Abstract
To research the paracrine role of adipose tissue-derived mesenchymal stem cells (ADMSCs) in promoting angiogenesis under the three-dimensional culture conditions consisting of a functionalized self-assembling peptide nanofiber hydrogel. ADMSCs were isolated, extracted, and then identified. Three kinds of peptides (RADAI-16, RGD, and KLT) were prepared, and a functionalized self-assembling peptide nanofiber hydrogel was produced by mixing RADAI-16, RGD, and KLT in a volume ratio 2:1:1. AFM was used to observe RADAI-16, RGD, KLT, and the functionalized self-assembling peptide nanofiber hydrogel. Then, ADMSCs were cultured under three-dimensional conditions consisting of the peptide nanofiber hydrogel, and AFM was used to observe cell migration. The ADMSCs in the common culture group (37°C, 5% CO2 cell culture box) and hypoxic culture group (37°C, 10% CO2, and 1% O2 hypoxic culture box) acted as controls. ADMSCs were three-dimensionally cultured in situ for 1 day, and then the concentrations of HGF and VEGF in the supernatant were determined by ELISA. Cells were extracted from the peptide nanofiber hydrogel, and HO-1 expression was detected by western blotting. ADMSCs have high expression levels of CD29, CD90, and CDl05 and low expression levels of CD34 and CD45. In addition, they can differentiate into adipocytes and osteocytes. The diameters of the fibers of RADAI-16, RGD, KLT, and the functionalized self-assembling peptide hydrogel are 17.34 ± 1.82, 15.50 ± 1.41, 13.77 ± 1.18, and 20.26 ± 1.25 nm, respectively. AFM indicated that cells in the functionalized self-assembling peptide nanofiber hydrogel migrated farther than those in RADAI-16. The concentrations of HGF under common, hypoxic, and three-dimensional culture conditions were 47.31 ± 6.75, 247.86 ± 17.59, and 297.25 ± 17.95 pg/mL, respectively, while the concentrations of VEGF were 218.30 ± 3.03, 267.13 ± 4.27, and 289.14 ± 3.11 pg/mL, respectively. Both HGF and VEGF were expressed more in the presence of the functionalized self-assembling peptide nanofiber hydrogel than in its absence (P < 0.05). Using western blotting, ADMSCs cultured under hypoxic and three-dimensional conditions were found to have high expression levels of HO-1. Culturing ADMSCs under three-dimensional conditions consisting of functionalized self-assembling peptide nanofiber hydrogels can promote their paracrine role in angiogenesis, such as HGF and VEGF, and hypoxia is one of the important elements.
1 Introduction
Mesenchymal stem cells (MSCs), namely, adult stem cells with high self-renewing ability and multidirectional differentiation potential from myeloblasts are extensively present in connective tissues and organ mesenchyme in the whole body. They were first found by Friedenstein in marrow-adhering cell culture at the earliest [1], and subsequently, similar MSCs were found in tissues such as cord blood, peripheral blood, muscle, and fat. By virtue of convenient acquisition, extensive sources, the high number of obtained cells, and minor damage to the donor site [2], adipose tissue-derived mesenchymal stem cells (ADMSC) have rapidly become widely accepted seed cells in tissue engineering.
Cells are in a three-dimensional microenvironment in vivo and are influenced by physical signals and bioactive signals, while it is difficult to provide a three-dimensional environment for ordinary cultures. In recent years, nano-polypeptide materials have made progress as biological scaffolds for three-dimensional cell culture. Liu et al. used a functional self-assembling nano-polypeptide hydrogel as a scaffold to culture ADMSCs and found that the expression of paracrine cytokines would increase during three-dimensional ADMSC culture conditions [3]; however, the reasons for this effect were not sufficiently explained. This experiment aims to explore the influencing factors of increasing the expression of ADMSC paracrine angiogenic growth factors under three-dimensional culture conditions in the presence of a functional self-assembling nano-polypeptide hydrogel.
2 Materials and methods
2.1 Materials
The following materials were used: DMEM/F12 (Gibco Corporation); fetal bovine serum (FBS, Gibco Corporation); double antibody (Gibco Corporation); pancreatin; edetic acid (EDTA, HyClone Corporation); I-type collagenase (Sigma Corporation); phosphate buffer solution (PBS, HyClone Corporation); ADMSC osteogenic differentiation induction solution (US Cyagen Corporation); ADMSC adipogenic differentiation induction solution (US Cyagen Corporation); oil red O dye liquor (US Cyagen Corporation); alizarin red S dye liquor (US Cyagen Corporation); alkaline phosphatase dye liquor (Nanjing Jiancheng Technology Co., Ltd); 4% paraformaldehyde (US Sigma Corporation); 0.1% Triton X-100 (US Sigma Corporation); 5% glutaraldehyde stationary liquid (US Sigma Corporation); sucrose (US Sigma Corporation); biologically sterile deionized water (US Sigma Corporation); RADAl6-I, RGD and KLT polypeptides (Hefei PopChem Biotech Co., Ltd); Transwell chambers (US Corning Corporation), an SDS-polyacrylamide gel electrophoresis (PAGE) gel configuration kit (Beyotime Corporation), anti-heme oxygenase-1 antibody (Abcam); horseradish enzyme-labeled goat anti-rabbit IgG (ZSGB-BIO); β-tubulin antibody (Shanghai BestBio Company); and an ELISA kit for HGF and VEGF detection (Shanghai Elisa Bio Co., Ltd).
2.2 Method
2.2.1 Separation and culture of ADMSC
The animal procedures and human participation/tissues in this study were carried out in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals, with approval from the Animal Ethics Committee of Shandong Academy of Medical Sciences.
Isolated fresh fat samples from patients with benign diseases (patients signed an informed consent form before operation) were transferred onto an ultraclean worktable within 30 min, megascopic blood vessels and connective tissues were washed and removed, I-type collagenase (m/v 0.1%) was added after the fats were cut into pieces (<1 mm3), and the samples were centrifuged at 1,500 rpm for 10 min after oscillation and digestion in a 37°C thermostatic water bath. Sediments at the substratum were resuspended using 10% FBS complete culture solution (10% FBS, 1% 100 µg/mL penicillin, and 100 µg/mL streptomycin) and were filtered by passing through a 100-mesh cell screen, and a complete culture solution was added until 5 mL. Then, the sediments were placed into a 25 cm2 cell incubator. The solution was replaced for the first time after 48 h, and then it was replaced once every 2–3 days. After primary cells were coated to approximately 90% density at the bottom, subculturing was carried out.
2.2.2 ADMSC osteoinductive differentiation and Alizarin Red S and alkaline phosphatase staining
Cell density was adjusted to 2 × 104/mL, and 2 mL of the cell suspension was inoculated into a six-well plate precoated with 0.1% gelatin. When the cell density was fused to 60–70%, the culture solution was removed, and the cells were washed. A total of 2 mL of osteoinductive differentiation complete culture solution was added to adult MSCs, the solution was replaced once every 3 days, and observation was conducted after 4 weeks. Alizarin S staining and alkaline phosphatase staining were also implemented.
2.2.3 ADMSC adipogenic differentiation and oil red O staining
The cell density was adjusted to 2 × 104/mL, and 2 mL of the cell suspension was added to a 6-well plate for culturing. The solution was replaced once every 2–3 days, and the culture medium was removed when the cell density was 100% or the cells were in the fusion state. Then, 2 mL of ADMSC adipogenesis-induced differentiation solution A was added. Three days later, the solution was removed, 2 mL of ADMSC adipogenesis-induced differentiation solution B was added, and the B solution was replaced by the A solution 24 h later. After 5 days of this alternate culturing, the B solution was continuously used to maintain culturing for 7 days, and the culture solution was removed until the fat droplets became large and round. The cells were washed, and fixation for 30 min using 4% paraformaldehyde and staining with 1 mL of oil red O solution for 30 min were performed. Then, the cells were washed and observed under a microscope.
2.2.4 ADMSC ordinary culture and cell supernatant and protein extraction under anaerobic culture conditions
The cell density was adjusted to 1.0 × 105/mL, and 200 µL of cells were placed in a 24-well plate. A total of 200 µL of the complete culture medium was added to each well, and after culturing in a 5% CO2 cell incubator at 37°C for 24 h, the cell culture supernatant was collected, centrifuged at 3,000 g/min for 10 min in a 4°C environment and preserved in an −80°C refrigerator for standby use. The cell total protein was extracted for the follow-up experiment. The cell supernatant and total protein under anoxic conditions (37°C, 10% CO2, and 1% O2 anoxic incubator) were obtained using the same method.
2.2.5 Preparation and detection of polypeptide solution
Ten milligrams each of RADA16-I, RGD, and KLT were dissolved in 1 mL of sterile deionized water in the ultraclean worktable, namely, they were completely dissolved through ultrasonic treatment for 30 min. The polypeptides were placed in a 4°C environment for standby use after sterilization using a 0.22 µm filter membrane. RADA16-I:RGD:KLT were blended in a proportion of 2:1:1 and subjected to ultrasonic blending, and the functional self-assembling polypeptide solution was obtained. After the above four types of polypeptide solutions were diluted 20 times, 5 µL of solutions were diluted and dropped on newly peeled mica sheets. They were gently washed using 100 µL of distilled water after standing for 10 s and then were observed under an atomic force microscope after airing.
2.2.6 Three-dimensional in situ culture of ADMSC and cell supernatant and protein extraction
First, 10% sterile sucrose solution was used to adjust the ADMSC density to 1.0 × 106/mL. A total of 20 µL of cell glucose solution was rapidly blended with 100 µL of functional self-assembling polypeptide solution, and the mixture was dropped into a Transwell chamber (the Transwell chamber was preplaced in a 24-well plate with each holder containing 400 µL of the complete culture medium). Then, 200 µL of the complete culture medium was gently dropped along the diagonal direction of the chamber, and the chamber was placed in a 5% CO2 cell incubator at 37°C. The solution was replaced after 15 min of culturing, and the culturing was continued for another 30 min. The chamber was transferred to a 12-hole incubator with 800 µL of the complete culture medium in each hole. After 1 day, the cell culture supernatants inside and outside the chamber and intracellular proteins in the hydrogel were collected.
2.2.7 Determination of VEGF and HGF concentrations using ELISA
ELISA was used to determine VEGF and HGF concentrations in cell supernatants in the common culture group, hypoxic culture group, and three-dimensional culture group.
2.2.8 Determination of the expression of intracellular heme oxygenase-1 (HO-1) under various conditions using a western blot method
ADMSCs cultured under common culture conditions, hypoxic culture conditions, and three-dimensional culture conditions for 1 day were extracted, and total proteins were obtained after pyrolysis, and protein concentration was determined. Equivalent amounts of proteins were taken and loaded, after which they were subjected to PAGE. After being transferred to polyvinylidene fluoride membranes, they were sealed for 1.5 h, and HO-1 primary antibody (1:1,000) was added and incubated overnight at 4°C. The secondary antibody (1:5,000) was added after washing the membranes, and then they were incubated at room temperature for 2 h, developed using an enhanced chemiluminescent agent, and finally imaged using a gel-imaging system.
2.2.9 Statistical method
Among the experimental results, all data were expressed as the mean ± standard deviation (
3 Results
ADMSC primary cells obtained through separation had slow growth and proliferation; they adhered to walls during the growth process and presented a fusiform shape. The cells increased about 7 days later and presented a long fusiform shape, and the cell growth presented a vortex shape with sizes of 30–50 µm (as shown in Figure 1). After osteoinductive differentiation of ADMSC, cells were transformed from the original long fusiform shape into triangular and polygonal shapes; particular matters were sedimented in the cells, and sediments increased continuously with time and finally presented a linear shape (as shown in Figure 2). After staining with alizarin red S, calcified nodes were seen in the cells, and black particular minerals were sedimented in cells by alkaline phosphatase staining. The above changes appeared in the control group. After adipogenesis induced differentiation of ADMSCs, cells were transformed from the original long fusiform shape into triangular or rectangular shapes, and circular vacuoles structures could be seen in cells with regular morphologies but unequal quantities and sizes. The spherical fat droplets were colored red in cells after oil red O staining with regular morphologies but were of unequal sizes. A similar change did not appear in the control group (as shown in Figure 3).
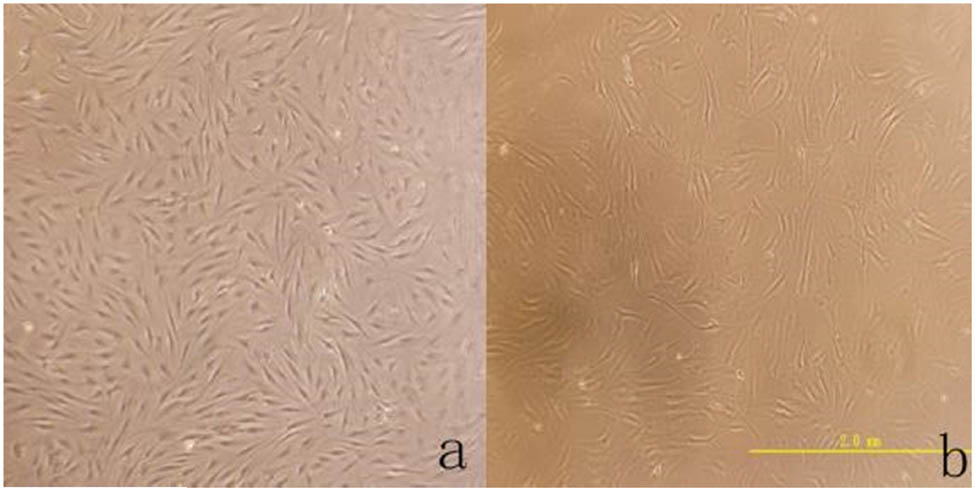
(a) The shape of the cells is fusiform, and the growth trend is swirled (×40). (b) Further observation of cell morphology and the size of the cell is about 30–50 μm (×200).

(a) After ADMSCs differentiate into osteoblasts, the cell morphology is no longer fusiform (×200). (b) After ADMSCs differentiate into osteoblasts, Alzheimer Red S staining revealed the deposition of red calcium nodules (×40). (c) After ADMSCs differentiate into osteoblasts, alkaline phosphatase staining showed brown calcium nodule precipitation (×40). (d) There was no obvious abnormality in ADMSCs without osteogenic differentiation (×40).
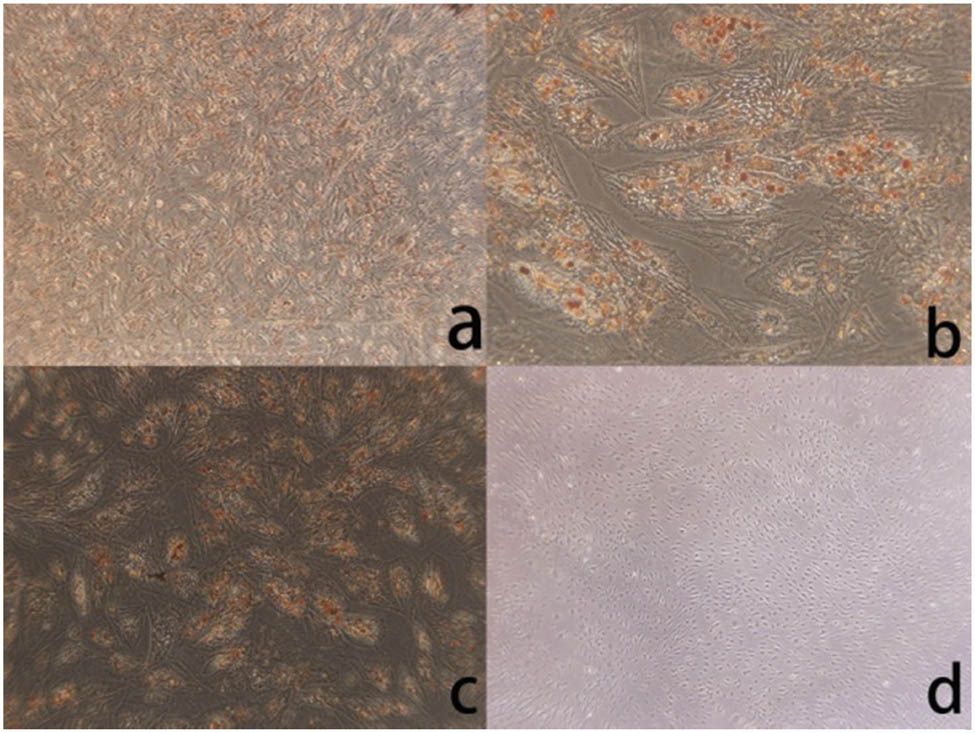
(a) After ADMSCs differentiate into adipose, the cell morphology is no longer fusiform (×40), and cell morphology changes. (b) After adipogenic differentiation of ADMSCs, the cells changed from spindle to triangle or irregular shape (×200). (c) After adipogenic differentiation of ADMSCs, oil red O staining showed intracellular red lipid droplets (×40). (d) There was no significant change without adipogenic differentiation cells (×40).
ADMSCs were placed in an anoxic incubator for 24 h culturing. Then they were observed under a microscope, and it was found that cells still presented “vortex”-shaped growth with morphologies of long fusiform shape retained. A small number of dead cells were floating on the surface of the complete culture solution, and there was no obvious difference in morphologies from cells in the ordinary culture group (as shown in Figure 4).

No morphological changes and cell growth trends were observed under hypoxic conditions, but the number of floating cells increased compared with that cultured under normal conditions: (a) ×40 and (b) ×200.
RADA16-I, RGD, KLT, and the self-assembly polypeptide solution were all colorless transparent liquids, and good self-assembly of the functional polypeptide solution could be observed under an atomic force microscope. As shown in Figure 5, RADA16-I, RGD, and KLT were all nanofiber shapes as observed under an atomic force microscope, with fiber diameters of 17.34 ± 1.82, 15.50 ± 1.41, and 13.77 ± 1.18 nm, respectively. The functional self-assembly polypeptide was also of nanofiber shape and the fiber diameter was 20.26 ± 1.25 nm. By the statistical analysis, as in Figure 6, the diameter of the self-assembly polypeptide increased when compared with those of the original three types of polypeptide fibers (P < 0.05).
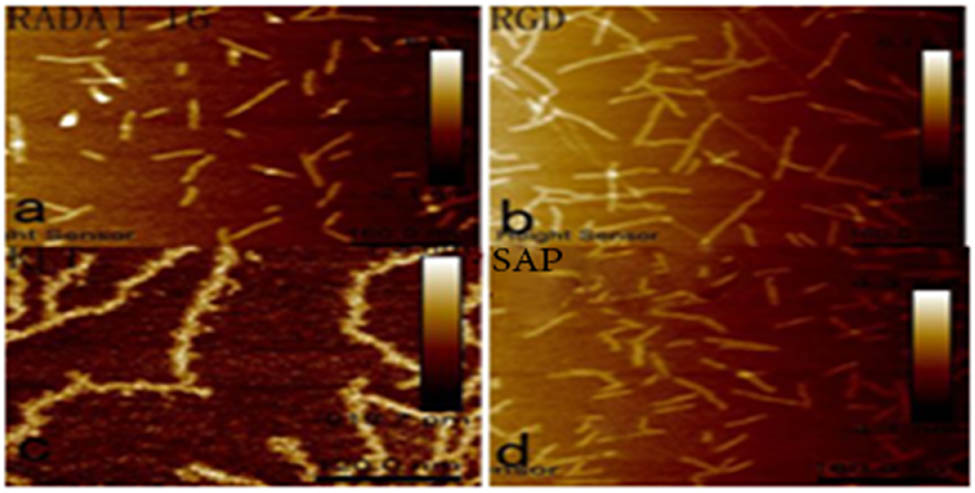
(a) RADAI-16, (b) RGD, (c) KLT, and (d) SAP (functionalized self-assembled polypeptide). Four polypeptide hydrogels were imaged under an AFM, and they were all nanofibers with different lengths and diameters.
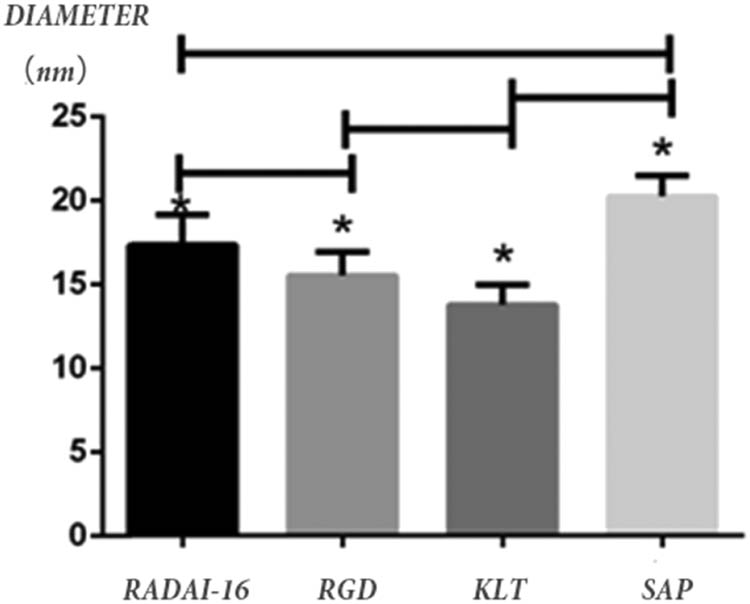
The diameters of RADA16-I, RGD, KLT, and functionalized self-assembled polypeptide (SAP) fibers were 17.34 ± 1.82, 15.50 ± 1.41, 13.77 ± 1.18, and 20.26 ± 1.25 nm, respectively, under AFM. *P < 0.05, with statistical significance.
The ELISA method was used to determine VEGF and HGF concentrations in the cell supernatant. The HGF concentrations in the common culture group, hypoxic culture group, and three-dimensional culture group were 47.31 ± 6.75, 247.86 ± 17.59, and 297.25 ± 17.95 pg/mL, respectively; their VEGF concentrations were 218.30 ± 3.03, 267.13 ± 4.27, and 289.14 ± 3.11 pg/mL, respectively. Thus, it could be seen that VEGF and HGF concentrations in the cell supernatant in both the hypoxic culture condition and three-dimensional culture condition obviously increased when compared with the common culture group (P < 0.05). The details can be seen in Figure 7.
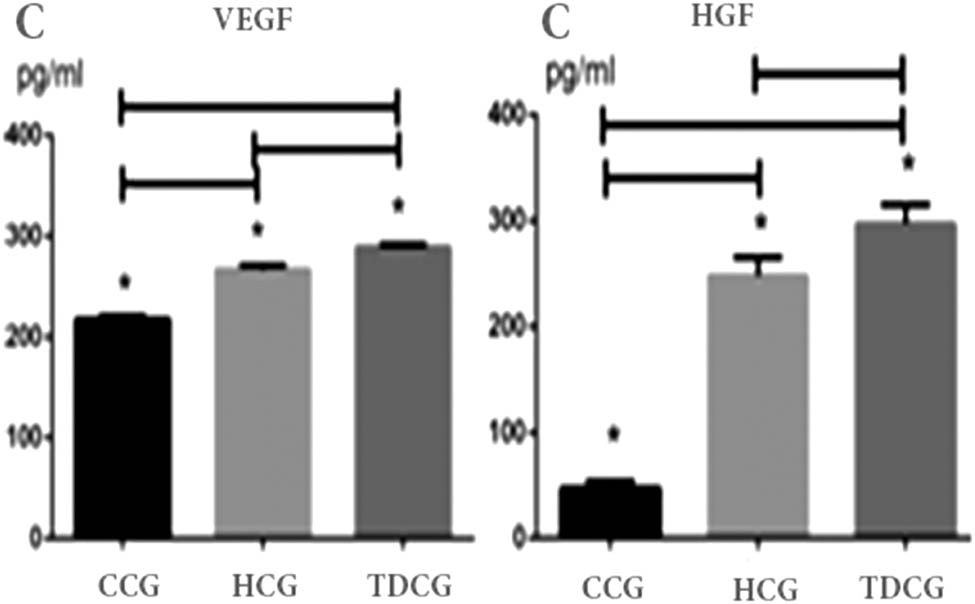
Laboratory tests by ELISA method for the concentration of HGF were found to be 47.31 ± 6.75, 247.86 ± 17.59, and 297.25 ± 17.95 pg/mL after one day under different culture conditions (the common culture, the hypoxic culture group, and the three-dimensional culture). Under the same conditions stated above, the concentrations of VEGF were 218.30 ± 3.03, 267.13 ± 4.27, and 289.14 ± 3.11 pg/mL. The concentrations of HGF and VEGF were highest in TDCG, closely followed by HCG. *P < 0.05. CCG: common culture group; HCG: hypoxic culture group; TDCG: three-dimensional culture group.
When cells were under the hypoxic culture environment, expression of the intracellular heme oxygenase (HO-1) would obviously increase. According to western blot results analysis, it could be seen that the HO-1 content expressed in cells in both the hypoxic culture group and three-dimensional culture group obviously increased when compared with the common culture condition group. The protein content expressed in the three-dimensional culture group was the highest (P < 0.05) (as shown in Figure 8).

The western-blot was used to examine the expression of the target protein. There was a high expression rate of the HO-1 in both the three-dimensional culture group and the hypoxic culture group. It also suggested that the expression rate of HO-1 was higher than the latter. *P < 0.05. CCG: common culture group; HCG: hypoxic culture group; TDCG: three-dimensional culture group; and HO-1: heme oxygenase-1.
4 Discussion
By virtue of convenient acquisition, extensive sources, the high number of obtained cells, and minor damage to the donor site [2], ADMSCs have rapidly become widely accepted seed cells in tissue engineering. Currently, it is believed that MSCs are a heterogeneous cell population, and their specific antigen phenotype has not yet been found. The International Society for Cellular Therapy (ISCT) proposed test criteria of MSCs in 2005, including cell adherence growth features, multidirectional differentiation features, and phenotyping features [4]. Adherent fusiform cells were obtained by the “adherence screening method,” and the cell growth trend presented a “vortex shape” [5]. ADMSCs are nonhematopoietic stem cells derived from myeloblasts with self-assembling and multidirectional differentiation potentials, and they can be differentiated into multiple types of cells from myeloblasts, such as bone, cartilage, and fat. By induced osteogenic differentiation, fusiform cells obtained through separation experienced changes in morphologies, and calcified nodes could be observed in cells by alizarin red S staining, and black particulate minerals were sedimented in cells after alkaline phosphatase staining. During induced adipogenic differentiation, quasi-circular vacuole structures could be observed in cells, and red spherical fat droplets were colored in cells through oil red O staining. Thus, the obtained cells could undergo favorable osteogenesis and adipogenic differentiation with multidirectional differentiation potential. With the combined features of the adherence screening method and multidirectional differentiation potential, fusiform and “vortex-shaped” cells obtained by separation were determined as ADMSCs from fats.
For the treatment of ischemic diseases, including ischemic heart disease and ischemic limb disease, stem cell treatment is a therapeutic method with prospects at present [6,7]. However, after treatment by inoculating stem cells, stem cells proliferate in the damaged part with a low growth rate, so the therapeutic effect cannot be achieved, which is the main disadvantage of restricting stem cell treatment [8,9]. Moreover, the existing studies have found that tumor risk exists after stem cell treatment [10,11]. One of the solutions to overcome the above disadvantage of stem cell treatment is to use the conditional culture medium for stem cell culturing to treat ischemic diseases. Stem cells can express, synthesize, and secrete cytokines and growth factors and regulate multiple types of bioactive factors such as polypeptides [12,13,14,15], especially ADMSCs, which can secrete proangiogenic factors and anti-apoptosis factors, including VEGF, HGF, bFGF, and TGF-b. Anoxia is an important factor influencing the secretion of bioactive factors by stem cells [16,17]. The concentration of bioactive factors secreted by stem cells after ordinary culture conditions is low, so it is difficult to exert a therapeutic effect. Recent studies have found that the VEGF concentration in the supernatant under ordinary culture conditions is 217 ± 97 pg/mL [18], while the concentration of VEGF with therapeutic significance is approximately 5,000 pg/mL [19,20]. Therefore, in vitro culturing of MSCs and enhancement in the ability of stem cells to secrete bioactive factors constitute the key to solving stem cell treatment problems. The three-dimensional culturing of ADMSCs by the “microsphere method” and by the use of supernatant obtained from culturing with the three-dimensional conditional culture medium can significantly ameliorate acute ischemic kidney diseases [21]. By three-dimensional culturing of ADMSCs using the “microsphere method,” bioactive factors secreted by stem cells will increase, which is closely related to the anoxic environment where the cells are located [22]. Moreover, three-dimensional culture conditions are similar to the in vivo environment within cells and can be better influenced by the physical environment and biological environment. Therefore, a functional self-assembling nano-polypeptide hydrogel was used in this experiment as a biological framework for the three-dimensional stem cell culture.
According to the previous literature reports, the self-assembling polypeptide RADA16-I can spontaneously form nanofibers with diameters of 10 nm, pore diameters of 5–200 nm, and abundant moisture [23,24,25]. The formed polypeptide nanofibers are extremely similar to the extracellular matrix and can act as a biological framework for the three-dimensional cell culture. The polypeptide RGD is a key integrin for cell adhesion and can facilitate cell adhesion [26,27]. The polypeptide KLT is a stimulatory factor of VEGF [28]. In this study, the above three types of polypeptide solutions were sufficiently blended in a volume ratio of 2:1:1, and then the colorless and transparent liquid was obtained. By observing the self-assembling polypeptide solution obtained after blending under an atomic force microscope, the solution still consisted of nanofiber structures. Compared with RADA16-I, the fiber diameter obtained through self-assembly was larger (thick). Thus, it can be seen that the self-assembling nano-polypeptide obtained by blending three types of polypeptides in a volume ratio of 2:1:1 could be very well assembled into a nanofiber structure with abundant moisture and could be used as a biological framework for the three-dimensional cell culture.
In this experiment, the in situ three-dimensional culture of ADMSCs was carried out using a functional self-assembling nano-polypeptide hydrogel, and the ELISA method was used to determine the VEGF and HGF concentrations in the supernatant of the culture medium under three-dimensional culture conditions and ordinary culture conditions. It was found that ADMSCs secreted pro-angiogenic factors more significantly after three-dimensional culture than under ordinary culture conditions. When cells are beyond the oxygen diffusion distance (generally 150–250 μm), they will be in an anoxic state [29]. Therefore, we believe that when a functional self-assembling polypeptide hydrogel is used for the three-dimensional culture, the anoxic state of cells is the influencing factor for increasing the secretion of pro-angiogenic factors. The results for the anoxic group in this study showed that under anoxic conditions, even an ordinary two-dimensional culture would result in an increase in the expression of pro-angiogenic factors by ADMSCs; however, the magnitude of this increase was obviously lower than that under three-dimensional culture conditions. Extensively existing in in vivo microsomal enzyme systems, HO, including three types of isozymes, HO-1, HO-2, and HO-3, participates in multiple philological and pathological processes in vivo. HO-1 can be activated by multiple oxidative stress factors in vivo and has important in vivo effects, such as antioxidation, anti-inflammatory reactions, and immune adjustment [30]. We found that HO-1 expression levels in cells in the three-dimensional culture group and the anoxic group were obviously higher than those in the ordinary culture group and so it is believed that the protein kinase (Akt) signal pathway is activated under three-dimensional culture conditions [16]. The expression of anoxic genes was upregulated inside ADMSCs, and as a result, the number of pro-angiogenic factors secreted by ADMSCs increased.
MSCs were obtained through isolated culture from fat tissues, and ADMSCs were validated by combining adherence screening and multidirectional differentiation potential. The mediated three-dimensional culture was carried out for ADMSCs with a functional self-assembling nano-polypeptide hydrogel, and it was found that the level of pro-angiogenic factors secreted by ADMSCs increased under three-dimensional culture conditions. Moreover, anoxia was validated as one of the important factors in increasing secretion. However, a further in-depth study is needed regarding whether the expression of anoxic genes in ADMSCs is upregulated by activating the Akt signal pathway during mediated three-dimensional ADMSC culture conditions using a functional self-assembling nano-polypeptide hydrogel.
5 Conclusion
In this experiment, MSCs were isolated and cultured from adipose tissue, and ADMSCs were confirmed by adherent screening and multidirectional differentiation potential. Three-dimensional culture of ADMSCs mediated by functionalized self-assembled nano-polypeptide hydrogel showed that ADMSCs secreted increased growth of vascular growth factors such as HGF and VEGF under three-dimensional culture conditions and confirmed that hypoxia was one of the important factors for its secretion. However, it is still necessary to further study the function of functional peptide nanometer hydrogels on ADMSC-mediated three-dimensional culture in order to upregulate the expression of Akt in ADMSCs.
Acknowledgement
The authors would like to express their gratitude to Dr Qian Wang for their assistance in performing the experiments.
-
Funding information: This study was supported by grants from the Natural Science Foundation of Shandong Province (ZR2017MH072), the Natural Science Foundation Program of Shandong Province (2016GSF201219), and the Livelihood Technology Project of Qingdao (18-6-1-90-nsh).
-
Author contributions: Ling Jianmin performed the experiments, analyzed, and interpreted the data, and wrote the manuscript; Tian Ailing and Yi Xin performed the analysis and interpretation of the data; Sun Nianfeng designed the study and revised the manuscript. All authors read and approved the final manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
-
Ethical approval: The procedures and participation/tissues of humans in this study were carried out in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory. All experimental protocols were approved by the Ethics Committee of Shandong Academy of Medical Sciences. All participants provided a statement of written informed consent.
References
[1] Friedenstein AJ . Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–59.10.1016/S0074-7696(08)60092-3Search in Google Scholar
[2] Zuk PA , Zhu M , Ashjian P . Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95.10.1091/mbc.e02-02-0105Search in Google Scholar PubMed PubMed Central
[3] Liu X , Wang X , Ren H . Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 2013;9(6):6798–805.10.1016/j.actbio.2013.01.027Search in Google Scholar PubMed
[4] Dominici M , Le Blanc K , Mueller I . Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7.10.1080/14653240600855905Search in Google Scholar PubMed
[5] Bunnell BA , Flaat M , Gagliardi C . Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–20.10.1016/j.ymeth.2008.03.006Search in Google Scholar PubMed PubMed Central
[6] Tateishi-Yuyama E , Matsubara H , Murohara T . Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–35.10.1016/S0140-6736(02)09670-8Search in Google Scholar PubMed
[7] Meyer GP , Wollert KC , Lotz J . Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30(24):2978–84.10.1093/eurheartj/ehp374Search in Google Scholar PubMed
[8] Menasche P . Stem cells for clinical use in cardiovascular medicine: current limitations and future perspectives. Thromb Haemost. 2005;94(4):697–701.10.1160/TH05-03-0218Search in Google Scholar PubMed
[9] Zhang M , Methot D , Poppa V . Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33(5):907–21.10.1006/jmcc.2001.1367Search in Google Scholar PubMed
[10] Gallagher G , Forrest DL . Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109(1):84–92.10.1002/cncr.22375Search in Google Scholar PubMed
[11] Friedman DL , Leisenring W , Schwartz JL . Second malignant neoplasms following hematopoietic stem cell transplantation. Int J Hematol. 2004;79(3):229–34.10.1532/IJH97.03178Search in Google Scholar PubMed
[12] Kinnaird T , Stabile E , Burnett MS . Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–85.10.1161/01.RES.0000118601.37875.ACSearch in Google Scholar PubMed
[13] Neuhuber B , Timothy Himes B , Shumsky JS . Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035(1):73–85.10.1016/j.brainres.2004.11.055Search in Google Scholar PubMed
[14] Takahashi M , Li T-S , Suzuki R . Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291(2):H886–93.10.1152/ajpheart.00142.2006Search in Google Scholar PubMed
[15] Rehman J , Traktuev D , Li J . Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8.10.1161/01.CIR.0000121425.42966.F1Search in Google Scholar PubMed
[16] Gnecchi M , He H , Noiseux N . Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20(6):661–9.10.1096/fj.05-5211comSearch in Google Scholar PubMed
[17] Wang M , Zhang W , Crisostomo P . STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42(6):1009–15.10.1016/j.yjmcc.2007.04.010Search in Google Scholar PubMed PubMed Central
[18] Potapova IA , Gaudette GR , Brink PR . Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25(7):1761–8.10.1634/stemcells.2007-0022Search in Google Scholar PubMed
[19] Silva E , Mooney D . Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5(3):590–8.10.1111/j.1538-7836.2007.02386.xSearch in Google Scholar PubMed
[20] Yuen WW , Du NR , Chan CH . Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci. 2010;107(42):17933–38.10.1073/pnas.1001192107Search in Google Scholar PubMed PubMed Central
[21] Xu Y , Shi T , Xu A . 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J Cell Mol Med. 2016;20(7):1203–13.10.1111/jcmm.12651Search in Google Scholar PubMed PubMed Central
[22] Angoulvant D , Ivanes F , Ferrera R . Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant. 2011;30(1):95–102.10.1016/j.healun.2010.08.023Search in Google Scholar PubMed
[23] Zhang S , Holmes T , Lockshin C . Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci. 1993;90(8):3334–8.10.1142/9789813272682_0052Search in Google Scholar
[24] Holmes TC , de Lacalle S , Su X . Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci. 2000;97(12):6728–33.10.1142/9789813272682_0054Search in Google Scholar
[25] Zhang S . Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21(10):1171–8.10.1038/nbt874Search in Google Scholar PubMed
[26] Sagnella S , Anderson E , Sanabria N . Human endothelial cell interaction with biomimetic surfactant polymers containing peptide ligands from the heparin binding domain of fibronectin. Tissue Eng. 2005;11(1–2):226–36.10.1089/ten.2005.11.226Search in Google Scholar PubMed PubMed Central
[27] Aguzzi MS , Giampietri C , De Marchis F . RGDS peptide induces caspase 8 and caspase 9 activation in human endothelial cells. Blood. 2004;103(11):4180–7.10.1182/blood-2003-06-2144Search in Google Scholar PubMed
[28] D’Andrea LD , Iaccarino G , Fattorusso R . Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc Natl Acad Sci USA. 2005;102(40):14215–20.10.1073/pnas.0505047102Search in Google Scholar PubMed PubMed Central
[29] Nichols M , Foster T . Oxygen diffusion and reaction kinetics in the photodynamic therapy of multicell tumour spheroids. Phys Med Biol. 1994;39(12):2161.10.1088/0031-9155/39/12/003Search in Google Scholar PubMed
[30] Araujo JA , Zhang M , Yin F . Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:181–5.10.3389/fphar.2012.00119Search in Google Scholar PubMed PubMed Central
© 2021 Jianmin Ling et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

