Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
-
Shanmugam Rajeshkumar
, Munusamy Tharani
Abstract
In the present investigation, silver nanoparticles (AgNPs) and silver nanoparticle-based chitosan nanocomposite were synthesized using Cissus arnottiana leaf extract. The biosynthesized nanoparticles and nanocomposites were characterized using SEM, TEM, and AFM to uncover the morphological characteristics such as size and shape. The SEM image depicts the size of the nanocomposite to be 30–40 nm and shape as spherical. The TEM results reveal the shape of the nanocomposite to be spherical and size around 10–60 nm. The XRD results show the crystalline nature of the AgNPs-based chitosan nanocomposite. The SAED analysis pattern seems to be concordant with the XRD results. The AFM image ensured the precise surface morphology of the synthesized silver nanocomposite in the 3-dimensional pattern. The antimicrobial efficacy of the biosynthesized AgNPs, AgNP nanocomposite, and chitosan nanoparticles was tested against oral pathogens. The results revealed a potential antimicrobial effect, which states that it must be converted into nanomedicine to meet future biomedical needs.
Graphical abstract
Antibacterial potential of silver nanoparticle-based chitosan nanocomposites.

1 Introduction
Recently, the field of nanotechnology arised to a greater extent due to its ablazing applications in biomedical field [1]. This attracts huge number of researchers to create different nanomaterials with specific functions to cure a disease or to enhance and perform in equipments or products such as health care products, cosmetics, household products, etc. The nanolevel requirement of these nanomaterials in living cells plays a significant role in outraging the disease-causing pathogens and organisms [2].
Nowadays, the disease-causing pathogens become more resistant to upcoming antibiotic drugs. To combat this issue, metallic nanoparticles like silver nanoparticles (AgNPs) have been reported in several studies by researchers as an effective antimicrobial agent [3,4,5]. The AgNPs are majorly used in treating burn and open injuries to avoid contamination from wound pathogens and other nosocomial pathogens [6]. AgNPs assume a significant part in science and medication due to their desirable physicochemical properties. AgNPs are known to have antifungal, anti-inflammatory, antiviral, antibacterial, antiangiogenesis, and antiplatelet properties [7,8,9].
In several studies, chitosan, a natural biopolymer, has been reported to enhance the antibacterial efficacy of the metallic nanoparticles [10,11,12]. In this present study, chitosan was added to Cissus arnottiana leaf extract-mediated AgNPs to attain as silver nanocomposite an increase in the potent antimicrobial efficacy of the AgNPs to a greater extent [13,14,15].
Cissus arnottiana is an erect woody shrub that has a place with the Vitaceae family distributed all through India. The plant roots are utilized as a remedy for rheumatic swellings. Phytochemical screening of Cissus arnottiana plant and fruit uncovered the presence of bioactive constituents, for example, tannins, phenols, terpenoids, flavonoids, glycosides, sugars, and saponins [16].
The AgNPs were incorporated with various polymers such as chitosan and its cross-linked polyvinyl pyrrolidone in gelatin, and polygalacturonic with hyaluronic acid-based silver nanofibers show good wound healing activity; the black berry-mediated silver, gold, and silver/gold bimetallic nanoparticles loaded with pectin show cardioprotective activity [17,18,19]. The AgNPs synthesized using various chemical methods show higher toxicity in animal model and cell line studies, but the AgNPs synthesized using biological agents like bacteria, plant and its parts, fungi, and algae show very good biomedical applications [20,21,22].
The current study deals with synthesizing AgNPs and silver chitosan nanocomposite using Cissus arnottiana extract as a stabilizing and reducing agent. The synthesized silver nanocomposite has been characterized using UV-double beam spectrophotometer, scanning electron microscope, transmission electron microscope, atomic force microscope, and X-ray diffraction analysis. The antimicrobial efficacy of biosynthesized silver nanocomposite was tested against oral pathogens such as Staphylococcus aureus, Streptococcus mutans, Enterococcus faecalis, and Candida albicans.
2 Materials and methods
2.1 Chemicals
The precursor silver nitrate was purchased from Sigma Aldrich chemicals Pvt. Ltd (India). Mueller Hinton Agar was received from Hi-Media, India. The leaves of Cissus arnottiana plant were localized in rural areas of Vellore, Tamilnadu, India. The bacterial cultures such as S. aureus, S. mutans, E. faecalis, and C. albicans were isolated and collected from Saveetha Dental College and Hospital, Poovirunthavalli Chennai.
2.2 Preparation of Cissus arnottiana leaf extract
The leaves of Cissus arnottiana were washed thoroughly under tap water and with Milli-Q water. Then the plant was shade-dried for 3–4 days. The dried plant was grounded into a fine powder and stored in an airtight container. 1 g of the dried powdered plant was added to 100 mL of double-distilled water. The mixture was then heated using a heating mantle at 70°C for 15 min. By this strategy, all phytochemical compounds present in the Cissus arnottiana plant get diffused in the aqueous solution. This final mixture was filtered by using filter paper (Whatman No.1) and the filtered leaf extract was stored in refrigerator for further use.
2.3 Nanoparticle synthesis
1 mM of precursor silver nitrate (AgNO3) was added to 90 mL of deionized water. 10 mL of Cissus arnottiana filtered plant extract was added. The mixture was kept on a magnetic stirrer for 600–700 rpm for 48 h. UV-Vis-double beam spectrophotometer was used to analyze the synthesis of AgNPs at regular time intervals from the start wavelength 360–500 rpm (revolutions per minute). To collect pellet from the aqueous reaction mixture, biosynthesized AgNPs were centrifuged at 8,000 rpm for 10 min. The supernatant was discarded, and pellet was washed thrice with ethanol followed by deionized water and kept inside hot air oven at 70°C for 2 h. And powdered form of AgNPs was stored in airtight Eppendorf tube for characterization studies.
2.4 Preparation of nanocomposites
0.5 g of chitosan was dissolved in 1 mL of 1% glacial acetic acid and 49 mL of deionized water. The synthesized AgNPs were added to the chitosan solution and kept in a magnetic stirrer for 3–4 h. After adding Cissus arnottiana-mediated AgNPs solution, a brown nanocomposites gel was obtained. The mixture was kept again in a magnetic stirrer for 48 h. Formation of silver nanocomposites was analyzed by UV-Vis spectrometry in the wavelength range of 360–500 nm. The synthesized silver nanocomposite was allowed for centrifugation at 10,000 rpm for 10 min. The nanocomposite pellet was suspended in deionized water, centrifuged again, and lyophilized. The lyophilized silver chitosan nanocomposites were dissolved in distilled water and used for different characterization studies.
2.5 Characterization of AgNPs and nanocomposites
The Cissus arnottiana-mediated AgNPs and nanocomposites were preliminarily characterized using UV-double beam spectrophotometer (UV-2450, Shimadzu) in the wavelength range of 360–500 nm. The morphology characteristics such as size and shape were studied using scanning electron microscope (SEM) and transmission electron microscope (TEM). The crystalline nature of the AgNPs-mediated chitosan nanoparticle was analyzed using X-ray diffraction. The atomic force microscope is used to study the 3-D structure of the biosynthesized AgNPs-mediated nanocomposites with sub-nanometer resolution.
2.6 Antimicrobial activity against oral pathogens
10 µL of fresh bacterial cultures such as S. aureus, S. mutans, E. faecalis, and C. albicans were inoculated in sterile Hi-veg broth and kept above orbital shaker for 18 h at 120–150 rpm. Mueller Hinton agar was prepared, and 5 mm wells were made using a sterile polystyrene tip. The antimicrobial activity was done to enable the efficacy of Cissus arnottiana-mediated AgNPs, biosynthesized AgNPs-mediated nanocomposite, and chitosan nanoparticle. Various concentrations such as 25, 50, and 100 µL of three samples were added to wells, and along with positive control, amoxyrite was added (except for C. albicans, fluconazole is used as a standard drug). The inoculated sample petriplates were kept inside a microbial incubator at 37°C for 24 h and the zone of inhibition zone was measured to compare and study the potential effect of Cissus arnottiana-mediated AgNP and nanocomposites and chitosan nanoparticle.
3 Results and discussion
3.1 Visual observation
Biosynthesis of nanoparticles by utilizing heterocyclic compounds acquires consideration because of their effortlessness and ecofriendly nature [23]. The color intensity of the Cissus arnottiana-mediated AgNPs solution mixture increased by an increase in time. Also, the reduction of silver nitrate to Ag0 by the reducing agent (Cissus arnottiana leaf extract) was indicated by an initial light yellow to final dark brown color change (Figure 1), which was further confirmed by UV-Vis, spectrophotometry analysis. The AgNPs synthesized using orchid leaf show the brown in color confirms the nanoparticles formation [15], the AgNPs with chitosan also formed color formation confirms the nanocomposite formation [13].
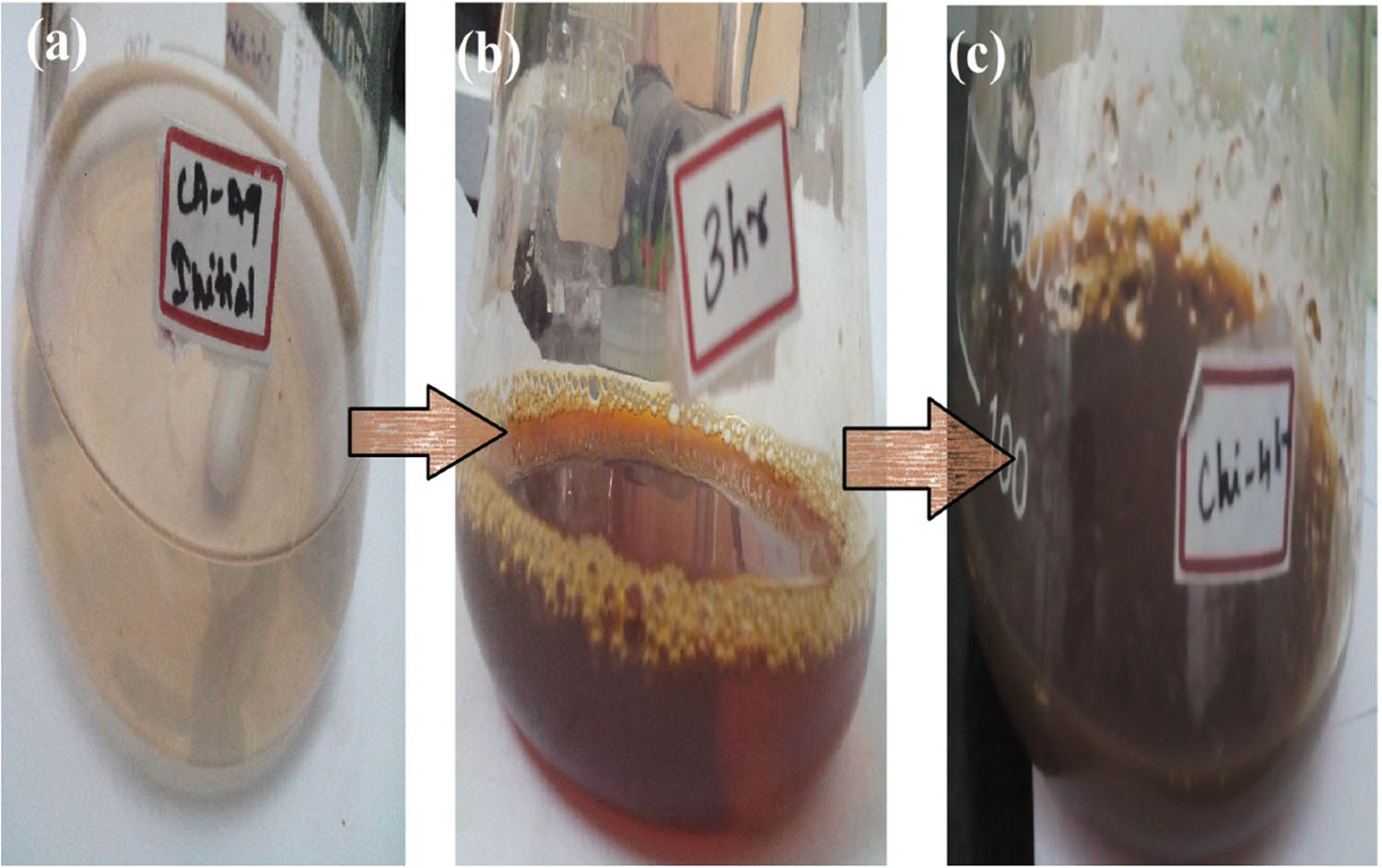
Visual observation: (a) silver nitrate with plant extract, (b) silver nanoparticles, and (c) AgNPs-based chitosan nanocomposites.
3.2 Optical analysis by UV-Vis spectrophotometer
UV-Vis spectroscopy is a significant step to assess the development and stability of nanoparticles [24]. Recent work such as that of Thamilarasan et al. [11] analyzed concordant results as attained in the current research work. The maximum absorption peak of AgNPs was observed at 420 nm. The broad peak found around 380–460 nm confirms the silver chitosan nanocomposite (Figure 2). The small peak variation in the UV-Vis spectroscopy absorbance confirms the stability of the AgNPs after treating with microwave irradiation. The peak around 400–440 nm confirms the AgNPs synthesis using leaf extracts of Clerodendrum inerme and Pedalium murex leaf extract [5,27].
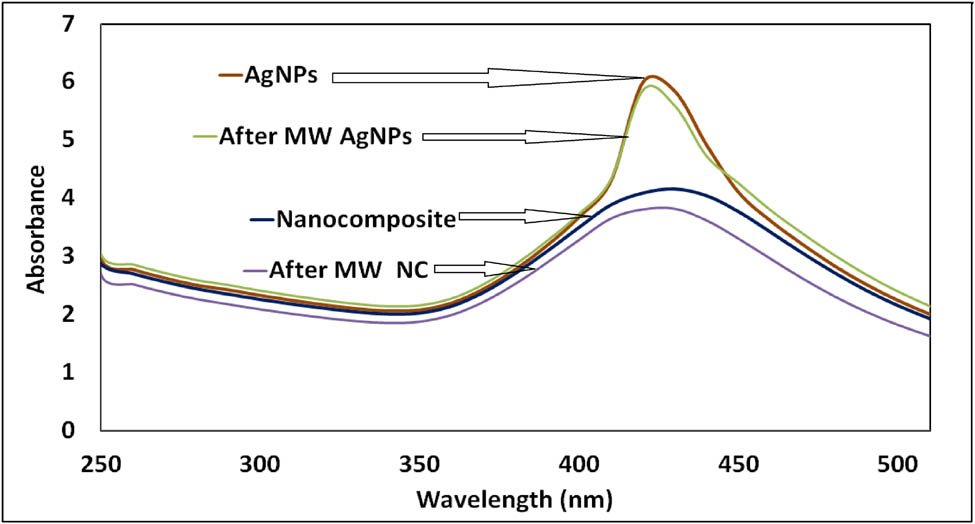
UV-Vis spectroscopic analysis of silver nanoparticles and AgNPs with chitosan.
3.3 SEM analysis
The SEM can be utilized to scan nanoscopic structures at high resolution [25]. The size of chitosan was depicted to be 90 nm and shape as pseudo-spherical. The size of Cissus arnottiana-mediated AgNPs embedded with chitosan was found to be 30–40 nm and it reveals its shape as cuboidal (Figure 3). Previous studies such as those of authors of [26] correlate with the SEM result of chitosan and Cissus arnottiana-mediated silver nanocomposites.
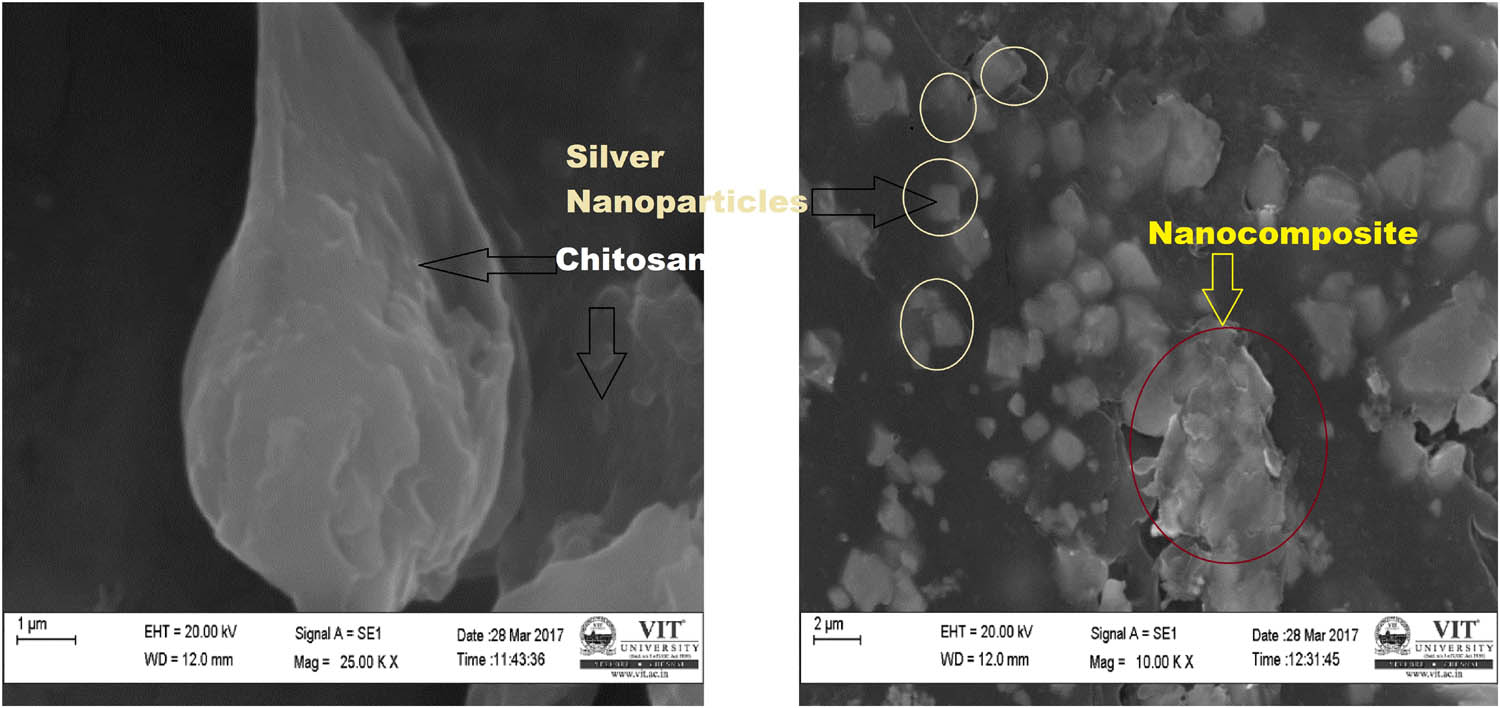
SEM images of chitosan and AgNPs embedded with chitosan.
3.4 XRD analysis
XRD spectra give an understanding of the crystallinity of nanoparticles. Figure 4 depicts XRD spectra of AgNPs embedded with chitosan synthesized using Cissus arnottiana leaf extract. The particle size was resolved using Debye–Scherrer equation and predicted as 23 nm. X-ray diffraction peaks acquired at 17.29°, 31.73°, 37.46°, and 40.64° were compared to the lattice plane of (4 6 7), (9 3 8), (6 4 9), and (5 7 0), which proposes the face-centered cubic (fcc) crystal structure of the silver nanocomposite. The stronger peaks predict presence of silver [27]. The results seem to agree with the previous studies reported [28].
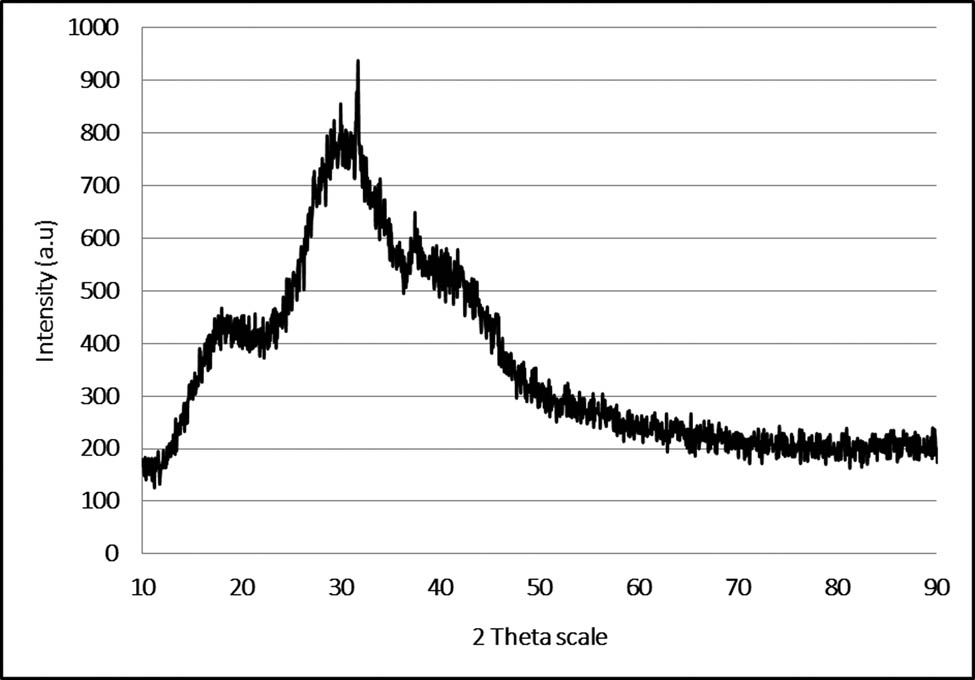
XRD pattern of AgNPs-based chitosan nanocomposite.
3.5 AFM analysis
The AFM image in Figure 5 ensured the precise surface morphology of the synthesized silver nanocomposite. The acquired image uncovered how the synthesized nanocomposite was almost spherical without other perceptible nanostructure morphologies as affirmed by absorbance range. The particles were not profoundly mono-scattered but instead appeared non-agglomerated. Previous studies such as those of Kalaivani et al. [29] synthesized silver nanocomposite and the AFM results are concordant with the current study.

AFM image of AgNPs-based chitosan nanocomposite.
3.6 TEM analysis
Figure 6a–c represents the TEM image and Figure 6d depicts the SAED analysis image of biosynthesized AgNP-based chitosan nanocomposite. The TEM results reveal the shape of the nanocomposite to be spherical and size around 10–60 nm. The SAED analysis pattern seems to agree with the XRD results. The TEM results of AgNPs-based chitosan nanocomposites were concordant with the earlier works, such as Ghadi et al. [30] stating the size of the nanocomposite to be 10–80 nm.
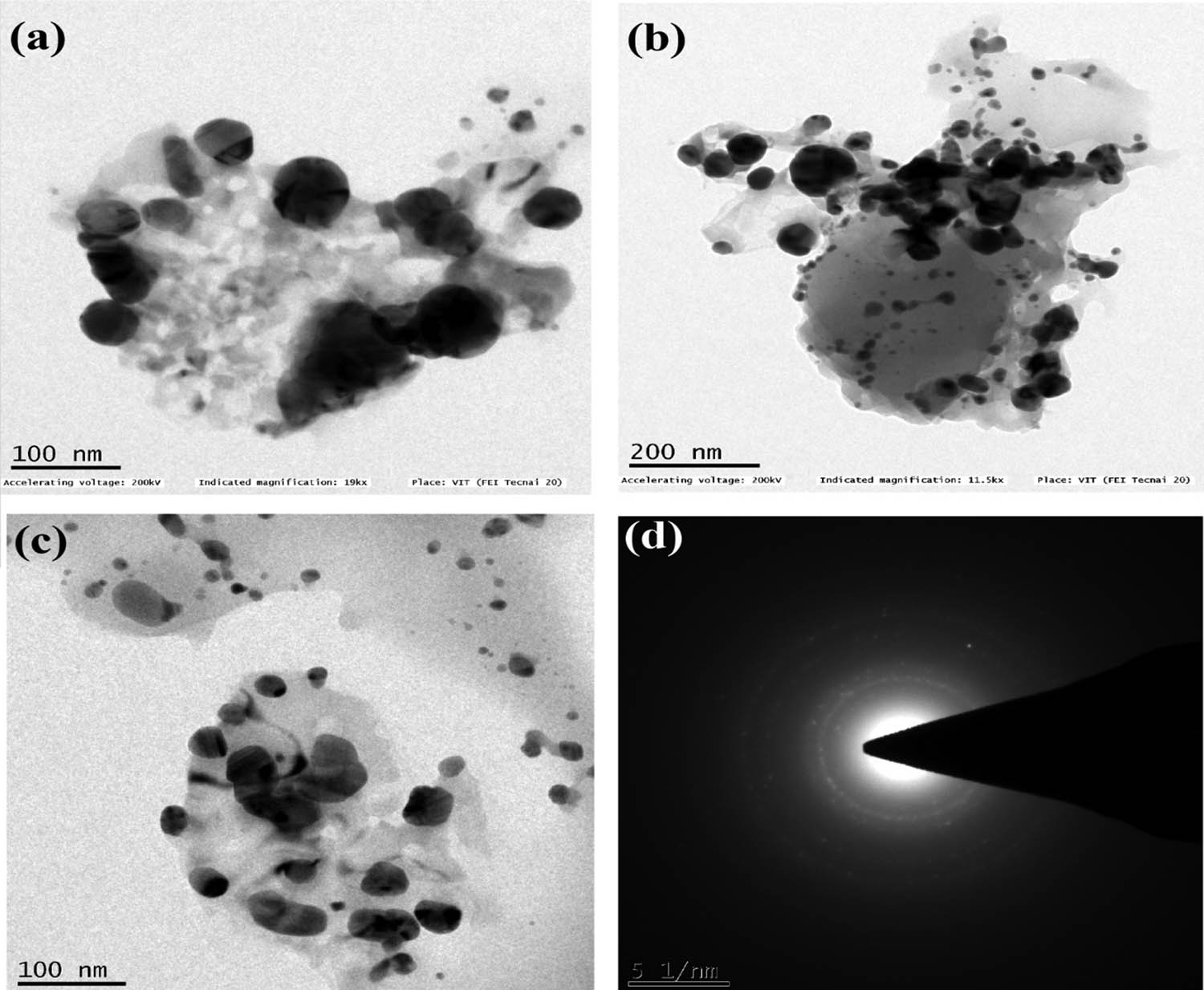
(a–c) TEM and (d) SAED analysis of Cissus arnotiana mediated AgNPs based chitosan nanocomposites.
3.7 Antimicrobial activity against oral pathogens
The antimicrobial activity of silver nanocomposite was tested by adopting agar well diffusion assay. Antibacterial effect of Cissus arnottiana-mediated AgNPs, chitosan NP, and AgNPs-based nanocomposite was visualized against four oral pathogens such as S. aureus, S. mutans, C. albicans, and E. faecalis, which was depicted in Figure 7. Amoxyrite was used as a standard control. Results demonstrate that biosynthesized AgNPs’ antibacterial efficacy, chitosan nanoparticles, and AgNP-based chitosan nanocomposite increase in a dose-dependent manner. The high inhibition zone was observed in gram-positive organism Staphylococcus aureus and also in opportunistic pathogenic yeast C. albicans. Minimum inhibition zone was noted in gram-negative organism E. faecalis. The biosynthesized AgNPs, chitosan nanoparticle, and AgNPs-based chitosan nanocomposite show the potent antimicrobial effect, which has to be utilized as a biomedical application in the future for desirable effects. [31,32] also reported that gram-positive organisms show higher sensitivity due to cell wall differences in bacteria. The AgNPs and its decorated cellulose and zinc oxide nanoparticles are showing lower toxicity and used in many biomedical applications [33,34].
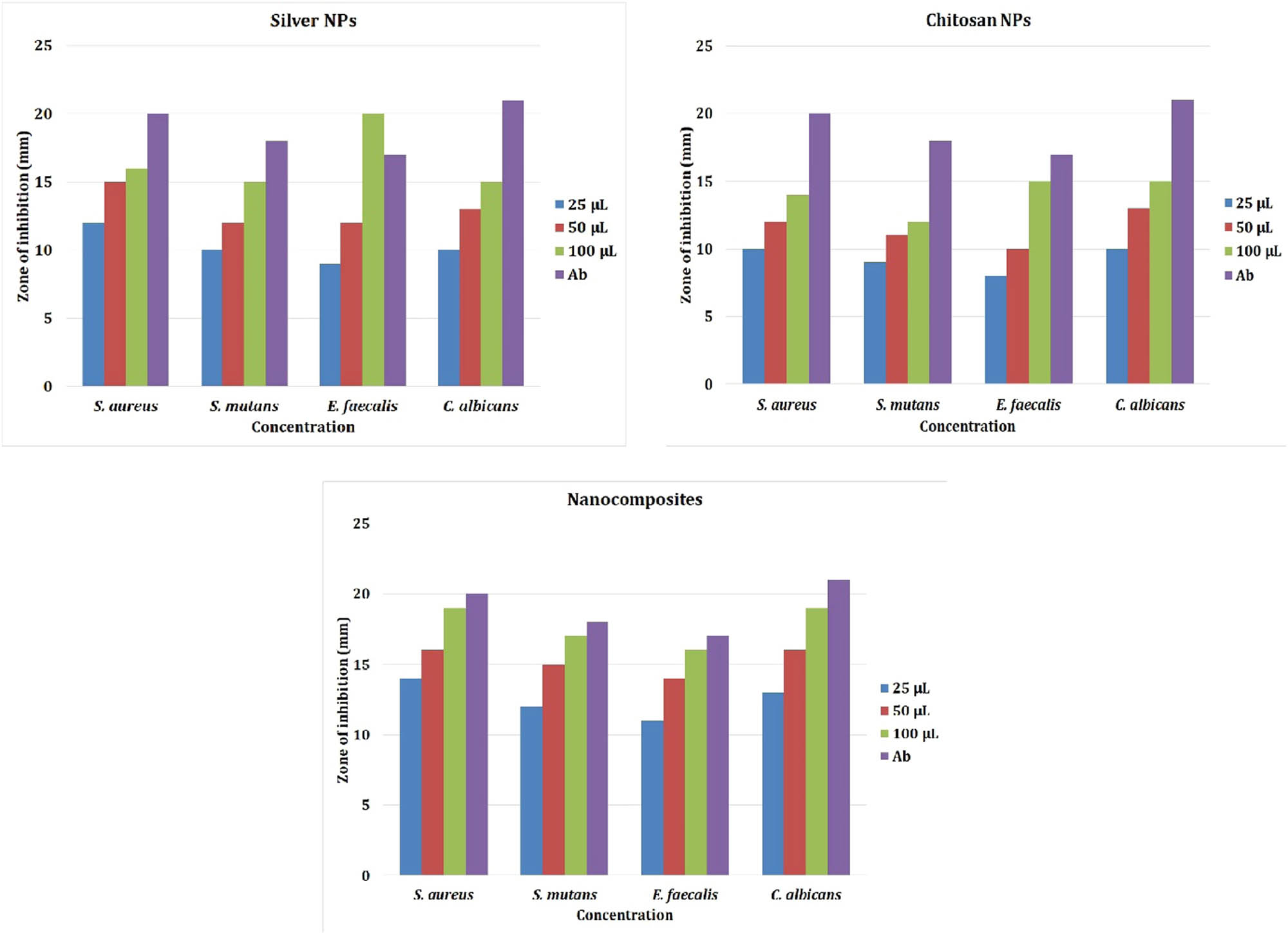
Antimicrobial activity of silver, chitosan nanoparticles, and nanocomposites.
4 Conclusion
In this research work, AgNPs and AgNP-based chitosan nanocomposite have been synthesized using Cissus arnottiana plant extract and the synthesized AgNPs and nanocomposite showed remarkable stability. The chitosan has been utilized as a potent stabilizing agent to combine with AgNPs to obtain Cissus arnottiana-mediated AgNPs-based chitosan nanocomposite. The results of AgNPs and nanocomposite were used as an effective antimicrobial material. The UV-Vis spectroscopy, SEM, and TEM analysis confirmed the existence of elemental silver in nanocomposite and its spherical form and size of about 30–40 nm. The synthesized AgNPs and AgNPs-based chitosan nanocomposite by Cissus arnottiana plant extract had been confirmed to show enhanced activity against oral pathogens. The present research is a cost-effective, eco-accomodating method for synthesizing silver nanocomposite. Therefore, the synthesized AgNPs and AgNPs-based chitosan nanocomposite can be utilized as an efficient antimicrobial material in future biomedical applications.
Acknowledgement
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/70), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: Researchers Supporting Project Number (RSP-2021/70), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Shanmugam Rajeshkumar: conceptualization, writing – original draft; Munusamy Tharani: formal analysis; Vijayarangan Devi Rajeswari: formal analysis; Naiyf S. Alharbi: resources, funding acquisition, validation; Shine Kadaikunnan: funding acquisition, formal analysis; Jamal M. Khaled: formal analysis; Kasi Gopinath: methodology, software; Natesan Vijayakumar: formal analysis; Marimuthu Govindarajan: writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Govindarajan M, Benelli G. A facile one-pot synthesis of eco-friendly nanoparticles using carissa carandas: ovicidal and larvicidal potential on malaria, dengue and filariasis mosquito vectors. J Cluster Sci. 2017;28(1):15–36.10.1007/s10876-016-1035-6Search in Google Scholar
[2] Goodsell DS. Bionanotechnology: lessons from nature. 2004;31–37.10.1002/0471469572Search in Google Scholar
[3] Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–20.10.1128/AEM.02218-06Search in Google Scholar PubMed PubMed Central
[4] Sap-Lam N, Homklinchan C, Larpudomlert R, Warisnoicharoen W, Sereemaspun A, Dubas ST. UV irradiation-induced silver nanoparticles as mosquito larvicides. J Appl Sci. 2010;10:3132–6.10.3923/jas.2010.3132.3136Search in Google Scholar
[5] Farooqui MA, Chauhan PS, Krishnamoorthy P, Shaik J. Extraction of silver nanoparticles from the leaf extracts of Clerodendrum inerme. Dig J Nanomater Biostructures. 2010;5:43–9.Search in Google Scholar
[6] Ip M, Lui SL, Poon VKM, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol. 2006;55:59–63.10.1099/jmm.0.46124-0Search in Google Scholar PubMed
[7] Wiley BJ, Im SH, Li ZY, McLellan J, Siekkinen A, Xia Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J Phys Chem B. 2006;110:15666–75.10.1021/jp0608628Search in Google Scholar PubMed
[8] Öztürk BY, Gürsu BY, Dağ İ. Antibiofilm and antimicrobial activities of green synthesized silver nanoparticles using marine red algae Gelidium corneum. Process Biochem. 2020;1(89):208–19.10.1016/j.procbio.2019.10.027Search in Google Scholar
[9] Dağlıoğlu Y, Öztürk BY. A novel intracellular synthesis of silver nanoparticles using Desmodesmus sp.(Scenedesmaceae): different methods of pigment change. Rend Lincei Sci Fis Nat. 2019;30(3):611–21.10.1007/s12210-019-00822-8Search in Google Scholar
[10] Susilowati E, Maryani A. Green synthesis of silver-chitosan nanocomposite and their application as antibacterial material. J Phys Conf Ser. 2019;1153:012135.10.1088/1742-6596/1153/1/012135Search in Google Scholar
[11] Thamilarasan V, Sethuraman V, Gopinath K, Balalakshmi C, Govindarajan M, Mothana RA, et al. Single step fabrication of chitosan nanocrystals using Penaeus semisulcatus: potential as new insecticides, antimicrobials and plant growth promoters. J Clust Sci. 2018;29:375–84.10.1007/s10876-018-1342-1Search in Google Scholar
[12] Shameli K, Ahmad MB, Zargar M, Yunus WM, Ibrahim NA, Shabanzadeh P, et al. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int J Nanomedicine. 2011;6:271–84.10.2147/IJN.S16043Search in Google Scholar PubMed PubMed Central
[13] Wei D, Sun W, Qian W, Ye Y, Ma X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr Res. 2009;344:2375–82.10.1016/j.carres.2009.09.001Search in Google Scholar PubMed
[14] Alharbi NS, Govindarajan M, Kadaikunnan S, Khaled JM, Almanaa TN, Alyahya SA, et al. Nanosilver crystals capped with Bauhinia acuminata phytochemicals as new antimicrobials and mosquito larvicides. J Trace Elem Med Biol. 2018;50:146–53.10.1016/j.jtemb.2018.06.016Search in Google Scholar PubMed
[15] Gopinath K, Devi NP, Govindarajan M, Bhakyaraj K, Kumaraguru S, Arumugam A, et al. One-Pot green synthesis of silver nanoparticles using the orchid leaf extracts of Anoectochilus elatus: growth inhibition activity on seven microbial pathogens. J Clust Sci. 2017;28:1541–50.10.1007/s10876-017-1164-6Search in Google Scholar
[16] Sama K, Sivaraj R. Pharmacognostical and phytochemical screening of fruit and leaves of Cissus arnottiana. Asian J Pharmaceautical Clin Res. 2012;5:64–6.Search in Google Scholar
[17] El-Aassar MR, Ibrahim OM, Fouda MM, Fakhry H, Ajarem J, Maodaa SN, et al. Wound dressing of chitosan-based-crosslinked gelatin/polyvinyl pyrrolidone embedded silver nanoparticles, for targeting multidrug resistance microbes. Carbohydr Polym. 2021;255:117484.10.1016/j.carbpol.2020.117484Search in Google Scholar PubMed
[18] El-Aassar MR, Ibrahim OM, Fouda MM, El-Beheri NG, Agwa MM. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: in-vitro and in-vivo studies. Carbohydr polym. 2020;238:116175.10.1016/j.carbpol.2020.116175Search in Google Scholar PubMed
[19] Hussein J, El-Naggar ME, Fouda MM, Morsy OM, Ajarem JS, Almalki AM, et al. The efficiency of blackberry loaded AgNPs, AuNPs and Ag@ AuNPs mediated pectin in the treatment of cisplatin-induced cardiotoxicity in experimental rats. Int J Biol Macromol. 2020;159:1084–93.10.1016/j.ijbiomac.2020.05.115Search in Google Scholar PubMed
[20] Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(1):1.10.1186/2228-5326-2-32Search in Google Scholar
[21] Roy N, Gaur A, Jain A, Bhattacharya S, Rani V. Green synthesis of silver nanoparticles: an approach to overcome toxicity. Environ Toxicol Pharmacol. 2013;36(3):807–12.10.1016/j.etap.2013.07.005Search in Google Scholar PubMed
[22] Rana A, Yadav K, Jagadevan S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: mechanism, application and toxicity. J Clean Prod. 2020;14:122880.10.1016/j.jclepro.2020.122880Search in Google Scholar
[23] Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502.10.1016/j.jcis.2004.03.003Search in Google Scholar PubMed
[24] Palem RR, Ganesh SD, Kronekova Z, Slávikov M, Saha N, Saha P. Green synthesis of silver nanoparticles and biopolymer nanocomposites: a comparative study on physico-chemical, antimicrobial and anticancer activity. Bull Mater Sci. 2018;4(12):1–11.10.1007/s12034-018-1567-5Search in Google Scholar
[25] Seyforth JA. Scanning electron microscopy (SEM): an introduction to the use of SEM for characterising the surface topology and composition of matter with further applications. Exp Tech Condens Matter Phys. 2015;6.Search in Google Scholar
[26] Khanmohammadi M, Elmizadeh H, Ghasemi K. Investigation of Size and morphology of chitosan nanoparticles used in drug delivery system employing chemometric technique. Iran J Pharm Res. 2015;14:665.Search in Google Scholar
[27] Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6(3):399–408.10.1007/s13204-015-0449-zSearch in Google Scholar
[28] Govindan S, Nivethaa EAK, Saravanan R, Narayanan V, Stephen A. Synthesis and characterization of chitosan–silver nanocomposite. Appl Nanosci. 2012;2(3):299–303.10.1007/s13204-012-0109-5Search in Google Scholar
[29] Kalaivani R, Maruthupandy M, Muneeswaran T, Hameedha Beevi A, Anand M, Ramakritinan CM, et al. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front Lab Med. 2018;2:30–5.10.1016/j.flm.2018.04.002Search in Google Scholar
[30] Ghadi A, Mahjoub S, Tabandeh F, Talebnia F. Synthesis and optimization of chitosan nanoparticles: potential applications in nanomedicine and biomedical engineering. Casp J Intern Med. 2014;5:156.Search in Google Scholar
[31] Mohsen E, El-Borady OM, Mohamed MB, Fahim IS. Synthesis and characterization of ciprofloxacin loaded silver nanoparticles and investigation of their antibacterial effect. Res Appl Sci. 2020;13:416–25.10.1080/16878507.2020.1748941Search in Google Scholar
[32] Balalakshmi C, Alharbi NS, Kadaikunnan S, Khaled JM, Alanzi KF, Gopinath K, et al. Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application. Green Process Synth. 2020;9(1):751–9.10.1515/gps-2020-0070Search in Google Scholar
[33] Abdelsalam NR, Fouda MM, Abdel-Megeed A, Ajarem J, Allam AA, El-Naggar ME. Assessment of silver nanoparticles decorated starch and commercial zinc nanoparticles with respect to their genotoxicity on onion. Int J Biol Macromol. 2019;133:1008–18.10.1016/j.ijbiomac.2019.04.134Search in Google Scholar PubMed
[34] Fouda MM, Abdelsalam NR, Gohar IM, Hanfy AE, Othman SI, Zaitoun AF, et al. Utilization of High throughput microcrystalline cellulose decorated silver nanoparticles as an eco-nematicide on root-knot nematodes. Colloids Surf B. 2020;188:110805.10.1016/j.colsurfb.2020.110805Search in Google Scholar PubMed
© 2021 Shanmugam Rajeshkumar et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

