Abstract
Using an easy mechanical agitation process at room temperature, a metal–organic framework (MOF) based on metallic Zn(ii), organic linker benzene-1,3,5-tricarboxylic acid (Zn-BTC), Fe3O4 nanoparticles, and nanocellulose are combined to create a novel composite material called Fe3O4/NC/MOF. Various tools were used to characterize the created composite. Congo red, Basic Blue 54 (BB 54), Basic Violet 14 (BV 14), and Acid red 88 (AR 88) dyes were effectively eliminated from water using Fe3O4/NC/MOF. A number of variables were investigated, including pH, temperature, contact time, initial dye concentration, and adsorbent dosage. To understand the specific adsorption process, a number of kinetic models were used, including the intra-particle diffusion model, Elovich’s kinetic model, pseudo-first-order, and pseudo-second-order kinetic models. The most accurate description of dye sorption kinetics comes from the pseudo-second-order kinetic model. Also, the Langmuir model is more accurate to describe isotherms than Freundlich and Temkin models. Furthermore, thermodynamic parameters were obtained and examined, including enthalpy (ΔH), Gibbs free energy (ΔG), and entropy (ΔS). After four cycles, the Fe3O4/NC/MOF demonstrated good recyclability. According to experimental research, this adsorbent is promising to enhance the quality of environmental water that has been tainted with organic dyes.
1 Introduction
The problem of water contamination has become increasingly urgent and dangerous as a result of the expansion of international trade and industrial activity, particularly in the leather tanning and textile industries [1,2]. These activities cause large volumes of organic dyes to be released into the environment, which has led to major pollution issues. Since most dyes are synthetic and include aromatic rings, they cannot biodegrade, are mutagenic and carcinogenic, and will not break down if they are dumped into waste streams [3,4]. They may have immediate or long-term effects on organisms that come into contact with them. Water-soluble dyes also absorb or reflect sunlight, preventing the growth of microorganisms [5]. Moreover, even in low quantities, they cause aesthetic pollution and are apparent. Consequently, it is crucial to significantly reduce the color of aquatic systems. To remove contaminants from water, a variety of treatments have been used, including chemical precipitation [6], coagulation [7], membrane filtration [8], adsorption [9,10], microbial degradation [11], and ion exchange [12,13]. Adsorption is one of these treatments that has received a lot of attention due to its easy to use, affordable, and highly effective nature [14,15].
Metal–organic frameworks (MOFs) are one-, two-, or three-dimensional porous crystalline polymer networks made up of metal nodes (metal ions or clusters) attached to multidentate organic linkers [16]. The linkers are then joined by strong covalent connections. Porous coordination networks and porous coordination polymers are other names for MOFs. They exhibit special qualities that go beyond what would be expected from a straightforward combination of these components, in addition to combining the advantageous aspects of organic and inorganic elements. MOFs have drawn particular attention for a variety of applications, including gas separation and storage [17], catalysis [18], adsorption [19], energy storage [20], drug delivery [21], and chemical sensing [22] because of their unique qualities, which include record-breaking surface areas, ultrahigh porosities, low density, high thermal stability, and tunable pore structures [23]. The two techniques that are now most frequently employed to recover MOFs from the reaction medium are filtration and centrifugation. MOFs can only be used on a limited scale due to their high cost, difficult separation methods, and relatively slow speed of separation. For this reason, creating a MOF material that is readily separated will be crucial to its eventual widespread use. To make their separation simple, the magnetization of MOF is the most applicable method.
Simple oscillation or agitation can be used to readily and uniformly spread magnetic nanoparticles (Fe3O4) in an aqueous or organic liquid phase. They may be directly separated from the liquid phase by applying an external magnetic field due to their high saturation magnetization, which eliminates the need for additional labor-intensive operating procedures like centrifugation and filtration [24]. These nanoparticles have the convenient ability to be reused. The applicability of Fe3O4 nanoparticles is limited due to the considerable particle aggregation that can occur from magnetic dipole interactions despite the comparatively straightforward production process. In order to compensate for this shortfall, cellulose nanocrystals [25], graphene [26], carbon nanotubes [27], and chitosan [28] have all been added to Fe3O4 nanoparticles.
Because of its special qualities, which include being affordable, renewable, degradable, plentiful, non-toxic, and ecologically benign, cellulose is a promising polymer for the creation of adsorbent compounds [29,30]. At the nanoscale, cellulose has a high aspect ratio and a large surface area, which makes adsorbent modification possible to attain superior adsorption [31,32]. Additionally desirable for the adsorbent reinforcement are nanocellulose’s (NC’s) extraordinary mechanical and chemical capabilities. The robustness and lifespan of adsorbents used in water treatment are further enhanced by these characteristics. These properties of NC allow their wide applications in adsorbents [33], flocculants [34], scaffolds [35], and drug carriers [36]. Our laboratory has utilized NC for the water treatment [37]. Also, many reported studies have combined NC and MOF. Using an in situ growing technique, Ma et al. created a ZIF-8@cellulose aerogel, which successfully adsorbed dyes and heavy metal ions [38]. Also, Wang et al. used UiO-66 on the cellulose aerogel [39] for adsorption applications. Zhu and colleagues blended cellulose aerogel with three distinct MOF materials (ZIF-8, UiO-66, and MIL-100(Fe)) to successfully adsorb heavy metals [40]. However, the high adsorption time and low adsorption capacity of these adsorbents need to be improved.
Herein, we aim to create a straightforward process for combining Fe3O4 nanoparticles, NC, and MOF to create a more powerful dye adsorbent (Fe3O4/NC/MOF) that is more effective. In this study, Zn(ii)-based MOF was produced on a magnetic NC surface, and characterized via different techniques. It was then evaluated as an adsorbent to various anionic and cationic types of dyes (Congo red [CR], BB 54, BV 14, and AR 88) from water under a range of experimental parameters, including temperature, contact time, pH, initial dye concentration, and adsorbent dose. Also, a thorough analysis of isotherms, kinetics, thermodynamics, and equilibrium was conducted to evaluate the performance of this adsorbent with target dyes. The industrial application of this hybrid adsorbent appears highly promising due to its high adsorption capacity, effectiveness, and simple synthesis.
2 Materials and methods
2.1 Chemicals
Trisodium citrate (Na3C6H5O7), iron(ii) sulfate heptahydrate (FeSO4·7H2O), NC, benzene-1,3,5-tricarboxylic acid (BTC), zinc acetate dehydrate, 2,2,6,6-tetramethyl piperidine-1-oxyl (TEMPO), triethylamine (Et3N), AR 88, BV 14, BB 54, and CR were supplied by Sigma-Aldrich. Sodium hydroxide (NaOH), nitric acid (HNO3), sodium bromide (NaBr), sodium hypochlorite (NaClO), and N,N-dimethylformamide (DMF) were supplied by El-Gomhuria Co., Egypt. The analytical grade chemical raw materials listed above are all usable without additional purification. The distilled water used in all of the experiments was self-made.
2.2 Synthesis of Fe3O4 nanoparticles
A technique called reverse co-precipitation was used to create Fe3O4 nanoparticles. This technique was slightly modified from the literature [41]. First, 50 mL of deionized water was combined with 1 mL of Na3C6H5O7 (1 mM) and 50 mL of NaOH (1 M). After that, the mixture was vigorously stirred for 10 min at room temperature and 55.6 mg of FeSO4·7H2O was added. Black precipitates were seen when the salts were added to the alkaline solution, which may indicate that Fe3O4 nanoparticles were formed. After the solution was cooled to ambient temperature, the precipitate was easily collected by a permanent magnet and microwave irradiated for up to 30 s. The black precipitates were air-dried overnight. After re-suspending in deionized water, the Fe3O4 nanoparticles were separated using a permanent magnet and centrifuged for 15 min at 6,000 rpm. After separating, the Fe3O4 nanoparticles were combined with 1 mL of deionized water to achieve a final concentration of 12 mg·mL−1. This mixture was then kept at room temperature until used.
2.3 Synthesis of the Fe3O4/NC nanocomposite
Using a homogenizer, about 1 g of NC was distributed in distilled water. Next, 0.125 g of sodium bromide (NaBr) and 0.0125 g of TEMPO were added to the suspension of NC and stirred at room temperature. About 9.0 mL of sodium hypochlorite (NaClO) was added dropwise to the NC suspension to start the oxidation process. The suspension was supplemented with 0.5 M sodium hydroxide (NaOH) to keep the pH between 10 and 11 during the process. To halt the reaction, around 10 mL of ethanol was injected once the pH of the suspension reached a steady level. Ultimately, NC was thoroughly washed with distilled water and then freeze-dried. Subsequently, 50 mg of NC was mixed with 50 mL of deionized water, and 10 mL of Fe3O4 nanoparticles was added to the mixture and vigorously agitated for 2 h at room temperature. After creating the hybrid nanocomposite, they were separated using an external magnet and then re-dispersed for future use in 50 mL of deionized water.
2.4 Synthesis of zinc-based MOF (Zn-BTC)
A minor modification was made to the published process for synthesizing MOF [42]. DMF (25 mL) was used to dissolve BTC (0.53 g, 2.5 mmol) and Et3N (2.7 mL). In addition, 0.72 g (3.3 mmol) of zinc acetate dihydrate was dissolved in the same volume of DMF. The two solutions were combined and swirled for 2.5 h. Centrifugation was used to recover the product, which was then cleaned thrice with DMF and twice with methanol. Ultimately, MOF was oven-dried at 70°C to get the desired dry product (0.9 g).
2.5 Synthesis of Fe3O4/NC/MOF nanocomposite
DMF (25 mL) was used to dissolve BTC (0.53 g, 2.5 mmol) and Et3N (2.7 mL). Before adding 1 g of Fe3O4/NC, zinc acetate dihydrate (0.72 g, 3.3 mmol) was also dissolved in 25 mL of DMF. These two solutions were combined and agitated mechanically for 30 min. The combined solution was then swirled for 2.5 h at room temperature. Following magnetic separation, the Fe3O4/NC/MOF nanocomposite was washed twice, alternatingly with DMF and methanol. Vacuum freeze-drying was then used to obtain the Fe3O4/NC/MOF nanocomposite. Scheme 1 illustrates the synthesis procedures and structure of the fabricated Fe3O4/NC/MOF nanocomposite.
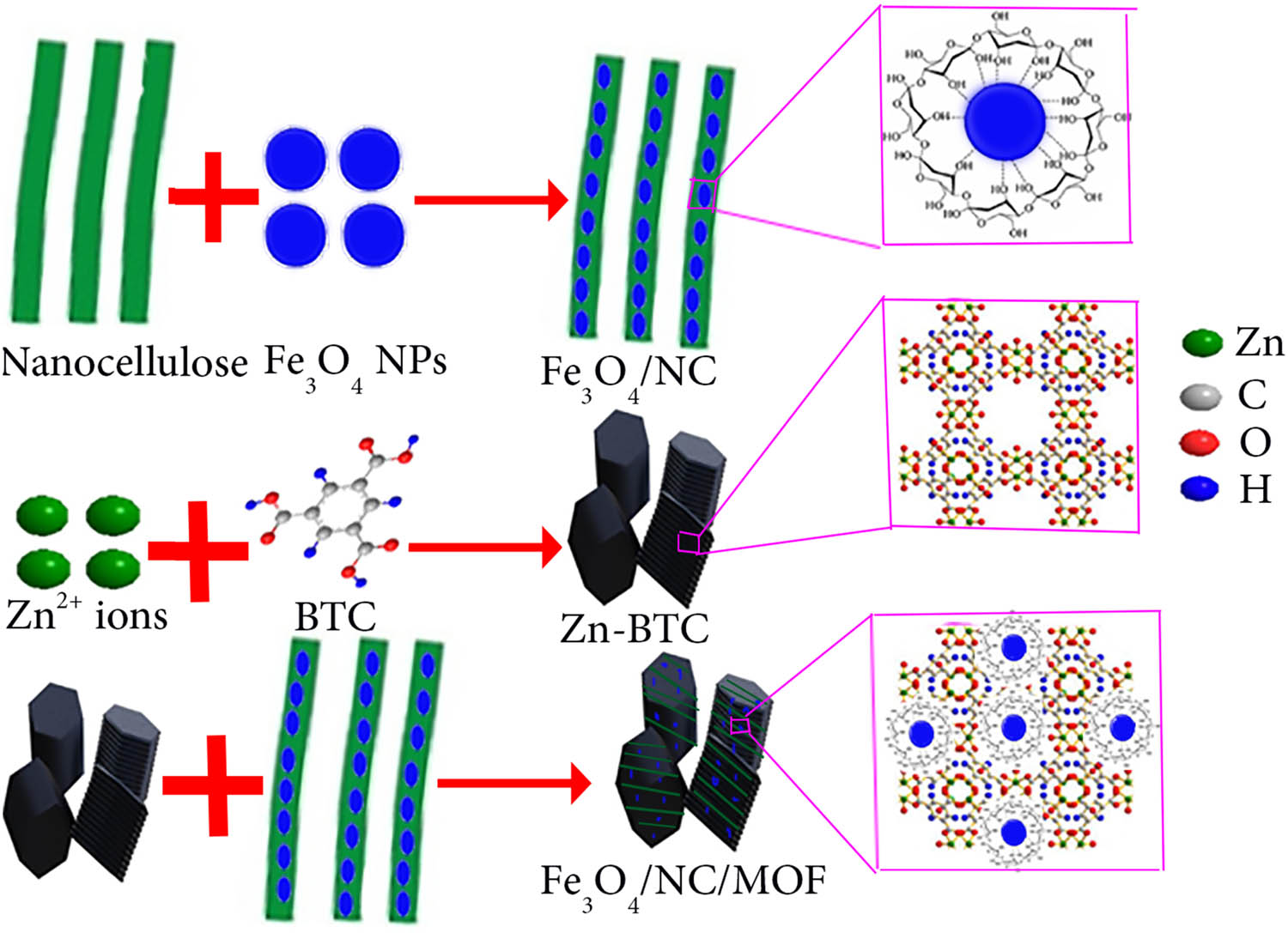
Synthesis procedures and the structure of the as-prepared materials.
2.6 Characterization of the as-prepared materials
Transmission electron microscopy (TEM) was performed using a JEOL JEM 2100 transmission electron microscope operating at 200 kV. The N2 adsorption–desorption isotherms were measured at 77 K by the Brunauer–Emmett–Teller method using a Micromeritics ASAP 2020 M + C analyzer. The materials’ surface morphology was investigated using a scanning electron microscope (SEM, S4800, Hitachi, Japan). X-ray diffraction (XRD) patterns of the produced materials were obtained using an X-ray diffractometer with Cu-Kα radiation (40 kV, 30 mA). With a scan step of 0.02°, the patterns were recorded in the 2θ region between 10° and 80°. Fourier-transform infrared (FTIR) spectra were recorded using a Perkin Elmer Spectrum 100 Fourier Transform Spectrometer. A vibrating sample magnetometer (VSM), a physical property measurement instrument, was used to measure the magnetic properties at 300 K as a function of the applied magnetic field, ranging from −80 to 80 kOe. X-ray photoelectron spectroscopy (XPS) (AXIS ULTRA DLD, Shimadzu, Japan) was performed to examine the materials’ surface constituents.
2.7 Adsorption of organic dyes
The synthesized adsorbent was investigated for the removal of different organic dyes AR 88, BV 14, BB 54, and CR. The molecular structures of the four dyes are presented in Scheme 2. In the adsorption process, the ideal parameters and influencing variables for Fe3O4/NC/MOF dye removal were examined. Using 0.008 g as the adsorbent dose, the impacts of the initial dye concentration (0.001–0.8 g·L−1) were examined for 20 min at pH = 2. Prior to mixing the dye solution with the adsorbent, 0.05 N hydrochloric acid or 0.05 N sodium hydroxide was added to the dye solution to correct the pH. The ideal amount of the adsorbent was found by mixing various quantities of the adsorbent (1 × 10−3 to 1 × 10−2 g) with 10.0 mL of polluted water for 20.0 min at 298 K, pH = 2–8, and an initial dye concentration of 20 mg·L−1 while stirring continuously at 500 rpm. The concentrations and adsorption of the dye solution were measured using a UV-Vis spectrophotometer. Subsequently, using the following equations, dye removal efficiency (%) and adsorption capacity at different time periods (q e, mg·g−1) were calculated:
where C e and C 0 (mg·L−1) are the equilibrium concentration and initial concentration of the dye, respectively. The mass of the adsorbent was expressed as m (g), and V was the dye solution’s volume. The dye concentration (0.01–0.8 g·L−1) and adsorbent dose (1, 0.08, 0.06, 0.02, and 0.01 g·L−1) were chosen for the investigations of adsorption isotherms and kinetics.
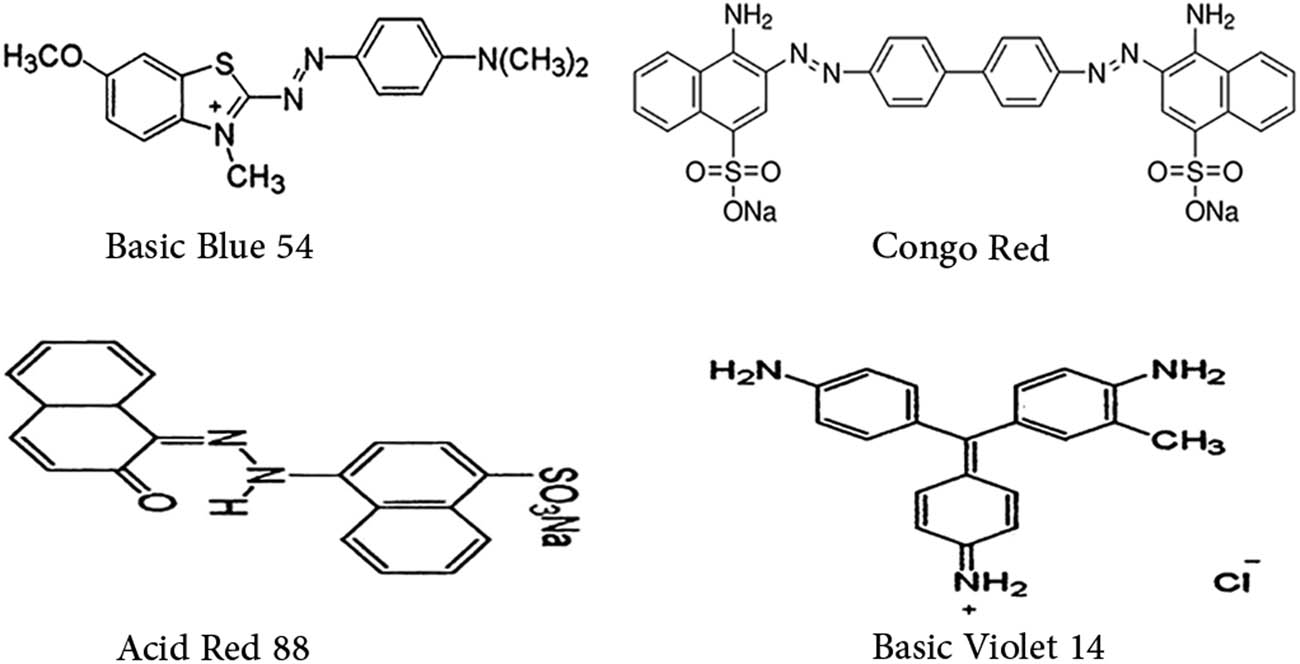
The molecular structures of the four dyes used in the adsorption experiments.
The recyclability of the synthesized Fe3O4/NC/MOF was investigated for the removal of organic dyes to determine its effectiveness as a reusable adsorbent. The reusability of Fe3O4/NC/MOF for dye adsorption was investigated under optimum conditions, which included an agitation rate of 500 rpm, a contact time of 20.0 min, pH of 2.0, a dosage of 0.08 g·L−1, and a dye concentration of 20.0 mg·L−1. The reusability study was investigated for up to four successive cycles. Each cycle consists of adsorption and desorption. In the adsorption part, the organic dyes were mixed with Fe3O4/NC/MOF under optimum conditions. After that, the adsorbent was collected using a magnet and reactivated. The reactivation process involved the desorption of adsorbed dyes from the surface of the adsorbent using a suitable eluent. Herein, the used eluent was ethanol 60% (10 mL) and NaOH 0.1 M (10 mL). After desorption, the adsorbent was washed using a mixed solvent of H2O/EtOH and dried by heating under a vacuum at 55°C for 55 min to be ready for the next cycle. After each cycle, UV-Vis spectroscopy was employed to examine for any remaining dye residues.
3 Results and discussion
3.1 Characterization of materials
Figure 1a displays the XRD patterns for MOF and Fe3O4/NC/MOF. At 15.8°, a distinctive peak for NC was observed. This peak shows the normal diffraction pattern of type I cellulose and correlates with the (101) reflection [43]. Magnetic Fe3O4 nanoparticles have six unique peaks at 2θ = 57.2, 53.7, 43.2, 35.6, and 30.3°, as shown in Figure 1a [44,45]. After MOF was coated by the Fe3O4/NC nanocomposite surface, the pattern seen for Fe3O4/NC/MOF additionally included the peak characteristic for MOF at 11.0° [46], as shown in Figure 1a. These XRD findings verify that the composite product (Fe3O4/NC/MOF) retains both the magnetic nanoparticles and the MOF crystal structure. FTIR spectra were used to characterize the structures of the produced materials. The MOF spectrum displays two peaks, corresponding to the asymmetric and symmetric carbonyl stretching vibrations of MOF, in the ranges of 1,619–1,540 and 1,438–1,371 cm−1 (Figure 1b) [47]. The Fe–O stretching vibration is represented by the peak at 582 cm−1 (Figure 1b) in the Fe3O4/NC nanocomposite spectrum, which is typical for Fe3O4 [48]. The 2,903 cm−1 band is associated with the C–H stretching vibration modes of NC, while the C–C bond vibrations are responsible for the bands at 1,161 and 1,064 cm−1 [49,50]. The vibration modes of O–H stretching are represented by the bands located at 3,416 cm−1 [51]. The FTIR spectrum of Fe3O4/NC/MOF also showed the typical peaks of MOF at 1,619–1,371 cm−1, suggesting that MOF was successfully bonded to the Fe3O4/NC nanocomposite surface. The presence of hydroxyl groups in Fe3O4/NC/MOF was shown by the broad band at 3,416 cm−1 induced by the O–H stretching vibration. FTIR results indicated that carboxyl groups are abundant on the surfaces of Fe3O4 nanoparticles and NC, and MOF has a significant amount of them as well. Because the oxygen atoms of carboxylic and hydroxyl groups have free pairs of electrons, they can interact with the adsorbate molecules to produce complexes that are mediated by coordinative bonds.
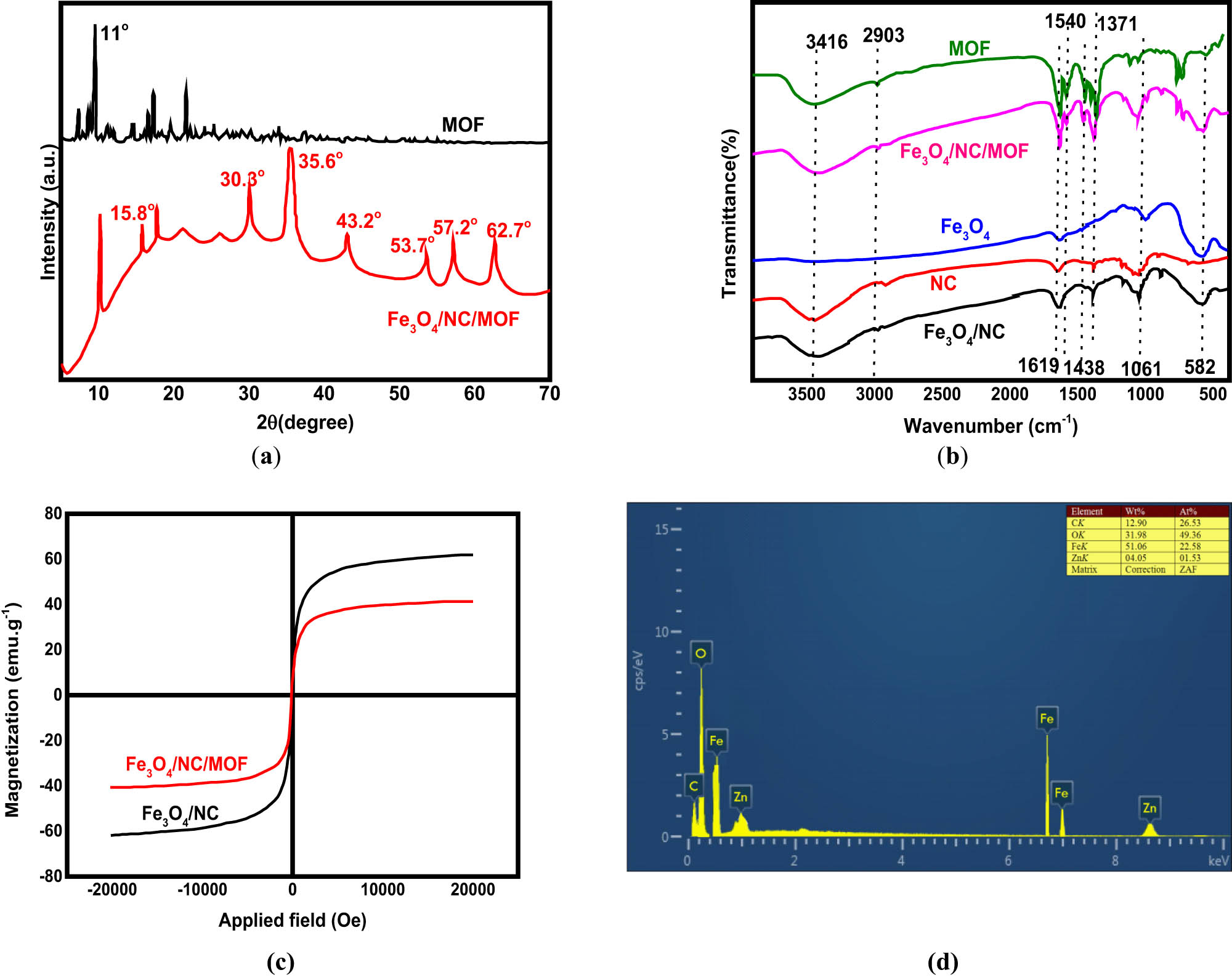
(a) XRD, (b) FTIR, (c) magnetization curves, and (d) EDX spectrum of the synthesized materials.
The magnetic characteristics of Fe3O4/NC and Fe3O4/NC/MOF were examined using a VSM. The magnetic hysteresis loops acquired for these two magnetic materials are displayed in Figure 1c. The saturation magnetization of Fe3O4/NC was 62.70 emu·g−1. This value dropped to 42.39 emu·g−1 with MOF loading. These findings suggest that the Fe3O4/NC/MOF can be quickly separated when a magnetic field is applied. After vigorous shaking, the Fe3O4/NC/MOF was separated by an external magnetic field in less than 40 s. Figure 1d presents the energy-dispersive X-ray spectra (EDX) distribution of C, O, Zn, and Fe in the Fe3O4/NC/MOF nanocomposite. The Fe/O ratio in the magnetic Fe3O4 nanoparticles (2.6) is higher than that in Fe3O4/NC/MOF (1.6), as shown in Figure 1d. This difference can be due to the increased oxygen concentration in MOF and NC, which suggests the production of Fe3O4/NC/MOF. Figure 2 displays the TEM images of NC, MOF, Fe3O4/NC, and Fe3O4/NC/MOF. Numerous hydroxyl (OH) groups on the NC surface (Figure 2a) can connect with one another through hydrogen bonding, which causes the cellulose rods to aggregate into spherical forms. Comparing the TEM image of NC (Figure 2a) with that of Fe3O4/NC (Figure 2c) in which NC was modified using magnetic nanoparticles, it is clear that the clustered NC has been distributed. The average length of the Zn-based MOF is shown in Figure 2b and d. It is also clear that Fe3O4/NC adhered to the surface of the prismatic MOF, indicating that the desired Fe3O4/NC/MOF was successfully fabricated.
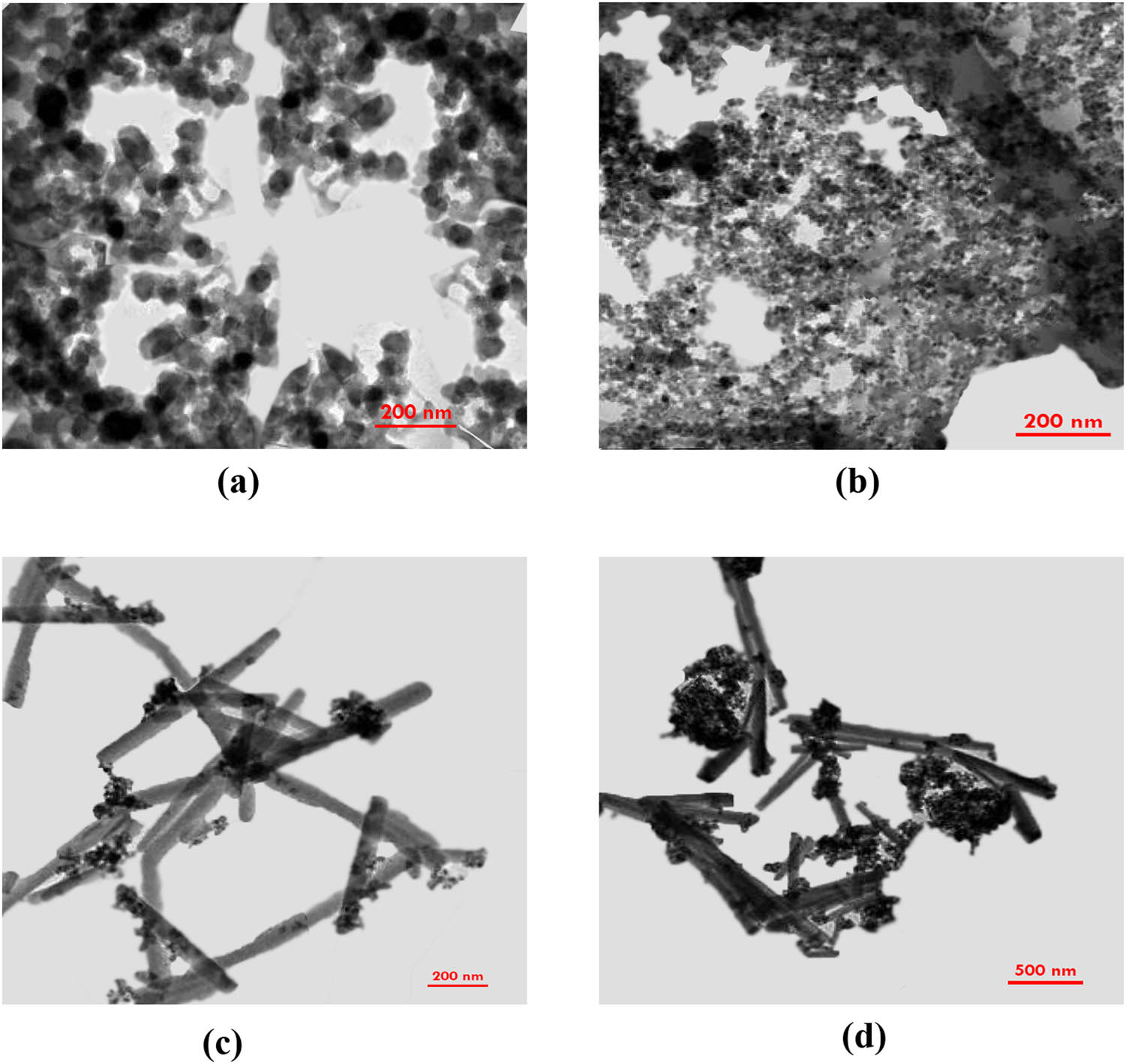
TEM images of (a) NC, (b) MOF, (c) Fe3O4/NC, and (d) Fe3O4/NC/MOF.
Figure 3 displays the SEM images of NC, MOF, Fe3O4/NC, and Fe3O4/NC/MOF. According to Figure 3a, partially aggregated spherical particles were observed for the NC surface. Furthermore, not many triangular or quadrangular structures were seen on the NC surface. MOF crystals appear as flawless, prismatic pillar-like crystals in the SEM image (Figure 3b).
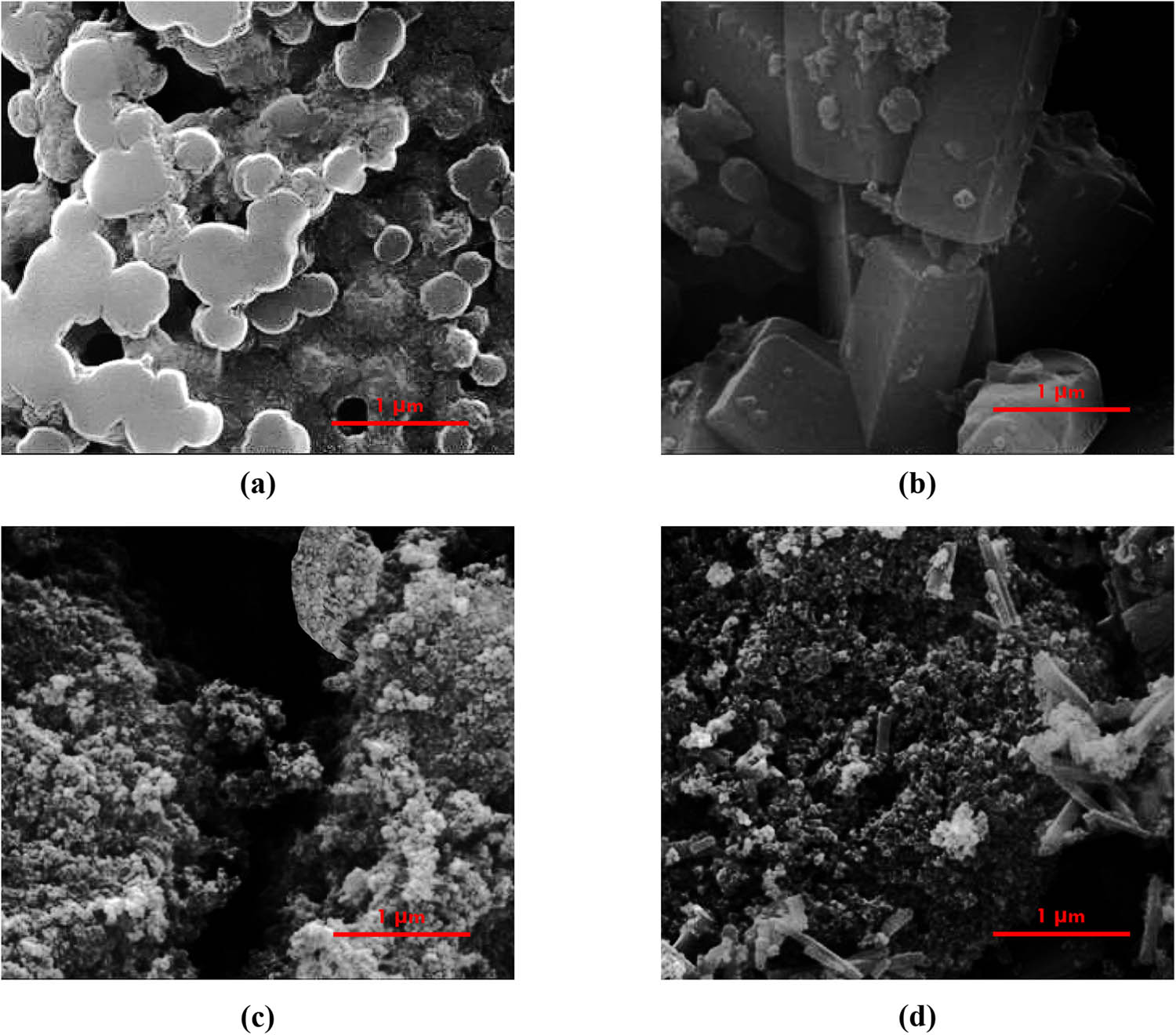
SEM images of (a) NC, (b) MOF, (c) Fe3O4/NC, and (d) Fe3O4/NC/MOF.
The surface of Fe3O4/NC showed irregularly shaped particles following treatment with the magnetic nanoparticles (Figure 3c). This discovery is explained by the interaction of the NC layer’s surface with magnetic Fe3O4 nanoparticles. Eventually, following MOF coating by Fe3O4/NC, pillar-like crystals were seen on the composite surface (Figure 3d), proving the effectiveness of the coating process. Such MOF-based adsorbents have a large number of pores, and the low-temperature nitrogen adsorption method can be used to measure the pore size distribution, pore volume, and specific surface area of these materials. Figure 4a displays the isothermal adsorption/desorption isotherms of Fe3O4/NC, NC, and Fe3O4/NC/MOF determined at 77 K. Type IV of the IUPAC classification was consistent with the nitrogen adsorption/desorption isotherms of NC, and H1 type hysteresis loops were observed. This suggested that the NC samples have a mesoporous structure. The monomolecular layer adsorption process was completed when P/P 0 < 0.1, as indicated by the first inflection point of the N2 adsorption–desorption isotherm of Fe3O4/NC/MOF. Further increase of the pressure causes the adsorption of the additional layer. There are an endless number of adsorption layers at the saturated vapor pressure. These are the type II isotherms for adsorption. Additionally, the specific surface area of Fe3O4/NC/MOF reached 66.3 m2·g−1, which might be attributable to the high specific surface area of MOF. In contrast, the BET-specific surface area of NC alone was 30.15 m2·g−1. This specific surface area of NC reached 40.45 m2·g−1 after the magnetization using Fe3O4 nanoparticles. The Barrett–Joyner–Halenda (BJH) technique yielded pore diameters of 3.85, 5.66, and 11.88 nm for Fe3O4/NC/MOF, Fe3O4/NC, and NC, respectively. The total pore volumes of Fe3O4/NC/MOF, Fe3O4/NC, and NC are 0.481, 0.067, and 0.813 cm2·g−1, respectively. The reason for this was that the pore diameter and pore volume significantly decreased as a result of MOF loading onto the NC’s surface and internal channel blockage. To ascertain the oxidation states of the novel nanocomposite Fe3O4/NC/MOF and chemical bonding, an XPS spectrum was generated. The components Zn, C, O, and Fe are present in the Fe3O4/NC/MOF, according to the survey XPS spectrum shown in Figure 4b.
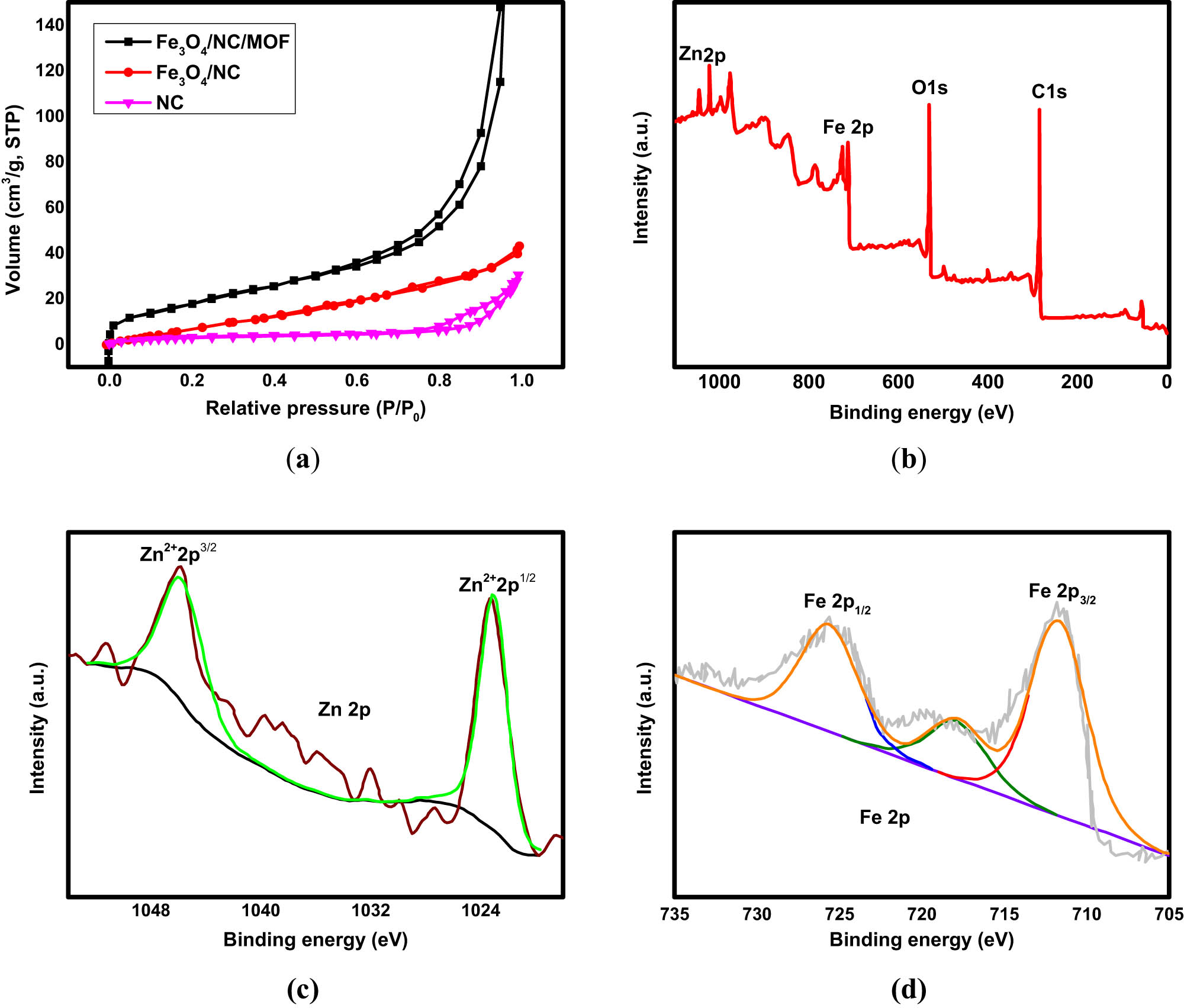
(a) N2 adsorption–desorption isotherm, (b) XPS spectra full survey, (c) XPS spectra of Zn 2p, and (d) XPS spectra of Fe 2p of the synthesized materials.
It was possible to demonstrate the oxidation states of zinc elements, Zn2+ 2p3/2 and Zn2+ 2p1/2, display significant peaks at 1,024 and 1046.55 eV, respectively, as shown in Figure 4c. The distinctive binding energies of Fe 2p3/2 (711.69 eV) and Fe 2p1/2 (725.42 eV) are responsible for the two peaks that make up the Fe 2p characteristic spectrum, as shown in Figure 4d. The XPS spectra results indicate the good incorporation between MOF and Fe3O4/NC in the nanocomposite confirming the successful fabrication of the desired adsorbent. We can conclude from the characterization results of XRD, FTIR, SEM, TEM, VSM, EDX, EBT, and XPS that the desired Fe3O4/NC/MOF nanocomposite was well constructed by the combination of MOF and the magnetic Fe3O4/NC nanocomposite.
3.2 Adsorption study
3.2.1 Optimization of adsorption parameters
First, the adsorption parameters for the removal of organic dyes CR, BB 54, BV 14, and AR 88 on the surface of the Fe3O4/NC/MOF nanocomposite were optimized. These adsorption parameters were optimized through variations in pH, contact time, adsorbent dosage, initial dye concentration, and temperature. The resistance of dye molecules to transfer from the liquid phase to the solid phase ascertains the effect of initial dye concentration, a very important factor [52]. Therefore, an increase in the initial dye concentration would increase the adsorption efficiency. However, a dye molecule can move from bulk liquids to the adsorbent’s external surface, suggesting that the concentration of dye molecules affects the amount of dye that is adsorbed onto the adsorbent [53]. As a result, the proportion of dye removed is higher at low initial concentrations and lower at high initial concentrations. The impact of dye concentration (ranging from 0.001 to 0.8 g·L−1) on removal capacity was examined in this study for 20.0 min at 298 K and 0.08 g·L−1 adsorbent dose. The Fe3O4/NC/MOF capacities of adsorption at equilibrium increase to 875.13, 877.71, 850.19, and 875.33 mg·g−1 from 13.0 mg·g−1 for AR 88, BB 54, BV 14, and CR, respectively; when the initial concentration of the organic dye is increased from 10 to 800 mg·L−1, as shown in Figure 5a.
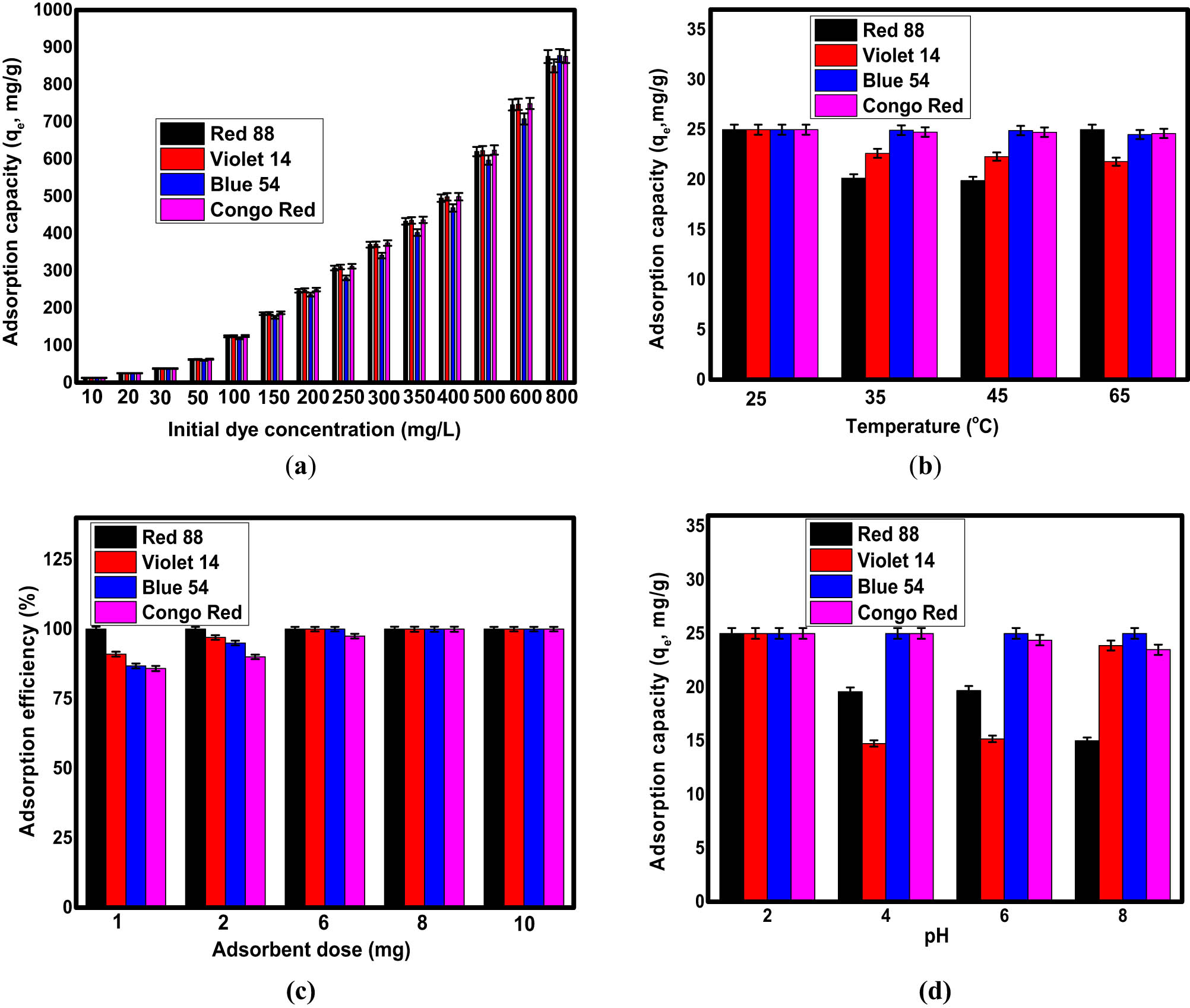
Effects of (a) initial dye concentration, (b) temperature, (c) adsorbent dose, and (d) pH for the removal of organic dyes using the Fe3O4/NC/MOF nanocomposite.
The primary adsorption parameter that may be crucial to the adsorption process is temperature. Temperature has a significant impact on both the viscosity of the solution and the capacity of adsorption [54]. Consequently, determining the ideal temperature for the adsorption process is essential. To determine the effect of temperature on the Fe3O4/NC/MOF behavior, adsorption was examined at an initial concentration of dye equal to 20.0 mg·L−1 under ideal conditions (stirring period: 20 min, initial dye concentrations: 20 mg·L−1, pH = 2). The adsorption capacities for the removal of CR, BB (54), BV (14), and AR (88) are shown against temperature in Figure 5b, in the range of 25–65°C. It is clear that the removal of dyes was adversely affected by the increase of temperature. The ideal temperature for the Fe3O4/NC/MOF to remove dyes was 25°C. Since it shows the maximum adsorption ability for a given initial concentration of dye, the adsorbent dose is a crucial parameter [55]. Adsorption studies utilizing an adsorbent amount of 1.0–1,000 mg·L−1 at a temperature of 298 K, pH 2.0, and an initial concentration of dye of 20 mg·L−1 for a contact time of 20.0 min were used to establish the ideal quantity of Fe3O4/NC/MOF to adsorb dyes. Figure 5c demonstrates that when 0.001 g of Fe3O4/NC/MOF was employed as an initial dose, 85.76, 86.79, 90, and 100% of CR, BB 54, BV 14, and AR 88 were eliminated from their aqueous solutions. BB 54, BV 14, and AR 88 required dosages of 0.006, 0.006, and 0.001 g in that order; however, CR was found to be best removed by the Fe3O4/NC/MOF at a dosage of 8.0 mg. It was found that the elimination efficiency (%) of every dye increased and eventually reached 100% when the Fe3O4/NC/MOF dosage was increased by 8.0 mg. Thus, the dosage of 8.0 mg was chosen because increasing the adsorbent dose (quantity) further did not affect the dye treatment. Furthermore, the removal rate (%) of dyes is presented in Figure 5c, which increases when the amount of the adsorbent is increased until an equilibrium is reached. It may be clarified that an increase in the number of adsorption sites resulted in an increase in the specific surface area of the adsorbent and the availability of additional binding sites for the adsorption of dye [56]. This study found that the ideal adsorbent dose of Fe3O4/NC/MOF was 0.008 g, which resulted in a complete dye removal rate. pH is important in dye adsorption because it controls the surface charge of the adsorbent and the level of ionization of the adsorptive molecules [57,58]. At several pH values (ranging from 2.0 to 8.0), the effect of the starting solution pH on the dyes’ ability to adsorb onto Fe3O4/NC/MOF was identified, with fixed values for the critical parameters (contact period of 20.0 min, dye concentration of 20 mg·L−1, and adsorbent dose of 0.008 g/10.0 mL). Based on Figure 5d, all colors comprising CR, BB 54, BV 14, and AR 88 had their maximum adsorption capacity at pH = 2.0. The adsorption capacity of the anionic dye Acid red 88 diminishes as the pH of the solution increases from 2.0 to 8.0. The pH increase had no discernible effect on the adsorption capability of the other colorants. This could be caused by the numerous functional groups of the dyes as well as various interactions including dipole–dipole forces and hydrogen bonds between the adsorbate and the adsorbent. Under ideal conditions (initial concentration of dye = 20.0 mg·L−1, 298 K, pH = 2.0, and adsorbent dose = 0.08 g·L−1), the influence of contact time on the Fe3O4/NC/MOF adsorption capacity and dye removal percent was assessed. The investigation of the effect of contact time on adsorption behavior was determined in this study, and the removal efficiency was ascertained for model organic dyes as a function of contact time alternating from 10.0 to 60.0 min. The adsorption capacity as well as removal percentage of the Fe3O4/NC/MOF show a significant increase at first, and then increase at a relatively slow pace till equilibrium is achieved. After 20 min of the dye interaction with Fe3O4/NC/MOF, the most significant adsorption efficiency was achieved. This is attributed to the fact that a large number of empty surfaces are initially available for adsorption [59]. The remaining empty active sites are difficult and harder to find after a while. As a result, 20 min was chosen as the equilibrium contact time in this investigation.
3.2.2 Adsorption kinetics
The adsorption kinetics of Fe3O4/NC/MOF were examined in order to comprehend the reaction pathways of the adsorption process and the adsorption effectiveness of the adsorbents. The chemical and physical properties of the adsorbent serve as the basis for chemical kinetic models, whose evaluation is essential for revealing the variables influencing the rate of reaction [60]. Four kinetic models (i.e., pseudo-first-order, pseudo-second-order, Elovich and intra-particle diffusion models) were used to study the adsorption kinetics of the Fe3O4/NC/MOF. The linear equations of the pseudo-first-order kinetic model [61] and the pseudo-second-order model [62] are shown in Eqs. (3) and (4), respectively:
where k 1 and k 2 are the rate constants for the pseudo-first-order (min−1) and pseudo-second-order adsorption kinetics (g·mg−1·min−1), respectively; q t (mg·g−1) and q e (mg·g−1) are the adsorption capacities at the reaction time t (min) and at equilibrium, respectively. The pseudo-first-order kinetic model sufficiency is represented by a straight line in the plot of ln(q e − q t) against t (Figure 6a), which shows a relationship between the intercept and slope. It is possible to ascertain the model parameters (k 1 and predicted q e). The compatibility of pseudo-second-order kinetics on the dye removal at different adsorbent doses from single systems was examined using a linear graph of t/q t against t. The slope of the t/q t against t plot (Figure 6b) yields the value of q e, and the initial adsorption rate value yields K 2.
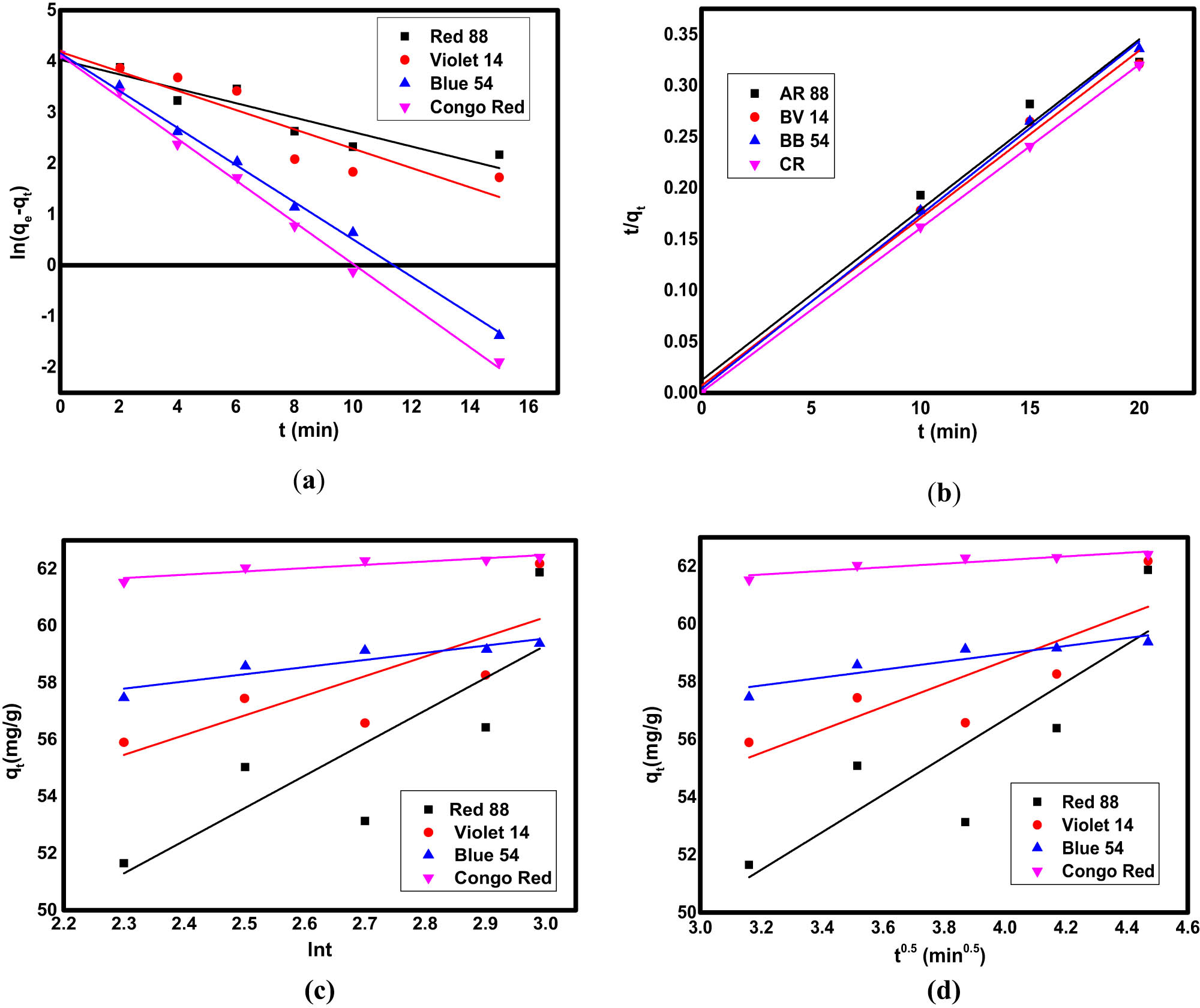
The fitting of experimental adsorption data to (a) pseudo-first-order, (b) pseudo-second-order, (c) Elovich, and (d) intra-particle diffusion kinetic models.
Eq. (5) represents the Elovich equation, which is another kinetic model equation related to the adsorption capacity [63]:
The Elovich constants α and β denote the initial adsorption rate (g·mg−1·min−1) and the desorption constant (mg·g−1·min−1), respectively. The plot of q t against ln t (Figure 6c) is used to determine the Elovich constants.
The adsorption mechanism was further examined using the kinetic intra-particle diffusion model (Eq. (6)) [64]. The linear form is displayed as follows:
where c is the thickness of the boundary layer (mg·g−1), k id is the rate constants for the intra-particle diffusion rate constants (mg·g−1·h−0.5), and q t (mg·g−1) is the adsorption capacity at the reaction time t (min). By plotting linearly q t versus t 0.5 (Figure 6d), the intra-particle diffusion kinetics constants (c and K id) were obtained. Table 1 displays the kinetic constants of the as-mentioned four models. The adsorption process, according to the results, more closely matched the pseudo-second-order model. In this model, Table 1 displays the linear plot with the highest correlation coefficients.
Kinetic parameters for the adsorption of different dyes on the surface of the Fe3O4/NC/MOF nanocomposite
| Model | Parameter | CR | BB 54 | BV 14 | AR 88 |
|---|---|---|---|---|---|
| Initial concentration (mg·L−1) | 50 | 50 | 50 | 50 | |
| Pseudo-first-order | q e,exp (mg·g−1) | 63.30 | 60.29 | 63.05 | 62.79 |
| q e,cal (mg·g−1) | 60.07 | 62.88 | 54.32 | 56.55 | |
| K 1 (min−1) | 0.4167 | 0.3505 | 0.1699 | 0.1380 | |
| R 2 | 0.9976 | 0.9979 | 0.9201 | 0.9445 | |
| Intra-particle diffusion | K id (g·min−1·mg−1) | 0.6798 | 1.5678 | 4.5889 | 7.5244 |
| C (mg·g−1) | 59.50 | 52.89 | 40.29 | 26.31 | |
| R 2 | 0.8975 | 0.8798 | 0.7778 | 0.7999 | |
| Pseudo-second-order | q e,cal (mg·g−1) | 63.40 | 59.76 | 61.88 | 60.99 |
| K 2(g·min−1·mg−1) | 0.3657 | 0.0741 | 0.0389 | 0.0330 | |
| R 2 | 1.0 | 0.9980 | 0.9931 | 0.9880 | |
| Elovich model | ß (g·mg−1) | 0.759 | 0.349 | 0.120 | 0.080 |
| α (mg·g−1·min−1) | 3.13456941 | 169642386.813 | 508.284 | 50.632 | |
| R 2 | 0.9129 | 0.9116 | 0.7446 | 0.7943 |
The pseudo-first-order postulates that a single mechanism or process working on a single class of adsorbing sites is the only one capable of limiting the absorption rate. Table 1 and Figure 6a indicate that compared to the experimental data, pseudo-first-order did not accurately compute the adsorption capacities for the manufactured material. Furthermore, the R 2 values deviate slightly from linearity. Because several sorption sites and mass transfer may be incorporated into the process mechanism, this model is therefore not suited to fully characterize the sorption processes onto heterogeneous surfaces. In comparison to the other three kinetic models, the adsorption processes more closely matched the pseudo-second-order reaction kinetic model when taking into account the coefficient of the determinant . The best fitting model for the higher correlation coefficients of CR, BB 54, BV 14, and AR 88 (R 2 = 1.0, 0.9980, 0.9931, and 0.9880) for the adsorption of dyes on Fe3O4/NC/MOF is the pseudo-second order model, as shown in Table 1. It appears from this that the adsorption mechanism is more similar to chemisorption. However, with R 2 better than 0.99, it is evident that the pseudo-first-order and pseudo-second-order models both fit the experimental data well of CR and BB 54 dyes. Based on these findings, we may conclude that the CR and BB 54 adsorption process onto Fe3O4/NC/MOF can be described by both pseudo-first-order and pseudo-second-order models. The adsorption of dye molecules to the surface of the solid adsorbent and enabling them to enter the pores is the slower step in the usually multi-step process of adsorption. So, the diffusion step is very important in the adsorption process. However, the diffusion mechanism is not identified by the first- and second-order kinetic models. Therefore, it is important to assess the experimental data using the intra-particle diffusion model (Eq. (6)). The intra-particle diffusion model suggested that the adsorption processes of dye molecules could occur via several steps [65]. These steps include the transport of the dyes in the bulk solution, film diffusion of these dyes at the boundary layer of the adsorbent, diffusion of dye molecules from the bulk solution to the external surface of the adsorbent material, and diffusion through small pores of the adsorbent. These steps are followed by the chemical binding of dyes with the adsorption sites. Finally, the equilibrium of adsorption was achieved and the maximum adsorption was obtained. Subsequently, based on the findings of the kinetic modeling, it was shown that surface chemisorptions that took place at the boundary layers of the developed material controlled the adsorption of dyes from the contaminated water onto the prepared MOF-based adsorbent.
3.2.3 Adsorption isotherm
Adsorption isotherms can reflect the surface characteristics of the adsorbent at the micro level. This is based on the interaction of the adsorbent with adsorbate molecules. This interaction determines the homogeneity or heterogeneity of the solid surface of the adsorbent and the type of coverage between the adsorbent and adsorbate. So, the isotherms of adsorption are important mathematical models to find the appropriate model that can be used for design purposes [66]. Adsorption isotherms also provide certain physicochemical details about how the adsorption takes place and how the reaction between the adsorbate and adsorbent surface occurs [67]. The equilibrium of dyes adsorbed by Fe3O4/NC/MOF was described in this work using the Freundlich, Langmuir, and Temkin isotherms. The adsorption data were fitted using the Freundlich model (Eq. (7)), Langmuir model (Eq. (8)), and Temkin model (Eq. (10)) as indicated in the following [68,69,70]:
where the adsorption intensity and adsorption capacity per unit concentration are related to the Freundlich constants 1/n and K F, respectively. The maximal adsorption capacity is denoted by q m (mg·g−1), and the Langmuir constant for the adsorbent and adsorbate requirements is described by (K L) (L·mg−1). K T is the equilibrium binding constant related to the first energy (L·g−1), and b T is the Temkin constant, which denotes the sorption point (kJ·mol−1). R L denotes the dimensionless separation constant and C o denotes the initial concentration of the dye. According to the Langmuir model, there is no adsorbent transmission through the surface; instead, adsorption takes place within homogeneous locations over the absorbent [71]. However, the reversible, monolayer, non-ideal adsorption that is necessary for the Freundlich isotherm model may be familiar with multiple-layer adsorption on heterogeneous surfaces with different affinities and adsorption energies [72]. According to the Temkin isotherm model, the adsorption heat decreased linearly as the absorbent surface’s coverage increased [73]. A consistent distribution of the binding energy is among the characteristics of this model. By plotting 1/C e against 1/q e linearly, the isotherm constants (q m and K L) in the Langmuir model were determined, as shown in Figure 7a. The Freundlich isotherm constants of dye removal were examined using a linear plot of log C e against log q e, as shown in Figure 7b. The Temkin isotherm was examined using the linear plot of C e against q e, as shown in Figure 7c, which is compatible with the dye adsorption data obtained using Fe3O4/NC/MOF. The isotherm constants of the three models are summarized in Table 2.
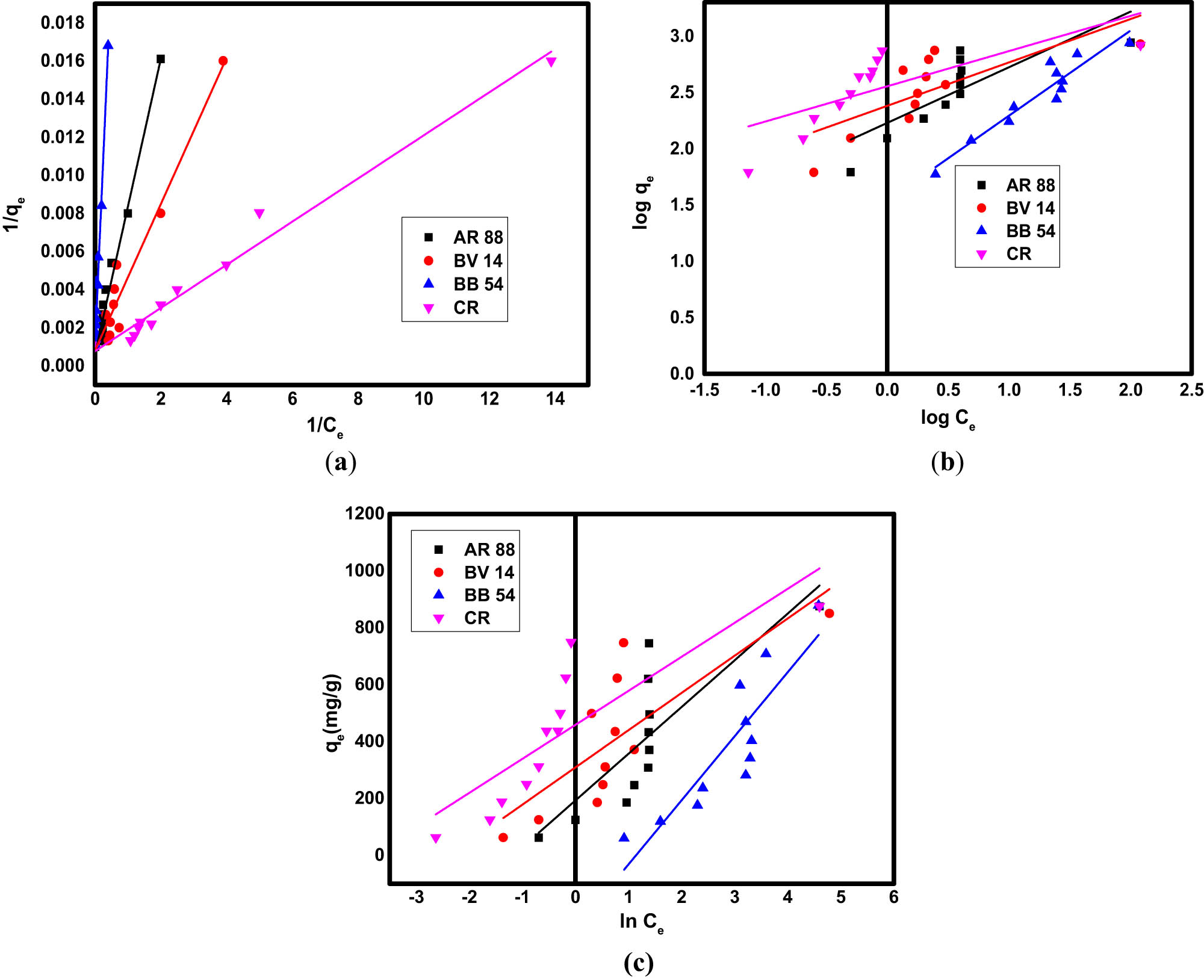
The fitting of experimental data to (a) Langmuir, (b) Freundlich, and (c) Temkin isotherm models for the adsorption of organic dyes on Fe3O4/NC/MOF.
Isotherm parameters for the removal of organic dyes on the Fe3O4/NC/MOF surface
| Model | Parameters | CR | BB 54 | BV 14 | AR 88 |
|---|---|---|---|---|---|
| Freundlich model | n | 3.05 | 1.35 | 2.61 | 2.04 |
| K f (mg·g−1) | 364.21 | 34.36 | 240.34 | 169.33 | |
| R 2 | 0.5590 | 0.9015 | 0.5478 | 0.6469 | |
| Langmuir model | K L (L·mg−1) | 0.730 | 0.030 | 0.209 | 0.080 |
| q m (mg·g−1) | 1249 | 999.0 | 1249 | 1667.55 | |
| R 2 | 0.9790 | 0.9700 | 0.950 | 0.9750 | |
| Temkin model | b T (J·mol−1) | 20.69 | 11.00 | 18.89 | 17.10 |
| K T (L·mg−1) | 46.20 | 3.15 | 10.60 | 3.30 | |
| R 2 | 0.6989 | 0.7810 | 0.6160 | 0.6720 | |
| Separation factor (R L) | C 0 (mg·L−1) | 0.027–0.002 | 0.445–0.048 | 0.088–0.006 | 0.207–0.016 |
According to Table 2, it is suggested that the adsorption isotherm data can be satisfactorily provided by the Langmuir model. The Langmuir adsorption isotherm was the most appropriate model of all. According to the correlation coefficients (R 2) of the isotherm models, the Langmuir isotherm has the highest correlation, indicating dye adsorption on a homogenous surface. The essential characteristics of the Langmuir isotherm model are expressed by the equilibrium parameter (R L) or dimensionless separation constant. The value of R L indicates whether the isotherm is favorable (0 < R L < 1), irreversible (R L = 0), linear (R L = 1), or unfavorable (R L > 1). Table 1 shows that all of the R L values were between 0 and 1, indicating that Fe3O4/NC/MOF adsorbed the dyes in a favorable manner. According to the Langmuir model, the highest monolayer adsorption capacities (q m) for the removal of CR, BB 54, BV 14, and AR 88, were 1,249, 999.0, 1,249, and 1667.55 mg·g−1, in that order. For comparison, the adsorption capacity of Fe3O4/NC/MOF was compared with that of the previously reported adsorbents, as listed in Table 3. The adsorption capacity of Fe3O4/NC/MOF was more significant (Table 3) than that in the majority of the other adsorbents’ reported values. The unique structure of Fe3O4/NC/MOF is responsible for its exceptional adsorption capabilities. This may be attributed to a large number of adsorption sites on its surface with large heterogeneity and diversity, which are energetically identical to provide homogeneous adsorption. That is, it offers organic framework adsorption sites in addition to Zn adsorption sites in addition to the active sites present on the surface of NC. Also, the ligands of MOF exhibit a π–π interaction with organic dyes because of their aromatic rings. Fe3O4/NC/MOF was discovered to have a significantly larger surface area than MOF alone that had just clumped together. The porous structure, their large specific extent, and more active adsorption sites are other factors that contribute to this adsorbent’s increased adsorption capacity. The Fe3O4/NC/MOF has been found to have a comparatively higher adsorption capacity, indicating its viability and suitability as an inexpensive, practical, and efficient adsorbent in the adsorption process to remove dyes. Scheme 3 illustrates the adsorption sites of the prepared Fe3O4/NC/MOF in which different sites participated in dye removal with a variety of interactions.
Comparison of the removal capacity of previously reported adsorbents with Fe3O4/NC/MOF for adsorption of organic dyes
| Adsorbent | Pollutant | Adsorption capacity (q m, mg·g−1) | Ref. |
|---|---|---|---|
| Fly ash | CR | 22.12 | [74] |
| Zeolite | CR | 9.23 | [75] |
| Padina gymnospora | CR | 12.38 | [75] |
| Zeolite/algae composite | CR | 12.25 | [75] |
| Physically activated bottom ash | CR | 106.61 | [76] |
| Ni/Co-LDH | CR | 909.2 | [77] |
| Ni/Zn MOF | CR | 460.90 | [78] |
| MOF-5/Cu | CR | 357.42 | [79] |
| Fe3O4/NC/MOF | CR | 1248 | This study |
| Theobroma cacao shells | BV 14 | 980.39 | [80] |
| Calophyllum inophyllum shells | BV 14 | 1416.43 | [80] |
| Curcuma angustifolia scales | BV 14 | 208.33 | [81] |
| Bottom ash | BV 14 | 6.39 | [82] |
| Fe3O4/NC/MOF | BV 14 | 1248.3 | This study |
| MNZnFe | AR 88 | 111.0 | [83] |
| Alunite | AR 88 | 21.0 | [84] |
| Anion membrane | AR 88 | 42.0 | [85] |
| Activated carbon | AR 88 | 109.0 | [86] |
| Fe3O4/NC/MOF | AR 88 | 1665.89 | This study |
| Sodium alginate (SA) | BB 54 | 254.0 | [87] |
| Na+-rectorite (Na+-REC) | BB 54 | 237.0 | [87] |
| SA/Na+ REC | BB 54 | 388.0 | [87] |
| Fe3O4/NC/MOF | BB 54 | 999.88 | This study |
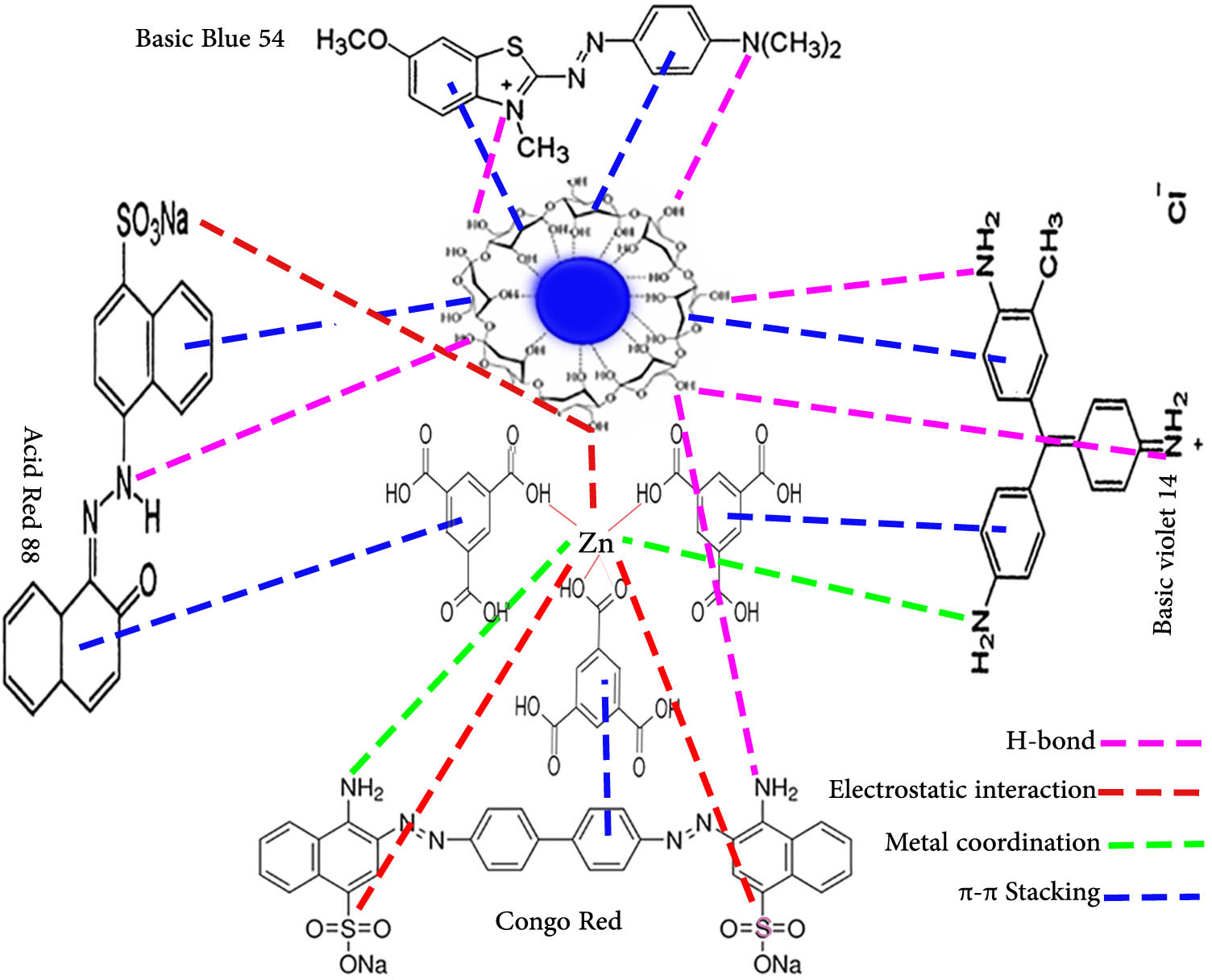
Suggested adsorption mechanism of the Fe3O4/NC/MOF nanocomposite towards organic dyes.
3.2.4 Thermodynamic parameters of adsorption
Experiments were conducted between 25 and 65°C to investigate the adsorption thermodynamics during the dye adsorption by the Fe3O4/NC/MOF nanocomposite. The orientation and viability of the Fe3O4/NC/MOF nanocomposite for dye adsorption were assessed using thermodynamic parameters. The following equations were used to calculate the thermodynamic parameters such as the change in the Gibbs free energy (ΔG, kJ·mol−1), enthalpy (ΔH, kJ·mol−1), and entropy (ΔS, J·mol−1·K−1):
Plotting ln(q e/c e) versus q e yields the sorption equilibrium constant, K d. R is the ideal gas constant (=8.314 J·mol−1·K−1). The linear plot of ln K d against 1/T (Van’t Hof plot) was used to investigate the values of ΔH and ΔS using the slope and intercept, as shown in Figure 8a. The thermodynamic parameters of adsorption are presented in Table 4. For all dyes, the spontaneous adsorption process is represented by the negative values of Gibb’s free energy, ΔG [88]. During the adsorption process, a decrease in randomness at the solid-solution interface is indicated by a negative value of entropy (ΔS) [89].
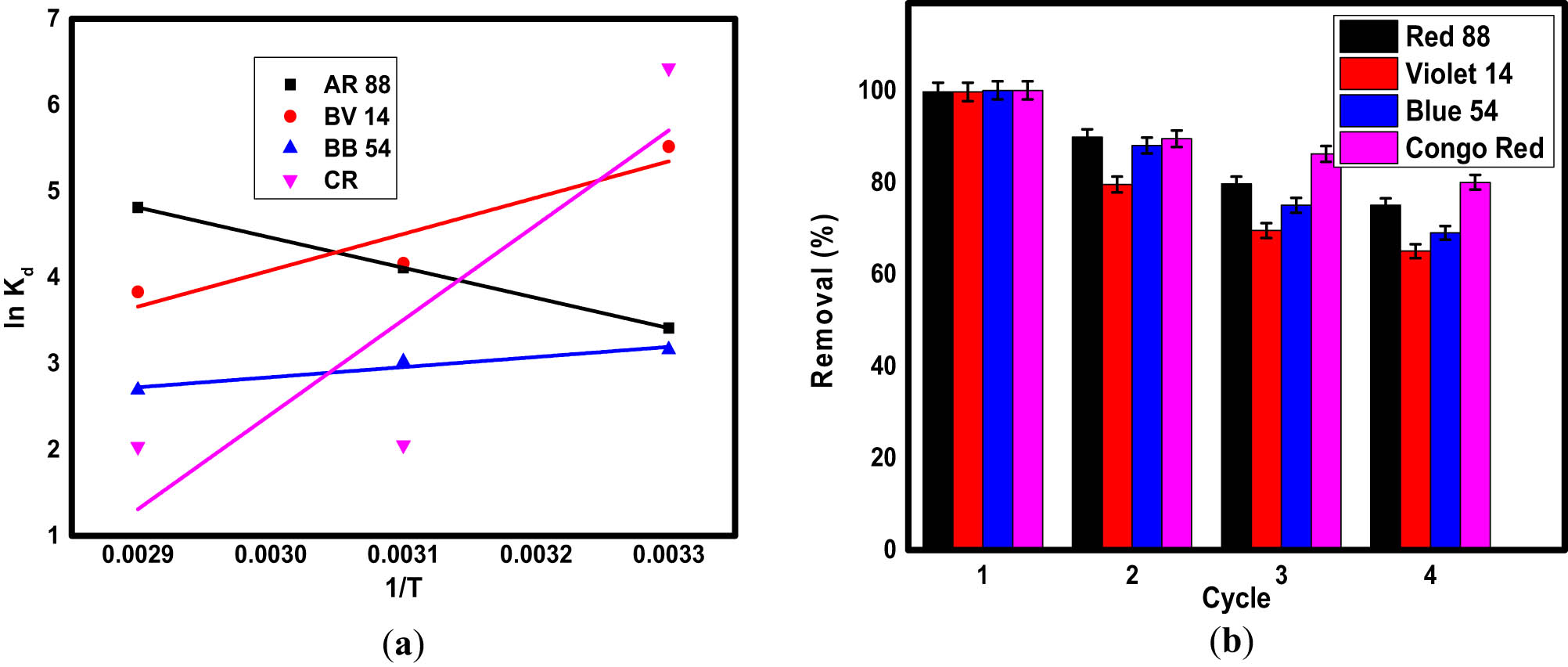
Van’t Hof plot (a) and reusability (b) for the adsorption of organic dyes on the surface of Fe3O4/NC/MOF.
Thermodynamic parameters for the adsorption of dyes on the surface of Fe3O4/NC/MOF
| Temperature (K) | CR | BB 54 | BV 14 | AR 88 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | |
| 298.18 | −15.95 | −7.84 | −13.67 | −8.46 | ||||||||
| 313.18 | −5.43 | −91.32 | −253.94 | −8.00 | −9.76 | −5.65 | −11.00 | −35.04 | −71.20 | −10.88 | 29.10 | 124.33 |
| 338.18 | 5.73 | −7.57 | −10.77 | −13.53 | ||||||||
The process involved increasing the temperature to enhance the mobility of the dye molecules, allowing them to move from the solid to the liquid phase. Furthermore, the exothermic adsorption of BV 14, BB 54, and CR onto Fe3O4/NC/MOF is shown by the negative value of enthalpy (ΔH) [90]. The adsorption process of AR 88 demonstrated endothermic qualities, as demonstrated by the positive value of ΔH [91]. On the other hand, as indicated by the positive value of ΔS, randomness increased at the solid-solution interface [92].
3.2.5 Reusability of Fe3O4/NC/MOF for dye adsorption
For commercial applications, the reusability of adsorbents is also a critical metric for assessing adsorption performance [93]. This contributes significantly to the decrease in the material cost of water treatment [94]. Adsorption–desorption tests were carried out in this work to investigate the adsorbent’s regeneration. The adsorption of dyes from water by Fe3O4/NC/MOF has good regeneration performance and stability even after 4 cycles, as seen in Figure 8b. It should be mentioned that the elimination percentage was still higher than 80% after three cycles. Conversely, the Fe3O4/NC/MOF’s regeneration rate of organic dyes decreased and lost its initial adsorption efficiency. This decrease shows that Fe3O4/NC/MOF has a high affinity toward organic dyes. This could be explained by the fact that the dye molecules can form very strong bonds with the functional groups on the surface of Fe3O4/NC/MOF. Also, the eluent could not totally desorb some of the dye-occupied active sites on the surface of Fe3O4/NC/MOF. However, the removal efficiency was still acceptable after the third cycle. Therefore, this adsorbent may be thought of as an effective and recyclable dye elimination option.
3.2.6 Application of Fe3O4/NC/MOF for wastewater sample
Further testing of the adsorption capabilities of Fe3O4/NC/MOF towards organic dyes in model wastewater (textile sample) was done to assess the technology’s potential for use in real-world applications. A membrane filter with a 0.45-mm pore size was used to filter the wastewater sample in order to eliminate the suspended particle debris. As a real sample, one solution with 50.0 mg·L−1 of CR in wastewater was made. Then, 0.016 g of the adsorbent was added to 100.0 mL of wastewater at pH = 2. The blended solution was shaken for 20 min. The starting and final concentrations of the target species were found using the conventional addition method, which was used to conduct the assays. Different volumetric flasks were filled with 20.0 mL of wastewater samples each in order to achieve this goal. To achieve different concentrations of the CR dye, the standard CR solutions were then added in various proportions (0, 0.25, 0.5, and 0.75 mL of 1,000 mg·L−1), and the solutions in the flasks were well mixed and diluted to an appropriate level. The UV-Vis spectrophotometry method was used to calculate the dye concentrations in the solution. Then, the concentration of dyes was plotted against the absorption to get a standard calibration curve. This curve was used to find the unknown concentration of the dye. The UV spectra of CR wastewater samples before and after adsorption are shown in Figure 9.
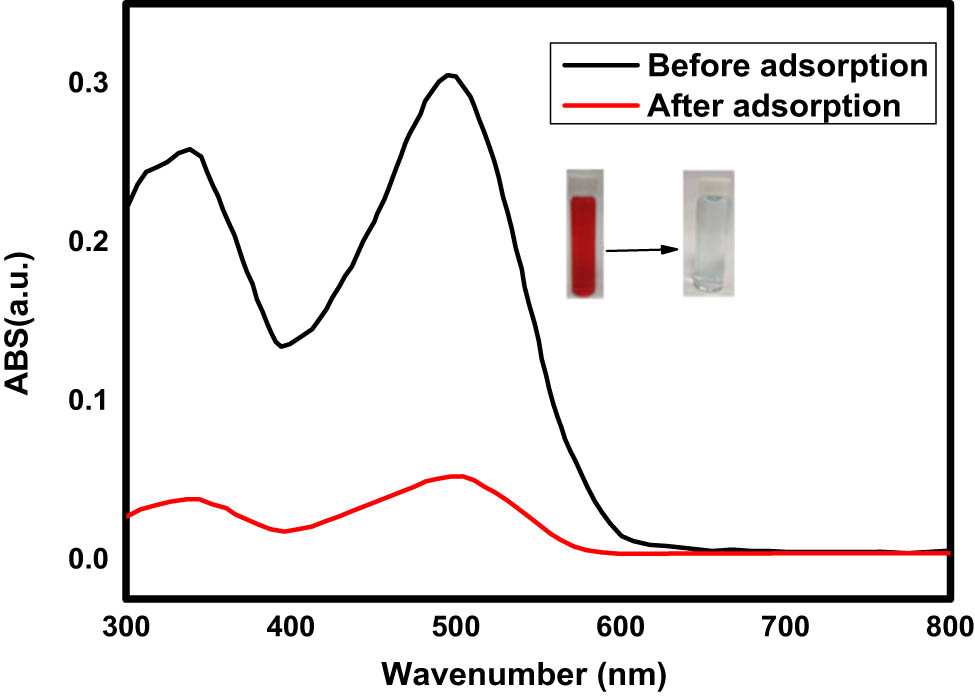
UV spectra before and after adsorption of CR on the surface of Fe3O4/NC/MOF.
According to the results, the Fe3O4/NC/MOF reduced the CR concentration from 50.0 to 3.5 mg·L−1, which represents 93% removal efficiency. These findings indicate that the suggested approach might be used effectively, and with a suitable degree of efficiency it removed acid red 88 from a real sample of textile effluent. These results showed that the presence of chemical and biological media, such as dissolved organic nitrogen, had no discernible impact on the dye adsorption processes, and they were quite similar to the removal rates found in synthetic wastewater.
Biodegradability and biological oxygen demand (COD and BOD) were assessed in a real wastewater sample. The amount of dissolved oxidizable organic matter, including non-biodegradable items, was indicated by the COD values. It is clear that a high COD value is frequently linked to wastewater’s high organic content, indicating a higher level of ecosystem pollution. BOD is the amount of oxygen needed for organic components in wastewater to break down. From the initial concentration of 1,899 mg·L−1, the COD level dropped to 59 mg·L−1. Parallel to this, the BOD level decreased with time from its initial concentration of 799.0 to 26.0 mg·L−1. This drop in COD and BOD values confirmed the effectiveness of this adsorbent to remove the organic dyes from water. Also, this gives the probability of dye removal from water though the mechanism of molecule breaks down besides the adsorption mechanism. The effectiveness of this adsorbent has been thoroughly assessed, and the treated effluent can be recycled, provided that it is utilized in accordance with the permissible levels of 200.0 and 100.0 mg·L−1, respectively, for irrigation and agricultural purposes. Consequently, the organic part of the dye molecules may be broken down, degraded, or even absorbed during the adsorption process. The above results suggest that Fe3O4/NC/MOF might be a good option for real wastewater treatment.
4 Conclusions
In this work, Fe3O4/NC was prepared by chemical co-precipitation, and then, under mild conditions, Zn-based MOF was coated by Fe3O4/NC utilizing an Et3N-catalyzed procedure. SEM, FTIR, XRD, XPS, and VSM were used to characterize the resultant Fe3O4/NC/MOF material, confirming its effective synthesis. Through a series of extensive tests, the adsorption conditions of this unique material were adjusted for removing dyes from the aqueous solution. The adsorption process conforms to the Langmuir isotherm model and follows pseudo-second-order kinetics, as indicated by the adsorption data. Regarding CR and BB 54 dyes, we may conclude that their adsorption process onto Fe3O4/NC/MOF can be described by both pseudo-first-order and pseudo-second-order models. The adsorption values were fitted to the Langmuir isotherm model, yielding q m values for the removal of CR, BB 54, BV 14, and AR 88, which were 1248, 999.88, 1248.3, and 1665.89 mg·g−1, in that order. The adsorption on Fe3O4/NC/MOF was both spontaneous and thermodynamically advantageous, according to the thermodynamic parameters. Fe3O4/NC/MOF exhibited endothermic adsorption for AR 88 and exothermic adsorption for CR, BB 54, and BV 14. Subsequently, Fe3O4/NC/MOF is an effective dye chelation material that may be applied in a wide range to eliminate pollutants from water because of its special qualities, which include maximum adsorption capacities, high removal efficiency, the number of aromatic rings for π–π interaction, and high recycling capacity.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Large Group Project under grant number RGP.2/174/44. This research was also funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R19), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Funding information: This research was funded by the Deanship of Scientific Research at King Khalid University under grant number RGP.2/174/44. Additionally, this research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R19), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Author contributions: N.S.A. and H.O. for conceptualization; N.S.A. for methodology; H.O. for software; H.O. for validation; H.O. for formal analysis; N.S.A. for investigation; N.S.A. for resources; A.A. for data curation; H.O. for writing – original draft preparation; N.S.A. for writing – review and editing; A.A. for visualization; M.A.T. for supervision; M.A.T. for project administration; and N.S.A. for funding acquisition. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Tkaczyk, A., K. Mitrowska, and A. Posyniak. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the Total Environment, Vol. 717, 2020, id. 137222.10.1016/j.scitotenv.2020.137222Suche in Google Scholar PubMed
[2] Pavithra, K. G. and V. Jaikumar. Removal of colorants from wastewater: A review on sources and treatment strategies. Journal of Industrial and Engineering Chemistry, Vol. 75, 2019, pp. 1–19.10.1016/j.jiec.2019.02.011Suche in Google Scholar
[3] Benkhaya, S., S. M’rabet, and A. El Harfi. A review on classifications, recent synthesis and applications of textile dyes. Inorganic Chemistry Communications, Vol. 115, 2020, id. 107891.10.1016/j.inoche.2020.107891Suche in Google Scholar
[4] Mehta, M., M. Sharma, K. Pathania, P. K. Jena, and I. Bhushan. Degradation of synthetic dyes using nanoparticles: A mini-review. Environmental Science and Pollution Research, Vol. 28, 2021, pp. 49434–49446.10.1007/s11356-021-15470-5Suche in Google Scholar PubMed
[5] Nachiyar, C. V., A. Rakshi, S. Sandhya, N. B. D. Jebasta, and J. Nellore. Developments in treatment technologies of dye-containing effluent: A review. Case Studies in Chemical and Environmental Engineering, Vol. 7, 2023, id. 100339.10.1016/j.cscee.2023.100339Suche in Google Scholar
[6] Shen, C., Y. Pan, D. Wu, Y. Liu, C. Ma, F. Li, et al. A crosslinking-induced precipitation process for the simultaneous removal of poly (vinyl alcohol) and reactive dye: the importance of covalent bond forming and magnesium coagulation. Chemical Engineering Journal, Vol. 374, 2019, pp. 904–913.10.1016/j.cej.2019.05.203Suche in Google Scholar
[7] Mcyotto, F., Q. Wei, D. K. Macharia, M. Huang, C. Shen, and C. W. Chow. Effect of dye structure on color removal efficiency by coagulation. Chemical Engineering Journal, Vol. 405, 2021, id. 126674.10.1016/j.cej.2020.126674Suche in Google Scholar
[8] Ma, J., X. Tang, Y. He, Y. Fan, and J. Chen. Robust stable MoS2/GO filtration membrane for effective removal of dyes and salts from water with enhanced permeability. Desalination, Vol. 480, 2020, id. 114328.10.1016/j.desal.2020.114328Suche in Google Scholar
[9] Alsaiari, N. S., A. Amari, K. M. Katubi, F. M. Alzahrani, F. B. Rebah, and M. A. Tahoon. The synthesis of magnetic nitrogen-doped graphene oxide nanocomposite for the removal of reactive orange 12 dye. Adsorption Science & Technology, Vol. 2022, 2022, pp. 1–14.10.1155/2022/9417542Suche in Google Scholar
[10] Alsaiari, N. S., A. Amari, K. M. Katubi, F. M. Alzahrani, F. B. Rebah, and M. A. Tahoon. Innovative magnetite based polymeric nanocomposite for simultaneous removal of methyl orange and hexavalent chromium from water. Processes, Vol. 9, No. 4, 2021, id. 576.10.3390/pr9040576Suche in Google Scholar
[11] Alsaiari, N. S., A. Amari, K. M. Katubi, F. M. Alzahrani, H. N. Harharah, F. B. Rebah, et al. The biocatalytic degradation of organic dyes using laccase immobilized magnetic nanoparticles. Applied Sciences, Vol. 11, No. 17, 2021, id. 8216.10.3390/app11178216Suche in Google Scholar
[12] Joseph, J., R. C. Radhakrishnan, J. K. Johnson, S. P. Joy, and J. Thomas. Ion-exchange mediated removal of cationic dye-stuffs from water using ammonium phosphomolybdate. Materials Chemistry and Physics, Vol. 242, 2020, id. 122488.10.1016/j.matchemphys.2019.122488Suche in Google Scholar
[13] Kumar, V. Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pb+2 ions from aqueous solutions by a hybrid ion-exchanger. Arabian Journal of Chemistry, Vol. 12, No. 3, 2019, pp. 316–329.10.1016/j.arabjc.2016.11.009Suche in Google Scholar
[14] Velusamy, S., A. Roy, S. Sundaram, and T. Kumar Mallick. A review on heavy metal ions and containing dyes removal through graphene oxide‐based adsorption strategies for textile wastewater treatment. The Chemical Record, Vol. 21, No. 7, 2021, pp. 1570–1610.10.1002/tcr.202000153Suche in Google Scholar PubMed
[15] Wang, L., C. Shi, L. Pan, X. Zhang, and J.-J. Zou. Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: A review. Nanoscale, Vol. 12, No. 8, 2020, pp. 4790–4815.10.1039/C9NR09274ASuche in Google Scholar PubMed
[16] Annamalai, J., P. Murugan, D. Ganapathy, D. Nallaswamy, R. Atchudan, S. Arya, et al. Synthesis of various dimensional metal organic frameworks (MOFs) and their hybrid composites for emerging applications–A review. Chemosphere, Vol. 298, 2022, id. 134184.10.1016/j.chemosphere.2022.134184Suche in Google Scholar PubMed
[17] Li, H., L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang, et al. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem, Vol. 1, No. 1, 2019, id. 100006.10.1016/j.enchem.2019.100006Suche in Google Scholar
[18] Wang, H. Nanostructure@ metal-organic frameworks (MOFs) for catalytic carbon dioxide (CO2) conversion in photocatalysis, electrocatalysis, and thermal catalysis. Nano Research, Vol. 15, No. 4, 2022, pp. 2834–2854.10.1007/s12274-021-3984-9Suche in Google Scholar
[19] Alsaiari, N. S., H. Osman, A. Amari, and M. A. Tahoon. The synthesis of metal–organic-framework-based ternary nanocomposite for the adsorption of organic dyes from aqueous solutions. Magnetochemistry, Vol. 8, No. 10, 2022, id. 133.10.3390/magnetochemistry8100133Suche in Google Scholar
[20] Lu, X. F., Y. Fang, D. Luan, and X. W. D. Lou. Metal–organic frameworks derived functional materials for electrochemical energy storage and conversion: a mini review. Nano Letters, Vol. 21, No. 4, 2021, pp. 1555–1565.10.1021/acs.nanolett.0c04898Suche in Google Scholar PubMed
[21] Sun, Y., L. Zheng, Y. Yang, X. Qian, T. Fu, X. Li, et al. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Letters, Vol. 12, 2020, pp. 1–29.10.1007/s40820-020-00423-3Suche in Google Scholar PubMed PubMed Central
[22] Olorunyomi, J. F., S. T. Geh, R. A. Caruso, and C. M. Doherty. Metal–organic frameworks for chemical sensing devices. Materials Horizons, Vol. 8, No. 9, 2021, pp. 2387–2419.10.1039/D1MH00609FSuche in Google Scholar PubMed
[23] Chen, Z., K. O. Kirlikovali, P. Li, and O. K. Farha. Reticular chemistry for highly porous metal–organic frameworks: The chemistry and applications. Accounts of Chemical Research, Vol. 55, No. 4, 2022, pp. 579–591.10.1021/acs.accounts.1c00707Suche in Google Scholar PubMed
[24] Zainal-Abidin, M. H., M. Hayyan, J. Matmin, A. M. Al-Fakih, N. Jamaluddin, W. M. A. W. Mahmood, et al. Greening industrial applications with magnetic-based deep eutectic solvents: A promising future. Journal of Industrial and Engineering Chemistry, Vol. 124, 2023, pp. 1–16.10.1016/j.jiec.2023.04.011Suche in Google Scholar
[25] Zhang, J., X. Feng, J. Wang, G. Fang, J. Liu, and S. Wang. Nano-crystalline cellulose-coated magnetic nanoparticles for affinity adsorption of glycoproteins. Analyst, Vol. 145, No. 9, 2020, pp. 3407–3413.10.1039/D0AN00442ASuche in Google Scholar
[26] Farooq, M. U. and M. I. Jalees. Application of magnetic graphene oxide for water purification: Heavy metals removal and disinfection. Journal of Water Process Engineering, Vol. 33, 2020, id. 101044.10.1016/j.jwpe.2019.101044Suche in Google Scholar
[27] Tang, Y., S. Zhang, Y. Su, D. Wu, Y. Zhao, and B. Xie. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chemical Engineering Journal, Vol. 406, 2021, id. 126804.10.1016/j.cej.2020.126804Suche in Google Scholar
[28] Niu, Y., J. Wu, Y. Kang, P. Sun, Z. Xiao, and D. Zhao. Recent advances of magnetic chitosan hydrogel: Preparation, properties and applications. International Journal of Biological Macromolecules, 2023, id. 125722.10.1016/j.ijbiomac.2023.125722Suche in Google Scholar PubMed
[29] Muthukumaran, P., P. S. Babu, S. Shyamalagowri, J. Aravind, M. Kamaraj, and M. Govarthanan. Polymeric biomolecules based nanomaterials: production strategies and pollutant mitigation as an emerging tool for environmental application. Chemosphere, Vol. 307, 2022, id. 136008.10.1016/j.chemosphere.2022.136008Suche in Google Scholar PubMed
[30] Hamidon, T. S., R. Adnan, M. M. Haafiz, and M. H. Hussin. Cellulose-based beads for the adsorptive removal of wastewater effluents: A review. Environmental Chemistry Letters, Vol. 20, No. 3, 2022, pp. 1965–2017.10.1007/s10311-022-01401-4Suche in Google Scholar
[31] Saya, L., Ratandeep, Bikaramjeet, Pooja. Cellulose-based nanosponges for wastewater remediation. Nanosponges for environmental remediation, Springer, Switzerland, 2023, pp. 355–382.10.1007/978-3-031-41077-2_16Suche in Google Scholar
[32] Rana, A. K., Y. K. Mishra, V. K. Gupta, and V. K. Thakur. Sustainable materials in the removal of pesticides from contaminated water: Perspective on macro to nanoscale cellulose. Science of The Total Environment, Vol. 797, 2021, id. 149129.10.1016/j.scitotenv.2021.149129Suche in Google Scholar PubMed
[33] Qiao, A., M. Cui, R. Huang, G. Ding, W. Qi, Z. He, et al. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydrate Polymers, Vol. 272, 2021, id. 118471.10.1016/j.carbpol.2021.118471Suche in Google Scholar PubMed
[34] Blockx, J., A. Verfaillie, O. Deschaume, C. Bartic, K. Muylaert, and W. Thielemans. Glycine betaine grafted nanocellulose as an effective and bio-based cationic nanocellulose flocculant for wastewater treatment and microalgal harvesting. Nanoscale Advances, Vol. 3, No. 14, 2021, pp. 4133–4144.10.1039/D1NA00102GSuche in Google Scholar
[35] Wu, Z., S. Xie, Y. Kang, X. Shan, Q. Li, and Z. Cai. Biocompatibility evaluation of a 3D-bioprinted alginate-GelMA-bacteria nanocellulose (BNC) scaffold laden with oriented-growth RSC96 cells. Materials Science and Engineering: C, Vol. 129, 2021, id. 112393.10.1016/j.msec.2021.112393Suche in Google Scholar PubMed
[36] Liu, Y., Q. Fan, Y. Huo, M. Li, H. Liu, and B. Li. Construction of nanocellulose-based composite hydrogel with a double packing structure as an intelligent drug carrier. Cellulose, Vol. 28, No. 11, 2021, pp. 6953–6966.10.1007/s10570-021-03978-5Suche in Google Scholar
[37] Alzahrani, F. M., A. Amari, K. M. Katubi, N. S. Alsaiari, and M. A. Tahoon. The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution. Open Chemistry, Vol. 20, No. 1, 2022, pp. 970–983.10.1515/chem-2022-0215Suche in Google Scholar
[38] Ma, S., M. Zhang, J. Nie, J. Tan, S. Song, and Y. Luo. Lightweight and porous cellulose-based foams with high loadings of zeolitic imidazolate frameworks-8 for adsorption applications. Carbohydrate polymers, Vol. 208, 2019, pp. 328–335.10.1016/j.carbpol.2018.12.081Suche in Google Scholar PubMed
[39] Wang, Z., L. Song, Y. Wang, X.-F. Zhang, D. Hao, Y. Feng, et al. Lightweight UiO-66/cellulose aerogels constructed through self-crosslinking strategy for adsorption applications. Chemical Engineering Journal, Vol. 371, 2019, pp. 138–144.10.1016/j.cej.2019.04.022Suche in Google Scholar
[40] Zhu, H., X. Yang, E. D. Cranston, and S. Zhu. Flexible and porous nanocellulose aerogels with high loadings of metal–organic‐framework particles for separations applications. Advanced materials, Vol. 28, No. 35, 2016, pp. 7652–7657.10.1002/adma.201601351Suche in Google Scholar PubMed
[41] Mahmed, N., O. Heczko, O. Söderberg, and S.-P. Hannula, editors. Room temperature synthesis of magnetite (Fe3− δO4) nanoparticles by a simple reverse co-precipitation Method. IOP Conference Series: Materials Science and Engineering, 2011. IOP Publishing.10.1088/1757-899X/18/3/032020Suche in Google Scholar
[42] Khan, I. A., M. Choucair, M. Imran, A. Badshah, and M. A. Nadeem. Supercapacitive behavior of microporous carbon derived from zinc based metal-organic framework and furfuryl alcohol. International Journal of Hydrogen Energy, Vol. 40, No. 39, 2015, pp. 13344–13356.10.1016/j.ijhydene.2015.08.053Suche in Google Scholar
[43] Huang, H., X. Wang, H. Ge, and M. Xu. Multifunctional magnetic cellulose surface-imprinted microspheres for highly selective adsorption of artesunate. ACS Sustainable Chemistry & Engineering, Vol. 4, No. 6, 2016, pp. 3334–3343.10.1021/acssuschemeng.6b00386Suche in Google Scholar
[44] Wang, L., G. Chen, H. Shu, X. Cui, Z. Luo, C. Chang, et al. Facile covalent preparation of carbon nanotubes/amine-functionalized Fe3O4 nanocomposites for selective extraction of estradiol in pharmaceutical industry wastewater. Journal of Chromatography A, Vol. 1638, 2021, id. 461889.10.1016/j.chroma.2021.461889Suche in Google Scholar PubMed
[45] Lin, X., X. Wang, Q. Zhou, C. Wen, S. Su, J. Xiang, et al. Magnetically recyclable MoS2/Fe3O4 hybrid composite as visible light responsive photocatalyst with enhanced photocatalytic performance. ACS Sustainable Chemistry & Engineering, Vol. 7, No. 1, 2018, pp. 1673–1682.Suche in Google Scholar
[46] Wang, X., X. Ma, H. Wang, P. Huang, X. Du, and X. Lu. A zinc(ii) benzenetricarboxylate metal organic framework with unusual adsorption properties, and its application to the preconcentration of pesticides. Microchimica Acta, Vol. 184, 2017, pp. 3681–3687.10.1007/s00604-017-2382-1Suche in Google Scholar
[47] Petit, C., B. Levasseur, B. Mendoza, and T. J. Bandosz. Reactive adsorption of acidic gases on MOF/graphite oxide composites. Microporous and Mesoporous Materials, Vol. 154, 2012, pp. 107–112.10.1016/j.micromeso.2011.09.012Suche in Google Scholar
[48] Ra’ad, Z., L. Al-Karam, and N. A. Alsahib, editors. Synthesis and characterization of dextran coated iron oxide nanoparticles as a T1 contrast agent. Journal of Physics: Conference Series, 2021. IOP Publishing.10.1088/1742-6596/2114/1/012037Suche in Google Scholar
[49] Hussin, F. N. N. M., N. Attan, and R. A. Wahab. Extraction and characterization of nanocellulose from raw oil palm leaves (Elaeis guineensis). Arabian Journal for Science and Engineering, Vol. 45, 2020, pp. 175–186.10.1007/s13369-019-04131-ySuche in Google Scholar
[50] Wang, N., R.-N. Jin, A. Omer, and X.-K. Ouyang. Adsorption of Pb(ii) from fish sauce using carboxylated cellulose nanocrystal: Isotherm, kinetics, and thermodynamic studies. International Journal of Biological Macromolecules, Vol. 102, 2017, pp. 232–240.10.1016/j.ijbiomac.2017.03.150Suche in Google Scholar PubMed
[51] Onkarappa, H., G. Prakash, G. Pujar, C. Rajith Kumar, V. Radha, and V. S. Betageri. Facile synthesis and characterization of nanocellulose from Zea mays husk. Polymer Composites, Vol. 41, No. 8, 2020, pp. 3153–3159.10.1002/pc.25606Suche in Google Scholar
[52] Chikri, R., N. Elhadiri, M. Benchanaa, and Y. Maguana. Efficiency of sawdust as low-cost adsorbent for dyes removal. Journal of Chemistry, Vol. 2020, 2020, pp. 1–17.10.1155/2020/8813420Suche in Google Scholar
[53] Yagub, M. T., T. K. Sen, S. Afroze, and H. M. Ang. Dye and its removal from aqueous solution by adsorption: a review. Advances in Colloid and Interface Science, Vol. 209, 2014, pp. 172–184.10.1016/j.cis.2014.04.002Suche in Google Scholar PubMed
[54] Qiu, B., Q. Shao, J. Shi, C. Yang, and H. Chu. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Separation and Purification Technology, Vol. 300, 2022, id. 121925.10.1016/j.seppur.2022.121925Suche in Google Scholar
[55] Mustapha, S., M. Ndamitso, A. Abdulkareem, J. Tijani, A. Mohammed, and D. Shuaib. Potential of using kaolin as a natural adsorbent for the removal of pollutants from tannery wastewater. Heliyon, Vol. 5, No. 11, 2019, id. e02923.10.1016/j.heliyon.2019.e02923Suche in Google Scholar PubMed PubMed Central
[56] Jawad, A. H. and A. S. Abdulhameed. Statistical modeling of methylene blue dye adsorption by high surface area mesoporous activated carbon from bamboo chip using KOH-assisted thermal activation. Energy, Ecology and Environment, Vol. 5, No. 6, 2020, pp. 456–469.10.1007/s40974-020-00177-zSuche in Google Scholar
[57] Alarifi, I. M., Y. O. Al-Ghamdi, R. Darwesh, M. O. Ansari, and M. K. Uddin. Properties and application of MoS2 nanopowder: Characterization, Congo red dye adsorption, and optimization. Journal of Materials Research and Technology, Vol. 13, 2021, pp. 1169–1180.10.1016/j.jmrt.2021.05.028Suche in Google Scholar
[58] Sultana, S., K. Islam, M. A. Hasan, H. J. Khan, M. A. R. Khan, A. Deb, et al. Adsorption of crystal violet dye by coconut husk powder: isotherm, kinetics and thermodynamics perspectives. Environmental Nanotechnology, Monitoring & Management, Vol. 17, 2022, id. 100651.10.1016/j.enmm.2022.100651Suche in Google Scholar
[59] Tian, X., R. Yang, T. Chen, Y. Cao, H. Deng, M. Zhang, et al. Removal of both anionic and cationic dyes from wastewater using pH-responsive adsorbents of L-lysine molecular-grafted cellulose porous foams. Journal of Hazardous Materials, Vol. 426, 2022, id. 128121.10.1016/j.jhazmat.2021.128121Suche in Google Scholar PubMed
[60] Ighalo, J. O., S. Rangabhashiyam, C. A. Adeyanju, S. Ogunniyi, A. G. Adeniyi, and C. A. Igwegbe. Zeolitic imidazolate frameworks (ZIFs) for aqueous phase adsorption–a review. Journal of Industrial and Engineering Chemistry, Vol. 105, 2022, pp. 34–48.10.1016/j.jiec.2021.09.029Suche in Google Scholar
[61] Mansor, E. S. and T. Aysha. Cerium organic frameworks as green pollution preventing materials for dye removal. Egyptian Journal of Chemistry, Vol. 65, No. 131, 2022, pp. 1217–1230.10.21608/ejchem.2022.168639.7084Suche in Google Scholar
[62] Alswieleh, A. M. Fabrication of carboxylic functionalized poly (methacrylic acid 2-(tert-butylamino) ethyl ester)-coated mesoporous silica nanoparticles and their application for removing ionic dyes from polluted water. Heliyon, Vol. 9, 2023, id. e23180.10.1016/j.heliyon.2023.e23180Suche in Google Scholar PubMed PubMed Central
[63] Sadiq, A. C., N. Y. Rahim, and F. B. M. Suah. Adsorption and desorption of malachite green by using chitosan-deep eutectic solvents beads. International Journal of Biological Macromolecules, Vol. 164, 2020, pp. 3965–3973.10.1016/j.ijbiomac.2020.09.029Suche in Google Scholar PubMed
[64] Fatombi, J. K., E. A. Idohou, S. A. Osseni, I. Agani, D. Neumeyer, M. Verelst, et al. Adsorption of indigo carmine from aqueous solution by chitosan and chitosan/activated carbon composite: kinetics, isotherms and thermodynamics studies. Fibers and Polymers, Vol. 20, 2019, pp. 1820–1832.10.1007/s12221-019-1107-ySuche in Google Scholar
[65] Shokry, H. and H. Hamad. Effect of superparamagnetic nanoparticles on the physicochemical properties of nano hydroxyapatite for groundwater treatment: adsorption mechanism of Fe(ii) and Mn(ii). RSC Advances, Vol. 6, No. 85, 2016, pp. 82244–82259.10.1039/C6RA14497GSuche in Google Scholar
[66] Al-Ghouti, M. A. and D. A. Da’ana. Guidelines for the use and interpretation of adsorption isotherm models: A review. Journal of hazardous materials, Vol. 393, 2020, id. 122383.10.1016/j.jhazmat.2020.122383Suche in Google Scholar PubMed
[67] Somashekara, D. and L. Mulky. Sequestration of contaminants from wastewater: a review of adsorption processes. ChemBioEng Reviews, Vol. 10, No. 4, 2023, pp. 491–509.10.1002/cben.202200050Suche in Google Scholar
[68] Shikuku, V. O. and T. Mishra. Adsorption isotherm modeling for methylene blue removal onto magnetic kaolinite clay: a comparison of two-parameter isotherms. Applied water science, Vol. 11, No. 6, 2021, id. 103.10.1007/s13201-021-01440-2Suche in Google Scholar
[69] Saxena, M., N. Sharma, and R. Saxena. Highly efficient and rapid removal of a toxic dye: adsorption kinetics, isotherm, and mechanism studies on functionalized multiwalled carbon nanotubes. Surfaces and Interfaces, Vol. 21, 2020, id. 100639.10.1016/j.surfin.2020.100639Suche in Google Scholar
[70] Alsaiari, N. S., M. S. Alsaiari, F. M. Alzahrani, A. Amari, and M. A. Tahoon. Synthesis, characterization, and application of the novel nanomagnet adsorbent for the removal of Cr (vi) ions. Reviews on Advanced Materials Science, Vol. 62, No. 1, 2023, id. 20230145.10.1515/rams-2023-0145Suche in Google Scholar
[71] Sharifi, M. J., A. Nouralishahi, and A. Hallajisani. Fe3O4-chitosan nanocomposite as a magnetic biosorbent for removal of nickel and cobalt heavy metals from polluted water. International Journal of Biological Macromolecules, Vol. 248, 2023, id. 125984.10.1016/j.ijbiomac.2023.125984Suche in Google Scholar PubMed
[72] Debnath, S. and R. Das. Strong adsorption of CV dye by Ni ferrite nanoparticles for waste water purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceramics International, Vol. 49, No. 10, 2023, pp. 16199–16215.10.1016/j.ceramint.2023.01.218Suche in Google Scholar
[73] Hidayat, A. R. P., D. O. Sulistiono, I. K. Murwani, B. F. Endrawati, H. Fansuri, L. L. Zulfa, et al. Linear and nonlinear isotherm, kinetic and thermodynamic behavior of methyl orange adsorption using modulated Al2O3@ UiO-66 via acetic acid. Journal of Environmental Chemical Engineering, Vol. 9, No. 6, 2021, id. 106675.10.1016/j.jece.2021.106675Suche in Google Scholar
[74] Harja, M., G. Buema, and D. Bucur. Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Scientific Reports, Vol. 12, No. 1, 2022, id. 6087.10.1038/s41598-022-10093-3Suche in Google Scholar PubMed PubMed Central
[75] Dryaz, A. R., M. Shaban, H. AlMohamadi, K. A. A. Al-Ola, A. Hamd, N. Soliman, et al. Design, characterization, and adsorption properties of Padina gymnospora/zeolite nanocomposite for Congo red dye removal from wastewater. Scientific Reports, Vol. 11, No. 1, 2021, id. 21058.10.1038/s41598-021-00025-ySuche in Google Scholar PubMed PubMed Central
[76] Saleh, S., H. Maarof, S. Rahim, and N. Nasuha. Adsorption of Congo red onto bottom ash. Journal of Applied Sciences, Vol. 12, No. 11, 2012, pp. 1181–1185.10.3923/jas.2012.1181.1185Suche in Google Scholar
[77] Hu, H., J. Liu, Z. Xu, L. Zhang, B. Cheng, and W. Ho. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr (VI) ions. Applied Surface Science, Vol. 478, 2019, pp. 981–990.10.1016/j.apsusc.2019.02.008Suche in Google Scholar
[78] Yang, M. and Q. Bai. Flower-like hierarchical Ni-Zn MOF microspheres: Efficient adsorbents for dye removal. Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 582, 2019, id. 123795.10.1016/j.colsurfa.2019.123795Suche in Google Scholar
[79] Mosavi, S. H., R. Zare‐Dorabei, and M. Bereyhi. Rapid and effective ultrasonic‐assisted adsorptive removal of congo red onto MOF‐5 modified by CuCl2 in ambient conditions: Adsorption isotherms and kinetics studies. ChemistrySelect, Vol. 6, No. 18, 2021, pp. 4432–4439.10.1002/slct.202100540Suche in Google Scholar
[80] Suresh, S. Removal of basic violet 14 from aqueous solution using sulphuric acid activated materials. SpringerPlus, Vol. 5, No. 1, 2016, pp. 1–14.10.1186/s40064-016-2294-2Suche in Google Scholar PubMed PubMed Central
[81] Suresh, S., R. W. Sugumar, and T. Maiyalagan. A low cost adsorbent prepared from Curcuma angustifolia scales for removal of Basic violet 14 from aqueous solution. Indian Journal of Chemical Technology, Vol. 21, 2014, pp. 368–378.Suche in Google Scholar
[82] Gupta, V., A. Mittal, V. Gajbe, and J. Mittal. Adsorption of basic fuchsin using waste materials—bottom ash and deoiled soya—as adsorbents. Journal of Colloid and Interface Science, Vol. 319, No. 1, 2008, pp. 30–39.10.1016/j.jcis.2007.09.091Suche in Google Scholar PubMed
[83] Konicki, W., I. Pełech, E. Mijowska, and I. Jasińska. Adsorption kinetics of acid dye acid red 88 onto magnetic multi‐walled carbon nanotubes‐Fe3C nanocomposite. Clean–Soil, Air, Water, Vol. 42, No. 3, 2014, pp. 284–294.10.1002/clen.201200458Suche in Google Scholar
[84] Soltani, R. D. C., A. Khataee, M. Safari, and S. Joo. Preparation of bio-silica/chitosan nanocomposite for adsorption of a textile dye in aqueous solutions. International Biodeterioration & Biodegradation, Vol. 85, 2013, pp. 383–391.10.1016/j.ibiod.2013.09.004Suche in Google Scholar
[85] Xing, T., H. Kai, and G. Chen. Study of adsorption and desorption performance of acid dyes on anion exchange membrane. Coloration Technology, Vol. 128, No. 4, 2012, pp. 295–299.10.1111/j.1478-4408.2012.00378.xSuche in Google Scholar
[86] Venkata Mohan, S., P. Sailaja, M. Srimurali, and J. Karthikeyan. Color removal of monoazo acid dye from aqueous solution by adsorption and chemical coagulation. Environmental Engineering and Policy, Vol. 1, 1998, pp. 149–154.10.1007/s100220050016Suche in Google Scholar
[87] Yang, L., X. Ma, and N. Guo. Sodium alginate/Na+ -rectorite composite microspheres: Preparation, characterization, and dye adsorption. Carbohydrate Polymers, Vol. 90, No. 2, 2012, pp. 853–858.10.1016/j.carbpol.2012.06.011Suche in Google Scholar PubMed
[88] Hou, F., D. Wang, X. Ma, L. Fan, T. Ding, X. Ye, et al. Enhanced adsorption of Congo red using chitin suspension after sonoenzymolysis. Ultrasonics Sonochemistry, Vol. 70, 2021, id. 105327.10.1016/j.ultsonch.2020.105327Suche in Google Scholar PubMed PubMed Central
[89] Sahmoune, M. N. Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environmental Chemistry Letters, Vol. 17, No. 2, 2019, pp. 697–704.10.1007/s10311-018-00819-zSuche in Google Scholar
[90] Chakraborty, S., A. Mukherjee, S. Das, N. R. Maddela, S. Iram, and P. Das. Study on isotherm, kinetics, and thermodynamics of adsorption of crystal violet dye by calcium oxide modified fly ash. Environmental Engineering Research, Vol. 26, No. 1, 2021, id. 190372.10.4491/eer.2019.372Suche in Google Scholar
[91] Quesada, H. B., L. F. Cusioli, O. de, C. Bezerra, A. T. Baptista, L. Nishi, et al. Acetaminophen adsorption using a low‐cost adsorbent prepared from modified residues of Moringa oleifera Lam. seed husks. Journal of Chemical Technology & Biotechnology, Vol. 94, No. 10, 2019, pp. 3147–3157.10.1002/jctb.6121Suche in Google Scholar
[92] Gómez-Avilés, A., M. Peñas-Garzón, C. Belver, J. Rodriguez, and J. Bedia. Equilibrium, kinetics and breakthrough curves of acetaminophen adsorption onto activated carbons from microwave-assisted FeCl3-activation of lignin. Separation and Purification Technology, Vol. 278, 2021, id. 119654.10.1016/j.seppur.2021.119654Suche in Google Scholar
[93] Chen, W., Z. Liu, Q. Tang, B. Du, X. Huang, Y. Mo, et al. Assessment of a novel aminated magnetic adsorbent with excellent adsorption capacity for dyes and drugs. Journal of Environmental Management, Vol. 293, 2021, id. 112809.10.1016/j.jenvman.2021.112809Suche in Google Scholar PubMed
[94] Fanourakis, S. K., J. Peña-Bahamonde, P. C. Bandara, D. F. Rodrigues. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. NPJ Clean Water, Vol. 3, No. 1, 2020, id. 1.10.1038/s41545-019-0048-8Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Review Articles
- Effect of superplasticizer in geopolymer and alkali-activated cement mortar/concrete: A review
- Experimenting the influence of corncob ash on the mechanical strength of slag-based geopolymer concrete
- Powder metallurgy processing of high entropy alloys: Bibliometric analysis and systematic review
- Exploring the potential of agricultural waste as an additive in ultra-high-performance concrete for sustainable construction: A comprehensive review
- A review on partial substitution of nanosilica in concrete
- Foam concrete for lightweight construction applications: A comprehensive review of the research development and material characteristics
- Modification of PEEK for implants: Strategies to improve mechanical, antibacterial, and osteogenic properties
- Interfacing the IoT in composite manufacturing: An overview
- Advances in processing and ablation properties of carbon fiber reinforced ultra-high temperature ceramic composites
- Advancing auxetic materials: Emerging development and innovative applications
- Revolutionizing energy harvesting: A comprehensive review of thermoelectric devices
- Exploring polyetheretherketone in dental implants and abutments: A focus on biomechanics and finite element methods
- Smart technologies and textiles and their potential use and application in the care and support of elderly individuals: A systematic review
- Reinforcement mechanisms and current research status of silicon carbide whisker-reinforced composites: A comprehensive review
- Innovative eco-friendly bio-composites: A comprehensive review of the fabrication, characterization, and applications
- Review on geopolymer concrete incorporating Alccofine-1203
- Advancements in surface treatments for aluminum alloys in sports equipment
- Ionic liquid-modified carbon-based fillers and their polymer composites – A Raman spectroscopy analysis
- Emerging boron nitride nanosheets: A review on synthesis, corrosion resistance coatings, and their impacts on the environment and health
- Mechanism, models, and influence of heterogeneous factors of the microarc oxidation process: A comprehensive review
- Synthesizing sustainable construction paradigms: A comprehensive review and bibliometric analysis of granite waste powder utilization and moisture correction in concrete
- 10.1515/rams-2025-0086
- Research Articles
- Coverage and reliability improvement of copper metallization layer in through hole at BGA area during load board manufacture
- Study on dynamic response of cushion layer-reinforced concrete slab under rockfall impact based on smoothed particle hydrodynamics and finite-element method coupling
- Study on the mechanical properties and microstructure of recycled brick aggregate concrete with waste fiber
- Multiscale characterization of the UV aging resistance and mechanism of light stabilizer-modified asphalt
- Characterization of sandwich materials – Nomex-Aramid carbon fiber performances under mechanical loadings: Nonlinear FE and convergence studies
- Effect of grain boundary segregation and oxygen vacancy annihilation on aging resistance of cobalt oxide-doped 3Y-TZP ceramics for biomedical applications
- Mechanical damage mechanism investigation on CFRP strengthened recycled red brick concrete
- Finite element analysis of deterioration of axial compression behavior of corroded steel-reinforced concrete middle-length columns
- Grinding force model for ultrasonic assisted grinding of γ-TiAl intermetallic compounds and experimental validation
- Enhancement of hardness and wear strength of pure Cu and Cu–TiO2 composites via a friction stir process while maintaining electrical resistivity
- Effect of sand–precursor ratio on mechanical properties and durability of geopolymer mortar with manufactured sand
- Research on the strength prediction for pervious concrete based on design porosity and water-to-cement ratio
- Development of a new damping ratio prediction model for recycled aggregate concrete: Incorporating modified admixtures and carbonation effects
- Exploring the viability of AI-aided genetic algorithms in estimating the crack repair rate of self-healing concrete
- Modification of methacrylate bone cement with eugenol – A new material with antibacterial properties
- Numerical investigations on constitutive model parameters of HRB400 and HTRB600 steel bars based on tensile and fatigue tests
- Research progress on Fe3+-activated near-infrared phosphor
- Discrete element simulation study on effects of grain preferred orientation on micro-cracking and macro-mechanical behavior of crystalline rocks
- Ultrasonic resonance evaluation method for deep interfacial debonding defects of multilayer adhesive bonded materials
- Effect of impurity components in titanium gypsum on the setting time and mechanical properties of gypsum-slag cementitious materials
- Bending energy absorption performance of composite fender piles with different winding angles
- Theoretical study of the effect of orientations and fibre volume on the thermal insulation capability of reinforced polymer composites
- Synthesis and characterization of a novel ternary magnetic composite for the enhanced adsorption capacity to remove organic dyes
- Couple effects of multi-impact damage and CAI capability on NCF composites
- Mechanical testing and engineering applicability analysis of SAP concrete used in buffer layer design for tunnels in active fault zones
- Investigating the rheological characteristics of alkali-activated concrete using contemporary artificial intelligence approaches
- Integrating micro- and nanowaste glass with waste foundry sand in ultra-high-performance concrete to enhance material performance and sustainability
- Effect of water immersion on shear strength of epoxy adhesive filled with graphene nanoplatelets
- Impact of carbon content on the phase structure and mechanical properties of TiBCN coatings via direct current magnetron sputtering
- Investigating the anti-aging properties of asphalt modified with polyphosphoric acid and tire pyrolysis oil
- Biomedical and therapeutic potential of marine-derived Pseudomonas sp. strain AHG22 exopolysaccharide: A novel bioactive microbial metabolite
- Effect of basalt fiber length on the behavior of natural hydraulic lime-based mortars
- Optimizing the performance of TPCB/SCA composite-modified asphalt using improved response surface methodology
- Compressive strength of waste-derived cementitious composites using machine learning
- Melting phenomenon of thermally stratified MHD Powell–Eyring nanofluid with variable porosity past a stretching Riga plate
- Development and characterization of a coaxial strain-sensing cable integrated steel strand for wide-range stress monitoring
- Compressive and tensile strength estimation of sustainable geopolymer concrete using contemporary boosting ensemble techniques
- Customized 3D printed porous titanium scaffolds with nanotubes loading antibacterial drugs for bone tissue engineering
- Facile design of PTFE-kaolin-based ternary nanocomposite as a hydrophobic and high corrosion-barrier coating
- Effects of C and heat treatment on microstructure, mechanical, and tribo-corrosion properties of VAlTiMoSi high-entropy alloy coating
- Study on the damage mechanism and evolution model of preloaded sandstone subjected to freezing–thawing action based on the NMR technology
- Promoting low carbon construction using alkali-activated materials: A modeling study for strength prediction and feature interaction
- Entropy generation analysis of MHD convection flow of hybrid nanofluid in a wavy enclosure with heat generation and thermal radiation
- Friction stir welding of dissimilar Al–Mg alloys for aerospace applications: Prospects and future potential
- Fe nanoparticle-functionalized ordered mesoporous carbon with tailored mesostructures and their applications in magnetic removal of Ag(i)
- Study on physical and mechanical properties of complex-phase conductive fiber cementitious materials
- Evaluating the strength loss and the effectiveness of glass and eggshell powder for cement mortar under acidic conditions
- Effect of fly ash on properties and hydration of calcium sulphoaluminate cement-based materials with high water content
- Analyzing the efficacy of waste marble and glass powder for the compressive strength of self-compacting concrete using machine learning strategies
- Experimental study on municipal solid waste incineration ash micro-powder as concrete admixture
- Parameter optimization for ultrasonic-assisted grinding of γ-TiAl intermetallics: A gray relational analysis approach with surface integrity evaluation
- Producing sustainable binding materials using marble waste blended with fly ash and rice husk ash for building materials
- Effect of steam curing system on compressive strength of recycled aggregate concrete
- A sawtooth constitutive model describing strain hardening and multiple cracking of ECC under uniaxial tension
- Predicting mechanical properties of sustainable green concrete using novel machine learning: Stacking and gene expression programming
- Toward sustainability: Integrating experimental study and data-driven modeling for eco-friendly paver blocks containing plastic waste
- A numerical analysis of the rotational flow of a hybrid nanofluid past a unidirectional extending surface with velocity and thermal slip conditions
- A magnetohydrodynamic flow of a water-based hybrid nanofluid past a convectively heated rotating disk surface: A passive control of nanoparticles
- Prediction of flexural strength of concrete with eggshell and glass powders: Advanced cutting-edge approach for sustainable materials
- Efficacy of sustainable cementitious materials on concrete porosity for enhancing the durability of building materials
- Phase and microstructural characterization of swat soapstone (Mg3Si4O10(OH)2)
- Effect of waste crab shell powder on matrix asphalt
- Improving effect and mechanism on service performance of asphalt binder modified by PW polymer
- Influence of pH on the synthesis of carbon spheres and the application of carbon sphere-based solid catalysts in esterification
- Experimenting the compressive performance of low-carbon alkali-activated materials using advanced modeling techniques
- Thermogravimetric (TG/DTG) characterization of cold-pressed oil blends and Saccharomyces cerevisiae-based microcapsules obtained with them
- Investigation of temperature effect on thermo-mechanical property of carbon fiber/PEEK composites
- Computational approaches for structural analysis of wood specimens
- Integrated structure–function design of 3D-printed porous polydimethylsiloxane for superhydrophobic engineering
- Exploring the impact of seashell powder and nano-silica on ultra-high-performance self-curing concrete: Insights into mechanical strength, durability, and high-temperature resilience
- Axial compression damage constitutive model and damage characteristics of fly ash/silica fume modified magnesium phosphate cement after being treated at different temperatures
- Integrating testing and modeling methods to examine the feasibility of blended waste materials for the compressive strength of rubberized mortar
- Special Issue on 3D and 4D Printing of Advanced Functional Materials - Part II
- Energy absorption of gradient triply periodic minimal surface structure manufactured by stereolithography
- Marine polymers in tissue bioprinting: Current achievements and challenges
- Quick insight into the dynamic dimensions of 4D printing in polymeric composite mechanics
- Recent advances in 4D printing of hydrogels
- Mechanically sustainable and primary recycled thermo-responsive ABS–PLA polymer composites for 4D printing applications: Fabrication and studies
- Special Issue on Materials and Technologies for Low-carbon Biomass Processing and Upgrading
- Low-carbon embodied alkali-activated materials for sustainable construction: A comparative study of single and ensemble learners
- Study on bending performance of prefabricated glulam-cross laminated timber composite floor
- Special Issue on Recent Advancement in Low-carbon Cement-based Materials - Part I
- Supplementary cementitious materials-based concrete porosity estimation using modeling approaches: A comparative study of GEP and MEP
- Modeling the strength parameters of agro waste-derived geopolymer concrete using advanced machine intelligence techniques
- Promoting the sustainable construction: A scientometric review on the utilization of waste glass in concrete
- Incorporating geranium plant waste into ultra-high performance concrete prepared with crumb rubber as fine aggregate in the presence of polypropylene fibers
- Investigation of nano-basic oxygen furnace slag and nano-banded iron formation on properties of high-performance geopolymer concrete
- Effect of incorporating ultrafine palm oil fuel ash on the resistance to corrosion of steel bars embedded in high-strength green concrete
- Influence of nanomaterials on properties and durability of ultra-high-performance geopolymer concrete
- Influence of palm oil ash and palm oil clinker on the properties of lightweight concrete
Artikel in diesem Heft
- Review Articles
- Effect of superplasticizer in geopolymer and alkali-activated cement mortar/concrete: A review
- Experimenting the influence of corncob ash on the mechanical strength of slag-based geopolymer concrete
- Powder metallurgy processing of high entropy alloys: Bibliometric analysis and systematic review
- Exploring the potential of agricultural waste as an additive in ultra-high-performance concrete for sustainable construction: A comprehensive review
- A review on partial substitution of nanosilica in concrete
- Foam concrete for lightweight construction applications: A comprehensive review of the research development and material characteristics
- Modification of PEEK for implants: Strategies to improve mechanical, antibacterial, and osteogenic properties
- Interfacing the IoT in composite manufacturing: An overview
- Advances in processing and ablation properties of carbon fiber reinforced ultra-high temperature ceramic composites
- Advancing auxetic materials: Emerging development and innovative applications
- Revolutionizing energy harvesting: A comprehensive review of thermoelectric devices
- Exploring polyetheretherketone in dental implants and abutments: A focus on biomechanics and finite element methods
- Smart technologies and textiles and their potential use and application in the care and support of elderly individuals: A systematic review
- Reinforcement mechanisms and current research status of silicon carbide whisker-reinforced composites: A comprehensive review
- Innovative eco-friendly bio-composites: A comprehensive review of the fabrication, characterization, and applications
- Review on geopolymer concrete incorporating Alccofine-1203
- Advancements in surface treatments for aluminum alloys in sports equipment
- Ionic liquid-modified carbon-based fillers and their polymer composites – A Raman spectroscopy analysis
- Emerging boron nitride nanosheets: A review on synthesis, corrosion resistance coatings, and their impacts on the environment and health
- Mechanism, models, and influence of heterogeneous factors of the microarc oxidation process: A comprehensive review
- Synthesizing sustainable construction paradigms: A comprehensive review and bibliometric analysis of granite waste powder utilization and moisture correction in concrete
- 10.1515/rams-2025-0086
- Research Articles
- Coverage and reliability improvement of copper metallization layer in through hole at BGA area during load board manufacture
- Study on dynamic response of cushion layer-reinforced concrete slab under rockfall impact based on smoothed particle hydrodynamics and finite-element method coupling
- Study on the mechanical properties and microstructure of recycled brick aggregate concrete with waste fiber
- Multiscale characterization of the UV aging resistance and mechanism of light stabilizer-modified asphalt
- Characterization of sandwich materials – Nomex-Aramid carbon fiber performances under mechanical loadings: Nonlinear FE and convergence studies
- Effect of grain boundary segregation and oxygen vacancy annihilation on aging resistance of cobalt oxide-doped 3Y-TZP ceramics for biomedical applications
- Mechanical damage mechanism investigation on CFRP strengthened recycled red brick concrete
- Finite element analysis of deterioration of axial compression behavior of corroded steel-reinforced concrete middle-length columns
- Grinding force model for ultrasonic assisted grinding of γ-TiAl intermetallic compounds and experimental validation
- Enhancement of hardness and wear strength of pure Cu and Cu–TiO2 composites via a friction stir process while maintaining electrical resistivity
- Effect of sand–precursor ratio on mechanical properties and durability of geopolymer mortar with manufactured sand
- Research on the strength prediction for pervious concrete based on design porosity and water-to-cement ratio
- Development of a new damping ratio prediction model for recycled aggregate concrete: Incorporating modified admixtures and carbonation effects
- Exploring the viability of AI-aided genetic algorithms in estimating the crack repair rate of self-healing concrete
- Modification of methacrylate bone cement with eugenol – A new material with antibacterial properties
- Numerical investigations on constitutive model parameters of HRB400 and HTRB600 steel bars based on tensile and fatigue tests
- Research progress on Fe3+-activated near-infrared phosphor
- Discrete element simulation study on effects of grain preferred orientation on micro-cracking and macro-mechanical behavior of crystalline rocks
- Ultrasonic resonance evaluation method for deep interfacial debonding defects of multilayer adhesive bonded materials
- Effect of impurity components in titanium gypsum on the setting time and mechanical properties of gypsum-slag cementitious materials
- Bending energy absorption performance of composite fender piles with different winding angles
- Theoretical study of the effect of orientations and fibre volume on the thermal insulation capability of reinforced polymer composites
- Synthesis and characterization of a novel ternary magnetic composite for the enhanced adsorption capacity to remove organic dyes
- Couple effects of multi-impact damage and CAI capability on NCF composites
- Mechanical testing and engineering applicability analysis of SAP concrete used in buffer layer design for tunnels in active fault zones
- Investigating the rheological characteristics of alkali-activated concrete using contemporary artificial intelligence approaches
- Integrating micro- and nanowaste glass with waste foundry sand in ultra-high-performance concrete to enhance material performance and sustainability
- Effect of water immersion on shear strength of epoxy adhesive filled with graphene nanoplatelets
- Impact of carbon content on the phase structure and mechanical properties of TiBCN coatings via direct current magnetron sputtering
- Investigating the anti-aging properties of asphalt modified with polyphosphoric acid and tire pyrolysis oil
- Biomedical and therapeutic potential of marine-derived Pseudomonas sp. strain AHG22 exopolysaccharide: A novel bioactive microbial metabolite
- Effect of basalt fiber length on the behavior of natural hydraulic lime-based mortars
- Optimizing the performance of TPCB/SCA composite-modified asphalt using improved response surface methodology
- Compressive strength of waste-derived cementitious composites using machine learning
- Melting phenomenon of thermally stratified MHD Powell–Eyring nanofluid with variable porosity past a stretching Riga plate
- Development and characterization of a coaxial strain-sensing cable integrated steel strand for wide-range stress monitoring
- Compressive and tensile strength estimation of sustainable geopolymer concrete using contemporary boosting ensemble techniques
- Customized 3D printed porous titanium scaffolds with nanotubes loading antibacterial drugs for bone tissue engineering
- Facile design of PTFE-kaolin-based ternary nanocomposite as a hydrophobic and high corrosion-barrier coating
- Effects of C and heat treatment on microstructure, mechanical, and tribo-corrosion properties of VAlTiMoSi high-entropy alloy coating
- Study on the damage mechanism and evolution model of preloaded sandstone subjected to freezing–thawing action based on the NMR technology
- Promoting low carbon construction using alkali-activated materials: A modeling study for strength prediction and feature interaction
- Entropy generation analysis of MHD convection flow of hybrid nanofluid in a wavy enclosure with heat generation and thermal radiation
- Friction stir welding of dissimilar Al–Mg alloys for aerospace applications: Prospects and future potential
- Fe nanoparticle-functionalized ordered mesoporous carbon with tailored mesostructures and their applications in magnetic removal of Ag(i)
- Study on physical and mechanical properties of complex-phase conductive fiber cementitious materials
- Evaluating the strength loss and the effectiveness of glass and eggshell powder for cement mortar under acidic conditions
- Effect of fly ash on properties and hydration of calcium sulphoaluminate cement-based materials with high water content
- Analyzing the efficacy of waste marble and glass powder for the compressive strength of self-compacting concrete using machine learning strategies
- Experimental study on municipal solid waste incineration ash micro-powder as concrete admixture
- Parameter optimization for ultrasonic-assisted grinding of γ-TiAl intermetallics: A gray relational analysis approach with surface integrity evaluation
- Producing sustainable binding materials using marble waste blended with fly ash and rice husk ash for building materials
- Effect of steam curing system on compressive strength of recycled aggregate concrete
- A sawtooth constitutive model describing strain hardening and multiple cracking of ECC under uniaxial tension
- Predicting mechanical properties of sustainable green concrete using novel machine learning: Stacking and gene expression programming
- Toward sustainability: Integrating experimental study and data-driven modeling for eco-friendly paver blocks containing plastic waste
- A numerical analysis of the rotational flow of a hybrid nanofluid past a unidirectional extending surface with velocity and thermal slip conditions
- A magnetohydrodynamic flow of a water-based hybrid nanofluid past a convectively heated rotating disk surface: A passive control of nanoparticles
- Prediction of flexural strength of concrete with eggshell and glass powders: Advanced cutting-edge approach for sustainable materials
- Efficacy of sustainable cementitious materials on concrete porosity for enhancing the durability of building materials
- Phase and microstructural characterization of swat soapstone (Mg3Si4O10(OH)2)
- Effect of waste crab shell powder on matrix asphalt
- Improving effect and mechanism on service performance of asphalt binder modified by PW polymer
- Influence of pH on the synthesis of carbon spheres and the application of carbon sphere-based solid catalysts in esterification
- Experimenting the compressive performance of low-carbon alkali-activated materials using advanced modeling techniques
- Thermogravimetric (TG/DTG) characterization of cold-pressed oil blends and Saccharomyces cerevisiae-based microcapsules obtained with them
- Investigation of temperature effect on thermo-mechanical property of carbon fiber/PEEK composites
- Computational approaches for structural analysis of wood specimens
- Integrated structure–function design of 3D-printed porous polydimethylsiloxane for superhydrophobic engineering
- Exploring the impact of seashell powder and nano-silica on ultra-high-performance self-curing concrete: Insights into mechanical strength, durability, and high-temperature resilience
- Axial compression damage constitutive model and damage characteristics of fly ash/silica fume modified magnesium phosphate cement after being treated at different temperatures
- Integrating testing and modeling methods to examine the feasibility of blended waste materials for the compressive strength of rubberized mortar
- Special Issue on 3D and 4D Printing of Advanced Functional Materials - Part II
- Energy absorption of gradient triply periodic minimal surface structure manufactured by stereolithography
- Marine polymers in tissue bioprinting: Current achievements and challenges
- Quick insight into the dynamic dimensions of 4D printing in polymeric composite mechanics
- Recent advances in 4D printing of hydrogels
- Mechanically sustainable and primary recycled thermo-responsive ABS–PLA polymer composites for 4D printing applications: Fabrication and studies
- Special Issue on Materials and Technologies for Low-carbon Biomass Processing and Upgrading
- Low-carbon embodied alkali-activated materials for sustainable construction: A comparative study of single and ensemble learners
- Study on bending performance of prefabricated glulam-cross laminated timber composite floor
- Special Issue on Recent Advancement in Low-carbon Cement-based Materials - Part I
- Supplementary cementitious materials-based concrete porosity estimation using modeling approaches: A comparative study of GEP and MEP
- Modeling the strength parameters of agro waste-derived geopolymer concrete using advanced machine intelligence techniques
- Promoting the sustainable construction: A scientometric review on the utilization of waste glass in concrete
- Incorporating geranium plant waste into ultra-high performance concrete prepared with crumb rubber as fine aggregate in the presence of polypropylene fibers
- Investigation of nano-basic oxygen furnace slag and nano-banded iron formation on properties of high-performance geopolymer concrete
- Effect of incorporating ultrafine palm oil fuel ash on the resistance to corrosion of steel bars embedded in high-strength green concrete
- Influence of nanomaterials on properties and durability of ultra-high-performance geopolymer concrete
- Influence of palm oil ash and palm oil clinker on the properties of lightweight concrete

