Abstract
Modified polyvinylidene fluoride (PVDF) membranes were prepared by the phase inversion method via blending in situ formed nanosilver (Ag) and nanozirconium dioxide (ZrO2). Scanning electron microscopy of the membranes revealed that the surface pore size of the membranes was increased and distributed widely with the addition of modified nanosilver (Ag) and nanozirconium dioxide (ZrO2). The pores of the membrane were reduced due to excessive modification of the material when the content of zirconium dioxide was increased to 0.4%. XRD characterization showed that in situ synthesis of nanosilver (Ag) and nanozirconium dioxide (ZrO2) had been successfully blended in the membranes. The contact angle of the modified membrane ranged from 82.72° to 67.37°, which showed that the hydrophilic properties of the membrane were improved. The pure water flux of the modified membrane increased from 28.43 to 143.2 L/m2 h, indicating that the hydrophilicity of the modified membrane was enhanced significantly. The flux recovery rate of the modified membrane was obviously increased in the fouling experiment with BSA as the source of organic pollutants. The antimicrobial contamination of the membrane was greatly enhanced with the E. coli microbial contamination experiment.
1 Introduction
Membrane separation technology, e.g., microfiltration [1,2], ultrafiltration [3,4], nanofiltration [5,6], and reverse osmosis [7,8] plays an important role in the sewage treatment technology, which has wide applications in many fields such as the industrial production separation [9,10], water and wastewater treatment [11], etc. Polyvinylidene fluoride (PVDF), a kind of organic polymer membrane material with the advantages of strong chemical stability, thermal stability, and high mechanical properties, has been used in membrane separation technology. However, the pristine PVDF membrane has strong hydrophobicity, which causes the adhered organic substance to form membrane contamination and then decreases its functionality. Membrane modification is an effective method to resist membrane fouling. The main methods of the hydrophilic modification of PVDF membrane are copolymerization modification and blending modification. Copolymerization is modified by chemical methods to endow the molecular chains of PVDF with hydrophilic groups or monomers to improve the hydrophilicity of the membrane. Zhang polymerized poly(acrylic acid) (PAA) side chains on the PVDF membrane with TiO2 by sol–gel preparation and found that the hydrophilicity and the antifouling abilities of the membrane were enhanced significantly [11]. PVDF was also blended with the hydrophilic material to improve the hydrophilicity of the membrane [12,13,14,15,16,17,18] with a simple and easy blending modification method. The main blending materials are hydrophilic polymers such as PMMA (poly(N-isopropyl-acrylamide)) [19], CA (cellulose acetate) [20], PU (polyurethane) [21], etc., or inorganic nanoscale particles such as silica [22], titanium dioxide [23], blending silver [24], alumina [4], etc. It can be found that via blending inorganic nanomaterials, not only the hydrophilic properties and the anti-pollution performances of the membrane can be improved, but the mechanical properties can also be enhanced. Pang modified the PVDF membrane with in situ formed nano-ZrO2 [25], and found that the hydrophilicity and the antifouling performances of the modified PVDF membrane were improved. In addition, ZrO2 had higher stability and was suitable for liquid-phase separation under extreme conditions. So, the anti-pollution performance of the anti-organic membrane could be improved by hydrophilic modification [26,27,28,29].
Biological fouling is a vital factor induced by the reduction of the membrane flux. Li employed in situ formed nanoscale Ag particles to modify the PVDF membrane and found that Ag played an important role in the antimicrobial properties and also could improve the hydrophilic properties of the membrane [30]. However, using Ag as a hydrophilic substance to improve the hydrophilic properties of the PVDF membrane was expensive. So, a cheaper and high efficiency method was developed by the modification of the membrane with two kinds of nanomaterials.
In this paper, a certain amount of AgNO3 as a raw material was used to synthesize nanoscale silver to improve the antimicrobial properties of the membrane, while ZrO2 as the main material was used to improve hydrophilic properties. The modified PVDF membrane was prepared by the phase inversion method via blending in situ formed nanosilver (Ag) and nano-zirconium dioxide (ZrO2) under ultrasonic dispersion. The membrane was further characterized by XRD and scanning electron microscopy (SEM). Hydrophilic and antifouling performances were also studied to obtain more insights into the membrane properties.
2 Experimental
2.1 Materials
Poly(vinylidene fluoride) (PVDF: CAS: 24937-79-9, FR904, Shanghai) was purchased from Sanaifu. Silver nitrate (AgNO3; analytical grade), and N,N-dimethylformamide (DMF; CAS: 68-12-2, d > 99%, reagent) were obtained from Jinhuada Co. Ltd of Guangzhou City; polyvinylpyrrolidone (PVP, CAS: 9003-39-8, reagent) was purchased from Zhengzhoupaini Co., Ltd; nano-zirconium dioxide (ZrO2) (CAS: 53801-45-9, 50 nm, Shanghai) was obtained from Macklin Biochemical Co., Ltd; and bovine serum albumin (BSA, CAS: 9048-46-8) protein was obtained from Biofroxx.
2.2 Preparation of the Ag/ZrO2-PVDF membrane
For the preparation of the Ag/ZrO2-PVDF membrane, AgNO3 (0.5%) was dissolved in an appropriate amount of DMF stirring until the solution changed from light yellow to brown and then nano-ZrO2 particles were added and dispersed ultrasonically for 30 min in a 50°C water bath. In another container, PVP (1%) was dissolved in DMF, and then PVDF (15%) was continuously added. The mixture was stirred in a water bath at 50°C to obtain a homogeneous clear solution. The Ag/ZrO2 solution was added into a uniform PVDF solution and stirred for 3 h at 50°C in a water bath to obtain a yellow casting solution, indicating that a well-dispersed Ag and ZrO2 solution was obtained, which was then dispersed ultrasonically for 30 min and allowed to stand one day for removing the bubbles and to obtain a homogeneous casting solution. The casting solution was poured into a glass plate that was washed with ethanol and deionized water, and then the membrane was scraped with a scratch knife until the plate was naturally dried. The glass was then put in deionized water to induce the liquid-liquid phase conversion to obtain the membrane PVDF and Ag/ZrO2-PVDF 0–4. PVDF membrane is a pristine membrane, Ag/ZrO2-PVDF 0–4 is a nanosized silver and nanoscale-zirconia modified membrane, where 0–4 refer to the modified membrane prepared under different concentrations of zirconium dioxide (Table 1).
Composition of the cast solution (mass%)
| PVDF | Ag/ZrO2-PVDF0 | Ag/ZrO2-PVDF1 | Ag/ZrO2-PVDF2 | Ag/ZrO2-PVDF3 | Ag/ZrO2-PVDF4 | |
|---|---|---|---|---|---|---|
| PVDF (%) | 15 | 15 | 15 | 15 | 15 | 15 |
| DMF (%) | 84 | 83.5 | 83.4 | 83.3 | 83.2 | 83.1 |
| PVP (%) | 1 | 1 | 1 | 1 | 1 | 1 |
| AgNO3 (%) | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| ZrO2 (%) | 0 | 0 | 0.1 | 0.2 | 0.3 | 0.4 |
2.3 Characterization of the Ag/ZrO2-PVDF membrane
X-ray diffractometer (XRD, Bruker AXS Co., Ltd., D8 ADVANCE) was used to analyze the surface compositions of the membranes with a copper Kα radiation source at a scan rate ranging from 10° to 90° at 2°/min. The chemical composition of the membrane was studied using Fourier transform infrared spectroscopy (FTIR-ATR, PerkinElmer Spectrum Two). After drying and spraying the membrane with gold, the surface morphology of the membrane was observed with an SEM (SEM, Hitachi S-4800). The contact angle was measured with a contact angle measuring instrument (Shanghai Zhongchen Digital Technology Equipment Co., Ltd., JC2000C). To measure the hydrophilicity of the membrane, 3 μL of water was dropped on the surface of the membrane, and the contact angle meter was used to record for 1 min. Each membrane was tested five times and the averaged data was obtained.
2.4 Flux experiment
The membrane flux and rejection were measured with a flux testing device under 0.1 MPa pressure and at room temperature. First, the membrane was cut into a disc of size 12.56 cm2 and pre-pressured with deionized water for 30 min to get a stable flux, J w. BSA rejection was measured at 0.1 MPa and prepressured with deionized water for 30 min; the water flux was recorded every 5 min. The deionized water was replaced by a BSA solution until the flux reached a stable value. The concentration of a certain period of penetration solution is calculated as the rejection R:
where J w is the pure water flux of the membrane (L/m2 h); V is the volume of penetrative water (L); A is the effective membrane area (m2); and t is the running time (h).
where R is the rejection of BSA (%); C P is the concentration of BSA in the permeation solution; and C f is the concentration of BSA in the feed solution.
2.5 Evaluation of antifouling properties
The multicycle filtration test was used to evaluate the antifouling properties of the membrane. First, the membrane was pre-pressured with deionized water for 30 min at 0.1 MPa and room temperature. The water flux J W was recorded, the deionized water was replaced by BSA solution, and the flux J p of the BSA solution was recorded by suction filtration at 0.1 MPa for 30 min. The water flux of the membrane was tested again after the membrane was rinsed with deionized water (J r). According to the multicycle filtration test, the membrane relative flux reduction and flux recovery ratio were calculated by the following equations:
2.6 Evaluation of antibacterial properties
The antibacterial properties of the membrane were evaluated by the inhibition zone method, and the size of the zone of inhibition was a direct indication of the ability to assess the antimicrobial contamination of the membrane. First, the Luria-Bertani (LB) broth in a certain ratio was processed in an autoclave. E. coli was inoculated into the liquid LB broth, which was incubated at 37°C for 24 h. Then, the LB agar was sterilized in a certain ratio in an autoclave and poured into a plate. Then, the cultured E. coli was inoculated into a solid medium and uniformly coated; then the membrane was cut into a disc of 12.56 cm2 and placed in a medium inoculated with E. coli cultivated for 24 h in an incubator at 37°C and the experiment result was recorded.
3 Results and discussion
3.1 Chemical constitution of the membrane surface
The XRD pattern of the modified PVDF membrane is shown in Figure 1. It can be seen that the diffraction peaks of PVDF appeared in the spectra 1–6 at 2θ around 20°, and the intensity of the diffraction peak weakened with the addition of the modified nanosilver and nano-ZrO2 in the membrane. For the 2–6 XRD pattern, a weak diffraction peak appeared around 2θ of 41°, corresponding to the diffraction peak of nano-Ag. The diffraction peaks of ZrO2 appeared at 27° and 30° at 2θ in spectra 3–6, corresponding to the monoclinic phases (111) and (−111), respectively. As the concentration of zirconia increased, the peak intensity increased. All the above results demonstrated that nanosilver and nanozirconia are successfully doped into PVDF membranes.
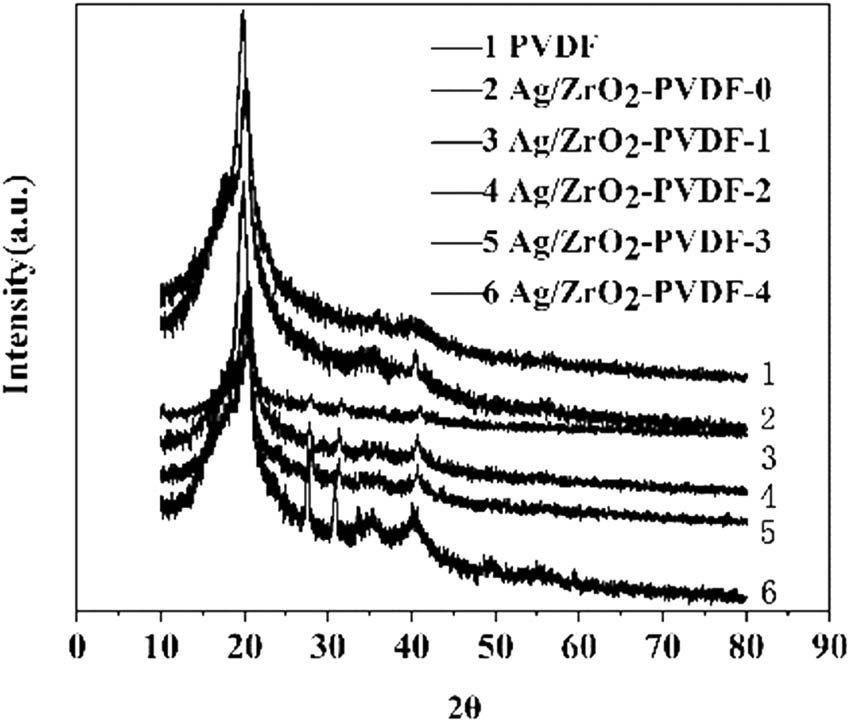
XRD patterns of PVDF, Ag/ZrO2-PVDF-0, Ag/ZrO2-PVDF-1, Ag/ZrO2-PVDF-2, Ag/ZrO2-PVDF-3, and Ag/ZrO2-PVDF-4.
As shown in Figure 2, 1–6 were the Fourier transform infrared spectra (FTIR-ATR) of PVDF, Ag/ZrO2-PVDF-0, Ag/ZrO2-PVDF-1, Ag/ZrO2-PVDF-2, Ag/ZrO2-PVDF-3, and Ag/ZrO2-PVDF-4, respectively. In the infrared spectrum of a pristine PVDF membrane, the absorption peak of C–C is at 879/cm, the deformation vibration absorption peak of –CH2 connected to –CF2 is at 1405.6/cm and the stretching vibration absorption peak of CF2 in PVDF is at 1173.1/cm; and the peak appearing at 1,667/cm corresponds to C═O, which is probably the chemical bond of DMF remaining in the film. The difference from the pristine PVDF spectrum is that the corresponding peak of Zr–O appears at 748.1/cm. The integrated XRD results show that it is ZrO2, and the intensity of the peak increases with the increase of the content of doped ZrO2.
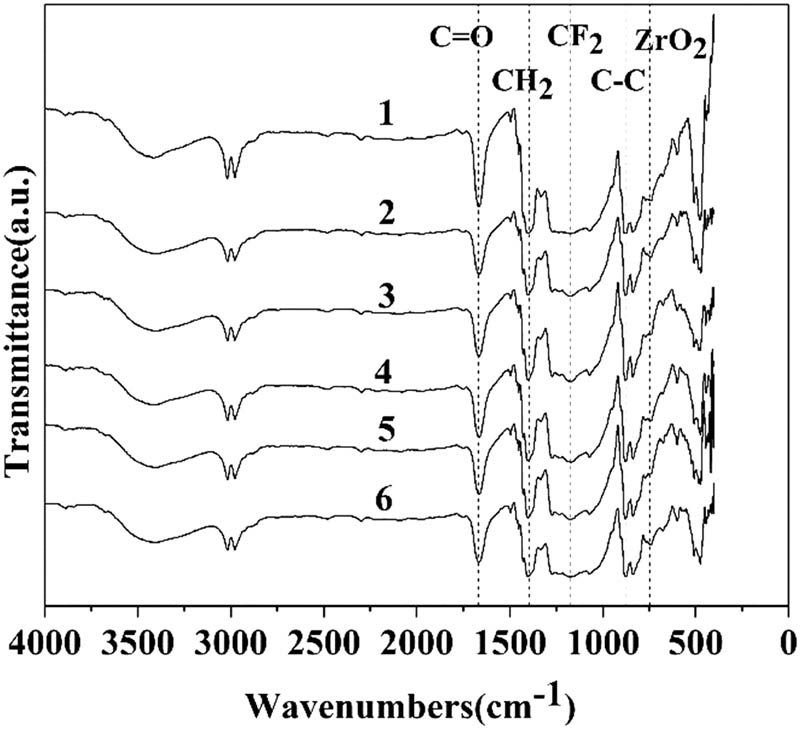
FTIR-ATR spectra of PVDF, Ag/ZrO2-PVDF-0, Ag/ZrO2-PVDF-1, Ag/ZrO2-PVDF-2, Ag/ZrO2-PVDF-3, and Ag/ZrO2-PVDF-4.
3.2 Membrane morphology
Scanning electron micrographs of the PVDF membrane and the modified membrane Ag/ZrO2-PVDF 0–4 to determine the surface morphology are shown in Figure 3. It can be seen that there are a few pores on the surface of the pristine PVDF membrane, which has a smaller pore size than the modified Ag/ZrO2-PVDF membrane. On increasing the concentration of ZrO2, the number of pores increased. When the mass of ZrO2 was increased to 0.4%, it could be seen that the number of pores was reduced due to the excessive amount of the modified material. The reason for a distinct difference between the pristine membrane and modified membrane was that the stronger repulsive force of PVDF and hydrophilic substance led to the shrinkage of the organic phase in the process of membrane modification. Meanwhile, the addition of hydrophilic materials accelerated the phase separation of the solvent and nonsolvent, resulting in the formation of porous structures. The casting solution became viscous; the speed of phase separation decreased as the content of ZrO2 increased, and when the content of ZrO2 increased to 0.4%, the membrane pores agglomerated, which resulted in the decrease in the number of the membrane pores, as shown in Figure 3f.
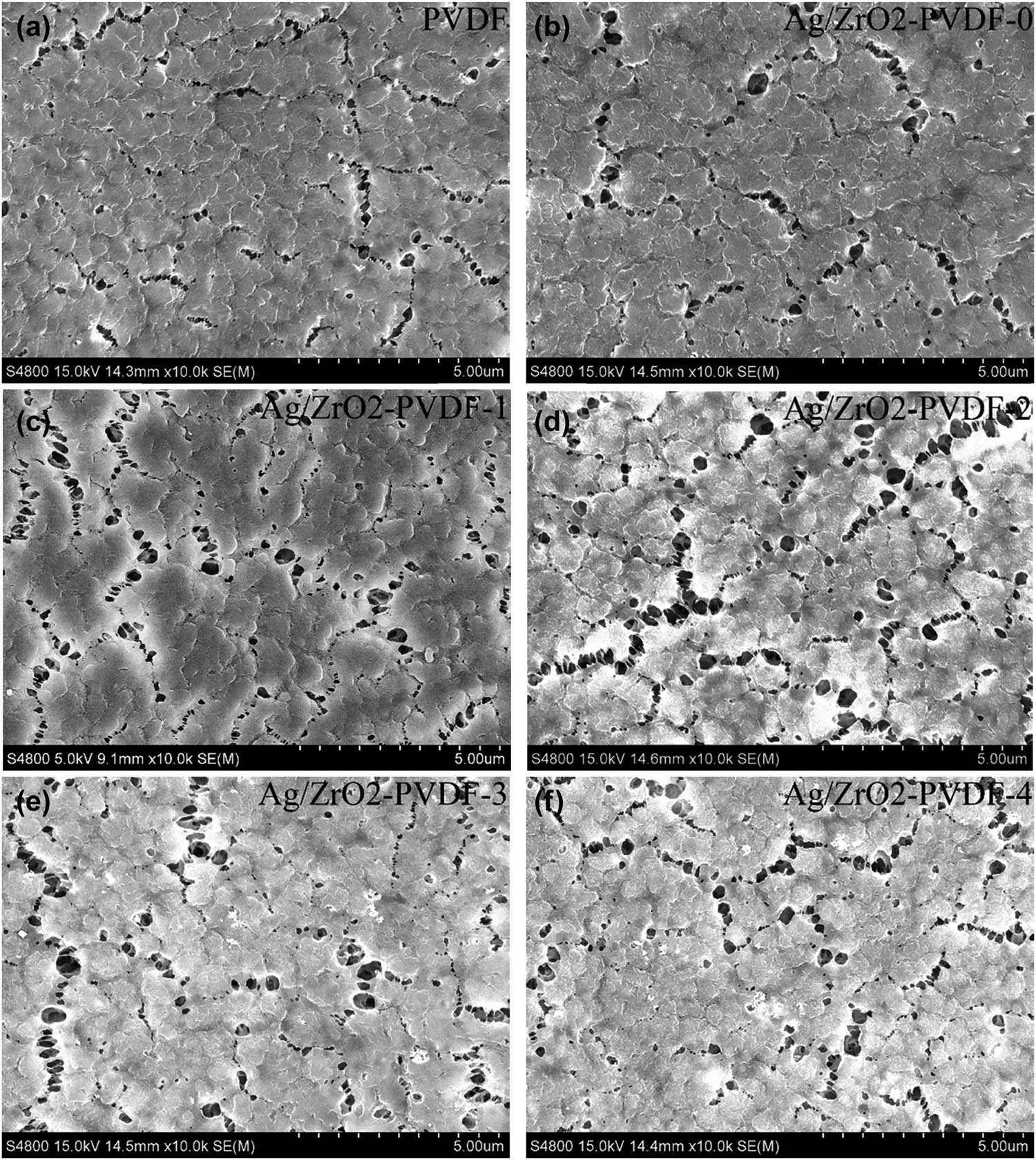
SEM images of membranes to determine the surface morphology: (a) PVDF, (b) Ag/ZrO2-PVDF-0, (c) Ag/ZrO2-PVDF-1, (d) Ag/ZrO2-PVDF-2, (e) Ag/ZrO2-PVDF-3, and (f) Ag/ZrO2-PVDF-4.
3.3 Membrane hydrophilicity
The contact angles of the PVDF membrane and Ag/ZrO2-PVDF membrane are shown in Figure 4. We evaluated the hydrophilicity of the membranes by comparing the contact angles. As shown, the contact angle decreased from 82.72° to 60.13° with the addition of the hydrophilic nanoscale Ag and the amount of nanoscale ZrO2, thereby increasing the hydrophilicity of the modified membrane. The contact angle of the Ag/ZrO2 PVDF membrane increased as the mass of ZrO2 reached 0.4%, possibly due to the agglomeration of ZrO2 on the membrane surface, which led to the increased roughness of the membrane surface thus increasing the contact angle. The contact angle of the membrane depends on the hydrophilicity of the membrane: the higher the hydrophilicity, the smaller the contact angle. At the same time, the roughness of the membrane surface also has a greater influence on the contact angle. From SEM characterization, it was evident that with the increase of the Ag/ZrO2, the pore size of the modified PVDF membrane increased. But when the amount of Ag/ZrO2 reached 1%, the pore size became smaller, and the water flux of the membrane tends to decrease thereby causing an increase in the retention rate. All of the above proved that the hydrophilic properties of the modified Ag/ZrO2-PVDF membrane increased with the increase of hydrophilic substances. The number of modified substances reached the breaking point when the content of Ag/ZrO2 is increased to 1%. The surface roughness of the subsequent PVDF membrane increases, which causes an increase in the contact angle.
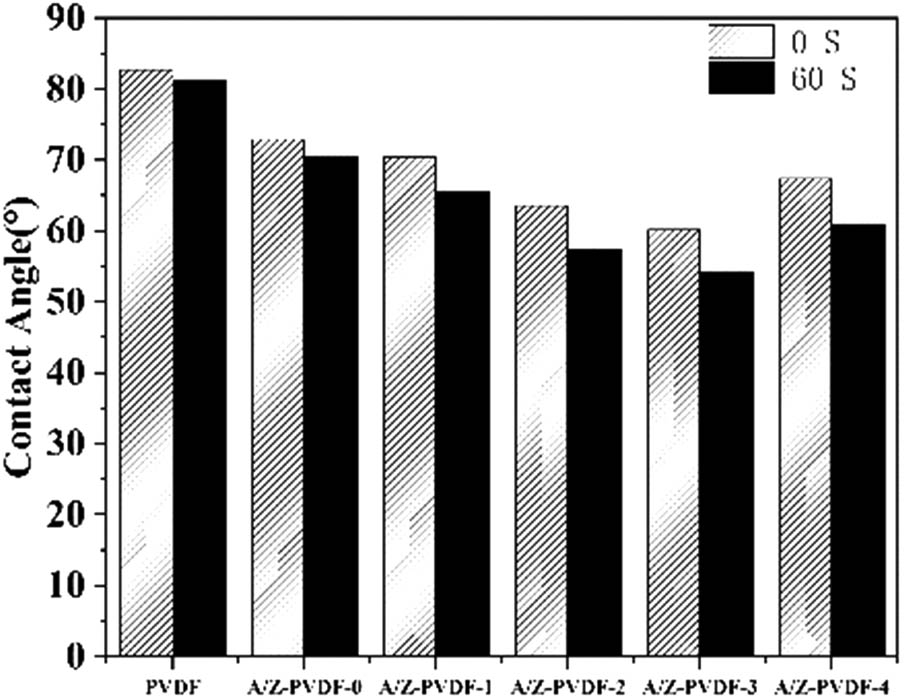
Contact angles of PVDF, Ag/ZrO2-PVDF-0, Ag/ZrO2-PVDF-1, Ag/ZrO2-PVDF-2, Ag/ZrO2-PVDF-3, and Ag/ZrO2-PVDF-4.
The changes in the contact angle of the PVDF membrane and the Ag/ZrO2-PVDF membranes were recorded in this experiment after dropping the water onto the membrane for 1 min. It revealed that the contact angle of the membrane decreased as the hydrophilic substance increased, indicating that the membrane hydrophilicity increases as the amount of hydrophilic material blend increases.
3.4 Filtration performance of the membrane
The water flux and the BSA rejection rate are shown in Figure 5. It can be seen that the water flux of the pristine membrane was lower (28.43 L/m2 h) compared with the modified membrane, which had obviously improved water flux. From SEM characterization, it was evident that with the increase of modified materials, the pore size and distribution of the membrane became larger and wider. The obvious difference between the pure PVDF membrane and Ag/ZrO2-PVDF membrane was due to the strong repulsion between the PVDF and hydrophilic materials, which led to the shrinkage of the organic phase in the process of membrane modification. At the same time, the addition of hydrophilic materials accelerated the phase separation of the solvent and nonsolvent, resulting in the formation of porous structures. In addition, the increase of the hydrophilic material had a great influence on the water flux. Therefore, the water flux of the pure PVDF membrane is much lower than that of the modified PVDF membrane.
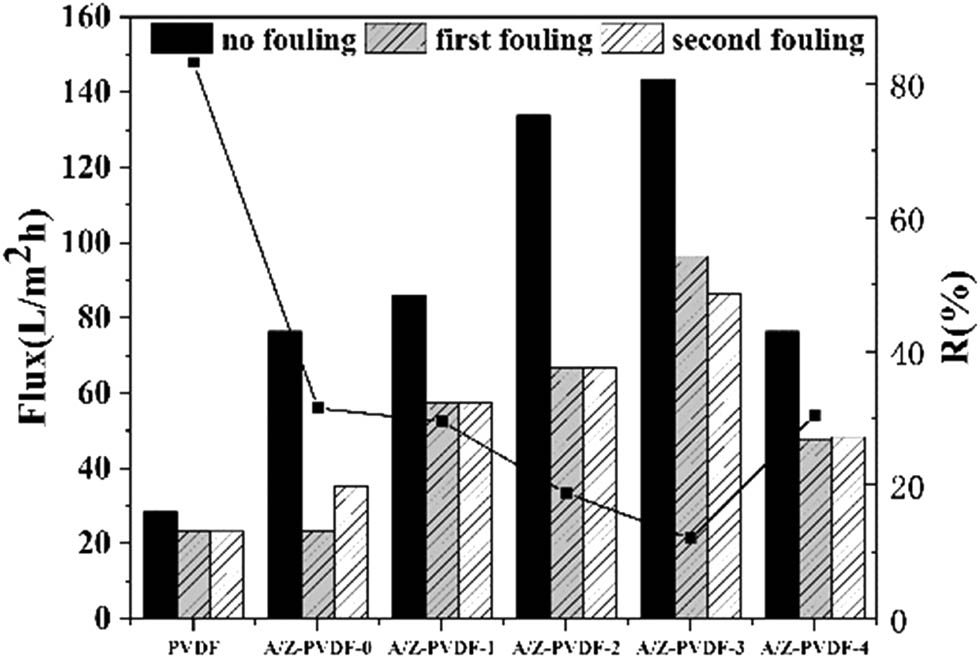
Water flux and BSA rejection rates of PVDF, Ag/ZrO2-PVDF-0, Ag/ZrO2-PVDF-1, Ag/ZrO2-PVDF-2, Ag/ZrO2-PVDF-3, and Ag/ZrO2-PVDF-4.
It was found that the membrane water flux decreased significantly after the membrane was contaminated by BSA, which was treated as organic pollutants in the experiment, and then the water flux of the membranes tends to be stable after the recycle contamination experiment. However, the water flux of the membrane tends to decrease when the content of ZrO2 was increased to 0.4%, which may be contributing to the change in the morphology of the membrane; due to the excessive amount, the blockage of the membrane pore made the water transport difficult. The PVDF membrane had the largest rejection among the membranes, and the reason for the change in the rejection rate of the membrane was due to the addition of the hydrophilic substance, which increased the pore diameter and decreased the adsorption of organic substances such as proteins, etc. However, the rejection rate increased when the ZrO2 was increased to 0.4%, which was consistent with the reason for the decrease in water flux. Above all, it is evident that the water flux could be increased by modifying the PVDF membrane to improve the hydrophilicity of the membrane.
3.5 Organic antifouling performance of the membrane
The pure water and BSA flux changes of the PVDF membrane and Ag/ZrO2-PVDF membrane are shown in Figure 6. After the membrane was contaminated with BSA, it was found that the water flux of the membrane dropped significantly, which indicated that BSA caused the membrane surface fouling and inner fouling. The membrane water flux recovers but does not return to the flux level prior to fouling after rinsing with deionized water. A further cycle of membrane fouling experiments revealed that the membrane water flux tended to be stable, which showed that BSA adsorption and diffusion to the surface of the membrane reached equilibrium. The relative flux reduction and flux recovery rate of the PVDF membrane and Ag/ZrO2-PVDF membrane were obtained through the calculation of two pollution cycle experiments. As shown in Figure 7, the pristine PVDF membrane had the highest RFR and the lowest FRR, which may be due to the strong hydrophobicity of the PVDF membrane and thus irreversible membrane fouling. Figure 7 shows that the flux decay rate of PVDF membranes decreased from 78% of pure PVDF membranes to 65% of Ag/ZrO2-PVDF (0.5%) membranes. This implies that the modified PVDF membrane in the BSA cyclic pollution experiment showed good resistance to protein pollution. The flux recovery rate also increased from 50% of pure PVDF membrane to about 70% of Ag/ZrO2-PVDF (0.5%) membrane, indicating that the modified membrane had better anti-pollution performance compared with the pure PVDF membrane. The strong hydrophobicity of the PVDF membrane can easily cause the blockage and fouling of BSA in the pores severely, and thus lead to irreversible membrane fouling. As the content of hydrophilic substances increased, a hydration layer was formed on the membrane surface, the relative flux of the membrane decreased, and the flux recovery rate increased. So, the hydrophilic modification can protect the membrane from organic pollution effectively.
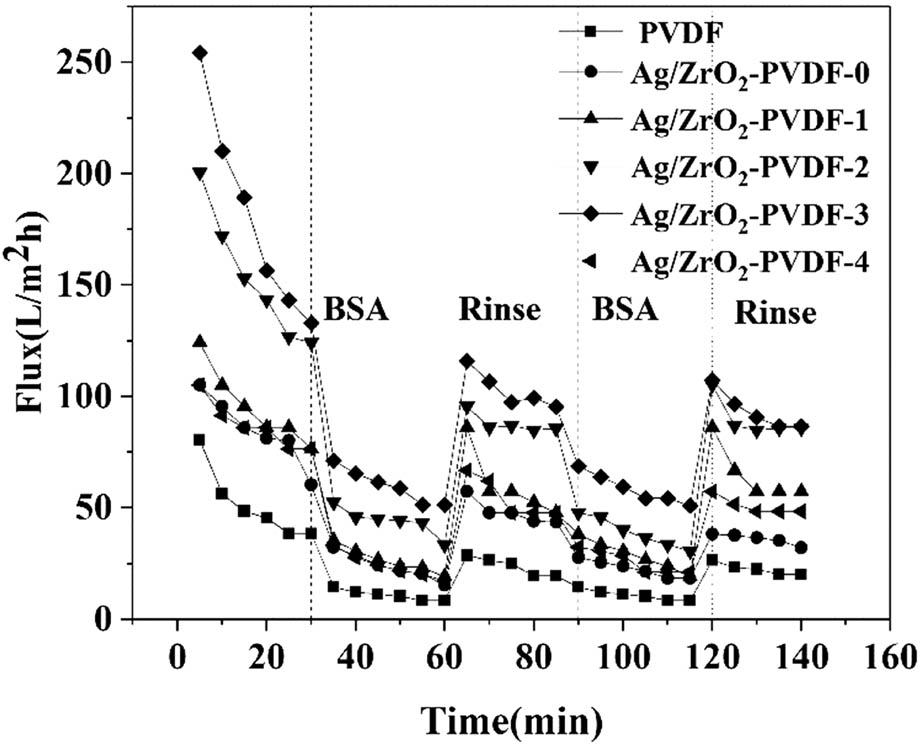
The changes in pure water and BSA flux of PVDF, Ag/ZrO2-PVDF-0, Ag/ZrO2-PVDF-1, Ag/ZrO2-PVDF-2, Ag/ZrO2-PVDF-3, and Ag/ZrO2-PVDF-4 with time.

RFR and FRR values of PVDF, Ag/ZrO2-PVDF-0, Ag/ZrO2-PVDF-1, Ag/ZrO2-PVDF-2, Ag/ZrO2-PVDF-3, and Ag/ZrO2-PVDF-4 with time.
Hydrophilic modification of the membrane revealed a decrease in the relative flux reduction of the membrane flux and an increase in the rate of flux recovery, indicating that the membrane can be more effectively protected against organic pollution by hydrophilic modification.
3.6 Bacterial antifouling performance of the membrane
In this experiment, the inhibition zone method was used to evaluate the antibacterial properties of the membrane. As shown in Figure 8, the PVDF membrane was contaminated during the cultivation of E. coli in Figure 8a, which illustrated that the pure PVDF membrane had no inhibitory effect on E. coli. It was clear that the zone of inhibition is formed around the PVDF membrane modified by in situ nanosilver blending in Figure 8b, which counteracted microbial contamination effectively. Meanwhile, the size of the suppression band formed in Figure 8c–f was almost the same as in Figure 8b, thus illustrating that the modification of the PVDF membrane by blending nanoscale-silver ions synthesized by in situ synthesis method was an effective method for protection from contamination by the antimicrobial.

The effect of Ag on the inhibition zone thickness of (a) PVDF, (b) Ag/ZrO2-PVDF-0, (c) Ag/ZrO2-PVDF-1, (d) Ag/ZrO2-PVDF-2, (e) Ag/ZrO2-PVDF-3, and (f) Ag/ZrO2-PVDF-4 with time.
4 Conclusion
In this experiment, the PVDF membrane was modified with nanoscale-zirconia and in situ synthesis of nanoscale-silver by the phase inversion method and blending modification.
It was found that the number and the size of pores were increased first and then decreased with the increase of hydrophilic materials, and the contact angle decreased by 15.35° with the increase of the content of hydrophilic polymer and the water flux significantly improved. This indicated that the surface morphology of the PVDF membrane was affected by the formed blending nanoscale-Ag and nanoscale-ZrO2, thus making a significant improvement in the hydrophilic properties of the membrane. After the membrane was contaminated with BSA as an organic pollutant, it revealed that the flux recovery of the membrane increased with the increase of hydrophilicity, indicating that the anti-fouling performance of the Ag/ZrO2-PVDF membrane after modification was improved. At the same time, the pristine PVDF membrane and Ag/ZrO2-PVDF membrane contaminated by E. coli indicated that the nanoscale-silver ions synthesized by the in situ synthesis method have a significant effect on the microbial antifouling performance of the membrane. In conclusion, the properties of the modified Ag/ZrO2-PVDF film have been greatly improved.
-
Funding information: This research was supported by the Natural Science Foundation of Shaanxi Province, China (2019JQ-838).
-
Author contributions: Yanjun Lu: writing – original draft, writing – review and editing, conceptualization, methodology, formal analysis, data curation, supervision, funding acquisition; Yuxuan Ma: writing – review and editing, resources, investigation; Tong Yang: resources, investigation, visualization; Jifeng Guo: writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wang X, Wang Z, Chen H, Wu Z. Removal of Cu(ii) ions from contaminated waters using a conducting microfiltration membrane. J Hazard Mater. 2017;339:182–90. 10.1016/j.jhazmat.2017.06.038.Search in Google Scholar
[2] Zhu L, Chen M, Dong Y, Tang CY, Huang A, Li L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016;90:277–85. 10.1016/j.watres.2015.12.035.Search in Google Scholar
[3] Bae TH, Tak TM. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J Membr Sci. 2005;249(1):1–8. 10.1016/j.memsci.2004.09.008.Search in Google Scholar
[4] Lu Y, Yu SL, Chai BX, Xianda S. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J Membr Sci. 2006;276(1):162–7. 10.1016/j.memsci.2005.09.044.Search in Google Scholar
[5] Cheng XQ, Shao L, Lau CH. High flux polyethylene glycol based nanofiltration membranes for water environmental remediation. J Membr Sci. 2015;476:95–104. 10.1016/j.memsci.2014.11.020.Search in Google Scholar
[6] Harisha RS, Hosamani KM, Keri RS, Nataraj SK, Aminabhavi TM. Arsenic removal from drinking water using thin film composite nanofiltration membrane. Desalination. 2010;252(1):75–80. 10.1016/j.desal.2009.10.022.Search in Google Scholar
[7] Cadott JE, Petersen RJ, Larson RE, Erickson EE. A new thin-film composite seawater reverse osmosis membrane. Desalination. 1980;32(1–3):25–31. 10.1016/S0011-9164(00)86003-8.Search in Google Scholar
[8] Konagaya S, Kuzumoto H, Watanabe O. New reverse osmosis membrane materials with higher resistance to chlorine. J Appl Polym Sci. 2000;75(11):1357–64. 10.1002/(SICI)1097-4628(20000314)75:11<1357:AID-APP6>3.0.CO;2-W.Search in Google Scholar
[9] Ghazali AA, Abd Rahman S, Abu Samah R. Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: a review. Green Process Synth. 2020;9(1):219–29. 10.1515/gps-2020-0023.Search in Google Scholar
[10] Marino T, Blefari S, Di Nicolo E, Figoli A. A more sustainable membrane preparation using triethyl phosphate as solvent. Green Process Synth. 2017;S16(3):295–300. 10.1515/gps-2016-0165.Search in Google Scholar
[11] Zhang F, Zhang W, Yu Y, Deng B, Li J, Jin J. Sol–gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved antifouling property. J Membr Sci. 2013;432(7):25–32. 10.1016/j.memsci.2012.12.041.Search in Google Scholar
[12] Rana D, Matsuura T, Narbaitz RM, Feng C. Development and characterization of novel hydrophilic surface modifying macromolecule for polymeric membranes. J Membr Sci. 2005;249(1–2):103–12. org/10.1016/j.memsci.2004.09.034.Search in Google Scholar
[13] Rana D, Matsuura T, Narbaitz RM. Novel hydrophilic surface modifying macromolecules for polymeric membranes: polyurethane ends capped by hydroxy group. J Membr Sci. 2006;282(1–2):205–16. 10.1016/j.memsci.2006.05.024.Search in Google Scholar
[14] Kim Y, Rana D, Matsuura T, Wook-Jin C. Influence of surface modifying macromolecules on the surface properties of poly (ether sulfone) ultra-filtration membranes. J Membr Sci. 2009;338(1–2):84–91. 10.1016/j.memsci.2009.04.017.Search in Google Scholar
[15] Kim Y, Rana D, Matsuura T, Chung WJ, Khulbe KC. Relationship between surface structure and separation performance of poly(ether sulfone) ultra-filtration membranes blended with surface modifying macromolecules. Sep Purif Tech. 2010;72(2):123–32. 10.1016/j.seppur.2010.01.006.Search in Google Scholar
[16] Rana D, Kim Y, Matsuura T, Arafat HA. Development of antifouling thin-film-composite membranes for seawater desalination. J Membr Sci. 2011;367(1–2):110–8. 10.1016/j.memsci.2010.10.050.Search in Google Scholar
[17] Rana D, Scheier B, Narbaitz RM, Matsuura T, Tabe S, Jasim SY, et al. Comparison of cellulose acetate (CA) membrane and novel CA membranes containing surface modifying macromolecules to remove pharmaceutical and personal care product micropollutants from drinking water. J Membr Sci. 2012;409–410:346–54. 10.1016/j.memsci.2012.04.005.Search in Google Scholar
[18] Rana D, Narbaitz RM, Garand-Sheridan AM, Westgate A, Matsuura T, Tabe S, et al. Development of novel charged surface modifying macromolecule blended PES membranes to remove EDCs and PPCPs from drinking water sources. J Mater Chem A. 2014;2(26):10059–72. 10.1039/c4ta01530d[17].Search in Google Scholar
[19] Ochoa NA, Masuelli M, Marchese J. Effect of hydrophilicity on fouling of an emulsified oil wastewater with PVDF/PMMA membranes. J Membr Sci. 2003;226(1):203–11. 10.1016/j.memsci.2003.09.004.Search in Google Scholar
[20] Razzaghi MH, Safekordi A, Tavakolmoghadm M, Rekabdar F, Hemmati M. Morphological and separation performance study of PVDF/CA blend membranes. J Membr Sci. 2014;470:547–57. 10.1016/j.memsci.2014.07.026.Search in Google Scholar
[21] Lü X, Wang X, Guo L, Zhang Q, Guo X, Li L. Preparation of PU modified PVDF antifouling membrane and its hydrophilic performance. J Membr Sci. 2016;520:933–40. 10.1016/j.memsci.2016.08.018.Search in Google Scholar
[22] Wu H, Mansouri J, Chen V. Silica nanoparticles as carriers of antifouling ligands for PVDF ultrafiltration membranes. J Membr Sci. 2013;433(15):135–51. 10.1016/j.memsci.2013.01.029.Search in Google Scholar
[23] Rahimpour A, Jahanshahi M, Rajaeian B, Rahimnejad M. TiO2, entrapped nano-composite PVDF/SPES membranes: preparation, characterization, antifouling and antibacterial properties. Desalination. 2011;278(1):343–53. 10.1016/j.desal.2011.05.049.Search in Google Scholar
[24] Kim Y, Rana D, Matsuura T, Chung WJ. Towards antibiofouling ultrafiltration membranes by blending silver containing surface modifying macromolecules. Chem Commun. 2012;48(5):693–5. 10.1039/c1cc16217a.Search in Google Scholar PubMed
[25] Pang RZ, Li X, Li JS, Lu ZY, Huang C, Sun XY. In situ preparation and antifouling performance of ZrO2/PVDF hybrid membrane. Acta Physico-Chimica Sin Acta Phys-Chim Sin. 2013;29(12):2592–8(7). 10.3866/PKU.WHXB201310291.Search in Google Scholar
[26] Kaur D, Bagga V, Behera N, Thakral B, Asija A, Kaur J, et al. SnSe/SnO2 nanocomposites: novel material for photocatalytic degradation of industrial waste dyes. Adv Compos Hybrid Mater. 2019;2(4):763–76. 10.1007/s42114-019-00130-7.Search in Google Scholar
[27] Zhao YY, Zhang YQ, Li FR, Bai YP, Pan YL, Ma J, et al. Ultra-robust superwetting hierarchical membranes constructed by coordination complex networks for oily water treatment. J Membr Sci. 2021;627(1):119234. 10.1016/j.memsci.2021.119234.Search in Google Scholar
[28] Zhang Y, Guo J, Han G, Bai Y, Ge Q, Ma J, et al. Molecularly soldered covalent organic frameworks for ultrafast precision sieving. Sci Adv. 2021;7(3):eabe8706. 10.1126/sciadv.abe8706.Search in Google Scholar PubMed PubMed Central
[29] Zhang Y, Cheng X, Jiang X, Urban JJ, Lau CH, Liu S, et al. Robust natural nanocomposites realizing unprecedented ultrafast precise molecular separations. Mater Today. 2020;36:40–7. 10.1016/j.mattod.2020.02.002.Search in Google Scholar
[30] Li X, Pang R, Li J, Sun X, Shen J, Han W. In situ formation of Ag nanoparticles in PVDF ultrafiltration membrane to mitigate organic and bacterial fouling. Desalination. 2013;324(14):48–56. 10.1016/j.desal.2013.05.021.Search in Google Scholar
© 2021 Yanjun Lu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

