Abstract
The use of enabling technologies, such as flow reactors, three-dimensional-printed devices, and electrochemistry, in the stereoselective synthesis of enantioenriched compounds is presented, with a special focus on the most significant contributions to the field reported in the last few years.
1 Introduction
Per Collins English dictionary, “Technology refers to methods, systems, and devices which are the result of scientific knowledge being used for practical purposes.” Hence, the application of science to develop new devices, systems, or methods is called technology. But the borders are not that clearly defined; indeed, science and technology are strictly related: technology may be considered the practical application of science, but, however, it can be used to do science.
Technology stimulates scientific progress by sustaining science with increasingly sophisticated devices and experimental equipment, but, at the same time, science continuously demands for revolutionary technologies. The importance of innovative technologies has been recognized also by the European Commission, which has identified key enabling technologies (KETs) as a priority within its Europe 2020 strategy. “KETs provide the basis for innovation in a range of products across all industrial sectors. They underpin the shift to a greener economy, are instrumental in modernizing Europe’s industrial base, and drive the development of entirely new industries” [1]. These considerations also apply to organic synthesis, where the advent of the so-called “enabling technologies” has brought unprecedented opportunities and offered the possibility to develop new, more efficient, or faster reactions [2,3,4,5].
Significant progress in organic synthesis has been realized through the integration of two or more of these enabling techniques, [6] for example, by the combination of supported catalysts, alternative solvents, flow chemistry (new reactors design), and alternative heating techniques (including microwave-assisted synthesis).
Considering the numerous reviews published in the last few years in the field of continuous-flow reactions, we have decided to focus the present contribution only on the in-flow technology-assisted enantioselective synthesis and to limit the discussion especially on the most recent publications reporting the stereoselective synthesis of enantiomerically enriched compounds. The presentation is focused only on homogenous reactions and more specifically on (a) in-flow asymmetric catalysis, (b) continuous-flow stereoselective organophotoredox transformations, and (c) in-flow electrochemical stereoselective organic reactions. The use of heterogenized catalysts and catalytic reactors, immobilized enzymes, and continuous-flow biocatalysis is not included in the present contribution.
2 Continuous-flow stereoselective reactions
Flow chemistry is now universally recognized as a main enabling technology as it can ensure facile automation, reproducibility, safety, and process reliability [7,8,9,10,11,12,13]. Moving from batch to in continuo processes can potentially solve the major issues that prevent the industrialization of some synthetic methodologies and may allow to develop new processes of less environmental impact and potential cost saving [12].
Recently, industries have shown an increasing interest in the switch from batch to in continuo processes, which can potentially solve some problems, especially safety issues, related to the use of toxic reagents or the application of hazardous technologies or dangerous synthetic methods. As a result of a better control on the reaction parameters, performing reactions in continuo can improve safety, selectivity, and productivity and can represent a green and sustainable approach for the process development.
Different equipment are commercially available to run in continuo transformations. Most popular equipment are coils, micro/mesochip, and catalytic reactors, but also continuous stirred tank reactors are, nowadays, available even for laboratory scale, while they are already widespread in industries for large-scale applications. Additionally, pharma industries are considering the superior performances of continuous-flow technology over traditional batch production (precise control of reaction parameters, higher productivity) [14,15,16]. Therefore, not surprisingly, a large community of chemists and engineers, either in universities or in industries, is routinely working with continuous-flow technologies.
In this review, continuous-flow stereoselective reactions promoted by chiral catalysts, both organocatalysts [17,18] and metal-based catalysts [19,20], for the synthesis of chiral molecules, are presented and discussed.
2.1 Homogenous asymmetric catalysis in flow
Despite the exponential growth of asymmetric organocatalysis in the last decades, and the advantages of merging it with flow chemistry, the area of continuous-flow asymmetric organocatalytic reactions remains relatively little explored and, until 2016, examples were mainly limited to the use of proline and its derivatives and of chiral phosphoric acids. Since asymmetric organocatalysis in flow was the objective of a recent review [18], we will here discuss only a few selected recent examples.
Last year, our group reported the stereoselective synthesis of trifluoromethylamine mimics of retro-thiorphan, an inhibitor of metalloproteinase neutral endopeptidase [21].
The crucial step is the stereoselective, catalytic trichlorosilane-mediated reduction of fluorinated enamine 3 in the presence of catalitic amounts of chiral picolinamide 4. By studying the synthetic sequence in flow, it was possible to synthetize an advanced intermediate of retro-thiorphan in a two-step process that affords the compound 5 without the need of purification procedures of the intermediates; chiral amine 5 was isolated in up to 87:13 diastereoisomeric ratio. The reaction steps performed under continuous-flow conditions are reported in Scheme 1. The commercially available fluorinated alkyne 1 was reacted with the O-allyl-protected phenyl alaninol ether 2 to afford enamine 3 with a complete conversion after 10 min residence time. Compound 3 was then reacted with trichlorosilane in the presence of the chiral Lewis base 4. The reduction step required long residence times to obtain reasonably good conversion; to increase the yield, the reactor was heated up to 35°C, obtaining compound 5 in up to 37% isolated yield, but with lower diasteroselectivity, whereas at room temperature, the product was isolated in 20% yield and 95:5 d.r. (diastereoisomeric ratio). Although yields and d.r. were not totally satisfying, this study represents an example of how the synthesis of enantiomerically pure, pharmaceutically relevant products could be achieved through a multistep continuous-flow process.
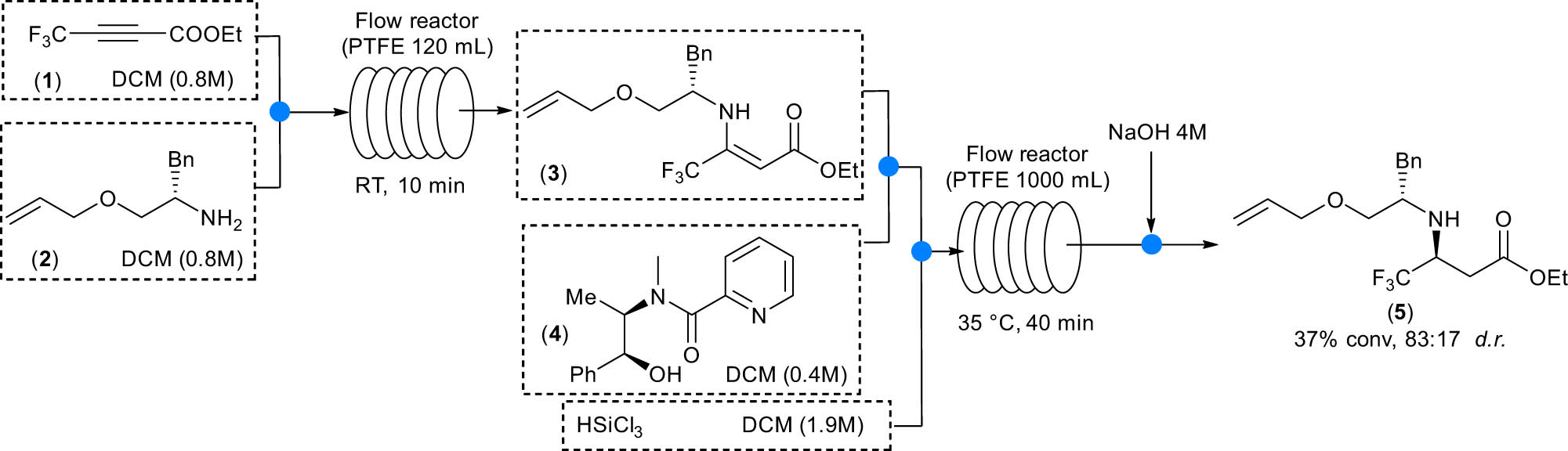
Continuous-flow synthesis of a precursor of retrothiorphan.
Recently, an in-flow asymmetric aldol reaction of 3-chloro-benzaldehyde 7 with acetone was reported to be catalyzed by prolinamide derivative 6 (Singh’s catalyst) used in a combination of buffer (pH 7)/2-propanol [22]. The project aims to realize a two-step one-flow process in an aqueous medium combining organo- and biocatalysis and the first example of a continuous-flow organocatalytic reaction with a hydrophobic substrate in water.
A long optimization work allowed us to find the correct conditions to afford the product in yield and enantiomeric excess (ee) comparable to the batch reaction. A solution of 3-chloro-benzaldehyde 2-propanol and phosphate buffer, mixed with the mixture of Singh’s catalyst, 2-propanol, acetone, and phosphate buffer, was pumped into a coil Teflon tube reactor. After equilibration of the reaction, the eluted sample was collected and quenched by dropwise addition into a solution of dichloromethane (DCM)/2.0 M HCl; after usual work up with DCM, product 8 was isolated in 74% conversion and 89% ee (Scheme 2a). One of the main issues associated with the use of homogeneous organocatalysts in flow is the high loading that these catalysts usually required to show good performances in terms of yields and stereoselectivity (20% for the initial reports, down to 1–5% that is currently used). Moreover, at the end of the reaction, a separation step is required to recover the product and, possibly, to recover and recycle the chiral catalyst.
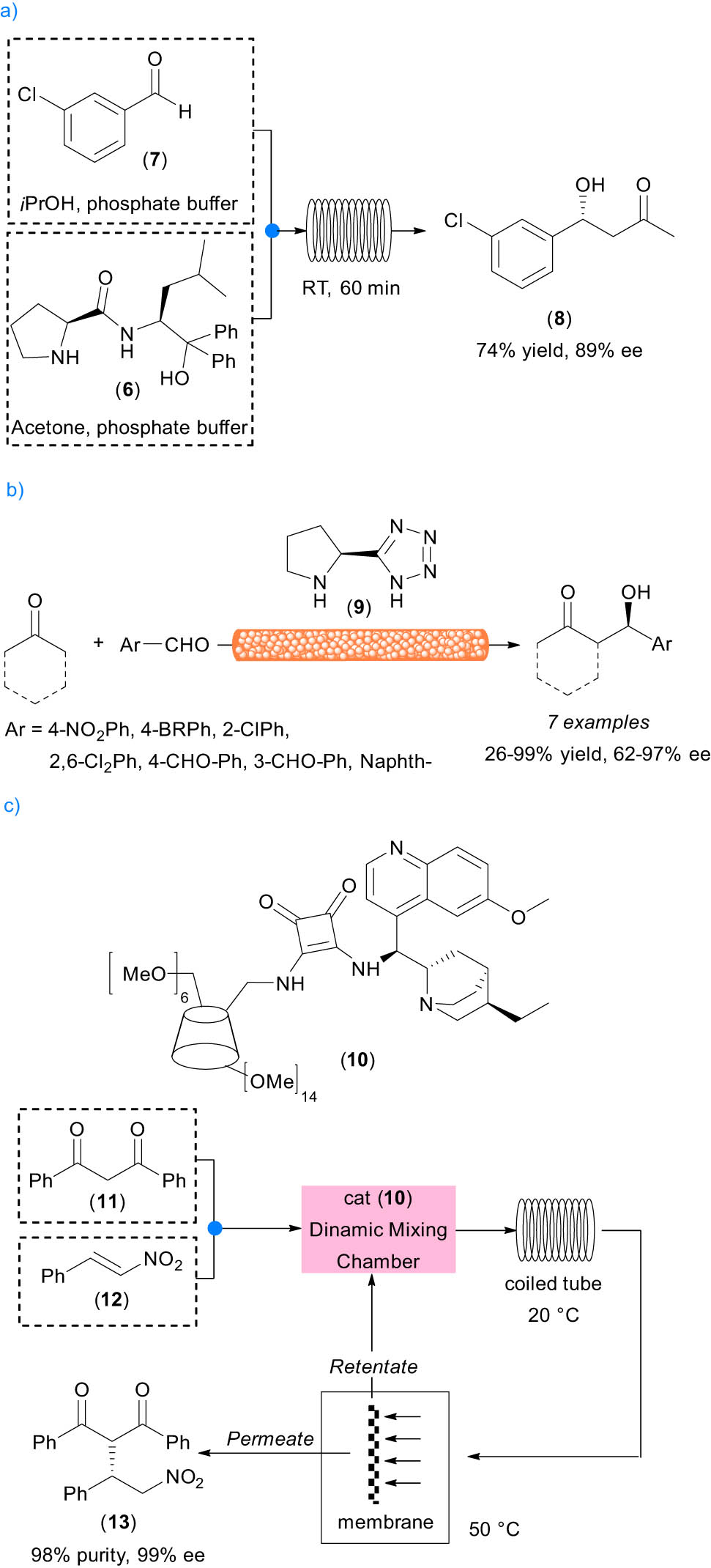
Examples of organocatalyzed transformations in flow. (a) Stereoselective aldol reaction in aqueous medium, (b) stereoselective aldol reaction with unsoluble catalyst, (c) stereoselective Michael addition with membrane separation.
Nakashima and Yamamoto reported a completely different approach for the use of nonsupported organocatalyst in flow [23]. Proline-tetrazole 9 packed into an empty column was used as a reactor; low polarity organic solvents, where the catalyst is not soluble in, were selected to run the reaction in continuo, without consumption and loss of catalytic activity (Scheme 2b). Therefore, the catalyst acts as a heterogeneous system as it is in a different phase than the reagents and the reaction solvents, but its immobilization on a solid support is not required.
The column was employed in the aldol reaction between ketones and different aromatic aldehydes in low-polar organic solvent (AcOEt), reused many times and used for reactions on 10 mmol scale, also in two different transformations, like nitro aldol and the Mannich reactions; it was observed that the system affords better ee with the addition of 3% water. Products were obtained often in higher than 70% yields. To assess the catalyst performances, the authors introduced a new reaction metric, the process catalyst mass efficiency (PCME), which is defined as the actual mass of catalyst used or catalyst consumption in the process relative to the moles of the desired product. Although the nonsupported proline tetrazole showed low PCME with respect to other solid-supported organocatalysts, this approach proved to be simple and efficient in the aldol reactions.
The last example discussed in this section allows us to introduce the concept of membrane separation. The membrane-assisted recovery of homogeneous catalysts [24] is sustainable with low-energy consumption, and its use in flow chemistry is feasible, scalable, and easily implemented in continuous-flow processes (Scheme 2c).
The molecular weight gap between the catalyst and the other components as well as the absolute catalyst retention by the membrane are the two main factors responsible for the efficiency of the strategy that usually requires a size enlargement of the catalyst, realized by conjugation to dendrimers or by embedding in soluble polymers. Kisszekely and Alammar prepared β-cyclodextrins functionalized with cinchona-thiourea and squaramide derivatives as enlarged organocatalysts for the addition of diketones to β-nitrostyrene [25].
After preliminary tests in batch, the homogeneous catalysts were used under optimized conditions in the model reaction in the continuous-flow reactor. The scheme of the continuous catalysis–separation platform is shown in Scheme 2c. Among the different membranes tested, the best performing system showed a 100% rejection of catalyst 10 and less than 5% rejection of the other species. This result was possible due to the large gap in the size of the catalyst and the reactants. During the integrated synthesis–separation process, the outcome of the flow reactor, containing the crude reaction mixture, was directed to a cross-flow membrane cell.
The permeate stream with high concentrations of product 13 (41 g·L−1) has a purity of 92%. The retentate stream in situ recycled 100% of catalyst 10 and 50% of the 2-MeTHF solvent. A fresh solution of diketone 11 and trans-β-nitrostrene 12 was pumped into a dynamic mixing chamber where it met the recycled stream. From the evaluation of the effect of recirculation on the productivity, purity, and concentrations, it turned out that the best results could be obtained with 50% recirculation, with the membrane thermoregulated at 50°C, to prevent the precipitation of the product. Product 13 was isolated by crystallization with 98% final purity and 99% e.e. The robustness and reusability of the catalyst were demonstrated over 18 days of operation, operating at up to 100°C in the flow reactor.
The three-dimensional (3D) printing technology represents a very promising tool that has found increasing applications in organic synthesis. The 3D printing is an additive manufacturing technique consisting in the production of 3D solid objects through the successive layering deposition of material [26]. It has been not only used for the realization of many fluidic devices [27] and catalyst-embedded devices to promote different chemical transformations [28,29] but also for pharmaceutical manufacturing [30]. At the moment, despite the numerous synthetic methodologies reported, only few of them are related to the synthesis of chiral molecules.
In 2017, our group reported the first stereoselective catalytic synthesis of norephedrine, metaraminol, and methoxamine accomplished under fluidic conditions using homemade fuse deposition modeling 3D-printed mesoreactors [31]. The common synthetic strategy for these products of pharmaceutical interest involves a catalytic, stereoselective nitroaldol reaction between benzaldehyde 14 and nitroethane performed under flow conditions in the presence of chiral copper complex catalyst obtained combining Cu(OAc)2·H2O with chiral ligand 15 derived from camphor. This transformation was performed using a poly lactic acid (PLA) 3D-printed mesoreactor. The crude nitro aldol 16 obtained from this process was then subjected to a short silica-pad filtration (to remove the copper catalyst that is not compatible with the hydrogenation process) before proceeding to an in-flow hydrogenation of the nitro group. The detailed process for the synthesis of norephedrine is summarized in Scheme 3. Many mesoreactors, differing in channel shapes and dimensions, as well as different copper complexes and reaction conditions were investigated. The best results were obtained when the synthesis of nitro alcohol 16 was performed using a PLA 3D-printed mesoreactor in the presence of complex [Cu]-15 at −20°C with a 30 min as residence time. The Pd/C catalyzed hydrogenation of the crude product performed in the presence of acetic acid afforded the desired norpehedrine 17 in 90% overall yield, 65:35 anti:syn ratio and with an enantiomeric excess of 80% for the anti-isomer.
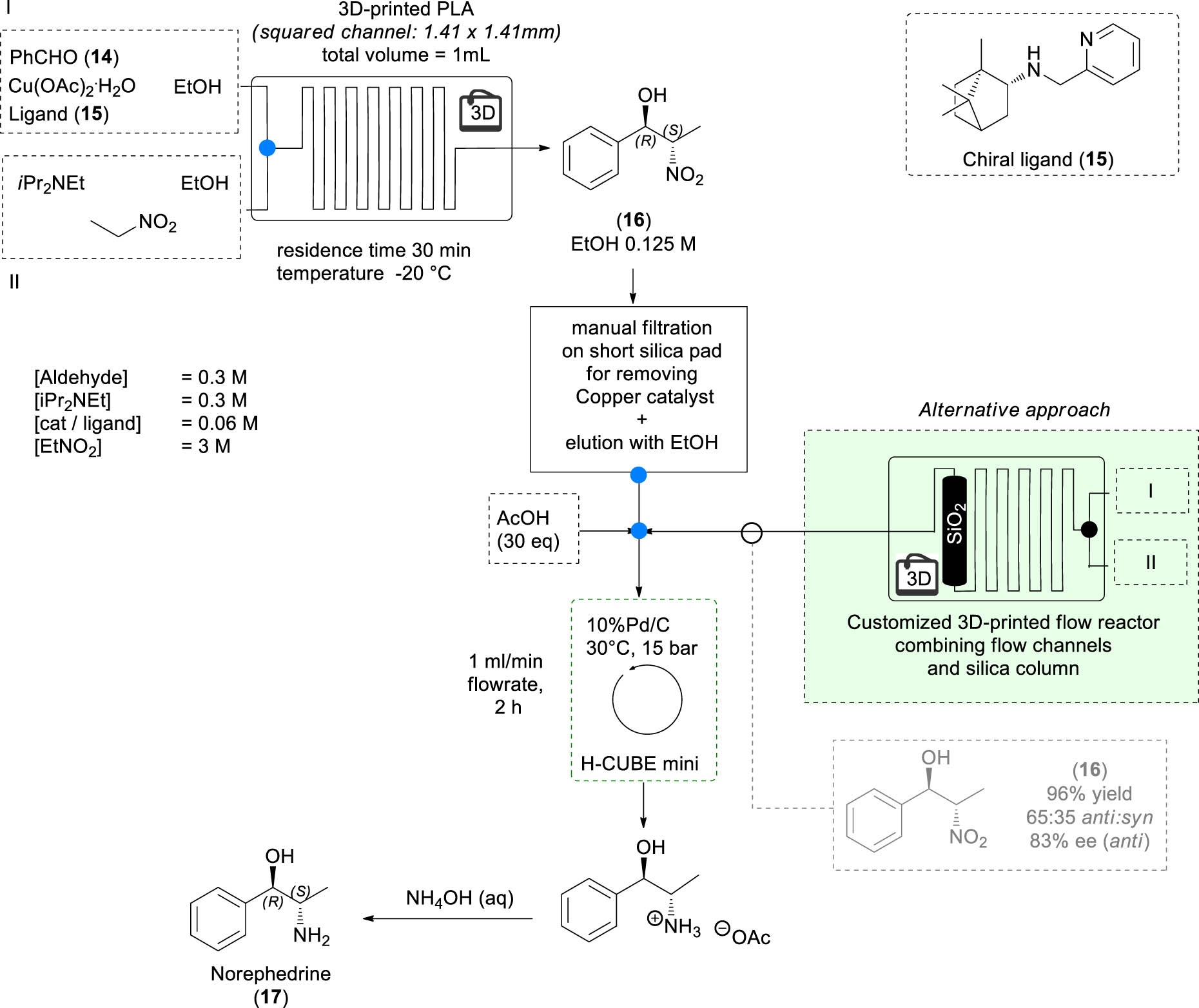
In-flow stereoselective organic synthesis in 3D-printed reactors.
Taking advantage of the powerful possibility offered by 3D printing technology to realize ad hoc devices, we described also the fabrication of a new fluidic device, in which the presence of flow channels (where the chemical transformation takes place) is combined with a short column of silica (necessary for the removal of the copper catalyst). In this case, the nitroaldol intermediate 16 was obtained in 96% yield, 65:35 anti:syn ratio, and 83% ee for the anti-isomer, which afforded norephedrine 17 after the earlier described in-flow hydrogenation process (Scheme 3, green box).
In the same year, Hilton group published the use of a 3D-printed polypropylene continuous-flow column reactor as component of a continuous-flow system for the synthesis of achiral bicyclic and tetracyclic heterocycles [32]. Among all the examples reported, it was shown that this fluidic device can be successfully used for the synthesis of compound 18, a precursor of the erythrina alkaloid family. In dichloroethane as a solvent of choice, the reaction proceeded in the presence of more than stoichiometric amounts of BF3·OEt2 at 0.1 mL·min−1 of flow rate, leading to the formation of 18 in 45% yield as a single diastereoisomer (Scheme 4a).
In 2019, Franzreb and coworkers developed 3D-printed hydrogel lattices for enzyme entrapment, which were inserted into 3D-printed reactor housings operating under continuous-flow condition to perform different enzymatic reactions [33].
An alcohol dehydrogenase from Lactobacillus brevis and a benzoylformate decarboxylase from Pseudomonas putida were entrapped in a crosslinked poly(ethylene glycol) diacrylate-based hydrogel and used to promote the synthesis of (R)-phenylethanol 19 and (1S,2S)-1-phenylpropane-1,2-diol 20, respectively (Scheme 4b). Stable product formation was observed over a period of 72 h for all enzymatic systems, even if the reaction rates of entrapped enzymes in the continuous-flow system were lower than those of the reactions performed under batch conditions.
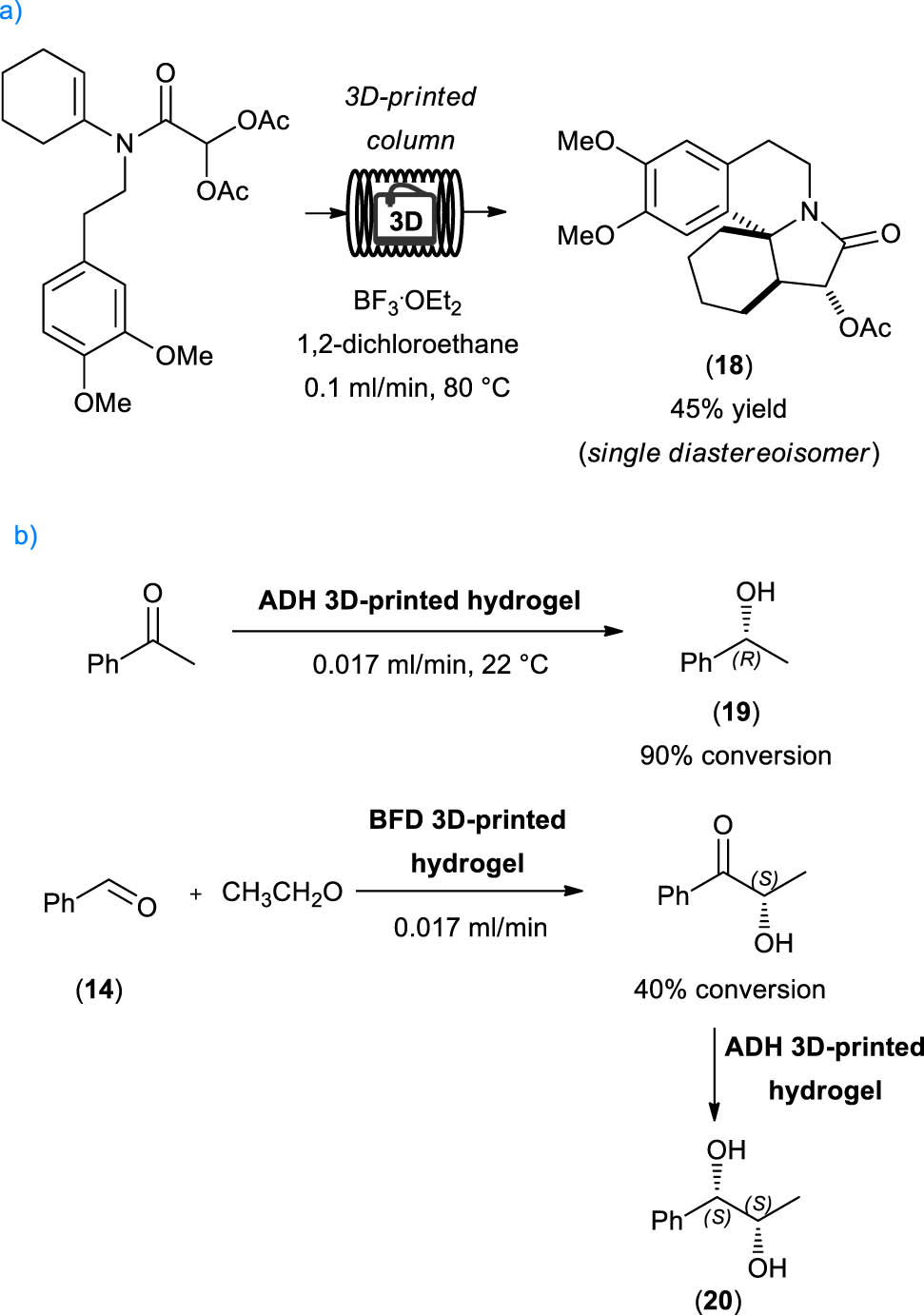
Examples of 3D printing applications to flow reactions. (a) Synthesis of erythratin core under flow condition using a 3D-printed column, (b) 3D-printed enzyme flow-through reactor.
2.2 Continuous-flow organophotoredox transformations
Dual catalysis of photoredox and organocatalysis has emerged in the last 10 years as a powerful synthetic tool. MacMillan and coworkers first demonstrated that unprecedented synthetic approaches could be opened to produce chiral molecules that are otherwise difficult to prepare with traditional strategies [34]. After the initial discoveries of MacMillan, a number of dual organophotoredox catalytic reactions were reported. Two reviews, by Yoon et al. and Bach et al., were published in the last 4 years, summarizing the findings [35,36].
To our knowledge, until today, only three successful continuous-flow examples of dual catalysis exist [37,38,39]. Those continuous-flow experiments showed a drastic increase in productivity, up to 100-fold. The high-molar extinction coefficients of organic dyes and metal complexes prevent most of the internal reactor volume to receive efficient irradiation: under batch conditions, most of the light is absorbed in the first few millimeters of the solution. In continuous flow, the increased surface-to-volume ratio of the microreactors is exploited to achieve much higher levels of irradiation.
Neumann and Zeitler reported the in-flow reaction between bromo-malonate 21 and octanal 22 catalyzed by MacMillan imidazolidinone (triflate salt) 23 in the presence of photocatalyst Eosin Y and 2,6-lutidine to afford α-alkylated aldehyde 24 [37]. Two different reactor setups were designed: one using glass microreactor technology together with light emitting diodes at 530 nm and the other using polyfluoroethylene high performance liquid chromatography (HPLC) tubing wrapped in coils around the 23 W compact fluorescent lamp household bulb, which was immersed in a cooling bath, as depicted in Scheme 5a. With this setup, an optimal irradiation was achieved with large reactor volumes and lengths (10.5 mL and 21 m of length). The reaction under flow condition still exhibits high enantioselectivity and high yields, leading to product 24 in up to 86% yield and up to 87% ee. Moreover, a 107-fold increase in productivity with respect to the batch conditions was achieved, due to the more efficient irradiation.
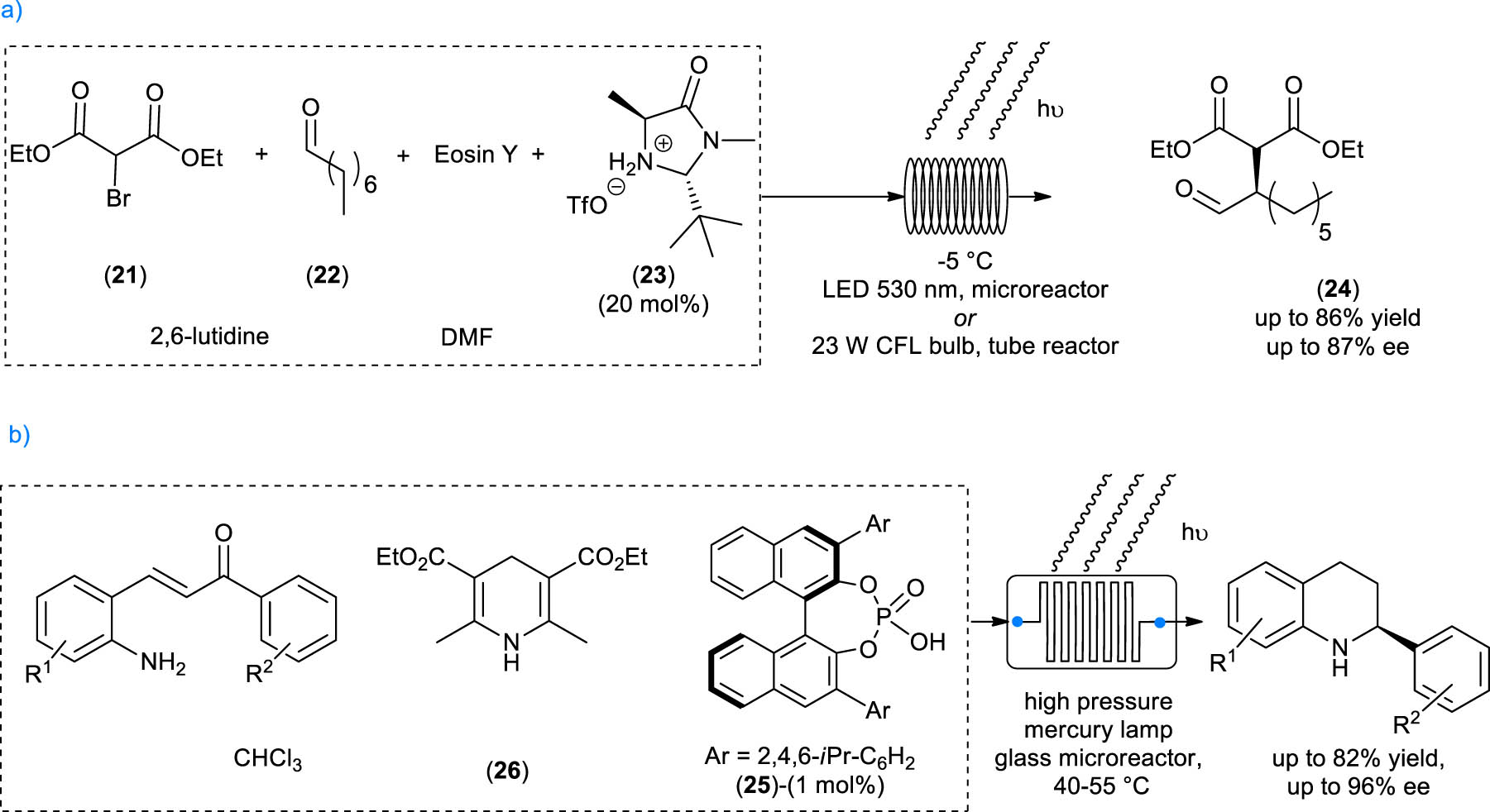
Examples of continuous-flow organophotoredox transformations. (a) MacMillan’s dual catalysis of α-alkylated aldehydes optimized by Zeitler and Neumann for continuous flow conditions, (b) Rueping and Sugiono's reductive cascade cyclization reaction flow setup.
An organocatalytic photocyclization–transfer hydrogenation cascade reaction was published by Rueping and Sugiono, using 2-amino-chalcones as the reagent, phosphoric acid 25 as the chiral catalyst, and Hantzsch ester 26 as the reducing agent [38].
For the continuous-flow setup, a glass microreactor immersed in a thermostated water bath was used. Directly next to it, a high-pressure mercury lamp irradiated the reactor from the side. This methodology allowed to prepare differently substituted isoquinolines in very high yield and ee, starting from readily available 2-aminochalcones (Scheme 5b). In particular, the flow setup showed a significant increase in productivity due to the more efficient irradiation. Moreover, the continuous removal of the product from the irradiation source avoided over-irradiation that can lead to undesired background reactions.
These two examples highlight some of the major advantages of conducting photoredox catalytic reactions in flow: the more efficient irradiation in the reaction vessel, the ease of scalability and the continuous removal of the product from the light source to avoid side-reactions (photodegradation and/or undesired reactions).
More recently, Meng et al. reported the use of photomicroreactors to perform the enantioselective photooxygenation of β-dicarbonyl compounds; in-batch and in-flow reactions were compared [39].
Differently modified cinchonine-derived phase-transfer catalysts were synthesized and used in the enantioselective photo-organocatalytic aerobic oxidation of β-dicarbonyl compounds with up to 97% yields and high enantioselectivities (up to 90% ee). Furthermore, when the reaction was carried out in a flow photomicroreactor, up to 97% yields and up to 86% ee were reached in less than 1 min.
In 2021, our group reported the stereoselective visible light catalytic cyclization of bis(enones), an easy entry to enantiomerically enriched cyclopentanes [40]. One of the more innovative strategies to build cyclic system is offered by the photoredox activation of aryl enones. We have studied the possibility to control the absolute stereochemistry of the final product, in a metal-free procedure, to generate enantioenriched cyclopentane rings.
The introduction of a chiral auxiliary, such as Evans’ oxazolidinones, into the bisenone chain offers a straightforward and convenient option to stereocontrol the light-driven cyclization. After the cyclization and the removal of the oxazolidinones that could recover, the functionalized 1,2-trans cyclopentane could be isolated in good yield and up to 83:17 enantiomeric ratio. When the reaction was performed in continuo, in a homemade coil photoreactor, high yields were observed. The cyclization was also successfully realized in a 3D-printed mesoreactor, without any change in the diastereoselectivity of the process (Scheme 6).
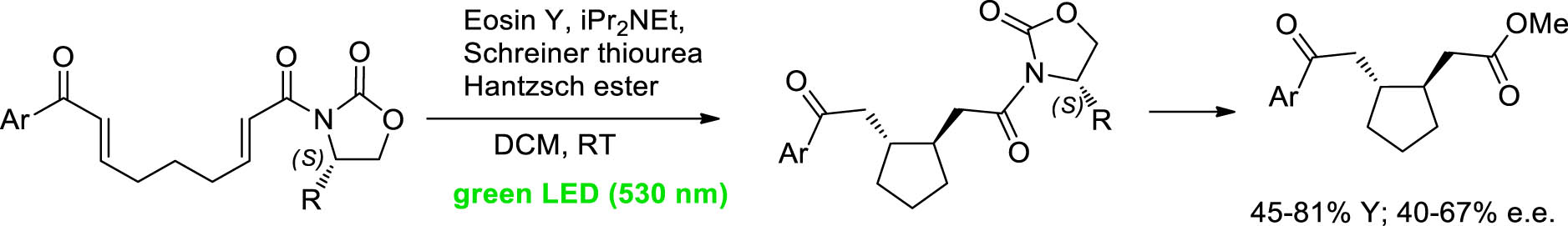
Continuous-flow organophotoredox cyclization reactions.
2.3 Electrochemistry
The application of electrochemical methods to the synthesis of organic molecules has witnessed a renaissance in the past few years. Undoubtedly, the replacement of oxidizing/reducing chemical agents by electricity is a great step toward the development of sustainable chemical processes. Despite the long history of electroorganic synthesis, this renewed interest in organic synthesis is relatively recent. This is probably due to a lack of equipment to develop standardized electrochemical protocols [41]. It is not surprising, then, that the development of equipment to perform organic reactions in flow helped the synthetic chemists to explore new possibilities with electrochemistry [42,43]. Continuous-flow electrolysis is performed by pumping the solution of the reagents between the two electrodes, with a typically small interelectrode gap. Usually, the reaction can be carried out with low concentrations of supporting electrolyte or even without supporting electrolyte at all, since the short distance between the two electrodes reduces the ohmic resistance. Moreover, the continuous removal of the reaction mixture minimizes the possibility of overoxidation, which is one of the main problems of electrolysis under batch conditions [44,45]. Finally, microreactors offer high surface-to-volume ratios and enable precise control over temperature, residence time, flow rate, and pressure. In addition, efficient mixing, enhanced mass and heat transfer, and handling of small volumes lead to simpler scaling-up protocols and minimize safety concerns. Nevertheless, achieving stereocontrol in the electrochemical process, where “electrons” are used as reagents, remains a major challenge [46].
The electrochemical transformation of an achiral material to a chiral (enantioenriched) molecule can be achieved, according to Rueping’s classification, by using chiral electrodes, chiral media, and chiral auxiliaries. As chiral media, we intend a chiral electrolyte, a chiral mediator, or a chiral catalyst (organic, enzyme, and metal-based). Since excellent recent reviews highlighted the modern aspects of electrochemistry, in this article, we will report few selected recent examples of electrochemical stereoselective transformations performed in flow to underline the potentialities of the technology.
Waldvogel and Opatz reported the total synthesis of (–)-oxycodone, a semisynthetic opioid derived from naturally occurring thebaine that presents a significantly higher oral bioavailability than morphine. It was prepared by a fully regio- and diastereoselective electrochemical 4a-2′-coupling of a 3′,4′,5′-trioxygenated laudanosine derivative [47]. The authors performed a first optimization study on the racemic compound 27 as starting material. Boron-doped diamond (BDD) anode in combination with a platinum cathode in acetonitrile containing a small amount of aqueous HBF4 as acidic electrolyte to prevent amine oxidation proved to be the best combination. It was observed that BDD showed higher performances for dehydrogenative couplings than other materials. They then performed an extensive screening of reaction parameters, including temperature, current density, reactant concentration, and stoichiometry of acidic additive, both in batch electrolysis and in continuous-flow mode. The desired product 28 was obtained in up to 69% yield in batch and up to 57% yield in flow. The same reaction was then carried out on the enantiopure compound 27 to afford enantiopure derivative 28 that was further elaborated to the desired (–)-oxycodone. Noteworthy, this complex transformation was operationally simple and afforded fully regio- and diastereoselectivity in good yields (Scheme 7a).
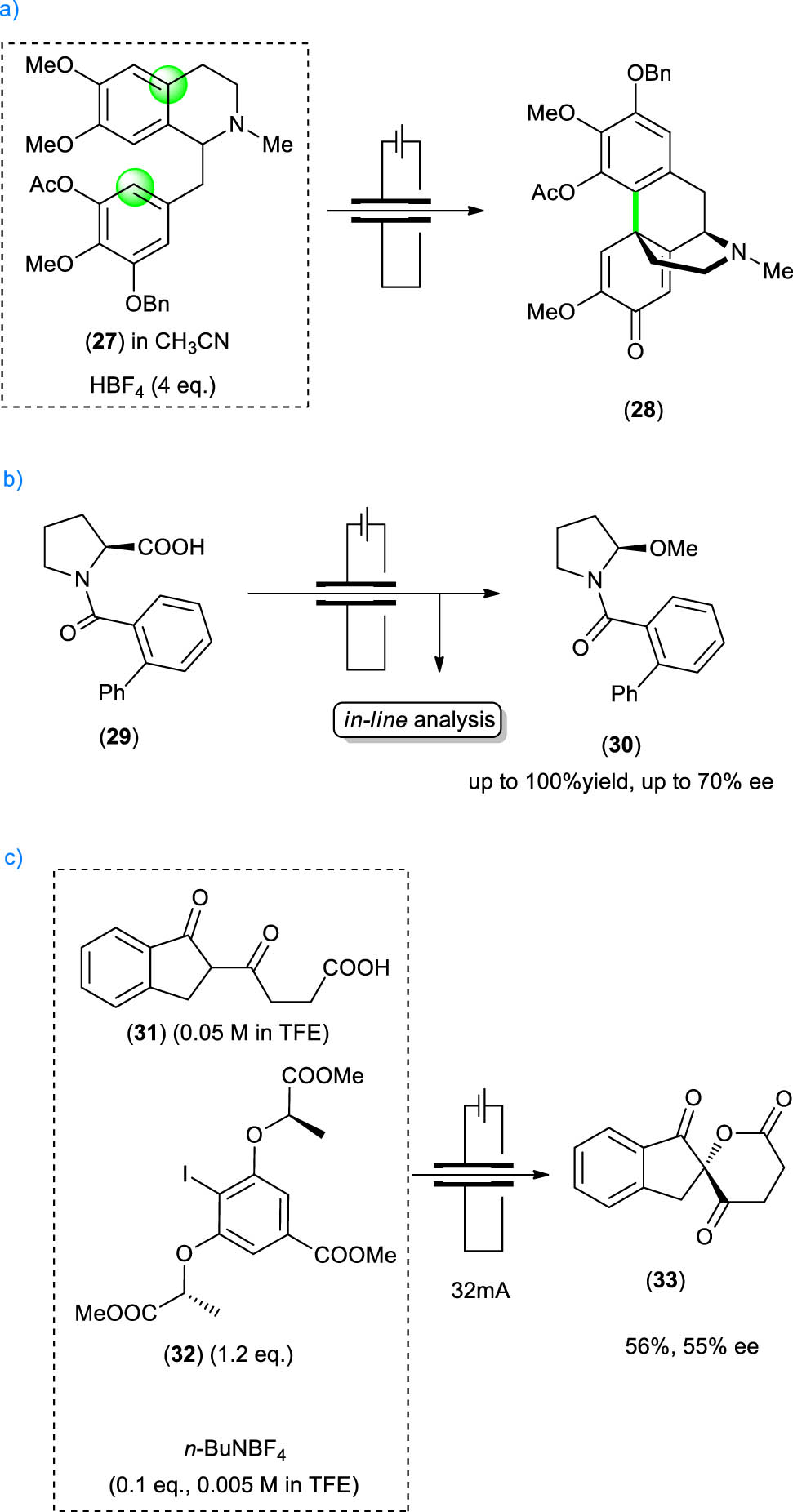
Stereoselective electrochemical transformations in flow. (a) Stereoselective aryl--aryl coupling, (b) electrolysis of proline 29, (c) electrochemical lactonization mediated by promoter 32.
Wirth described the electrolysis of N-(2-phenyl)benzoyl proline 29 through an acyliminium ion intermediate to afford an enantiomerically enriched α-alkoxyamino compound. The reaction proceeds via the electrochemical decarboxylation of substrate 29 and oxidation of the resulting radical to the iminium ion, which is trapped by methanol. The presence of the bulky substituent at the nitrogen atom is responsible for the face selectivity of the nucleophilic addition and leads to the formation of product 30 that maintains the same configuration of the starting material at the stereocenter [48]. The authors also developed a rapid and reliable analytical method to evaluate the outcome of the stereoselective electrochemical alkoxylation by coupling the flow system to a 2D-HPLC. This allowed to obtain the percentage yield and the percentage enantiomeric excess of each experiment within 15 min. The possibility of obtaining the desired information directly from the reaction mixture, without isolation or purification step, can dramatically speed up the optimization process and is an additional benefit of performing the reaction in flow. The authors made an extensive optimization of reaction parameters, such as material of the anode, reaction temperature, flow rates, distance of the electrodes, and charge. Product 30 was obtained in high yields (up to 100%) and enantioselectivities up to 70% ee within a short period. The reaction was also scaled-up, and the products were isolated without loss in enantioselectivity (Scheme 7 b).
The same group reported the enantioselective electrochemical lactonization of diketo acid derivatives, using chiral iodoarenes as redox mediators [49]. Hypervalent iodine(iii) reagents are generated via anodic oxidation and can be used to realize several transformations without using a stoichiometric oxidant, such as mCPBA, 3-chloroperbenzoic acid. After extensive optimization in batch, the authors investigated the lactonization of diketo acid 31 to lactone 33 in the presence of the chiral iodoarene 32 and n-Bu4NI as mediators, using platinum as anode and cathode materials, in 2,2,2-trifluoroethanol as a solvent as fluorinated solvents are known to stabilize iodine(iii) reagents. They obtained lactone 33 in 56% yield and 55% ee in the first enantioselective reaction with iodine(iii) reagents generated in an electrochemical flow microreactor, as shown in Scheme 7c.
3 Conclusion
The advent and the full development to a mature age of several, new or rediscovered, technologies have given a new impulse to the organic synthesis and offered incredibly powerful tools at the service of the creativity of the synthetic chemist. Alternative and more efficient heating systems, microwave, and ultrasound technologies, mechanochemistry, electroorganic synthesis, flow chemistry, and photoredox catalysis were all successfully used to speed up reactions or simplify the isolation and/or the analysis of the product, or to efficiently perform nonconventional transformations that would be impossible to realize without the decisive support of a new technique or an innovative technology. Safer processes could be developed taking advantage of continuous-flow technologies [50].
However, in the synthesis of an enantiomerically pure chiral molecule, not only the chemical efficiency and the chemoselectivity of the process should be reached but also the stereochemical outcome of the reaction must be efficiently controlled. One of the major challenges in the field is to develop telescoped stereoselective processes, thus avoiding isolation of intermediates and maximizing efficiency, practicality, and environmental impact [51,52,53,54,55,56]. Moreover, in this area, much space for improvement still exists; for example, the introduction of automation in organic chemistry is a hot topic, and in the last years, extraordinary achievements in automated synthesis have been reported [57,58,59].
It is easy to predict in a near future, the publication of novel applications and significant breakthroughs in new, challenging, but highly rewarding, fields [60,61].
-
Funding information: MB and SR thank Università degli Studi di Milano (grant PSR 2019). AP thanks Università degli Studi di Milano (Transition Grant 2019). MB and FH thank ITN-EID project Marie Sklodowska-Curie Actions Innovative Training Network – TECHNOTRAIN H2020-MSCA-ITN-2018 Grant Agreement 812944 (www.technotrain-ITN.eu).
-
Author contributions: Alessandra Puglisi: writing – original draft, writing – review and editing, Sergio Rossi: writing – original draft, visualization, project administration; Fabian Herbrik: writing – original draft; Fabrizio Medici: writing – original draft; Maurizio Benaglia: writing – original draft, writing – review and editing, resources.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Communication from the commission to the European parliament, the council, the european economic and social committee and the committee of the regions ‘A European strategy for Key Enabling Technologies – A bridge to growth and jobs – Document 52012DC0341.Search in Google Scholar
[2] Fitzpatrick DE, Battilocchio C, Ley SV. Enabling technologies for the future of chemical synthesis. ACS Cent Sci. 2016;2:131–8.10.1021/acscentsci.6b00015Search in Google Scholar PubMed PubMed Central
[3] Knox ST, Warren NJ. Enabling technologies in polymer synthesis: accessing a new design space for advanced polymer materials. React Chem Eng. 2020;5:405–23.10.1039/C9RE00474BSearch in Google Scholar
[4] de Almeida AF, Moreira R, Rodrigues T. Synthetic organic chemistry driven by artificial intelligence. Nat Rev Chem. 2019;3:589–604.10.1038/s41570-019-0124-0Search in Google Scholar
[5] Trobe M, Burke MD. Automated synthesis of small molecules. Angew Chem Int Ed. 2018;57:4192–214.10.1002/anie.201710482Search in Google Scholar PubMed PubMed Central
[6] Kirschning A, Solodenko W, Mennecke K. Combining enabling techniques in organic synthesis: continuous flow processes with heterogenized catalysts. Chem Eur J. 2006;12:5972–90.10.1002/chem.200600236Search in Google Scholar PubMed
[7] Plutschack MB, Pieber B, Gilmore K, Seeberger PH. The Hitchhiker’s guide to flow chemistry. Chem Rev. 2017;117:11796–893.10.1021/acs.chemrev.7b00183Search in Google Scholar PubMed
[8] Yu T, Ding Z, Nie W, Jiao J, Zhang H, Zhang Q, et al. Recent advances in continuous flow enantioselective catalysis. Chem Eur J. 2020;26:5729–47.10.1002/chem.201905151Search in Google Scholar PubMed
[9] Yoo W-JYoo, Ishitani H, Laroche B, Kobayashi S. Reworking organic synthesis for the modern age: synthetic strategies on continuous-flow addition and condensation reactions with heterogeneous catalysts. J Org Chem. 2020;85:5132–45.10.1021/acs.joc.9b03416Search in Google Scholar PubMed
[10] Pastre JC, Browne DL, Ley SV. Flow chemistry syntheses of natural products. Chem Soc Rev. 2013;42:8849–69.10.1039/c3cs60246jSearch in Google Scholar PubMed
[11] Wirth T. Microreactors in organic synthesis and catalysis. 2nd ed. Weinheim: Wiley-VCH; 2013.10.1002/9783527659722Search in Google Scholar
[12] Hartman RL, McMullen JP, Jensen KF. Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angew Chem Int Ed. 2011;50:7502–19.10.1002/anie.201004637Search in Google Scholar PubMed
[13] Baumann M, Moody TS, Smyth M, Wharry S. A perspective on continuous flow chemistry in the pharmaceutical industry. Org Process Res Dev. 2020;24:1802–13.10.1021/acs.oprd.9b00524Search in Google Scholar
[14] May SA. Flow chemistry, continuous processing, and continuous manufacturing: a pharmaceutical perspective. J Flow Chem. 2017;7:137–45.10.1556/1846.2017.00029Search in Google Scholar
[15] Porta R, Benaglia M, Puglisi A. Flow chemistry: recent developments in the synthesis of pharmaceutical products. Org Process Res Dev. 2016;20:2–25.10.1021/acs.oprd.5b00325Search in Google Scholar
[16] Gutmann B, Cantillo D, Kappe CO. Continuous-flow technology–a tool for the safe manufacturing of active pharmaceutical ingredients. Angew Chem Int Ed. 2015;54:6688–728.10.1002/anie.201409318Search in Google Scholar PubMed
[17] Puglisi A, Benaglia M, Chiroli V. Stereoselective organic reactions promoted by immobilized chiral catalysts in continuous flow systems. Green Chem. 2013;15:1790–813.10.1039/c3gc40195bSearch in Google Scholar
[18] Atodiresei I, Vila C, Rueping M. Asymmetric organocatalysis in continuous flow. ACS Catal. 2015;5:1972–85.10.1021/acscatal.5b00002Search in Google Scholar
[19] Tsubogo T, Ishiwata T, Kobayashi S. Asymmetric carbon-carbon bond formation under continuous-flow conditions with chiral heterogeneous catalysts. Angew Chem Int Ed. 2013;52:6590–604.10.1002/anie.201210066Search in Google Scholar PubMed
[20] Zhao D, Ding K. Recent advances in asymmetric catalysis in flow. ACS Catal. 2013;3:928–44.10.1021/cs300830xSearch in Google Scholar
[21] Pirola M, Puglisi A, Raimondi L, Forni A, Benaglia M. Evaluation of in-batch and in-flow synthetic strategies towards the stereoselective synthesis of a fluorinated analogue of retro-thiorphan. Molecules. 2019;24:2260–9.10.3390/molecules24122260Search in Google Scholar PubMed PubMed Central
[22] Schober L, Ratnam S, Yamashita Y, Adebar N, Pieper M, Berkessel A, et al. An asymmetric organocatalytic Aldol reaction of a hydrophobic aldehyde in aqueous medium running in flow mode. Synthesis. 2019;51:1178–84.10.1055/s-0037-1610404Search in Google Scholar
[23] Nakashima E, Yamamoto H. Process catalyst mass efficiency by using proline tetrazole column-flow system. Chem Eur J. 2018;24:1076–9.10.1002/chem.201705982Search in Google Scholar PubMed
[24] Ormerod D, Lefevre N, Dorbec M, Eyskens I, Vloemans P, Duyssens K, et al. Potential of homogeneous Pd catalyst separation by ceramic membranes. Application to downstream and continuous flow processes. Org Process Res Dev. 2016;20:911–20.10.1021/acs.oprd.5b00418Search in Google Scholar
[25] Kisszekely P, Alammar A, Kupai J, Huszthya P, Barabasa J, Holtzlac T, et al. Asymmetric synthesis with cinchona-decorated cyclodextrin in a continuous-flow membrane reactor. J Catal. 2019;371:255–61.10.1016/j.jcat.2019.01.041Search in Google Scholar
[26] Capel AJ, Rimington RP, Lewis MP, Christie SDR. 3D printing for chemical, pharmaceutical and biological applications. Nat Rev Chem. 2018;2:422–36.10.1038/s41570-018-0058-ySearch in Google Scholar
[27] Rossi S, Puglisi A, Benaglia M. Additive manufacturing technologies: 3D printing in organic synthesis. Chem Cat Chem. 2018;10:1512–25.10.1002/cctc.201701619Search in Google Scholar
[28] Rossi S, Puglisi A, Raimondi L, Benaglia M. Stereolitography 3D-printed catalytically active devices in organic synthesis. Catalysts. 2020;10:109–17.10.3390/catal10010109Search in Google Scholar
[29] Hilton S;, Penny M;, Dos Santos BS;, Patel B. Three-dimensional printing of impregnated plastic for chemical reactions; Patent application WO2017158336A1. 21 September 2017.Search in Google Scholar
[30] Icten E, Giridhar A, Taylor LS, Nagy ZK, Reklaitis GV. Dropwise additive manufacturing of pharmaceutical products for melt-based dosage forms. J Pharm Sci. 2015;104:1641–9.10.1002/jps.24367Search in Google Scholar PubMed
[31] Rossi S, Porta R, Brenna D, Puglisi A, Benaglia M. Stereoselective catalytic synthesis of active pharmaceutical ingredients in homemade 3D-printed mesoreactors. Angew Chem Int Ed. 2017;56:4290–4.10.1002/anie.201612192Search in Google Scholar PubMed
[32] Rao ZX, Patel B, Monaco A, Cao Z, Barniol-Xicota M, Pichon E, et al. 3D-Printed polypropylene continuous-flow column reactors: exploration of reactor utility in SNAr reactions and the synthesis of bicyclic and tetracyclic heterocycles. Eur J Org Chem. 2017;44:6499–504.10.1002/ejoc.201701111Search in Google Scholar
[33] Schmieg B, Dobber J, Kirschhofer F, Pohl M, Franzreb M. Advantages of hydrogel-based 3D-printed enzyme reactors and their limitations for biocatalysis. Front Bioeng Biotechnol. 2018;6:211–9.10.3389/fbioe.2018.00211Search in Google Scholar PubMed PubMed Central
[34] Nicewicz DA, MacMillan DWC. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science. 2008;322:77–80.10.1126/science.1161976Search in Google Scholar PubMed PubMed Central
[35] Skubi KL, Blum TR, Yoon TP. Dual catalysis strategies in photochemical synthesis. Chem Rev. 2016;116:10035–74.10.1021/acs.chemrev.6b00018Search in Google Scholar PubMed PubMed Central
[36] Zou YQ, Hormann FM, Bach T. Iminium and enamine catalysis in enantioselective photochemical reactions. Chem Soc Rev. 2018;47:278–90.10.1039/C7CS00509ASearch in Google Scholar PubMed PubMed Central
[37] Neumann M, Zeitler K. Application of microflow conditions to visible light photoredox catalysis. Org Lett. 2012;14:2658–61.10.1021/ol3005529Search in Google Scholar PubMed
[38] Sugiono E, Rueping M. A combined continuous microflow photochemistry and asymmetric organocatalysis approach for the enantioselective synthesis of tetrahydroquinolines. Beilstein J Org Chem. 2013;9:2457–62.10.3762/bjoc.9.284Search in Google Scholar PubMed PubMed Central
[39] Tang XF, Zhao JN, Wu YF, Zheng ZH, Feng SH, Yu ZY, et al. Enantioselective photooxygenation of β-dicarbonyl compounds in batch and flow photomicroreactor. Org Biomol Chem. 2019;17:7938–42.10.1039/C9OB01379BSearch in Google Scholar PubMed
[40] Medici F, Resta S, Presenti P, Caruso L, Puglisi A, Raimondi L, et al. Stereoselective visible-light catalyzed cyclization of bis(enones): a viable approach to the synthesis of enantiomerically enriched cyclopentane rings. Eur J Org Chem. 2021;4521–4.10.1002/ejoc.202100397Search in Google Scholar
[41] Möhle S, Zirbes M, Rodrigo E, Gieshoff T, Wiebe A, Waldvogel SR. Modern electrochemical aspects for the synthesis of value-added organic products. Angew Chem Int Ed. 2018;57:6018–41.10.1002/anie.201712732Search in Google Scholar PubMed PubMed Central
[42] Atobe M, Tateno H, Matsumura Y. Applications of flow microreactors in electrosynthetic processes. Chem Rev. 2018;118:4541–72.10.1021/acs.chemrev.7b00353Search in Google Scholar PubMed
[43] Kingston C, Palkowitz MD, Takahira Y, Vantourout JC, Peters BK, Kawamata Y, et al. A survival guide for the “Electro-curious”. Acc Chem Res. 2020;53:72–83.10.1021/acs.accounts.9b00539Search in Google Scholar PubMed PubMed Central
[44] Elsherbini M, Wirth T. Electroorganic synthesis under flow conditions. Acc Chem Res. 2019;52:3287–96.10.1021/acs.accounts.9b00497Search in Google Scholar PubMed
[45] Noel T, Cao Y, Laudadio G. The fundamentals behind the use of flow reactors in electrochemistry. Acc Chem Res. 2019;52:2858–69.10.1021/acs.accounts.9b00412Search in Google Scholar PubMed PubMed Central
[46] Ghosh M, Shinde VS, Rueping M. A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions. Beilstein J Org Chem. 2019;15:2710–46.10.3762/bjoc.15.264Search in Google Scholar PubMed PubMed Central
[47] Lipp A, Selt M, Ferenc D, Schollmeyer D, Waldvogel SR, Opatz T. Total synthesis of (−)-oxycodone via anodic Aryl–Aryl coupling. Org Lett. 2019;21:1828–31.10.1021/acs.orglett.9b00419Search in Google Scholar PubMed
[48] Santi M, Seitz J, Cicala R, Hardwick T, Ahmed N, Wirth T. Memory of chirality in flow electrochemistry: fast optimisation with DoE and online 2D-HPLC. Chem Eur J. 2019;25:16230–35.10.1002/chem.201904711Search in Google Scholar PubMed
[49] Gao WC, Xiong ZY, Pirhaghani S, Wirth T. Enantioselective electrochemical lactonization using chiral iodoarenes as mediators. Synthesis. 2018;51:276–84.10.1055/s-0037-1610373Search in Google Scholar
[50] Sagandira CR, Watts P. Safe and highly efficient adaptation of potentially explosive azide chemistry involved in the synthesis of Tamiflu using continuous-flow technology. Beilstein J Org Chem. 2019;15:2577–89.10.3762/bjoc.15.251Search in Google Scholar PubMed PubMed Central
[51] Britton J, Raston CL. Multi-step continuous-flow synthesis. Chem Soc Rev. 2017;46:1250–71.10.1039/C6CS00830ESearch in Google Scholar
[52] Vasudevan N, Sharma MK, Reddy DS, Kulkarni AA. A multi-step continuous flow synthesis of the cystic fibrosis medicine ivacaftor. React Chem Eng. 2018;3:520–6.10.1039/C8RE00025ESearch in Google Scholar
[53] Gilmore K, Kopetzki D, Lee JW, Horváth Z, McQuade DT, Seidel-Morgenstern A, et al. Continuous synthesis of artemisinin-derived medicines. Chem Commun. 2014;50:12652–55.10.1039/C4CC05098CSearch in Google Scholar PubMed
[54] Murray PRD, Browne DL, Pastre JC, Butters C, Guthrie D, Ley SV. Continuous flow-processing of organometallic reagents using an advanced peristaltic pumping system and the telescoped flow synthesis of (E/Z)-Tamoxifen. Org Process Res Dev. 2013;17:1192–208.10.5151/chempro-15bmos-BMOS2013_2013914162643Search in Google Scholar
[55] Jiao J, Nie W, Yu T, Yang F, Zhang Q, Aihemaiti F, et al. Multi-step continuous-flow organic synthesis: opportunities and challenges. Chem Eur J. 2021;27:4817–38.10.1002/chem.202004477Search in Google Scholar PubMed
[56] Ötvös SB, Llanes P, Pericas MA, Kappe CO. Telescoped continuous flow synthesis of optically active γ-nitrobutyric acids as key intermediates of baclofen, phenibut, and fluorophenibut. Org Lett. 2020;22:8122–6.10.1021/acs.orglett.0c03100Search in Google Scholar PubMed PubMed Central
[57] Chatterjee S, Guidi M, Seeberger PH, Gilmore K. Automated radial synthesis of organic molecules. Nature. 2020;57:379–84.10.1038/s41586-020-2083-5Search in Google Scholar PubMed
[58] Adamo A, Beingessner RL, Behnam M, Chen J, Jamison TF, Jensen KF, et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science. 2016;352:61–7.10.1126/science.aaf1337Search in Google Scholar PubMed
[59] Bédard A-C, Adamo A, Aroh KC, Russell MG, Bedermann AA, Torosian J, et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science. 2018;361:1220–5.10.1126/science.aat0650Search in Google Scholar PubMed
[60] Sivo A, de Souza Galaverna R, Gomes GR, Pastre JC, Vilé G. From circular synthesis to material manufacturing: advances, challenges, and future steps for using flow chemistry in novel application area. React Chem Eng. 2021;6(5):756–86. 10.1039/D0RE00411A. For very recent review highlighting novel applications of flow chemistry in different fields.Search in Google Scholar
[61] Gioiello A, Piccinno A, Lozza AM, Cerra B. The medicinal chemistry in the era of machines and automation: recent advances in continuous flow technology. J Med Chem. 2020;63:6624–47.10.1021/acs.jmedchem.9b01956Search in Google Scholar PubMed PubMed Central
© 2021 Alessandra Puglisi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Articles in the same Issue
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

