Abstract
C17H22N2S, orthorhombic, Pbca (no. 61), a = 12.0579(7) Å, b = 11.12133(5) Å, c = 21.9741(13) Å, V = 2946.7(3) Å3, Z = 8, Rgt(F) = 0.0470, wRref(F2) = 0.1125, T = 100 K.
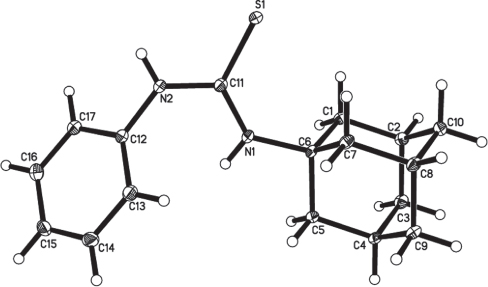
The crystal structure is shown in the figure. Tables 1–3 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | colourless, block, size 0.256×0.263×0.398 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.12 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II D8 venture, φ and ω scans |
| 2θmax: | 66.44° |
| N(hkl)measured, N(hkl)unique: | 75607, 5647 |
| N(param)refined: | 189 |
| Programs: | Bruker programs [21], SHELX [22] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(1A) | 8c | 0.1761 | 1.0092 | 0.3019 | 0.015 |
| H(1B) | 8c | 0.2275 | 0.8773 | 0.2932 | 0.015 |
| H(2A) | 8c | 0.2662 | 0.9974 | 0.2053 | 0.016 |
| H(3A) | 8c | 0.4024 | 0.8491 | 0.2310 | 0.017 |
| H(3B) | 8c | 0.4620 | 0.9633 | 0.2007 | 0.017 |
| H(4A) | 8c | 0.5603 | 0.9046 | 0.2907 | 0.015 |
| H(5A) | 8c | 0.4694 | 0.9182 | 0.3870 | 0.017 |
| H(5B) | 8c | 0.4068 | 0.8219 | 0.3450 | 0.017 |
| H(7A) | 8c | 0.3900 | 1.1240 | 0.4017 | 0.015 |
| H(7B) | 8c | 0.2762 | 1.1614 | 0.3690 | 0.015 |
| H(8A) | 8c | 0.4286 | 1.2449 | 0.3141 | 0.016 |
| H(9A) | 8c | 0.5649 | 1.0971 | 0.3389 | 0.018 |
| H(9B) | 8c | 0.5603 | 1.1148 | 0.2664 | 0.018 |
| H(10A) | 8c | 0.2709 | 1.1887 | 0.2545 | 0.018 |
| H(10B) | 8c | 0.3817 | 1.1707 | 0.2152 | 0.018 |
| H(13A) | 8c | 0.3606 | 0.8937 | 0.5161 | 0.020 |
| H(14A) | 8c | 0.4562 | 0.7413 | 0.5673 | 0.023 |
| H(15A) | 8c | 0.3605 | 0.5779 | 0.6081 | 0.024 |
| H(16A) | 8c | 0.1674 | 0.5679 | 0.5987 | 0.024 |
| H(17) | 8c | 0.0720 | 0.7140 | 0.5434 | 0.021 |
| H(1N2) | 8c | 0.078(1) | 0.921(2) | 0.5072(8) | 0.023(4) |
| H(1N1) | 8c | 0.291(1) | 0.868(2) | 0.4235(7) | 0.023(4) |
Fractional atomic coordinate and displacement parameters (Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| S(1) | 8c | 0.08030(2) | 1.07375(3) | 0.41559(2) | 0.0116(1) | 0.0181(2) | 0.0144(1) | 0.0040(1) | 0.0035(1) | 0.0036(1) |
| N(1) | 8c | 0.26034(8) | 0.9295(1) | 0.41040(5) | 0.0125(4) | 0.0155(5) | 0.0141(4) | 0.0037(4) | 0.0042(4) | 0.0052(4) |
| N(2) | 8c | 0.14348(8) | 0.9049(1) | 0.49190(5) | 0.0110(4) | 0.0201(5) | 0.0144(5) | 0.0034(4) | 0.0048(4) | 0.0052(4) |
| C(1) | 8c | 0.24619(9) | 0.9634(1) | 0.29833(5) | 0.0099(4) | 0.0130(5) | 0.0134(5) | −0.0011(4) | 0.0006(4) | −0.0021(4) |
| C(2) | 8c | 0.31196(9) | 1.0079(1) | 0.24289(5) | 0.0123(5) | 0.0163(5) | 0.0109(5) | 0.0007(4) | −0.0005(4) | −0.0012(4) |
| C(3) | 8c | 0.4199(1) | 0.9353(1) | 0.23670(5) | 0.0154(5) | 0.0146(5) | 0.0135(5) | 0.0010(4) | 0.0038(4) | −0.0028(4) |
| C(4) | 8c | 0.49018(9) | 0.9521(1) | 0.29439(5) | 0.0102(5) | 0.0141(5) | 0.0142(5) | 0.0022(4) | 0.0037(4) | 0.0005(4) |
| C(5) | 8c | 0.42416(9) | 0.9084(1) | 0.34975(5) | 0.0112(5) | 0.0159(6) | 0.0148(5) | 0.0033(4) | 0.0030(4) | 0.0036(4) |
| C(6) | 8c | 0.31567(9) | 0.9803(1) | 0.35617(5) | 0.0095(5) | 0.0133(5) | 0.0095(5) | 0.0001(4) | 0.0027(4) | 0.0009(4) |
| C(7) | 8c | 0.34492(9) | 1.1135(1) | 0.36445(5) | 0.0111(5) | 0.0141(5) | 0.0123(5) | −0.0012(4) | 0.0019(4) | −0.0034(4) |
| C(8) | 8c | 0.41048(9) | 1.1578(1) | 0.30882(5) | 0.0120(5) | 0.0109(5) | 0.0171(5) | −0.0014(4) | 0.0034(4) | −0.0011(4) |
| C(9) | 8c | 0.51822(9) | 1.0858(1) | 0.30224(6) | 0.0099(5) | 0.0170(6) | 0.0168(5) | −0.0015(4) | 0.0027(4) | −0.0006(4) |
| C(10) | 8c | 0.3401(1) | 1.1413(1) | 0.25103(6) | 0.0147(5) | 0.0142(5) | 0.0148(5) | 0.0026(4) | 0.0024(4) | 0.0032(4) |
| C(11) | 8c | 0.16826(9) | 0.9648(1) | 0.43974(5) | 0.0109(5) | 0.0142(5) | 0.0124(5) | −0.0005(4) | 0.0014(4) | 0.0004(4) |
| C(12) | 8c | 0.20689(9) | 0.8168(1) | 0.52299(5) | 0.0127(5) | 0.0164(6) | 0.0109(5) | 0.0019(4) | 0.0021(4) | 0.0011(4) |
| C(13) | 8c | 0.3215(1) | 0.8261(1) | 0.53153(5) | 0.0142(5) | 0.0211(6) | 0.0136(5) | −0.0016(4) | 0.0004(4) | 0.0007(4) |
| C(14) | 8c | 0.3780(1) | 0.7362(1) | 0.56271(6) | 0.0143(5) | 0.0280(7) | 0.0141(5) | 0.0026(5) | −0.0015(4) | −0.0022(5) |
| C(15) | 8c | 0.3214(1) | 0.6394(1) | 0.58715(6) | 0.0215(6) | 0.0247(7) | 0.0130(5) | 0.0074(5) | −0.0003(4) | 0.0026(5) |
| C(16) | 8c | 0.2069(1) | 0.6327(1) | 0.58079(6) | 0.0212(6) | 0.0204(6) | 0.0194(6) | 0.0002(5) | 0.0024(5) | 0.0064(5) |
| C(17) | 8c | 0.1500(1) | 0.7201(1) | 0.54835(6) | 0.0129(5) | 0.0215(6) | 0.0173(6) | −0.0002(4) | 0.0019(4) | 0.0043(5) |
Discussion
The adamantane nucleus represent an important structural motif in several biologically and pharmaceutically active drugs [1, 2]. Several adamantane-based drugs are currently used as efficient medications against influenza viral infections [3–5], malaria infections [6], central nervous disorders [7, 8], hyperglycaemia [9] and drug-resistant TB strain infections [10]. In addition, thiourea derivatives are recognized as potent anti-Malaria [11], anti-HIV [12], anticancer [13] and antimicrobial [14] agents. As a part of an ongoing research interest in the chemotherapeutic [15–17] and structure [18–20] of adamantane derivatives, we report herein, the crystal structural of the title compound. In the title structure the asymmetric unit of the crystal structure contains one molecule. The title molecule is a functionalized thiourea with an adamantyl and phenyl substituents attached to the two nitrogen atoms, N1 and N2. The molecules packing in the crystal structure is stabilized via one intermolecular hydrogen bond, of which S1 acts as the hydrogen bond acceptor and the NH group of N2 acts as hydrogen bond donor. The distance of the interactions between N2—H1N2⋯S1i is 2.554 (17) Å and the angle is 158.6(15) Symmetry code: (i) -x, -y+2, -z+1.
Experimental details
Cell refinement and data reduction were carried out by Bruker SAINT [21]. All hydrogen atoms were idealized and refined usind a riding model (AFIX 13, 23 or 43 option of the SHELX program [22]).
Source of material
A mixture of phenyl isothiocyanate (1.35 g, 0.01 mol) and 1-adamantylamine (1.51 mg, 0.01 mol), in ethanol (10 mL), was heated under reflux for 4 h. After cooling, the precipitated crude product was filtered, dried and crystallized from ethanol to yield 2.18 mg (76%) of the title compound (C17H22N2S) as transparent block crystals. M.P.: 444–446 K. Single crystals suitable for X-ray diffraction were obtained by slow evaporation of a solution in EtOH/CHCl3 (1:1) at room temperature. 1H NMR (DMSO-d6, 500.13 MHz): δ 1.66–1.69 (m, 6H, Adamantane-H), 2.02 (s, 3H, Adamantane-H), 2.14–2.18 (m, 6H, Adamantane-H), 6.95 (d, 2H, Ar—H, J = 7.0 Hz), 7.25–7.27 (m, 1H, Ar—H), 7.41 (s, 1H, NH), 9.34 (s, 1H, NH). 13C NMR (DMSO-d6, 125.76 MHz): δ 28.50, 34.96, 41.82, 51.22 (Adamantane-C), 123.22, 126.84, 130.32, 138.74 (Ar—C), 180.26 (C = S). ESI-MS, m/z: 285.3 (M—H)−.
Acknowledgements:
The authors would like to extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project no. RG-1436–024.
References
1. Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R.: The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 46 (2011) 1949–1963.10.1016/j.ejmech.2011.01.047Search in Google Scholar
2. Lamoureux, G.; Artavia, G.: Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 17 (2010) 2967–2978.10.2174/092986710792065027Search in Google Scholar
3. Rabinovich, S.; Baldini, J. T.; Bannister, R.: Treatment of influenza. The therapeutic efficacy of rimantadine HCl in a naturally occurring influenza A2 outbreak. Am. J. Med. Sci. 257 (1969) 328–335.10.1097/00000441-196905000-00005Search in Google Scholar
4. Davies, W. L.; Grunnert, R. R.; Haff, R. F.; McGahen, J. W.; Neumeyer, E. M.; Paulshock, M.; Watts, J. C.; Wood, T. R.; Hermann, E. C.; Hoffmann, C. E.: Antiviral activity of 1-adamantamine (amantadine). Science 144 (1964) 862–863.10.1126/science.144.3620.862Search in Google Scholar
5. Hayden, F. G.; Gwaltney, J. M. I.; Van, C. R. L.; Adams, K. F.; Giordani, B. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrob. Agents Chemother. 19 (1981) 226–233.10.1128/AAC.19.2.226Search in Google Scholar
6. Wang, X.; Dong, Y.; Wittlin, S.; Charman, S. A.; Chiu, F. C. K.; Chollet, J.; Katneni, K.; Mannila, J.; Morizzi, J.; Ryan, E.; Scheurer, C.;Steuten, J.; Tomas, J. S.; Snyder, C.; Vennerstrom, J. L.: Comparative antimalarial activities and ADME profiles of ozonides (1,2,4-trioxolanes) OZ277, OZ439, and their 1,2-dioxolane, 1,2,4-trioxane, and 1,2,4,5-tetraoxane isosteres. J. Med. Chem. 56 (2013) 2547–2555.10.1021/jm400004uSearch in Google Scholar
7. Bormann, J.: Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur. J. Pharmacol. 166 (1989) 591–592.10.1016/0014-2999(89)90385-3Search in Google Scholar
8. Abou-Gharbia, M. A.; Childers, W. E.; Fletcher, H.; McGaughey, G.; Patel, U.; Webb, M. B.; Yardley, J.; Andree, T.; Boast, C.; Kucharik, R. J.; Marquis, K.; Morris, H.; Scerni, R.; Moyer, J. A.: Synthesis and SAR of adatanserin: novel adamantly aryl- and heteroarylpiperazines with dual serotonin 5-HT1A and 5-HT2 activity as potential anxiolytic and antidepressant agents. J. Med. Chem. 42 (1999) 5077–5094.10.1021/jm9806704Search in Google Scholar PubMed
9. Villhauer, E. B.; Brinkman, J. A.; Naderi, G. B.; Burkey, B. F.; Dunning, B. E.; Prasad, K.; Mangold, B. L.; Russell, M. E.; Hughes, T. E.: 1-(3-Hydroxy-1-adamantyl)aminoacetyl-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 46 (2003) 2774–2789.10.1021/jm030091lSearch in Google Scholar PubMed
10. Jia, L.; Tomaszewski, J. E.; Hanrahan, C.; Coward, L.; Noker, P.; Gorman, G.; Nikonenko, B.; Protopopova, M.: Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Brit. J. Pharmacol. 144 (2005) 80–87.10.1038/sj.bjp.0705984Search in Google Scholar PubMed PubMed Central
11. Verlinden, B. K.; Niemand, J.; Snyman, J.; Sharma, S. K.; Beattie, R. J.; Woster, P. M.; Birkholtz, L. M.: Discovery of novel alkylated (bis)urea and (bis)thiourea polyamine analogues with potent antimalarial activities. J. Med. Chem. 54 (2011) 6624–6633.10.1021/jm200463zSearch in Google Scholar PubMed PubMed Central
12. Venkatachalam, T. K.; Mao, C.; Uckum, F. M.: Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 12 (2004) 4275–4284.10.1016/j.bmc.2004.04.050Search in Google Scholar PubMed
13. Li, H. Q.; Yan, T.; Yang, Y.; Shi, L.; Zhou, C. F.; Zhu, H. L.: Synthesis and structure-activity relationships of N-benzyl-N-(X-2-hydroxybenzyl)-N′-phenylureas and thioureas as antitumor agents. Bioorg. Med. Chem. 18 (2010) 305–313.10.1016/j.bmc.2009.10.054Search in Google Scholar PubMed
14. Vega-Pérez, J. M.; Perinán, I.; Argandona, M.; Vega-Holm, M.; Palo-Nieto, C.; Burgos-Morón, E.; López-Lázaro, M.; Vargas, C.; Nieto, J. J.; Iglesias-Guerra, F.: Isoprenyl-thiourea and urea derivatives as new farnesyl diphosphate analogues: Synthesis and in vitro antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 58 (2012) 591–612.10.1016/j.ejmech.2012.10.042Search in Google Scholar PubMed
15. El-Emam, A. A.; Al-Deeb, O. A.; Al-Omar, M. A.; Lehmann, J.: Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 12 (2004) 5107–5113.10.1016/j.bmc.2004.07.033Search in Google Scholar PubMed
16. El-Emam, A. A.; Al-Tamimi, A.-M. S.; Al-Omar, M. A.; Al-Rashood, K. A.; Habib, E. E.: Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur. J. Med. Chem. 68 (2013) 96–102.10.1016/j.ejmech.2013.07.024Search in Google Scholar PubMed
17. Kadi, A. A.; Al-Abdullah, E. S.; Shehata, I. A.; Habib, E. E.; Ibrahim, T. M.; El-Emam, A. A.: Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole Derivatives. Eur. J. Med. Chem. 45 (2010) 5006–5011.10.1016/j.ejmech.2010.08.007Search in Google Scholar PubMed
18. Al-Omary, F. A. M.; Mary, Y. S.; Panicker, C. Y.; El-Emam, A. A.; Al-Swaidan, I. A.; Al-Saadi, A. A.; Van Alsenoy, C.: Spectroscopic investigations, NBO, HOMO-LUMO, NLO analysis and molecular docking of 5-(adamantan-1-yl)-3-anilinomethyl-2,3-dihydro-1,3,4-oxadiazole-2-thione, a potential bioactive agent. J. Mol. Struct. 1096 (2015) 1–14.10.1016/j.molstruc.2015.03.049Search in Google Scholar
19. Almutairi, M. S.; Alanazi, A. M.; Al-Abdullah, E. S.; El-Emam, A. A.; Pathak, S. K.; Srivatava, R.; Prasad, O.; Sinha, L.: FT-IR and FT-Raman spectroscopic signatures, vibrational assignments, NBO, NLO analysis and molecular docking study of 2-5-(adamantan-1-yl)-4-methyl-4H-1,2,4-triazol-3-yl]sulfanyl-N,N-dimethylethanamine. Spectrochim. Acta A140 (2015) 1–14.10.1016/j.saa.2014.12.064Search in Google Scholar PubMed
20. Haress, N. G.; Al-Omary, F.; El-Emam, A. A.; Mary, Y. S.; Panicker, C. Y.; Al-Saadi, A. A.; War, J. A.; van Alsenoy, C.: Spectroscopic investigation (FT-IR and FT-Raman), vibrational assignments, HOMO-LUMO analysis and molecular docking study of 2-(adamantan-1-yl)-5-(4-nitrophenyl)-1,3,4-oxadiazole. Spectrochim. Acta A 135 (2015) 973–983.10.1016/j.saa.2014.07.077Search in Google Scholar PubMed
21. Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, 2009.Search in Google Scholar
22. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2016 Lamya H. Al-Wahaibi et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of fac-hexacarbonylbisμ2-(3-carboxy-3′-carboxylato-2,2′-bipyridine)-κ3N,N′:O-dirhenium(I) tetrahydrate, C30H22N4O18Re2
- The crystal structure of bis(4-(2,4-dimethylphenyl)piperazin-1-yl)methane, C25H36N4
- Crystal structure of bis(triphenylphosphine-κP)bis(μ2-1H,1′H-2,2′-biimidazole-κ3N,N′:N′)disilver(I) bis(tetrafluoroborate), C48H42Ag2B2F8N8P2
- The crystal structure of 1,2-bis(2-pyrazinecarboxamido)-benzene, C16H12N6O2
- Redetermination of the crystal structure of 3-bromobenzoic acid, C7H5BrO2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocuprate(II), (C20H20P)2[CuBr4]
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-2,3-dihydro-1H-perimidine, C19H17BrN2
- Crystal structure of trans-diaqua-bis(3-(pyrazin-2-yl)-5-(pyridin-4-yl)1,2,4-triazol-1-ido-κ2N,N′)-cobalt(II),C22H18CoN12O2
- Crystal structure of hexaaquamanganese(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MnN6O12S2
- Crystal structure of butyl 2-(3,5-dimethyl-1,1-dioxido-2H-1,2,6-thiadiazin-4-yl)benzoate, C16H20N2O4S
- Crystal structure of hexaaquabis(μ2-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)bis(μ3-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)tetra-manganese(II) dihydrate, C48H40Mn4N24O16
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmate(II)] bis(2-aminoisonicotinate) tetrahydrate, C38H50CdN8O10
- Crystal structure of succinic acid — 4-((pyridin-4-ylmethyl)sulfanylpyridine (1/1), C15H16N2O4S
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)bis(2-((pyridin-4-ylmethyl)sulfanyl)pyridine-κN)dicopper(II), C30H32N4O8S2Cu2
- Crystal structure of 1-((2R,3S)-2,3-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-phenoxyethan-1-one, C18H19NO3
- Crystal structure of tetramethylammonium sulfanilate, C10H18N2O3S
- Crystal structure of triethylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate, C20H25NO4
- Crystal structure of 2-(4-acetyl-2,6-dimethylphenyl)-5,6-dichloro-1H-isoindole-1,3(2H)-dione, C18H13Cl2NO3
- Crystal structure of diaqua-bis(μ3-2-methyl-6-oxidopyridinium-4-carboxylato-κ3O:O′:O′′)neodymium(III) chloride, C14H16ClN2O8Nd
- Crystal structure of 5-methoxy-4-methyl-2-(2-methylbenzyl)-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H15N3O2
- The crystal structure of 1-(4-(2-chloroethoxy)phenyl)ethanone
- Crystal structure of (E)-4-(2-(4-(diethylamino)phenyl)diazen-1-ium-1-yl)benzenesulfonate monohydrate
- Crystal structure of 2,2′-[(1E)-prop-1-ene-1,2-diyldisulfanediyl]bis(5-methyl-2,5-dihydro-1,3,4-thiadiazole, C9H10N4S4
- Crystal structure of poly [μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)cerium(III)] monohydrate (C10H13O9Ce)
- Crystal structure of tris((2-(2,2-dicyanovinyl)phenoxy)ethyl)amine, C36H27N7O3
- Crystal structure of catena[diaqua-bis(μ2-1,3-bis((1H-tetrazol-1-yl)methyl)benzene-κ2N:N′)copper(II)] dinitrate, C20H24CuN18O8
- Crystal structure of (4-(1H-imidazol-5-yl)benzoic acid-κN) (4-(1H-imidazol-5-yl)benzoato-κN)silver(I), C20H15N4O4Ag
- Crystal structure of 2-amino-3-cyano-7,7-dimethyl-5-oxo-4-(3,4,5-trifluorophenyl)-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C18H15F3N2O2
- Crystal structure of (2-(2-chlorophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol hemihydrate, C13H17ClO3 · 0.5 H2O
- Crystal structure of catena-poly[diaqua-bis(benzene-1,2,4,5-tetracarboxylato-κN)(m2-2-(1H-1,2,4-trizol-1-ylmethyl)-1H-3,1-benzimidazol-3-ium-κ2O:O′)zinc(II)] dihydrate, C30H30N10O12Zn
- Crystal structure of [2,2′-((((ethane-1,2-diylbis(oxy-κ2O,O′))bis(2,1-phenylene))bis(azanylylidene-κ2N,N′))bis(methanylylidene))diphenolato-κ2O′′,O′′′]zinc(II), C28H22N2O4Zn
- Crystal structure of (E)-1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C13H17NO3
- Crystal structure of bis(2-fluoro-4-nitrophenyl) terephthalate C20H10F2N2O8
- Crystal structure of catena-[aqua((4-carboxyphenyl)acetato-κO)(μ2-(4-carboxyphenyl)acetato-κ2O:O′)bis(4,4′-ethene-1,2-diyldipyridine-κN)manganese(II)], C42H36N4O9Mn
- Crystal structure of N′-(2-hydroxybenzylidene)-3,4-dimethyl-1H-pyrrole-2-carbohydrazide, C14H15N3O2
- Crystal structure of bis(8-ethyl-5-oxo-2-(piperazin-4-ium-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylato-κ2O,O′)copper(II) benzene-1,4-dicarboxylate dihydrate, C36H42CuN10O12
- Crystal structure of diaquabis(μ2-biphenyl-2,2′-dicarboxylato-κ2O:O′)bis(1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylato)zinc(II), C60H56N6Zn2O16F2
- Crystal structure of 2-amino-4-(4-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- The crystal structure of hexaqua(μ2-3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-1κ2O,O′;2κO′)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κO)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κ2O,O′)digadolinium(III) octahydrate, C60H76Gd2N18O32
- Crystal structure of hexacarbonyl bis(μ2-2-methoxybenzenethiolato-κ2S)pyridine(triphenylphosphane)dirhenium(I), C43H34NO8PS2Re2
- Crystal structure of 14-((1-(benzyloxycarbonyl-amino)-2-methylpropan-2-yl)sulfanyl)acetate Mutilin, C34H49NO6S
- Crystal structure of 2-methoxy-6-(((2-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)imino)methyl)phenol — ethanol (1/1), C24H25N3O3
- Crystal structure of 2-(bis(methylthio)methylene)-1-phenylbutane-1,3-dione, C13H14O2S2
- Crystal structure of (E)-2-(bis(methylthio)methylene)-1-phenyl-3-(2-phenylhydrazono)butan-1-one, C19H20N2OS2
- Crystal structure of dichlorido(2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline-κ2N,N′)zinc(II)
- Crystal structure of bis(1-methyl-1H-tetrazole-5-thiolato)mercury(II)
- Crystal structure of (E)-2-styryl-1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C24H19FeN3O2S
- Crystal structure of aquabis(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-4-ium-1-yl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)copper(II) thiophene-2,5-dicarboxylate trihydrate, [Cu(C17H18N3FO3)2(H2O)](C6H2SO4)·3(H2O)
- Crystal structure of 2-amino-4-(2,6-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16Cl2N2O2
- Crystal structure of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5·5·0·05·9·03·11]dodecane 1/3 hydrate, C6H8N12O13
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ2O,O′)(triphenylarsine-κAs)rhodium(I), C25H20AsN2O3Rh
- The crystal structure of 1-(4-(4-chlorophenoxy)-2-chlorophenyl)ethanone, C14H10Cl2O2
- Crystal structure of N,N-diethyl-2-(2-(6-(4-methoxybenzyl)-7-oxo-7H-thiazolo[3,3-b][1,2,4]triazin-3-yl)phenoxy)acetamide, C25H26N4O4S
- Crystal structure of tetraqua((E)-4,4′-(diazene-1,2-diyl)bis(5-oxo-4,5-dihydro-1,2,4-triazol-1-ide)-κ2N:O)barium(II), C4H10N8O6Ba
- Crystal structure of 2-amino-4-(3-phenoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C22H18N2O3
- Crystal structure of diethylammonium 5-((4-fluorophenyl)(6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate, C23H30FN5O6
- Crystal structure of 2-amino-4-(3,5-difluoro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12F2N2O2
- Crystal structure of tris(N-nitroso-N-oxyanilino-κ2O, O′) oxidoniobium(V), C18H15N6O7Nb
- Crystal structure of 1-(5-benzoyl-4-methyl-2-(phenylamino)thiophen-3-yl)ethan-1-one, a structure with Z′ = 6, C20H17NO2S
- Crystal structure of diethyl-3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarboxylate, C19H18O4S2
- Crystal structure of 3,5-dicarboxybenzoate — benzene-1,3,5-tricarboxylic acid (1/1), C24H22N2O12
- The crystal structure of 2-chloro-1,3-bis(2,4,6-trimethylphenyl)-4,4-dimethyl-1,3,2λ3,4-diazaphosphasiletidine
- Crystal structure of hexaquamanganese(II) bis(hexaborato-κ3O,O′,O′′)manganese(II) dihydrate, B12H28Mn2O34
- Crystal structure of 1-propyl-3-methylimidazolium pentaborate, [C7H13N2][B5O6(OH)4]
- Crystal structure of 13-(4-fluorophenyl)-11,13-dihydro-1H-benzo[h]indazolo[6,7-b] [1, 6]naphthyridin-12(6H)-one — dimethylformamide — water (1/2/1), C29H31FN6O4
- Crystal structure of 1-(2-chlorophenyl)-2-(2-nitrophenyl)ethan-1-ol, C14H12ClNO3
- Crystal structure of (Z)-2-((2-bromo-1-phenylvinyl)oxy)benzonitrile, C15H10BrNO
- Crystal structure of tetrachlorido(1E,1′E)-N,N′-((1,4-phenylenebis(propane-2,2-diyl))bis(4,1-phenylene))bis(1-(pyridin-2-yl-κN)methanimine-κN)dizinc(II), C36H34N4Zn2Cl4
- Crystal structure of 2,6-bis(3-methylpyridinyl)hexahydro-4,8-ethenopyrrolo-[3,4-f]isoindole-1,3,5,7(2H,6H)-tetrone, C24H20N4O4
- Crystal structure of trans-bis(2-methylmaleato-κ2O,O′) bis(piperazinium-κN) cobalt(II) trihydrate, C18H36CoN4O11
- Crystal structure of (E)-4-chloro-N′-(4-(diethylamino)benzylidene)benzohydrazide, C18H20ClN3O
- Crystal structure of 3,6-di(1H-imidazol-1-yl)-9H-carbazole, C18H13N5
- Crystal structure of 4-(4-pyridinyl)-1-naphthoic acid, C16H11NO2
- Crystal structure of 1,1′-diformyl-4,4′-(6H,12H-5,11-methano-dibenzo[b,f][11,5]diazocine-2,8-diyl)dibenzene, C29H22N2O2
- Crystal structure of N′-(adamantan-2-ylidene)pyridine-3-carbohydrazide, C16H19N3O
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(5-methyl-1H-pyrazole-3-carbaldehyde), C14H18N4O2
- Crystal structure of methyl 8-hydroxy-3-isopropyl-5a,8-dimethyl-2,3,4,5,5a,6,7,8,10a,10b-decahydrocyclohepta[e]indene-3a(1H)-carboxylate, C21H34O3
- Crystal structure of 6-oxo-4-propyl-2-(propylthio)-1,6-dihydropyrimidine-5-carbonitrile, C11H15N3OS
- Crystal structure of poly[diacetato(μ2-1,4-bis(1H-imidazol-1-yl)benzene-κ2N:N′)nickel(II)], C26H22N8NiO4
- Crystal structure of bis(2,4-dibromo-6-{(E)[(4-fluorobenzyl)imino]methyl}phenolato-κ2N,O) copper(II), C28H18Br4F2N2O2Cu
- Crystal structure of 1-(adamantan-1-yl)-3-phenylthiourea, C17H22N2S
- Crystal structure of 3-(6-(5-amino-1-phenyl-1H-pyrazol-3-yl)pyridin-2-yl)-1-phenyl-1H-pyrazol-5-amine – dioxan (2/1), C25H23N7O
- Crystal structure of 5-ethyl-6-[(3-methylphenyl)sulfanyl]pyrimidine-2,4(1H,3H)-dione, C13H14N2O2S
- Crystal structure of (((1E,1′E)-(cyclohexane-1,2-diylbis(azanylylidene-κ2N,N′))bis(methanylylidene))bis(2,1-phenylene))bis((2,6-diisopropylphenyl)amide-κ2N′′,N′′′)manganese(II), C44H54N4Mn
- Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4
- Crystal structure of bis(μ2-2-((3-methylphenyl)imino)methylphenolato-κ2N,O:O)hexacarbonyldimanganese(I), C34H24Mn2N2O8
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxybut-2-en-1-one C9H12N2O2
- Crystal structure of 2,2′-[pentane-1,5-diylbis(oxy)]dibenzaldehyde, C19H20O4
- Crystal structure of 2-phenyl-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,3]oxazin-4-one, C16H13NO2S
- Crystal structure of (E)-1-(2-chlorophenyl)-N-(4-chlorophenyl)methanimine, C13H9Cl2N
- Crystal structure of ethyl 2-amino-5-bromothiazole-4-carboxylate, C6H7BrN2O2S
- Crystal structure of 2-benzylisothiouronium tetraphenylborate, C32H31BN2S
- Crystal structure of poly[(μ2-biphenyl-2,2′-dicarboxylato-κ4O,O′:O′′,O′′′)(μ2-4,4′-bipyridine-κ2N:N′)copper(II)], C24H16CuN2O4
- Crystal structure of (η5-pentamethylcyclopentadienyl)titanium(III)dichloride (THF), C14H23Cl2OTi
- Crystal structure of 3-ferrocenylsulfonyl-2-(4-methoxyphenyl)-3H-imidazo[4,5-b]pyridine, C23H19FeN3O3S
- Crystal structure of 2-benzoyl-3-(4-fluorophenyl)cyclopropane-1,1-dicarbonitrile, C18H11FN2O
- Crystal structure of 1,6-ditosyl-1,6-diazecane, C22H30N2O4S2
- Crystal structure of N-phenyl-2-(pyridin-4-ylcarbonyl)hydrazinecarboxamide with Z′ = 4, C13H12N4O2
- Crystal structure of N,N-bis(diphenylphosphanyl)cyclohexylamine, C30H31NP2
- Crystal structure of 3-(4-hydroxy-3-methoxyphenyl)-N-phenylpropanamide, C16H17NO3
- Crystal structure of 6-(2-fluorophenyl)-3-phenyl-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazole, C15H9FN4S
- Crystal structure of catena-poly[aqua(dicyanoazanido-2κN-μ2-dicyanoazanido-1κN:2κN′)(μ2-2-methoxy-6-(((2-((3-methoxy-2-oxidobenzylidene)amino)ethyl)imino)methyl)phenolato-1κ2N,N′,2κ2O,O′,1κ2O′′,O′′′:2κ2O′′,O′′′)cadmium(II)copper(II)], C22H20CdCuN8O5
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of fac-hexacarbonylbisμ2-(3-carboxy-3′-carboxylato-2,2′-bipyridine)-κ3N,N′:O-dirhenium(I) tetrahydrate, C30H22N4O18Re2
- The crystal structure of bis(4-(2,4-dimethylphenyl)piperazin-1-yl)methane, C25H36N4
- Crystal structure of bis(triphenylphosphine-κP)bis(μ2-1H,1′H-2,2′-biimidazole-κ3N,N′:N′)disilver(I) bis(tetrafluoroborate), C48H42Ag2B2F8N8P2
- The crystal structure of 1,2-bis(2-pyrazinecarboxamido)-benzene, C16H12N6O2
- Redetermination of the crystal structure of 3-bromobenzoic acid, C7H5BrO2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocuprate(II), (C20H20P)2[CuBr4]
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-2,3-dihydro-1H-perimidine, C19H17BrN2
- Crystal structure of trans-diaqua-bis(3-(pyrazin-2-yl)-5-(pyridin-4-yl)1,2,4-triazol-1-ido-κ2N,N′)-cobalt(II),C22H18CoN12O2
- Crystal structure of hexaaquamanganese(II) bis((E)-4-((4-(dimethylamino)phenyl)diazenyl)benzenesulfonate), C28H40MnN6O12S2
- Crystal structure of butyl 2-(3,5-dimethyl-1,1-dioxido-2H-1,2,6-thiadiazin-4-yl)benzoate, C16H20N2O4S
- Crystal structure of hexaaquabis(μ2-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)bis(μ3-3-(6-carboxylatopyridin-2-yl)-5-(pyrazin-2-yl)-1,2,4-triazol-1-ido)tetra-manganese(II) dihydrate, C48H40Mn4N24O16
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(pyridin-4-yl)propane-κ2N:N′)cadmate(II)] bis(2-aminoisonicotinate) tetrahydrate, C38H50CdN8O10
- Crystal structure of succinic acid — 4-((pyridin-4-ylmethyl)sulfanylpyridine (1/1), C15H16N2O4S
- Crystal structure of tetrakis(μ2-acetato-κ2O:O′)bis(2-((pyridin-4-ylmethyl)sulfanyl)pyridine-κN)dicopper(II), C30H32N4O8S2Cu2
- Crystal structure of 1-((2R,3S)-2,3-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-phenoxyethan-1-one, C18H19NO3
- Crystal structure of tetramethylammonium sulfanilate, C10H18N2O3S
- Crystal structure of triethylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate, C20H25NO4
- Crystal structure of 2-(4-acetyl-2,6-dimethylphenyl)-5,6-dichloro-1H-isoindole-1,3(2H)-dione, C18H13Cl2NO3
- Crystal structure of diaqua-bis(μ3-2-methyl-6-oxidopyridinium-4-carboxylato-κ3O:O′:O′′)neodymium(III) chloride, C14H16ClN2O8Nd
- Crystal structure of 5-methoxy-4-methyl-2-(2-methylbenzyl)-2,4-dihydro-3H-1,2,4-triazol-3-one, C12H15N3O2
- The crystal structure of 1-(4-(2-chloroethoxy)phenyl)ethanone
- Crystal structure of (E)-4-(2-(4-(diethylamino)phenyl)diazen-1-ium-1-yl)benzenesulfonate monohydrate
- Crystal structure of 2,2′-[(1E)-prop-1-ene-1,2-diyldisulfanediyl]bis(5-methyl-2,5-dihydro-1,3,4-thiadiazole, C9H10N4S4
- Crystal structure of poly [μ2-acetato-κ3-O,O′:O′)diaqua(μ3-isophthalato-κ4O,O′:O′′:O′′′)cerium(III)] monohydrate (C10H13O9Ce)
- Crystal structure of tris((2-(2,2-dicyanovinyl)phenoxy)ethyl)amine, C36H27N7O3
- Crystal structure of catena[diaqua-bis(μ2-1,3-bis((1H-tetrazol-1-yl)methyl)benzene-κ2N:N′)copper(II)] dinitrate, C20H24CuN18O8
- Crystal structure of (4-(1H-imidazol-5-yl)benzoic acid-κN) (4-(1H-imidazol-5-yl)benzoato-κN)silver(I), C20H15N4O4Ag
- Crystal structure of 2-amino-3-cyano-7,7-dimethyl-5-oxo-4-(3,4,5-trifluorophenyl)-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C18H15F3N2O2
- Crystal structure of (2-(2-chlorophenyl)-5-ethyl-1,3-dioxan-5-yl)methanol hemihydrate, C13H17ClO3 · 0.5 H2O
- Crystal structure of catena-poly[diaqua-bis(benzene-1,2,4,5-tetracarboxylato-κN)(m2-2-(1H-1,2,4-trizol-1-ylmethyl)-1H-3,1-benzimidazol-3-ium-κ2O:O′)zinc(II)] dihydrate, C30H30N10O12Zn
- Crystal structure of [2,2′-((((ethane-1,2-diylbis(oxy-κ2O,O′))bis(2,1-phenylene))bis(azanylylidene-κ2N,N′))bis(methanylylidene))diphenolato-κ2O′′,O′′′]zinc(II), C28H22N2O4Zn
- Crystal structure of (E)-1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C13H17NO3
- Crystal structure of bis(2-fluoro-4-nitrophenyl) terephthalate C20H10F2N2O8
- Crystal structure of catena-[aqua((4-carboxyphenyl)acetato-κO)(μ2-(4-carboxyphenyl)acetato-κ2O:O′)bis(4,4′-ethene-1,2-diyldipyridine-κN)manganese(II)], C42H36N4O9Mn
- Crystal structure of N′-(2-hydroxybenzylidene)-3,4-dimethyl-1H-pyrrole-2-carbohydrazide, C14H15N3O2
- Crystal structure of bis(8-ethyl-5-oxo-2-(piperazin-4-ium-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylato-κ2O,O′)copper(II) benzene-1,4-dicarboxylate dihydrate, C36H42CuN10O12
- Crystal structure of diaquabis(μ2-biphenyl-2,2′-dicarboxylato-κ2O:O′)bis(1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylato)zinc(II), C60H56N6Zn2O16F2
- Crystal structure of 2-amino-4-(4-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13FN2O2
- The crystal structure of hexaqua(μ2-3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-1κ2O,O′;2κO′)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κO)(3-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)propanoato-κ2O,O′)digadolinium(III) octahydrate, C60H76Gd2N18O32
- Crystal structure of hexacarbonyl bis(μ2-2-methoxybenzenethiolato-κ2S)pyridine(triphenylphosphane)dirhenium(I), C43H34NO8PS2Re2
- Crystal structure of 14-((1-(benzyloxycarbonyl-amino)-2-methylpropan-2-yl)sulfanyl)acetate Mutilin, C34H49NO6S
- Crystal structure of 2-methoxy-6-(((2-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)imino)methyl)phenol — ethanol (1/1), C24H25N3O3
- Crystal structure of 2-(bis(methylthio)methylene)-1-phenylbutane-1,3-dione, C13H14O2S2
- Crystal structure of (E)-2-(bis(methylthio)methylene)-1-phenyl-3-(2-phenylhydrazono)butan-1-one, C19H20N2OS2
- Crystal structure of dichlorido(2-(1-methyl-1H-benzo[d]imidazol-2-yl)aniline-κ2N,N′)zinc(II)
- Crystal structure of bis(1-methyl-1H-tetrazole-5-thiolato)mercury(II)
- Crystal structure of (E)-2-styryl-1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C24H19FeN3O2S
- Crystal structure of aquabis(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-4-ium-1-yl)-1,4-dihydroquinoline-3-carboxylato-κ2O,O′)copper(II) thiophene-2,5-dicarboxylate trihydrate, [Cu(C17H18N3FO3)2(H2O)](C6H2SO4)·3(H2O)
- Crystal structure of 2-amino-4-(2,6-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C18H16Cl2N2O2
- Crystal structure of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5·5·0·05·9·03·11]dodecane 1/3 hydrate, C6H8N12O13
- Crystal structure of monocarbonyl(N-nitroso-N-oxido-phenylamine-κ2O,O′)(triphenylarsine-κAs)rhodium(I), C25H20AsN2O3Rh
- The crystal structure of 1-(4-(4-chlorophenoxy)-2-chlorophenyl)ethanone, C14H10Cl2O2
- Crystal structure of N,N-diethyl-2-(2-(6-(4-methoxybenzyl)-7-oxo-7H-thiazolo[3,3-b][1,2,4]triazin-3-yl)phenoxy)acetamide, C25H26N4O4S
- Crystal structure of tetraqua((E)-4,4′-(diazene-1,2-diyl)bis(5-oxo-4,5-dihydro-1,2,4-triazol-1-ide)-κ2N:O)barium(II), C4H10N8O6Ba
- Crystal structure of 2-amino-4-(3-phenoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C22H18N2O3
- Crystal structure of diethylammonium 5-((4-fluorophenyl)(6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate, C23H30FN5O6
- Crystal structure of 2-amino-4-(3,5-difluoro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H12F2N2O2
- Crystal structure of tris(N-nitroso-N-oxyanilino-κ2O, O′) oxidoniobium(V), C18H15N6O7Nb
- Crystal structure of 1-(5-benzoyl-4-methyl-2-(phenylamino)thiophen-3-yl)ethan-1-one, a structure with Z′ = 6, C20H17NO2S
- Crystal structure of diethyl-3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarboxylate, C19H18O4S2
- Crystal structure of 3,5-dicarboxybenzoate — benzene-1,3,5-tricarboxylic acid (1/1), C24H22N2O12
- The crystal structure of 2-chloro-1,3-bis(2,4,6-trimethylphenyl)-4,4-dimethyl-1,3,2λ3,4-diazaphosphasiletidine
- Crystal structure of hexaquamanganese(II) bis(hexaborato-κ3O,O′,O′′)manganese(II) dihydrate, B12H28Mn2O34
- Crystal structure of 1-propyl-3-methylimidazolium pentaborate, [C7H13N2][B5O6(OH)4]
- Crystal structure of 13-(4-fluorophenyl)-11,13-dihydro-1H-benzo[h]indazolo[6,7-b] [1, 6]naphthyridin-12(6H)-one — dimethylformamide — water (1/2/1), C29H31FN6O4
- Crystal structure of 1-(2-chlorophenyl)-2-(2-nitrophenyl)ethan-1-ol, C14H12ClNO3
- Crystal structure of (Z)-2-((2-bromo-1-phenylvinyl)oxy)benzonitrile, C15H10BrNO
- Crystal structure of tetrachlorido(1E,1′E)-N,N′-((1,4-phenylenebis(propane-2,2-diyl))bis(4,1-phenylene))bis(1-(pyridin-2-yl-κN)methanimine-κN)dizinc(II), C36H34N4Zn2Cl4
- Crystal structure of 2,6-bis(3-methylpyridinyl)hexahydro-4,8-ethenopyrrolo-[3,4-f]isoindole-1,3,5,7(2H,6H)-tetrone, C24H20N4O4
- Crystal structure of trans-bis(2-methylmaleato-κ2O,O′) bis(piperazinium-κN) cobalt(II) trihydrate, C18H36CoN4O11
- Crystal structure of (E)-4-chloro-N′-(4-(diethylamino)benzylidene)benzohydrazide, C18H20ClN3O
- Crystal structure of 3,6-di(1H-imidazol-1-yl)-9H-carbazole, C18H13N5
- Crystal structure of 4-(4-pyridinyl)-1-naphthoic acid, C16H11NO2
- Crystal structure of 1,1′-diformyl-4,4′-(6H,12H-5,11-methano-dibenzo[b,f][11,5]diazocine-2,8-diyl)dibenzene, C29H22N2O2
- Crystal structure of N′-(adamantan-2-ylidene)pyridine-3-carbohydrazide, C16H19N3O
- Crystal structure of 1,1′-(butane-1,4-diyl)bis(5-methyl-1H-pyrazole-3-carbaldehyde), C14H18N4O2
- Crystal structure of methyl 8-hydroxy-3-isopropyl-5a,8-dimethyl-2,3,4,5,5a,6,7,8,10a,10b-decahydrocyclohepta[e]indene-3a(1H)-carboxylate, C21H34O3
- Crystal structure of 6-oxo-4-propyl-2-(propylthio)-1,6-dihydropyrimidine-5-carbonitrile, C11H15N3OS
- Crystal structure of poly[diacetato(μ2-1,4-bis(1H-imidazol-1-yl)benzene-κ2N:N′)nickel(II)], C26H22N8NiO4
- Crystal structure of bis(2,4-dibromo-6-{(E)[(4-fluorobenzyl)imino]methyl}phenolato-κ2N,O) copper(II), C28H18Br4F2N2O2Cu
- Crystal structure of 1-(adamantan-1-yl)-3-phenylthiourea, C17H22N2S
- Crystal structure of 3-(6-(5-amino-1-phenyl-1H-pyrazol-3-yl)pyridin-2-yl)-1-phenyl-1H-pyrazol-5-amine – dioxan (2/1), C25H23N7O
- Crystal structure of 5-ethyl-6-[(3-methylphenyl)sulfanyl]pyrimidine-2,4(1H,3H)-dione, C13H14N2O2S
- Crystal structure of (((1E,1′E)-(cyclohexane-1,2-diylbis(azanylylidene-κ2N,N′))bis(methanylylidene))bis(2,1-phenylene))bis((2,6-diisopropylphenyl)amide-κ2N′′,N′′′)manganese(II), C44H54N4Mn
- Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4
- Crystal structure of bis(μ2-2-((3-methylphenyl)imino)methylphenolato-κ2N,O:O)hexacarbonyldimanganese(I), C34H24Mn2N2O8
- Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxybut-2-en-1-one C9H12N2O2
- Crystal structure of 2,2′-[pentane-1,5-diylbis(oxy)]dibenzaldehyde, C19H20O4
- Crystal structure of 2-phenyl-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,3]oxazin-4-one, C16H13NO2S
- Crystal structure of (E)-1-(2-chlorophenyl)-N-(4-chlorophenyl)methanimine, C13H9Cl2N
- Crystal structure of ethyl 2-amino-5-bromothiazole-4-carboxylate, C6H7BrN2O2S
- Crystal structure of 2-benzylisothiouronium tetraphenylborate, C32H31BN2S
- Crystal structure of poly[(μ2-biphenyl-2,2′-dicarboxylato-κ4O,O′:O′′,O′′′)(μ2-4,4′-bipyridine-κ2N:N′)copper(II)], C24H16CuN2O4
- Crystal structure of (η5-pentamethylcyclopentadienyl)titanium(III)dichloride (THF), C14H23Cl2OTi
- Crystal structure of 3-ferrocenylsulfonyl-2-(4-methoxyphenyl)-3H-imidazo[4,5-b]pyridine, C23H19FeN3O3S
- Crystal structure of 2-benzoyl-3-(4-fluorophenyl)cyclopropane-1,1-dicarbonitrile, C18H11FN2O
- Crystal structure of 1,6-ditosyl-1,6-diazecane, C22H30N2O4S2
- Crystal structure of N-phenyl-2-(pyridin-4-ylcarbonyl)hydrazinecarboxamide with Z′ = 4, C13H12N4O2
- Crystal structure of N,N-bis(diphenylphosphanyl)cyclohexylamine, C30H31NP2
- Crystal structure of 3-(4-hydroxy-3-methoxyphenyl)-N-phenylpropanamide, C16H17NO3
- Crystal structure of 6-(2-fluorophenyl)-3-phenyl-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazole, C15H9FN4S
- Crystal structure of catena-poly[aqua(dicyanoazanido-2κN-μ2-dicyanoazanido-1κN:2κN′)(μ2-2-methoxy-6-(((2-((3-methoxy-2-oxidobenzylidene)amino)ethyl)imino)methyl)phenolato-1κ2N,N′,2κ2O,O′,1κ2O′′,O′′′:2κ2O′′,O′′′)cadmium(II)copper(II)], C22H20CdCuN8O5


