystal structure of ethyl 3-(4-methoxyphenyl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-pyrazole-5-carboxylate, C22H22N2O5
- Crystal structure of 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate methanol solvate, C37H35N1O10
- The crystal structure of the inner salt of 2-[(aminoiminomethyl)amino]ethylcarbamic acid [systematic name: (2-((diaminomethylene)ammonio)ethyl)carbamate], C4H10N4O2
- Crystal structure of (8-hydroxy-5-nitroquinolinium) perchlorate – 8-hydroxy-5-nitroquinoline (1/1), C18H13ClN4O10
- The crystal structure of (5-methyl-1,2,4-oxadiazol-3-yl)ferrocene, C13H12FeN2O
- Crystal structure of methyl 1-(2-(fluorosulfonyl)ethyl)-2-oxocyclopentanecarboxylate, C9H13FO5S
- Crystal structure of the triclinic modification of 1-methyl-4-nitroimidzole, C4H5N3O2
- Corrigendum
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O

