Abstract
This study was performed to validate the previous antimicrobial and cytotoxic data on the influence of ionic liquids as coatings of silver nanoparticles (AgNPs). The antibacterial and cytotoxicity assessments were carried out against different microorganisms and a cancerous cell line. AgNPs with two different ionic-liquid coatings and hydrocarbon chains were synthesized and characterized. We tested the antibacterial activity of these NPs against Salmonella typhi, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Candida albicans in planktonic forms and against Enterococcus faecalis and Escherichia coli in biofilm forms. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay was employed for toxicity evaluation. The antimicrobial activity of NPs with 12 carbons was significantly higher than those with 18 carbons. Furthermore, NPs with 12 carbons were also effective against bacterial biofilms. All of the NPs tested had good cell viability at different antimicrobial concentrations. The length of the hydrocarbon chain is an essential factor in determining the antimicrobial activity of ionic-liquid-coated AgNPs. The variation in ionic-liquid coatings was not as effective as other influencing factors. Evaluation of AgNPs using other alkyl chain lengths to find the optimal size is recommended.
1 Introduction
Owing to the indiscriminate use of antibiotics and the related multiple antibiotic resistance of pathogenic microorganisms, the need to search for new strategies to restrain these pathogens is essential [1,2].
Among different nanoparticles, silver nanoparticles (AgNPs) have shown antimicrobial features against a broad range of microorganisms, including Gram-positive bacteria, Gram-negative bacteria, and fungi [3,4,5,6]. AgNP antimicrobials refer to any silver-containing antimicrobial agent with nanoscale particles (smaller than 100 nm), with an enhanced activity because of their small particle sizes and significant active surface area [7]. It is shown that these agents demonstrate superior properties when particle sizes range between 5 and 50 nm [8].
The main proposed mechanisms of action for antimicrobial activity of AgNPs are the release of silver ions, generation of reactive oxygen, and damage to the cell membrane [9,10,11]. The positively charged NPs tend to attract negatively charged bacterial cell walls, causing them to accumulate inside the membrane, penetrate the cells, and eventually damage the bacterial cell walls [12,13].
Ionic liquids (ILs) are categorized as organic salts and are used as solvents consisting of polyatomic inorganic anions and organic cations. However, they are also used as electrolytes, and, therefore, considering their dual role, they can be marked as an environmental molecule with tunable ions. The application of ILs in green chemistry has been largely addressed in many aspects recently [14], where NPs are synthesized in organic solvents, achieving a good level of monodispersity and synthesis of particles with the same size and shape is highly probable. Moreover, the physical and chemical properties of ILs, such as hydrophobicity, viscosity, and polarity, can be changed by modifying their cations or anions, and the attached substituents such as alkyl chains. As an example, a strong association between the length of the alkyl chain in their structure and the level of polarity has been reported [15].
The coating of the AgNP surface with organic compounds such as ILs can inhibit particle aggregation and, therefore, enhance the positive charges at the surface of NPs thereby causing changes in the zeta potential value, which is defined as the potential difference between the surface of a solid particle immersed in a conducting liquid and the bulk of the liquid [16].
Considering these hypotheses, we initially examined the effect of charges on the surface of the imidazolium (Im)-coated AgNPs on their level of antimicrobial activity against different microorganisms. We found the positively charged Im-based AgNPs to be favorable antimicrobial agents with a low toxicity level [17,18]. Later, we tested another IL salt as a coating agent [pyridinium (Py)] in terms of antimicrobial activity and found that this substance was also able to change the bioactivity of AgNPs against Enterococcus faecalis [13].
Furthermore, we investigated the effect of the length and the number of alkyl chains attached to the IL coatings. We found that these parameters are capable of changing the characteristics of AgNPs, including the amount of surface charge, size, and bioactivity against Enterococcus faecalis. As a result, it was speculated that the regulation of the size of silver nuclei might be modulated by the alkyl chains [16,19].
It is known that the increase in the alkyl chain length can influence lipophilicity. Having said that, the higher lipophilicity may result in easier penetration of AgNPs into the cell membrane of the prokaryotic or eukaryotic cells, which, in turn, can affect the antibacterial activity and toxicity of NPs [20]. In other words, the greater repulsion between AgNPs with longer alkyl chains may lead to an increase in interparticle distances, decreasing aggregation and eventually promoting the efficacy of NPs [20].
Inspired by the promising results of Im-based AgNPs as a root canal irrigant, we also examined other features of this antimicrobial agent [21,22,23]. However, since our previous antimicrobial studies mainly were performed on Enterococcus faecalis, it was found relevant to validate our earlier results by observing the behavior of different AgNPs with positive charges, different coatings, and different alkyl chain lengths against a panel of microorganisms, including bacteria and a fungus. Therefore, this study was designed to validate the influence of the size of alkyl chain (12 hydrocarbons vs 18 hydrocarbon chain lengths) with different IL coatings on AgNP surfaces on antibacterial activity against a wide range of bacteria and fungi in both planktonic and biofilm forms and their relevant toxicity in human cells.
2 Materials and methods
2.1 Synthesis of NPs
The synthesis of ILs was carried out by reacting 1-methylimidazolium/pyridine with 1-chlorododecane/1-chlorooctane with no extra solvent in a flask fitted with a reflux condenser by heating and stirring the mixture at 70°C for about 48–72 h. Then, the temperature of the resultant liquid was decreased to 24°C and was washed using diethyl ether to obtain the ILs for the present experiment as 1-dodecyl-3-methylimidazolium chloride, 1-octadecyl-3-methylimidazolium chloride, 1-dodecyl pyridinium chloride, and 1-octadecyl pyridinium chloride.
For the synthesis of NPs, 1.0 mL of an aqueous solution of AgNO3 (0.01 M) was mixed with 20 mL of each IL (6.2 mM) and stirred vigorously. The prepared 0.4 M NaBH4 aqueous solution was then instantly added to the stirred solution drop by drop until a golden color was obtained. After that, the colloidal solutions were centrifuged for about 20 min to remove the unreacted ILs. The initial concentrations of all NP solutions before the commencement of the experiments were set as 1,024 µg/mL.
The four NP solutions prepared for this experiment were Im- and Py-protected with two different alkyl chains (C12, C18). AgNPs were characterized via a spectrophotometer (Ultrospec 3000 UV-Visible; Biochrom Ltd, Cambridge, UK) at a resolution of 1 nm. Additionally, transmission electron microscopy (TEM) analyses were performed on AgNPs at 200 kV, and then the average sizes of 250 particles were documented. The surface charges were also calculated via a zeta potential analyzer (Zeta Plus, Brookhaven Instruments, NY, USA).
2.2 Antimicrobial assessment
The test organisms were Salmonella typhi (PTCC 1609), Bacillus subtilis (PTCC 1720), Staphylococcus aureus (ATCC 29737), Escherichia coli (ATCC 15224), Enterococcus faecalis (PTCC1394), and Candida albicans (PTCC 5027).
Several antimicrobial assays including the zone of inhibition, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum biofilm inhibitory concentration (MBIC) were determined to assess the antibacterial activity. All experiments were performed in triplicate according to previous studies and based on the guidelines of the Clinical and Laboratory Standards Institute (CLSI) standards [24,25].
For the disc diffusion assay, 100 µL of each microbial suspension (1.5 × 108 CFU/mL) was separately spread on BHI (brain heart infusion) agar plates. After an incubation period of 24 h, 50 µL of each experimental solution was added in sterile blank 6-mm filter paper discs and lodged on the plates. After incubation for 24 h, the zone of inhibition was measured. Sterile normal saline was used as the negative control group.
For the MIC test, the two-fold serial dilution of all solutions up to seven times was prepared and poured into a 96-well microplate with Muller–Hinton broth (MHB) medium enriched with calcium (25 mg/L) and magnesium (12.5 mg/L) to a final volume of 90 μL. Afterward, 10 μL suspension of the test microorganism matching the turbidity of 2 McFarland standards was introduced to each microplate and subsequently incubated for 24 h at 37°C. The optical density of each microplate was determined using an ELISA reader (Biotek, Winooski, VT, USA) at an optical density of 600 nm. The minimum inhibitory concentration (MIC90) was calculated where 90% of the bacterial growth was inhibited compared to the growth of the control group. The positive and negative control groups were microorganism-inoculated culture media and sterile culture media, respectively.
For the MBC test, the obtained concentrations of each solution those equal to or higher than MIC were selected to be spread on BHI agar plates. They were incubated overnight at 37°C, and the colonies were counted consequently.
The most effective NPs (NPs with 12 or 18 hydrocarbon chain lengths) were selected for a biofilm inhibition test and examined on a Gram-positive and a -negative bacterial species (Enterococcus faecalis and Escherichia coli). Microorganisms in BHI were contacted with a series of 2-fold dilutions in 96-well plates. After 24/48 h, the plates were rinsed two times with saline and 10% formaldehyde followed by a rewash with saline. Then, the biofilms were stained for 30 min using 0.5% crystal violet. The plates were cured with 200 µL of 2-propanol for 1 hand read via a microplate reader spectrophotometer at an optical density of 490 nm (VITA Easyshade; VITA Zahnfabrik, Bäd Sackingen, Germany). The absorbance of crystal violet is considered an indicator of the presence of a bacterial biofilm.
2.3 Cytotoxicity assessment
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] test was used to determine the cytotoxicity of the NP solutions on the MCF-7 cell line. Concisely, MCF-7 cells in PRMI1640 media were moved into a 96-well cell culture plate and incubated for 24 h at 37°C in 95% air in the presence of 5% CO2.
The media was then refreshed with100 μL of each NP solution dissolved in RPMI1640 and incubated at 37°C for the second time. After 24 h, 25 μL of MTT solution (Sigma-Aldrich Co., St. Louis, MO, USA) was added to each well and incubated again for another 4 h with the previous experimental parameters. Furthermore, 100 μL of dimethyl sulfoxide was added to each well and incubated for 10 min. An ELISA plate reader (Biotek, Winooski, VT, USA) was employed to read the absorption of each NP solution at an optical density of 540 nm. The negative and positive control groups were culture media and hydrogen peroxide at a concentration of 35%, respectively. Cytotoxicity of Im and Py was also evaluated. All procedures were repeated three times for each group.
2.4 Statistical analysis
Statistical analysis was carried out using SPSS software (v 17.0) (SPSS Inc., Chicago, IL, USA).
The data from agar diffusion assays were analyzed using the Kruskal–Wallis test and then the Mann–Whitney U test for post hoc comparisons.
Cytotoxicity-related data were analyzed using one-way analysis of variance (ANOVA), and if the intergroup comparison test showed a significant difference, Tukey’s Honestly Significant Difference (HSD) was carried out as a post hoc test. For all tests, the significance level was 5%.
3 Results
3.1 Characterization of NPs
In the UV-visible spectroscopy of the synthesized NPs (Figure 1), a characteristic peak at around λ 450 nm is seen which confirms the formation of AgNPs due to the reduction of Ag+ ions into Ag0.

UV-visible spectroscopy of different ionic liquid-coated Ag nanoparticles.
The zeta potential distribution and the TEM images of the synthesized NPs are illustrated in Figures 2 and 3. The surface charge density values (zeta/radius) of C12 Im, C18 Im, C12 Py, and C18 Py NPs were 5.55, 6.76, 1.35, and 8.58, respectively. Furthermore, the calculated sizes of particles were 9, 8.6, 18.49, and 6.71 nm for C12 Im, C18 Im, C12 Py, and C18 Py, respectively.

Zeta potential distribution of different ionic liquid-coated Ag nanoparticles.

TEM images of nanoparticles: (a) C12 Im, (b) C18 Im, (c) C12 Py, and (d) C18 Py.
3.2 Antimicrobial activity of NPs
The disk diffusion test results revealed that C12 NPs had greater inhibition zones than C18 NPs, and the control group for all microorganisms was evaluated. The details are shown in Table 1.
Diameter of the inhibition zone in mm (median) against each microorganism
| C12 Im | C18 Im | C12 Py | C18 Py | p-value | |
|---|---|---|---|---|---|
| Staphylococcus aureus | 28A | 9B | 26A | 9B | <0.01 |
| Bacillus subtilis | 25A | 8.5B | 23A | 10B | 0.01 |
| Escherichia coli | 20A | 4B | 16A | 0B | <0.01 |
| Salmonella typhi | 17A | 0B | 18A | 0B | 0.04 |
| Candida albicans | 21A | 9.5B | 20A | 10B | 0.06 |
Different letters in a row indicate the presence of a statistically significant difference.
Table 2 indicates the results of MIC and MBC of the tested NPs against each microorganism. The microdilution broth test verified that the C12 NPs killed planktonic microorganisms at lower concentrations compared to C18 NPs. Moreover, C12 NPs killed more than 90% of Gram-positive and -negative bacteria in the form of biofilms after 24 and 48 h, respectively (Figures 4–7).
The MIC and MBC of the tested nanoparticles against each microorganism
| C12 Im (µg/mL) | C18 Im (µg/mL) | C12 Py (µg/mL) | C18 Py (µg/mL) | ||
|---|---|---|---|---|---|
| Staphylococcus aureus | MIC | 16 | 256 | 16 | 128 |
| MBC | 16 | 256 | 16 | 128 | |
| Bacillus subtilis | MIC | 16 | 512 | 16 | 256 |
| MBC | 16 | 512 | 16 | 256 | |
| Escherichia coli | MIC | 16 | 512 | 16 | 256 |
| MBC | 16 | 512 | 16 | 256 | |
| Salmonella typhi | MIC | 128 | 256 | 64 | >512 |
| MBC | 128 | 256 | 64 | >512 | |
| Candida albicans | MIC | 32 | 256 | 64 | 256 |
| MBC | 32 | 256 | 64 | 256 | |

Inhibitory activity of C12 NPs at different concentrations against the biofilm formation of Escherichia coli in 24 h.
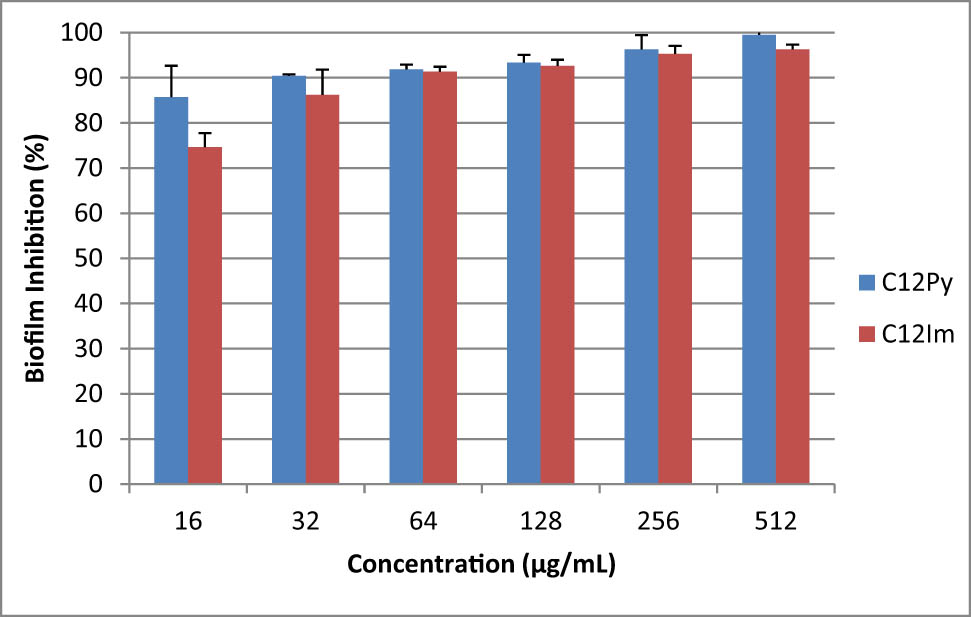
Inhibitory activity of C12 NPs at different concentrations against the biofilm formation of Escherichia coli in 48 h.
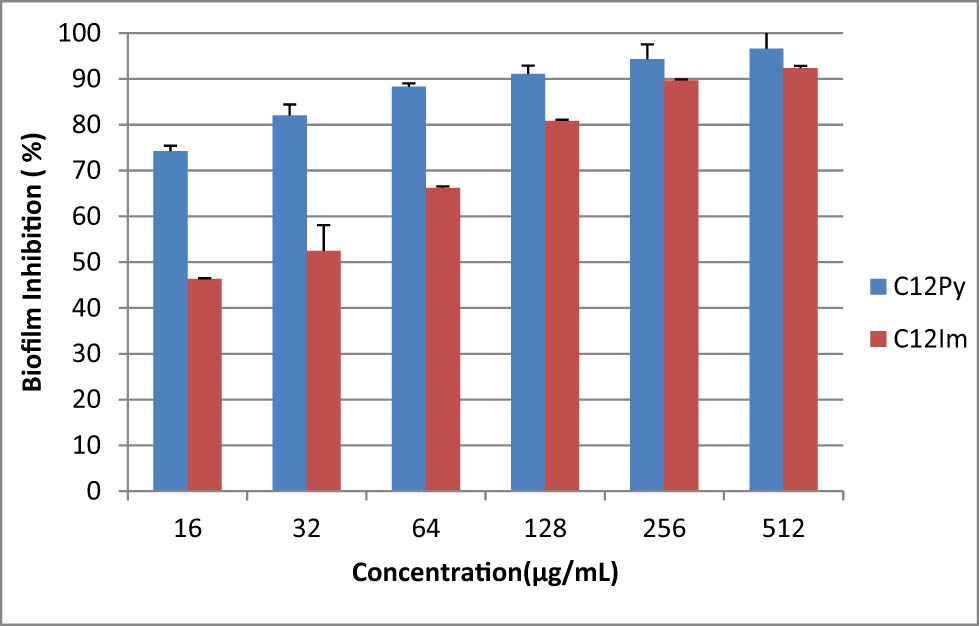
Inhibitory activity of C12 NPs at different concentrations against the biofilm formation of Enterococcus faecalis in 24 h.
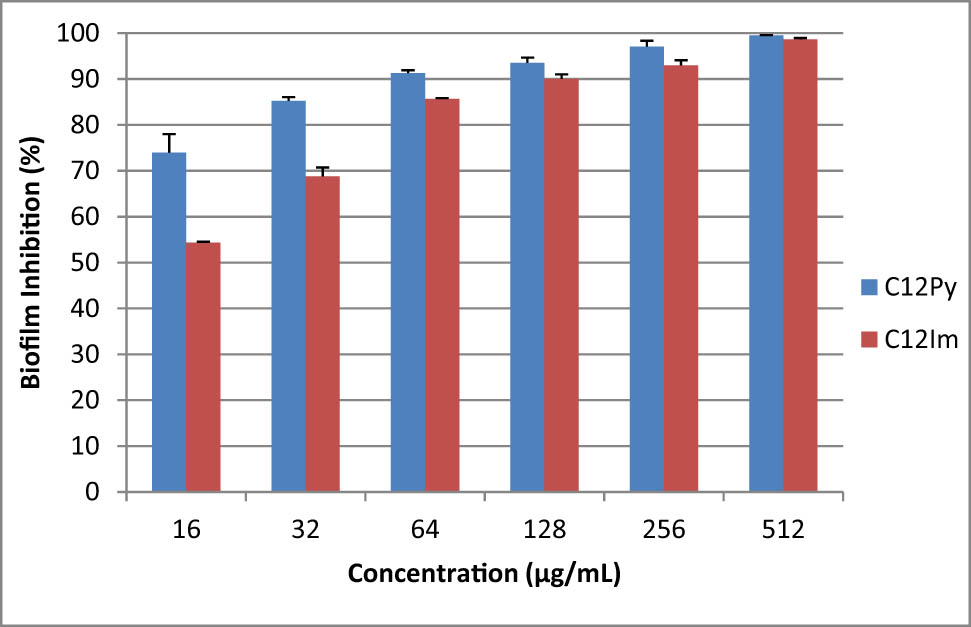
Inhibitory activity of C12 NPs at different concentrations against the biofilm formation of Enterococcus faecalis in 48 h.
3.3 Cytotoxic activity of NPs
The results exhibited that the level of cytotoxicity was concentration-dependent for all tested solutions (Table 3). NPs at 128 µg/mL or lower could retain the viability of 90% of MCF-7 cells in all groups. At higher concentrations, Py-based NPs showed more cytotoxicity, but the ANOVA test shows that the difference was not statistically significant for the concentrations examined.
The viability of MCF-7 cells (%) when treated with different concentrations of the tested nanoparticles (mean ± SD)
| Concentration (µg/mL) | C12 Im | C18 Im | C12 Py | C18 Py | p-value |
|---|---|---|---|---|---|
| 512 | 94.46 ± 14.42 | 85.82 ± 13.89 | 74.46 ± 7.40 | 69.04 ± 14.68 | 0.151 |
| 256 | 95.12 ± 11.46 | 89.51 ± 13.59 | 88.67 ± 9.34 | 79.34 ± 12.29 | 0.472 |
| 128 | 97.58 ± 5.15 | 93.25 ± 12.11 | 94.42 ± 5.22 | 87.71 ± 5.33 | 0.488 |
| 64 | 106.61 ± 6.22 | 102.09 ± 7.93 | 97.66 ± 12.90 | 93.78 ± 18.59 | 0.633 |
| 32 | 101.55 ± 8.25 | 101.50 ± 4.09 | 102.40 ± 14.59 | 99.14 ± 11.58 | 0.982 |
| 16 | 101.22 ± 7.21 | 103.80 ± 9.91 | 102.12 ± 11.56 | 101.57 ± 3.04 | 0.982 |
4 Discussion
The study was performed to validate the previous data on the antimicrobial effect of alkyl chain length on the surface of AgNPs, along with the number of particle charges and its density (zeta/radius). The antibacterial activity of NPs was assessed against a panel of Gram-positive and -negative bacteria and a fungus. Besides, we evaluated their level of cytotoxicity in a mammalian cancer cell line. We verified that the length of the alkyl chain is an essential factor in determining antimicrobial activity, and the increase in the magnitude of the positive charge of AgNPs does not necessarily elevate the antimicrobial properties. Besides, all tested AgNPs were not cytotoxic to the MCF-7 cell line; however, at high concentrations, Im-coated NPs showed a lower cytotoxicity level than Py-coated NPs.
Resistance to antimicrobial agents, including antibiotics, has become a significant health problem in recent years [1]. To solve this problem, metallic NPs have been extensively studied for their antimicrobial properties. They have a high specific surface area and a high fraction of surface atoms, endowing them with unique physicochemical properties, including catalytic activity and electronic and magnetic properties [26]. Furthermore, the chance that bacteria develop resistance against metallic NPs is extremely less compared to other conventional and narrow-spectrum antibiotics [27]. It is because metals, especially silver, can act on a broad range of targets in microorganisms, and several mutations must occur in their genome to change this trait [27].
AgNPs should possess hydrophobicity to enhance their interactions with the cell membrane. On the other hand, hydrophilicity is also crucial for a steady dispersion in an aqueous solution to prevent aggregation [28]. As a result, a balance between these two features is essential for a medicament to be effective against microorganisms. To gain hydrophobic characteristics, an alkyl chain was added to AgNPs. It should be noted that as the attached alkyl chain to AgNPs becomes longer, the hydrophobicity of NPs will increase. Furthermore, since the cell membrane is composed of phospholipids, containing a head group and a fatty acid tail that is roughly 10–20 hydrocarbons long [29], to obtain optimal results, the alkyl chain length was selected to be within this range.
We found that AgNPs with a shorter hydrocarbon length (C12) had increased antibacterial activity than longer-chain substitutes (C18) against all tested microorganisms. Agar diffusion and microdilution broth tests revealed that C12 AgNPs had a more significant inhibition zone and could kill microorganisms at lower concentrations. This finding was regardless of the type of IL used. This effect might be due to the low hydrophobicity of C18 particles, which had an adverse effect on their dispersion and, in turn, their antibacterial efficacy.
Given that the cellular membrane of the bacteria has a negative charge due to the presence of phosphate, carboxyl, and amino groups, particles with positive charge have more tendency to attack the bacterial membrane [12,30]. However, we found that the increase in the positive charge of AgNPs does not necessarily elevate antimicrobial properties. Although C12 Py-coated NPs had the least surface area and density of charge among the tested AgNPs, they showed better antimicrobial activity than other NPs in general. This may be interpreted due to the fact that an increase in the number of particles with the same charge can result in the repulsion of the particles from each other and an increase in the distance between them, and, therefore, it may have an adverse effect on the level of antimicrobial activity. Hence, we propose that a balance between lipophilicity and hydrophobicity is a more influential factor for antibacterial activity and might be a more critical factor than the density of surface charges.
Previous studies have confirmed that in the case where NPs bear surface groups with a positive charge, even a slight change in surface functionalities can change the degree of hydrophobicity, resulting in varying amounts of internalization in the cells [31,32]. For instance, Zue et al. [31] demonstrated that among gold NPs that had positive charges, most hydrophobic NPs were the least efficient NPs taken up by the monkey kidney cells. This was inconsistent with our findings that hydrophobic AgNPs with more extended chain coatings were less effective and could be because they were less taken up by microbial cells.
Our research team previously found that AgNPs coated with Py killed Enterococcus faecalis better than those coated with Im. However, in the current study, the antimicrobial test results were dissimilar in terms of the microorganisms tested and alkyl chain lengths. AgNPs with a 12-hydrocarbon chain length showed enhanced antimicrobial activity. Considering these results together, the type of IL coating might not be as effective as the alkyl chain length in determining antimicrobial features of AgNPs.
In this study, only C12 NPs were assessed for antibacterial activity against biofilms as they are more hydrophilic than C18 counterparts and had better results against planktonic bacteria. Given that the virulent biofilms are the cause of the majority of persistent human infectious diseases [33], and the extracellular polymeric substance (EPS) matrix is strongly anionic, positive NPs can penetrate this matrix more efficiently compared to the uncharged or anionic counterparts based on a catch-and-release phenomenon [34]. Furthermore, when NPs enter the biofilm, their hydrophilicity is crucial because microorganisms take up hydrophobic NPs, but hydrophilic cationic particles remain bound to the EPS [34].
Although the Py-coated NPs are less cationic than Im-coated ones, for both Escherichia coli and Enterococcus faecalis, Py-coated NPs inhibited the formation of their biofilms more effectively than Im-coated ones after 24 h, especially at lower concentrations. Nevertheless, after 48 h, the difference narrowed, but Py-coated NPs were still more effective. As discussed earlier, this can be interpreted by the fact that when molecules become more cationic, they repel each other more strongly. On the other hand, they attach more tightly to EPS and make their release to bacterial cells more difficult.
The toxicity of an antimicrobial agent is also an important aspect that needs to be evaluated because these agents are in direct contact with human tissues. This evaluation is even more critical for cationic NPs because they distribute more through nonphagocytic cells and are more toxic [35]. However, the current study showed that the alteration in the magnitude of the positive charges on the surface of AgNPs may not result in an increase in the level of cytotoxicity. For example, C12 Py-coated NPs, at high concentrations (512 µg/mL), did not show lower toxicity than other tested NPs. However, they have higher zeta potential values compared to other NPs. Although the differences were not statistically significant at higher concentrations, Im-coated NPs showed lower toxicity than Py-coated counterparts. In vivo studies are required to evaluate tissue reaction to these materials and to validate our findings.
Future studies can be targeted at the synthesis of NPs coated with a combination of hydrophobic and hydrophilic carriers to enable them to penetrate the cell membrane and easily dissolve in water.
5 Conclusion
Within the limitations of this study, we found that the alkyl length is an essential factor in determining the antimicrobial activity of AgNPs. However, modification in IL coatings was not as effective as other influencing factors considered in this study. We suggest evaluating NPs with different alkyl chain lengths, such as NPs with 10, 14, or 16 hydrocarbons, to find the optimal hydrocarbon chain length.
Acknowledgment
The authors thank the Vice-Chancellery of Shiraz University of Medical Sciences for supporting this research (Grant #23092). The authors would also like to thank Dr Mehrdad Vosooghi of the Research Development Center for the statistical analysis.
-
Funding information: This research received funding from the Shiraz University of Medical Sciences.
-
Author contributions: Ahmad Gholami: conceptualization, data curation, methodology, visualization, project administration, writing – original draft, writing – review and editing; Mahdi Sedigh Shams: conceptualization, data curation, writing – original draft, formal analysis, visualization, project administration; Abbas Abbaszadegan: conceptualization, data curation, writing – original draft, writing – review and editing, visualization, project administration; Mohammad Reza Nabavizadeh: resources, data curation, methodology, validation, writing – original draft, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during the current study are available from the corresponding author on reasonable request.
References
[1] Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227.10.2147/IJN.S121956Suche in Google Scholar PubMed PubMed Central
[2] Moazami F, Gholami A, Mehrabi V, Ghahramani Y. Evaluation of the antibacterial and antifungal effects of ProRoot MTA and nano-fast cement: an in vitro study. J Contemp Dent Pract. 2020;21(7):761.10.5005/jp-journals-10024-2877Suche in Google Scholar
[3] Al-thabaiti SA, Khan Z, Manzoor N. Biosynthesis of silver nanoparticles and its antibacterial and antifungal activities towards Gram-positive, Gram-negative bacterial strains and different species of Candida fungus. Bioprocess Biosyst Eng. 2015;38(9):1773–81.10.1007/s00449-015-1418-3Suche in Google Scholar PubMed
[4] Mohammadi F, Abbaszadegan A, Gholami A. Recent advances in nanodentistry: a special focus on endodontics. Micro Nano Lett. 2020;15(12):812–6.10.1049/mnl.2019.0747Suche in Google Scholar
[5] Chartarrayawadee W, Charoensin P, Saenma J, Rin T, Khamai P, Nasomjai P, et al. Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity. Green Process Synth. 2020;9(1):107–18.10.1515/gps-2020-0012Suche in Google Scholar
[6] Kumar A, Madhu G, John E, Kuttinarayanan S, Nair S. Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey. Green Process Synth. 2020;9(1):268–74.10.1515/gps-2020-0029Suche in Google Scholar
[7] Miernicki M, Hofmann T, Eisenberger I, von der Kammer F, Praetorius A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat Nanotechnol. 2019;14(3):208–16.10.1038/s41565-019-0396-zSuche in Google Scholar PubMed
[8] Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, et al. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25(18):4383–91.10.1016/j.biomaterials.2003.10.078Suche in Google Scholar PubMed
[9] Durán N, Durán M, De Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 2016;12(3):789–99.10.1016/j.nano.2015.11.016Suche in Google Scholar PubMed
[10] Marambio-Jones C, Hoek EM. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res. 2010;12(5):1531–51.10.1007/s11051-010-9900-ySuche in Google Scholar
[11] Gholami A, Mohammadi F, Ghasemi Y, Omidifar N, Ebrahiminezhad A. Antibacterial activity of SPIONs versus ferrous and ferric ions under aerobic and anaerobic conditions: a preliminary mechanism study. IET Nanobiotechnol. 2019;14(2):155–60.10.1049/iet-nbt.2019.0266Suche in Google Scholar PubMed PubMed Central
[12] Abbaszadegan A, Ghahramani Y, Gholami A, Hemmateenejad B, Dorostkar S, Nabavizadeh M, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015;2015:720654.10.1155/2015/720654Suche in Google Scholar
[13] Abbaszadegan A, Nabavizadeh M, Gholami A, Aleyasin Z, Dorostkar S, Saliminasab M, et al. Positively charged imidazolium‐based ionic liquid‐protected silver nanoparticles: a promising disinfectant in root canal treatment. Int Endod J. 2015;48(8):790–800.10.1111/iej.12377Suche in Google Scholar
[14] Mallakpour S, Dinari M. Ionic liquids as green solvents: progress and prospects. In: Mohammad A, Inamuddin D, editors. Green solvents II. Dordrecht: Springer; 2012. p. 1–32.10.1007/978-94-007-2891-2_1Suche in Google Scholar
[15] Carmichael AJ, Seddon KR. Polarity study of some 1-alkyl-3-methylimidazolium ambient-temperature ionic liquids with the solvatochromic dye, Nile Red. J Phys Org Chem. 2000;13:591–5.10.1002/1099-1395(200010)13:10<591::AID-POC305>3.0.CO;2-2Suche in Google Scholar
[16] Yamamoto M, Kashiwagi Y, Nakamoto M. Size-controlled synthesis of monodispersed silver nanoparticles capped by long-chain alkyl carboxylates from silver carboxylate and tertiary amine. Langmuir. 2006;22(20):8581–6.10.1021/la0600245Suche in Google Scholar
[17] Nabavizadeh M, Abbaszadegan A, Gholami A, Kadkhoda Z, Mirhadi H, Ghasemi Y, et al. Antibiofilm efficacy of positively charged imidazolium-based silver nanoparticles in Enterococcus faecalis using quantitative real-time PCR. Jundishapur J Microbiol. 2017;10:10.10.5812/jjm.55616Suche in Google Scholar
[18] Nabavizadeh M, Ghahramani Y, Abbaszadegan A, Jamshidzadeh A, Jenabi P, Makarempour A. In vivo biocompatibility of an ionic liquid-protected silver nanoparticle solution as root canal irrigant. Iran Endod J. 2018;13(3):293.Suche in Google Scholar
[19] Kashiwagi Y, Yamamoto M, Nakamoto M. Facile size-regulated synthesis of silver nanoparticles by controlled thermolysis of silver alkylcarboxylates in the presence of alkylamines with different chain lengths. J Colloid Interface Sci. 2006;300(1):169–75.10.1016/j.jcis.2006.03.041Suche in Google Scholar
[20] Abbaszadegan A, Gholami A, Abbaszadegan S, Aleyasin ZS, Ghahramani Y, Dorostkar S, et al. The effects of different ionic liquid coatings and the length of alkyl chain on antimicrobial and cytotoxic properties of silver nanoparticles. Iran Endod J. 2017;12(4):481.Suche in Google Scholar
[21] Abbaszadegan A, Ghahramani Y, Farshad M, Sedigh-Shams M, Ghomali A, Jamshidzadeh A. In vitro evaluation of dynamic viscosity, surface tension and dentin wettability of silver nanoparticles as an irrigation solution. Iran Endod J. 2018;14(1):23–7.Suche in Google Scholar
[22] Adl A, Abbaszadegan A, Gholami A, Parvizi F, Ghahramani Y. Effect of a new imidazolium-based silver nanoparticle irrigant on the bond strength of epoxy resin sealer to root canal dentine. Iran Endod J. 2019;14(2):122–5.Suche in Google Scholar
[23] Ghahramani Y, Yaghoubi F, Motamedi R, Jamshidzade A, Abbaszadegan A. Effect of endodontic irrigants and medicaments mixed with silver nanoparticles against biofilm formation of enterococcus faecalis. Iran Endod J. 2018;13(4):559–64.Suche in Google Scholar
[24] Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI document M100-S24. Wayne, PA: Clinical and Laboratory Standard Institute; 2014.Suche in Google Scholar
[25] Abbaszadegan A, Sahebi S, Gholami A, Delroba A, Kiani A, Iraji A, et al. Time‐dependent antibacterial effects of Aloe vera and Zataria multiflora plant essential oils compared to calcium hydroxide in teeth infected with Enterococcus faecalis. J Investig Clin Dent. 2016;7(1):93–101.10.1111/jicd.12123Suche in Google Scholar
[26] Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3(2):168–71.10.1016/j.nano.2007.02.001Suche in Google Scholar
[27] Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–20.10.1128/AEM.02218-06Suche in Google Scholar
[28] Fratoddi I. Hydrophobic and hydrophilic Au and Ag nanoparticles. Breakthroughs and perspectives. Nanomaterials. 2018;8(1):11.10.3390/nano8010011Suche in Google Scholar PubMed PubMed Central
[29] Milo R, Phillips R. Cell biology by the numbers. New York , USA: Garland Science; 2015.10.1201/9780429258770Suche in Google Scholar
[30] Gholami A, Shahin S, Mohkam M, Nezafat N, Ghasemi Y. Cloning, characterization and bioinformatics analysis of novel cytosine deaminase from Escherichia coli AGH09. Int J Pept Res Ther. 2015;21(3):365–74.10.1007/s10989-015-9465-9Suche in Google Scholar
[31] Zhu Z-J, Ghosh PS, Miranda OR, Vachet RW, Rotello VM. Multiplexed screening of cellular uptake of gold nanoparticles using laser desorption/ionization mass spectrometry. J Am Chem Soc. 2008;130(43):14139–43.10.1021/ja805392fSuche in Google Scholar PubMed PubMed Central
[32] Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small. 2010;6(1):12–21.10.1002/smll.200901158Suche in Google Scholar PubMed
[33] Benoit DS, Sims Jr KR, Fraser D. Nanoparticles for oral biofilm treatments. ACS Nano. 2019;13(5):4869–75.10.1021/acsnano.9b02816Suche in Google Scholar PubMed PubMed Central
[34] Li X, Yeh Y-C, Giri K, Mout R, Landis RF, Prakash Y, et al. Control of nanoparticle penetration into biofilms through surface design. ChemComm. 2015;51(2):282–5.10.1039/C4CC07737GSuche in Google Scholar
[35] Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed. 2012;7:5577.10.2147/IJN.S36111Suche in Google Scholar PubMed PubMed Central
© 2021 Ahmad Gholami et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

