Abstract
Baeyer–Villiger (BV) oxidation of cyclohexanone to ε-caprolactone was studied by a co-precipitation method using Fe–Sn–O catalysts in an O2/benzaldehyde system. The effects of the Fe:Sn ratio, calcination temperature, calcination time, and reaction conditions on the catalytic performance were investigated. The catalysts present the best activity when it is prepared at a Fe:Sn ratio of 1:1, calcination temperature of 850°C, and calcination time of 5 h. Under these conditions, catalysts form a large number of small prisms, which result in a larger specific surface area and enhanced catalytic activity. The optimum reaction conditions for the synthesis of ε-caprolactone in the presence of the Fe–Sn–O catalyst are as follows: catalyst (0.12 g), 1,2-dichloroethane (30 mL), O2 flow rate of 25 mL min−1, cyclohexanone to benzophenone of 3:1, reaction temperature of 60°C, and reaction time of 5 h. The conversion of cyclohexanone and the average yield of ε-caprolactone are determined at 98.96% and 83.36%, respectively.
1 Introduction
ε-Caprolactone is a new type of polyester monomer that has been utilized in a variety of applications. It is predominantly used in the synthesis of poly-ε-caprolactone or modification by blending or copolymerization with other esters [1,2]. The key methods for the synthesis of ε-caprolactone include oxidation of cyclohexanone by the Baeyer–Villiger (BV) reaction, intramolecular condensation of 6-hydroxycaproic acid, catalytic dehydrogenation of 1,6-hexanediol, and diester hydrogenation of hexamethylene glycol. Particularly, the BV oxidation of cyclohexanone is the most widely employed approach [3]. BV oxidation is a very important reaction in the organic synthesis for designing value-added lactone/esters from the respective carbonyl compounds. Usually an active metal-containing material catalyzes this reaction in the presence of peroxides [4,5,6]. According to the utilized raw materials, BV oxidation of cyclohexanone can include H2O2, O2/air, peroxyacid, or biological oxidation.
Currently, ε-caprolactone is mainly prepared by the peroxyacid oxidation method [7,8]. The approach is widely used in the industry to produce ε-caprolactone using organic peroxic acids, such as peroxyformic [9], peracetic [10], peroxybenzoic [11], 3-chloroperoxybenzoic [12], and trifluoro-peracetic acids [13]. Nevertheless, organic peroxy acids are expensive and are associated with certain risks, particularly during the purification and concentration processes. In addition, acidic waste is a serious environmental pollutant. Recycling carboxylic acids and separation of by-products in the later stages of the synthesis is challenging.
In contrast, bio-oxidation approaches utilize the high specificity and efficiency of enzymes. Such methods are more environmentally friendly and can be conducted under relatively mild reaction conditions. However, enzymes exhibit poor stability, and the production cost is high. Moreover, bio-oxidation is associated with significant environmental requirements and risks. Furthermore, the method involving H2O2 oxidation uses low concentration H2O2 as the oxidant, and the main by-product is water. Nonetheless, to improve the performance, the use of a catalyst displaying high activity is required. Commonly employed catalysts include Lewis acids, metal ion-loaded polymers, and metal oxides. Due to the low polarity of cyclohexanone, the addition of an organic solvent is typically necessary. Unfortunately, organic solvents can participate in the reaction mixture and co-oxidize with H2O2.
The O2/air oxidation method appears to be the most optimal; however, it has been conducted only on a laboratory scale [14]. O2 is a relatively clean oxidant; therefore, utilizing it for the oxidation of cyclohexanone to ε-caprolactone would be environmentally friendly, economical, and safe. An approach involving O2/air oxidation would also generate fewer by-products. Similar to H2O2, O2 is not a sufficiently strong oxidant; hence, the addition of a co-oxidant would be necessary for the effective oxidation of cyclohexanone. Zhou et al. [15] demonstrated that the catalytic activity of iron tetraphenylporphyrin (Fe-TCPP) in the synthesis of ε-caprolactone by the O2/air oxidation method was better than that of Ru, Co, and Mn phenyl porphyrin catalysts. In addition, Belaroui et al. [16] reported loading iron phthalocyanine (Fe-Phtal) on SiO2 to obtain a Fe-Phtal/SiO2 catalyst, which was employed in the O2/air oxidation of cyclohexanone to ε-caprolactone, affording the product in a 61% yield. Jeong et al. [17] prepared a Fe-TCPP-PMO catalyst by loading Fe-TCPP on organo-energetic mesoporous silica. The generated catalyst displayed higher activity in the O2/air oxidation than pure Fe-TCPP. Numerous other catalysts, including those loaded on molecular sieves, transition metal-based materials, metal oxides, non-metal oxides (e.g., graphene) [18], Cu-MCM-41 [19,20], SnO2, and CuO [21,22], have been reported. Among them, benzaldehyde is the most frequently used catalyst [23,24,25].
During the reaction, O2 first oxidizes benzaldehyde to benzoic acid, which subsequently oxidizes cyclohexanone to ε-caprolactone. However, as mentioned earlier, the oxidation ability of O2 is weak; thus, the use of highly active metal oxide or composite metal oxide catalysts is essential. Specifically, catalysts containing Fe and Sn metals are often used in this process [17,26,27,28,29]. In the present study, we prepared an Fe-Sn composite metal oxide catalysts [30,31,32], referred to as Fe–Sn–O, which is used to catalyze the synthesis of ε-caprolactone in the presence of a O2/benzaldehyde system. The effects of different calcination temperatures, calcination times, and metal ratios on the performance of the catalyst are investigated. A feasible green synthesis method for the preparation of ε-caprolactone is proposed.
2 Experimental
2.1 Preparation of the Fe–Sn–O catalyst
A 5% solution of Na2CO3 was added to a three-necked flask and heated at 60°C. A total of 0.15 mol L−1 Fe(NO3)3‧9H2O and 0.15 mol L−1 SnCl4·5H2O precursor solutions were added dropwise to the flask with stirring [33,34]. Then, 2 mol L−1 NaOH was then used to adjust the pH to 10. The reaction mixture was stirred for 2 h and then placed in an electrically heated blast drying oven set to 60°C for 48 h. The reaction mixture was washed with deionized water until pH 7. Subsequently, the co-precipitate was dried in an electrically heated blast drying oven at 100°C for 24 h, crushed, and calcined in a muffle furnace at a heating rate of 5°C min−1 up to a certain temperature for a specific amount of time. After cooling the calcined product to room temperature, metal oxide particles of 40–60 mesh were sieved to obtain the Fe–Sn–O catalyst.
2.2 BV co-oxidation reaction of cyclohexanone
The BV reaction was carried out in a 100 mL three-necked flask. Briefly, 0.15 g of the catalyst and 35 mL of 1,2-dichloroethane were added to the flask in the presence of oxygen at a flow rate controlled at 20 mL min−1. After 15 min, 0.01 mol cyclohexanone and 0.04 mol benzaldehyde were added sequentially. The reaction mixture was heated to 55°C and stirred for 5 h. The reaction mixture was then cooled for 1 h and the catalyst was separated by filtration.
GC-2014C (SHIMADZU, Japan) was equipped with an FID detector. The temperature program mode (120°C for 3 min, 10°C min−1 to 240°C for 15 min) was used to analyze the extracted reaction solution. The chromatographic column was HP-5 50 m × 0.200 mm. N2 was used as carrier gas at the nitrogen pressure of 0.5 MPa. The detector temperature was 260°C, and injection port temperature was 260°C. The injection quantity was 0.3 μL.
2.3 Characterization of the Fe–Sn–O catalyst
Figure 1 illustrates the X-ray diffraction (XRD) spectra of Fe–Sn–O prepared at different calcination temperatures. The spectrum of the Fe–Sn–O catalyst exhibits three characteristic peaks (i.e., 2θ = 26.693°, 33.960°, and 51.860°), which are consistent with the (110), (101), and (211) crystal planes of SnO2 (PDF#41-1445), respectively. In addition, the peaks at 2θ = 33.160°, 35.621°, and 54.120° are consistent with the crystal planes of Fe2O3 (PDF#33-0664), and the peaks at 2θ = 32.976°, 45.186°, and 60.176° are consistent with the crystal planes of α-Fe2O3 (PDF#39-0238). Hence, the catalyst is a composite metal oxide composed of Fe2O3 and SnO2. Notably, the higher the calcination temperature, the stronger the characteristic diffraction peaks and the better the crystallinity.

XRD spectrum of the Fe–Sn–O catalyst at different calcination temperatures of 700°C, 750°C, 800°C, and 850°C.
Figure 2 shows the scanning electron microscopy (SEM) images of the Fe–Sn–O catalyst at different calcination temperatures. From Figure 2, at a calcination temperature of 700°C, Fe–Sn–O is a sparse and porous agglomerate formed by the accumulation of smaller particles. Furthermore, at a calcination temperature of 750°C, the surface particles begin to grow, and the surface of the agglomerates becomes smooth. When the calcination temperature reached 800°C, the surface particles grow into small prisms, which are less obvious. The catalyst is formed by the accumulation of these small prismatic particles. The appearance of prismatic particles on the surface is detected at a calcination temperature of 850°C. A detailed qualitative inspection of nanostructured materials is obtained from the TEM and HRTEM images shown in Figure 3. In Figure 3a, it can be seen that the distribution of nanoparticles in the sample is the same as the SEM result. The Fe–Sn–O catalyst is composed of prismatic particles at a calcination temperature of 850°C. In Figure 3b, some lattice fringes can be observed, in which the grain boundary spacing d = 0.159 nm and d = 0.176 nm are attributed to the (002) and (211) crystal planes of SnO2, respectively, which is the same as 57.818° and 51.780° in XRD. The peaks coincide with each other; the grain boundary spacing d = 0.152 nm and d = 0.270 nm are attributed to the (611) crystal plane of α-Fe2O3 and the (104) crystal plane of Fe2O3, respectively, which are consistent with the peaks at 60.676° and 33.152° in XRD. All in all, Fe–Sn–O catalyst shows the best activity when calcined at 850°C. This is due to the accumulation of prismatic particles, resulting in a sparser structure than in the case of smooth particles. Consequently, the Fe–Sn–O catalyst displays a larger specific surface area and pore volume, leading to enhanced activity.
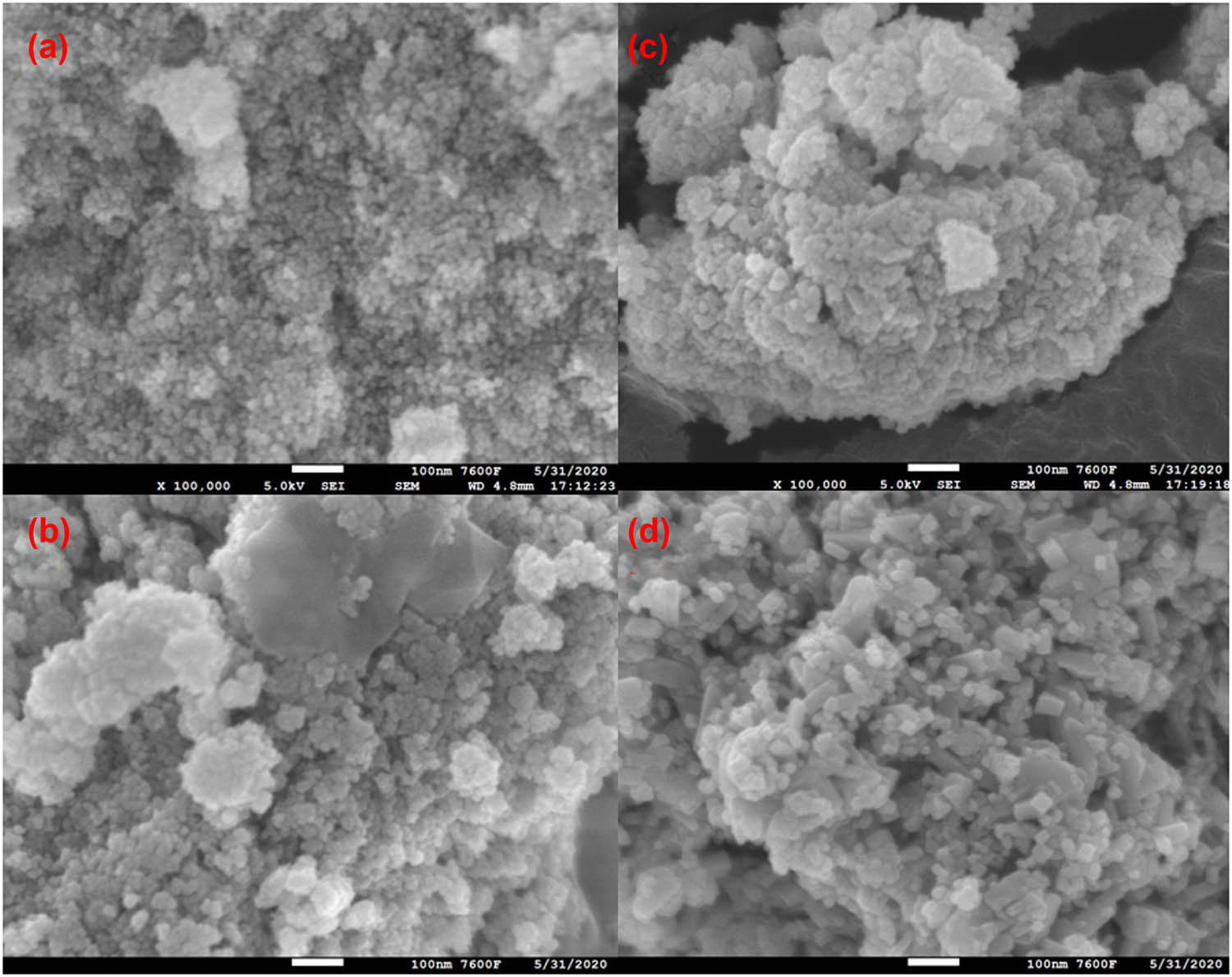
SEM images of the Fe–Sn–O catalyst at different calcination temperatures of (a) 700°C, (b) 750°C, (c) 800°C, and (d) 850°C.

TEM image (a) and HRTEM image (b) of Fe–Sn–O catalyst calcined at 850°C.
Figure 4a illustrates the Fourier transform infrared (FTIR) spectra of the Fe–Sn–O catalyst at different calcination temperatures. The broad absorption peak at ∼3,460 cm−1 corresponds to the O–H bond of the hydroxyl molecules on the surface of Fe–Sn–O or the stretching vibration of water molecules adsorb on the surface of the catalyst. The absorption peak attributed to the vibration of the Fe–O bond is detected in the fingerprint region at ∼500 cm−1. Finally, the absorption peak between ∼1,500 and 1,700 cm−1 is ascribed to the vibration of the Sn–O bond.

FTIR spectra (a) and H2-TPR diagram (b) of the Fe–Sn–O catalyst prepared at different calcination temperatures.
Figure 4b demonstrates the H2 temperature-programmed reduction (TPR) diagrams of the Fe–Sn–O catalysts prepared at different calcination temperatures. In the figure, the peak at around 710°C corresponds to reduction of Sn4+ to Sn, while that at 520°C indicates the reduction of Fe2O3. Moreover, the reduction temperature is higher during calcination at 800°C and 850°C.
Based on the Brunauer–Emmett–Teller (BET) analysis, the specific surface area of the Fe–Sn–O catalyst is found to decrease with the increasing calcination temperature (see Table 1). Fe–Sn–O also displays the better activity at this temperature range. Moreover, the increase in the calcination temperature leads to the agglomeration of crystal grains on the surface of the catalyst. This results in a smaller specific surface area, which is consistent with the SEM evaluation. In addition, as the calcination temperature increases, the pore volume and pore diameter increase, which makes it easier for reactants and products with larger molecular diameters to enter and leave the catalyst. This is also an important reason for the stronger catalyst activity at this calcination temperature.
Specific surface area, pore volume, and pore size distribution of the Fe–Sn–O catalyst at different calcination temperatures
| Calcination temperature (°C) | 700 | 750 | 800 | 850 |
| Specific surface area (m2 g−1) | 35.352 | 35.102 | 16.152 | 13.387 |
| Pore volume (mL g−1) | 0.062 | 0.054 | 0.046 | 0.087 |
| Pore size (nm) | 5.568 | 5.689 | 7.452 | 9.635 |
3 Results and discussion
3.1 The Fe–Sn–O preparation conditions
3.1.1 Effect of the metal ratio on the catalytic performance of Fe–Sn–O
The metal ratio in the composite metal oxide has a significant effect on the reaction. The Fe–Sn–O catalysts exhibiting Fe:Sn ratios of 0.5:1, 1:1, 1.5:1, and 2:1 are prepared by calcination at 600°C for 4 h.
As it can be seen in Figure 5, when the n(Fe):n(Sn) ratio is increased from 0.5:1 to 1:1, the cyclohexanone conversion rate and the yield of ε-caprolactone slightly increase. In contrast, the conversion rate and yield decrease with the increase of Sn in the Fe–Sn–O catalyst. Generally, Fe is a key for the improvement of the reaction conversion rate, while Sn plays a role in enhancing the selectivity. Thus, finding the most optimal Fe:Sn ratio is essential to achieve high yields. In addition, the phases and structures of the catalysts exhibiting different metal ratios might also affect their activity. In the present, the most suitable metal ratio is established to be n(Fe):n(Sn) = 1:1.
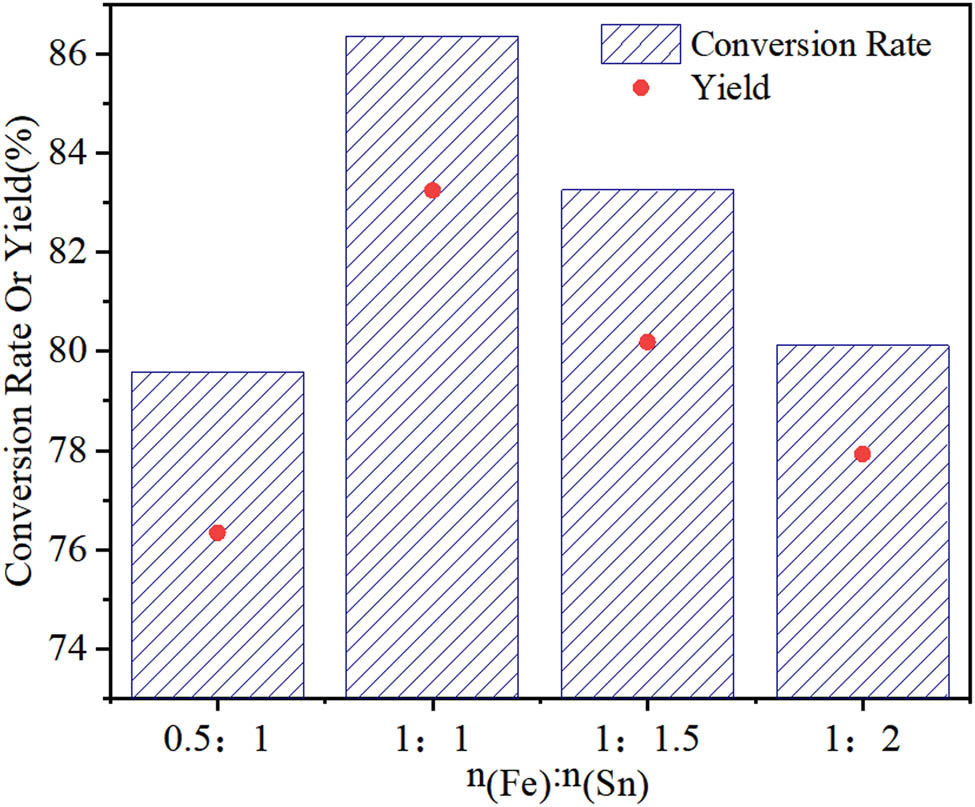
Effect of the metal ratio on the catalytic performance of Fe–Sn–O.
3.1.2 Effect of the calcination temperature on the catalytic performance of Fe–Sn–O
The catalyst calcination temperature also affects its structure, leading to varying activity. Different Fe–Sn–O catalysts are prepared by calcination at 700°C, 750°C, 800°C, and 850°C for 4 h at the n(Fe):n(Sn) ratio of 1:1. The activities of the resulting catalysts are evaluated.
As it can be seen in Figure 6, an increase in the calcination temperature to 850°C results in increases in both the cyclohexanone conversion rate and the ε-caprolactone yield. As the temperature continues to increase, the conversion rate and yield decrease. This is because the Fe2O3 and SnO2 pores generated at a low calcination temperature are not sufficiently developed. In addition, the specific surface area and volume of the catalyst are not large enough, which results in poor catalytic activity, low cyclohexanone conversion rate, and low ε-caprolactone yield. When the calcination is conducted at 900°C, the activity of the catalyst begins to decrease. Hence, 850°C is the most suitable catalyst calcination temperature.

Effect of the calcination temperature on the catalytic performance of Fe–Sn–O.
3.1.3 Effect of calcination time on the catalytic performance of the Fe–Sn–O catalyst
The Fe–Sn–O catalyst is calcined for 2, 3, 4, and 5 h at a n(Fe):n(Sn) ratio of 1:1 and calcination temperature of 850°C. The effect of the calcination time on the catalytic performance of the Fe–Sn–O catalyst is investigated.
As shown in Figure 7, when the calcination time is less than 5 h, the activity of the Fe–Sn–O catalyst increases with an increase of the calcination time. In contrast, when the calcination time is more than 5 h, the activity of the catalyst remains nearly unchanged. This is because the Fe(OH)3 and Sn(OH)4 species in the precursor are only partially decomposed at short calcination times, resulting in lower catalyst activity. The metal hydrates completely decompose into metal oxides after 5 h of calcination. Under these conditions, the activity of the catalyst is the highest. When the catalyst calcination time is further increased, the metal oxide and catalyst activity remain unchanged. Hence, the most suitable calcination time is 5 h.
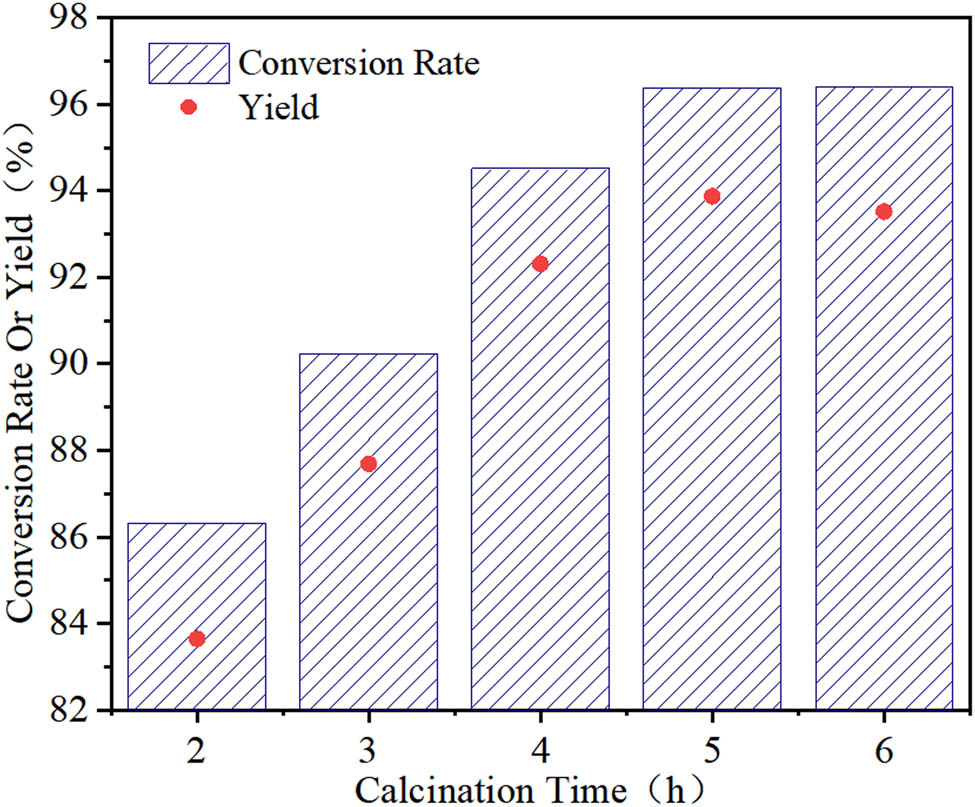
Effect of the calcination time on the catalytic performance of Fe–Sn–O.
3.2 Benzaldehyde oxidation reaction conditions
3.2.1 Effect of the O2 amount on the reaction
The amount of oxidant used in the investigated reaction is a key factor affecting its outcome. As it can be seen in Figure 8, the cyclohexanone conversion rate and ε-caprolactone yield initially increase with the increase of the oxygen flow rate. When the flow rate is over 25 mL min−1, the reaction performance reaches a maximum. According to Henry’s law, when the temperature and pressure are constant, the solubility of O2 in the reaction solution is also constant. Thus, when the flow rate is continuously increased, the concentration of O2 in the reaction solution remains unchanged. Correspondingly, the cyclohexanone conversion rate and ε-caprolactone yield keep constant during the reaction. Hence, the optimal O2 flow rate is 25 mL min−1.
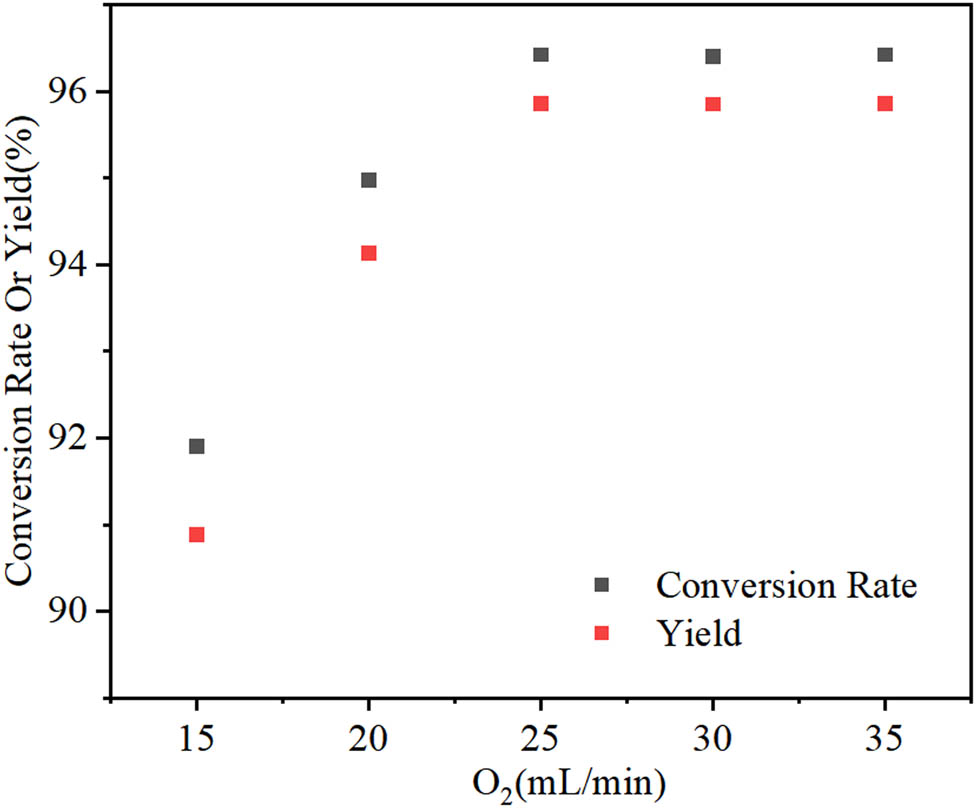
Effect of the O2 amount on the ε-caprolactone yield. Reaction conditions: cyclohexanone (0.02 mol), benzaldehyde (0.06 mol), 1,2-dichloroethane (30 mL), Fe–Sn–O (0.12 g), 55°C, 5 h.
3.2.2 Effect of the amount of benzaldehyde on the reaction
During the reaction, benzaldehyde participates and acts as a co-oxidant to oxidize cyclohexanone in the presence of O2. Thus, its amount in the reaction also affects the cyclohexanone conversion rate and ε-caprolactone yield (see Figure 9). From Figure 9, the cyclohexanone conversion rate and ε-caprolactone yield initially increase with the increase of n (cyclohexanone):n (benzaldehyde) and then slowly decrease. The most optimal ratio of n (cyclohexanone):n (benzaldehyde) is established to be 1:3. Under these conditions, the cyclohexanone conversion rate and ε-caprolactone yield are 96.43% and 95.87%, respectively. This is because the concentration of the cyclohexanone substrate in the reaction is constant. When excess benzaldehyde is used, the substrate concentration decreases, leading to a decrease in the reaction rate.

The effect of the amount of benzaldehyde on the ε-caprolactone yield. Reaction conditions: cyclohexanone (0.02 mol), 1,2-dichloroethane (30 mL), Fe–Sn–O (0.12 g), O2 (20 mL min−1), 55°C, 5 h.
3.2.3 Effect of the reaction temperature
According to Henry’s law, high temperatures reduce the solubility of gases in the solution of reaction, which can affect its reaction performance. Moreover, the temperature also has an effect on the number of activated molecules in the reaction. From Figure 10, the cyclohexanone conversion rate and ε-caprolactone yield initially increase with the increase of the temperature and reach the highest values at 60°C (98.97% conversion and 98.34% yield, respectively). Conversely, the cyclohexanone conversion rate and ε-caprolactone yield decrease at the reaction temperature of 65°C. This is attributed to the reduced solubility of O2 in the reaction solution due to the high reaction temperature. Thus, the optimal reaction temperature is determined to be 60°C.

Effect of the reaction temperature on the ε-caprolactone yield. Reaction conditions: cyclohexanone (0.02 mol), benzaldehyde (0.06 mol), 1,2-dichloroethane (30 mL), Fe–Sn–O (0.12 g), O2 (20 mL min−1), 5 h.
3.2.4 Effect of the reaction time on the reaction
As shown in Figure 11, the cyclohexanone conversion rate and ε-caprolactone yield initially increase with the reaction time and reach a maximum of 98.97% and 98.34%, respectively, at 5 h. Furthermore, it is found that the conversion rate and yield started to gradually decrease after 5 h. This is because the amount of cyclohexanone in the reaction continued to decrease as the reaction progressed until reaching equilibrium at 5 h. Hence, the optimal reaction time is 5 h.

Effect of the reaction time on the ε-caprolactone yield. Reaction conditions: cyclohexanone (0.02 mol), benzaldehyde (0.06 mol), 1,2-dichloroethane (30 mL), Fe–Sn–O (0.12 g), O2 (20 mL min−1), 60°C.
3.2.5 Investigation of the reaction stability
Using the optimized reaction conditions, specifically Fe–Sn–O catalyst (0.12 g), 1,2-dichloroethane (30 mL), O2 (25 mL min−1 flow rate), n (cyclohexanone):n (benzaldehyde) ratio of 3:1, reaction temperature of 60°C, and reaction time of 5 h, three parallel experiments are used to investigate the reaction stability. The results are obtained in Table 2.
Investigation of the reaction stability
| Serial number | Cyclohexanone conversion rate (%) | Yield of ε-caprolactone (%) |
|---|---|---|
| 1 | 98.95 | 98.33 |
| 2 | 98.96 | 98.35 |
| 3 | 98.98 | 98.39 |
| Average | 98.96 | 98.36 |
4 Conclusion
In the present study, we prepared a Fe–Sn–O composite catalyst by a co-precipitation method using Fe(NO3)3‧9H2O and SnCl4‧5H2O as the precursors and Na2CO3 as the co-precipitation agent. The reaction conditions are investigated in detail, and the obtained Fe–Sn–O catalysts are characterized by XRD, SEM, BET, FTIR, and H2-TPR analyses. The effects of the structural characteristics and surface morphology on the catalytic performance of the synthesized material are evaluated. The catalyst is used to catalyze a BV oxidation of cyclohexanone to ε-caprolactone in the presence of an O2/benzaldehyde system. We examined the reaction conditions such as the amount of the Fe–Sn–O catalyst, O2, and benzaldehyde as well as the reaction temperature and time. It is found that the catalyst exhibits the best activity at a Fe:Sn ratio of 1:1, calcination temperature of 850°C, and calcination time of 5 h. Under these conditions, the surface of the Fe–Sn–O catalyst appears as an accumulation of small prisms, which results in a large specific surface area and pore volume. The BET analysis demonstrates that the maximum specific surface area is 207.813 m2 g−1, while the maximum pore size is 0.193 nm. In addition, the XRD evaluation reveals the best crystallinity of the material at a calcination temperature of 850°C. The optimal reaction conditions are as follows: Fe–Sn–O catalyst (0.12 g), 1,2-dichloroethane (30 mL), O2 (25 mL min−1), n (cyclohexanone):n (benzaldehyde) = 3:1, reaction temperature of 60°C, and reaction time of 5 h. Under these conditions, the average cyclohexanone conversion rate reaches 98.96%, whereas the average ε-caprolactone yield is established at 98.36%. Hence, in the current work, we achieved oxidation of cyclohexanone to ε-caprolactone using a BV oxidation reaction under mild conditions. Importantly, the described method is inexpensive and environmentally friendly.
-
Funding information: Financial supported by the National Natural Science Foundation of China (91634101) and The Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20180508).
-
Author contributions: Jingjing Sun: writing – original draft, writing – review and editing, methodology, and formal analysis; Qianqian Zhu: writing – original draft, formal analysis, andconceptualization; Xiaoyan Guo: writing – review and editing, formal analysis, and investigation; Haibo Jin: writing – review and editing, resources, and funding acquisition; Guangxiang He: resources and methodology; Lei Ma: supervision; Rongyue Zhang: validation; Qingyang Gu: formal analysis; Suohe Yang: software.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Reference
[1] Shinzaburo O. Studies on lactone formation in vapor phase. II. Synthesis of ε-caprolactone. B Chem Soc Jpn. 1962;35(4):562–6. 10.1246/bcsj.35.562.Suche in Google Scholar
[2] Mckay AF, Tarlton EJ, Petri SI, Steyermmark PR, Mosley MA. Amino acids. V. 1,3-Di-(ι-carboxyalkyl)-thioureas and their chemistry. J Am Chem Soc. 1958;80(6):1510–7. 10.1021/ja01539a058.Suche in Google Scholar
[3] Lu QS, Xiao Y, Xie HH, Huang CM, Li Y, Yao XS. Progress in the production process of ε-caprolactone. Mod Chem Ind. 2015;35(2):42–5. org/10.1002/ceat.202070065.Suche in Google Scholar
[4] Asim B, Prashant K, Rajiv K. Baeyer-Villiger rearrangement catalyzed by titanium silicate molecular sieve (TS-1)/H2O2 system. Catal Lett. 1996;40(1–2):47–50. 10.1007/BF00807456.Suche in Google Scholar
[5] Manidipa P, Nabanita P, John M, Manickam S, Asim B. New mesoporous magnesium–aluminum mixed oxide and its catalytic activity in liquid phase Baeyer-Villiger oxidation reaction. Chem Eng Sci. 2012;71:564–72. 10.1016/j.ces.2011.11.038.Suche in Google Scholar
[6] Sumbul R, Nagasuresh E, Ruth G, Asim B, Debasis S, Mazumdar S, et al. Aerobic Baeyer–Villiger oxidation of cyclic ketones over periodic mesoporous silica Cu/Fe/Ni/Co-HMS-X. Appl Catal A-gen. 2015;505:515–23. 10.1016/j.apcata.2015.03.014.Suche in Google Scholar
[7] Baj S, Chrobok A, Siewniak A. New and efficient technique for the synthesis of ε-caprolactone using KHSO5 as an oxidising agent in the presence of a phase transfer catalyst. Appl Catal A-gen. 2011;395(1–2):49–52. 10.1016/j.apcata.2011.01.022.Suche in Google Scholar
[8] Zhu QQ, Jin HB, Guo XY, He GX, Ma L, Zhuang RY, et al. Study on synthesis of ε-caprolactone with MgO catalysis by Baeyer-Villiger green oxidation of cyclohexanone in H2O2/acetonitrile system. Ciesc J. 2021;72(5):2638–46. 10.11949/0438-1157.20201245.Suche in Google Scholar
[9] Lambert A, Elings JA, Macquarrie JD, Carr G, Clark HJ. The Baeyer-Villiger oxidation of ketones using HMS supported peroxycarboxylic acids. Synlett. 2000;7:1052–4. 10.1055/s-2000-6666.Suche in Google Scholar
[10] Koo DH, Kim M, Chang S. WO3 Nanoparticles on MCM-48 as a highly selective and versatile heterogeneous catalyst for the oxidation of olefins, sulfides, and cyclic ketones. Org Lett. 2005;22(7):5015–8. 10.1021/ol052019i.Suche in Google Scholar PubMed
[11] Luo WP, Yan MQ, Tao B, Shi SL, Chen B. Solubility of succinic acid, glutaric acid and adipic acid in propionic acid + ε-caprolactone + water mixtures: Experimental measurement and thermodynamic modeling. J Chem Thermodyn. 2019;138:332–44. 10.1016/j.jct.2019.06.033.Suche in Google Scholar
[12] Kotsuki H, Arimura K, Araki T, Shinohara T. Sc(OTf)3- and TfOH- catalyzed Baeyer-Villiger oxidation of carbonyl compounds with m-chloroperbenzoic acid. Synlett. 1999;4:462–4. 10.1055/s-1999-2648.Suche in Google Scholar
[13] Choudary BM, Sridhar C, Sateesh M, Sreedhar B. Microencapsulated bismuth(iii) triflate catalyst for organic transformations. J Mol Catal A: Chem. 2004;212(1/2):237–43. 10.1016/j.molcata.2003.10.037.Suche in Google Scholar
[14] Li Y. Catalytic oxidation of the epsilon-caprolactone for the synthesis of cyclohexanone. China: Wuhan University of Technology; 2017.Suche in Google Scholar
[15] Zhou XT, Ji HB, Yuan QL. Baeyer-Villiger oxidation of ketones catalyzed by iron(iii) meso-tetraphenyl porphyrin chloride in the presence of molecular oxygen. J Porphyr Phthalocyanines. 2018;12(2):94–100. 10.1142/S1088424608000121.Suche in Google Scholar
[16] Belaroui LS, Sorokin AB, Figueras F, Bengueddach A, Milletet MJ. Comparative Baeyer-Villiger oxidation of cyclohexanone on Fe-pillared clays and iron tetrasulfophthalocyanine covalently supported on silica. Cr Chim. 2010;13(4):466–72. 10.1016/j.crci.2010.01.007.Suche in Google Scholar
[17] Jeong EY, Ansari MB, Park SE. Aerobic Baeyer–Villiger oxidation of cyclic ketones over metalloporphyrins bridged periodic mesoporous organosilica. ACS Catal. 2011;1(8):855–63. 10.1021/cs200163r.Suche in Google Scholar
[18] Zheng C, Chang S, Yang C, Lian D, Ma C, Zhang C, et al. Enhanced shape selective catalysis of mixed cyclic ketones in aerobic Baeyer-Villiger oxidation with magnetic Cu-Fe3O4 supported mesoporous silica microspheres. Tetrahedron. 2011;74(21):2608–16. 10.1016/j.tet.2018.04.009.Suche in Google Scholar
[19] Zhou Z, Wang J, Qin J, Yu Y, Wu WL. The multifunctional mesoporous Sn-Cu-Ti catalysts for the B-V oxidation of cyclohexanone by molecular oxygen. J Porous Mat. 2018;25(3):835–43. 10.1007/s10934-017-0496-9.Suche in Google Scholar
[20] Ma Y, Liang Z, Feng S, Zhang Y. Baeyer–Villiger oxidation of cyclohexanone by molecular oxygen with Fe–Sn–O mixed oxides as catalysts. Appl Organomet Chem. 2015;29(7):450–5. 10.1002/aoc.3314.Suche in Google Scholar
[21] Zhang XY, Yang HL, Yang GX, Li SW, Wang X, Ma JT. Metal-free mesoporous SiO2 nanorods as a highly efficient catalyst for the Baeyer-Villiger oxidation under mild conditions. ACS Sustain Chem Eng. 2018;6(5):5868–76. 10.1021/acssuschemeng.7b04167.Suche in Google Scholar
[22] Li YF, Guo MQ, Yin SF, Chen L, Zhou YB, Qiu RH, et al. Graphite as a highly efficient and stable catalyst for the production of lactones. Carbon. 2013;55(2):269–75. 10.1016/j.carbon.2012.12.036.Suche in Google Scholar
[23] Subramanian H, Koodali RT. Baeyer-Villiger oxidation of cyclic ketones over iron-containing mesoporous MCM-48 silica materials. React Kinet Catal L. 2008;95(2):239–45. 10.1007/s11144-008-5352-0.Suche in Google Scholar
[24] Kawabata T, Ohishi Y, Itsuki S, Fujisaki N, Shishido T, Takaki K, et al. Iron-containing MCM-41 catalysts for Baeyer–Villiger oxidation of ketones using molecular oxygen and benzaldehyde. J Mol Catal A-Chem. 2005;236(1–2):99–106. 10.1016/j.molcata.2005.03.027.Suche in Google Scholar
[25] Zang J, Ding JY, Li Y, Wang T, Li Y, Gong LF. Highly efficient and reusable Cu-MCM-41 catalyst for the Baeyer–Villiger oxidation of cyclohexanone. Catal Commun. 2014;51:24–8. 10.1016/j.catcom.2014.03.022.Suche in Google Scholar
[26] Chen SY, Zhou X, Li Y, Luo RC, Ji HB. Biomimetic Baeyer-Villiger oxidation of ketones with SnO2 as cocatalyst, features in activating carbonyl group of substrates. Chem Eng J. 2014;241:138–44. 10.1016/j.cej.2013.12.027.Suche in Google Scholar
[27] Chen SY, Zhou TX, Ji HB. Insight into the cocatalyst effect of 4A molecular sieve on Sn(II) porphyrin-catalyzed BV oxidation of cyclohexanone. Catal Today. 2015;264:191–7. 10.1016/j.cattod.2015.07.051.Suche in Google Scholar
[28] Zhou Z, Wang J, Qin J, Yu Y, Wu WL. The multifunctional mesoporous Sn–Cu–Ti catalysts for the BV oxidation of cyclohexanone by molecular oxygen. J Porous Mat. 2018;25(3):835–43. 10.1007/s10934-017-0496-9.Suche in Google Scholar
[29] Huo H, Wu L, Ma J, Yang HL, Zhang L, Yang YY, et al. Fabrication of Fe3O4-l-dopa-CuII/SnIV@ Micro-Mesoporous-SiO2 catalyst applied to Baeyer-Villiger oxidation reaction. ChemCatChem. 2016;8(4):779–86. 10.1002/cctc.201501107.Suche in Google Scholar
[30] Carballeira JD, Alvarez E, Sinisterra JV. Biotransformation of cyclohexanone using immobilized Geotrichum candidum NCYC49: factors affecting the selectivity of the process. J Mol Catal B-enzym. 2004;28(1):25–32. 10.1016/j.molcatb.2004.01.009.Suche in Google Scholar
[31] Bornadel A, Hatti‐Kaul R, Hollmann F, Kara S. A Bi‐enzymatic convergent cascade for ε‐caprolactone synthesis employing 1,6‐hexanediol as a ‘Double‐Smart Cosubstrate’. ChemCatChem. 2015;7(16):2442–5. 10.1002/cctc.201500511.Suche in Google Scholar
[32] Romero E, Rubén GC, Mattevi A, Fraaije MW. Characterization and crystal structure of a robust cyclohexanone monooxygenase. Angew Chem Int Ed. 2016;55(51):15852–5. 10.1002/ange.201608951.Suche in Google Scholar
[33] Yang Z, Niu L, Ma Z, Ma HC, Lei ZQ. Fabrication of highly active Sn/W mixed transition-metal oxides as solid acid catalysts. Transit Met Chem. 2011;36(3):269–74. 10.1007/s11243-011-9465-3.Suche in Google Scholar
[34] Zhang GX, Ren XC, Zhang HB, Yu P, Gui SY. MgO/SnO2/WO3 as catalysts for synthesis of ε-caprolactone over oxidation of cyclohexanone with peracetic acid. Catal Commun. 2015;58:59–63. 10.1016/j.catcom.2014.08.031.Suche in Google Scholar
© 2021 Jingjing Sun et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis
Artikel in diesem Heft
- Research Articles
- MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses
- Effect of CaO on catalytic combustion of semi-coke
- Studies of Penicillium species associated with blue mold disease of grapes and management through plant essential oils as non-hazardous botanical fungicides
- Development of leftover rice/gelatin interpenetrating polymer network films for food packaging
- Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract
- Green synthesized silver and copper nanoparticles induced changes in biomass parameters, secondary metabolites production, and antioxidant activity in callus cultures of Artemisia absinthium L.
- Gold nanoparticles from Celastrus hindsii and HAuCl4: Green synthesis, characteristics, and their cytotoxic effects on HeLa cells
- Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies
- One-step preparation of metal-free phthalocyanine with controllable crystal form
- In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres
- Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs
- Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater
- Feasibility of fly ash as fluxing agent in mid- and low-grade phosphate rock carbothermal reduction and its reaction kinetics
- Three combined pretreatments for reactive gasification feedstock from wet coffee grounds waste
- Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent
- Combustion and gasification characteristics of low-temperature pyrolytic semi-coke prepared through atmosphere rich in CH4 and H2
- Microwave-assisted reactions: Efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating
- New approach in process intensification based on subcritical water, as green solvent, in propolis oil in water nanoemulsion preparation
- Continuous sulfonation of hexadecylbenzene in a microreactor
- Synthesis, characterization, biological activities, and catalytic applications of alcoholic extract of saffron (Crocus sativus) flower stigma-based gold nanoparticles
- Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress
- Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4
- Silica extraction from bauxite reaction residue and synthesis water glass
- Metal–organic framework-derived nanoporous titanium dioxide–heteropoly acid composites and its application in esterification
- Highly Cr(vi)-tolerant Staphylococcus simulans assisting chromate evacuation from tannery effluent
- A green method for the preparation of phoxim based on high-boiling nitrite
- Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus
- Mixed gel electrolytes: Synthesis, characterization, and gas release on PbSb electrode
- Supported on mesoporous silica nanospheres, molecularly imprinted polymer for selective adsorption of dichlorophen
- Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater
- Development of a continuous PET depolymerization process as a basis for a back-to-monomer recycling method
- Green synthesis of ZnS nanoparticles and fabrication of ZnS–chitosan nanocomposites for the removal of Cr(vi) ion from wastewater
- Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure
- Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus
- Variability and improvement of optical and antimicrobial performances for CQDs/mesoporous SiO2/Ag NPs composites via in situ synthesis
- Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications
- Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia
- Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers
- Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications
- Digera muricata (L.) Mart. mediated synthesis of antimicrobial and enzymatic inhibitory zinc oxide bionanoparticles
- Aqueous synthesis of Nb-modified SnO2 quantum dots for efficient photocatalytic degradation of polyethylene for in situ agricultural waste treatment
- Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid
- Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity
- Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens
- Hydrophilic modification of PVDF membranes by in situ synthesis of nano-Ag with nano-ZrO2
- Paracrine study of adipose tissue-derived mesenchymal stem cells (ADMSCs) in a self-assembling nano-polypeptide hydrogel environment
- Study of the corrosion-inhibiting activity of the green materials of the Posidonia oceanica leaves’ ethanolic extract based on PVP in corrosive media (1 M of HCl)
- Callus-mediated biosynthesis of Ag and ZnO nanoparticles using aqueous callus extract of Cannabis sativa: Their cytotoxic potential and clinical potential against human pathogenic bacteria and fungi
- Ionic liquids as capping agents of silver nanoparticles. Part II: Antimicrobial and cytotoxic study
- CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes
- Corylus avellana leaf extract-mediated green synthesis of antifungal silver nanoparticles using microwave irradiation and assessment of their properties
- Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations
- Fe3+ and Ce3+ modified nano-TiO2 for degradation of exhaust gas in tunnels
- Analysis of enzyme activity and microbial community structure changes in the anaerobic digestion process of cattle manure at sub-mesophilic temperatures
- Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens
- Baeyer–Villiger co-oxidation of cyclohexanone with Fe–Sn–O catalysts in an O2/benzaldehyde system
- Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts
- Study on titanium dioxide nanoparticles as MALDI MS matrix for the determination of lipids in the brain
- Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential
- Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi
- Antibacterial greener silver nanoparticles synthesized using Marsilea quadrifolia extract and their eco-friendly evaluation against Zika virus vector, Aedes aegypti
- Optimization for simultaneous removal of NH3-N and COD from coking wastewater via a three-dimensional electrode system with coal-based electrode materials by RSM method
- Effect of Cu doping on the optical property of green synthesised l-cystein-capped CdSe quantum dots
- Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan
- Biosynthesis of silver nanoparticles using leaves of Mentha pulegium, their characterization, and antifungal properties
- A study on the coordination of cyclohexanocucurbit[6]uril with copper, zinc, and magnesium ions
- Ultrasound-assisted l-cysteine whole-cell bioconversion by recombinant Escherichia coli with tryptophan synthase
- Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy
- Preparation and characterization of sodium alginate/acrylic acid composite hydrogels conjugated to silver nanoparticles as an antibiotic delivery system
- Synthesis of tert-amylbenzene for side-chain alkylation of cumene catalyzed by a solid superbase
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities
- Simulation and improvement of the separation process of synthesizing vinyl acetate by acetylene gas-phase method
- Review Articles
- Carbon dots: Discovery, structure, fluorescent properties, and applications
- Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives
- Review on functionalized magnetic nanoparticles for the pretreatment of organophosphorus pesticides
- Extraction and modification of hemicellulose from lignocellulosic biomass: A review
- Topical Issue: Recent advances in deep eutectic solvents: Fundamentals and applications (Guest Editors: Santiago Aparicio and Mert Atilhan)
- Delignification of unbleached pulp by ternary deep eutectic solvents
- Removal of thiophene from model oil by polyethylene glycol via forming deep eutectic solvents
- Valorization of birch bark using a low transition temperature mixture composed of choline chloride and lactic acid
- Topical Issue: Flow chemistry and microreaction technologies for circular processes (Guest Editor: Gianvito Vilé)
- Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
- In-flow enantioselective homogeneous organic synthesis

