Abstract
C42H55NSiTi, triclinic, P1̅ (no. 2), a = 9.2556(4) Å, b = 12.5048(5) Å, c = 15.7350(7) Å, α = 69.6151(13)°, β = 85.3502(14)°, γ = 83.8861(14)°, V = 1695.51(13) Å3, Z = 2, Rgt(F) = 0.0334, wRref(F2) = 0.0885, T = 100(2) K.
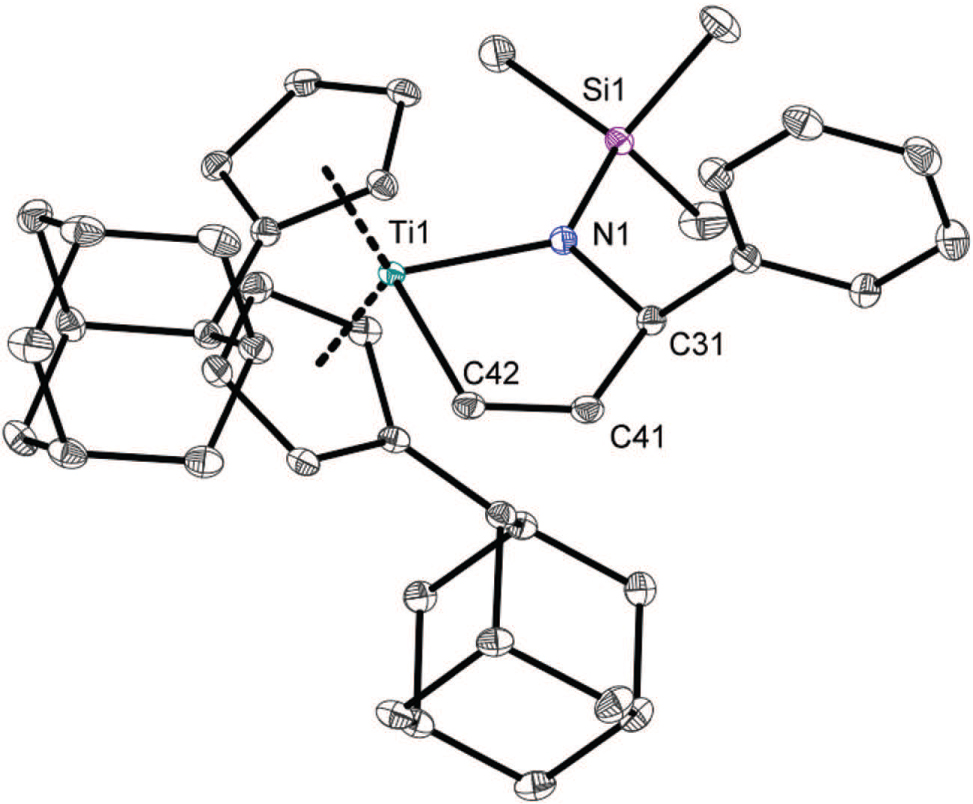
The asymmetric unit of the title crystal structure is shown in the figure. Hydrogen atoms are omitted for clarity. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green block |
| Size: | 0.36 × 0.24 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.2 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 70°, >99% |
| N(hkl)measured, N(hkl)unique: | 32114, 32114 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 28454 |
| N(param)refined: | 418 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Ti1 | 0.35939(2) | 0.25596(2) | 0.27314(2) | 0.00837(3) |
| Si1 | 0.62020(3) | 0.37181(2) | 0.33914(2) | 0.01040(5) |
| N1 | 0.44596(8) | 0.37739(6) | 0.30401(5) | 0.01022(11) |
| C1 | 0.28537(9) | 0.24738(7) | 0.12846(5) | 0.01080(13) |
| C2 | 0.39936(9) | 0.16056(7) | 0.15786(6) | 0.01219(14) |
| H2 | 0.3919 | 0.0810 | 0.1720 | 0.015* |
| C3 | 0.52689(9) | 0.21123(8) | 0.16296(6) | 0.01355(14) |
| H3 | 0.6177 | 0.1716 | 0.1839 | 0.016* |
| C4 | 0.49456(9) | 0.33129(8) | 0.13116(6) | 0.01312(14) |
| H4 | 0.5611 | 0.3869 | 0.1243 | 0.016* |
| C5 | 0.34655(9) | 0.35377(7) | 0.11154(6) | 0.01199(14) |
| H5 | 0.2955 | 0.4275 | 0.0905 | 0.014* |
| C6 | 0.12832(9) | 0.23142(7) | 0.11884(6) | 0.01076(13) |
| H6 | 0.0757 | 0.2215 | 0.1788 | 0.013* |
| C7 | 0.05216(9) | 0.33657(7) | 0.04764(6) | 0.01313(14) |
| H7 | 0.0634 | 0.4069 | 0.0622 | 0.016* |
| C8 | −0.11038(10) | 0.31994(8) | 0.04948(6) | 0.01599(15) |
| H8A | −0.1553 | 0.3114 | 0.1105 | 0.019* |
| H8B | −0.1601 | 0.3880 | 0.0049 | 0.019* |
| C9 | −0.12868(10) | 0.21289(8) | 0.02625(6) | 0.01504(15) |
| H9 | −0.2345 | 0.2021 | 0.0277 | 0.018* |
| C10 | −0.05203(10) | 0.10865(8) | 0.09668(6) | 0.01559(15) |
| H10A | −0.0647 | 0.0386 | 0.0833 | 0.019* |
| H10B | −0.0962 | 0.0997 | 0.1580 | 0.019* |
| C11 | 0.11101(9) | 0.12405(7) | 0.09462(6) | 0.01308(14) |
| H11 | 0.1602 | 0.0552 | 0.1401 | 0.016* |
| C12 | 0.11990(10) | 0.35046(9) | −0.04759(6) | 0.01712(16) |
| H12A | 0.2244 | 0.3623 | −0.0497 | 0.021* |
| H12B | 0.0713 | 0.4184 | −0.0928 | 0.021* |
| C13 | −0.05990(11) | 0.22650(10) | −0.06878(6) | 0.01944(18) |
| H13A | −0.1093 | 0.2935 | −0.1145 | 0.023* |
| H13B | −0.0719 | 0.1576 | −0.0839 | 0.023* |
| C14 | 0.17929(10) | 0.13840(9) | −0.00047(7) | 0.01726(16) |
| H14A | 0.1696 | 0.0688 | −0.0152 | 0.021* |
| H14B | 0.2842 | 0.1487 | −0.0020 | 0.021* |
| C15 | 0.10269(10) | 0.24305(10) | −0.07083(6) | 0.01853(17) |
| H15 | 0.1475 | 0.2523 | −0.1327 | 0.022* |
| C16 | 0.26417(9) | 0.17175(7) | 0.43676(5) | 0.01074(13) |
| C17 | 0.40601(9) | 0.12254(7) | 0.42704(6) | 0.01205(14) |
| H17 | 0.4883 | 0.1289 | 0.4568 | 0.014* |
| C18 | 0.40638(10) | 0.06196(7) | 0.36568(6) | 0.01368(14) |
| H18 | 0.4883 | 0.0217 | 0.3468 | 0.016* |
| C19 | 0.26247(10) | 0.07250(7) | 0.33782(6) | 0.01382(14) |
| H19 | 0.2296 | 0.0385 | 0.2983 | 0.017* |
| C20 | 0.17656(9) | 0.14250(7) | 0.37912(6) | 0.01259(14) |
| H20 | 0.0764 | 0.1663 | 0.3700 | 0.015* |
| C21 | 0.21769(9) | 0.24104(7) | 0.49726(6) | 0.01104(13) |
| H21 | 0.2553 | 0.3181 | 0.4678 | 0.013* |
| C22 | 0.05111(9) | 0.25946(7) | 0.51189(6) | 0.01295(14) |
| H22 | 0.0059 | 0.2946 | 0.4517 | 0.016* |
| C23 | 0.01917(11) | 0.34082(8) | 0.56658(7) | 0.01754(16) |
| H23A | 0.0606 | 0.4146 | 0.5330 | 0.021* |
| H23B | −0.0873 | 0.3562 | 0.5745 | 0.021* |
| C24 | 0.08556(10) | 0.28741(8) | 0.66007(6) | 0.01574(15) |
| H24 | 0.0636 | 0.3408 | 0.6951 | 0.019* |
| C25 | 0.25063(10) | 0.26704(8) | 0.64621(6) | 0.01523(15) |
| H25A | 0.2953 | 0.2330 | 0.7059 | 0.018* |
| H25B | 0.2924 | 0.3409 | 0.6133 | 0.018* |
| C26 | 0.28435(9) | 0.18613(7) | 0.59168(6) | 0.01133(13) |
| H26 | 0.3921 | 0.1722 | 0.5835 | 0.014* |
| C27 | −0.01438(10) | 0.14560(8) | 0.56502(6) | 0.01439(15) |
| H27A | −0.1210 | 0.1593 | 0.5737 | 0.017* |
| H27B | 0.0045 | 0.0925 | 0.5304 | 0.017* |
| C28 | 0.02163(10) | 0.17311(8) | 0.71235(6) | 0.01608(15) |
| H28A | −0.0848 | 0.1861 | 0.7222 | 0.019* |
| H28B | 0.0649 | 0.1383 | 0.7724 | 0.019* |
| C29 | 0.21932(10) | 0.07186(7) | 0.64333(6) | 0.01286(14) |
| H29A | 0.2408 | 0.0193 | 0.6084 | 0.015* |
| H29B | 0.2640 | 0.0357 | 0.7028 | 0.015* |
| C30 | 0.05427(10) | 0.09197(8) | 0.65778(6) | 0.01349(14) |
| H30 | 0.0130 | 0.0173 | 0.6919 | 0.016* |
| C31 | 0.34483(9) | 0.47561(7) | 0.30921(6) | 0.01141(13) |
| H31 | 0.3393 | 0.4739 | 0.3734 | 0.014* |
| C32 | 0.38784(9) | 0.59398(7) | 0.24793(6) | 0.01142(13) |
| C33 | 0.42535(10) | 0.61815(8) | 0.15569(6) | 0.01477(15) |
| H33 | 0.4283 | 0.5591 | 0.1304 | 0.018* |
| C34 | 0.45840(10) | 0.72734(8) | 0.10045(6) | 0.01726(16) |
| H34 | 0.4855 | 0.7422 | 0.0381 | 0.021* |
| C35 | 0.45181(11) | 0.81519(8) | 0.13659(7) | 0.02050(18) |
| H35 | 0.4735 | 0.8901 | 0.0989 | 0.025* |
| C36 | 0.41346(12) | 0.79252(8) | 0.22781(7) | 0.02068(18) |
| H36 | 0.4083 | 0.8521 | 0.2526 | 0.025* |
| C37 | 0.38233(10) | 0.68227(8) | 0.28334(7) | 0.01584(15) |
| H37 | 0.3571 | 0.6673 | 0.3459 | 0.019* |
| C38 | 0.73137(10) | 0.23770(8) | 0.33748(8) | 0.01997(18) |
| H38A | 0.6724 | 0.1722 | 0.3633 | 0.030* |
| H38B | 0.8171 | 0.2256 | 0.3735 | 0.030* |
| H38C | 0.7626 | 0.2454 | 0.2748 | 0.030* |
| C39 | 0.73053(11) | 0.49011(9) | 0.26585(7) | 0.02071(18) |
| H39A | 0.7355 | 0.4910 | 0.2031 | 0.031* |
| H39B | 0.8290 | 0.4777 | 0.2880 | 0.031* |
| H39C | 0.6848 | 0.5636 | 0.2680 | 0.031* |
| C40 | 0.61472(12) | 0.37536(11) | 0.45757(7) | 0.0241(2) |
| H40A | 0.5626 | 0.4472 | 0.4591 | 0.036* |
| H40B | 0.7142 | 0.3702 | 0.4766 | 0.036* |
| H40C | 0.5644 | 0.3106 | 0.4989 | 0.036* |
| C41 | 0.19439(9) | 0.46138(7) | 0.28591(6) | 0.01302(14) |
| H41 | 0.1172(15) | 0.5245(12) | 0.2843(9) | 0.018(3)* |
| C42 | 0.17073(9) | 0.37173(7) | 0.26282(6) | 0.01207(14) |
| H42 | 0.0735(16) | 0.3664(13) | 0.2483(10) | 0.022(4)* |
Source of material
All reactions were carried out under a dry nitrogen atmosphere using Schlenk-technique. Bis(adamantylidenepentafulvene)titanium was prepared by procedures reported previously [3, 4] . Bis(adamantylcyclopentadienyl)titanium-η2(N-benzylidenetrimethylsilylamine) was prepared by reaction of bis(adamantylidenepentafulvene)titanium with N-benzyltrimethylsilylamine as described for N-methylanilines at 60 °C for 3 d [4]. For the target compound a suspension of bis(adamantylcyclopentadienyl)titanium-η2-(N-benzylidenetrimethylsilylamine) (250 mg, 0.385 mmol) in 10 mL n-hexane was stirred under acetylene (1 atm.) for 15 min at room temperature, forming a brown suspension. The brown product was separated, washed with n-hexane and dried in vacuum.
Experimental details
The measured crystal was twinned non-merohedrally. The data were processed accordingly and refined against F2 in the HKLF5 format of the SHELX program [2]. All hydrogen atoms were located in the difference Fourier syntheses, and subsequently fixed to geometric positions using appropiate riding models.
Comment
Titanaaziridines are used in a variety of organic synthesis methods [5, 6] . In this context, the hydroaminoalkylation of alkenes, in which the insertion of the alkene into the Ti—C bond of the titanaaziridine is supposed to be the C—C forming step [7], [8], [9], [10], is likely the most important one. To the best of our knowledge, reactions of titanaaziridines and alkynes in the catalytic hydroaminoalkylation have not been reported until now. However, in stochiometric reactions there are a few examples of 5-membered ring insertion products [4, 11, 12]. Usually, such products are generated from mono and doubly aryl, alkyl- and trimethylsilyl-substituted alkynes. Here, we present the crystal structure of a dihydroisotitanazole, which was synthesized from bis(adamantylcyclopentadienyl)titanium-η2(N-benzylidene-trimethylsilylamine) and acetylene. This is the first structurally characterized dihydroisotitanazole employing the smallest possible substituted alkyne acetylene.
The Ti1—N1 (2.0049(7) Å) and the Ti1—C42 (2.1248(9) Å) bonds are in the expected range of dihydroisotitanazoles [4, 12] . The former acetylene C—C triple bond is now elongated to a typical C—C double bond with 1.3363(12) Å between C41—C42 in the insertion product [13]. The newly formed bond C31—C41 (1.5120(12) Å) is in accordance with a single bond [13]. The 5-membered ring (Ti1-N1-C31-C32-C42) is almost planar. Due to the strain of the ring, the angle N1—Ti1—C42 (81.34(3)°) differs significantly from the angle of an ideal pentagon (108°). Consequently, the titanium center has a distorted tetrahedral coordination.
References
1 Bruker. APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA (2012).Suche in Google Scholar
2 Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
3 Diekmann, M.; Bockstiegel, G.; Lützen, A.; Friedemann, M.; Haase, D.; Saak, W.; Beckhaus, R.: Chiral bis(η5:η1-pentafulvene)titanium complexes. Organometallics 25 (2006) 339–348.10.1021/om050815mSuche in Google Scholar
4 Manßen, M.; Lauterbach, N.; Dörfler, J.; Schmidtmann, M.; Saak, W.; Doye, S.; Beckhaus, R.: Efficient access to titanaaziridines by C—H activation of N-methylanilines at ambient temperatures. Angew. Chem. Int. Ed. 54 (2015) 4383–4384.10.1002/anie.201500796Suche in Google Scholar PubMed
5 See Ref. 4. and references therein.Suche in Google Scholar
6 Loose, F.; Plettenberg, I.; Haase, D.; Saak, W.; Schmidtmann, S.; Schäfer, A.; Müller, T.; Beckhaus, R.: Aromatic imines in the titanocene coordination sphere − titanaaziridine vs 1-aza-2-titanacyclopent-4-ene structures. Organometallics 33 (2014) 6785–6795.10.1021/om500750ySuche in Google Scholar
7 Chong, E.; Garcia, P.; Schafer, L. L.: Hydroaminoalkylation: early-transition-metal-catalyzed α-alkylation of amines. Synthesis 21 (2014) 2884–2896.10.1055/s-0034-1379216Suche in Google Scholar
8 Prochnow, I.; Kubiak, R.; Frey, O. N.; Beckhaus, R.: Tetrabenzyltitanium: an improved catalyst for the activation of sp3 C—H bonds adjacent to nitrogen atoms. ChemCatChem 1 (2009) 162–172.10.1002/cctc.200900092Suche in Google Scholar
9 Prochnow, I.; Zark, P.; Müller, T.; Doye, S.: The mechanism of the titanium-catalyzed hydroaminoalkylation of alkenes. Angew. Chem. Int. Ed. 50 (2011) 6401–6405.10.1002/anie.201101239Suche in Google Scholar PubMed
10 Roesky, P. W.: Catalytic hydroaminoalkylation. Angew. Chem. Int. Ed. 48 (2009) 4892–4894.10.1002/anie.200900735Suche in Google Scholar PubMed
11 See Ref. 10. and references therein.Suche in Google Scholar
12 Loose, F.; Schmidtmann, M.; Saak, W.; Beckhaus, R.: Imines in the titanium coordination sphere: highly reactive titanaa-ziridines and larger titanacycles formed by subsequent C—C coupling reactions. Eur. J. Inorg. Chem. 2015 (2015) 5171–5187.10.1002/ejic.201500805Suche in Google Scholar
13 March, J.: Advanced organic chemistry, 4th ed. Wiley, New York (1992).Suche in Google Scholar
©2017 Manfred Manßen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)manganese(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2) C42H40Cl2MnN6O10S2
- The crystal structure of 1,2-bis[2-methyl-5-(3-cyanophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H16F6N2S2
- Crystal structure of the first characterized polymeric copper and sodium complex diaqua-(tris-acetato-κO,O′)(μ2-acetato-κO′′)dinatrium copper(II) monohydrate, C8H18CuNa2O11
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-3-yl-methylene)aniline, C18H22N2
- Crystal structure of methyl 2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22N2O5
- Crystal structure of bis(1,3-bis(diphenylphosphino)propane-κ2P,P′)silver(I) trifluorosulfonate–methanol (1:0.5), [Ag(C27H26P2)]SO3CF3⋅0.5CH3OH
- Crystal structure of μ-1,4-bis(diphenylphosphine)butane-2,9-dimethyl-1,10-phenanthroline-κ2N:N′-bis(cyano-κC)dicopper(I)]-water, C58H56Cu2N6O2P2
- Crystal structure of N2,N6-bis(1-hydrazinyl-2-methyl-1-oxopropan-2-yl) pyridine-2,6-dicarboxamide, C15H23N7O4
- Crystal structure of 1,3-dimethyl-2-phenyl-1H-perimidin-3-ium iodide, C19H17IN2
- Crystal structure of diaqua-(2,2′-(butane-1,4-diyl)-bis(5-carboxy-1H-imidazole-4-carboxylato)-κ4O,O′,N,N′)cadmium(II) monohydrate, C14H18O11N4Cd
- Crystal structure of ethyl (E)-3-(cyclopropylamino)-2-(2,4-dichloro-5-fluorobenzoyl) acrylate, C15H14Cl2FNO3
- Crystal structure of bis-(1-(4-chlorophenyl)-3-phenyl-4-thenoyl-1H-pyrazol-5-ol-κ2O,O′)-(N,N-dimethylformamide)zinc(II), C43H31Cl2N5O5S2Zn
- Crystal structure of tetrakis(μ3-2-(N-(2-hydroxyethyl)amino)ethoxo)-tetrachloro-tetra-cobalt(II) methanol solvate, C17H44Cl4Co4N4O9
- Crystal structure of 3-amino-1-(4-bromophenyl)-9-methoxy-1H-benzo[f]chromene-2-carbonitrile, C21H15BrN2O2
- Crystal structure of 2-amino-4-(4-isopropyl-phenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2-c]chromene, C22H18N2O3
- Crystal structure of 3-amino-8-methoxy-1-(4-methoxy phenyl)-1H-benzo[f]chromene-2-carbonitrile, C22H18N2O3
- Crystal structure of monoaqua-[6,6′-((1E,1′E)-(1,2-phenylene bis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-nitrophenolato-κ4N,N′,O,O′)]zinc(II), C20H12Br2N4O7Zn
- Crystal structure of 11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one – acetonitril (1/2), C33H41N3O2
- Crystal structure of tert-butyl (phenylsulfinyl)carbamate, C11H15NO3S
- Crystal structure of catena-poly{diaqua-bis(3-(1H-1,2,4-triazol-1-yl)benzoato-κ2O:N)copper(II)} monohydrate, C18H18CuN6O7
- Crystal structure of tetraaqua-bis(3-(4H-1,2,4-triazol-4-yl)benzoato-κN)cobalt(II), C18H20CoN6O8
- The crystal structure of 4-bromo-N-cyclopropyl-2,5-difluorobenzenesulfonamide, C9H8BrF2NO2S
- Crystal structure of ([3,3′-bipyridine]-6,6′-dicarboxylato-κ2O:O′)-bis(1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) dihydrate, C37H35Ag2N5O6
- The crystal structure of (4-(1H-1,2,4-triazol-1-yl)benzoato-κN)-[4-(1H-1,2,4-triazol-1-yl)benzoic acid-κN]silver(I), C18H13AgN6O4
- Crystal structure of 3-(4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one, C17H16O2
- Crystal structure of bis(2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-methoxyphenolato-κ2O,N)copper(II), C36H38CuN4O6
- Crystal structure of poly[1,2-bis(1,2,4-triazol-4-yl)ethane-κ2N:N′]silver(I) bromate monohydrate]silver(I), C6H10AgBrN6O4
- The crystal structure of 2,3,5-triphenyl-2,3-dihydro-1H-tetrazol-1-ium 2,3-dioxoindoline-5-sulfonate, C27H19N5O5S
- Crystal structure of 3-(2-amino-1,3-selenazol-4-yl)-2H-chromen-2-one – dimethylformamide (1/1), C15H15N3O3Se
- Crystal structure of diethyl 3,3′-(diazene-1,2-diyl)(E)-dibenzoate, C18H18N2O4
- Crystal structure of cis-bis((1H-benzimidazol-2-yl)methanol-κN,O)-bis(isothiocyanato-κN)nickel(II), C18H16N6NiO2S2
- Crystal structure of bis(2-(2′-hydroxy-5′-methoxyphenyl)-1H-benzimidazole)boron – tetrahydrofuran (1/1), C36H37N4O6B
- Crystal structure of poly[aqua-bis(nitrato-κ2O,O′)-(μ3-1,3-benzimidazol-3-ium-1,3-diacetato-κ4O,O′:O′′:O′′′)dysprosium(III)], C11H11DyN4O11
- Crystal structure of n-butyl-tris(dicyclo-hexylamido)hafnium(IV), C40H75HfN3
- Crystal structure of (E)-1-[1-(3-chloro-4-fluoro-phenyl)ethylidene]-2-(2,4-dinitrophenyl)hydrazine, C28H20Cl2F2N8O8
- The crystal structure of ethyl 4-((2-hydroxybenzyl)amino)benzoate, a Schiff base, C16H17NO3
- Crystal structure of 3-(2-(4-isobutylphenyl)propanoyl)-1-methylimidazolidine-2,4-dione, C17H22N2O3
- Crystal structure of poly[(μ3-2-(pyrazin-2-ylthio)acetato-κ3N:O:S)silver(I)], C6H5AgN2O2S
- Crystal structure of (E)-1-(3-((E)-((2-hydroxynaphthalen-1-yl)methylene)amino)phenyl)ethanone O-benzyl oxime, C26H22N2O2
- Crystal structure of (E)-2,3-dihydroxybenzaldehyde O-(2-((((E)-1-(2,5-dihydroxyphenyl)ethylidene)amino)oxy)ethyl) oxime monohydrate, C17H20N2O7
- Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4
- Crystal stucture of 4-((10H-phenothiazin-10-yl)methyl)-2,6-di-tert-butylphenol, C27H31NOS
- The crystal structure of ethyl 1-(4-nitrophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C13H10F3N3O4
- Crystal structure of catena-poly[di-(μ3-oxido-κ3O:O:O)-tetraoxido-(μ2-5′-(pyrazin-2-yl)-1H,2′H-3,3′-bi(1,2,4-triazole)-κ2N:N′)dimolybdenum(VI)], C8H6Mo2N8O6
- Crystal structure of (3,6-dioxocyclohexa-1,4-diene-1,4-bis(olato)-κ4O,O′:O′′,O′′′)-bis(tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′))-dizinc(II) bis(hexafluorophosphate(V)), C42H38F12N8O4P2Zn2
- Crystal structure of catena-poly[hexakis(μ2-2-acetylphenolato-κ3O:O,O′)trimanganese(II)], C48H42Mn3O12
- The crystal structure of 2,2-difluoro-4-(trifluoromethyl)-2,5-dihydro-[1,3,2]dioxaborinino[5,4-c]chromen-3-ium-2-uide, C11H6BF5O3
- Crystal structure of methyl (E)-2-(4-(diethylamino)-2-hydroxybenzylidene)hydrazine-1-carboxylate, C13H19N3O3
- The crystal structure of tert-butyl 2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate, C10H15N3O2
- Crystal structure of 1,1-bis(η5-adamantylcyclopentadienyl)-3-phenyl-2-trimethylsilyl-2,3-dihydroisotitanazole, C42H55NSiTi
- Crystal structure of 2-(4-(2-(4-benzylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C28H29N3O2
- Important impurity of Flupirtine – a single crystal study on ethyl (6-amino-5-((ethoxycarbonyl)amino)pyridin-2-yl)(4-fluorobenzyl)carbamate, C18H21FN4O4
- Crystal structure of ethyl 3-(4-methoxyphenyl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-pyrazole-5-carboxylate, C22H22N2O5
- Crystal structure of 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate methanol solvate, C37H35N1O10
- The crystal structure of the inner salt of 2-[(aminoiminomethyl)amino]ethylcarbamic acid [systematic name: (2-((diaminomethylene)ammonio)ethyl)carbamate], C4H10N4O2
- Crystal structure of (8-hydroxy-5-nitroquinolinium) perchlorate – 8-hydroxy-5-nitroquinoline (1/1), C18H13ClN4O10
- The crystal structure of (5-methyl-1,2,4-oxadiazol-3-yl)ferrocene, C13H12FeN2O
- Crystal structure of methyl 1-(2-(fluorosulfonyl)ethyl)-2-oxocyclopentanecarboxylate, C9H13FO5S
- Crystal structure of the triclinic modification of 1-methyl-4-nitroimidzole, C4H5N3O2
- Corrigendum
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine-κ2N:N′)manganese(II)] bis(4-chlorobenzenesulfonate) – 4,4′-bipyridine – water (1/1/2) C42H40Cl2MnN6O10S2
- The crystal structure of 1,2-bis[2-methyl-5-(3-cyanophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluoro-cyclopent-1-ene, C29H16F6N2S2
- Crystal structure of the first characterized polymeric copper and sodium complex diaqua-(tris-acetato-κO,O′)(μ2-acetato-κO′′)dinatrium copper(II) monohydrate, C8H18CuNa2O11
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-3-yl-methylene)aniline, C18H22N2
- Crystal structure of methyl 2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C20H22N2O5
- Crystal structure of bis(1,3-bis(diphenylphosphino)propane-κ2P,P′)silver(I) trifluorosulfonate–methanol (1:0.5), [Ag(C27H26P2)]SO3CF3⋅0.5CH3OH
- Crystal structure of μ-1,4-bis(diphenylphosphine)butane-2,9-dimethyl-1,10-phenanthroline-κ2N:N′-bis(cyano-κC)dicopper(I)]-water, C58H56Cu2N6O2P2
- Crystal structure of N2,N6-bis(1-hydrazinyl-2-methyl-1-oxopropan-2-yl) pyridine-2,6-dicarboxamide, C15H23N7O4
- Crystal structure of 1,3-dimethyl-2-phenyl-1H-perimidin-3-ium iodide, C19H17IN2
- Crystal structure of diaqua-(2,2′-(butane-1,4-diyl)-bis(5-carboxy-1H-imidazole-4-carboxylato)-κ4O,O′,N,N′)cadmium(II) monohydrate, C14H18O11N4Cd
- Crystal structure of ethyl (E)-3-(cyclopropylamino)-2-(2,4-dichloro-5-fluorobenzoyl) acrylate, C15H14Cl2FNO3
- Crystal structure of bis-(1-(4-chlorophenyl)-3-phenyl-4-thenoyl-1H-pyrazol-5-ol-κ2O,O′)-(N,N-dimethylformamide)zinc(II), C43H31Cl2N5O5S2Zn
- Crystal structure of tetrakis(μ3-2-(N-(2-hydroxyethyl)amino)ethoxo)-tetrachloro-tetra-cobalt(II) methanol solvate, C17H44Cl4Co4N4O9
- Crystal structure of 3-amino-1-(4-bromophenyl)-9-methoxy-1H-benzo[f]chromene-2-carbonitrile, C21H15BrN2O2
- Crystal structure of 2-amino-4-(4-isopropyl-phenyl)-3-cyano-5-oxo-4H,5H-pyrano[3,2-c]chromene, C22H18N2O3
- Crystal structure of 3-amino-8-methoxy-1-(4-methoxy phenyl)-1H-benzo[f]chromene-2-carbonitrile, C22H18N2O3
- Crystal structure of monoaqua-[6,6′-((1E,1′E)-(1,2-phenylene bis(azanylylidene))bis(methanylylidene))bis(4-bromo-2-nitrophenolato-κ4N,N′,O,O′)]zinc(II), C20H12Br2N4O7Zn
- Crystal structure of 11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one – acetonitril (1/2), C33H41N3O2
- Crystal structure of tert-butyl (phenylsulfinyl)carbamate, C11H15NO3S
- Crystal structure of catena-poly{diaqua-bis(3-(1H-1,2,4-triazol-1-yl)benzoato-κ2O:N)copper(II)} monohydrate, C18H18CuN6O7
- Crystal structure of tetraaqua-bis(3-(4H-1,2,4-triazol-4-yl)benzoato-κN)cobalt(II), C18H20CoN6O8
- The crystal structure of 4-bromo-N-cyclopropyl-2,5-difluorobenzenesulfonamide, C9H8BrF2NO2S
- Crystal structure of ([3,3′-bipyridine]-6,6′-dicarboxylato-κ2O:O′)-bis(1,2-bis(4-pyridyl)ethane-κ2N:N′)disilver(I) dihydrate, C37H35Ag2N5O6
- The crystal structure of (4-(1H-1,2,4-triazol-1-yl)benzoato-κN)-[4-(1H-1,2,4-triazol-1-yl)benzoic acid-κN]silver(I), C18H13AgN6O4
- Crystal structure of 3-(4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one, C17H16O2
- Crystal structure of bis(2-((E)-((4-((E)-1-(ethoxyimino)ethyl)phenyl)imino)methyl)-5-methoxyphenolato-κ2O,N)copper(II), C36H38CuN4O6
- Crystal structure of poly[1,2-bis(1,2,4-triazol-4-yl)ethane-κ2N:N′]silver(I) bromate monohydrate]silver(I), C6H10AgBrN6O4
- The crystal structure of 2,3,5-triphenyl-2,3-dihydro-1H-tetrazol-1-ium 2,3-dioxoindoline-5-sulfonate, C27H19N5O5S
- Crystal structure of 3-(2-amino-1,3-selenazol-4-yl)-2H-chromen-2-one – dimethylformamide (1/1), C15H15N3O3Se
- Crystal structure of diethyl 3,3′-(diazene-1,2-diyl)(E)-dibenzoate, C18H18N2O4
- Crystal structure of cis-bis((1H-benzimidazol-2-yl)methanol-κN,O)-bis(isothiocyanato-κN)nickel(II), C18H16N6NiO2S2
- Crystal structure of bis(2-(2′-hydroxy-5′-methoxyphenyl)-1H-benzimidazole)boron – tetrahydrofuran (1/1), C36H37N4O6B
- Crystal structure of poly[aqua-bis(nitrato-κ2O,O′)-(μ3-1,3-benzimidazol-3-ium-1,3-diacetato-κ4O,O′:O′′:O′′′)dysprosium(III)], C11H11DyN4O11
- Crystal structure of n-butyl-tris(dicyclo-hexylamido)hafnium(IV), C40H75HfN3
- Crystal structure of (E)-1-[1-(3-chloro-4-fluoro-phenyl)ethylidene]-2-(2,4-dinitrophenyl)hydrazine, C28H20Cl2F2N8O8
- The crystal structure of ethyl 4-((2-hydroxybenzyl)amino)benzoate, a Schiff base, C16H17NO3
- Crystal structure of 3-(2-(4-isobutylphenyl)propanoyl)-1-methylimidazolidine-2,4-dione, C17H22N2O3
- Crystal structure of poly[(μ3-2-(pyrazin-2-ylthio)acetato-κ3N:O:S)silver(I)], C6H5AgN2O2S
- Crystal structure of (E)-1-(3-((E)-((2-hydroxynaphthalen-1-yl)methylene)amino)phenyl)ethanone O-benzyl oxime, C26H22N2O2
- Crystal structure of (E)-2,3-dihydroxybenzaldehyde O-(2-((((E)-1-(2,5-dihydroxyphenyl)ethylidene)amino)oxy)ethyl) oxime monohydrate, C17H20N2O7
- Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4
- Crystal stucture of 4-((10H-phenothiazin-10-yl)methyl)-2,6-di-tert-butylphenol, C27H31NOS
- The crystal structure of ethyl 1-(4-nitrophenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C13H10F3N3O4
- Crystal structure of catena-poly[di-(μ3-oxido-κ3O:O:O)-tetraoxido-(μ2-5′-(pyrazin-2-yl)-1H,2′H-3,3′-bi(1,2,4-triazole)-κ2N:N′)dimolybdenum(VI)], C8H6Mo2N8O6
- Crystal structure of (3,6-dioxocyclohexa-1,4-diene-1,4-bis(olato)-κ4O,O′:O′′,O′′′)-bis(tris(2-pyridylmethyl)amine-κ4N,N′,N′′,N′′′))-dizinc(II) bis(hexafluorophosphate(V)), C42H38F12N8O4P2Zn2
- Crystal structure of catena-poly[hexakis(μ2-2-acetylphenolato-κ3O:O,O′)trimanganese(II)], C48H42Mn3O12
- The crystal structure of 2,2-difluoro-4-(trifluoromethyl)-2,5-dihydro-[1,3,2]dioxaborinino[5,4-c]chromen-3-ium-2-uide, C11H6BF5O3
- Crystal structure of methyl (E)-2-(4-(diethylamino)-2-hydroxybenzylidene)hydrazine-1-carboxylate, C13H19N3O3
- The crystal structure of tert-butyl 2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate, C10H15N3O2
- Crystal structure of 1,1-bis(η5-adamantylcyclopentadienyl)-3-phenyl-2-trimethylsilyl-2,3-dihydroisotitanazole, C42H55NSiTi
- Crystal structure of 2-(4-(2-(4-benzylpiperazin-1-yl)ethyl)benzyl)isoindoline-1,3-dione, C28H29N3O2
- Important impurity of Flupirtine – a single crystal study on ethyl (6-amino-5-((ethoxycarbonyl)amino)pyridin-2-yl)(4-fluorobenzyl)carbamate, C18H21FN4O4
- Crystal structure of ethyl 3-(4-methoxyphenyl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-pyrazole-5-carboxylate, C22H22N2O5
- Crystal structure of 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-olate methanol solvate, C37H35N1O10
- The crystal structure of the inner salt of 2-[(aminoiminomethyl)amino]ethylcarbamic acid [systematic name: (2-((diaminomethylene)ammonio)ethyl)carbamate], C4H10N4O2
- Crystal structure of (8-hydroxy-5-nitroquinolinium) perchlorate – 8-hydroxy-5-nitroquinoline (1/1), C18H13ClN4O10
- The crystal structure of (5-methyl-1,2,4-oxadiazol-3-yl)ferrocene, C13H12FeN2O
- Crystal structure of methyl 1-(2-(fluorosulfonyl)ethyl)-2-oxocyclopentanecarboxylate, C9H13FO5S
- Crystal structure of the triclinic modification of 1-methyl-4-nitroimidzole, C4H5N3O2
- Corrigendum
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O

