Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
-
Yosuke Ashikari
Abstract
Owing to their recyclability, heterogeneous transition metal catalysts represent a means of conserving depletable resources for the synthesis of pharmaceutical, agricultural, and functional chemicals. We recently developed a novel heterogeneous palladium catalyst and demonstrated its synthetic availability for Suzuki–Miyaura cross-coupling. Herein, we report the further application of the present catalyst to cross-coupling reactions in batch and flow, as well as a hydrogenative reduction reaction in flow. We demonstrate the flow synthesis for useful material, a liquid crystal, and a 1 h sequential operation of the coupling reaction and hydrogenation reaction.
Graphical abstract
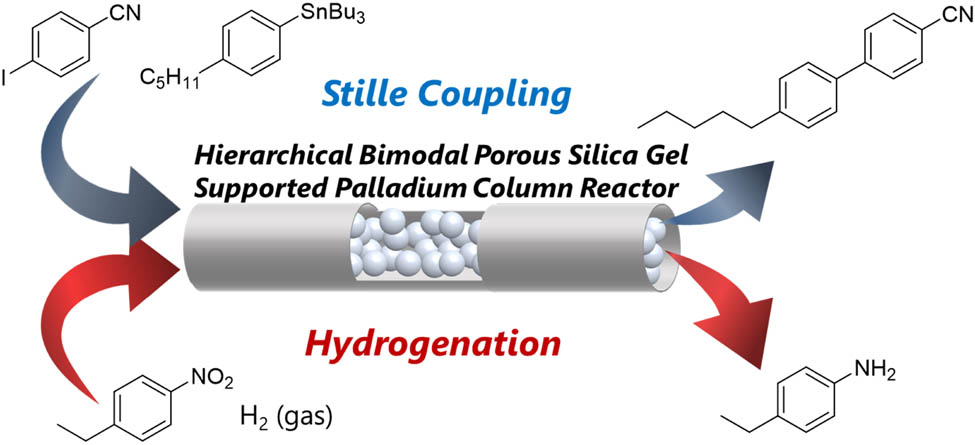
1 Introduction
Reuse of reactants is an important means of achieving green processes in organic synthesis, especially given the Sustainable Development Goal 12 (responsible consumption and production) established by the United Nations. In this context, heterogeneous catalysts have attracted considerable interest in both academic and industrial fields because of their ease of recovery and reuse. A wide range of reactions catalyzed by heterogeneous catalysts have been developed to produce useful organic compounds, including pharmaceutical and functional chemicals [1].
One notable advantage of heterogeneous catalysts is their applicability to flow synthesis, which is usually performed using channel- or tube-type reactors [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16], as heterogeneous column reactors [17,18,19,20,21,22,23]. In addition to the advantages of excellent heat- and mass-transfer ability and precise reaction-time controllability, flow synthesis enables continuous operation; this is beneficial as increasing the operational time increases the synthesis productivity [24,25]. With respect to heterogeneous column reactors, continuous operation can be considered to be a form of catalyst recycling. To recycle a heterogeneous catalyst in batch reactions, the catalyst should be separated (typically by filtration) and used for the next reaction. By contrast, in a flow reaction, multiplying the volume of the reactant solution is equivalent to performing multiple reaction operations (Figure 1). Thus, enhancing the continuous operability of the heterogeneous column reactor can increase the recyclability of the catalyst.
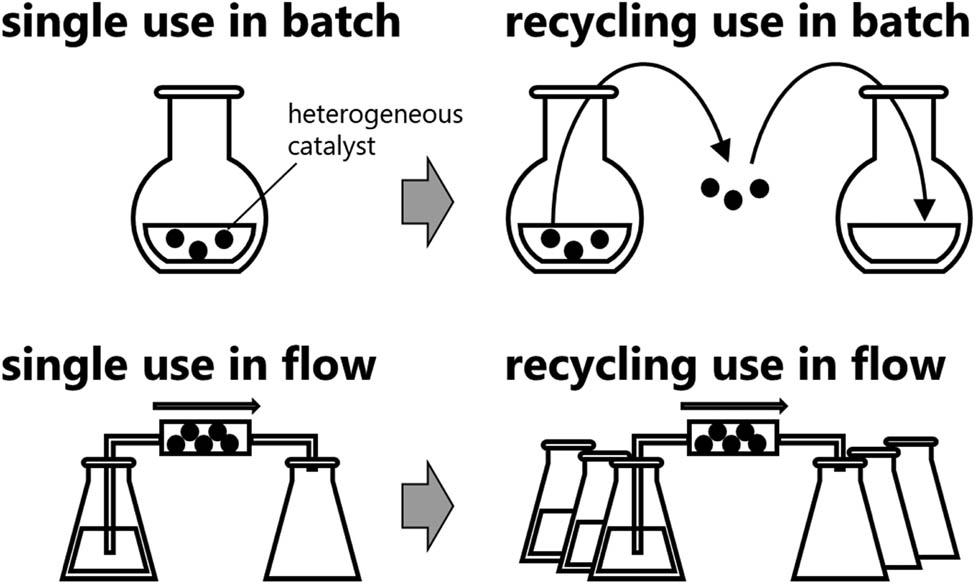
Schematic for recycling a heterogeneous catalyst in batch and flow.
Based on this idea, we recently developed a palladium catalyst supported on a bimodal porous silica gel and reported its synthetic application in Suzuki–Miyaura cross-coupling [26]. The advantage of this catalyst is that the hierarchical macro–mesoporous structure of the silica gel provides a low back pressure [27,28]; this allows a higher flow rate without an exceedance of the pump pressure. Moreover, the low back pressure helps to avoid reactor clogging, which would stop the operation and ruin the recyclability of the catalyst. Using our heterogeneous palladium catalyst, we demonstrated a 1-h operation of flow Suzuki coupling without any decrease in the product yield. Given the high usability of our immobilized palladium catalyst, we considered its further applications in organic synthesis. In addition to its application to Suzuki coupling, we envisaged that it could be adapted to other palladium-catalyzed cross-coupling reactions. Herein, we report the application of a hierarchical bimodal porous-silica-gel-supported palladium catalyst for cross-coupling reactions in batch and flow, as well as a hydrogenative reduction in flow.
2 Materials and methods
All chemicals were purchased from Fujifilm Wako Pure Chemical Corporation, Tokyo Chemical Industry Co., Ltd., Kanto Chemical Co., Inc., or Aldrich, and used without any further purification. Gas chromatography (GC) analysis was performed on a Shimadzu GC-2014 instrument equipped with a flame ionization detector using a fused silica capillary column (column, CBP1; 0.22 mm × 25 m). X-ray fluorescence (XRF) analyses were performed using a Shimadzu EDX-8000 system. For flow reactions, a syringe pump (Harvard PHD 2000 or PHD ULTRA) equipped with a gastight syringe (purchased from SGE) or a plunger pump (Shimadzu LC-20AR) was used to introduce solutions into the column reactor. To introduce gases, a mass flow controller (Brooks Instrument SLA5850S) was used.
2.1 Immobilization of palladium on silica gel
Palladium nanoparticles were immobilized in the pores of a dual-pore silica gel (DualPoreTM) [29] using a supercritical carbon dioxide/acetone solution at 20 MPa and 70°C for 24 h. The vessel was depressurized to atmospheric pressure and the contents were removed. After the removal of acetone, the precipitate was reduced by H2 and N2 at 300°C for 5 h. TEM analyses indicated that palladium was widely dispersed on the DualPore (see Supporting Information). Pd@DualPore was placed in stainless steel (SUS316) tubes.
2.2 Coupling reactions in batch
2.2.1 Stille coupling
The following were added to a Schlenk tube capped by a septum: 4-iodobenzonitrile (1, 0.1 mmol), tri-n-butylphenylstannane (2, 1.5 eq), lithium chloride (3 eq), Pd@DualPore (2 mol%), and dimethylformamide (3 mL). The mixture was stirred at 120°C in an argon atmosphere for 18 h. The solution was mixed with aqueous ammonium chloride, n-hexane, ethyl acetate, and n-tridecane as internal standards. The organic phase was analyzed by GC to determine the yield of the desired product 3.
2.2.2 Heck and Sonogashira coupling
The starting materials, Pd@DualPore (2 mol%), and dimethylformamide (3 mL) were added to a Schlenk tube capped by a septum. The mixture was stirred at 120°C in an argon atmosphere for 18 h. After extraction, the crude mixture was analyzed by NMR.
2.3 Typical procedure for Stille coupling in flow
A methanol/dimethyl sulfoxide solution (MeOH/DMSO) 1 (0.033 M), arylstannane (1.5 eq), and lithium chloride (3 eq) were introduced into a column reactor filled with Pd@DualPore using a syringe pump or a plunger pump. The column reactor was heated at 97°C in a water bath; after it had reached a steady state, the solution was collected for 10 min. The solution was extracted and analyzed by GC to determine the yield of the product.
2.4 Typical procedure for hydrogenation in flow
A methanol solution containing 1-ethyl-4-nitrobenzene (11, 1.25 M) was introduced into the column reactor using a plunger pump. In addition, hydrogen gas was introduced using a mass flow controller. The column reactor was dipped in a water bath to maintain its temperature at 25°C. After it had reached a steady state, the solution was collected for 10 min. The solution was analyzed by GC to determine the yield of the desired product 12.
3 Results and discussion
First, we tested the feasibility of the immobilized catalyst for cross-coupling reactions in a batch process. As shown in Table 1, we investigated three types of coupling reactions, in which aryl iodides (1 and 4) were reacted with arylstannane 2 (Stille coupling [30]), alkene 5 (Heck coupling [31]), or alkyne 7 (Sonogashira coupling [32,33]) in the presence of a 2-mol% immobilized palladium catalyst. From the Stille coupling (Table 1, entry 1), the corresponding biphenyl 3 was obtained in a high yield. The Heck and Sonogashira couplings (entries 2 and 3) resulted in full conversions of 4. In particular, after the Heck and Sonogashira couplings, filtration, and extraction, pure products 6 and 8 were obtained, respectively.
Immobilized Pd-catalyzed coupling reactions in batcha
| Entry | Aryl iodide | Coupling partner | Additive | Product | Conversion (%) |
|---|---|---|---|---|---|
| 1 |
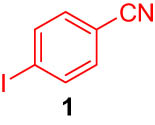
|
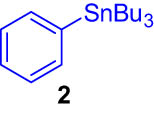
|
LiCl |
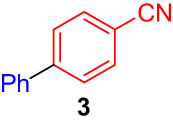
|
80b |
| 2 |
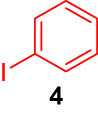
|
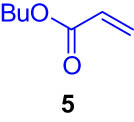
|
iPr2NEt |
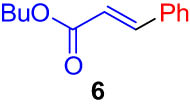
|
quant |
| 3 |
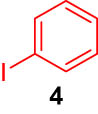
|
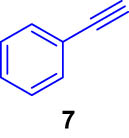
|
iPr2Net CuI |
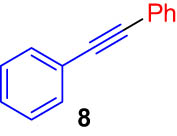
|
quant |
a The reactions were carried out at 97°C for 18 h. b The product yield was determined by GC. See the supporting information for details. Bu: n-butyl group, iPr: isopropyl group, Ph: phenyl group.
Having confirmed the good tolerance of our immobilized palladium catalyst, we attempted to utilize it under flow conditions. We chose Stille coupling for demonstration purposes (Table 2). First, we applied the previous Suzuki coupling condition [26] to the flow Stille coupling using a co-solvent of methanol and tetrahydrofuran (THF) at 97°C. When tetra-n-butylammonium fluoride (TBAF) was used as an additive (Table 2, entry 1), the reaction yield was low. The reaction without the additive did not significantly change the result (entry 2), indicating that TBAF is not suitable for this reaction. Thus, we decided to use lithium chloride as an additive. As LiCl is not soluble in THF, we changed the co-solvent to methanol with DMSO. The use of LiCl increased the product yield (entry 3). By contrast, the reaction in MeOH/DMSO without the additive resulted in a dismal yield (entry 4), indicating that the addition of LiCl was crucial for this Stille coupling, presumably because the acceleration of the transmetallation step varies according to the strength of the Stannane–halogen bond
Investigation for flow Stille couplinga
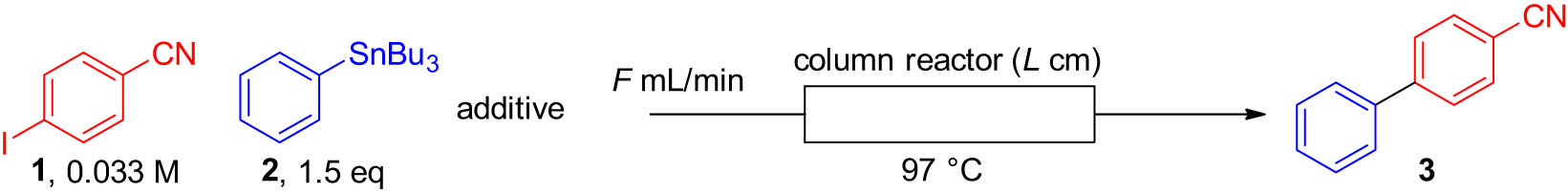
|
||||||
|---|---|---|---|---|---|---|
| Entry | Solvent | Additiveb | F (mL·min−1) | L (cm) | Yield (%) | Productivity (mg·h−1) |
| 1 | MeOH/THF (2/8) | TBAF | 1.0 | 10 | 17 | 60 |
| 2 | None | 10 | 35 | |||
| 3 | MeOH/DMSO (2/8) | LiCl | 29 | 103 | ||
| 4 | None | 10 | 35 | |||
| 5 | MeOH/DMSO (2/8) | LiCl | 0.3 | 10 | 44 | 47 |
| 6 | 0.1 | 72 | 26 | |||
| 7 | MeOH/DMSO (3/7) | LiCl | 0.1 | 10 | 79 | 28 |
| 8 | MeOH/DMSO (5/5) | 43 | 15 | |||
| 9 | MeOH | Trace | <0.1 | |||
| 10 | MeOH/DMSO (3/7) | LiCl | 1.0 | 25 | 84 | 298 |
- a
The reaction solutions were collected for 10 min and analyzed by GC.
- b
TBAF: 1.8 eq, LiCl: 3.0 eq.
To increase the yield, we decreased the flow rate of the substrate solution to prolong the reaction time. When a flow rate of 0.3 mL·min−1 was applied (the previous flow rate was 1.0 mL·min−1), the yield increased (entry 5), and a further decrease in the flow rate to 0.1 mL·min−1 led to a high yield of 72% (entry 6). After investigating the effects of the solvent ratio (entries 7–9), we found that 30% methanol with DMSO gave the best yield (79%, entry 7). However, although the yield was satisfactory, the lower flow rate resulted in lower productivity, calculated as the weight of the generated product (3) per time unit. Thus, we increased the flow rate to 1.0 mL and increased the length of the column reactor from 10 to 25 cm to achieve a sufficient reaction time. As anticipated, the longer column helped to maintain the product yield with a higher flow rate (entry 10), increasing the productivity tenfold.
Having confirmed the usability of the palladium catalyst immobilized on DualPore, we decided to demonstrate the synthetic utility of this heterogeneous Stille coupling by producing a functional molecule. As a target, we selected 4-cyano-4′-n-pentylbiphenyl (10), named 5CB, which is a commonly used liquid crystal [34], because cross-coupling reactions have made important contributions in this field [35]. Using optimized conditions (Table 2, entry 10), we reacted tri-n-butyl-4-n-pentylphenylstannane (9) with 1. As shown in Scheme 1, from a 60-min operation, 10 was successfully obtained in a high yield. It is noteworthy that after the 1-hour experiment, the weight difference of the palladium on the DualPore was only 7% even though the reaction was performed with the highly polar solvents. This strongly supports that this flow process can tolerate a long-time operation to increase productivity.
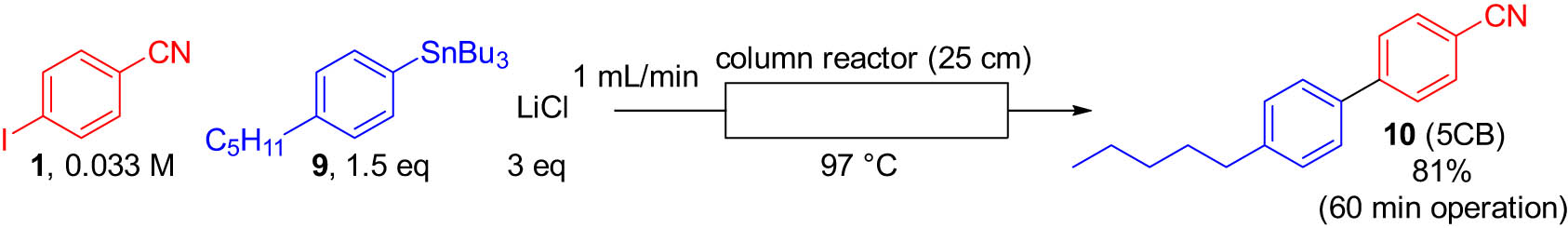
Flow synthesis for liquid crystal 5CB.
Having demonstrated the flow cross-coupling of the palladium catalyst immobilized on DualPore, we next focused on reactions using gases. Owing to the ubiquity of the nitro reduction process in organic synthesis [36], we decided to investigate the hydrogenation of nitro compounds to amines. We had previously shown that this transformation could be catalyzed by Pd@DualPore but only under batch conditions [37]. Thus, we attempted the flow hydrogenation of nitro compounds to demonstrate the utility and recyclability of the catalyst.
We chose to study the reduction of 1-ethyl-4-nitrobenzene (11); the results are summarized in Table 3. First, the substrate solution was mixed with hydrogen gas at a flow rate of 10 mL·min−1 and passed through the column reactor at 25°C. Under these conditions, we obtained the desired amine product 12, albeit in low yield (Table 3, entry 1). Having confirmed the feasibility of the flow hydrogenation of nitro groups, we optimized the flow rate of the hydrogen gas (entries 1–5) and found that using 50 mL·min−1 of hydrogen gas afforded 80% yield (entry 5). To increase the product yield, we optimized the flow rate of the solution of 11 (entries 5–8). When the flow rate was decreased, the product yield increased (entries 6 and 7); however, the productivity was diminished. As a higher flow rate (entry 8) resulted in lower productivity, we concluded that the condition given in entry 5 was the best one.
Optimization of flow hydroxylation of nitro compoundsa
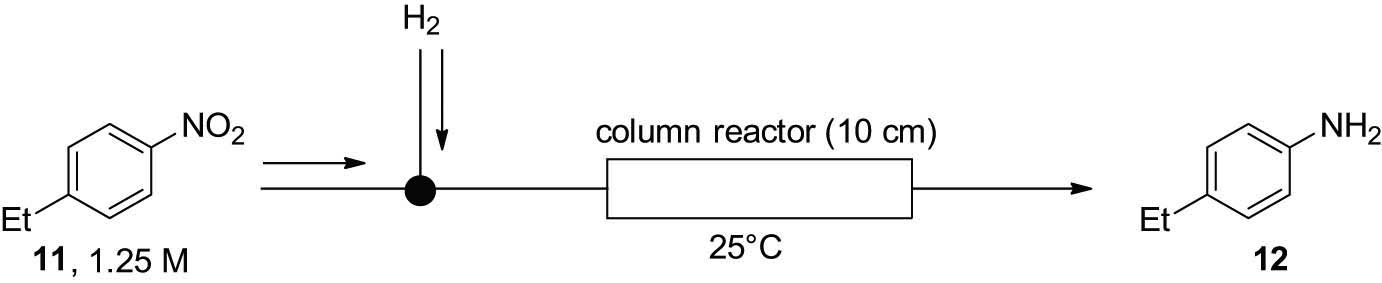
|
||||
|---|---|---|---|---|
| Entry | H2 flow rate (mL·min−1) | 11 flow rate (mL·min−1) | Yield (%) | Productivity (g·h−1) |
| 1 | 10 | 0.8 | 22 | 1.6 |
| 2 | 20 | 41 | 3.0 | |
| 3 | 30 | 54 | 3.9 | |
| 4 | 40 | 70 | 5.1 | |
| 5 | 50 | 80 | 5.8 | |
| 6 | 50 | 0.5 | 99 | 4.5 |
| 7 | 0.7 | 90 | 5.7 | |
| 8 | 1.0 | 61 | 5.5 | |
- a
The reaction solutions were collected for 10 min and analyzed by GC.
Finally, once the flow Stille coupling and hydrogenation catalyzed by the palladium catalyst immobilized on DualPore had been achieved, we attempted to demonstrate a long-timescale operation. When the flow Stille cross-coupling was continued for 60 min, we found that the yield did not diminish (Figure 2a). Similarly, a 60-min operation for the nitro group reduction was also successfully carried out without any decrease in the yield (Figure 2b). These results strongly indicate the high recyclability of the immobilized catalyst under a wide range of reaction conditions.
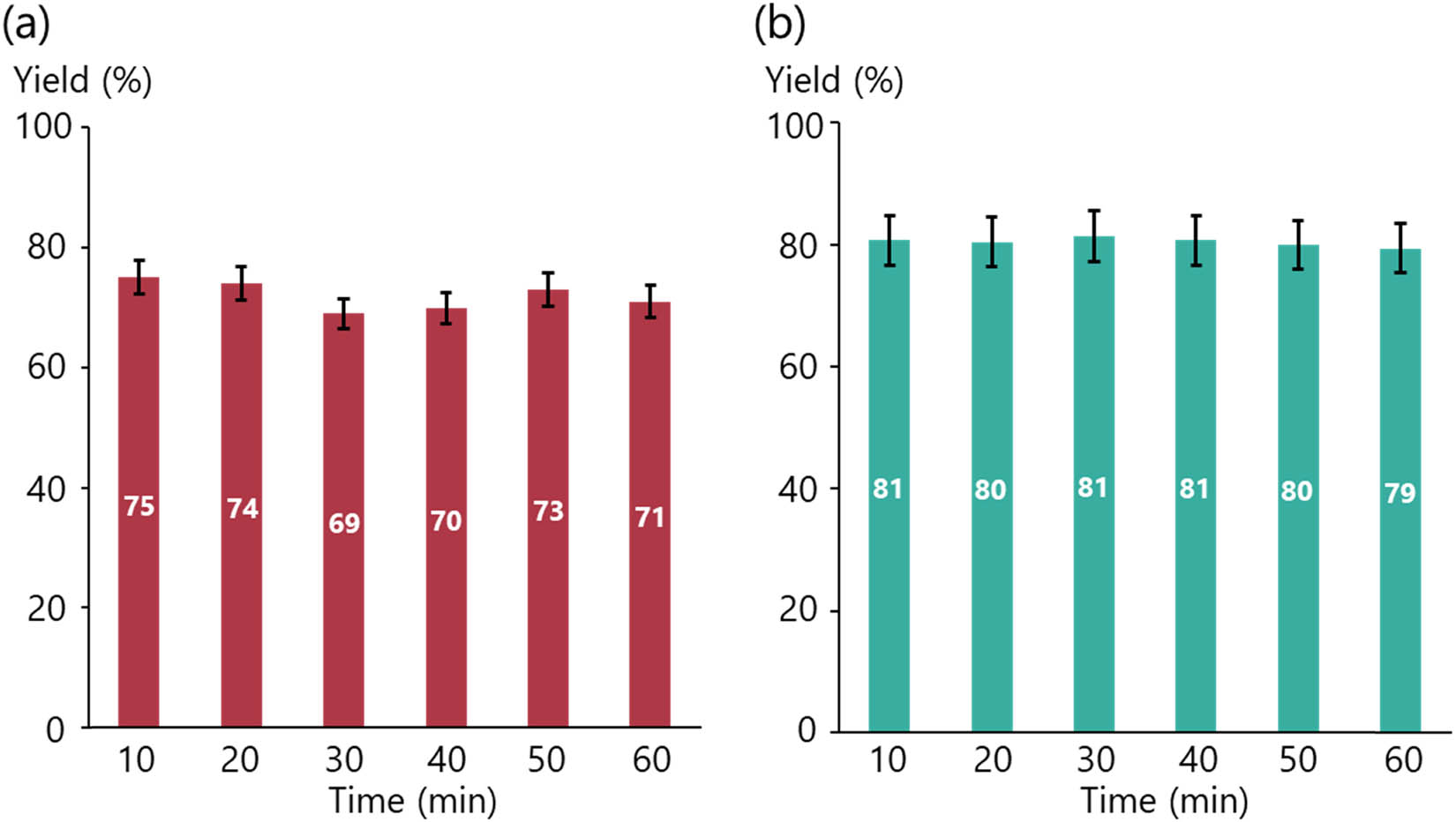
Long time operation for the flow reactions: (a) Stille cross-coupling and (b) hydrogenation. Error bars indicate the standard deviation of the measurements.
4 Conclusion
We demonstrated the utility of our palladium catalyst immobilized on dual-pore silica gel for coupling reactions under both batch and flow conditions, as well as for flow hydrogenation of the nitro compound. Although homogeneous flow cross-couplings have been widely developed and utilized [38,39], heterogeneous coupling reactions have great benefits, including the high recyclability of the catalyst. In particular, heterogeneous catalytic reactions promote the recyclability of the catalyst during long-timescale reactions, as proven by the 60-min operation demonstrated here. Further investigations are underway; these include optimizing the structure of DualPore to increase immobilized palladium, synthesizing important molecules, and integrating [40] the coupling reactions with hydrogenation reactions using the same column reactor.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
-
Funding information: This work was supported by JSPS KAKENHI Grant Numbers, JP17K06910, JP20K15276, JP20KK0121 and JP21H01936. This work was also partially supported by AMED (JP20ak0101090 and JP21ak0101156), JST A-step program (18067420), CREST (JPMJCR18R1), NEDO (JPNP19004), the Kyoto Technoscience Center, and the Ogasawara Foundation.
-
Author contributions: Yosuke Ashikari: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, visualization, writing – original draft, writing – review and editing; Kei Maekawa: investigation, resources; Chiemi Fujita: investigation; Kiyonari Shiosaki: investigation; Hongzhi Bai: resources; Kiyoshi Matsuyama: conceptualization, formal analysis, investigation, methodology, resources, writing – review and editing; Aiichiro Nagaki: conceptualization, funding acquisition, project administration, supervision, writing – review and editing.
-
Conflict of interest: Hongzhi Bai is working at DPS. Inc., and provided DualPoreTM.
-
Data availability statement: All data generated or analyzed during this study are included in this published article and the supporting material.
References
[1] McNamara CA, Dixon MJ, Bradley M. Recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem Rev. 2002;102(10):3275–300. 10.1021/cr0103571. and references therein.Suche in Google Scholar
[2] Kobayashi S. Flow “Fine” synthesis: high yielding and selective organic synthesis by flow methods. Chem Asian J. 2016;11(4):425–36. 10.1002/asia.201500916.Suche in Google Scholar PubMed PubMed Central
[3] Plutschack MB, Pieber B, Gilmore K, Seeberger PH. The Hitchhiker’s guide to flow chemistry. Chem Rev. 2017;117(18):11796–893. 10.1021/acs.chemrev.7b00183.Suche in Google Scholar PubMed
[4] Sivo A, Galaverna RdS, Gomes GR, Pastre JC, Vilé G. From circular synthesis to material manufacturing: advances, challenges, and future steps for using flow chemistry in novel application area. React Chem Eng. 2021;6(5):756–86. 10.1039/D0RE00411A.Suche in Google Scholar
[5] Nagaki A, Ashikari Y, Takumi M, Tamaki T. Flash chemistry makes impossible organolithium chemistry possible. Chem Lett. 2021;50(3):485–92. 10.1246/cl.200837.Suche in Google Scholar
[6] Ashikari Y, Saito K, Nokami T, Yoshida J, Nagaki A. Oxo-thiolation of cationically polymerizable alkenes using flow microreactors. Chem Eur J 2019;25(67):15239–43. 10.1002/chem.201903426.Suche in Google Scholar PubMed
[7] Elsherbini M, Huynh F, Dunbabin A, Allemann RK, Wirth T. Selective hydroboration–oxidation of terminal alkenes under flow conditions. Chem Eur J. 2020;26(50):11423–5. 10.1002/chem.202001650.Suche in Google Scholar PubMed PubMed Central
[8] Nagaki A, Yamashita H, Hirose K, Tsuchihashi Y, Yoshida J. Alkyllithium compounds bearing electrophilic functional groups: a flash chemistry approach. Angew Chem Int Ed. 2019;58(12):4027–30. 10.1002/anie.201814088.Suche in Google Scholar PubMed
[9] Fukazawa A, Minoshima J, Tanaka K, Hashimoto Y, Kobori Y, Sato Y, et al. A new approach to stereoselective electrocatalytic semihydrogenation of alkynes to Z-alkenes using proton-exchange membrane reactor. ACS Sustain Chem Eng. 2019;7(13):11050–5. 10.1021/acssuschemeng.9b01882.Suche in Google Scholar
[10] Ichinari D, Ashikari Y, Mandai K, Aizawa Y, Yoshida J, Nagaki A. A novel approach to functionalization of aryl azides through the generation and reaction of organolithium species bearing masked azides in flow microreactors. Angew Chem Int Ed. 2020;59(4):1567–71. 10.1002/anie.201912419.Suche in Google Scholar PubMed
[11] Colella M, Tota A, Takahashi Y, Higuma R, Ishikawa S, Degennaro L, et al. Fluoro-substituted methyllithium chemistry: external quenching method using flow microreactors. Angew Chem Int Ed. 2020;59(27):10924–8. 10.1002/anie.202003831.Suche in Google Scholar PubMed
[12] Otake Y, Shibata Y, Hayashi Y, Kawauchi S, Nakamura H, Fuse S. N-Methylated peptide synthesis via generation of an acyl N-methylimidazolium cation accelerated by a Brønsted acid. Angew Chem Int Ed. 2020;59(31):12925–30. 10.1002/anie.202002106.Suche in Google Scholar PubMed
[13] Prieschl M, Cantillo D, Kappe CO. A continuous flow bromodimethylsulfonium bromide generator: application to the synthesis of 2-arylaziridines from styrenes. J Flow Chem. 2021;11(2):117–25. 10.1007/s41981-020-00125-2.Suche in Google Scholar
[14] Ahn G-N, Sharma BM, Lahore S, Yim S-J, Vidyacharan S, Kim D-P. Flow parallel synthesizer for multiplex synthesis of aryl diazonium libraries via efficient parameter screening. Commun Chem. 2021;4:53. 10.1038/s42004-021-00490-6.Suche in Google Scholar
[15] Ashikari Y, Kawaguchi T, Mandai K, Aizawa Y, Nagaki A. A synthetic approach to dimetalated arenes using flow microreactors and the switchable application to chemoselective cross-coupling reactions. J Am Chem Soc. 2020;142(40):17039–47. 10.1021/jacs.0c06370.Suche in Google Scholar PubMed
[16] Watanabe E, Chen Y, May O, Ley SV. A practical method for continuous production of sp3-rich compounds from (Hetero)aryl halides and redox-active esters. Chem Eur J. 2020;26(1):186–91. 10.1002/chem.201905048.Suche in Google Scholar PubMed
[17] Nagaki A, Hirose K, Moriwaki Y, Mitamura K, Matsukawa K, Ishizuka N, et al. Integration of borylation of aryllithiums and Suzuki–Miyaura coupling using monolithic Pd catalyst. Catal Sci Technol. 2016;6(13):4690–2. 10.1039/C5CY02098K.Suche in Google Scholar
[18] Nagaki A, Hirose K, Tonomura O, Taniguchi S, Taga T, Hasebe S, et al. Design of a numbering-up system of monolithic microreactors and its application to synthesis of a key intermediate of valsartan. Org Process Res Dev. 2016;20(3):687–91. 10.1021/acs.oprd.5b00414.Suche in Google Scholar
[19] Masui S, Manabe Y, Hirao K, Shimoyama A, Fukuyama T, Ryu I, et al. Kinetically controlled Fischer glycosidation under flow conditions: a new method for preparing furanosides. Synlett. 2019;30(4):397–400. 10.1055/s-0037-1611643.Suche in Google Scholar
[20] Nagaki A, Hirose K, Moriwaki Y, Takumi M, Takahashi Y, Mitamura K, et al. Suzuki–Miyaura coupling using monolithic Pd reactors and scaling-up by series connection of the reactors. Catalysts. 2019;9(3):300. 10.3390/catal9030300.Suche in Google Scholar
[21] Harenberg JH, Weidmann N, Wiegand AJ, Hoefer CA, Annapureddy RR, Knochel P. (2-Ethylhexyl)sodium: a hexane-soluble reagent for Br/Na-exchanges and directed metalations in continuous flow. Angew Chem Int Ed. 2021;60(26):14296–301. 10.1002/anie.202103031.Suche in Google Scholar PubMed PubMed Central
[22] Park K, Ito N, Yamada T, Sajiki H. Efficient continuous-flow H-D exchange reaction of aromatic nuclei in D2O/2-PrOH mixed solvent in catalysts cartridge packed with platinum on carbon beads. Bull Chem Soc Jpn. 2021;94(2):600–5. 10.1246/bcsj.20200325.Suche in Google Scholar
[23] Yue C, Yamashita Y, Kobayashi S. Highly enantioselective immobilized prolinamide-catalyzed aldol reactions in continuous-flow systems: effect of water on the catalyst lifetime and application in the synthesis of a chiral fenpentadiol analogue. Green Chem. 2021;23(5):1989–94. 10.1039/D0GC04202A.Suche in Google Scholar
[24] Nagaki A, Nakahara Y, Furusawa M, Sawaki T, Yamamoto T, Toukairin H, et al. Feasibility study on continuous flow controlled/living anionic polymerization processes. Org Process Res Dev. 2016;20(7):1377–82. 10.1021/acs.oprd.6b00158.Suche in Google Scholar
[25] Nakahara Y, Furusawa M, Endo Y, Shimazaki T, Ohtsuka K, Takahashi Y, et al. Practical continuous-flow controlled/living anionic polymerization. Chem Eng Technol. 2019;42(10):2154–63. 10.1002/ceat.201900160.Suche in Google Scholar
[26] Ashikari Y, Maekawa K, Takumi M, Tomiyasu N, Fujita C, Matsuyama K, et al. Flow grams-per-hour production enabled by hierarchical bimodal porous silica gel supported palladium column reactor having low pressure drop. Catal Today. in press 2020. 10.1016/j.cattod.2020.07.014.Suche in Google Scholar
[27] Ishizuka N, Minakuchi H, Nakanishi K, Soga N, Nagayama H, Hosoya K, et al. Performance of a monolithic silica column in a capillary under pressure-driven and electrodriven conditions. Anal Chem. 2000;72(6):1275–80. 10.1021/ac990942q.Suche in Google Scholar PubMed
[28] Matsuyama K, Tanaka S, Kato T, Okuyama T, Muto H, Miyamoto R, et al. Supercritical fluid-assisted immobilization of Pd nanoparticles in the mesopores of hierarchical porous SiO2 for catalytic applications. J Supercrit Fluids. 2017;130:140–6. 10.1016/j.supflu.2017.07.032.Suche in Google Scholar
[29] Manufactured by DPS. Inc.: http://www.dps-inc.co.jp/en/ (Accessed on 6th August, 2021).Suche in Google Scholar
[30] Jana S, Haldar S, Koner S. Heterogeneous Suzuki and Stille coupling reactions using highly efficient palladium(0) immobilized MCM-41 catalyst. Tetrahedron Lett. 2009;50(34):4820–3. 10.1016/j.tetlet.2009.05.098.Suche in Google Scholar
[31] Beletskaya IP, Cheprakov AV. The heck reaction as a sharpening stone of Palladium catalysis. Chem Rev. 2000;100(8):3009–66. 10.1021/cr9903048.Suche in Google Scholar PubMed
[32] Chinchilla R, Nájera C. The Sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem Rev. 2007;107(3):874–922. 10.1021/cr050992x.Suche in Google Scholar PubMed
[33] Fukuyama T, Rahman MT, Sumino Y, Ryu I. 100 Gram scale synthesis of a key intermediate of matrix metalloproteinase inhibitor in a continuous-flow system based on a copper-free sonogashira reaction using an ionic liquid as a catalyst support. Synlett. 2012;23(15):2279–83. 10.1055/s-0031-1290456.Suche in Google Scholar
[34] Seo DS, Matsuda H, Oh-ide T, Kobayashi S. Alignment of nematic liquid crystal (5CB) on the treated substrates: characterization of orientation films, generation of pretilt angles, and surface anchoring strength. Mol Cryst Liq Cryst. 1993;224(1):13–31. 10.1080/10587259308032475.Suche in Google Scholar
[35] Hird M, Gray GW, Toyne KJ. Cross-coupling reactions in the synthesis of liquid crystals. Mol Cryst Liq Cryst. 1991;206(1):187–204. 10.1080/00268949108037730.Suche in Google Scholar
[36] Orlandi M, Brenna D, Harms R, Jost S, Benaglia M. Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines. Org Process Res Dev. 2018;22(4):430–45. 10.1021/acs.oprd.6b00205.Suche in Google Scholar
[37] Yamada T, Ogawa A, Masuda H, Teranishi W, Fujii A, Park K, et al. Pd catalysts supported on dual-pore monolithic silica beads for chemoselective hydrogenation under batch and flow reaction conditions. Catal Sci Technol. 2020;10(18):6359–67. 10.1039/D0CY01442G.Suche in Google Scholar
[38] Sugisawa N, Nakamura H, Fuse S. Recent advances in continuous-flow reactions using metal-free homogeneous catalyst. Catalysts. 2020;10(11):1321. 10.3390/catal10111321.Suche in Google Scholar
[39] Ashikari Y, Nagaki A. Homogeneous catalyzed Aryl–Aryl cross-couplings in flow. Synthesis. 2021;53(11):1879–88. 10.1055/a-1360-7798.Suche in Google Scholar
[40] Fukase K, Doi T, editors. Middle molecular strategy: flow synthesis to functional molecules. Heidelberg: Springer; 2021.10.1007/978-981-16-2458-2Suche in Google Scholar
© 2021 Yosuke Ashikari et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

