Abstract
The removal of organosulfurs from liquid fuel has become a hot issue due to the serious environmental pollution by sulfur oxide gases. In this study, the removal of thiophene from model oil was carried out using polyethylene glycol (PEG). It was found that deep eutectic solvents formed by PEG as hydrogen bond donor and thiophene as hydrogen bond acceptor could efficiently separate thiophene from model oil. The influencing parameters in this process were discussed, such as extraction time, temperature, mass ratio of PEG to oil, and initial sulfur concentration. The results showed that the single extraction efficiency of PEG-200 and PEG-400 could reach up to 89.0% and 97.1% in optimal conditions, respectively. The extraction efficiency as high as 95.7% and 99.9% could be achieved after three extraction cycles. The kinetic equation of the extractive desulfurization was studied by in situ infrared (IR), and the kinetic constant k 1 of PEG-400 and PEG-200 was compared (k 1(PEG-400) > k 1(PEG-200)). The desulfurization mechanism of PEG was studied by IR, 1H NMR spectra, and density functional theory (DFT). The results showed that the hydrogen bond formed between hydroxyl hydrogen in PEG and sulfur atom in thiophene accounted for the high extraction efficiency.
1 Introduction
SOx emissions from the combustion of sulfur-containing liquid fuel are an important cause of environmental problems such as acid rain and smog [1]. In order to eliminate pollution from the source, stringent legislation has been implemented all over the world to regulate the sulfur content of transportation fuel [2,3]. Therefore, the study on the removal of organosulfurs from liquid fuel has important application value and theoretical significance.
At present, hydrogenation desulfurization (HDS) is the most commonly used technology for the removal of organosulfurs from liquid fuel. HDS can effectively remove aliphatic sulfides such as mercaptans, thioethers, and tetrahydrothiophene [4,5], but aromatic sulfides such as thiophene, benzothiophene, and dibenzothiophene (DBT) are especially difficult to eliminate by this process [6]. In addition, HDS requires extreme conditions and the operating costs are also high. Therefore, various new desulfurization technologies have been investigated comprising adsorption desulfurization [7,8], biological desulfurization [9,10], oxidative desulfurization [11,12], and extractive desulfurization [13,14,15]. Among them, extractive desulfurization has the advantages of mild operating conditions, relatively simple processes, and low energy consumption. So extractive desulfurization is considered to be one of the most promising desulfurization technologies. The most important thing for extractive desulfurization is the choice of extractant. In previous studies, various traditional extractants such as dimethyl formamide, acetonitrile, dimethyl sulfoxide, pyrrolidone, and other volatile organic solvents had been used in the extractive desulfurization [16,17]. However, the organic solvents pose challenges such as volatile, highly toxic, difficult to regenerate, and the extraction efficiency is not significant [18]. Therefore, it has always been a research hotpot to find a green, environmentally friendly, low-cost, and high efficiency extractant.
In recent years, green solvents-ionic liquids (ILs) and deep eutectic solvents (DESs) as new non-volatile solvents have been widely investigated as alternative solvents in organic synthesis [19,20], chemical separation [21,22], material preparation [23,24], etc. They also have attracted much attention in desulfurization. ILs, especially functionalized ILs, can be effectively applied in desulfurization and overcome the drawbacks of conventional organic solvents [25,26]. However, ILs also have certain limits, for instance, the poor extraction efficiencies and some of the ILs are hazardous and toxic. Thus, DESs have been gaining more attention due to their versatility, simple and low cost, easy availability, and simple synthesis process [27]. DESs are eutectics formed by two or three kinds of green, inexpensive components combined with each other through hydrogen bonds. Quaternary ammonium salts are mainly selected as hydrogen bond acceptors (HBAs), and carboxylic acids, alcohols, and amides are usually used as hydrogen bond donors (HBDs) [28,29]. At present, a series of different DESs have been used to extract aromatic sulfides from liquid fuel. They have achieved ideal desulfurization performance and realized the “green” desulfurization [30,31,32,33,34,35,36]. It is generally believed that the hydrogen bond between DESs and aromatic sulfides is the main driving force for the desulfurization process [34,35,36]. The mechanism of HBA and HBD formed DES and the desulfurization mechanism of DES are all based on the hydrogen bond. The aromatic sulfide has an electron-rich aromatic ring, which means that the aromatic sulfide has the condition to act as HBA in DES. The single-component green, cheap solvent as HBD can be interacted with aromatic sulfide via forming DES. Therefore, in preliminary study, single-component solvents were selected for the extractive desulfurization. The study found that the single extraction efficiency of PEG-400 could reach up to 99.99% for DBT in optimal conditions [37]. PEG has several advantages, such as non-toxic, odorless, nonvolatile, and non-irritating, which can meet the requirements of green desulfurization [38].
At present, our research on PEG desulfurization is not detailed enough. In continuation with our previous works [37], this work investigated the application of PEG-200 and PEG-400 in the extractive desulfurization of model oil that contains thiophene as model sulfur compound. The aim of the work is to lower the sulfur content of liquid fuel to below the environmental regulation using PEG in a process with low energy requirement. The effects of the extraction time, temperature, mass ratio of PEG to oil, and initial sulfur concentration on the extraction efficiency were investigated. The kinetics and mechanism of the desulfurization process were discussed in detail.
2 Experimental methods
2.1 Extractive desulfurization process
Some important parameters that affect the extraction efficiency were investigated in detail, such as extraction time, temperature, mass ratio of PEG to oil, initial sulfur concentration, etc., to get the optimal conditions. According to the methods of literature [37], the model oil was prepared by dissolving thiophene in n-octane, and the initial sulfur concentration was 1,600 mg·L−1. Extractive desulfurization process was carried out in self-made equipment, and the equipment was immersed in a water bath. First, PEG and model oil were placed in a home-made 25 mL pear-shaped flask. The mass ratio of PEG to model oil was 1:1 except for otherwise defined and then they were stirred magnetically at 800 rpm at a certain temperature in a water bath. After stirring for a definite time, the two phases settled down for 30 min. Finally, the upper oil phase was taken and analyzed by gas chromatography.
The sulfur concentration of model oil was measured by Agilent 7890A gas chromatography using an area unitary method with a flame ionization detector. The GC conditions are listed below:
chromatogram column: HP-5
injection volume: 0.6 µL
carrier gas (N2): 210 mL·min−1
H2: 35 mL·min−1
air: 350 mL·min−1
flux: 1.6 mL·min−1, constant flow mode
inlet temperature: 250°C
detector temperature: 250°C
column temperature: heating from 60°C to 270°C with 5°C·min−1 increase.
The sulfur concentration was measured by internal standard method, using n-hexadecane as the internal standard solvent. The analysis of standard curve was shown in Figure A1 (in Appendix). The correlation coefficient achieved was 0.99993. The extraction efficiency (E%) was obtained using below Eq. 1:
where C i is the initial sulfur concentration in the model oil, and C f is the final sulfur concentration in model oil after extraction. All the experiments in this study were performed in duplicate to determine reproducibility, and the parallel errors were within 3%.
2.2 Kinetic experiment
The desulfurization rate of PEG was analyzed by kinetic equation. The kinetic research was carried out on METTLER TOLEDO React-IR15 in situ infrared instrument using self-made device (Figure 1). 6.0 g PEG-200/PEG-400 was added to the ① part and 8.0 g model oil was added to the ② part of the self-made device ensuring that the mass ratio of extractant to oil was 1:1 during the experiment. The probe was placed in the upper layer to monitor the stretching vibration of S–C at 719 cm−1 in real time. Thus, the reduction of sulfur concentration in the model oil was got and the kinetic equation of PEG desulfurization was obtained by fitting the experimental data.
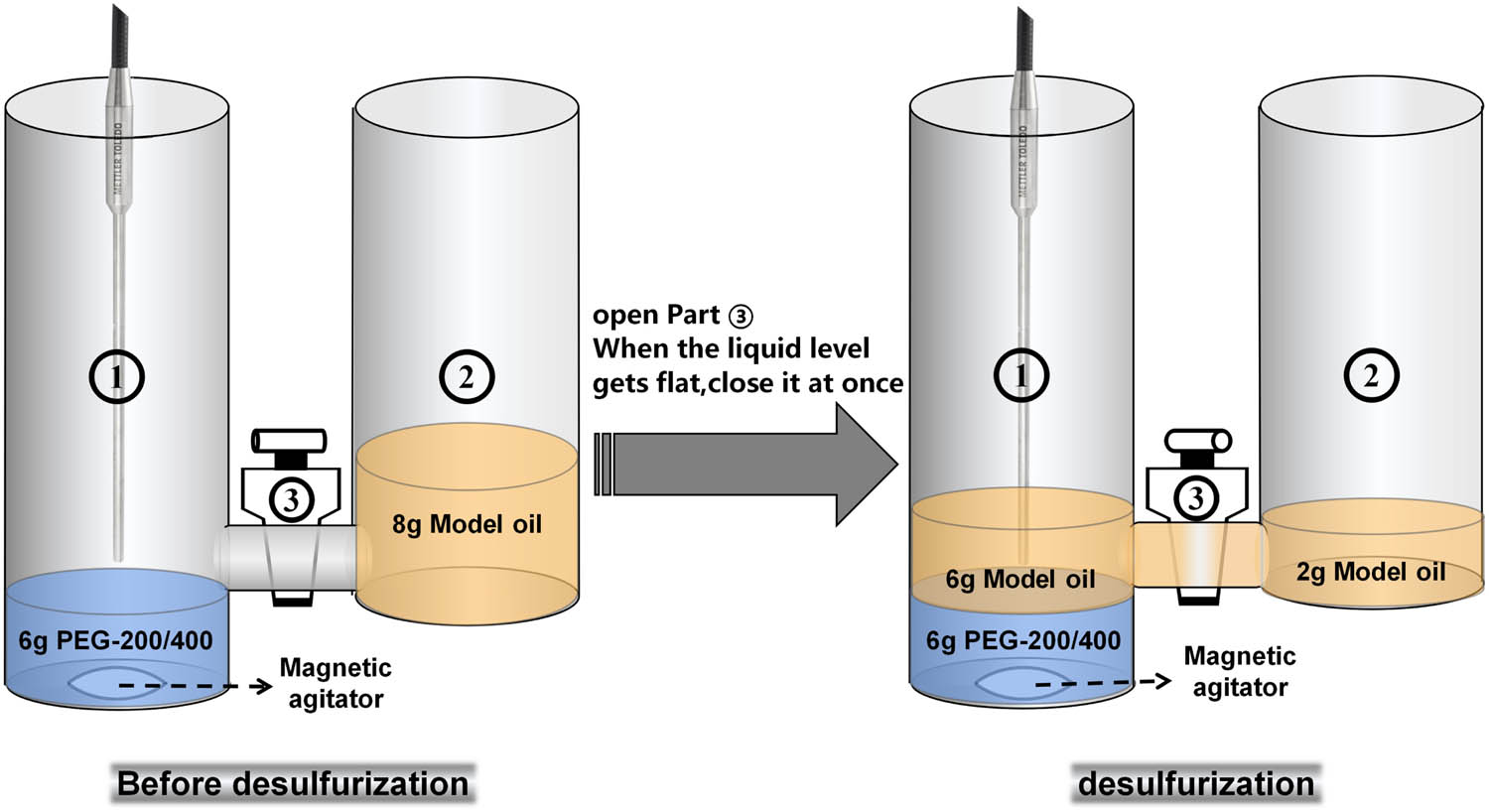
Schematic diagram of dynamic experimental device.
2.3 Mechanism study
Our previous research showed that hydrogen bond played a vital role in the extractive desulfurization process [37]. In order to further explore the hydrogen bond site, the extraction mechanism was investigated using infrared (IR), 1H NMR spectra, and density functional theory (DFT).
1H NMR spectrum were obtained using Ascend 500 spectrometer (Bruker). The geometries, vibrational frequencies, and 1H NMR chemical shifts of the simplified model of PEG and thiophene were calculated by DFT method using the Gaussian 09 program package. The B3LYP functional as a typical hybrid functional of DFT was used with a basis set of 6-31+G*.
3 Results and discussion
3.1 Optimization of the extraction parameters
In order to determine the time needed to reach maximum extraction efficiency, the experiments were carried out at different periods of time, ranging from 5 to 40 min. As shown in Figure 2, the extraction efficiency of PEG increased with the increase in time, the extraction efficiency of PEG-400 increased from 82.2% at 5 min to 97.1% at 30 min. The forming of hydrogen bond between PEG and thiophene required a short time. However, 30 min was chosen as optical time throughout the investigation to achieve an abundant equilibrium. Meanwhile, it can be seen that the extraction efficiency of PEG-400 was higher than that of PEG-200 at the same time. PEG with smaller molar mass implied lower extraction efficiency maybe due to the intermolecular hydrogen bond. The smaller the PEG molar mass was, the shorter the PEG chain was, the hydroxyl groups were easy to get close to each other, resulting in an intermolecular hydrogen bond, thus blocking hydrogen bond with thiophene.
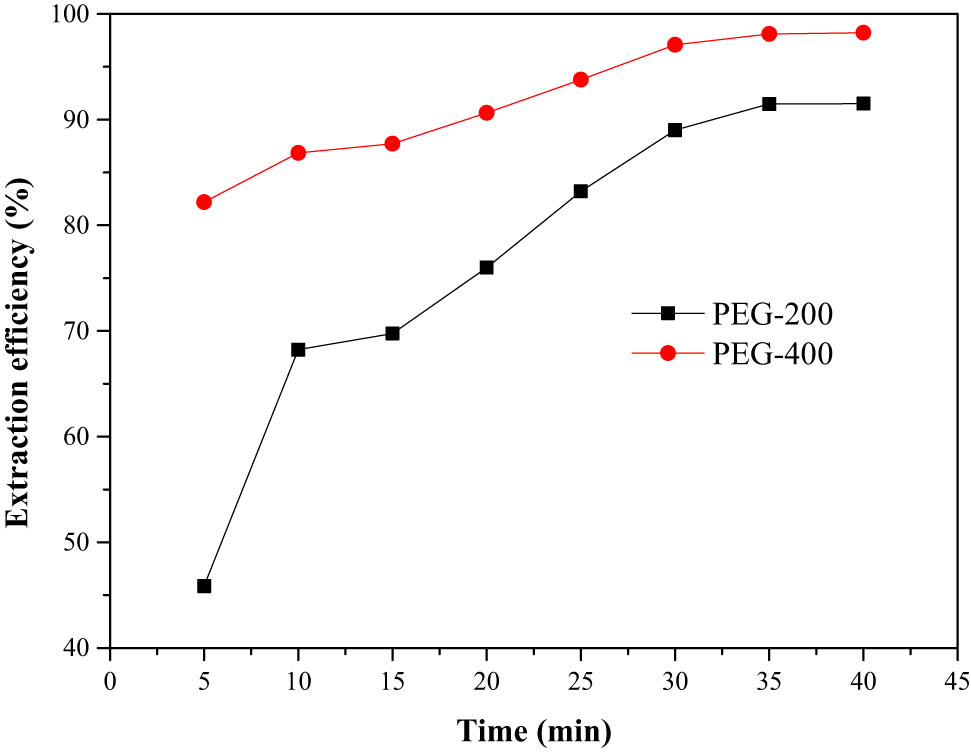
Effect of extraction time on extraction efficiency.
From a cost perspective, temperature was another important factor in the desulfurization process. The effects of temperature on the extraction efficiency had been investigated from 20°C to 60°C (Figure 3). It can be seen that the extraction efficiencies for both PEG-200 and PEG-400 remained unchanged with temperature. A possible explanation for this phenomenon was that temperature had little influence on the formation of DES by PEG and thiophene. The hydrogen bond formed between PEG and thiophene was not affected by temperature. Therefore, a relatively low temperature was suitable for the extractive process with PEG, which was also one of the advantages in the extractive desulfurization. Considering energy consumption, room temperature (25°C) was chosen as operation temperature in the following experiment. Room temperature was beneficial for the practical application.
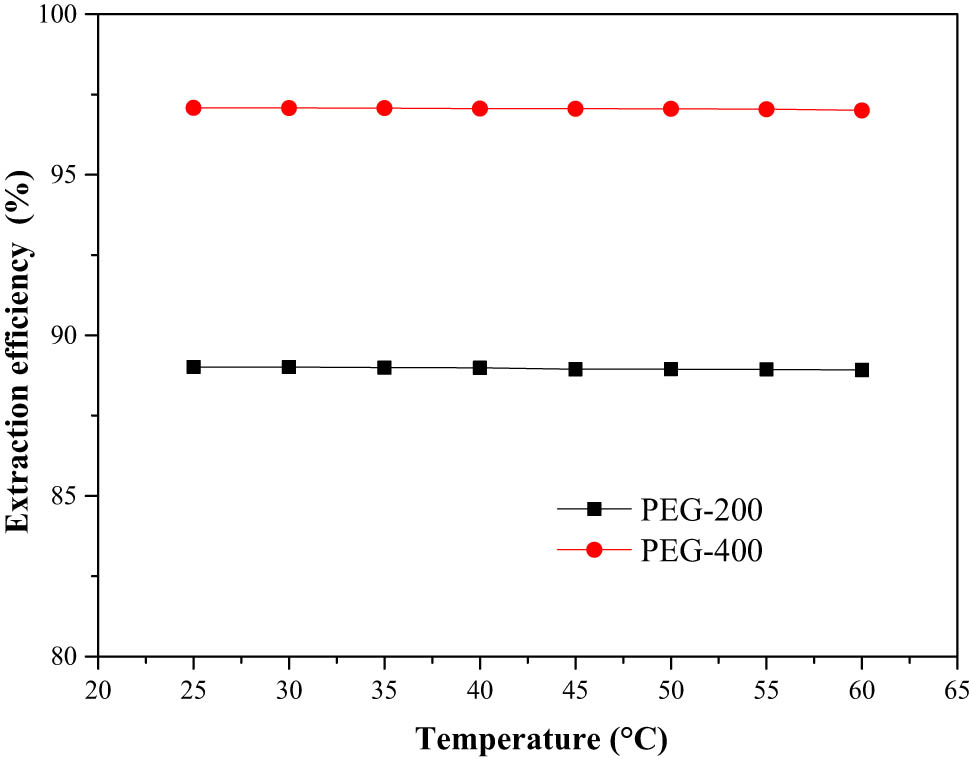
Effect of system temperature on extraction efficiency.
In the extraction process, the amount of extractant had an apparent influence on the extraction efficiency, which also related to the economic cost of the application. Hence, the effect of mass ratio of PEG to model oil on extraction efficiency was investigated (Figure 4). The increase in the amount of PEG was a direct method to increase the extraction efficiency. When the mass ratio of PEG-200/PEG-400 to oil was 3:1, the extraction efficiencies of 98.0% and 99.1% could be achieved, respectively. However, the increase in PEG did not result in the increase in the extraction efficiency sharply. The probable reason was that the content of PEG increased, but the amount of thiophene in model oil was tiny, and hence the contact probability of PEG and thiophene was decreased. Considering the extraction efficiency and energy consumption comprehensively, the mass ratio of PEG to oil was chosen as 1:1 in the following experiment process.
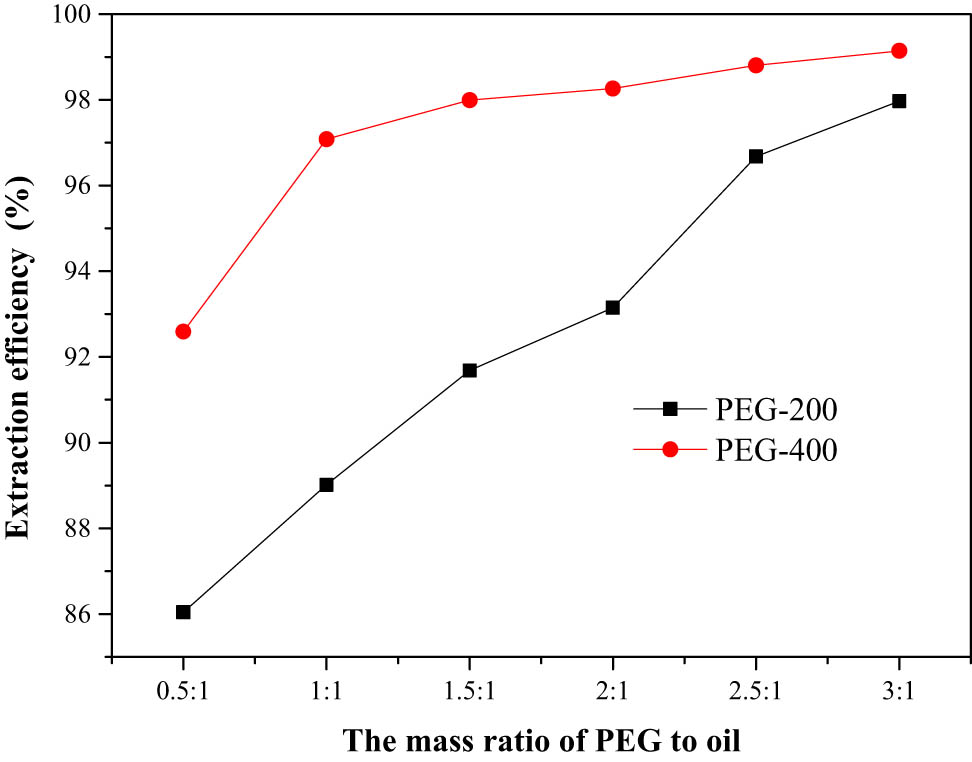
Effect of mass ratio of PEG to oil on extraction efficiency.
In the industrial production process, sulfur-containing liquid fuel with different sulfur concentrations will be processed. Therefore, it was of great significance to study the effect of initial sulfur concentration on the extraction efficiency. The effect of the initial sulfur concentration on the extraction efficiency was shown in Figure 5. The extraction efficiency increased with the increase in the initial sulfur concentration. The extraction efficiency of PEG-400 was only 38.6%, when initial sulfur concentration was 200 mg·L−1. However, when the initial sulfur concentration was 1,600 mg·L−1, extraction efficiency of 97.1% could be achieved. The concentration of thiophene in model oil was high, PEG was more likely to combine with it to form DES. From these observations, it can be summarized that PEG was more suitable for liquid fuel with higher thiophene concentration.
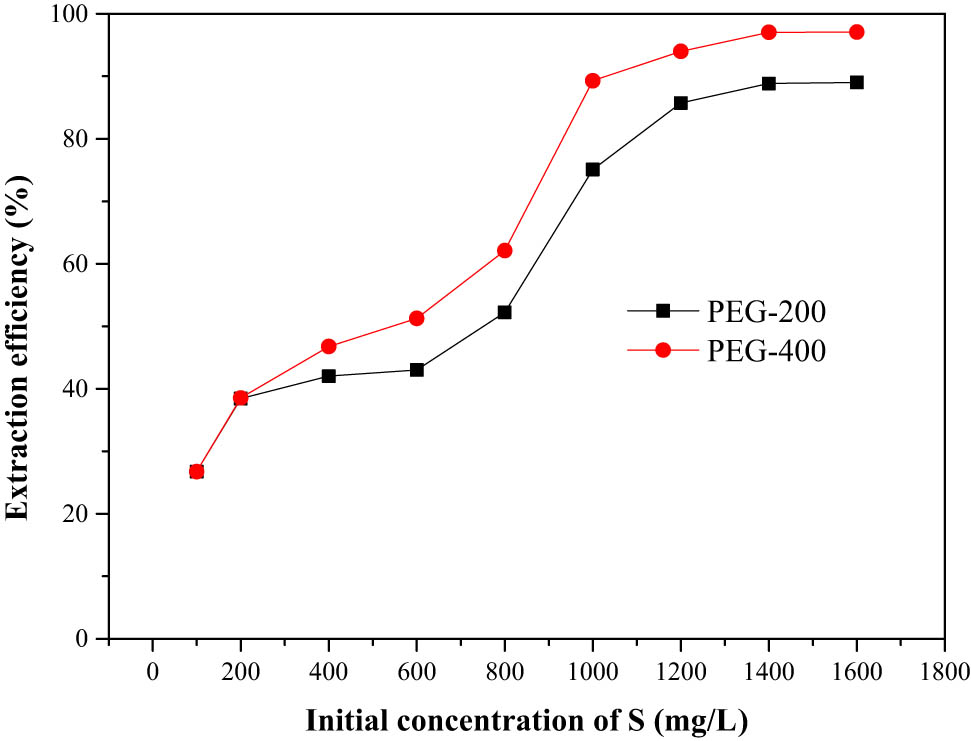
Effect of initial sulfur concentration on extraction efficiency.
3.2 Multiple time extraction
Considering the cost factor of subsequent industrial production, the multiple time removal of thiophene by PEG were also investigated in spite of the relatively high extraction efficiency of PEG, which was displayed in Figure 6. The extraction efficiency of the third cycle by PEG-400 could be up to 99.9%, while the extraction efficiency of PEG-200 was 99.9% after the fifth cycle, which meant that deep desulfurization could be realized successfully after the third or fifth extraction cycle. Through multiple extraction methods, the deep desulfurization was achieved. While this specific process was more suitable for the preparation of low-sulfur liquid fuel, the extractant was not necessary to be recycled due to the limitation of operation space. The inexpensive PEG could be used for the removal of aromatic sulfur compounds from liquid fuel to below environmental set limits in mild operating conditions that could lead to huge savings in energy requirement.
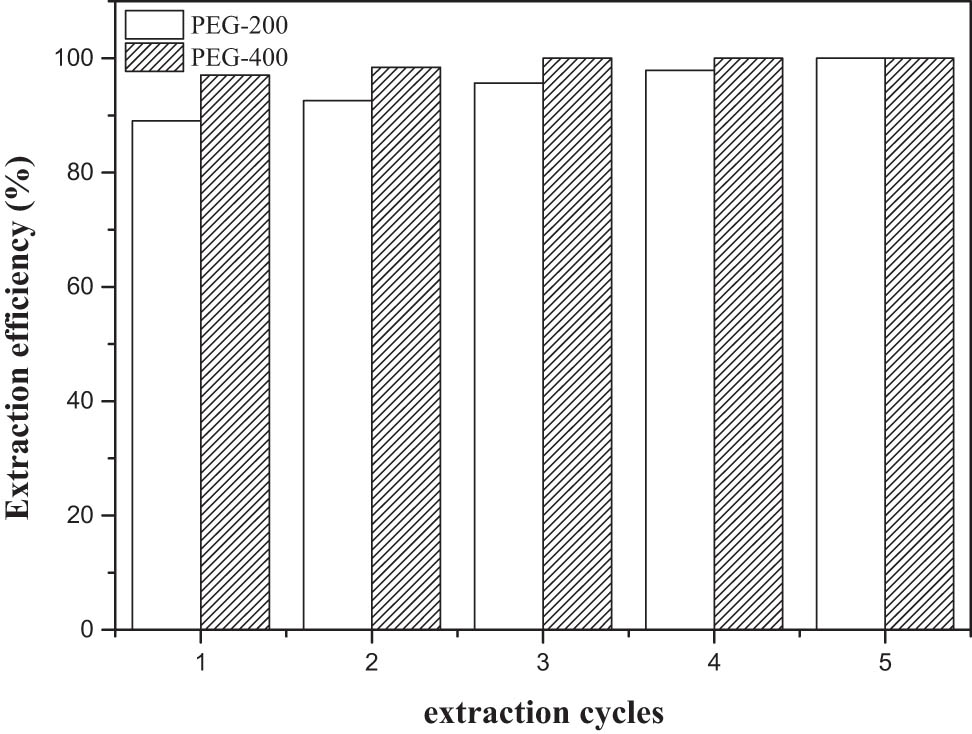
Effect of extraction cycles on extraction efficiency.
3.3 Kinetic analysis
Reaction kinetics is the study of the rate and mechanism of chemical reactions. It is science and strictness with the combination of theoretical method and experimental method. Therefore, the reaction kinetics was studied using in situ IR spectroscopy. Due to the influence of the size of the in situ IR probe, the experiment was performed with a magnification of six times. In addition, the magnetic stirring speed was also reduced to 500 rpm in order to protect the probe and maintain a stable measurement liquid level. The extraction efficiency of PEG-200 was shown in Figure 7. The curve had a high slope from 0 min to 25 min, the extraction efficiency of 59.9% was obtained in 25 min. The slope of the curve decreased, and the desulfurization rate dropped when the time was longer than 25 min, the extraction efficiency slowly increased to 70.5% at 40 min. This trend of change was consistent with the previous experimental rule (Figure 2).

The extraction efficiency of thiophene with PEG-200 in the in situ infrared experiment.
According to the online monitoring results, the first-order kinetic equation (Eq. 2) and the second-order kinetic equation (Eq. 3) were used to fit the curves:
where c 0 is the initial sulfur concentration (mg·L−1), c t is the residual sulfur concentration (mg·L−1), k 1 is the kinetic constant for the first-order reaction (min−1), k 2 is the kinetic constant for the second-order reaction (min·L·mg−1), and t is the desulfurization time (min).
The fitting of the experimental data showed that the kinetic constant at the value of k 1 = 0.0404 min−1 and k 2 = 4.050 × 10−5 min·L·mg−1, respectively. The first-order kinetics fitting curve for removal of thiophene with PEG-200 was shown in Figure 8. The correlation coefficient R 2 of the first-order kinetics was 0.9940, which was significantly higher than that of the second-order kinetics (0.9851), indicating that the desulfurization of PEG-200 was closer to the first-order kinetics.
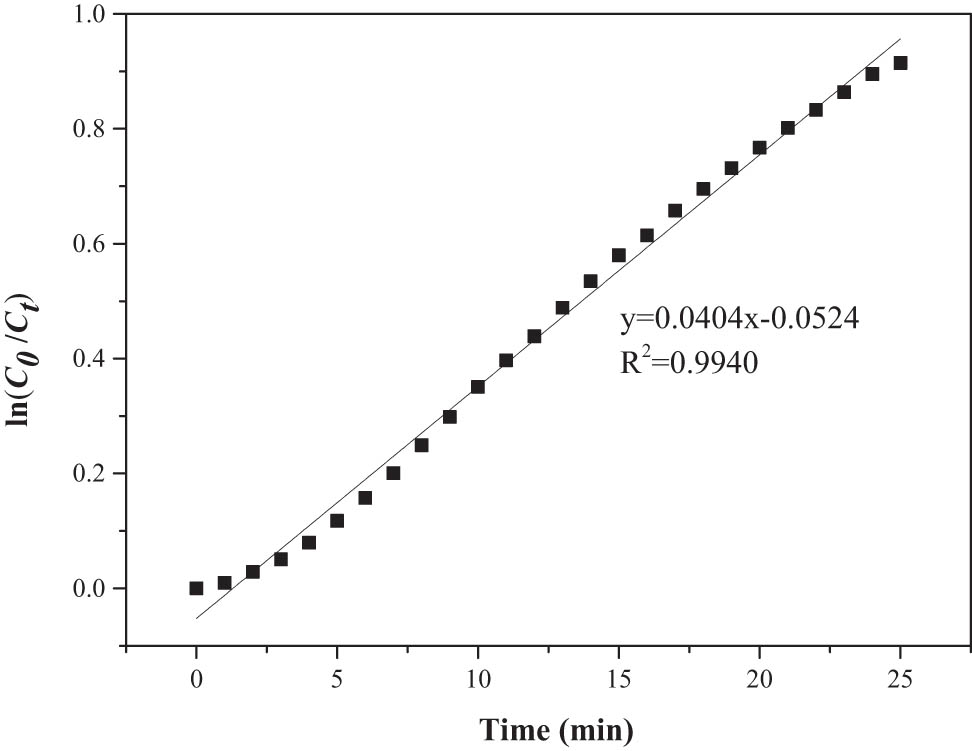
First-order kinetics fitting curve for removal of thiophene with PEG-200.
The extraction efficiency of PEG-400 obtained from the online experiment was shown in Figure A2. According to the data in Figure A2, the first-order kinetic equation of PEG-400 was also obtained, as shown in Figure 9. The kinetic constant k 1 was 0.0416 min−1, and the correlation coefficient R 2 was 0.9720, which indicated a satisfactory linear relationship. It can be concluded that the desulfurization rate of PEG-400 was greater than PEG-200 by comparing the kinetic constant k 1. The theory was in agreement with the experiment. Therefore, the desulfurization of PEG followed first-order kinetics.
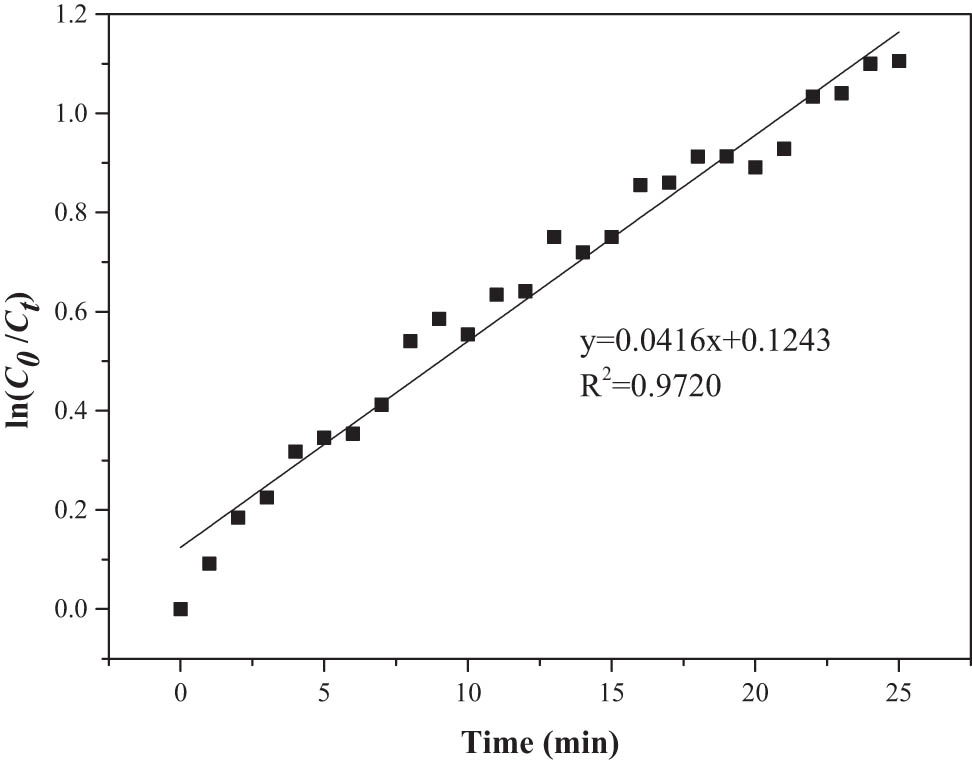
First-order kinetics fitting curve for removal of thiophene with PEG-400.
3.4 Extraction mechanisms
The IR spectrum and 1H NMR chemical shifts of thiophene were listed in Table 1. The computing model was simplified by using diethylene glycol ether instead of PEG to optimize the interaction between thiophene and PEG (Figure 10). The calculation results showed that the sulfur atom in thiophene formed a hydrogen bond with the hydrogen in hydroxyl group. The length of the hydrogen bond is 2.71 Å and the angle of ∠SHO is 175.11°. It has almost a linear hydrogen bond indicating that a strong hydrogen bond is formed.
Observed and calculated IR and 1H NMR spectra
| Assignments | Entry | Experiment values | Calculated values |
|---|---|---|---|
| ν(C–S) | T | 719 cm−1 | 724 cm−1 |
| T-PEG | 731 cm−1 | 728 cm−1 | |
| 1H NMR | T | α-H 7.57 β-H 7.16 | α-H 7.17 β-H 7.14 |
| T-PEG | α-H 7.53 β-H 7.15 | α-H 7.04 7.05 β-H 7.14 7.15 |

Optimized structure of diethylene glycol ether and thiophene.
A comparison of the calculated vibrational frequencies and the 1H NMR shifts of thiophene in simplified model with the observed in situ infrared and 1H NMR spectra was also made. It can be seen from Table 1 that the ν(C–S) vibration of pure thiophene observed at 719 cm−1 shifted to 731 cm−1 after PEG was added. When sulfur atom interacted with the hydroxyl hydrogen, a consistent change in IR spectrum (from 724 to 728 cm−1) was obtained. 1H NMR was also investigated in order to prove the hydrogen bond interaction. The electron density of the thiophene ring was reduced when DES was formed with PEG, thereby reducing the chemical shift of H. In addition, being affected by induction and conjugation effects, the chemical shift change in α-H was greater than that of β-H. The same results were obtained by theoretical calculation and experiment. Only one hydrogen bond of a pair of molecules was simulated, and the reduction in α-H chemical shift was more obvious than that of the real system. The calculation of a more complex system is underway.
4 Conclusion
PEG-200 and PEG-400 were used to extract thiophene from a model oil of thiophene and n-octane. The results showed that the extraction efficiencies of 89.0% and 97.1% were, respectively, achieved with PEG-200 and PEG-400 as extractant in a single run extraction. Further investigations revealed that PEG-400 showed 99.9% extraction efficiency when three extraction cycles were used, PEG-200 exhibited extraction efficiency of up to 99.9% in five extraction cycles. Moreover, the removal of thiophene by PEG followed first-order kinetics, the kinetic constant k 1 of PEG-400 was greater than that of PEG-200, which was consistent with the actual experimental results. Finally, the extraction mechanism was investigated systematically by IR, 1H NMR spectra, and density functional theory (DFT). The results suggested that the hydrogen bond formed between hydroxyl hydrogen in PEG and sulfur atom in thiophene accounted for the higher extraction efficiencies.
-
Funding information: This work was supported by the National Natural Science Foundation of China (52070042) and PhD Research Startup Foundation of Dalian University (No. 2021QL12).
-
Author contributions: Yingna Cui: writing – original draft, writing – review and editing, methodology, and formal analysis; Wenqing Xu: methodology and formal analysis; Yingping Jia: data curation and formal analysis; Shenmin Li: writing – review and editing; Jingmei Yin: resources and formal analysis.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
-
Supplementary data: Supplementary data is available in Appendix: analysis of standard curve and the extraction efficiency of PEG-400 obtained from the online experiment.
Appendix
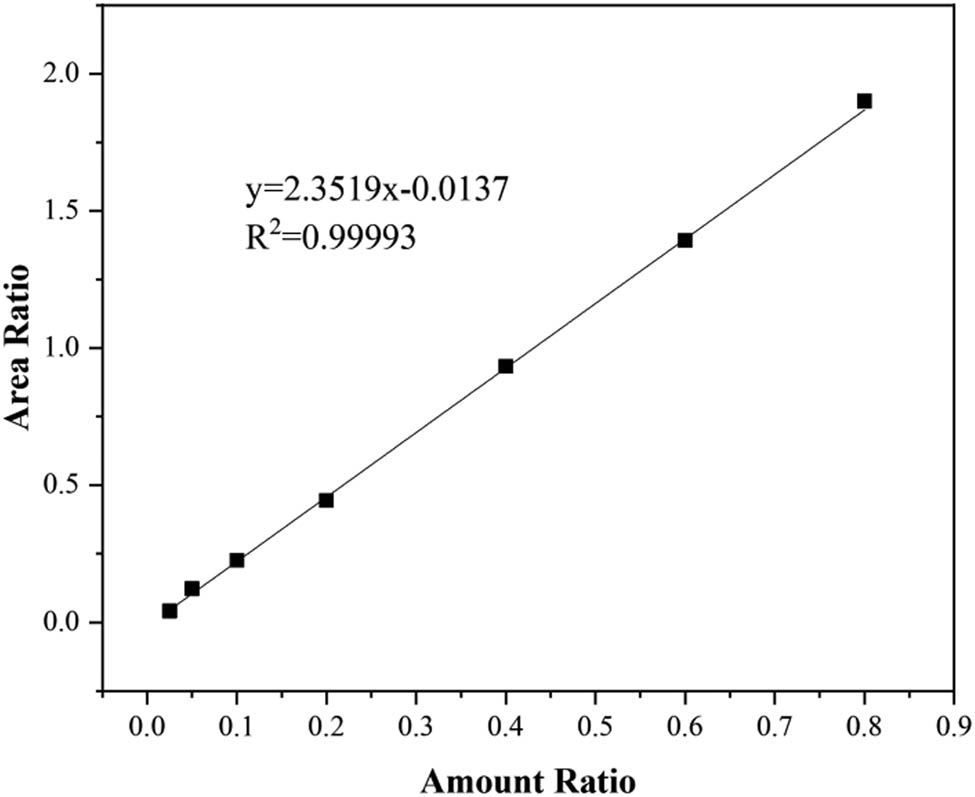
Analysis of standard curve.

The extraction efficiency of thiophene with PEG-400 in the in situ infrared experiment.
References
[1] Kulkarni PS, Afonso CAM. Deep desulfurization of diesel fuel using ionic liquids: current status and future challenges. Green Chem. 2010;12:1139–49.10.1039/c002113jSearch in Google Scholar
[2] Dharaskar SA, Wasewar KL, Varma MN, Shende DZ, Tadi KK, Yoo CK. Synthesis, characterization, and application of novel trihexyl tetradecyl phosphonium bis (2,4,4-trimethylpentyl) phosphinate for extractive desulfurization of liquid fuel. Fuel Process Technol. 2014;123:1–10.10.1016/j.fuproc.2014.02.001Search in Google Scholar
[3] Mao CF, Zhao RX, Li XP, Gao XH. Trifluoromethanesulfonic acid-based DESs as extractants and catalysts for removal of DBT from model oil. RSC Adv. 2017;7:12805–11.10.1039/C6RA28448ESearch in Google Scholar
[4] Rychlewska K, Konieczny K, Bodzek M. Pervaporative desulfurization of gasoline-separation of thiophene/n-heptane mixture/perwaporacyjne odsiarczanie benzyny-separacja mieszanin tiofen/n-heptan. Arch Environ Prot. 2015;41:3–11.10.1515/aep-2015-0013Search in Google Scholar
[5] Srivastava VC. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012;2:759–83.10.1039/C1RA00309GSearch in Google Scholar
[6] Javadli R, de Klerk A. Desulfurization of heavy oil. Appl Petrochem Res. 2012;1:3–19.10.1007/s13203-012-0006-6Search in Google Scholar
[7] Kwon JM, Moon JH, Bae YS, Lee DG, Sohn HC, Lee CH. Adsorptive desulfurization and denitrogenation of refinery fuels using mesoporous silica adsorbents. ChemSusChem. 2008;1:307–9.10.1002/cssc.200700011Search in Google Scholar PubMed
[8] Dehghan R, Anbia M. Zeolites for adsorptive desulfurization from fuels: a review. Fuel Process Technol. 2017;167:99–116.10.1016/j.fuproc.2017.06.015Search in Google Scholar
[9] Bhatia S, Sharma DK. Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3. Biochem Eng J. 2010;50:104–9.10.1016/j.bej.2010.04.001Search in Google Scholar
[10] Alves L, Paixão SM. Enhancement of dibenzothiophene desulfurization by Gordonia alkanivorans 642 strain 1B using sugar beet molasses as alternative carbon source. Appl biochem biotech. 2014;172:3297–305.10.1007/s12010-014-0763-zSearch in Google Scholar PubMed
[11] Wang JY, Zhang LH, Sun YL, Jiang B, Chen Y, Gao X, et al. Deep catalytic oxidative desulfurization of fuels by novel Lewis acidic ionic liquids. Fuel Process Technol. 2018;177:81–8.10.1016/j.fuproc.2018.04.013Search in Google Scholar
[12] Gao Y, Lv ZY, Gao RM, Zhang G, Zheng Y, Zhao JS. Oxidative desulfurization process of model fuel under molecular oxygen by polyoxometalate loaded in hybrid material CNTs@MOF-199 as catalyst. J Hazard Mater. 2018;359:258–65.10.1016/j.jhazmat.2018.07.008Search in Google Scholar PubMed
[13] Kianpour E, Azizian S, Yarie M, Zolfigol MA, Bayat M. A task-specific phosphonium ionic liquid as an efficient extractant for green desulfurization of liquid fuel: An experimental and computational study. Chem Eng J. 2016;295:500–8.10.1016/j.cej.2016.03.072Search in Google Scholar
[14] Yu FL, Liu CY, Yuan B, Xie PH, Xie CX, Yu ST. Energy-efficient extractive desulfurization of gasoline by polyether-based ionic liquids. Fuel. 2016;177:39–45.10.1016/j.fuel.2016.02.063Search in Google Scholar
[15] Gao JJ, Meng H, Lu YZ, Zhang HX, Li CX. A carbonium pseudo ionic liquid with excellent extractive desulfurization performance. AIChE J. 2013;59:948–58.10.1002/aic.13869Search in Google Scholar
[16] Ban LL, Liu P, Ma CH, Dai B. Deep extractive desulfurization of diesel fuels by FeCl3/ionic liquids. Chinese. Chem Lett. 2013;24:755–8.10.1016/j.cclet.2013.04.031Search in Google Scholar
[17] Kianpour E, Azizian S. Polyethylene glycol as a green solvent for effective extractive desulfurization of liquid fuel at ambient conditions. Fuel. 2014;137:36–40.10.1016/j.fuel.2014.07.096Search in Google Scholar
[18] Otsuki S, Nonaka T, Takashima N, Qian WH, Ishihara A, Imai T, et al. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energ Fuel. 2000;14:1232–9.10.1021/ef000096iSearch in Google Scholar
[19] Xiong XQ, Yi C, Liao X, Lai SL. A practical multigram-scale method for the green synthesis of 5-substituted-1H-tetrazoles in deep eutectic solvent. Tetrahedron Lett. 2019;60:402–6.10.1016/j.tetlet.2018.12.037Search in Google Scholar
[20] Zhang WH, Chen MN, Hao Y, Jiang X, Zhou XL, Zhang ZH. Choline chloride and lactic acid: a natural deep eutectic solvent for one-pot rapid construction of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines]. J Mol Liq. 2019;278:124–9.10.1016/j.molliq.2019.01.065Search in Google Scholar
[21] Trujillo-Rodríguez MJ, Pino V, Miró M. High-throughput microscale extraction using ionic liquids and derivatives: a review. J Sep Sci. 2020;43:1890–907.10.1002/jssc.202000045Search in Google Scholar PubMed
[22] Wang Q, Zhang T, Zhang SL, Fan YC, Chen B. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids. Sep Purif Technol. 2020;231:115923.10.1016/j.seppur.2019.115923Search in Google Scholar
[23] Cooper ER, Andrews CD, Wheatley PS, Webb PB, Wormald P, Morris RE. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature. 2004;430:1012–6.10.1038/nature02860Search in Google Scholar PubMed
[24] Sasikumar B, Arthanareeswaran G, Ismail AF. Recent progress in ionic liquid membranes for gas separation. J Mol Liq. 2018;266:330–41.10.1016/j.molliq.2018.06.081Search in Google Scholar
[25] Raj JJ, Magaret S, Pranesh M, Lethesh KC, Devi WC, Mutalib MIA. Dual functionalized imidazolium ionic liquids as a green solvent for extractive desulfurization of fuel oil: toxicology and mechanistic studies. J Clean Prod. 2019;213:989–98.10.1016/j.jclepro.2018.12.207Search in Google Scholar
[26] Li E, Zhu YY, Xu Y, Zhang YH, Yao P. Desulfurization of gasoline by [C4, 6, 8mim]Br/FeCl3 ILs collaboration with CTAB. Sep Sci Technol. 2021;56:310–21.10.1080/01496395.2020.1713817Search in Google Scholar
[27] Paiva A, Craveiro R, Aroso I, Martins M, Reis RL, Duarte ARC. Natural deep eutectic solvents-solvents for the 21st century. ACS Sustain Chem Eng. 2014;2:1063–71.10.1021/sc500096jSearch in Google Scholar
[28] Nkuku CA, LeSuer RJ. Electrochemistry in deep eutectic solvents. J Phy Chem B. 2007;111:13271–77.10.1021/jp075794jSearch in Google Scholar PubMed
[29] Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc. 2004;126:9142–7.10.1021/ja048266jSearch in Google Scholar PubMed
[30] Almashjary KH, Khalid M, Dharaskar S, Jagadish P, Walvekar R, Gupta TCSM. Optimisation of extractive desulfurization using choline chloride-based deep eutectic solvents. Fuel. 2018;234:1388–400.10.1016/j.fuel.2018.08.005Search in Google Scholar
[31] Xu H, Zhang DD, Wu FM, Wei XF, Zhang J. Deep desulfurization of fuels with cobalt chloride-choline chloride/polyethylene glycol metal deep eutectic solvents. Fuel. 2018;225:104–10.10.1016/j.fuel.2018.03.159Search in Google Scholar
[32] Makoś P, Boczkaj G. Deep eutectic solvents based highly efficient extractive desulfurization of fuels-eco-friendly approach. J Mol Liq. 2019;296:111916.10.1016/j.molliq.2019.111916Search in Google Scholar
[33] Shirazinia SR, Semnani A, Nekoeinia M, Shirani M, Akbari A. Novel sustainable metal complex based deep eutectic solvents for extractive desulphurisation of fuel. J Mol Liq. 2020;301:112364.10.1016/j.molliq.2019.112364Search in Google Scholar
[34] Wang X, Jiang W, Zhu WS, Li HP, Yin S, Chang YH, et al. A simple and cost-effective extractive desulfurization process with novel deep eutectic solvents. RSC Adv. 2016;6:30345–52.10.1039/C5RA27266ASearch in Google Scholar
[35] Jiang W, Li HP, Wang C, Liu W, Guo T, Liu H, et al. Synthesis of ionic-liquid-based deep eutectic solvents for extractive desulfurization of fuel. Energ Fuel. 2016;30:8164–70.10.1021/acs.energyfuels.6b01976Search in Google Scholar
[36] Tang XD, Zhang YF, Li JJ, Zhu YQ, Qing DY, Deng YX. Deep extractive desulfurization with arenium ion deep eutectic solvents. Ind Eng Chem Res. 2015;54:4625–32.10.1021/acs.iecr.5b00291Search in Google Scholar
[37] Li Z, Cui YN, Li CP, Shen YM. Deep desulfurization of fuels based on deep eutectic theory. Sep Sci Technol. 2019;219:9–15.10.1016/j.seppur.2019.03.003Search in Google Scholar
[38] Hiebela MA, Berteina-Raboin S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015;17:937–44.10.1039/C4GC01462FSearch in Google Scholar
© 2021 Yingna Cui et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

