Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
-
Sabah Ahmed Al-Zahrani
, Sarah A. Al Rashed
Abstract
Marine green algae are rich in various bioactive components with known anticancer activity. Some anticancer drugs present in green algae are in clinical trials nowadays. Algae-mediated silver nanoparticles (AgNPs) have been of a great interest in cancer treatment due to their unique physico-chemical properties. In this study, we evaluate the anticancer efficiency of marine alga Chaetomorpha ligustica collected from the Arabian Gulf against colon cancer cell lines HT29 and HCT116. The anticancer potential of biosynthesized AgNPs from C. ligustica extract is also reported. Fourier transform infrared (FTIR) spectroscopy and gas chromatography-mass spectrometry analyses were used to identify the phytoconstituents present in algae extract. The synthesized AgNPs were confirmed via UV-Vis spectroscopy, whereas their morphology and stability were recorded by transmission electron microscopy (TEM), zeta potential, and zetasizer. We recorded absorption peak at 420 nm; TEM images showed an average size of 8.8 nm, whereas zeta potential and zetasizer study showed aggregation of nanoparticles. FTIR spectroscopy peaks of C. ligustica AgNPs were a little different from those of the C. ligustica extract. Both extracts showed cytotoxicity against cancer cell lines in a dose-dependent manner, but nanoparticles were found to be more toxic than algae extract. HT29 was found to be more sensitive than HCT116. For the first time, species of C. ligustica have been used and reported for the synthesis of nanoparticles. C. ligustica and its biogenic nanoparticles need to be scaled up for many biomedical applications especially in cancer research.
1 Introduction
Herbal medicine in the form of extracts, powders, brews, or gels has been used to treat various ailments both in humans and animals [1]. Primary health care through herbal medicine is a very common practice in developed and in developing countries [2]. Cancer is a major cause of death in humans, and colorectal cancer is the third most diagnosed form of this disease all over the world [3]. A large number of preventive therapeutic studies are going on all over the world to find a way to ameliorate cancer incidence and/or reduce cancer mortality in human populations [4]. An enhanced search for natural compounds that can help in preventing cancer is being explored keenly. Most of the herbal medicines are derived from plants, but some algae are being focused on due to their novel bioactive compounds [5]. Algae are heterogeneous organisms, existing as brown (Phaeophyta), red (Rhodophyta), and green (Chlorophyta) and are mainly divided as microalgae and macroalgae. Various bioactive metabolites with potent cytotoxicity against different kinds of cancers are being explored from marine macroalgae or seaweeds [6]. Some recent studies reported many novel anticancer drugs present in seaweeds [7,8,9,10] and Kahalalide F, an antitumor marine drug, present in green algae are in clinical trials nowadays [11].
Chaetomorpha, one of the genera of green algae, are macroscopic unbranched filaments of cylindrical-shaped cells and are mostly found in the intertidal zone of the seas. The Chaetomorpha sp. are a rich source of antioxidants and possess drug properties against various human diseases [12]. Currently, many biomedical compounds present in Chaetomorpha sp. are being explored for various cancer therapeutic treatments such as chemo-protective agents, anticancer and drug-delivery systems [13,14]. Chaetomorpha sp. are a rich source of proteins, carbohydrates, fatty acids, pigments, and secondary metabolites, such as phenols, sulfated polysaccharides, and halogenated compounds [15]. Besides being strong antitumor, antiviral, antibacterial, and cytotoxic agents, these compounds have the ability to convert metal ions into metal nanoparticles due to their reducing and capping properties [16].
Biological methods of preparing nanoparticles are less toxic with low cost as compared to physical or chemical methods [17]. Being rich in bioactive compounds, plants are preferred for the green synthesis of metallic nanoparticles [18]. Plant-mediated metal nanoparticles are either synthesized intracellular, that is, inside plant tissue or extracellular by using plant extract [19,20]. Biomolecules present in plants significantly influence the quality and quantity of metal nanoparticles and thus determine their application approach. Due to unique surface chemistry, charge, energy, and spatial dimensions, metal nanoparticles offer outstanding applications in many fields of biomedicine [21]. They are widely used in drug-delivery systems, in vitro diagnostic tests, as biosensors and antimicrobial and anticancer agents [22,23]. Metal nanostructures are used as cargo molecules for imaging and gene delivery in many anticancer therapies. Metal nanoparticles can hinder some signal cascades that are responsible for the development and pathogenesis of tumors. Biogenic metal nanoparticles are found to be more cytotoxic against human cancer cells as compared to normal cells [22].
Algae-mediated silver nanoparticles (AgNPs) have been of a great interest in biomedicine especially cancer treatment and for targeted delivery of various anticancerous drugs [16]. This study examined the in vitro anticancer potential of marine alga Chaetomorpha ligustica collected from the Arabian Gulf against two colon cancer cell lines HCT116 and HT 29. Biologically synthesized AgNPs mediated by water extract of C. ligustica were also reported in this study.
2 Materials and methods
2.1 Collection of algae
The green algae, C. ligustica, was collected from the shore of the Arabian Gulf. It was washed properly with distilled water and then shade dried at room temperature. Once dry, it was powdered in an electric blender. One gram of powder was dissolved in 100 mL of sterile autoclaved water and left on a magnetic stirrer for 48 h at 4°C. Then, the mixture was filtered by using Whitman No. 1 filter paper. The obtained extract was stored at −80°C for further experiments.
2.2 Fourier transformed infrared (FTIR)
Perkin Elmer FTIR-Spectrometer Spectrum (Spectrum BX, USA) was used to identify the functional groups present in algae extract. FTIR was measured in the range from 4,400 to 400 cm−1. The spectra (16 scans per spectrum) were collected with a spectral resolution of 16.0 cm−1 with an interval of 2.0 cm−1.
2.3 Gas chromatography-mass spectrometry (GC-MS)
The phytochemical analysis of hexane extract of C. ligustica was accomplished by GC-MS analysis. GC Column TRACE™ TR-35MS (Thermo Fisher Scientific, USA) with the film thickness of 0.25 µm, length of 30 m, and diameter of 0.25 mm was used. The carrier gas was helium (99.9999% purity) at a flow rate of 1.2 mL·min−1. The injector temperature was set to 250°C. The injection mode was split less, and the injection volume was 1.0 μL. The GC column temperature was programed as an initial temperature of 60°C, held for 3 min, increased at a rate of 10°C·min−1 to 300°C, and held for 5 min.
2.4 Biogenically synthesized AgNPs from C. ligustica extract
AgNPs were synthesized by dissolving silver nitrate (AgNO3) solution in C. ligustica extract with the concentration of 5 mM AgNO3 in the mixture. The reduction of silver ions to AgNPs was observed by a color change from light green to dark brown.
2.5 Characterization of biogenically synthesized AgNPs
Prepared nanoparticles were characterized as described below before treating the cells.
2.5.1 UV-Vis spectrophotometer
Prepared nanoparticles were initially confirmed by observing a UV-Visible spectrophotometer from 300 to 600 nm with a peak at 420 nm by using the UV-Vis spectrophotometer (Thermo Scientific 1500, USA).
2.5.2 Transmission electron microscopy (TEM)
The shape and size of the nanoparticles were determined by using TEM. A grid coated with copper was used to place the silver nanoparticle sample to be imaged under TEM (JEOL JEM-1400 Plus, Japan).
2.5.3 Zetasizer and zeta potential
The average size of nanoparticles was recorded by Zetasizer Nano Series ZS (ZEN3600), UK by using the technique of dynamic light scattering (DLS) and zeta potential by electrophoretic light scattering (ELS).
2.6 Cell lines
In this study, two types of colon cancer cell lines, HT 29 and HCT116, were included. According to ATCC, the Global Bio-resource Center, HCT116 is derived from carcinoma-primary and HT29 adenocarcinoma-primary.
2.7 Cell treatment
2.7.1 Cell viability
Cell lines were divided into three groups depending upon the treatment: control or untreated, C. ligustica extract (10, 20, 50, and 100 µg) treated, and C. ligustica AgNPs (10, 20, 50, and 100 µg) treated for 48 h.
2.7.2 Expression level of genes
Cells were divided into three groups as control or untreated, C. ligustica extract (50% cytotoxic) treated, and C. ligustica AgNPs (50% cytotoxic) treated for 24 h.
2.8 Determination of cell viability
Cell viability was measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Absorbance was measured at 490 nm using a scanning microliter-well spectrophotometer. The color intensity is correlated with the number of healthy living cells.
2.9 Determining the expressional level of ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3 related (ATR), checkpoint kinase (CHK1), and CHK2 using RT-PCR
2.9.1 Nucleic acid isolation
After treatment, RNA samples were extracted from two different colon cancer cell lines by using an RNeasy mini kit (Qiagen, DE). All extractions were performed following the manufacturer’s procedure.
2.9.2 cDNA preparation
cDNA synthesis was performed by reverse transcription of total purified by using the high-capacity cDNA reverse transcription kit (Applied Biosystems, USA). The cDNA synthesis was performed according to the manufacturer’s procedure. The obtained cDNA samples were stored at −20°C.
2.9.3 Expressional level of genes
Power SYBR® Green PCR Master Mix 2X from Applied Biosystems® (Life Technologies, USA) was used to perform quantitative real-time PCR. All colon cancer cell lines were analyzed in triplicate, and gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH); a nontemplate negative control was included in each run to check background signal. Primer sequences and annealing temperature are listed in Table 1.
Primer sequences of ATM, ATR, CHK1, CHK2, and GAPDH
| Primer | Sequence | Annealing temperature (°C) |
|---|---|---|
| ATM | Forward: 5ʹ-GCA GAT GAC CAA GAA TGC AA-3ʹ | 60 |
| Reverse: 5ʹ-GGC CTG CTG TAT GAG CAA AT-3ʹ | ||
| ATR | Forward: 5′-GGGATGCCACTGCTTGTTATGAC-3′ | 60 |
| Reverse: 5′-CTGTCCACTCGGACCTGTTAGC-3′ | ||
| CHK1 | Forward: 5′-CTTTGGCTTGGCAACAGT-3′ | 60 |
| Reverse: 5′-CCAGTCAGAATACTCCTG-3′ | ||
| CHK2 | Forward: 5′-CTC GGG AGT CGG ATG TTG AG-3′ | 57 |
| Reverse: 5′-CCAGTCAGAATACTCCTG-3′ | ||
| GAPDH | Forward: 5′-GGTATCGTGGAAGGACTCATGAC-3′ | 60 |
| Reverse: 5′-ATGCCAGTGAGCTTCCGTTCAGC-3′ |
2.10 Statistical analysis
Statistical analyses were carried out using GraphPad Prism® 9.0 statistical software (GraphPad Inc., USA) and Microsoft Excel®. An independent t-test was used to evaluate the difference in gene expression between the different stages of colon cancer cell lines and treated and untreated colon cancer cell lines. The level of significance used throughout was p < 0.05.
3 Results
3.1 FTIR of C. ligustica extracts
FTIR spectroscopy was measured for C. ligustica extract as shown in Figure 1. The 3403.66, 2924.81, and 2554.91 cm−1 absorption peaks were assigned for the OH bond of phenols, the N–H bond of amines, and S, O–H bond of carboxylic acid, respectively, for lipids. The 1707.76 cm−1 absorption peak was assigned for the C═O stretch of ketone and C═C stretch of benzene; 1,655 cm−1 absorption peak was assigned for N–H, and 1578.41 and 1543.89 cm−1 absorption peaks were assigned for C–C stretch for amides in proteins. The 1464.40 cm−1 absorption peak was assigned for N–H bond (nitro compounds), C–O stretch (amides), C═C (benzenes), and C═O (ketones) in carbohydrates. 1085.29 for S═O stretch (sulfoxides), C–N stretch (amines), C–O stretch (esters, ether, alcohol), and ═C–H bend (alkenes). The 856.37 cm−1 absorption peak was assigned for C═C–H bend (alkenes) in pectin. The 697.86 and 462.24 cm−1 absorption peaks were assigned for C–N stretch (amines), ═C–H bend (benzene), and C–C stretch (chlorides) for cell wall components.
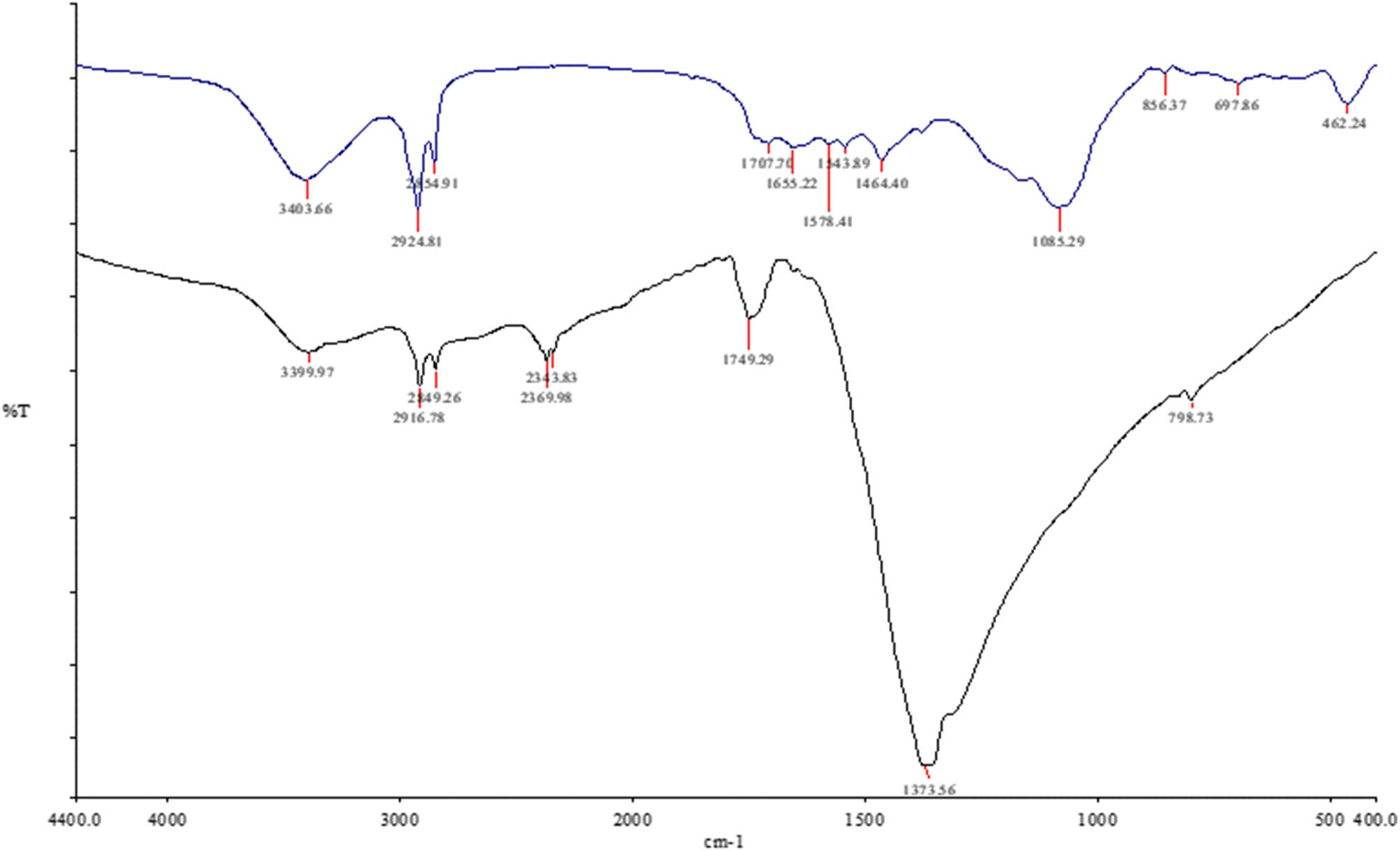
IR spectra of C. ligustica extract (blue color) and C. ligustica AgNPs (black color).
3.2 GC-MS analysis of C. ligustica extracts
GC-MS analysis of C. ligustica extracts leads to the identification of many compounds with anticancer properties. The components present in C. ligustica extract with anticancer properties detected by the GC-MS are shown in Figure 2. Antitumor compounds mainly detected in C. ligustica extract include methanone, propanoic acid, milbemycin B, eucalyptol, and fucoxanthin. Other than these cyclohexane; heptane; 2,4,6,8,10-tetradecapentaenoic acid; benzene; docosanoic acid; 1,2,3-propanetriyl ester; 3,8,12-tri-O-acetoxy-7-desoxyingol-7-one; trisiloxane; 12,15-octadecadiynoic acid; methyl ester; cyclopentasiloxane; decamethyl; cyclohexasiloxane; dodecamethyl; bicyclo[2.2.1]heptan-2-one; 1,7,7-trimethyl-cycloheptasiloxane; tetradecamethyl; benzaldehyde; 2,5-dimethyl; (3,4,trans-6-trimethyl-3-cyclohexenyl)formaldehyde 2,4-dinitrophenylhydrazone; D-homo-24-nor-17-oxachola-20,22-diene-3,16-dione; methyl glycocholate; 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione; ethyl iso-allocholate; 7,8-epoxylanostan-11-ol; and 3-acetoxy were present in C. ligustica extracts.
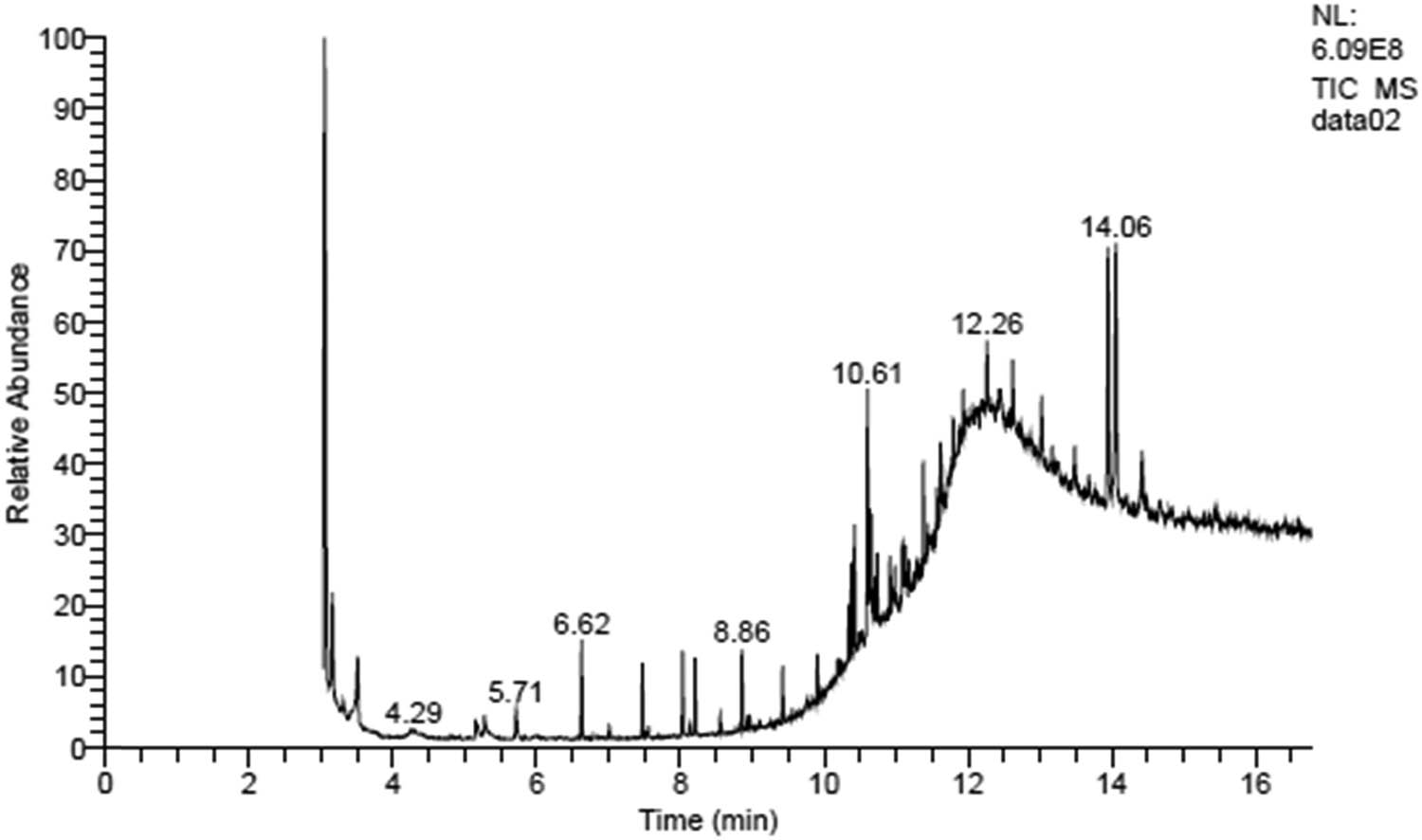
GC-MS analysis to detect the anticancer compound in C. ligustica extract.
3.3 Characterization of C. ligustica AgNPs
Figure 3a presents the UV spectra of C. ligustica AgNPs recorded at 3 h duration from the initiation of reaction between C. ligustica extract and AgNO3 solution with a constant maximum absorption at 420 nm, which represents silver nanospheres. Results from TEM images showed the average size of 8.8 nm (Figure 3b); however, DLS analysis showed size as 1,800 nm (Figure 3c), indicating aggregation of nanoparticles. ELS analysis showed the zeta potential of 2.36 mV. FTIR spectroscopy result of C. ligustica AgNPs was a little different from that of the C. ligustica extract as some of the peaks belonging to functional groups of carbohydrate and proteins were not found as shown in Figure 1.
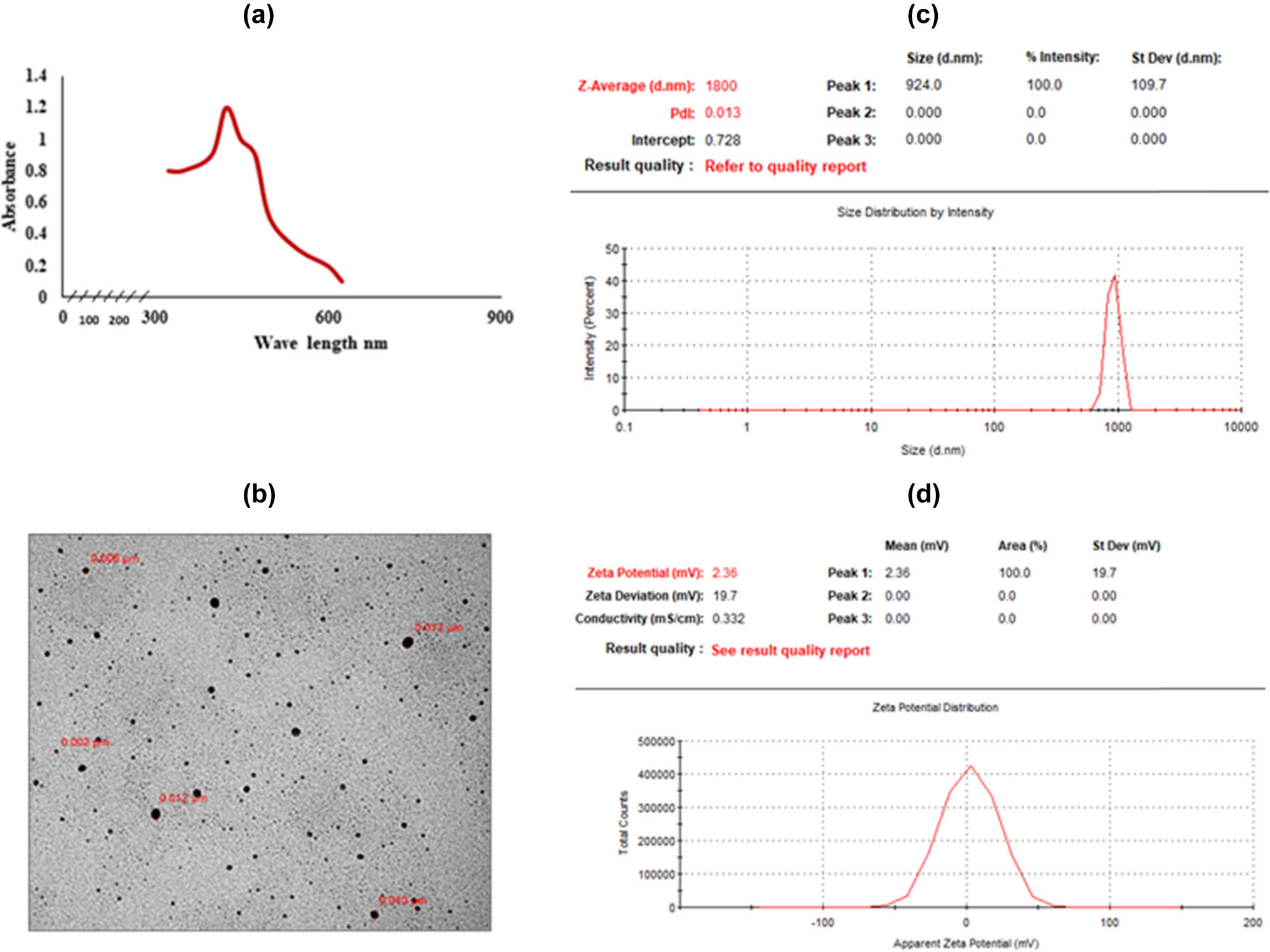
Characterization of biologically synthesized C. ligustica AgNPs: (a) UV-Vis spectra showing absorbance peak, (b) TEM micrograph, (c) zetasizer – DLS, and (d) zeta potential distribution – ELS.
3.4 Cell viability
Results of cytotoxic effects of C. ligustica extract and C. ligustica AgNPs against human colon cancer cell lines HT29 and HCT116 after treatment are presented in Figure 4. C. ligustica extract was found more toxic at 10 μg·mL−1 as compared to C. ligustica AgNPs, but with the increase in concentration C. ligustica AgNPs showed higher cytotoxicity against both the cell lines.
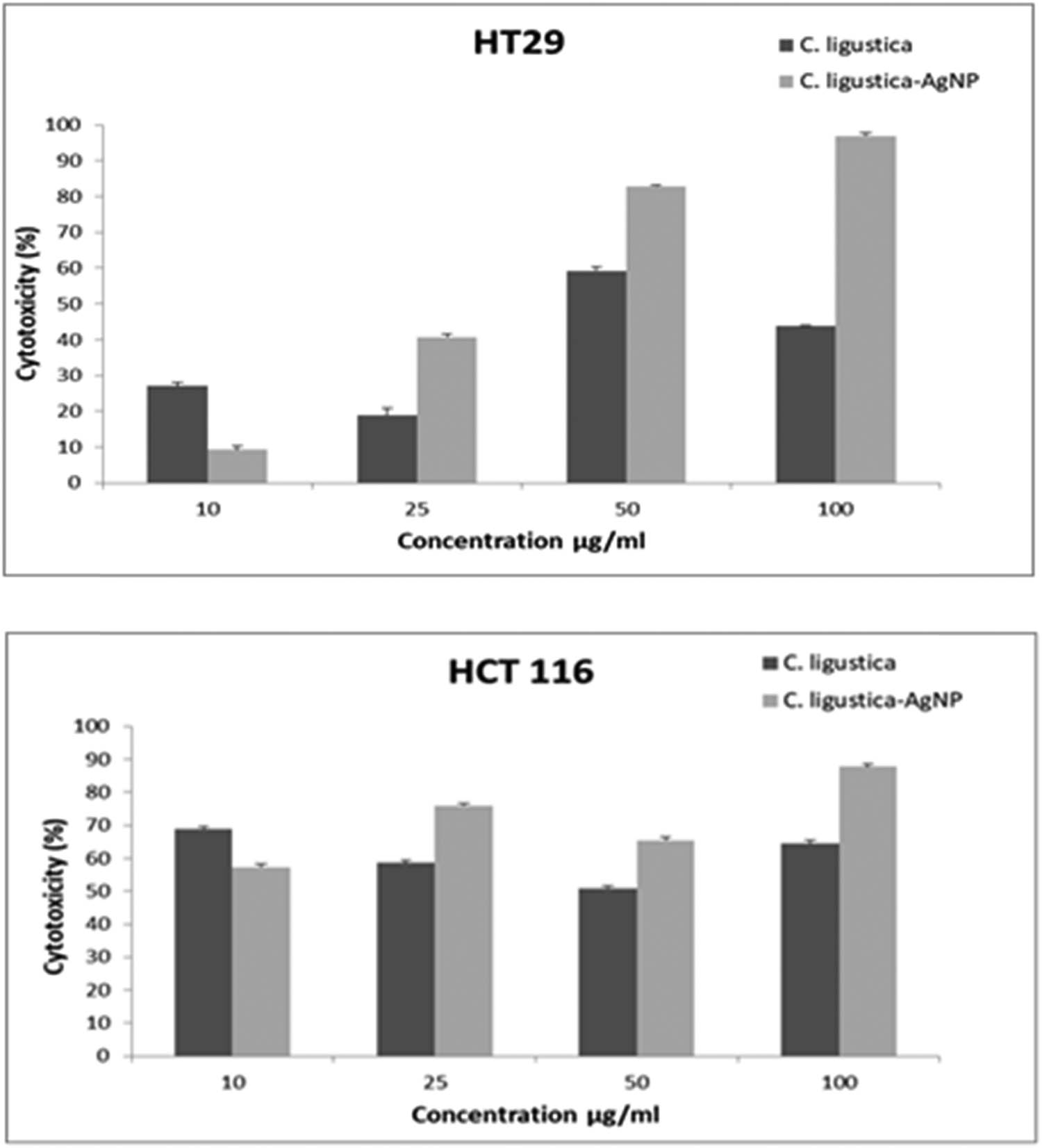
Cytotoxicity of C. ligustica extract and C. ligustica AgNPs against human colon cancer cell lines HT29 and HCT116.
3.5 Expressional level of ATM, ATR, CHK1, and CHK2 in cell lines treated with C. ligustica extract and C. ligustica AgNPs extract
As shown in Figure 5, the expression level of all the four genes (ATM, ATR, CHK1, and CHK2) was increased with treatment in HT29. In HCT116, the expression level of ATM was decreased with C. ligustica extract and AgNP treatment, but we found a slight increase in gene expression levels of other genes on treatment as shown in Figure 5.

Gene expression fold change in cell lines treated with C. ligustica extract and C. ligustica AgNPs.
4 Discussion
Green algae are being explored for drug properties for the fight against many diseases, including colorectal cancers, as they are found rich in many therapeutic compounds that can induce apoptosis through different molecular mechanisms [24,25]. The cytotoxic potential of various marine macroalgae against the growth of many kinds of cancer cell lines has been reported in the literature [25,26,27,28,29]. We explored both C. ligustica extract and C. ligustica AgNPs for cytotoxicity. FTIR and GC-MS analyses were used to identify the phytoconstituents present in algae extract.
4.1 FTIR of C. ligustica extracts
FTIR analysis is one of the most reliable and sensitive methods used by researchers to identify the biomolecules involved in the green synthesis of nanoparticles from metal ions [30,31]. C. ligustica extract was found rich in active metabolites (Figure 1) as shown by IR spectrum peaks, which may have supported the green synthesis process of AgNPs [15]. Our results suggested that compounds with hydroxyl, alkene, carboxyl, and amide groups of the protein, polysaccharides, and phenols present in C. ligustica extract were likely involved in the synthesis process as some peak shifts were seen in C. ligustica AgNPs spectral at 2369.98 specific for O–H stretch (carboxylic acids), C–H stretch (alkenes); 1749.29 specific for N–H bend (nitro compounds, amides), C–C stretch (amides); and 1373.36 specific for S (═O) 2 stretch (sulfones), N–O stretch (nitro compounds), O–H bend (carboxylic acids, alcohols), which are mainly assigned for proteins, polysaccharides, and polyphenols. Our results are in agreement with some earlier findings showing hydroxyl and carbonyl groups of algal biomass as reducing and capping agents for the successful synthesis of metal nanoparticles [32].
4.2 GC-MS analysis of C. ligustica extracts
C. ligustica extract showed antitumor properties against both the cell lines, which shows its potential as an antitumor drug. The GC-MS profile of the C. ligustica extracts showed the presence of some anticancer compounds. Fucoxanthin is reported to induce G1 cell-cycle arrest and apoptosis in various cancer cell lines and in animal models of cancer [33]. Eucalyptol is an active anticancer compound that induces cell death through reactive oxygen species-mediated apoptosis [34,35]. Propanoic acid, milbemycin B, and rhodopin detected in C. ligustica extract are achieving a lot of interest due to their cytotoxic properties against various cancer cell lines [36,37,38]. Several biochemical and microscopic experiments revealed the anticancer potential of methanone [39].
4.3 Characterization of C. ligustica AgNPs
AgNPs were successfully synthesized from C. ligustica extract. It is well known that the formation of AgNPs is initially confirmed by recording a sharp surface plasmon resonance (SPR) in the range from 350 to 500 nm; however, the position of the peak is decided by the shape and size of the nanoparticle formed [40,41]. The absorption peak at 420 nm (Figure 3a) confirms SPR in AgNPs [41]. These results confirm the particle size below 100 nm with a small degree of polydispersity, which is in agreement with our zetasizer and TEM results. Overall, the size of the nanoparticle was satisfactory as the polydispersity index was 0.013, indicating little aggregation. The TEM is a perfect tool to study the morphology of particles at the nanoscale [42]. Our TEM images were clearly showing spherical shapes with particle sizes in the range from 2 to 12 nm.
4.4 Cell viability
Both C. ligustica extract and its biogenic AgNPs showed cytotoxicity against cancer cell lines in a dose-dependent manner, but nanoparticles were found to be more toxic than the algae extract. Our results generally agree with many studies that highlight the efficacy of AgNPs against different types of cancer cell lines [26,27]. HT29 was found to be more sensitive than HCT116. Our results are well supported by Gurunathan et al. [28] reporting plant-based nanoparticles are more effective against HCT116 as compared to HT29.
4.5 Expressional level of ATM, ATR, CHK1, and CHK2 in cell lines treated with C. ligustica extract and C. ligustica AgNPs extract
We analyzed the gene expression of ATM, ATR, CHK1, and CHK2 in both the cell lines after treating the cells with C. ligustica extract and its biogenic AgNPs. All these genes are involved in DNA damage response and cell cycle checkpoints. The gene expression of ATM was significantly increased in HT29 but slightly decreased in HCT116 after treatment. The ATM gene expression is considered a predictive biomarker in colorectal cancer. Decreased expression levels of ATM are associated with poor survival in colon cancer patients [43]. The loss of ATM expression is directly related to chromosomal instability and worse survival in colorectal cancer associated [44]. The expression level of ATR, CHK1, and CHK2 was significantly increased in both cell lines, but it was more prominent in HT29. All cell lines have their unique characteristics depending on the origin from tumor tissue samples of a patient [45,46]. HT29 is derived from a female adenocarcinoma-primary, whereas HCT116 is derived from male carcinoma-primary [47]. HT29 has a deficient p53 gene expression, but HCT116 has mutations in PI3KCA and K‑RAS genes [48]. Recent report by Khorrami et al. states that biogenic nanoparticles can interrupt the metabolic pathway of cancer cells even at the very lowest concentration [49].
5 Conclusion
To the best of our knowledge, bio-reduction of silver ions by C. ligustica aqueous extract has been reported for the first time. The produced AgNPs show peak at 420 nm in the UV-Vis spectra; TEM images showed particle size in the range from 2 to 12 nm. Quick formation of AgNPs in presence of C. ligustica extract showed the presence of active metabolites, which was confirmed by FTIR and GC-MS analyses. GC-MS profile showed some anticancer compounds in C. ligustica extracts. C. ligustica and its biogenic nanoparticles have good potential to be explored for anticancer compounds. C. ligustica and its biosynthesized AgNPs hold huge potential for pharmaceuticals mainly for cancer therapies.
Acknowledgements
The author would like to thank Deanship of Scientific Research in King Saud University for funding and supporting this research through the initiative of DSR Graduate Students Research Support (GSR).
-
Funding information: Deanship of Scientific Research in King Saud University funded and supported this research through the initiative of DSR Graduate Students Research Support (GSR).
-
Author contributions: Ramesa Shafi Bhat: writing – original draft, writing – review and editing; Sabah Ahmed Al-Zahra, Sarah A. Al Rashed, Amer Mahmood, Ahmed Al Fahad, Ghadah Alamro, and Jamilah Almusallam: methodology; Roua Al Subki and Raha Orfali: resources; Sooad Al Daihan: project administration, resources, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Tugume P, Nyakoojo C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement Altern Med. 2019;19:353. 10.1186/s12906-019-2763-6.Search in Google Scholar PubMed PubMed Central
[2] Al Shehri SD, Abdul Hameed RM, Taha AZ, Almusalmi AM, Almulaify MS, Alkhabbaz FL. Complementary and alternative medicine practice and perceptions of attendees of primary care centers in Eastern Saudi Arabia. J Family Community Med. 2020;27(2):120–4. 10.4103/jfcm.JFCM_218_19.Search in Google Scholar PubMed PubMed Central
[3] Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. 10.5114/pg.2018.81072.Search in Google Scholar PubMed PubMed Central
[4] Sheikh I, Sharma V, Tuli HS, Aggarwal D, Sankhyan A, Vyas P, et al. Cancer chemoprevention by flavonoids, dietary polyphenols and terpenoids. Biointerface Res Appl Chem. 2020;11:8502–37.10.33263/BRIAC111.85028537Search in Google Scholar
[5] Vasas G. Microalgae as the source of natural products. Orv Hetil. 2018 May;159(18):703–8. 10.1556/650.2018.30956.Search in Google Scholar PubMed
[6] Pradhan B, Nayak R, Patra S, Jit BP, Ragusa A, Jena M. Bioactive metabolites from marine algae as potent pharmacophores against oxidative stress-associated human diseases: a comprehensive review. Molecules. 2020;26(1):37. 10.3390/molecules26010037.Search in Google Scholar PubMed PubMed Central
[7] Senthilkumar K, Manivasagan P, Venkatesan J, Kim SK. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int J Biol Macromol. 2013;60:366–74.10.1016/j.ijbiomac.2013.06.030Search in Google Scholar PubMed
[8] Newman DJ, Cragg GM. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar Drugs. 2014;12:255–78.10.3390/md12010255Search in Google Scholar PubMed PubMed Central
[9] Murphy C, Hotchkiss S, Worthington J, McKeown S. The potential of seaweed as a source of drugs for use in cancer chemotherapy. J Appl Phycol. 2014;26:1–54.10.1007/s10811-014-0245-2Search in Google Scholar
[10] Kwak JY. Fucoidan as a marine anticancer agent in preclinical development. Mar Drugs. 2014;12:851–70.10.3390/md12020851Search in Google Scholar PubMed PubMed Central
[11] Varshney A, Singh V. Effects of algal compounds on cancer cell line. J Exp Biol. 2013;1:337–52.Search in Google Scholar
[12] Ferdous UT, Yusof ZNB. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front Pharmacol. 2021;12:593116. 10.3389/fphar.2021.593116.Search in Google Scholar PubMed PubMed Central
[13] Jiang J, Shi S. Seaweeds and cancer prevention. Bioactive Seaweeds for Food Applications. Academic Press, Elsevier Inc.; 2018. p. 269–90.10.1016/B978-0-12-813312-5.00014-5Search in Google Scholar
[14] Rocha DHA, Seca AML, Pinto DCGA. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar Drugs. 2018;16:410.10.3390/md16110410Search in Google Scholar PubMed PubMed Central
[15] Salehi B, Sharifi-Rad J, Seca AML, Pinto DCGA, Michalak I, Trincone A, et al. Current trends on seaweeds: looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules. 2019;24(22):4182. 10.3390/molecules24224182.Search in Google Scholar PubMed PubMed Central
[16] Chaudhary R, Nawaz K, Khan AK, Hano C, Abbasi BH, Anjum S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules. 2020;10(11):1498. 10.3390/biom10111498.Search in Google Scholar PubMed PubMed Central
[17] Heinemann MG, Rosa CH, Rosa GR, Dias D. Biogenic synthesis of gold and silver nanoparticles used in environmental applications: a review, Trends Environ Anal Chem. 2021;30:e00129.10.1016/j.teac.2021.e00129Search in Google Scholar
[18] Naikoo GA, Mustaqeem M, Hassan IU, Awan T, Arshad F, Salim H, et al. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: a critical review, J Saudi Chem Soc. 2021;25(9):101304.10.1016/j.jscs.2021.101304Search in Google Scholar
[19] Raju D, Mehta UJ, Ahmad A. Simple recovery of intracellular gold nanoparticles from peanut seedling roots. J Nanosci Nanotechnol. 2015;15:1575–81.10.1166/jnn.2015.9046Search in Google Scholar PubMed
[20] Ali MA, Ahmed T, Wu W, Hossain A, Hafeez R, Islam Masum MM, et al. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials (Basel). 2020;10(6):1146.10.3390/nano10061146Search in Google Scholar PubMed PubMed Central
[21] Ramos AP, Cruz MAE, Tovani CB, Ciancaglini P. Biomedical applications of nanotechnology. Biophys Rev. 2017 9(2):79–89.10.1007/s12551-016-0246-2Search in Google Scholar PubMed PubMed Central
[22] Saravanan M, Vahidi H, Medina Cruz D, Vernet-Crua A, Mostafavi E, Stelmach R, et al. Emerging antineoplastic biogenic gold nanomaterials for breast cancer therapeutics: a systematic review. Int J Nanomed. 2020;19(15):3577–95.10.2147/IJN.S240293Search in Google Scholar PubMed PubMed Central
[23] Saravanan M, Barabadi H, Vahidi H, Webster TJ, Medina-Cruz D, Mostafavi E, et al. Emerging theranostic silver and gold nanobiomaterials for breast cancer: present status and future prospects, Handbook on nanobiomaterials for therapeutics and diagnostic applications. Vol. 19. Elsevier B.V.; 2021. p. 439–56.10.1016/B978-0-12-821013-0.00004-0Search in Google Scholar
[24] Abd El-Hack ME, Abdelnour S, Alagawany M, Abdo M, Sakr MA, Khafaga AF, et al. Microalgae in modern cancer therapy: current knowledge. Biomed Pharmacother. 2019;111:42–50.10.1016/j.biopha.2018.12.069Search in Google Scholar PubMed
[25] Moussavou G, Kwak DH, Obiang-Obonou BW, Maranguy CA, Dinzouna-Boutamba SD, Lee DH, et al. Anticancer effects of different seaweeds on human colon and breast cancers. Mar Drugs. 2014;12(9):4898–911. 10.3390/md12094898.Search in Google Scholar PubMed PubMed Central
[26] Daghestani M, Al Rashed SA, Bukhari W, Al-Ojayan B, Ibrahim EM, Al-Qahtani AM et al. Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves. Green Process Synth. 2020;9:230–6.10.1515/gps-2020-0025Search in Google Scholar
[27] Ratan ZA, Haidere MF, Nurunnabi M, Shahriar SM, Ahammad AJS, Shim YY, et al. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers (Basel). 2020;12(4):855. 10.3390/cancers12040855.Search in Google Scholar PubMed PubMed Central
[28] Gurunathan S, Qasim M, Park C, Yoo H, Kim JH, Hong K. Cytotoxic potential and molecular pathway analysis of silver nanoparticles in human colon cancer cells HCT116. Int J Mol Sci. 2018;19(8):2269.10.3390/ijms19082269Search in Google Scholar PubMed PubMed Central
[29] Sathishkumar RS, Sundaramanickam A, Srinath R, Ramesh T, Saranya K, Meena M, et al., Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J Saudi Chem Soc. 2019;23(8):1180–91.10.1016/j.jscs.2019.07.008Search in Google Scholar
[30] Grace CE, Lakshmi PK, Meenakshi S, Vaidyanathan S, Srisudha S, Mary MB. Biomoleculartransitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochimica Acta A Mol Biomol Spectrosc. 2020;224:117382.10.1016/j.saa.2019.117382Search in Google Scholar PubMed
[31] Marslin G, Siram K, Maqbool Q, Selvakesavan RK, Kruszka D, Kachlicki P, et al. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials (Basel). 2018;11(6):940.10.3390/ma11060940Search in Google Scholar PubMed PubMed Central
[32] Chugh D, Viswamalya VS, Das B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J Genet Eng Biotechnol. 2021;19:126.10.1186/s43141-021-00228-wSearch in Google Scholar PubMed PubMed Central
[33] Satomi Y. Antitumor and cancer-preventative function of fucoxanthin: a marine carotenoid. Anticancer Res. 2017;37(4):1557–62. 10.21873/anticanres.11484.Search in Google Scholar PubMed
[34] Boukhatem MN, Sudha T, Darwish NHE, Chader H, Belkadi A, Rajabi M, et al. A new eucalyptol-rich lavender (Lavandula stoechas L.) essential oil: emerging potential for therapy against inflammation and cancer. Molecules. 2020;25(16):3671. 10.3390/molecules25163671.Search in Google Scholar PubMed PubMed Central
[35] Sampath S, Veeramani V, Krishnakumar GS, Sivalingam U, Madurai SL, Chellan R. Evaluation of in vitro anticancer activity of 1,8-Cineole-containing n-hexane extract of Callistemon citrinus (Curtis) Skeels plant and its apoptotic potential. Biomed Pharmacother. 2017;93:296–307. 10.1016/j.biopha.2017.06.056.Search in Google Scholar PubMed
[36] Li X, Jiang Z, Feng J, Zhang X, Wu J, Chen W. 2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino) phenyl carbamoylsulfanyl] propionic acid, a glutathione reductase inhibitor, induces G2/M cell cycle arrest through generation of thiol oxidative stress in human esophageal cancer cells. Oncotarget. 2017;8(37):61846–60. 10.18632/oncotarget.18705.Search in Google Scholar PubMed PubMed Central
[37] Faith-Anthony AO, Ibrahim N, Hamzah A. Clinical potentials of bacteriocarotenoids: Rhodopin and β-carotene from phototrophic Rhodopseudomonas palustris. IOSR J Dental Med Sci. 2014;13(12):52–58.Search in Google Scholar
[38] Li XQ, Yue CW, Xu WH, Lü YH, Huang YJ, Tian P, et al. A milbemycin compound isolated from Streptomyces Sp. FJS31-2 with cytotoxicity and reversal of cisplatin resistance activity in A549/DDP cells, Biomed Pharmacotherapy. 2020;128:110322.10.1016/j.biopha.2020.110322Search in Google Scholar PubMed
[39] Islam K, Pal K, Debnath U, Sidick Basha R, Khan AT, Jana K, et al. Anti-cancer potential of (1,2-dihydronaphtho[2,1-b]furan-2-yl) methanone derivatives. Bioorg Med Chem Lett. 2020;30(20):127476. 10.1016/j.bmcl.2020.127476.Search in Google Scholar PubMed
[40] Ider M, Abderrafi K, Eddahbi A, Ouaskit S, Kassiba A. Silver metallic nanoparticles with surface plasmon resonance: synthesis and characterizations. J Clust Sci 2017;28:1051–69.10.1007/s10876-016-1080-1Search in Google Scholar
[41] Ashkarran AA, Bayat A. Surface plasmon resonance of metal nanostructures as a complementary technique for microscopic size measurement. Int Nano Lett 2013;3:50.10.1186/2228-5326-3-50Search in Google Scholar
[42] Eskandari MJ, Gostariani R, Asadabad MA. Transmission electron microscopy of nanomaterials. In: Electron Crystallography. UK: Intech Open; 2020. p. 9.10.5772/intechopen.92212Search in Google Scholar
[43] Morio T, Ku KH. Artemis and ataxia-telangiectasia-mutated: signaling networks in DNA damage. Int J Biochem Cell Biol. 2008;4(40):598–603.10.1016/j.biocel.2007.12.007Search in Google Scholar PubMed
[44] Beggs AD, Domingo E, McGregor M, Presz M, Johnstone E, Midgley R, et al. Loss of expression of the double strand break repair protein ATM is associated with worse prognosis in colorectal cancer and loss of Ku70 expression is associated with CIN. Oncotarget 2012;3:11.10.18632/oncotarget.694Search in Google Scholar PubMed PubMed Central
[45] Kodack DP, Farago AF, Dastur A, Held MA, Dardaei L, Friboulet L, et al. Primary patient-derived cancer cells and their potential for personalized cancer patient care. Cell Rep. 2017;21(11):3298–309.10.1016/j.celrep.2017.11.051Search in Google Scholar PubMed PubMed Central
[46] Salvadores M, Fuster-Tormo F, Supek F. Matching cell lines with cancer type and subtype of origin via mutational, epigenomic, and transcriptomic patterns. Sci Adv. 2020;6(27):eaba1862.10.1126/sciadv.aba1862Search in Google Scholar PubMed PubMed Central
[47] Davidson D, Coulombe Y, Martinez Marignac V, Amrein L, Grenier J, Hodkinson K, et al. Irinotecan and DNA PKcs inhibitors synergize in killing of colon cancer cells. Invest N Drugs. 2012;30(3):1248–56.10.1007/s10637-010-9626-9Search in Google Scholar PubMed
[48] Wang J, Kuropatwinski K, Hauser J, Ross MR, Zhou Y, Conway A, et al. Colon carcinoma cells harboring PIK3CA mutations display resistance to growth factor deprivation induced apoptosis. Mol Cancer Ther. 2007;6(3):1143–50.10.1158/1535-7163.MCT-06-0555Search in Google Scholar PubMed
[49] Khorrami S, Kamali F, Zarrabi A. Bacteriostatic activity of aquatic extract of black peel pomegranate and silver nanoparticles biosynthesized by using the extract. Biocat Agric Biotech. 2020;25:101620.10.1016/j.bcab.2020.101620Search in Google Scholar
© 2021 Sabah Ahmed Al-Zahrani et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

